Submitted:
08 September 2025
Posted:
11 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Samples
2.1. Moisture Content Determination
2.2. Oil Content Determination
2.3. Factorial Design of Experiment
2.4. Pretreatment of Samples and Oil Extraction Procedure
2.5. Measurement of Absorbance-Wavelength Values
2.7. Calculated Responses from the Oil Extraction Process
2.7.1. Mass of Extracted Crude Oil
2.7.1. Mass of Extracted Oil and Seedcake Sediments
2.7.2. Percentage Oil Yield
2.7.3. Percentage Oil Oil Expression Efficiency
2.7.4. Extraction Loss
2.7.5. Throughput
2.6. Statistical Analysis
3. Results
3.1. Determined Moisture and Oil Contents
3.2. Evaluation of the Determined Parameters of Yellowish Sesame

|
(g) |
Input factor | Input factor | Coded Factor | Coded Factor | |||||||
| 100 | 40 | 15 | -1 | -1 | 99.55 | 0.45 | 266 | 0.37 | 64.20 | 34.92* 34.23** 33.90*** |
99.12* 98.43** 98.10*** |
| 100 | 40 | 30 | -1 | 0 | 99.28 | 0.72 | 251 | 0.40 | 66.13 | 32.38* 31.73** 31.26*** |
98.51* 97.86** 97.39*** |
| 100 | 40 | 45 | -1 | 1 | 99.07 | 0.93 | 247 | 0.40 | 66.81 | 31.86* 31.38** 31.52*** |
98.67* 98.19** 98.33*** |
| 100 | 50 | 15 | 0 | -1 | 99.00 | 1.00 | 243 | 0.41 | 64.86 | 33.41* 32.77** 32.80*** |
98.27* 97.63** 97.66*** |
| 100 | 50 | 30 | 0 | 0 | 98.81 | 1.19 | 243 | 0.41 | 65.73 | 32.39* 31.80** 31.88*** |
98.12* 97.53** 97.61*** |
| 100 | 50 | 45 | 0 | 1 | 97.71 | 2.29 | 237 | 0.41 | 66.22 | 31.30* 31.07** 30.91*** |
97.13* 97.29** 97.13*** |
| 100 | 60 | 15 | 1 | -1 | 98.81 | 1.19 | 241 | 0.41 | 68.32 | 30.29* 30.20** 30.29*** |
98.45* 98.52** 98.61*** |
| 100 | 60 | 30 | 1 | 0 | 98.21 | 1.79 | 237 | 0.41 | 68.46 | 29.41* 28.81** 28.43*** |
97.87* 97.27** 96.89*** |
| 100 | 60 | 45 | 1 | 1 | 98.08 | 1.92 | 246 | 0.40 | 68.90 | 28.94* 28.36** 28.41*** |
97.84* 97.26** 97.31*** |
| 100 | 50 | 30 | 0 | 0 | 98.87 | 1.13 | 247 | 0.40 | 66.74 | 32.08* 31.56** 31.30*** |
98.82* 98.30** 98.04*** |
| 100 | 50 | 30 | 0 | 0 | 99.00 | 1.00 | 244 | 0.41 | 65.80 | 32.20* 31.86** 30.80*** |
98.00* 97.66** 96.60*** |
| 100 | 50 | 30 | 0 | 0 | 98.88 | 1.12 | 246 | 0.40 | 66.09 | 32.62* 32.47** 30.72*** |
98.71* 98.56** 96.81*** |
| 100 | 50 | 30 | 0 | 0 | 98.89 | 1.11 | 238 | 0.42 | 66.11 | 32.72* 32.24** 31.40*** |
98.83* 98.35** 97.51*** |
|
(g) |
Input factor | Input factor | Coded Factor | Coded Factor | |||||||
| 100 | 40 | 15 | -1 | -1 | 6.95 | 26.95 | 27.07 | 69.90 | (0.43)A 0.43* |
(1.12)B 1.13** |
(1.45)C 1.46*** |
