Submitted:
11 August 2025
Posted:
27 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Numerical and Experimental Methods
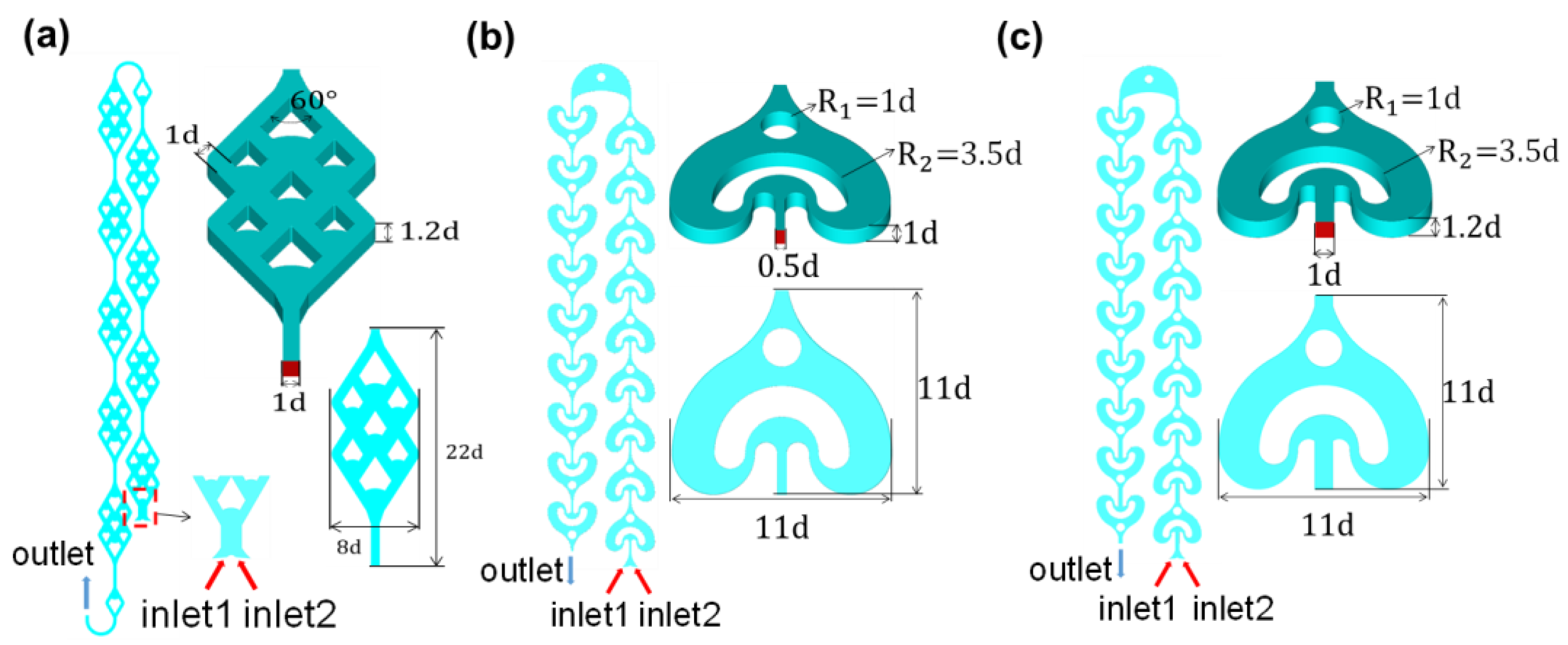
2.1. Basic Configurations of New Microreactor Design
