Introduction
Interleukin-17A (IL-17A) has been recognized as an inflammatory cytokine that plays a central role in immune responses and the maintenance of tissue homeostasis since it was first identified by Rouvier et al. in 1995 [
1]. IL-17A is a representative member of the IL-17 family of cytokines (IL-17A-F) and has a wide range of physiological functions, from infection defense to tissue repair. In particular, it is known to play an essential role in the body’s defense against bacteria and fungi. On the other hand, its excessive activation is deeply involved in the pathogenesis of autoimmune diseases such as psoriasis and rheumatoid arthritis.
In recent years, the function of IL-17A has attracted attention not only in the immune system but also in the central nervous system [
2,
3,
4]. In particular, IL-17A has been linked to neurological and psychiatric disorders such as multiple sclerosis, autism spectrum disorder, and schizophrenia, and its potential as a new therapeutic target is being investigated. In fact, the development of IL-17A-targeted therapeutic agents is progressing steadily, and in the field of autoimmune diseases, anti-IL-17A antibody drugs have already been commercialized and demonstrated high therapeutic efficacy.
This review provides a comprehensive overview of IL-17A, covering its molecular structure, intracellular signaling pathways, physiological functions, and associations with diseases, incorporating the latest findings. Particular emphasis is placed on areas of rapid progress, such as transcription control mechanisms, cell-specific receptor expression, and functions in the central nervous system. These findings are expected to provide essential insights for the development of novel therapeutic strategies targeting IL-17A.
Molecular Structure and Expression Regulation of IL-17A
IL-17A is a representative member of the IL-17 family (IL-17A–F), a group of proinflammatory cystine knot-type cytokines. A distinctive cystine-binding pattern and functions characterize this family as homodimers or heterodimers [
5]. IL-17A is a glycoprotein that functions as a homodimer with a molecular weight of approximately 35 kDa. It forms intramolecular disulfide bonds via four cysteine residues and an intermolecular disulfide bond via a single cysteine residue. Its three-dimensional structure exhibits a unique folding pattern distinct from other cystine knot-type cytokines (e.g., NGF, TGF-β) [
6]. The gene structure is composed of three exons and two introns, and contains a highly conserved regulatory region [
7]. The first exon encodes the signal peptide, whereas the second and third exons encode the mature protein. Within the promoter region, binding sites for transcription factors, including RORγt and STAT3, are present, and the gene expression is precisely regulated at multiple levels—transcriptional, translational, and post-translational. IL-17A is glycosylated post-translationally, a modification that plays a pivotal role in controlling its secretion efficiency and biological activity. The conservation of the N-linked glycosylation site among species underscores the functional significance of this modification [
8].
Transcriptional Regulation of IL-17A
The nuclear receptor RORγt constitutes the central regulator of IL-17A transcription. By directly binding to the promoter region of the IL-17A gene, RORγt drives transcriptional activation [
9]. This process is orchestrated by STAT3 signaling, which induces RORγt expression, with cytokines such as IL-6 and IL-23 acting as indispensable upstream mediators [
10]. IRF4 and BATF function in a cooperative manner, engaging enhancer elements to remodel chromatin architecture, thereby promoting transcriptional activation [
11]。In addition, transcription factors such as c-Maf and AHR have also been identified as important contributors to the regulation of IL-17A gene expression [
11,
12]. Epigenetic mechanisms are increasingly appreciated as essential regulators of IL-17A expression. The histone acetyltransferase p300 and the methyltransferase EZH2 govern chromatin configuration at the IL-17A locus, thereby modulating its accessibility. Notably, the dynamic balance between H3K27ac, an activating epigenetic mark, and H3K27me3, a repressive counterpart, serves as a pivotal determinant of IL-17A transcriptional output [
13].
