Submitted:
19 June 2023
Posted:
20 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and method
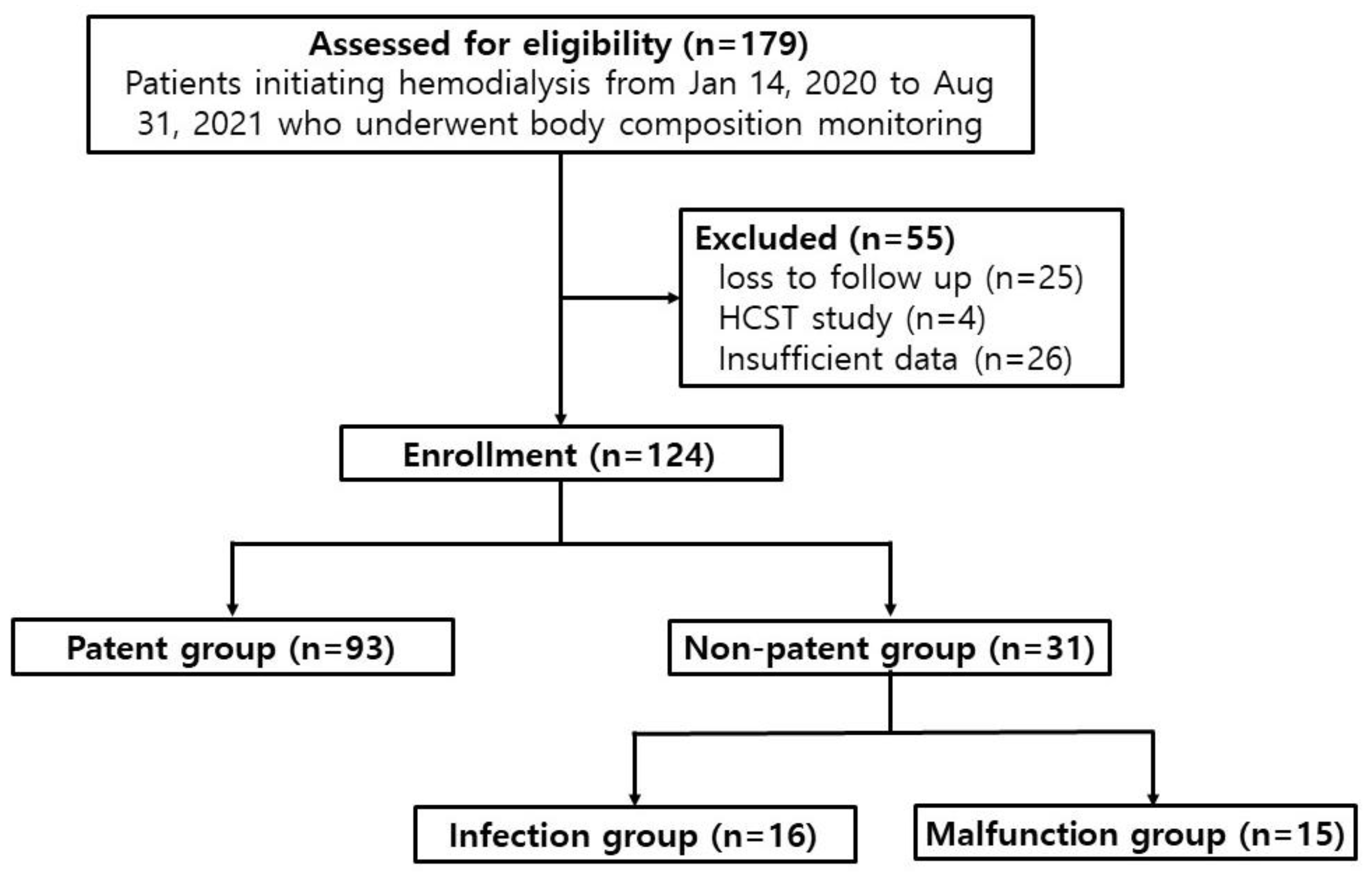
2.1. Study design and participants
2.2. Definitions
2.2.1. Infection of permanent catheter
- -
- redness, tenderness, warmth, or pus was observed at the catheter exit site, and infection was simultaneously suspected from laboratory tests.
- -
- no infection was evident before catheter insertion, but symptoms of infection such as fever began within five days after insertion, infection was confirmed on blood culture, and no prominent infection in other organs was evident.
2.2.2. Malfunction of permanent catheter
- -
- an extracorporeal blood flow sufficient to perform HD (<300 mL/min) could not be attained or maintained.
- -
- catheter dysfunction unrelated to catheter tip malposition or mechanical kinking of the catheter occurred.
