Submitted:
04 September 2025
Posted:
05 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Mathematical Preliminaries
- Linearity: ,
- Composition of integrals: ,
- Commutativity: .
3. Mathematical Structure of the Model
3.1. Formulation of the Anthroponotic Cutaneous Leishmaniasis (ACL) Model
3.2. Positivity of Solutions
- The factor is positive and thus does not alter the sign structure of the system.
- All right-hand sides contain non-negative inflows (, , or transfers from other non-negative compartments).
- All loss terms are proportional to the variable itself (linear decay or bilinear incidence), so they cannot produce negativity.
3.3. Boundedness of Solutions
3.4. Existence of Disease-Free Equilibrium (DFE)
3.5. Basic Reproduction Number
3.6. Existence of Endemic Equilibrium (EE)
3.7. Stability Analysis of Equilibria
3.7.1. Stability of the DFE
3.7.2. Stability of the EE
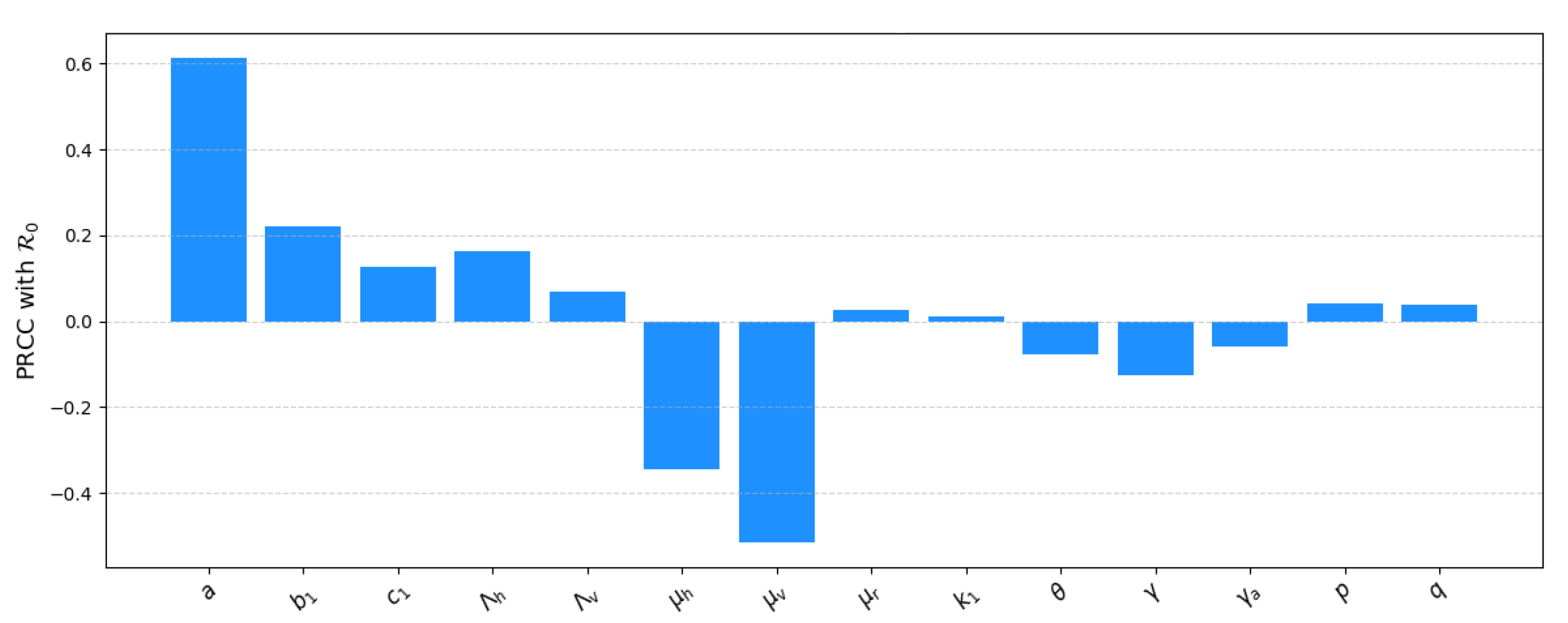
3.8. Sensitivity Analysis
4. Objective Functional and Constraints
4.1. Pontryagin’s Maximum Principle
5. Numerical Technique
| Algorithm 1 (Forward–backward sweep method for solving the fractional optimal control problem) |
|
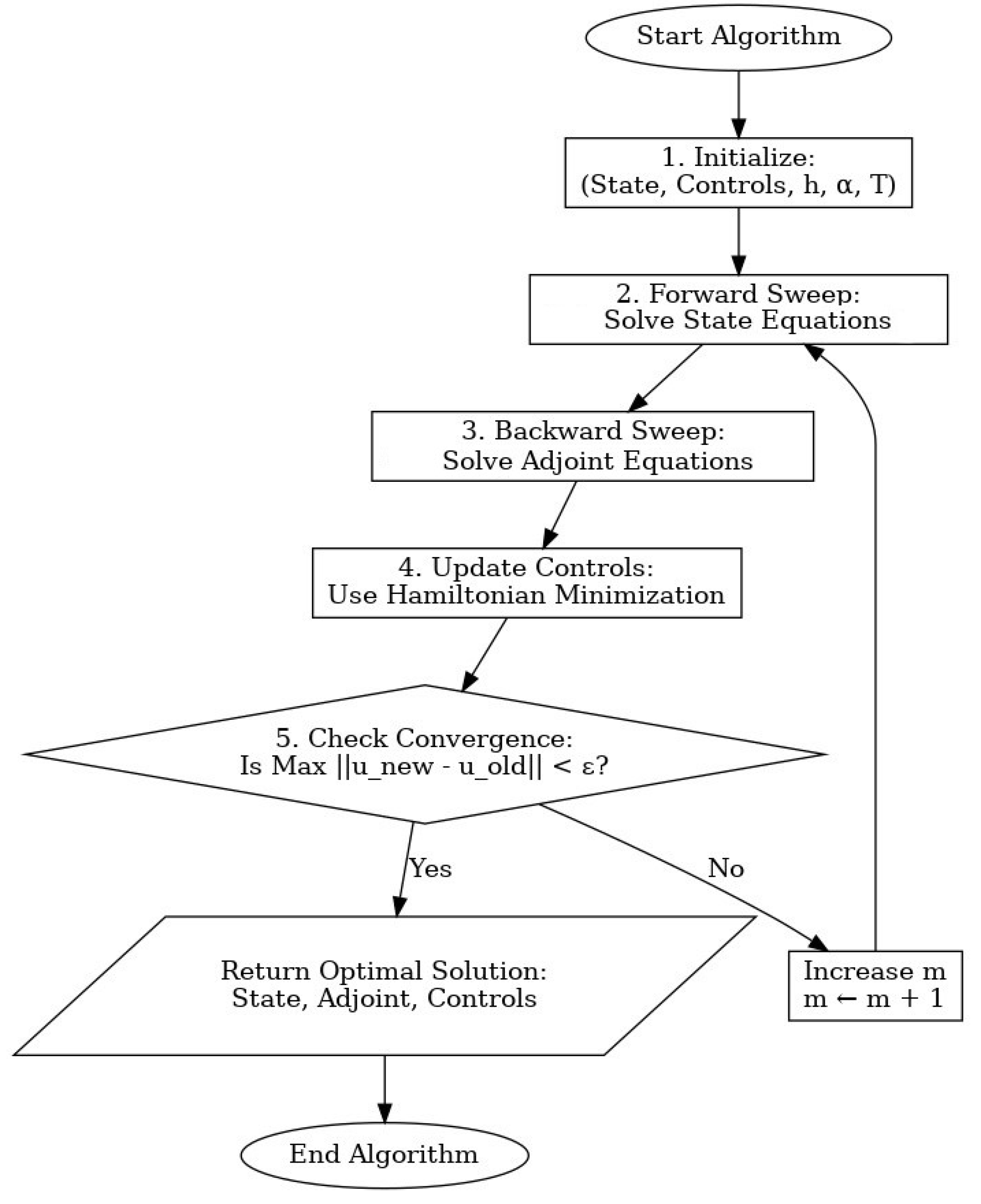
5.1. Convergence Analysis
6. Numerical Results
6.1. Simulation Scenarios
-
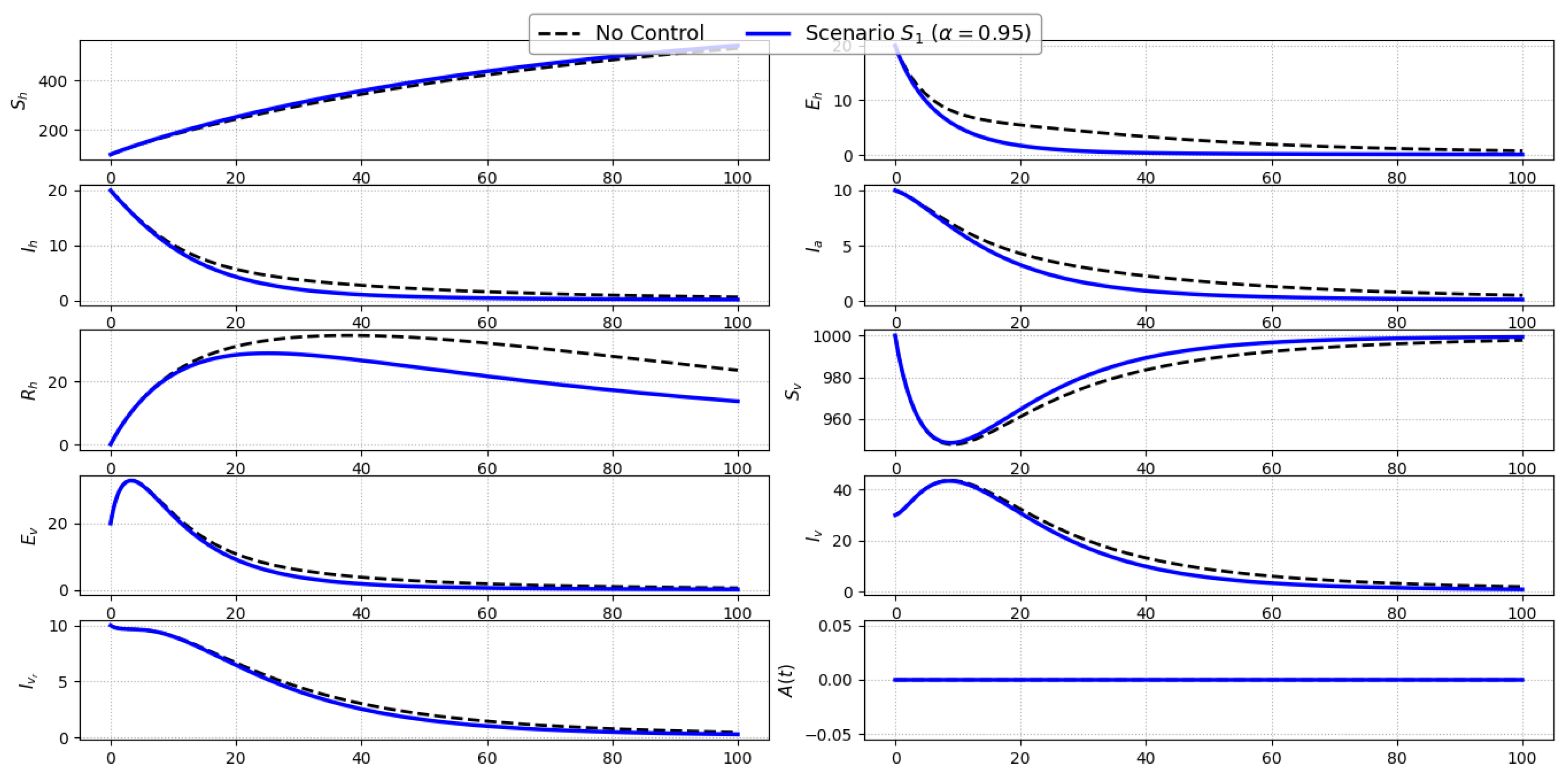
Scenario S1 (Vector Avoidance Only): Figure 3 illustrates the temporal evolution of all ten state variables under Scenario , where only the personal protection control (e.g., use of bed nets and repellents) is active, and compares it to the uncontrolled baseline. The fractional order is fixed at to account for memory effects inherent in both the biological and behavioral dynamics of the system. The implementation of significantly reduces the force