1. Introduction
In the Peruvian high-Andean zone, at altitudes above 3,000 m, unique vegetation types are found, such as Polylepis (Rosaceae) forests, the highest on the planet, as well as pajonal grasslands, high-Andean shrublands, Puya (Bromeliaceae) clusters, bofedales, cushion plant communities, and yaretales, as well as vegetation in cryoturbated soils [
1,
2]. These vegetation formations offer numerous potential substrates for the development of myxomycetes, which have remained poorly studied for a long time. The first published studies mentioning the presence of myxomycetes in these habitats were those of Wrigley de Basanta et al. [
3,
4,
5], who identified three new species to science (
Didymium xerophilum, D. azorellae and Licea aurea) and highlighted the remarkable diversity of myxomycetes in these extreme environments.
Subsequently, Treviño-Zevallos & Lado [
6] conducted the first inventory of myxomycetes in the humid montane forests of Peru, contributing to the understanding of the diversity of these organisms in this little-explored region. The research, conducted at the Wayqecha Biological Station, adopted a systematic approach to characterize species composition and abundance, analyze occurrence by substrate type, and compared diversity in the dry and wet seasons. The study documented 81 taxa belonging to six orders, 10 families, and 22 genera, highlighting the richness and complexity of the myxomycete community in the area.
Treviño-Zevallos et al. [
2] recorded three additional species from the Peruvian Andes:
Arcyodes incarnata (Alb. & Schwein.) O.F. Cook, cited for the first time for the Southern Hemisphere, at an altitude of 3505 m;
Metatrichia floripara (Rammeloo) Rammeloo, at 4020 m, representing the third record in the world, previously cited only for Rwanda, at 2400 m, and for Brazil, at 841 m [
7]; and
Trichia mirabilis Nann.-Bremek., at 4874 m, representing one of the highest altitudes ever recorded for the occurrence of Myxomycetes. Furthermore, the authors comment that the sporocarps of
T. mirabilis were collected already developed in the field, while previous reports included only specimens derived from humid chamber culture.
In a study of the myxobiota of the high elevations of the Tropical Andes of Peru by Lado et al. [
8], specimens were collected from 11 different locations between 3,400 and 4,150 m above sea level, including a new species,
Diachea mitchellii (Lado & Treviño), associated with
Polylepis (Rosaceae) forests, on the bark of this species.
The most recent and comprehensive study of myxomycetes from the Peruvian Andes was conducted by Treviño-Zevallos et al. [
9] with a survey based on 3,352 collections resulting from sampling during six field expeditions of the Myxotropic Project, using humid chamber culture and the review of specimens deposited in the HSP herbarium. In total, the authors reported 178 species, representing 31 genera, with 10 species considered new records for South America or the Neotropics, and 53 additional records for Peru. The authors suggest that the Andes Mountains may act as a species reservoir or center of diversification, emphasizing the importance of cataloging Myxomycete biodiversity in these extreme, high-altitude environments.
Therefore, the present study, in addition to expanding knowledge about the species of Myxomycetes occurring in Peru, provides information on the distribution patterns of the species across different altitudinal ranges and vegetation types, obtained in an area of the Cuzco department, in the Peruvian Andes. Furthermore, three species are cited for the first time in Peru.
2. Materials and Methods
2.1. Study Area
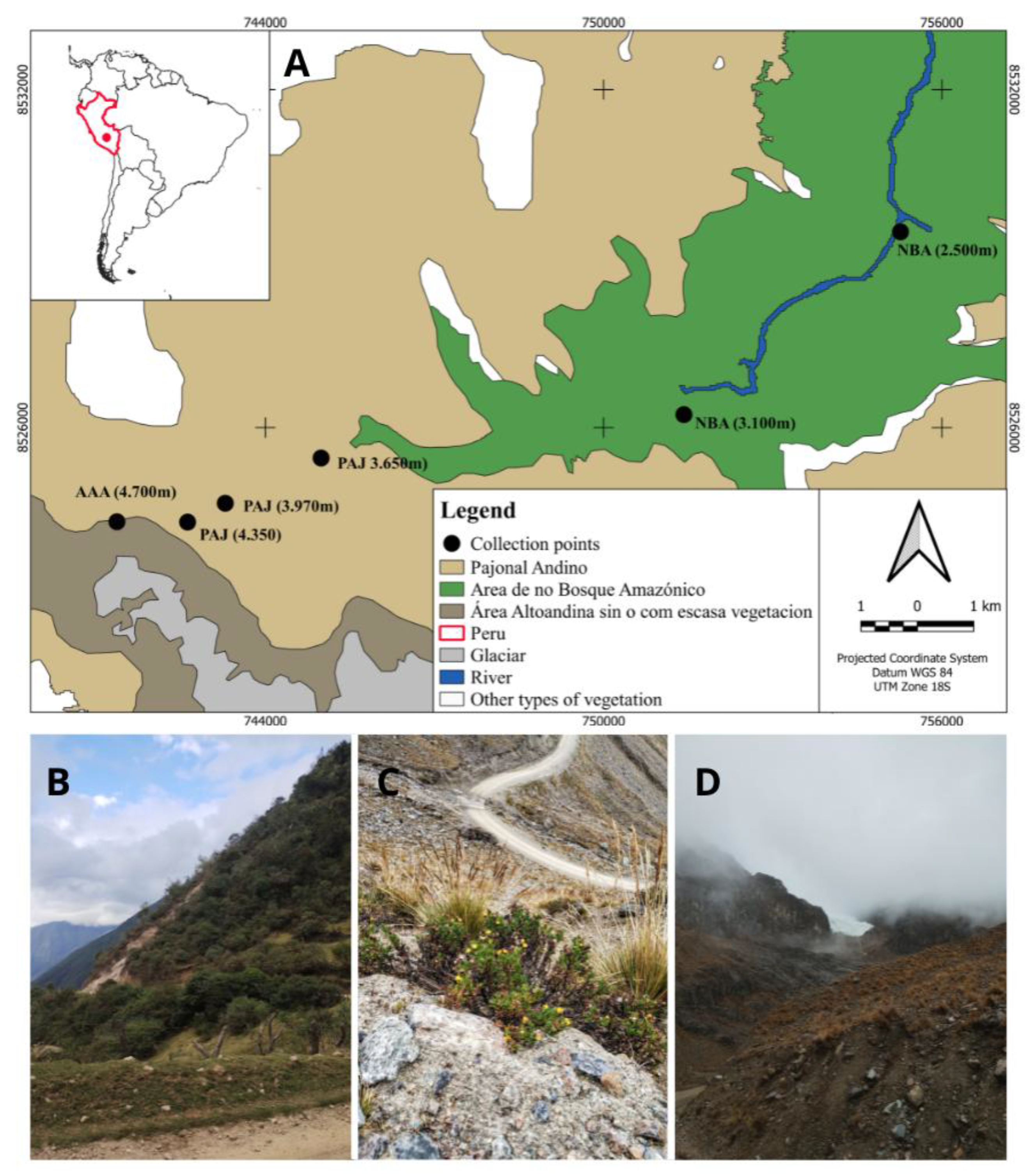
The work was carried out in the Cuzco department, in the southern region of Peru, during late July and early August 2024. Six points were explored, distributed across three distinct vegetation formations (MINAM 2015): NAF = Non-Amazonian Forest (Point 1, 2500m, -13.291007, -72.643868; Point 2, 3100m, -13.320648, -72.679027), PAJ = Pajonal ecosystem (Point 3, 3650m, -13.328151, -72.738344; Point 4, 3970m, -13.338679, -72.771676; Point 5, 4350m, -13.338633,-72.760102), HAZ = High Andean zones with and without plant cover (Point 6, 4700m, -13.335552,-72.753960) (
Figure 1).
