1. Introduction
The process of fabricating a full-arch implant-supported prosthesis involves a series of critical steps, each capable of introducing variables that may influence the final fit and accuracy of the prosthetic outcome. Among these, the accurate recording of implant positions is essential and traditionally depends on the chosen impression material, technique, implant depth, angulation, and spatial distribution [
1]. Conventional approaches have included open- and closed-tray impression techniques using polyvinyl siloxane or polyether, often supplemented by splinting methods to enhance stability [
2,
3]. Recent advancements in digital impression techniques, particularly with intraoral scanners, have the potential to improve accuracy and efficiency in capturing implant positions for full-arch prostheses [
4,
5,
6]. However, the effectiveness of these digital methods compared to traditional techniques remains a topic of ongoing research and debate [
7,
8]. While digital implant impressions offer promising benefits, studies indicate that conventional techniques may still provide superior accuracy in certain contexts, necessitating further investigation to establish definitive conclusions [
7,
9,
10]. Consequently, understanding the comparative accuracy of various impression techniques is crucial for optimising the fabrication process of implant-supported prostheses.
In recent years, digital workflows employing intraoral scanners (IOS) have transformed clinical protocols in implant dentistry. These digital alternatives offer increased patient comfort, enhanced communication with laboratories, and more streamlined clinical steps [
11]. Although splinted open-tray impressions continue to be considered the clinical reference standard, numerous studies have validated that intraoral scanners can yield comparable levels of accuracy in both partially and fully edentulous implant cases [
12,
13]. Further research is essential to determine the long-term reliability and clinical applicability of IOS technologies in various implant scenarios, especially concerning complex anatomical considerations [
14]. Intraoral scanners, such as the Medit i900, have demonstrated significant potential in achieving high precision and trueness for full-arch implant impressions, warranting further exploration in clinical settings [
15].
Moreover, the integration of advanced technologies such as stereophotogrammetry and machine learning algorithms into the digital impression workflow has the potential to further enhance the accuracy and precision of implant positioning [
16]. Stereophotogrammetry, for instance, has been shown to exhibit superior trueness and precision compared to conventional methods, meeting the misfit thresholds necessary for effective implant-supported prostheses [
17]. Additionally, the implementation of automated analysis tools could mitigate human error during the scanning process, optimising the workflow and potentially leading to improved patient outcomes. As the field continues to evolve, it is essential to rigorously compare these modern techniques against traditional methods, not only to validate their effectiveness but also to explore their implications for clinical practice and patient satisfaction. Thus, ongoing research is crucial to fully understand how these innovations can be seamlessly integrated into existing protocols to maximise both efficiency and accuracy in implant dentistry.
Despite these advancements, the accuracy of IOS-based scans can be affected by a range of patient and operator dependent factors, including arch morphology, implant spacing, scanning sequence, scanner calibration, ambient conditions, software algorithms, and scan body design [
18,
19]. Traditionally, photogrammetry has emerged as a reliable chairside method for transferring implant positions due to its high accuracy and minimal operator influence [
20]. However, the clinical adoption of photogrammetry systems is limited by their high cost, steep learning curve, and complexity, restricting widespread use [
21]. In contrast, intraoral scanners like the Medit scanners are becoming increasingly popular due to their ability to simplify the impression process while maintaining accuracy, thus enhancing clinical workflows [
22,
23]. This study aims to evaluate the trueness and precision of the Medit SmartX AI workflow in comparison to traditional impression techniques, particularly focusing on its effectiveness in full-arch implant scanning accuracy. The findings will contribute to understanding how digital implant impression techniques using artificial intelligence can be optimised for clinical use, potentially reshaping implant prosthodontics.
The Medit SmartX protocol, recently introduced for use in Medit Link Scanning software for Medit i600/i700/i900 intraoral scanners, aims to democratise access to accurate full-arch implant scanning through real-time library matching and artificial intelligence-based recognition of scan bodies. This system digitally identifies and superimposes idealised virtual representations of scan bodies during the scan process, eliminating the need for external library alignment or costly photogrammetry hardware. The SmartX system uses AI-trained geometric matching to register scan body positions with high fidelity. This innovative approach not only streamlines the scanning process but also enhances the overall accuracy of full-arch implant impressions, making it a promising alternative to traditional techniques.
A recent study evaluated the in vitro performance of the Scan Ladder system used with two intraoral scanners (Primescan and Medit i900), comparing results against an intraoral photogrammetry device in edentulous patient cases. Their findings showed that the inclusion of Scan Ladder scan bodys significantly enhanced scan accuracy—achieving trueness and precision that were statistically indistinguishable from the photogrammetry gold standard [
24].
