1. Introduction
Severe acute pancreatitis (SAP) is a highly complex clinical condition affecting approximately 20–30% of patients with acute pancreatitis, with an in-hospital mortality rate of 15%, which may exceed 35% in the presence of infected necrosis or multiorgan failure (MODS) [
1].
SAP has a characteristic biphasic course. In the early phase (first 1–2 weeks from onset), pathogenesis is dominated by a marked sterile systemic inflammatory response syndrome (SIRS), in which sepsis is rare. In severe SIRS, massive activation of pro-inflammatory mediators (TNF-α, IL-1β, IL-6) can cause endothelial damage, diffuse oedema, and early MODS involving the respiratory, cardiovascular, renal, and hepatic systems. Pancreatic necrosis, which may develop within the first 96 hours, tends to evolve over the first two weeks; its extent is not necessarily correlated with the presence or severity of SIRS. However, in patients with severe early organ dysfunction, necrosis is frequently detected on computed tomography. Peripancreatic fluid collections are common in this phase.
In the late phase (> 1–2 weeks), there is a shift from a pro-inflammatory response to a state of relative immunosuppression, favouring bacterial translocation due to impaired intestinal barrier function. This results in a high risk of infection of pancreatic necrotic areas and peripancreatic collections.
Mortality in SAP therefore shows two distinct temporal peaks: an early peak, related to severe SIRS and MOF, and a late peak, attributable to infected necrosis complicated by sepsis [
2,
3,
4].
The most widely used clinical tools for assessing disease severity include the APACHE II and SOFA prognostic scores, in line with the 2012 Atlanta Classification criteria, which define SAP as acute pancreatitis associated with persistent organ failure for more than 48 hours [
2,
3,
5].
Currently, SAP management is based on intensive support — including haemodynamic, ventilatory, and nutritional support — close monitoring of local and systemic complications, and surgical intervention in the most complex cases refractory to conservative therapy. However, there are no specific therapies capable of effectively modulating the systemic inflammatory response [
6].
In this context, extracorporeal blood purification techniques have generated increasing interest. In particular, continuous veno-venous hemofiltration (CVVH), especially at high volume (HVHF), can remove inflammatory mediators of intermediate molecular weight (such as IL-6 and TNF-α), potentially reducing the cytokine storm and improving survival, although clinical data remain partial and heterogeneous [
7].
The oXiris filter is a CRRT membrane designed to combine renal support and extracorporeal immunomodulation in a single device. Its structure is based on AN69 (acrylonitrile–metallyl sulfonate sodium copolymer), which is biocompatible and offers excellent permeability for convective/diffusive clearance; an internal surface layer of polyethyleneimine (PEI), a cationic polymer that, due to its positive charge, enhances electrostatic adsorption of endotoxins and pro-inflammatory cytokines; and a heparinised blood-contacting surface, reducing contact activation of coagulation and improving tolerability during prolonged sessions [
8,
9,
10].
The result is a “four-in-one” cartridge: renal replacement, cytokine removal, endotoxin removal, and reduced surface thrombogenicity. Operationally, oXiris functions like a standard CRRT hemofilter but, compared with conventional filters, adds a direct adsorption component allowing rapid “debulking” of circulating mediators during hyperinflammatory phases. It can also be used in patients at higher bleeding risk, as it often allows minimal anticoagulation protocols [
11], and is compatible with major dialysis platforms (e.g., Prismaflex/Prismax).
Adsorptive capacity is greatest in the first hours and progressively saturates; to maintain clinical efficacy, many centres replace the set every 12–24 hours. During use, a gradual increase in transmembrane pressure (TMP) may occur. The average functional lifespan is similar, sometimes slightly shorter, than that of conventional filters. As with all adsorptive systems, the action is not completely selective: in addition to endotoxins and cytokines, beneficial molecules (such as certain drugs or nutrients) may be removed, requiring close clinical monitoring, electrolyte assessment — particularly phosphate — and adjustment of therapeutic dosages if necessary.
In septic settings, several studies have demonstrated superiority over standard filters in reducing IL-6, TNF-α, IL-8, serum lactate levels, and vasopressor requirements [
12,
13,
14].
The aim of the present study was therefore to evaluate the efficacy and safety of continuous hemofiltration with the oXiris filter in patients with severe acute pancreatitis complicated by organ dysfunction and septic status, analysing its impact on inflammatory and prognostic parameters.
