Submitted:
25 August 2025
Posted:
26 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Callus Induction and Maintenance
2.2. Extracellular Vesicles Extraction
2.3. Transmission Electron Microscopy
2.4. Nanoparticle Track Analysis
2.5. In Vitro Transcription of Fluorescent RNA
2.6. Loading Assays
2.7. Uptake Assays
3. Results
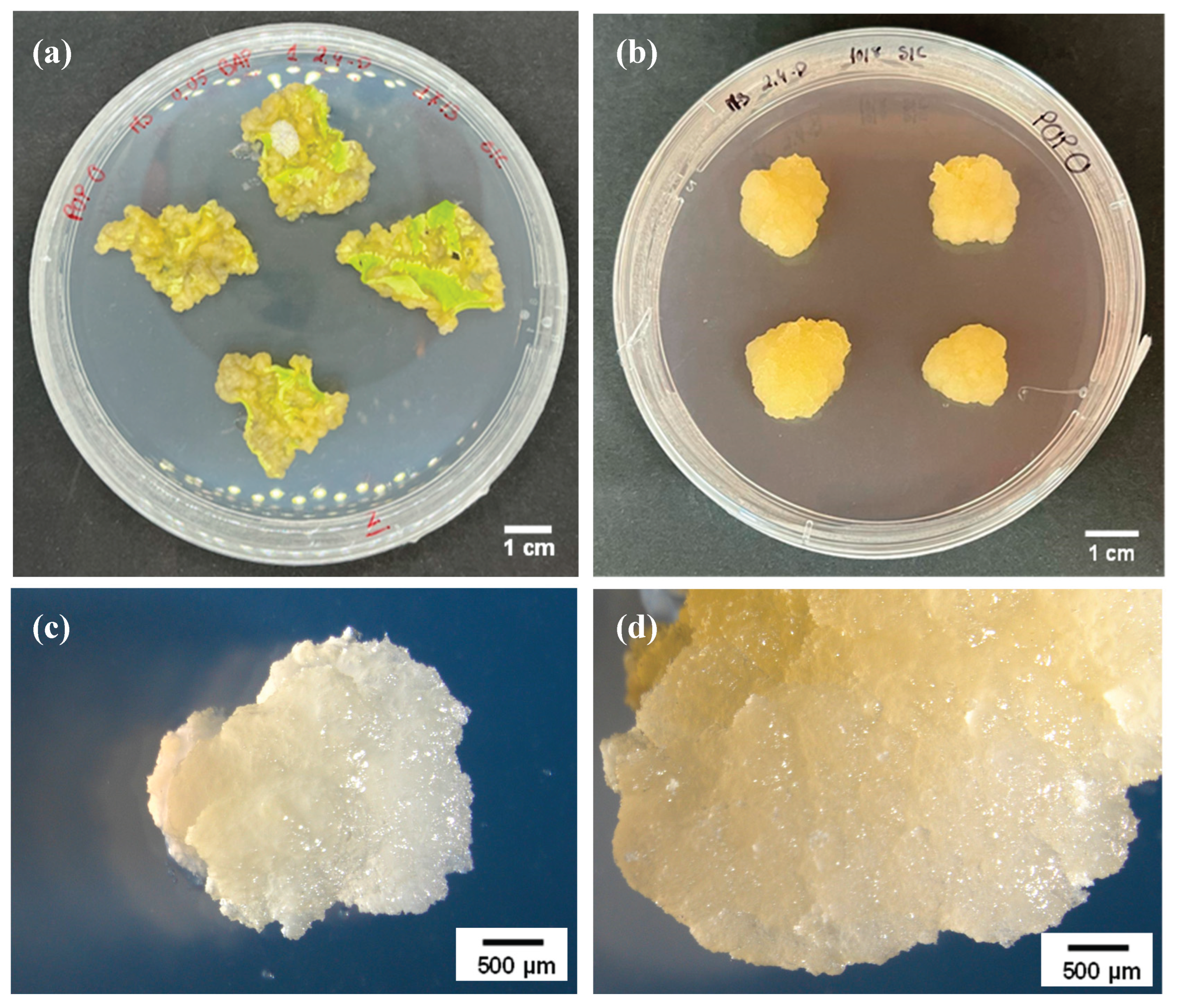
3.1. Callus Induction and Mass Propagation
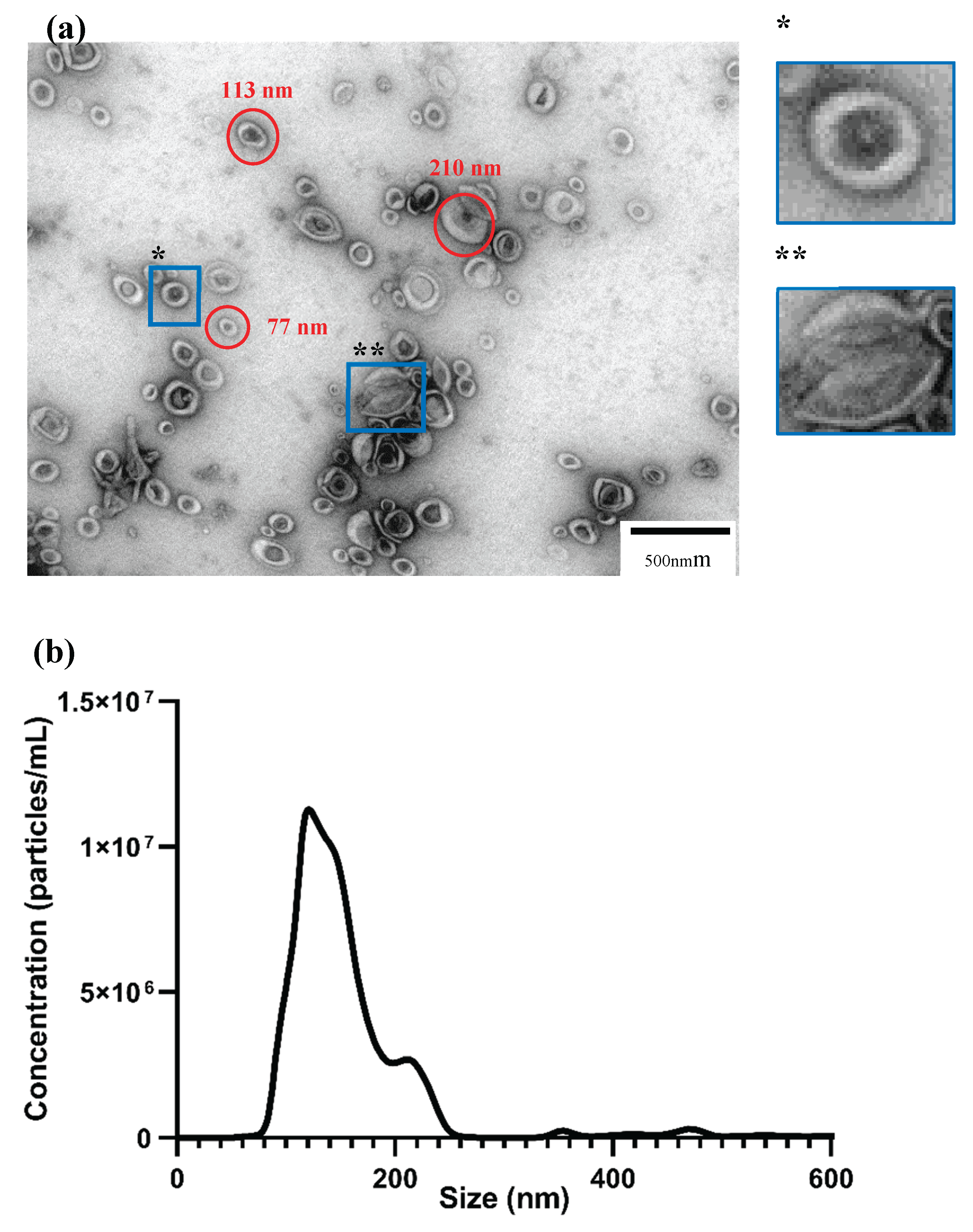
3.2. EV Isolation and Characterization
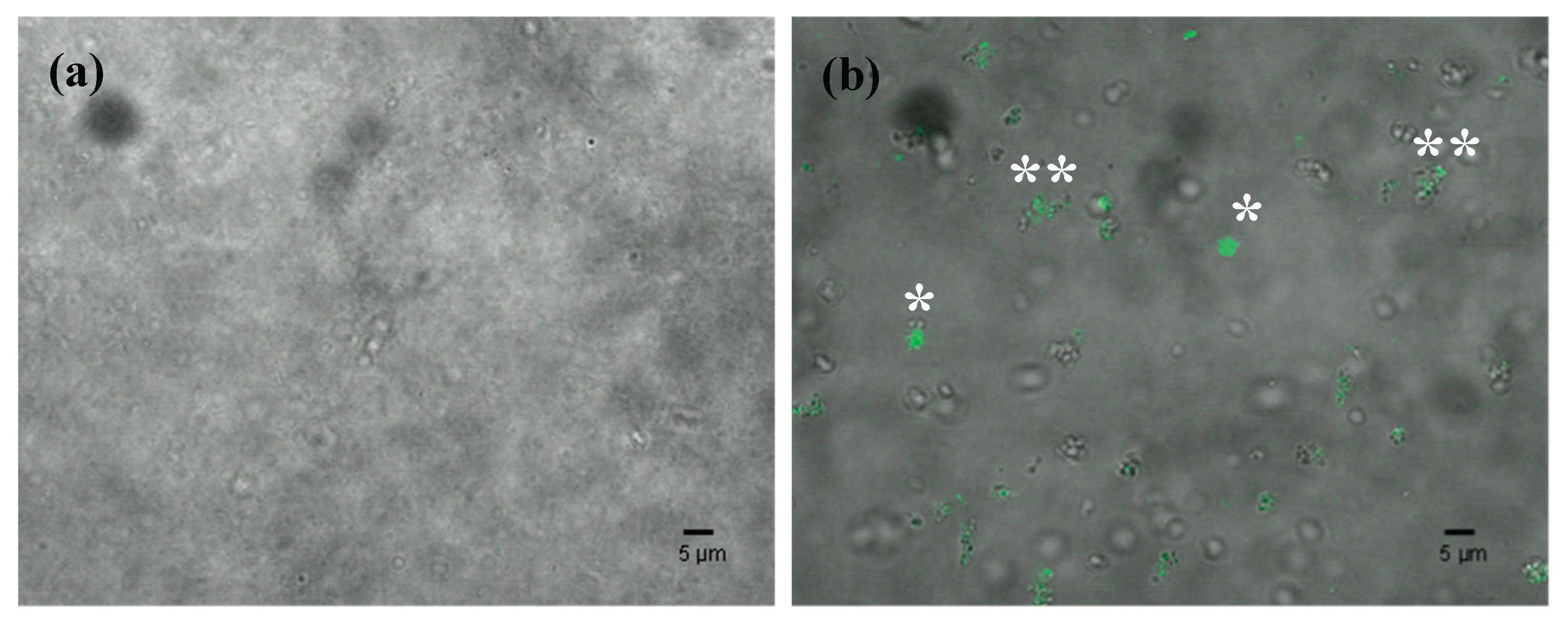
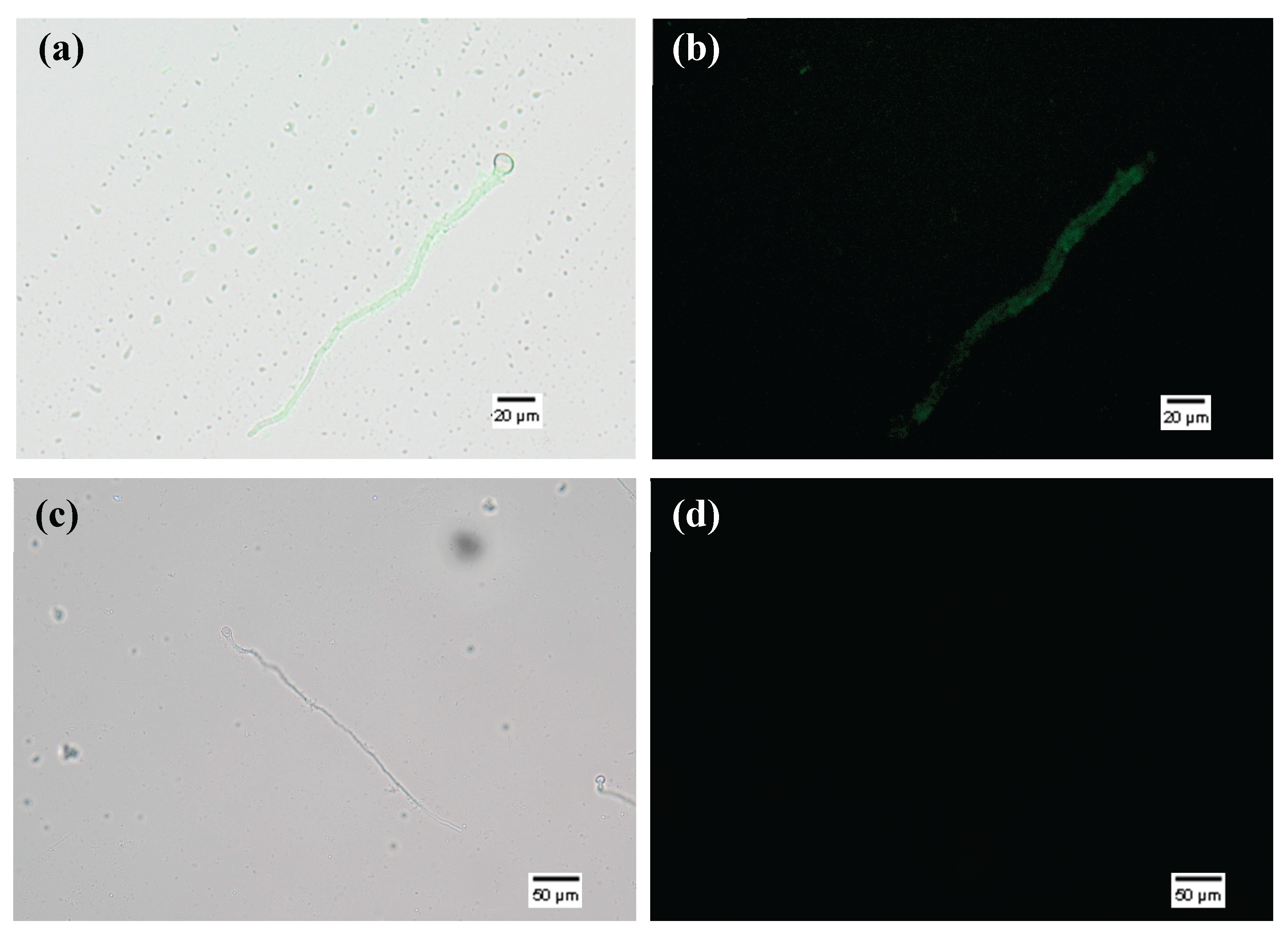
3.2. Loading and Uptake Assays
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RNAi | RNA interference |
| siRNA | small interfering RNAs |
| miRNA | micro RNAs |
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| BAP | 6-Benzylaminopurine |
| VIB | Vesicle Isolation Buffer |
| AWF | Apoplastic Washing Fluid |
| NTA | Nanoparticle Track analysis |
| TEM | Transmission Electron Microscopy |
| MNase | Micrococcal Nuclease |
References
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50-59. [CrossRef]
- Long, Y.; Yang, Y.; Pan, G.; Shen, Y. New insights into tissue culture plant-regeneration mechanisms. Front. Plant Sci. 2022, 13, 926752. [CrossRef]
- Nic-Can, G.I.; Avilez-Montalvo, J.R.; Aviles-Montalvo, R.N.; Márquez-López, R.E.; Mellado-Mojica, E.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M. The relationship between stress and somatic embryogenesis. In Somatic embryogenesis: fundamental aspects and applications, 1st ed.; Loyola-Vargas, V., Ochoa-Alejo, N. Eds.; Springer: Cham, 2016; pp. 151-170. [CrossRef]
- Wawrosch, C.; Zotchev, S.B. Production of bioactive plant secondary metabolites through in vitro technologies—status and outlook. Appl. Microbiol. Biotechnol. 2021, 105, 6649-6668. [CrossRef]
