1. Introduction
Cancer biomarkers are molecular indicators that reflect the biological state of tumors, offering valuable insight into cancer development, progression, and therapeutic responsiveness [
1]. In clinical oncology, biomarkers are essential tools for stratifying patients, predicting disease prognosis, and guiding personalized treatment strategies. The sensitivity and specificity of cancer biomarker detection can greatly influence the efficacy of diagnostic and therapeutic decisions [
2]. Detection of cancer biomarkers enables early diagnosis, which significantly improves patient prognosis and survival rates. The sensitivity and specificity of cancer biomarker detection can have a significant impact on the efficacy of diagnostic and therapeutic decisions. Despite remarkable advances in cancer treatment, the lack of early and accurate detection methods remains a major challenge, highlighting the need for sensitive biomarker detection platforms [
3].
Alpha-fetoprotein (AFP), a glycoprotein produced primarily in the fetal liver and yolk sac, is one of the most widely used biomarkers for hepatocellular carcinoma (HCC) and germ cell tumors (GCTs). Although elevated levels of AFP are associated with malignancies, their presence in small quantities in adult serum necessitates highly sensitive and selective detection methods for clinical application [
4,
5]. Conventional immunoassays, such as enzyme-linked immunosorbent assays (ELISAs), though widely used, often lack the sensitivity required for early-stage cancer detection or for samples with low AFP concentrations. Therefore, biosensing platforms with enhanced sensitivity and signal amplification capabilities are of growing importance [
6,
7,
8].
Quartz crystal microbalance (QCM) biosensors have attracted significant attention as mass-sensitive transducers capable of label-free detection of biomolecular interactions. QCM sensors operate based on the piezoelectric properties of quartz crystals, where the resonance frequency shifts in response to changes in mass on the crystal surface, as described by the Sauerbrey equation. As QCM is capable of detecting real-time mass changes at the sub-nanogram level, it has found broad applications in immunosensing, DNA hybridization, and molecular interaction studies. However, the limited sensitivity of QCM, most notably at low analyte concentrations, has led to the widespread adoption of signal amplification approaches [
9,
10,
11,
12].
Recent advances in nanotechnology have opened new possibilities for improving biosensor performance. Among these, nanoparticle-mediated signal amplification has shown remarkable potential. Gold nanoparticles (AuNPs) have been widely employed to enhance the QCM signal by increasing the mass on the sensor surface through sandwich-type immunoassay configurations [
13,
14,
15,
16,
17]. Furthermore, enhancement techniques such as gold staining have enabled even greater signal magnitudes by catalytically growing metal layers around nanoparticle cores. Building on this foundation, alternative approaches utilizing photocatalytic deposition of metals such as silver on nanoparticle surfaces are being investigated for greater signal amplification and cost-effectiveness.
In particular, tungsten(IV) oxide (WO₃) nanoparticles have emerged as a promising photocatalytic material due to their tunable bandgap, chemical stability, and strong oxidative power under UV or visible light. When irradiated, WO₃ generates electron-hole pairs capable of driving redox reactions, such as the reduction of metal ions (e.g., Ag⁺) into metallic deposits on the nanoparticle surface [
18,
19,
20,
21]. This photocatalytic property can be harnessed to amplify the detectable mass on QCM biosensors. By functionalizing WO₃ nanoparticles with antibodies and exposing them to silver ions under UV light, a silver shell can be deposited onto the WO₃ particles, resulting in a significant increase in overall mass and corresponding frequency shift in QCM measurements. Although silver has a lower atomic density and mass than gold, which may result in a relatively smaller signal amplification effect, its photo-induced deposition eliminates the need for additional reducing agents, enabling a more controlled and environmentally benign process [
22,
23]. Importantly, WO₃ nanoparticles offer a dual functionality, acting both as photocatalysts and as nanocarriers for antibody immobilization, thereby streamlining sensor design and enhancing overall assay performance.
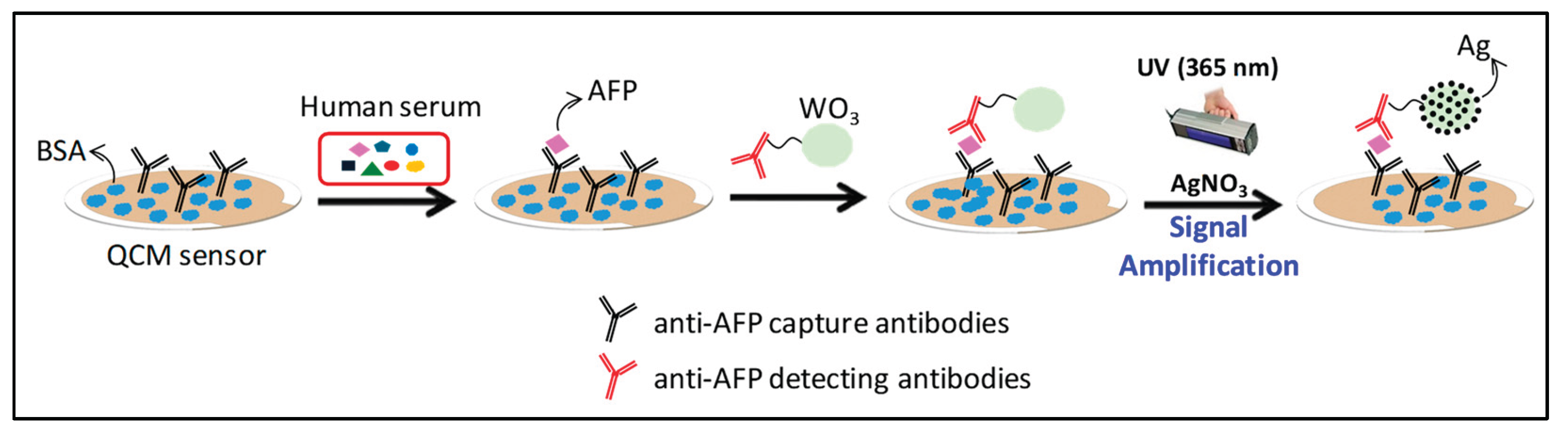
In this study, we report a novel QCM immunosensor for AFP detection that integrates photocatalytic signal amplification via silver deposition on WO₃ nanoparticles. The biosensor is constructed by covalently attaching anti-AFP capture antibodies onto a 3-Glycidyloxypropyltrimethoxysilane (3-GPTMS)-functionalized surface, which promotes stable and efficient antibody binding. A sandwich-type immunoassay is then conducted using AFP-containing serum and detection antibodies conjugated to WO₃ nanoparticles. Upon UV irradiation in the presence of Ag⁺, photocatalytic silver deposition occurs selectively on the nanoparticle surface, resulting in a marked increase in resonator mass and corresponding resonance frequency shift. This photocatalytic amplification method represents a significant advancement in QCM biosensing, offering improved sensitivity and lowering detection limits for AFP. Our approach not only broadens the scope of nanoparticle-based signal enhancement strategies but also demonstrates the potential of integrating semiconductor photocatalysts into label-free biosensing platforms. Ultimately, this technology could be extended to other clinically relevant biomarkers, paving the way for broader applications in early disease diagnostics and point-of-care testing.