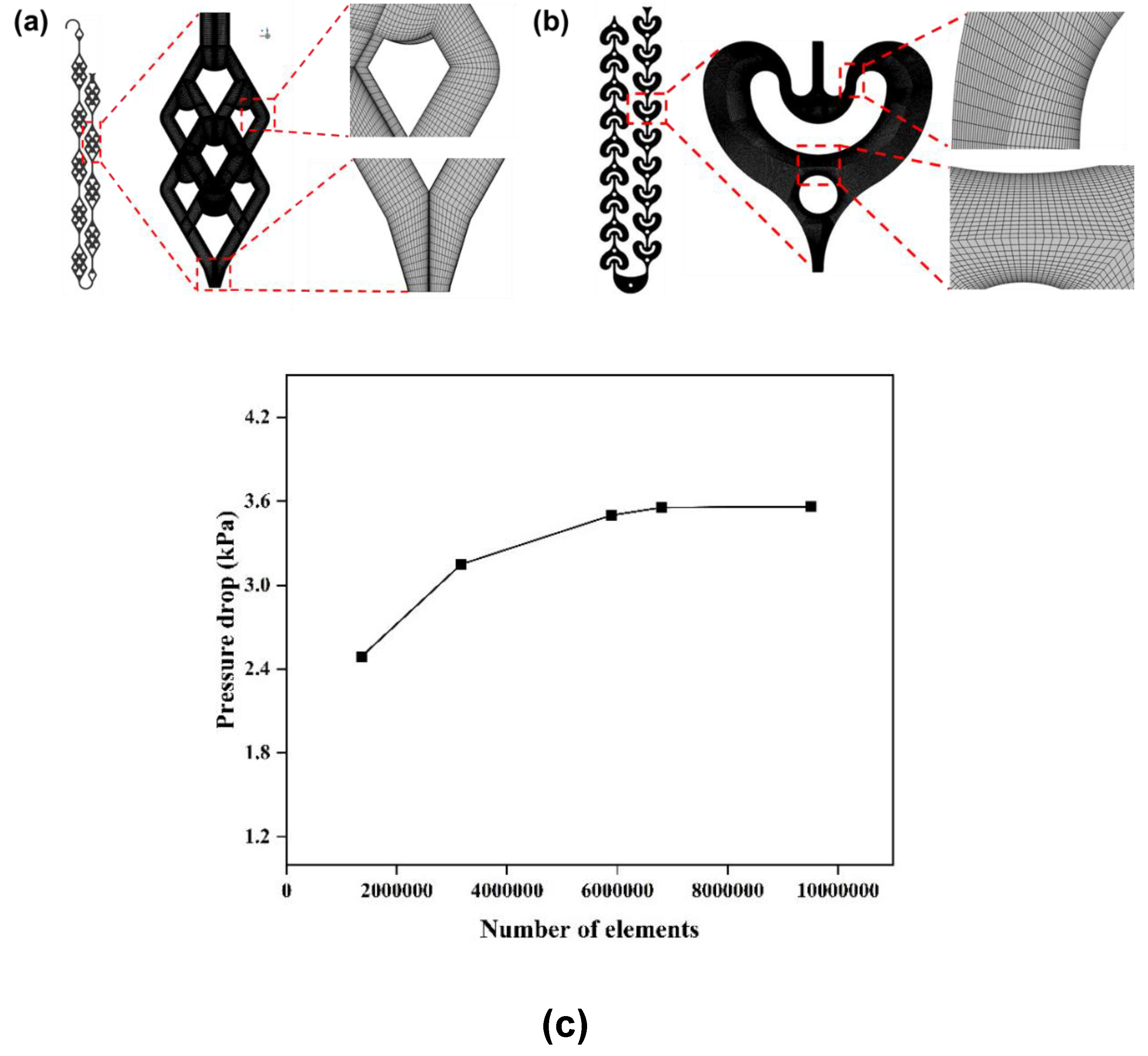
2.2. Computational Fluid Dynamics (CFD) Simulation
2.3. Parallel Competing Reaction System of Villermaux-Dushman Test
2.4. Synthesis of BaSO4 Nanoparticles
