Introduction
Vaccinating children against multiple diseases have dramatically decreased the incidence of these diseases for these children. Public health systems include database systems like Vaccine Adverse Events Reporting System (VAERS) for passive identification of unknown safety signals through retrospective studies. Three recent studies have identified safety signals for infants associated with a set of vaccines and combinations of specific vaccines. In the first study, epilepsy adverse events (AEs) were detected with higher frequencies for infants less than one year of age compared to older children for specific vaccines [
1]; statistically different frequencies were observed for the same vaccine from different manufactures were observed [
1]. In the second study, bradycardia and cardiac arrest AEs were detected with higher frequencies for infants less than one year of age compared to infants age 1 for similar set of vaccines and vaccine combinations [
2]. In the third study, similar safety signals were identified associating specific vaccines with Kawasaki disease in infants [
3]. Does this candidate safety signal pattern extend to other AEs with higher frequencies for infants less than a year old?
Note that AEs and serious adverse events (SAEs) are referred to as AEs following immunization (AEFI) [
4]. AEFIs are represented by a combination of background AEs and (when they occur) vaccine-associated AEs. For infants less than one year of age, multiple AEs occur including collapse (hypotonic-hyporesponsive episode – HHE), infantile spasms, and sudden infant death syndrome (SIDS) or sudden unexpected infant death (SUID). Observation of these AEs may represent background AEs with possible additional vaccine-associated AEs. The effect of age on the risk of collapse (hypotonic-hyporesponsive episode – HHE) has been observed for younger infants based on age at immunization for infants less than 1 year of age [
5]. In a risk-benefit comparison, the risk of pertussis for infants is high compared to risks of expected AEs and SAEs associated with immunizations. Overall, SIDS mortality is decreasing and it is inversely related to immunization coverage [
6,
7]. A possible association of SIDS with bacterial toxins has been previously proposed [
8]. This observation is supported by the observation that the incidence of SIDS decreased after the introduction of Haemophilus influenzae type b (Hib) vaccine to Hungarian infants [
9]. In the United States, a retrospective study of VAERS reported no unexpected safety concerns for Hib vaccines [
10]. Likewise, infantile spasms (onset 3 to 10 months) overlaps with timing of infant immunizations [
11]. AEs needed to be considered in the context of likely temporal proximity for some vaccine associations. Evidence of coincidental temporal proximity does not establish causation of AEs and SAEs.
Additional AEs are observed in infants post immunization. AEs reported post DTP-HB-Hib immunization included diarrhea (2.95%), vomiting (1.88%), and hypotonic hyporesponsive episode (0.36%) [
12]. A case of a 2-mo-old infant developing myocarditis and HHE after immunization with pneumococcal + Haemophilus B conjugate + polio vaccine + DTP vaccine has been reported [
13]. HHE has been reported following 13-valent pneumococcal conjugate vaccine (PCV13) [
14] and hexavalent vaccine (DTP, hepatitis B, inactivated poliovirus, & Haemophilus influenzae type-b conjugate vaccine) [
15]. An infant developed hypotonic-hyporesponsive episode AE after receipt of multiple vaccines; poliovirus, DTP, Haemophilus influenzae type b-hepatitis B virus, and pneumococcal vaccines [
16]. Multiple AEs and SAEs can occur post immunization with rare frequencies.
The risks associated with pathogen infections should be considered in the context of risks associated with vaccines in risks versus benefits for patients. Patients and parents need to be fully informed of both pathogen risks versus risks associated with each vaccine in the principal of informed consent; while compulsory vaccines can increase population coverage [
17], it is inconsistent with informed consent. In general, public health officials consider the disease risks to outweigh the risks of possible AEs and SAEs. Increasing public outreach and improving local supply chains can also improve population immunization coverage [
18]. Monitoring AEs is important for maintaining the public trust of national vaccination programs [
19]. The risks associated with different vaccines targeting the same pathogen(s) may be similar or different. Note that the background occurrences of any specific AE are related to the period of time examined with no relationship to specific vaccines; any differences in AE frequencies for two vaccines examined for the same period of time will be due to random variations and vaccine-associated AE occurrences.
Hypothesis 1
– the observed safety signal for infants less than a year of age extends to additional AEs and SAEs beyond epilepsy, bradycardia, cardiac arrest, and Kawasaki disease AEs.
Hypothesis 2
– observed statistically significant increases in normalized frequencies of AEFIs for infants less one year of age are associated with vaccine dosage, concomitant administration of specific vaccines, one or more vaccine components, excipients, and/or possibly manufacturing contaminants.
Herein, the VAERS was retrospectively examined for several AEs for elevated normalized frequencies of AEFIs for infants less than a year of age. Multiple safety signals were observed; results support multiple causative factors including, live attenuated vaccines, lack of dosage adjustment of child body weight, concomitant administration of specific vaccines, vaccine components, and possibly manufacturing contaminants. The majority of these infant AEFIs are reported within days of immunization.
Results
The VAERS database [
20] was data mined slice4.rb [
22] for multiple AEs in addition to epilepsy [
1], bradycardia [
2], and cardiac arrest [
2]. Results were summarized by either singly administered vaccines or concomitantly administered vaccine combinations for infant ages 0 and 1. These results summaries adverse event reports to the VAERS database and do not include asymptomatic vaccinees. Estimates of background AE normalized frequencies were determined for comparisons to identify candidate safety signals.
Background adverse events rate estimates
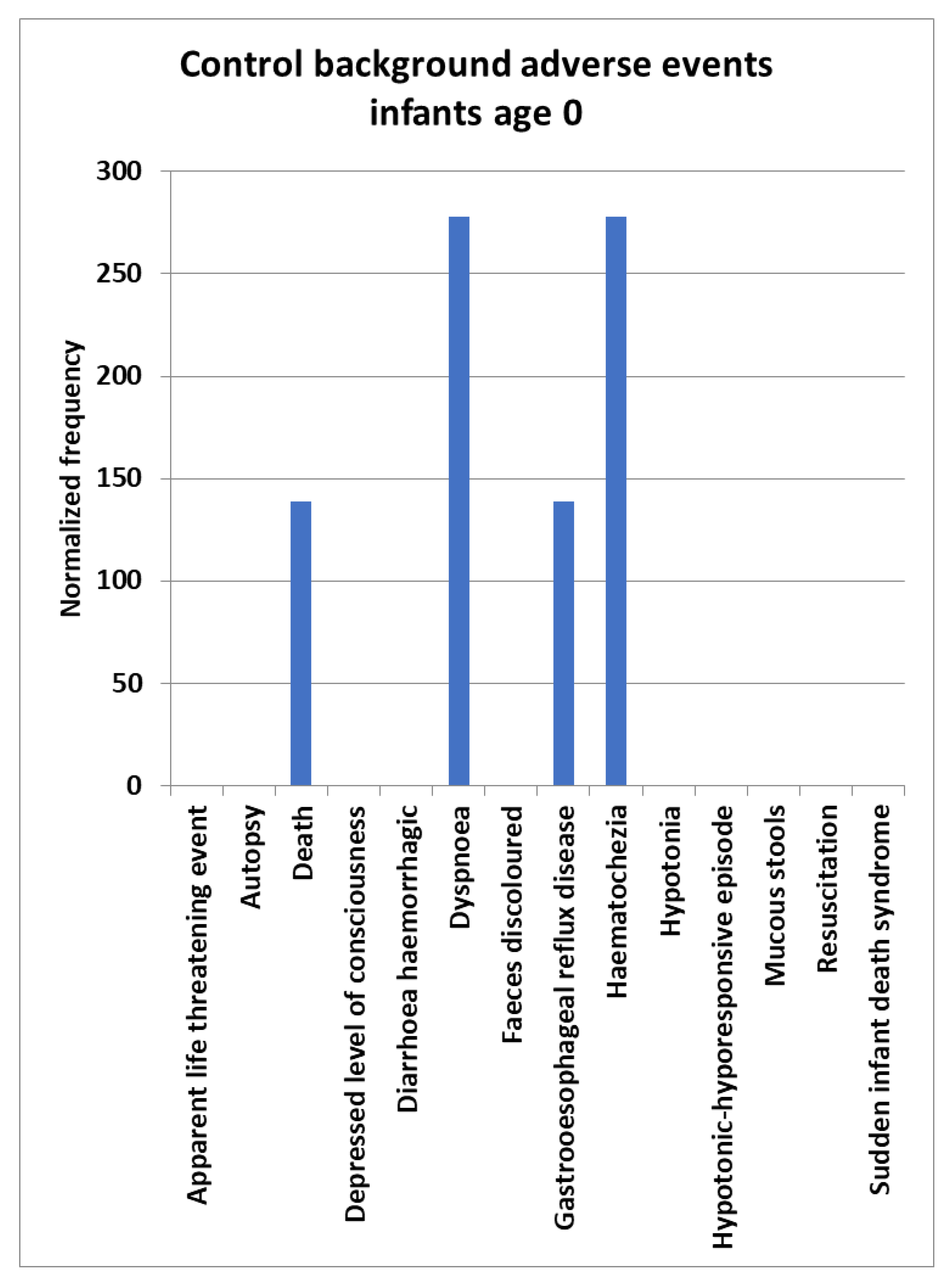
Background AEs normalized frequencies for infants age 0 were estimated by combining AEs for three vaccines Hepatitis A (Hep A: Havrix) (143 VAERS reports), Pneumo (Pneumovax) (236 VAERS reports), and Varicella (Varivax) (341 VAERS reports) vaccines (
Figure 1); assuming no vaccine associated safety signals for these three vaccines,
equation II applies for estimating the AE background rate normalized frequencies. Two AEs for Dyspnoea, Hematochezia and one AE for Death were reported to VAERS for these three vaccines for infants age 0 (
Figure 1). No AEs were reported for Apparent life-threatening event (ALTE), Autopsy, Depressed level of consciousness, Diarrhoea haemorrhagic, Faeces discoloured, Hypotonia, Hypotonic-hyporesponsive episode, Mucous stools, Resuscitation, and Sudden infant death syndrome for these three vaccines for infants age 0 (
Figure 1). Control background population rates for the AEs examined are likely in the range between 0 and 300 per 100,000 VAERS reports.