The Cellular Heterogeneity Underlying IL-17A Production
IL-17A production arises from multiple cellular sources, each governed by characteristic regulatory mechanisms. The most critical producers are Th17 cells, which differentiate from naïve CD4
+ T cells under the influence of cytokines including TGF-β, IL-6, and IL-23. This differentiation program is driven by STAT3 signaling, which promotes the induction of RORγt and endows cells with IL-17A–producing potential [
14]. γδ T cells constitute another critical source of IL-17A. Of particular interest is a distinct subset that intrinsically acquires IL-17A–producing potential during thymic ontogeny. These cells display tissue-resident properties and contribute indispensably to the initiation of immune responses during the early phases of infection. [
15]. Among innate lymphoid cells, group 3 ILCs (ILC3s) represent a principal source of IL-17A in mucosal tissues. These cells promptly secrete IL-17A upon stimulation with IL-23 and IL-1β, playing an indispensable role in sustaining mucosal barrier function [
16]. Among innate lymphoid cells, group 3 ILCs (ILC3s) represent a principal source of IL-17A in mucosal tissues. These cells promptly secrete IL-17A upon stimulation with IL-23 and IL-1β, playing an indispensable role in sustaining mucosal barrier function [
17].
Regulatory Mechanisms and Functions of IL-17 Receptors
The IL-17 receptor family comprises five members, IL-17RA to IL-17RE. A heterodimeric complex of IL-17RA and IL-17RC mediates effective IL-17A signaling. IL-17RA itself is a ~90-kDa type I transmembrane protein, harboring a cytoplasmic SEFIR domain that is indispensable for propagating downstream signaling events [
18]。IL-17RA is broadly expressed across nearly all tissues; however, its expression levels exhibit marked variability depending on cellular identity and physiological context. Transcriptional regulation of IL-17RA is mediated by an array of transcription factors, notably NF-κB, AP-1, C/EBPβ, and C/EBPδ [
19]. By contrast, the expression of IL-17RC displays greater tissue specificity, and several isoforms arising from alternative splicing have been described [
7]。
The Cellular Heterogeneity of IL-17 Receptor Expression
IL-17 receptor–expressing cells are widely distributed across tissues, with functional outcomes that vary according to cellular context. Epithelial cells, notably those of the airway, intestinal tract, and skin keratinocytes, exhibit high expression of IL-17RA and IL-17RC. In these epithelial compartments, IL-17 signaling is essential for barrier maintenance, primarily through the induction of antimicrobial peptides, regulation of tight junction–associated molecules, and the production of chemokines [
20]。
Synovial and dermal fibroblasts constitutively express IL-17 receptors, and IL-17 signaling in these cells contributes to tissue remodeling through the production of inflammatory mediators and the induction of matrix metalloproteinases. In particular, in rheumatoid arthritis, upregulation of IL-17 receptor expression on synovial fibroblasts has been shown to play a critical role in disease pathogenesis [
21]. Expression of IL-17 receptors on vascular endothelial cells has also attracted attention, as IL-17 signaling is implicated in the regulation of angiogenesis and vascular permeability. During inflammation, endothelial IL-17 receptor expression is upregulated, thereby contributing to the induction of adhesion molecules and promoting the transendothelial migration of inflammatory cells [
22].
The IL-17 Signaling Pathway
Signal transduction by IL-17A commences with the assembly of its receptor complex. Engagement of IL-17A with the IL-17RA/RC heterodimer induces conformational rearrangements that facilitate the association of the adaptor Act1 (TRAF3IP2) with the receptor’s cytoplasmic domains. Beyond its role as a scaffold, Act1 exerts ubiquitin ligase activity, which drives the activation of downstream mediators including TRAF6 [
22,
23].
