Introduction
Chronic kidney disease (CKD) represents a significant global health burden, characterized by progressive loss of kidney function and associated with various cardiovascular and metabolic complications (Cockwell & Fisher, 2020). In chronic kidney disease, inadequate activation of Nrf2 is frequently associated with oxidative stress and NF-κB signaling, triggering inflammatory cell accumulation in renal tissue and promoting glomerulosclerosis, interstitial fibrosis, and progressive loss of kidney function (Ruiz et al., 2013). CKD severity is commonly assessed using low estimated glomerular filtration rate (eGFR) to reflect kidney filtration and elevated urinary albumin-to-creatinine ratio as an indicator of kidney damage (Cockwell & Fisher, 2020).
The management of CKD remains challenging, necessitating innovative therapeutic approaches to improve patient outcomes. Oxyhydrogen (HHO) nanobubble therapy has emerged as a novel therapeutic modality in various medical fields (Khan et al., 2018; Russel & Nenov, 2024). This technology combines the potential benefits of molecular hydrogen, known for its antioxidant properties (Ohta, 2012), and oxygen, known for its role in cellular respiration and metabolic support (Mohanto et al., 2025), with nanoscale bubble delivery systems. The nanoscale size of these bubbles (approximately 100 nm in diameter) enables enhanced tissue penetration and potentially improved therapeutic efficacy (Cavalli et al., 2016).
Research has shown that HHO nanobubble therapy may reduce oxidative stress and inflammation, which are critical factors in the progression of CKD (Nakayama et al., 2016), thereby offering a promising avenue for treatment. In our previous case report involving three CKD patients, we suggested that intravenous oxyhydrogen nanobubble therapy may improve renal function and overall well-being, as indicated by increases in eGFR, decreases in serum creatinine, and subjective symptom relief (Indrajani et al., 2025). Additionally, molecular hydrogen capsule therapy led to improved renal function and reduced fatigue in an elderly patient with CKD and multiple comorbidities, accompanied by favorable immunomodulatory effects (Lin et al., 2025). As studies continue to explore the mechanisms underlying its effects, HHO nanobubble therapy could pave the way for new strategies in managing chronic kidney disease and improving overall renal function.
Building upon this potential, this retrospective study analyzed the impact of intravenous (IV) HHO nanobubble administration in CKD patients by examining changes across various physiological markers, including eGFR, blood pressure, hematological indices, lipid profile, electrolytes, and metabolic parameters. This retrospective study aimed to evaluate the physiological effects of intravenous HHO nanobubble therapy by analyzing changes in renal function and related clinical parameters in CKD patients before and after treatment. This retrospective investigation represents one of the first systematic evaluations of intravenous HHO nanobubble treatment in CKD patients, with potential implications for developing new therapeutic strategies in nephrology.
Methods
This retrospective observational study was based on data from patients who received intravenous HHO nanobubble therapy as part of routine clinical care at the Reverse Aging and Homeostasis (RAHO) Club, a clinical wellness center that integrates complementary approaches to support organ function and systemic health. Each therapy session delivered approximately 500 million HHO nanobubbles suspended in 500 mL of normal saline into the circulation. Data were retrospectively extracted from electronic medical records and analyzed between January and July 2025. Of the 152 patients with CKD available in the database, 47 patients had complete baseline and follow-up data and met the inclusion criteria for this study.
Estimated glomerular filtration rate (eGFR) was designated as the primary outcome variable. eGFR was calculated using the CKD-EPI 2009 creatinine-based equation, which incorporates serum creatinine, age, sex, and race as variables (Levey et al., 2009). The specific equations for different subgroups are summarized in
Table 1. Treatment response was first categorized into Improved, Stable, or Worsened groups based on changes in eGFR (ΔeGFR) and CKD staging. ΔeGFR was defined as the difference between follow-up and baseline eGFR values. Patients were categorized as Improved if their eGFR increased or remained unchanged with stable staging, or if both eGFR values and CKD stage improved. Patients were classified as Stable if their eGFR declined but CKD stage remained the same, and as Worsened if both eGFR values and CKD stage declined. The percentage distribution of patients across CKD stages before and after therapy was illustrated using bar charts. Stage-specific changes in ΔeGFR were further analyzed using boxplots stratified by baseline CKD stage. Clinical, hematological, and biochemical parameters were systematically evaluated before and after the therapy.
Paired t-tests were applied to determine the statistical significance of baseline and follow-up differences in blood pressure, hematological indices, lipid profile, electrolytes, minerals, and metabolic parameters. Additionally, Pearson correlation analysis was performed to explore associations between ΔeGFR and various clinical, hematological, or biochemical markers. A significance threshold of p < 0.05 was applied for all analyses.