2. Materials and Methods
2.1. Study Design and Clinical Setting
A retrospective, single-centre observational study was conducted at the Unit of General Surgery and Pancreatic Diseases, Policlinico Umberto I, Rome, a tertiary university hospital. The study included patients treated between January 2000 and July 2022.
All patients provided written informed consent for the use of their clinical data for research purposes. Clinical procedures and data collection were conducted in accordance with the ethical principles of the Declaration of Helsinki (2013) and current regulations for retrospective research.
2.2. Patient Selection Criteria
A total of 48 consecutive adult patients (age ≥ 18 years) with a diagnosis of severe acute pancreatitis (SAP), defined according to the 2012 revised Atlanta Classification as the presence of persistent organ failure for more than 48 hours and an APACHE II score ≥ 19, were included. Inclusion required the presence of systemic inflammatory response syndrome (SIRS) and multiorgan dysfunction unresponsive to conventional intensive treatment, with an indication for urgent surgical intervention due to infected pancreatic necrosis or abdominal compartment syndrome.
In the study cohort, the predominant etiology of severe acute pancreatitis was biliary lithiasis, observed in 34 of 48 patients (70.8%). Alcohol-induced pancreatitis was identified in 8 patients (16.7%), whereas hypertriglyceridemia accounted for 3 cases (6.3%). In an additional 3 patients (6.3%), pancreatitis was attributed to asparaginase-related toxicity during treatment for acute lymphoblastic leukemia (ALL).
Exclusion criteria were: pregnancy, age < 18 years, chronic renal failure on replacement therapy, end-stage liver disease, known immunodeficiency, or refusal of consent.
2.3. Preoperative Assessment and Surgical Procedure
Upon hospital admission, all patients underwent detailed medical history taking, physical examination, and complete laboratory evaluation, including complete blood count, pancreatic enzymes (amylase, lipase), inflammatory markers (CRP, procalcitonin), arterial blood gas analysis, serum creatinine, and electrolytes. Clinical severity was quantified using APACHE II and SOFA scores at admission and reassessed daily.
All patients underwent urgent exploratory laparotomy with opening of retroperitoneal spaces and debridement of pancreatic necrosis, thorough peritoneal lavage, and placement of multiple intra-peritoneal drains.
2.4. Hemofiltration with oXiris Filter
CVVH using a Prismax machine with oXiris filter began within 12 hours post-surgery. Main settings included:
Filter: oXiris (AN69 membrane modified with polyethyleneimine coating and heparinised), with adsorption capacity for endotoxins and cytokines.
Mode: continuous convective hemofiltration (pre-dilution).
Blood flow rate: ≥ 75 mL/min (standard 180 mL/min).
Ultrafiltration dose: 35 mL/kg/h, delivered with balanced replacement solution (PrismaSol) in pre-dilution.
Anticoagulation: low-molecular-weight heparin continuous infusion (5–10 U/kg/h) or citrate.
Filter replacement: every 24 hours or earlier in the event of circuit clotting.
Treatment duration: minimum 72 hours, extended until haemodynamic stabilisation and reduction of inflammatory markers (mean 5 days, range 3–7 days).
Cytokines adsorbed (TNF-α, IL-6) were measured in serum and ultrafiltrate at baseline and every 24 hours thereafter.
2.5. Outcome Measures and Data Collection
Clinical and laboratory parameters were recorded at predetermined intervals (T0 = pre-CVVH; T24, T48, T72, T96 hours). Recorded variables included:
Demographic and clinical data: age, sex, aetiology of pancreatitis, comorbidities.
Inflammatory markers: leukocyte count, serum C-reactive protein (CRP), procalcitonin (PCT), TNF-α and IL-6 measured in serum, peritoneal lavage fluid, and CVVH ultrafiltrate.
Haemodynamic parameters: heart rate, mean arterial pressure (MAP), lactate levels, pH, and base excess.
Organ function indices: serum creatinine, PaO₂/FiO₂ ratio, intra-abdominal pressure.
Intraoperative microbiological cultures from intra-abdominal collections.
Prognostic scores: APACHE II and SOFA, calculated daily.
Adverse events: hypotension, filter clotting, electrolyte disturbances.
The primary outcome was the change in TNF-α and IL-6 levels between T0 and T96 hours. Secondary outcomes included changes in APACHE II and SOFA scores, haemodynamic parameters, and incidence of adverse events. Twenty-eight-day survival was recorded as a descriptive endpoint.