Immediate onset AEs
Background AEFIs should occur evenly over examined time periods. Both background and vaccine associated AEFIs are subject to reporting bias that increases with elapsed time. For any selected three days, the observed background AEFIs should have very low rates.
Table 1 illustrates the observed normalized frequencies for the first three days post immunization for the AEs examined. Low normalized frequencies are consistent with possibly representing background rates. Higher normalized frequencies are supportive evidence of vaccine associations. The normalized frequencies in
Table 1 represent individual day frequencies compared to the cumulative background rates reported for each vaccine (
Figure 1).
Adverse events inverse age observations
Vaccines generally are administered to children following recommended vaccine schedules (e.g., [
24]). The normalized frequencies for multiple AEs were observed to be higher for infants age 0 than infants age 1 for some vaccines and also some concomitant vaccine combinations, see
Table 2; all of the infant age 0 AEs are higher than infant age 0, 63 were at least 500 higher, 47 at least 1,000 higher, and 8 at least 5,000 higher per 100,000 VAERS reports (
Table 2).
Apparent life-threatening event (ALTE) for infants age 0
ALTE is defined as the combination of clinical presentations such as marked change in skin and muscle tone, apnea, gagging, or choking [
25]. Normalized frequencies for ALTE AEs for infants age 0 were observed for DTaP+IPV+HEPB+HIB (Infanrix hexa)+pneumococcal (Prevnar) dose 2 at 1,626 (chi-squared 2x2 p=.003962), and DTaP+IPV+HEPB+HIB (Infanrix hexa)+pneumococcal (Prevnar13)+rotavirus (Rotarix) dose 3 at 1,479 (p=.007243). Note that Infanrix hexa represents six different vaccines with including Prevnar or Prevnar13 plus Rotarix increases the total vaccines to seven and eight, respectively. Possible causative factors to consider include manufacturing contaminants (e.g., endotoxin), combined adjuvant level (e.g., aluminum), and also inclusion of live attenuated virus (rotavirus).
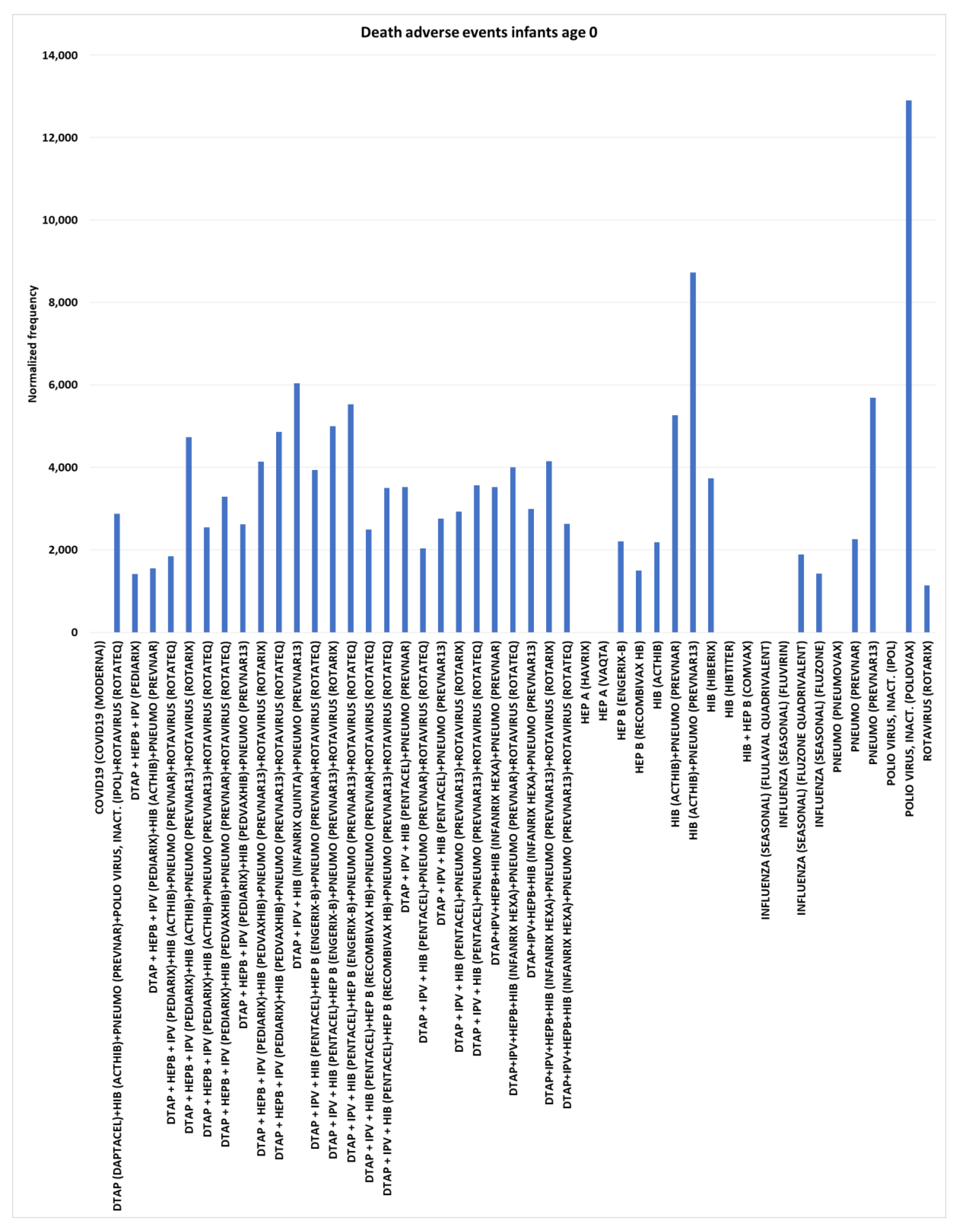
Death adverse events for infants age 0
For infants age 0, multiple vaccines were observed with death AEs normalized frequencies of zero: COVID-19 (Moderna), HEP A (Havrix), HEP A (Vaqta), HIB (Hibtiter), HIB + HEP B (Comvax), influenza (seasonal) (Flulaval quadrivalent), influenza (seasonal) (Fluvirin), pneumococcal (Pneumovax), and polio virus, inact. (IPOL) (
Figure 2). The background normalized rate is estimated to be between 0 and 150 (
< 150) (
Figure 1). In contrast to IPOL at 0 death reports for 311 total VAERS reports, polio virus, inact. (Poliovax) had 24 dose 1 AE death reports for 186 total Poliovax VAERS reports with a normalized frequency of 12,903 (chi-square 2x2 p<0.00001) (
Figure 2). Similar discrepancies were observed for HEP B (Engerix-B) dose 1 at 2,208 (p=0.000287) and HEP B (Recombivax HB) dose 1 at 1,501 (p=0.00409) compared to HIB + HEP B (Comvax) at 0 and also HIB (Acthib) at 2,180 (p=0.000356) and HIB (Hiberix) dose 1 at 3,731 (p<0.00001) versus HIB (Hibtiter) at 0 and also HIB + HEP B (Comvax) at 0 (
Figure 2).
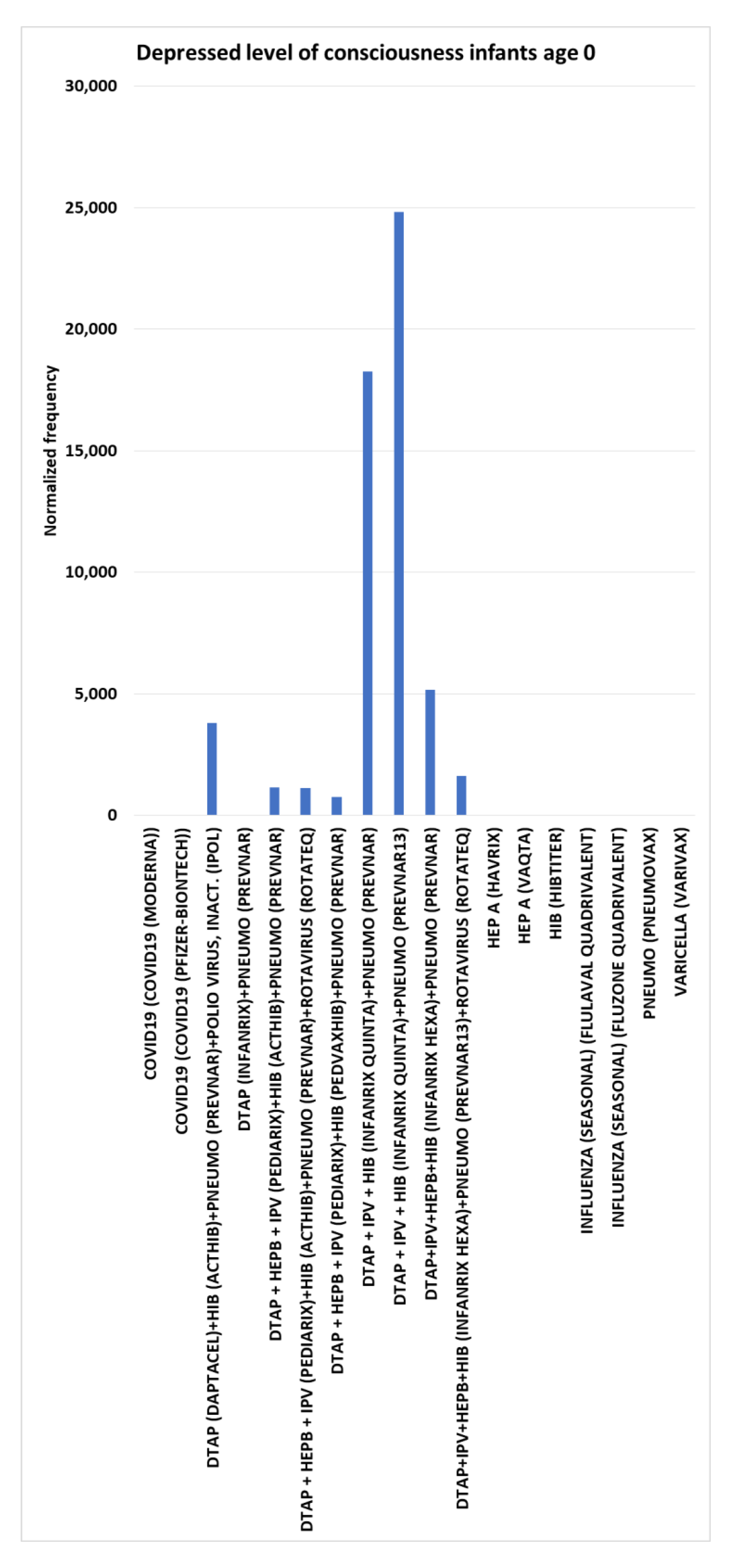
Depressed level of consciousness (DLoC),
For infants age 0, 10 vaccines or vaccine combinations were observed with no depressed level of consciousness AEs, hence (
= 0). For infants age 0, seven vaccine concomitant combinations were observed greater than 1,000 per 100,000 VAERS reports and four highest with high normalized frequencies for depressed level of consciousness: DTaP (Daptacel)+HIB (Acthib)+pneumococcal (Prevnar)+polio virus, inact. (IPOL) dose 4 at 3,803 (synergy) (chi-square 2x2 p<0.00001), DTaP + IPV + HIB (Infanrix quinta)+pneumococcal (Prevnar) dose 2 at 18,254 (high synergy) (p<0.00001), DTaP + IPV + HIB (Infanrix quinta)+pneumococcal (Prevnar13) dose 2 at 24,832 (high synergy) (p<0.00001), and DTaP+IPV+HEPB+HIB (Infanrix hexa)+pneumococcal (Prevnar) dose 2 at 5,149 (synergy) (p<0.00001) (
Figure 3).