of infection between humans and vectors by lowering effective contact rates. As a result, a modest yet consistent decline is observed in the number of exposed () and symptomatic infected humans () throughout the simulation period, relative to the no-control scenario. The impact on asymptomatic infections () is less pronounced, given their indirect dependence on exposure rather than contact intensity. For the vector population, a slight reduction in exposed () and infected vectors (, ) is observed due to the reduced likelihood of acquiring infection from protected human hosts. However, since vector dynamics are not directly targeted in this scenario, the changes are relatively subdued. Importantly, the awareness function shows no deviation between the two cases, as no awareness-related control is employed.Overall, Scenario demonstrates that personal protection can contribute meaningfully to reducing human infections, especially during the early and middle phases of the outbreak. However, its isolated application yields limited control over the broader transmission cycle, suggesting that more comprehensive strategies are required to achieve substantial disease suppression.
-
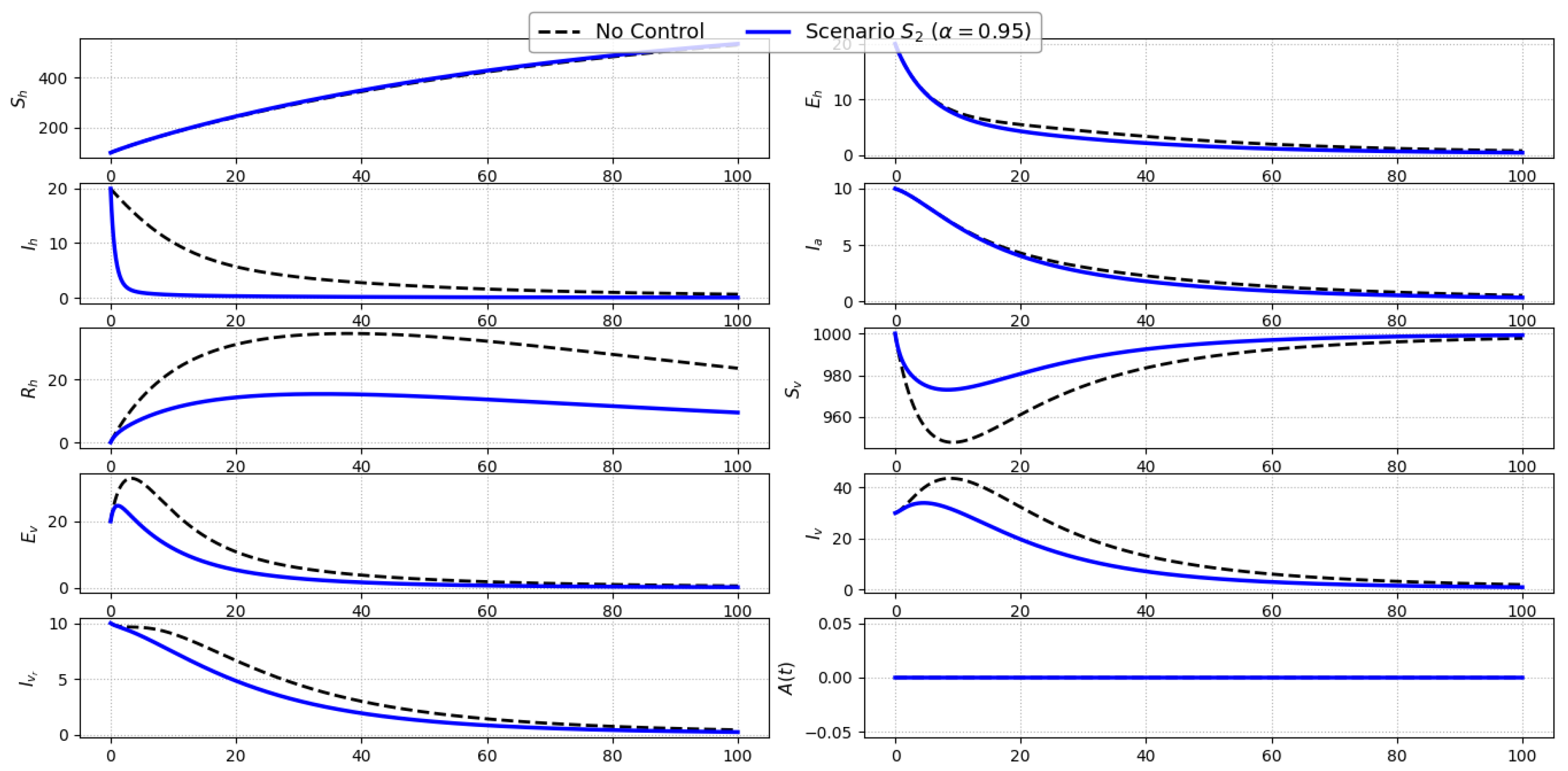
Scenario S2 (Treatment Only): Figure 4 presents the model dynamics under Scenario , where treatment of symptomatic individuals via control is the sole intervention. Compared to the baseline, a marked reduction in the symptomatic infected population () is achieved, particularly evident during the early and mid stages of the epidemic. This decline results from a shortened infectious period, which also indirectly reduces secondary infections.Consequently, a delayed but observable decline in the number of exposed humans () emerges due to