2. Materials and Methods
2.1. Reagent and Materials
Recombinant Human AFP (ab114216) and two anti-AFP monoclonal antibodies (ab242532 for capture, ab242780 for detector) were purchased from Abcam (Cambridge, UK). AFP-free serum (P61000) was also obtained from BioPacific (Emeryville, CA, USA). Tungsten(IV) oxide (WO3) nanopowder (< 100 nm particle size) and silver nitrate (AgNO3) for photocatalytic silver deposition, 3-Glycidyloxypropyltrimethoxysilane (3-GPTMS) for surface modification were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) and used without further purification. Bovine serum albumin (BSA) and 10X phosphate-buffered saline (PBS, pH 7.4) was also purchased from Sigma-Aldrich Chemical Co. A 1X PBS solution was prepared by diluting 10X PBS with double-distilled water. All organic solvents were purchased from Samchun Chemical Co., Ltd. (Seoul, South Korea). All aqueous solutions were prepared in double-distilled water from a Milli-Q water-purifying system (18 MΩ cm).
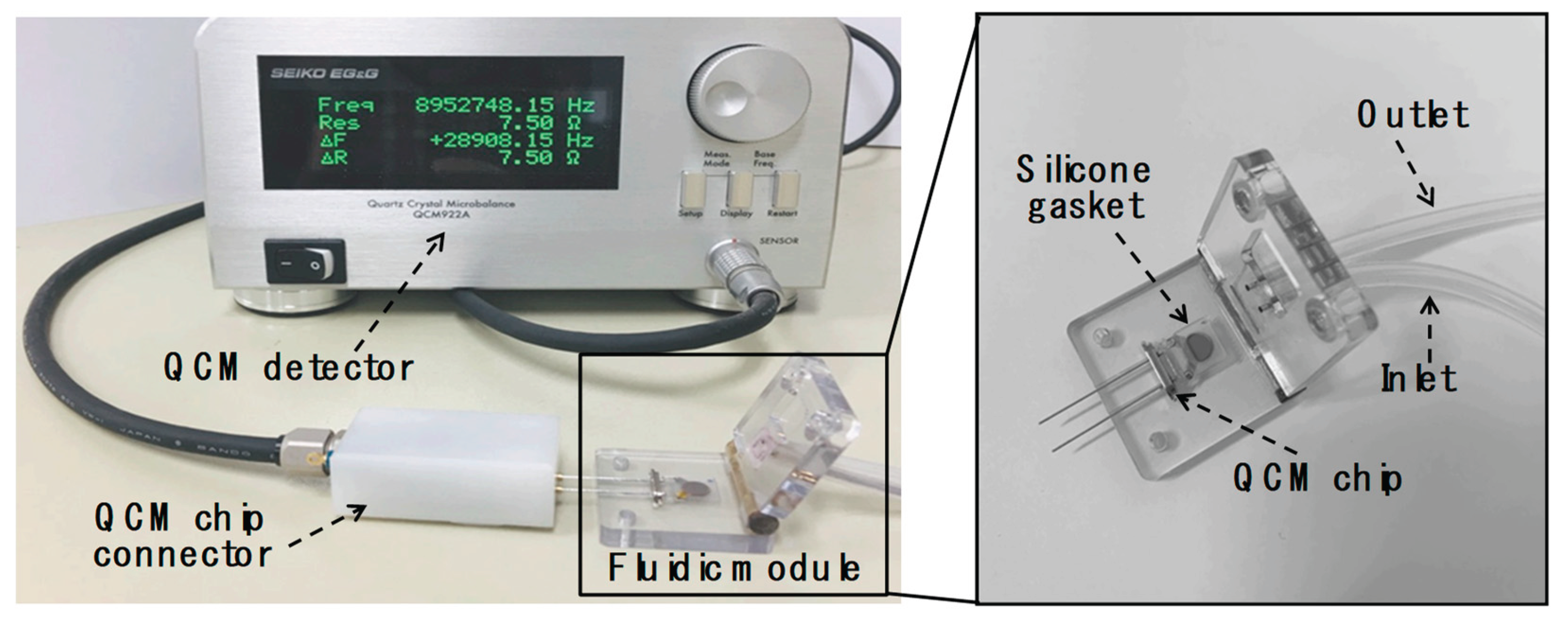
2.2. Design and Configuration of the QCM Measurement System
The QCM measurement system consisted of fluidic and detection modules designed to provide real-time sensor data during assays. Mass changes on silicon dioxide-coated QCM resonators (5 mm diameter; 9 MHz resonance frequency) were monitored by tracking shifts in the resonance frequency of bulk acoustic waves using a QCM922A (Princeton Applied Research, SEIKO EG&G, Tokyo, Japan). The flow cell comprised a peristaltic pump (ISM597; ISMATEC, Glattbrugg, Switzerland), a custom-fabricated fluidic block, and a silicone rubber gasket. Precise fluid control is critical for reliable and user-friendly biomolecular detection in liquid environments. To address this, we developed a specialized fluidic module as shown in
Figure 1. The top section of the fluidic block included recessed areas for reaction chambers and gaskets to prevent leakage caused by hydrodynamic pressure, along with integrated fluidic connectors enabling flow across the QCM sensors. Sample and buffer solutions were delivered to the reaction chambers via the peristaltic pump at a constant flow rate of 1.0 mL/min. Each reaction chamber had a volume of 20 µL. After each experiment, the chambers and gaskets were thoroughly rinsed with deionized water followed by 0.05% Tween 20 (Sigma-Aldrich, MO, USA) in PBS. Teflon
® tubing (0.032 in. I.D.; The Lee Company, CT, USA) was used to interconnect the fluidic and detection components.
2.3. X-Ray Diffraction (XRD) Analysis
Samples for XRD analysis were prepared by repeated centrifugation and decantation of WO₃ (1.5 × 10⁻³ M) and Ag-doped WO₃ nanoparticles, both synthesized via photocatalytic silver staining in double-distilled water. The collected residues were oven-dried and analyzed using a Rigaku Miniflex 600 diffractometer (Wilmington, MA, USA). The XRD spectra were recorded in 2-thetha range between 5° and 90°. For TEM imaging, highly diluted suspensions of WO₃ nanoparticles (1.0 × 10⁻⁵ M) and WO₃/Ag hybrid nanostructures were prepared in double-distilled water. These samples were sonicated for 2 hours in an ultrasonic bath (Branson Ultrasonics Corp., Danbury, CT, USA) prior to imaging.
2.4. UV–Vis Spectroscopic Analysis of Silver-Doped WO₃ Nanostructures
To evaluate the photocatalytic activity of WO₃ nanoparticles in the reduction of silver ions, the nanoparticles were dispersed in aqueous AgNO₃ solutions with concentrations ranging from 0 to 20 mM. The mixtures were subjected to UV irradiation at a wavelength of 365 nm for durations ranging from 0 to 20 min using a handheld UV lamp. Upon irradiation, the solutions progressively developed a dark gray coloration, indicative of the formation of silver nanoparticles via photocatalytic reduction. The extent of silver nanoparticle generation was quantitatively assessed using UV–visible absorption spectroscopy. Absorbance measurements were performed in 96-well microplates (Thermo Fisher Scientific, Bremen, Germany) using an Epoch 2 microplate spectrophotometer (BioTek Instruments, Winooski, VT, USA). Each experimental condition was tested in quintuplicate to ensure reproducibility and statistical reliability.