2.3. Statistical analysis
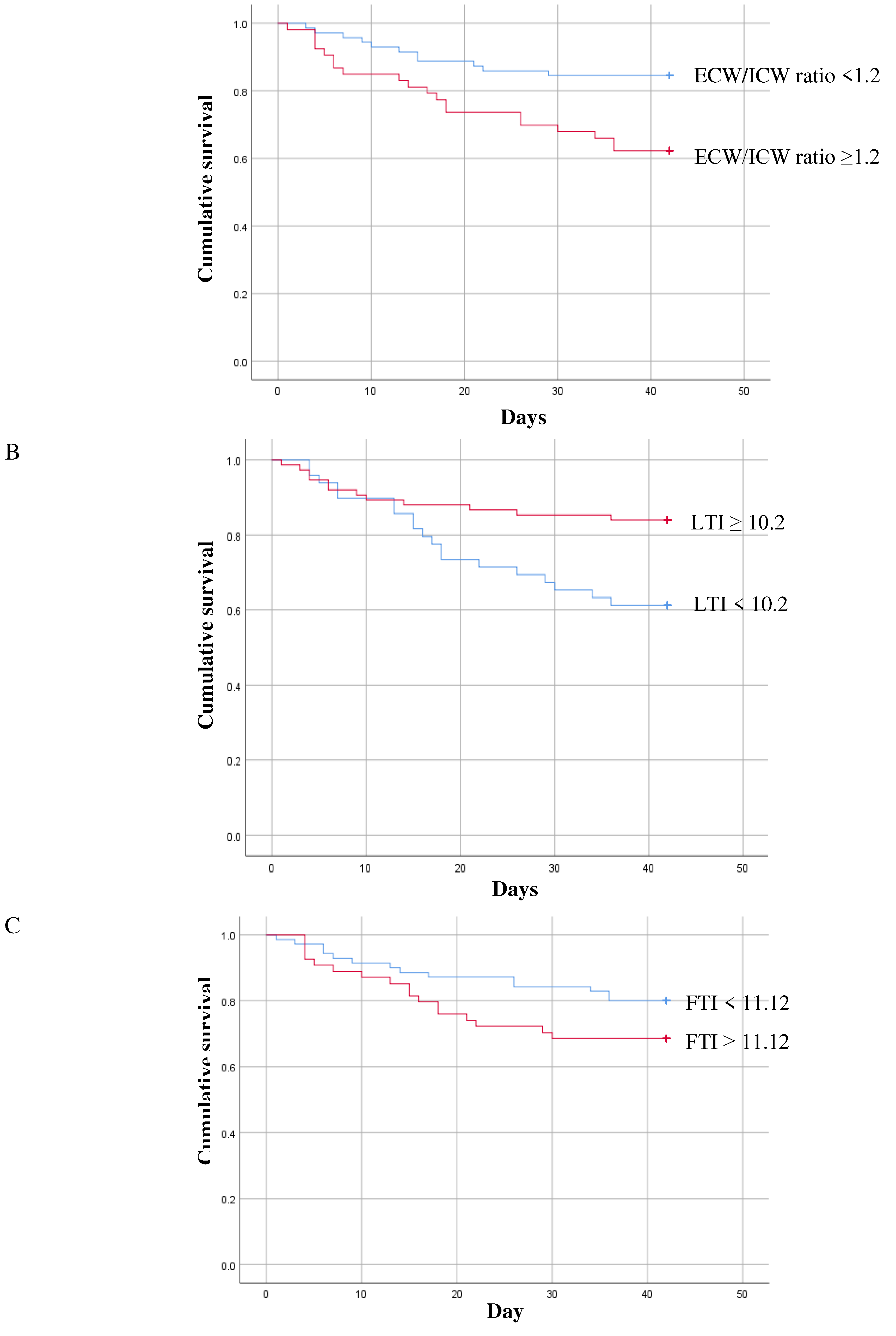
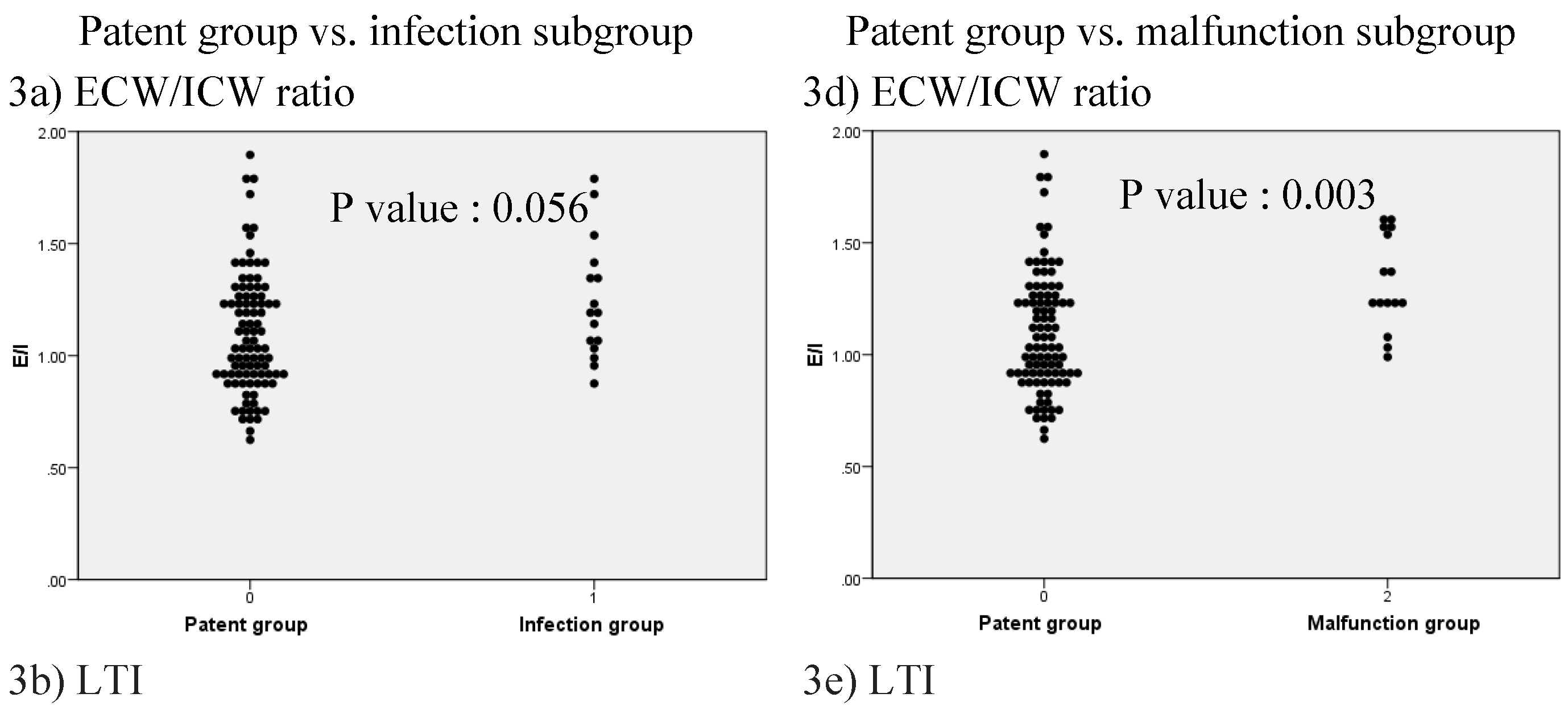
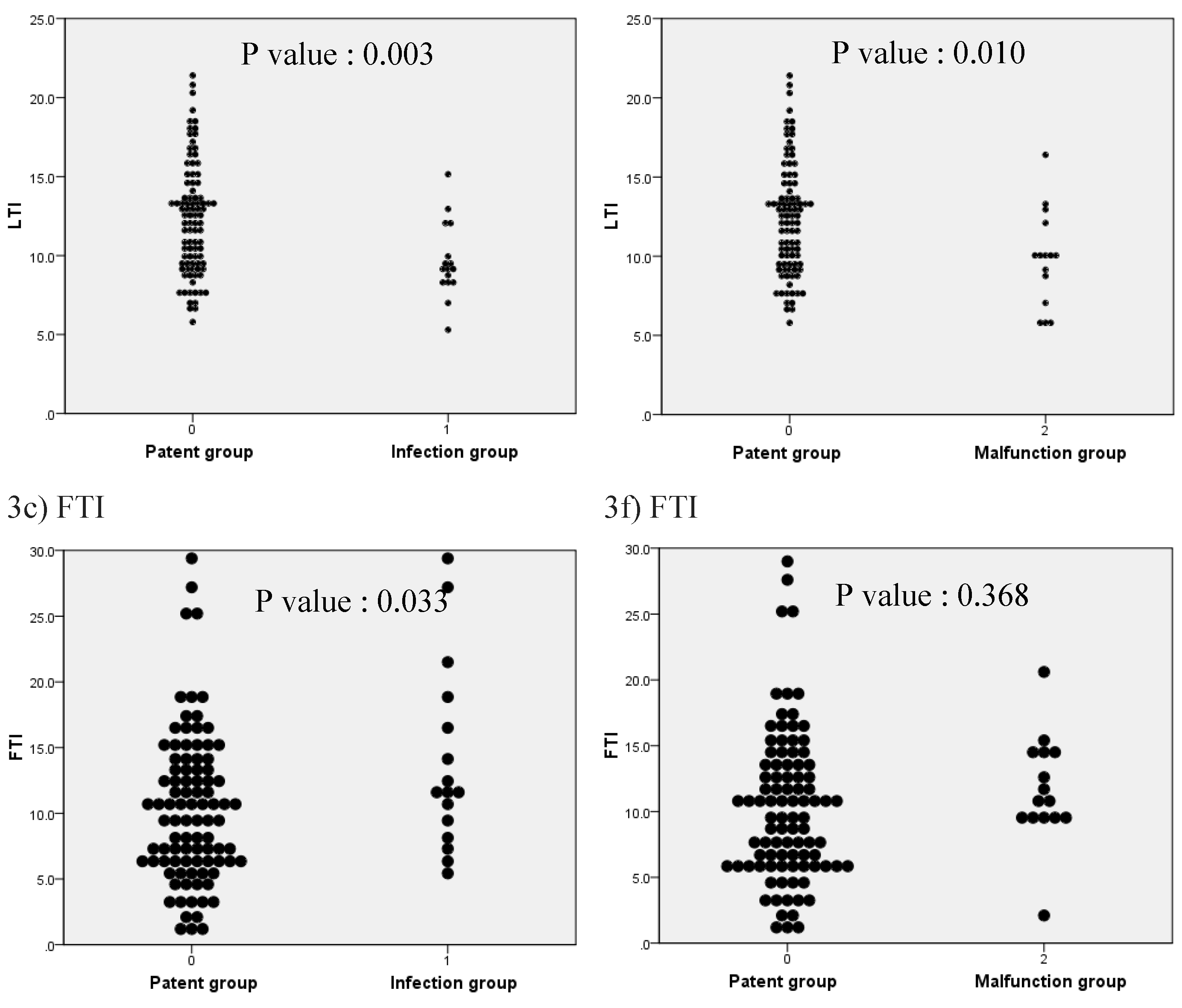
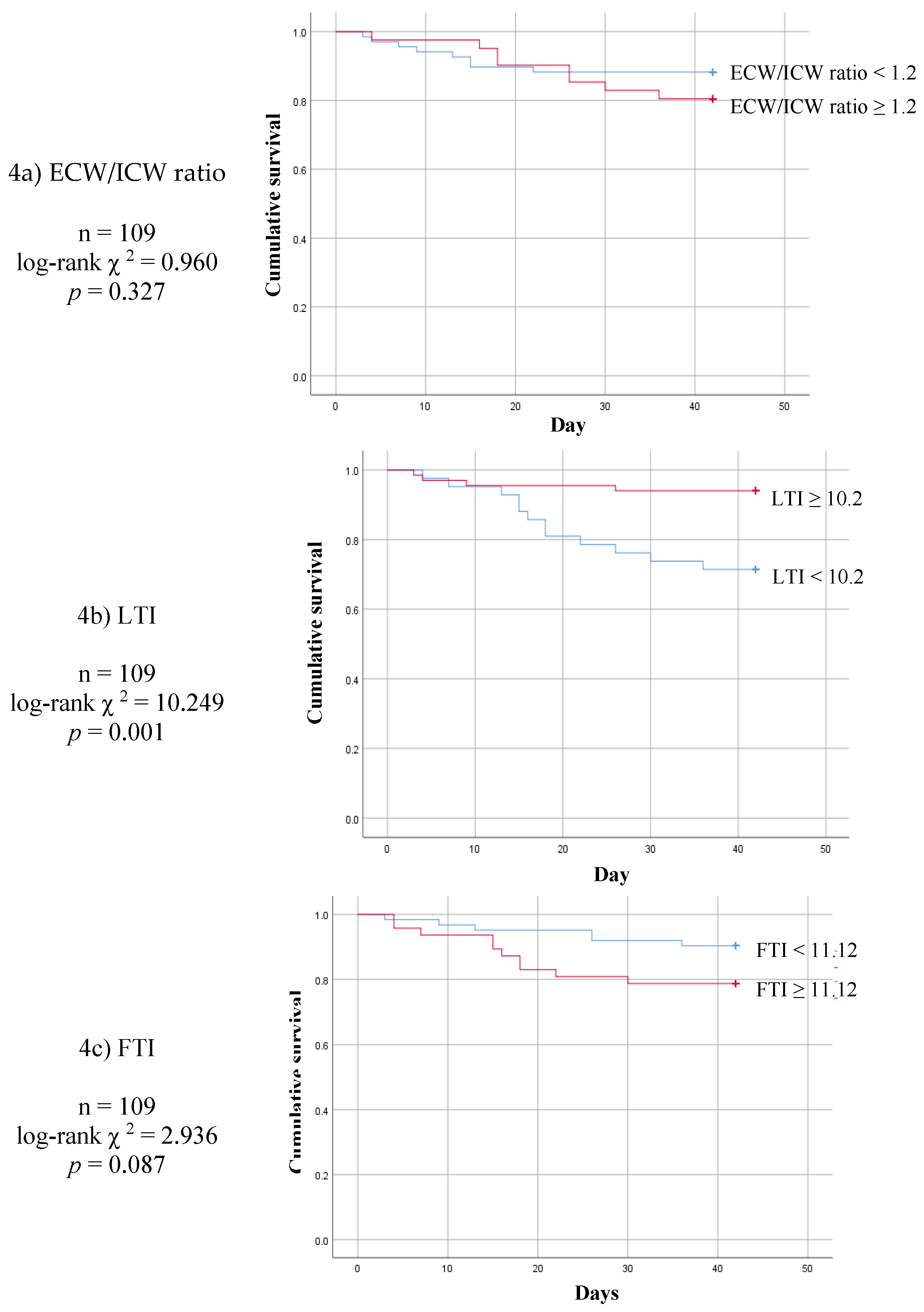
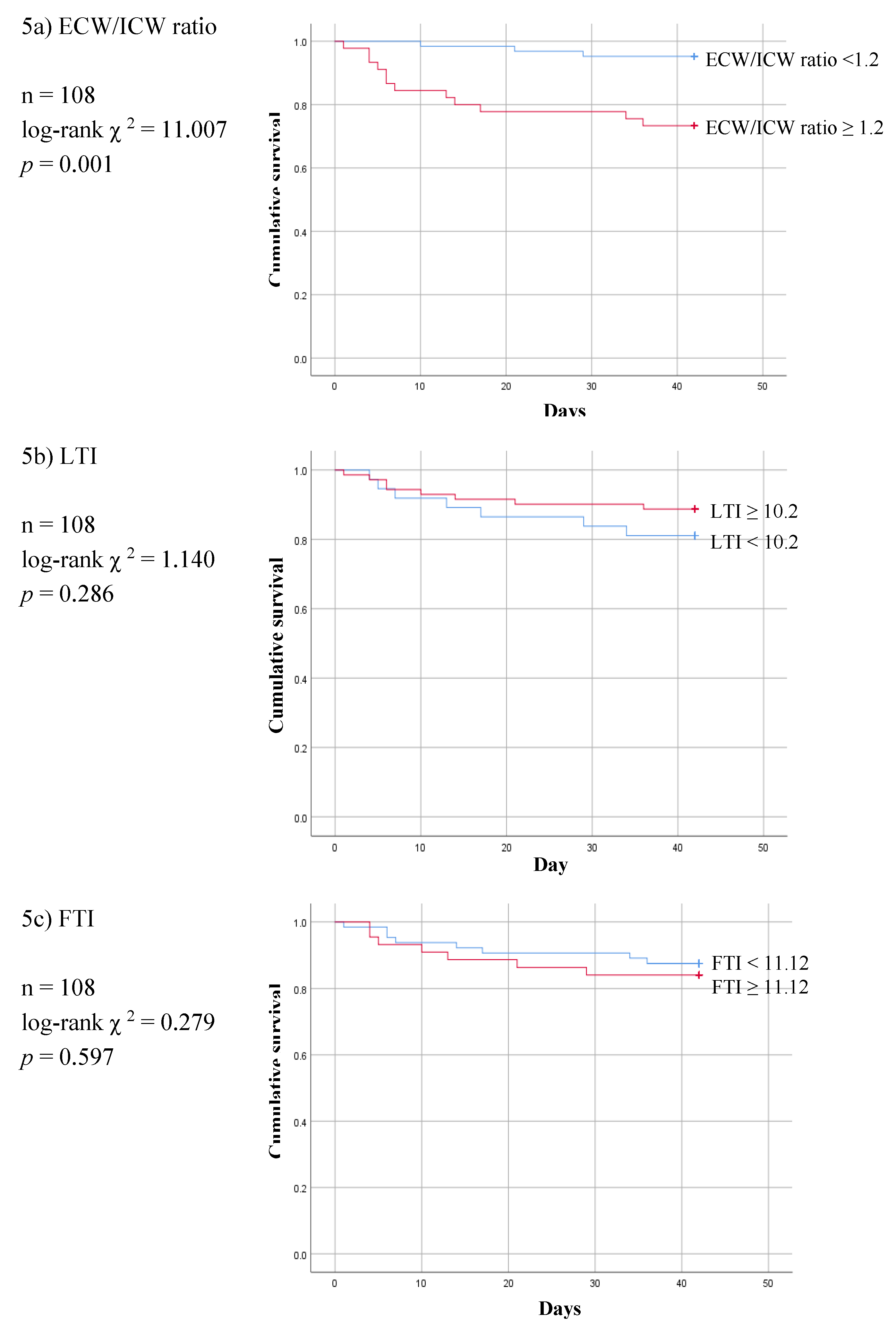
3. Results
4. Discussion
5. Conclusions
Authors Contributions
Fundings
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Broers, N.J.; Martens, R.J.; Cornelis, T.; Diederen, N.M.; Wabel, P.; van der Sande, F.M.; Leunissen, K.M.; Kooman, J.P. Body composition in dialysis patients: a functional assessment of bioimpedance using different prediction models. J Ren Nutr 2015, 25, 121-128. [CrossRef]
- Chen, H.S.; Lee, K.C.; Cheng, C.T.; Hou, C.C.; Liou, H.H.; Lin, C.J.; Lim, P.S. Application of Bioimpedance Spectroscopy in Asian Dialysis Patients (ABISAD): serial follow-up and dry weight evaluation. Clin Kidney J 2013, 6, 29-34. [CrossRef]
- Mamat, R.; Kong, N.C.; Ba'in, A.; Shah, S.A.; Cader, R.; Wong, V.; Mohd, R.; Abdul Gafor, A.H.; Ismail, R. Assessment of body fluid status in hemodialysis patients using the body composition monitor measurement technique. J Clin Nurs 2012, 21, 2879-2885. [CrossRef]
- Docktor, B.L.; Sadler, D.J.; Gray, R.R.; Saliken, J.C.; So, C.B. Radiologic placement of tunneled central catheters: rates of success and of immediate complications in a large series. AJR Am J Roentgenol 1999, 173, 457-460. [CrossRef]
- Delistefani, F.; Wallbach, M.; Muller, G.A.; Koziolek, M.J.; Grupp, C. Risk factors for catheter-related infections in patients receiving permanent dialysis catheter. BMC Nephrol 2019, 20, 199. [CrossRef]