- Kankaanpää, S.; Väisänen, E.; Goeminne, G.; Soliymani, R.; Desmet, S.; Samoylenko, A.; Vainio, S.; Wingsle, G.; Boerjan, W.; Vanholme, R.; Kärkönen, A. Extracellular vesicles of Norway spruce contain precursors and enzymes for lignin formation and salicylic acid. Plant Physiol. 2024, 196, 788–809. [CrossRef]
- Kırbaş, O.K.; Sağraç, D.; Çiftçi, Ö.C.; Özdemir, G.; Öztürkoğlu, D.; Bozkurt, B.T.; Derman, Ü.C.; Taşkan, E.; Taşlı, P.N.; Özdemir, B.S; Şahin, F. Unveiling the potential: Extracellular vesicles from plant cell suspension cultures as a promising source. BioFactors 2025, 51, e2090. [CrossRef]
- Park, H. Y.; Kang, M. H.; Lee, G.; Kim, J. W. Enhancement of skin regeneration through activation of TGF-β/SMAD signaling pathway by Panax ginseng meyer non-edible callus-derived extracellular vesicles. J. Ginseng Res. 2024, 49, 34-41. [CrossRef]
- Yugay, Y.; Tsydeneshieva, Z.; Rusapetova, T.; Grischenko, O.; Mironova, A.; Bulgakov, D.; Silant’ev, V.; Tchernoded, G.; Bulgakov, V.; Shkryl, Y. Isolation and Characterization of Extracellular Vesicles from Arabidopsis thaliana Cell Culture and Investigation of the Specificities of Their Biogenesis. Plants 2023, 12, 3604. [CrossRef]
- Kocholatá, M.; Prusova, M.; Malinska, H. A.; Maly, J.; Janouskova, O. Comparison of two isolation methods of tobacco-derived extracellular vesicles, their characterization and uptake by plant and rat cells. Sci. Rep. 2022, 12, 19896. [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148-156. [CrossRef]
