1. Introduction
The hyoid bone stands out in the craniofacial skeleton as the only anatomic structure that remains unattached to neighboring bones, maintaining its position through muscular and ligamentous support. With its U-shaped or horseshoe-like configuration, the hyoid rests in the front of the neck, positioned between the lower jaw and the larynx, roughly aligning with the third cervical vertebra [
1]. Positioned just above the thyroid cartilage, the hyoid is suspended within a complex system of muscles and connective tissues rather than being anchored to adjacent bones [
2].
Structurally, the hyoid consists of three main components: a central segment known as the body, and two projections—greater horns (cornu majus) and lesser horns (cornu minus) [
3]. Developmentally, the upper portion of the hyoid body together with the lesser horns originates from the second pharyngeal arch, whereas the lower body region and the greater horns arise from the third pharyngeal arch [
4]. Through multiple muscular connections, the hyoid maintains functional and structural associations with the skull base, mandible, tongue, scapula, sternum, pharynx, and thyroid cartilage [
5].
Since the hyoid bone lacks direct bony connections, its spatial position is maintained exclusively by muscular forces. This position is influenced by several factors, including the elastic properties of the laryngeal and tracheal membranes, gravitational effects on the larynx, and the activity and length of surrounding muscles. Superiorly, muscles link the hyoid to the mandible and cranial structures, while inferior connections extend to the scapula, thyroid cartilage, and manubrium sterni. These muscular attachments regulate both the movement and stabilization of the hyoid and larynx [
6]. Based on their location, these muscles are grouped as suprahyoid or infrahyoid. Located above the hyoid, the suprahyoid muscle group includes the digastric, stylohyoid, mylohyoid, and geniohyoid muscles, all of which work collectively to lift the hyoid bone during functions such as swallowing. Situated beneath the hyoid, the infrahyoid muscles—namely sternohyoid, sternothyroid, omohyoid, and thyrohyoid—primarily act to lower the bone, assisting in coordinated neck and swallowing movements [
7].
Functionally, the hyoid bone is integral to essential orofacial activities such as maintaining cranial and cervical posture, ensuring airway patency, supporting tongue function, and contributing to phonation via its association with the larynx [
8]. In addition, the hyoid contributes to key processes such as swallowing, chewing, breathing, and safeguarding against the backward flow of ingested material [
9]. The anatomical position of the hyoid can be affected by head posture, sex differences, jaw relationships, and craniofacial growth patterns.
Previous researches have demonstrated correlations between hyoid positioning and mandibular position, with subsequent implications for airway configuration [
10,
11,
12,
13]. In forensic and anthropological studies, variations in hyoid shape and structure have been utilized as indicators for determining an individual’s sex and estimating age [
14,
15,
16]. Additionally, an individual’s predominant breathing mode (oral vs. nasal) has been suggested to influence hyoid location [
17,
18,
19,
20,
21].
Although numerous studies have investigated the relationship between hyoid bone position and skeletal or respiratory characteristics, the findings remain inconclusive. Variations in methodology, population, and measurement parameters have led to conflicting results regarding the role of age, sex, skeletal class, and breathing pattern. Furthermore, many studies have relied on two-dimensional cephalometry or limited subgroup analyses, which may not fully capture the complex interplay of factors influencing hyoid morphology [
22,
23].
Given this gap, the present study aims to comprehensively evaluate the horizontal and vertical positions of the hyoid bone, as well as its angular relationships, in association with skeletal and breathing characteristics using cone-beam computed tomography (CBCT) imaging. Uniquely, this study applies a three-way robust ANOVA model to simultaneously analyze the effects and interactions of age, sex, skeletal class, and breathing mode—offering a statistically rigorous and multifactorial approach rarely used in the field. We hypothesize that hyoid morphology differs significantly by breathing pattern, skeletal classification, and age group, with sex-specific patterns, and that a multivariable model will reveal interactions not captured in single-variable studies.
2. Materials and Methods
Ethical Approval
Ethics committee approval for this retrospective study was obtained by Necmettin Erbakan University Faculty of Dentistry, Faculty of Dentistry, Drug and Non-Medical Device Research Ethics Committee on 25.05.2023 with protocol number 2023/299, and the scientific and ethical appropriateness of the study was approved.
Study Population and Power Calculation
CBCT images obtained for diagnostic purposes of patients who applied to Necmettin Erbakan University Faculty of Dentistry, Department of Oral, Dental and Maxillofacial Radiology between February 2019 and October 2024 with various dental complaints were retrospectively analyzed.