Downstream signaling of IL-17A predominantly engages the NF-κB, MAPK, and C/EBP pathways. The NF-κB axis encompasses both canonical and non-canonical branches. Canonical NF-κB activation involves stimulation of the IKK complex, leading to IκB phosphorylation and degradation, and consequent nuclear translocation of p65/p50. The non-canonical branch, in contrast, depends on NIK activation, which drives p100 processing and the nuclear accumulation of p52/RelB.The MAPK pathway is characterized by the activation of p38 MAPK, ERK1/2, and JNK. p38 MAPK is critical for transcriptional regulation of inflammatory genes as well as mRNA stabilization; ERK1/2 governs proliferative responses and cytokine production; and JNK activation regulates gene expression primarily through AP-1 [
24]. Within the C/EBP pathway, activation of C/EBPβ and C/EBPδ is induced, and these transcription factors orchestrate the transcriptional regulation of numerous target genes. Notably, they are pivotal in governing the expression of antimicrobial peptides and inflammatory cytokines.
Evolutionary Conservation of IL-17 and Its Signaling Pathways
IL-17A exhibits remarkable evolutionary conservation across vertebrates. The maintenance of its core structural and functional features from fish to mammals underscores the critical physiological significance of this molecule [
25,
26]. Of particular significance, the domains responsible for receptor engagement and signal transduction exhibit strong conservation across species, ensuring preservation of the core immunological functions of IL-17A. Genomic features, including exon–intron architecture and the spatial organization of regulatory regions, are likewise highly conserved. Promoter elements containing critical transcription factor–binding motifs, such as RORγt and STAT3 response elements, are maintained across vertebrate lineages, highlighting the evolutionary importance of conserved regulatory networks in governing IL-17A expression. Nonetheless, species-specific variation in expression patterns and functional roles has been documented, reflecting adaptive pressures and co-evolution with pathogens. For instance, in fish, a distinctive expression pattern in the gills has been observed, potentially serving as a specialized defense mechanism tailored to the aquatic environment.
In mammalian systems, IL-17A has undergone functional diversification beyond its ancestral role in antimicrobial defense. While its protective functions against extracellular pathogens are preserved, IL-17A also participates in diverse processes, including epithelial barrier maintenance, tissue repair, and modulation of the microbiota. Importantly, dysregulated IL-17A responses have been implicated in the etiology of multiple autoimmune and inflammatory diseases, such as psoriasis, rheumatoid arthritis, and inflammatory bowel disease. These findings illustrate the evolutionary trajectory of IL-17A toward expanded immunological functions in mammals, reflecting a fine balance between beneficial host defense and detrimental immunopathology.
Functional Implications of IL-17 Signaling in the CNS
IL-17A has recently garnered significant attention for its roles within the central nervous system. In the healthy brain, IL-17A production has been demonstrated not only in infiltrating Th17 cells but also in resident microglia, astrocytes, and subsets of neurons. Physiologically, IL-17A has been implicated in modulating neuronal plasticity and synaptogenesis, as well as regulating the proliferation and differentiation of neural stem cells [
27]。
A hallmark of IL-17 signaling within the central nervous system lies in the broad diversity of its receptor expression. Astrocytes, microglia, oligodendrocytes, and neurons have all been shown to express IL-17 receptors. Among these, astrocytic expression of IL-17 receptors has emerged as a key determinant in the pathophysiology of autoimmune neuroinflammatory diseases, most prominently multiple sclerosis [
28].