| 100 | 40 | 30 | -1 | 0 | 6.31 | 24.95 | 25.13 | 64.89 | (0.77)A 0.78* |
(1.42)B 1.43** |
(1.89)C 1.90*** |
| 100 | 40 | 45 | -1 | 1 | 5.87 | 25.65 | 25.89 | 66.85 | (0.40)A 0.40* |
(0.88)B 0.89** |
(0.74)C 0.75*** |
| 100 | 50 | 15 | 0 | -1 | 5.39 | 27.41 | 27.69 | 71.49 | (0.73)A 0.74* |
(1.37)B 1.38** |
(1.34)C 1.35*** |
| 100 | 50 | 30 | 0 | 0 | 5.49 | 26.39 | 26.71 | 68.96 | (0.69)A 0.70* |
(1.28)B 1.30** |
(1.20)C 1.21*** |
| 100 | 50 | 45 | 0 | 1 | 6.54 | 24.37 | 24.94 | 64.40 | (0.19)A 0.19* |
(0.42)B 0.43** |
(0.58)C 0.59*** |
| 100 | 60 | 15 | 1 | -1 | 8.47 | 21.82 | 22.08 | 57.02 | (0.20)A 0.20* |
(0.29)B 0.29** |
(0.20)C 0.20*** |
| 100 | 60 | 30 | 1 | 0 | 5.74 | 22.69 | 23.10 | 59.65 | (0.34)A 0.35* |
(0.94)B 0.96** |
(1.32)C 1.34*** |
| 100 | 60 | 45 | 1 | 1 | 6.28 | 22.13 | 22.56 | 58.26 | (0.24)A 0.24* |
(0.82)B 0.84** |
(0.77)C 0.79*** |
| 100 | 50 | 30 | 0 | 0 | 5.97 | 25.33 | 25.62 | 66.15 | (0.05)A 0.05* |
(0.57)B 0.58** |
(0.83)C 0.84*** |
| 100 | 50 | 30 | 0 | 0 | 5.90 | 24.90 | 25.15 | 64.94 | (1.00)A 1.01* |
(1.34)B 1.35** |
(2.40)C 2.42*** |
| 100 | 50 | 30 | 0 | 0 | 5.14 | 25.58 | 25.87 | 66.80 | (0.17)A 0.17* |
(0.32)B 0.32** |
(2.07)C 2.09*** |
| 100 | 50 | 30 | 0 | 0 | 6.18 | 25.22 | 25.50 | 65.85 | (0.06)A 0.06* |
(0.54)B 0.55** |
(1.38)C 1.40*** |
3.3. Evaluation of the Determined Parameters of Blackish Sesame
| (g) | * | * | |||||
| 100 | 100 | 100 | 242.33 ± 11.02 | 0.41 ± 0.02 | 66.46 ± 0.30 | 32.06 ± 0.15* 31.49 ± 0.14** 31.15 ± 0.23*** |
98.52 ± 0.20* 97.95 ± 0.20** 97.61 ± 0.14*** |
| (g) | * | ||||||
| 100 | 4.12 ± 0.85 |
27.03 ± 0.75 | 27.03 ± 0.75 | 59.66 ± 1.66 | (1.48 ± 0.20)A 1.48 ± 0.20* |
(2.05 ± 0.20)B 2.05 ± 0.20** |
(2.39 ± 0.14)C 2.45 ± 0.15*** |
|
(g) |
Input factor | Input factor | Coded Factor | Coded Factor | |||||||
| 100 | 40 | 15 | -1 | -1 | 98.52 | 1.48 | 225 | 0.44 | 69.21 | 28.50* 28.10** 27.81*** |
97.71* 97.31** 97.02*** |
| 100 | 40 | 30 | -1 | 0 | 98.34 | 1.66 | 222 | 0.44 | 69.35 | 28.14* 27.57** 27.52*** |
97.49* 96.92** 96.87*** |
| 100 | 40 | 45 | -1 | 1 | 98.14 | 1.86 | 226 | 0.43 | 68.72 | 28.37* 27.93** 27.81*** |
97.09* 96.65** 96.53*** |
| 100 | 50 | 15 | 0 | -1 | 98.08 | 1.92 | 224 | 0.44 | 69.51 | 27.96* 27.55** 27.56*** |
97.47* 97.06** 97.07*** |
| 100 | 50 | 30 | 0 | 0 | 97.4 | 2.6 | 226 | 0.43 | 69.37 | 27.48* 27.01** 26.90*** |
96.85* 96.38** 96.27*** |
| 100 | 50 | 45 | 0 | 1 | 97.16 | 2.84 | 223 | 0.44 | 69.27 | 27.38* 26.98** 26.98*** |
96.65* 96.25** 96.25*** |
| 100 | 60 | 15 | 1 | -1 | 97.08 | 2.92 | 224 | 0.43 | 69.31 | 27.28* 26.87** 26.51*** |