- Thomson, P.; Stirling, C.; Traynor, J.; Morris, S.; Mactier, R. A prospective observational study of catheter-related bacteraemia and thrombosis in a haemodialysis cohort: univariate and multivariate analyses of risk association. Nephrol Dial Transplant 2010, 25, 1596-1604. [CrossRef]
- Miller, L.M.; Clark, E.; Dipchand, C.; Hiremath, S.; Kappel, J.; Kiaii, M.; Lok, C.; Luscombe, R.; Moist, L.; Oliver, M.; et al. Hemodialysis Tunneled Catheter-Related Infections. Can J Kidney Health Dis 2016, 3, 2054358116669129. [CrossRef]
- Miller, L.M.; MacRae, J.M.; Kiaii, M.; Clark, E.; Dipchand, C.; Kappel, J.; Lok, C.; Luscombe, R.; Moist, L.; Oliver, M.; et al. Hemodialysis Tunneled Catheter Noninfectious Complications. Can J Kidney Health Dis 2016, 3, 2054358116669130. [CrossRef]
- Perez-Morales, R.; Donate-Correa, J.; Martin-Nunez, E.; Perez-Delgado, N.; Ferri, C.; Lopez-Montes, A.; Jimenez-Sosa, A.; Navarro-Gonzalez, J.F. Extracellular water/total body water ratio as predictor of mortality in hemodialysis patients. Ren Fail 2021, 43, 821-829. [CrossRef]
- Nakayama, Y.; Yamada, Y.; Ishii, S.; Hitaka, M.; Yamazaki, K.; Masai, M.; Joki, N.; Sakai, K.; Ohashi, Y. Association between Intra- and Extra-Cellular Water Ratio Imbalance and Natriuretic Peptides in Patients Undergoing Hemodialysis. Nutrients 2023, 15. [CrossRef]
- Zhou, C.; Lin, X.; Ma, G.; Yuan, J.; Zha, Y. Increased Predialysis Extracellular to Intracellular Water Ratio Is Associated With Sarcopenia in Hemodialysis Patients. J Ren Nutr 2023, 33, 157-164. [CrossRef]