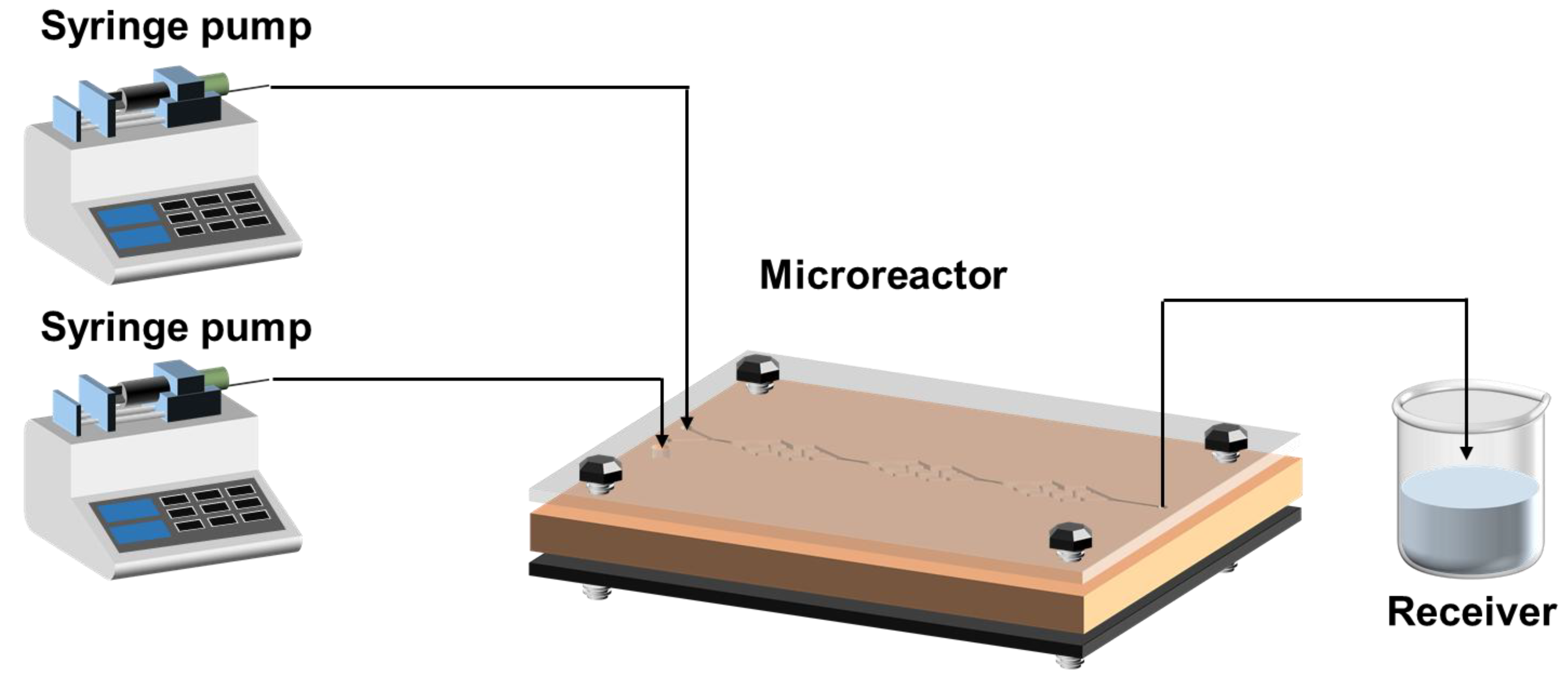
2.5. Materials
3. Results and Discussion
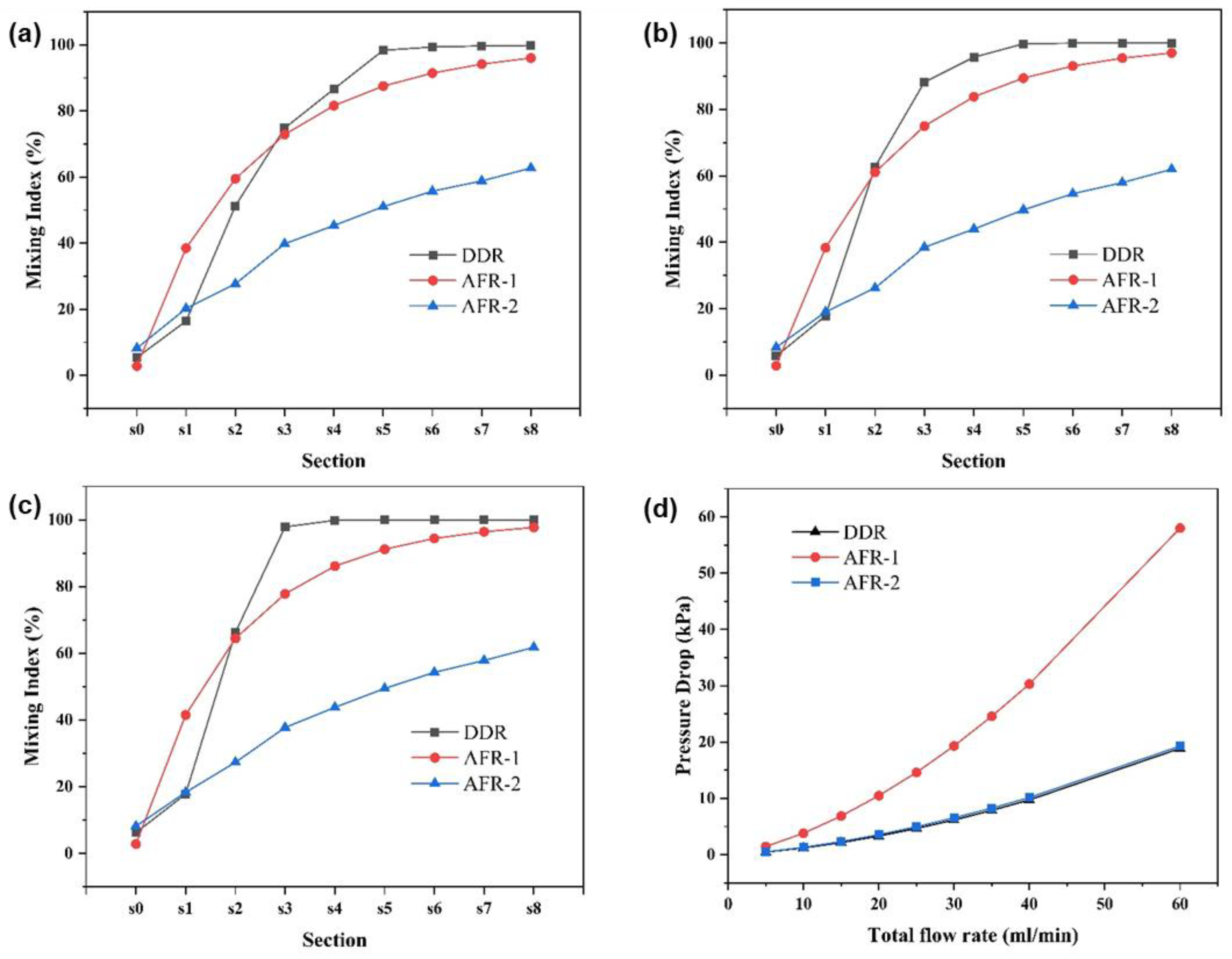
3.1. Flow Characteristics and Mixing Behavior Based on CFD Analysis
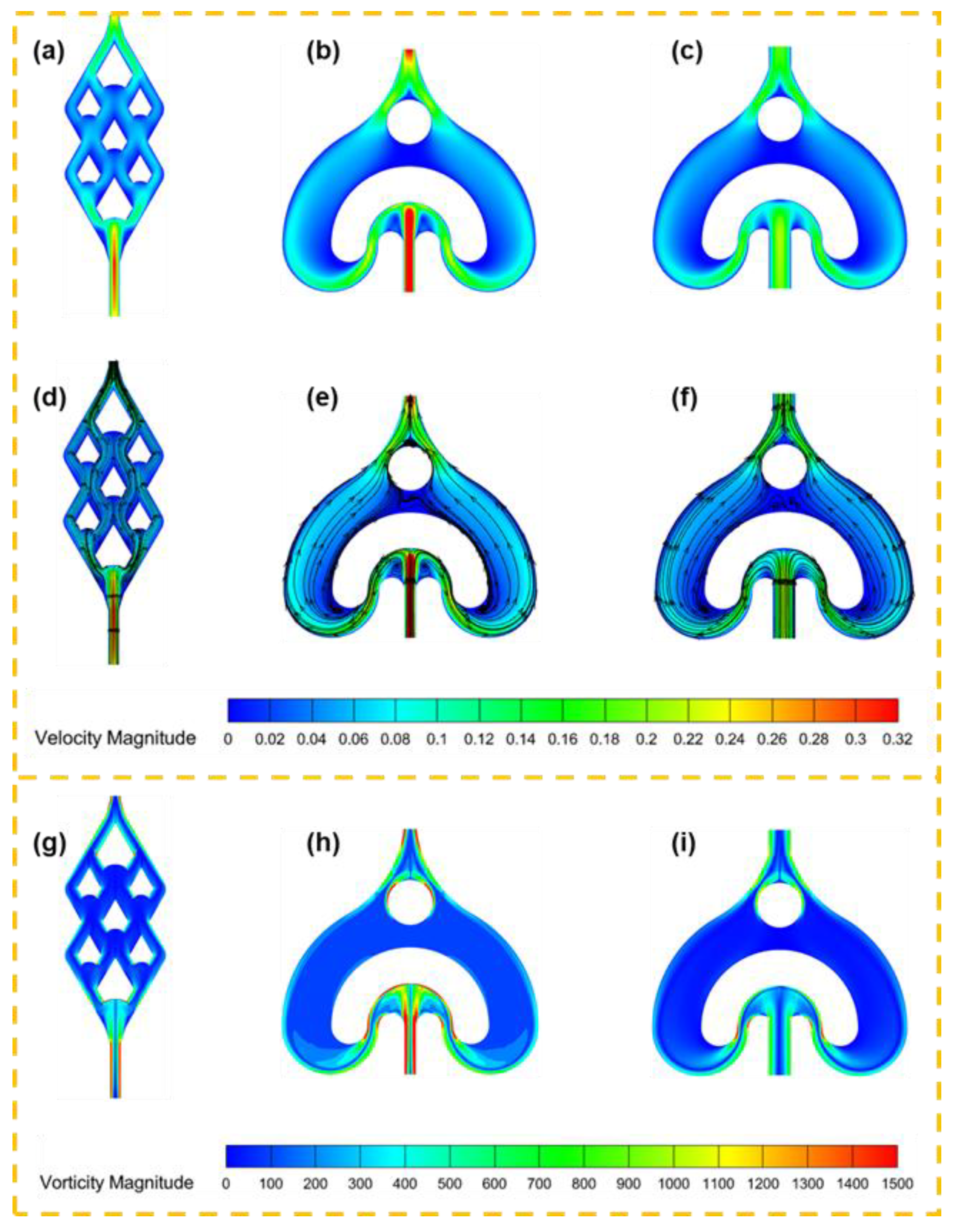
