1. Introduction
Although present in plants in only trace quantities, micronutrients are indispensable for sustaining the metabolic processes that drive cotton growth, reproduction, and fiber development. Out of nearly 90 elements occurring naturally in soils, only a limited number are classified as essential, and among them, micronutrients such as Fe, Zn, Mn, B, copper (Cu), molybdenum (Mo), and chlorine (Cl) play particularly critical roles. Despite their minute concentration requirements compared with macronutrients, these elements regulate key physiological functions including enzyme activation, photosynthesis, and reproductive development making them fundamental for realizing cotton’s genetic yield and quality potential [
1,
2]. Unlike macronutrients, which are supplied in large quantities, micronutrients often exist in soils at sufficient total concentrations but with limited bioavailability due to pH, organic matter, redox potential, and interactions with other nutrients [
3].
Among these, Fe stands out as particularly critical for cotton development, yet its management has long been overshadowed by emphasis on N, P, K, and S [
3,
4]. Iron plays a fundamental role in photosynthesis, chlorophyll biosynthesis, electron transport, and enzyme activation, directly influencing boll retention, flowering, and fiber development [
5,
6,
7]. Deficiencies manifest as interveinal chlorosis, stunted growth, and poor boll formation, conditions increasingly reported in calcareous, sodic, and alkaline soils across major cotton-growing regions [
8]. Although Fe and other micronutrients are abundant in the earth’s crust their solubility and plant availability decline sharply over time especially in alkaline conditions, resulting in hidden deficiencies or hidden hunger that are often overlooked in cotton fertility programs [
9,
10].
Other micronutrients such as Zn, B, and Mn also exert critical influences on cotton physiology, each contributing to distinct but interconnected processes that regulate plant growth and yield formation. Zinc for example is fundamental to auxin biosynthesis, protein metabolism, and membrane integrity, and it activates a wide array of enzymes involved in carbohydrate and nucleic acid metabolism. In cotton, adequate Zn supply promotes balanced vegetative growth, proper leaf expansion, and reproductive development, while deficiencies result in shortened internodes, small, deformed leaves, delayed flowering, and reduced boll set [
11]
Boron, though required in very small quantities, is indispensable for cell wall stability, sugar transport, and reproductive processes. In cotton, B deficiency disrupts pollen germination and tube elongation, leading to poor fertilization, floral deformities, and premature boll shedding [
12,
13]. Its role in maintaining membrane function and assimilate partitioning is especially critical during the flowering and boll-filling stages, when demand is high. Manganese, on the other hand, is a cofactor in numerous enzymatic systems and plays a pivotal role in photosystem II function, aiding in water-splitting and oxygen evolution. It also contributes to lignin biosynthesis, disease resistance, and the detoxification of reactive oxygen species [
14,
15]. Cotton grown in Mn-deficient soils exhibits interveinal chlorosis, leaf speckling, and impaired photosynthetic activity, which collectively reduce biomass accumulation and reproductive success.
Despite their importance, these micronutrients have historically received limited attention compared with macronutrients. Fertilizer recommendations, research priorities, and extension programs worldwide have largely focused on N-P-K and, more recently, S, leaving critical gaps in understanding the role of these micronutrients in sustaining cotton yields, and profitability. This imbalance in nutrient management has likely contributed to unexplained yield gaps, with emerging evidence suggesting that micronutrient deficiencies can constrain cotton productivity even when macronutrient levels are optimal. Given the global significance of cotton as both an economic and industrial crop, it is imperative to reevaluate the current advances, mechanisms and the contribution of B, Fe, Mn, and Zn in cotton growth and yield formation. This review examines their roles in cotton growth, physiology, soil-plant dynamics, and agronomic performance across diverse environments, while also identifying the opportunities and limitations of current research in the understanding of these elements. By synthesizing recent findings, this work highlights how unbalanced nutrient management perpetuates hidden yield losses and the need for integrated approaches that incorporate micronutrient nutrition into sustainable cotton production systems.
2. Effects of Micronutrients of B, Fe, Mn, and Zn in Cotton Growth Phenotypic Traits
Cotton growth and yield depend not only on adequate macronutrients but also on the balanced supply of micronutrients such as B, Fe, Mn, and Zn [
16]. Each plays a unique physiological role that directly influences growth traits such as vegetation, nodes, height of the plant, flowering, flower retention, boll retention, and fiber development. Boron is essential for cell wall formation, membrane integrity, and reproductive structures formation, particularly during pollination, pollen germination and tube growth, which ultimately ensures successful boll formation, and boll setting growth stages [
12,
17]. Iron regulates chlorophyll biosynthesis, photosynthetic electron transport, and respiration, making it indispensable for leaf expansion and energy metabolism [
5,
18]. Research shows that when Iron is applied to cotton, it enhances its tolerance to ionic stress from metals such as Lead and downregulates the effect of macronutrient deficiency [
19]. Manganese functions as a cofactor for enzymes in photosystem II and is critical for lignin biosynthesis and oxidative stress regulation. Zinc contributes to auxin synthesis, enzyme activation, and reproductive organ development. Optimal supply of these nutrients sustains both vegetative vigor and reproductive success, while their deficiencies impose severe limitations on cotton physiology and yield.
Figure 1.
Showing the visible symptoms of Zn deficiency in cotton. Image courtesy of Krishi Bazaar.
Figure 1.
Showing the visible symptoms of Zn deficiency in cotton. Image courtesy of Krishi Bazaar.
2.1. Plant Growth and Development
Cotton’s growth phases from seedling, root development, vegetative, and reproductive growth and development are highly sensitive to the availability of all nutrients including the micronutrients especially B, Fe, Mn, and Zn. The deficiencies in these nutrients exist synergistically and antagonistically and often interacting to exacerbate plant stress. For example, B deficiency impairs pollen tube growth during flowering, affecting flower fertilization. This negatively affects the square, boll numbers, sizes of individual boll, and micronaires in cotton lint. These come up due to the resultant distorted flowers, boll shedding, and poor fiber initiation. For the case of Iron, its deficiency, commonly expressed as interveinal chlorosis of young leaves, reduces chlorophyll content, photosynthetic efficiency, and ultimately boll retention [
20]. Manganese shortages impair enzyme activity and photosynthetic water-splitting, causing leaf speckling, reduced vigor, and susceptibility to oxidative stress. Zn deficiency leads to shortened internodes, stunted growth, smaller leaves, and poor boll development.
These micronutrient deficiencies not only reduce crop performance directly but also diminish the efficiency of applied macronutrients such as N and P. For instance, Fe and Zn are essential for nitrate reductase activity and protein synthesis [
21], meaning that their absence reduces the efficiency of N fertilization. This assertion is in line with several studies that observed yield reduction when these micronutrients are deficient compared to fertilized plots [
22,
23,
24]. Similarly, B deficiency interferes with carbohydrate transport and reproductive growth, lowering the effectiveness of P and K applications [
25,
26]. Interactions among micronutrients further complicate cotton nutrition; for example, excess Zn can induce Fe deficiency in soybeans, while high soil pH reduces the availability of both Fe and Mn [
26,
27]. Such imbalances frequently result in unexplained yield gaps, where macronutrient inputs are adequate, but cotton fails to reach its genetic potential. The emerging evidence demonstrates the importance of integrating B, Fe, Mn, and Zn management into fertility programs to ensure efficient nutrient use, resilience under stress, and sustainable yield gains in cotton production systems.
2.2. Effects of Micronutrients on Cotton Physiological Stress Indicators
Boron plays a crucial role in pollen germination and carbohydrate translocation, key processes for reproductive development and assimilate partitioning in cotton [
28]. Increased B availability promotes square retention, boll set, and efficient utilization of nitrogen and potassium, enhancing photosynthetic rates and chlorophyll content through improved nutrient mobilization from leaves to developing bolls [
29]. Low B levels disrupt cell wall integrity and accelerate square abscission, leading to reduced leaf area and photosynthetic capacity, while excessive B can induce toxicity that lowers chlorophyll levels and alters antioxidant enzyme activities [
30,
31]. Adequate B supplementation, often via foliar application, delays leaf senescence and improves yields by sustaining photosynthetic activity [
32].