- Paganini, C.; Capasso Palmiero, U.; Pocsfalvi, G.; Touzet, N.; Bongiovanni, A.; Arosio, P. Scalable production and isolation of extracellular vesicles: available sources and lessons from current industrial bioprocesses. Biotechnol. J. 2019, 14, 1800528. [CrossRef]
- Giancaterino, S.; Boi, C. Alternative biological sources for extracellular vesicles production and purification strategies for process scale-up. Biotechnol. Adv. 2023, 63, 108092. [CrossRef]
- Rome, S. Biological properties of plant-derived extracellular vesicles. Food Funct. 2019, 10, 529-538. [CrossRef]
- van Niel, G.; d'Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213-228. [CrossRef]
- Cui, Y.; Gao, J.; He, Y.; Jiang, L. Plant extracellular vesicles. Protoplasma 2020, 257, 3–12. [CrossRef]
- Lian, M.Q.; Chng, W.H.; Liang, J.; Yeo, H.Q.; Lee, C.K.; Belaid, M.; Tollemeto, M.; Wacker, M.G.; Czarny, B.; Pastorin, G. Plant-derived extracellular vesicles: Recent advancements and current challenges on their use for biomedical applications. J. Extracell. Vesicles 2022, 11, 12283. [CrossRef]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: a novel nanomedicine approach with advantages and challenges. Cell Commun. Signal 2022, 20, 69. [CrossRef]
- Woith, E.; Guerriero, G.; Hausman, J.F.; Renaut, J.; Leclercq, C.C.; Weise, C.; Legay, S.; Weng, A.; Melzig, M.F. Plant extracellular vesicles and nanovesicles: focus on secondary metabolites, proteins and lipids with perspectives on their potential and sources. Int. J. Mol. Sci. 2021, 22, 3719. [CrossRef]
- Qiang, W.; Li, J.; Ruan, R.; Li, Q.; Zhang, X.; Yan, A.; Zhu, H. Plant-derived extracellular vesicles as a promising anti-tumor approach: a comprehensive assessment of effectiveness, safety, and mechanisms. Phytomedicine 2024, 130, 155750. [CrossRef]