2.2. Collection Methodology
During collections, substrates present at the sites, within a 50-meter radius, were examined for myxomycete sporophores and plasmodia. The collected material was glued into small cardboard boxes. Substrate samples were also collected for the preparation of humid chambers.
Humid chamber cultures were prepared as described by Wrigley de Basanta & Estrada-Torres [
10]. The pH of each culture was determined after 24 hours, and the cultures were maintained at room temperature (21–25°C) in diffuse daylight and examined at regular intervals for up to four months. All sporophores of the same species that developed in the same culture represented a single record. Specimens obtained in the field or sporulated in a humid chamber were identified using Martin & Alexopoulos [
11], Farr [
12], Poulain et al. [
13] and Treviño-Zevallos [
2], and, whenever necessary, the binomials were updated according to the instructions in Lado [
14]. Exsiccates were incorporated into the collection of the Bruno Edgar Irgang Herbarium (HBEI), at the Universidade Federal do Pampa, São Gabriel municpality campus, in southern Brazil.
2.3. Data Analysis
Several stages of data processing, visualization, and statistical analysis were performed, primarily using the tidyverse packages (including dplyr, ggplot2, and tidyr), as well as specific packages for multivariate analysis such as FactoMineR and factoextra, using Rstudio software version 4.4.2 [
15,
16,
17,
18,
19].
To explore the species composition in each environment, a clustered bar graph was generated, where species were ordered according to the sum of their occurrences, using the functions of the dplyr package [
15] for data manipulation and ggplot2 [
16] for visualization. The plot also allowed a visual comparison of the abundance of each species between environments.
To ensure proper ordering of the points, an ordered factor with specific levels was configured. Data with richness equal to zero were filtered to focus only on substrates where myxomycetes were found. The resulting visualization was a dot plot, where the size of the points represented the substrate richness, and the color indicated the substrate type, facilitating the interpretation of spatial variation in substrate richness.
To explore the relationships between environmental variables (such as altitude and vegetation types) and species distribution, a Principal Component Analysis (PCA) was conducted. Two analyses were performed: one with the covariance matrix and the other with the correlation matrix, the latter being the primary analysis to standardize the variables prior to analysis. The PCA results, including eigenvalues and eigenvectors, were visualized using functions from the factoextra package [
19] allowing the interpretation of the variables' contributions and the representation of individuals (species) in the principal component space. The points were colored according to substrate categories, facilitating the analysis of spatial and environmental associations.
To compare species richness and abundance across collection points, a combined visualization was constructed using ggplot2 [
16]. The richness and abundance curves were plotted, normalizing abundance for compatibility across the graph scale, and using secondary axes to facilitate visual comparison.
3. Results
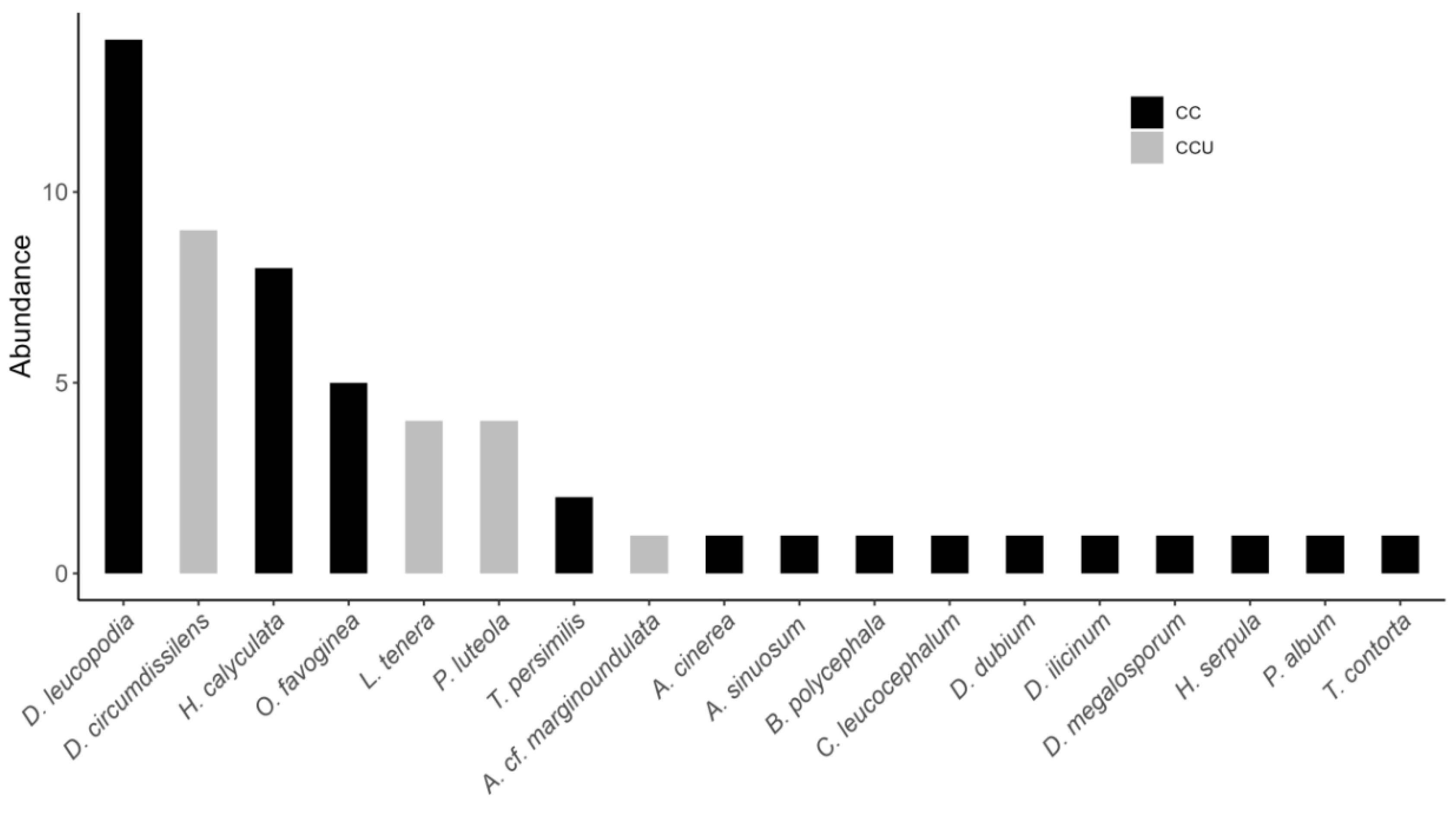
Fifty-six specimens were obtained, 38 from field collections (CC) and 18 from humid chamber cultures (CCU), distributed among 18 species and 11 genera (
Figure 3). Of these,
Diderma circumdissiliens Flatau & Schirmer,
Licea tenera E. Jahn, and
Perichaena luteola (Kowalski) Gilert are recorded for the first time in Peru. With the exception of a single representative of the order Liceales (
Licea tenera), only the orders Physarales, with nine species (50%), and Trichiales, with eight species (44.4%), were sampled in this study, totaling 30 and 22 specimens, respectively. The vegetation type NBA, with 12 species, showed the greatest richness, while the type AAA had only six species. Among the substrates, DB, with six species, had the greatest richness, while DL had the lowest, with only two species (
Table 1).
