1. Introduction
The persistence of plastic waste, particularly polyethylene-based polymers, has become one of the most pressing environmental challenges of the 21st century. Among these, linear low-density polyethylene (LLDPE) is widely used in flexible packaging, agricultural films, and consumer products due to its mechanical properties and chemical resistance. However, this same resistance renders it highly recalcitrant in natural environments, with degradation timelines that span centuries [
1].
Conventional disposal strategies—including landfilling, incineration, and mechanical recycling—have proven insufficient to address the escalating accumulation of polyethylene-based plastics. Consequently, alternative biodegradation pathways are under active investigation. In recent years, particular interest has focused on the capacity of invertebrates to ingest and potentially transform synthetic polymers, a phenomenon initially observed in larvae of
Tenebrio molitor (mealworms) and other insects [
2,
3].
Several studies have reported that mealworms can fragment and partially mineralize polystyrene (PS) and low-density polyethylene (LDPE), likely through the combined action of gut microbiota and endogenous enzymatic activity [
2,
3]. However, the biodegradation of LLDPE, which exhibits a higher degree of crystallinity and thermal stability than LDPE, remains far less understood. In particular, the extent to which ingested LLDPE is chemically transformed, metabolized, or excreted intact is still under debate.
Frass—the excrement produced by insects—offers a unique matrix to assess the fate of ingested plastics. It may contain unaltered polymer residues, degradation byproducts, or even biotransformed compounds. Therefore, accurately quantifying the amount of residual plastic in frass is essential for evaluating the efficacy of insect-mediated plastic degradation. This requires highly sensitive and selective analytical techniques capable of resolving polymer signals within a complex organic background.
Previous analytical approaches have included thermal decomposition analysis, gas chromatography–mass spectrometry (GC-MS), and Fourier-transform infrared spectroscopy (FTIR) to detect plastic residues in biological matrices [
4,
5,
6]. However, these techniques often lack sufficient resolution to identify LLDPE at low concentrations, particularly in heterogeneous substrates like frass.
Solid-state ¹³C nuclear magnetic resonance (NMR) spectroscopy with cross-polarization and magic angle spinning (CP-MAS NMR) has recently emerged as a promising method for analyzing synthetic polymers in environmental samples [
7]. It allows for non-destructive, label-free detection of characteristic aliphatic carbon signals and can differentiate between synthetic and biogenic carbon structures. When combined with chemometric modeling, such as interval partial least squares (iPLS), this technique enables precise quantification of plastic residues even at trace levels [
8,
9].
In this study, we present a multitechnique analytical strategy to detect and quantify LLDPE in frass samples from T. molitor. Artificial mixtures of frass and cryogenically milled LLDPE were analyzed using thermal analysis (under both oxidizing and inert atmospheres), coupled FTIR and mass spectrometry, and solid-state CP-MAS ¹³C NMR. iPLS-based chemometric models were applied to improve sensitivity and predictive accuracy. Our goal is to establish a robust analytical framework for future in vivo experiments investigating the fate of plastic polymers in insect digestion systems and to evaluate the biochemical impact of plastic ingestion in T. molitor.
2. Materials and Methods
2.1. Tenebrio Culture and Sample Preparation
We used the
T. molitor frass obtained in the trial carried out in another parallel study [
10]. In that study, a control culture of
T. molitor was grown and fed only wheat bran. The frass obtained was stored refrigerated and then used in the present study.
Linear low-density polyethylene (LLDPE) standard was purchased from Sigma–Aldrich (Sigma-Aldrich, St. Louis, MO, USA). The LLDPE standard was mechanically milled using a Cryogenic Mixer Mill CryoMill (Retsch, Haan, Germany) to obtain cryo-milled LLDPE dust of 200 μm. Artificial mixtures of frass and LLDPE were prepared at gravimetric concentrations of 0.096%, 0.51%, 1.44%, 3.12%, 6.97% and 10.41% w/w. Mixtures were homogenized manually to simulate expected compositions in future feeding experiments.
2.2. Thermal Analysis Coupled with FTIR and MS in Oxidant Atmosphere
Thermal analysis under oxidizing conditions was conducted using a coupled TG-FTIR-MS system. A STA 449 F5 Jupiter thermobalance (NETZSCH) was used in combination with a quadrupole mass spectrometer (Aeolos QMS 403 Quadro, NETZSCH) and an FTIR spectrometer (Invenio, Bruker) (Bruker, Rheinstetten, Germany) equipped with a TGA-IR gas cell.
Approximately 5 mg of each sample was placed in an open Al₂O₃ crucible and heated from 25 °C to 700 °C at a constant rate of 10 °C/min under a dynamic gas mixture (20% O₂ / 80% N₂) flowing at 100 mL/min. The system was calibrated automatically using standard reference materials. The evolved gases were analyzed simultaneously via FTIR and MS.
En atmósfera inerte (N2), the thermal analyses were performed with a METTLER TOLEDO (TGA/SDTA851e/LF/1600) and PFEIFFER VACUUM (THERMOSTAR GSD301T). All samples were decomposed with a stream of N2, a gas flow 100 ml min-1 within a temperature range from 25 to 700 ºC, a heating rate 10 ºC min-1, a sample weight about 5 mg, Al2O3 pan, and self-controlled calibration.