the weakened forward transmission. The asymptomatic class (), unaffected directly by treatment, exhibits minor differences from the baseline. For the vector populations, a mild reduction in exposed and infected compartments (, , ) occurs, driven by the lowered human-to-vector transmission intensity. No change is observed in the awareness level (), as no awareness-related intervention is employed. Overall, Scenario proves effective in rapidly reducing symptomatic infections and partially suppressing the transmission cycle, but it does not fully disrupt vector-driven dynamics without complementary controls
-
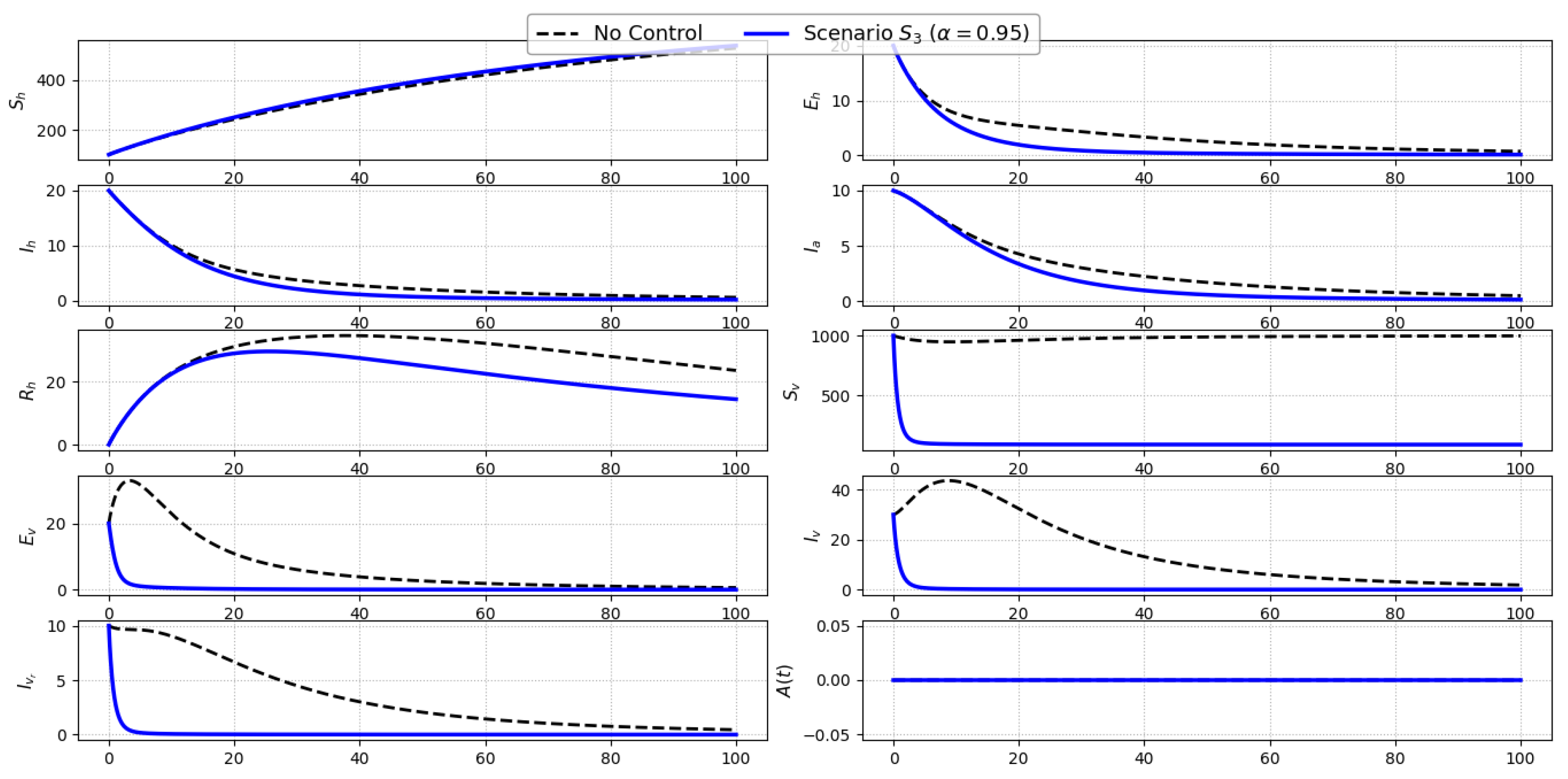
Scenario S3 (Vector Spraying Only): Figure 5 depicts the evolution of state variables under Scenario , where insecticide spraying () is the only active control. The most pronounced impact is observed in the vector-related compartments, with substantial reductions in susceptible (), exposed (), and both infected classes (, ), indicating the direct mortality effect of the intervention on the vector population. This reduction in vector density effectively lowers the force of infection toward humans, leading to a delayed but meaningful decline in exposed () and symptomatic infected humans (). The asymptomatic class (), being indirectly affected, also exhibits a slight downward shift compared to the no-control case.Since treatment and awareness controls are inactive, the recovery and awareness levels (, ) follow trajectories similar to the baseline. Overall, vector spraying in isolation demonstrates notable efficiency in disrupting vector dynamics and indirectly suppressing human transmission, though it falls short of completely halting disease propagation in the absence of host-directed interventions.