- Gracia-Iguacel, C.; Gonzalez-Parra, E.; Mahillo, I.; Ortiz, A. Low Intracellular Water, Overhydration, and Mortality in Hemodialysis Patients. J Clin Med 2020, 9. [CrossRef]
- Taniguchi, M.; Ikezoe, T.; Kamitani, T.; Tsuboyama, T.; Ito, H.; Matsuda, S.; Tabara, Y.; Matsuda, F.; Ichihashi, N.; Nagahama Study, G. Extracellular-to-intracellular water ratios are associated with functional disability levels in patients with knee osteoarthritis: results from the Nagahama Study. Clin Rheumatol 2021, 40, 2889-2896. [CrossRef]
- Nakajima, H.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Takahashi, F.; Yoshimura, Y.; Bamba, R.; Okamura, T.; Kitagawa, N.; Majima, S.; et al. Impact of extracellular-to-intracellular fluid volume ratio on albuminuria in patients with type 2 diabetes: A cross-sectional and longitudinal cohort study. J Diabetes Investig 2021, 12, 1202-1211. [CrossRef]
- Ishani, A.; Collins, A.J.; Herzog, C.A.; Foley, R.N. Septicemia, access and cardiovascular disease in dialysis patients: the USRDS Wave 2 study. Kidney Int 2005, 68, 311-318. [CrossRef]
- Beathard, G.A. Catheter thrombosis. Semin Dial 2001, 14, 441-445. [CrossRef]
- Little, M.A.; O'Riordan, A.; Lucey, B.; Farrell, M.; Lee, M.; Conlon, P.J.; Walshe, J.J. A prospective study of complications associated with cuffed, tunnelled haemodialysis catheters. Nephrol Dial Transplant 2001, 16, 2194-2200. [CrossRef]
- Kang, S.S.; Chang, J.W.; Park, Y. Nutritional Status Predicts 10-Year Mortality in Patients with End-Stage Renal Disease on Hemodialysis. Nutrients 2017, 9. [CrossRef]