96.59* 96.18** 95.82*** |
| 100 | 60 | 30 | 1 | 0 | 96.74 | 3.26 | 223 | 0.43 | 67.29 | 28.68* 28.26** 28.18*** |
95.97* 95.55** 95.47*** |
| 100 | 60 | 45 | 1 | 1 | 96.46 | 3.54 | 221 | 0.44 | 67.88 | 28.23* 27.67** 27.55*** |
96.11* 95.55** 95.43*** |
| 100 | 50 | 30 | 0 | 0 | 97.51 | 2.49 | 225 | 0.43 | 70.39 | 25.85* 25.48** 25.52*** |
96.24* 95.87** 95.91*** |
| 100 | 50 | 30 | 0 | 0 | 97.36 | 2.64 | 224 | 0.43 | 69.77 | 27.55* 27.10** 26.84*** |
97.32* 96.87** 96.61*** |
| 100 | 50 | 30 | 0 | 0 | 97.38 | 2.62 | 225 | 0.43 | 69.14 | 27.60* 27.25** 27.26*** |
96.74* 96.39** 96.40*** |
| 100 | 50 | 30 | 0 | 0 | 97.64 | 2.36 | 223 | 0.44 | 67.49 | 29.08* 28.64** 28.38*** |
96.57* 96.13** 95.87*** |
|
(g) |
Input factor | Input factor | Coded Factor | Coded Factor | |||||||
| 100 | 40 | 15 | -1 | -1 | 3.33 | 24.48 | 24.85 | 54.84 | (0.81)A 0.82* |
(1.21)B 1.23** |
(1.50)C 1.52*** |
| 100 | 40 | 30 | -1 | 0 | 3.11 | 24.41 | 24.82 | 54.78 | (0.85)A 0.86* |
(1.42)B 1.44** |
(1.47)C 1.49*** |
| 100 | 40 | 45 | -1 | 1 | 3.64 | 24.17 | 24.63 | 54.35 | (1.05)A 1.07* |
(1.49)B 1.52** |
(1.61)C 1.64*** |
| 100 | 50 | 15 | 0 | -1 | 3.85 | 23.71 | 24.17 | 53.35 | (0.61)A 0.62* |
(1.02)B 1.04** |
(1.01)C 1.03*** |
| 100 | 50 | 30 | 0 | 0 | 4.09 | 22.81 | 23.42 | 51.69 | (0.55)A 0.56* |
(1.02)B 1.05** |
(1.13)C 1.16*** |
| 100 | 50 | 45 | 0 | 1 | 5.48 | 21.5 | 22.13 | 48.84 | (0.51)A 0.52* |
(0.91)B 0.94** |
(0.91)C 0.94*** |
| 100 | 60 | 15 | 1 | -1 | 4.94 | 21.57 | 22.22 | 49.04 | (0.49)A 0.50* |
(0.90)B 0.93** |
(1.26)C 1.30*** |
| 100 | 60 | 30 | 1 | 0 | 5.73 | 22.45 | 23.21 | 51.22 | (0.77)A 0.80* |
(1.19)B 1.23** |
(1.27)C 1.31*** |
| 100 | 60 | 45 | 1 | 1 | 5.75 | 21.80 | 22.60 | 49.88 | (0.35)A 0.36* |
(0.91)B 0.94** |
(1.03)C 1.07*** |
| 100 | 50 | 30 | 0 | 0 | 5.77 | 19.75 | 20.25 | 44.70 | (1.27)A 1.30* |
(1.64)B 1.68** |
(1.60)C 1.64*** |
| 100 | 50 | 30 | 0 | 0 | 5.99 | 20.85 | 21.42 | 47.26 | (0.04)A 0.04* |
(0.49)B 0.50** |
(0.75)C 0.77*** |
| 100 | 50 | 30 | 0 | 0 | 5.98 | 21.28 | 21.85 | 48.23 | (0.64)A 0.66* |
(0.99)B 1.02** |
(0.98)C 1.01*** |
| 100 | 50 | 30 | 0 | 0 | 5.89 | 22.49 | 23.03 | 50.84 | (1.07)A 1.10* |
(1.51)B 1.55** |
(1.77)C 1.81*** |
3.4. Comparison of Oil Output Parameters of Sesame Varieties
3.5. Comparison of Extraction Losses of Sesame Varieties
3.6. Comparison of Seedcake, Sediments, and Throughput of Sesame Varieties
3.7. Comparison of Weight Losses of Sesame Varieties
