1. Introduction
The skin serves as a defensive barrier against pathogen invasion. The alteration of the normal anatomical structure due to surgical procedures or chemical, physical, mechanical, and thermal events, leading to a disruption of skin functions, results in a wound [
1]. Wound healing is intricate and entails a sequence of coordinated and overlapping phases that must converge to restore skin integrity. The stages include a variety of discrete but frequently interconnected events, including coagulation, inflammation, migration, proliferation, regeneration, and remodeling of the extracellular matrix (ECM). Wound healing initiates with hemostasis, continued by an inflammatory phase, a proliferative phase ending in re-epithelization, and a remodeling phase wherein the scar develops [
2,
3]. Once the skin is compromised, typical microorganisms from the normal skin flora, along with exogenous bacteria and fungus, can rapidly infiltrate the underlying tissues, which provide a moist, warm, and nutrient-rich environment [
4]. Staphylococcus aureus, Methicillin resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa are the dominant microbial strains present in patients with infected wounds [
5]. As antibiotics are increasingly being tolerated by pathogenic strains Individuals are currently utilizing the extensive array of bioresources. These consist primarily of herbs, but may also incorporate animal and mineral components [
6]. Skin regeneration and infection prevention are complicated procedures in wound healing. Both local and systemic therapies are employed in wound healing. However, localized treatment may be a more advantageous approach to reduce side effects, enhance efficacy, and overcome antibiotic resistance [
7]. Current wound healing therapies typically fail to achieve favorable clinical results, either structurally (e.g., wound re-epithelialization, fluid loss management) or functionally (e.g., histological characteristics affecting elasticity, durability, sensitivity, etc.). Therefore, nanotechnology, due to its diverse physicochemical features, is a dependable field of research for wound-healing therapies. By altering the material type, size, and electrical charge of the nanoparticles, their biochemical characteristics, including hydrophobicity, interaction with biological targets, and tissue penetration depth, may be readily optimized for various wound types [
8]
Chitin and its deacetylated derivative, chitosan, include a series of linear polysaccharides comprised of differing quantities of (β1→4) linked residues of Nacetyl-2-amino-2-deoxy-D-glucose (glucosamine, GlcN) and 2-amino-2-deoxy-Dglucose (N-acetyl-glucosamine, GlcNAc) residues. Chitosan dissolves in aqueous acidic environments by the protonation of primary amines. Chitin is a prevalent biopolymer present in the exoskeletons of crustaceans, the cuticles of insects, algae, and the cell walls of fungi. Chitosan rarely occurs in nature, primarily in some fungi of the Mucoraceae family. Historically, commercial chitosan samples were predominantly derived from the chemical deacetylation of chitin sourced from crustaceans. Chitosan derived from fungi is increasingly attracting market interest [
9]. Chitosan demonstrates numerous biological activities, including antitumoral, antibacterial, antioxidant, and anti-inflammatory properties, making it suitable for application as therapeutic polymers [
10].
Lavender (Lavandula angustifolia Mill.) is among the most valued plants utilized in cosmetics and aromatherapy. It possesses deodorizing, calming, antibacterial, and anti-inflammatory properties [
11]. The most valuable component of the plant are the flowers, which contain up to 4.5% essential oil. The primary constituents of the oil include esterified and free linalool, camphor, and cineole [
12]. the leaves generally possess higher concentrations of camphor and cineole compared to the flowers, resulting in a more potent leaf oil. Alongside the oil, there are components like tannins, phenolic acids, flavones, anthocyanidins, saponins, polyphenols and minerals [
13].
This study was conducted to evaluate the efficacy of Chitosan nanoparticles loaded hydrogel, Lavandula angustifolia extract and their combination on the rate of wound healing.
2. Materials and Methods
An experimental, randomized, controlled animal study was conducted at (The Department of Pharmacology, College of Medicine/ Baghdad University) and (The Iraqi Center for Cancer Research and Medical Genetics/ Mustansiriyah University). This research was conducted from October 2024 to August 2025.
All the experimental work was implemented following the protocol reviewed by the Iraqi Board Review of the College of Medicine/ Baghdad university after being approved by the scientific committee of the Department of Pharmacology at the College of Medicine/ Baghdad University
2.1. Lavandula Angustifolia Collection and Preparation of the Essential Oil
Lavandula angustifolia leaves was collected from the mountains of Sulaimani, Kurdistan region of Iraq, in September 2024, the collection was carried out in the field involving species of the plant under the supervision of researcher to avoid contaminants, the collected plant was identified and confirmed at Baghdad University/Iraq Natural History Research Center and Museum, the plant was cleaned, dried in the shade at room temperature and extracted in the Ministry of Science and Technology (Environment, Water and Renewable Energy Directorate). Essential oil of L. angustifolia was extracted using hydro distillation of every 100 g of dried crushed leaves, in 500 mL of distilled water utilizing a Clevenger-type apparatus for 4 hours. Every 100 g of the plant produce about 5ml of the oil. The essential oil was extracted, centrifuged, separated from the upper layer and preserved in amber bottles at +4 °C until use [
14].
2.2. Preparation of Chitosan Nanoparticles Loaded Hydrogel
Preparation of chitosan nanoparticles-loaded hydrogel involved reacting chitosan using a Dean–Stark (Clevenger) apparatus with xylene to extract water. The chitosan amide was separated by filtration, washed with methanol, hot distilled water, and ethanol, then dried at 50°C. Chitosan nanoparticles (Cs NPs) were synthesized via ionic gelation using TPP and chitosan. Chitosan was dissolved in 1% acetic acid until clear, and then TPP was added at a 1:2.5 (w/w) ratio with continuous stirring for 6 hours at room temperature. The resulting nanoparticles were separated by centrifugation, washed multiple times, and the supernatant discarded, leaving the precipitate which was re-suspended in water [
15,
16].
2.3. Preparation of Chitosan-Lavandula angustifolia Nano Emulsion
The preparation involved reacting chitosan using a Dean–Stark (Clevenger) apparatus with xylene to extract water. The chitosan amide product was separated by filtration, washed with methanol, hot distilled water, and ethanol, and then dried at 50°C and weighed [
15,
16]. Chitosan was dissolved in 1% acetic acid until clear, and Tween 80 (1%) was added as a surfactant. Lavender oil was gradually incorporated with continuous stirring to form a stable emulsion. TPP solution was added dropwise under high-speed stirring, where ionic interactions crosslinked chitosan chains, encapsulating the lavender oil into nanoparticles. Sonication was used to reduce particle size and ensure uniformity [
17]. The characterization of the chitosan nanoparticles-loaded hydrogel and nano emulsion was performed by the Ministry of Science and Technology-Iraq, using techniques such as UV-Vis spectroscopy, FTIR, SEM, EDX, AFM, and XRD to confirm their nano properties.
2.3. Preparation of Staphylococcus aureus Suspension
Staphylococcus aureus was sourced from a patient with infected wound in Ghazi Al-Hariri hospital for surgical specialties, bacteria were cultivated on nutrient agar for 24 hours at 37º C under aerobic conditions. Following the confirmation of their viability and purity by Vitek2 system (Bio number is 050602062361231). A pure colony was aseptically transferred into BHI broth and incubated at 37ºC for 18-24 hours, resulting in turbid broth indicating bacterial growth. The turbidity was adjusted to the 0.5 McFarland standard (1×10^8 CFU/mL) [
18].
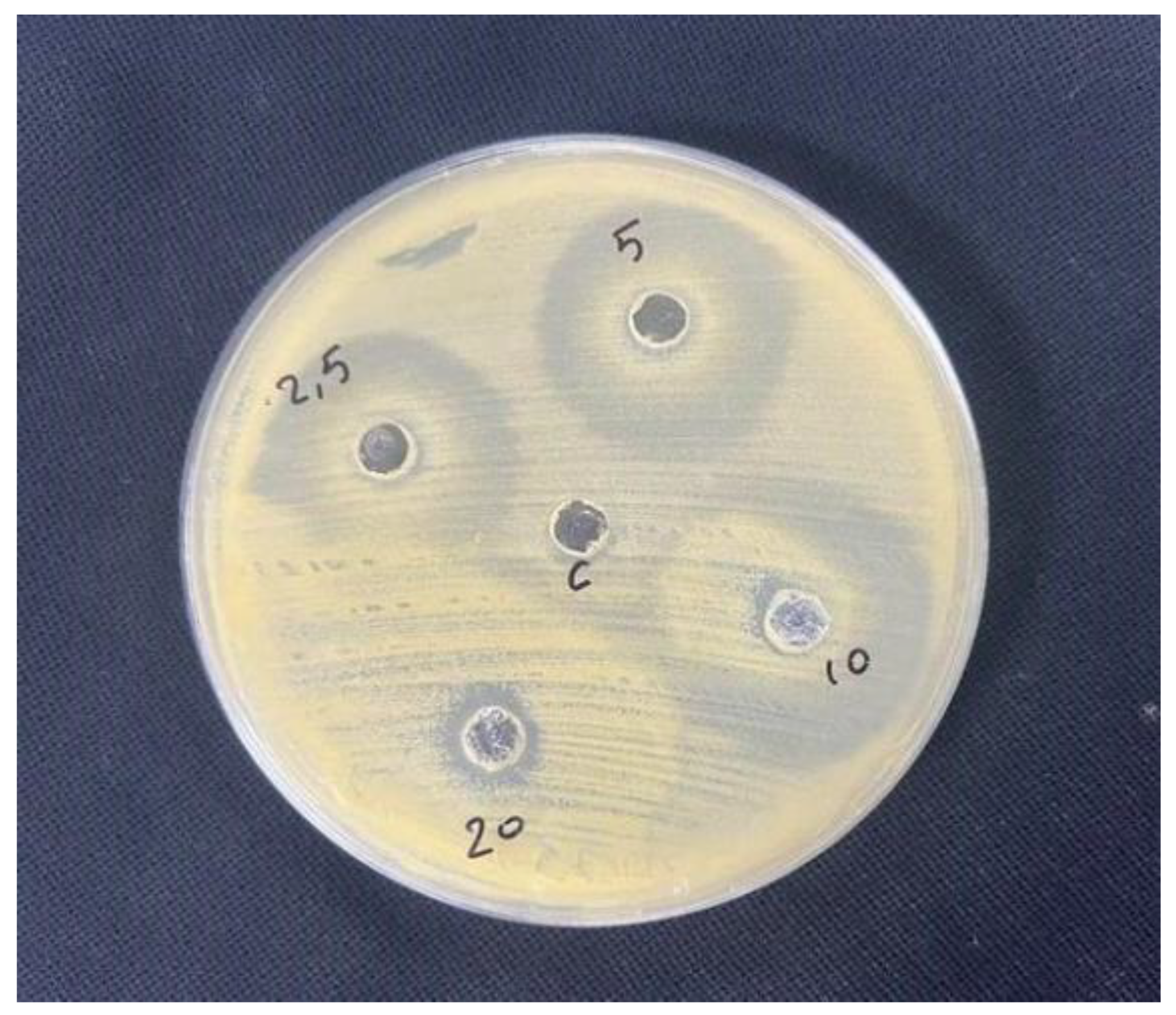
2.4. In Vitro Antimicrobial Test of Lavandula angustifolia Extract Using Agar Well Diffusion Method
For in vitro antimicrobial testing, Mueller-Hinton agar plates were evenly inoculated with the bacterial suspension. Wells of 6–8 mm diameter were punched into the agar, and 20–100 µL of Lavandula angustifolia extract at various concentrations (2.5%, 5%, 10%, 20%) in Dimethyl Sulfoxide (DMSO) were added. The plates were incubated, allowing the extract to diffuse and inhibit bacterial growth. Zones of inhibition were measured to determine the effective concentration [
19].