- Alves, F.C.; Sun, J.; Qureshi, A.R.; Dai, L.; Snaedal, S.; Barany, P.; Heimburger, O.; Lindholm, B.; Stenvinkel, P. The higher mortality associated with low serum albumin is dependent on systemic inflammation in end-stage kidney disease. PLoS One 2018, 13, e0190410. [CrossRef]
- Wizemann, V.; Wabel, P.; Chamney, P.; Zaluska, W.; Moissl, U.; Rode, C.; Malecka-Masalska, T.; Marcelli, D. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 2009, 24, 1574-1579. [CrossRef]
- Ohashi, Y.; Joki, N.; Yamazaki, K.; Kawamura, T.; Tai, R.; Oguchi, H.; Yuasa, R.; Sakai, K. Changes in the fluid volume balance between intra- and extracellular water in a sample of Japanese adults aged 15-88 yr old: a cross-sectional study. Am J Physiol Renal Physiol 2018, 314, F614-F622. [CrossRef]
- Dekker, M.J.E.; Konings, C.; Canaud, B.; van der Sande, F.M.; Stuard, S.; Raimann, J.G.; Ozturk, E.; Usvyat, L.; Kotanko, P.; Kooman, J.P. Interactions Between Malnutrition, Inflammation, and Fluid Overload and Their Associations With Survival in Prevalent Hemodialysis Patients. J Renal Nutr 2018, 28, 435-444. [CrossRef]
- Kim, E.J.; Choi, M.J.; Lee, J.H.; Oh, J.E.; Seo, J.W.; Lee, Y.K.; Yoon, J.W.; Kim, H.J.; Noh, J.W.; Koo, J.R. Extracellular Fluid/Intracellular Fluid Volume Ratio as a Novel Risk Indicator for All-Cause Mortality and Cardiovascular Disease in Hemodialysis Patients. PLoS One 2017, 12, e0170272. [CrossRef]