2.4. Thermal Analysis under Inert Atmosphere
Thermal degradation under inert conditions was performed using a TGA/SDTA851e thermobalance (Mettler Toledo) coupled to a Thermostar GSD301T quadrupole mass spectrometer (Pfeiffer Vacuum). Approximately 5 mg of sample was placed in an Al₂O₃ pan and heated from 25 °C to 700 °C at 10 °C/min under a continuous flow of high-purity nitrogen (100 mL/min). The instrument was calibrated with certified standards to ensure accurate temperature and mass measurements.
2.3. Solid-State 13C NMR
Cross-Polarization Magic Angle Spinning Carbon-13 Nuclear Magnetic Resonance (CPMAS 13C-NMR) experiments were performed on a BRUKER AVANCE DRX500 (Bruker, Rheinstetten, Germany) operating at 125.75 MHz for
13C. Samples were packed into a 4 mm diameter cylindrical zirconia rotor with Kel-F end-caps and spun at 2000 ± 100 Hz. A conventional cross polarization/magic-angle-spinning (CP-MAS) pulse sequence [
11] was used with a 1.0 ms contact time. Between 2000 and 5000 scans were accumulated with a pulse delay of 1.5 s. Line broadening was adjusted to 50 Hz. Spectra were processed using Bruker TopSpin software (Bruker, Rheinstetten, Germany).
2.4. Chemometric Analysis
The iPLS algorithm, especially useful to wavelength selection, has been used. These algorithms have already been described and the reader is referred to these papers for more details [
12,
13,
14]. In order to reach the best model with the lowest cross-validated error, the models and preprocessing methods (none, scaling, multiplicative scatter correction (MSC), and extended multiplicative signal correction (EMSC)) (39) were tested. MATLAB version 2024 (MathWorks, Natick, MA, USA) is used for the calculations, and the iToolbox is available at
https://ucphchemometrics.com/186-2/algorithms/ (accessed on 17 August 2025).
3. Results
3.1. Thermal Analysis Coupled with FTIR and MS in Oxidant Atmosphere