3.8. Determined Regression Models of Dependent Parameters of Sesame Varieties
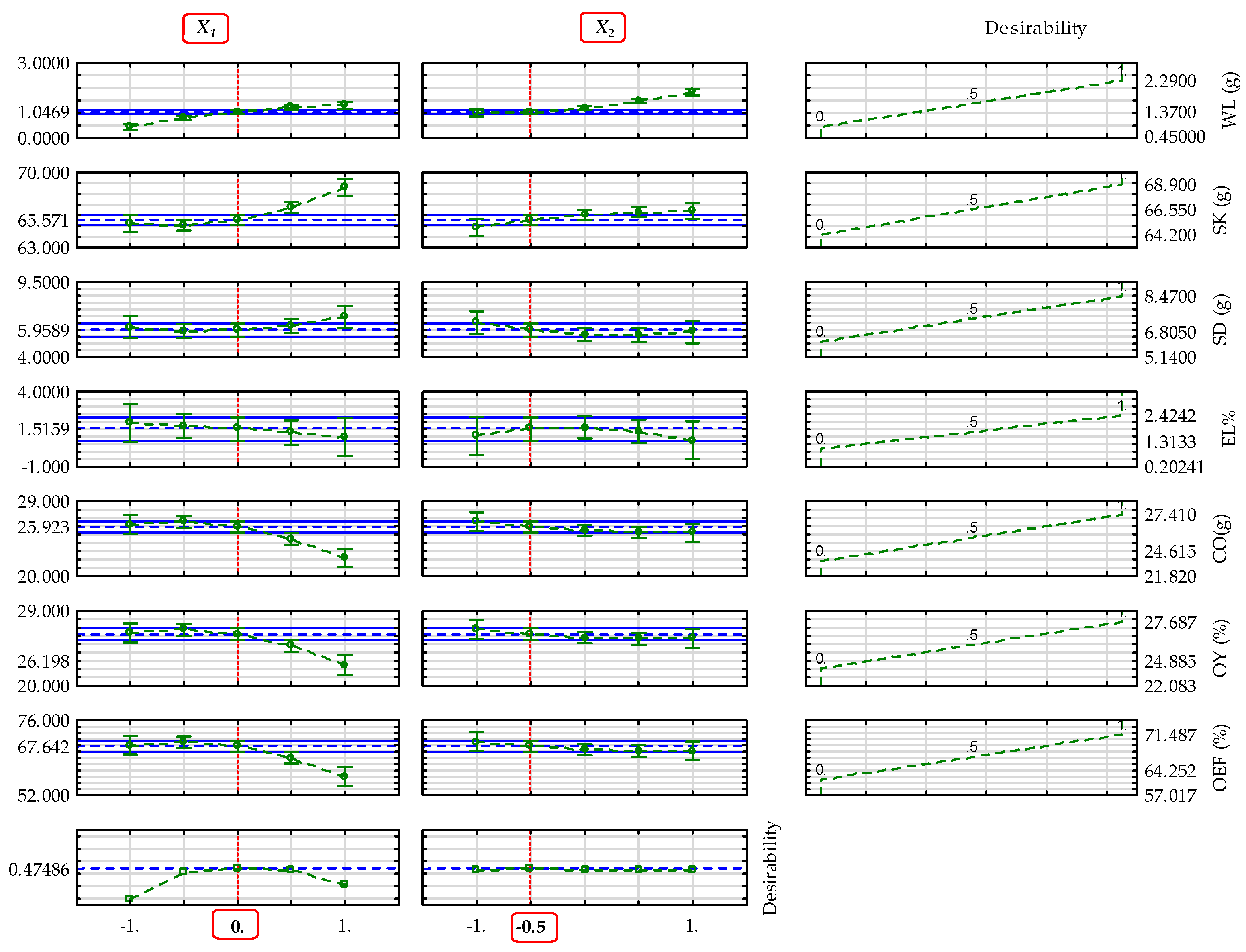
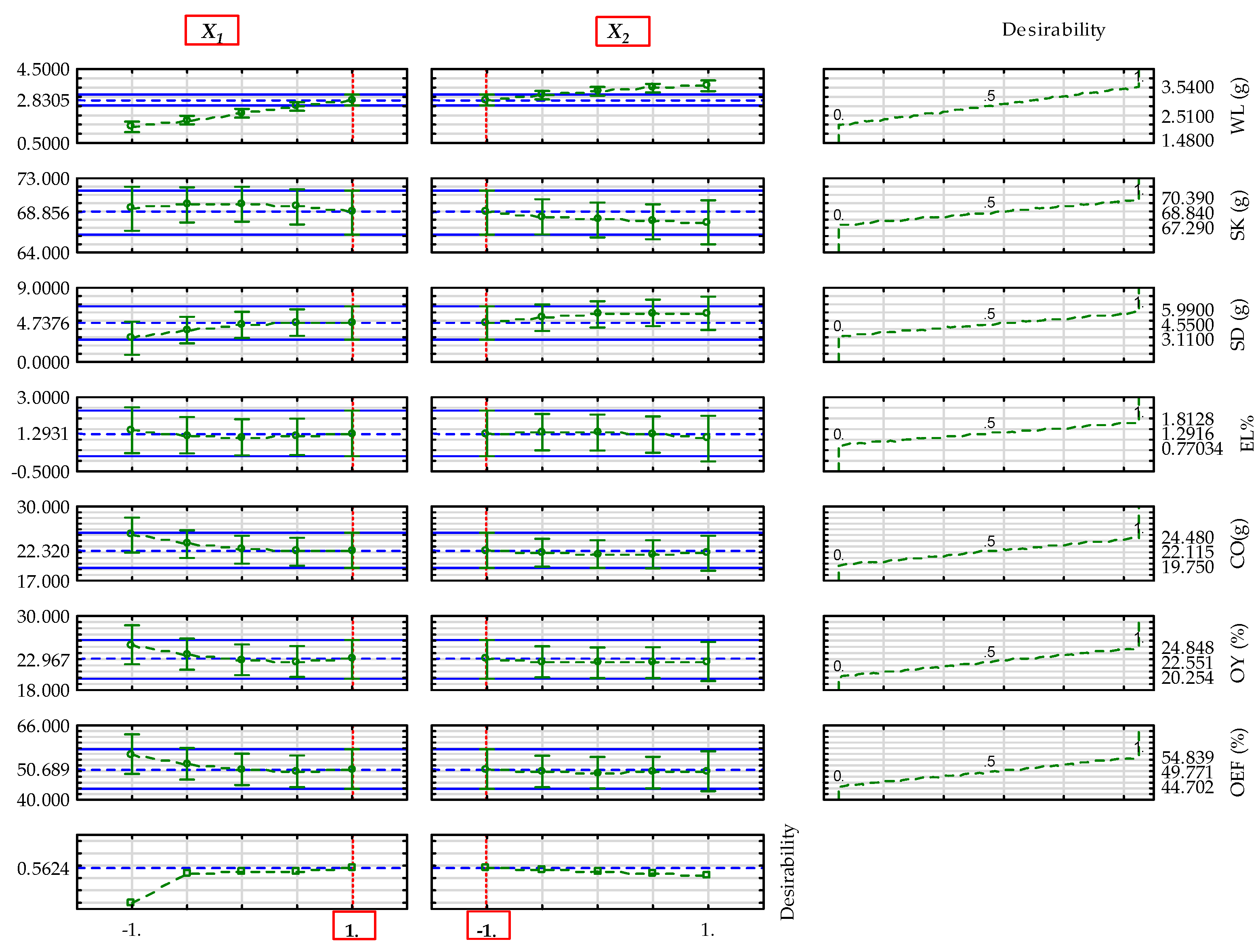
3.9. Determined Optimal Input Factor-Levels of the Parameters of Sesame Varieties
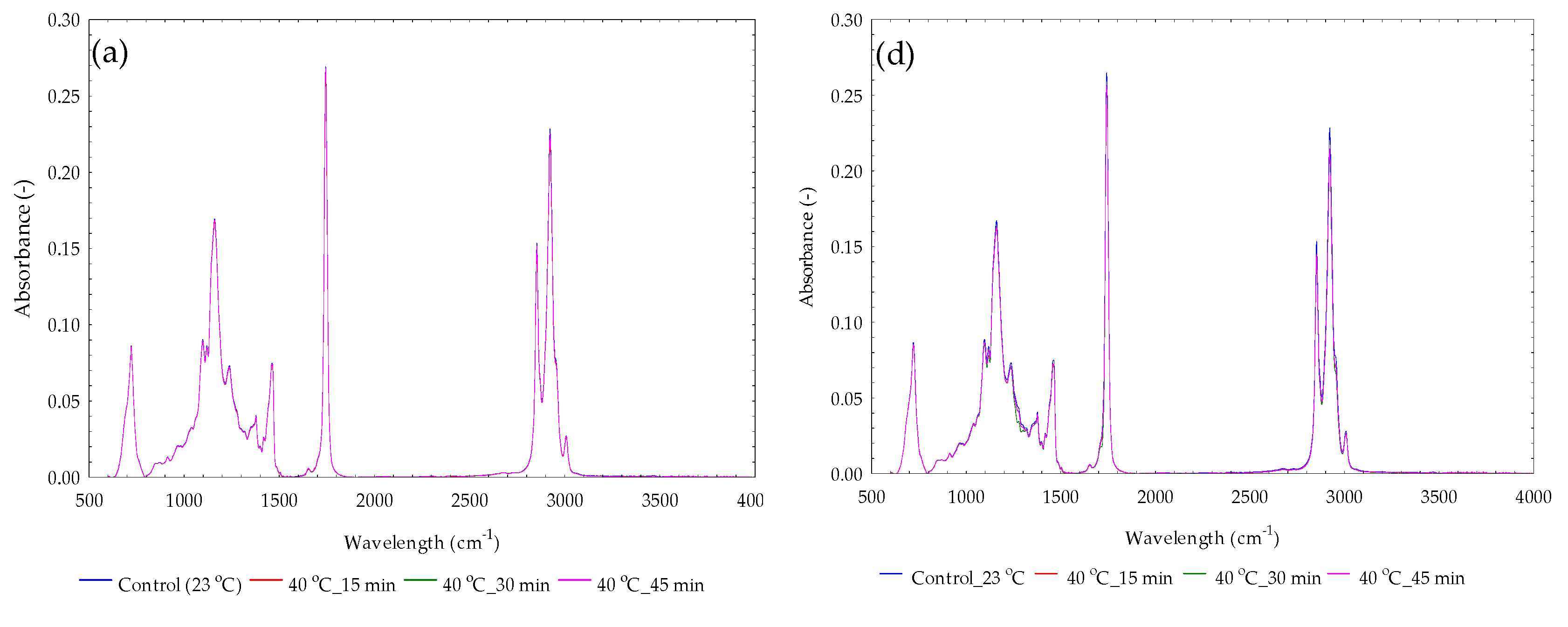
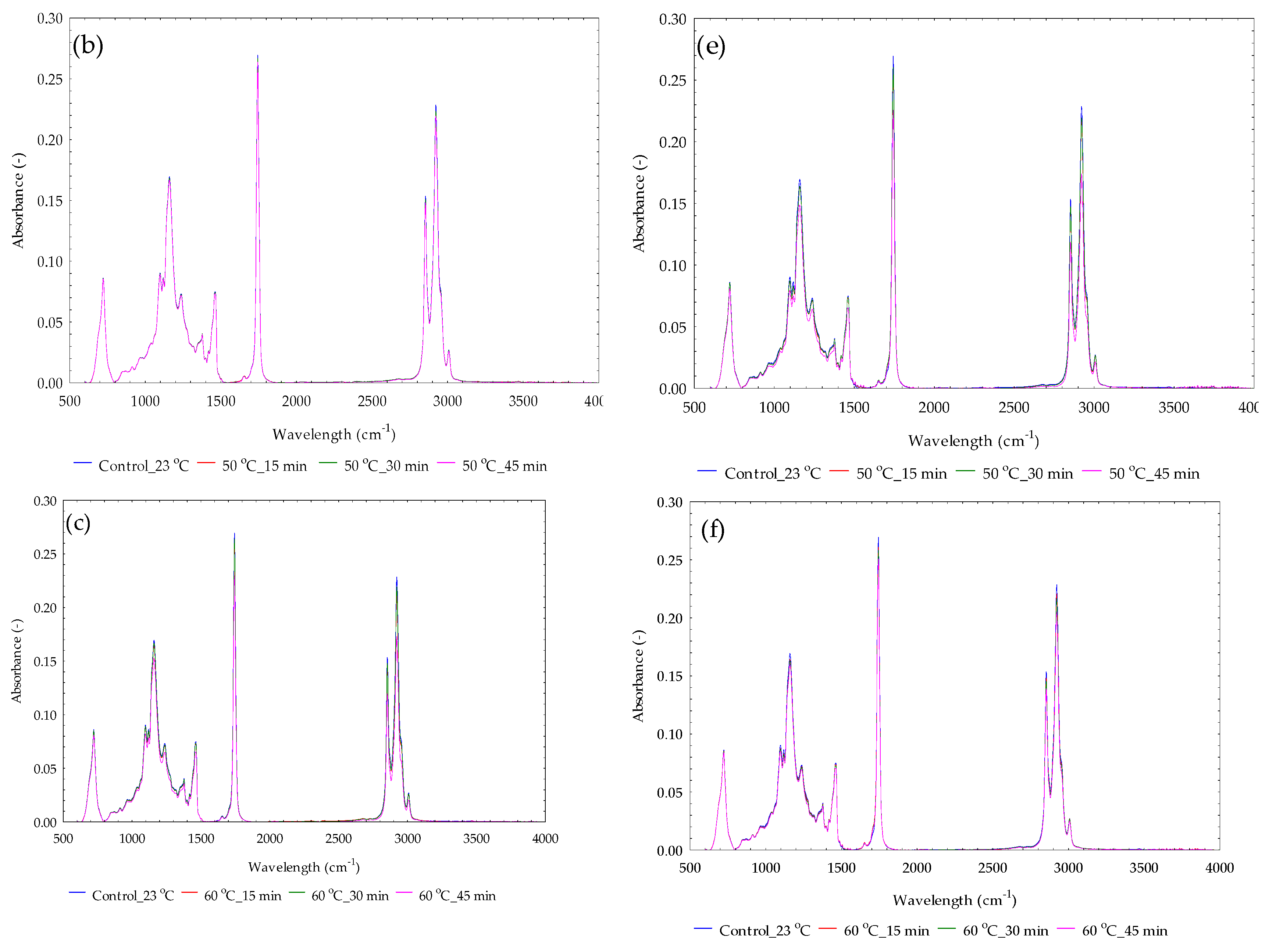
3.10. Absorbance Spectral Curves for Yellowish Sesame Oils
3.11. Absorbance Spectral Curves for Blackish Sesame Oils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Liu, W.; Xiao, L.; Zhao, J.; Chen, Y.; Zhang, L.; Li, P.; Perez-Marin, D.; Wang, X. The application of emerging technologies for the quality and safety evaluation of oilseeds and edible oils. Food Chem.: X. 2025, 25, 102241. [Google Scholar] [CrossRef] [PubMed]
- Slawinska, N.; Olas, B. Effect of thermal processing on the antioxidant activity of oilseeds used in bakery products: A systematic review. Ind. Crops. Prod. 2025, 233, 121419. [Google Scholar] [CrossRef]
- De Lamo, B.; Gomez, M. Bread enrichment with oilseeds. A review. Foods. 2018, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Esrafil, M.; Akter, S.; Alam, M.J.; Alim, M.A.; Reza, M.S.A.; Zubair, M.A.; Joy, P.R. .; Jahan, M.; Khatun, M. Development and quality evaluation of novel biscuits by utilizing fruits and vegetables seed. Food Res. 2024, 8, 181–189. [Google Scholar] [CrossRef]
- Agirbas, H.E.T.; Yavuz-Duzgun, M.; Ozcelik, B. Valorization of fruit seed flours: rheological characteristics of composite dough and cake quality. J. Food Meas. Charact. 2022, 16, 3117–3129. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X.; Zhou, S. Effect of flaxseed marc flour on high-yield wheat bread production: Comparison in baking, staling, antioxidant and digestion properties, LWT – Food. Sci. Technol. 2022, 169, 113979. [Google Scholar] [CrossRef]
- Tanska, M.; Rotkiewicz, D. Quality of fat from oilseeds used to produce selected kinds of bread. Zywn. Nauka Technol. Jakosc Food Sci. Technol. 2011, 18, 62–67. [Google Scholar]
- Khan, M.S.U.; Rahman, M.M.; Basak, A.R.; Angon, P.B.; Ritu, S.A.; Kobir, M.; Islam, M.R. Evaluation of different sesame varieties cultivated under saline conditions in the southwestern coastal region of Bangladesh. Crop Des. 2025, 4. [Google Scholar] [CrossRef]
- Moisa, M.B.; Merga, B.B.; Gabissa, B.T.; Gemeda, D.O. Assessment of land suitability for oilseeds crops (sesame and groundnut) using geospatial techniques: In the case of Diga district, East Wollega zone, western Ethiopia. Oil Crop Sci. 2022, 7, 127–134. [Google Scholar] [CrossRef]