2.6. Statistical Analysis
Data were analysed using SPSS Statistics v.27 (IBM, Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range) depending on distribution, assessed with the Shapiro–Wilk test. Categorical variables were reported as number and percentage. Comparisons between pre- and post-treatment values were performed using the paired Student’s t-test or Wilcoxon signed-rank test for non-parametric data. A p-value < 0.05 was considered statistically significant.
2.7. Ethical Considerations
The study did not require formal approval from the Ethics Committee as it was a retrospective observational analysis. All patients provided informed consent for the use of clinical data. Data were collected and processed in anonymised and aggregated form to ensure confidentiality and privacy protection.
3. Results
3.1. Population and Clinical Characteristics
Between January 2000 and July 2022, of 135 patients hospitalised for acute pancreatitis, 48 (35.6%) presented with severe acute pancreatitis (SAP) with an APACHE II score ≥ 19 and persistent multiorgan failure unresponsive to conventional intensive care. The cohort included 30 women (62.5%) and 18 men (37.5%), with a mean age of 60.4 ± 18 years.
At the time of surgical assessment, 39 patients (81.3%) had abdominal compartment syndrome and 9 (18.8%) presented with septic shock refractory to intensive care. All underwent urgent laparotomy with thorough peritoneal lavage, pancreatic necrosectomy, and placement of multiple abdominal drains, followed by transfer to the intensive care unit and initiation of CVVH with the oXiris (AN69-based) filter (surface area 1.2 m²) within 12 hours postoperatively. Treatment parameters included a blood flow rate of 75 mL/min, an ultrafiltration dose of 35 mL/kg/h (pre-dilution, PrismaSol), anticoagulation with low-molecular-weight heparin, and filter replacement every 24 hours. Mean CVVH duration was 6 days (range 3–8) (