Results
This retrospective study included 47 participants. The mean age of participants was 64.81 ± 15.31 years, ranging from 20 to 91 years. Among the 47 participants, 31.9% were female and 68.1% were male. Participants received an average of 20.53 ± 12.40 intravenous (IV) treatments, with the number of treatments ranging from 2 to 43 sessions.
Changes in Renal Function (eGFR) Following IV HHO Nanobubble Therapy
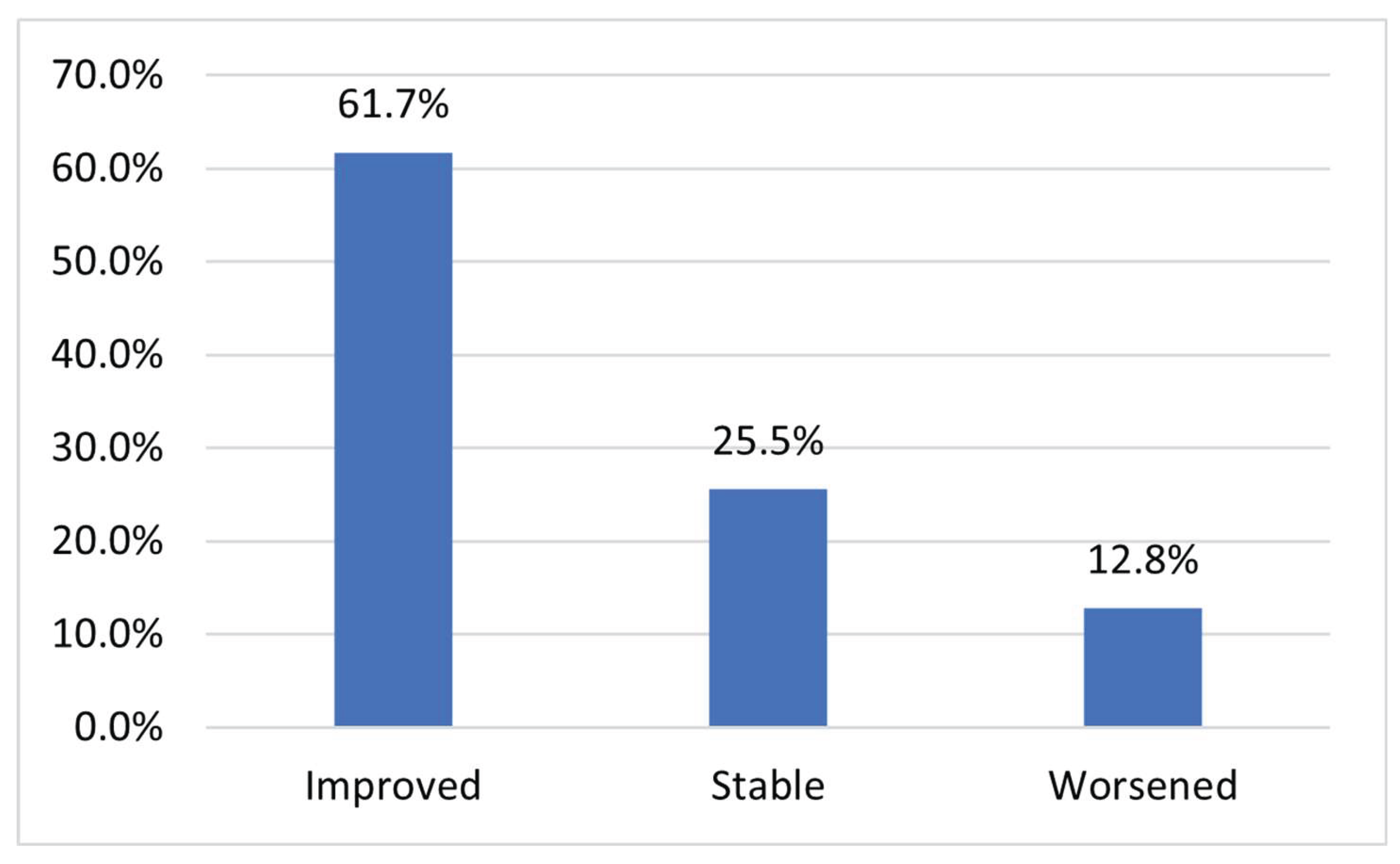
Patients were classified into three categories based on changes in their estimated glomerular filtration rate (ΔeGFR) values and CKD stages before and after receiving nanobubble therapy. Patients were categorized as Improved if their eGFR values increased or remained unchanged with stable CKD stage, or if both eGFR values and CKD stage improved. Patients were categorized as Stable if their eGFR values declined but their CKD stage remained the same, indicating no clinically significant deterioration. Finally, patients were classified as Worsened if both their eGFR values and CKD stage declined, reflecting progression of kidney dysfunction. Based on this classification, out of the total patient population, 29 patients (61.7%) demonstrated improvement, 12 patients (25.5%) remained stable, and 6 patients (12.8%) exhibited worsening of their kidney function following infusion therapy (
Figure 1). These findings suggest that the majority of patients experienced either improvement or stabilization in renal function, with a smaller proportion showing disease progression.