Scan Ladder scan bodies, as shown in
Figure 1, are uniquely suited for this workflow and feature novel attributes. Unlike traditional scan bodies, each Scan Ladder component is geometrically distinct in shape and length, allowing clinicians to customise positioning for each patient case to reduce gingival interference and improve scanning efficiency. The irregular surface geometry facilitates reliable AI recognition during scanning, while the shared library model standardises digital matching across all scan body positions. Furthermore, the Scan Ladder’s horizontal alignment structure reduces the frame distance between scan bodies, minimising distortion introduced during stitching and the irregular design and random surfaces unique to Scan Ladder which aims to enable precise cross-arch calibration. This study will assess the trueness and precision of the Medit SmartX using the Scan Ladder system as seen during scan in
Figure 2, comparing its performance against traditional methods in capturing implant positions.The results of this study are anticipated to provide valuable insights into the effectiveness of AI-driven scanning techniques in enhancing the accuracy of full-arch implant prostheses and will question the concept that carefully designed scan body geometry, combined with appropriate digital workflows using artificial intelligence, can substantially close the accuracy gap between standard IOS systems and photogrammetric approaches.
To date, no published data exist comparing the trueness and precision of the SmartX real-time library matching protocol against validated reference data in full-arch implant models. In this study, a model with six implants restored using Scan Ladder scan bodies was scanned using a 3Shape E2 desktop scanner to establish a high-resolution reference model, representing the gold standard based on a clinically verified multi-unit jig. This was compared with twenty digital scans performed using the Medit i900 under SmartX protocol conditions.
This in vitro investigation aims to evaluate the spatial accuracy of the Medit SmartX scanning protocol in terms of both trueness and precision, as defined by ISO 5725-1 standards [
25,
26]. The null hypothesis is that no significant difference exists between the implant positions generated by Medit SmartX and those captured by the E2 desktop scanner. The findings of this study may clarify whether the SmartX system can serve as a clinically viable alternative to photogrammetry and traditional methods for recording full-arch implant positions.
2. Materials and Methods
2.1. Model Preparation
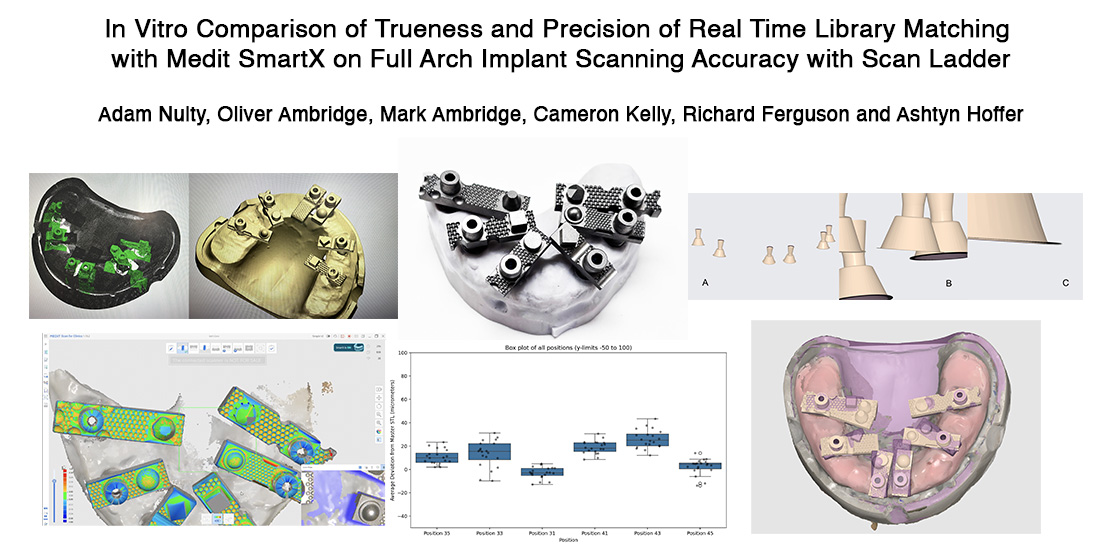
This in vitro study was conducted using a custom-fabricated mandibular full-arch model simulating an edentulous clinical scenario. The model incorporated six implant analogues, positioned to replicate a typical All-on-X distribution at sites 35, 33, 31, 41, 43, and 45. Multi-Unit Abutments (MUAs) were placed on each analogue, and Scan Ladder scan bodies were secured to the MUAs in their definitive positions.