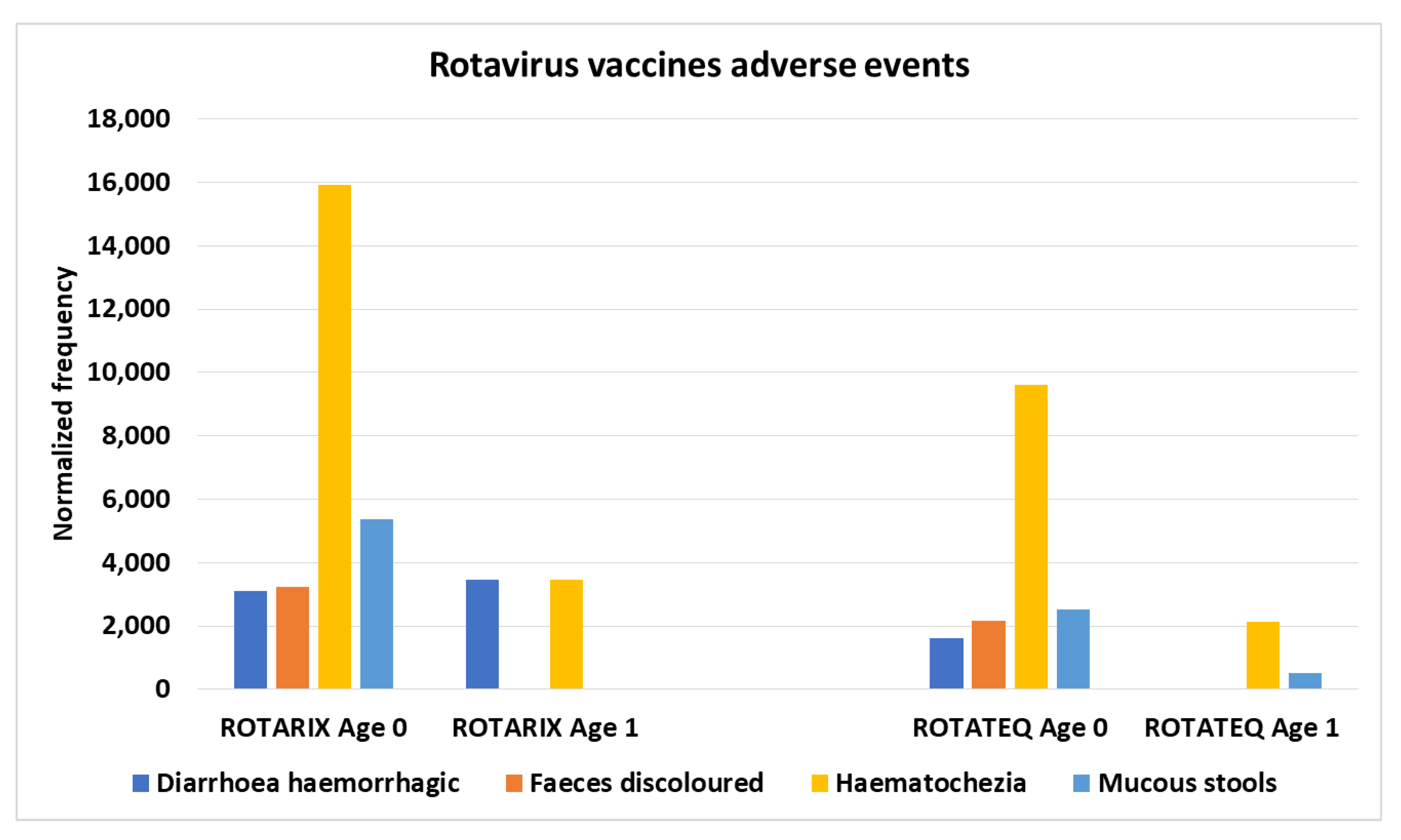
Diarrhoea haemorrhagic, Faeces discoloured, Haematochezia, and Mucous stools adverse events infants age 0 and 1
The normalized frequencies for diarrhoea haemorrhagic, faeces discoloured, haematochezia (red blood in stool), and mucous stools adverse events were dominantly associated with rotavirus vaccines (
Figure 4) and concomitant combinations including a rotavirus vaccine. The normalized frequencies were observed to be higher for infants age 0 than infants age 1 (
Figure 4) consistent with possible minimum infectious units (IU) being too high for some infants for their age (and hence body size/weight).
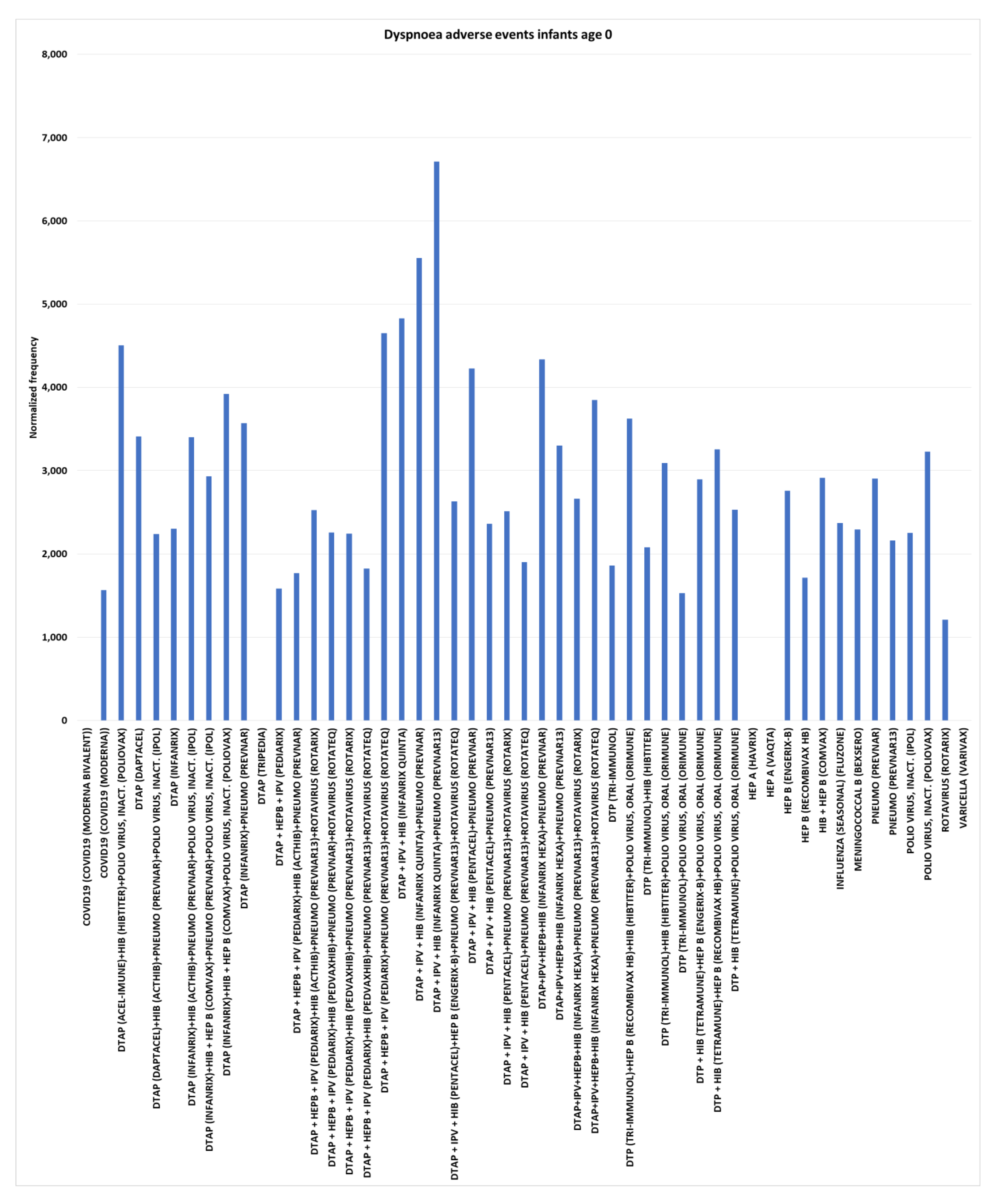
Dyspnoea adverse events for infants age 0
No dyspnoea (shortness of breath or difficulty breathing) AEs were observed for COVID-19 (Moderna bivalent), DTaP (Tripedia), HEP A (Havrix), HEP A (Vaqta), and Varicella (Varivax) for infants age 0 (
Figure 5). By
equation II, the background rate can be estimated to be less than 300 (
< 300) (
Figure 1). The three highest normalized frequencies observed were DTaP + IPV + HIB (Infanrix quinta) dose 1 at 4,831 (chi-square 2x2 p<0.00001), DTaP + IPV + HIB (Infanrix quinta)+pneumococcal (Prevnar) dose 2 at 5,556 (p<0.00001), and DTaP + IPV + HIB (Infanrix quinta)+pneumococcal (Prevnar13) dose 2 at 6,711 (p<0.00001).