Figure 1.
Antimicrobial effect of different concentrations of Lavandula angustifolia extract.
Figure 1.
Antimicrobial effect of different concentrations of Lavandula angustifolia extract.
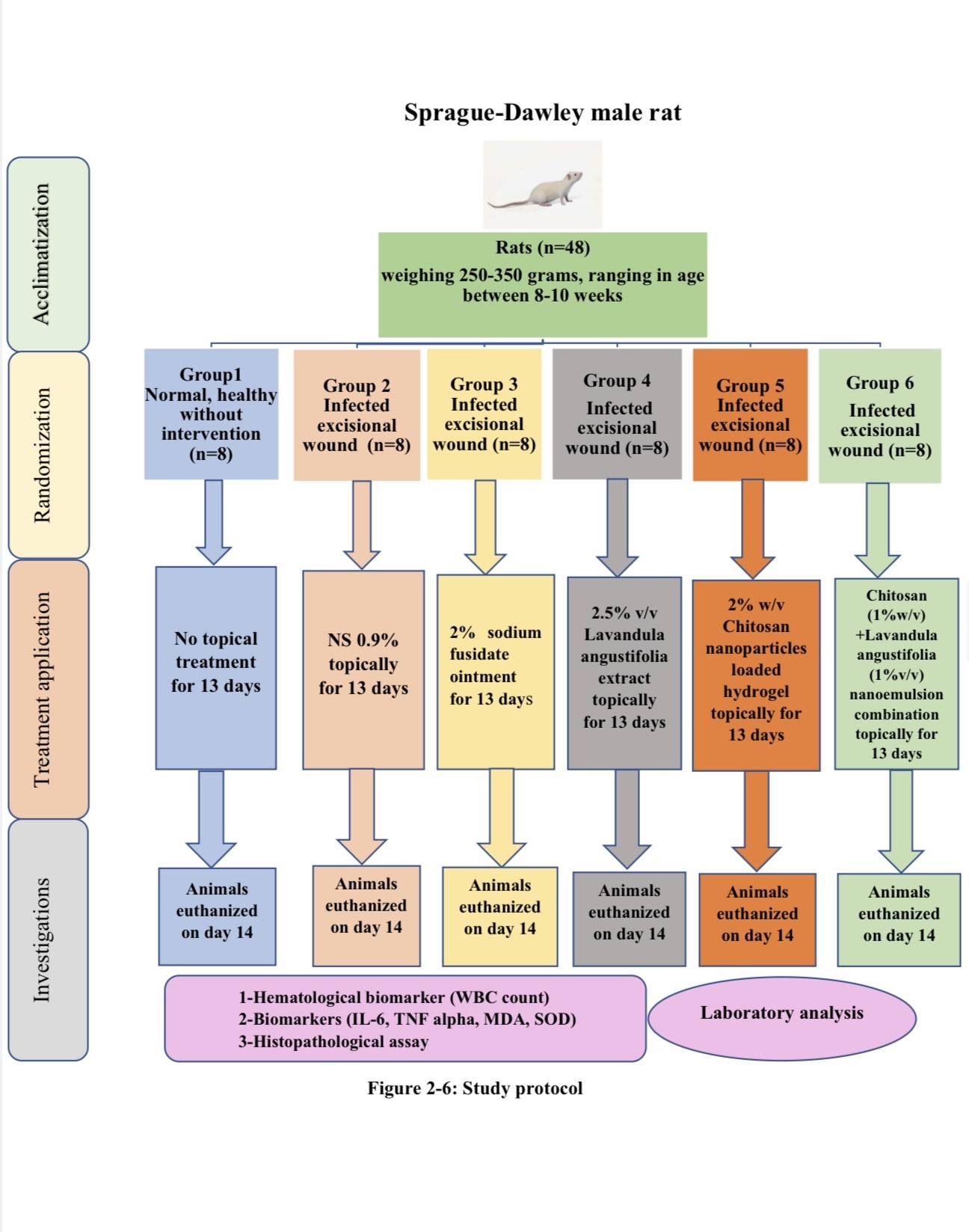
2.5. Experimental Design and Preparation of Experimental Animals
Forty-eight Sprague-Dawley Male Rats (weighing 250-350 grams, ranging in age between 8-10 weeks). Animals were obtained from the Iraqi Center for Cancer Research and Medical Genetics. Before the experiment, the animals were acclimatized for two weeks at the same location where they were obtained. The animals were housed in separate polypropylene cages with consistent 12-hour light/dark cycles, temperature and humidity levels (40-60%). Water and standard food pellets were provided ad libitum. Each animal was anaesthetized with an intramuscular injection of a ketamine and xylazine mixture, at dosages of 20 mg/kg and 100 mg/kg, respectively [
20]. The dorsal region of the animals had been cleaned with 70% ethanol and shaved. A full thickness circular excision wound (1.5 cm in diameter) was created using a sterilized surgical blade [
21]. Ten microliters of the bacterial suspension that is previously prepared were applied on the wound and spread with the aid of a sterile swab to wounds of infected animal groups [
18]
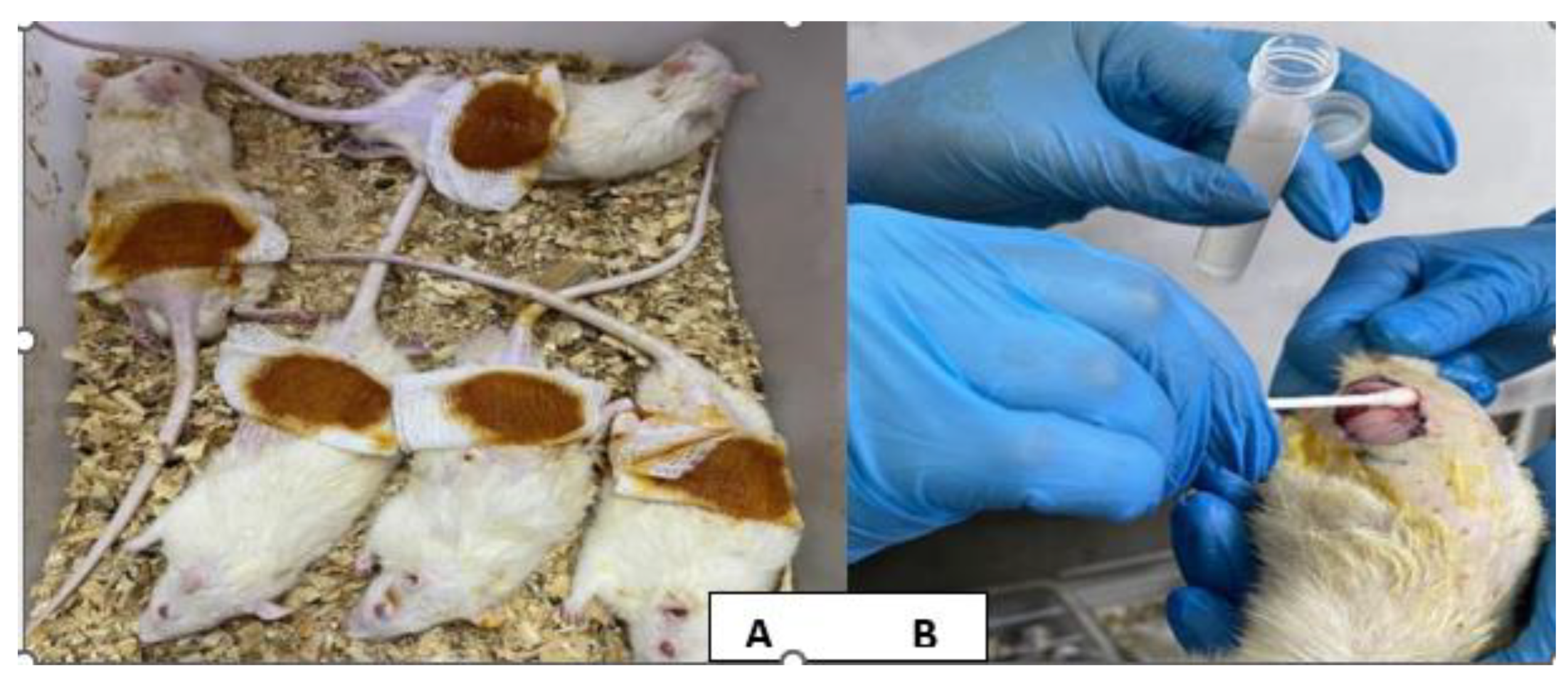
Figure 2.
2.6. Induction of Staphylococcus aureus Infected Excisional Wound
Each animal was anaesthetized with an intramuscular injection of a ketamine and xylazine mixture, at dosages of 20 mg/kg and 100 mg/kg, respectively [
20]. The dorsal region of the animals had been cleaned with 70% ethanol and shaved. A full thickness circular excision wound (1.5 cm in diameter) was created using a sterilized surgical blade [
21]. Ten microliters of the bacterial suspension that is previously prepared were applied on the wound and spread with the aid of a sterile swab to wounds of infected animal groups. (110) (18), (
Figure 2 A & B).
Study groups
After confirming the induction of excisional wound infection, a total of 48 rats were divided randomly into six groups (each group consists of 8 rats) as follows: -
1-Group 1: - Members of this group are normal, healthy without any intervention.
2-Group 2: - Infected excisional wounds were treated only with normal saline 0.9% topically.
3-Group 3: - Infected excisional wounds were treated with 2% Sodium Fusidate Ointment topically for 13 days.
4-Group 4: - Infected excisional wounds were treated with 2.5% v/v Lavandula angustifolia extract topically for 13 days.
5-Group 5: - Infected excisional wounds were treated with 2% w/v Chitosan nanoparticles loaded hydrogel topically for 13 days.
6-Group 6: - Infected excisional wounds were treated with Chitosan (1%w/v) +Lavandula angustifolia (1%v/v) nano emulsion combination topically for 13 days.
All topical formulations were applied two times daily for 13 days duration. At the end of the experiment, the animals were humanely euthanized at day 14 in accordance with [ethical approval number]. Immediately following euthanasia, full thickness skin samples were removed, fixed in 10% formalin for 24–48 hours, and processed for histological examination.
2.6. Tissue Sampling and Processing for Histopathological Study
Tissue sampling and processing for histopathological study were performed according to Bancroft’s Theory and Practice of Histological Techniques [
22]. A piece of healed skin was excised from each animal after confirmation on day 14. The specimens were fixed in 10% formalin for 24 hours, and then dehydrated by sequential immersion for 2 hours in each concentration of ethanol (70%, 80%, 95%, and 100%). The specimens were cleared from ethanol by immersing them in xylene for an additional 2 hours. Paraffin impregnation, infiltration, and penetration were carried out using molten paraffin (55-60°C) for 2 hours. The specimens were then cooled in an L-shaped metal template. Upon solidification, the paraffin blocks were sectioned using a rotary microtome, obtaining sections of 5 μm thickness. The sections were attached to slides after being floated on a water bath (45-48°C) and then allowed to settle on clean glass slides. Deparaffinization was performed by removing excess paraffin from the sections after 30 minutes in an oven at 65°C.
