Submitted:
28 August 2025
Posted:
29 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Drug Substances
2.1.1. FAPI-46
2.1.2. Gallium-68
2.2. Investigational Medicinal Product under Test (IMP)
2.2.1. Description and Composition of the IMP
2.2.1. Description and Composition of the IMP
2.3. Quality Controls
2.3.1. Acceptance Criteria
2.3.2. Validation of the Analytical Procedures
2.3.3. Bioburden
2.3.4. Batch Analysis and Process Validation
2.3.5. Stability
3. Discussion
4. Materials and Methods
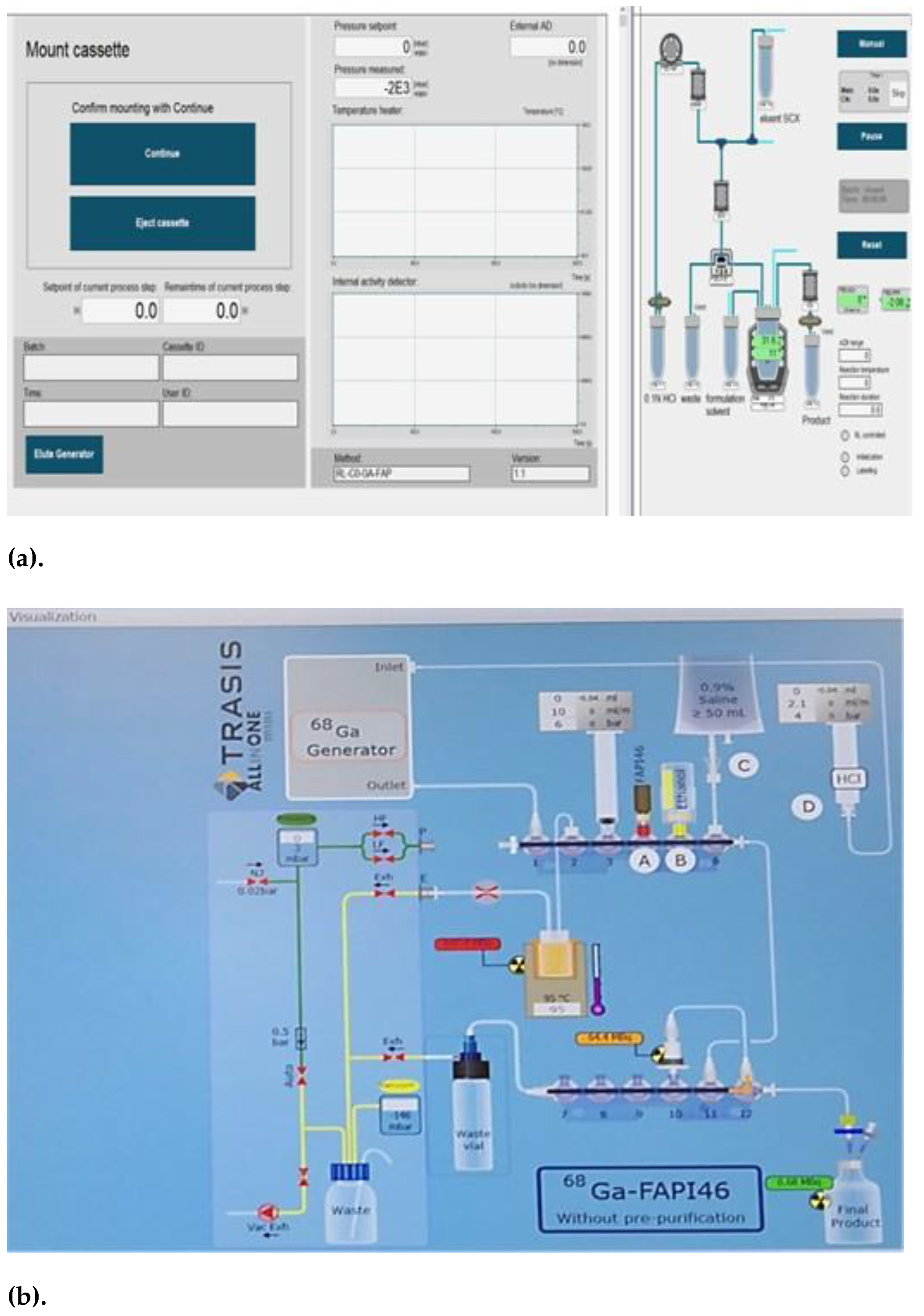
4.1. Description of [⁶⁸Ga]Ga-FAPI-46 Manufacturing Process
4.1.1. Set up of Radiosynthesizer
4.1.2. Reagents
-
Reagent set product by ABX (Heinrich-Glaeser- Straße 10-14, 01454 Radeberg, Germany) composed by:
- ○
- Vial 1 (EZ-102-V1) containing 5 ml of NaCl 5M/HCl 30%
- ○
- Vial 2 (EZ-102-V2) containing 680 mg of sodium acetate trihydrate
- ○
- Vial 5 (EZ-102-V5) containing 3 mg of ascorbic acid
- Water for injectable preparations (100 ml bottles) with MA was purchased from Monico S.p.A. Venezia/Mestre, Italy
- Sodium Chloride 0,9% 100 ml with a MA was purchased form Fresenius Kabi S.r.l. Isola della Scala, Italy.
- Single use sterile cassettes were produced by Eckert & Ziegler Eurotope GmbH (Robert Rossle- Straße 10, Berlin, Germany) provided by Radius (RADIUS s.r.l. Via Luigi Menarini, 31-40054 Budrio-BO).