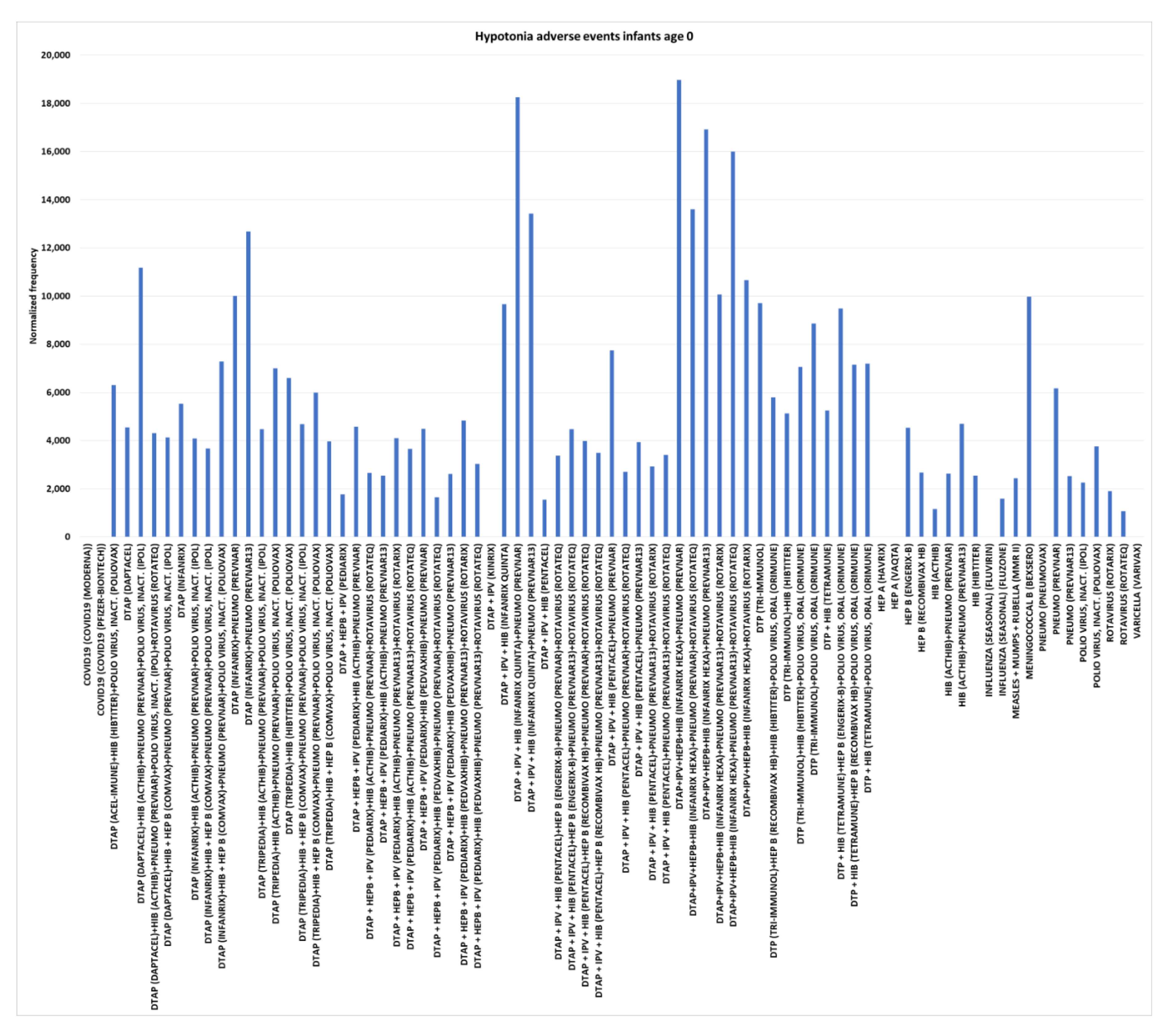
Hypotonia adverse events for infants age 0
Hypotonia is defined as defined by decreased muscle tone. Multiple vaccines had zero normalized frequencies for hypotonia (low muscle tone, resulting in weakness and floppiness) for infants age 0: COVID-19 (Moderna), COVID-19 (Pfizer-BioNTech), DTaP + IPV (Kinrix), HEP A (Havrix), HEP A (Vaqta), influenza (seasonal) (Fluvirin), pneumococcal (Pneumovax), and Varicella (Varivax) (
Figure 6); hence background (
= 0). The highest normalized frequencies were observed for combinations of DTaP+IPV+HEPB+HIB (Infanrix hexa) or DTaP + IPV + HIB (Infanrix quinta) with pneumococcal (Prevnar) or pneumococcal (Prevnar13) dose 2 ranging from 10,059 (chi-square 2x2 p<0.00001) to 18,970 (p<0.00001), DTaP (Infanrix)+pneumococcal (Prevnar13) dose 2 at 12,676 (p<0.00001), DTaP (Daptacel)+HIB (Acthib)+pneumococcal (Prevnar)+polio virus, inact. (IPOL) dose 4 at 11,186, meningococcal B (Bexsero) dose 1 at 9,968, DTP (Tri-Immunol) dose 1 at 9,711, DTaP + IPV + HIB (Infanrix quinta) dose 1 at 9,662, and DTP + HIB (Tetramune)+HEP B (Engerix-B)+polio virus, oral (Orimune) dose 3 at 9,486.
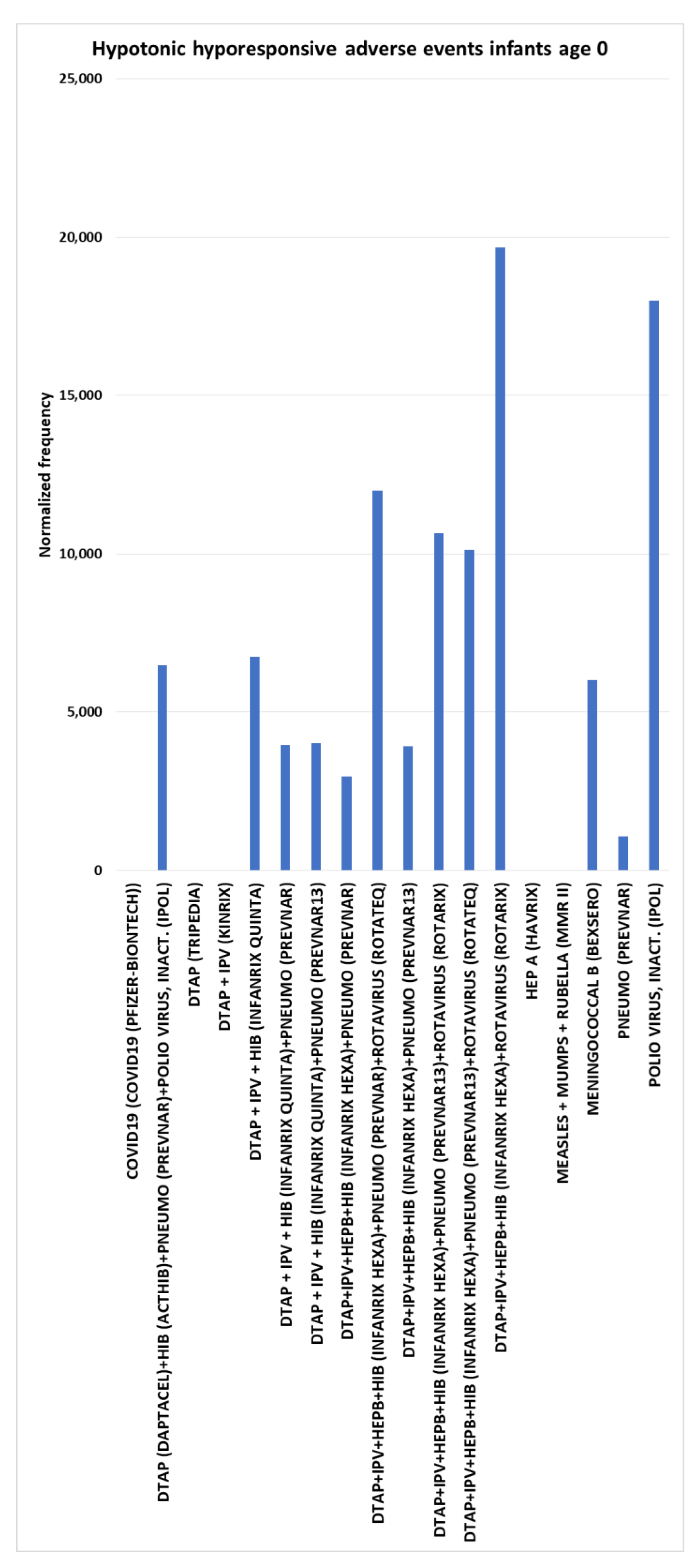
Hypotonic-hyporesponsive episode (HHE) adverse events for infants age 0
No HHE (reduced muscle tone, decreased responsiveness to stimuli, and pale or bluish skin) AEs were observed for multiple vaccines: COVID-19 (Pfizer-BioNTech), DTaP (Tripedia), DTaP + IPV (Kinrix), HEP A (Harvix), and Measles + Mumps + Rubella (MMR II); hence, background
= 0. For hypotonic-hyporesponsive episode AEs, multiple synergy concomitant combinations were observed combining DTaP+IPV+HEPB+HIB (Infanrix hexa) with one of the rotavirus vaccines (dose 2) (
Figure 7); other observations include polio virus, inact. (IPOL) dose 1 at 18,006, DTaP + IPV + HIB (Infanrix quinta) dose 1 at 6,763, meningococcal B (Bexsero) dose 1 at 6,013 plus two by two combinations of Infanrix quinta or Infanrix hexa with dose 2 Prevnar or dose 2 Prevnar13 (
Figure 7).