-
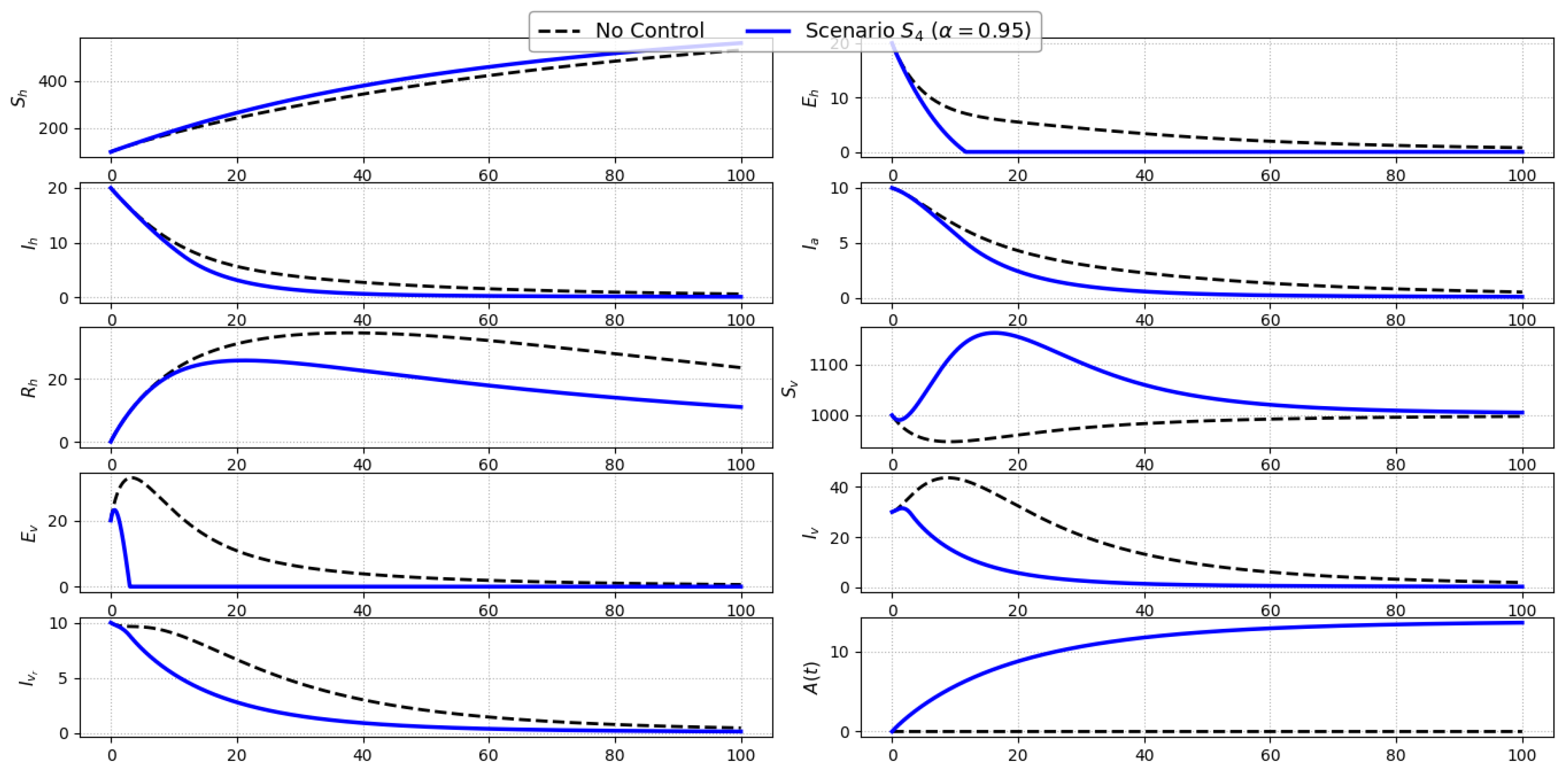
Scenario S4 (Awareness Campaign Only): Figure 6 shows the system trajectories under Scenario , where only public awareness efforts () are implemented. As expected, the awareness level exhibits a sustained and significant increase over time, reflecting the direct effect of the control. This rise in awareness reduces the effective contact rate between humans and vectors, which in turn mildly suppresses transmission. The impact on epidemiological compartments is modest but consistent: small reductions are observed in the exposed (), symptomatic (), and asymptomatic () human classes, along with slight decreases in infected vectors (, ). However, due to the indirect nature of this intervention and the absence of direct treatment or vector-killing measures, the declines are not pronounced.Overall, Scenario highlights the role of behavioral modification as a supportive control. While awareness alone does not significantly alter the disease trajectory, it contributes to reducing transmission intensity, especially when integrated with other strategies.
-
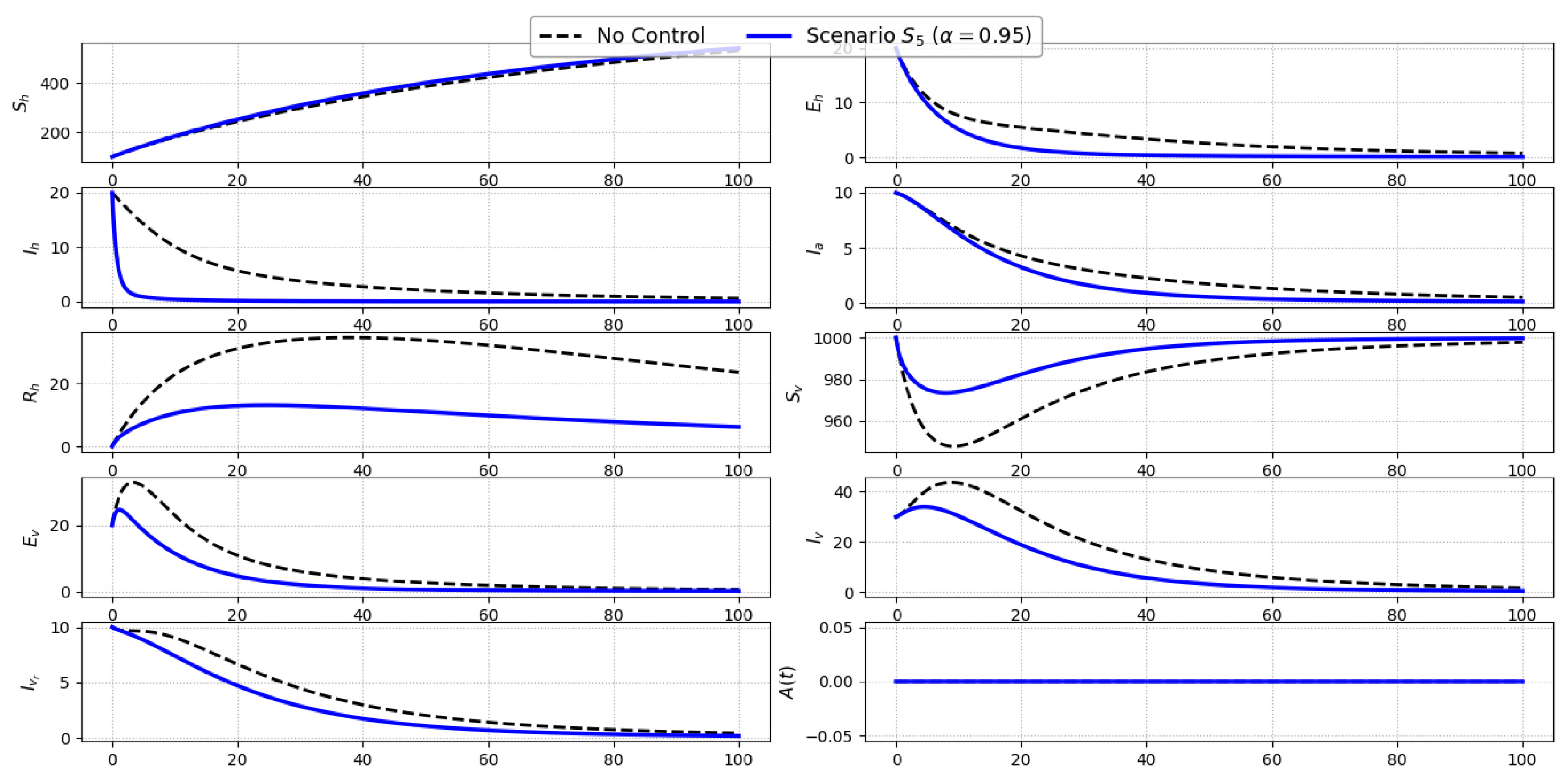
Scenario S5 (Combined Treatment and Personal Protection): Figure 7 illustrates the dynamics under Scenario , which combines personal protection () and treatment of symptomatic individuals (). This dual intervention targets both the infection source and the transmission pathway, leading to a more pronounced suppression of the epidemic compared to single-control strategies. A significant reduction in symptomatic infections () is observed, resulting from both shortened infectious periods and decreased exposure risk. The exposed class () also declines notably, benefiting from the synergistic effect of reduced contact and diminished secondary transmission. Asymptomatic infections (), while not directly treated, follow a downward trend as a result of reduced upstream exposure. In the vector population, moderate declines are observed in exposed and infected compartments due to the reduced infectivity of human hosts. Since no vector-targeted control or awareness campaign is applied, the susceptible vector pool () and awareness level () remain largely unchanged.Overall, Scenario demonstrates the enhanced effectiveness of integrated host-based strategies. The simultaneous use of repellents and treatment significantly weakens transmission feedback loops and offers a balanced approach to disease mitigation
-
Scenario S6 (Combined Repellent and Spraying): Figure 8 presents the evolution of the system under Scenario , which integrates personal protection () with insecticide spraying (). This combined strategy simultaneously reduces human-vector contact and vector abundance, resulting in a strong and rapid suppression of disease transmission. The vector-related compartments (, , , ) show substantial declines due to direct vector mortality induced by spraying. Additionally, the reduction in human exposure—achieved through repellents and bed nets—further limits the replenishment of the infected vector pool. On the human side, exposed (), symptomatic (), and asymptomatic () infections are all significantly reduced relative to the baseline. These improvements stem from the combined impact of fewer infectious bites and diminished forward transmission pressure. However, since no treatment or awareness interventions are applied, recovery dynamics and the awareness level remain nearly identical to the uncontrolled case.In summary, Scenario effectively disrupts both the environmental and behavioral transmission routes. It proves particularly valuable in settings where vector control and personal-level measures can be deployed in tandem, even in the absence of clinical or educational interventions.