- Akondo, M.R.I.; Uddin, F.J.; Islam, M.M.; Adhikary, S.; Rana, M.S. Yield comparison of bina developed four sesame varieties under the agro-ecological conditions of gopalganj district of Bangladesh. Res. Agric. Livestock Fish. 2022, 9, 247–251. [Google Scholar] [CrossRef]
- Myint, D.; Gilani, S.A.; Kawase, M.; Watanabe, K.N. Sustainable sesame (Sesamum indicum L.) production through improved Technology: an overview of production, challenges and opportunities in Myanmar. Sustainability. 2020, 12. [Google Scholar] [CrossRef]
- Bedigian, D.; Harlan, J.R. Evidence for cultivation of sesame in the ancient world. Econ. Bot. 1986, 40, 137–154. [Google Scholar] [CrossRef]
- Lukurugu, G.A.; Nzunda, J.; Kidunda, B.R.; Chilala, R.; Ngamba, Z.S.; Minja, A.; Kapinga, F.A. Sesame production constraints, variety traits preference in the Southeastern Tanzania: Implication for genetic improvement. J. Agric. Food Res. 2023, 14, 100665. [Google Scholar] [CrossRef]
- https://essfeed.com/top-10-sesame-seed-producing-countries-in-the/.
- Song, G.; Wang, J.; He, Y.; Sun, Y.; Huang, J.; Sun, Q.; Deng, Z. Roasting increases the oil yield of sesame seeds by altering the amino acid composition and secondary structure of the sesame protein. Food Biosci. 2025, 68, 106491. [Google Scholar] [CrossRef]
- He, S.; Pan, T.; Zhang, Z.; Wu, Y.; Sun, H.; Ma, Y.; Zhang, Y. Interactive effect of hot air roasting processes on the sensory property, allergenicity, and oil extraction of sesame (Sesamum indicum L.) seeds. Grain Oil Sci. Technol. 2023, 6, 71–81. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Uslu, N.; Ozcan, M.M.; Juhaimi, F.A.; Ghafoor, K.; Babiker, E.E.; Osman, M.A.; Alqah, H.A.S. Effect of conventional oven roasting treatment on the physicochemical quality attributes of sesame seeds obtained from different regions. Food Chem. 2021, 338, 128109. [Google Scholar] [CrossRef] [PubMed]
- Elkhaleefa, A.; Shigidi, I. Optimization of sesame oil extraction process conditions. ACES. 2015, 305–310. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Zhang, J.; Ge, Y.; He, L.; Kang, W.; Huang, W.; Sun, J.-L.; Chen, Y. Effect of roasting on the conformational structure and IgE binding of sesame allergens. Effect of roasting on the conformational structure and IgE binding of sesame allergens. J. Agric. Food Chem. 2022, 70(30), 9442–9450. [Google Scholar] [CrossRef]
- IS:3579; Indian Standard Methods for Analysis of Oilseeds. Indian Standard Institute: New Delhi, India, 1996.