Table 1.)
3.2. Primary and Secondary Outcomes
Tolerability and survival
All patients tolerated the treatment well. Overall in-hospital survival was 97.9% (47/48), with a single death (2.1%) due to septic shock from Acinetobacter baumannii. The only surgical complication reported was an enteric fistula, managed conservatively. No major bleeding or ischaemic complications occurred.
Hospital stay
Mean total hospital stay was 28.5 ± 19 days, with a mean intensive care unit stay of 13.3 ± 11 days. Intraoperative cultures yielded Enterococcus spp. in 30 patients (62.5%), Escherichia coli in 10 (20.8%), Pseudomonas aeruginosa in 5 (10.4%), and Acinetobacter baumannii in 1 (2.1%); cultures were sterile in 2 patients (4.2%).
Changes in inflammatory biomarkers and clinical scores
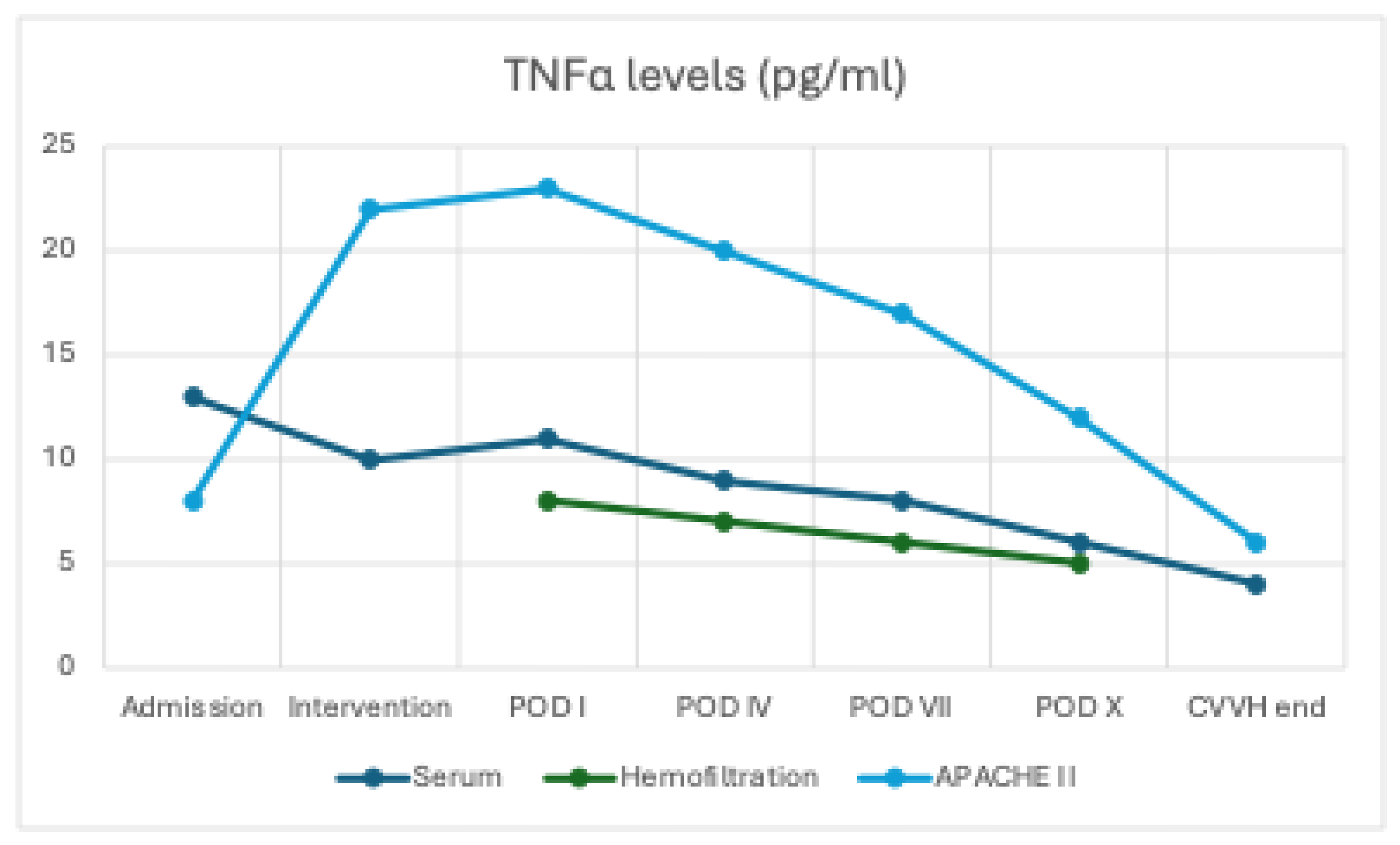
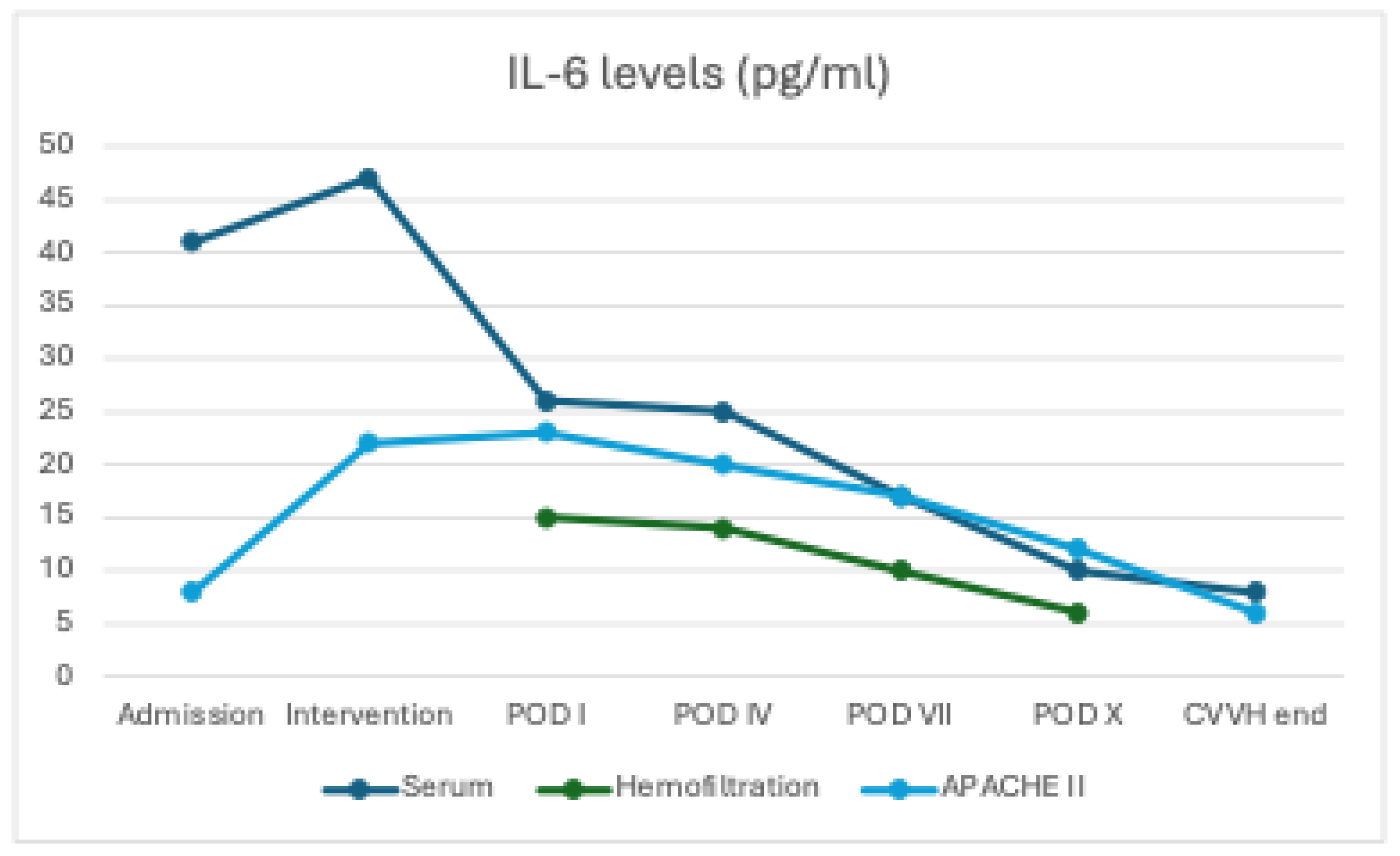
At baseline (T0), serum concentrations of IL-6 (40.8 ± 8 pg/mL) and TNF-α (13.5 ± 2.1 pg/mL) were elevated in all patients. During CVVH treatment with the oXiris membrane, both cytokines were also detected in the ultrafiltrate, initially at high levels (IL-6: 15.3 ± 5 pg/mL; TNF-α: 8.0 ± 2.0 pg/mL at intervention) and subsequently showing a progressive decline over time (IL-6: 7.3 ± 2 pg/mL and TNF-α: 4.6 ± 1.4 pg/mL at POD X), indicating effective removal through convection and adsorption.
In parallel, serum concentrations of both IL-6 and TNF-α exhibited a clear downward trend (IL-6 decreased from 40.8 ± 8 to 10 ± 3 pg/mL, p = 0.08; TNF-α from 13.5 ± 2.1 to 5.5 ± 1.5 pg/mL, p = 0.310), although without reaching statistical significance, likely due to the ongoing endogenous production of pro-inflammatory mediators (
Figure 1 and
Figure 2)
Filter performance