Before starting the study, a power analysis was performed by ANOVA method using the G*Power v3.1.9.7 program to determine the number of individuals to be included in the groups. According to the 95% confidence level (1-α), 95% test power (1-β), Cohen's f=0.303 effect size and multi-way analysis of variance parameters, it was determined that a minimum of 240 individuals should be included in the study, with at least 4 cases (209/60 ≅ 4) in each group. In order to increase the statistical power of the study, it was decided to include more records than the minimum number of cases initially determined.
Inclusion Criteria:
The study included patients aged ≥ 8 years with no congenital or acquired craniofacial anomalies, history of craniofacial trauma or surgery, cleft lip/palate, systemic syndromes, or missing teeth affecting occlusion, and with CBCT images of optimal diagnostic quality allowing clear identification of reference points.
Exclusion Criteria:
Patients were excluded if they had congenital or acquired craniofacial anomalies, a history of craniofacial trauma or surgery, cleft lip/palate, systemic syndromes, missing teeth affecting occlusion, suboptimal CBCT image quality, or unclear reference points.
CBCT Acquisition Protocol
In this retrospective study, CBCT images of 560 male and female patients aged 8-73 years with high diagnostic quality who met the inclusion criteria were analyzed. The CBCT images used in the study were obtained with Morita 3D Accuitomo 170 and NewTom GiANO 3D devices. The Morita 3D Accuitomo 170 (J Morita MFG Corp., Kyoto, Japan) was operated at 90 kVp and 5 mA, with an irradiation time of 17.5 seconds, 0.25 mm voxel size, 140 mm × 100 mm FOV, 360° data acquisition and no additional filtering. The NewTom GiANO 3D device (Verona, Italy) was operated at 90 kVp and 10 mA, with an irradiation time of 18 seconds, 0.15 mm voxel size, 140 mm × 100 mm FOV, 360° data acquisition and no additional filtering. Both devices were calibrated before each patient and radiographs were taken by the same technician according to standard protocols.
Measurement Procedure
The included CBCT images were reviewed by a single observer with three years of experience in oral diagnosis and maxillofacial radiology (BO). Image data were saved in DICOM (Digital Imaging and Communications in Medicine) format and measurements were performed using Dolphin 3D Imaging Software (Dolphin Imaging & Management Solutions®, Chatsworth, CA, USA). Mark points and reference planes were checked in axial, coronal and sagittal planes, and precise measurements were made on three-dimensional models. The observer was blinded to participants' skeletal classification and breathing pattern to minimize measurement bias.
