Submitted:
15 September 2025
Posted:
16 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Overview of Gut Microbiota and Microecology
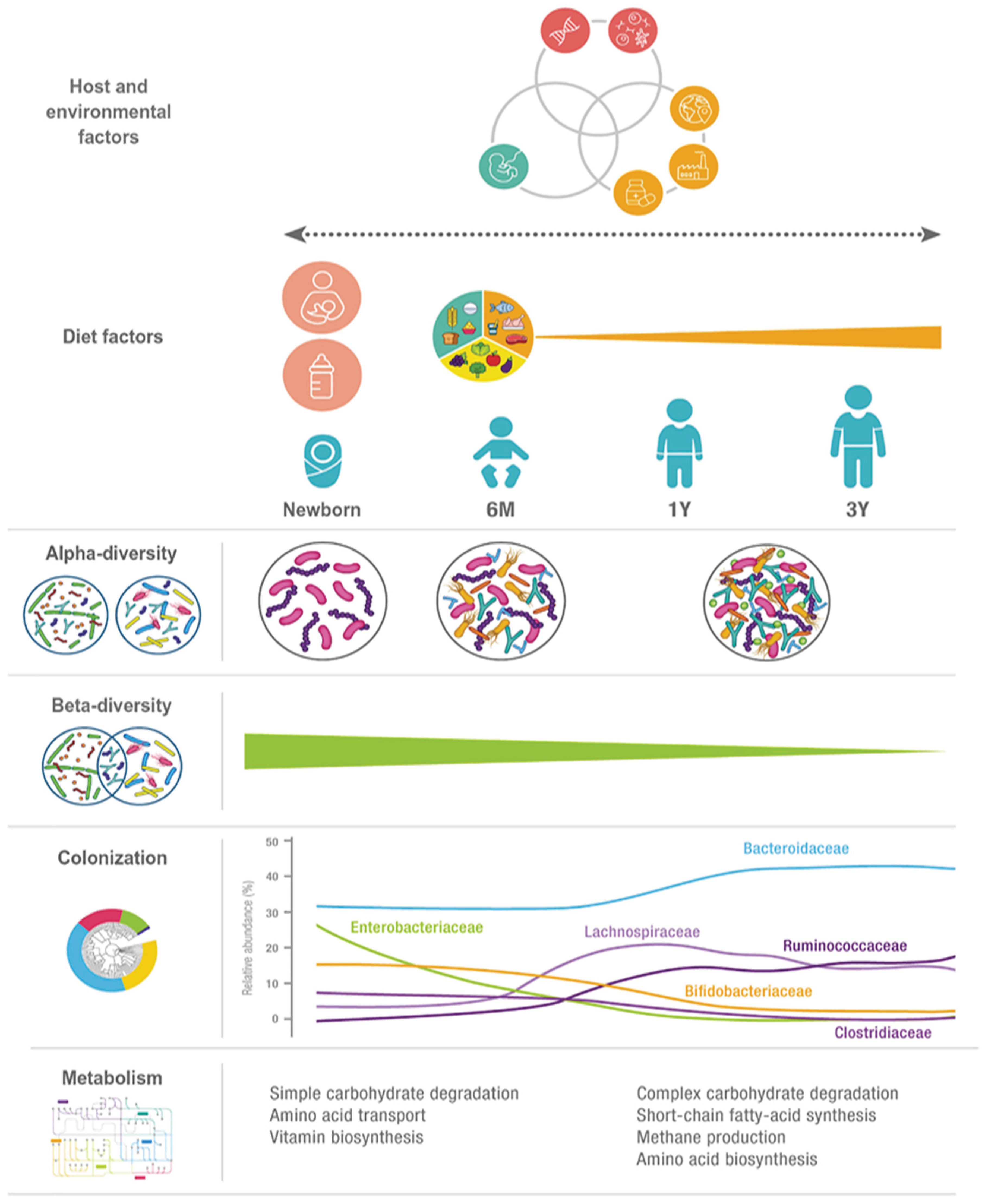
1.2. Microbial Diversity and Composition of Gut Microbiota
1.2.1. Diversity and Complexity
1.2.2. Composition and Variability
- Diet: Nutrient intake influences microbial diversity; fiber-rich diets promote beneficial bacteria, while high-fat or processed diets encourage the growth of potentially pathogenic species.
- Age: The microbiome undergoes dynamic shifts across the human lifespan, from infancy to old age.
- Antibiotic Use: Antibiotics can profoundly disrupt microbial balance, decreasing diversity and promoting dysbiosis.
- Environmental Exposures: Factors such as geography, hygiene, lifestyle, and contact with animals impact microbial acquisition and composition.
1.3. Aims and Significance of Microbiome Research
- Understanding Disease Mechanisms: Investigating how microbial communities influence the onset and progression of diseases.
- Developing Therapeutic Strategies: Exploring the potential of probiotics, prebiotics, fecal microbiota transplantation (FMT), and microbial-based drug formulations for gut microbiota modulation (Jaswal et al., 2025).
- Advancing Precision Medicine: Integrating microbiome profiles with metagenomics and multi-omics technologies to develop personalized healthcare strategies.
- Identifying Research Gaps: Synthesizing current knowledge to illuminate areas requiring further investigation and innovation.
- Enhancing Cancer Treatment: Studying how the gut microbiota affects the tumor microenvironment and modulates the efficacy of immunotherapy and chemotherapy.