The consistently high cytokine concentration in the hemofiltration effluent confirmed the AN69 filter’s efficacy in adsorbing pro-inflammatory mediators. Paired data analysis demonstrated a significant correlation between cytokine reduction and clinical improvement (decrease in APACHE II score).
Prognostic scores
The APACHE II score was 23 on the first postoperative day. It decreased to 18.6 by day 4 (−19.1%) and reached 8 by the second week (−65.2%; p = 0.013). The reduction in APACHE II scores was significantly correlated with decreases in serum IL-6 and TNF-α levels (non-parametric correlation, p < 0.05).
Adverse events
The only adverse event directly attributable to CVVH was a febrile episode due to central venous catheter infection, resolved after catheter removal. Two patients (4.2%) developed hypophosphataemia, corrected with targeted supplementation (
Table 2).
4. Discussion
In agreement with prior studies on CVVH in SAP [
2,
16,
17,
18,
23,
25,
26,
28,
29] and with the evidence supporting the adsorptive oXiris membrane in sepsis [
7,
8,
9,
10,
11,
12,
15], our study demonstrates that early use of continuous veno-venous hemofiltration (CVVH) with the oXiris filter in patients with severe acute pancreatitis (SAP) complicated by organ dysfunction and sepsis refractory to intensive care is associated with a high short-term survival rate (97.9%), a significant reduction in APACHE II score, and a marked decrease in serum pro-inflammatory cytokines (IL-6, TNF-α). The progressive increase in cytokine concentration in the dialysis effluent, coupled with their reduction in the blood circuit and systemically, suggests effective extracorporeal removal of inflammatory mediators, supporting the hypothesis of a genuine immunomodulatory effect of the treatment.
These results are consistent with international literature.