3.1.1. Flow Field
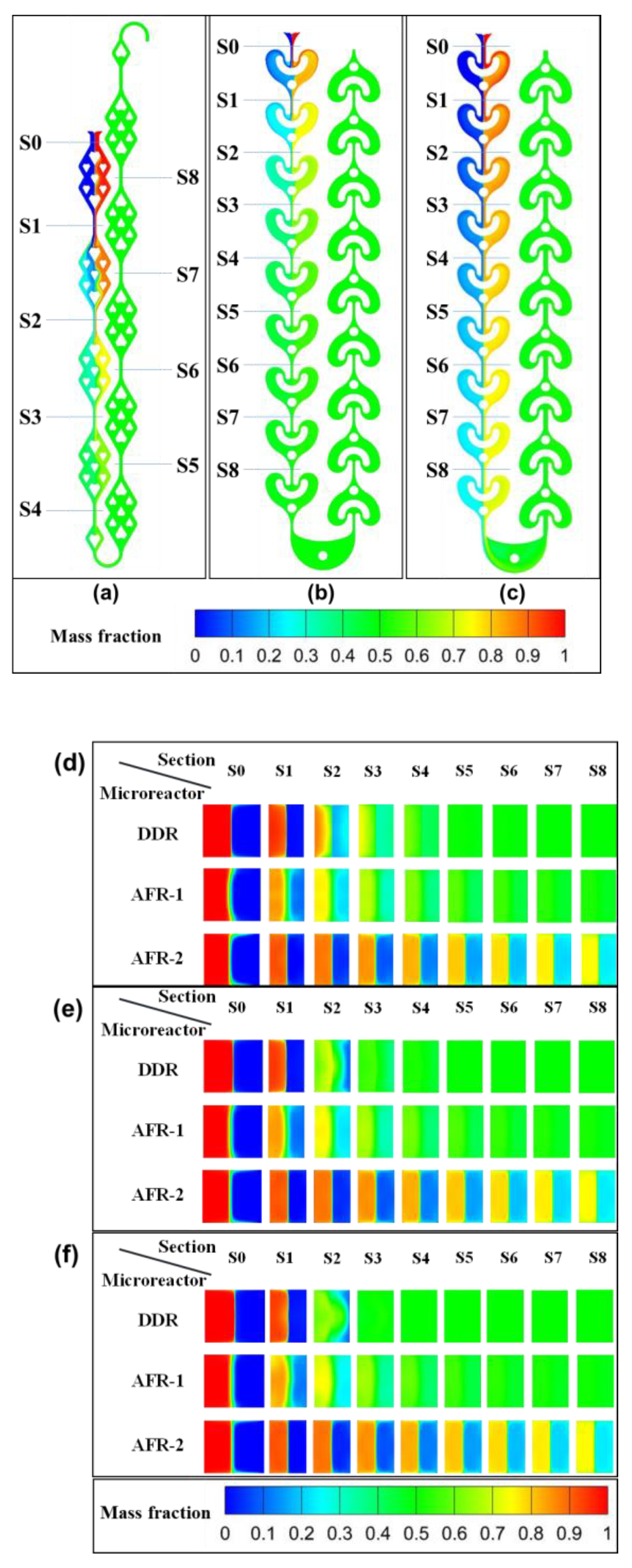
3.1.2. Concentration Field
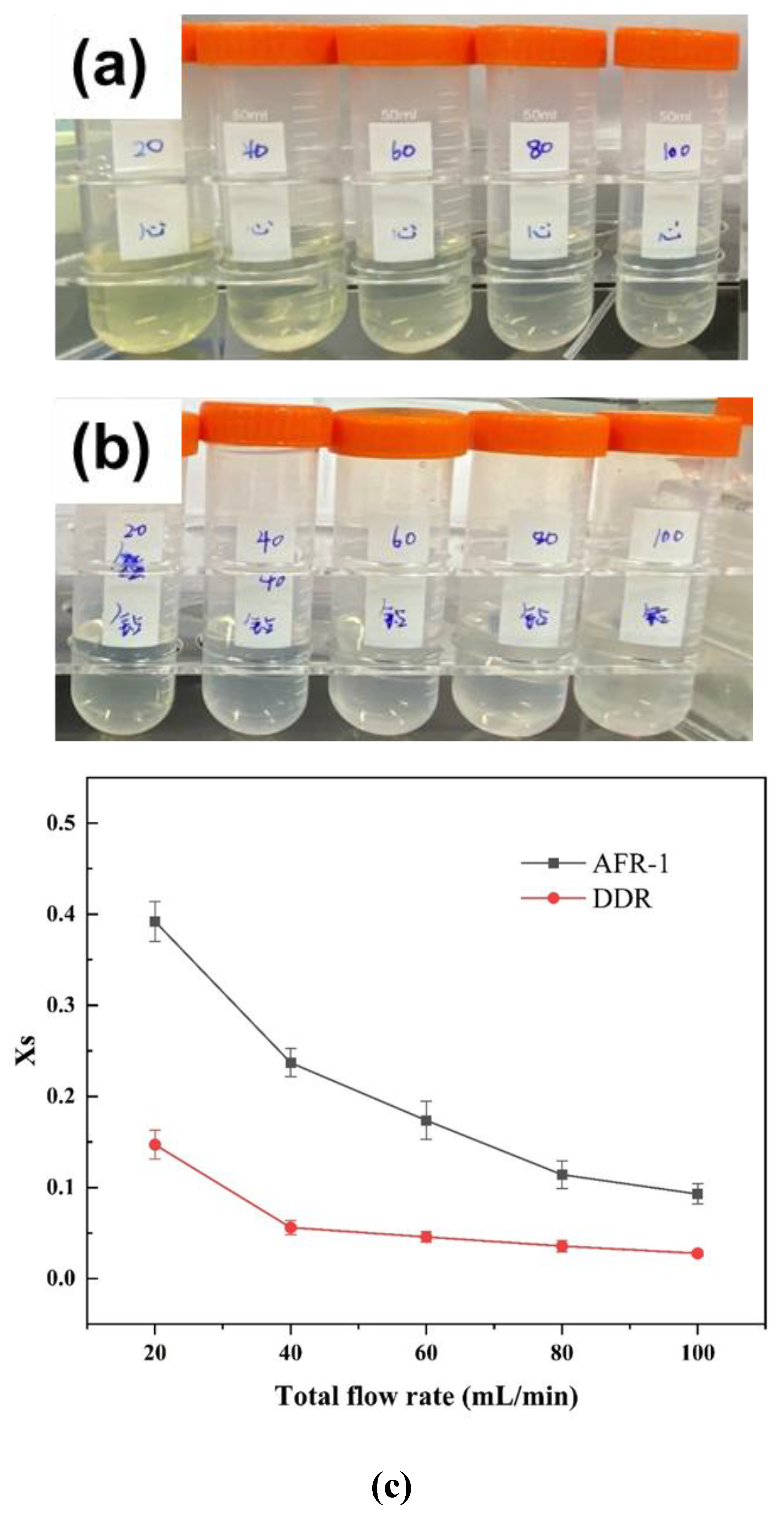
3.2. Validation on Mixing Performance by Villermaux-Dushman Experiments