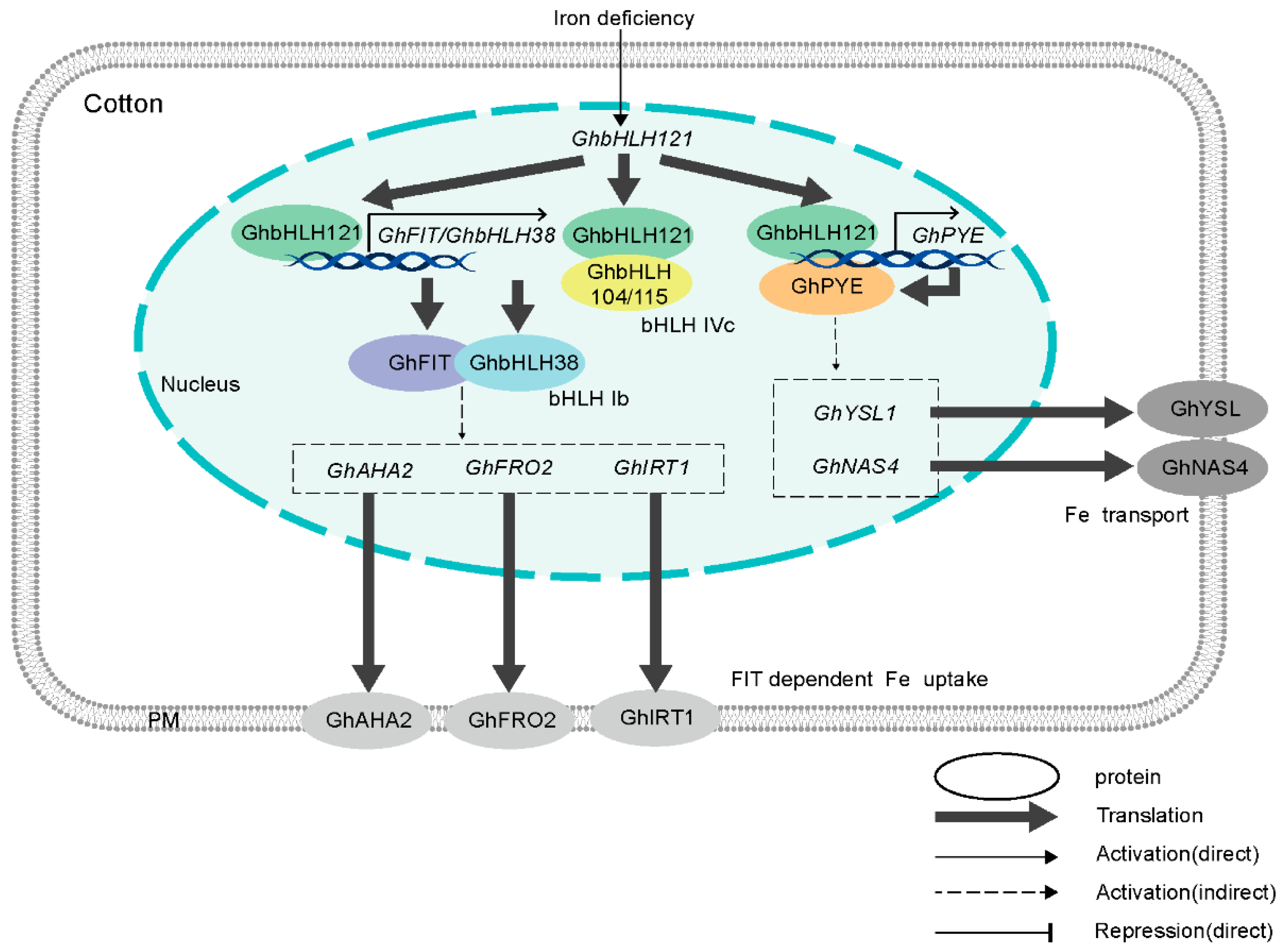
Iron is integral to chlorophyll biosynthesis and electron transport in photosynthesis, with deficiencies severely disrupting chloroplast structure and function in cotton. Increased Fe availability supports chlorophyll production and maintains photosynthetic efficiency, promoting leaf expansion and overall plant vigor. Low Fe levels, common in high pH calcareous soils, accelerate chlorosis through reduced chlorophyll synthesis and impaired photosystem activity, leading to yellowing of young leaves and decreased net photosynthetic rates. Adequate Fe supplementation enhances enzyme activities involved in respiration and nitrogen assimilation, ensuring sustained crop development and higher biomass accumulation (Rout and Sahoo 2015). This is as a result GhbHLH121 gene expression which is upregulated under of Under Fe-deficient conditions cotton growing conditions. The gene GhbHLH121 forms heterodimers with GhbHLH104 or GhbHLH115 both independently activating the downstream expression of the three genes GhbHLH38, GhFIT, and GhPYE [
20].
Figure 2.
A working model of GhbHLH121-mediated regulation under iron deficiency in cotton [
20]
.
Figure 2.
A working model of GhbHLH121-mediated regulation under iron deficiency in cotton [
20]
.
Manganese functions as a cofactor in photosynthetic enzymes, particularly in photosystem II, and supports chlorophyll synthesis and nitrogen metabolism in cotton [
33,
34]. Increased Mn availability promotes chlorophyll content and stomatal conductance, enhancing the net photosynthetic rate and carbohydrate accumulation. Low Mn levels disrupt oxygen evolution in photosynthesis and reduce enzyme activities such as superoxide dismutase, leading to interveinal chlorosis and accelerated leaf senescence [
34]. Excessive Mn can induce toxicity, lowering chlorophyll levels and inhibiting growth, but foliar application at optimal rates increases chlorophyll by up to 17% and improves yield quality on calcareous soils [
33,
35,
36].
Zinc is essential for enzyme activation in carbon fixation and protein synthesis, playing a key role in photosynthesis and hormone regulation in cotton. Increased Zn availability boosts carbonic anhydrase activity and chlorophyll concentration, with critical leaf levels around 13-14 μg/g dry weight required for maximum photosynthetic rates and chlorophyll synthesis [
37]. Low Zn levels accelerate chlorophyll degradation and reduce stomatal conductance, disrupting cell proliferation and leading to stunted growth and lower leaf area. Adequate Zn supplementation, particularly in combination with B, enhances antioxidant enzyme activities and improves yields by maintaining sustained photosynthetic performance [
38,
39].
3. Impact of Micronutrients on Cotton Yield and Quality Traits
3.1. Nutrient Dynamics and Cotton Yield
Cotton production hinges on two key elements: the availability and timing of nutrient delivery to the plant, and the plant’s ability to store and utilize these nutrients effectively [
40]. The first factor ties closely to the plant’s ability to photosynthesize efficiently and the activity within its root zone, where nutrients are absorbed. The second factor relates to the development of reproductive structures, such as flowers, buds, and bolls, and the efficient movement of energy-rich compounds produced during photosynthesis to these areas [
1,
13,
40].
3.1.1. Boron’s Role in Cotton Development
Boron is a critical player in achieving high cotton yields, as it helps strike a delicate balance between vegetative growth and the formation of fibers. When boron levels are just right, it supports robust boll and fiber development, leading to heavier bolls and better fiber with optimum micronaires and seed production [
29,
39,
41]. However, too much B can throw this balance off, causing toxicity that manifests as fewer bolls, stunted fiber growth, and weaker plant tissues, which often results in increased boll shedding and lower overall yield due to poor light energy use [
42]. On the flip side, too little boron hampers plant growth, reduces the weight of individual bolls, and impairs seed development, leading to thicker boll shells and heavier stalks that compromise fiber quality [
13]. Research highlights that adequate boron fosters healthy plant growth, boosts dry matter accumulation, and increases the number of mature seeds, while excessive boron can delay crop maturity and diminish fiber quality.
3.1.2. Iron’s Influence on Growth and Productivity of Cotton
Iron deficiency can significantly stunt cotton plants, leading to shorter plants with smaller leaves compared to those with sufficient iron [
43]. While iron doesn’t directly impact lint percentage, its application boosts the number and weight of bolls, ultimately increasing the cotton yield of seed. This improvement largely comes from reduced shedding of buds and bolls and better nutrient delivery to developing bolls. When soil iron levels are low during the reproductive phase, plants may prematurely shift nutrients from leaves to bolls, which lowers photosynthetic efficiency and cuts into yields [
13,
44].
