Submitted:
20 August 2025
Posted:
22 August 2025
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
1.1. How Can Subjective Continuity be Connected to the Branching, Probabilistic Nature of Reality?
1.2. Model Scope and Limitations
2. Definitions
2.1. Assumptions of the FSM
- Emergent Consciousness: Consciousness arises from sufficiently complex, integrated physical systems such as biological neural networks.
- Decoherence-Defined Frame Boundaries: Hyperframes are delineated by quantum decoherence events that collapse superpositions into classically distinguishable states, particularly within consciousness-relevant structures.
- Binary Survival Outcomes: Each Hyperframe F is assigned a binary survival value A(F) ∈ {0,1}, determining whether subjective awareness persists or terminates.
- Observer-Relative Survival Paths: Each conscious agent experiences a private survival path through Hyperframes, independent of the experiences of other agents.
- Standard Quantum Mechanics Framework: The model operates within the standard interpretation of quantum mechanics involving decoherence without invoking additional hidden variables, objective collapse mechanisms, or postulated metaphysical structures.
2.2. Hyperframes
2.3. Conscious Agents
2.4. Dead Frame (0-Frame)
2.5. Alive Frame (1-Frame)
2.5.1. Note on Digital or Artificial Agents
2.6. Survival Function
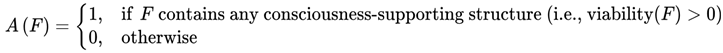
2.7. Survival Path (Timeline)
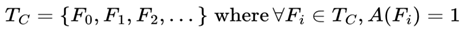
2.8. Frame Transition
2.8.1. Frame Chunking and Temporal Coherence
2.9. Observer-Relative Continuity
2.10. Decoherence-Defined Frame Resolution
- The rate at which consciousness-relevant systems decohere.
- The emergence of new distinguishable macrostates capable of sustaining awareness.
2.11. Decoherence Event Index (e) and Frame Index (t)
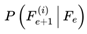 overall decohered candidate next frames using the full transition equation (see Section 3.13). The selected frame Fe+1 becomes the new present, and the subjective time advances by τe (with the corresponding frame rate re = rmax × τe).
overall decohered candidate next frames using the full transition equation (see Section 3.13). The selected frame Fe+1 becomes the new present, and the subjective time advances by τe (with the corresponding frame rate re = rmax × τe).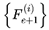 is evaluated, and one is selected according to the transition probabilities. The chosen subjective survival path is a sequence of such frames. Each step is determined by the outcome of the decoherence-resolved transition at that event.
is evaluated, and one is selected according to the transition probabilities. The chosen subjective survival path is a sequence of such frames. Each step is determined by the outcome of the decoherence-resolved transition at that event.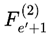 and
and 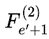 to represent identical physical configurations, the selected frame of each event is indexed according to its unique position and context within the chosen survival path. For a given run, every assigned Fe is treated as a distinct, context-specific moment in subjective experience, even if candidate frames overlap across events.
to represent identical physical configurations, the selected frame of each event is indexed according to its unique position and context within the chosen survival path. For a given run, every assigned Fe is treated as a distinct, context-specific moment in subjective experience, even if candidate frames overlap across events.- In theory, n can be set as low as 1 (comparing just two consecutive frames for “instantaneous” variance), although this may be too noisy for most cognitive or phenomenological timescales.
- n can also be set higher, up to the maximum subjective frame rate (approximately 50 Hz for humans, corresponding to n = 50), which would analyze a 1-second window of continuous experience.
- For nonhuman or digital agents, n can be tuned to match the agent’s substrate or phenomenological requirements—shorter windows for fast digital minds and longer windows for slower systems.
2.12. Decoherence Timescale
- Decoherence timescales are defined when frames become distinct and navigable.
- Consciousness-relevant systems decohere at rates that set the subjective transition speed between frames.
2.13. Decoherence-Conditioned Survival Probability
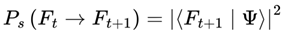
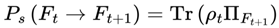
- |Ψ⟩: is the predecoherence quantum state of the system; it represents the full quantum information about all possible outcomes before decoherence or measurement occurs.
- |Ft+1⟩: denotes a specific possible outcome frame — a definite, classical state into which the system could “collapse” after decoherence. In this model, it can represent a specific conscious brain state or configuration of the observer’s world at the next step.
- ρt is the density matrix representing the (mixed) quantum state of the system at time t, just prior to decoherence—a situation common in open or realistic systems subject to environmental interactions.
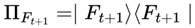 is the projection operator onto the frame ∣Ft+1⟩; for mixed states, this operator is used in the density matrix [8].
is the projection operator onto the frame ∣Ft+1⟩; for mixed states, this operator is used in the density matrix [8].
2.13.1. Steps for Extracting the Density Matrix from a Hyperframe
- Identify the conscious-relevant subsystem: For a human brain or a biological brain, this might be a functionally integrated neural circuit or large-scale brain network; for a digital agent, it could be a memory register or computational process.