Sections were examined using a light microscope at different magnifications (10X and 40X) to evaluate histopathological changes according to the semi-quantitative wound scoring system [
23]. The healing process was scored into four categories (scores 0, 1, 2, 3, and 4) based on the level of the following essentials of healing: re-epithelialization, granulation tissue formation, collagen deposition, inflammatory cell infiltration, angiogenesis, hemorrhage, and necrosis/degeneration.
2.7. Statistical Analysis
All analyses were conducted using SPSS v.26. Data was represented as tables and figures. All continuous variables were non-normally distributed, so they were reported as median with interquartile range (IQR). For within-group comparisons of these non-normally distributed variables, we used Mann-Whitney U test instead of independent t test; Kruskal-Wallis Test instead of one-way ANOVA test, Wilcoxon signed-rank test instead of paired t-test. P value < 0.05 was considered statistically significant.
3. Results
This study included six groups; 8 rats in each group, these groups were: apparently healthy (Control group), Induced non-treated group, induced treated topically with Fucidin ointment 2% (sodium fusidate 20mg/g) twice daily for 14 days (Fucidin group), induced treated topically with Lavandula angustifolia extract 2.5 mg/ml twice daily for 14 days (Lavandula group), induced treated topically with Chitosan nanoparticles hydrogel 2% W/V twice daily for 14 days (Chitosan group), and induced treated topically with Chitosan nanoparticles and Lavandula angustifolia nano emulsion combination applied twice daily for 14 days (Chitosan & Lavandula group).
Comparison among the studied groups was done with Hematological biomarker White Blood Cell (WBC) count; skin tissue homogenate (TNF-α, IL-6, MDA, and SOD); Wound contraction percentage; and semi quantitative wound scoring system (Reepithelization, Granulation Tissue Formation, Collagen deposition, Inflammatory Infiltrate, Angiogenesis, Hemorrhage, and Necrosis/Degeneration.
Comparison between apparently healthy control group and induced non- treated group in relation to different measured parameters
Induced non-treated group shows that level of WBC at day 7 and 14, and (ELISA Biomarkers at day 14: TNF-α, IL-6, and MDA, were significantly highly increased in comparison with apparently healthy control group, P<0.001.
While level of SOD was significantly highly decreased among Induced non- treated group in comparison with apparently healthy control group, P<0.001.
Table 1.
Induced non-treated group shows that Granulation Tissue Formation, Inflammatory Infiltrate, Angiogenesis, Hemorrhage, and Necrosis/Degeneration were significantly highly increased in comparison with apparently healthy control group, P<0.001.
While Reepithelization and Collagen Deposition were significantly highly decreased among Induced non-treated group in comparison with apparently healthy control group, P<0.001.
Table 2.
Regarding induced then treated groups, Chitosan & Lavandula group shows that level of WBC at day 7 and 14, and ELISA Biomarkers at day 14: TNF-α, IL-6, and MDA were significantly highly decreased in comparison with other groups, P<0.05.
While level of SOD was significantly highly increased among Chitosan & Lavandula group in comparison with other groups, P<0.001.
Table 3.
Regarding Semi quantitative wound scoring system plus Wound contraction % among all induces then treated groups, Chitosan group and Chitosan & Lavandula group shows that Reepithelization Granulation Tissue Formation, Collagen Deposition, and Wound contraction % at days 3, 7, 14 were significantly highly increased in comparison with Fucidin group and Lavandula group, P<0.001.
Also; Inflammatory Infiltrate and Angiogenesis were significantly highly decreased among Chitosan group and Chitosan & Lavandula group in comparison with Fucidin group and Lavandula group, P<0.001.
Table 4. While Hemorrhage and Necrosis/Degeneration were significantly highly decreased among all induces then treated groups, P<0.001.
Table 4.
The comparison between induced non-treated group and induced then treated groups was shown in
Table 5.
At day 7 and 14; the lowest WBC level was observed among Chitosan & Lavandula group with significant difference from other groups, P<0.001.
Similarly; the lowest LISA Biomarkers level at day 14: TNF-α, IL-6, and MDA were observed among Chitosan & Lavandula group with significant difference from other groups, P<0.001.
Also; the highest level of SOD was significantly observed among Chitosan & Lavandula group in comparison with other groups, P<0.001.
Table 5.
Semi quantitative wound scoring system shown that Reepithelization, Granulation Tissue Formation, and Collagen Deposition were significantly higher among both Chitosan group and Chitosan & Lavandula group in comparison to other groups after 14 days of therapy; P<0.001.
Also; Inflammatory Infiltrate and Angiogenesis were significantly lower among both Chitosan group and Chitosan & Lavandula group in comparison to other groups after 14 days of therapy; P<0.001.
Hemorrhage and Necrosis/Degeneration were significantly decreased among all induced then treated groups in comparison to induced non-treated group, P<0.001.
Wound contraction % at days 3, 7, and 14 of therapy was significantly increased among all induced then treated groups in comparison to induced non-treated group, but highest increased was observed among Chitosan & Lavandula group, P<0.001.
Table 6.
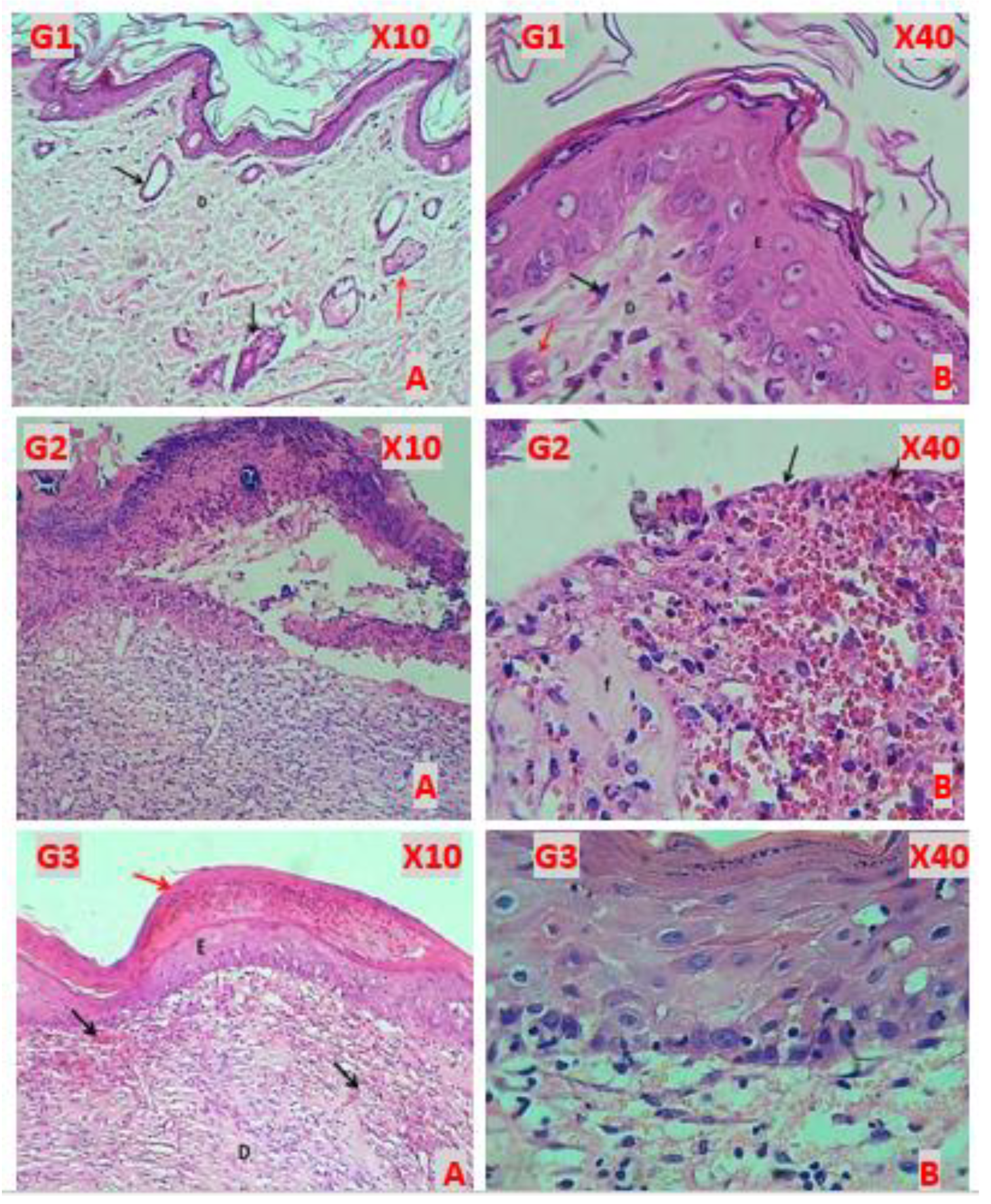
3.1. Histopathological Findings
G1 (Healthy control)
The histopathological figures of skin revealed normal appearance of epidermis lining epithelial cells, normal appearance dermal collagen fibers with blood vessels, fibroblasts and fibrocytes contents with normal sweat glands and hair follicle with related sebaceous glands (
Figure 3, G1A & G1B)
G2 (Induction)
All histopathological figures of the skin revealed sever ulcerative dermatitis that characterized by depressed area of skin and completely loss of the epidermis, the dermis revealed hemorrhagic ulcer with fibrin deposition, degeneration with necrosis of fibrous connective tissue, vascular congestion and infiltration of mononuclear leukocytes (
Figure 3, G2A & G2B).
G3 (Fucidin treatment)
Almost histopathological figures of the skin revealed normal epidermis keratinized stratified squamous epithelium (complete epithelization). The dermis revealed immature fibrous tissue with marked angiogenesis and little sub epidermis infiltration of leukocytes. Some figure revealed hyperkeratosis with well reepithelization of epidermis and the dermis comprised of immature fibrous tissue with marked angiogenesis (
Figure 3, G3A & G3B).
G4 (Lavandula treatment)
Almost histopathological figures of the skin revealed incomplete reepithelization of epidermis non keratinized stratified squamous epithelium that showed mitotic figures of stratum basalis. The dermis comprised of immature dermal collagen fibers had marked angiogenesis and little infiltrated mononuclear leukocytes (
Figure 4, G4A & G4B).
G5 (Chitosan treatment)
Almost histopathological figures of the skin revealed normal epidermis keratinized stratified squamous epithelium which little mitotic figures. The dermis comprised of mature fibrous tissue characterized by marked angiogenesis (numerous blood capillaries) and no infiltration of leukocytes. Other figures revealed well keratinized stratified epithelial cells of epidermis (
Figure 4, G5A & G5B).
G6 (Chitosan & Lavandula Treatment)
Histopathological examination of the skin sections revealed normal epidermis with keratinized stratified squamous epithelium showing complete epithelialization. The dermis comprised mature fibrous connective tissue with abundant collagen bundles and numerous blood capillaries indicating marked angiogenesis. Sweat glands and sebaceous glands appeared normal. There was no significant infiltration of inflammatory cells. The overall tissue architecture appeared well-organized, suggesting effective healing response post-treatment (
Figure 4, G6A & G6B).