- Lykogianni, M.; Bempelou, E.; Karamaouna, F.; Aliferis, K.A. Do pesticides promote or hinder sustainability in agriculture? The challenge of sustainable use of pesticides in modern agriculture. Sci. Total Environ. 2021, 795, 148625. [CrossRef]
- Niu, D.; Hamby, R.; Sanchez, J.N.; Cai, Q.; Yan, Q.; Jin, H. RNAs—a new frontier in crop protection. Curr. Opin. Biotechnol. 2021, 70, 204-212. [CrossRef]
- Kong, X.; Yang, M.; Le, B.H.; He, W.; Hou, Y. The master role of siRNAs in plant immunity. Mol. Plant Pathol. 2022, 23, 1565-1574. [CrossRef]
- Rodrigues, T.B.; Mishra, S.K.; Sridharan, K.; Barnes, E.R.; Alyokhin, A.; Tuttle, R.; Kokulapalan, W.; Garby, D.; Skizim, N.J.; Tang, Y.W.; Manley, B. First sprayable double-stranded RNA-based biopesticide product targets proteasome subunit beta type-5 in Colorado potato beetle (Leptinotarsa decemlineata). Front. Plant Sci. 2021, 12, 728652. [CrossRef]
- Zhao, J.H.; Liu, Q.Y.; Xie, Z.M.; Guo, H.S. Exploring the challenges of RNAi-based strategies for crop protection. Adv. Biotechnol. 2024, 2, 23. [CrossRef]
- Zhao, Y.; Zhou, Y.; Xu, J.; Fan, S.; Zhu, N.; Meng, Q.; Dai, S.; Yuan, X. Cross-Kingdom RNA Transport Based on Extracellular Vesicles Provides Innovative Tools for Plant Protection. Plants 2024, 13, 2712. [CrossRef]
- Niño-Sánchez, J.; Sambasivam, P.T.; Sawyer, A.; Hamby, R.; Chen, A.; Czislowski, E.; Li, P.; Manzie, N.; Gardiner, D.M.; Ford, R.; Xu, Z.P. BioClay™ prolongs RNA interference-mediated crop protection against Botrytis cinerea. J. Integr. Plant Biol. 2022, 64, 2187-2198. [CrossRef]
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing softly: a roadmap of Botrytis cinerea pathogenicity. Trends Plant Sci. 2023, 28, 211-222. 10.1016/j.tplants.2022.08.024.