3.1.3. Manganese’s Contribution to Yield and Fiber Quality
Manganese is vital for maximizing cotton yield and enhancing fiber quality. Applying manganese through foliar sprays or seed treatments promotes boll formation and strengthens fiber properties [
1]. A lack of manganese can cause yellowing between leaf veins (interveinal chlorosis), lower boll retention, and reduced seed cotton yields. In contrast, sufficient manganese increases boll weight and improves fiber strength. However, excessive manganese can lead to toxicity, marked by bronzed leaves and reduced yields [
45]. Balanced manganese application, often paired with other micronutrients, supports higher lint percentages and overall productivity [
46]. Zinc nutrition plays a critical role in cotton yield formation, as optimal Zn levels promote boll development and fiber maturation. Zinc deficiency results in stunted growth, fewer bolls per plant, and reduced fiber length and strength, leading to significant yield losses. Adequate Zn application, particularly foliar sprays, increases seed cotton yield by enhancing boll set and weight, while also improving fiber quality parameters such as micronaire and uniformity. However, excessive Zn can interact negatively with other nutrients like B, potentially reducing uptake and affecting yield, though combined applications often mitigate such effects and boost overall efficiency [
47].
4. Physiological and Molecular Mechanisms of B, Fe, Mn, and Zn Utilization in Cotton
Micronutrients like B, Fe, Mn, and Zn are like tiny sparks that ignite cotton growth, drive higher yields and supporting global food security. However, heavy reliance on micronutrient fertilizers can harm the environment, deplete soils and cause contamination [
48]. To grow more cotton with less environmental cost, improving micronutrient use efficiency (MUE), the plant’s ability to make the most of these nutrients, is critical [
49]. While smarter farming practices help, the real game-changer lies in breeding cotton varieties that naturally excel at using these nutrients efficiently [
18,
50,
51]. Over the past 30 years, selective breeding has made great strides in enhancing how cotton plants handle B, Fe, Mn, and Zn [
20,
52]. Looking ahead, boosting nutrient redistribution within the plant will be key to unlocking even greater yields.
When boron runs low in the soil, cotton plants increase boron uptake efficiency (BUE) through root extensions to absorb more and fine-tuning internal processes to make better use of what’s available. These adaptations to boron uptake are driven by active root systems and optimized cellular functions that help maintain a healthy boron balance in the soil systems, especially with varieties bred for high BUE [
3,
26,
50,
53,
54]. Similarly, when iron is deficient in the soil, cotton plants release phytosiderophores, chemical “magnets” that help to chelate iron from the soil and boost the activity of iron transporters to pull it into the plant [
20]. Below is a table (
Table 1) summarizing iron uptake strategies in various crops, including upland cotton (dicotyledonous plant), employing iron utilization. The commonest strategy is reduction-based, where it acidifies the rhizosphere, reducing Fe3+ to Fe2+, and transporting it via specific transporters like IRT1. Cotton for example uses genes like GhbHLH121 to regulate responses to iron deficiency, like Arabidopsis (
Arabidopsis thaliana (L.) Heynh). The alternative pathway is chelation.
For manganese and zinc shortages, cotton plants increase the production of metal tolerance proteins and ZIP transporters, which act like gatekeepers to regulate the uptake and balance of these nutrients, ensuring the plant thrives despite limited supply [
33].
Boron is a cornerstone of cotton’s growth that strengthens cell walls and assists the plant in transporting sugars around the plant. Enzymes like pectin methylesterases and expansins work together to keep cell walls sturdy enhancing resistance to lodging [
12,
41,
50]. Boron helps in maintaining cell wall structure and function through its involvement in the cross-linking of rhamnogalacturonan II (RG-II), a process mediated by enzymes such as UDP-glycosyltransferases that strengthen cell wall integrity. Concurrently, xyloglucan endotransglucosylases contribute to cell wall remodeling during growth and development [
41,
62]. Beyond structural functions, boron also modulates signaling pathways, where reactive oxygen species (ROS) generated by oxidases act as key regulators of developmental processes and stress adaptation in cotton [
54,
63].
Iron underpins energy metabolism by driving photosynthesis and respiration, with ferric reductases converting Fe
3+ to the more soluble Fe
2+ under deficiency conditions to enhance uptake [
6,
21,
60,
64]. Within the plant, enzymes such as ferredoxin and heme oxygenase sustain iron mobilization and availability, ensuring continued photosynthetic efficiency and growth despite limited supply [
51]. Manganese is equally critical, functioning as a cofactor in photosystem II to maintain efficient light harvesting and energy conversion. Its role extends to stress defense, where manganese superoxide dismutase (MnSOD) mitigates reactive oxygen species damage, while incorporation into metalloproteins supports boll development and overall growth [
33,
34]. Zinc contributes to both metabolic regulation and genetic stability, serving as a cofactor for enzymes such as carbonic anhydrase and alcohol dehydrogenase, which facilitate CO
2 fixation and energy metabolism. Under deficiency, alkaline phosphatase and zinc finger proteins become central to mobilizing zinc, regulating gene expression, and sustaining DNA replication and protein synthesis processes essential for fiber quality and seed development [
21,
22,
65].
5. B, Fe, Mn, and Zn Efficiency Genes and Hormonal Regulatory Mechanisms in Plants
Boron B uptake in plants is primarily mediated by the boron transporter (BOR) and nodulin 26-like intrinsic protein (NIP) families, with functional variation observed among species. In cotton (Gossypium), BOR family members have been identified to contribute to B export and tolerance. Specific transporters such as BOR1 and NIP5;1 are directly involved in B uptake, while others regulate B translocation and distribution [
5,
20,
63,
66]. Similarly, Fe, Mn, and Zn homeostasis in cotton is mediated by zinc-regulated/iron-regulated transporter-like proteins (ZIP) and natural resistance-associated macrophage proteins (NRAMP) [
21,
67]. Across plant species related to cotton, 14, 22, and 18 ZIP genes have been identified for Fe, Mn, and Zn transport, respectively, with collinearity analyses highlighting structural and functional conservation among these transporters [
21].
Reactive oxygen species (ROS) further regulate micronutrient balance, with endogenous ROS accumulation enhancing tolerance under low-micronutrient conditions. Adaptive responses to micronutrient stress also include modifications in root morphology, exudation of organic acids, enhanced membrane and intracellular transport, and induction of high-affinity transporter genes, all of which facilitate improved uptake and utilization [
66]. In Arabidopsis for example, transcription factors such as FIT, and bHLH regulate Fe deficiency when activated by MYB proteins including MYB30 and MYB121 to enhance its distribution in the plants [
68]. Homologous genes in cotton, including GhbHLH121, GhZIP, and GhNRAMP, have been identified as key regulators of micronutrient acquisition and utilization, underscoring conserved regulatory mechanisms across species [
20,
42,
55].
5.1. Hormonal Regulation of B, Fe, Mn, and Zn
Plant hormones act as master regulators of cotton growth and development, coordinating diverse physiological responses to environmental and nutritional signals. Among them, nine major groups, auxin, cytokinins (CTKs), abscisic acid (ABA), ethylene (ETH), gibberellins (GAs), brassinosteroids (BRs), jasmonic acid (JAs), salicylic acid (SA), and strigolactones (SL), play central roles in mediating cotton’s adaptation to the availability of B, Fe, Mn, and Zn [
69,
70,
71,
72,
73]. ABA has been shown to regulate Fe-driven lateral root formation, with ABA-dependent signaling pathways promoting root development and enhancing Fe redistribution from roots to shoots in both cotton and model systems like
Arabidopsis thaliana (L.) Heynh [
71]. CTKs serve as long-distance messengers of nutrient status, relaying Zn and Fe utilization signals via the xylem. Genes such as AtIPT3 and AtIPT5, which govern CTK biosynthesis under Zn and Fe stress in Arabidopsis, appear to function through similar pathways in cotton roots which influences nutrient uptake [
72].