Thermal analysis coupled with mass spectrometry (MS) and Fourier-transform infrared spectroscopy (FTIR) was initially employed to investigate the thermal decomposition profiles of frass samples containing linear low-density polyethylene (LLDPE). Samples were artificially prepared by mixing oat bran-based frass from Tenebrio molitor with cryogenically milled LLDPE in proportions ranging from 0.096% to 10% w/w.
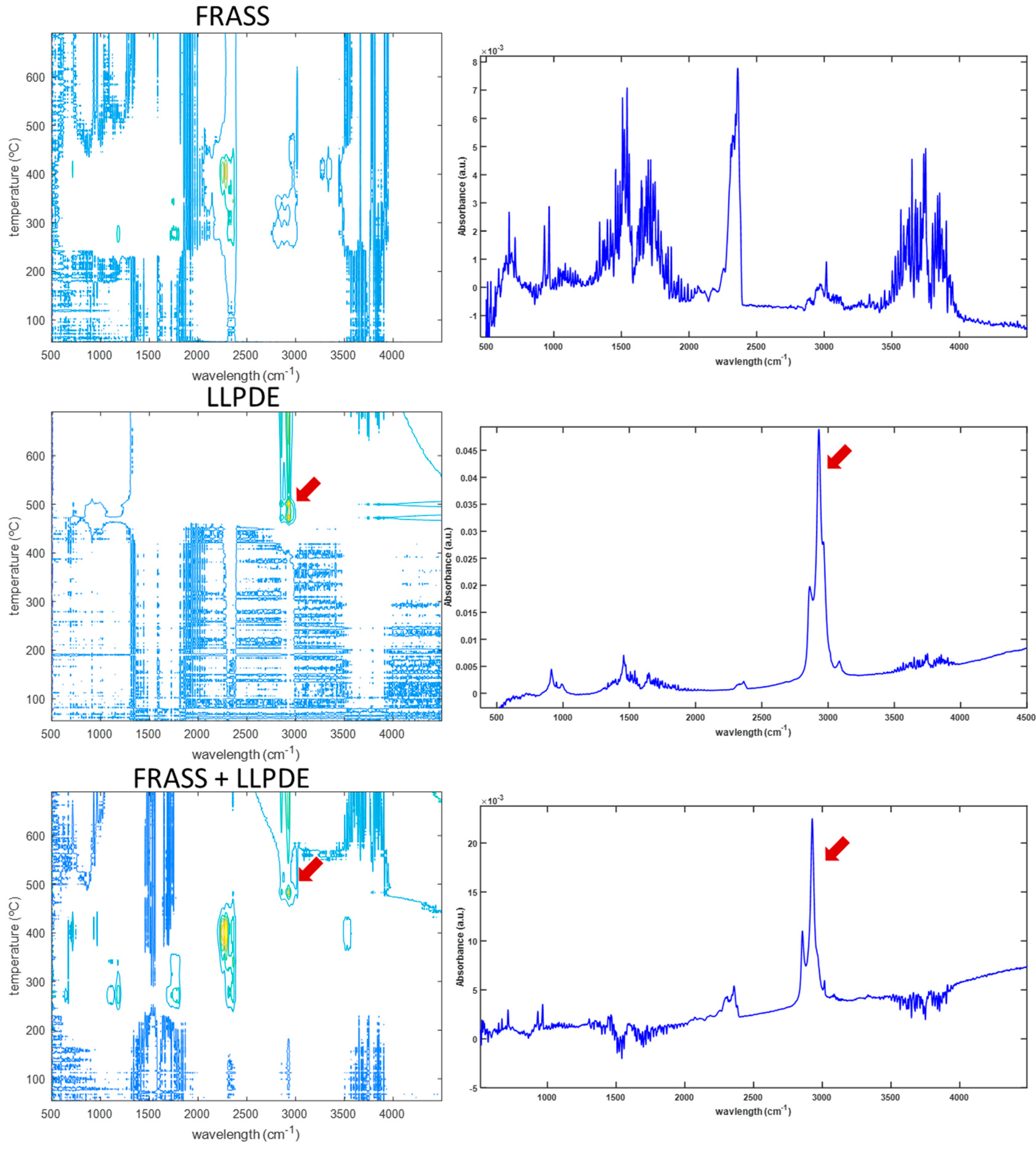
The FTIR and MS signals revealed distinct decomposition profiles for frass and LLDPE, particularly at elevated temperatures. LLDPE exhibited thermal degradation at significantly higher temperatures compared to frass, which allowed for differentiation based on specific vibrational bands and ion fragmentation patterns (
Figure 1). Notably, strong absorbance bands at 2928 and 2855 cm⁻¹—corresponding to C–H stretching in methyl and methylene groups of aliphatic chains—were observed during the thermal degradation of pure LLDPE and in the 10% LLDPE-frass blend, particularly at 483 °C (
Figure 1, right panel). These bands were absent in frass-only samples and in mixtures with lower LLDPE content, indicating a detection limit based on FTIR signal intensity. The persistence of these bands in an oxidizing atmosphere suggests incomplete combustion of LLDPE and the release of unoxidized volatile aliphatic fragments.
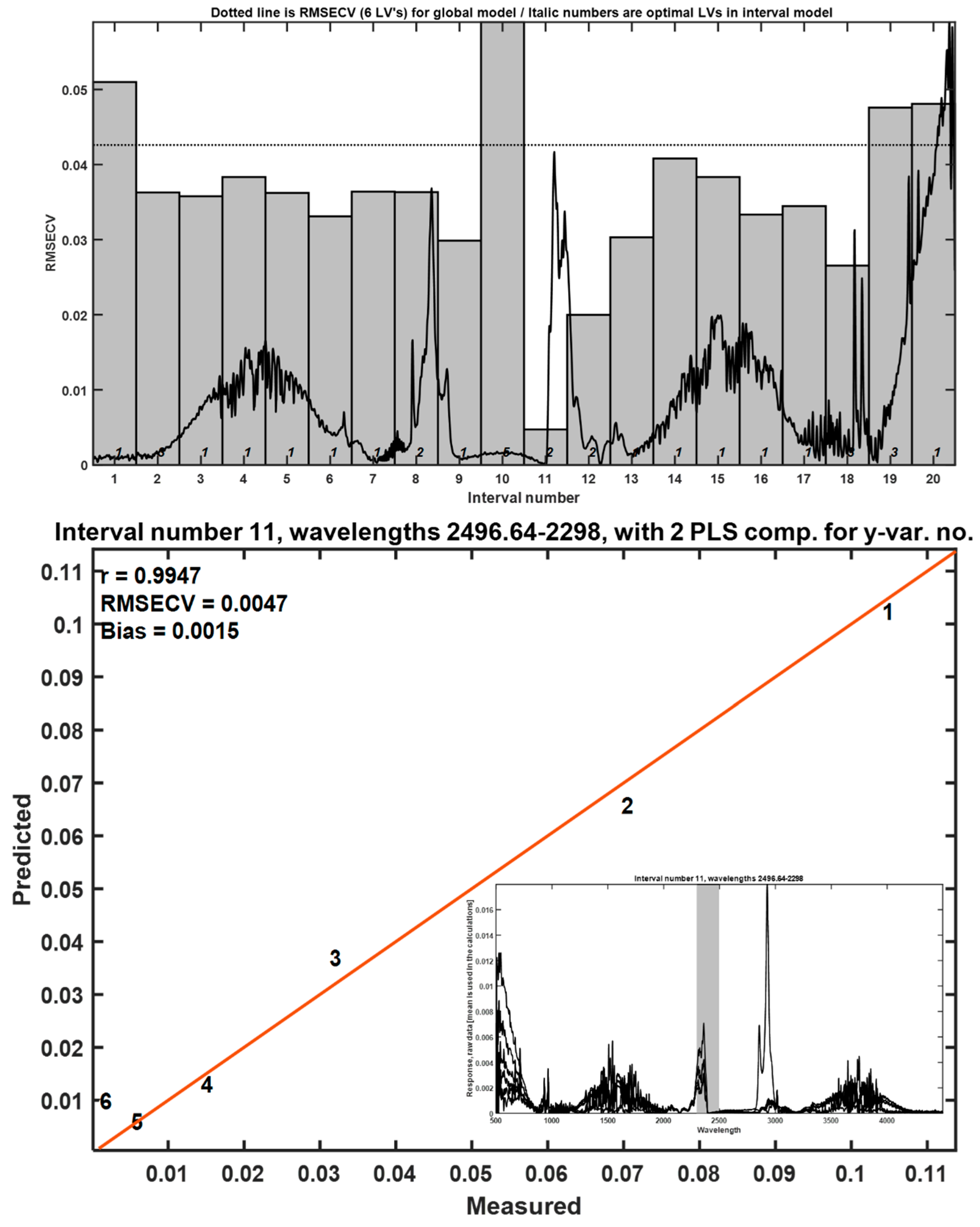
To assess the potential of FTIR spectra for quantifying LLDPE, interval partial least squares (iPLS) models were constructed using spectra collected at 483 °C. Among 20 tested intervals, a single predictive region yielded a reliable model with good fit and minimal cross-validation error, as shown in
Figure 2. This supports the utility of FTIR-based chemometrics for semi-quantitative analysis of LLDPE in complex organic matrices, though sensitivity is limited at lower concentrations.