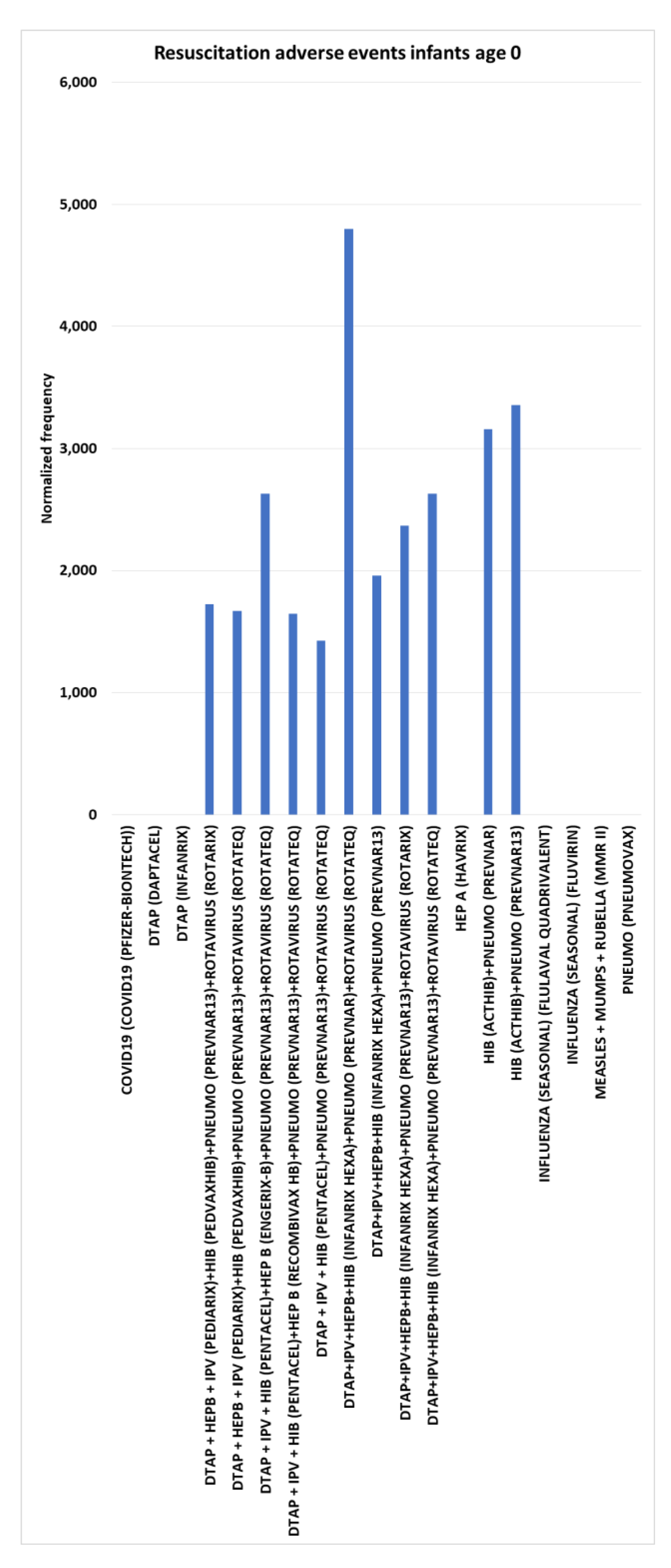
Resuscitation adverse events for infants age 0
Multiple vaccines have no resuscitation adverse events for infants age 0, including DTaP (Daptacel), DTaP (Infanrix), HEP A (Havrix), pneumococcal (Pneumovax), etc. (hence,
= 0) (
Figure 8). Normalized frequencies for resuscitation adverse events for infants age 0 include DTaP+IPV+HEPB+HIB (Infanrix hexa)+pneumococcal (Prevnar)+rotavirus (RotaTeq) at 4,800, HIB (Acthib)+pneumococcal (Prevnar13) dose 3 at 3,356, HIB (Acthib)+pneumococcal (Prevnar) dose 2 at 3,158, and concomitant combinations including Infanrix hexa or Pediarix, or Pentacel (
Figure 8).
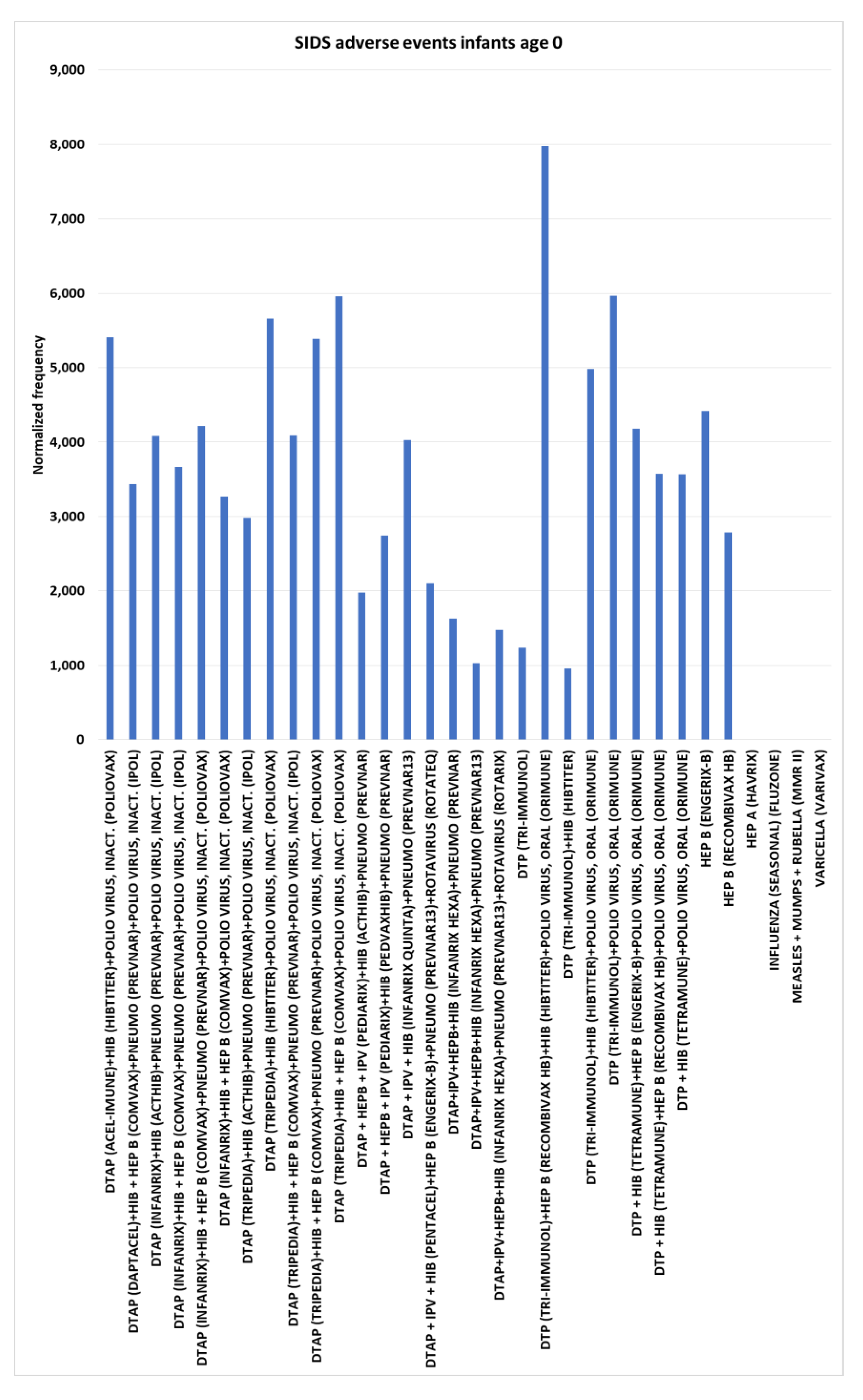
Sudden infant death syndrome (SIDS) adverse events
For SIDS normalized frequencies for infants age 0 include rotavirus (Rotarix) at 69, rotavirus (RotaTeq) at 38, and HEP A (Havrix) at 0; these low normalized frequencies negate possible temporal proximity associations for the following results. In contrast six concomitant combinations including polio virus, oral (Orimune) (dose 2 or dose 3) ranged from 3,567 (chi-square 2x2 p<0.00001) to 7,971 (synergy) (p<0.00001) (
Figure 9). An additional six concomitant combinations including polio virus, inact. (Poliovax) ranged from (dose 4) 3,268 (p=0.00003) to 5,960 (p<0.00001) and five concomitant combinations including polio virus, inact. (IPOL) ranged from (dose 4) 2,985 (p=00004) to 4,094 (p<0.00001) (
Figure 9). In contrast to these polio vaccine results, with only 1 SIDS AE for 1,033 VAERS reports, DTaP + IPV + HIB (Pentacel) has a normalized frequency of 97 and 1 SIDS AE for 567 VAERS reports for DTaP + HEPB + IPV (Pediarix) at 176. Four Infanrix hexa concomitant combinations ranged from (dose 2 or 3 by combination) 1,032 to 1,626 and DTaP + IPV + HIB (Infanrix quinta)+pneumococcal (Prevnar13) dose 2 at 4,027. Single vaccines of note include Hepatitis B vaccines HEP B (Engerix-B) dose 1 at 4,415 (p<0.00001), HEP B (Recombivax HB) dose 1 at 2,787 (p=0.00003), and DTP (Tri-Immunol) dose 1 at 1,240 (p=0.01443) (
Figure 9).