Of the 60 humidity chambers set up, 24 tested positive, but 83.3% of them did not produce sporulation. Fifteen species were collected from field collections (FC), and only four sporulated in humidity chambers (CCU): Diderma circumdissiliens, Licea tenera, and Perichaena luteola, which developed on herbivore dung (Bos taurus and Lagidium peruanum), and Arcyria cf. marginondulata, which was collected on mosses.
Diachea leucopodia was the most abundant species, with 14 specimens, followed by Diderma circumdissiliens, with eight specimens, while Angioridium sinuosum, Arcyria cinerea, and Arcyria cf. marginondulata, Badhamia polycephala, Craterium leucocephalum, Didymium dubium, D. illicinum, D. megalosporum, Hemitrichia serpula, Physarum album, and Trichia contorta were represented by only one specimen (
Figure 2).
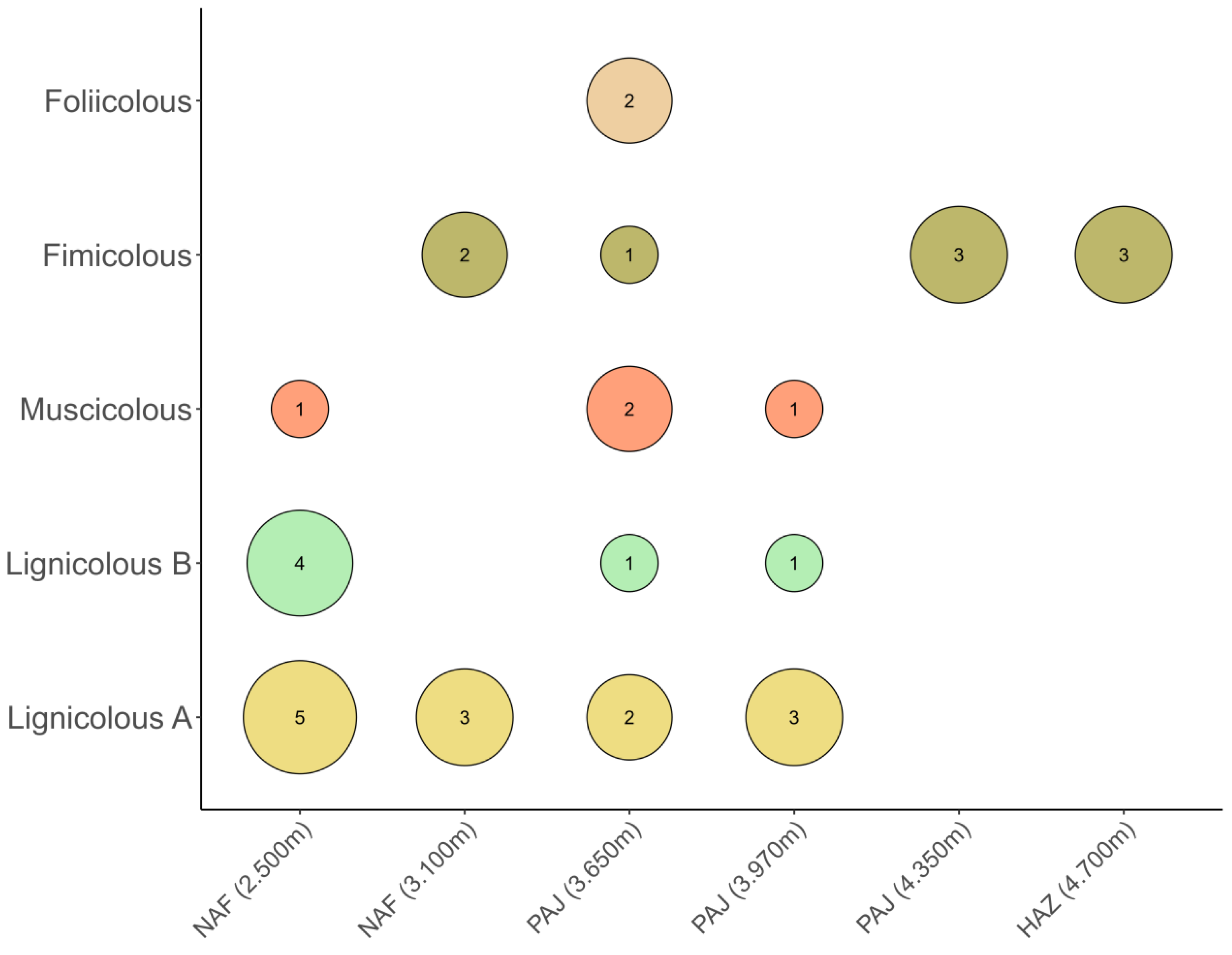
The substrate with the greatest species richness was dead branches (lignicolous A), with six species, followed by twigs (lignicolous B), with five species, bryophytes (muscicolous), herbivore dung (fimicolous), with three species each, and soil leaf litter (foliicolous), with two species. Fimicolous myxomycetes were the only ones present at the highest point of the Pajonal ecosystem (4,350 m) and in the High Andean zones with and without plant cover (4,700 m), where lignified material, an abundant substrate at lower altitudes, is no longer found. The ecological types lignicolous A and lignicolous B are those with the greatest species richness in non-Amazonian Forest, which have lower altitudes (2500 m), with five species and four species, respectively (
Figure 3).
Figure 3.