In multiple sclerosis (MS) and in experimental autoimmune encephalomyelitis (EAE), IL-17A has been identified as a pivotal mediator of disease pathogenesis. Mechanistically, IL-17A promotes blood–brain barrier disruption, augments the infiltration of inflammatory leukocytes into the central nervous system, induces injury to oligodendrocytes, and accelerates demyelination, thereby driving disease progression [
29]. Collectively, these observations highlight the importance of IL-17A as a potential therapeutic target in multiple sclerosis. Emerging evidence further implicates IL-17A in ischemic brain injury, where it contributes to disease progression by amplifying acute inflammatory responses, aggravating neuronal death, increasing blood–brain barrier permeability, and promoting the activation of glial cells
Links Between IL-17A and Psychiatric Disorders
An emerging body of evidence has highlighted a potential connection between IL-17A and psychiatric disorders, with particular focus on autism spectrum disorder (ASD). Studies employing maternal immune activation (MIA) models have revealed that inflammation-induced elevations of IL-17A during gestation perturb fetal brain development, thereby promoting ASD-like behavioral phenotypes [
30]. Accumulating evidence indicates that several pathological mechanisms are implicated in this process, including disrupted cortical development, defective neural circuit assembly, and alterations in synaptic function. Of particular note, impaired differentiation of neural stem cells and incomplete formation of neuronal networks are proposed to underlie the emergence of core ASD phenotypes. In clinical settings, dysregulated levels of inflammatory cytokines—including IL-17A—have been documented in patients with ASD. Increases in peripheral blood IL-17A concentrations have been observed, and such elevations correlate with the severity of clinical manifestations [
31]. Although inflammatory cytokines have been implicated in the pathophysiology of schizophrenia and depression, evidence specifically addressing the role of IL-17A is still scarce at present [
32,
33,
34]. Increased serum concentrations of IL-17 have been documented in patients with acute-phase schizophrenia and in individuals with major depressive disorder [
33,
35]. Nevertheless, the pathophysiological relevance of these observations remains to be fully elucidated. Taken together, current findings indicate that IL-17A could serve as a critical target, both for deepening our understanding of psychiatric disorders, especially ASD, and for guiding the development of innovative therapeutic interventions.
Pathophysiological Insights and Therapeutic Target Potential of IL-17A
IL-17A has been intensively investigated as a potential therapeutic target across multiple disease contexts. Notably, in autoimmune conditions such as psoriasis, psoriatic arthritis, and ankylosing spondylitis, anti-IL-17A monoclonal antibodies—such as secukinumab and ixekizumab—have been successfully translated into clinical application, where they exhibit robust therapeutic benefit [
36]. The clinical success of these agents underscores the therapeutic efficacy of IL-17A–targeted strategies. Extending beyond autoimmune diseases of the periphery, therapeutic applications in central nervous system disorders are now under active investigation [
4]. Clinical trials evaluating anti-IL-17A antibody therapy are in progress for multiple sclerosis, yet significant technical challenges persist, notably concerning blood–brain barrier permeability and optimization of administration routes. Moreover, in the context of neurodegenerative and psychiatric disorders, the therapeutic application of IL-17A blockade remains an area requiring further investigation, with particular interest in its potential for preventive interventions
Conclusions and Prospects
IL-17A is a key molecule involved in a wide range of physiological and pathological processes, from immune responses to central nervous system function. Its evolutionary conservation, complex signaling mechanisms, and association with various diseases highlight its importance in host defense and disease pathogenesis. Notably, IL-17A exhibits cell type-specific and context-dependent effects. Despite being the same molecule, it shows different, and sometimes opposing, effects depending on cell type and context, suggesting both its potential as a therapeutic target and the challenges in its regulation.
Future research will focus on elucidating the cell-type-specific regulatory mechanisms of signal transduction pathways. In particular, detailed analysis of its functions in the central nervous system holds promise for the development of novel therapies for neurodegenerative and psychiatric disorders.
Additionally, elucidating the mechanisms underlying the association with mental disorders may reveal new aspects of the neuro-immune connection, which has not been fully understood to date. Furthermore, exploring its potential as a biomarker could open new avenues for early diagnosis and monitoring of treatment efficacy. The success of anti-IL-17A antibody drugs already in clinical use demonstrates the efficacy of therapeutic strategies targeting this molecule.
However, there are still technical challenges to be addressed in the application of IL-17A to central nervous system diseases, such as the permeability of the blood-brain barrier and the optimization of administration routes. Progress in research addressing these challenges is likely to lead to the development of more effective and safer treatment strategies. Furthermore, as a preventive medicine approach, findings related to maternal immune activation suggest the possibility of a new treatment paradigm for the prevention of neurodevelopmental disorders.