To establish a gold standard reference geometry, a verification titanium bar jig was employed during analogue placement, ensuring consistent spacing and alignment across all implant sites. A silicone gingival mask was applied to the model to replicate soft tissue contours and introduce the scanning challenges encountered in vivo, such as sub-gingival interference and limited access around angled abutments.
Unlike retrospective clinical data analysis, this investigation involved direct experimental measurement under controlled conditions. A total of 20 independent intraoral scans were performed using a Medit i900 scanner equipped with the SmartX real-time library matching protocol. Each scan captured all six Scan Ladder positions, and the datasets were subsequently compared to a laboratory-grade reference scan generated with a 3Shape (Copenhagen, Denmark) E2 structured light scanner.
The study was designed in accordance with international standards for laboratory-based accuracy testing of intraoral scanners (ISO 12836). As no human participants were involved, no ethical approval was required.
2.2. Master STL Creation
A gold standard reference model was created using a mandibular full-arch model embedded with six implant analogues at positions 35, 33, 31, 41, 43, and 45. Multi-Unit Abutments (MUAs) were placed on each analogue, and Scan Ladder scan bodies were securely attached to replicate the definitive clinical scanning condition.
The model was digitised with a 3Shape E2 desktop structured light scanner as seen in
Figure 3 below. (3Shape, Copenhagen, Denmark), which is accredited for high-precision dental metrology under ISO 12836 standards. The E2 scanner has a trueness specification of ≤7 μm and was therefore considered an appropriate reference for evaluating intraoral scanner accuracy.
The resulting STL dataset, representing the exact geometry and spatial relationships of the six implant positions, was designated as the Master STL. This file served as the gold standard against which all subsequent intraoral scans were compared.
The aim of creating the Master STL was to provide a validated, reproducible reference geometry for assessing the trueness (closeness to the reference) and precision (repeatability across scans) of the Medit SmartX protocol in conjunction with the Scan Ladder system. By ensuring that all deviations were measured against this high-fidelity reference, the study could isolate and quantify the contribution of the SmartX + Scan Ladder workflow to full-arch digital implant accuracy.
2.3. Scanning Procedure
All experimental scans were performed on the mandibular full-arch model with six implant analogues fitted with Multi-Unit Abutments and Scan Ladder scan bodies. Scanning was carried out using the Medit i900 intraoral scanner (Medit Corp., Seoul, South Korea) under the SmartX real-time library matching protocol.
Figure 4.
All Scans, Master and Test Data, Aligned and overlaid to view within Medit Link.
Figure 4.
All Scans, Master and Test Data, Aligned and overlaid to view within Medit Link.
The Scan Ladder system provides detailed guidance for both the centralised positioning of scan bodies along the arch and for the recommended scanning sequence to optimise digital capture. These instructions were strictly adhered to in this study. The scan bodies were positioned in their prescribed horizontal alignment, ensuring their centroids were placed within a common reference plane. This arrangement is a defining feature of the Scan Ladder system and is designed to reduce cumulative stitching error during full-arch scanning.
For each acquisition, a standardised scan path was followed in accordance with the Scan Ladder protocol. Scanning commenced at the right posterior quadrant and proceeded sequentially across the occlusal surfaces towards the contralateral posterior region. Additional sweeps were made across buccal and lingual surfaces to capture gingival contours and ensure full visibility of each scan body. This workflow is specifically designed to maintain consistent reference points and optimise AI-based library matching within the SmartX system.