3.2. Wound Contraction
Wound contraction percentage at day 3, day 7, and day 14 of therapy was significantly higher among Chitosan & Lavandula group in comparison to other groups, P<0.001. wound contraction % at day 14 of therapy was significantly increased from that at day 3, and day 7 of therapy among all study groups, P<0.001, (
Table 4 and
Table 7 &
Figure 5).
4. Discussion
Wound healing is a natural physiological response to tissue damage. Wound healing is a complex process that includes a complex interaction among many cell types, cytokines, mediators, and the vascular system. The sequence of initial vasoconstriction of blood vessels and platelet aggregation aims to stop hemorrhaging. This is succeeded by an influx of different inflammatory cells, beginning with the neutrophil. These inflammatory cells subsequently secrete various mediators and cytokines to facilitate angiogenesis, thrombosis, and re-epithelialization. The fibroblasts subsequently release extracellular components that function as Wound healing is a natural physiological response to tissue damage [
24]. Wound infections resulting from the colonization of bacteria and other microorganisms at the wound site present a significant challenge to wound treatment, as they induce severe inflammatory reactions that impede healing and may lead to wound deteriorating [
25,
26,
27].
The current study investigates various wound healing processes across multiple groups, including those with untreated wounds compared to induced treatment groups. These treatment groups consist of Lavandula group, Chitosan group, and a combination of both Lavandula and chitosan group, all compared to a positive control group treated with Fucidin group.
The current study compared hematological biomarker: (WBC count at day 7 & day 14) and ELISA Bi-o markers at day 14: TNF-α, IL-6, MDA, and SOD between apparently healthy control group and induced non-treated group. The significant elevation in WBC count observed on days 7 and 14 indicates a persistent immune response during the inflammatory phase of wound healing. This response is further supported by the marked increase in the pro-inflammatory cytokines TNF-α and IL-6, and the oxidative stress marker MDA at day 14. These biomarkers are critical indicators of the inflammatory and oxidative status of the wound envi-ronment and suggest that the healing process is still in an active or prolonged inflammatory phase. This can influence tissue remodeling and may delay progression to the proliferative and maturation phases of healing.
These findings align with recent studies indicating that as a result of skin injury, various chemoattractant chemicals are released into the circulation, prompting the migration and recruitment of neutrophils initially and macrophages later. Recruited leukocytes phagocytize necrotic tissues and secrete cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6, as well as growth factors which mediate and promote healing [
28,
29]. Interleukin (IL)-6 is essential for the prompt resolution of wound healing and plays a key role in acute inflammation. IL-6, which is released early in response to damage, causes tissue-resident macrophages, keratinocytes, endothelial cells, and stromal cells to release proinflammatory cytokines. Leu-kocyte chemotaxis into a wound has also been observed in response to IL-6 .IL-6 signaling is responsible for the switch to a reparative environment. Controlling the healing process of wounds is essential. Inappropriate proinflammatory signaling can lead to wounds that are more prone to infection and take quite longer to heal [
30].
TNF-α, a type of tumor necrosis factor, is crucial in the inflammatory phase of wound healing. TNF-α not only activates the immune response but also attracts immune cells to the site of injury. Furthermore, TNF-α stimulates the proliferation of fibroblasts and angiogenesis, the differentiation of keratinocytes, and the production of growth factors [
31].
Oxidative stress is an internal imbalance between the body's pro-oxidants and antioxidants. During oxidative stress, the generation of reactive oxygen species (ROS) increases, which is crucial for wound healing [
32]. Superoxide dismutase (SOD) functions as an antioxidant enzyme that reduces oxygen radicals via oxida-tion/reduction cycles at an exceptionally rapid response rate [
33].
4.1. Rationale for Using Chitosan-Based Nanoparticles in Wound Healing
Chitosan-based nanoparticles (CSNPs) have emerged as a novel approach in wound healing due to their ca-pacity to accurately deliver therapeutic agents for enhanced tissue repair. The sustained release of encapsu-lated pharmaceuticals, growth hormones, or antibacterial agents is facilitated by their small size, allowing for effective infiltration and retention at the wound location. The targeted delivery reduces systemic side effects and sustains optimal local therapeutic concentrations, which is essential for effective healing. (34)
The biocompatibility of chitosan arises from its natural origin, non-toxic breakdown products, and capacity to facilitate cellular functions without triggering negative immunological responses. It stimulates fibroblast proliferation, facilitates collagen production, and accelerates tissue regeneration by mimicking extracellular matrix components, making it optimal for wound healing. Its cationic properties enhance interactions with negatively charged cell membranes and extracellular components, boosting adhesion and migration while preserving a non-immunogenic character [
35].
4.2. Anti-Inflammatory Effects of Different Treatment Groups
The Chitosan group and Chitosan & Lavandula combination group demonstrated a significant reduction in WBC counts at days 7 and 14, along with decreased TNF-α and IL-6 levels at day 14 compared with other treated groups (p<0.05). This indicates an accelerated resolution of infection-induced inflammation due to their nanoparticles properties.
Chitosan regulates the inflammatory phase of wound healing by reducing pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). It promotes the resolution of inflammation by stimulating macrophage polarization towards the anti-inflammatory M2 phenotype [
36].
Lavandula angustifolia decreased the expression of inflammatory mediators including IL-6, and TNF-α; the anti-inflammatory properties of lavender are attributed to its essential oil constituents, non-volatile terpe-noids, and polyphenols [
37].
Linalool and linalyl acetate components exhibit anti-inflammatory properties via diminishing the phosphory-lation of the p65 and p50 NFκB transcription factors, hence reducing the synthesis of pro-inflammatory cy-tokines IL-6 and TNFα. α-terpineol reduces the synthesis of the pro-inflammatory cytokine IL-6 [
38].
Lavandula angustifolia extract low chemical stability, poor water solubility, and volatile nature leading to poor lasting effects, this gives rise to combine the lavender essential oil with chitosan in emulsion form [
39] this explains why it’s less effective than other treatment groups when used alone.
Together, the synergistic effect of the Chitosan & Lavandula combination group likely explains the pro-nounced decline in systemic and local inflammatory markers among other groups observed in this study. Fucidin have demonstrated anti-inflammatory properties, particularly by diminishing the secretion of tumor necrosis factor alpha (TNF-α&IL-6). It has an inhibitory effect associated with the inhibition of 12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced upregulation of the pro-inflammatory cytokines IL-1β, TNF-α, and COX-2 [
40].
Members of this group show less anti-inflammatory action than nanoparticles treatment groups and more an-ti-inflammatory action than Lavandula angustifolia extract group.
4.3. Modulation of Oxidative Stress
At day 14, MDA levels were significantly reduced, whereas SOD activity was markedly increased in the Chitosan & Lavandula group (p<0.001). This suggests improved redox balance and enhanced antioxidant defense.
Chitosan demonstrates redox-regulatory function by inhibiting reactive oxygen species (ROS) formation, preventing lipid oxidation through significantly reducing serum free fatty acids and malondialdehyde levels, and enhancing intracellular antioxidant enzyme activity in biological systems. Chitosan exhibits an effective capacity for metal-ion chelation, indicating its promise as a natural product antioxidant [
41].
The antioxidant effectiveness of polyphenols in Lavandula angustifolia comes from their capacity to inhibit the formation of free radicals or to neutralize reactive oxygen species. They can give a hydrogen atom or an electron, exhibiting reducing properties [
42]. These chemicals can inhibit oxidation processes by activating antioxidant enzymes such as superoxide dismutase, glutathione dismutase, glutathione peroxidase, and cata-lase, as well as by their chelating activity with metal ions [
43,
44].
The combination of Chitosan & Lavandula agents likely exerts an additive antioxidant effect, contributing to accelerated tissue repair.
Chitosan and Fucidin groups have higher antioxidant effect than Lavandula angustifolia group due to the nano particles size of Chitosan group, stronger anti-oxidant properties and better stability with longer lasting effect of both Fucidin and Chitosan groups
4.4. Antimicrobial Synergy Against Staphylococcus aureus
The lowest WBC level was observed among Chitosan & Lavandula combination group with significant dif-ference from other groups (P<0.001). The enhanced outcomes in the Chitosan & Lavandula combination group are also attributed to dual antimicrobial activity.
The cationic properties of chitosan enable it to engage with negatively charged microbial membranes, de-stroying their structural integrity and inhibiting the growth of bacteria and fungi. Moreover, chitosan's anti-bacterial efficacy has been evidenced against a wide array of microorganisms, including those frequently as-sociated with chronic wound infections especially staphylococcus aureus [
36].
The main chemical ingredients of L. angustifolia essential oil, including linalool, linalyl acetate, and ter-pinen-4-ol, suggest that their method of action mostly involves disrupting the lipid bilayer of the cell mem-brane, leading to bacterial cell leakage [
45,
46,
47].
Fucidin group members also show significant reduction in WBC count (p<0.001) in comparison with in-duced non treated group. It blocks bacterial protein synthesis by binding to elongation factor G (EF-G) on ribosomes, hence preventing the elongation of nascent polypeptides [
48].
The enhanced outcomes observed in the Chitosan and Lavandula group can be attributed to a multifaceted mechanism involving reduced bacterial load, downregulation of pro-inflammatory cytokines, and strength-ened antioxidant defenses. These synergistic actions account for the decreases in WBC, TNF-α/IL-6, and MDA levels, alongside the elevation of SOD activity, underscoring the therapeutic promise of integrating natural extracts with biomaterials to optimize infected wound healing.
4.5. Histopathological Findings
Histopathological analysis revealed comprehensive evidence of tissue-level healing among the groups: His-tological evaluation clearly revealed the gradual variations in tissue repair among the groups. In the untreated wounds, persistent epidermal loss, necrotic dermal tissue, vascular congestion, and significant leukocyte in-filtration indicated prolonged inflammation and delayed advancement to subsequent healing stages. These pathological characteristics align with chronic wound conditions in which ongoing infection and oxidative stress hinder fibroblast migration and collagen production [
49].
Treatment with Fucidin facilitated partial healing, as seen by re-epithelialization, immature dermal tissue, and angiogenesis; nevertheless, residual inflammation persisted, signifying an incomplete progression to tissue remodeling [
50]. Similarly, the use of Lavandula angustifolia extract enhanced epithelial and vascular re-sponses; but the presence of immature collagen bundles and minor inflammatory infiltrates indicated only a slight improvement in healing. This is in agreement with previous research indicating that Lavandula extracts decrease inflammatory cytokine expression and enhance fibroblast activity, although may be inadequate as a standalone treatment for complex infected wounds [
51].
In contrast, chitosan therapy resulted in significant enhancements, including the restoration of keratinized epithelium, organized dermal fibrous tissue, and heightened capillary density, suggestive of enhanced prolif-erative activity and decreased inflammation. Chitosan is recognized for its ability to induce macrophage po-larization, fibroblast proliferation, and collagen deposition, thus expediting the proliferative process to the remodeling phase transition [
49,
52].
The most remarkable result was noted in the combination of Chitosan and Lavandula, which resulted in complete tissue restoration—normal epidermis, mature dermal connective tissue abundant in collagen, exten-sive neovascularization, and fully intact cutaneous tissues, free of inflammatory infiltrates. These data vali-date that the combination not only improves structural recovery but also restores tissue integrity, a hallmark of real tissue regeneration rather than simple repair. Recent findings have established that the synergistic ef-fect of chitosan scaffolds infused with essential oils greatly accelerated granulation, collagen remodeling, and re-epithelialization compared to the use of each agent independently [
53].