2. Composition and Function of the Gut Microbiota
2.1. Significant Microbial Phyla
2.2. Functions of the Gut Microbiota
2.2.1. Nutrient Metabolism and SCFA Production
2.2.2. Immune System Modulation
2.2.3. Protection Against Pathogens
2.2.4. Gut–Organ Communication Axes
-
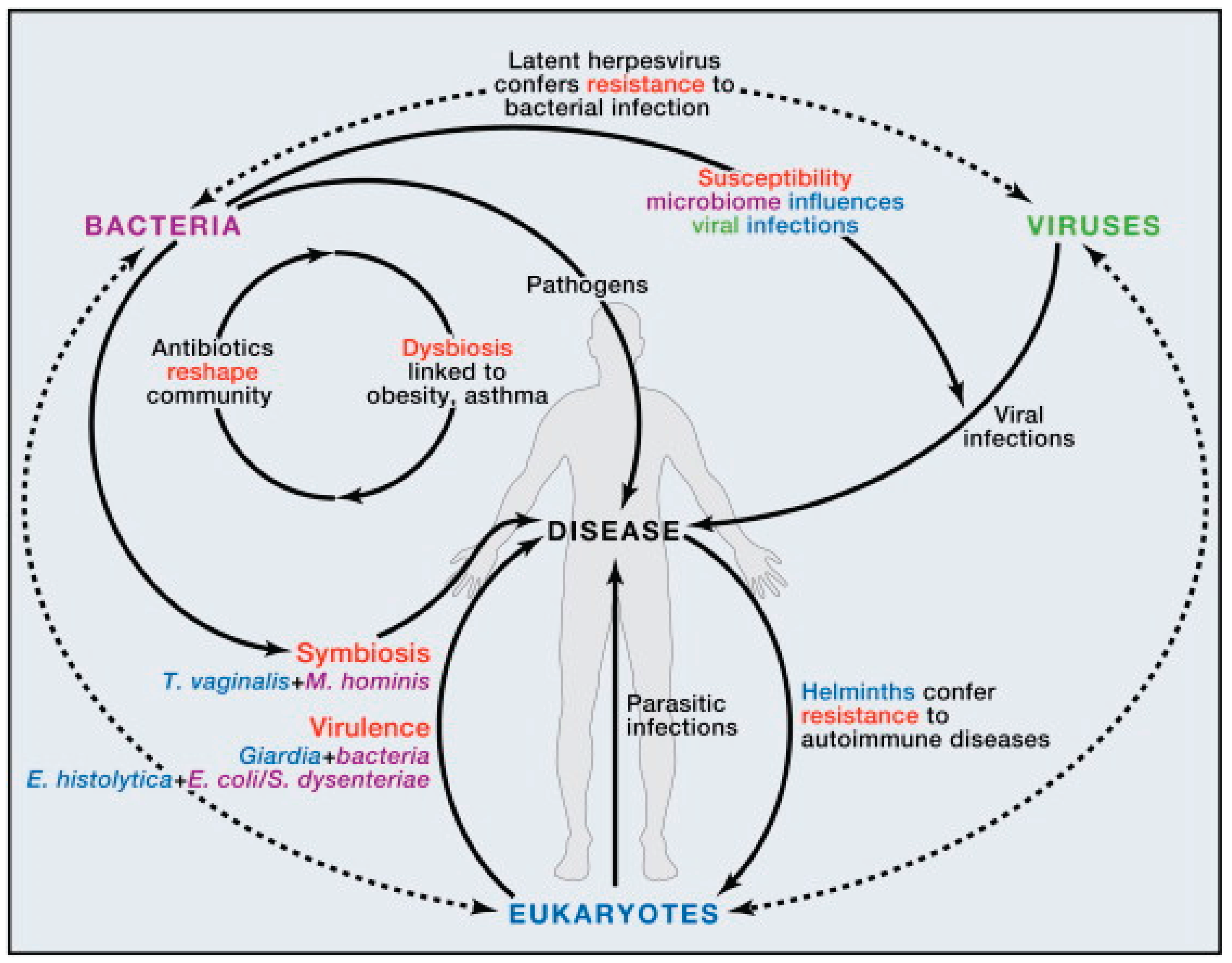
Gut–Brain Axis:The gut–brain axis represents a complex, bidirectional communication system that operates through neural, hormonal, metabolic, and immune pathways. Gut microbes synthesize a variety of neuroactive compounds, including serotonin, dopamine precursors, γ-aminobutyric acid (GABA), and short-chain fatty acids (Dicks L. M., 2022), all of which influence gastrointestinal motility, stress response, mood regulation, and cognitive processes. Disruptions in this axis have been linked to neuropsychiatric and neurodegenerative disorders. For example, alterations in microbial composition have been associated with heightened risk of Parkinson’s disease and Alzheimer’s disease, where gut-derived signals may influence neuroinflammation and protein aggregation in the brain (Giau et al., 2018).
-
Gut–Liver Axis:The liver and gut are intimately connected, as nearly 70% of hepatic blood flow is supplied directly from the gut via the portal vein. This anatomical relationship exposes the liver to microbial metabolites and components such as lipopolysaccharides (LPS), peptidoglycans, and bile acids. The gut microbiota plays a critical role in bile acid metabolism (Figure 2), which in turn modulates immune cell function in the liver, particularly natural killer T (NKT) cells (Scarpellini et al., 2020). Interestingly, primary bile acids can support protective immune activity and even suppress tumor growth by enhancing NKT responses, while secondary bile acids, produced by microbial transformation, may contribute to chronic inflammation and carcinogenesis. This delicate balance illustrates how gut–liver interactions can determine outcomes ranging from metabolic homeostasis to liver disease progression (Song et al., 2024).
-
Gut–Skin Axis:The relationship between the gut microbiota and skin health has gained increasing attention, especially in the context of chronic inflammatory skin conditions. Dysbiosis in the gut can lead to systemic immune dysregulation (|Figure 1), increased intestinal permeability, and circulation of pro-inflammatory metabolites that affect skin physiology (Munteanu et al., 2025). Conditions such as psoriasis, rosacea, and atopic dermatitis have all been linked to gut microbial imbalances. Likewise, gut-derived metabolites, including short-chain fatty acids, can influence skin barrier integrity, hydration, and immune responses, underscoring the systemic nature of microbial communication. These insights highlight that maintaining gut microbial balance is not only essential for internal organ function but also for visible markers of health such as skin integrity and appearance (Khan et al., 2024).