- Sterilizing filter 0.22 mm (product code: SY25PL-S-MDI Advanced Microdevices PVT LTD 21 Ind. AreaAmbala Canti – 133006 India)
- For Laboratory 2 reagent set included:
-
Reagent set produced by Trasis (Rue Gilles Magnée 90 4430, Ans, Belgium), composed by:
- ○
- Part 1: Syringe containing E&Z Eluent; Syringe containing acetate buffer; Ethanol vial; Sodium Chloride 0,9 % (BBraun – Melsungen AG 34209 Melsungen, Germany).
- ○
- Part 2: Sodium ascorbate
- Single use sterile cassette product by Medline Liége Science Park-Rue des Gardes-Frontiére 5, 4031 Angleur Belgium, distributed by Trasis (Rue Gilles Magnée 90 4430, Ans, Belgium). The cassette includes a Solid Phase extraction (SPE) cartridge Oasis HLB Plus Short Cartridge, 225 mg sorbent per cartridge, 60 mm, 50 /pk
- Sterilizing filter 0.22 mm (product code: 6764192 PALL Medical Avenue de Tivoli 3, CH-1700 Fribourg Switzerland).
4.1.3. Process Description
4.2. Quality Control
4.2.1. Standard Procedures
- The pH value of the formulation was determined by pH strips (Merck pH indicator strip, Acilit, increment 0,5 pH unit).
- The Endotoxin test was performed by the Limulus Amebocyte Lysate test (LAL test), on an Endosafe Nexgen-PTSTM (Charles River Laboratories, 26866 Sant’Angelo Lodigiano (LO), Italy).
-
As required by national regulations on quality assurance (NBP MN), since this is a preparation that cannot be subjected to terminal sterilization, it should be sterilized by filtration with a sterile disposable membrane having pores of nominal diameter 0.22 µm. Filter integrity must be checked by bubble point test before release:
- ○
- for Laboratory 1 the Bubble point test was performed on an Integritest 4 system (Merck Millipore KgaA, Darmstadt, Germany).
- ○
- for Laboratory 2 the bubble point test is performed automatically by the synthesis module (Mini All-in-One Trasis – Hot Cell H700).
- Sterility test was performed by an external Laboratory for both Laboratory 1 and Laboratory 2.
- Radionuclidic purity is assessed in both laboratories by measuring the half-life and identifying characteristic emission peaks for Gallium-68, in accordance with the current European Pharmacopoeia Monograph 2.2.66 “Detection and Measurement of radioactivity.”
4.2.2. HPLC Analysis
4.2.3. Thin Layer Chromatography (TLC)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edosada, C.Y.; Quan, C.; Wiesmann, C.; Tran, T.; Sutherlin, D.; Reynolds, M.; Elliott, J.M.; Raab, H.; Fairbrother, W.; Wolf, B.B. Selective Inhibition of Fibroblast Activation Protein Protease Based on Dipeptide Substrate Specificity. J. Biol. Chem. 2006, 281, 7437–7444. [CrossRef]
- Park, J.E.; Lenter, M.C.; Zimmermann, R.N.; Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Fibroblast Activation Protein, a Dual Specificity Serine Protease Expressed in Reactive Human Tumor Stromal Fibroblasts. J. Biol. Chem. 1999, 274, 36505–36512. [CrossRef]
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteom. – Clin. Appl. 2014, 8, 454–463. [CrossRef]
- Zi, F.; He, J.; He, D.; Li, Y.; Yang, L.; Cai, Z. Fibroblast activation protein α in tumor microenvironment: Recent progression and implications (Review). Mol. Med. Rep. 2015, 11, 3203–3211. [CrossRef]
- Lindner, T.; Loktev, A.; Giesel, F.; Kratochwil, C.; Altmann, A.; Haberkorn, U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm. Chem. 2019, 4, 16. [CrossRef]
- Altmann, A.; Haberkorn, U.A.; Siveke, J. The Latest Developments in Imaging of Fibroblast Activation Protein. J. Nucl. Med. 2020, 62, 160–167. [CrossRef]
- Gascard, P.; Tlsty, T.D. Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes Dev. 2016, 30, 1002–1019. [CrossRef]
- Brennen, W.N.; Isaacs, J.T.; Denmeade, S.R. Rationale Behind Targeting Fibroblast Activation Protein–Expressing Carcinoma-Associated Fibroblasts as a Novel Chemotherapeutic Strategy. Mol. Cancer Ther. 2012, 11, 257–266. [CrossRef]
- Wikberg, M.L.; Edin, S.; Lundberg, I.V.; Van Guelpen, B.; Dahlin, A.M.; Rutegård, J.; Stenling, R.; Öberg, Å.; Palmqvist, R. High intratumoral expression of fibroblast activation protein (FAP) in colon cancer is associated with poorer patient prognosis. Tumor Biol. 2013, 34, 1013–1020. [CrossRef]
- Bauer, S.; Jendro, M.C.; Wadle, A.; Kleber, S.; Stenner, F.; Dinser, R.; Reich, A.; Faccin, E.; Gödde, S.; Dinges, H.; et al. Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res. Ther. 2006, 8, R171–R171. [CrossRef]
- Windisch, P.; Zwahlen, D.R.; Koerber, S.A.; Giesel, F.L.; Debus, J.; Haberkorn, U.; Adeberg, S. Clinical Results of Fibroblast Activation Protein (FAP) Specific PET and Implications for Radiotherapy Planning: Systematic Review. Cancers 2020, 12, 2629. [CrossRef]