Discussion
Current vaccines are protecting children against multiple diseases with well-established efficacy. The results observed in this study identify possible patterns of safety signals with associations for specific vaccines and specific concomitantly administered vaccines. The observed safety signals may enable possible adjustments to further improve safety profiles for these vaccines while reducing AEs and SAEs in vaccinated children.
Adverse events inverse age observations
For infants age 0, the vaccine dosage levels may be too high for specific vaccines and concomitant vaccine combinations (
Table 2). Supporting Hypothesis 1 and 2, the observed results (
Table 1 and
Table 2 and
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8 and
Figure 9) are consistent with the detection of safety signals for multiple specific vaccines and concomitant vaccine combinations for infants less than 1 year of age.
The vaccinees body weight is a crucial consideration in determination of treatment dosages. AEFI evidence of infants age 0 having higher AEFI normalized frequencies than infants age 1 is suggestive that the dosage of infant vaccines likely needs to be adjusted (i.e., reduced) for smaller (younger) infants (
Table 2).
Hypothesis 3
: multiple AEs and SAEs in infants are resulting from vaccine dosage levels being too high for these infants with body weight/age being a factor.
Apparent life-threatening event (ALTE) infants age 0
The estimated control background AE normalized frequency for ALTE is close to zero for infants age 0 (
Figure 1). This AE is not list in any of the FDA package insert documents for the vaccines examined. Immediate onset of this AE is observed for the three vaccines (Infanrix quinta, Infanrix hexa, and HIB Hiberix) shown in
Table 1. Co-administered Infanrix hexa and Prevnar vaccines have a normalized frequency of 1626 per 100,000 VAERS reports for infants age 0 (p=.003962) but none for infants age 1,
Table 2). This represents a candidate infant age 0 safety signal.
Death adverse events infants age 0
The estimated control background AE normalized frequency for death AEs is approximately 150 per 100,000 VAERS reports (
Figure 1). The death AE is included in the FDA package insert documents for Fluvirin, IPOL, Pediarix, Pentacel, Prevnar, Prevnar13, Rotarix, and Rotateq; for Rotateq, the reported rate matched that for the placebo control. Death after vaccination may be by temporal association rather than by any causal relationship [
26]. Immediate onset of death AE is observed for the three vaccines (Infanrix quinta, Infanrix hexa, and HIB Hiberix) shown in
Table 1. Infants age 0 have higher normalized frequencies than infants age 0 for eight vaccines shown in
Table 2. In
Figure 2, 38 vaccines have normalized frequencies for death AEs of 1142 (chi-square 2x2 p=0.0132) to 12,903 (p<0.00001) per 100,000 VAERS reports and also nine vaccines with no reported death AEs. These represent infant age 0 candidate safety signals. A previous VAERS retrospective study also identified an infant age and mortality linear relationship [
27].
Depressed level of consciousness,
The estimated control background AE normalized frequency for Depressed level of consciousness is close to zero for infants age 0 (
Figure 1). This AE is listed in the Post-marketing adverse events for vaccines Pediarix and Pentacel. Immediate onset for day 0 and day 1 are observed for three vaccines (
Table 1). Higher normalized frequencies were observed for Recombivax HB and Acthib for infants age 0 compared to age 1 (
Table 2). Seven vaccine combinations have normalized frequencies greater than 1,000 per 100,000 VAERS reports with Infanrix quinta & Prevnar (18,254) (chi-square 2x2 p<0.00001) and Infanrix quinta & Prevnar13 (24,832) (p<0.00001) (
Figure 3). These higher AE rates could be reduced by not co-administering these specific vaccine combinations with higher normalized frequencies. Alternatively, adjusting the schedule for some of these vaccines to age 1 would also likely reduce these observed normalized frequencies.
Diarrhoea haemorrhagic, Faeces discoloured, Haematochezia, and Mucous stools adverse events infants age 0 and 1
The estimated control background AE normalized frequencies for Diarrhoea haemorrhagic, Faeces discoloured, Haematochezia, and Mucous stools are close to 0, 0, 300, and 0, respectively (
Figure 1). Immediate onset was observed for Haematochezia (
Table 1). Twelve vaccines or vaccine combinations were observed with higher normalized frequencies for infants age 0 compared to age 1 for Diarrhoea haemorrhagic (
Table 2). For Haematochezia and Mucous stools AEs, infants age 0 have higher normalized frequencies than infants age 1 for the rotavirus Rotateq vaccine (
Table 2). While 19 FDA package inserts list diarrhea AE, none of the vaccines examined list Diarrhoea haemorrhagic, Faeces discoloured, or mucous stools AE; rotavirus vaccines RotaTeq and Rotarix both list Haematochezia AE in post-marketing AEs.
Observed gastrointestinal AEFIs may be associated with immunizations of live attenuated rotavirus vaccines. Rotavirus infections can cause gastroenteritis, characterized by severe diarrhea and vomiting. An estimated 2.7 million rotavirus infectious were estimated to occur annually in the United States prior to rotavirus vaccinations [
28]. The top AEs following human rotavirus vaccine (HRV) immunization include: diarrhea, vomiting, hematochezia, abdominal pain, intussusception, decreased appetite, pallor, discomfort, gastroenteritis, dehydration, hypotonia, mucous stools, flatulence, feces discolored, seizure, abnormal feces, abdominal pain upper, general physical health deterioration, cyanosis, diarrhea haemorrhagic, and hypotonic-hyporesponsive episode [
29]. Increased risk of intussusception (IS) following HRV immunization has been reported [
30] but not in clinical trial data [
31].
Hypothesis 4
: live attenuated rotavirus vaccines (Vrotavirus) are contributing to multiple AEFIs in some infants.
The AEs associated with live attenuated rotavirus vaccines is supportive that either the live attenuated vaccine itself may be contributing to the AEs (
Figure 4) or both rotavirus vaccines contain unknown manufacturing contaminants that are causative. By Occam’s razor reasoning, the live attenuated rotavirus is the obvious candidate. The minimum infectious units may need to be adjusted to infant body size/weight for infants age 0. The goal is to reduce unnecessary associated safety signals while also protecting infants from rotavirus infections.
Dyspnoea adverse events infants age 0
The estimated control background AE normalized frequency for Dyspnoea is close to 300 per 100,000 VAERS reports for infants age 0 (
Figure 1). Seven FDA package inserts for vaccines examined include the Dyspnoea AE (4 in post-marketing AEs). Immediate onset for Dyspnoea AE is shown in
Table 1 for three vaccines. Higher normalized frequencies for infants age 0 compared to age 1 are illustrated in Table2.
Figure 5 illustrates five vaccines with no reported Dyspnoea AEs for infants age 0 and 45 vaccines and vaccine combinations with normalized frequencies above 12000. These represent infant age 0 candidate safety signals.
Hypotonia adverse events infants age 0
The estimated control background AE normalized frequency for Hypotonia AE is close to zero for infants age 0 (
Figure 1).
Table 1 illustrates immediate onset Hypotonia AEs for three vaccines. Higher normalized frequencies were observed for 24 vaccines and vaccine combinations for infants age 0 compared to age 1 (
Table 2). Five FDA package inserts include the Hypotonia AE (4 post-marketing and 1 vaccine combination). Infant age 0 safety signals are illustrated in
Figure 6 with 8 vaccines with no reported Hypotonia AEs, 71 vaccines and vaccine combinations with normalized frequencies greater than 1,000, and 11 with normalized frequencies greater than 10,000 per 100,000 VAERS reports. These represent infant age 0 candidate safety signals.
Hypotonic-hyporesponsive episode (HHE) adverse events infants age 0
The estimated control background AE normalized frequency for HHE AE is close to zero for infants age 0 (
Figure 1). Immediate onset HHE AEs are illustrated for three vaccines in
Table 1. Normalized frequencies are higher for five vaccines for infants age 0 compare to age 1 (
Table 2). Seven (3 post-marketing AE) FDA package inserts include the HHE AE. For DTaP, the rate for HHE has been reported at 22.8 per 100,000 doses and 60.0 per 100,000 doses for DTaP-Hep B-IPV [
32]. Infant age 0 safety signals are illustrated in
Figure 7 with five vaccines with no reported HHE AEs, 13 vaccines and vaccine combinations with normalized frequencies above 10000 (11 vaccines with chi-square 2x2 p<0.00001), and 5 above 10,000 (all p<0.00001) per 100,000 VAERS reports. These represent infant age 0 candidate safety signals.
Resuscitation adverse events infants age 0
The estimated control background AE normalized frequency for Resuscitation AE is close to zero for infants age 0 (
Figure 1). Immediate onset for Resuscitation AE is shown for three vaccines (
Table 1). The normalized frequency for infants age 0 compared to age 1 was higher for four vaccines and combinations for Resuscitation AE (
Table 2). Resuscitation AE was not observed to be included in FDA package insert AEs reported. Eight vaccines for infants age 0 have no Resuscitation AEs reported to VAERS and 11 vaccines and combinations have normalized frequencies above 1950 per 100,000 VAERS reports (
Figure 8).