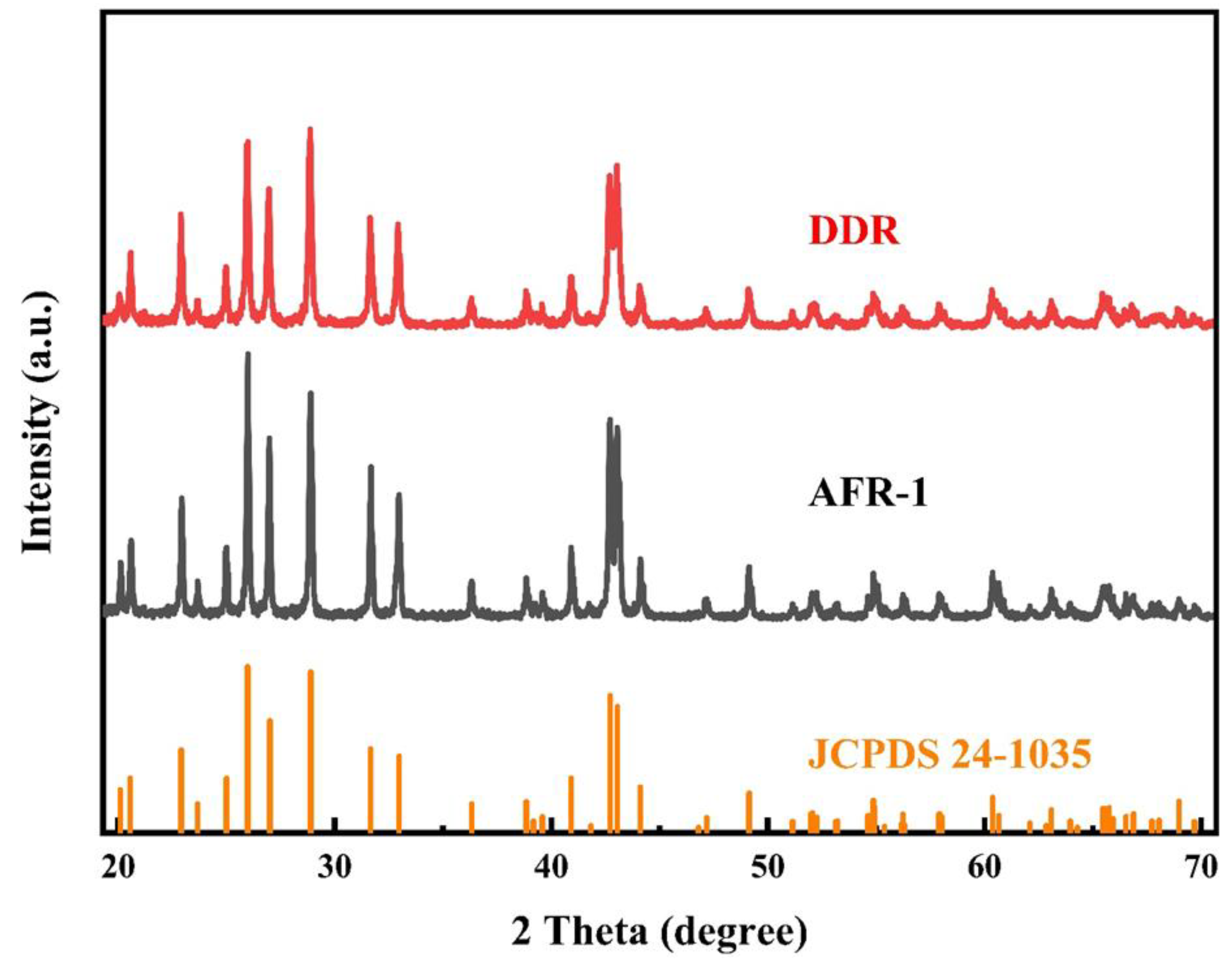
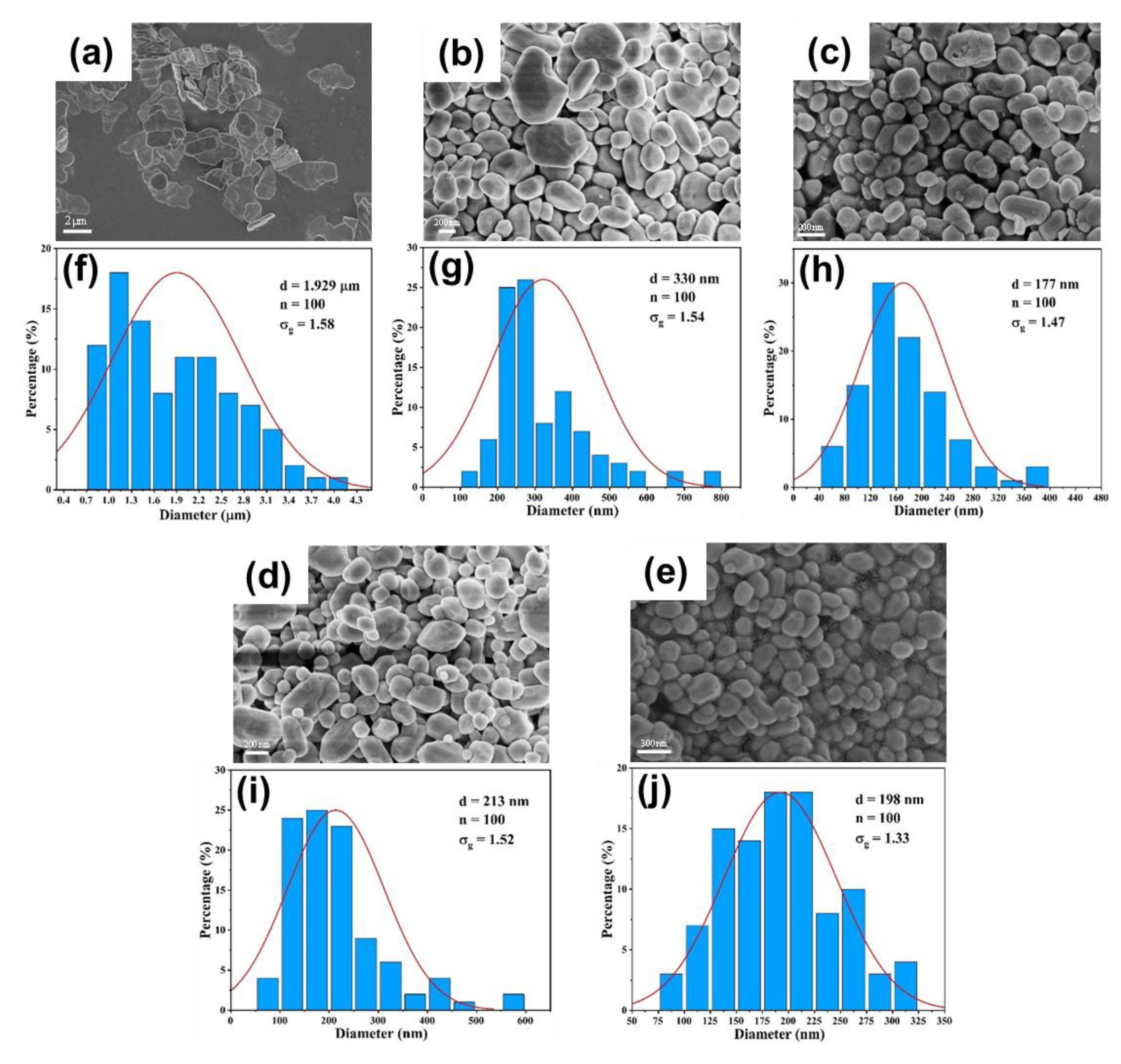
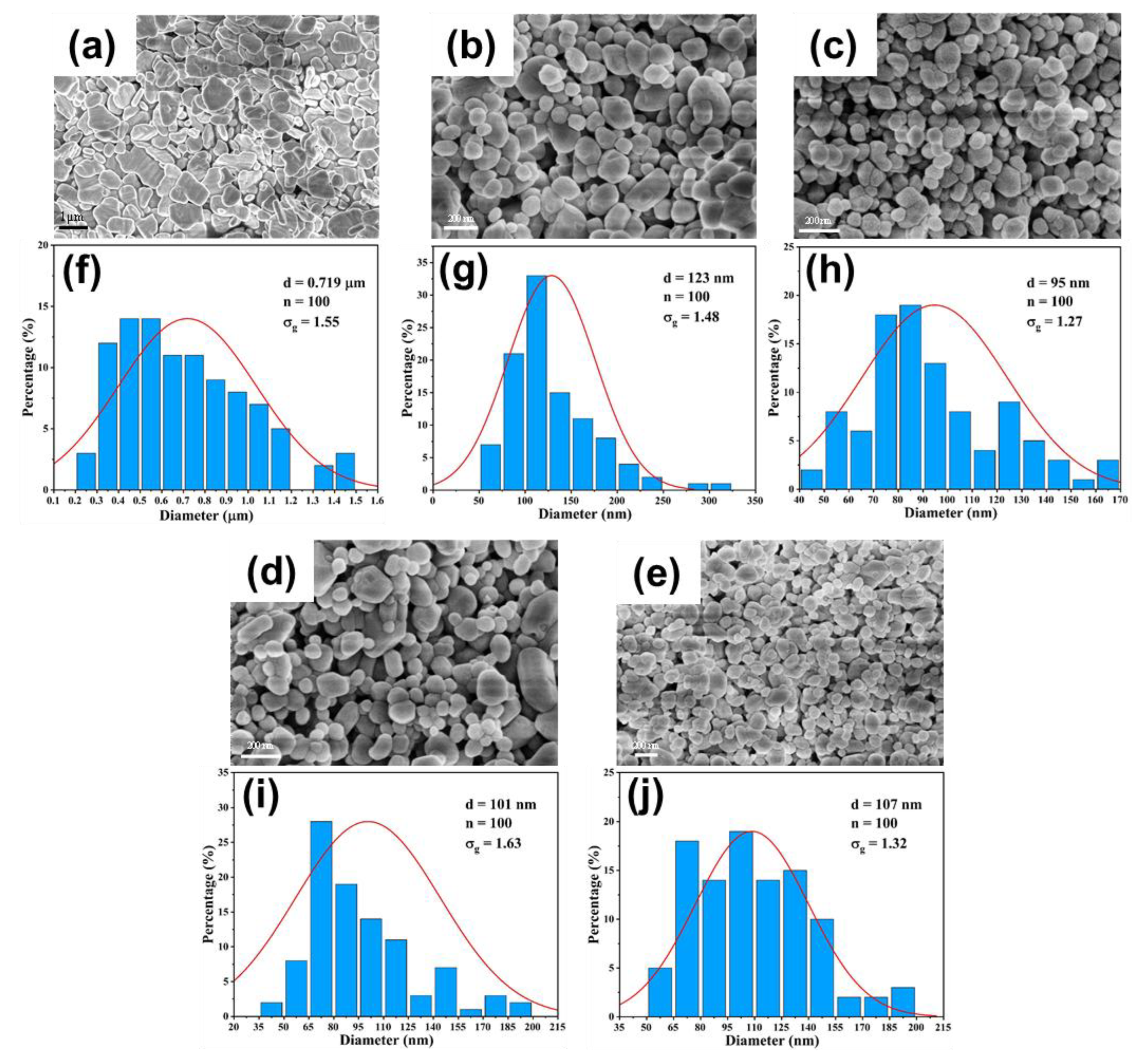
3.3. Validation on Capability of Continuous Synthesis of BaSO4 Nanoparticles