- Chen, W.; Guo, L.J.; Wang, T. Extracellular water/intracellular water is a strong predictor of patient survival in incident peritoneal dialysis patients. Blood Purif 2007, 25, 260-266. [CrossRef]
- Chan, M.R. Hemodialysis Central Venous Catheter Dysfunction. Semin Dialysis 2008, 21, 516-521. [CrossRef]
- Forauer, A.R.; Theoharis, C. Histologic changes in the human vein wall adjacent to indwelling central venous catheters. J Vasc Interv Radiol 2003, 14, 1163-1168. [CrossRef]
- Rymarz, A.; Gibinska, J.; Zajbt, M.; Piechota, W.; Niemczyk, S. Low lean tissue mass can be a predictor of one-year survival in hemodialysis patients. Ren Fail 2018, 40, 231-237. [CrossRef]
- Wang, L.; Jia, L.; Jiang, A. Pathology of catheter-related complications: what we need to know and what should be discovered. J Int Med Res 2022, 50, 3000605221127890. [CrossRef]
| Characteristic | Overall (N = 124) |
Catheter group |
P Value |
|
| Patent (N = 93) |
Non-patent (N = 31) |
|||
| Age (median [range] years) | 72.0 [26–93] | 69.0 [26–93] | 75.0 [50–92] | 0.003 |
| Sex [n (%)] | ||||
| Men | 68 (54.8) | 54 (58.1) | 14 (45.2) | 0.214 |
| Women | 56 (45.2) | 39 (41.9) | 17 (54.8) | |
| Comorbidities [n (%)] | ||||
| Diabetes mellitus | 89 (71.8) | 69 (74.2) | 20 (64.5) | 0.304 |
| Hypertension | 96 (77.4) | 68 (73.1) | 28 (90.3) | 0.048 |
| Heart failure | 41 (33.1) | 31 (33.3) | 10 (32.3) | 0.913 |
| Ischemic heart disease | 34 (27.4) | 28 (30.1) | 6 (19.4) | 0.249 |
| Atrial fibrillation | 24 (19.4) | 15 (16.1) | 9 (29.0) | 0.117 |
| Cerebral infarction | 19 (15.3) | 10 (10.8) | 9 (29.0) | 0.014 |