The interplay between Fe and GAs has been widely reported, with growth regulator factor 4 (GRF4) identified as a key player in Fe uptake and biomass partitioning in rice; comparable functions are proposed for cotton [
73]. BR signaling kinase 3 (BSK3), essential for root elongation under Fe deficiency in Arabidopsis, points to potential BR–Fe crosstalk in shaping cotton root architecture under nutrient stress [
35,
73]. Likewise, the application of ethephon, an ETH-releasing compound, improves Zn utilization efficiency by enhancing enzyme activity linked to Zn metabolism, thereby increasing Zn and protein concentrations in cotton tissues. Peptide hormones such as CEPD1 and CEPD2, which stimulate IRT1 expression and promote Fe uptake in Arabidopsis, may also have analogous functions in cotton nutrient transport. Boron availability strongly interacts with hormonal pathways regulating root growth and cell wall dynamics. Under B deficiency, SA, ABA, and JA converge on WRKY75, a transcription factor that modulates auxin-mediated lateral root elongation and density, ultimately affecting cell wall stability and root morphology in cotton [
69]. In similar way, excess Mn disrupts IAA balance, accelerating IAA oxidation and altering root development, underscoring the crosstalk between hormones and micronutrient signaling [
33,
34,
35], and more details in
Table 2.
5.2. B, Fe, Mn, and Zn Signal Integration
The coordinated utilization of B, Fe, Mn, and Zn is essential for sustaining plant growth and maximizing crop productivity. While the signaling pathways of these micronutrients have been extensively studied individually, their interactions remain poorly understood [
3,
48,
80]. Emerging evidence indicates that the integration of B, Fe, Mn, and Zn uptake represents an evolutionary adaptation that enables plants to maintain a balanced nutrient profile [
36,
43]. Significant progress has been achieved in identifying the key regulatory components governing B–Fe–Mn–Zn interactions in model species such as Arabidopsis and rice [
80]. Although research in cotton is limited, available molecular insights suggest promising opportunities to enhance boron use efficiency, iron use efficiency (IUE), manganese use efficiency (MUE), and zinc use efficiency (ZUE) [
20,
21,
34,
35,
46]. Current findings reveal a complex network that integrates B, Fe, Mn, and Zn signaling pathways, offering a clearer understanding of their interdependence (
Table 3).
5.3. B–Fe–Mn–Zn Signaling Integration in Root Development
The availability of B, Fe, Mn, and Zn influences root architecture, allowing plants to adapt to diverse environmental conditions. Local B supply promotes lateral root elongation [
75], while excess Mn induces short, highly branched roots in cotton, altering nutrient uptake [
47]. Boron deficiency can have a two-way effect on plant growth, where by it can either promotes or suppresses taproot growth, with inhibition linked to blue light–induced Fe redox reactions near roots that generate reactive oxygen species (ROS), particularly under combined B and Fe deficiency in calcareous soils [
42,
50,
82].
B–Zn interactions further regulate root nutrient dynamics. Adequate B enhances Mn and Fe accumulation while maintaining balanced Mn/Fe ratios, supporting root health. In contrast, B deficiency upregulates ACS11, increasing ethylene biosynthesis, auxin-mediated inhibition of root elongation, and ROS production. Zn supplementation, including ZnO nanoparticles, alleviates B toxicity by improving root biomass, reducing ROS, and enhancing Fe and Mn transporter activity [
83]. At the molecular level, the Arabidopsis transcription factor (AtNIGT1) integrates B and Zn signals, with homologs likely functioning in cotton [
74,
83]. B induces AtNIGT1 via BOR1, while Zn deficiency reduces its stability [
84]. This dual regulation modulates taproot growth, with AtNIGT1 suppressing taproot elongation under B deficiency but only in the presence of Zn, coordinating with Fe and Mn signals to maintain root structure. The BOR1-AtNIGT1 module thus links B and Zn cues to downstream regulation of root development [
84]. In cotton, B deficiency also enhances Cu, Mn, and Fe accumulation to sustain root function [
26]. High planting density reduces root growth due to competition, but B supplementation and growth regulators such as mepiquat chloride improve Mn and Zn uptake, enhancing root morphology [
85]
.
5.4. B-Fe-Mn-Zn Signaling Integration to Regulate Cotton Boll Development
Boron, iron, manganese, and zinc availability plays a pivotal role in boll formation and maturation in cotton, influencing reproductive structures and yield. Adequate boron supply enhances boll weight and reduces boll shedding, while manganese excess can disrupt iron homeostasis, leading to reduced boll nutrient content, as seen in studies where high Mn/Fe ratios impair fiber quality [
3,
21,
22]. Low Zn conditions impair boll development by limiting enzyme activities, with deficiencies exacerbating iron and manganese imbalances that affect cell wall integrity in bolls, particularly under calcareous soils where Zn availability is low [
3,
11].
In cotton, the interaction between B and Zn affects Fe and Mn content in bolls, with B promoting the transport of Fe and Mn to reproductive tissues, as evidenced by increased boll micronutrient levels under combined B-Zn application [
3,
47]. Boron deficiency inhibits pollen tube growth and boll setting, but zinc supplementation can mitigate this by stabilizing membrane functions and hormone signaling, leading to higher boll retention and seed quality [
21]. Transcription factors like GhbHLH121 integrate Fe and Zn signals to regulate boll gene expression under deficiency, coordinating with boron transporters for nutrient allocation and influencing fiber elongation [
20,
66]. Optimal B-Zn ratios ensure balanced Mn/Fe uptake, preventing oxidative stress in bolls and improving fiber quality, with foliar applications of Zn, Fe, and B significantly boosting boll yield and micronutrient concentrations.
5.5. B-Fe-Mn-Zn Signaling Integration to Regulate Cotton Response to Stress
Boron, iron, manganese, and zinc are integral to cotton’s stress response mechanisms, modulating antioxidant defenses and nutrient homeostasis under abiotic stresses like toxicity or deficiency [
12,
19,
86,
87]. Boron toxicity elevates ROS levels, but zinc alleviates this by enhancing SOD, POD, and CAT activities, reducing H2O2 and MDA in roots and leaves, as demonstrated in cotton seedlings where ZnO nanoparticles improve tolerance [
65,
69,
88]. Manganese and iron interactions under stress influence redox balance, with excess Mn disrupting Fe uptake and exacerbating oxidative damage, particularly in acidic soils where Mn toxicity is prevalent in cotton fields [
35].
In cotton, ZnO nanoparticles upregulate ABC transporter genes and photosynthesis pathways to counter boron stress, integrating signals for nutrient redistribution and stress tolerance [
65,
86]. Boron interacts with Zn to modulate hormone signaling (e.g., jasmonic acid), enhancing resilience to toxicity, with foliar Zn reducing B-induced growth inhibition. Under calcareous soil stress, balanced B-Zn-Fe-Mn ratios improve membrane integrity and reduce susceptibility to environmental cues like waterlogging or salinity, as nano-Zn applications mitigate salt stress by maintaining ionic homeostasis [
23]. Heat stress responses are alleviated by micronutrient sprays, including Mn and Zn, which upregulate defense genes and reduce oxidative damage in cotton [
1]. More details are available on
Table 4.
5.6. B–Fe–Mn–Zn Signaling Integration Regulating Nutrient Uptake
The AtNIGT1s family (AtNIGT1.1–1.4) plays a central role in nutrient regulation by repressing B-starvation–induced genes (e.g.,
BOR1,
NIP5;1) and controlling Zn-starvation–responsive genes (
ZIP2/4,
HMA2/4), thereby enhancing Zn utilization. AtNIGT1 expression is induced by high B and Zn deficiency and regulated by transcription factors AtbHLH121 and AtMYB13, linking B and Zn signaling pathways [
41,
79,
89]. Boron directly promotes Zn utilization [
3,
47]. In rice and cotton, the B transporter
BOR1 activates Zn-starvation induced (ZSI) genes by interacting with SPX4-like proteins, leading to SPX4 degradation, MYB translocation into the nucleus, and induction of Zn-responsive genes[
42,
53,
74]. This BOR1–SPX4–MYB axis exemplifies B–Zn crosstalk, where boron signaling co-activates both B- and Zn-responsive pathways.
The bHLH transcription factors also contribute to B signaling via cytoplasmic–nuclear shuttling, while SPX4 coordinates MYB13 (Zn signaling) and bHLH121 (B signaling), forming a unified BOR1–SPX4–bHLH121–MYB13 cascade that integrates B and Zn responses [
20,
66]. BOR1 and SPX4 are regulated through ubiquitination by plasma membrane–localized E3 ubiquitin ligases. Under toxic B conditions, BOR1 is targeted for degradation, preventing excess B accumulation while linking B perception to Zn signaling [
65,
69,
88]. This BOR1–SPX module transduces B signals to core transcription factors, enabling synergistic regulation of B- and Zn-responsive genes [
90]. Downstream, MYB, bHLH, and NIGT1 sustain B–Zn homeostasis under fluctuating environments [
91].