Myxomycete species richness according to ecological types and altitude. NAF = Non- Amazonian Forest; PAJ = Pajonal ecosystem; HAZ = High Andean zones with and without plant cover.
Figure 3.
Myxomycete species richness according to ecological types and altitude. NAF = Non- Amazonian Forest; PAJ = Pajonal ecosystem; HAZ = High Andean zones with and without plant cover.
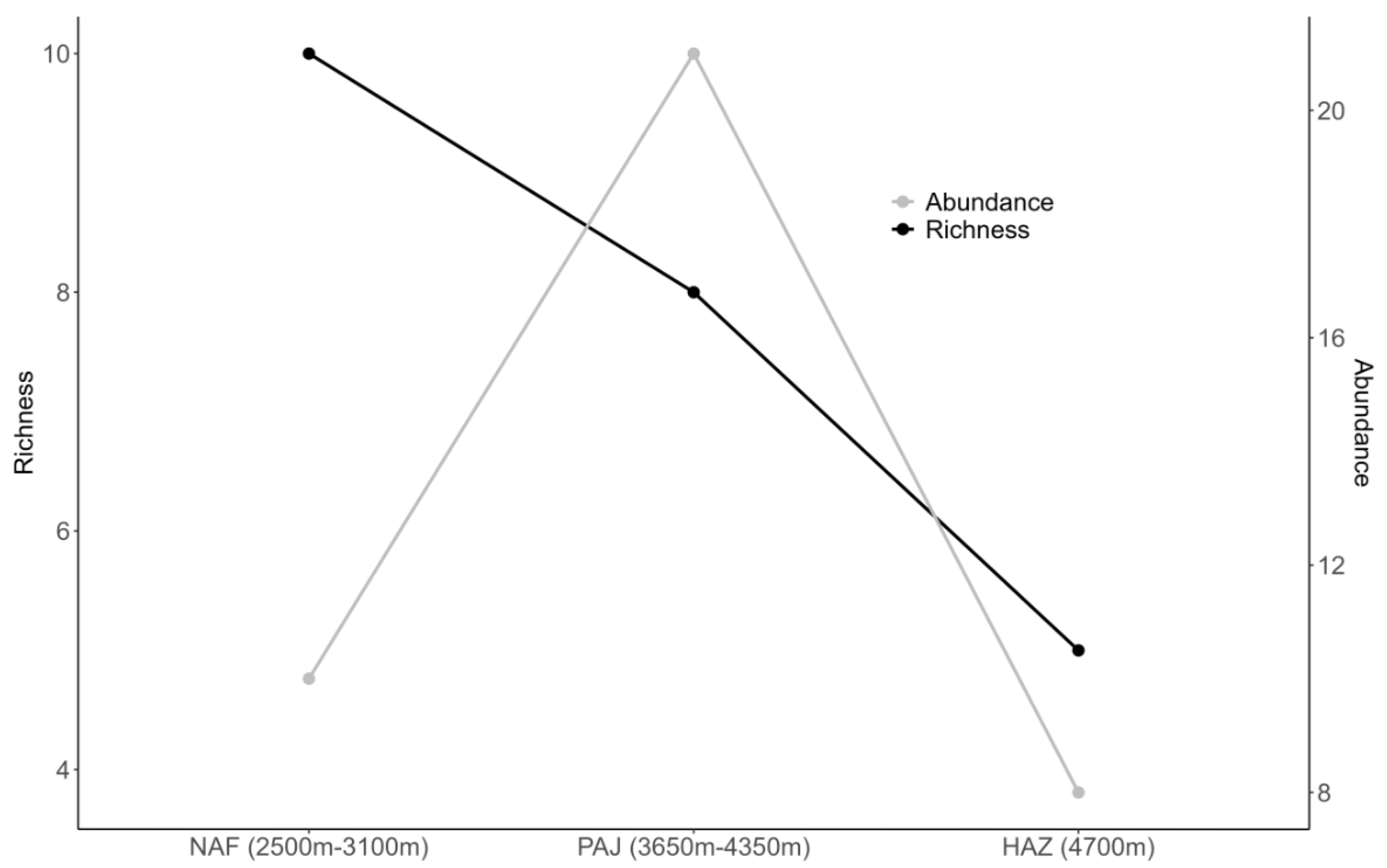
When accounting for recorded species according to vegetation type and altitude, it is observed that richness decreases as altitude increases, and tree and shrub vegetation becomes scarce. The NAF areas (2500m–3100m) have 10 species, while the PAJ areas (3650m–4350m) have eight species, and HAZ (4700m) only five species. Abundance remains relatively similar in NBA and AAA, with 10 and eight specimens, respectively, and 21 specimens in the PAJ areas (
Figure 4).
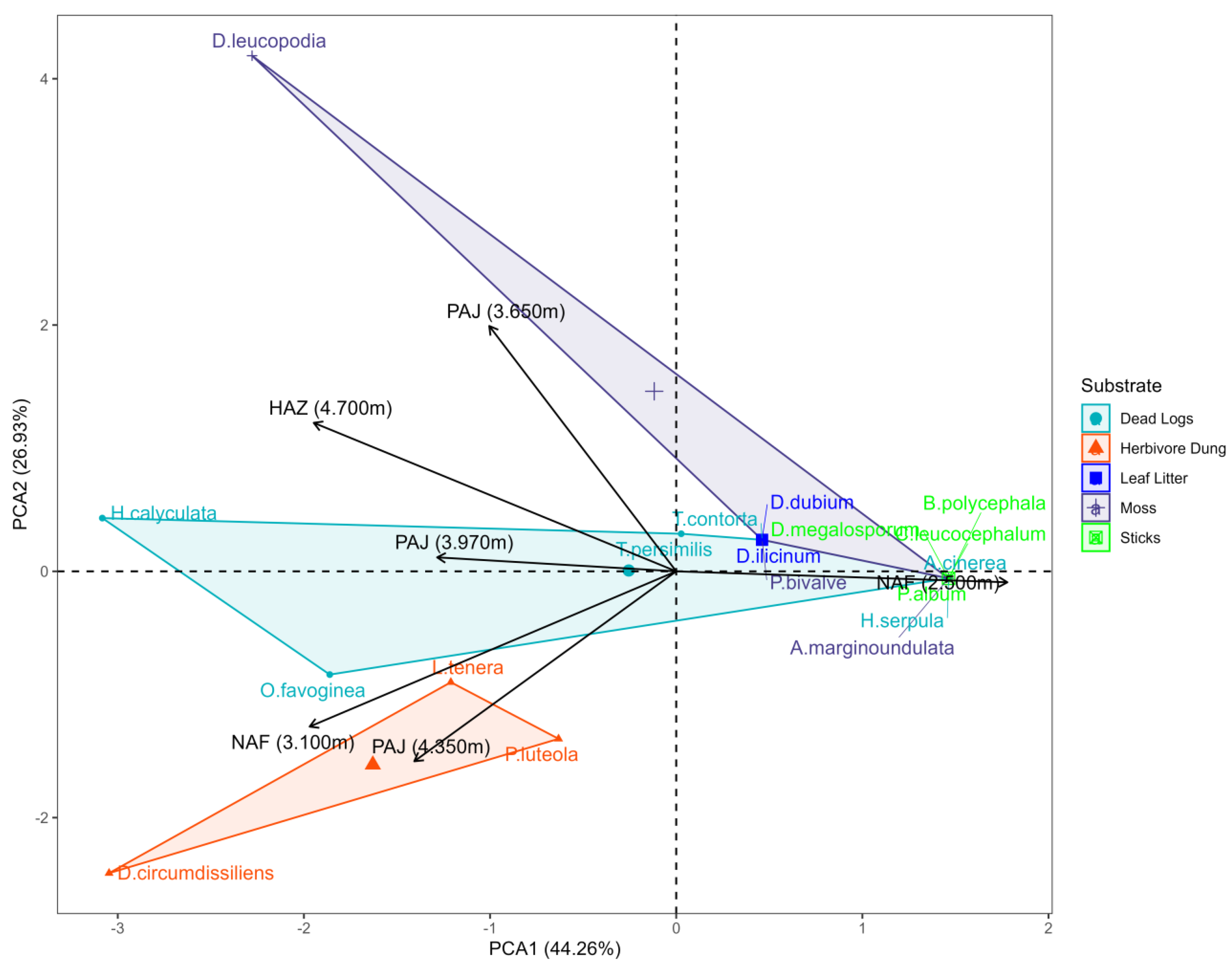
Principal component analysis (PCA) reveals a strong distinction in the myxobiota of NAF (2500 m) compared to the other sites. Although NAF (3100 m) has a similar floristic composition, its myxomycete community is more closely related to that of higher altitude areas. A clear division is observed between the ecological groups of myxomycetes, according to substrates: fimicolous, muscicolas, and lignicolous. The three fimicolous species,
D. circumdissilens,
L. tenera, and
P. luteola, are strongly associated with environments in NAF (3100 m) and PAJ (4350 m).
A. cinerea,
A. marginondulata,
B. polycephala,
C. leucocephalum,
D. melanosporum and
H. serpula are more related to the NAF environment (2500 m), which has forest, but at lower altitudes (
Figure 5).
3.1. New Occurrences for Peru
3.3.1. Diderma circumdissilens Flatau & Schirmer (Figure 6)
Sporophores clustered, rounded, ovate to short plasmodiocarps, with a flat base, 0.4 to 1.4 mm long, 0.2 to 0.35 mm high, off-white, darker at the base; hypothallus inconspicuous; columella flat, like a thickening of the base, orange to reddish-brown; peridium double, the outer calcareous layer strongly adhered to the inner membranous layer; dehiscence circumcised, but without forming a cap, leaving the base with the margin folded inwards; capillitium profuse, with fine, smooth, sinuous filaments, light brown to hyaline, poorly anastomosed; spore in mass dark brown to black; spores globose, reddish brown by transmitted light, warty, with irregularly distributed warts, 7.75-11.1 µm diam.
Figure 6.
New occurrences for Peru. A-B. Diderma circumdissilens. A. Sporangia. B. Spores and capillitium. C-E. Licea tenera. C. Sporangia. D. Spores. E. Peridium. F-H. Perichaena luteola. F. Sporangia. G. Spores and capillitium. H. Spores.
Figure 6.