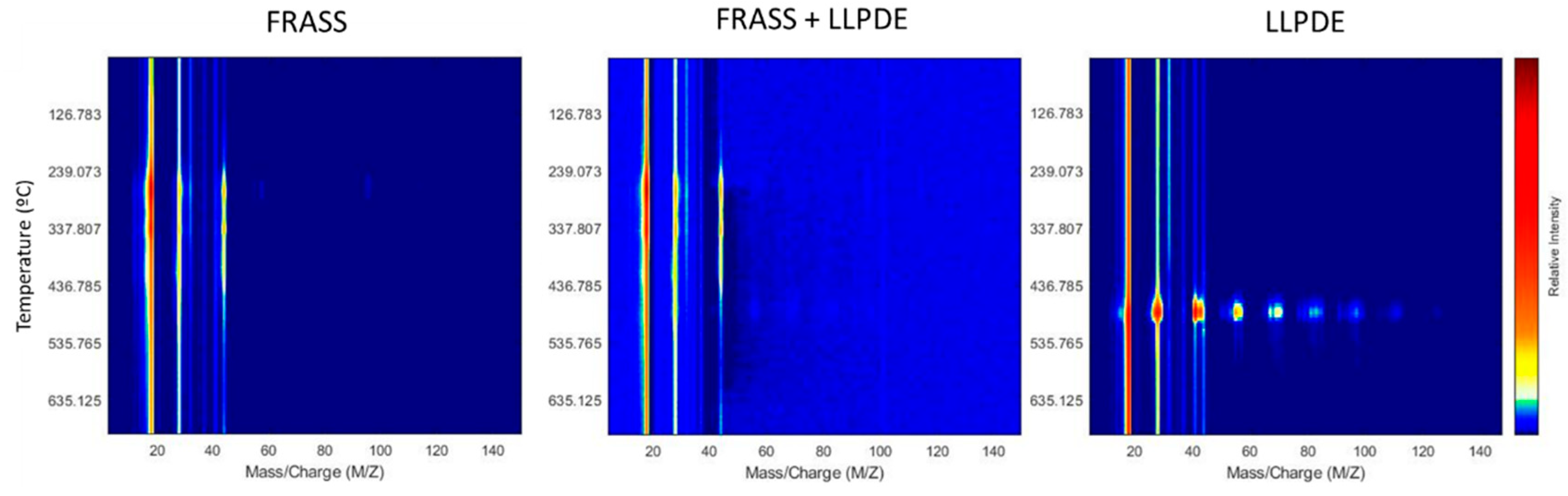
Mass spectrometry further supported these findings by identifying specific decomposition products in the evolved gas phase (
Figure 3). The total ion chromatogram (TIC) showed characteristic ion fragments, and the ion with m/z = 28 provided a strong correlation with LLDPE concentration in the temperature range of 439.7–499.0 °C (R = 0.9785,
Figure 4). Similarly, m/z = 44 yielded a higher correlation (R = 0.9864), although the relevant thermal window (607.35–632.0 °C) exceeded the expected decomposition range for LLDPE, suggesting the formation of CO₂ from secondary reactions or matrix combustion.
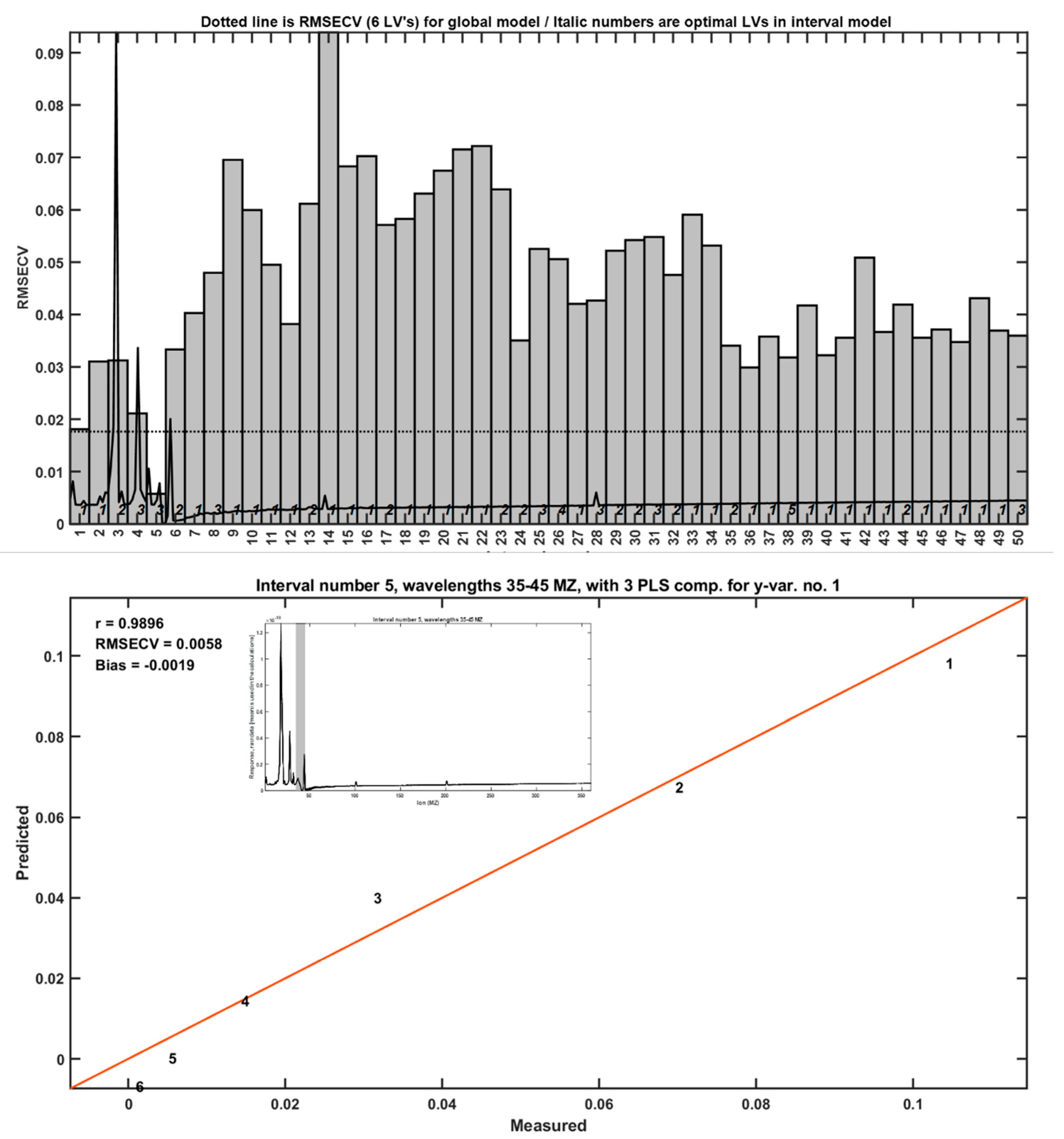
A comprehensive TIC-based iPLS analysis demonstrated the highest correlation (R = 0.9896) when summing ion signals in the m/z 35–45 range (
Figure 5). This finding indicates that, despite the complexity of combustion products, specific ion clusters can serve as proxies for LLDPE quantification.
Overall, the combination of thermal analysis, FTIR, and MS proved capable of identifying LLDPE in frass samples with reasonable precision at high concentrations. The iPLS approach allowed for selection of optimal spectral or mass regions most correlated with plastic content. However, at concentrations below 1%, the sensitivity of this method decreased substantially, limiting its utility for detecting trace amounts of LLDPE. Moreover, the requirement for advanced instrumentation and multi-modal coupling presents a barrier to broader application in routine analysis.
Therefore, while the oxidative TGA–FTIR–MS approach offers valuable insights into decomposition behavior and volatile profiles, further analytical refinement or alternative techniques are needed for more accurate quantification of low-level LLDPE residues in biological matrices.
3.2. Thermal Analysis with Inert Atmosphere
Thermal analysis under inert conditions (N₂ atmosphere) was carried out to evaluate whether the absence of oxidative reactions enhances the detection and quantification of LLDPE in frass mixtures. Thermogravimetric (TG) and derivative thermogravimetric (DTG) profiles were obtained for mixtures containing 0.096% to 10% w/w LLDPE using a METTLER TOLEDO TGA/SDTA851e system, as described in Materials and Method Section.