The elevated collagen levels observed in the treated groups indicate that the formulations enhance the heal-ing process, as the rise in collagen content correlates with tissue maturation, a reduction in total cellularity (indicative of diminished inflammation), and the formation of blood vessels [
54].
Together, these histological results highlight the enhanced regenerating capacity of the Chitosan–Lavandula formulation, which combines the structural scaffold characteristics of chitosan with the anti-inflammatory and antioxidant properties of lavender. This dual action targets both the microbial/inflammatory load and the necessity for strong matrix formation, therefore facilitating systematic and comprehensive wound healing.
4.6. Wound Contraction Percentage %
Wound contraction is essential for the closure of full-thickness wounds, as the surrounding skin is drawn into the defect by forces produced within the granulation tissue. The myofibroblast, a distinct fibroblast type marked by cytoplasmic stress fibers abundant in α smooth muscle actin (SMA), is recognized as the cell phenotype accountable for wound contraction. The organization of the freshly generated connective tissue matrix into denser collagen fiber bundles generates tension, which facilitates the conversion of fibroblasts into myofibroblasts. TGFβ1 biochemically facilitates the conversion of fibroblasts into myofibroblasts in monolayer culture. Promoting the myofibroblast phenotype will help to enhance wound contraction; conversely, ob-structing the emergence of myofibroblasts is expected to impede or inhibit wound contraction [
55].
Wound contraction percentages at days 3, 7, and 14 were significantly higher in the Chitosan & Lavandula group compared to other groups (p<0.001), with day 14 showing the greatest contraction across all groups.
Chitosan offers a biocompatible scaffold that facilitates tissue regeneration, while volatile oils give antibac-terial and anti-inflammatory advantages, resulting in a multifunctional strategy for wound management. The incorporation of Lavandula into a Chitosan matrix could improve the antibacterial and anti-inflammatory properties of the mixture, demonstrating efficacy in facilitating wound healing and tissue regeneration (56).
The results together demonstrate a progressive improvement from untreated wounds to single-agent therapies (Fucidin, Lavandula, Chitosan), with the Chitosan and Lavandula combination yielding superior systemic, biochemical, histological, and functional effects. This combined therapy accelerates the resolution of in-flammation, increases antioxidant activity, and stimulates
Collagen deposition, angiogenesis, re-epithelialization, and wound contraction promote ordered tissue re-generation and efficient wound closure. The synergistic interaction of chitosan and Lavandula is a promising approach for the management of infected wounds, combining structural support, antibacterial properties, an-ti-inflammatory effects, and antioxidant capabilities.
5. Conclusions
The integration of chitosan nanoparticles-loaded hydrogel with Lavandula angustifolia extract demonstrates a potent synergistic effect in enhancing wound healing in S. aureus-infected wounds. By improving hematological and biochemical profiles, promoting favorable histopathological changes, and accelerating wound contraction, this combination therapy represents a promising alternative strategy for managing infected wounds. Future studies should focus on mechanistic insights and clinical translation to validate its efficacy in humans.
Author Contributions
Conceptualization, F.F.M.Z, and M.Q.Y.M.A.A,; Methodology F.F.M.Z, and M.Q.Y.M.A.A.; Software, F.F.M.Z; Validation, M.Q.Y.M.A.A.; FFMZ Investigations; F.F.M.Z; resources, F.F.M.Z.,; data curation, F.F.M.Z; writing original draft preparation F.F.M.Z; writing review and editing, F.F.M.Z; visualization M.Q.Y.M.A.A; supervision M.Q.Y.M.A.A ; project administration M.Q.Y.M.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This research was conducted from October 2024 to August 2025. All the experimental work was implemented following the protocol reviewed by the Iraqi Board Review of the College of Medicine/ Baghdad university after being approved by the scientific committee of the Department of Pharmacology at the College of Medicine/ Baghdad University. Number 36 On 2024/10/6.
Data Availability Statement
Data available with the researchers on reasonable demand, and the majority of the data were utilized in the manuscript.
Acknowledgments
Great and deep thanks to Prof. Dr. Faraedon M. Zardawi for his endless assistance, advising and encouragement during this research. Special thanks to Assist. Prof. Dr. Labeeb Ahmed Kadhim Al-zubaidi and Assist. Prof. Dr. Suha Mohammed Ibrahim/Ministry of Science and Technology for their assistance in the extraction and nano-materials preparation. special thanks to Assist. Prof. Dr. Adnan Khazaal Ajeel/Iraqi Center for cancer and medical Genetic researches, University of Mustansiriyah) for his assistances during this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ECM |
Extracellular Matrix |
| MRSA |
Methicillin resistant Staphylococcus aureus |
| GlcN |
Nacetyl-2-amino-2-deoxy-D-glucose glucosamine |
| Cs NPs |
Chitosan nanoparticles |
| WBC |
White Blood Cells |
| TNF-α |
Tumor Necrosis Factor-alpha |
| IL-6 |
Interleukin-6 |
| MDA |
Malondialdehyde |
| SOD |
Superoxide Dismutase |
| ecSOD |
Extracellular Super-oxide Dis-mutase |
| UV-Vis spectroscopy |
Ultraviolet–Visible Spectroscopy |
| FTIR |
Fourier Transform Infrared Spectroscopy |
| SEM |
Scanning Electron Microscopy |
| EDX |
Energy Dispersive X-ray Spectroscopy |
| AFM |
Atomic Force Microscopy |
| XRD |
X-ray Diffraction |
| TLR4 |
Toll-Like Receptor 4 |
| NF-κB |
Nuclear Factor kappa-light-chain-enhancer of activated B cell |
| Nrf2 |
Nuclear factor erythroid 2–related factor 2 |
| DMSO |
Dimethyl Sulfoxide |
References
- Maillard J.Y., Kampf G., Cooper R. Antimicrobial stewardship of antiseptics that are pertinent to wounds: The need for a united approach. JAC Antimicrob. Resist. 2021; 3:dlab 027. doi: 10.1093/jacamr/dlab027. [DOI] [PMC free article] [PubMed] [Google Scholar].
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef] [Green Version].
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed].
- Sarheed, O.; Ahmed, A.; Shouqair, D.; Boateng, J. Antimicrobial dressings for improving wound healing. In Wound Healing-New Insights into Ancient Challenges; Alexandrescu, V., Ed.; InTech: London, UK, 2016; pp. 373–398. ISBN 978-953-51-2679-9. [Google Scholar].
- Cardona, A.F.; Wilson, S.E. Skin and soft-tissue infections: A critical review and the role of telavancin in their treatment. Clin. Infect. Dis. 2015, 61, S69–S78. [Google Scholar] [CrossRef] [PubMed].
- Shrestha, G.; Raphael, J.; Leavitt, S.D.; St Clair, L.L. In vitro evaluation of the antibacterial activity of ex-tracts from 34 species of North American lichens. Pharm. Biol. 2014, 52, 1262–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version].
- Gao W., Chen Y., Zhang Y., Zhang Q., Zhang L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018;127:46–57. doi: 10.1016/j.addr.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar].
- Hamdan S., Pastar I., Drakulich S., Dikici E., Tomic-Canic M., Deo S., Daunert S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017;3:163–175. doi: 10.1021/acscentsci.6b00371. [DOI] [PMC free article] [PubMed] [Google Scholar].
- Ghormade V., Pathan E.K., Deshpande M.V. Can fungi compete with marine sources for chitosan produc-tion? Int. J. Biol. Macromol. 2017;104:1415–1421. doi: 10.1016/j.ijbiomac.2017.01.112. [DOI] [PubMed] [Google Scholar].
- Aranaz I, Alcántara AR, Civera MC, Arias C, Elorza B, Heras Caballero A, Acosta N. Chitosan: An Overview of Its Properties and Applications. Polymers (Basel). 2021 Sep 24;13(19):3256. doi: 10.3390/polym13193256. PMID: 34641071; PMCID: PMC8512059.
- Angelova, V.R.; Grekov, D.F.; Kisyov, V.K.; Ivanov, K.I. Potential of Lavender (Lavandula vera L.) for Phytoremediation of Soils Contaminated with Heavy Metals. Int. J. Sci. Res. Innov. 2015, 9, 465–472. [Google Scholar].
- Dobros, N.; Zawada, K.D.; Paradowska, K. Phytochemical Profiling, Antioxidant and AntiInflammatory Activity of Plants Belonging to the Lavandula Genus. Molecules 2023, 28, 256. [Google Scholar] [CrossRef] [PubMed].
- Tăbăraşu, A.-M.; Anghelache, D.-N.; Găgeanu, I.; Biris, S.-S.; Vlădut, N.-V. Considerations on the Use of Active Compounds Obtained from Lavender. Sustainability 2023, 15, 8879. [Google Scholar] [CrossRef].
- Kırkıncı, S., Gercek, Y.C., Baştürk, F.N. et al. Evaluation of lavender essential oils and byproducts using microwave hydrodistillation and conventional hydrodistillation. Sci Rep 14, 20922 (2024). https://doi.org/10.1038/s41598-024-71115-w.
- El- Ghaffar, M. A. A. and Hashem, M. S. Chitosan and its amino acids condensation adducts as reactive natural polymer supports for cellulase immobilization. Carbohydrate Polymers, 2010, 81 (3): 507–516. http://doi.org/10.1016/j.carbpol. 2010. 02. 025.
- El- Ghaffar, M. A. and Hashem, M. S. Immobilization of α-amylase onto chitosan and its amino acid condensation adducts. Journal of Applied Polymer Science, 2009 112 (2): 805-14.
- Tan WN, Samling BA, Tong WY, Chear NJ, Yusof SR, Lim JW, Tchamgoue J, Leong CR, Ramanathan S. Chitosan-Based Nanoencapsulated Essential Oils: Potential Leads against Breast Cancer Cells in Preclinical Studies. Polymers (Basel). 2024 Feb 8;16(4):478. doi: 10.3390/polym16040478. PMID: 38399856; PMCID: PMC10891598.
- Silva DC, Plapler H, Costa MM, Silva SR, Sá Mda C, Silva BS. Low level laser therapy (AlGaInP) applied at 5J/cm2 reduces the proliferation of Staphylococcus aureus MRSA in infected wounds and intact skin of rats. An Bras Dermatol. 2013 Jan Feb; 88 (1):50-5. doi: 10.1590/s0365 05962013000100005. PMID: 23539003; PMCID: PMC3699931.
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016 Apr;6(2):71-79. doi:10.1016/j.jpha.2015.11.005. Epub 2015 Dec 2. PMID: 29403965; PMCID: PMC5762448.
- de Moura Estevão, Lígia Reis, et al. "Morphological evaluation of wound healing event the excisional wound healing model in rats." Bio-protocol 9.13 (2019): e3285-e3285.
- Chinko, Bruno Chukwuemeka, and Awosebiotonke Dolly Precious-Abraham. "Wound healing activity of hydromethanolic Dioscorea bulbifera extract on male wistar rat excision wound models." Pharmacological Research-Modern Chinese Medicine 11 (2024): 100425.
- Survana Kim Suvarna, Layton, C. and Bancroft, J.D. Bancroft’s theory and practice of histological techniques. Oxford: Elsevier, 2019. 8th ed :672.