Sudden infant death syndrome (SIDS) adverse events
The risk for SIDS or sudden unexpected infant death (SUID) is highest for infants less than 1 year of age with peak risk window overlapping the recommended timing of Diphtheria-tetanus toxoids-pertussis (DTP) immunization. While, DTP vaccination association with SIDS was observed [
33], this observation is not supported by longitudinal evidence [
34] and a case-control study [
35] does not support an association between SIDS and vaccination. In contrast, a study of 70 SIDS cases found 2/3 had been immunized with DTP prior to death [
36]. Association with diphtheria, tetanus, and whole-cell pertussis (DTwP) but not with acellular pertussis (DTaP) with SIDS was also reported [
37]. It is suggested that DTP associations with SIDS are expected due to temporal proximity to receipt of DTP by simple chance [
38]. Association of vaccine hexavalent vaccine and Infanrix hexa with SIDS have also been reported [
39,
40,
41]. While discordant with their conclusions, increased numbers of SIDS and SUD cases were observed in the first five days following immunization of Infanrix hexa vaccine for infants less than 1 year of age [
42]. The candidate association signal detected in Germany [
41] was not reproduced in an Italian case series for hexavalent vaccines [
43]. The estimated control background AE normalized frequency for SIDS AE is close to zero for infants age 0 per 100,000 VAERS reports (
Figure 1). Immediate onset SIDS AEs are illustrated for three vaccines (
Table 1). Seven (1 post-marketing) FDA package insert documents include the SIDS AE with two being single SIDS reports not thought to be associated with the vaccine. Published studies report lower incidence of SIDS for vaccinated infants compared to unvaccinated infants [
7,
44]. In a study of 50 SIDS cases, seven (21.9%) had received immunization within 7 days of death [
45]. For infants age 0, four vaccines have no VAERS reports for SIDS AEs and 28 have normalized frequencies above 1000 per 100,000 VAERS reports, with chi-square 2x2 p<0.00001 for 18 of these vaccines. Of these 28 vaccines, 26 (93%) include DTP or DTaP vaccines, 24 (86%) include Polio vaccines, 24 (86%) include Haemophilus influenza type b vaccines, 19 (68%) include Hepatitis B vaccines, and 14 (50%) include Pnemococcal vaccines (
Figure 9). These combinations of vaccines may be exceeding a tolerance threshold for some infants with six of these combinations representing more than 1 in 20 (> 5%) (all p<0.00001) of the VAERS reports for the co-administered vaccines. A previous VAERS retrospective study of SIDS also detected safety signals (p<0.00001) [
46].
Possible causative components
Hypothesis 5
: some vaccines may be contaminated with manufacturing contaminants (e.g., endotoxins) that may be contributing to observed AEFIs.
The vaccines Infanrix quinta and Infanrix hexa appeared in combination with pneumococcal (Prevnar) and pneumococcal (Prevnar13) across multiple AEs examined for infants age 0. Also of note, HIB (Acthib), HEP B (Engerix-B), meningococcal B (Bexsero), etc. appeared for multiple AEs. Likely, some unknown component(s), concentration of excipient (e.g., aluminum), and/or possible manufacturing contaminant(s) (e.g., endotoxins) are worthy of future characterization and quantification for these vaccines. Characterization of vaccine lots to identify or exclude potential manufacturing contaminations with techniques like mass spectrometry is recommended.
Figure 1.
Control background normalized frequencies for infants age 0. Control background estimated from combination of Heptatis A (Hep A Havrix) (143 VAERS reports), Pneumococcal (Pneumo Pneumovax) (236 VAERS reports), and Varicella (Varivax) (341 VAERS reports) vaccines for infants aged 0.
Figure 1.
Control background normalized frequencies for infants age 0. Control background estimated from combination of Heptatis A (Hep A Havrix) (143 VAERS reports), Pneumococcal (Pneumo Pneumovax) (236 VAERS reports), and Varicella (Varivax) (341 VAERS reports) vaccines for infants aged 0.
Figure 2.
Death adverse events normalized frequency per 100K VAERS reports for infants age 0 (minimum of 100 VAERS reports per vaccine or combination and minimum of 5 death reports; equation III). ). Chi-square p-values (left to right for non-zero columns): 0.00008, 0.00598, 0.00324, 0.00111, < 0.00001, 0.00010, < 0.00001, 0.00020, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, 0.00030, < 0.00001, 0.00001, 0.00078, 0.00009, 0.00005, < 0.00001, < 0.00001, < 0.00001, 0.00002, < 0.00001, 0.00008, 0.00029, 0.00409, 0.00036, < 0.00001, < 0.00001, < 0.00001, 0.00172, 0.00638, < 0.00001, < 0.00001, and 0.01321.
Figure 2.
Death adverse events normalized frequency per 100K VAERS reports for infants age 0 (minimum of 100 VAERS reports per vaccine or combination and minimum of 5 death reports; equation III). ). Chi-square p-values (left to right for non-zero columns): 0.00008, 0.00598, 0.00324, 0.00111, < 0.00001, 0.00010, < 0.00001, 0.00020, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, 0.00030, < 0.00001, 0.00001, 0.00078, 0.00009, 0.00005, < 0.00001, < 0.00001, < 0.00001, 0.00002, < 0.00001, 0.00008, 0.00029, 0.00409, 0.00036, < 0.00001, < 0.00001, < 0.00001, 0.00172, 0.00638, < 0.00001, < 0.00001, and 0.01321.
Figure 3.
Depressed level of consciousness adverse events normalized frequency per 100K VAERS reports for infants age 0 (minimum of 100 VAERS reports per vaccine or combination and minimum of 5 AE reports; equation III). Chi-square p-values (left to right for non-zero columns): < 0.00001, 0.01610, 0.01724, < 0.00001, < 0.00001, < 0.00001, and 0.00341.
Figure 3.
Depressed level of consciousness adverse events normalized frequency per 100K VAERS reports for infants age 0 (minimum of 100 VAERS reports per vaccine or combination and minimum of 5 AE reports; equation III). Chi-square p-values (left to right for non-zero columns): < 0.00001, 0.01610, 0.01724, < 0.00001, < 0.00001, < 0.00001, and 0.00341.
Figure 4.
Rotavirus vaccines adverse events normalized frequency for infants ages 0 and 1 (equation III). Chi-squared 2x2 p-values for control backgrounds compared to Rotarix age 0: < 0.00001, < 0.00001, < 0.00001, and 0.01681 and RotaTeq age 0: 0.00205, 0.00023, < 0.00001, and 0.00006.
Figure 4.
Rotavirus vaccines adverse events normalized frequency for infants ages 0 and 1 (equation III). Chi-squared 2x2 p-values for control backgrounds compared to Rotarix age 0: < 0.00001, < 0.00001, < 0.00001, and 0.01681 and RotaTeq age 0: 0.00205, 0.00023, < 0.00001, and 0.00006.
Figure 5.
Dyspnoea adverse events normalized frequency for infants age 0 (minimum of 100 VAERS reports per vaccine or combination and minimum of 5 dyspnoea reports; equation III). Chi-square p-values (left to right for non-zero columns): 0.01387, < 0.00001, 0.00005, 0.00145, 0.00117, 0.00015, 0.00023, 0.00003, 0.00010, 0.12068, 0.00464, 0.00077, 0.00124, 0.00114, 0.00472, < 0.00001, < 0.00001, < 0.00001, < 0.00001, 0.00043, 0.00001, 0.00179, 0.00118, 0.00252, < 0.00001, 0.00002, 0.00044, < 0.00001, 0.00514, 0.00009, 0.00192, 0.00003, 0.01432, 0.00010, 0.00002, 0.00021, 0.00013, 0.00571, 0.00067, 0.00059, 0.00006, 0.00093, 0.00202, 0.00017, and 0.02715.
Figure 5.
Dyspnoea adverse events normalized frequency for infants age 0 (minimum of 100 VAERS reports per vaccine or combination and minimum of 5 dyspnoea reports; equation III). Chi-square p-values (left to right for non-zero columns): 0.01387, < 0.00001, 0.00005, 0.00145, 0.00117, 0.00015, 0.00023, 0.00003, 0.00010, 0.12068, 0.00464, 0.00077, 0.00124, 0.00114, 0.00472, < 0.00001, < 0.00001, < 0.00001, < 0.00001, 0.00043, 0.00001, 0.00179, 0.00118, 0.00252, < 0.00001, 0.00002, 0.00044, < 0.00001, 0.00514, 0.00009, 0.00192, 0.00003, 0.01432, 0.00010, 0.00002, 0.00021, 0.00013, 0.00571, 0.00067, 0.00059, 0.00006, 0.00093, 0.00202, 0.00017, and 0.02715.
Figure 6.
Hypotonia adverse events normalized frequency for infants age 0 (minimum of 100 VAERS reports per vaccine or combination and minimum of 5 hypotonia reports; equation III). The highest chi-squared 2x2 p-values are for rotavirus RotaTeq, HIB Acthib, DTAP+IPV+HIB Pentacel, and influenza (seasonal) Fluzone: 0.01675, 0.01665, 0.00329, and 0.00315; the remaining p-values decrease from 0.00185.
Figure 6.
Hypotonia adverse events normalized frequency for infants age 0 (minimum of 100 VAERS reports per vaccine or combination and minimum of 5 hypotonia reports; equation III). The highest chi-squared 2x2 p-values are for rotavirus RotaTeq, HIB Acthib, DTAP+IPV+HIB Pentacel, and influenza (seasonal) Fluzone: 0.01675, 0.01665, 0.00329, and 0.00315; the remaining p-values decrease from 0.00185.
Figure 7.
Hypotonic hyporesponsive adverse events normalized frequency for infants age 0 (minimum of 100 VAERS reports per vaccine or combination and minimum of 5 AE reports; equation III). Chi-square p-values (left to right for non-zero columns): < 0.00001, < 0.00001, < 0.00001, < 0.00001, 0.000028, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, 0.018253, < 0.00001.
Figure 7.
Hypotonic hyporesponsive adverse events normalized frequency for infants age 0 (minimum of 100 VAERS reports per vaccine or combination and minimum of 5 AE reports; equation III). Chi-square p-values (left to right for non-zero columns): < 0.00001, < 0.00001, < 0.00001, < 0.00001, 0.000028, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, 0.018253, < 0.00001.
Figure 8.
Resuscitation adverse events normalized frequency for infants age 0 (minimum of 100 VAERS reports per vaccine or combination and minimum of 5 AE reports; equation III). Chi-square p-values (left to right for non-zero columns): 0.00212, 0.00246, 0.00010, 0.00311, 0.00502, < 0.00001, 0.00071, 0.00028, 0.00008, 0.00003, and 0.00002.
Figure 8.
Resuscitation adverse events normalized frequency for infants age 0 (minimum of 100 VAERS reports per vaccine or combination and minimum of 5 AE reports; equation III). Chi-square p-values (left to right for non-zero columns): 0.00212, 0.00246, 0.00010, 0.00311, 0.00502, < 0.00001, 0.00071, 0.00028, 0.00008, 0.00003, and 0.00002.
Figure 9.
Sudden infant death syndrome adverse events normalized frequency for infants age 0 (minimum of 100 VAERS reports per vaccine or combination and minimum of 5 SIDS reports; equation III). Chi-square 2x2 p-values (left to right): < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, 0.00003, 0.00004, < 0.00001, < 0.00001, < 0.00001, < 0.00001, 0.00067, 0.00004, < 0.00001, 0.00066, 0.00396, 0.02480, 0.00720, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, and 0.00003.