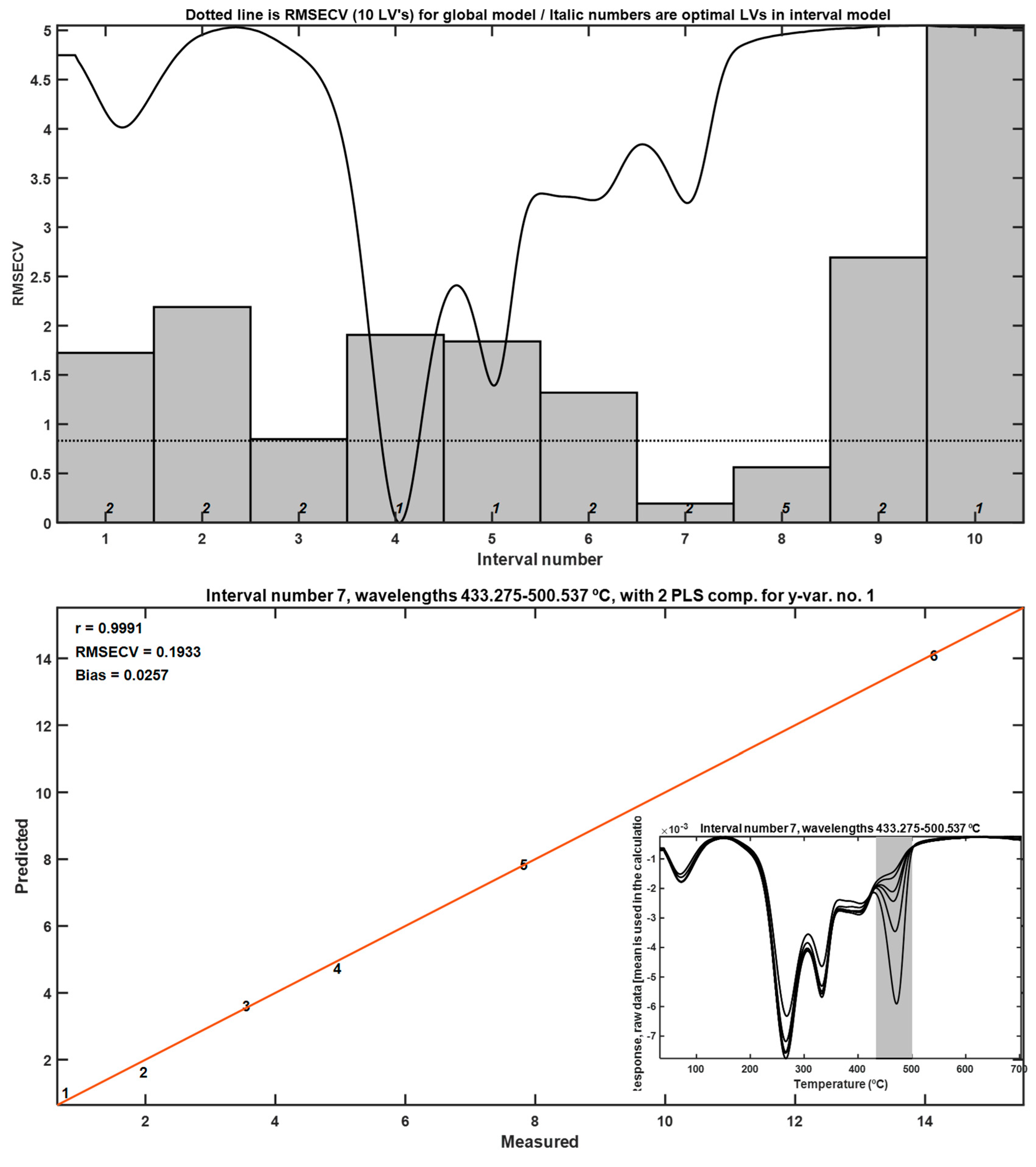
DTG curves revealed a distinct peak corresponding to the thermal degradation of LLDPE, with a maximum at 472 °C (
Figure 6). This peak was well separated from the broader degradation events associated with the frass matrix, which occurred at lower temperatures. Application of interval partial least squares (iPLS) regression to the DTG data enabled the identification of the most predictive thermal regions for quantifying LLDPE content. The most informative temperature interval was 433.27–500.54 °C, which corresponds precisely to the onset and peak of LLDPE decomposition.
The iPLS calibration model based on DTG curves achieved an excellent correlation coefficient (R = 0.9991), with minimal cross-validation error, indicating a highly accurate predictive model. The lowest detectable concentration using this method was approximately 0.096% w/w, establishing a practical detection threshold for future biodegradation assays involving frass.
The use of an inert atmosphere provided several analytical advantages. First, it avoided complications arising from exothermic oxidative reactions that may obscure or shift the thermal signature of LLDPE. Second, the high thermal stability of LLDPE in nitrogen allowed for clear discrimination from the more labile organic components of frass. These findings are consistent with prior studies that highlight the utility of inert-atmosphere TGA for polymer quantification in heterogeneous matrices, particularly for polyethylene derivatives [
4,
5].
In addition, the high sensitivity of the TGA instrument used enabled the generation of well-defined DTG curves, further enhancing model performance. Overall, thermal analysis under inert conditions emerged as a robust and accessible method for quantifying LLDPE in complex biological samples, especially when high concentrations or rapid screening are required.