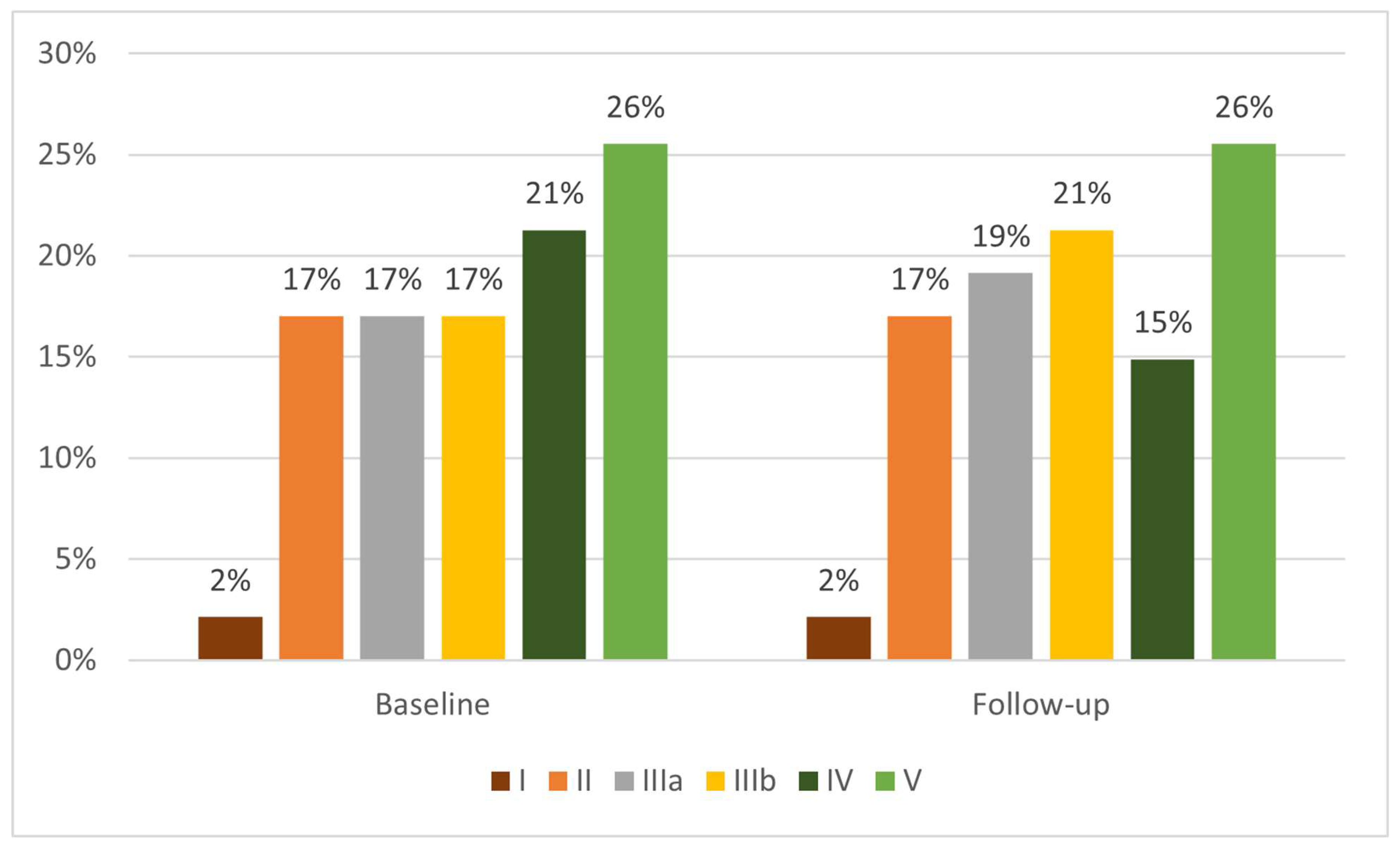
The bar chart illustrates the distribution of patients across chronic kidney disease (CKD) stages before and after intravenous HHO nanobubble therapy (
Figure 2). At baseline, the largest proportion of patients was classified in Stage V (26%), followed by Stage IV (21%), and equal proportions in Stages II, IIIa, and IIIb (each 17%), while only 2% were in Stage I. After treatment, the proportion of Stage V patients remained unchanged at 26%, whereas the proportion of Stage IV patients declined from 21% to 15%. In contrast, Stage IIIb patients increased from 17% to 21%, while Stage IIIa patients showed a modest increase from 17% to 19%. Stages II and I remained stable at 17% and 2%, respectively (
Figure 2). These shifts suggest that IV HHO nanobubble therapy may have contributed to stage reclassification in some CKD patients, particularly by reducing the proportion of individuals with advanced disease (Stage IV) and increasing those in the intermediate stage (IIIb), indicating a potential stabilization or modest improvement in renal function across the cohort.