2.5. Preparation of Antibody-Conjugated WO₃ Nanoparticles for AFP Biosensing
WO₃ nanoparticles (average diameter ~21 nm) were initially purified by sequential washing with methanol and deionized water, followed by drying in an oven at 80 °C. Surface activation was achieved by immersing the nanoparticles in 10 mL of freshly prepared piranha solution (H₂SO₄/H₂O₂ = 3:1, v/v) for 10 min. After thorough rinsing with deionized water, the activated nanoparticles were functionalized by treatment with 5% (v/v) 3-glycidyloxypropyltrimethoxy-silane (3-GPTMS) in methanol for 1 h at room temperature. The GPTMS-treated nanoparticles were subsequently washed with methanol (2 min), dried under a nitrogen (N₂) stream, and cured at 110 °C for 1 h in a dry oven. Post-curing, the particles were again washed with methanol and dried under N₂ to remove any unbound silane. For antibody conjugation, 10 μL of anti-AFP detecting antibody (1 mg/mL) was added to 1 mL of the GPTMS-modified WO₃ nanoparticle suspension. The mixture was gently agitated at room temperature for 1 h to facilitate covalent binding. To block residual active sites and minimize nonspecific binding, 0.1 mL of 1% bovine serum albumin (BSA) in 1× phosphate-buffered saline (PBS, pH 7.4) was added, followed by incubation for 30 min at room temperature. The resulting antibody-conjugated WO₃ nanoparticles were collected by centrifugation at 13,000 rpm for 10 min at 4 °C. After discarding the supernatant, the pellet was resuspended in 0.5 mL of 1× PBS containing 0.1% BSA and stored for subsequent use.
2.6. WO₃-Mediated Photocatalytic Silver Staining in Sandwich Immunoassay for AFP Detection
The SiO₂-coated QCM sensor surface was first cleaned by sequential rinsing with deionized water and absolute ethanol, followed by nitrogen (N₂) drying. The cleaned chip was then subjected to surface activation using UV/ozone treatment for 10 min. Subsequently, the activated surface was functionalized by immersion in 5% (v/v) 3-glycidyloxypropyl-trimethoxysilane (3-GPTMS) in methanol for 1 h at room temperature. Afterward, the chip was rinsed with methanol (2 min), dried under N₂, and cured at 110 °C for 1 h. A final methanol rinse and N₂ drying completed the silanization process. To immobilize the capture antibodies, 30 μL of anti-AFP antibody solution (100 μg/mL in PBS, pH 7.4) was deposited onto the GPTMS-modified QCM surface and incubated for 1 h in a humidity chamber to prevent evaporation. Unreacted surface sites were subsequently blocked with 3% bovine serum albumin (BSA) in PBS (pH 7.4) to minimize nonspecific binding during subsequent assay steps. Following surface preparation, 50 μL aliquots of AFP-spiked AFP-free human serum at various concentrations were applied to the sensor and incubated for 30 min to allow antigen binding. The surface was then washed with PBS (1 min), and 50 μL of WO₃ nanoparticle-conjugated anti-AFP detecting antibody was introduced. After a further 30 min incubation, the chip was again washed with PBS (1 min). For signal amplification, 5 mM AgNO₃ solution was added, and the sensor surface was irradiated with UV light (365 nm) using a 4 W handheld UV lamp (Vilber Lourmat, France) for 10 min to initiate photocatalytic silver deposition mediated by the WO₃ nanoparticles. The sensor was finally washed with PBS to remove excess reagents. All measurements were performed in triplicate for each AFP concentration, with independent replicates conducted on different days to ensure reproducibility. In addition, a blind test was conducted using five serum samples spiked with unknown concentrations of AFP. Each 1 mL sample was analyzed using both the developed QCM sensor platform and the commercial VIDAS® immunoassay system (BioMérieux, Marcy L’Étoile, France) to assess comparative performance.
3. Results and Discussion
3.1. Strategies for Sensitive AFP Immunoassay: Detection and Signal Amplification Approaches