As described above, research on IL-17A is making steady progress in a wide range of fields, from basic molecular biology research to clinical applications. With the accumulation of new knowledge, deeper understanding, and the development of innovative treatments are expected in the future. In particular, further elucidation of the regulatory mechanisms of IL-17A signaling will be necessary for the realization of precision medicine tailored to the individual patient’s condition.
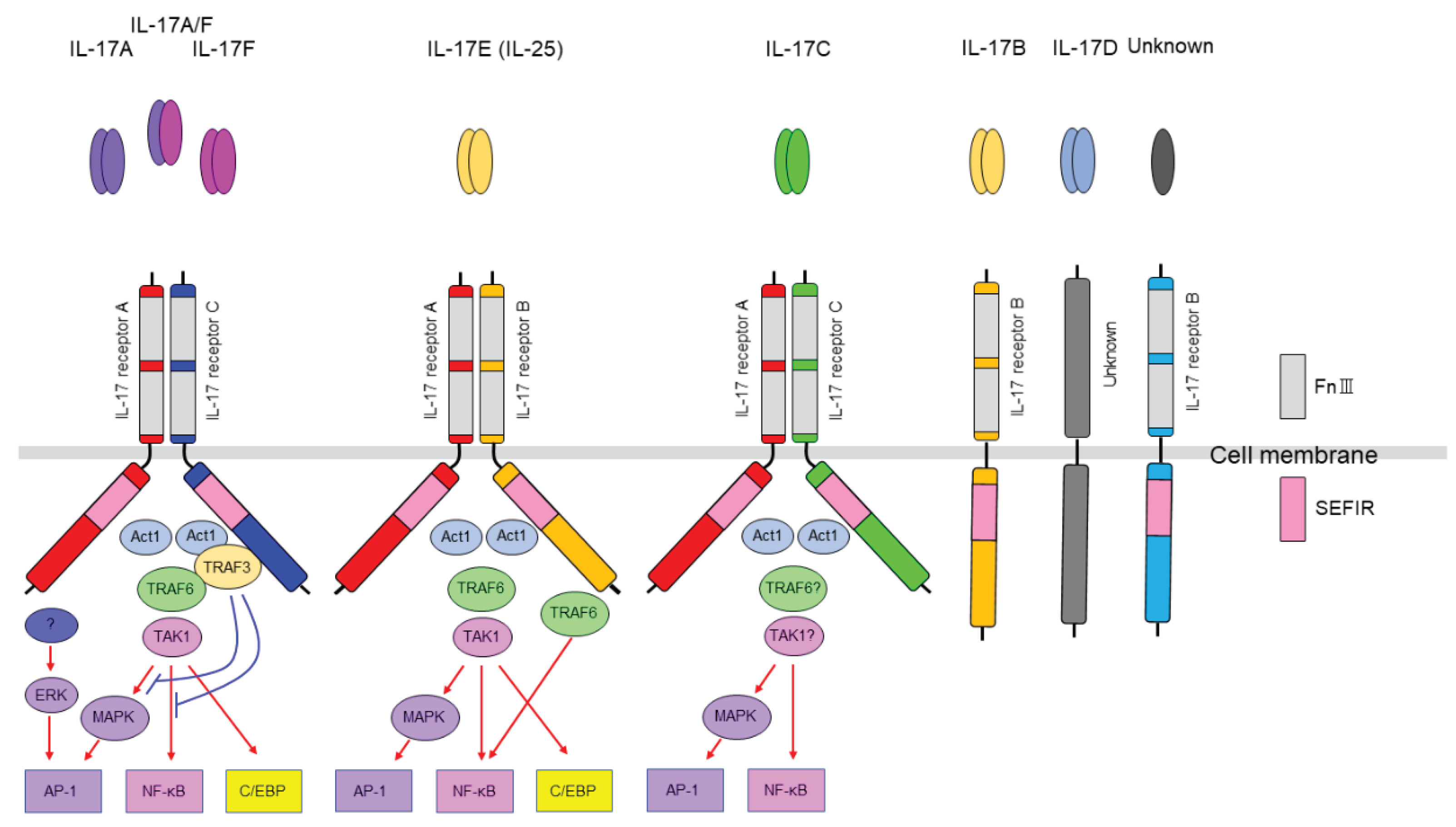
Figure 1.