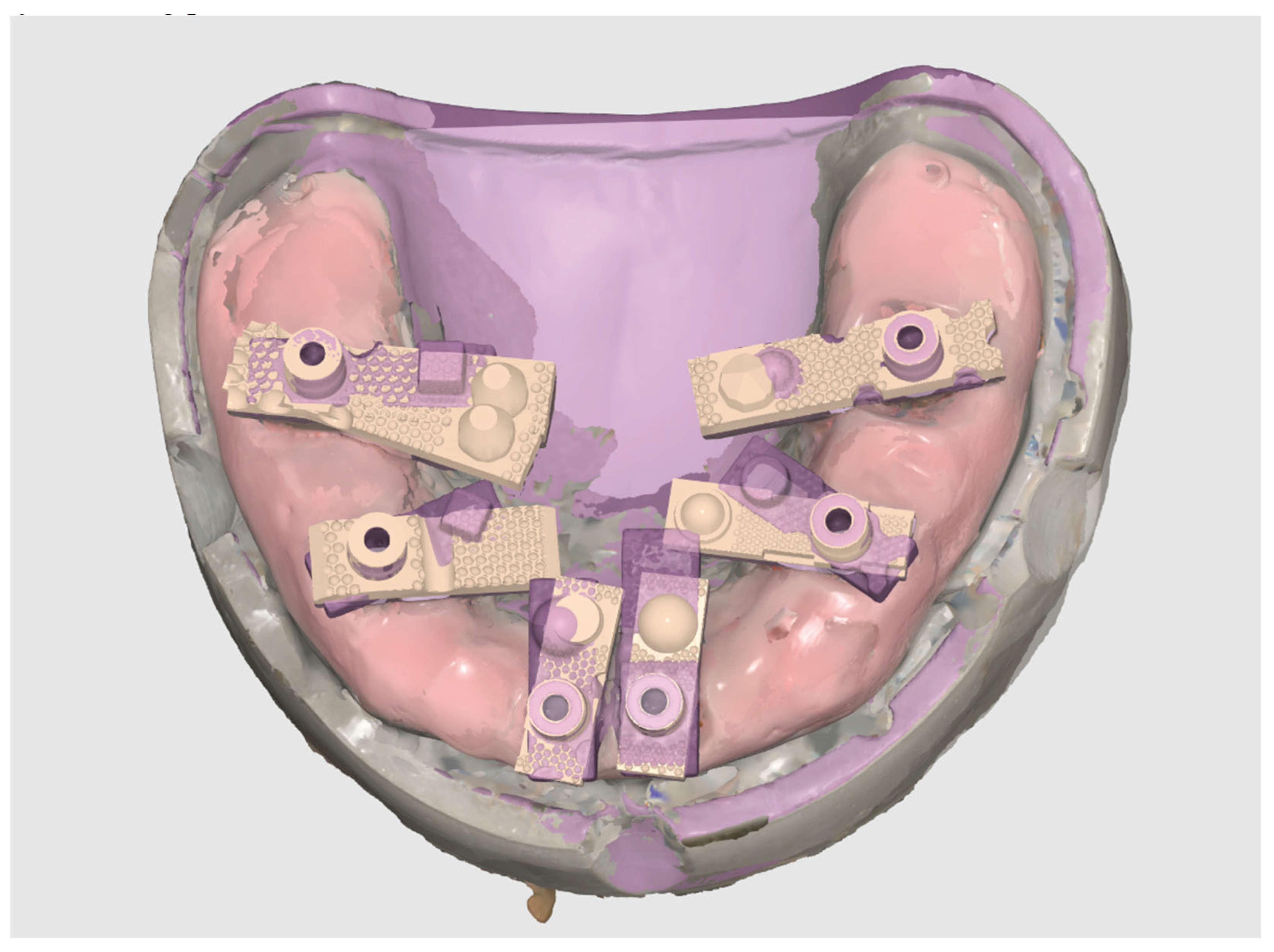
A total of 20 independent full-arch scans were acquired under identical conditions by a single experienced operator. Each SmartX dataset was automatically processed in real time, with virtual Multi-Unit Abutments (MUAs) replacing the physical Scan Ladder geometries during scanning. The resulting digital models were exported as STL files and imported into Exocad DentalCAD (Exocad GmbH, Darmstadt, Germany). Within Exocad, the six virtual MUAs were isolated and saved individually, creating 120 site-specific STL files for subsequent accuracy analysis.
Accuracy assessment was performed by importing each test STL alongside the Master STL (generated with the 3Shape E2 desktop scanner; see
Section 2.2) into CloudCompare (3D Systems, Rock Hill, SC, USA), an open-source point-cloud and mesh processing software originally developed by Daniel Girardeau-Montaut at Télécom ParisTech/EDF R&D and now maintained by a global community under the GPL license. No best-fit superimposition was executed, and the average surface deviation (μm) was calculated between each test implant position and the reference in their relative 3D position as exported from exocad. This process produced one average deviation value per implant position per scan, yielding 20 values per site and 120 measurements in total.
Figure 5.
Aligning Scan Ladder Implant Library in Exocad.
Figure 5.
Aligning Scan Ladder Implant Library in Exocad.
Figure 6.
Zoom in of all Test Sets viewed overlaid. A. Whole Scene. B. Zoom into Multi Unit. C. Close up of all data.