New occurrences for Peru. A-B. Diderma circumdissilens. A. Sporangia. B. Spores and capillitium. C-E. Licea tenera. C. Sporangia. D. Spores. E. Peridium. F-H. Perichaena luteola. F. Sporangia. G. Spores and capillitium. H. Spores.
Geographic distribution:
Diderma circumdissilens Flatau & Schinner was described based on material collected in the Utwald Sababurg Nature Reserve (Germany), at approximately 300 meters above sea level, sporulating on the bark of
Carpinus betulus L. The type specimens and
Supplementary Materials (LF 3397, PS 2562, PS 2746, PS 2747) were obtained between 2001 and 2002 and are preserved in European collections, including the Herbarium of the Botanical Garden Berlin-Dahlem [
20]. Initially found in Germany, the known distribution of this species was expanded thanks to recent records from France and the Netherlands [
21]. In addition to these, the record presented in this work, the first for South America, significantly expands the known geographic range of the species and highlights a likely more extensive distribution.
Material examined: Peru, Department of Cuzco, La Convención, Area de Conservación Regional Choquequirao, -13,320, -72,680, in cattle dung (Bos taurus L.), 19/X/2024, col. Velloso, J.R.P, det. Velloso, J.R.P, Cavalcanti, L.H., Treviño-Zevallos, I., from wet chamber, HBEI; Choquequirao Regional Conservation Area, -13,343, -72, 773, in viscacha dung (Lagidium peruanum L.), 23/X/2024, col. Velloso, J.R.P, det. Velloso, J.R.P, Cavalcanti, L.H., Treviño-Zevallos, I., from wet chamber, HBEI; Peru, Department of Cuzco, La Convención, Area de Conservación Regional Choquequirao, -13,339, -72, 760, in viscacha dung (Lagidium peruanum L.), 23/X/2024, col. Velloso, J.R.P, det. Velloso, J.R.P, Cavalcanti, L.H., Treviño-Zevallos, I. from humid chamber, HBEI.
3.3.2. Licea tenera E. Jahn (Figure 6)
Sporophores sessile, black, solitary, globose, 0.1-0.8 mm diâm.; hypothallus inconspicuous; peridium membranous, dark yellow under transmitted light, up to 1.44 µm thick, dehiscence irregular; spore mass golden yellow; spores globose, light yellow under transmitted light, minutely warty, 10.2-11.61 µm diam.
Geographic distribution: According to Poulain et al. [
13], this is a cosmopolitan species, widely distributed in different biomes and continents. According to updated data from the GBIF portal, which includes observations and preserved material [
22], L. tenera has been recorded in countries in Africa (Kenya), Asia (India, Pakistan), Europe (France, Russia, Sweden, Turkey, Ukraine), Oceania (Australia), and the Americas (Brazil, Canada, United States, Mexico, Uruguay). This information confirms its presence in both temperate and tropical regions and supports its ecologically generalist nature. However, to date, there have been no documented records of the species in Peru, so its discovery represents a novelty for the national microbiota.