2.2.5. Gut Barrier Integrity
2.3. The Role of Gut Microbiota in Human Health
2.3.1. The Microbiota as a Central Regulator of Host Physiology
2.3.2. Key Functional Contributions
- I. Nutrient Absorption and Metabolism: Gut microbes facilitate the breakdown of dietary fibers and complex carbohydrates, leading to SCFA production, which fuels colonocytes, modulates immune signaling, and contributes to anti-inflammatory processes (Fernández et al., 2016).
- II. Immune System Modulation: The gut microbiota plays a critical role in educating and shaping the host immune system. It stimulates the secretion of protective mucins and antimicrobial peptides, while also fostering immune tolerance to harmless antigens. Through interactions with macrophages, dendritic cells, and regulatory T cells (Tregs), commensal microbes ensure a balanced immune response that is effective against pathogens but restrained enough to prevent excessive inflammation or autoimmunity (Fan et al., 2024). This immunomodulatory role is central to preventing conditions such as allergies, autoimmune diseases, and chronic inflammatory disorders.
- III. Barrier Integrity and Pathogen Defense: Commensal microbes maintain the gut mucosal barrier and inhibit pathogenic overgrowth by producing antimicrobial peptides and modifying the intestinal environment (Baindara & Mandal, 2023).
- IV. Xenobiotic and Drug Metabolism: Gut microbes also influence the fate of therapeutic drugs and xenobiotics. Microbial enzymes can activate, deactivate, or even toxify pharmaceutical compounds, thereby altering their bioavailability and efficacy. For example, certain bacteria metabolize digoxin, reducing its therapeutic effect (Pant et al., 2023), while others enhance the activity of specific chemotherapeutic agents.
2.4. Importance of Gut Microbiota Balance
| Microbial Factor/ Species | Immune-Metabolic Function | Disease Context / Effect | Reference |
|---|---|---|---|
| Faecalibacterium prausnitzii | Produces butyrate and anti-inflammatory metabolites; induces IL-10 and Treg cells; suppresses NF-κB signaling | Mitigates colitis inflammation and enhances gut barrier integrity | (Garabatos et al., 2025; Bastida et al., 2023). |
| F. prausnitzii (live strain) | Reduces expression of pro-inflammatory cytokines (IFN-γ, TNF-α, IL-6, IL-12); inhibits IL-8 via NF-κB | Reduces severity in IBD models, stabilizes gut homeostasis | (Olteanu et al. 2024). |
| Butyrate (SCFA) | Inhibits histone deacetylases (HDACs); downregulates NF-κB and TNF expression in immune cells | Reduces intestinal and systemic inflammation | (Portincasa et al., 2022). |
| Akkermansia muciniphila | Supports mucosal integrity; modulates metabolic inflammation | Linked to improved obesity and metabolic health outcomes | (Wang et al., 2024; Jiang & Zhang, 2024). |
| SCFAs & metabolites | Signal through GPCRs; support gut barrier, immune balance, and systemic metabolism | Protective in asthma, IBD, and colon inflammation | (Munteanu & Schwartz, 2024). |
| Bacteroides species (e.g., B. uniformis) | Ferment carbohydrates into acetate/propionate; regulate gut microenvironment and metabolism | Potential obesity alleviation and immune modulation | Jyoti & Dey, 2025). |
| Dysbiosis (Microbial Imbalance) | Loss of beneficial organisms, overgrowth of pathobionts, reduced diversity (Figure 2) | Associated with IBD, obesity, cancer, and neuro-inflammation | (Munteanu et al., 2025; Garabatos et al., 2025) |
3. The Gut Microbiome and Host Systems
3.1. Overview of Host–Microbiota Interactions
3.2. Immune System Crosstalk
- Recognition of Microbial Signals: The host immune system detects microbial presence via pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) and NOD-like receptors (NLRs). These receptors recognize microbial-associated molecular patterns (MAMPs) such as lipopolysaccharides (LPS), flagellin, and peptidoglycans, initiating tailored immune responses that distinguish between beneficial commensals and potential pathogens (Tiruvayipati et al., 2022).
- Mucosal Immunity: Commensal microbes actively promote the secretion of antimicrobial peptides (AMPs), immunoglobulin A (IgA), and mucus, forming a multifaceted defense that protects against invading pathogens while maintaining immune tolerance to harmless microbes. These mechanisms establish a controlled environment that balances protection with symbiosis (Tonetti et al., 2024).
- Immune Homeostasis: Gut microbial signals modulate the equilibrium between pro-inflammatory and regulatory pathways. They influence the differentiation and function of T cells—including regulatory T cells (Tregs)—as well as dendritic cells and macrophages, shaping immune responses that are precise and proportionate (Brescia et al., 2024).
- Epithelial Barrier Support: The microbiota reinforces gut barrier integrity by enhancing tight junction protein expression and stimulating mucus production (Table 1), preventing the translocation of pathogens and their toxins into systemic circulation (Shu et al., 2023).
3.3. Microbial Metabolites and Host Physiology
- Short-Chain Fatty Acids (SCFAs): Fermentation of dietary fibers generates SCFAs—primarily butyrate, acetate, and propionate—that serve as energy sources for colonocytes, modulate immune signaling (e.g., Treg differentiation), and strengthen epithelial barrier function (Martin-Gallausiaux et al., 2021).
- Bile Acid Metabolites: Microbial transformation of primary bile acids into secondary bile acids influences lipid metabolism, immune pathways, and hepatic function through receptors such as FXR and TGR5. These metabolites play key roles in the gut–liver axis and in systemic metabolic regulation (Tong & Lou, 2025).
- Tryptophan Derivatives: Indole and related compounds activate the aryl hydrocarbon receptor (AhR), contributing to mucosal immunity, epithelial repair, and modulation of inflammatory responses (Marafini et al., 2024).
- Vitamins: Certain gut bacteria synthesize essential vitamins, including vitamin K and B-group vitamins, supporting coagulation, DNA synthesis, energy metabolism, and overall cellular function (Tarracchini et al., 2025).