IL-17 family and IL-17 receptors. IL-17 receptors are constitutively expressed in various cells. Like ligands, receptors also form families, with extracellular domains containing FnIII (fibronectin III)-like domains and intracellular domains containing SEFIR (Similar expression to fibroblast growth factor/IL-17R) domains corresponding to the TIR (Toll/IL-1R) domains of the IL-1/Toll-like receptor family.IL-17RA functions as a direct receptor for the IL-17 ligand or as a co-receptor, acting as an organizing hub for IL-17 family signaling on the membrane. IL-17A and IL-17F bind to the receptor and activate NF-κB, MAPK,C/EBP pathways. However, this process requires the SEFIR domains of the receptor and Act1 (Act 1 adaptor protein) to interact, thereby recruiting TRAF6 (TNF receptor-associated factor 6) and TAK1 (TGFβ-activated kinase 1).
Figure 1.
IL-17 family and IL-17 receptors. IL-17 receptors are constitutively expressed in various cells. Like ligands, receptors also form families, with extracellular domains containing FnIII (fibronectin III)-like domains and intracellular domains containing SEFIR (Similar expression to fibroblast growth factor/IL-17R) domains corresponding to the TIR (Toll/IL-1R) domains of the IL-1/Toll-like receptor family.IL-17RA functions as a direct receptor for the IL-17 ligand or as a co-receptor, acting as an organizing hub for IL-17 family signaling on the membrane. IL-17A and IL-17F bind to the receptor and activate NF-κB, MAPK,C/EBP pathways. However, this process requires the SEFIR domains of the receptor and Act1 (Act 1 adaptor protein) to interact, thereby recruiting TRAF6 (TNF receptor-associated factor 6) and TAK1 (TGFβ-activated kinase 1).
Acknowledgements
We are grateful to Kenyu Nakamura and Kyoko Kishi for their valuable discussions and critical reading of this manuscript. This work was supported by Grants-in-Aid for Scientific Research C (KAKENHI Nos. 19K08065, 22K07611), and Grant-in-Aid for Scientific Research on Innovative Areas “Multiscale Brain” (No. 19H05201) from The Ministry of Education, Culture, Sports, Science and Technology MEXT Japan. T.S. was also supported by the Foundation for Advanced Medical Research, the Naito Foundation, the Takeda Science Foundation, the Kawano Masanori Memorial Public Interest Incorporated Foundation for Promotion of Pediatrics, the Taiju Life Social Welfare Foundation, the Life Science Foundation of Japan, the Nakatomi Foundation, the Mishima Kaiun Memorial Foundation, the Kanehara Ichiro Memorial Foundation for Medical Science and Medical Care, the Foundation for Pharmaceutical Research. Part of this work was supported by the NIBB Collaborative Research Program and Advanced Animal Model Support (16H06276) of Grant-in-Aid for Scientific Research on Innovative Areas to T.S.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Johansen, C.; Usher, P.; Kjellerup, R.; Lundsgaard, D.; Iversen, L.; Kragballe, K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br. J. Dermatol. 2009, 160, 319–324. [Google Scholar] [CrossRef]
- Kamiya S, Sanaka S, Kubo A, Higuchi K, Nakamura K, Sasaki T. Psoriasis and ASD: Revolutionary Insights into Disease Mechanisms through IL-17. The Allergy in Practice. 2024 Nov;44(12):70–1.
- Kamiya S, Sasaki T. Fetal Environment and Neurodevelopment: The Role of Maternal Immune System and Microbiota in Autism Spectrum Disorder. Reproductive Immunology and Biology. 2024 Dec;39(1):21–5.