Material examined: Peru, Department of Cuzco, La Convención, Area de Conservación Regional Choquequirao, -13,320, -72,680, in cattle dung (Bos taurus L.), 11/X/2024, col. Velloso, J.R.P, det. Velloso, J.R.P, Cavalcanti, L.H., Treviño-Zevallos, I., from wet chamber, HBEI; Peru, Department of Cuzco, La Convención, Area de Conservación Regional Choquequirao, -13,343, -72, 773, in viscacha dung (Lagidium peruanum L.), 19/X/2024, col. Velloso, J.R.P, det. Velloso, J.R.P, Cavalcanti, L.H., Treviño-Zevallos, I., from wet chamber, HBEI; Peru, Department of Cuzco, La Convención, Area de Conservación Regional Choquequirao, -13,339, -72, 760, in viscacha dung (Lagidium peruanum L.), 05/XI/2024, col. Velloso, J.R.P, det. Velloso, J.R.P, Cavalcanti, L.H., Treviño-Zevallos, I., from wet chamber, HBEI.
3.3.3. Perichaena luteola (Kowalski) Gilert (Figure 6)
Sporophores are sessile, globose, gregarious, orange-yellow in color, 0.2-0.4 mm in diameter; peridium is simple, membranous, minutely warty on the inner surface, with irregular dehiscence; capillitium is yellow, with cylindrical filaments, presenting some constrictions, warty, 1.5-3 μm in diameter; spore is yellow; spores are globose to slightly subglobose, pale yellow under transmitted light, spinulose, appearing warty at 400x magnification, 11.1-12.6 μm in diameter.
Geographic distribution: Traditionally, the species has been considered typical of the European continent, where it has been widely reported in several mycological studies (Poulain et al., 2011). However, its current distribution far exceeds the European range. Specimens have been documented in 16 countries, distributed across several continents: Australia, Argentina, Brazil, Cyprus, Spain, the United States, France, Italy, Lithuania, New Zealand, Madagascar, Mexico, the United Kingdom, Russia, Sweden, and Ukraine [
23]. This distribution is complemented by a new record in Peru, which represents the first confirmed record for the country and confirms its presence in South American countries. This geographic range reflects P. luteola's ability to establish itself in diverse environmental conditions, from temperate forests to tropical and subtropical zones.
Material examined: Peru, Department of Cuzco, La Convención, Area de Conservación Regional Choquequirao, -13,320, -72,680, in cattle dung (Bos taurus L.), 19/X/2024, col. Velloso, J.R.P, det. Velloso, J.R.P, Cavalcanti, L.H., Treviño-Zevallos, I., from wet chamber, HBEI; Peru, Department of Cuzco, La Convención, Area de Conservación Regional Choquequirao, -13,343, -72, 773, in viscacha dung (Lagidium peruanum L.), 05/XI/2024, col. Velloso, J.R.P, det. Velloso, J.R.P, Cavalcanti, L.H., Treviño-Zevallos, I., from wet chamber, HBEI; Peru, Department of Cuzco, La Convención, Area de Conservación Regional Choquequirao, -13,339, -72, 760, in viscacha dung (Lagidium peruanum L.), 11/X/2024, col. Velloso, J.R.P, det. Velloso, J.R.P, Cavalcanti, L.H., Treviño-Zevallos, I., from wet chamber, HBEI.
4. Discussion
Although myxomycetes are found in terrestrial ecosystems globally, there are few studies on their distribution across high elevations, and these results are ambiguous. Research indicates that myxomycete diversity tends to decrease with altitude in temperate [
24,
25] and tropical forests [
26,
27,
28,
29,
30]. However, Rojas et al. [
31] argue that by increasing sampling precision and considering common floristic elements, elevation may not be such a relevant factor in the distribution of myxomycetes in tropical forests. They emphasize that differences in vegetation between high and low altitudes reflect natural structural and floristic variations, and that the observed patterns may be a consequence of these differences rather than a direct influence of altitude on homogeneous units.
The myxomycete species richness observed in this study decreases with increasing altitude, likely due to more extreme environmental conditions such as lower temperatures, lower humidity, higher UV radiation, and the scarcity of woody substrates, essential for many lignicolous species. In the Andean Altiplano (4,700 m), 50% of the species (
D. circumdissiliens, L. tenera, and
P. luteola) are fimicolous, occurring in Viscacha (
Lagidium peruanum) dung, while
D. leucopodia was found on mosses. At this altitude, two strictly lignicolous species (
H. calyculata and
O. favogineum) were found in a single fragment of a dead branch. Some authors comment that herbivore feces, in general, are not a very productive substrate [
32,
33], with open environments such as deserts and grasslands being the most notable exceptions. Diversity in this substrate appears to be associated with cold, dry climates, where prevailing conditions slow fecal decomposition [
34,
35,
36,
37,
38].
The NBA vegetation area (2500–3100 m) has greater species diversity (66.6%) due to the availability of varied substrates, such as dead branches and logs, unlike the pajonal and Andean highlands, where other substrates predominate. In the tropical montane forests of the Dalat Plateau in Vietnam, at approximately 1500 m altitude, a similar situation occurs, where species diversity decreases from mid-altitude deciduous forest, through mixed broadleaf and coniferous forest, to mid-altitude pyrogenic coniferous forest, and to high-altitude cloud forest [
39]. The authors note that as altitude increases in this area, tree diversity decreases, with a predominance of more specific microhabitats, with myxomycete communities more specialized to certain substrates.
In the pajonal and Andean highlands, climatic conditions such as high altitude, low temperatures, and snow accumulation limit the vegetative growth period to a few months [
33]. The Peruvian highlands, located above 3,000 meters above sea level, are home to vegetation types unique to the region, such as
Polylepis forests, the grasslands known as “puna”, the high-Andean scrublands, the
Puya peaks, the aquatic plant communities known as “bofedales”, the vegetation cover formations called “yaretales”, and vegetation growing in cryoturbated soils. These vegetation formations offer diverse potential substrates for the development of myxomycetes, which have remained little studied [
9]. Prior to the study by Treviño-Zevallos [
9], there were doubts about the ability of these organisms to survive in extreme environmental conditions. However, this hypothesis was refuted, as several previously unexplored natural habitats and substrates for myxomycetes were identified. This discovery demonstrated that the tropical Andes of Peru also harbor a rich biodiversity of these organisms, as is the case in Argentina and Chile [
40,
41].
The distribution patterns found are consistent with previous studies conducted in the Peruvian Andes, where greater diversity of myxomycetes was observed in areas with greater vegetation cover and a variety of microhabitats. For example, a study conducted at the Wayqecha Biological Station, located on the eastern slope of the Andes, recorded 81 myxomycete taxa, with a predominance of the orders Physarales and Trichiales, attributed to the diversity of microhabitats present in the region [
6]. In contrast, in higher altitude regions with sparse vegetation, such as the Andean highlands, myxomycete diversity tends to be lower, possibly due to more extreme environmental conditions and the limited availability of suitable substrates for their development. Furthermore, research in arid zones of Peru conducted by Lado et al. [
42,
43] demonstrated that, despite extreme conditions, there is a remarkable diversity of myxomycetes, with records of new species and expansions in their geographic distribution. These findings highlight the adaptability of myxomycetes to diverse environments and the importance of conducting studies in different ecosystems to better understand their distribution and ecology.