- Jiang, G.-M.; Xu, W.; Du, J.; Zhang, K.-S.; Zhang, Q.-G.; Wang, X.-W.; Liu, Z.-G.; Liu, S.-Q.; Xie, W.-Y.; Liu, H.-F.; et al. The application of the fibroblast activation protein α-targeted immunotherapy strategy. Oncotarget 2016, 7, 33472–33482. [CrossRef]
- Puré, E.; Lo, A. Can Targeting Stroma Pave the Way to Enhanced Antitumor Immunity and Immunotherapy of Solid Tumors?. Cancer Immunol. Res. 2016, 4, 269–278. [CrossRef]
- Meletta, R.; Herde, A.M.; Chiotellis, A.; Isa, M.; Rancic, Z.; Borel, N.; Ametamey, S.M.; Krämer, S.D.; Schibli, R. Evaluation of the Radiolabeled Boronic Acid-Based FAP Inhibitor MIP-1232 for Atherosclerotic Plaque Imaging. Molecules 2015, 20, 2081–2099. [CrossRef]
- Imlimthan, S.; Moon, E.S.; Rathke, H.; Afshar-Oromieh, A.; Rösch, F.; Rominger, A.; Gourni, E. New Frontiers in Cancer Imaging and Therapy Based on Radiolabeled Fibroblast Activation Protein Inhibitors: A Rational Review and Current Progress. Pharmaceuticals 2021, 14, 1023. [CrossRef]
- Jansen, K.; Heirbaut, L.; Cheng, J.D.; Joossens, J.; Ryabtsova, O.; Cos, P.; Maes, L.; Lambeir, A.-M.; De Meester, I.; Augustyns, K.; et al. Selective Inhibitors of Fibroblast Activation Protein (FAP) with a (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine Scaffold. ACS Med. Chem. Lett. 2013, 4, 491–496. [CrossRef]
- Syed, M.; Flechsig, P.; Liermann, J.; Windisch, P.; Staudinger, F.; Akbaba, S.; Koerber, S.A.; Freudlsperger, C.; Plinkert, P.K.; Debus, J.; et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur. J. Nucl. Med. 2020, 47, 2836–2845. [CrossRef]
- Terry, S.Y.; Koenders, M.I.; Franssen, G.M.; Nayak, T.K.; Freimoser-Grundschober, A.; Klein, C.; Oyen, W.J.; Boerman, O.C.; Laverman, P. Monitoring Therapy Response of Experimental Arthritis with Radiolabeled Tracers Targeting Fibroblasts, Macrophages, or Integrin αvβ3. J. Nucl. Med. 2015, 57, 467–472. [CrossRef]
- van der Geest, T.; Roeleveld, D.M.; Walgreen, B.; Helsen, M.M.; Nayak, T.K.; Klein, C.; Hegen, M.; Storm, G.; Metselaar, J.M.; Berg, W.B.v.D.; et al. Imaging fibroblast activation protein to monitor therapeutic effects of neutralizing interleukin-22 in collagen-induced arthritis. Rheumatology 2018, 57, 737–747. [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.-M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marme, F.; Jäger, D.; Mier, W.; et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [CrossRef]
- Meyer, C.; Dahlbom, M.; Lindner, T.; Vauclin, S.; Mona, C.; Slavik, R.; Czernin, J.; Haberkorn, U.; Calais, J. Radiation Dosimetry and Biodistribution of 68Ga-FAPI-46 PET Imaging in Cancer Patients. J. Nucl. Med. 2019, 61, 1171–1177. [CrossRef]
- Sharma, P.M.; Singh, S.S.D.; Gayana, S. Fibroblast Activation Protein Inhibitor PET/CT: A Promising Molecular Imaging Tool. Clin. Nucl. Med. 2020, 46, e141–e150. [CrossRef]
- Rubira, L.; Torchio, J.; Fouillet, J.; Vanney, J.; Fersing, C. GMP-Compliant Automated Radiolabeling and Quality Controls of [68Ga]Ga-FAPI-46 for Fibroblast Activation Protein-Targeted PET Imaging in Clinical Settings. Chem. Pharm. Bull. 2024, 72, 1014–1023. [CrossRef]
- Rosenberg, A.J.; Cheung, Y.-Y.; Liu, F.; Sollert, C.; Peterson, T.E.; Kropski, J.A. Fully automated radiosynthesis of [68Ga]Ga-FAPI-46 with cyclotron produced gallium. EJNMMI Radiopharm. Chem. 2023, 8, 1–11. [CrossRef]
- Meisenheimer, M.; Saenko, Y.; Eppard, E. Gallium-68: Radiolabeling of Radiopharmaceuticals for PET Imaging—A Lot to Consider. In: Naqvi S.A.R., Imrani M.B., editors. Medical Isotopes. IntechOpen; London, UK: 2019.
- Boschi, S.; Malizia, C.; Lodi, F. Overview and perspectives on automation strategies in (68)Ga radiopharmaceutical preparations. Recent Results Cancer Res. 2013;194:17-31. PMID: 22918752. [CrossRef]
- Bauwens, M.; Chekol, R.; Vanbilloen, H.; Bormans, G.; Verbruggen, A. Optimal buffer choice of the radiosynthesis of 68Ga–Dotatoc for clinical application. Nucl. Med. Commun. 2010, 31, 753–758. [CrossRef]
- Rubira, L.; Donzé, C.; Fouillet, J.; Algudo, B.; Kotzki, P.O.; Deshayes, E.; Fersing, C. [68Ga]Ga-FAPI-46 synthesis on a GAIA® module system: Thorough study of the automated radiolabeling reaction conditions. Appl. Radiat. Isot. 2024, 206, 111211. [CrossRef]
- Velikyan, I. 68Ga-Based Radiopharmaceuticals: Production and Application Relationship. Molecules 2015, 20, 12913–12943. [CrossRef]
- https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2010-11-23&atto.codiceRedazionale=10A13706&elenco30giorni=false. Consultato 19 luglio 2025.