Figure 6.
Zoom in of all Test Sets viewed overlaid. A. Whole Scene. B. Zoom into Multi Unit. C. Close up of all data.
2.3.1. Scanners in the Study
The scanners used in the present in vitro study are summarised in Table 1.
Table 1.
The Digital Scanners Used In This Study.
Table 1.
The Digital Scanners Used In This Study.
| Name |
Manufacturer |
Technology |
STL Export |
PLY/OBJ Colour Export |
Photogrammetry |
| E2 |
3Shape, Copenhagen, Denmark |
Structured white light desktop scanner. |
YES |
NO |
NO |
| i900 |
Medit, Seongbuk-gu, Seoul, Korea |
Structured light-Active
Speed 3D Video™ |
YES |
YES |
NO |
2.4. Design of the Study
2.4.1. Overview
This in vitro study evaluated the accuracy—defined in terms of trueness (closeness to the reference model) and precision(repeatability across scans)—of the Medit i900 intraoral scanner (Medit Corp., Seoul, South Korea) operating under the SmartX real-time library matching protocol in combination with Scan Ladder scan bodies.
The digital outputs from 20 repeated SmartX scans were compared against a high-accuracy Master STL generated using a 3Shape E2 desktop scanner (3Shape, Copenhagen, Denmark), accredited under ISO 12836 and specified to achieve ≤7 μm accuracy. The Master STL was considered the gold standard for all comparative analyses.
In total, 120 deviation measurements were collected across six implant positions (35, 33, 31, 41, 43, 45). This sample size was sufficient to provide robust statistical analysis, enabling evaluation of both systematic error (trueness) and variability across repeated acquisitions (precision).
2.5. Data Processing and Analysis
Following the scanning procedures, each SmartX-generated dataset was exported in STL format. Within Exocad DentalCAD (Exocad GmbH, Darmstadt, Germany), the automatically aligned virtual Multi-Unit Abutments (MUAs) were isolated from the full-arch model. Each implant position was exported as a separate STL file, resulting in six implant-specific files per scan and a total of 120 STL datasets across all 20 scans. This systematic segmentation ensured that each implant site could be assessed independently while maintaining consistent alignment with the overall arch geometry.
Each test STL was then imported into CloudCompare (3D Systems, Rock Hill, SC, USA) alongside the corresponding implant position from the Master STL (generated with the 3Shape E2 desktop scanner;
Section 2.2). A best-fit alignment was performed using the software’s iterative closest point (ICP) algorithm. The average surface deviation (μm) between the test and reference STL was calculated for each implant site. This yielded 20 deviation values per implant position, providing a robust dataset for statistical evaluation of both trueness and precision.
2.5.1. Evaluating Trueness
Trueness was defined as the closeness of agreement between the test scan and the Master STL reference. For each implant position, the mean average deviation across the 20 scans was calculated, representing how accurately the SmartX workflow reproduced the known implant geometry. Descriptive statistics (mean, standard deviation, minimum, maximum) were compiled for each implant site as well as for the pooled dataset.
2.5.2. Evaluating Precision
Precision was defined as the repeatability of deviation measurements across multiple scans under identical conditions. For each implant site, the dispersion of deviation values across the 20 replicates was used to assess consistency. A lower standard deviation indicated higher precision.
2.5.3. Statistical Analysis
Statistical analysis was performed using SPSS version 22 (IBM, Chicago, IL, USA). A two-way analysis of variance (ANOVA) was applied to evaluate the effects of implant position and scan iteration on deviation values. Where significant effects were detected, post hoc multiple comparisons were conducted using Tukey’s HSD test, with the significance level set at α = 0.05. The analysis was designed to determine whether systematic differences existed between anterior and posterior implant positions and whether scan repetition introduced additional variability.
This methodology provided a rigorous framework for quantifying both trueness and precision of the Medit SmartX protocol with Scan Ladder scan bodies, ensuring reproducibility and comparability with existing literature on digital implant scanning accuracy.
3. Results
A total of 120 measurements (six implant positions across 20 Medit SmartX scans) were analysed and compared with the reference positions obtained using the 3Shape E2 desktop scanner. The reference STL file, generated with the aid of a verification jig, was treated as the gold standard against which all intraoral scanner-derived datasets were assessed.
Table 1.