Several studies have addressed the use of hemofiltration with oXiris in severe sepsis, a pathophysiological setting closely related to severe SAP. These studies have shown that the oXiris filter removes endotoxins and cytokines more effectively than standard filters, with haemodynamic benefits and lactate reduction [
7,
8,
9,
14,
15]. Observational studies [
12] confirm improvements in SOFA score, haemodynamic stability, and reduced vasopressor requirements within the first 24 hours. However, evidence remains heterogeneous across studies and systematic reviews, and not all analyses demonstrate a survival advantage [
16,
18,
25].
Evidence specific to SAP is more limited but promising. The meta-analysis by Guo et al. [
17], involving over 1,200 patients, demonstrated that CVVH reduces mortality, APACHE II score, and inflammatory markers compared to standard treatment, particularly when initiated within a few days of onset. Huang et al. [
18] confirmed that high-volume hemofiltration reduces early mortality and infections, although it does not significantly affect the incidence of MODS or overall hospital stay. Additional evidence comes from studies in hyperlipidaemic or septic pancreatitis, where the combination of high-volume hemofiltration and haemoperfusion significantly reduced APACHE II and SOFA scores and improved haemodynamic stability [
19,
20].
Cui et al. showed, in patients with SAP complicated by ARDS, not only a significant reduction in IL-6 and TNF-α after just 24 hours of treatment, but also improvement in ARDS severity and blood oxygenation [
21]. Some studies also suggest a potential advantage of early CVVH in reducing the incidence of pancreatic pseudocysts [
22].
Clinical data also indicate that timing is crucial: Jiang et al. [
23] demonstrated that initiation within 48 hours, particularly with high filtration volumes, improves survival (94% vs. 68%) and more effectively reduces TNF-α, IL-1β, and IL-6 compared to delayed or standard-volume CVVH. Xu et al. [
5] found that early CVVH in patients with SAP and abdominal compartment syndrome rapidly reduces intra-abdominal pressure, improves organ function, and decreases mortality.
Although literature specific to SAP remains limited, available evidence is encouraging, showing substantial reductions in inflammatory biomarkers, improvement in haemodynamic status, and better metabolic parameters within 24 hours of initiating CRRT [
17,
24,
25,
26,
29]. Regarding the specific use of oXiris in SAP, only one recent case report [
27] exists, describing complete clinical recovery following combined CVVH with the oXiris filter in a paediatric patient with drug-induced severe acute pancreatitis and multiorgan failure. Our results align with these findings, documenting the filter’s efficacy in adsorbing inflammatory cytokines and contributing to early clinical stabilisation.
4.1. Pathophysiological Mechanisms Involved
The beneficial effect of hemofiltration in SAP can be attributed to a dual mechanism: convective removal of intermediate-molecular-weight molecules (such as IL-6, IL-1β, TNF-α) and, in the case of adsorptive filters like oXiris, selective elimination of endotoxins and cytokines via surface interactions. This strategy helps attenuate the hyperactive systemic immune response (SIRS) and prevents progression to multiorgan dysfunction syndrome (MODS). Our study reinforces these hypotheses, demonstrating a direct correlation between cytokine reduction and improvement in APACHE II scores.
Overall, available data suggest that the efficacy of hemofiltration in SAP depends on key variables: timing of initiation, filtration volume, treatment duration, and type of filter used. In our patients, the choice to initiate CVVH early, employ a high-adsorptive-capacity filter, and maintain an adequate convective dose may have contributed to the positive outcomes observed.
5. Conclusions
Early initiation of continuous veno-venous hemofiltration (CVVH) with adsorptive AN69-based oXiris filter in patients with severe acute pancreatitis (SAP) and organ dysfunction was associated, in our experience, with significant clinical benefits. We observed a marked reduction in pro-inflammatory cytokines (IL-6 and TNF-α) both in serum and in the hemofiltration effluent, a significant improvement in APACHE II score, and a very high short-term survival rate (97.9%).
These findings support the hypothesis of a tangible immunomodulatory effect of CVVH with the oXiris filter, potentially capable of attenuating the “cytokine storm” characteristic of the most severe forms of SAP. Furthermore, the treatment demonstrated a favourable safety profile, with rare and manageable complications.
Consistent with prior reports [
2,
16,
17,
18,
23,
25,
26,
28], our findings support the effectiveness of CVVH as an adjunct when instituted early in SAP. However, the optimal timing and duration remain to be defined, and confirmation in prospective, randomised, multicentre trials is needed to determine the effects on mortality, organ-failure resolution, and health-related quality of life.