Scheme 1 presents the overall strategy for AFP detection, which is based on a conventional sandwich immunoassay format coupled with a photocatalytic signal amplification mechanism. In this approach, anti-AFP capture antibodies were covalently immobilized onto a QCM sensor surface pre-functionalized with 3-glycidyloxypropyltrimethoxysilane (3-GPTMS). Upon exposure to serum containing AFP, the target proteins specifically bound to the immobilized antibodies, forming the primary immune complex. Subsequently, detection antibodies conjugated to WO₃ nanoparticles were introduced to form a complete sandwich structure. To amplify the sensor signal, the bound WO₃ nanoparticles underwent a photocatalytic silver staining reaction, which increased their size and mass, thereby enhancing the resonance frequency shift measured by the QCM. This signal amplification was achieved through UV-induced reduction of silver ions (Ag⁺) on the WO₃ nanoparticle surface, resulting in the formation of a WO₃/Ag composite. The silver deposition step was critical for achieving sufficient signal intensity, as the resonance frequency shift without this amplification was negligible and inadequate for reliable AFP quantification. These results indicate that the majority of the observed frequency change during AFP detection originated from the photocatalytic silver staining process.
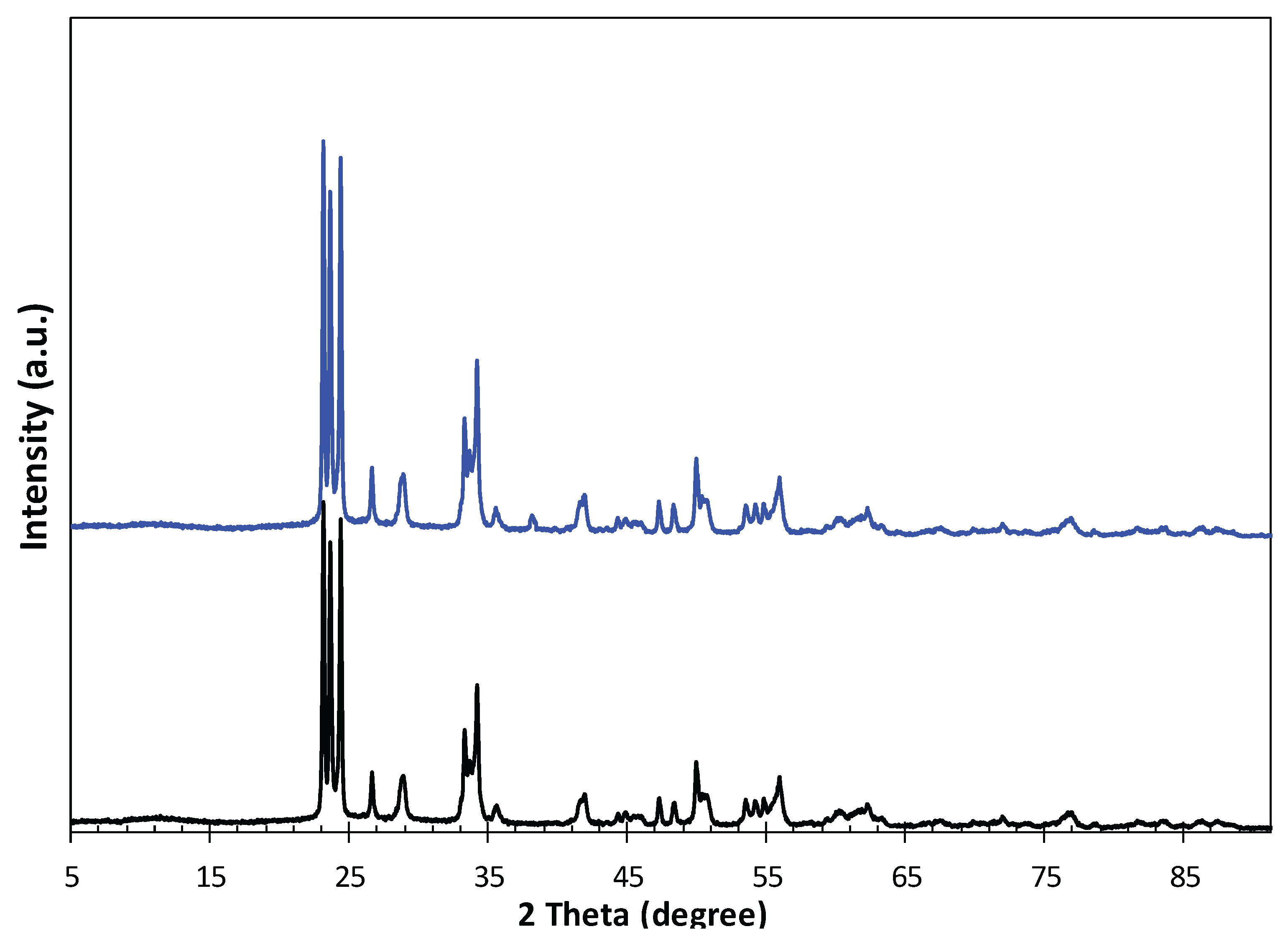
To confirm the successful formation of the WO₃/Ag composite, X-ray diffraction (XRD) analysis was conducted to investigate the crystal structures of WO₃ and Ag-doped WO₃ nanostructures, as well as to confirm the presence of silver particles on the WO₃ surface following photocatalytic silver deposition. For comparison, the XRD pattern of pristine WO₃ was also recorded. The diffraction results for both WO₃ and Ag/WO₃ specimens are presented in
Figure 2. As shown in
Figure 2, the typical diffraction spectrum of WO₃ crystals exhibits the characteristic peaks of tungsten oxide. The XRD patterns of Ag-loaded WO₃ clearly display the same set of peaks observed for pure WO₃, confirming the incorporation of silver into the tungsten oxide lattice. A distinct peak appearing at approximately 38.6° was identified as the (111) main reflection of metallic silver, which was absent in the pristine WO₃ sample. These XRD results validate the successful fabrication of Ag/WO₃ nanostructures through photocatalytic deposition of silver.
3.2. Signal Amplification Process: WO₃-Based Photocatalytic Silver Staining
To improve the detection sensitivity of the QCM-based sandwich immunoassay, we performed a photocatalytic silver staining reaction, which is crucial for amplifying the mass-induced resonance shift of the QCM sensor. Given that the silver staining process relies on UV-induced reduction of Ag⁺ ions in aqueous AgNO₃ solution in the presence of WO₃ nanoparticles, two critical factors were investigated: AgNO₃ concentration and UV irradiation time. Throughout this process, the intensity of UV light was held constant to exclude its influence on reaction kinetics. First, the effect of UV irradiation duration was assessed by dispersing WO₃ nanoparticles in 10 mM AgNO₃ solution and monitoring the absorption band appeared in the visible region, which is a typical phenomenon associated with silver formation [
24]. As shown in
Figure 3(a), the UV–vis spectra recorded at various time points revealed a progressive increase in absorbance at 400 nm with prolonged UV exposure. Based on the rate of absorbance increase and the overall immunoassay time for AFP detection, we determined that 5 minutes of UV irradiation was sufficient. Next, the influence of AgNO₃ concentration was investigated under the fixed condition of 10 minutes UV exposure. As shown in
Figure 3(b), the absorbance at 400 nm increased with increasing AgNO₃ concentration up to 20 mM. A concentration of 10 mM was considered sufficient to achieve uniform and stable silver deposition. At higher concentrations, excessive nucleation of silver could lead to uncontrolled nanoparticle growth and potential detachment from the WO₃ surface. This was further evidenced in QCM experiments, where sensor instability and signal deterioration were observed due to silver nanoparticle aggregation and shedding from the surface. Based on these observations, the conditions for WO₃-mediated photocatalytic silver staining were established as 10 mM AgNO₃ and 5 minutes of UV irradiation. These parameters were employed in all subsequent signal amplification steps within the AFP sandwich immunoassay.
3.3. Real-Time QCM Sensor Response for AFP Detection
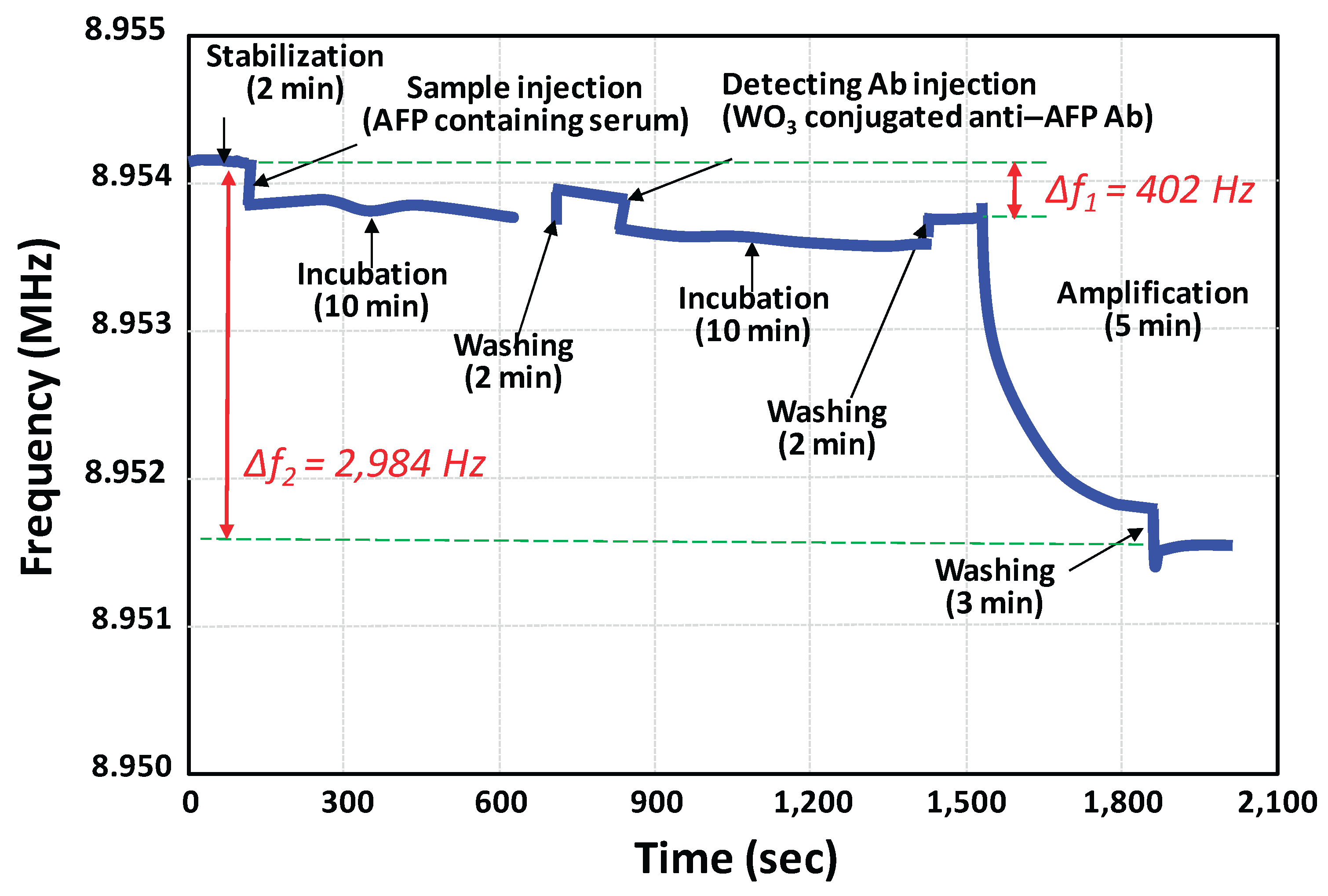