- Kubo A, Sasaki T. IL-17 signaling and NEUROIMMUNOLOGY: Psoriasis to Autism Spectrum Disorder [Internet]. Jxiv; 2024. Available from. [CrossRef]
- Wright, J.F.; Bennett, F.; Li, B.; Brooks, J.; Luxenberg, D.P.; Whitters, M.J.; Tomkinson, K.N.; Fitz, L.J.; Wolfman, N.M.; Collins, M.; et al. The Human IL-17F/IL-17A Heterodimeric Cytokine Signals through the IL-17RA/IL-17RC Receptor Complex. J. Immunol. 2008, 181, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Hymowitz, S.G.; Filvaroff, E.H.; Yin, J.; Lee, J.; Cai, L.; Risser, P.; Maruoka, M.; Mao, W.; Foster, J.; Kelley, R.F.; et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001, 20, 5332–5341. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Ely, L.K.; Fischer, S.; Garcia, K.C. Structural basis of receptor sharing by interleukin 17 cytokines. Nat. Immunol. 2009, 10, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The Orphan Nuclear Receptor RORγt Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell. 2006 Sep 22;126(6):1121–33.
- Durant, L.; Watford, W.T.; Ramos, H.L.; Laurence, A.; Vahedi, G.; Wei, L.; Takahashi, H.; Sun, H.-W.; Kanno, Y.; Powrie, F.; et al. Diverse Targets of the Transcription Factor STAT3 Contribute to T Cell Pathogenicity and Homeostasis. Immunity 2010, 32, 605–615. [Google Scholar] [CrossRef]
- Ciofani, M.; Madar, A.; Galan, C.; Sellars, M.; Mace, K.; Pauli, F.; Agarwal, A.; Huang, W.; Parkurst, C.N.; Muratet, M.; et al. A Validated Regulatory Network for Th17 Cell Specification. Cell 2012, 151, 289–303. [Google Scholar] [CrossRef]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008 May 1;453(7191):65–71.
- Wei, G.; Wei, L.; Zhu, J.; Zang, C.; Hu-Li, J.; Yao, Z.; Cui, K.; Kanno, Y.; Roh, T.-Y.; Watford, W.T.; et al. Global Mapping of H3K4me3 and H3K27me3 Reveals Specificity and Plasticity in Lineage Fate Determination of Differentiating CD4+ T Cells. Immunity 2009, 30, 155–167. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Papotto PH, Ribot JC, Silva-Santos B. IL-17+ γδ T cells as kick-starters of inflammation. Nat Immunol. 2017 May 18;18(6):604–11.
- Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015 Jan 15;517(7534):293–301.
- Taylor PR, Roy S, Leal SM Jr, Sun Y, Howell SJ, Cobb BA, et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nat Immunol. 2014 Feb;15(2):143–51.
- Gu C, Wu L, Li X. IL-17 family: cytokines, receptors and signaling. Cytokine. 2013 Nov;64(2):477–85.
- Shen, F.; Li, N.; Gade, P.; Kalvakolanu, D.V.; Weibley, T.; Doble, B.; Woodgett, J.R.; Wood, T.D.; Gaffen, S.L. IL-17 Receptor Signaling Inhibits C/EBPβ by Sequential Phosphorylation of the Regulatory 2 Domain. Sci. Signal. 2009, 2, ra8–ra8. [Google Scholar] [CrossRef]
- Ramirez-Carrozzi, V.; Sambandam, A.; Luis, E.; Lin, Z.; Jeet, S.; Lesch, J.; Hackney, J.; Kim, J.; Zhou, M.; Lai, J.; et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 2011, 12, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Hot, A.; Lenief, V.; Miossec, P. Combination of IL-17 and TNFα induces a pro-inflammatory, pro-coagulant and pro-thrombotic phenotype in human endothelial cells. Ann. Rheum. Dis. 2012, 71, 768–776. [Google Scholar] [CrossRef]
- Liu, C.; Qian, W.; Qian, Y.; Giltiay, N.V.; Lu, Y.; Swaidani, S.; Misra, S.; Deng, L.; Chen, Z.J.; Li, X. Act1, a U-box E3 Ubiquitin Ligase for IL-17 Signaling. Sci. Signal. 2009, 2, ra63–ra63. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Liu, C.; Hartupee, J.; Altuntas, C.Z.; Gulen, M.F.; Jane-Wit, D.; Xiao, J.; Lu, Y.; Giltiay, N.; Liu, J.; et al. The adaptor Act1 is required for interleukin 17–dependent signaling associated with autoimmune and inflammatory disease. Nat. Immunol. 2007, 8, 247–256. [Google Scholar] [CrossRef]
- Song, X.; Qian, Y. IL-17 family cytokines mediated signaling in the pathogenesis of inflammatory diseases. Cell. Signal. 2013, 25, 2335–2347. [Google Scholar] [CrossRef]
- Lethenteron japonicum) IL-17 upregulated by LPS-stimulation in the skin cells.