- Gupta A, Kumar P. Assessment of the histological state of the healing wound. Plast Aesthet Res. 2015;2:239-42. http://dx.doi.org/10.4103/2347-9264.158862.
- Ozgok Kangal MK, Regan JP. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): May 1, 2023. Wound Healing. [PubMed].
- Cefalu, J.E.; Barrier, K.M.; Davis, A.H. Wound infections in critical care. Crit. Care Nurs. Clin. N. Am. 2017, 29, 81. [Google Scholar] [CrossRef].
- Han, D.; Li, Y.; Liu, X.; Li, B.; Han, Y.; Zheng, Y.; Yeung, K.; Li, C.; Cui, Z.; Liang, Y. Rapid bacteria trapping and killing of metal-organic frameworks strengthened photo-responsive hydrogel for rapid tissue repair of bacterial infected wounds. Chem. Eng. J. 2020, 396, 125194. [Google Scholar] [CrossRef].
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment strategies for infected wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef].
- Tejada, S.; Batle, J.M.; Ferrer, M.D.; Busquets-Cortés, C.; Monserrat-Mesquida, M.; Nabavi, S.M.; Del Mar Bibiloni, M.; Pons, A.; Sureda, A. Therapeutic Effects of Hyperbaric Oxygen in the Process of Wound Healing. Curr. Pharm. Des. 2019, 25, 1682–1693. [Google Scholar] [CrossRef].
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef].
- Johnson BZ, Stevenson AW, Prêle CM, Fear MW, Wood FM. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines. 2020 Apr 30;8(5):101. doi: 10.3390/biomedicines8050101. PMID: 32365896; PMCID: PMC7277690.
- Yusuf K. The Role of TNF-Alpha in the Wound Healing Process: Molecular and Clinical Perspectives-A Systematic Literature Review. Jurnal RSMH Palembang. 2022;3(2):222-8.
- Dunnill C, Patton T, Brennan J, et al. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017;14(1):89–96. doi:10.1111/iwj.12557.
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Su-peroxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. https://doi.org/10.3390/antiox12091675.
- Naskar, A.; Kim, K.-s. Recent Advances in Nanomaterial-Based Wound-Healing Therapeutics. Pharma-ceutics 2020, 12, 499. https://doi.org/10.3390/pharmaceutics12060499.
- Korniienko, V.; Husak, Y.; Diedkova, K.; Varava, Y.; Grebnevs, V.; Pogorielova, O.; Bērtiņš, M.; Korniienko, V.; Zandersone, B.; Ramanaviciene, A.; et al. Antibacterial Potential and Biocompatibility of Chitosan/Polycaprolactone Nanofibrous Membranes Incorporated with Silver Nanoparticles. Polymers 2024, 16, 1729. https://doi.org/10.3390/polym16121729.
- Fong D, Hoemann CD. Chitosan immunomodulatory properties: perspectives on the impact of structural properties and dosage. Future Sci OA. 2017;4(1). doi:10.4155/fsoa-2017-0064.
- Lagouri, Vasiliki, et al. "Lamiaceae Family Plants as Natural Solutions for Inflammation and Blood Sugar Management." ESI Preprints (European Scientific Journal, ESJ) 20.30 (2024): 34-34.
- Pandur E, Balatinácz A, Micalizzi G, Mondello L, Horváth A, Sipos K, Horváth G. Anti-inflammatory effect of lavender (Lavandula angustifolia Mill.) essential oil prepared during different plant phenophases on THP-1 macrophages. BMC Complement Med Ther. 2021 Nov 24;21(1):287. doi: 10.1186/s12906-021-03461-5. PMID: 34819075; PMCID: PMC8611982.
- Danila A, Muresan EI, Ibanescu SA, Popescu A, Danu M, Zaharia C, Türkoğlu GC, Erkan G, Staras AI. Preparation, characterization, and application of polysaccharide-based emulsions incorporated with lavender essential oil for skin-friendly cellulosic support. International Journal of Biological Macromolecules. 2021 Nov 30;191:405-13.
- Wu P. P., He H., Hong W. D., Wu T. R., Huang G. Y., Zhong Y. Y., et al. (2018a). The Biological Evaluation of Fusidic Acid and its Hydrogenation Derivative as Antimicrobial and Anti-inflammatory Agents. Infect. Drug Resist. 11, 1945–1957. 10.2147/IDR.S176390 [DOI] [PMC free article] [PubMed] [Google Scholar][Ref list].
- Ivanova DG, Yaneva ZL. Antioxidant Properties and Redox-Modulating Activity of Chitosan and Its Derivatives: Biomaterials with Application in Cancer Therapy. Biores Open Access. 2020 Mar 12;9(1):64-72. doi: 10.1089/biores.2019.0028. PMID: 32219012; PMCID: PMC7097683.
- Boligon A.A., Machado M.M., Athayde M.L. Technical evaluation of antioxidant activity. Med Chem. 2014;4:517–522. doi: 10.4172/2161-0444.1000188. [DOI] [Google Scholar][Ref list]37. Moon, T., Wilkinson, J. M., & Cavanagh, H. M. A. Antibacterial activity of essential oils in combination with conventional antibi-otics. Phytomedicine, 2020, 67, 153165.
- Tsao R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar][Ref list].
- De Lima Cherubim D.J., Buzanello Martins C.V., Oliveira Fariña L., da Silva de Lucca R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020;19:33–37. doi: 10.1111/jocd.13093. [DOI] [PubMed] [Google Scholar][Ref list].
- Carson C. F., Mee B. J., Riley T. V. Mechanism of action of Melaleuca alternifolia (Tea Tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron micros-copy. Antimicrobial Agents and Chemotherapy. 2002;46(6):1914–1920. doi: 10.1128/aac.46.6.1914-1920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar].
- Trombetta D., Castelli F., Sarpietro M. G., et al. Mechanisms of antibacterial action of three monoterpenes. Antimicrobial Agents and Chemotherapy. 2005;49(6):2474–2478. doi: 10.1128/AAC.49.6.2474-2478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar].
- Lopez-Romero J. C., González-Ríos H., Borges A., Simões M. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus . Evidence-Based Complementary and Alternative Medicine. 2015;2015:9. doi: 10.1155/2015/795435.795435 [DOI] [PMC free article] [PubMed] [Google Scholar].
- Naseef, H.; Sahoury, Y.; Farraj, M.; Qurt, M.; Abukhalil, A.D.; Jaradat, N.; Sabri, I.; Rabba, A.K.; Sbeih, M. Novel Fusidic Acid Cream Containing Metal Ions and Natural Products against Multidrug-Resistant Bacteria. Pharmaceutics 2022, 14, 1638. https://doi.org/10.3390/pharmaceutics14081638.
- Bektas N, Şenel B, Yenilmez E, Özatik O, Arslan R. Evaluation of wound healing effect of chitosan-based gel formulation containing vitexin. Saudi Pharmaceutical Journal. 2020 Jan 1;28(1):87-94.
- Gurel MS, Naycı S, Turgut AV, Bozkurt ER. Comparison of the effects of topical fusidic acid and ri-famycin on wound healing in rats. International Wound Journal. 2015 Feb;12(1):106-10.
- Koca Kutlu A, Çeçen D, Gürgen SG, Sayın O, Çetin F. A comparison study of growth factor expression following treatment with transcutaneous electrical nerve stimulation, saline solution, Povidone-iodine, and lavender oil in wounds healing. Evidence-Based Complementary and Alternative Medicine. 2013;2013(1):361832.
- Mohamed N, Al Balah OF, Refat M, Badr AM, Afifi A. Low-level laser and chitosan nanoparticles therapy speeds up the process of skin wound healing in mice: histological, hematological, and proinflammatory cyto-kines assessment. The Journal of Basic and Applied Zoology. 2025 Mar 2;86(1):17.
- Osanloo, M., Noori, F., Varaa, N. et al. The wound healing effect of polycaprolactone-chitosan scaffold coated with a gel containing Zataria multiflora Boiss. volatile oil nanoemulsions. BMC Complement Med Ther 24, 56 (2024). https://doi.org/10.1186/s12906-024-04352-1.
- Oryan A, Mohammadalipour A, Moshiri A, Tabandeh MR. Topical Application of Aloe vera Accelerated Wound Healing, Modeling, and Remodeling. Ann Plast Surg [Internet]. 2016;77(1):37-46. Available from: https://doi.org/10.1097/SAP.0000000000000239.
- Au K, Ehrlich HP. When the Smad signaling pathway is impaired, fibroblasts advance open wound con-traction. Experimental and molecular pathology. 2010 Dec 1;89(3):236-40.
- Nabil, Yassmin, et al. "Progress in chitosan/essential oil/ZnO nanobiocomposites fabrication for wound healing applications: A review." International Journal of Biological Macromolecules (2025): 145123.
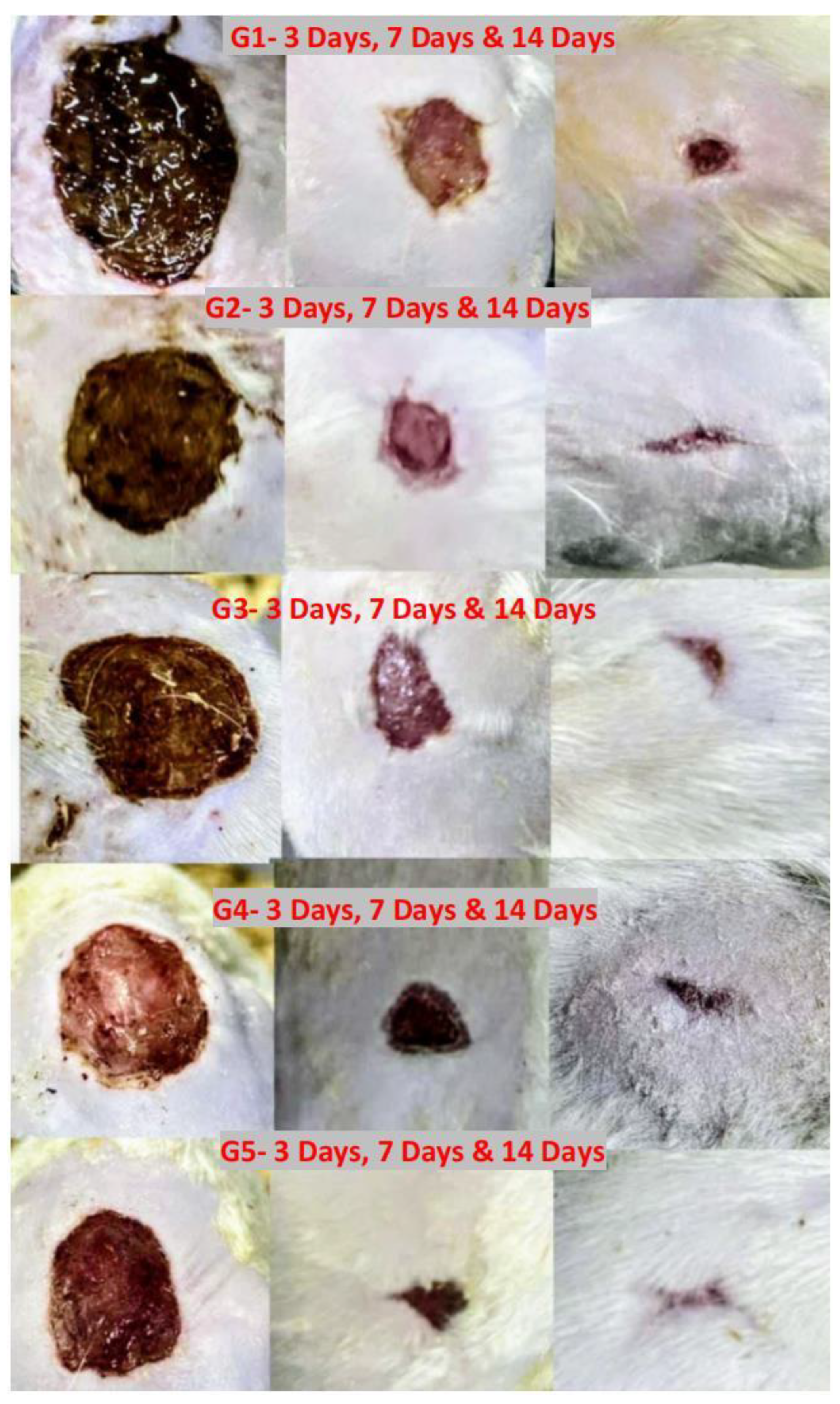
Figure 2.