- Requirements to the Chemical and Pharmaceutical Quality Documentation Concerning Investigational Medicinal Products in Clinical Trials - Scientific Guideline | European Medicines Agency (EMA). 31 marzo 2006. https://www.ema.europa.eu/en/requirements-chemical-pharmaceutical-quality-documentation-concerning-investigational-medicinal-products-clinical-trials-scientific-guideline.
- Peppicelli, S.; Andreucci, E.; Ruzzolini, J.; Bianchini, F.; Calorini, L. FDG uptake in cancer: a continuing debate. Theranostics 2020, 10, 2944–2948. [CrossRef]
- Alavi, A.; Saboury, B.; Nardo, L.; Zhang, V.B.; Wang, M.; Li, H.; Raynor, W.Y.; Werner, T.J.M.; Høilund-Carlsen, P.F.M.; Revheim, M.-E.M. Potential and Most Relevant Applications of Total Body PET/CT Imaging. Clin. Nucl. Med. 2022, 47, 43–55. [CrossRef]
- Abbasi, S.; Dehghani, M.; Khademi, S.; Irajirad, R.; Parizi, Z.P.; Sahebi, M.; Sadeghi, M.; Montazerabadi, A.; Tavakoli, M. Revolutionizing cancer diagnosis and dose biodistribution: a meta-analysis of [68ga] FAPI- 46 vs. [18f] FDG imaging. Syst. Rev. 2025, 14, 1–20. [CrossRef]
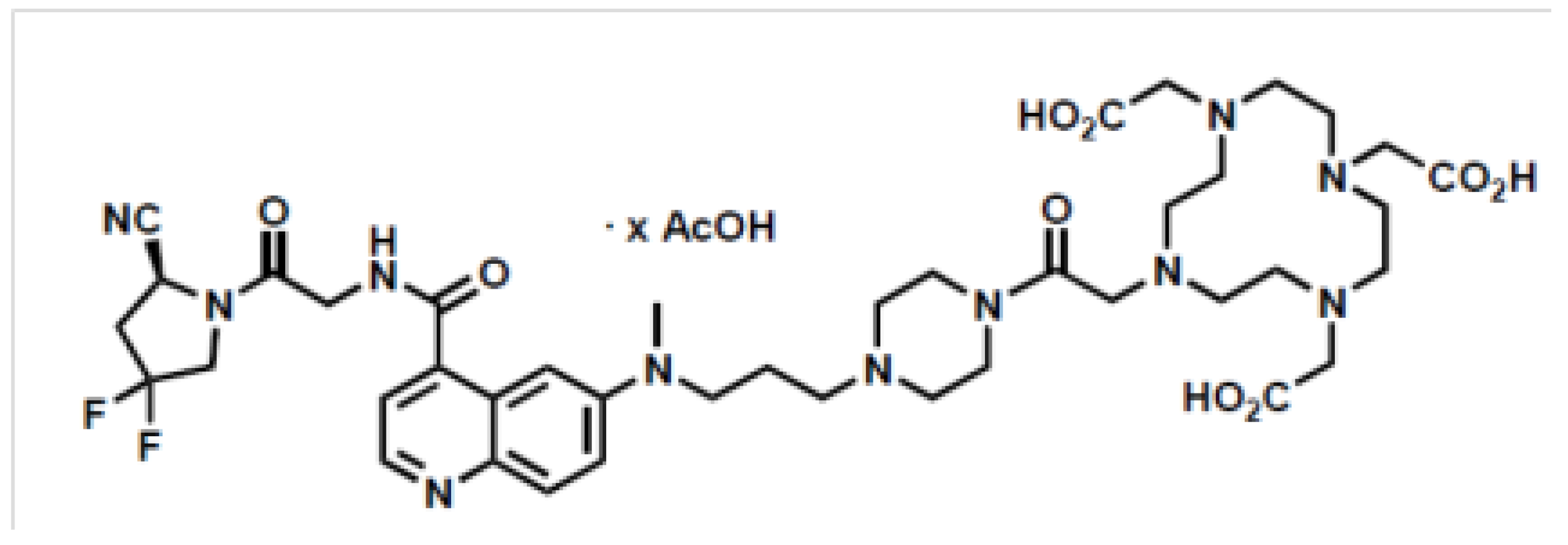
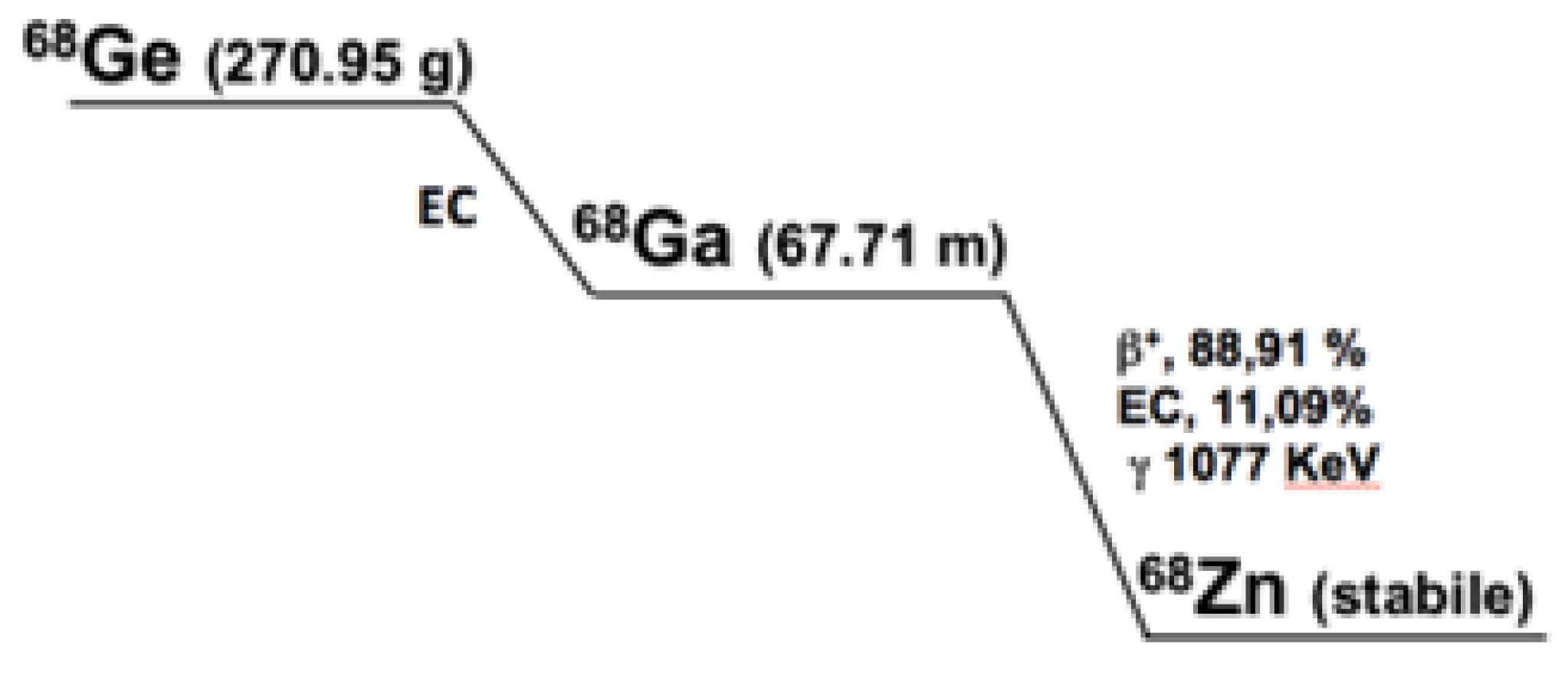
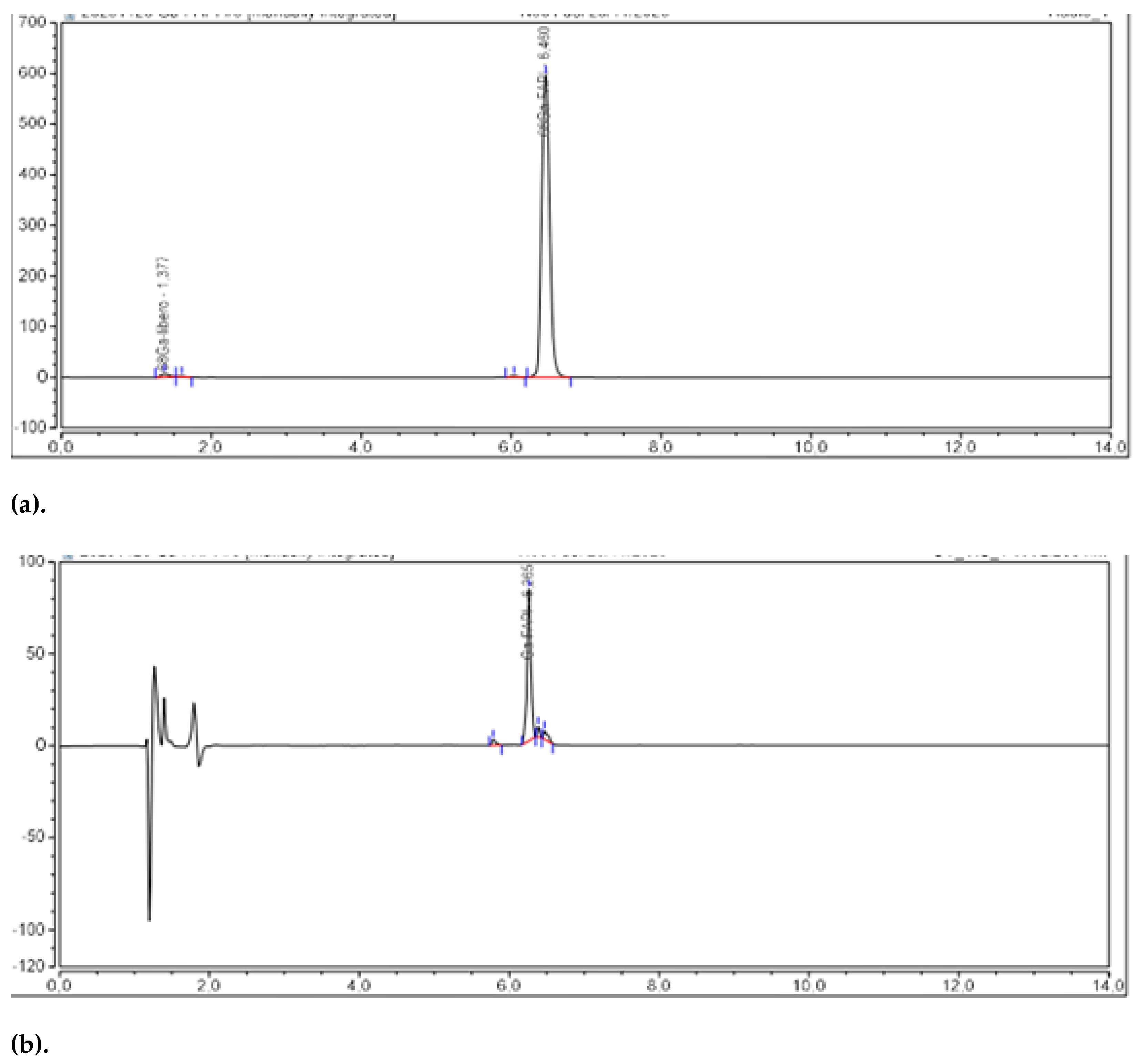
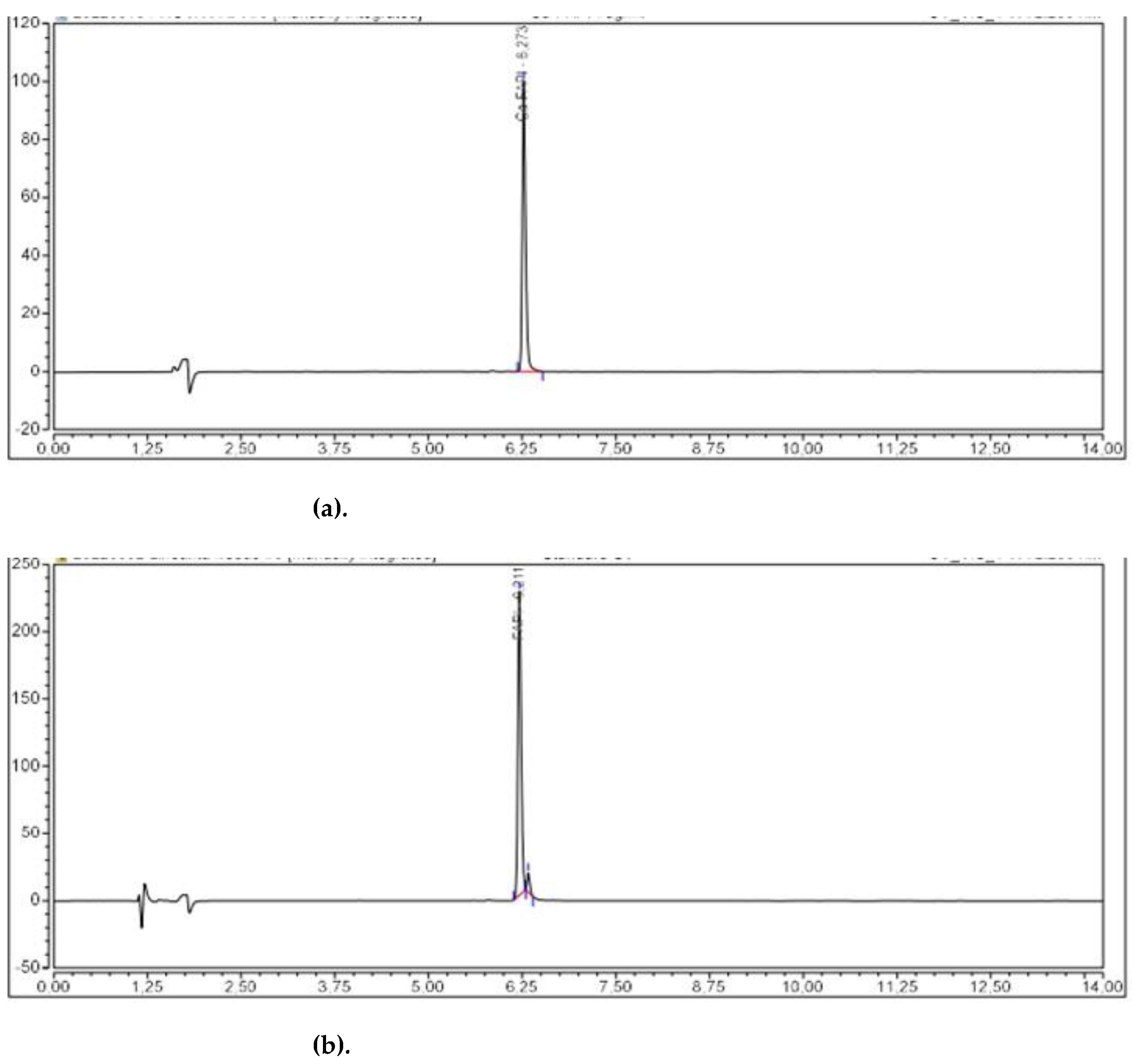
| Parameters | Acceptance Criteria |
| Appareance | Clear and colorless solution |
| Radionuclidic Putity | ≥ 99.9 % |
| 68Ge breakthorough | ≤ 0,001 % |
| Non-radioactive metals (ICP-EOS) | Iron: < 10 <g/GBq Zinc: < 10 <g/GBq |
| Identity | Gamma spectrometry: 0.511; 1.077 MeV (a sum peak may be observed at 1.022 MeV) |
| Half Life | 62-74 minute |
| Radiochemical purity | ITLC-SG; mobile phase: Methanol/ammonium acetate (1:1) ≥ 95 % |
| pH | ≤ 2 |
| Endotoxin Level | ≤ 175 EU/V |
| Components | Function | Amount/Activity |