-
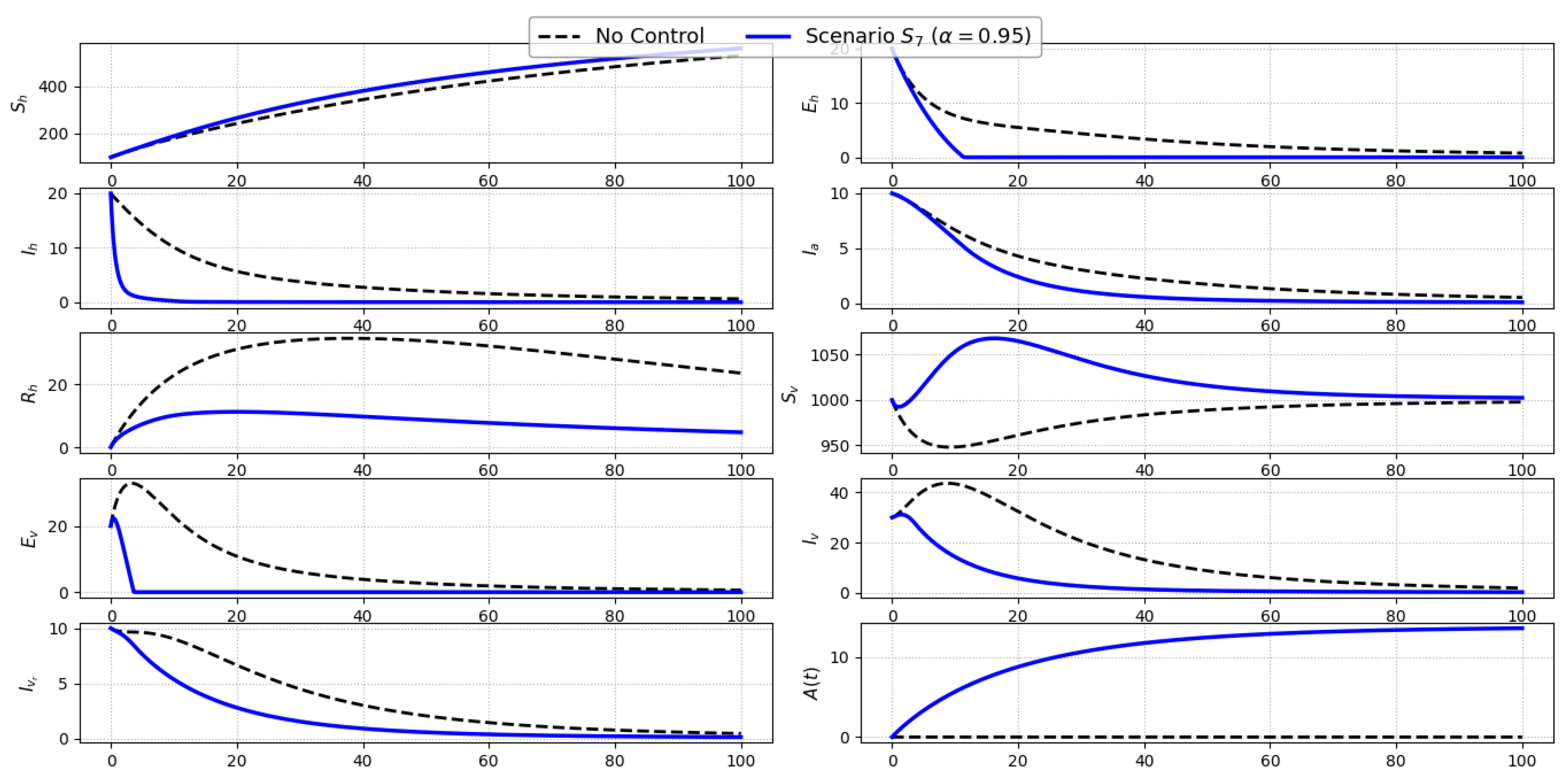
Scenario S7 (Combined Treatment and Awareness): Figure 9 depicts the model behavior under Scenario , which combines treatment of symptomatic individuals () with public awareness campaigns (). This hybrid approach merges direct clinical intervention with indirect behavioral modification, resulting in meaningful reductions across several compartments. Symptomatic infections () decline sharply due to active treatment, which shortens the infectious period and interrupts human-to-vector transmission. At the same time, the awareness function increases steadily, decreasing exposure risk by reducing contact rates. These complementary effects yield a visible drop in the number of exposed humans (), as well as a moderate decline in asymptomatic infections (), which are indirectly influenced by reduced incidence. Although the vector compartments are not targeted directly, a slight reduction in infected vectors (, ) emerges due to decreased transmission from human hosts. As expected, the susceptible vector population () remains stable in the absence of vector-specific controls.Scenario illustrates the added value of combining behavioral and therapeutic strategies. The synergy between heightened public awareness and timely clinical response provides a two-pronged mechanism for curbing transmission, especially in human-dominated settings.
-
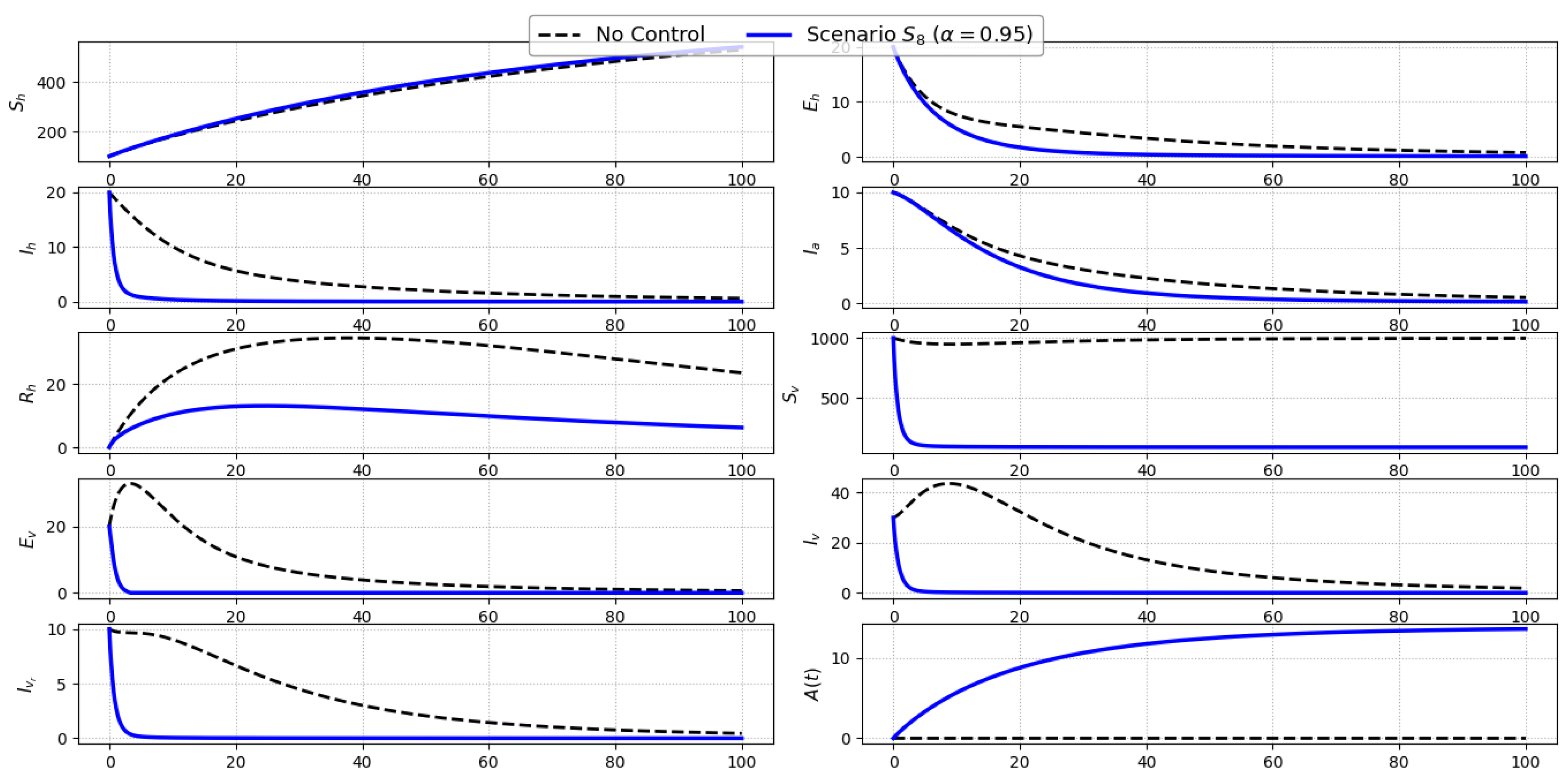
Scenario S8 (Full Control Strategy): Figure 10 shows the dynamics of the full intervention scenario , in which all four controls—personal protection (), treatment (), insecticide spraying (), and public awareness ()—are simultaneously applied. This comprehensive strategy exerts maximal pressure on the disease system by targeting both human and vector dynamics as well as behavioral responses. The result is a pronounced and rapid decline in all key epidemiological compartments. Symptomatic () and asymptomatic () infections are significantly reduced due to the combined effects of reduced exposure, enhanced recovery, and increased awareness. The exposed human class () also decreases sharply, reflecting strong suppression of transmission at early stages. Vector-related states—including , , , and —drop substantially as a consequence of direct vector mortality (via ) and indirect transmission reductions. Moreover, the awareness level rises steadily, further reinforcing the behavioral shift needed to sustain control measures.Overall, Scenario delivers the most effective outcome among all considered strategies. By synchronizing environmental, clinical, and educational interventions, it disrupts multiple feedback loops in the transmission cycle and highlights the power of integrated public health approaches in managing ACL.