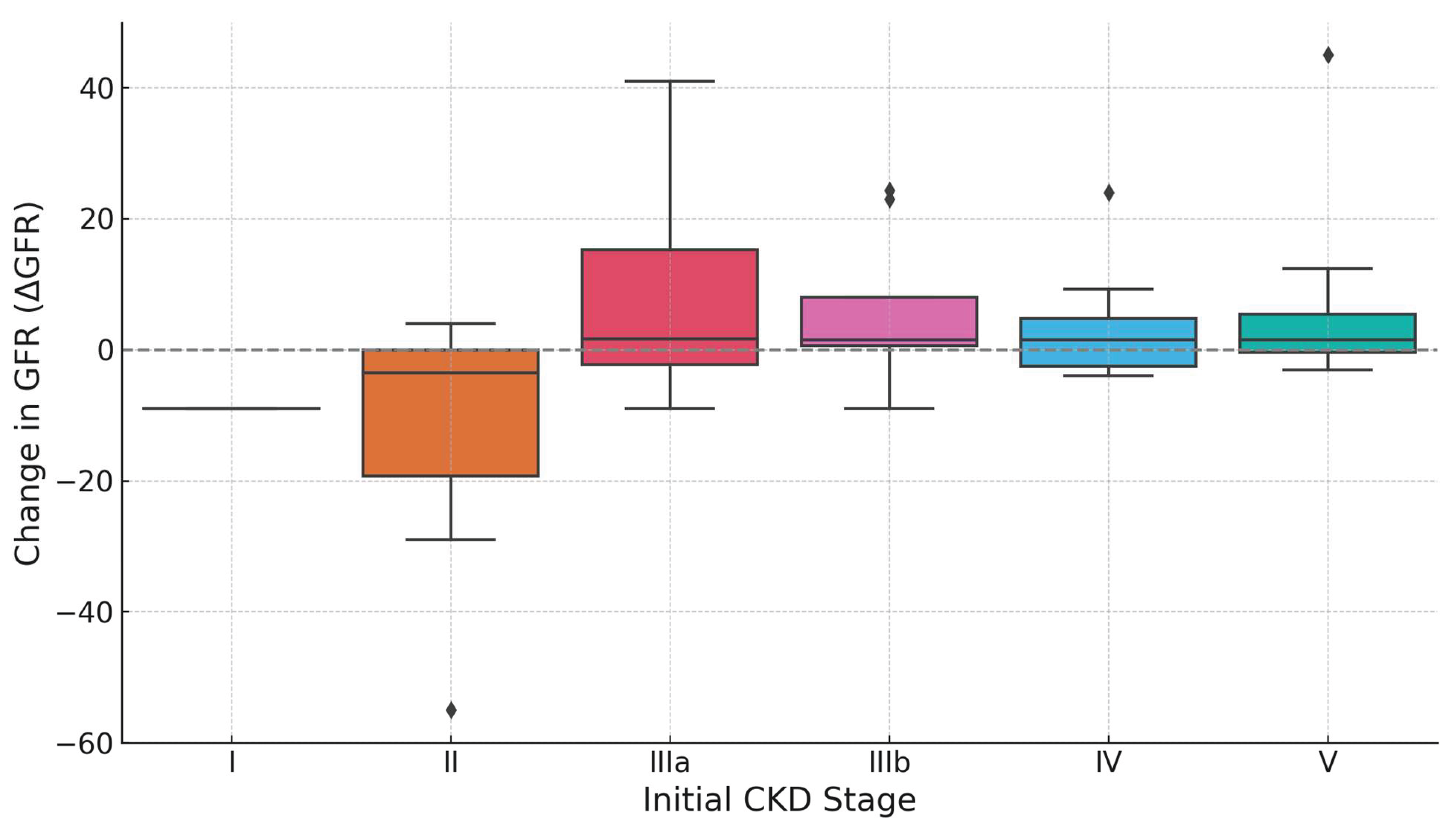
The boxplot illustrates the distribution of changes in estimated glomerular filtration rate (ΔeGFR) following IV treatment, stratified by initial CKD stage. Each box represents the interquartile range (IQR) of ΔeGFR values, with the horizontal line indicating the median change and whiskers extending to reflect the data spread outside the IQR (
Figure 3). Outliers are shown as individual points. Patients in Stage IIIa exhibited the greatest median improvement, with a broad IQR suggesting variable individual responses. Stage IIIb also showed a positive median ΔeGFR, although with less dispersion. Stages IV and V presented modest but positive median improvements, accompanied by relatively narrow IQRs, indicating more consistent responses within these groups. In contrast, patients in Stage II experienced a negative median ΔeGFR and wide variability, including one extreme outlier with substantial decline. Only one patient was recorded in Stage I, limiting interpretability (
Figure 3). Overall, the plot suggests that IV treatment yields the most substantial and variable benefits in moderate CKD (Stage III), while responses in advanced stages are smaller but more uniform.