The discovery of
Diderma circumdissilens,
Licea tenera, and
Perichaena luteola in the department of Cusco represents the first confirmed record of these species in Peru, significantly expanding knowledge about their geographic distribution. Previously documented in Europe, North America, and South America [
13,
21,
22,
23], the three species demonstrate a remarkable ability to adapt to highland ecosystems, characterized by extreme climatic conditions and a scarcity of suitable substrates. Their detection in high-altitude environments in the Andes Mountains confirms that these areas constitute a potential hotspot for the development and diversification of myxomycetes, highlighting the importance of continuing to explore these poorly sampled ecosystems to better understand the true distribution of this group of protists. Therefore, further studies are needed in these environments, including collections during the rainy season, the use of molecular biology to confirm the identity of specimens, and long-term studies to assess the effects of climate change on the diversity of myxomycetes in Andean environments.
5. Conclusions
This study significantly contributes to the understanding of the distribution and diversity of myxomycetes in high-altitude environments in the Peruvian Andean region. The results indicate that, although species diversity decreases with increasing altitude, these organisms demonstrate a remarkable capacity to adapt to extreme environmental conditions such as low temperatures, low humidity, and limited availability of suitable substrates. The predominance of fimicolous and lignicolous species at different altitudes reflects the importance of microhabitat and substrate variability in shaping myxomycete communities.
The discovery of Diderma circumdissilens, Licea tenera, and Perichaena luteola for Peru represents a significant advancement in understanding the geographic distribution of these species, expanding knowledge beyond previously documented areas. These findings reinforce the idea that high-altitude regions, often underexplored, can serve as biodiversity hotspots for myxomycetes, challenging the perception that these organisms are limited to milder environments.
Furthermore, the results support previous studies in different regions, demonstrating that factors such as substrate availability and microhabitat diversity are primary determinants of the richness and composition of these communities, rather than altitude alone. The need for additional research—including molecular approaches, sampling across different seasons, and long-term studies—is evident to better understand the effects of environmental and climate changes on these organisms.
Finally, this work highlights the importance of expanding research efforts in high mountain ecosystems, fostering a deeper understanding of the ecology, distribution, and biodiversity potential of myxomycetes, thereby contributing to the conservation and recognition of the biological complexity of these regions.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Conception, JRPV, LHC, ITZ, JP; methodology, JRPV, LHC, ITZ, JP; software, JRPV; validation, JRPV, LHC, ITZ, JP, CEGRS, MFR; formal analysis, JRPV, LHC, ITZ, JP, CEGRS, MFR; investigation, JRPV, LHC, ITZ; resources, JRPV, LHC, ITZ, JP, CEGRS, MFR; data curation, JRPV, LHC, ITZ; original draft preparation, JRPV, LHC, ITZ; review and editing, JRPV, LHC, ITZ; visualization, JRPV, LHC, ITZ, JP, CEGRS, MFR; supervision, JRPV, LHC, ITZ, JP, CEGRS, MFR; project administration, JRPV, LHC, ITZ, JP, CEGRS, MFR; funding acquisition, JRPV, JP, CEGRS, MFR. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CNPq, grant number 440.910/2023-4” and “The APC was funded by XXX”. Check carefully that the details given are accurate and use the standard spelling of funding agency names at
https://search.crossref.org/funding. Any errors may affect your future funding.
Data Availability Statement
All data supporting the findings of this study are available in the present paper. Additional information regarding the conduction of the study is available from the corresponding author upon reasonable request.
Acknowledgments
During the preparation of this study, the authors used the QGIS software (version 3.16 Hannover) and RStudio (version 4.3.2) to create the location map of the study area and for data analysis and visualization. The authors reviewed and edited the output and assume full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- MINAM 2015. Mapa nacional de cobertura vegetal: memoria descriptiva. Lima, Dirección General de Evaluación, Valoración y Financiamiento del Patrimonio Natural. 105 pp.
- Trevino-Zevallos, I.; Garcia-Cunchillos, I.; Lado, C. New records of Myxomycetes (Amoebozoa) from the tropical Andes. Phytot. 2021, 522, 231–239. [Google Scholar] [CrossRef]
- Wrigley de Basanta, D.; Lado, C.; García-Martín, J.; Estrada-Torres, A. Didymium xerophilum, a new myxomycete from the tropical Andes. Mycol. 2015, 107, 157–158. [Google Scholar] [CrossRef]
- Wrigley de Basanta, D.; Estrada-Torres, A.; García-Cunchillos, I.; Cano Echevarría, A.; Lado, C. Didymium azorellae, a new myxomycete from cushion plants of cold arid areas of South America. Mycol. 2018, 109, 993–1002. [Google Scholar] [CrossRef]
- Wrigley De Basanta, D.; Estrada-Torres, A.; Lado, C. Licea aurea a new Myxomycete from the Peruvian Andes. Phytot. 2019, 391, 218–224. [Google Scholar] [CrossRef]
- Treviño-Zevallos, I.F.; Lado, C. Myxomycete diversity in a humid montane forest on the eastern slopes of the Peruvian Andes. Pl. Ecol. Evol. 2020, 153, 390–398. [Google Scholar] [CrossRef]
- Xavier de Lima, V.; Cavalcanti, L.D.H. Second world record of Metatrichia floripara (Trichiaceae, Myxomycetes), found in Brazil. Phytot. 2016, 247, 292–294. [Google Scholar] [CrossRef]
- Lado, C.; Treviño-Zevallos, I.; García-Martín, J.M.; Wrigley de Basanta, D. Diachea mitchellii: A new myxomycete species from high elevation forests in the tropical Andes of Peru. Mycol. 2022, 114, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Treviño-Zevallos, I.; García-Cunchillos, I.; de Basanta, D.W.; Lado, C. Diversity of Myxomycetes from Peru part III: the high andes and the altiplano. Phytot 2023, 624, 1–92. [Google Scholar] [CrossRef]
- Wrigley de Basanta, D.; Estrada-Torres, A. 2017. Techniques for Recording and Isolating Myxomycetes. In Myxomycetes. Biology, Systematics, Biogeography, and Ecology; Stephenson, S.L. & Rojas, C. (Eds.). Academic Press, London, pp. 333–363. [CrossRef]
- Martin, G.W.; Alexopoulos, C.J. .The Myxomycetes. University of Iowa Press, Iowa, USA., 1969, 560 pp.
- Farr, M.L. Myxomycetes. pp. 1–305, in CT Rogerson (org.). Flora neotropica Monograph 16. New York, New York Botanical Garden. 1976, 304p.