| [68Ga]Ga-FAPI-46 | Active Pharmaceutical ingredient | 620-697 MBq ART |
| Sodium Chloride NaCl ≥ 99.99% Suprapur | Eluent | 312.4 mg |
| Chloridric acid HCl 30% TraceSELECT Ultra | 15 µl | |
| Ultrapure water TraceSELECT Ultra, ACS Reagent | 1.08 ml | |
| Chloridric acid HCl 30% TraceSELECT Ultra | Reaction Buffer | 8.8 µl |
| Acetic Acid ≥ 99.5% | 20 µl | |
| Sodium acetate trihydrate BioUltra ≥ 99.5% | 60.4 mg | |
| Ultrapure water TraceSELECT Ultra, ACS Reagent | 0.37 ml | |
| Ascorbic Acid | Radical Scavanger | 0.3 mg |
| Sodium Chloride 0.9% | Diluent/Excipient | 7.5 ml |
| Components | Function | Amount/Activity |
| [68Ga]Ga-FAPI-46 | Active Pharmaceutical ingredient | 500-700 MBq ART |
| Ethanol Absolute 100%, EMSURE ACS, ISO, Eur Ph Reag. | Eluent | 0.7 ml |
| Sodium Ascorbate Ph Eur | Radical Scavanger | 0.1 g |
| Sodium chloride 0.9 % | Diluent/Excipient | 9.5 ml |
| Parameter | Method | Acceptance criteria | Pre/Post Release |
| [68Ga]Ga-FAPI-46 activity | Dose calibrator | Lab 1:620-697 MBq Lab 2: 500-700 MBq |
pre |
| Radioactive concentration | Dose calibrator | Lab 1:68-78 MBq/ml Lab 2: 50-70 MBq/ml |
pre |
| Appearance | Visual inspection | Clear and colorless solution | pre |
| Identification | -spectrometry | Peaks at 0,511 and 1022 Mev | pre |
| Half-life | 62-74 min. | ||
| Identification | HPLC | TR [68Ga]Ga-FAPI-46 ± 0,2 min TR natGa-FAPI-46 | pre |
| Radiohemical purity | TLC | [68Ga]Ga-FAPI-46 ≥ 95% - [68Ga]Ga3+ ≤ 3% | pre |
| Radiohemical purity | HPLC | [68Ga]Ga-FAPI-46 ≥ 95% - [68Ga]Ga3+ and other radiolysis products ≤ 5% of which [68Ga]Ga3+ ≤ 2% | pre |
| System suitability | HPLC | symmetry factor [68Ga]Ga-FAPI-46 ≤ 2,5 | pre |
| pH | pH strips | 4.0 – 8.0 | pre |
| Filter Integrity | Bubble Point Test | ≥ 50 psi | pre |