- Wang, M.; Weiberg, A.; Lin, F.M.; Thomma, B.P.; Huang, H.D.; Jin, H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. plants 2016, 2, 1-10. [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 1962, 15, 473-497. 10.1111/j.1399-30541962tb08052.x.
- Rutter, B. D.; Innes, R. W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017, 173, 728–741. [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3-22. [CrossRef]
- Hamby, R.; Wang, M.; Qiao, L.; Jin, H. Synthesizing Fluorescently Labeled dsRNAs and sRNAs to Visualize Fungal RNA Uptake. In RNA Tagging: Methods and Protocols, 1st ed.; Heinlein, M., Eds.; Springer US: NY, USA, 2020; Vol. 2166, pp . 215–225. [CrossRef]
- Aranda, P. S.; LaJoie, D. M.; Jorcyk, C. L. Bleach Gel: A Simple Agarose Gel for Analyzing RNA Quality. Electrophoresis 2012, 33, 366–369. [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; Hingtgen, S.D. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655-664. [CrossRef]
- Yang, P.; Zhao, Z.; Virag, A.; Becker, T.; Zhao, L.; Liu, W.; Xia, Y. Botrytis cinerea in vivo Inoculation Assays for Early-, Middle-and Late-stage Strawberries. Bio-protocol 2023, 13, e4859. [CrossRef]
- Michler, C.H.; and Bauer, E.O. High frequency somatic embryogenesis from leaf tissue of Populus spp. Plant Sci. 1991, 77, 111-118. [CrossRef]
- Lelu-Walter, M.A.; Thompson, D.; Harvengt, L.; Sanchez, L.; Toribio, M.; Pâques, L.E. Somatic embryogenesis in forestry with a focus on Europe: state-of-the-art, benefits, challenges and future direction. Tree Genet. Genomes 2013, 9, 883-899. [CrossRef]
- Arencibia, A.D.; Gómez, A.; Poblete, M.; Vergara, C. High-performance micropropagation of dendroenergetic poplar hybrids in photomixotrophic Temporary Immersion Bioreactors (TIBs). Ind. Crops Prod. 2017, 96, 102-109. [CrossRef]