Study Results.
| Block Number |
Position 35 |
Position 33 |
Position 31 |
Position 41 |
Position 43 |
Position 45 |
| 1 |
3.18 |
25.12 |
-3.19 |
23.06 |
26.17 |
0.98 |
| 2 |
18.43 |
15.23 |
-4.70 |
8.58 |
19.86 |
4.79 |
| 3 |
10.10 |
31.28 |
-6.33 |
17.42 |
43.46 |
7.05 |
| 4 |
12.87 |
4.05 |
2.61 |
13.70 |
18.93 |
3.54 |
| 5 |
9.92 |
15.77 |
-2.75 |
30.69 |
23.54 |
-5.96 |
| 6 |
7.25 |
9.75 |
0.85 |
9.84 |
32.06 |
4.93 |
| 7 |
4.91 |
22.25 |
4.25 |
13.59 |
34.90 |
5.26 |
| 8 |
7.71 |
22.24 |
1.27 |
21.42 |
12.10 |
-0.77 |
| 9 |
11.36 |
-1.70 |
1.17 |
23.19 |
25.56 |
14.07 |
| 10 |
16.52 |
10.81 |
-6.07 |
18.65 |
24.36 |
4.78 |
| 11 |
20.62 |
16.73 |
-3.36 |
16.20 |
21.85 |
8.47 |
| 12 |
6.08 |
-8.94 |
-4.71 |
22.88 |
21.64 |
3.87 |
| 13 |
19.00 |
14.21 |
-10.99 |
23.50 |
16.48 |
5.15 |
| 14 |
6.92 |
15.82 |
-1.14 |
14.63 |
37.62 |
6.59 |
| 15 |
2.04 |
17.68 |
-12.77 |
15.54 |
17.84 |
-13.88 |
| 16 |
5.67 |
27.43 |
-2.26 |
20.90 |
35.72 |
2.10 |
| 17 |
2.04 |
25.55 |
5.00 |
17.62 |
29.29 |
-11.88 |
| 18 |
11.68 |
11.77 |
-3.16 |
22.60 |
29.70 |
-0.56 |
| 19 |
23.44 |
1.53 |
-11.08 |
27.17 |
28.85 |
8.81 |
| 20 |
13.16 |
-9.78 |
-2.62 |
17.86 |
20.41 |
2.32 |
3.1. Trueness
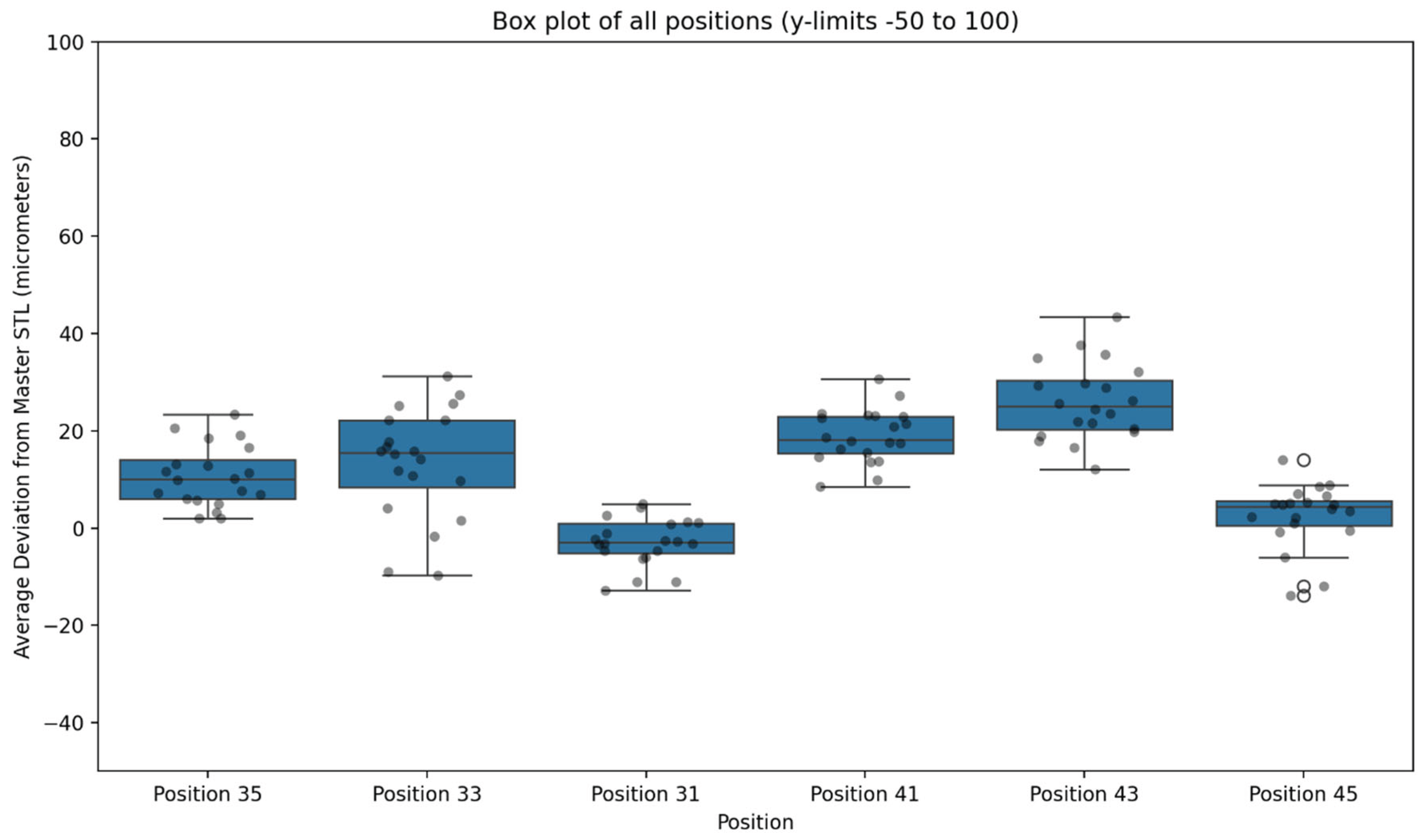
When all positions were pooled, the Medit SmartX + Scan Ladder workflow achieved a mean average deviation of 11.41 μm with a standard deviation of 12.16 μm. These findings indicate exceptionally high trueness, with all values falling within a narrow range well below the widely accepted ±150 μm clinical threshold.