- Blahovec, J. Agromaterials Study Guide; Czech University of Life Sciences Prague: Prague, Czech Republic, 2008. [Google Scholar]
- Danlami, J.M.; Arsad, A.; Zaini, M.A.A. Characterization and process optimization of castor oil (Ricinus communis L.) extracted by the Soxhlet method using polar and non-polar solvents. J. Taiwan Inst. Chem Eng. 2015, 47, 99–104. [Google Scholar] [CrossRef]
- Niu, L.; Li, J.; Chen, M.-S.; Xu, Z.-F. Determination of oil contents in Sacha inchi (Plukenetia volubilis) seeds at different developmental stages by two methods: Soxhlet extraction and time-domain nuclear magnetic resonance. Ind Crop. Prod. 2014, 56, 187–190. [Google Scholar] [CrossRef]
- Gürdil, G.A.K.; Kabutey, A.; Selvi, K.Ç.; Mizera, Č.; Herák, D.; Fraňková, A. Evaluation of Postharvest Processing of Hazelnut Kernel Oil Extraction Using Uniaxial Pressure and Organic Solvent. Processes 2020, 8, 957. [Google Scholar] [CrossRef]
- Ocholi, O.; Menkiti, M.; Auta, M.; Ezemagu, I. Optimization of the operating parameters for the extractive synthesis of biolubricant from sesame seed oil via response surface methodology. Egypt. J. Pet. 2018, 27, 265–275. [Google Scholar] [CrossRef]
- Demirel, C.; Kabutey, A.; Herák, D.; Sedlaček, A.; Mizera, Č.; Dajbych, O. Using Box–Behnken Design Coupled with Response Surface Methodology for Optimizing Rapeseed Oil Expression Parameters under Heating and Freezing Conditions. Processes 2022, 10, 490. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Optimization of ultrasound-assisted extraction of oil from olive pomace using response surface technology: Oil recovery, unsaponifiable matter, total phenol content and antioxidant activity. LWT – Food Sci. Technol. 2017, 79, 178–189. [Google Scholar] [CrossRef]
- Yuan, L.; Meng, X.; Xin, K.; Ju, Y.; Zhang, Y.; Yin, C.; Hu, L. A Comparative Study on Classification of Edible Vegetable Oils by Infrared, near Infrared and Fluorescence Spectroscopy Combined with Chemometrics. Spectrochim. Acta, Part A: Mol. Biomol. Spectrosc. 2023, 288, 122120. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Khorrami, M.K.; Vatani, A.; Ghasemzadeh, H.; Vatanparast, H.; Bahramian, A.; Fallah, A. Rapid Determination and Classification of Crude Oils by ATR-FTIR Spectroscopy and Chemometric Methods. Spectrochim. Acta, Part A: Mol. Biomol. Spectrosc., 2020, 232, 118157. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, T.A.d; Lopes, T.I.B.; Nazario, C.E.D.; Oliveira, S.L.; Alcantara, G.B. Vegetable oils: Are they true? A point of view from ATR-FTIR, 1H NMR, and regiospecific analysis by 13 C NMR. Food Res. Int., 2021, 144, 110362. [Google Scholar] [CrossRef]
- Deli, S.; Farah Masturah, M.; Tajul Aris, Y.; Wan Nadiah, W.A. The effects of physical parameters of the screw press oil expeller on oil yield from Nigella sativa L. seeds. Int. Food Res. J. 2011, 18, 1367–1373. [Google Scholar]
- Hernandez-Santos, B.; Rodriguez-Miranda, J.; Herman-Lara, E.; Torruco-Uco, J.G.; Carmona-Garcia, R.; Juarez-Barrientos, J.M.; Chavez-Zamudio, R.; Martinez-Sanchez, C.E. Effect of oil extraction assisted by ultrasound on the physicochemical properties and fatty acid profile of pumpkin seed oil (Cucurbita pepo). Ultrason. Sonochem. 2016, 31, 429–436. [Google Scholar] [CrossRef]
- Alenyorege, E.A.; Hussein, Y.A.; Adongo, T.A. Extraction yield, efficiency and loss of the traditional hot water floatation (HWF) method of oil extraction from the seeds of allanblackia floribunda. Int. J. Sci. Technol. Res. 2015, 4(2), 1–4. [Google Scholar]
- Karaj, S.; Muller, J. Optimizing mechanical oil extraction of Jatropha curcas L. seeds with respect to press capacity, oil recovery and energy efficiency. Ind. Crops Prod. 2011, 34, 1010–1016. [Google Scholar] [CrossRef]
- Statsoft Inc. STATISTICA for Windows; Statsoft Inc.: Tulsa, OK, USA, 2013. [Google Scholar]
- Wooldridge, J.M. What is standard error? (And how should we compute it?). J. Econom. 2023, 237. [Google Scholar]
- Powell, J.L. Discussion of ‘’What is a standard error?’’. J. Econom. 2023, 237(4), 105518. [Google Scholar] [CrossRef]
- Zambrano, M.V.; Dutta, B.; Mercer, D.G.; MacLean, H.L.; Touchie, M.F. Assessment of moisture content measurement methods of dried food products in small-scale operations in developing countries: A review. Trends Food Sci. Technol. 2019, 88, 484–496. [Google Scholar] [CrossRef]
- Meng, X.; Sedman, J.; van de Voort, F.R. Improving the determination of moisture in edible oils by FTIR spectroscopy using acetonitrile extraction. Food Chem. 2012, 135(2), 722–729. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Zhao, Y.; Cui, R.; Wu, H.; Xu, M.; Liu, W.; Liu, R.; Xu, L.; Song, L. Effect of different heating pretreatment methods on physicochemical properties of pressed walnut oils and functional properties of walnut protein isolates. LWT – Food Sci. Technol. 2025, 225, 117885. [Google Scholar] [CrossRef]