| Liver cirrhosis | 6 (4.8) | 1 (1.1) | 5 (16.1) | 0.001 |
| Medication [n (%)] | ||||
| Aspirin | 29 (23.4) | 20 (21.5) | 9 (29.0) | 0.395 |
| Clopidogrel | 33 (26.6) | 24 (25.8) | 9 (29.0) | 0.727 |
| Warfarin | 6 (4.8) | 3 (3.2) | 3 (9.7) | 0.150 |
| Cilostazol | 7 (5.6) | 5 (5.4) | 2 (6.5) | 0.824 |
| NOAC | 8 (6.5) | 6 (6.5) | 2 (6.5) | 1.000 |
| Statin | 61 (49.2) | 46 (49.5) | 15 (48.4) | 0.918 |
| Laboratory data (median [range]) | ||||
| Hemoglobin (g/dL) | 9.6 [4.8–14.4] | 9.5 [4.8–16.1] | 9.9 [6.1–13.0] | 0.158 |
| Total lymphocyte count (103/µL) | 1160.0 [240–11390] | 1270.0 [240–11390] | 1000.0 [340–3220] | 0.254 |
| Platelet (000s) | 189.0 [12–820] | 191.0 [12–820] | 167.0 [20–329] | 0.221 |
| CRP (mg/dL) | 1.8 [0.1–40] | 1.1 [0.1–40] | 6.7 [0.1–40] | 0.003 |
| Total protein (g/dL) | 6.1 [4.1–8.4] | 6.1 [3.2–8.4] | 5.9 [4.1–7.9] | 0.383 |
| Albumin (g/dL) | 3.0 [1.6–4.4] | 3.0 [1.5–4.4] | 2.8 [1.6–3.8] | 0.009 |
| BUN (mg/dL) | 54.3 [11.9–177.8] | 53.0 [13.9–184] | 54.9 [11.9–143.4] | 0.944 |
| Creatinine (mg/dL) | 4.8 [0.7–31.4] | 5.1 [0.7–32.9] | 4.3 [0.9–11.7] | 0.021 |
| Total cholesterol (mg/dL) | 127.0 [45–494] | 126.0 [45–494] | 135.0 [52–290] | 0.835 |
| Total calcium (mg/dL) | 7.7 [5.0–10.5] | 7.7 [5.0–10.4] | 7.7 [5.3–10.5] | 0.330 |
| Phosphorus (mg/dL) | 4.2 [1.5 –12.6] | 4.1 [1.5–12.6] | 4.2 [1.7–9.9] | 0.606 |
| Sodium (mEq/L) | 136.5 [122–153.6] | 136.6 [122–153] | 136.4 [124–153.6] | 0.471 |
| Potassium (mEq/L) | 4.3 [2.8–7.8] | 4.3 [2.8–7.8] | 4.3 [2.8–6.2] | 0.976 |