Figure 4 illustrates the real-time changes in resonance frequency and resistance of the QCM sensor during the stepwise detection of AFP. The sensing process comprises three main stages: (1) immobilization and binding of AFP to surface-anchored capture antibodies, (2) formation of sandwich complexes through the introduction of WO₃-conjugated detection antibodies, and (3) signal amplification via photocatalytic silver staining, leading to size enhancement of the WO₃ nanoparticles. The entire process takes approximately 30 min. As anticipated, the resonance frequency decreased progressively due to mass accumulation on the sensor surface during each sequential step, while the resonance resistance concurrently increased, reflecting energy dissipation associated with the growing viscoelastic layer [
25]. The blue bold line in
Figure 4 represents the temporal decrease in resonance frequency observed during AFP detection at a concentration of 10 ng/mL. Notably, the most significant shifts in both parameters occurred during the photocatalytic silver deposition phase. This amplification step, in which metallic silver was selectively deposited onto the surface of WO₃ nanoparticles, substantially increased the total mass bound to the sensor, resulting in a pronounced frequency drop. These findings underscore the critical role of the silver staining step in enhancing signal magnitude. In the absence of this step, the sensor exhibits a markedly weaker response, thereby compromising detection sensitivity. Thus, the incorporation of photocatalytic silver amplification is essential for achieving ultrasensitive AFP detection using the QCM platform. The substantial increase in mass observed on the QCM sensor surface following the photocatalytic deposition of metallic silver onto WO₃ nanoparticles resulted in a pronounced decrease in resonance frequency, as described by the Sauerbrey equation (Equation (1)) [
26]:
where f
0 is the fundamental resonance frequency (8.953 × 10⁶ Hz), Δf is the change in frequency (Hz), Δm is the change in mass (g), A is the active area of the piezoelectric quartz crystal (0.196 cm²), and ρ
q and μ
q represent the mass density (2.648 g/cm³) and shear modulus (2.947 × 10¹¹ g·cm⁻¹·s⁻²) of AT-cut quartz, respectively.
3.4. Improving AFP Detection Sensitivity Using WO₃-Mediated Photocatalytic Silver Staining
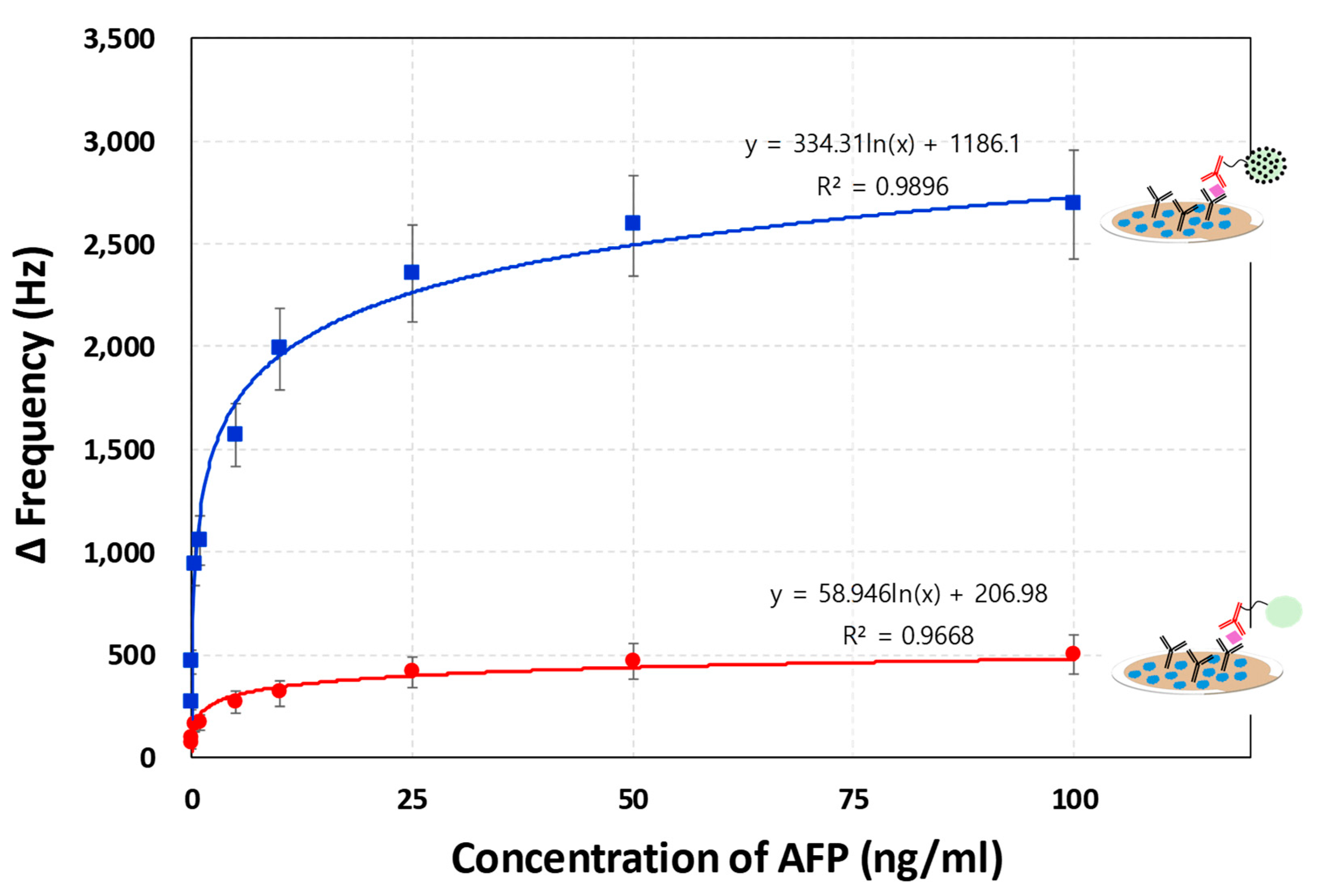
We investigated the effect of varying concentrations of alpha-fetoprotein (AFP) ranging from 50 pg/mL to 100 ng/mL in troponin-free human serum on the resonance frequency shifts of a QCM sensor during a sandwich immunoassay followed by photocatalytic silver staining. For each AFP concentration, the average frequency shift was calculated from three independent experiments. As depicted in
Figure 5, the resonance frequency shift increased logarithmically with increasing AFP concentration. At higher AFP concentrations, a greater amount of the target protein was captured by the immobilized paired antibodies, resulting in an increased accumulation of WO₃ nanoparticles conjugated to the detection antibody on the QCM sensor surface. This increase in surface-bound nanoparticles contributed to the mass loading on the sensor and was further amplified by the enlargement of the WO₃ nanoparticles via the photocatalytic silver deposition process. To isolate the mass-loading effect from other confounding factors, frequency shifts at each concentration were corrected by subtracting the blank values. This correction was essential to eliminate the influence of non-specific factors such as solution viscosity and temperature fluctuations, which were introduced during the silver staining process involving the addition of AgNO₃ and UV irradiation. It is well known that in most QCM-based biomolecular detection systems, the thickness of the adsorbed biomolecular layer is typically much smaller than the viscous penetration depth in the liquid phase. Therefore, viscosity changes can significantly affect the resonance frequency. However, in this study, the presence of a signal amplification step involving photocatalytic nanoparticle growth necessitated rigorous blank subtraction to ensure that the observed frequency changes reflected only the mass-loading effect due to AFP binding.