Statistical Analysis and Interpretation of Biomarkers in CKD: Insights from T-Tests, P-Values, and Correlations with ΔeGFR
A total of 26 clinical and laboratory parameters were analyzed before and after intervention to evaluate potential changes and their correlation with ΔeGFR. Systolic and diastolic blood pressure exhibited statistically significant reductions following the intervention (SBP: 143.65 ± 20.09 vs. 130.54 ± 19.69 mmHg, p < 0.001; DBP: 82.04 ± 12.30 vs. 77.61 ± 11.39 mmHg, p = 0.046). However, these changes were not significantly correlated with ΔeGFR (SBP: r = -0.12, p = 0.43; DBP: r = -0.15, p = 0.33) (
Table 2).
In hematological parameters, significant changes were observed in granulocyte percentage (66.37 ± 9.23% to 61.25 ± 12.67%, p = 0.04), lymphocytes (24.27 ± 8.92% to 28.88 ± 10.03%, p = 0.01), and red cell distribution width (RDW) (13.67 ± 1.18% to 14.02 ± 1.44%, p = 0.04). Other hematological indices, including erythrocyte count, hemoglobin, hematocrit, leukocytes, MCV, MCH, MCHC, and ESR, did not show statistically significant changes (
Table 2). Lymphocyte percentage exhibited a moderate positive correlation with ΔeGFR (r = 0.26, p = 0.10), although not statistically significant.
Within the lipid profile, a significant reduction in total cholesterol was observed (183.75 ± 47.58 mg/dL to 166.62 ± 42.07 mg/dL, p = 0.04), while LDL, HDL, and triglyceride levels remained statistically unchanged (
Table 2). Notably, HDL was positively correlated with ΔeGFR (r = 0.54, p = 0.0018), suggesting a potential association between HDL improvement and renal function enhancement. Electrolyte and mineral parameters, including potassium, sodium, chloride, ionized calcium, and total calcium, showed no significant differences post-intervention, nor did they exhibit notable correlations with ΔeGFR. Similarly, metabolic markers such as albumin and uric acid remained unchanged and were not significantly correlated with changes in GFR (
Table 2).
Discussion
This study evaluated the effects of intravenous HHO nanobubble therapy in patients with CKD, based on a range of clinical parameters related to kidney health. Among the cohort, 61.7% of patients demonstrated improvement in eGFR following repeated HHO nanobubble therapy, with only a small minority showing progression of disease. The percentage distribution across CKD stages indicated a modest reduction in the proportion of patients in Stage IV with a corresponding increase in Stage IIIb after therapy, suggesting a potential shift toward less advanced disease stages. Stratified boxplot analysis further supported this observation, highlighting that patients with CKD Stage III (both IIIa and IIIb) exhibited the most pronounced and variable improvements in ΔeGFR, while those in more advanced stages showed smaller but consistent responses. In Sharma et al. (2010), it was reported that in patients with CKD stage III, 27% showed no decline in eGFR over 10 years (as cited in Eriksen & Ingebretsen, 2006), and two other studies reported that ≥80% did not experience worsening of CKD stage within 5 years of follow-up (as cited in Khatami et al., 2007; Orlando et al., 2007). The results suggest that patients in moderate stages of CKD possess a greater potential for renal functional recovery in response to therapies targeting oxidative and metabolic stress.