3.4. Host Regulation of Microbiota
- Diet: Macronutrient composition and fiber intake strongly influence microbial diversity and metabolic activity. High-fiber diets enrich SCFA-producing bacteria, while high-fat or low-fiber diets may promote dysbiosis and inflammation (Mantri et al., 2024).
- Immune Regulation: Host defenses, including IgA secretion, antimicrobial peptide production, and continuous epithelial renewal, maintain microbial balance and prevent overgrowth of potentially harmful organisms.
- Medications: Antibiotics, nonsteroidal anti-inflammatory drugs (NSAIDs), proton pump inhibitors, and other pharmaceuticals can disrupt microbial ecosystems, often leading to reduced diversity and altered metabolic outputs (Fan et al., 2024).
- Environmental and Lifestyle Factors: Urbanization, stress, hygiene practices, toxin exposure, and early-life microbial exposures shape the microbiota, influencing immune development and long-term health outcomes.
- Genetic Background: Host genetics determine susceptibility to microbial colonization, immune responsiveness, and predisposition to dysbiosis, highlighting the interplay between hereditary factors and microbial ecology (Wang et al., 2024).
4. Gut Microbial Symbiosis and the Health Consequences of Dysbiosis
4.1. The Functional Benefits of Gut Symbiosis
4.1.1. Nutrient Metabolism and Energy Production
- Energy Supply: Butyrate acts as a primary energy source for colonocytes, supporting epithelial health and promoting efficient nutrient absorption.
- Immune Modulation: SCFAs influence the differentiation and function of immune cells, including regulatory T cells, helping to maintain a balanced inflammatory response (Paddison Rees N., 2024).
- Metabolic Regulation: These metabolites play key roles in lipid metabolism and glucose homeostasis, contributing to overall energy balance and metabolic health.
4.1.2. Immune System Development and Regulation
4.1.3. Maintenance of Gut Barrier Integrity and Pathogen Defense
4.1.4. Gut–Brain Axis and Mental Health
4.1.5. Metabolite Production and Systemic Regulation
4.1.6. Drug Metabolism and Therapeutic Response
4.1.7. Modulation of the Tumor Microenvironment (TME)
4.2. Gut Dysbiosis: Etiology and Clinical Consequences
4.2.1. Definition and Causes
4.2.2. Disease Associations
5. Therapeutic Interventions Targeting the Gut Microbiota
5.1. Prebiotics
5.2. Probiotics
5.3. Synbiotics
5.4. Fecal Microbiota Transplantation (FMT)
5.5. Dietary Modifications
5.6. Microbiome-Based Drug Development
6. Gut Microbiota in Cancer and Immunotherapy
6.1. Influence on Immune Checkpoint Inhibitors (ICIs) and Immunotherapy Response
6.2. Key Bacteria Associated with Improved Cancer Treatment Outcomes
6.3. Role of Microbial Metabolites in Tumor Progression and Therapy
6.4. Antibiotic Impact on Cancer Therapy
| Strategy | Mechanism of Action | Immune Effects | Metabolic Effects | References |
|---|---|---|---|---|
| Probiotics (Bifidobacterium, Lactobacillus, Akkermansia) | Enhance gut barrier, modulate cytokine production, compete with pathogens | Increase Treg activation, reduce pro-inflammatory cytokines (IL-6, TNF-α), promote antitumor immunity | Improve insulin sensitivity, lipid metabolism, and SCFA production. | (Shahini & Shahini, 2023). |
| Prebiotics (inulin, resistant starch, fibers) | Provide substrates for beneficial microbes, increase SCFA production | Promote anti-inflammatory immune responses; enhance IgA secretion | Boost SCFA levels (butyrate, propionate), improve glucose and lipid metabolism. | (Sheng et al., 2023). |
| Synbiotics (Probiotic + Prebiotic) | Synergistic effect improving colonization of beneficial microbes (Figure 2) | Enhance immune tolerance and lower inflammatory markers | Support nutrient absorption and improve metabolic homeostasis. | (Al-Habsi et al., 2024) |
| Fecal Microbiota Transplantation (FMT) | Restores microbial diversity using donor stool | Re-establishes immune balance, restores Treg/Th17 ratio, improves response to immunotherapy | Enhances metabolic function, reduces endotoxemia and systemic inflammation. | (Jandhyala et al., 2015) |
| Dietary Interventions (Mediterranean diet, fermented foods) | Increase microbial richness and diversity | Promote immune tolerance, decrease pro-inflammatory pathways | Increase SCFA levels, improve cardiovascular and metabolic outcomes. | David & Lev-Ari, 2024). |
| Microbiome-based Drugs (live biotherapeutics, engineered bacteria) | Target-specific pathways with microbial strains or engineered metabolites | Modulate tumor immunity, balance immune dysregulation | Correct metabolic disorders by modulating bile acids and SCFAs. | (Desai et al., 2025). |
| Antibiotic Stewardship | Prevents broad-spectrum disruption of microbiota | Reduces dysbiosis-related immune dysfunction | Preserves metabolic stability by maintaining microbial diversity | Dongre et al., 2025 |
7. The Role of Gut Microbiota in Other Health Conditions
7.1. Cardiovascular Diseases
7.2. Neurological Disorders
7.3. Psoriasis and Skin Disorders
7.4. Hormonal Regulation
8. Emerging Trends and Future Perspectives
8.1. Microbiome Editing (CRISPR, Synthetic Biology)
8.2. Personalized Microbiome-Based Therapies
8.3. Ethical and Regulatory Challenges in Microbiome Research
8.4. Long-Term Implications of Gut Microbiota Modulation
9. Conclusion
Acknowledgements
Conflict of Interest
References
- Hugon, P.; Lagier, J.C.; Colson, P.; Bittar, F.; Raoult, D. Repertoire of human gut microbes. Microbial pathogenesis 2017, 106, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Symphony of Digestion: Coordinated Host–Microbiome Enzymatic Interplay in Gut Ecosystem. Biomolecules 2025, 15, 1151. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World journal of gastroenterology: WJG 2015, 21, 8787. [Google Scholar] [CrossRef]
- Virgo, M.; Mostowy, S.; Ho, B.T. Emerging models to study competitive interactions within bacterial communities. Trends in microbiology 2025, 33, 688–700. [Google Scholar] [CrossRef]
- Moran, N.A.; Ochman, H.; Hammer, T.J. Evolutionary and ecological consequences of gut microbial communities. Annual Review of Ecology, Evolution, and Systematics 2019, 50, 451–475. [Google Scholar] [CrossRef] [PubMed]
- Matijašić, M.; Meštrović, T.; Čipčić Paljetak, H.; Perić, M.; Barešić, A.; Verbanac, D. Gut microbiota beyond bacteria—mycobiome, virome, archaeome, and eukaryotic parasites in IBD. International journal of molecular sciences 2020, 21, 2668. [Google Scholar] [CrossRef] [PubMed]
- Deering, K.E.; Devine, A.; O’Sullivan, T.A.; Lo, J.; Boyce, M.C.; Christophersen, C.T. Characterizing the composition of the pediatric gut microbiome: a systematic review. Nutrients 2019, 12, 16. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Tremaroli, V.; Bäckhed, F. (2013). The gut microbiota. In The prokaryotes (pp. 3-24). Springer, Berlin, Heidelberg.