3.2. Solid-State CP-MAS 13C NMR Spectroscopy
To enhance the sensitivity and selectivity in detecting residual LLDPE within frass matrices, we employed solid-state 13C nuclear magnetic resonance (NMR) spectroscopy with cross-polarization and magic angle spinning (CP-MAS). Samples of frass mixed with cryogenically milled LLDPE were prepared in concentrations ranging from 0.96% to 10% w/w. Spectra were acquired for each mixture and compared with those of pure frass and pure LLDPE under identical acquisition parameters.
The CP-MAS
13C NMR spectra (
Figure 7) revealed characteristic resonances between 10 and 45 ppm, attributed to aliphatic methylene and methyl groups (–CH₂– and –CH₃–) that are typical of polyethylene chains. These signals were clearly identifiable even at the lowest LLDPE concentration tested (0.096%), demonstrating the high sensitivity of this technique. In contrast, spectra from frass alone were dominated by broader peaks centered near 72 ppm, corresponding to O-alkyl carbons in polysaccharides such as cellulose and hemicellulose, consistent with the insect’s oat bran-based diet.
Chemometric analysis using interval partial least squares (iPLS) confirmed that the most predictive spectral region for LLDPE quantification was the aliphatic zone (10–45 ppm). The optimized model showed excellent linear correlation (R = 0.9972) with low cross-validation error (RMSECV = 0.3426), indicating robust predictive performance across the tested concentration range. The observed calibration performance highlights the technique's potential not only for qualitative detection but also for precise quantification of microplastic residues in biological matrices.
Importantly, CP-MAS 13C NMR offers additional advantages over thermal methods. It is non-destructive, requires minimal sample preparation, and provides direct molecular-level information without chemical derivatization or pyrolysis. Moreover, the spectral resolution achieved under MAS conditions enables discrimination between polyethylene signals and other organic components of frass, even in complex mixtures.