| Radionuclidic purity | -spectrometry | ≤ 0,001% | post |
| Sterility | Sterility Test (Ph.Eur) | Sterile | post |
| Bacterial Endotoxin | Eur.Ph. | ≤ 175 EU/V | pre |
| Chemical Purity UV Detector | |||
| Parameters | Acceptance Criteria | Lab 1 results | Lab 2 results |
| Specificity | Rs natGa-FAPI-46 and FAPI-46 Rs ≥ 1,5 |
Comply | Comply |
| Precision | CV% FAPI-46 ≤ 5% CV% natGa-FAPI-46 ≤ 5% |
2% 2,6% |
2,3% 2,4% |
| Linearity | R2 FAPI-46 ≥ 0,99 R2 natGa-FAPI-46 ≥ 0,99 |
0,999 0,998 |
0,998 0,999 |
| Limit of quantification LOQ (g/ml) |
Experimental | FAPI-46 = 0,39 natGa-FAPI-46 = 0,74 |
FAPI-46 = 1,91 natGa-FAPI-46 = 0,58 |
| Limit of detection LOD (g/ml) |
Experimental | FAPI-46 = 0,13 natGa-FAPI-46 = 0,39 |
FAPI-46 = 0,63 natGa-FAPI-46 = 0,19 |
| Range Accuracy | Avarage bias < 5% | Comply | Comply |
| Radiochemical Purity Radiodetector | |||
| Parameters | Acceptance Criteria | Lab 1 results | Lab 2 results |
| Specificity | Not applicabile | NA | NA |
| Precision | CV% ≤ 5% | 3,1 % | 3,1% |
| Linearity | R2 ≥ 0,99 | 0,9983 | 0,992 |
| Limit of quantification LOQ (MBq/ml) |
Experimental | 30,01 | 4,5 |
| Limit of detection LOD (MBq/ml) |
Experimental | 9,9 | 1,5 |
| Range Accuracy | Avarage bias < 5% | Comply | Comply |
| Parameters | Method | Acceptance criteria | Laboratory 1 | Laboratory 2 | ||||
| Batch 1 08/04/2022 | Batch 2 12/05/2022 | Batch 3 18/05/2022 |
Batch 1 08/08/2023 |
Batch 2 10/08/2023 | Batch 3 11/08/2023 | |||
| [⁶⁸Ga]Ga-FAPI-46 activity | Dose calibrator | Lab 1:620-697 MBq Lab 2: 500-700 MBq |
628 MBq | 662 MBq | 697 MBq | 644 MBq | 662 MBq | 593 MBq |
| Radioactive concentration | Dose calibrator | Lab 1: 68-78 MBq Lab 2:50-70 MBq |
69,7 MBq/ml | 73,5 MBq/ml | 77.4 MBq/ml | 64.4 MBq/ml | 66.2 MBq/ml | 59,3 MBq/ml |