- Khalil, M.; Di Ciaula, A.; Mahdi, L.; Jaber, N.; Di Palo, D.M.; Graziani, A.; Portincasa, P.; et al. Unraveling the role of the human gut microbiome in health and diseases. Microorganisms 2024, 12, 2333. [Google Scholar] [CrossRef]
- Olteanu, G.; Ciucă-Pană, M.A.; Busnatu, Ș.S.; Lupuliasa, D.; Neacșu, S.M.; Mititelu, M.; Boroghină, S.C.; et al. Unraveling the microbiome–human body axis: a comprehensive examination of therapeutic strategies, interactions and implications. International Journal of Molecular Sciences 2024, 25, 5561. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World journal of gastroenterology: WJG 2015, 21, 8787. [Google Scholar]
- Ahlawat, S.; Asha, N.; Sharma, K.K. Gut–organ axis: a microbial outreach and networking. Letters in applied microbiology 2021, 72, 636–668. [Google Scholar]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Di Ciaula, A.; et al. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. International journal of molecular sciences 2022, 23, 1105. [Google Scholar]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut microbiota and immune system interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [PubMed]
- Zhang, X.; Zhang, K.; Yan, L.; Wang, P.; Zhao, F.; Hu, S. The role of toll-like receptors in immune tolerance induced by Helicobacter pylori infection. Helicobacter 2023, 28, e13020. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Flores, G.; Pickard, J.M.; Núñez, G. Microbiota-mediated colonization resistance: mechanisms and regulation. Nature Reviews Microbiology 2023, 21, 347–360. [Google Scholar] [CrossRef]
- Rose, A.E.; Fansler, R.T.; Zhu, W. Commensal resilience: ancient ecological lessons for the modern microbiota. Infection and Immunity 2025, 93, e00502–24. [Google Scholar] [CrossRef]
- Shang, Z.; Pai, L.; Patil, S. Unveiling the dynamics of gut microbial interactions: a review of dietary impact and precision nutrition in gastrointestinal health. Frontiers in Nutrition 2024, 11, 1395664. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nature medicine 2016, 22, 1079–1089. [Google Scholar] [PubMed]
- Dicks, L.M. Gut bacteria and neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Giau, V.V.; Wu, S.Y.; Jamerlan, A.; An, S.S.A.; Kim, S.; Hulme, J. Gut microbiota and their neuroinflammatory implications in Alzheimer’s disease. Nutrients 2018, 10, 1765. [Google Scholar] [CrossRef]
- Scarpellini, E.; Fagoonee, S.; Rinninella, E.; Rasetti, C.; Aquila, I.; Larussa, T.; Abenavoli, L.; et al. Gut microbiota and liver interaction through immune system cross-talk: a comprehensive review at the time of the SARS-CoV-2 pandemic. Journal of Clinical Medicine 2020, 9, 2488. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lau, H.C.; Zhang, X.; Yu, J. Bile acids, gut microbiota, and therapeutic insights in hepatocellular carcinoma. Cancer Biology & Medicine 2024, 21, 144–162. [Google Scholar]
- Munteanu, C.; Turti, S.; Marza, S.M. Unraveling the Gut–Skin Axis: The Role of Microbiota in Skin Health and Disease. Cosmetics 2025, 12, 167. [Google Scholar] [CrossRef]
- Khan, I.M.; Nassar, N.; Chang, H.; Khan, S.; Cheng, M.; Wang, Z.; Xiang, X. The microbiota: A key regulator of health, productivity, and reproductive success in mammals. Frontiers in Microbiology 2024, 15, 1480811. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Internal and emergency medicine 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Alonso-Cotoner, C.; Abril-Gil, M.; Albert-Bayo, M.; Mall, J.P.G.; Expósito, E.; Gonzalez-Castro, A.M.; Santos, J.; et al. The role of purported mucoprotectants in dealing with irritable bowel syndrome, functional diarrhea, and other chronic diarrheal disorders in adults. Advances in therapy 2021, 38, 2054–2076. [Google Scholar] [CrossRef]
- Caballero-Flores, G.; Pickard, J.M.; Núñez, G. Microbiota-mediated colonization resistance: mechanisms and regulation. Nature Reviews Microbiology 2023, 21, 347–360. [Google Scholar]
- Zaidi, S.; Ali, K.; Khan, A.U. It's all relative: analyzing microbiome compositions, its significance, pathogenesis and microbiota derived biofilms: challenges and opportunities for disease intervention. Archives of Microbiology 2023, 205, 257. [Google Scholar]
- Fernández, J.; Redondo-Blanco, S.; Gutiérrez-del-Río, I.; Miguélez, E.M.; Villar, C.J.; Lombo, F. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: A review. Journal of Functional Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Fan, J.; Zhu, J.; Xu, H. Strategies of Helicobacter pylori in evading host innate and adaptive immunity: insights and prospects for therapeutic targeting. Frontiers in Cellular and Infection Microbiology 2024, 14, 1342913. [Google Scholar] [CrossRef] [PubMed]
- Baindara, P.; Mandal, S.M. Gut-antimicrobial peptides: synergistic co-evolution with antibiotics to combat multi-antibiotic resistance. Antibiotics 2023, 12, 1732. [Google Scholar] [CrossRef]
- Pant, A.; Maiti, T.K.; Mahajan, D.; Das, B. Human gut microbiota and drug metabolism. Microbial Ecology 2023, 86, 97–111. [Google Scholar] [CrossRef]
- Jena, R.; Singh, N.A.; Ahmed, N.; Choudhury, P.K. Bifidobacteria in antibiotic-associated dysbiosis: restoring balance in the gut microbiome. World Journal of Microbiology and Biotechnology 2025, 41, 297. [Google Scholar] [CrossRef]
- Wu, S.; Bu, X.; Chen, D.; Wu, X.; Wu, H.; Caiyin, Q.; Qiao, J. Molecules-mediated bidirectional interactions between microbes and human cells. npj Biofilms and Microbiomes 2025, 11, 38. [Google Scholar] [CrossRef]
- Wang, L.; Cao, Z.M.; Zhang, L.L.; Li, J.M.; Lv, W.L. The role of gut microbiota in some liver diseases: from an immunological perspective. Frontiers in immunology 2022, 13, 923599. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Somaratne, G. (2023). Functional food and nutraceuticals for the prevention of gastrointestinal disorders. In Industrial Application of Functional Foods, Ingredients and Nutraceuticals (pp. 501-534). Academic Press.