Three-Dimensional Cephalometric Measurements
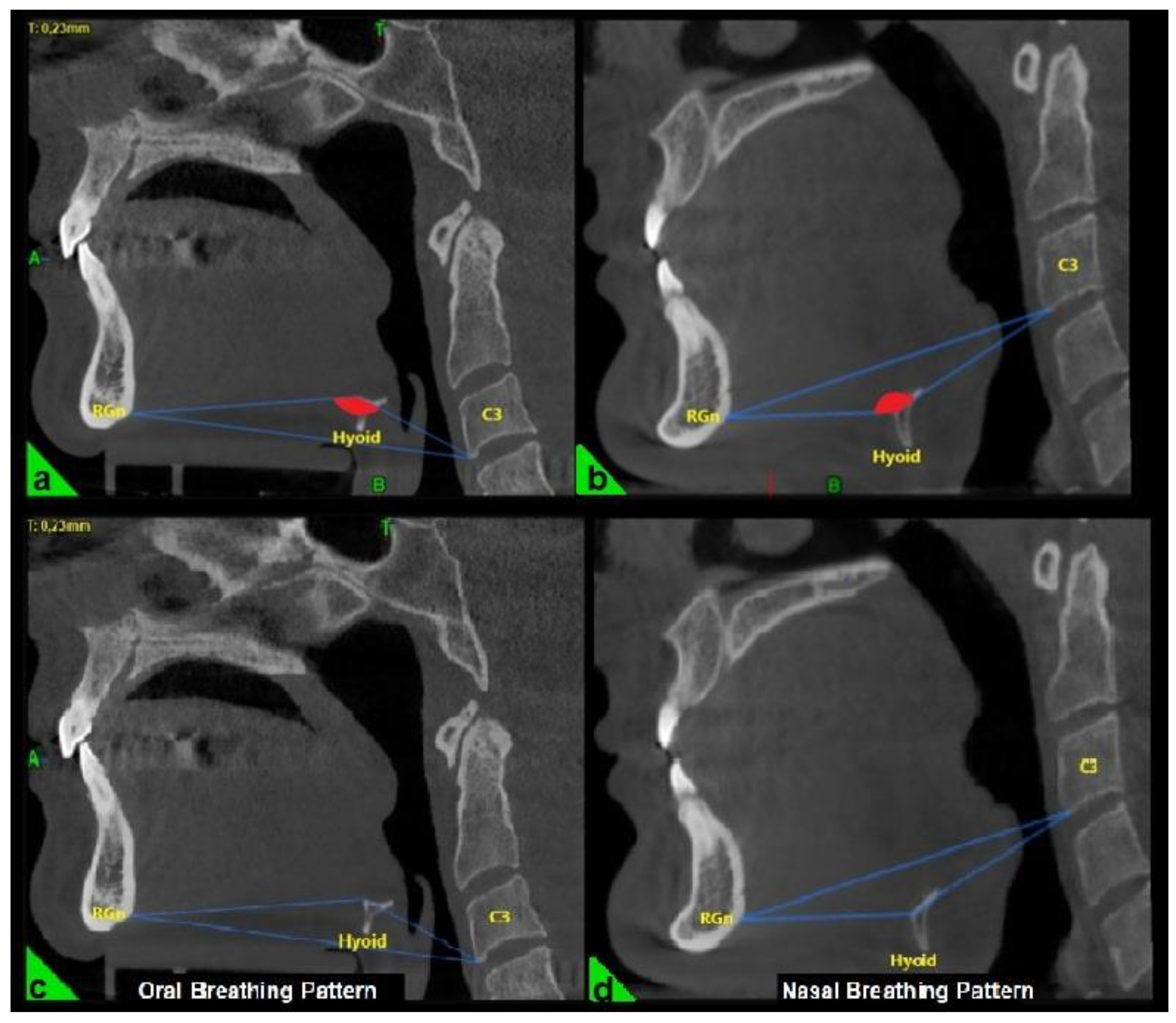
In the three-dimensional cephalometric analysis, three key measurements were performed to evaluate the position and morphology of the hyoid bone. The hyoid angle was defined as the angle formed by two lines: one extending from the anterior-inferior point of the third cervical vertebra (C3) to the most anterior point of the hyoid bone, and the second from this point to the retrognathion (RGn), which is the most posterior-inferior point of the mandibular symphysis (
Figure 1a, b) [
22].
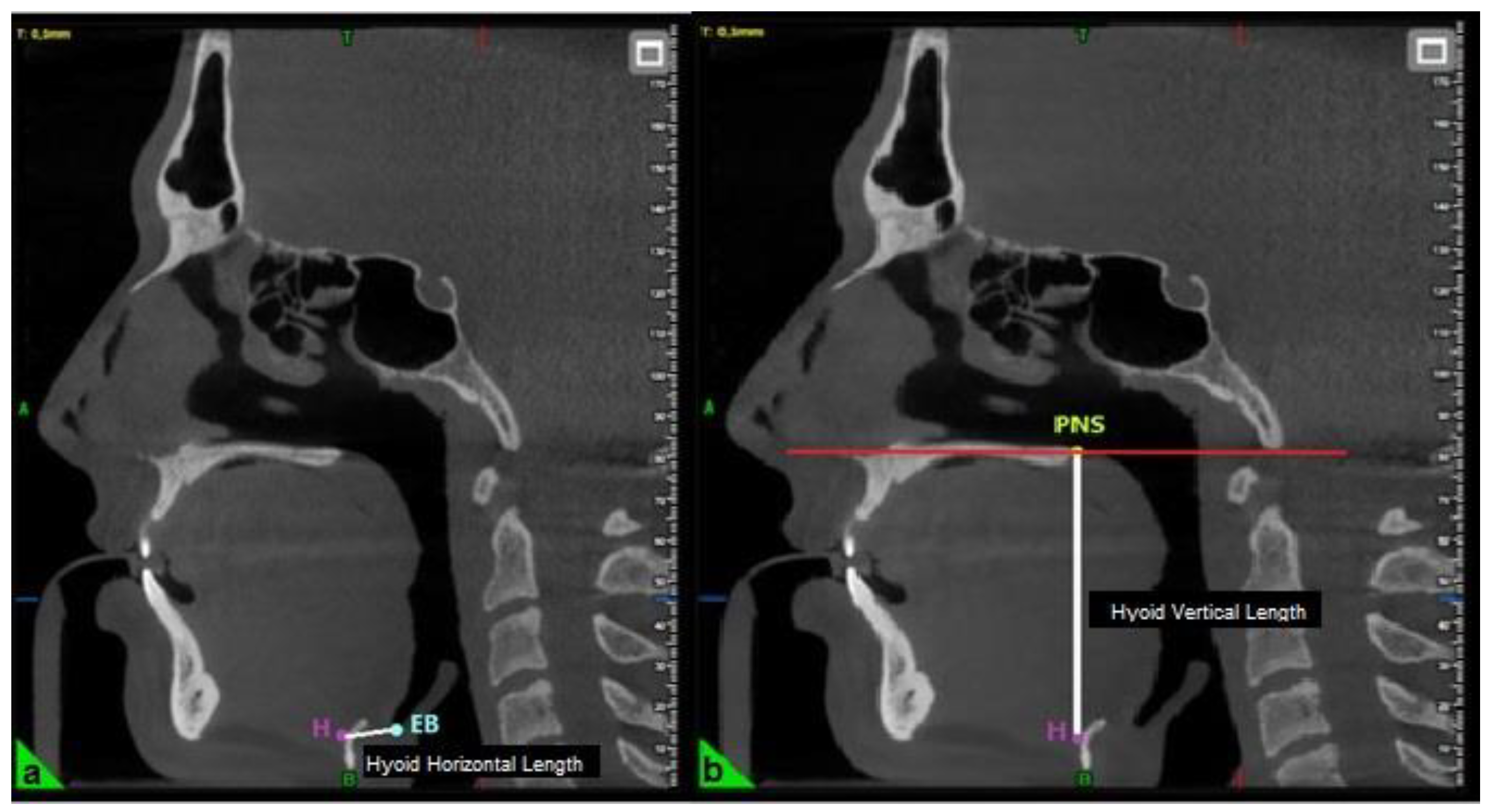
The horizontal length of the hyoid bone was measured as the linear distance between point H (the superior point of the hyoid bone) and point EB (the most inferior point on the posterior surface of the epiglottis) (
Figure 2) [
23]. The vertical length was determined by measuring the perpendicular distance from the palatal plane (PL) to point H (
Figure 2) [
19].
Participant Grouping Criteria
Sex-based categorization was applied in this study. A total of 560 CBCT scans were analyzed, comprising 295 female and 265 male patients. Participants ranged in age from 8 to 73 years and were stratified into five age groups for subgroup analysis: 8–18 years, 19–29 years, 30–40 years, 41–51 years, and over 52 years.
Skeletal classification was determined by calculating the ANB angle on sagittal sections of the CBCT images using Dolphin 3D software (Dolphin Imaging & Management Solutions®, Chatsworth, CA, USA), based on the principles of Steiner analysis [
24]. The ANB angle was obtained by subtracting the SNB (sella–nasion–point B) angle from the SNA (sella–nasion–point A) angle, which reflect the anteroposterior relationship of the maxilla and mandible to the cranial base. All measurements were performed on mid-sagittal slices aligned with standard cephalometric reference planes. Based on the measured ANB values, patients were categorized into three skeletal classes: Class I (ANB angle between 0° and 4°), Class II (ANB > 4°), and Class III (ANB < 0°).