| BMI (kg/m2) | 23.5 [15.1–41.9] | 23.4 [15.1–41.9] | 25.0 [16.0–39.2] | 0.339 |
| Body composition (median [range]) | ||||
| ECW (L) | 16.2 [8.4–32.5] | 17.4 [8.4–32.5] | 12.2 [10.3–30.7] | 0.371 |
| ICW (L) | 15.3 [7.8–28.0] | 15.8 [7.9–28.0] | 12.5 [7.8–22.2] | 0.000 |
| TBW (L) | 31.8 [16.3–56.4] | 33.8 [16.3–56.4] | 27.7 [19.7–52.9] | 0.013 |
| ECW/ICW ratio | 1.13 [0.62–1.90] | 1.05 [0.62–1.89] | 1.22 [0.87–1.78] | 0.001 |
| LTI (kg/m2) | 11.6 [5.3–21.4] | 12.4 [5.8–21.4] | 9.4 [5.3–16.4] | 0.000 |
| FTI (kg/m2) | 10.7 [0.9–29.8] | 9.7 [0.9–29.0] | 11.2 [2.1–29.8] | 0.042 |
| LTM (kg) | 28.2 [2.0–59.3] | 30.7 [2.0–59.3] | 22.9 [12.5–51.3] | 0.013 |
| ATM (kg) | 24.8 [2.3–66.9] | 24.0 [2.3–64.5] | 29.5 [2.4–66.9] | 0.222 |
| FAT (kg) | 18.4 [1.7–49.2] | 17.6 [1.7–47.4] | 21.7 [4.9–49.2] | 0.042 |
| Body cell mass | 15.4 [2.3–36.1] | 16.8 [2.3–36.1] | 11.7 [4.1–29.6] | 0.001 |
| Dry weight (kg) | 57.6 [33.5–99.7] | 58.5 [33.5–99.7] | 53.3 [37.2–90.9] | 0.228 |
| Hazard ratio | 95% CI of difference | P value | ||
| Lower | Upper | |||
| Model 1 | ||||
| ECW/ICW ratio | 6.016 | 1.895 | 19.094 | 0.002 |
| Model 2 | ||||
| ECW/ICW ratio | 6.615 | 1.958 | 22.346 | 0.002 |
| Age | 1.049 | 1.016 | 1.083 | 0.004 |
| Sex | 0.950 | 0.458 | 1.971 | 0.890 |
| Model 3 | ||||
| ECW/ICW ratio | 4.792 | 1.225 | 18.747 | 0.024 |
| Age | 1.043 | 1.006 | 1.080 | 0.021 |
| Sex | 1.211 | 0.566 | 2.592 | 0.622 |
| LTI | 0.907 | 0.768 | 1.071 | 0.248 |
| FTI | 1.031 | 0.962 | 1.104 | 0.393 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