- Construct the physical state: Given complete knowledge of all degrees of freedom, specify the quantum state |Ψ⟩ of the total system (or the classical-probabilistic equivalent for nonquantum agents).
- Reduce to the subsystem: Take the partial trace over everything not included in the consciously relevant system, yielding the reduced density matrix ρF.
- Plug into the model: Use this ρF for transition probabilities, coherence calculations, etc., as per the equations in the main text.
2.13.2. Representative Survival Probability (Ps) Values
| Transitions/Risk Scenario | Survival Probability (Ps) |
|---|---|
| Routine, everyday transitions | Ps ≈ 1.0 |
| Mild risk/moderate stress | Ps ≈ 0.9-0.95 |
| Significant risk (medical trauma, accident) | Ps ≈ 0.5-0.9 |
| Lethal threat/near-death | Ps ≪ 1.0 |
| Frames in which survival is impossible | Ps = 0 (these are pruned from the survival path) |
2.13.3. Flexibility in Defining Survival Probability (Ps)
2.14. Information Coherence Function
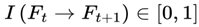
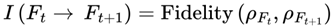
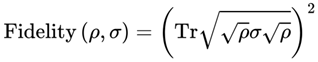
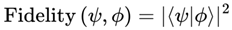 .
.2.14.1. Optional Flexibility
2.14.2. Representative Information Coherence (I) Values for Phenomenological States
| Cognitive State/Condition | Information Coherence (I) |
|---|---|
| Flow/Peak Focus | I ≈ 0.9-1.0 |
| Normal Alertness | I ≈ 0.8-0.9 |
| Routine/Mild Distraction | I ≈ 0.7-0.8 |
| Sleep/Fatigue, Drugs, Shock/Trauma | I ≈ 0.5-0.7 |
| Coma/Unconsciousness | I ≲ 0.5 |
| Frames in which survival is impossible | I = 0 (these are pruned from the survival path) |
| Symbol/Notation | Name/Description | Typical values/Domain | Section/Context |
|---|---|---|---|
| F, Ft | Hyper frame (Frame): Complete macro state at an instant in spacetime | – | Def. 2.2, throughout |
| C | Conscious agent: Coherent info-processing structure in frame F | – | Def. 2.3 |
| t, e | Frame index/Decoherence event index (discrete time step) |
t = 0,1,2,… e = 0,1,2,… |
Def. 2.11 |
| A(F) | Survival function: Binary indicator if F is “alive” (supports consciousness) | [0,1] | Def. 2.4–2.7, throughout |
| Φ | Integrated information: Degree of integration in a frame | [0, ∞) (the max is dependent on substrate and species) | Def. 2.5 |
| Φcritical | Minimum threshold for conscious frame | Context-dependent | Def. 2.5 |
| S(F) | Agentic preference weight: Transition bias/intent for frame F | +, ≥ 1 | Sec. 3.9 |
| N | Set/Number of candidate next frames at a transition | 1 to large (106+) | Def. 2.15 |
| TC | Survival path: Sequence of alive frames for agent C | – | Def. 2.7, 2.9 |
| τ | Decoherence timescale: Time for a quantum system to decohere | 10–50 ms (humans, could be higher or lower in different substrates) | Def. 2.12, Sec 3.4 |
| r, rmax, rrel | Frame rate, maximum, and relativistic frame rate | 1–50 Hz (humans, substrate dependent) | Sec. 3.4, 4.12 |
| I(Ft →Ft+1) | Information coherence function: Similarity between frames | [0,1] | Def. 2.14 |
| Ps(Ft → Ft+1) | Survival probability: Likelihood of decoherence into next frame | [0,1] | Def. 2.13 |
| w(Ft →Ft+1) | Transition weight: Unnormalized likelihood for next frame | – | Sec. 3.11 |
| P(Ft+1 | Ft), Pbase, Pchunk | Transition probability: Normalized probability for next frame | [0,1] | Sec. 3.6, 3.12 |
| Varcog | Cognitive variance: Instability/divergence for a frame’s next states | [0,1] | Sec. 3.4, 3.12.2, 4.12 |
| Varwindow | Windowed cognitive Variance: Mean Var across window of n frames | [0,1] | Sec. 3.12.2 |
| n | Window size (number of recent frames for chunking/variance) | 10–30 (humans, substrate dependent) | Def. 2.11, 3.4, 3.12.2, 4.12 |
| at | Chunk confidence coefficient: Weight for chunk-based transitions | [0,1] | Sec. 3.12.2 |
| k | Integration timescale constant (min. frame update time) | 0.01–0.1 s (depends on the substrate) tunable | Sec. 3.4, 4.12 |
| B | Scaling constant for variance to match frame rates | ∼5 (humans, substrate dependent), tunable | Sec. 3.4, 4.12 |
| ϵ | Saturation/nonlinearity parameter in frame rate | ∼27 (humans, substrate dependent) tunable | Sec. 3.4, 4.12 |
| γ | Lorentz factor (relativity) | ≥ 1 | Sec. 4.12 |
| μ | Mean frame index (for entropy/variance metrics) | – | Sec. 3.10 |
| σ2 | Chunk “tightness” parameter | Tunable | Sec. 3.12.1 |
| Z, Zchunk | Normalization constants for probability distributions | – | Sec. 3.12, 3.12.1 |
| λ | Sensitivity to cognitive variance in chunking(αt) | +−3, tunable | Sec. 3.12.1 |
| κ | Sensitivity to coherence loss in chunk confidence (αt) | 0–3 | Sec. 3.12.1 |
| i | Index for summing over candidate frames in variance/averaging formulas | – | Sec. 2.11, 3.1, 3.4, 3.12.2, 4.12 |
2.14.3. Relationship Between Φ and I
2.15. Definition of N: The Set of Possible Next Frames
- Physically possible: Frames in which Ps(Ft+1) > 0, i.e., transitions that are not forbidden by quantum law and system dynamics.