The breathing pattern of each participant was classified as either nasal or oral based on the “hyoid triangle” technique, applied to sagittal CBCT sections using Dolphin 3D software (Dolphin Imaging & Management Solutions®, Chatsworth, CA, USA). This method involves constructing a triangle using three anatomical landmarks: the anterior-inferior point of the third cervical vertebra (C3), the most anterior point of the hyoid bone, and the retrognathion (RGn). In this configuration, the RGn–C3 line serves as the base, and a triangle is formed by connecting these three points. When the hyoid bone is positioned above the RGn–C3 plane, the triangle has a superior vertex and is considered a “negative triangle,” indicating an oral breathing pattern (
Figure 1a, c). Conversely, when the hyoid lies below the RGn–C3 line, the triangle takes on a “positive” configuration, which is indicative of nasal breathing (
Figure 1b, d) [
22].
To assess measurement reliability, 50 CBCT scans were randomly selected for both intra- and inter-observer evaluations. Intra-observer reliability was assessed by having the primary examiner (BO), with three years of experience in oral and maxillofacial radiology, repeat all measurements after a two-week interval. For inter-observer reliability analysis, a second examiner (GM), with sixteen years of experience in the same field, independently performed the same measurements on the selected scans. Both observers followed identical measurement protocols. Intraclass correlation coefficients (ICC) were calculated for both assessments, and values above 0.90 were considered indicative of excellent agreement [
25].
Statistical Analysis
Data were analyzed using the JAMOVI V2.3.22 program. Compliance with normal distribution was evaluated by Shapiro-Wilk test. For the comparison of non-normally distributed data according to age, respiratory and skeletal pattern groups, three-way Robust ANOVA was applied using the Walrus package. Multiple comparisons were performed with Bonferroni test. Analysis results are reported as pruned mean ± standard error of the mean for quantitative data. Statistical significance level was accepted as p<0.05.
3. Results
The average age of the 560 participants was 33.74 ± 14.96 years. When the sex distribution is analyzed, 52.7% of the participants were female (295 people) and 47.3% were male (265 people), and in terms of breathing pattern, 28.6% were oral (160 people) and 71.4% were nasal (400 people) (
Table 1).