Author Contributions
Conceptualization, P.S. and P.C.; methodology, P.S.; validation, R.C., A.M.A, A.M. and P.C.; formal analysis, P.S. ; investigation, P.S., R.C. and G.L.; resources, G.L.; data curation, P.S. and V.R.; writing—original draft preparation, P.S.; writing—review and editing, P.S. and P.C.; visualization, A.M.A.; supervision, A.M. and P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not require formal approval from the Ethics Committee as it was a retrospective observational analysis.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SAP |
Severe acute pancreatitis |
| MODS |
Multiple Organ Dysfunction Syndrome |
| SIRS |
Systemic Inflammatory Response Syndrome |
| CVVH |
Continuous Veno-Venous Hemofiltration |
| HVHF |
High-Volume Hemofiltration |
| CRRT |
Continuous Renal Replacement Therapy |
| AN69 |
Acrylonitrile–metallyl sulfonate sodium copolymer 69 |
| PEI |
Polyethyleneimine |
| TMP |
TransMembrane Pressure |
| APACHE II |
Acute Physiology And Chronic Health Evaluation II |
| SOFA |
Sequential Organ Failure Assessment |
| ICU |
Intensive Care Unit |
| POD |
Post-Operative Day |
| CVC |
Central Venous Catheter |
| COPD |
Chronic Obstructive Pulmonary Disease |
| CKD |
Chronic Kidney Disease |
| DM |
Diabetes Mellitus |
| ALL |
Acute Lymphoblastic Leukemia |
| CRP |
C-Reactive Protein |
| PCT |
Procalcitonin |
| MAP |
Mean Arterial Pressure |
| ARDS |
Acute Respiratory Distress Syndrome |
References
- Leppäniemi A, Tolonen M, Tarasconi A, et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27. doi:10.1186/s13017-019-0247-0.
- Pupelis G, Plaudis H, Grigane A, Zeiza K, Purmalis G. Continuous veno-venous haemofiltration in the treatment of severe acute pancreatitis: 6-year experience. HPB (Oxford). 2007;9(4):295-301. doi:10.1080/13651820701329225.
- Hu Y, Xiong W, Li C, Cui Y. Continuous blood purification for severe acute pancreatitis: A systematic review and meta-analysis. Medicine (Baltimore). 2019;98(12):e14873. doi:10.1097/MD.0000000000014873.
- Zerem E. Treatment of severe acute pancreatitis and its complications. World J Gastroenterol. 2014;20(38):13879-13892. doi:10.3748/wjg.v20.i38.13879.
- Xu J, Yang H, Tian X. Effects of early hemofiltration on organ function and intra-abdominal pressure in severe acute pancreatitis patients with abdominal compartment syndrome. Clin Nephrol. 2019;91(4):237-243. doi:10.5414/CN109435.
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139(3):813-820. doi:10.1053/j.gastro.2010.06.010.
- Li Y, Sun P, Chang K, et al. Effect of continuous renal replacement therapy with the oXiris hemofilter on critically ill patients: A narrative review. J Clin Med. 2022;11(22):6719. doi:10.3390/jcm11226719.
- Wang G, He Y, Guo Q, Zhao Y, He J, Chen Y, et al. Continuous renal replacement therapy with the adsorptive oXiris filter may be associated with the lower 28-day mortality in sepsis: A systematic review and meta-analysis. Crit Care. 2023;27:54. doi:10.1186/s13054-023-04555-x.
- Zhou Y, Wu C, Ouyang L, Peng Y, Zhong D, Xiang X, Li J. Application of oXiris-continuous hemofiltration adsorption in patients with sepsis and septic shock: A single-centre experience in China. Front Public Health. 2022;10:1012998. doi:10.3389/fpubh.2022.1012998.
- Tang J, Cao T. CRRT with the oXiris filter attenuates IL-6 in a patient with severe COVID-19. J Am Soc Nephrol. 2021;32:10S1103C. doi:10.1681/ASN.20213210S1103C.
- Wong E, Ong V, Remani D, Wong W, Haroon S, Lau T, et al. Filter life and safety of heparin-grafted membrane for continuous renal replacement therapy: A randomized controlled trial. Semin Dial. 2021;34(4):300-308. doi:10.1111/sdi.12951.
- Broman ME, Hansson F, Vincent JL, Bodelsson M. Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: A randomized crossover double-blind study. PLoS One. 2019;14(8):e0220444. doi:10.1371/journal.pone.0220444.