(A) Excisional wound of the rat at the first day, (B) Application of the bacterial suspension to the wound.
Figure 2.
(A) Excisional wound of the rat at the first day, (B) Application of the bacterial suspension to the wound.
Figure 3.
(G1A-X10, Normal): normal appearance of epidermis lining epithelial cells (E), normal dermal collagen fibers and cells (D), sweat gland with duct (black arrow) & sebaceous glands (red arrow). (G1B-X40) lining epithelial cells (E), normal dermal collagen fibers with capillaries (D) and fibroblasts (black arrow) & blood vessel (red arrow). (G2A-X10-Induction) shows: skin ulcer covered by thick layer of necrotic tissue, thick fibrin deposition, degeneration with necrosis of fibrous tissue and infiltration of MNLs. (G2B-X40) shows hemorrhagic dermal ulcer (Black arrows), fibrin deposition (f) & degeneration with necrosis of fibrous tissue. (G3A-X10 Fucidin) shows hyperkeratosis (red arrow), well reepithelization (E), and immature fibrous tissue (D) with marked angiogenesis (black arrows). (G3B-X40) well regenerated epidermis epithelium, sub epithelial angiogenesis & little infiltration of leukocytes.
Figure 3.
(G1A-X10, Normal): normal appearance of epidermis lining epithelial cells (E), normal dermal collagen fibers and cells (D), sweat gland with duct (black arrow) & sebaceous glands (red arrow). (G1B-X40) lining epithelial cells (E), normal dermal collagen fibers with capillaries (D) and fibroblasts (black arrow) & blood vessel (red arrow). (G2A-X10-Induction) shows: skin ulcer covered by thick layer of necrotic tissue, thick fibrin deposition, degeneration with necrosis of fibrous tissue and infiltration of MNLs. (G2B-X40) shows hemorrhagic dermal ulcer (Black arrows), fibrin deposition (f) & degeneration with necrosis of fibrous tissue. (G3A-X10 Fucidin) shows hyperkeratosis (red arrow), well reepithelization (E), and immature fibrous tissue (D) with marked angiogenesis (black arrows). (G3B-X40) well regenerated epidermis epithelium, sub epithelial angiogenesis & little infiltration of leukocytes.
Figure 4.
(G4A-X40 Lavandula) revealed marked dermal papilla within epidermis, with immature dermal collagen fibers and marked angiogenesis. (G4B-X40). revealed well regenerated epidermis that showed little intra epithelial leukocytes (Red arrow), immature dermal collagen fibers (D) with marked angiogenesis (Black arrows). (G5A-X10 Chitosan), well reepithelization of epidermis, dermis comprised of mature fibrous tissue with marked angiogenesis and no infiltration of leukocytes. (G5B-X40) well reepithelization of epidermis (Red arrow), dermis comprised of mature fibrous tissue (D) with marked angiogenesis (Black arrows) and no infiltration of leukocytes. (G6A-X10 Chitosan &Lavandula). epidermal reepithelization with keratinized stratified squamous epithelium (black arrow). Matured granulation tissue formation (yellow arrow), with more prominent fibroblasts’ proliferation and collagen fibers deposition, with more mature collagen fibers deposition (white arrow). Moderate angiogenesis with very few Inflammatory cells infiltrates (green arrow). (G6B-X40), showing matured dermal granulation tissue showing more matured fibrous tissue, with more prominent proliferative fibroblasts (yellow arrow), and coarse collagen fibers deposition (green arrows). Angiogenesis (black arrow).
Figure 4.
(G4A-X40 Lavandula) revealed marked dermal papilla within epidermis, with immature dermal collagen fibers and marked angiogenesis. (G4B-X40). revealed well regenerated epidermis that showed little intra epithelial leukocytes (Red arrow), immature dermal collagen fibers (D) with marked angiogenesis (Black arrows). (G5A-X10 Chitosan), well reepithelization of epidermis, dermis comprised of mature fibrous tissue with marked angiogenesis and no infiltration of leukocytes. (G5B-X40) well reepithelization of epidermis (Red arrow), dermis comprised of mature fibrous tissue (D) with marked angiogenesis (Black arrows) and no infiltration of leukocytes. (G6A-X10 Chitosan &Lavandula). epidermal reepithelization with keratinized stratified squamous epithelium (black arrow). Matured granulation tissue formation (yellow arrow), with more prominent fibroblasts’ proliferation and collagen fibers deposition, with more mature collagen fibers deposition (white arrow). Moderate angiogenesis with very few Inflammatory cells infiltrates (green arrow). (G6B-X40), showing matured dermal granulation tissue showing more matured fibrous tissue, with more prominent proliferative fibroblasts (yellow arrow), and coarse collagen fibers deposition (green arrows). Angiogenesis (black arrow).
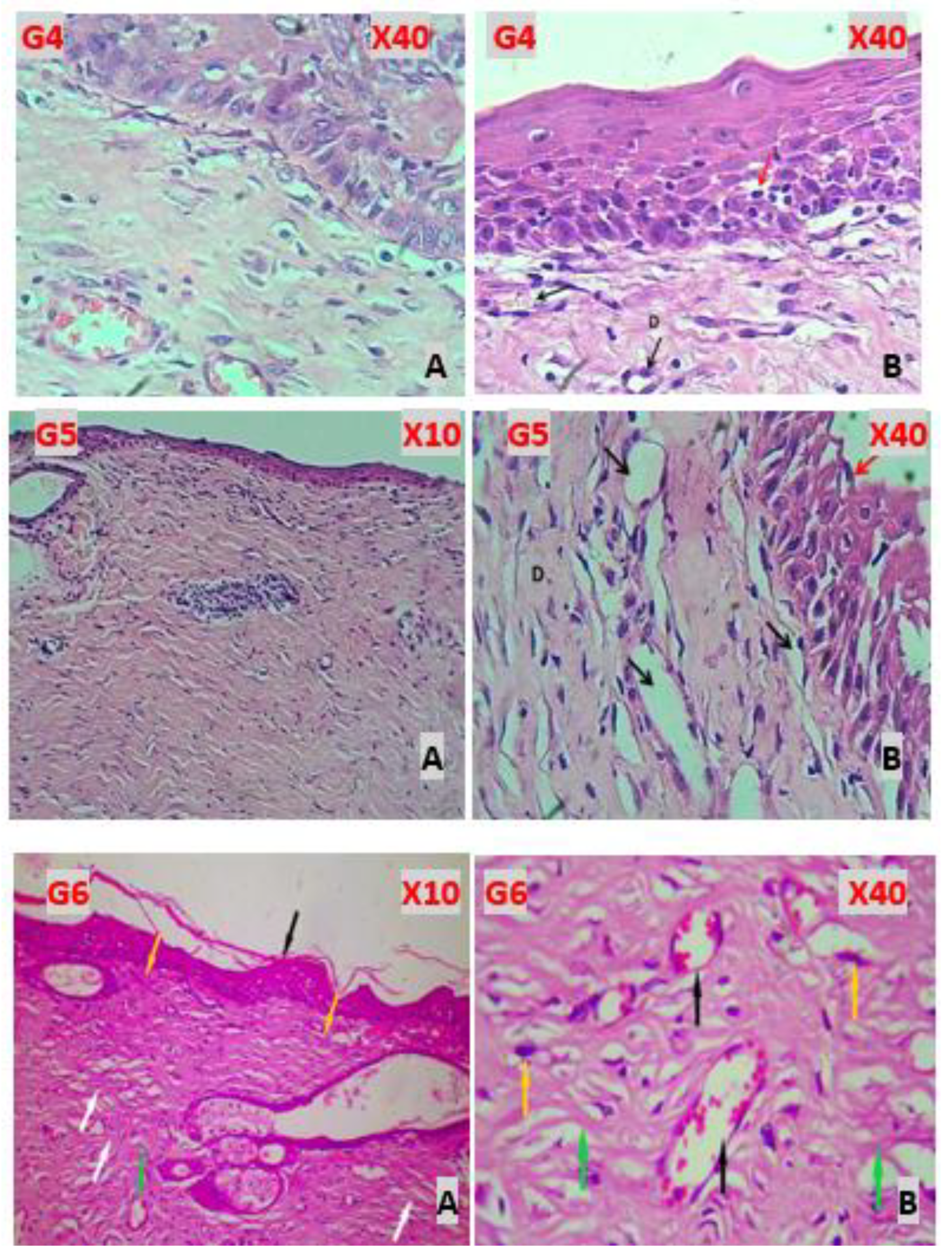
Figure 5.
Wound contraction percentage for Groups (2 to 6) at day 3, day 7, and day 14 of therapy. G1 Induced non-treated Group, G2 Fucidin group, G3 Lavandula group, G4 Chitosan group, and G5 Chitosan & Lavandula group.
Figure 5.
Wound contraction percentage for Groups (2 to 6) at day 3, day 7, and day 14 of therapy. G1 Induced non-treated Group, G2 Fucidin group, G3 Lavandula group, G4 Chitosan group, and G5 Chitosan & Lavandula group.
Table 1.
Comparison between apparently healthy control group and induced non-treated group in relation to different measured parameters (Hematological biomarker: WBC count) and (ELISA Biomarkers at day 14: TNF-α, IL-6, MDA, and SOD).
Table 1.
Comparison between apparently healthy control group and induced non-treated group in relation to different measured parameters (Hematological biomarker: WBC count) and (ELISA Biomarkers at day 14: TNF-α, IL-6, MDA, and SOD).
| Parameters |
Groups |
P* value |
| Control Group |
Induced non-treated |
| Median (IQR) |
Median (IQR) |
| WBC count × 10⁹/L. at day 7 |
5.85 (1.63) |
20.1 (1.75) |
<0.001 |
| WBC count × 10⁹/L. at day 14 |
5.8 (1.7) |
14.4 (1.27) |
<0.001 |
| TNF-alpha pg/ml |
60.67 (25.85) |
379.66 (92.71) |
<0.001 |
| IL-6 pg/ml |
95.71 (23.2) |
299.15 (20.1) |
<0.001 |
| MDA ng/ml |
94.87 (21.7) |
336.07 (103.41) |
<0.001 |
SOD ng/ml |
3.73 (1.37) |
0.88 (0.13) |
<0.001 |
Table 2.