In
Table 2, the morphometric measurements of the participants are presented in detail with descriptive statistics. The mean hyoid angle was 88.79° ± 130.86°, hyoid horizontal length 14.08 mm ± 3.08 mm and hyoid vertical length 62.87 mm ± 8.14 mm.
Among female participants, hyoid angle values demonstrated a statistically significant difference based on breathing pattern (p < 0.001), independent of age or skeletal pattern classification. No significant effects were observed for age group (p = 0.32), skeletal pattern (p = 0.998), or the two-way interactions of age and breathing (p = 0.605), age and skeletal pattern (p = 0.600), or breathing and skeletal pattern (p = 0.319). Additionally, the three-way interaction did not reach statistical significance (p = 0.088). In contrast, male participants exhibited a significant effect of age group on hyoid angle (p = 0.011), with post-hoc comparisons revealing notable differences particularly between the 19–29 and 41–51 age categories. Breathing pattern also had a significant effect (p < 0.001), as did the interaction between age and breathing pattern (p = 0.002), indicating consistent differences between oral and nasal breathers across age groups. However, skeletal pattern (p = 0.377), age–skeletal interaction (p = 0.359), and breathing–skeletal interaction (p = 0.676) was not statistically significant. Notably, a significant three-way interaction among age, breathing pattern, and skeletal classification was observed in males (p = 0.029) (
Table 3).
Among female participants, hyoid horizontal length varied significantly across age groups (p = 0.009), regardless of breathing pattern or skeletal classification. Post-hoc comparisons indicated that this difference was particularly pronounced between the 8–18 age group and both the 30–40 and over 52 age groups. No statistically significant differences were observed in relation to breathing pattern (p = 0.920), skeletal pattern (p = 0.940), or any two-way interactions, including age–breathing (p = 0.709), age–skeletal (p = 0.175), and breathing–skeletal (p = 0.369) combinations. The three-way interaction was also not significant (p = 0.290). In male participants, no significant differences were found in hyoid horizontal length across any of the examined variables or interactions. Specifically, there were no statistically significant effects of age group (p = 0.170), breathing pattern (p = 0.170), or skeletal classification (p = 0.588), nor were there any significant two-way or three-way interactions (all p > 0.05) (
Table 4).
In female participants, hyoid vertical height differed significantly among age groups (p < 0.001), with post-hoc analyses revealing marked differences between the 8–18 and 19–29 age groups compared to the older cohorts. Breathing pattern also had a significant effect on hyoid vertical height (p < 0.001). Additionally, a significant interaction between breathing pattern and skeletal classification was found (p = 0.029), indicating notable differences in vertical height between oral and nasal breathers within each skeletal class. No significant differences were observed for skeletal classification alone (p = 0.947), nor for the age–breathing (p = 0.232), age–skeletal (p = 0.388), or three-way interaction (p = 0.122). Among male participants, hyoid vertical height also varied significantly across age groups (p < 0.001), particularly between the 8–18 age group and all older groups, as well as between the 19–29 and ≥52 age groups. Breathing pattern was again significantly associated with hyoid vertical height (p < 0.001). Moreover, skeletal classification showed a significant effect (p = 0.008), with a notable difference identified between Class I and Class III. No statistically significant effects were found for age–breathing (p = 0.109), age–skeletal (p = 0.127), breathing–skeletal (p = 0.536), or three-way interaction (p = 0.373) terms (
Table 5).
4. Discussion
This study comprehensively assessed hyoid bone morphology—including angular, horizontal, and vertical parameters—across skeletal classifications, age groups, sex, and breathing patterns using CBCT imaging. Key findings demonstrated that age and breathing pattern exerted significant effects on hyoid position, while skeletal pattern influenced specific metrics, particularly in males. These results reaffirm the dynamic nature of the hyoid bone, which—unlike other craniofacial bones—does not articulate directly with any other bone and is thus sensitive to functional and postural alterations in surrounding musculature and airway anatomy [
2,
7].
While the literature remains inconsistent regarding sex-based differences in hyoid position—some reporting no differences [
8,
26,
27,
28], others indicating a more anterior-inferior placement in males [
12,
29,
30,
31]—this study found age-related increases in vertical hyoid distance in both sexes, suggesting inferior displacement over time. Notably, in females, horizontal distance increased significantly with age, especially between younger (8–18) and older (>30) cohorts. These trends mirror longitudinal findings by Kollias and Krogstad [
30], who observed continued craniofacial growth and inferior hyoid displacement into adulthood. , who observed continued craniofacial growth and inferior hyoid displacement into adulthood.