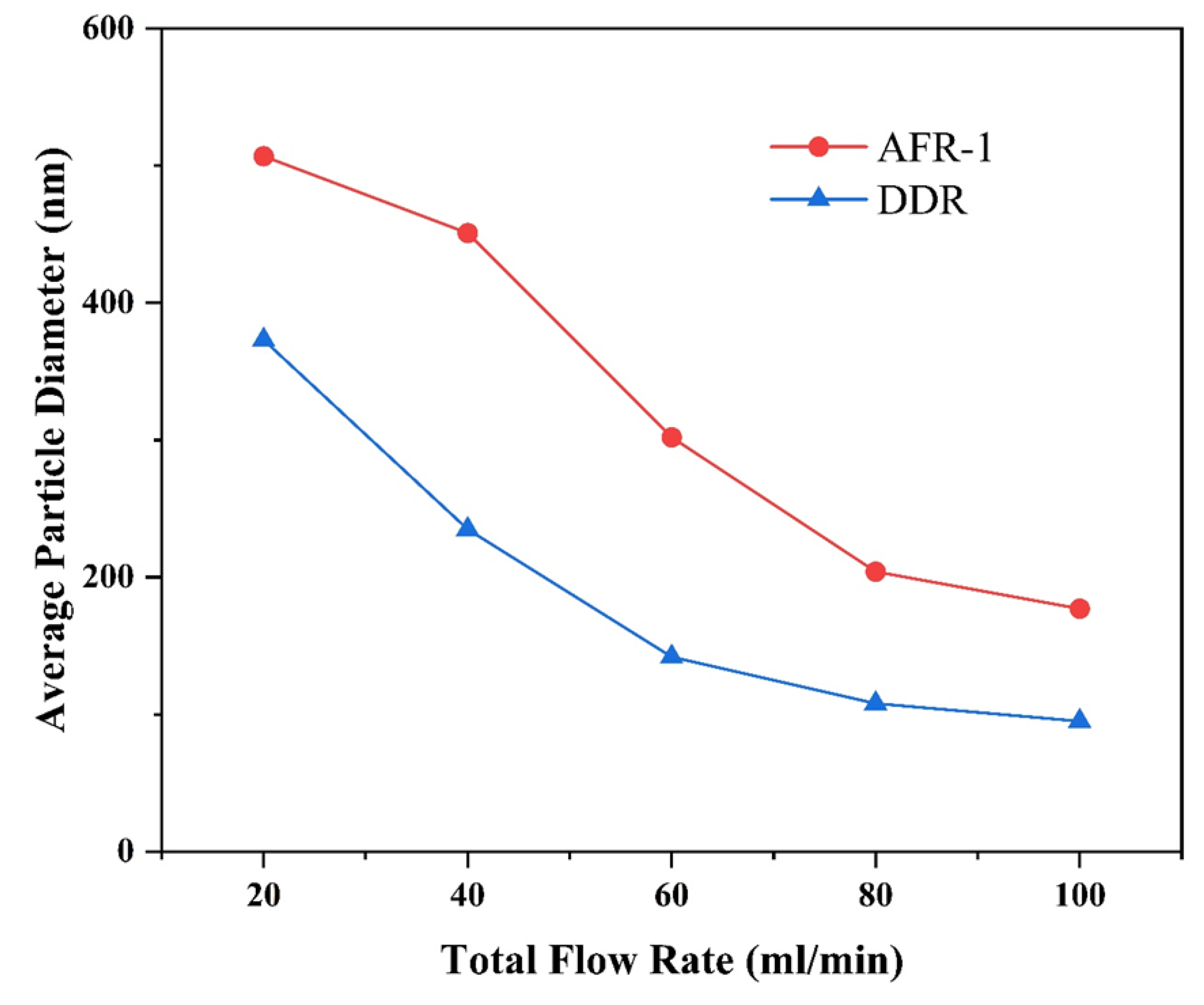
3.3.1. Effect of Flow Rate
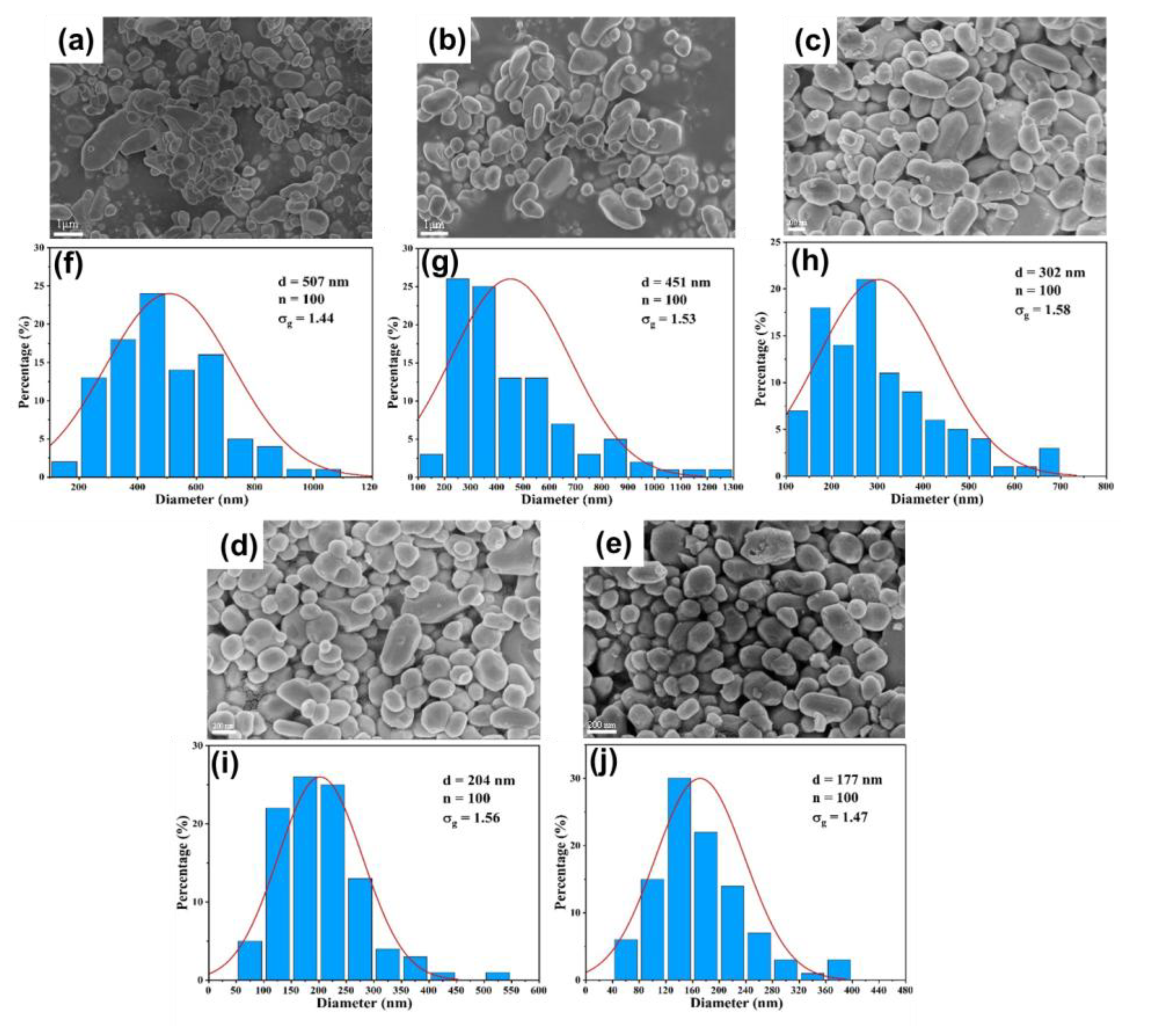
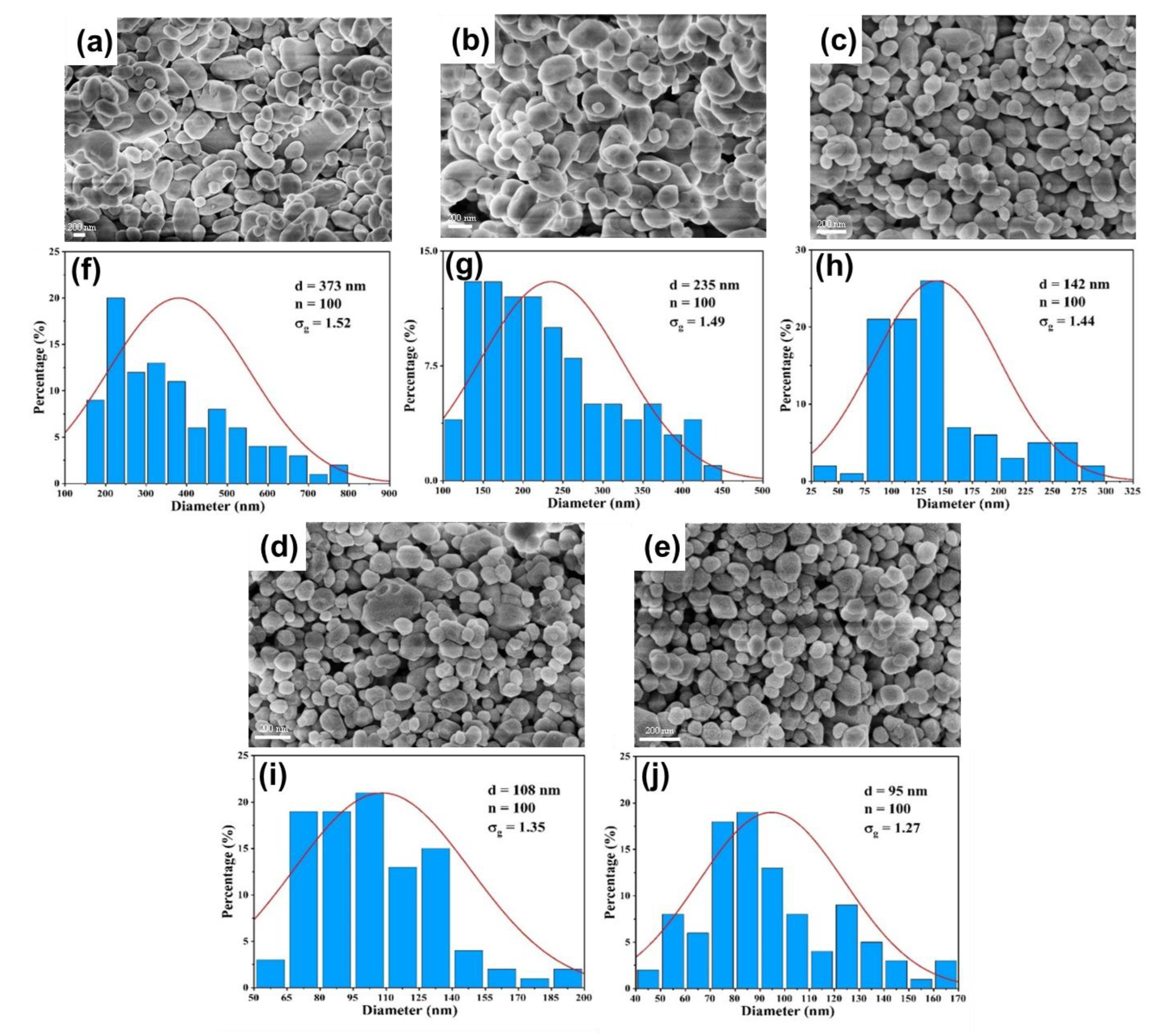
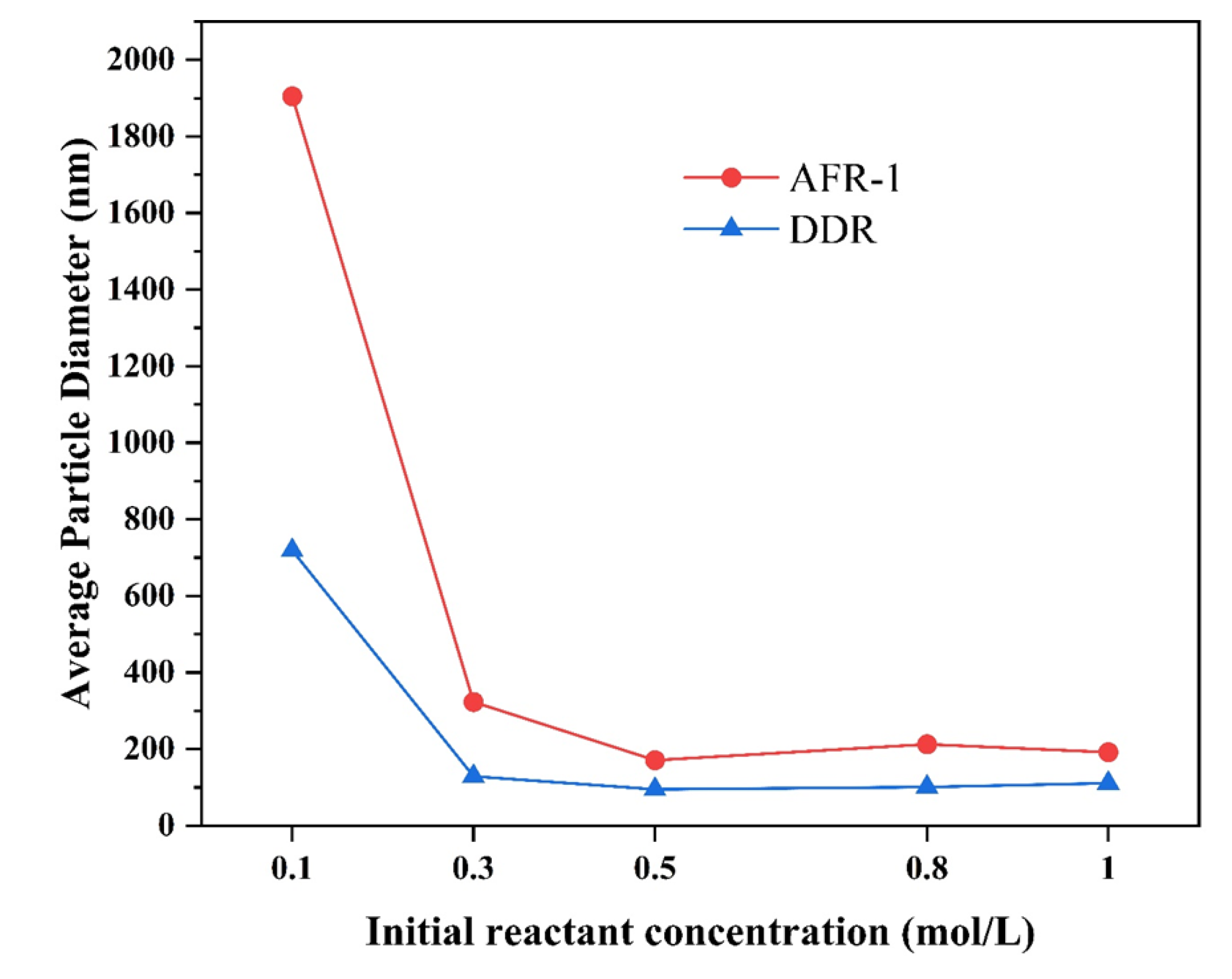
3.3.2. Effect of Reactant Concentration
4. Conclusions
References
- Ouyang, Y.; Xiang, Y.; Zou, H.; Chu, G.; Chen, J. Flow characteristics and micromixing modeling in a microporous tube-in-tube microchannel reactor by CFD. Chem. Eng. J. (2017), 321, 533.