Beyond Zn, B also influences Mn utilization by regulating Mn concentration and assimilation. Boron reduces free Mn levels, represses
NRAMP1, and activates
MTP11, while stimulating Mn superoxide dismutase activity. This demonstrates B’s role in linking Mn and Zn regulation [
92]. In cotton, B–Fe–Mn–Zn integration extends to macronutrient uptake. B enhances N and K absorption but reduces P and Fe translocation under high B. Zn promotes N and K uptake while antagonizing P and Fe, affecting S metabolism through enzyme activation [
3,
15]. Although the molecular basis of B–Fe–Mn–Zn signaling in cotton remains limited and unclear, evidence from model plants shows these micronutrients function both independently and interactively, coordinating with macronutrient (N, P, K, S) uptake (
Table 5).
6. Genomic and Phenotyping Approaches for Breeding Nutrient-Efficient Cotton Varieties
Rising cotton yields have been accompanied by greater inputs of micronutrients such as boron B, Fe, Mn, and Zn, creating challenges including soil imbalances, potential toxicity, and low nutrient use efficiency (NUE), which increases production costs. Natural variation in micronutrient efficiency among cotton genotypes provides an opportunity to breed varieties with both high yield potential and improved NUE. High-throughput phenotyping, particularly using unmanned aerial vehicles (UAVs) equipped with multispectral and hyperspectral sensors, now enables precise assessment of chlorophyll content, biomass, and micronutrient status [
93]. These technologies allow detection of nutrient deficiency symptoms, such as B-induced boll shedding or Fe-related chlorosis and support the identification of nutrient-efficient genotypes [
93]. However, cotton’s complex canopy and inconsistent correlations between biomass and yield under variable micronutrient conditions remain challenges [
94]. Advances in UAV imaging and precision cameras are expected to overcome these limitations, enabling more accurate trait measurement [
95,
96].
Integrating UAV-based phenotyping with genome-wide association studies (GWAS) facilitates the discovery of genes controlling B, Fe, Mn, and Zn efficiency [
93]. Field trials with gradient micronutrient treatments can further improve screening accuracy while conserving resources. Genes such as
GhbHLH121 (Fe regulation) and
GhZIP3 (Zn uptake) enhance NUE and yield stability under deficiency, while
BOR1 and
NRAMP1 regulate B and Mn transport, influencing boll development and nutrient allocation [
20,
66].
Breeding strategies focus on incorporating such genes into elite cotton lines. For example, overexpression of
BOR1 reduces B deficiency–related pod abortion, while enhanced
IRT1 expression improves Fe and Zn uptake under calcareous soils [
42,
53,
74,
97]. While marker-assisted selection (MAS), CRISPR-based editing, and transgenic approaches targeting genes such as
GhMTP11 (Mn homeostasis) are producing varieties with balanced micronutrient uptake and improved fiber quality [
81]. Lessons from model plants like
Arabidopsis and rice continue to inform these efforts (
Table 6).
7. Future Research Directions for B, Fe, Mn, and Zn Efficiency in Cotton
Although the physiology of B, Fe, Mn, and Zn uptake, assimilation signaling, and use efficiency in cotton is increasingly getting attention, their genetic, hormonal pathways and technological advances remain underexplored. Insights from other plants highlight that these could improve cotton’s nutrient use efficiency (NUE) and yield stability. The future research should focus on high-throughput phenotyping using UAV-based imaging, combined with genome-wide association studies (GWAS), among other molecular techniques in identifying nutrient-efficient genotypes. Breeding strategies incorporating marker-assisted selection and CRISPR editing, for instance, overexpressing BOR1 or IRT1, or editing GhMTP11, can enhance micronutrient uptake, fiber quality, and stress tolerance. Research focusing on nutrient interactions with macronutrients (N, P, K, S), adaptation to environmental stresses, and sustainable nutrient management is highly recommended. Integrating genetic improvements with precision fertilization, biofortification, and sensor-guided application can optimize nutrient efficiency, reduce environmental impact, and support high-yield, resilient cotton production
8. Conclusions
In conclusion, this review synthesizes the pivotal roles of boron, iron, manganese, and zinc in cotton physiology, signaling integration, and nutrient use efficiency, addressing overlooked yield gaps in macronutrient-focused programs. Key findings demonstrate that these micronutrients regulate photosynthesis, hormone signaling, stress responses, and reproductive success, with deficiencies exacerbated by soil pH and interactions causing hidden hunger; genes such as BOR1, IRT1, and GhZIP3 provide targets for optimization. Future directions include leveraging microRNAs, CRISPR gene editing, UAV phenotyping, and precision nutrition to enhance efficiency, mitigate environmental challenges, and sustain high-yield cotton production.
Author Contributions
Conceptualization, Unius Arinaitwe, and Abraham Hangamaisho.; methodology, Unius Arinaitwe.; software, Unius Arinaitwe.; validation, Unius Arinaitwe., Abraham Hangamaisho.; formal analysis, Unius Arinaitwe and Abraham Hangamaisho.; investigation, Unius Arinaitwe.; resources, Unius Arinaitwe.; data curation; Unius Arinaitwe and Abraham Hangamaisho.; writing original draft preparation, Unius Arinaitwe.; writing review and editing, Unius arinaitwe and Abraham Hangamaisho.; supervision, Unius Arinaitwe.; project administration, Unius Arinaitwe.; funding acquisition, n/a. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data for this article is contained in it. For additional information, contact the corresponding author.
Acknowledgments
During the preparation of this manuscript, the author(s) took full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| NUE |
Nutrient Use Efficiency |
| BUE |
Boron Use Efficiency |
| IUE |
Iron Use Efficiency |
| MUE |
Manganese Use Efficiency |
| ZUE |
Zinc Use Efficiency |
| N, P, K, S |
Nitrogen, Phosphorus, Potassium, Sulfur |
| |
Molecular/Genetic Acronyms |
| IRT1 |
Iron-Regulated Transporter 1 |
| BOR1 |
Boron Transporter 1 |
| NIP5;1 |
Nodulin 26-like Intrinsic Protein 5;1 |
| ZIP |
Zinc/Iron-Regulated Transporter-like Protein family |
| NRAMP1 |
Natural Resistance-Associated Macrophage Protein 1 |
| MTP11 |
Metal Tolerance Protein 11 |
| HMA2/HMA4 |
Heavy Metal ATPase 2/4 |
| GhbHLH121 |
Basic Helix-Loop-Helix transcription factor in cotton |
| GhZIP3 |
Zinc Transporter gene in cotton |
| GhMTP11 |
Cotton Metal Tolerance Protein gene |
| miRNAs |
MicroRNAs |
| miR169, miR398, miR408 |
Specific microRNAs involved in nutrient regulation |
| NFYA |
Nuclear Factor Y subunit A |
| ROS |
Reactive Oxygen Species |
| RG-II |
Rhamnogalacturonan II |
| SOD |
Superoxide Dismutase |
| POD |
Peroxidase |
| CAT |
Catalase |
| GhbHLH121 |
Basic Helix-Loop-Helix transcription factor in cotton |
| GhZIP3 |
Zinc Transporter gene in cotton |
| GhMTP11 |
Cotton Metal Tolerance Protein gene |
| |
|
| |
Hormone and Signaling Acronyms |
| ABA |
Abscisic Acid |
| CTK / CTKs |
Cytokinins |
| ETH |
Ethylene |
| GA / GAs |
Gibberellins |
| BR / BRs |
Brassinosteroids |
| JA / JAs |
Jasmonic Acid |
| SA |
Salicylic Acid |
| SL |
Strigolactones |
| IAA |
Indole-3-Acetic Acid (Auxin) |
| |
Technology Acronyms |
| UAVs |
Unmanned Aerial Vehicles |
| scRNA-seq |
Single-Cell RNA Sequencing |
| GWAS |
Genome-Wide Association Studies |
| MAS |
Marker-Assisted Selection |
| CRISPR |
Clustered Regularly Interspaced Short Palindromic Repeats |
References
- C. Dordas, “Foliar application of manganese increases seed yield and improves seed quality of cotton grown on calcareous soils,” Journal of Plant Nutrition, vol. 32, no. 1, pp. 160-176, 2009. [CrossRef]
- G. J. Kidron and A. Zilberman, “Low cotton yield is associated with micronutrient deficiency in West Africa,” Agronomy Journal, vol. 111, no. 4, pp. 1977-1984, 2019. [CrossRef]
- É. de Oliveira Araújo, É. Ferreira Dos Santos, and M. A. Camacho, “Boron-zinc interaction in the absorption of micronutrients by cotton,” Agronomía Colombiana, vol. 36, no. 1, pp. 51-57, 2018. [CrossRef]
- H. Xu, X. Yang, and Y. Zhu, “Research on the Role of Micronutrient Management in Improving Cotton Fiber Quality,” Cotton Genomics and Genetics, vol. 16, 2025. [CrossRef]
- G. R. Rout and S. Sahoo, “Role of iron in plant growth and metabolism,” Reviews in agricultural science, vol. 3, pp. 1-24, 2015. [CrossRef]
- A. F. López-Millán, D. Duy, and K. Philippar, “Chloroplast iron transport proteins–function and impact on plant physiology,” Frontiers in plant science, vol. 7, p. 178, 2016. [CrossRef]
- G. E. Kroh and M. Pilon, “Regulation of iron homeostasis and use in chloroplasts,” International Journal of Molecular Sciences, vol. 21, no. 9, p. 3395, 2020. [CrossRef]
- J. Xiao and X. Yin, “Nutrient Management in cotton,” Cotton Production, pp. 61-83, 2019. [CrossRef]
- K. S. Smith and H. L. Huyck, “An overview of the abundance, relative mobility, bioavailability, and human toxicity of metals,” 1997. [CrossRef]
- J. Moraghan and H. Mascagni Jr, “Environmental and soil factors affecting micronutrient deficiencies and toxicities,” Micronutrients in agriculture, vol. 4, pp. 371-425, 1991. [CrossRef]
- M. Ashraf et al., “Zinc nutrition optimization for better cotton productivity on alkaline calcareous soil,” Journal of Cotton Research, vol. 8, no. 1, p. 14, 2025. [CrossRef]
- C. A. Rosolem and J. C. Bogiani, “Physiology of boron stress in cotton,” Stress physiology in cotton. The Cotton Foundation, Cordova, TN, USA, pp. 113-124, 2011.