The blue plot in
Figure 5 illustrates the results of a WO₃ nanoparticle-conjugated sandwich immunoassay without signal amplification, while the upper red plot shows the enhanced response achieved through photocatalytic silver staining-mediated signal amplification. As previously described, the silver staining process significantly contributed to the overall decrease in resonance frequency due to the increased mass loading and nanoparticle growth on the sensor surface. Signal amplification using photocatalytic silver staining (red plot) substantially improved the sensor’s sensitivity, achieving a limit of detection (LOD) for AFP of 43.7 pg/mL. In contrast, the LODs for the assays without amplification were 286 pg/mL (blue plot). In all cases, the LOD, calculated according to Shrivastava and Gupta [
27], was defined as follows
Owing to the enhanced signal intensity from silver deposition, the detectable AFP concentration was significantly reduced—representing approximately 6.54-fold improvements in LOD compared to the blue plot. Furthermore, the linear correlation (R²) between AFP concentration and resonance frequency improved with signal amplification. The correlation coefficient increased from 0.9668 (red plot) to 0.9896 (blue plot) with silver staining (
Figure 5), indicating better linearity and quantitative reliability. The coefficient of variation (CV), a key factor for reproducibility, was 30.6% at the lowest AFP concentration (50 pg/mL) for the red plot. However, when the same assay was followed by photocatalytic silver staining, the CV values across all tested concentrations were reduced to below 13.6%, demonstrating significantly improved assay precision (
Supplementary material S1). Taken together, these findings indicate that the QCM-based AFP immunosensor employing WO₃ nanoparticle-mediated photocatalytic silver staining provides a highly sensitive and reproducible platform, with an LOD of 43.7 pg/mL. This performance is comparable to, or better than, several recently reported nanomaterial-based AFP biosensors. A comparative summary of LODs from various detection methods, including the present study, is provided in
Table 1.
3.5. Evaluation of QCM Sensor Performance Using Blind-Spiked AFP Samples
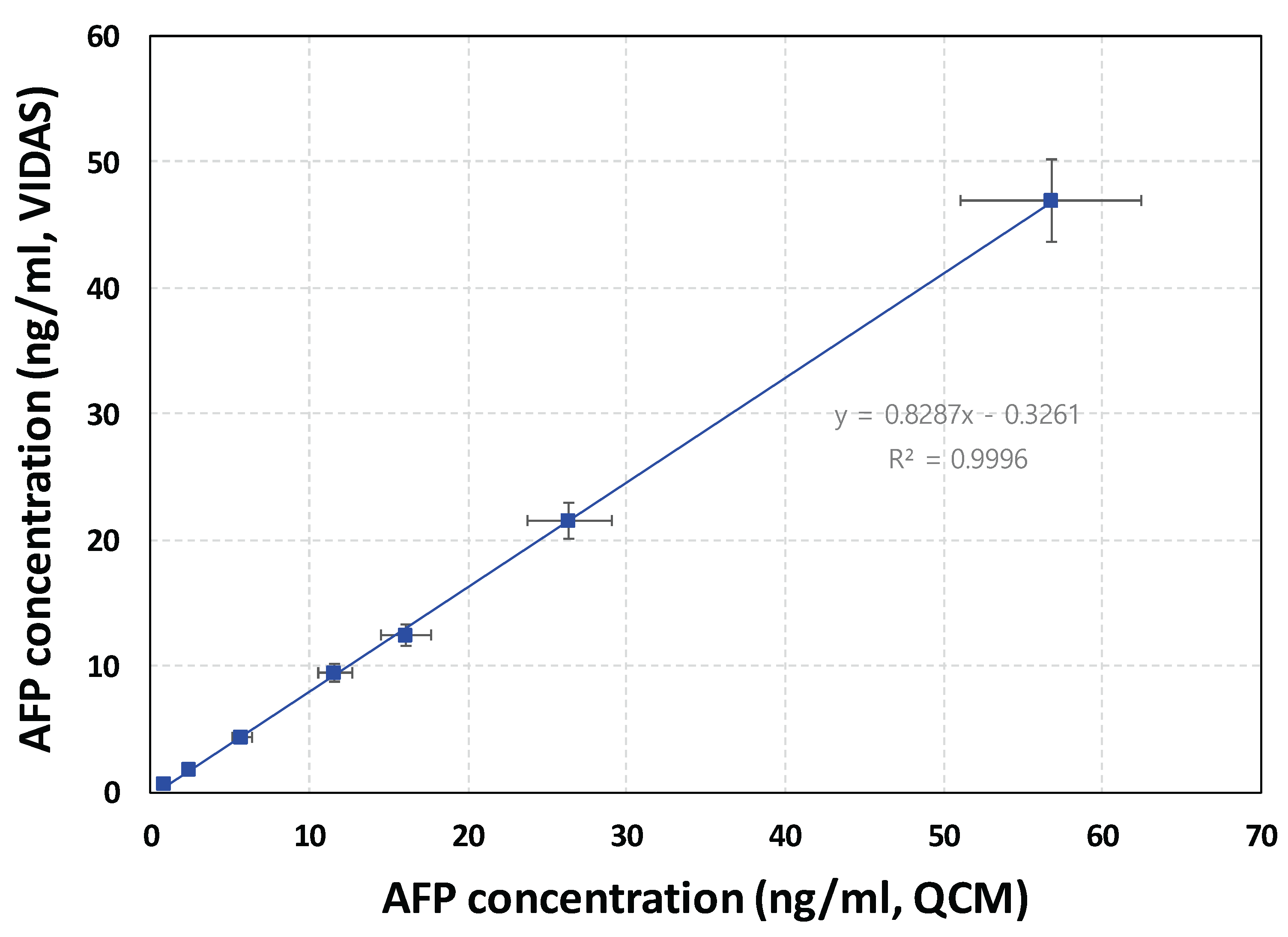
To validate the practical utility and analytical accuracy of our QCM-based immunoassay for AFP detection, a blind-spiked sample analysis was conducted using both our QCM sensor platform and a commercial reference system, the VIDAS
® immunoassay (bioMérieux, Marcy L’Étoile, France). Seven serum samples were independently prepared by spiking an undisclosed amount of AFP into AFP-free human serum (1 mL each). These blind samples were analyzed using the two systems, and the results were compared. In the QCM system, the resonance frequency shifts measured for the blind samples were converted into AFP concentrations using the standard calibration curve derived from the upper (blue) plot in
Figure 5. The AFP concentrations obtained from both the QCM and VIDAS
® systems are presented in
Supplementary material S2. For the QCM system, the mean value of three replicate measurements was used, while the VIDAS
® results represent the average of two replicates. Relative errors were calculated as the percentage difference between the QCM and VIDAS
® results, normalized to the VIDAS
® values. Although the AFP concentrations measured by the QCM system were slightly lower overall compared to those from the clinically validated VIDAS
® system, the relative errors ranged between 20–35 %. Despite these deviations, the two methods showed acceptable agreement across the entire AFP concentration range tested (0.5 ng/mL to 50 ng/mL), as illustrated in
Figure 6. Deming regression analysis revealed a strong correlation between the two methods, with a correlation coefficient (R) of 0.9996 and a slope of 0.8287. Considering the methodological and calibration differences between the two systems, the proportional bias indicated by the slope was deemed acceptable [
39]. Additionally, the recovery rates of AFP spiked into human serum at three concentrations (5, 10, and 50 ng/mL) were assessed using the QCM immunoassay with photocatalytic silver staining. The observed recovery rates ranged from 116% to 123%, further supporting the quantitative performance of the developed assay (
Supplementary material S3).