Beyond quantification, the spectral data also revealed compositional shifts in frass with increasing LLDPE content. Notably, the intensity of carbohydrate-associated signals decreased in parallel with an increase in peaks around 45–55 ppm, which may correspond to proteinaceous or lipid-related carbon environments. These shifts could reflect metabolic changes, altered digestion, or microbial colonization induced by LLDPE ingestion, warranting further biochemical investigation.
Taken together, CP-MAS 13C NMR emerges as a powerful analytical tool for detecting and quantifying polyethylene-based microplastics in insect-derived matrices, with clear advantages in resolution, sensitivity, and mechanistic insight compared to thermal or spectroscopic methods alone.
4. Discussion
This study evaluated and compared three complementary analytical techniques—thermal analysis coupled with FTIR and MS under oxidizing atmosphere, thermal analysis under inert atmosphere, and solid-state CP-MAS ¹³C NMR spectroscopy—to determine their efficacy in detecting and quantifying linear low-density polyethylene (LLDPE) in complex biological matrices derived from Tenebrio molitor frass.
The thermal analysis coupled with FTIR and MS offered valuable initial insights into the decomposition pathways of frass–LLDPE mixtures. Specific FTIR bands associated with aliphatic C–H stretching vibrations (2928 and 2855 cm⁻¹), as well as mass spectral fragments (e.g., m/z 28 and 44), enabled partial discrimination between organic and plastic components. However, the method exhibited significant limitations at low LLDPE concentrations (<1%), primarily due to spectral overlap and the influence of matrix background signals. The high complexity and cost of the coupled instrumentation further reduce its practicality for routine quantification, particularly when high-throughput or field-relevant analysis is required. Similar limitations have been reported in other studies using evolved gas analysis for microplastic detection in organic-rich matrices [
4,
5].
In contrast, thermogravimetric analysis under an inert atmosphere provided more robust and interpretable data. The DTG profiles clearly separated the degradation events of frass from those of LLDPE, with the latter showing a well-defined thermal event at ~472 °C. Application of iPLS modeling enabled accurate calibration, with high correlation (R = 0.9991) and low RMSECV. The reduced interference from oxidative reactions simplified spectral interpretation and improved sensitivity. This method requires less specialized instrumentation than the FTIR–MS setup, making it more accessible. These findings are in line with previous studies highlighting the advantages of inert-atmosphere TGA for polymer analysis in heterogeneous samples [
5,
6].
Among the techniques tested, solid-state CP-MAS ¹³C NMR proved to be the most sensitive and informative. Characteristic aliphatic carbon signals (10–45 ppm) associated with polyethylene chains were clearly detected even at the lowest tested concentration (0.096% w/w). Chemometric modeling based on iPLS provided excellent quantitative performance (R = 0.9972; RMSECV = 0.3426), and the method also revealed compositional shifts in the frass matrix potentially linked to metabolic responses. Unlike thermal methods, CP-MAS NMR is non-destructive and provides direct molecular-level insight, with minimal sample preparation. Its capability to distinguish between overlapping organic and synthetic signals without chemical pretreatment underscores its analytical strength [
7,
8,
9].
Overall, while all three techniques provide complementary information, CP-MAS ¹³C NMR offers the most reliable and precise quantification of LLDPE in frass. Thermal methods remain valuable for rapid screening or high-temperature behavior analysis, but they lack the specificity and resolution required for low-concentration detection. Combining these analytical approaches with chemometric tools like iPLS significantly enhances interpretability and predictive accuracy, particularly in heterogeneous biological systems [
12,
13,
14].
These results lay the foundation for future experiments aimed at evaluating LLDPE degradation in vivo. By establishing baseline quantification techniques, it becomes possible to assess whether ingested plastic is excreted unchanged, partially depolymerized, or metabolically transformed. This analytical framework also opens the door to broader applications in environmental monitoring, biodegradation research, and insect-based plastic valorization.
5. Conclusions
This study demonstrates the utility of a multitechnique analytical approach for detecting and quantifying linear low-density polyethylene (LLDPE) in insect-derived frass. Among the techniques evaluated, solid-state CP-MAS ¹³C NMR provided the most accurate and sensitive quantification of LLDPE, detecting characteristic aliphatic carbon signals even at low concentrations (0.096% w/w). The chemometric modeling using interval partial least squares (iPLS) further enhanced the precision of the calibration, enabling reliable quantification in complex organic matrices.
Thermal analysis under inert atmosphere also showed strong potential for LLDPE quantification, with DTG profiles offering clear thermal discrimination and robust regression models. In contrast, thermal analysis coupled with FTIR and MS under oxidizing conditions, while informative in characterizing evolved gases, was less sensitive at low plastic concentrations and required complex instrumentation.
Taken together, these findings provide a validated analytical framework to quantify synthetic polymer residues in insect frass. This is a critical step toward assessing the biodegradation potential of insects such as Tenebrio molitor when fed plastic-containing diets. Furthermore, the combination of spectroscopy and chemometrics opens avenues for high-resolution analysis of polymer–biomass interactions and supports the development of bio-based microplastic monitoring strategies.
Future work should focus on applying this methodology to in vivo feeding experiments to determine the actual fate of ingested LLDPE, evaluate metabolic transformation products, and investigate the biochemical and ecological implications of plastic ingestion by insects.