- Poulain, M.; Meyer, M.; Bozonnet, J. Les Myxomycètes. Delémont: Fédération Mycologique et Botanique Dauphiné-Savoie, 2011, pp. 1112.
- Lado, C. 2005-2025. An online nomenclatural information system of Eumycetozoa. (https://eumycetozoa.com). Access: 15/05/2025.
- Wickham, H. dplyr: A Grammar of Data Manipulation. R package version 1.0.10. 2022. Available from: https://CRAN.R-project.org/package=dplyr.
- Wickham, H. ggplot2: Elegant graphics for data analysis. Springer-Verlag New York; 2016. Available from: https://CRAN.R-project.org/package=ggplot2.
- Wickham, H.; Henry, L. tidyr: Tidy Messy Data. R package version 1.2.1. 2021. Available from: https://CRAN.R-project.org/package=tidyr.
- Lê, S.; Husson, F.; Pagès, J. FactoMineR: An R package for multivariate analysis. J Stat Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package version 1.0.7. 2020. Available from: https://CRAN.R-project.org/package=factoextra.
- Flatau, L.; Schinner, P. Diderma circundissilens, eine neue Art der Myxomyceten. Z. Mykol. 2002, 68, 73–80. [Google Scholar]
- GBIF.org (16 May 2025a). GBIF Occurrence Download – Licea tenera. [CrossRef]
- GBIF.org (16 May 2025b). GBIF Occurrence Download – Diderma circundissilens. [CrossRef]
- GBIF.org (16 May 2025c). GBIF Occurrence Download – Perichaena luteola. [CrossRef]
- Takahashi, K. Altitudinal distribution patterns of myxomycete species growing on bark of Cryptomeria japonica tree in warm temperate zone of Japan. RKBAA 2017, 17, 207–217. [Google Scholar]
- Takahashi, K.; Harakon, Y.; Degawa, Y. Myxomycetes distribution along an elevational gradient on coniferous woody debris in the mountains of Central Japan. Slime Molds 2024, 4, 1–16. [Google Scholar]
- Stephenson, S.L.; Landolt, J.C.; Moore, D.L. Protostelídeos, dictiostelídeos e mixomicetos no microhabitat da serapilheira da Floresta Experimental de Luquillo, Porto Rico. Mycol Res 1999, 103, 209–214. [Google Scholar] [CrossRef]
- Stephenson, S.L.; Schnittler, M.; Lado, C. Caracterização ecológica de uma assembleia tropical de mixomicetos: Reserva Florestal Nublada de Maquipucuna, Equador. Mycol. 2004, 96, 488–497. [Google Scholar] [CrossRef]
- Schnittler, M.; Stephenson, S.L. Biodiversidade de mixomicetos em quatro diferentes tipos de floresta na Costa Rica. Mycol. 2000, 92, 626–637. [Google Scholar] [CrossRef]
- Rojas, C.; Stephenson, S.L. Myxomycete ecology along an elevation gradient on Cocos Island, Costa Rica. Fungal Divers. 2008, 29, 117–127. [Google Scholar]
- Rojas, C.; Valverde, R.; Stephenson, S.L.; Vargas, M.J. Padrões ecológicos de mixomicetos da Costa Rica. Fungal Ecol. 2010, 3, 139–147. [Google Scholar] [CrossRef]
- Rojas, C.; Valverde, R.; Calvo, E. Does elevation influence the distributional patterns of tropical myxomycetes? A case study in Costa Rica. Mycology 2016, 7, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, S.L. Distribution and ecology of myxomycetes in temperate forests. II. Patterns of occurence on bark surface of living trees, leaf litter, and dung. Mycol. 1989, 81, 608–621. [Google Scholar] [CrossRef]
- Novozhilov, Y.K.; Rollins, A.W.; Shchepin, O.N.; Schnittler, M. Ecology and distribution of Myxomycetes. In Myxomycetes. Academic Press: 2022; pp. 325–376.
- Novozhilov, T.K.; Schnittler, M. Myxomycete diversity in arid regions of the Great Lake Basin of western Mongolia. Fungal Divers. 2008, 30, 97–119. [Google Scholar]
- Novozhilov, Y.K.; Zemlyanskaya, I.; Schnittler, M.; Stephenson, S.L. Myxomycete diver sity and ecology in the arid regions of the Lower Volga River Basin (Russia). Fungal Divers. 2006, 23, 193–241. [Google Scholar]
- Novozhilov, Y.K.; Schnittler, M.; Vlasenko, A.V.; Fefelov, K.A. Myxomycete diversity of the Altay Mts. (southwestern Siberia, Russia). Mycotaxon 2010, 111, 91–94. Available from: http://www.mycotaxon.com/resources/checklists/novozhilov-v111-checklist.pdf. [CrossRef]
- Schnittler, M.; Novozhilov, Y.K. Myxomycetes of the winter-cold desert in western Kazakhstan. Mycotaxon 2000, 74, 267–285. [Google Scholar] [CrossRef]
- Novozhilov, Y.K.; Rollins, A.W.; Schnittler, M. Ecology and Distribution of Myxomycetes. In Myxomycetes. In Myxomycetes. Biology, Systematics, Biogeography, and Ecology; Stephenson, S.L., Rojas, C., Eds.; Academic Press: London, UK, 2017; pp. 253–297. [Google Scholar]
- Novozhilov, Y.K.; Erastova, D.A.; Shchepin, O.N.; Schnittler, M.; Aleksandrova, A.V.; Popov, E.S.; Kuznetsov, A.N. Myxomycetes associated with monsoon lowland tropical for ests in southern Vietnam. Nova Hedwig 2017, 104, 143–182. [Google Scholar] [CrossRef]
- Lado, C.; Wrigley de Basanta, D.; Estrada-Torres, A.; Stephenson, S.L. The biodiversity of myxomycetes in central Chile. Fungal Divers. 2013, 59, 3–32. [Google Scholar] [CrossRef]
- Wrigley de Basanta, D.; Lado, C.; Estrada-Torres, A.; Stephenson, S. Biodiversity of myxomycetes in subantarctic forests of Patagonia and Tierra del Fuego, Argentina. Nova Hedwig. 2010, 90, 45–79. [Google Scholar] [CrossRef]
- Lado, C.; Wrigley de Basanta, D.; Estrada-Torres, A.; Stephenson, S.L. Myxomycete diversity in the coastal desert of Peru with emphasis on the lomas formations. An. Jard. Bot. Madr. 2016, 73, 1–27. [Google Scholar] [CrossRef]
- Lado, C.; Wrigley de Basanta, D.; Estrada-Torres, A.; Stephenson, S.L.; Treviño-Zevallos, I. Diversity of Myxomycetes in arid zones of Peru part II: the cactus belt and transition zones. An. Jard. Bot. Madr. 2019, 76, 083. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).