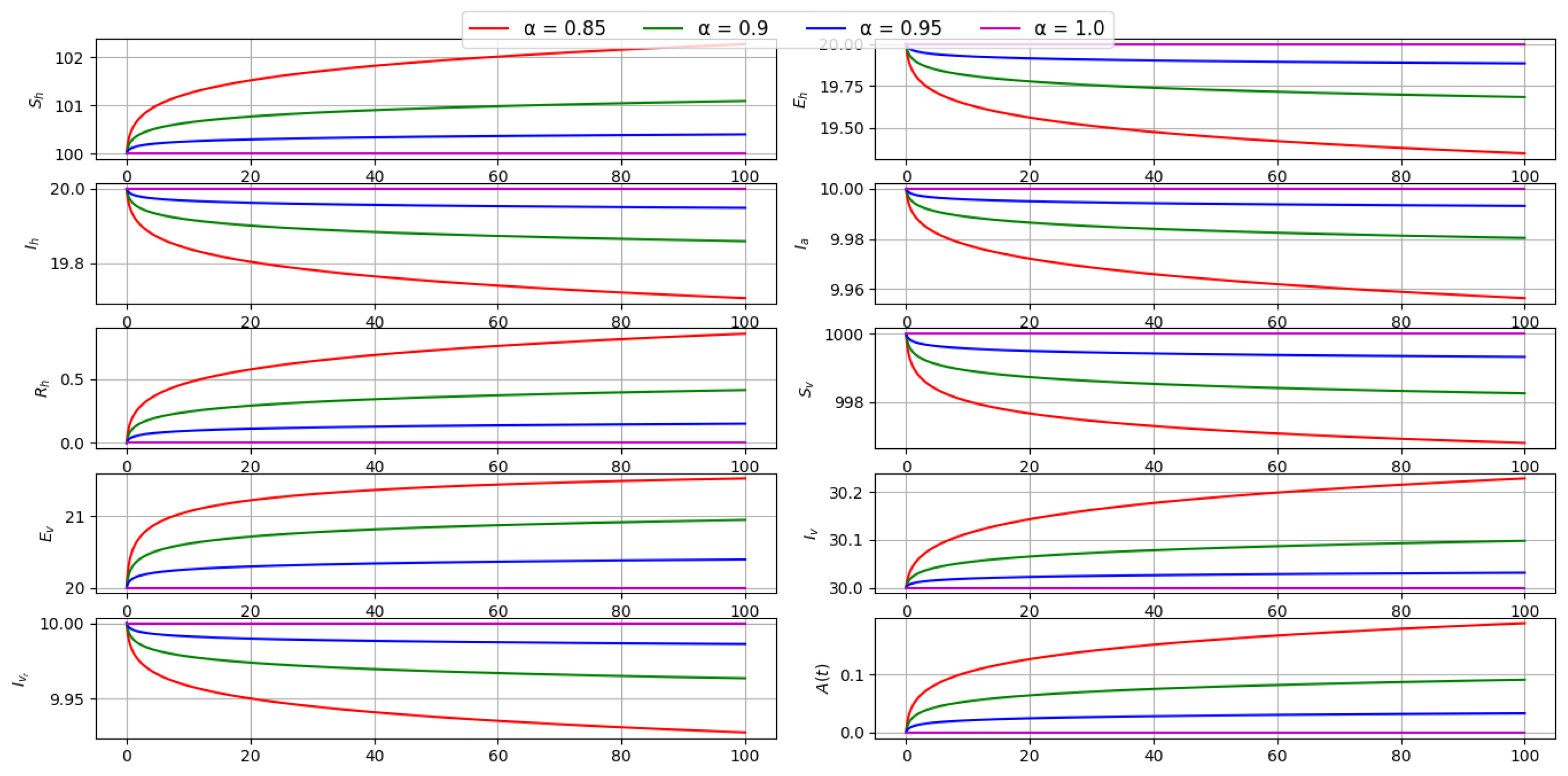
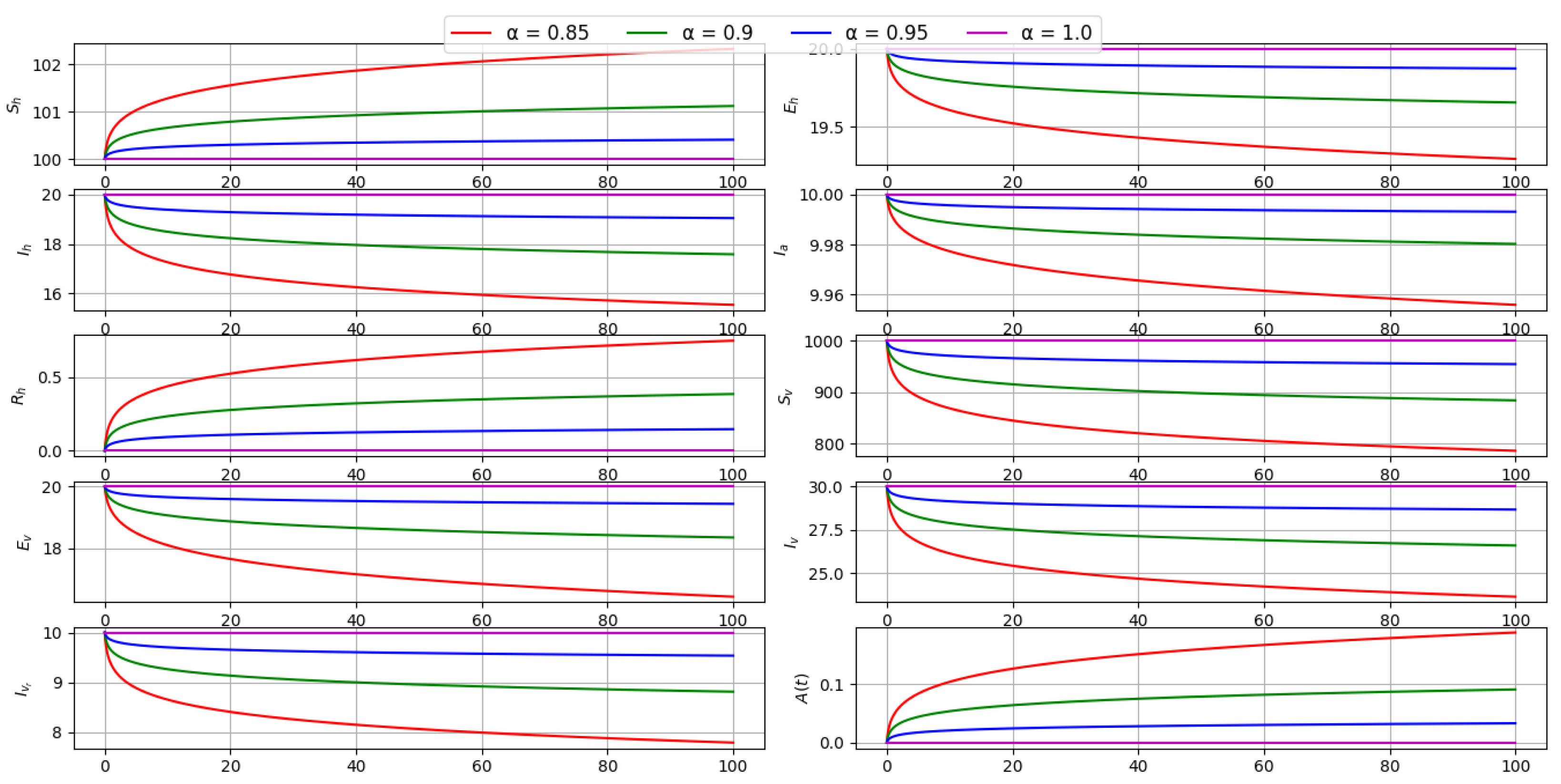
6.2. Effect of Fractional Order
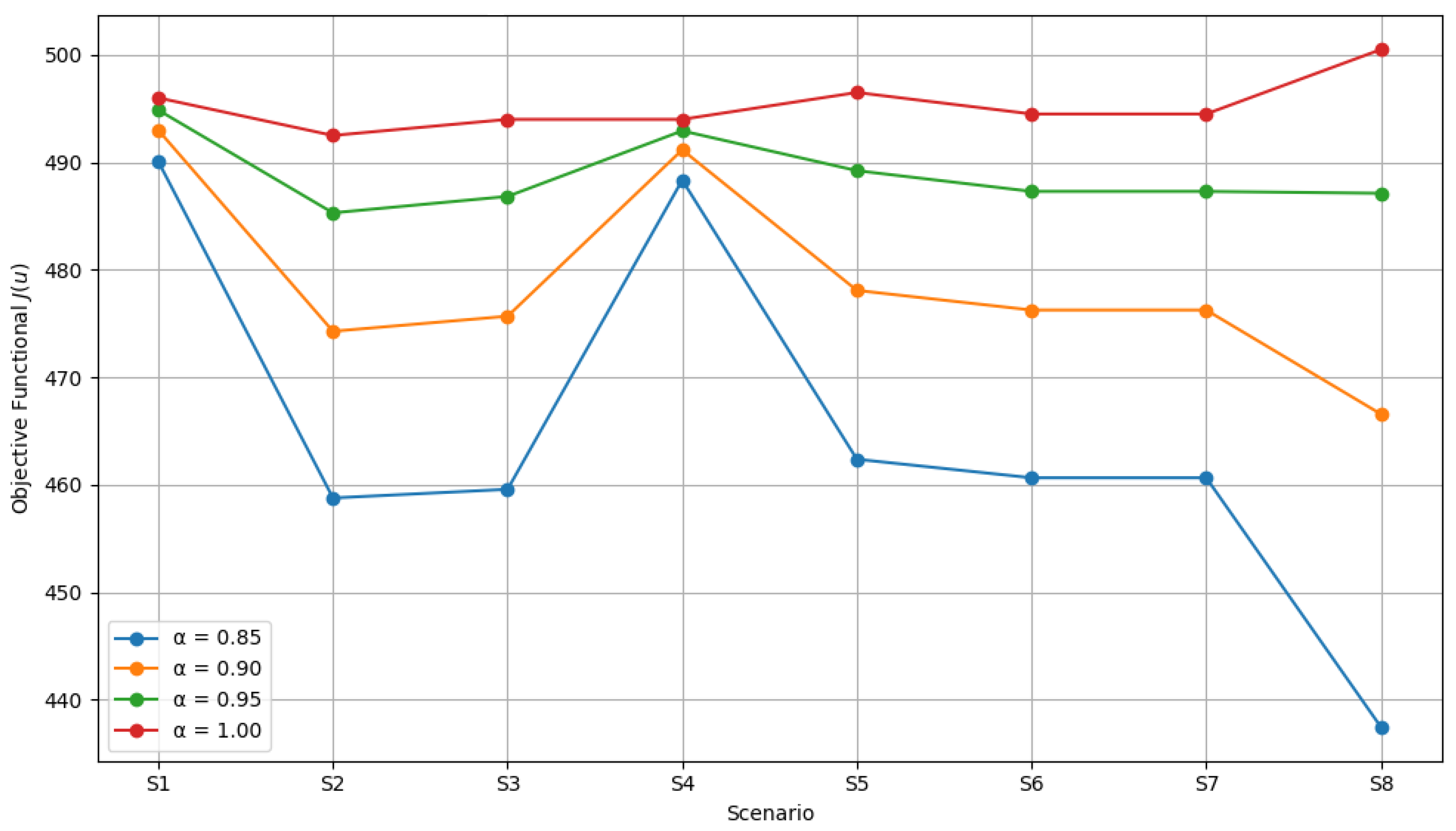
6.3. Optimal Scenario Identification
6.4. Scientific Interpretation, Policy and Public Health Implications, and Comparison with Classical Control Models
7. Conclusion and Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alvar, J. , Vélez, I. D., Bern, C., Herrero, M., Desjeux, P., Cano, J.,... & the WHO Leishmaniasis Control Team, Leishmaniasis worldwide and global estimates of its incidence, PLoS One, 7(5) (2012) e35671.
- Cosma, C. , Maia, C., Khan, N., Infantino, M., & Del Riccio, M., Leishmaniasis in humans and animals: A one health approach for surveillance, prevention and control in a changing world, Tropical Medicine and Infectious Disease, 9(11) (2024) 258.
- Almeida-Souza, F. , Calabrese, K. D. S., Abreu-Silva, A. L., &; Cardoso, F., Leishmania Parasites: Epidemiology, Immunopathology and Hosts, BoD–Books on Demand, 2024. [Google Scholar]
- Khater, H. F. , & Ramadan, M. Y., Control of phlebotomine sandflies (Diptera: Psychodidae) with insecticides: A review of current status and future prospects, Journal of Vector Ecology, 38(1) (2013) 1–9.
- Hethcote, H. W. , The mathematics of infectious diseases, SIAM Review, 42(4) (2000) 599-653.
- Van den Driessche, P. , & Watmough, J., Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission, Mathematical Biosciences, 180(1–2) (2002) 29-48. [CrossRef]
- Safan, M. , & Altheyabi, A., Mathematical analysis of an anthroponotic cutaneous leishmaniasis model with asymptomatic infection, Mathematics, 11(10) (2023) 2388. [CrossRef]
- Sinan, M. , Ansari, K. J., Kanwal, A., Shah, K., Abdeljawad, T., & Abdalla, B., Analysis of the mathematical model of cutaneous leishmaniasis disease, Alexandria Engineering Journal, 72 (2023) 117-134. [CrossRef]
- Biswas, D. , Dolai, S., Chowdhury, J., Roy, P. K., & Grigorieva, E. V., Cost-effective analysis of control strategies to reduce the prevalence of cutaneous leishmaniasis, based on a mathematical model, Mathematical and Computational Applications, 23(3) (2018) 38. [CrossRef]
- Diethelm, K. , The Analysis of Fractional Differential Equations: An Application-Oriented Exposition Using Differential Operators of Caputo Type, Springer, 2010.