| Volume | - | Lab 1: 9 ml Lab 2: 10 ml |
Comply | comply | comply | comply | comply | Comply |
| Appearance | Visual test | Clear and colorless solution | Comply | comply | comply | comply | comply | Comply |
| Identification | HPLC | TR [⁶⁸Ga]Ga-FAPI-46 ± 0,2 min TR natGa-FAPI-46 | 0,157 min | 0,172 min | 0,144 min | 0,141 min | 0,160 min | 0,156 min |
| Radionuclidic identity | γ-spectrometry | Peaks at 0,511 and 1022 Mev | Comply | comply | comply | comply | comply | Comply |
| Half-life | 62-74 min. | 67,85 min | 69,39 min | 67,83 min | 68,2 min | 68,9 min. | 66,9 min. | |
| Radiohemical purity | TLC | [⁶⁸Ga]Ga-FAPI-46 ≥ 95% - [68Ga]Ga3+ ≤ 3% | 99,6 % | 99.9 % | 99.2 % | 99,2 % | 99,7 % | 99,6 % |
| 0,4 % | 0,1 % | 0,8 % | 0,8 % | 0,3 % | 0,4 % | |||
| Radiohemical purity | HPLC | [⁶⁸Ga]Ga-FAPI-46 ≥ 95% | 98,1 % | 99,0 % | 97,1 % | 99,9 % | 99,7 % | 99,9 % |
| [68Ga]Ga3+ and other radiolysis products ≤ 5% | 1,9 % | 1,0 % | 2,9 % | 0,1 % | 0,3 % | 0.0 % | ||
| [68Ga]Ga3+ ≤ 2% | 1,6 % | 0,7 % | 2.0 % | 0,1% | 0,3 % | 0,0 % | ||
| System suitability | HPLC | symmetry factor [⁶⁸Ga]Ga-FAPI-46 ≤ 2,5% | comply | comply | comply | comply | comply | Comply |
| pH | pH strips | 4.0 – 8.0 | 4.5 | 4.5 | 4.5 | 5.8 | 6.0 | 5.9 |
| Filter Integrity | Bubble Point Test | ≥ 50 psi | ≥ 50 psi | ≥ 50 psi | ≥ 50 psi | ≥ 50 psi | ≥ 50 psi | ≥ 50 psi |
| Radionuclidic purity | γ-spectrometry | ≤ 0,001% | 0,000035 % | 0,000018 % | 0,000034 % | < 2 . 10-5 % | < 2 . 10-5 % | < 2 . 10-5 % |
| Sterility | Sterility Test (Ph.Eur) | Sterile | comply | comply | comply | comply | comply | Comply |
| Bacterial Endotoxin | Eur.Ph. | ≤ 175 EU/V | comply | comply | comply | comply | comply | Comply |
| 1 h Stability test | ||||||||