At the level of individual implant sites, the smallest mean deviations were observed at position 31 (4.7 μm) and position 45 (6.1 μm). The largest deviations occurred at position 43 (22.5 μm), with other posterior sites also demonstrating slightly greater error compared with anterior positions. Nevertheless, no mean site deviation exceeded 25 μm, and no individual test scan result exceeded 45 μm.
3.2. Precision
Repeated scans demonstrated strong reproducibility across the dataset. The pooled standard deviation of 12.16 μm reflects high precision, with tight clustering of values across the 20 replicate scans per position. Anterior positions in particular showed minimal variation, while posterior sites displayed slightly broader distributions yet remained within clinically acceptable limits.
3.3. Statistical Analysis
Two-way ANOVA confirmed that implant position significantly influenced deviation values (P < .001). Posterior implants yielded higher mean deviations than anterior sites, with the greatest discrepancies observed at position 43. In contrast, scan iteration (repeated scanning) showed no significant effect (P > .05), confirming reproducibility across all repeated SmartX scans.
No significant interaction was identified between implant position and scan iteration (P > .05), indicating that deviations were primarily influenced by anatomical and scanning-span factors rather than operator- or sequence-related variability.The results suggest that the Medit SmartX protocol, particularly when combined with Scan Ladder scan bodies, offers a promising alternative to traditional methods for achieving high trueness and precision in full-arch implant scanning.
The findings of this study may significantly influence the adoption of AI-driven scanning techniques in clinical practice, potentially reshaping standards for full-arch implant prosthetics.