Author Contributions
Funding obtained: F.C.M.-E. Conceived and designed the experiments: R. G.-A., R.P., M.R., J.A.P., R.M. and F.C.M.-E. Performed the analysis and figures: R. G.-A. and F.C.M.-E. Analyzed the data: R. G.-A. and F.C.M.-E. Wrote the manuscript: R. G.-A. and F.C.M.-E. Reviewed the manuscript: R. G.-A., R.P., M.R., J.A.P., R.M. and F.C.M.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to thank PhD Emilio Lorenzo and PhD Ion Such (SSTTI of University of Alicante) for technical assistance with NMR and Thermal analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Giaganini, G., M. Cifelli, D. Biagini, S. Ghimenti, A. Corti, V. Castelvetro, V. Domenici and T. Lomonaco. "Multi-analytical approach to characterize the degradation of different types of microplastics: Identification and quantification of released organic compounds." Molecules 28 (2023). [CrossRef]
- Peng, B.-Y., Y. Sun, X. Zhang, J. Sun, Y. Xu, S. Xiao, J. Chen, X. Zhou and Y. Zhang. "Unveiling the residual plastics and produced toxicity during biodegradation of polyethylene (pe), polystyrene (ps), and polyvinyl chloride (pvc) microplastics by mealworms (larvae of tenebrio molitor)." Journal of Hazardous Materials 452 (2023): 131326. https://www.sciencedirect.com/science/article/pii/S0304389423006088. [CrossRef]
- Yang, W.-M. Wu, J. Zhao, Y. Song, L. Gao, R. Yang and L. Jiang. "Biodegradation and mineralization of polystyrene by plastic-eating mealworms: Part 1. Chemical and physical characterization and isotopic tests." Environmental Science & Technology 49 (2015): 12080-86. [CrossRef]
- Dümichen, E., P. Eisentraut, C. G. Bannick, A.-K. Barthel, R. Senz and U. Braun. "Fast identification of microplastics in complex environmental samples by a thermal degradation method." Chemosphere 174 (2017): 572-84. https://www.sciencedirect.com/science/article/pii/S0045653517301881. [CrossRef]
- Martín de la Fuente, A., F. C. Marhuenda-Egea, M. Ros, J. A. Pascual, J. A. Saez-Tovar, E. Martinez-Sabater and R. Peñalver. "Thermogravimetry coupled with mass spectrometry successfully used to quantify polyethylene and polystyrene microplastics in organic amendments." Environmental Research 213 (2022): 113583. https://www.sciencedirect.com/science/article/pii/S0013935122009100. [CrossRef]
- Mostefaoui, O., Z. Iannuzzi, D. Lopez, E. Mignot, G. Lipeme-Kouyi, R. Bayard, V. Massardier-Nageotte and B. Mourier. "Quantitative study of microplastic degradation in urban hydrosystems: Comparing in situ environmentally aged microplastics vs. Artificially aged materials generated via accelerated photo-oxidation." Journal of Hazardous Materials 486 (2025): 137087. https://www.sciencedirect.com/science/article/pii/S0304389424036689. [CrossRef]
- Xochitl, Q. P., H. B. María Del Consuelo, M. S. María Del Consuelo, E. V. Rosa María and V. M. Alethia. "Degradation of plastics in simulated landfill conditions." Polymers (Basel) 13 (2021). [CrossRef]
- Papini, G., G. Petrella, D. O. Cicero, C. Boglione and A. Rakaj. "Identification and quantification of polystyrene microplastics in marine sediments facing a river mouth through nmr spectroscopy." Marine Pollution Bulletin 198 (2024): 115784. https://www.sciencedirect.com/science/article/pii/S0025326X23012195. [CrossRef]
- Schmidt, J. Haave and W. Wang. "Overcoming the challenge of quantifying aged microplastic by qnmr spectroscopy." Environmental Science: Processes & Impacts (2025): 10.1039/D5EM00393H. [CrossRef]
- Orts, J. M., J. Parrado, J. A. Pascual, A. Orts, J. Cuartero, M. Tejada and M. Ros. Polyurethane foam residue biodegradation through the tenebrio molitor digestive tract: Microbial communities and enzymatic activity. 15. 2023.
- Nelson, P. N. and J. A. Baldock. "Estimating the molecular composition of a diverse range of natural organic materials from solid-state 13c nmr and elemental analyses." Biogeochemistry 72 (2005): 1-34. ://WOS:000229218300001. [CrossRef]
- Kristensen, M., F. Savorani, G. Ravn-Haren, M. Poulsen, J. Markowski, F. Larsen, L. Dragsted and S. Engelsen. "Nmr and interval pls as reliable methods for determination of cholesterol in rodent lipoprotein fractions." Metabolomics 6 (2010): 129-36. [CrossRef]
- Petersen, M., M. Dyrby, S. Toubro, S. B. Engelsen, L. Nørgaard, H. T. Pedersen and J. Dyerberg. "Quantification of lipoprotein subclasses by proton nuclear magnetic resonance–based partial least-squares regression models." Clinical Chemistry 51 (2005): 1457-61. [CrossRef]
- Norgaard, L., A. Saudland, J. Wagner, J. P. Nielsen, L. Munck and S. B. Engelsen. "Interval partial least-squares regression (ipls): A comparative chemometric study with an example from near-infrared spectroscopy." Applied Spectroscopy 54 (2000): 413-19. https://opg.optica.org/as/abstract.cfm?URI=as-54-3-413.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).