Molecular hydrogen exerts its renoprotective effects by activating the Keap1/Nrf2 and PTEN–AKT–mTOR pathways, which enhance antioxidant defenses and reduce fibrosis in kidney tissues (Cheng et al., 2023). It also inhibits the NF-κB signaling pathway, thereby suppressing inflammatory cytokines such as IL-6 and TNF-α (Ohta, 2012; Cheng et al., 2023). In addition, hydrogen promotes p53/DRAM-mediated autophagy and preserves the expression of Klotho, a key anti-aging and anti-fibrotic protein (Cheng et al., 2023). The nanoscale size of the HHO bubbles may allow for enhanced delivery and distribution within the renal tissue. The nanobubble formulation could potentially enhance the bioavailability and efficacy of the dissolved gases (Tekile et al., 2016). Nanoparticles have shown promise in targeting specific kidney regions and improving drug delivery in CKD (Williams et al., 2016).
The statistically significant reductions in both systolic and diastolic blood pressure post-intervention (SBP: p < 0.001; DBP: p = 0.046) underscore one of the most clinically relevant outcomes of this therapy. Elevated blood pressure is both a cause and consequence of CKD progression (Burnier & Damianaki, 2023), and effective control is essential to preserving renal function. Previous studies using hydrogen-enriched dialysate solution have similarly reported significant reductions in systolic blood pressure in hemodialysis patients (Nakayama et al., 2016). Moreover, recent evidence suggests that hydrogen–oxygen inhalation may exert its antihypertensive effects in part by modulating the renin–angiotensin–aldosterone system (RAAS) and the levels of stress hormones, such as cortisol and aldosterone (Liu et al., 2022). A recent study demonstrated that inhibition of chemokine receptor type 2 reduces macrophage infiltration into the vasculature and contributes to blood pressure reduction (Nakayama et al., 2018).
Granulocytes significantly decreased (p = 0.04), while lymphocytes increased (p = 0.01), indicating a shift in white cell dynamics potentially reflective of reduced systemic inflammation (Yuan et al., 2019). The neutrophil-to-lymphocyte ratio (NLR) is an inflammatory marker reflecting the ratio between neutrophil-driven inflammation and lymphocyte-mediated immunity. As neutrophils are the most abundant type of granulocytes, an elevated NLR—indicating increased granulocytic activity and reduced lymphocytes—has been linked to a worse prognosis for CKD (Yuan et al., 2019). HHO therapy may contribute to regulating the chronic low-grade inflammation characteristic of CKD by decreasing granulocyte predominance and promoting a higher proportion of lymphocytes.
Given the small but statistically significant increase in red cell distribution width (RDW) following therapy (p = 0.04), this change may not necessarily indicate ongoing pathology. Instead, it could reflect underlying recovery processes, highlighting a potential shift in the physiological state post-intervention. An increase in red cell distribution width (RDW) may indicate the recovery of previously suppressed erythropoiesis associated with inflammatory conditions (Eldem et al., 2023). The significant increase in red cell distribution width is noteworthy, and the relatively low variability in both baseline and follow-up measurements suggests that this change was consistently observed across patients. RDW is distinct in that its elevation may signal recovery rather than disease severity (Eldem et al., 2023).
The reduction in total cholesterol (p = 0.04) is noteworthy. Based on a previous study conducted by Hernowo et al. (2024), in rats subjected to a high-fat diet with baseline cholesterol levels of 207.75 ± 2.89 mg/dL, treatment with hydrogen nanobubbles reduced total cholesterol dramatically to 99 ± 0 mg/dL, demonstrating its strong lipid-lowering effect. HDL levels demonstrated a strong positive correlation with ΔeGFR (r = 0.54, p = 0.0018), although the mean change in HDL levels between baseline and follow-up was not statistically significant. Supporting this interpretation, Yadegar et al. (2023) demonstrated that after adjusting for key risk factors, HDL-C showed a significant positive association with eGFR, suggesting that higher HDL-C levels are linked to better kidney function. As reported by Todorovic et al. (2023), hydrogen-rich water (HRW) supplementation has been shown to significantly reduce total cholesterol, triglyceride, and LDL levels, with small to moderate effect sizes, while HDL levels remained largely unchanged. In CKD, especially in hemodialysis patients, oxidized LDL (oxLDL) accumulates and contributes to atherosclerosis, while HDL function is significantly impaired due to both decreased levels and structural modifications of ApoA1 and ApoA2 (Florens et al., 2016; Vaziri, 2013). The proposed mechanism of HRW involves activation of PGC-1α, which enhances mitochondrial biogenesis and stimulates FGF-21 production, thereby improving insulin sensitivity and lipid metabolism; HRW also upregulates UCP1 expression in brown adipose tissue (BAT), promoting energy expenditure (Todorovic et al., 2023). Additionally, HRW may stimulate the release of ghrelin, a hormone involved in energy regulation and lipid metabolism (Todorovic et al., 2023).