Skeletal classification did not consistently emerge as a dominant determinant of hyoid position across all parameters. While some studies found retruded hyoid positions in Class II patterns [
12] , others—including Kocakara et al. [
11]—reported no significant association when respiratory mode was controlled. In this study, skeletal class did not significantly affect horizontal metrics; however, in males, vertical hyoid height decreased from Class I to Class III, suggesting more superior placement in more prognathic skeletal types. These results align with findings by Adamidis and Spyropoulos [
32] found significant differences in hyoid position and angles between Class I and Class III malocclusions, with Class III subjects—particularly males—showing more anterior positioning and reduced hyoid angles. Tallgren and Solow [
7] demonstrated that hyoid position is influenced by mandibular position, cervical inclination, and craniocervical angulation. In our findings, skeletal pattern did not significantly affect horizontal distances in either sex. However, in males, vertical distance decreased from Class I to Class III, indicating a more superior hyoid placement in advanced skeletal classes. This suggests that skeletal morphology can influence vertical positioning, at least in males.
Hyoid bone position is influenced not only by anatomical structures but also by functional factors such as breathing type. Prior studies have yielded contradictory findings: some observed a more superior hyoid in oral breathers [
21,
33], while others reported an inferior displacement[
17,
18]. In the present study, nasal breathers—both male and female—exhibited greater vertical hyoid distances (inferior placement), whereas oral breathers had shorter distances (superior placement). Among females, hyoid angle was significantly influenced only by breathing pattern, with nasal breathing associated with more positive angles and oral breathing with more negative angles. Among males, hyoid angle was influenced by both age and breathing type. These results indicate that functional factors, particularly breathing patterns, may exert a greater influence on hyoid morphology than skeletal factors. When age, breathing pattern, and skeletal pattern were analyzed jointly, no significant combined effects were found for females. In contrast, in males, these three variables significantly influenced hyoid angle, highlighting the multifactorial determinants of hyoid positioning. From a clinical standpoint, changes in hyoid angle may impact upper airway patency and respiratory function, and thus should be considered in individualized orthodontic and functional treatment planning [
19,
34]. Oral breathing has been linked to alterations in craniofacial morphology and upper airway function, increasing susceptibility to obstructive sleep apnea syndrome [
35]. Chang and Shiao [
36] demonstrated a positive correlation between mandibular plane–hyoid distance and the apnea-hypopnea index, with greater distances associated with more pronounced daytime sleepiness. In our study, individuals diagnosed with obstructive sleep apnea syndrome were excluded to isolate the effects of physiological breathing patterns. Furthermore, studies have reported positional changes in the hyoid bone after orthodontic or functional appliance therapy [
37,
38].
This study has several limitations that should be considered when interpreting the results. First, its retrospective and cross-sectional design limits causal inference and does not allow for evaluation of longitudinal changes in hyoid bone positioning. Second, all CBCT scans were obtained in a static seated position, which fails to capture dynamic functional activities such as swallowing, phonation, or postural adjustments—factors that can influence hyoid morphology in real-time contexts. Additionally, although the hyoid triangle technique was used to categorize breathing patterns, no objective respiratory assessments such as airflow analysis or rhinomanometry were performed, potentially introducing classification bias. The exclusion of individuals with craniofacial anomalies or obstructive sleep apnea allowed for a focus on physiological variation, but this may also limit the generalizability of findings to more clinically relevant populations. Moreover, factors such as tongue posture, head position, and neck muscle tone—known to affect hyoid location—were not evaluated, potentially confounding the observed relationships. While standardized protocols were followed, the use of two different CBCT machines with different voxel sizes may have introduced minor variability in imaging resolution. Finally, the study was conducted in a single institution with a demographically homogeneous population, which may limit its external validity across different ethnic or geographic cohorts.
5. Conclusions
This study demonstrates that the position and dimensions of the hyoid bone are significantly influenced by breathing pattern, age, and skeletal morphology, with notable variations between sexes. Among these factors, breathing type consistently impacted both hyoid angle and vertical displacement, while age showed stronger associations with horizontal and vertical distances. Skeletal classification exhibited limited influence overall, with statistically significant effects observed primarily in male subgroups. These findings underscore the multifactorial nature of hyoid morphology and highlight the importance of integrating functional variables—particularly respiratory patterns—into individualized orthodontic, orthopedic, and surgical treatment planning. Accounting for age-related and sex-specific changes in hyoid position may contribute to improved airway management and craniofacial balance in clinical practice.