| Parameters | Method | Acceptance criteria | Laboratory 1 | Laboratory 2 | ||||
| Batch 1 08/04/2022 | Batch 2 12/05/2022 | Batch 3 18/05/2022 |
Batch 1 08/08/2023 |
Batch 2 10/08/2023 | Batch 3 11/08/2023 | |||
| Appearance | Visual test | Clear and colorless solution | comply | comply | comply | comply | comply | Comply |
| Radiohemical purity | TLC | [⁶⁸Ga]Ga-FAPI-46 ≥ 95% - [68Ga]Ga3+ ≤ 5% | 99,6 % | 99.9 % | 99.6 % | 98,9 % | 99,6 % | 99,5 % |
| 0,4 % | 0,1 % | 0,4 % | 1,1 % | 0,4 % | 0,5 % | |||
| Radiohemical purity | HPLC | [⁶⁸Ga]Ga-FAPI-46 ≥ 95% | 98,0 % | 98,7 % | 97, % | 99,8 % | 99,7 % | 99,8 % |
| [68Ga]Ga3+ and other radiolysis products ≤ 5% | 2,0 % | 1,3 % | 3,0 % | 0,2 % | 0,3 % | 0.2 % | ||
| [68Ga]Ga3+ ≤ 2% | 1,7 % | 0,8 % | 2.0 % | 0,1% | 0,3 % | 0,1 % | ||
| pH | pH strips | 4.0 – 8.0 | 4.5 | 4.5 | 4.5 | 5.8 | 5.9 | 5.9 |
| 2 h Stability test | ||||||||
| Parameters | Method | Acceptance criteria | Laboratory 1 | Laboratory 2 | ||||
| Batch 1 08/04/2022 | Batch 2 12/05/2022 | Batch 3 18/05/2022 |
Batch 1 08/08/2023 |
Batch 2 10/08/2023 | Batch 3 11/08/2023 | |||
| Appearance | Visual test | Clear and colorless solution | comply | comply | comply | comply | comply | Comply |
| Radiohemical purity | TLC | [⁶⁸Ga]Ga-FAPI-46 ≥ 95% - [68Ga]Ga3+ ≤ 5% | 99,5 % | 99.7 % | 99.5 % | 98,6 % | 99,5 % | 99,3 % |
| 0,5 % | 0,3 % | 0,5 % | 1,3 % | 0,5 % | 0,6 % | |||
| Radiohemical purity | HPLC | [⁶⁸Ga]Ga-FAPI-46 ≥ 95% | 97,5 % | 98,5 % | 96,7 % | 99,3 % | 98,5 % | 99,3 % |
| [68Ga]Ga3+ and other radiolysis products ≤ 5% | 2,5 % | 1,5 % | 3,3 % | 0,7 % | 0,6 % | 0.7 % | ||
| [68Ga]Ga3+ ≤ 2% | 1,7 % | 0,8 % | 2.0 % | 0,6 % | 0,1 % | 0,1 % | ||
| pH | pH strips | 4.0 – 8.0 | 4.5 | 4.5 | 4.5 | 5.8 | 5.8 | 5.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