- Kim, S.; Kim, Y.O.; Lee, Y.; Choi, I.; Joshi, C.P.; Lee, K.; Bae, H.J. The transgenic poplar as an efficient bioreactor system for the production of xylanase. Biosci. Biotechnol. Biochem. 2012, 76, 1140-1145. [CrossRef]
- Verdú-Navarro, F.; Moreno-Cid, J.A.; Weiss, J.; Egea-Cortines, M. The advent of plant cells in bioreactors. Front. Plant Sci. 2023, 14, 1310405. [CrossRef]
- Martínez, M.; Corredoira, E. Recent Advances in Plant Somatic Embryogenesis: Where We Stand and Where to Go? Int. J. Mol. Sci. 2024, 25, 8912. [CrossRef]
- Kocholatá, M.; Malý, J.; Kříženecká, S.; Janoušková, O. Diversity of extracellular vesicles derived from calli, cell culture and apoplastic fluid of tobacco. Sci. Rep. 2024, 14, 1-13. [CrossRef]
- Rutter, B. D.; Innes, R.W. Growing pains: addressing the pitfalls of plant extracellular vesicle research. New Phytol. 2020, 228, 1505-1510. [CrossRef]
- Rikkert, L.G.; Nieuwland, R.; Terstappen, L.W.M.M.; Coumans, F.A.W. Quality of extracellular vesicle images by transmission electron microscopy is operator and protocol dependent. J. Extracell. Vesicles 2019, 8, 1555419. [CrossRef]
- Duan, X.; Chen, L.; Liu, Y.; Chen, H.; Wang, F.; Hu, Y. Integrated physicochemical, hormonal, and transcriptomic analysis reveals the underlying mechanism of callus formation in Pinellia ternata hydroponic cuttings. Front. Plant Sci. 2023, 14, p.1189499. [CrossRef]
- Wen, S.S.; Ge, X.L.; Wang, R.; Yang, H.F.; Bai, Y.E.; Guo, Y.H.; Zhang, J.; Lu, M.Z.; Zhao, S.T.; Wang, L.Q. An efficient Agrobacterium-mediated transformation method for hybrid poplar 84K (Populus alba× P. glandulosa) using calli as explants. Int. J. Mol. Sci. 2022. 23, 2216. [CrossRef]