- Tiruvayipati, S.; Hameed, D.S.; Ahmed, N. Play the plug: How bacteria modify recognition by host receptors? Frontiers in Microbiology 2022, 13, 960326. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, F.R.; Eguileor, A.; Llorente, C. Goblet cells: guardians of gut immunity and their role in gastrointestinal diseases. Egastroenterology 2024, 2. [Google Scholar] [CrossRef]
- Brescia, C.; Audia, S.; Pugliano, A.; Scaglione, F.; Iuliano, R.; Trapasso, F.; Amato, R.; et al. Metabolic drives affecting Th17/Treg gene expression changes and differentiation: impact on immune-microenvironment regulation. Apmis 2024, 132, 1026–1045. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.Z.; Ding, Y.D.; Xue, Q.M.; Cai, W.; Deng, H. Direct and indirect effects of pathogenic bacteria on the integrity of intestinal barrier. Therapeutic advances in gastroenterology 2023, 16, 17562848231176427. [Google Scholar] [CrossRef]
- Bastida, G.; Mínguez, A.; Nos, P.; Moret-Tatay, I. Immunoepigenetic regulation of inflammatory bowel disease: current insights into novel epigenetic modulations of the systemic immune response. Genes 2023, 14, 554. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: mechanisms and functional importance in the gut. Proceedings of the Nutrition Society 2021, 80, 37–49. [Google Scholar]
- Tong, Y.; Lou, X. Interplay Between Bile Acids, Gut Microbiota, and the Tumor Immune Microenvironment: Mechanistic Insights and Therapeutic Strategies. Frontiers in Immunology 2025, 16, 1638352. [Google Scholar] [CrossRef]
- Marafini, I.; Monteleone, I.; Laudisi, F.; Monteleone, G. Aryl hydrocarbon receptor signalling in the control of gut inflammation. International Journal of Molecular Sciences 2024, 25, 4527. [Google Scholar] [CrossRef]
- Tarracchini, C.; Lordan, C.; Milani, C.; Moreira, L.P.; Alabedallat, Q.M.; de Moreno de LeBlanc, A.; Ventura, M.; et al. Vitamin biosynthesis in the gut: interplay between mammalian host and its resident microbiota. Microbiology and Molecular Biology Reviews 2025, 89, e00184-23. [Google Scholar] [CrossRef]
- Mantri, A.; Klümpen, L.; Seel, W.; Krawitz, P.; Stehle, P.; Weber, B.; Simon, M.C.; et al. Beneficial effects of Synbiotics on the gut microbiome in individuals with low Fiber intake: secondary analysis of a double-blind, randomized controlled trial. Nutrients 2024, 16, 2082. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, Y.; Xiao, H.; Sun, H. Advancements in understanding the role of intestinal dysbacteriosis mediated mucosal immunity in IgA nephropathy. BMC nephrology 2024, 25, 203. [Google Scholar]
- Wang, S.; Cui, J.; Jiang, S.; Zheng, C.; Zhao, J.; Zhang, H.; Zhai, Q. Early life gut microbiota: consequences for health and opportunities for prevention. Critical Reviews in Food Science and Nutrition 2024, 64, 5793–5817. [Google Scholar] [PubMed]
- Pires, L.; Gonzalez-Paramás, A.M.; Heleno, S.A.; Calhelha, R.C. Gut microbiota as an endocrine organ: Unveiling its role in human physiology and health. Applied Sciences 2024, 14, 9383. [Google Scholar] [CrossRef]
- Munteanu, C.; Schwartz, B. Interactions between dietary antioxidants, dietary Fiber and the gut microbiome: their putative role in inflammation and Cancer. International Journal of Molecular Sciences 2024, 25, 8250. [Google Scholar] [CrossRef] [PubMed]
- Paddison Rees, N. (2024). Investigating the potential senomorphic properties of SCFA butyrate on aged T cells (Doctoral dissertation, University of Birmingham).