4. Conclusions
In this study, we successfully developed a sandwich immunoassay combined with a WO₃-mediated photocatalytic silver staining technique for signal amplification, enabling highly sensitive and reproducible detection of AFP in human serum. The substantial enhancement in resonance frequency shift induced by the signal amplification markedly improved both the sensitivity and reproducibility of the assay. Given these advantages, the proposed QCM sensing platform holds great promise for broad applications in immunoassays and biomarker detection. Future work will focus on advancing the QCM biosensor system towards point-of-care testing by integrating automated fluidics and multiplexed target detection. In this context, a cartridge-based device incorporating a QCM sensor chip and a UV irradiation module capable of automated fluid control is envisioned, with materials such as quartz that allow efficient transmission of 365 nm UV light being essential for optimal performance.
Supplementary Materials
The following supporting information can be downloaded at website of this paper posted on Preprints.org, Table S1: QCM signal intensities (changes in resonance frequency) and % CV values; Table S2: Blind-spiked AFP sample tests using our QCM sensing system and the VIDAS® immunoassay system; Table S3: Recovery rate of spiked AFP in human serum tested by our QCM sensing system
Author Contributions
Conceptualization, S.L.; methodology, H.K.; software, H.K.; validation, S.L., H.K.; formal analysis, H.K.; investigation, S.L.; resources, S.L.; data curation, H.K. and Y.C; writing—original draft preparation, S.H.; writing—review and editing, H.K.; visualization, Y.C.; supervision, S.L.; project administration, S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2022R1F1A1067428).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This work was supported by Soonchunhyang University. This work was also the results of a study on the “Regional Innovation System & Education (RISE)” Project, supported by the Ministry of Education of Korea and Chungnam RISE.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Passaro, A.; Bakir, M.A.; Hamilton, E.G.; Diehn, M.; Andre, F.; Roy-Chowdhuri, S.; Mountzios, G.; Wistuba, I.I.; Swanton, C.; Peters, S. Cancer biomarkers: Emerging trends and clinical implications for personalized treatment. Cell 2024, 187, 1617–1635. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dey, M.K.; Devireddy, R.; Gartia, M.R. Biomarkers in cancer detection, diagnosis, and prognosis. Sensors 2023, 24, 37. [Google Scholar] [CrossRef]
- Mustafa, S.K.; Khan, M.F.; Sagheer, M.; Kumar, D.; Pandey, S. Advancements in biosensors for cancer detection: revolutionizing diagnostics. Med.l Oncol. 2024, 41, 73. [Google Scholar] [CrossRef]
- Kim, H.; Jang, M.; Kim, E. Exploring the Multifunctional Role of Alpha-Fetoprotein in Cancer Progression: Implications for Targeted Therapy in Hepatocellular Carcinoma and Beyond. Int. J. Mol. Sci. 2025, 26, 4863. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, B.; li, M. The role of alpha-fetoprotein in the tumor microenvironment of hepatocellular carcinoma. Front. Oncol. 2024, 14, 1363695. [Google Scholar] [CrossRef]
- Niaki, N.M.; Hatefnia, F.; Heidari, M.M.; Tabean, M.; Mobed, A. Alpha-Fetoprotein (AFP) biosensors. Clin. Chim. Acta 2025, 573, 120293. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhao, H.; Huang, S.; Yin, F.; Wang, W.; Hu, Q.; Wang, Y.; Feng, B. Rapid and portable detection of hepatocellular carcinoma marker alpha-fetoprotein using a droplet evaporation-based biosensor. Talanta 2025, 294, 128189. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, L.; Rub, F.A.; Hajja, A.; Alodhaibi, I.; Arai, M.; Alfuwais, M.; Makhzoum, T.; Yaqinuddin, A.; Al-Kattan, K.; . Assiri, A.M.; Broering, D.C.; Chinnappan, R.; Mir, T.A.; Mani, N.K. Biosensing of Alpha-Fetoprotein: A Key Direction toward the Early Detection and Management of Hepatocellular Carcinoma. Biosensors 2024, 14, 235. [Google Scholar] [CrossRef]
- Alanazi, M.; Almutairi, M.; Alodhayb, A.N. A Review of quartz crystal microbalance for chemical and biological sensing applications. Sens Imaging 2023, 24, 10. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Özgür, E.; Denizli, A. Recent Advances in Quartz Crystal Microbalance Biosensors Based on the Molecular Imprinting Technique for Disease-Related Biomarkers. Chemosensors 2022, 10, 106. [Google Scholar] [CrossRef]
- Lino, C.; Barrias, S.; Chaves, R.; Adega, F.; Fernandes, J.R.; Martine-Lopes, P. Development of a QCM-based biosensor for the detection of non-small cell lung cancer biomarkers in liquid biopsies. Talanta 2023, 260, 124624. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Lee, S.S. Quartz crystal microbalance cardiac Troponin I immunosensors employing signal amplification with TiO2 nanoparticle photocatalyst. Talanta 2021, 228, 122233. [Google Scholar] [CrossRef] [PubMed]
- Safari, Z.; Cohan, R.A.; Sepahi, M.; Sadeqi, M.; Khoobi, M.; Fard, M.H.; Ghavidel, A.; Amiri, F.B.; Aghasadeghi, M.R.; Norouzian, D. Signal amplifcation of a quartz crystal microbalance immunosensor by gold nanoparticles-polyethyleneimine for hepatitis B biomarker detection. Sci. Reports 2023, 13, 21851. [Google Scholar]
- Pohanka, M. Quartz crystal microbalance biosensor for the detection of procalcitonin. Talanta 2023, 257, 124325. [Google Scholar] [CrossRef]
- Min, H.J.; Mina, H.A.; Deering, A.J.; Robinson, J.P.; Bae, E. Detection of Salmonella Typhimurium with gold nanoparticles using quartz crystal microbalance biosensor. Sensors 2022, 22, 8928. [Google Scholar] [CrossRef]