Author Contributions
B.O. carried out the CBCT measurements, performed intra-observer reliability tests, conducted statistical analyses, and drafted the initial version of the manuscript. G.M. was responsible for the overall study conception and design, supervised the methodological framework, performed inter-observer measurements, and contributed substantially to the interpretation of the findings. M.Y. assisted in data collection, organization of patient records, and contributed to the literature review. A.E. provided methodological input, guided the structuring of the results and discussion sections, and critically revised the manuscript for intellectual content. All authors read, revised, and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Institutional Review Board Statement
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments. The study was approved by the Ethics Committee of the Faculty of Dentistry, Necmettin Erbakan University (Approval No: 2023/299).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to institutional data privacy policies but are available from the corresponding author on reasonable request.
Acknowledgments
This study constitutes a part of the specialty thesis submitted by Busra Ozturk to the Department of Oral and Maxillofacial Radiology, Faculty of Dentistry, Necmettin Erbakan University.
Conflicts of Interest
The authors declare no competing interests.
Abbreviations
The following abbreviations are used in this manuscript:
| CBCT |
Cone Beam Computed Tomography |
| ICC |
Intraclass Correlation Coefficient |
| RGn |
Retrognathion |
| PL |
Palatal Plane |
| C3 |
Third Cervical Vertebra |
| ANB |
A–Nasion–B Angle |
| SNA |
Sella-Nasion-A Angle |
| SNB |
Sella-Nasion-B Angle |
References
- Jose, N.P.; Shetty, S.; Mogra, S.; Shetty, V.S.; Rangarajan, S.; Mary, L. Evaluation of hyoid bone position and its correlation with pharyngeal airway space in different types of skeletal malocclusion. Contemp. Clin. Dent. 2014, 5, 187–9. [Google Scholar] [CrossRef] [PubMed]
- Auvenshine, R.C.; Pettit, N.J. The hyoid bone: an overview. Cranio® 2018, 38, 6–14. [Google Scholar] [CrossRef] [PubMed]
- de Bakker BS, de Bakker HM, Soerdjbalie-Maikoe V, Dikkers FGJTL: The development of the human hyoid–larynx complex revisited. 2018, 128, 1829–1834.
- Fisher, E.; Austin, D.; Werner, H.M.; Chuang, Y.J.; Bersu, E.; Vorperian, H.K. Hyoid bone fusion and bone density across the lifespan: prediction of age and sex. Forensic Sci. Med. Pathol. 2016, 12, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Bibby R, Preston CJAJoO: The hyoid triangle. 1981, 80, 92–97.
- Parsons FJJoa, physiology: The topography and morphology of the human hyoid bone. 1909, 43(Pt 4):279.
- Tallgren A, Solow BJTEJoO: Hyoid bone position, facial morphology and head posture in adults. 1987, 9, 1–8.
- Graber LWJTAO: Hyoid changes following orthopedic treatment of mandibular prognathism. 1978, 48, 33–38.
- AlJulaih GH, Menezes RG: Anatomy, head and neck: Hyoid bone. In: StatPearls [Internet]. edn.: StatPearls Publishing; 2023.
- Cheng, J.-H.; Hsiao, S.-Y.; Chen, C.-M.; Hsu, K.-J. Relationship between hyoid bone and pharyngeal airway in different skeletal patterns. J. Dent. Sci. 2020, 15, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Kocakara, G.; Buyukcavus, M.H.; Orhan, H. Evaluation of pharyngeal airway dimensions and hyoid bone position according to craniofacial growth pattern. Cranio® 2020, 40, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi S, Asghari-Moghaddam H, Dehghani M, Aboutorabzade M, Yaloodbardan B, Tohidi E, Hoseini-Zarch S-HJJoc, dentistry e: Hyoid bone position in different facial skeletal patterns. 2018, 10, e346.
- Nejaim, Y.; Aps, J.K.; Groppo, F.C.; Neto, F.H. Evaluation of pharyngeal space and its correlation with mandible and hyoid bone in patients with different skeletal classes and facial types. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Balseven-Odabasi, A.; Yalcinozan, E.; Keten, A.; Akçan, R.; Tumer, A.R.; Onan, A.; Canturk, N.; Odabasi, O.; Dinc, A.H. Age and sex estimation by metric measurements and fusion of hyoid bone in a Turkish population. J. Forensic Leg. Med. 2013, 20, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Kindschuh SC, Dupras TL, Cowgill LWJAjopa: Determination of sex from the hyoid bone. 2010, 143, 279–284.