Figure 9.
Sudden infant death syndrome adverse events normalized frequency for infants age 0 (minimum of 100 VAERS reports per vaccine or combination and minimum of 5 SIDS reports; equation III). Chi-square 2x2 p-values (left to right): < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, 0.00003, 0.00004, < 0.00001, < 0.00001, < 0.00001, < 0.00001, 0.00067, 0.00004, < 0.00001, 0.00066, 0.00396, 0.02480, 0.00720, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, < 0.00001, and 0.00003.
Table 1.
Day 0, 1, and 2 onset normalized frequencies (per 100K VAERS reports for each vaccine; equation III); expected background values for each day was 0 or near 0 for each adverse event.
Table 1.
Day 0, 1, and 2 onset normalized frequencies (per 100K VAERS reports for each vaccine; equation III); expected background values for each day was 0 or near 0 for each adverse event.
| Symptom |
DTaP + IPV + HIB Infanrix quinta |
DTaP+IPV+HEPB+HIB Infanrix hexa |
HIB Hiberix |
| Onset Day |
Day 0 |
Day 1 |
Day 2 |
Day 0 |
Day 1 |
Day 2 |
Day 0 |
Day 1 |
Day 2 |
| Apparent life-threatening event |
206 |
138 |
275 |
174 |
218 |
44 |
170 |
463 |
245 |
| Autopsy |
138 |
0 |
481 |
131 |
218 |
65 |
70 |
70 |
70 |
| Death |
619 |
138 |
206 |
501 |
609 |
283 |
348 |
522 |
70 |
| Depressed level of consciousness |
2,201 |
344 |
69 |
827 |
196 |
22 |
940 |
0 |
70 |
| Dyspnoea |
1,857 |
275 |
138 |
1,654 |
326 |
174 |
1,393 |
348 |
0 |
| Haematochezia |
413 |
69 |
0 |
696 |
566 |
283 |
244 |
174 |
35 |
| Hypotonia |
6,259 |
1,513 |
206 |
6,812 |
1,306 |
696 |
5,571 |
1,219 |
244 |
| Hypotonic-hyporesponsive episode |
3,989 |
1,110 |
344 |
4,331 |
609 |
261 |
3,586 |
244 |
35 |
| Resuscitation |
481 |
206 |
275 |
413 |
370 |
152 |
279 |
35 |
70 |
| Sudden infant death syndrome |
206 |
138 |
275 |
174 |
218 |
44 |
104 |
209 |
70 |
Table 2.
Infant adverse events normalized frequency (per 100K VAERS reports; equation III) comparisons for infants age 0 (minimum of 100 VAERS reports per vaccine or combination, age 0, and minimum of 5 AE reports) and infants age 1 (minimum of 100 VAERS reports age 1 with normalized frequency difference ≥ 500).
Table 2.
Infant adverse events normalized frequency (per 100K VAERS reports; equation III) comparisons for infants age 0 (minimum of 100 VAERS reports per vaccine or combination, age 0, and minimum of 5 AE reports) and infants age 1 (minimum of 100 VAERS reports age 1 with normalized frequency difference ≥ 500).
| Adverse Event |
Combination |
Age 0 |
Age 1 |
| Diarrhoea haemorrhagic |
COVID-19 (COVID-19 (Moderna)) |
1,569 |
429 |
| Diarrhoea haemorrhagic |
DTaP (Daptacel) |
3,409 |
482 |
| Hypotonia |
DTaP (Daptacel) |
4,546 |
965 |
| Diarrhoea haemorrhagic |
DTaP (Infanrix) |
2,304 |
469 |
| Hypotonia |
DTaP (Infanrix) |
5,530 |
1,218 |
| Diarrhoea haemorrhagic |
DTaP (Infanrix) & pneumococcal (Prevnar) |
3,571 |
0 |
| Hypotonia |
DTaP (Infanrix) & pneumococcal (Prevnar) |
10,000 |
714 |
| Death |
DTaP + HEPB + IPV (Pediarix) |
1,411 |
0 |
| Hypotonia |
DTaP + HEPB + IPV (Pediarix) |
1,764 |
0 |
| Resuscitation |
DTaP + HEPB + IPV (Pediarix) |
1,058 |
0 |
| Diarrhoea haemorrhagic |
DTaP + IPV + HIB (Infanrix quinta) |
4,831 |
758 |
| Hypotonia |
DTaP + IPV + HIB (Infanrix quinta) |
9,662 |
3,030 |
| Hypotonic-hyporesponsive episode |
DTaP + IPV + HIB (Infanrix quinta) |
6,763 |
4,546 |
| Diarrhoea haemorrhagic |
DTaP + IPV + HIB (Pentacel) |
968 |
402 |
| Hypotonia |
DTaP + IPV + HIB (Pentacel) |
1,549 |
1,409 |
| Death |
DTaP + IPV + HIB (Pentacel) & pneumococcal (Prevnar13) |
2,756 |
0 |
| Diarrhoea haemorrhagic |
DTaP + IPV + HIB (Pentacel) & pneumococcal (Prevnar13) |
2,362 |
709 |
| Hypotonia |
DTaP + IPV + HIB (Pentacel) & pneumococcal (Prevnar13) |
3,937 |
2,837 |
| Apparent life-threatening event |
DTaP+IPV+HEPB+HIB (Infanrix hexa) & pneumococcal (Prevnar13) |
826 |
0 |
| Diarrhoea haemorrhagic |
DTaP+IPV+HEPB+HIB (Infanrix hexa) & pneumococcal (Prevnar13) |
3,302 |
2,116 |
| Hypotonia |
DTaP+IPV+HEPB+HIB (Infanrix hexa) & pneumococcal (Prevnar13) |
16,925 |
5,820 |
| Hypotonic-hyporesponsive episode |
DTaP+IPV+HEPB+HIB (Infanrix hexa) & pneumococcal (Prevnar13) |
3,922 |
2,646 |
| Diarrhoea haemorrhagic |
DTP (Tri-Immunol) |
1,860 |
730 |
| Hypotonia |
DTP (Tri-Immunol) |
9,711 |
3,650 |
| Hypotonia |
DTP (Tri-Immunol) & HIB (Hibtiter) |
5,120 |
4,237 |
| Diarrhoea haemorrhagic |
DTP (Tri-Immunol) & HIB (Hibtiter) & polio virus, oral (Orimune) |
3,093 |
1,563 |
| Hypotonia |
DTP (Tri-Immunol) & HIB (Hibtiter) & polio virus, oral (Orimune) |
7,064 |
2,344 |
| Hypotonia |
DTP (Tri-Immunol) & polio virus, oral (Orimune) |
8,859 |
5,802 |
| Hypotonia |
DTP + HIB (Tetramune) |
5,251 |
4,167 |
| Death |
HEP B (Engerix-B) |
2,208 |
0 |
| Diarrhoea haemorrhagic |
HEP B (Engerix-B) |
2,759 |
1,695 |
| Hypotonia |
HEP B (Engerix-B) |
4,525 |
1,130 |
| Resuscitation |
HEP B (Engerix-B) |
993 |
0 |
| Death |
HEP B (Recombivax HB) |
1,501 |
0 |
| Depressed level of consciousness |
HEP B (Recombivax HB) |
1,715 |
0 |
| Depressed level of consciousness |
HIB (Acthib) |
1,163 |
470 |
| Hypotonia |
HIB (Acthib) |
1,163 |
470 |
| Resuscitation |
HIB (Acthib) |
1,017 |
0 |
| Death |
HIB (Acthib) & pneumococcal (Prevnar13) |
8,725 |
1,626 |
| Hypotonia |
HIB (Acthib) & pneumococcal (Prevnar13) |
4,698 |
1,626 |
| Resuscitation |
HIB (Acthib) & pneumococcal (Prevnar13) |
3,356 |
0 |
| Hypotonia |
HIB (Hibtiter) |
2,532 |
1,376 |
| Dyspnoea |
HIB (PEDVAXHIB) |
1,061 |
465 |
| Death |
influenza (seasonal) (FLUZONE quadrivalent) |
1,881 |
1,010 |
| Death |
influenza (seasonal) (FLUZONE) |
1,422 |
184 |
| Hypotonia |
influenza (seasonal) (FLUZONE) |
1,580 |
917 |
| Hypotonia |
Measles + Mumps + Rubella (MMR II) |
2,429 |
1,750 |
| Hypotonia |
meningococcal B (Bexsero) |
9,968 |
3,672 |
| Hypotonic-hyporesponsive episode |
meningococcal B (Bexsero) |
6,013 |
2,808 |
| Dyspnoea |
pneumococcal (Prevnar) |
2,905 |
2,026 |
| Hypotonia |
pneumococcal (Prevnar) |
6,173 |
2,345 |
| Hypotonic-hyporesponsive episode |
pneumococcal (Prevnar) |
1,089 |
107 |
| Death |
pneumococcal (Prevnar13) |
5,688 |
3,338 |
| Dyspnoea |
pneumococcal (Prevnar13) |
2,160 |
1,252 |
| Hypotonia |
pneumococcal (Prevnar13) |
2,520 |
1,669 |
| Dyspnoea |
polio virus, inact. (IPOL) |
2,251 |
0 |
| Hypotonia |
polio virus, inact. (IPOL) |
2,251 |
971 |
| Hypotonic-hyporesponsive episode |
polio virus, inact. (IPOL) |
18,006 |
971 |
| Diarrhoea haemorrhagic |
rotavirus (RotaTeq) |
1,606 |
0 |
| Dyspnoea |
rotavirus (RotaTeq) |
516 |
0 |
| Faeces discoloured |
rotavirus (RotaTeq) |
2,180 |
0 |
| Haematochezia |
rotavirus (RotaTeq) |
9,618 |
2,128 |
| Hypotonia |
rotavirus (RotaTeq) |
1,071 |
532 |
| Mucous stools |
rotavirus (RotaTeq) |
2,524 |
532 |