- M. Mehran et al., “Growth, yield and fiber quality characteristics of Bt and non-Bt cotton cultivars in response to boron nutrition,” Journal of Cotton Research, vol. 6, no. 1, p. 1, 2023. [CrossRef]
- E. J. Martinez-Finley, C. E. Gavin, M. Aschner, and T. E. Gunter, “Manganese neurotoxicity and the role of reactive oxygen species,” Free radical biology and medicine, vol. 62, pp. 65-75, 2013. [CrossRef]
- M. D. Khan, L. Mei, B. Ali, Y. Chen, X. Cheng, and S. Zhu, “Cadmium-Induced Upregulation of Lipid Peroxidation and Reactive Oxygen Species Caused Physiological, Biochemical, and Ultrastructural Changes in Upland Cotton Seedlings,” BioMed research international, vol. 2013, no. 1, p. 374063, 2013. [CrossRef]
- N. Ahmed, M. A. Ali, S. Hussain, W. Hassan, F. Ahmad, and S. Danish, “Essential micronutrients for cotton production,” in Cotton production and uses: Agronomy, crop protection, and postharvest technologies: Springer, 2020, pp. 105-117. [CrossRef]
- A. Nesic, S. Meseldzija, A. Onjia, and G. Cabrera-Barjas, “Impact of crosslinking on the characteristics of pectin monolith cryogels,” Polymers, vol. 14, no. 23, p. 5252, 2022. [CrossRef]
- J. Li et al., “Iron deficiency leads to chlorosis through impacting chlorophyll synthesis and nitrogen metabolism in Areca catechu L,” Frontiers in Plant Science, vol. 12, p. 710093, 2021. [CrossRef]
- S. Zhang, Z. Zhang, X. Qin, and Z. Zhang, “Engineered sulfur and iron nanoparticles enhance cotton tolerance to lead stress via distinct molecular and physiological strategies,” Industrial Crops and Products, vol. 233, p. 121343, 2025. [CrossRef]
- J. Li et al., “The molecular mechanism of GhbHLH121 in response to iron deficiency in cotton seedlings,” Plants, vol. 12, no. 10, p. 1955, 2023. [CrossRef]
- Q. An, C. Cui, N. Muhammad Khan, G. Zhou, and Y. Wan, “Genome-wide investigation of ZINC-IRON PERMEASE (ZIP) genes in Areca catechu and potential roles of ZIPs in Fe and Zn uptake and transport,” Plant Signaling & Behavior, vol. 16, no. 12, p. 1995647, 2021. [CrossRef]
- N. Ahmed, F. Ahmad, M. Abid, and M. A. Ullah, “Impact of zinc fertilization on gas exchange characteristics and water use efficiency of cotton crop under arid environment,” Pak. J. Bot, vol. 41, no. 5, pp. 2189-2197, 2009.
- X. Yin, O. Gwathmey, C. Main, and A. Johnson, “Effects of Sulfur Application Rates and Foliar Zinc Fertilization on Cotton Lint Yields and Qualities,” Agronomy Journal, vol. 103, no. 6, pp. 1794-1803, 2011. [CrossRef]
- S. Caliskan, I. Ozkaya, M. Caliskan, and M. Arslan, “The effects of nitrogen and iron fertilization on growth, yield and fertilizer use efficiency of soybean in a Mediterranean-type soil,” Field Crops Research, vol. 108, no. 2, pp. 126-132, 2008. [CrossRef]
- J. C. Bogiani, A. C. E. Amaro, and C. A. Rosolem, “Carbohydrate production and transport in cotton cultivars grown under boron deficiency,” Scientia agrícola, vol. 70, pp. 442-448, 2013. [CrossRef]
- Y. Long and J. Peng, “Interaction between boron and other elements in plants,” Genes, vol. 14, no. 1, p. 130, 2023. [CrossRef]
- N. T. de Oliveira et al., “Iron counteracts zinc-induced toxicity in soybeans,” Plant Physiology and Biochemistry, vol. 194, pp. 335-344, 2023. [CrossRef]
- M. Aasim, Ö. Akgür, and B. Yıldırım, “An overview on boron and pollen germination, tube growth and development under in vitro and in vivo conditions,” Boron in Plants and Agriculture, pp. 293-310, 2022. [CrossRef]
- N. Y. Ahmed and A. H. Noaman, “The role of boron in the vegetative growth characteristics of several cotton (Gossypium hirsutum L.) cultivars,” in IOP Conference Series: Earth and Environmental Science, 2024, vol. 1371, no. 5: IOP Publishing, p. 052056. [CrossRef]
- D. Jin et al., “Chemical defoliant promotes leaf abscission by altering ROS metabolism and photosynthetic efficiency in Gossypium hirsutum,” International journal of molecular sciences, vol. 21, no. 8, p. 2738, 2020. [CrossRef]
- H. Yu, Y. Luo, N. Cao, S. Wang, Z. Zhou, and W. Hu, “Drought-induced cell wall degradation in the base of pedicel is associated with accelerated cotton square shedding,” Plant Physiology and Biochemistry, vol. 214, p. 108894, 2024. [CrossRef]
- M. A. Shallan, H. M. Hassan, A. A. Namich, and A. A. Ibrahim, “Effect of sodium nitroprusside, putrescine and glycine betaine on alleviation of drought stress in cotton plant,” Am Eurasian J Agric Environ Sci, vol. 12, no. 9, pp. 1252-1265, 2012. [CrossRef]
- S. Alejandro, S. Höller, B. Meier, and E. Peiter, “Manganese in plants: from acquisition to subcellular allocation,” Frontiers in plant science, vol. 11, p. 300, 2020. [CrossRef]
- S. Mukhopadhyay, S. K. Mandal, S. Bhaduri, and W. H. Armstrong, “Manganese clusters with relevance to photosystem II,” Chemical Reviews, vol. 104, no. 9, pp. 3981-4026, 2004. [CrossRef]
- Y. Liu, M. Zhao, J. Chen, S. Yang, J. Chen, and Y. Xue, “Comparative transcriptome analysis reveals complex physiological response and gene regulation in peanut roots and leaves under manganese toxicity stress,” International Journal of Molecular Sciences, vol. 24, no. 2, p. 1161, 2023. [CrossRef]
- Y. Jia et al., “Physiological and transcriptomic analyses reveal the roles of secondary metabolism in the adaptive responses of Stylosanthes to manganese toxicity,” BMC genomics, vol. 21, no. 1, p. 861, 2020. [CrossRef]
- M. Solanki, “The Zn as a vital micronutrient in plants,” Journal of microbiology, biotechnology and food sciences, vol. 11, no. 3, pp. e4026-e4026, 2021. [CrossRef]
- K. Ohki, “Lower and upper critical zinc levels in relation to cotton growth and development,” Physiologia Plantarum, vol. 35, no. 2, pp. 96-100, 1975. [CrossRef]
- K. Ohki, “Mn and B Effects on Micronutrients and P in Cotton 1,” Agronomy Journal, vol. 67, no. 2, pp. 204-207, 1975. [CrossRef]
- I. J. Rochester, G. A. Constable, D. M. Oosterhuis, and M. Errington, “Nutritional requirements of cotton during flowering and fruiting,” Flowering and fruiting in cotton. Cordova: The cotton foundation, pp. 35-50, 2012.