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: metabolism of nutrients and other food components. European journal of nutrition 2018, 57, 1–24. [Google Scholar]
- Pandiyan, P.; Bhaskaran, N.; Zou, M.; Schneider, E.; Jayaraman, S.; Huehn, J. Microbiome dependent regulation of Tregs and Th17 cells in mucosa. Frontiers in immunology 2019, 10, 426. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Q. Gut microbiota influences the efficiency of immune checkpoint inhibitors by modulating the immune system. Oncology Letters 2024, 27, 87. [Google Scholar] [CrossRef]
- Shu, L.Z.; Ding, Y.D.; Xue, Q.M.; Cai, W.; Deng, H. Direct and indirect effects of pathogenic bacteria on the integrity of intestinal barrier. Therapeutic advances in gastroenterology 2023, 16, 17562848231176427. [Google Scholar] [CrossRef]
- Dey, P.; Ray Chaudhuri, S. The opportunistic nature of gut commensal microbiota. Critical reviews in microbiology 2023, 49, 739–763. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.; Takeda, K. (2025, December). The role of the mucosal barrier system in maintaining gut symbiosis to prevent intestinal inflammation. In Seminars in Immunopathology (Vol. 47, No. 1, p. 2). Berlin/Heidelberg: Springer Berlin Heidelberg.
- Aburto, M.R.; Cryan, J.F. Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota–gut–brain axis. Nature reviews Gastroenterology & hepatology 2024, 21, 222–247. [Google Scholar]
- Tao, W.; Yu, Y.; DANNI, T.; Huang, X.; Huang, J.; Lin, C.; Yu, R. Microbiota and enteric nervous system crosstalk in diabetic gastroenteropathy: bridging mechanistic insights to microbiome-based therapies. Frontiers in Cellular and Infection Microbiology 2025, 15, 1603442. [Google Scholar] [CrossRef] [PubMed]
- Dezfouli, M.A.; Rashidi, S.K.; Yazdanfar, N.; Khalili, H.; Goudarzi, M.; Saadi, A.; Kiani Deh Kiani, A. The emerging roles of neuroactive components produced by gut microbiota. Molecular Biology Reports 2025, 52, 1. [Google Scholar] [CrossRef]
- Müller, L.; Di Benedetto, S. Neuroimmune crosstalk in chronic neuroinflammation: microglial interactions and immune modulation. Frontiers in Cellular Neuroscience 2025, 19, 1575022. [Google Scholar] [CrossRef]
- Kustrimovic, N.; Balkhi, S.; Bilato, G.; Mortara, L. Gut microbiota and immune system dynamics in Parkinson’s and Alzheimer’s diseases. International Journal of Molecular Sciences 2024, 25, 12164. [Google Scholar] [CrossRef]
- Anwer, E.K.; Ajagbe, M.; Sherif, M.; Musaibah, A.S.; Mahmoud, S.; ElBanbi, A.; Abdelnaser, A. Gut microbiota secondary metabolites: key roles in GI tract cancers and infectious diseases. Biomedicines 2025, 13, 100. [Google Scholar] [CrossRef]
- Mafe, A.N.; Büsselberg, D. The Effect of Microbiome-Derived Metabolites in Inflammation-Related Cancer Prevention and Treatment. Biomolecules 2025, 15, 688. [Google Scholar] [CrossRef] [PubMed]
- Marroncini, G.; Naldi, L.; Martinelli, S.; Amedei, A. Gut–liver–pancreas axis crosstalk in health and disease: from the role of microbial metabolites to innovative microbiota manipulating strategies. Biomedicines 2024, 12, 1398. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.A.; Patel, V.P.; Bhosle, K.P.; Nagare, S.D.; Thombare, K.C. The tumor microenvironment: shaping cancer progression and treatment response. Journal of Chemotherapy 2025, 37, 15–44. [Google Scholar] [CrossRef]
- Nishida, A.; Andoh, A. The role of inflammation in cancer: mechanisms of tumor initiation, progression, and metastasis. Cells 2025, 14, 488. [Google Scholar] [CrossRef]
- Mc Neil, V.; Lee, S.W. Advancing Cancer Treatment: A Review of Immune Checkpoint Inhibitors and Combination Strategies. Cancers 2025, 17, 1408. [Google Scholar] [CrossRef]
- Dey, P.; Ray Chaudhuri, S. The opportunistic nature of gut commensal microbiota. Critical reviews in microbiology 2023, 49, 739–763. [Google Scholar] [CrossRef]
- Garabatos, N.; Angelats, E.; Santamaria, P. Mechanistic and therapeutic advances in immune-mediated gastrointestinal disorders. Journal of Allergy and Clinical Immunology 2025. [Google Scholar] [CrossRef]
- Pignatelli, P.; Nuccio, F.; Piattelli, A.; Curia, M.C. The role of Fusobacterium nucleatum in oral and colorectal carcinogenesis. Microorganisms 2023, 11, 2358. [Google Scholar] [CrossRef]
- Peterson, C.T. Dysfunction of the microbiota-gut-brain axis in neurodegenerative disease: the promise of therapeutic modulation with prebiotics, medicinal herbs, probiotics, and synbiotics. Journal of evidence-based integrative medicine 2020, 25, 2515690X20957225. [Google Scholar] [CrossRef]
- Jaswal, A.S.; Mishra, S.; Elangovan, R. Prebiotic Oligosaccharide Production in Microbial Cells. Microbial Nutraceuticals: Products and Processes 2025, 81–113. [Google Scholar]
- Shah, A.M.; Tarfeen, N.; Mohamed, H.; Song, Y. Fermented foods: their health-promoting components and potential effects on gut microbiota. Fermentation 2023, 9, 118. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- Amen, R.A.; Hassan, Y.M.; Essmat, R.A.; Ahmed, R.H.; Azab, M.M.; Shehata, N.R.; El-Sayed, W.M.; et al. Harnessing the Microbiome: CRISPR-Based Gene Editing and Antimicrobial Peptides in Combating Antibiotic Resistance and Cancer. Probiotics and Antimicrobial Proteins 2025, 1–31. [Google Scholar] [CrossRef]