Beyond the observed clinical improvements, this study positions HHO nanobubble therapy as a potentially valuable adjunct in the broader landscape of CKD management—one that complements existing strategies by actively targeting systemic oxidative stress and microinflammatory processes. The unique integration of gas biology and nanoscale delivery offers a novel, non-pharmacological platform that aligns with emerging interests in redox modulation and regenerative approaches for chronic disease. These findings also contribute to a growing body of evidence supporting the therapeutic relevance of molecular hydrogen, not only in renal protection but also in cardiovascular and metabolic regulation. To fully realize its clinical potential, future research should bridge molecular mechanisms and patient-centered outcomes through robust translational and controlled studies—thereby establishing a clearer role for HHO nanobubble therapy in modern nephrology.
Conclusion
This retrospective observational study demonstrates that intravenous HHO nanobubble treatment may hold potential as a novel therapeutic approach for CKD. Notably, 61.7% of participants experienced improvements in eGFR, particularly those in Stage III, indicating a higher potential for renal functional recovery in moderate CKD. Statistically significant reductions in systolic and diastolic blood pressure, along with favorable changes in lipid profile and hematologic-inflammatory markers, were observed based on baseline and follow-up comparisons. These findings suggest a potential modulatory effect of HHO therapy on cardiovascular and metabolic stressors associated with CKD progression. While the outcomes are encouraging, the retrospective design, limited sample size, short follow-up period, and absence of a control group highlight the need for cautious interpretation. Further prospective and controlled studies are warranted to validate efficacy, explore underlying mechanisms, and optimize treatment protocols.
Ethical Clearance
This study was conducted retrospectively using data obtained from routine intravenous HHO nanobubble therapy administered at the RAHO Club. Prior to undergoing infusion therapy, all patients had received detailed information regarding the procedure and provided written informed consent as part of routine clinical care. The present analysis involved only secondary use of de-identified data collected during routine treatment. The study was performed in accordance with the ethical principles of the Declaration of Helsinki.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cockwell, P., & Fisher, L.-A. (2020). The global burden of chronic kidney disease. The Lancet, 395, 662-663. [CrossRef]
- Ruiz, S., Pergola, P. E., Zager, R. A., & Vaziri, N. D. (2013). Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney International, 83(6), 1029–1041. [CrossRef]
- Khan, M. S., Hwang, J., Seo, Y., Shin, K. R., Lee, K., Park, C., Choi, Y., Hong, J. W., & Choi, J. (2018). Engineering oxygen nanobubbles for the effective reversal of hypoxia. Artificial Cells, Nanomedicine, and Biotechnology, 46(sup3), 318-327. [CrossRef]
- Russell, G., & Nenov, A. (2024). Oxyhydrogen gas: A promising therapeutic approach for lung, breast and colorectal cancer. Oxygen, 4, 338–350. [CrossRef]
- Ohta, S. (2012). Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochimica et Biophysica Acta, 1820, 586-594. [CrossRef]
- Mohanto, N., Mondal, H., Park, Y.-J., & Jee, J.-P. (2025). Therapeutic delivery of oxygen using artificial oxygen carriers demonstrates the possibility of treating a wide range of diseases. Journal of Nanobiotechnology 23(25), 1-49. [CrossRef]
- Cavalli, R., Soster, M., & Argenziano, M. (2016). Nanobubbles: a promising efficient tool for therapeutic delivery. Therapeutic Delivery. [CrossRef]
- Nakayama, M., Kabayama, S., & Ito, S. (2016). The hydrogen molecule as antioxidant therapy: Clinical application in hemodialysis and perspectives. Renal Replacement Therapy, 2(23), 1-10. [CrossRef]
- Indrajani, O., Hernowo, A. T., Ekasari, C. P., Yudhanata, R., & Sumitro, S. B. (2025). The effect of intravenous oxyhydrogen nanobubble on chronic kidney disease: Case series. Clinical Therapeutics. Advance online publication. [CrossRef]
- Lin, Y.-T., Lu, J.-W., Ho, Y.-J., Lui, S.-W., Hsieh, T.-Y., Wang, K.-Y., & Liu, F.-C. (2025). Molecular hydrogen as a potential adjunctive therapy to improve renal function and reduce fatigue in an elderly patient with chronic comorbidities: A case report. In Vivo, 39(1), 572–576. [CrossRef]