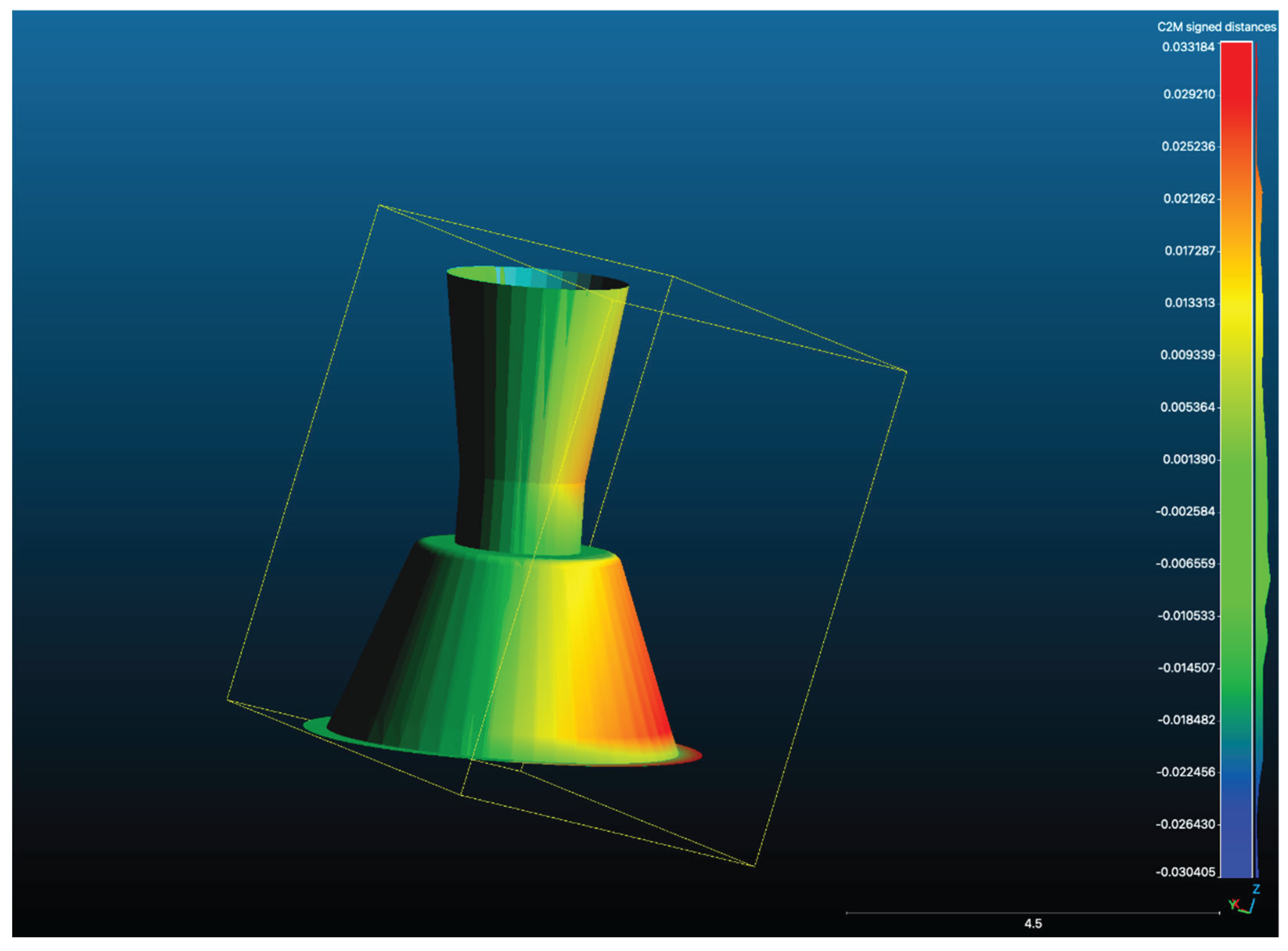
Figure 7.
A Scan Overlay of one test scan STL from one position with the Master STL showing deviation between the two scans.
Figure 7.
A Scan Overlay of one test scan STL from one position with the Master STL showing deviation between the two scans.
Figure 8.
Box Plot of Deviation in μm for Each Data Set for Each Scan Type in the Present Study.
Figure 8.
Box Plot of Deviation in μm for Each Data Set for Each Scan Type in the Present Study.
5. Conclusions
This in vitro study demonstrated that the Medit SmartX real-time library matching protocol, when used in conjunction with Scan Ladder scan bodies, can achieve trueness and precision closely comparable to a laboratory-grade desktop scanner. Across all implant positions, the workflow produced a mean average deviation of 11.41 μm with a standard deviation of 12.16 μm, with no individual site exceeding 25 μm. These values place the system well within the sub-20 μm range typically associated with photogrammetry, the current gold standard for full-arch implant position transfer.
The findings support the clinical potential of SmartX as a simplified and cost-effective alternative to photogrammetry for full-arch digital implant scanning. The distinctive attributes of the Scan Ladder system—variable scan body geometries, a unified virtual library, and horizontally optimised positioning—appear to contribute substantially to the observed accuracy and reproducibility.
While these results highlight the promise of this approach, further clinical studies are required to confirm performance under real-world conditions, where biological and environmental variables may affect outcomes. Such investigations will be essential to validate whether SmartX with Scan Ladder can reliably deliver high-fidelity digital impressions in routine implant practice. The potential for integrating AI-driven technologies in dental workflows not only enhances accuracy but may also streamline the overall clinical process, ultimately benefiting patient care.