- Yaqub, M.O.; Jain, A.; Joseph, C.E.; Edison, L.K. Microbiome-driven therapeutics: from gut health to precision medicine. Gastrointestinal Disorders 2025, 7, 7. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Q. Gut microbiota influences the efficiency of immune checkpoint inhibitors by modulating the immune system. Oncology Letters 2024, 27, 87. [Google Scholar] [CrossRef]
- Ciernikova, S.; Sevcikova, A.; Novisedlakova, M.; Mego, M. Insights into the Relationship Between the Gut Microbiome and Immune Checkpoint Inhibitors in Solid Tumors. Cancers 2024, 16, 4271. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, B.; Zhang, Y.; Chen, G.; Zhao, P.; Gao, Q.; Yuan, L. The role of intestinal flora on tumorigenesis, progression, and the efficacy of PD-1/PD-L1 antibodies in colorectal cancer. Cancer Biology & Medicine 2024, 21, 65–82. [Google Scholar]
- Xin, Y.; Peng, G.; Song, W.; Zhou, X.; Huang, X.; Cao, X. Gut microbiota as a prognostic biomarker for unresectable hepatocellular carcinoma treated with anti-PD-1 therapy. Frontiers in Genetics 2024, 15, 1366131. [Google Scholar] [CrossRef]
- Cao, Q.; Yang, M.; Chen, M. Metabolic interactions: how gut microbial metabolites influence colorectal cancer. Frontiers in Microbiology 2025, 16, 1611698. [Google Scholar] [CrossRef]
- Mafe, A.N.; Büsselberg, D. Microbiome integrity enhances the efficacy and safety of anticancer drug. Biomedicines 2025, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Cheemala, S.C.; Syed, S.; Bibi, R.; Suhail, M.B.; Dhakecha, M.D.; Subhan, M.; Islam, R. Unraveling the gut microbiota: key insights into its role in gastrointestinal and cardiovascular health. Journal of Advances in Medicine and Medical Research 2024, 36, 34–47. [Google Scholar] [CrossRef]
- Leng, X.; Wei, X.; Wang, J.; Yao, X.; Zhang, M.; Sun, D.; Cheng, Y.; et al. Impacts of intestinal microbiota metabolite trimethylamine N-oxide on cardiovascular disease: a bibliometric analysis. Frontiers in Microbiology 2025, 15, 1491731. [Google Scholar] [CrossRef]
- Mak, K.M.; Shekhar, A.C. Lipopolysaccharide, arbiter of the gut–liver axis, modulates hepatic cell pathophysiology in alcoholism. The Anatomical Record 2025, 308, 975–1004. [Google Scholar]
- Mehta, I.; Juneja, K.; Nimmakayala, T.; Bansal, L.; Pulekar, S.; Duggineni, D.; Younas, S. Gut Microbiota and Mental Health: A Comprehensive Review of Gut-Brain Interactions in Mood Disorders. Cureus 2025, 17. [Google Scholar] [CrossRef]
- Munteanu, C.; Turti, S.; Marza, S.M. Unraveling the Gut–Skin Axis: The Role of Microbiota in Skin Health and Disease. Cosmetics 2025, 12, 167. [Google Scholar] [CrossRef]
- Larnder, A.H.; Manges, A.R.; Murphy, R.A. The estrobolome: Estrogen-metabolizing pathways of the gut microbiome and their relation to breast cancer. International Journal of Cancer 2025. [Google Scholar]
- Babu, A.; Devi Rajeswari, V.; Ganesh, V.; Das, S.; Dhanasekaran, S.; Usha Rani, G.; Ramanathan, G. Gut microbiome and polycystic ovary syndrome: interplay of associated microbial-metabolite pathways and therapeutic strategies. Reproductive Sciences 2024, 31, 1508–1520. [Google Scholar] [CrossRef] [PubMed]
- Amen, R.A.; Hassan, Y.M.; Essmat, R.A.; Ahmed, R.H.; Azab, M.M.; Shehata, N.R.; El-Sayed, W.M.; et al. Harnessing the Microbiome: CRISPR-Based Gene Editing and Antimicrobial Peptides in Combating Antibiotic Resistance and Cancer. Probiotics and Antimicrobial Proteins 2025, 1–31. [Google Scholar] [CrossRef]
- Shukla, V.; Singh, S.; Verma, S.; Verma, S.; Rizvi, A.A.; Abbas, M. Targeting the microbiome to improve human health with the approach of personalized medicine: Latest aspects and current updates. Clinical Nutrition ESPEN 2024, 63, 813–820. [Google Scholar] [CrossRef]
- Maslowski, K.M. Metabolism at the centre of the host–microbe relationship. Clinical & Experimental Immunology 2019, 197, 193–204. [Google Scholar]
- Jyoti; Dey, P. Mechanisms and implications of the gut microbial modulation of intestinal metabolic processes. npj Metabolic Health and Disease 2025, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Shahini, A.; Shahini, A. Role of interleukin-6-mediated inflammation in the pathogenesis of inflammatory bowel disease: focus on the available therapeutic approaches and gut microbiome. Journal of cell communication and signaling 2023, 17, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Ji, G.; Zhang, L. Immunomodulatory effects of inulin and its intestinal metabolites. Frontiers in immunology 2023, 14, 1224092. [Google Scholar] [CrossRef]
- Al-Habsi, N.; Al-Khalili, M.; Haque, S.A.; Elias, M.; Olqi, N.A.; Al Uraimi, T. Health benefits of prebiotics, probiotics, synbiotics, and postbiotics. Nutrients 2024, 16, 3955. [Google Scholar] [CrossRef]
- David, A.; Lev-Ari, S. Targeting the Gut Microbiome to Improve Immunotherapy Outcomes: A Review. Integrative Cancer Therapies 2024, 23, 15347354241269870. [Google Scholar] [CrossRef]
- Dongre, D.S.; Saha, U.B.; Saroj, S.D. Exploring the role of gut microbiota in antibiotic resistance and prevention. Annals of Medicine 2025, 57, 2478317. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: an integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Alvarez, A.S.; de Vos, W.M. The gut microbiota in the first decade of life. Trends in microbiology 2019, 27, 997–1010. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