- Urbanová, P.; Hejna, P.; Zátopková, L.; Šafr, M. The morphology of human hyoid bone in relation to sex, age and body proportions. HOMO 2013, 64, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Behlfelt, K.; Linder-Aronson, S.; Neander, P. Posture of the head, the hyoid bone, and the tongue in children with and without enlarged tonsils. Eur. J. Orthod. 1990, 12, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, A.M.; Lotti, M.; Caradonna, D. Oral Breathing and Head Posture. Angle Orthod. 2008, 78, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Suga, H.; Yanagisawa-Minami, A.; Sato, H.; Sato-Hashiguchi, M.; Shirazawa, Y.; Tsujii, T.; Yamamoto, Y.; Kanomi, R.; Yamasaki, Y. Relationships among tongue volume, hyoid position, airway volume and maxillofacial form in paediatric patients with Class-I, Class-II and Class-III malocclusions. Orthod. Craniofacial Res. 2018, 22, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.S.; Habumugisha, J.; Cheng, B.; Zhao, M.; Guo, Y.; Zou, R.; Wang, F. Three-dimensional evaluation of hyoid bone position in nasal and mouth breathing subjects with skeletal Class I, and Class II. BMC Oral Heal. 2022, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Muñoz ICL, Orta PBJIjopo: Comparison of cephalometric patterns in mouth breathing and nose breathing children. 2014, 78, 1167–1172.
- da Costa, E.D.; Roque-Torres, G.D.; Brasil, D.M.; Bóscolo, F.N.; de Almeida, S.M.; Ambrosano, G.M.B. Correlation between the position of hyoid bone and subregions of the pharyngeal airway space in lateral cephalometry and cone beam computed tomography. Angle Orthod. 2017, 87, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Jiang Y-YJIjoo, surgery m: Correlation between hyoid bone position and airway dimensions in Chinese adolescents by cone beam computed tomography analysis. 2016, 45, 914–921.
- Steiner CCJAjoo: Cephalometrics for you and, me. 1953, 39, 729–755.
- Babu N, Kohli P: Commentary: Reliability in research. Indian Journal of Ophthalmology 2023, 71, 400–401. [CrossRef] [PubMed]
- Bench RWJAJoO: Growth of the cervical vertebrae as related to tongue, face, and denture behavior. 1963, 49, 183–214.
- Kim Y-J, Hong J-S, Hwang Y-I, Park Y-HJAJoO, Orthopedics D: Three-dimensional analysis of pharyngeal airway in preadolescent children with different anteroposterior skeletal patterns. 2010, 137, 306. e301-306. e311.
- Sarı Z, Uysal T, Çatalbağ B, Üşümez S, Bağçiftçi FJTOD: Sınıf I, Sınıf II D II maloklüzyona sahip bireylerde hyoid kemik pozisyonu. 2003, 16:95-101.
- Ceylan IJAUSBEOAD: Degisik ANB Acılarında Dogal BasKonumu ve Hyoid Kemiginin Konumunun Incelenmesi. 1990.
- Kollias I, Krogstad OJTEJoO: Adult craniofacial and pharyngeal changes-a longitudinal cephalometric study between 22 and 42 years of age. Part II: morphological uvulo-glossopharyngeal changes. 1999, 21, 345–355.
- Sağlam AMŞ, Şenışık NEJSDÜSBD: Upper airway morphology and head posture in healthy men and women. 2017, 8, 12–17.
- Adamidis IP, Spyropoulos MNJAJoO, Orthopedics D: Hyoid bone position and orientation in Class I and Class III malocclusions. 1992, 101, 308–312.
- Chaves, T.C.; Silva, T.S.d.A.e.; Monteiro, S.A.C.; Watanabe, P.C.A.; Oliveira, A.S.; Grossi, D.B. Craniocervical posture and hyoid bone position in children with mild and moderate asthma and mouth breathing. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Harari, D.; Redlich, M.; Miri, S.; Hamud, T.; Gross, M. The effect of mouth breathing versus nasal breathing on dentofacial and craniofacial development in orthodontic patients. Laryngoscope 2010, 120, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Juliano ML, Machado MAC, Carvalho LBCd, Prado LBFd, Prado GFdJAdn-p: Mouth breathing children have cephalometric patterns similar to those of adult patients with obstructive sleep apnea syndrome. 2009, 67:860-865.
- Chang E-T, Shiao G-MJSM: Craniofacial abnormalities in Chinese patients with obstructive and positional sleep apnea. 2008, 9, 403–410.
- Ulusoy, C.; Bavbek, N.C.; Tuncer, B.B.; Tuncer, C.; Turkoz, C.; Gencturk, Z. Evaluation of airway dimensions and changes in hyoid bone position following class II functional therapy with activator. Acta Odontol. Scand. 2014, 72, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Baka ZM, Fidanboy MJAJoO, Orthopedics D: Pharyngeal airway, hyoid bone, and soft palate changes after Class II treatment with Twin-block and Forsus appliances during the postpeak growth period. 2021, 159, 148–157.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).