- Zhou Y, Liu MJ, Lin X, Jiang JH, Zhuo HC. Comparative efficacy of two hemopurification filters for treating intra-abdominal sepsis: A retrospective study. Chin J Traumatol. 2025;28(2):82-88. doi:10.1016/j.cjtee.2024.12.003.
- Wei T, Chen Z, Li P, et al. Early use of endotoxin absorption by oXiris in abdominal septic shock: A case report. Medicine (Baltimore). 2020;99(28):e19632. doi:10.1097/MD.0000000000019632.
- Wang J, Wei SR, Ding T, et al. Continuous renal replacement therapy with oXiris® in patients with hematologically malignant septic shock: A retrospective study. World J Clin Cases. 2023;11(26):6073-6082. doi:10.12998/wjcc.v11.i26.6073.
- Chen X, Sun M, Mao X, Liu X, Sun W. Effectiveness of continuous veno-venous hemofiltration in the treatment of severe acute pancreatitis. Exp Ther Med. 2019;17(4):2720-2724. doi:10.3892/etm.2019.7192.
- Guo Y, Cao F, Li C, et al. Continuous hemofiltration reduces mortality in severe acute pancreatitis: A meta-analysis. Emerg Med Int. 2020;2020:6474308. doi:10.1155/2020/6474308.
- Huang H, Zhou Q, Chen M. High-volume hemofiltration reduces short-term mortality with no influence on the incidence of MODS, hospital stay, and hospitalization cost in patients with severe acute pancreatitis: A meta-analysis. Artif Organs. 2021;45(12):1411-1424. doi:10.1111/aor.14016.
- Wang Y, Dai GF, Xiao WB, et al. Effects of continuous venous-venous hemofiltration with or without hemoperfusion on patients with hypertriglyceride acute pancreatitis. Clin Res Hepatol Gastroenterol. 2025;49(5):102572. doi:10.1016/j.clinre.2025.102572.
- Sun S, He L, Bai M, et al. High-volume hemofiltration plus hemoperfusion for hyperlipidemic severe acute pancreatitis: A controlled pilot study. Ann Saudi Med. 2015;35(5):352-358. doi:10.5144/0256-4947.2015.352.
- Cui HX, Xu JY, Li MQ. Efficacy of continuous renal replacement therapy in the treatment of severe acute pancreatitis associated acute respiratory distress syndrome. Eur Rev Med Pharmacol Sci. 2014;18(17):2523-2526.
- Yadav SC, Zhang B. Effect of early continuous veno-venous haemofiltration in severe acute pancreatitis for the prevention of local pancreatic complications. Gastroenterol Res Pract. 2022;2022:7575231. doi:10.1155/2022/7575231.
- Jiang HL, Xue WJ, Li DQ, et al. Influence of continuous veno-venous hemofiltration on the course of acute pancreatitis. World J Gastroenterol. 2005;11(31):4815-4821. doi:10.3748/wjg.v11.i31.4815.
- Mielnicki W, Dyla A, Zając M, et al. Does continuous renal replacement therapy with oXiris in septic shock have any positive impact? Single-centre experience with oXiris therapy in septic shock patients. J Clin Med. 2024;13(24):7527. doi:10.3390/jcm13247527.
- Lin Y, He S, Gong J, et al. Continuous veno-venous hemofiltration for severe acute pancreatitis. Cochrane Database Syst Rev. 2019;10(10):CD012959. doi:10.1002/14651858.CD012959.pub2.
- Xie Y, Yuan Y, Su W, et al. Effect of continuous hemofiltration on severe acute pancreatitis with different intra-abdominal pressure: A cohort study. Medicine (Baltimore). 2021;100(44):e27641. doi:10.1097/MD.0000000000027641.
- Wang Y, Gao Y, Zhao L, Kang K. Continuous renal replacement therapy in combination with oXiris haemofilter in a paediatric patient with sodium valproate-induced acute pancreatitis. BMJ Case Rep. 2025;18:e258126. doi:10.1136/bcr-2023-258126.
- Caronna R, Benedetti M, Morelli A, et al. Clinical effects of laparotomy with perioperative continuous peritoneal lavage and postoperative hemofiltration in patients with severe acute pancreatitis. World J Emerg Surg. 2009;4:45. Published 2009 Dec 16. doi:10.1186/1749-7922-4-45.
- Guo J, Huang W, Yang XN, et al. Short-term continuous high-volume hemofiltration on clinical outcomes of severe acute pancreatitis. Pancreas. 2014;43(2):250-254. doi:10.1097/01.mpa.0000437321.06857.fc.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).