- Ge, F.; Hu, H.; Huang, X.; Zhang, Y.; Wang, Y.; Li, Z.; Zou, C.; Peng, H.; Li, L.; Gao, S.; Pan, G. Metabolomic and proteomic analysis of maize embryonic callus induced from immature embryo. Sci. Rep. 2017, 7, 1004. [CrossRef]
- Kumari, A.; Ray, K.; Sadhna, S.; Pandey, A. K.; Sreelakshmi, Y.; Sharma, R. Metabolomic homeostasis shifts after callus formation and shoot regeneration in tomato. PloS One 2017, 12, e0176978. 10.1371/journal.pone.0176978.
- Amiri, A.; Bagherifar, R.; Ansari Dezfouli, E.; Kiaie, S.H.; Jafari, R.; Ramezani, R. Exosomes as bio-inspired nanocarriers for RNA delivery: preparation and applications. J. Transl. Med. 2022, 20, 125. [CrossRef]
- Donoso-Quezada, J.; Ayala-Mar, S.; González-Valdez, J. State-of-the-art exosome loading and functionalization techniques for enhanced therapeutics: a review. Crit. Rev. Biotechnol. 2020, 40, 804-820. [CrossRef]
- Nordmeier, S.; Ke, W.; Afonin, K. A.; Portnoy, V. Exosome mediated delivery of functional nucleic acid nanoparticles (NANPs). Nanomedicine 2020, 30, 102285.
- Iqbal, Z.; Rehman, K.; Mahmood, A.; Shabbir, M.; Liang, Y.; Duan, L.; Zeng, H. Exosome for mRNA delivery: strategies and therapeutic applications. J. Nanobiotechnology 2024, 22, 395. [CrossRef]
- de Voogt, W.S.; Tanenbaum, M.E.; Vader, P. Illuminating RNA trafficking and functional delivery by extracellular vesicles. Adv. Drug Deliv. Rev. 2021, 174, 250-264. [CrossRef]
| Primer Name | Sequence |
|---|---|
| TaPDS Frw | TTTGCTCCAGCAGAGGAATGG |
| TaPDS Frw T7pol | TAATACGACTCACTATAGGTTTGCTCCAGCAGAGGAATGG |
| TaPDS Rev | AAACCCTTCGATCGGTGATCG |
| TaPDS Rev T7pol | TAATACGACTCACTATAGGAAACCCTTCGATCGGTGATCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