Comparison between apparently healthy control group and induced non-treated group in relation to Semi quantitative wound scoring system:.
Table 2.
Comparison between apparently healthy control group and induced non-treated group in relation to Semi quantitative wound scoring system:.
| Parameters |
Groups |
P* value |
| Control Group |
Induced non-treated |
| Median (IQR) |
Median (IQR) |
| Reepithelization |
3.0 (0) |
0.0 (0) |
<0.001 |
| Granulation Tissue Formation |
0.0 (0) |
1.0 (0) |
<0.001 |
| Collagen Deposition |
3.0 (0) |
0.0 (0) |
<0.001 |
| Inflammatory Infiltrate |
0.0 (0) |
3.0 (0) |
<0.001 |
| Angiogenesis |
0.0 (0) |
3.0 (0) |
<0.001 |
| Hemorrhage |
0.0 (0) |
3.0 (0) |
<0.001 |
| Necrosis/Degeneration |
0.0 (0) |
3.0 (0) |
<0.001 |
Table 3.
Comparison between all treated groups (Fucidin group, Lavandula group, Chitosan group, and Chitosan & Lavandula group) in relation to different measured parameters (Hematological biomarker: WBC count) and (ELISA Biomarkers at day 14: TNF-α, IL-6, MDA, and SOD).
Table 3.
Comparison between all treated groups (Fucidin group, Lavandula group, Chitosan group, and Chitosan & Lavandula group) in relation to different measured parameters (Hematological biomarker: WBC count) and (ELISA Biomarkers at day 14: TNF-α, IL-6, MDA, and SOD).
| Parameters |
Fucidin group |
Lavandula group |
Chitosan group |
Chitosan & Lavandula group |
P* value |
| Median (IQR) |
Median (IQR) |
Median (IQR) |
Median (IQR) |
| WBC count × 10⁹/L. at day 7 |
14.15 (3.15) |
17.9 (1.65) |
12.3 (1.72) |
10.65 (1.4) |
<0.001 |
| WBC count × 10⁹/L. at day14 |
7.95 (2.15) |
9.15 (1.55) |
7.0 (1.5) |
6.0 (1.17) |
<0.001 |
| TNF-alpha pg/ml |
94.34 (17.32) |
167.52 (100.5) |
74.46 (9.45) |
65.36 (4.15) |
<0.001 |
| IL-6 pg/ml |
106.14 (82.29) |
147.39 (105.3) |
96.15 (10.5) |
90.11 (17.6) |
0.004 |
| MDA ng/ml |
158.33 (87.35) |
195.68 (96.1) |
118.18 (43.93) |
99.67 (9.0) |
<0.001 |
| SOD ng/ml |
2.52 (1.03) |
1.81 (0.46) |
2.42 (0.59) |
3.86 (1.21) |
<0.001 |
Table 4.
Comparison between all treated groups (Fucidin group, Lavandula group, Chitosan group, and Chitosan & Lavandula group) in relation to Semi quantitative wound scoring system.
Table 4.
Comparison between all treated groups (Fucidin group, Lavandula group, Chitosan group, and Chitosan & Lavandula group) in relation to Semi quantitative wound scoring system.
| Parameters |
Fucidin group |
Lavandula group |
Chitosan group |
Chitosan & Lavandula group |
P value |
| Median (IQR) |
Median (IQR) |
Median (IQR) |
Median (IQR) |
| Reepithelization |
3.0 (0) |
2.0 (0) |
3.0 (0) |
3.0 (0) |
<0.001 |
| Granulation Tissue Formation |
3.0 (0) |
2.0 (0) |
3.0 (0) |
3.0 (0) |
<0.001 |
| Collagen Deposition |
2.0 (0) |
2.0 (0) |
3.0 (0) |
3.0 (0) |
<0.001 |
| Inflammatory Infiltrate |
1.0 (0) |
1.0 (0) |
0.0 (0) |
0.0 (0) |
<0.001 |
| Angiogenesis |
3.0 (0) |
3.0 (0) |
2.0 (0) |
2.0 (0) |
<0.001 |
| Hemorrhage |
0.0 (0) |
0.0 (0) |
0.0 (0) |
0.0 (0) |
1 |
| Necrosis/Degeneration |
0.0 (0) |
0.0 (0) |
0.0 (0) |
0.0 (0) |
1 |
| Wound contraction % day 3 |
38.0 (0) |
37.36 (0) |
38.85 (0) |
40.12 (0) |
<0.001 |
| Wound contraction % day 7 |
56.05 (0) |
52.01 (0) |
57.53 (0) |
58.81 (0) |
<0.001 |
| Wound contraction % day 14 |
92.14 (0) |
89.38 (0) |
93.41 (0) |
94.69 (0) |
<0.001 |
Table 5.
Comparison between induced groups (Induced non-treated Group, Fucidin group, Lavandula group, Chitosan group, and Chitosan & Lavandula group) in relation to different measured parameters (Hematological biomarker: WBC count) and (ELISA Biomarkers at day 14: TNF-α, IL-6, MDA, and SOD).
Table 5.
Comparison between induced groups (Induced non-treated Group, Fucidin group, Lavandula group, Chitosan group, and Chitosan & Lavandula group) in relation to different measured parameters (Hematological biomarker: WBC count) and (ELISA Biomarkers at day 14: TNF-α, IL-6, MDA, and SOD).
| Parameters |
Induced non-treated Group |
Fucidin group |
Lavandula group |
Chitosan group |
Chitosan & Lavandula group |
P* value |
| Median (IQR) |
Median (IQR) |
Median (IQR) |
Median (IQR) |
Median (IQR) |
| WBC count × 10⁹/L. at day 7 |
20.1 (1.75) |
14.15 (3.15)
a** |
17.9 (1.65)
a* b** |
12.3 (1.72)
a** b* c** |
10.65 (1.4)
a** b* c** d* |
<0.001 |
| WBC count × 10⁹/L. at day 14 |
14.4 (1.27) |
7.95 (2.15)
a** |
9.15 (1.55)
a** b* |
7.0 (1.5)
a** bNS c** |
6.0 (1.17)
a** b* c** d* |
<0.001 |
| TNF-alpha pg/ml |
379.66 (92.71) |
94.34 (17.32)
a** |
167.52 (100.5)
a** b* |
74.46 (9.45)
a** b* c** |
65.36 (4.15)
a** b** c** d** |
<0.001 |
| IL-6 pg/ml |
299.15 (20.1) |
106.14 (82.29)
a** |
147.39 (105.3)
a** bNS |
96.15 (10.5)
a** bNS c* |
90.11 (17.6)
a** b* c* dNS |
<0.001 |
| MDA ng/ml |
336.07 (103.41) |
158.33 (87.35)
a** |
195.68 (96.1)
a** bNS |
118.18 (43.93)
a** b* c* |
99.67 (9.0)
a** b** c** dNS |
<0.001 |
| SOD ng/ml |
0.88 (0.13) |
2.52 (1.03)
a** |
1.81 (0.46)
a** b* |
2.42 (0.59)
a** bNS c* |
3.86 (1.21)
a** b* c** d* |
<0.001 |
Table 6.
Comparison between induced groups (Induced non-treated Group, Fucidin group, Lavandula group, Chitosan group, and (Chitosan & Lavandula group) in relation to Semi quantitative wound scoring system and Wound contraction %:.
Table 6.
Comparison between induced groups (Induced non-treated Group, Fucidin group, Lavandula group, Chitosan group, and (Chitosan & Lavandula group) in relation to Semi quantitative wound scoring system and Wound contraction %:.
| Parameters |
Groups |
P* value |
| Induced non-treated Group |
Fucidin group |
Lavandula group |
Chitosan group |
Chitosan & Lavandula group |
| Median (IQR) |
Median (IQR) |
Median (IQR) |
Median (IQR) |
Median (IQR) |
| Reepithelization |
0.0 (0) |
3.0 (0)
a** |
2.0 (0)
a** b** |
3.0 (0)
a** bNS c** |
3.0 (0)
a** bNS c** dNS |
<0.001 |
| Granulation Tissue Formation |
1.0 (0) |
3.0 (0)
a** |
2.0 (0)
a** b** |
3.0 (0)
a** bNS c** |
3.0 (0)
a** bNS c** dNS |
<0.001 |
| Collagen Deposition |
0.0 (0) |
2.0 (0)
a** |
2.0 (0)
a** bNS |
3.0 (0)
a** b** c** |
3.0 (0)
a** b** c** dNS |
<0.001 |
| Inflammatory Infiltrate |
3.0 (0) |
1.0 (0)
a** |
1.0 (0)
a** bNS |
0.0 (0)
a** b** c** |
0.0 (0)
a** b** c** dNS |
<0.001 |
| Angiogenesis |
3.0 (0) |
3.0 (0)
aNS |
3.0 (0)
aNS bNS |
2.0 (0)
a** b** c** |
2.0 (0)
a** b** c** dNS |
<0.001 |
| Hemorrhage |
3.0 (0) |
0.0 (0)
a** |
0.0 (0)
a** bNS |
0.0 (0)
a** bNS cNS |
0.0 (0)
a** bNS cNS dNS |
<0.001 |
| Necrosis/Degeneration |
3.0 (0) |
0.0 (0)
a** |
0.0 (0)
a** bNS |
0.0 (0)
a** bNS cNS |
0.0 (0)
a** bNS cNS dNS |
<0.001 |
| Wound contraction % day 3 |
5.52 (0) |
38.0 (0)
a** |
37.36 (0)
a** b** |
38.85 (0)
a** b** c** |
40.12 (0)
a** b** c** d** |
<0.001 |
| Wound contraction % day 7 |
36.09 (0) |
56.05 (0)
a** |
52.01 (0)
a** b** |
57.53 (0)
a** b** c** |
58.81 (0)
a** b** c** d** |
<0.001 |
| Wound contraction % day 14 |
81.52 (0) |
92.14 (0)
a** |
89.38 (0)
a** b** |
93.41 (0)
a** b** c** |
94.69 (0)
a** b** c** d** |
<0.001 |
Table 7.
Comparison between induced groups (Induced non-treated Group, Fucidin group, Lavandula group, Chitosan group, and Chitosan & Lavandula group) in relation to Wound contraction % through different time of therapy.
Table 7.
Comparison between induced groups (Induced non-treated Group, Fucidin group, Lavandula group, Chitosan group, and Chitosan & Lavandula group) in relation to Wound contraction % through different time of therapy.
| Groups |
Wound contraction % day 3 |
Wound contraction % day 7 |
Wound contraction % day 14 |
P* value |
| Median (IQR) |
Median (IQR) |
|
| Induced non-treated Group |
5.52 (0) |
36.09 (0) |
81.52 (0) |
<0.001 |
| Fucidin group |
38.0 (0) |
56.05 (0) |
92.14 (0) |
<0.001 |
| Lavandula group |
37.36 (0) |
52.01 (0) |
89.38 (0) |
<0.001 |
| Chitosan group |
38.85 (0) |
57.53 (0) |
93.41 (0) |
<0.001 |
| Chitosan & Lavandula group |
40.12 (0) |
58.81 (0) |
94.69 (0) |
<0.001 |
| P** among all groups |
<0.001 |
<0.001 |
<0.001 |
- |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).