- Dun, Q.; Yao, L.; Deng, Z.; Li, H.; Li, J.; Fan, Y.; Zhang, B. Effects of hot and cold-pressed processes on volatile compounds of peanut oil and corresponding analysis of characteristic flavor components. LWT – Food Sci. Technol. 2019, 112, 107648. [Google Scholar] [CrossRef]
- Liu, X.; Jin, Q.; Liu, Y.; Huang, J.; Wang, X.;Mao, W; Wang, S. Changes in volatile compounds of peanut oil during the roasting process for production of aromatic roasted peanut oil. Changes in volatile compounds of peanut oil during the roasting process for production of aromatic roasted peanut oil. J. Food Sci. 2011, 76(3), C404–C412. [Google Scholar] [CrossRef]
- Zheng, L.; Ren, J.; Su, G.; Yang, B.; Zhao, M. Comparison of in vitro digestion characteristics and antioxidant activity of hot-and cold-pressed peanut meals. Food Chem. 2013, 141(4), 4246–4252. [Google Scholar] [CrossRef]
- Parker, T.D.; Adams, D.A.; Zhou, K.; Harris, M.; Yu, L. Fatty acid composition and oxidative stability of cold-pressed edible seed oils. J. Food Sci. 2010, 68(4), 1240–1242. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Ozcan, M.M.; Osman, M.A.; Gassem, M.A.; Salih, H.A.A. Effects of cold-press and Soxhlet extraction systems on antioxidant activity, total phenol contents, fatty acids, and tocopherol contents of walnut kernel oils. J. Oleo Sci. 2019, 68(2), 167–173. [Google Scholar] [CrossRef]
- Bagheri, H.; Kashaninejad, M.; Ziaiifar, A.M.; Aalami, M. Textural, color and sensory attributes of peanut kernels as affected by infrared roasting method. Inf. Process. Agric 2019, 6, 255–264. [Google Scholar] [CrossRef]
- Willems, P.; Kuipers, N.; De Haan, A. Hydraulic pressing of oilseeds: experimental determination and modelling of yield and pressing rates. J. Food Eng. 2008, 89, 8–16. [Google Scholar] [CrossRef]
- Chakraborty, D.; Das, J.; Das, P.K.; Bhattacharjee, S.C.; Das, S. Evaluation of the parameters affecting the extraction of sesame oil from sesame (Sesamum indicum L.) seed using soxhlet apparatus. Int. Food Res. J. 2017, 24, 691–695. [Google Scholar]
- Amit, R.J.; Kumari, S.; Dhaulaniya, A.S.; Balan, B.; Singh, D.K. Application of ATR-FTIR spectroscopy along with regression modelling for the detection of adulteration of virgin coconut oil with paraffin oil. LWT – Food Sci. Technol., 2020, 118, 108754. [Google Scholar] [CrossRef]
- Uncu, O.; Ozen, B. A Comparative study of mid-infrared, UV-Visible and fluorescence spectroscopy in combination with chemometric for the detection of adulteration of fresh olive oils with old olive oils. Food Control. 2019, 105, 209–218. [Google Scholar] [CrossRef]
- Ozulku, G.; Yildirim, R.M.; Toker, O.S.; Karasu, S.; Durak, M.Z. Rapid detection of adulteration of cold pressed sesame oil adulterated with hazelnut, canola, and sunflower oils using ATR-FTIR spectroscopy combined with chemometric. Food Control. 2017, 82, 212–216. [Google Scholar] [CrossRef]
- Rizki, H.; Terouzi, W.; Kzaiber, F.; Hanine, H.; Oussama, A. Quantification of adulterations in sesame oil with inferior edible oils by using ATR-FTIR coupled to chemometrics. J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 138–145. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta. 2006, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.F.; Ting, W. An automated approach for analysis of Fourier Transform Infrared (FTIR) spectra of edible oils. Talanta, 2012, 88, 537–534. [Google Scholar] [CrossRef]
- Rolim, C.S.d.S.; Freire, J.O.; Muniz, I.d.C.B.; Nunomura, R.d.C.S.; Santos, L.S.; Bauer, L.C.; Lamarao, C.V.; Bonomo, R.C.F. Characterization of physiochemical and thermos oxidative properties of inaja fruit oil (Maximiliana maripa). Food Sci. 2024, 60, 104191. [Google Scholar] [CrossRef]

|
Runs |
Input factor, | Input factor, | Coded factor, | Coded factor, |
| 1 | 40 | 15 | -1 | -1 |
| 2 | 40 | 30 | -1 | 0 |
| 3 | 40 | 45 | -1 | 1 |
| 4 | 50 | 15 | 0 | -1 |
| 5 | 50 | 30 | 0 | 0 |
| 6 | 50 | 45 | 0 | 1 |
| 7 | 60 | 15 | 1 | -1 |
| 8 | 60 | 30 | 1 | 0 |
| 9 | 60 | 45 | 1 | 1 |
| 10 | 50 | 30 | 0 | 0 |
| 11 | 50 | 30 | 0 | 0 |
| 12 | 50 | 30 | 0 | 0 |
| 13 | 50 | 30 | 0 | 0 |
| (g) | * | * | |||||
| 100 | 100 | 100 | 255.33 ± 7.23 | 0.39 ± 0.01 | 69.41 ± 0.17 | 29.77 ± 0.23* 29.11 ± 0.24** 28.93 ± 0.21*** |
99.18 ± 0.39* 98.52**± 0.41** 98.34 ± 0.33*** |
| (g) | * | ||||||
| 100 | 4.13 ± 1.30 |
24.80 ± 1.30 | 24.80 ± 1.30 | 64.04 ± 3.37 | (0.82 ± 0.39)A 0.82 ± 0.39* |
(1.48 ± 0.41)B 1.48 ± 0.41** |
(1.66 ± 0.33)C 1.69 ± 0.34*** |
|
Dependent parameters |
Mean |
Std. Dev. |
N |
Mean Diff. |
t-value |
df. |
P-value |
| 31.735 | 1.646 | 14 | |||||
| 28.154 | 1.369 | 14 | 3.581 | 6.257 | 26 | < 0.05 | |
| 24.871 | 1.672 | 14 | |||||
| 22.736 | 1.877 | 14 | 2.135 | 3.177 | 26 | < 0.05 | |
| 25.152 | 1.627 | 14 | |||||
| 23.259 | 1.737 | 14 | 1.892 | 2.975 | 26 | < 0.05 | |
| 64.942 | 4.201 | 14 | |||||
| 51.334 | 3.833 | 14 | 13.607 | 8.953 | 26 | < 0.05 |