- P. Vera-Maldonado et al., “Role of boron and its interaction with other elements in plants,” Frontiers in Plant Science, vol. 15, p. 1332459, 2024. [CrossRef]
- K. Kasai, J. Takano, K. Miwa, A. Toyoda, and T. Fujiwara, “High boron-induced ubiquitination regulates vacuolar sorting of the BOR1 borate transporter in Arabidopsis thaliana,” Journal of Biological Chemistry, vol. 286, no. 8, pp. 6175-6183, 2011. [CrossRef]
- X. Zhang, D. Zhang, W. Sun, and T. Wang, “The adaptive mechanism of plants to iron deficiency via iron uptake, transport, and homeostasis,” International journal of molecular sciences, vol. 20, no. 10, p. 2424, 2019. [CrossRef]
- L. F. dos Santos Cordeiro, C. F. dos Santos Cordeiro, and S. Ferrari, “Cotton yield and boron dynamics affected by cover crops and boron fertilization in a tropical sandy soil,” Field Crops Research, vol. 284, p. 108575, 2022. [CrossRef]
- F. Adams and J. I. Wear, “Manganese toxicity and soil acidity in relation to crinkle leaf of cotton,” Soil Science Society of America Journal, vol. 21, no. 3, pp. 305-308, 1957. [CrossRef]
- K. Ohki, “Manganese Nutrition of Cotton Under Two Boron Levels I. Growth and Development,” Agronomy Journal, vol. 65, no. 3, pp. 482-485, 1973. [CrossRef]
- S. Hosseini, M. Maftoun, N. Karimian, A. Ronaghi, and Y. Emam, “Effect of zinc× boron interaction on plant growth and tissue nutrient concentration of corn,” Journal of plant nutrition, vol. 30, no. 5, pp. 773-781, 2007. [CrossRef]
- S. Wang, L. Xu, and M. Hao, “Impacts of long-term micronutrient fertilizer application on soil properties and micronutrient availability,” International Journal of Environmental Research and Public Health, vol. 19, no. 23, p. 16358, 2022. [CrossRef]
- T. Aftab and K. R. Hakeem, Plant Micronutrients (no. 1). Springer, 2020.
- J. Li et al., “Physiological and molecular bases of the boron deficiency response in tomatoes,” Horticulture Research, vol. 10, no. 12, p. uhad229, 2023. [CrossRef]
- M. Nazari et al., “Enhancing photosynthesis and plant productivity through genetic modification,” Cells, vol. 13, no. 16, p. 1319, 2024. [CrossRef]
- J. Zhang and T. Wedegaertner, “Genetics and breeding for glandless upland cotton with improved yield potential and disease resistance: A review,” Frontiers in Plant Science, vol. 12, p. 753426, 2021. [CrossRef]
- F. Kurt and A. Aydın, “An in-silico study: interaction of BOR1-type boron (B) transporters with a small group of functionally unidentified proteins under various stresses in potato (Solanum tuberosum),” Commagene Journal of Biology, vol. 4, no. 2, pp. 134-139, 2020. [CrossRef]
- Z. Jiang, L. Liu, S. Wang, X. Ye, Z. Liu, and F. Xu, “Transcriptional Analysis Reveals the Differences in Response of Floral Buds to Boron Deficiency Between Two Contrasting Brassica napus Varieties,” Plants, vol. 14, no. 6, p. 859, 2025. [CrossRef]
- G. Vert et al., “IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth,” The plant cell, vol. 14, no. 6, pp. 1223-1233, 2002. [CrossRef]
- Y.-T. Chen, Y. Wang, and K.-C. Yeh, “Role of root exudates in metal acquisition and tolerance,” Current opinion in plant biology, vol. 39, pp. 66-72, 2017. [CrossRef]
- K. Bashir et al., “Paralogs and mutants show that one DMA synthase functions in iron homeostasis in rice,” Journal of Experimental Botany, vol. 68, no. 7, pp. 1785-1795, 2017. [CrossRef]
- A. Martín-Barranco, S. Thomine, G. Vert, and E. Zelazny, “A quick journey into the diversity of iron uptake strategies in photosynthetic organisms,” Plant Signaling & Behavior, vol. 16, no. 11, p. 1975088, 2021. [CrossRef]
- J. Y. Yan et al., “A WRKY transcription factor regulates Fe translocation under Fe deficiency,” Plant Physiology, vol. 171, no. 3, pp. 2017-2027, 2016. [CrossRef]
- E. Aksoy, “Barley preferentially activates strategy-II iron uptake mechanism under iron deficiency,” Biotech Studies, vol. 33, no. 1, pp. 23-32, 2024. [CrossRef]
- G. Saridis, S. N. Chorianopoulou, Y. E. Ventouris, P. P. Sigalas, and D. L. Bouranis, “An exploration of the roles of ferric iron chelation-strategy components in the leaves and roots of maize plants,” Plants, vol. 8, no. 5, p. 133, 2019. [CrossRef]
- J. P. de Souza Júnior et al., “Addition of silicon to boron foliar spray in cotton plants modulates the antioxidative system attenuating boron deficiency and toxicity,” BMC Plant Biology, vol. 22, no. 1, p. 338, 2022. [CrossRef]
- X. Song et al., “Transcriptome analysis reveals the molecular mechanism of boron deficiency tolerance in leaves of boron-efficient Beta vulgaris seedlings,” Plant Physiology and Biochemistry, vol. 168, pp. 294-304, 2021. [CrossRef]
- G. Liang, “Iron uptake, signaling, and sensing in plants,” Plant Communications, vol. 3, no. 5, 2022. [CrossRef]
- I. S. Nassarawa et al., “Zinc oxide nanoparticles and zinc sulfate alleviate boron toxicity in cotton (Gossypium hirsutum L.),” Plants, vol. 13, no. 9, p. 1184, 2024. [CrossRef]
- X. Wei et al., “GhRCD1 promotes cotton tolerance to cadmium by regulating the GhbHLH12–GhMYB44–GhHMA1 transcriptional cascade,” Plant Biotechnology Journal, vol. 22, no. 7, pp. 1777-1796, 2024. [CrossRef]
- S. Thomine and J. I. Schroeder, “Plant metal transporters with homology to proteins of the NRAMP family,” The NRAMP family. Edited by M. Cellier and P. Gros. Molecular Biology Intelligence Unit, Andes/Kluwer Series, pp. 113-121, 2004.