- Hu, X. Yang, F. Zhao, H. Guo, M. Wang, Y. Design and Evaluation of Three-Dimensional Zigzag Chaotic Micromixers for Biochemical Applications.Ind. Eng. Chem. Res. (2021), 60,16116–16125.
- Sun, H.; Ren, Y.; Tao, Y.; Jiang, T.; Jiang, H. Three-Fluid Sequential Micromixing-Assisted Nanoparticle Synthesis Utilizing Alternating Current Electrothermal Flow. Ind. Eng. Chem. Res. (2020) ,59,12514-12524.
- Z. Liu, M. Yang, Q. Zhao, C. Yao, G. Chen, Scale-up of antisolvent precipitation process with ultrasonic microreactors: Cavitation patterns, mixing characteristics and application in nanoparticle manufacturing. Chem. Eng. J. 475 (2023) 146040.
- P.A. Petrini, D.R. Lester, G. Rosengarten, Enhanced laminar heat transfer via magnetically driven ferrofluids, Int. J. Heat Mass Transf., 217 (2023) 124703.
- H. Shi, K. Nie, B. Dong, L.Chao, F. Gao, M. Ma, M. Long, Z. Liu, Mixing enhancement via a serpentine micromixer for real-time activation of carboxyl, Chem. Eng. J. 392 (2020) 123642.
- T. Sheu, S. Chen, J. Chen, Mixing of a split and recombine micromixer with tapered curved microchannels, Chem. Eng. Sci. 71, (2012) 321-332.
- Feng, X.; Ren, Y.; Hou, L.; Tao, Y.; Jiang, T.; Li, W.; Jiang, H. Tri-fluid mixing in a microchannel for nanoparticle synthesis. Lab Chip (2019) , 19, 2936−2946.
- Ansari, M. A.; Kim, K. Y.; Kim, S. M. Numerical and Experimental Study on Mixing Performances of Simple and Vortex Micro T-Mixers. Micromachines (2018), 9, 204.
- Bannon R., Morrison G., Smyth M., Moody T.S., Wharry S., Roth P.M.C., Gauron G., Baumann M. Continuous Flow Approach for Benzylic Photo-oxidations Using Compressed Air. Org. Process Res. Dev. 28 (2024) 3307-3312.
- Gaikwad SM, Jolhe PD, Bhanvase BA, et al. Process intensification for continuous synthesis of performic acid using Corning advanced-flow reactors. Green Process Synthesis (2017); 6:189–96.
- M. Peer, N. Weeranoppanant, A. Adamo, Y. Zhang, K.F. Jensen, Biphasic catalytic hydrogen peroxide oxidation of alcohols in flow: scale-up and extraction, Org. Process. Res. Dev. 20 (2016) 1677–1685.
- Suranani, S.; Maralla, Y.; Gaikwad, S. M.; Sonawane, S. H. Process intensification using corning® advanced-flowTM reactor for continuous flow synthesis of biodiesel from fresh oil and used cooking oil. Chem. Eng. Process. (2018), 126, 62−73.
- Nieves Remacha, M. J.; Jensen, K. F. Mass transfer characteristics of ozonolysis in microreactors and advanced-flow reactors. J. Flow Chem. (2015), 53, 160−165.
- Chen H., He W., Zhao W., Feng H., Zhu C., Fu T., Ma Y. Dynamics of Bubble Breakup in Improved AFRs with a Sharp Corner Structure. Ind. Eng. Chem. Res. (2024), 63 , 6435-6445.
- Zheng M, Li H, Chen C, et al. Breakup of bubbles in advanced-flowreactor at low Reynolds numbers. J Taiwan Inst Chem Eng. (2022);139:104506.
- Y. Zhang, S.C. Born, K.F. Jensen, Scale-up investigation of the continuous phasetransfer-catalyzed hypochlorite oxidation of alcohols and aldehydes, Org. Process Res. Dev. 18 (2014) 1476–1481.
- K.J. Wu, V. Nappo, S. Kuhn, Hydrodynamic study of single- and two-phase flow in an advanced-flow reactor, Ind. Eng. Chem. Res. 54 (2015) 7554–7564.
- N.R.M. Jos’e, A.A. Kulkarni, K.F. Jensen, OpenFOAM computational fluid dynamic simulations of single-phase flows in an advanced-flow reactor, Ind. Eng. Chem. Res. 54 (2015) 7543–7553.
- P.L. Suryawanshi, S.H. Sonawane, B.A. Bhanvase, M. Ashokkumar, M. S. Pimplapure, P.R. Gogate, Synthesis of iron oxide nanoparticles in a continuous flow spiral microreactor and Corning® advanced flowTM reactor, Green Process. Synth. 7 (1) (2018) 1–11.
- Qi, D. W.; Xie, L. M.; Yang, M.; Meng, X. Q.; Yi, Y.-Q.-Q.; Hao, Y. F.; Su, W. M.; Xu, L.; Gai, Y. Q.; Cui, Z. Finely Controlled Synthesis of Zn1−xMgxO Nanoparticles with Uniform Size Distribution Used as Electron Transport Materials for Red QLEDs. ACS Appl. Electron. Mater. (2022), 4 (4), 1875−1881.
- M.C. Fournier, L. Falk, J. Villermaux. A new parallel competing reaction system for assessing micromixing efficiency—Experimental approach, Chem. Eng. Sci. 51 (1996) 5053-5064.
- M.C. Fournier, L. Falk, J. Villermaux. A new parallel competing reaction system for assessing micromixing efficiency—Determination of micromixing time by a simple mixing model. Chem. Eng. Sci. 51 (1996) 5187-5192.
- Guo, M.; Hu, X.; Yang, F.; Jiao, S.; Wang, Y.; Zhao, H.; Luo, G.; Yu, H. Mixing performance and application of a three-dimensional serpentine microchannel reactor with a periodic vortex-inducing structure. Ind. Eng. Chem. Res. (2019), 58, 13357– 13365.
- Bourne, J. R. Comments on the iodide/iodate method for characterising micromixing. Chem. Eng. J. (2008), 140, 638−641.
- Pierrette, G.; Laurent, F.; Jacques, V. Characterisation of micromixing efficiency by the iodide−iodate reaction system. Part II: kinetic study. Chem. Eng. Sci., 55 (2000) 4245−4253.
- W. Wang, M. Li, C. Xu, Selective scaling-up of oscillating feedback micromixer: Characteristics and scaling criteria, Chem. Eng. Sci., 282 (2023) 119332.
- Kolbl, A.; Kraut, M.; Schubert, K. The iodide lodate method to characterize microstructured mixing devices. AIChE J., (2008), 54, 639.
- Palmer DA, Ramette RW, Mesmer RE. Triiodide ion formation equilibrium and activity coefficient in aqueous solution. J. Sol. Chem. (1984);13:673–683.
- N.T.K. Thanh, N. Maclean, S. Mahiddine, Mechanisms of Nucleation and Growth of Nanoparticles in Solution, Chem. Rev. 114 (2014) 7610–7630.
- Chen GG, Luo GS, Xu JH, Wang JD. Membrane dispersion precipitation method to prepare nanoparticles. Powder Tech. (2004);139: 180–185.
- Li SW, Xu JH, Wang YJ, Luo GS. Liquid-liquid two-phase flow in pore array microstructured devices for scaling-up of nanoparticle preparation. AIChE J. (2009);55:3041-3051.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