|
Dependent parameters |
Mean |
Std. Dev. |
N |
Mean Diff. |
t-value |
df. |
P-value |
| 0.439 | 0.313 | 14 | |||||
| 0.765 | 0.382 | 14 | –0.326 | –2.468 | 26 | < 0.05 | |
| 0.923 | 0.431 | 14 | |||||
| 1.222 | 0.393 | 14 | –0.299 | –1.919 | 26 | > 0.05 | |
| 1.289 | 0.616 | 14 | |||||
| 1.367 | 0.436 | 14 | –0.078 | –0.388 | 26 | > 0.05 |
|
Dependent parameters |
Mean |
Std. Dev. |
N |
Mean Diff. |
t-value |
df. |
P-value |
| 66.698 | 1.533 | 14 | |||||
| 68.797 | 1.098 | 14 | –2.099 | –4.164 | 26 | < 0.05 | |
| 6.026 | 0.979 | 14 | |||||
| 4.833 | 1.089 | 14 | 1.192 | 3.047 | 26 | < 0.05 | |
| 0.403 | 0.011 | 14 | |||||
| 0.434 | 0.007 | 14 | –0.031 | –9.302 | 26 | < 0.05 |
|
Dependent parameters |
Mean |
Std. Dev. |
N |
Mean Diff. |
t-value |
df. |
p-value |
| 98.879 | 0.581 | 14 | |||||
| 97.701 | 0.885 | 14 | 1.168 | 4.125 | 26 | < 0.05 |
|
Effect |
Yellowish Sesame: Parameter Regression Model Coefficients based on (Equation (4)) | ||||||
| (g) | (g) | (g) | (%) | (g) | (%) | (%) | |
| Intercept | 1.202 | 66.050 | 5.648 | 1.622 | 25.496 | 25.806 | 66.631 |
| (L) | 0.467 | 1.423 | 0.227 | –0.296 | –1.818 | –1.724 | –4.451 |
| (Q) | –0.177 | 1.354 | 0.597 | –0.069 | –1.707 | –1.778 | –4.591 |
| (L) | 0.417 | 0.758 | –0.353 | –0.148 | –0.672 | –0.574 | –1.483 |
| (Q) | 0.213 | –0.401 | 0.537 | –0.719 | 0.363 | 0.419 | 1.081 |
| (L) by (Q) | 0.063 | –0.508 | –0.278 | 0.323 | 0.403 | 0.415 | 1.072 |
| R2 | 0.835 | 0.959 | 0.494 | 0.605 | 0.867 | 0.859 | 0.859 |
| R | 0.914 | 0.979 | 0.703 | 0.778 | 0.931 | 0.927 | 0.927 |
|
Effect |
Blackish Sesame: Parameter Regression Model Coefficients based on (Eq.4) | ||||||
| (g) | (g) | (g) | (%) | (g) | (%) | (%) | |
| Intercept | 2.521 | 69.190 | 5.423 | 1.240 | 21.656 | 22.215 | 49.029 |
| (L) | 0.787 | –0.467 | 1.057 | –0.163 | –1.207 | –1.045 | –2.307 |
| (Q) | –0.009 | –0.766 | –0.702 | 0.258 | 1.224 | 1.248 | 2.755 |
| (L) | 0.320 | –0.360 | 0.458 | –0.034 | –0.382 | –0.314 | –0.693 |
| (Q) | –0.089 | 0.304 | –0.457 | –0.163 | 0.399 | 0.385 | 0.851 |
| (L) by (Q) | 0.060 | –0.235 | 0.125 | –0.087 | 0.135 | 0.150 | 0.332 |
| R2 | 0.971 | 0.400 | 0.746 | 0.322 | 0.627 | 0.584 | 0.584 |
| R | 0.985 | 0.633 | 0.864 | 0.567 | 0.792 | 0.764 | 0.764 |
| Dependent parameters (Blackish sesame) |
Optimal factor levels |
Desirability value |
Profiles predicted value* |
Model predicted value** |
|
| (°C) | (min) | ||||
| +1 (60) | +1 (45) | 0.942 | 2.183 | 2.085 | |
| +1 (60) | +0.5 (37.5) | 0.989 | 68.853 | 68.953 | |
| +1 (60) | –1 (15) | 0.751 | 7.639 | 5.648 | |
| –1 (40) | –0.5 (22.5) | 0.766 | 1.904 | 1.622 | |
| –0.5 (45) | –1 (15) | 0.965 | 27.215 | 27.259 | |
| –0.5 (45) | –1 (15) | 0.953 | 27.424 | 27.458 | |
| –0.5 (45) | –1 (15) | 0.953 | 70.809 | 74.125 | |
| Dependent parameters (Blackish sesame) |
Overall optimal factor levels |
Desirability value |
Profiles predicted value* |
Model predicted value** |
|
| (°C) | (min) | ||||
|
0 (50) |
–0.5 (22.5) |
0.475 |
1.047 | 0.994 | |
| 65.571 | 65.671 | ||||
| 5.959 | 5.648 | ||||
| 1.516 | 1.622 | ||||
| 25.923 | 25.496 | ||||
| 26.198 | 25.806 | ||||
| 67.642 | 66.631 | ||||
| Dependent parameters (Blackish sesame) |
Optimal factor levels |
Desirability value |
Profiles predicted value* |
Model predicted value** |
|
| (°C) | (min) | ||||
| +1 (60) | +1 (45) | 1.000 | 3.591 | 3.628 | |
| 0 (50) | –1 (15) | 0.827 | 69.854 | 69.190 | |
| +1 (60) | +0.5 (37.5) | 0.988 | 5.955 | 6.480 | |
| –1 (40) | 0 (30) | 0.855 | 1.661 | 1.240 | |
| –1 (40) | –1 (15) | 1.000 | 25.003 | 22.863 | |
| –1 (40) | –1 (15) | 1.000 | 25.359 | 22.215 | |
| –1 (40) | –1 (15) | 1.000 | 55.967 | 49.029 | |
| Dependent parameters (Blackish sesame) |
Overall optimal factor levels |
Desirability value |
Profiles predicted value* |
Model predicted value** |
|
| (°C) | (min) | ||||
|
+1 (60) |
–1 (15) |
0.562 |
2.831 | 2.988 | |
| 68.856 | 69.190 | ||||
| 4.738 | 6.480 | ||||
| 1.293 | 1.240 | ||||
| 22.320 | 20.449 | ||||
| 22.967 | 22.215 | ||||
| 50.689 | 49.029 | ||||
|
Effect |
Degree of freedom | Sum of squares | Mean Squares |
F-value |
P-value |
| Intercept | 1 | 0.56573 | 0.565732 | 507.742 | 0.000000* |
| Wavelength | 1 | 1.44365 | 1.443649 | 1295.669 | 0.000000* |
| Temperature | 1 | 0.00229 | 0.002286 | 2.052 | 0.152048** |
| Time | 1 | 0.00113 | 0.001126 | 1.010 | 0.314829** |
| Residual | 14891 | 16.59171 | 0.001114 | ||
| Total | 14894 | 18.03878 |
|
Effect |
Degree of freedom | Sum of squares | Mean Squares |
F-value |
P-value |
| Intercept | 1 | 0.48081 | 0.480807 | 456.499 | 0.000000* |
| Wavelength | 1 | 1.39028 | 1.390277 | 1319.991 | 0.000000* |
| Temperature | 1 | 0.00005 | 0.000050 | 0.048 | 0.152048** |
| Time | 1 | 0.00179 | 0.001788 | 1.697 | 0.314829** |
| Residual | 14891 | 15.68391 | 0.001053 | ||
| Total | 14894 | 17.07603 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