- Wang, T.; Johansson, P.; Abós, B.; Holt, A.; Tafalla, C.; Jiang, Y.; Wang, A.; Xu, Q.; Qi, Z.; Huang, W.; et al. First in-depth analysis of the novel Th2-type cytokines in salmonid fish reveals distinct patterns of expression and modulation but overlapping bioactivities. Oncotarget 2016, 7, 10917–10946. [Google Scholar] [CrossRef]
- Benedetti F, Poletti S, Hoogenboezem TA, Mazza E, Ambrée O, de Wit H, et al. Inflammatory cytokines influence measures of white matter integrity in Bipolar Disorder. J Affect Disord. 2016 Sep;202:1–9.
- Kang, Z.; Altuntas, C.Z.; Gulen, M.F.; Liu, C.; Giltiay, N.; Qin, H.; Liu, L.; Qian, W.; Ransohoff, R.M.; Bergmann, C.; et al. Astrocyte-Restricted Ablation of Interleukin-17-Induced Act1-Mediated Signaling Ameliorates Autoimmune Encephalomyelitis. Immunity 2010, 32, 414–425. [Google Scholar] [CrossRef]
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediat. Inflamm. 2017, 2017, 3908061. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dang, Y.; Yan, Y. Serum interleukin-17 A and homocysteine levels in children with autism. BMC Neurosci. 2024, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boerrigter, D.; Weickert, T.W.; Lenroot, R.; O’dOnnell, M.; Galletly, C.; Liu, D.; Burgess, M.; Cadiz, R.; Jacomb, I.; Catts, V.S.; et al. Using blood cytokine measures to define high inflammatory biotype of schizophrenia and schizoaffective disorder. J. Neuroinflammation 2017, 14, 1–15. [Google Scholar] [CrossRef]
- El Kissi, Y.; Samoud, S.; Mtiraoui, A.; Letaief, L.; Hannachi, N.; Ayachi, M.; Ali, B.B.H.; Boukadida, J. Increased Interleukin-17 and decreased BAFF serum levels in drug-free acute schizophrenia. Psychiatry Res. 2015, 225, 58–63. [Google Scholar] [CrossRef]
- Debnath, M.; Berk, M. Th17 Pathway-Mediated Immunopathogenesis of Schizophrenia: Mechanisms and Implications. Schizophr. Bull. 2014, 40, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Davami, M.H.; Baharlou, R.; Vasmehjani, A.A.; Ghanizadeh, A.; Keshtkar, M.; Dezhkam, I.; Atashzar, M.R. Elevated IL-17 and TGF-β Serum Levels: A Positive Correlation between T-helper 17 Cell-Related Pro-Inflammatory Responses with Major Depressive Disorder. Basic Clin. Neurosci. J. 2016, 7, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Papp, K.A.; Matheson, R.T.; Tu, J.H.; Bissonnette, R.; Bourcier, M.; Gratton, D.; Kunynetz, R.A.; Poulin, Y.; Rosoph, L.A.; et al. Evidence that a neutrophil–keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis. Exp. Dermatol. 2015, 24, 529–535. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).