- R. Lei et al., “bHLH121 functions as a direct link that facilitates the activation of FIT by bHLH IVc transcription factors for maintaining Fe homeostasis in Arabidopsis,” Molecular plant, vol. 13, no. 4, pp. 634-649, 2020. [CrossRef]
- X. Chen, S. M. Smith, S. Shabala, and M. Yu, “Phytohormones in plant responses to boron deficiency and toxicity,” Journal of Experimental Botany, vol. 74, no. 3, pp. 743-754, 2023. [CrossRef]
- A. Sharma et al., “The role of salicylic acid in plants exposed to heavy metals,” Molecules, vol. 25, no. 3, p. 540, 2020. [CrossRef]
- G. J. Lei, X. F. Zhu, Z. W. Wang, F. Dong, N. Y. Dong, and S. J. Zheng, “Abscisic acid alleviates iron deficiency by promoting root iron reutilization and transport from root to shoot in A rabidopsis,” Plant, cell & environment, vol. 37, no. 4, pp. 852-863, 2014. [CrossRef]
- K. Takei et al., “AtIPT3 is a key determinant of nitrate-dependent cytokinin biosynthesis in Arabidopsis,” Plant and Cell Physiology, vol. 45, no. 8, pp. 1053-1062, 2004. [CrossRef]
- P. He et al., “Gibberellic acid promotes single-celled fiber elongation through the activation of two signaling cascades in cotton,” Developmental Cell, vol. 59, no. 6, pp. 723-739. e4, 2024. [CrossRef]
- J. Takano, K. Miwa, L. Yuan, N. von Wirén, and T. Fujiwara, “Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability,” Proceedings of the National Academy of Sciences, vol. 102, no. 34, pp. 12276-12281, 2005. [CrossRef]
- A. González-Fontes, M. Herrera-Rodríguez, E. M. Martín-Rejano, M. Navarro-Gochicoa, J. Rexach, and J. J. Camacho-Cristóbal, “Root responses to boron deficiency mediated by ethylene,” Frontiers in plant science, vol. 6, p. 1103, 2016. [CrossRef]
- J. Takano, M. Wada, U. Ludewig, G. Schaaf, N. von Wirén, and T. Fujiwara, “The Arabidopsis major intrinsic protein NIP5; 1 is essential for efficient boron uptake and plant development under boron limitation,” The Plant Cell, vol. 18, no. 6, pp. 1498-1509, 2006. [CrossRef]
- C. Lucena, F. J. Romera, M. J. García, E. Alcántara, and R. Pérez-Vicente, “Ethylene participates in the regulation of Fe deficiency responses in Strategy I plants and in rice,” Frontiers in Plant Science, vol. 6, p. 1056, 2015. [CrossRef]
- P. W. Morgan, H. E. Joham, and J. Amin, “Effect of manganese toxicity on the indoleacetic acid oxidase system of cotton,” Plant Physiology, vol. 41, no. 4, pp. 718-724, 1966. [CrossRef]
- V. Escudero et al., “Arabidopsis thaliana Zn2+-efflux ATPases HMA2 and HMA4 are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina BMM,” Journal of Experimental Botany, vol. 73, no. 1, pp. 339-350, 2022. [CrossRef]
- A. G. Assunção, I. Cakmak, S. Clemens, M. González-Guerrero, A. Nawrocki, and S. Thomine, “Micronutrient homeostasis in plants for more sustainable agriculture and healthier human nutrition,” Journal of experimental botany, vol. 73, no. 6, pp. 1789-1799, 2022. [CrossRef]
- Y. Liu et al., “Transcriptome sequencing analysis of root in soybean responding to Mn poisoning,” International Journal of Molecular Sciences, vol. 24, no. 16, p. 12727, 2023. [CrossRef]
- L. Chu, C. C. Schäfer, and M. S. Matthes, “Molecular mechanisms affected by boron deficiency in root and shoot meristems of plants,” Journal of Experimental Botany, vol. 76, no. 7, pp. 1866-1878, 2025. [CrossRef]
- J. J. Camacho-Cristóbal, E. M. Martín-Rejano, M. B. Herrera-Rodríguez, M. T. Navarro-Gochicoa, J. Rexach, and A. González-Fontes, “Boron deficiency inhibits root cell elongation via an ethylene/auxin/ROS-dependent pathway in Arabidopsis seedlings,” Journal of experimental botany, vol. 66, no. 13, pp. 3831-3840, 2015. [CrossRef]
- A. Medici et al., “AtNIGT1/HRS1 integrates nitrate and phosphate signals at the Arabidopsis root tip,” Nature communications, vol. 6, no. 1, p. 6274, 2015. [CrossRef]
- K. Murtza et al., “Effect of mepiquat chloride on phenology, yield and quality of cotton as a function of application time using different sowing techniques,” Agronomy, vol. 12, no. 5, p. 1200, 2022. [CrossRef]
- M. Hussein and N. Abou-Baker, “The contribution of nano-zinc to alleviate salinity stress on cotton plants,” Royal Society open science, vol. 5, no. 8, p. 171809, 2018. [CrossRef]
- D.-D. Li et al., “Expressions of three cotton genes encoding the PIP proteins are regulated in root development and in response to stresses,” Plant Cell Reports, vol. 28, no. 2, pp. 291-300, 2009. [CrossRef]
- I. E. Erkan and U. C. Akcay, “Overexpression of miR408 influences the cotton response to boron toxicity,” Chilean journal of agricultural research, vol. 84, no. 2, pp. 236-245, 2024. [CrossRef]
- X. Fan, X. Zhou, H. Chen, M. Tang, and X. Xie, “Cross-talks between macro-and micronutrient uptake and signaling in plants,” Frontiers in Plant Science, vol. 12, p. 663477, 2021. [CrossRef]
- M. Nezamivand-Chegini et al., “New insights into the evolution of SPX gene family from algae to legumes; a focus on soybean,” BMC genomics, vol. 22, no. 1, p. 915, 2021. [CrossRef]
- M. Pireyre and M. Burow, “Regulation of MYB and bHLH transcription factors: a glance at the protein level,” Molecular plant, vol. 8, no. 3, pp. 378-388, 2015. [CrossRef]
- L. Ren et al., “Plant availability of boron doped on iron and manganese oxides and its effect on soil acidosis,” Geoderma, vol. 151, no. 3-4, pp. 401-406, 2009. [CrossRef]
- I. L. B. Pabuayon, Y. Sun, W. Guo, and G. L. Ritchie, “High-throughput phenotyping in cotton: a review,” Journal of Cotton Research, vol. 2, no. 1, p. 18, 2019. [CrossRef]
- U. Arinaitwe, “Optimizing Corn and Cotton Performance with Adaptive Management Systems and Subsurface Drip Irrigation in the Mid-Atlantic USA,” 2025.
- J. L. Boggs, T. D. Tsegaye, T. L. Coleman, K. Reddy, and A. Fahsi, “Relationship between hyperspectral reflectance, soil nitrate-nitrogen, cotton leaf chlorophyll, and cotton yield: a step toward precision agriculture,” Journal of Sustainable Agriculture, vol. 22, no. 3, pp. 5-16, 2003. [CrossRef]
- J. A. Prananto, B. Minasny, and T. Weaver, “Rapid and cost-effective nutrient content analysis of cotton leaves using near-infrared spectroscopy (NIRS),” PeerJ, vol. 9, p. e11042, 2021. [CrossRef]
- B. H. Thurtle-Schmidt and R. M. Stroud, “Structure of Bor1 supports an elevator transport mechanism for SLC4 anion exchangers,” Proceedings of the National Academy of Sciences, vol. 113, no. 38, pp. 10542-10546, 2016. [CrossRef]
- W. Park, B. E. Scheffler, P. J. Bauer, and B. T. Campbell, “Identification of the family of aquaporin genes and their expression in upland cotton (Gossypium hirsutum L.),” BMC plant biology, vol. 10, no. 1, p. 142, 2010. [CrossRef]
- Y. Ishimaru et al., “Mutational reconstructed ferric chelate reductase confers enhanced tolerance in rice to iron deficiency in calcareous soil,” Proceedings of the National Academy of Sciences, vol. 104, no. 18, pp. 7373-7378, 2007. [CrossRef]
- L. Pan, C. Huang, R. Li, and Y. Li, “The bHLH transcription factor PhbHLH121 regulates response to iron deficiency in Petunia hybrida,” Plants, vol. 13, no. 23, p. 3429, 2024. [CrossRef]
- L. Castaings, C. Alcon, T. Kosuth, D. Correia, and C. Curie, “Manganese triggers phosphorylation-mediated endocytosis of the Arabidopsis metal transporter NRAMP1,” The Plant Journal, vol. 106, no. 5, pp. 1328-1337, 2021. [CrossRef]
- N. Grotz, T. Fox, E. Connolly, W. Park, M. L. Guerinot, and D. Eide, “Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency,” Proceedings of the National Academy of Sciences, vol. 95, no. 12, pp. 7220-7224, 1998. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).