- Sharma, P., McCullough, K., Scotland, G., McNamee, P., Prescott, G., MacLeod, A., Fluck, N., Smith, W. C., & Black, C. (2010). Does stage-3 chronic kidney disease matter? British Journal of General Practice, 60(576), e266–e276. [CrossRef]
- Eriksen, B. O., & Ingebretsen, O. C. (2006). The progression of chronic kidney disease: A 10-year population-based study of the effects of gender and age. Kidney International, 69(2), 375–382. [CrossRef]
- Khatami, Z., Handley, G., Narayanan, K., & Weaver, J. U. (2007). Applicability of estimated glomerular filtration rate in stratifying chronic kidney disease. Scandinavian Journal of Clinical and Laboratory Investigation, 67(3), 297–305. [CrossRef]
- Orlando, L. A., Owen, W. F., & Matchar, D. B. (2007). Relationship between nephrologist care and progression of chronic kidney disease. North Carolina Medical Journal, 68(1), 9–16. [CrossRef]
- Cheng, J., Shi, M., Sun, X., & Lu, H. (2023). Therapeutic effect of hydrogen and its mechanisms in kidney disease treatment. Medical Gas Research, 14(2), 48–53. [CrossRef]
- Tekile, A., Kim, I., & Lee, J. H. (2016). Extent and persistence of dissolved oxygen enhancement using nanobubbles. Environmental Engineering Research, 21(4), 427-435. [CrossRef]
- Williams, R. M., Jaimes, E. A., & Heller, D. A. (2016). Nanomedicines for kidney diseases. Kidney International, 90, 740–745. [CrossRef]
- Burnier, M., & Damianaki, A. (2023). Hypertension as cardiovascular risk factor in chronic kidney disease. Circulation Research, 132, 1050–1063. [CrossRef]
- Liu, B., Jiang, X., Xie, Y., Jia, X., Zhang, J., Xue, Y., & Qin, S. (2022). The effect of a low dose hydrogen-oxygen mixture inhalation in midlife/older adults with hypertension: A randomized, placebo-controlled trial. Frontiers in Pharmacology, 13(1025487), 1-10. [CrossRef]
- Nakayama, M., Itami, N., Suzuki, H., Hamada, H., Yamamoto, R., Tsunoda, K., Osaka, N., Nakano, H., Maruyama, Y., Kabayama, S., Nakazawa, R., Miyazaki, M., & Ito, S. (2018). Novel haemodialysis (HD) treatment employing molecular hydrogen (H2)-enriched dialysis solution improves prognosis of chronic dialysis patients: A prospective observational study. Scientific Reports, 8(254), 1-10. [CrossRef]
- Yuan, Q., Wang, J., Peng, Z., Zhou, Q., Xiao, X., Xie, Y., Wang, W., Huang, L., Tang, W., Sun, D., Zhang, L., Wang, F., Zhao, M. H., Tao, L., He, K., Xu, H., & C-STRIDE study group. (2019). Neutrophil-to-lymphocyte ratio and incident end-stage renal disease in Chinese patients with chronic kidney disease: Results from the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). Journal of Translational Medicine, 17(86), 1-8. [CrossRef]
- Eldem, I., Almekdash, M. H., Almadani, O., Levent, F., & Al-Rahawan, M. M. (2023). Red blood cell distribution width as a potential inflammatory marker in pediatric osteomyelitis. Proceedings (Baylor University Medical Center), 36(4), 443–447. [CrossRef]
- Hernowo, A. T., Widyarti, S., & Aristyani, S. (2024). Evaluating the safety and therapeutic efficacy of intravenous hydrogen nanobubble infusions in a hypercholesterolemic rat model. Berkala Penelitian Hayati, 30(1), 40-47. [CrossRef]
- Yadegar, A., Mohammadi, F., Rabizadeh, S., Ayati, A., Seyedi, S. A., Nabipoorashrafi, S. A., Esteghamati, A., & Nakhjavani, M. (2023). Correlation between different levels and patterns of dyslipidemia and glomerular filtration rate in patients with type 2 diabetes: A cross-sectional survey of a regional cohort. Journal of Clinical Laboratory Analysis, 37(e24954), 1-10. [CrossRef]
- Todorovic, N., Fernández-Landa, J., Santibañez, A., Kura, B., Stajer, V., Korovljev, D., & Ostojic, S. M. (2023). The effects of hydrogen-rich water on blood lipid profiles in clinical populations: A systematic review and meta-analysis. Pharmaceuticals, 16(142), 1-14. [CrossRef]
- Florens, N., Calzada, C., Lyasko, E., Juillard, L., & Soulage, C. O. (2016). Modified lipids and lipoproteins in chronic kidney disease: A new class of uremic toxins. Toxins, 8(376), 1–27. [CrossRef]
- Vaziri, N. D. (2013). Role of dyslipidemia in impairment of energy metabolism, oxidative stress, inflammation and cardiovascular disease in chronic kidney disease. Clinical and Experimental Nephrology. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).