- Lim, J.Y.; Lee, S.S. A QCM immunosensor employing signal amplification strategies by enlarging the size of nanoparticles using gold or silver staining. Analyst 2022, 147, 5725–5731. [Google Scholar] [CrossRef]
- Kwak, J.; Lee, S.S. Highly sensitive piezoelectric immunosensors employing signal amplification with gold nanoparticles. Nanotechnology 2019, 30, 445502. [Google Scholar] [CrossRef]
- Ghazal, S.; Mirzaee, M.; Darroudi, M.; Sabouri, Z.; Khadempir, S. Chitosan-based synthesis of silver-doped tungsten oxide nanoparticles and assessment of its cytotoxicity and photocatalytic performance. J. Photochem. Photobiol. A Chem. 2024, 448, 115323. [Google Scholar] [CrossRef]
- Loka, C.; Lee, K.-S. Dewetted silver nanoparticle-dispersed WO3 heterojunction nanostructures on glass fibers for efficient visible-light-active photocatalysis by magnetron sputtering. ACS Omega 2022, 7, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Matalkeh, M.; Nasrallah, G.; Shurrab, F.M.; Al-Absi, E.S.; Mohammed, W.; Elzatahry, A.; Saoud, L.M. Visible light photocatalytic activity of Ag/WO3 nanoparticles and its antibacterial activity under ambient light and in the dark. Results Eng. 2022, 13, 100313. [Google Scholar] [CrossRef]
- Pourcin, F; Bernardini, S.; Videlot-Ackermann, C.; Nambiema, N.; Liu, J.; Liu, L.; Aguir, K.; Margeat, O.; Ackermann., J. Silver Growth on Tungsten Oxide Nanowires for Nitrogen Dioxide Sensing at Low Temperature. Proceedings 2018, 2, 946. [Google Scholar] [CrossRef]
- Cao, X.; Tan, B.; Zhang, B.; Huang, G.; Xu, H.; Song, Z.; Yan, J. Integration of silver nanoparticles into carbon-encapsulated tungsten oxide promoting visible-light-driven photocatalytic degradation efficiency. Appl. Surf. Sci. 2024, 678, 161112. [Google Scholar] [CrossRef]
- Can, F.; Courtois, X.; Duprez, D. Tungsten-Based Catalysts for Environmental Applications. Catalysis 2021, 11, 703. [Google Scholar] [CrossRef]
- Hussain, A.; Fiaz, S.; Almohammedi, A; Waqar, A. Optimizing photocatalytic performance with AG-doped ZNO nanoparticles: Synthesis and characterization. Heliyon, 2024,10, e35725.
- Muramatsu, H.; Kawamura, M.; Tanabe, S.; Suda, M. Basic characteristics of quartz crystal sensor with interdigitated electrodes. Anal. Chem. Res. 2016, 7, 23–30. [Google Scholar] [CrossRef]
- Cimmino, W.; Raucci, A.; Grosso, S.P.; Normanno, N.; Cinti, S. Enhancing sensitivity towards electrochemical miRNA detection using an affordable paper-based strategy. Anal. Bioanal. Chem. 2024, 416, 4227–4239. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, N.; Yang, A.; Xu, Z.; Zhang, W.; Liu, H.; Law, H.K. Ultrasensitive detection of ribonucleic acid biomarkers using portable sensing platforms based on organic electrochemical transistors. Anal. Chem. 2021, 93, 14359–14364. [Google Scholar] [CrossRef]
- Peng, J.; He, T.; Sun, Y.; Liu, Y.; Cao, Q.; Wang, Q.; Tang, H. A Route to the colorimetric detection of alpha-fetoprotein based on a smartphone. Micromachines 2024, 15, 1116. [Google Scholar] [CrossRef]
- Zhu, D.; Hu, Y.; Zhang, X.-J.; Yang, X.-T.; Tang, Y.-Y. Colorimetric and Fluorometric Dual-Channel Detection of α-Fetoprotein Based on the Use of ZnS-CdTe Hierarchical Porous Nanospheres. Microchim. Acta 2019, 186, 124. [Google Scholar] [CrossRef]
- Sun, C.; Pan, L.; Zhang, L.; Huang, J.; Yao, D.; Wang, C.-Z.; Zhang, Y.; Jiang, N.; Chen, L.; Yuan, C. A Biomimetic Fluorescent Nanosensor Based on Imprinted Polymers Modified with Carbon Dots for Sensitive Detection of Alpha-Fetoprotein in Clinical Samples. Analyst 2019, 144, 6760–6772. [Google Scholar] [CrossRef]
- Xie, B.; Wang, H.; Mochiwa, Z.O.; Zhou, D.; Gao, L. Highly sensitive detection of alpha-fetoprotein using sandwich sensors. RSC Adv. 2024, 14, 34661–34667. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, Y.; Cao, T.; Luo, C.; Wei, Q. A Chemiluminescence Sensor for the Detection of α-Fetoprotein and Carcinoembryonic Antigen Based on Dual-Aptamer Functionalized Magnetic Silicon Composite. Anal. Chem. 2023, 95, 7387–7395. [Google Scholar] [CrossRef]
- Liu, J.; Jing, T.; Qi, H.; Zhao, Y.; Wu, M. A nano-silver modified heterojunction of bismuth sulfide nanoflowers–titania nanorods for sensitively detecting alpha-fetoprotein. New J. Chem. 2025, 49, 7918–7927. [Google Scholar] [CrossRef]
- Su, X.; Chem. J.; Wu, S.; Qiu, Y.; Pan, Y.. A Signal-On Microelectrode Electrochemical Aptamer Sensor Based on AuNPs–MXene for Alpha-Fetoprotein Determination. Sensors 2024, 24, 7878.
- Thakur, B.; Anbalagan, A.C.; Koyande, P.; Sawant, S.M. Electrochemical surface plasmon resonance based biosensor for α-fetoprotein detection via different coupling strategies. Sci. Rep. 2025, 15, 16902. [Google Scholar] [CrossRef]
- Ma, H.; Sun, X.; Chen, L.; Cheng, W.; Han, X.X.; Zhao, B.; He, C. Multiplex Immunochips for High-Accuracy Detection of AFP-L3% Based on Surface-Enhanced Raman Scattering: Implications for Early Liver Cancer Diagnosis. Anal. Chem. 2017, 89, 8877–8883.
- Cong, B.; Liang, W.; Lai, W.; Jiang, M.; Ma, C.; Zhao, C.; Jiang, W.; Zhang, S.; Li, H.; Hong, C. A signal amplification electrochemiluminescence biosensor based on Ru (bpy)32+ and β-cyclodextrin for detection of AFP. Biochem. 2024, 156, 108626. [Google Scholar] [CrossRef]
- Lim, J.Y.; Lee, S.S. A QCM immunosensor employing signal amplification strategies by enlarging the size of nanoparticles using gold or silver staining. Analyst 2022, 147, 5725–5731. [Google Scholar] [CrossRef]
- Stevens, R.J.; Poppe, K.K. Validation of clinical prediction models: what does the “calibration slope” really measure? J. Clin. Epidemiol. 2020, 118, 93–99. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).