- Baleanu, D. , Losada, J., & Jarad, F., Nonlocal modeling and analysis of the memory-dependent SIR epidemic system, Applied Mathematical Modelling, 65 (2019) 123-137.
- Li, Q. , Peng, Y., & Bai, X., Memory-dependent fractional-order model of COVID-19 with public psychological effects, Applied Mathematical Modelling, 90 (2021) 1062–1080.
- Yoon, J. , Saha, S., & Kalantari, A., Bayesian parameter estimation for fractional-order epidemiological models: A case study of COVID-19, Mathematics, 10(5) (2022) 822.
- Funk, S. , Gilad, E., & Jansen, V. A. A., The spread of awareness and its impact on epidemic outbreaks, Proceedings of the National Academy of Sciences, 106(16) (2009) 6872-6877. [CrossRef]
- Verelst, F. , Willem, L., &; Beutels, P., Behavioural change models for infectious disease transmission: A systematic review (2010–2015), Journal of the Royal Society Interface, 13(125) (2016) 20160820. [Google Scholar]
- Van Bavel, J. J. , Baicker, K., Boggio, P. S., Capraro, V., Cichocka, A., Cikara, M.,... & Willer, R., Using social and behavioural science to support COVID-19 pandemic response, Nature Human Behaviour, 4(5) (2020) 460-471. [CrossRef]
- Lenhart, S. , & Workman, J. T., Optimal Control Applied to Biological Models, Chapman & Hall/CRC, 2007.
- Pantha, B. , Agusto, F. B., & Elmojtaba, I. M., Optimal Control Applied to a Visceral Leishmaniasis Model, Technical Report, Department of Mathematics, Texas State University, 2020. [CrossRef]
- Nadeem, F. , Zamir, M., & Tridane, A., Modeling and control of zoonotic cutaneous leishmaniasis, Punjab University Journal of Mathematics, 51(2) (2019) 105-121.
- Zamir, M. , Zaman, G., & Alshomrani, A. S., Sensitivity analysis and optimal control of anthroponotic cutaneous leishmania, PLoS One, 11(8) (2016) e0160513.
- Kilbas, A. A. , Srivastava, H. M., & Trujillo, J. J., Theory and Applications of Fractional Differential Equations, Elsevier, 2006.
- Machado, J. A. T. , Kiryakova, V., & Mainardi, F., Recent history of fractional calculus, Communications in Nonlinear Science and Numerical Simulation, 16(3) (2011) 1140–1153. [CrossRef]
- Mainardi, F. , Fractional Calculus and Waves in Linear Viscoelasticity: An Introduction to Mathematical Models, Imperial College Press, 2010.
- Toufga, H. , Sakkoum, A., Benahmadi, L., & Lhous, M., Analysis of the dynamics and optimal control of cutaneous Leishmania during human immigration, Iranian Journal of Numerical Analysis and Optimization, 15(1) (2025) 311-345.
- Khan, A. , Zarin, R., Inc, M., Zaman, G., & Almohsen, B., Stability analysis of Leishmania epidemic model with harmonic mean type incidence rate, European Physical Journal Plus, 135(6) (2020) 528. [CrossRef]
- Podlubny, I. , Fractional Differential Equations: An Introduction to Fractional Derivatives, Fractional Differential Equations, to Methods of Their Solution and Some of Their Applications, Academic Press, 1999.
- Marino, S. , Hogue, I. B., Ray, C. J., & Kirschner, D. E., A methodology for performing global uncertainty and sensitivity analysis in systems biology, Journal of Theoretical Biology, 254(1) (2008) 178-196. [CrossRef]
- Belser, A. B. , McKay, C. L., McMahon, D. K., & Hittner, J. B., Global sensitivity analysis: Concepts, methods, and applications in public health modeling, BMC Public Health, 22 (2022) 1-14.
- Wu, J. , Dhingra, R., Gambhir, M., &; Remais, J. V., Sensitivity analysis of infectious disease models: Methods, advances and their application, Journal of the Royal Society Interface, 10(86) (2013) 20121018. [Google Scholar]
- Saltelli, A. , Ratto, M., Andres, T., Campolongo, F., Cariboni, J., Gatelli, D.,... &; Tarantola, S., Global Sensitivity Analysis: The Primer, John Wiley & Sons, 2008. [Google Scholar]
- Sobie, E. A. , Parameter sensitivity analysis in electrophysiological models using multivariable regression, Biophysical Journal, 96(4) (2009) 1264-1274. [CrossRef]
- World Health Organization, Vector control in leishmaniasis: Current strategies and future directions, WHO Technical Report, 2020.
- Kiss, I. Z. , Miller, J. C., &; Simon, P. L., Mathematics of Epidemics on Networks: From Exact to Approximate Models, Springer, 2017. [Google Scholar]
- Allen, L. J. S. , A primer on stochastic epidemic models: Formulation, numerical simulation, and analysis, Infectious Disease Modeling, 2(2) (2017) 128-142. [CrossRef]
- Pastor-Satorras, R. , Castellano, C., Van Mieghem, P., & Vespignani, A., Epidemic processes in complex networks, Reviews of Modern Physics, 87(3) (2015) 925–979.
- Ferrer, R. A. , &; Klein, W. M., Examining the impact of co-infection in neglected tropical diseases: A theoretical modeling approach, PLoS Neglected Tropical Diseases, 15(4) (2021) e0009333. [Google Scholar]

| Variable | Description | Initial value |
|---|---|---|
| Number of susceptible humans | 100 | |
| Number of exposed humans (infected but not yet infectious) | 20 | |
| Number of symptomatic infected humans | 20 | |
| Number of asymptomatic infected humans | 10 | |
| Number of recovered humans | 10 | |
| Number of susceptible vectors | 1000 | |
| Number of exposed vectors | 20 | |
| Number of infected vectors (non-resistant) | 30 | |
| Number of infected insecticide-resistant vectors | 10 | |
| Level of awareness in the human population | 0.1 |
| Parameter | Description | Value (per day) | Source |
|---|---|---|---|
| Recruitment rate of humans | 10 | [18] | |
| Recruitment rate of vectors | 100 | [18] | |
| Natural death rate of humans | 0.014 | [19] | |
| Natural death rate of vectors | 0.1 | [19] | |
| Death rate of resistant vectors | 0.07 | Assumed | |
| a | Average biting rate of vectors | 0.3 | [24] |
| Transmission probability from vector to human | 0.2 | [24] | |
| Transmission probability from human to vector | 0.25 | [24] | |
| Incubation rate in humans | 0.1 | [25] | |
| Incubation rate in vectors | 0.2 | [19] | |
| Recovery rate of symptomatic individuals | 0.1 | [24] | |
| Recovery rate of asymptomatic individuals | 0.08 | Assumed | |
| Natural recovery rate from exposed class | 0.02 | [24] | |
| Awareness increase rate due to campaigns () | 0.7 | Assumed | |
| Awareness decay rate | 0.05 | Assumed | |
| p | Proportion of exposed humans becoming asymptomatic | 0.4 | [24] |
| q | Proportion of vectors becoming insecticide-resistant | 0.1 | Assumed |
| Fractional-order derivative (Caputo) | 0.95 | Assumed |
| a | p | q | |||||||||||
| 0.5916 | 0.2454 | 0.2126 | 0.2369 | 0.1153 | 0.0100 | 0.0746 | 0.0070 | 0.0606 |
| Variable | Description |
|---|---|
| Use of bed nets and repellents | |
| Treatment effort for symptomatic humans | |
| Insecticide spraying | |
| Public awareness campaigns |
| Scenario | J | E |
|---|---|---|
| No Control | 490.9330 | 1995.7209 |
| Only | 494.8612 | 1995.7189 |
| Only | 481.1943 | 1919.9739 |
| Only | 492.9306 | 1995.7209 |
| 491.2249 | 1919.9719 | |
| 481.1943 | 1919.9739 | |
| Only | 486.8301 | 1995.7209 |
| All Controls | 487.1221 | 1919.9719 |
| Scenario | ICER |
|---|---|
| Only | 1901.0400 |
| Only | -0.1286 |
| Only | 36595.7979 |
| 0.0039 | |
| -0.1286 | |
| Only | -13442860.2455 |
| All Controls | -0.0503 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