- Informationally coherent: Frames in which I(Ft → Ft+1) > 0, i.e., transitions that are not maximally incoherent or disconnected from the current frame.
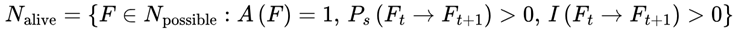
3. Theoretical Framework of the FSM
3.1. Conscious Survival as Frame Navigation
3.2. Binary Outcome Model: Survival (1) and Death (0)
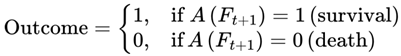
3.3. Formal Description of Frame Transitions
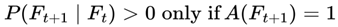
- P(Ft+1 ∣ Ft) is the probability of transitioning from Ft to Ft+1,
- A(Ft+1) determines whether the frame supports conscious awareness.
3.4. Frame Transition Rate and Decoherence Dynamics
- rt: the frame rate at time (or step) t along the subjective survival path.
- rmax: the maximum possible frame rate for any particular substrate.
- τeff,t : the effective decoherence timescale governing the progression of subjective moments, determined both by the fastest physical decoherence possible in the local environment and by ongoing changes in cognitive stability and informational coherence.
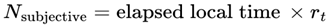
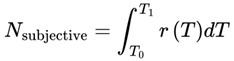
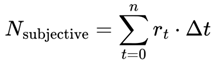
- Integral/sum: Both expressions accumulate the total number of moments experienced by summing the “fraction of a moment” contributed by each tiny time interval, weighted by the local decoherence timescale. If τeff is constant, this divides the total time by τeff; if it varies, the moments are “packed” more tightly or spread out as τeff shrinks or grows.
- Nsubjective: The total number of subjective moments (or “conscious frames”) experienced over the interval. This represents the “ticks” of conscious awareness, which might differ from objective clock time if the conditions for consciousness change.
- T0,T1: The start and end times of the interval being considered (can be measured in seconds, minutes, etc., depending on context).
- T: Elapsed local (proper) time for the agent, measured according to the agent’s own subjective/process clock. T can be in seconds, minutes, hours, or any unit appropriate for the time scale of the process under consideration. In relativistic or time-dilated scenarios, T always refers to the local time actually experienced by the agent, not the time measured by an external or distant observer.
- d: denotes the index of the last alive frame before subjective death, that is, the endpoint of a lifetime survival path, such that A(Fd) = 1 and A(Fd+1) = 0. When specifically calculating the total number of subjective moments up to the agent’s final alive frame, d is used.
- r(T): The instantaneous subjective frame rate at each moment. This value determines how rapidly conscious frames occur—a higher r means that moments are packed closely together (time feels “fast” or “full”); a lower r means that moments are more spaced out (time feels “slow” or “fragmented”).
- Δt: The size of each time step in the simulation or dataset. For real neural or behavioral data, this might be the sampling interval; for a theoretical model, it is typically set to “1” for event-by-event counting.
- n: denotes the final event or frame index in the interval considered (for example, the endpoint of a simulation run, time segment, or experiment—not necessarily related to death). Use n, t, or e for general sums over any period.
3.4.1. Cognitive Variance Interpretation of τeff,t
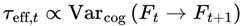
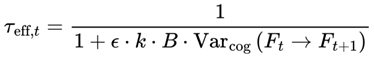
- Varcog: represents the variance or instability across a local window of n consecutive frames—either the most recent n frames or a moving window within a chunk.
- k is a proportionality constant that determines the scaling between the cognitive variance and the subjective frame transition timescale. (state dependent, e.g., 0.01–0.1 s for humans).
- ϵ: a saturating parameter (set here, ϵ = 27 for humans).
- B: The scaling constant is empirically set so that the model’s predicted variance matches the observed subjective frame rates in both high-focus and unconscious states (e.g., 5 for humans).
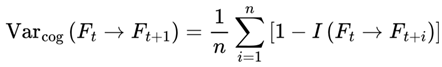
- Varcog(Ft → Ft+1): The cognitive variance between the current frame Ft and its possible future frames.
- n: The number of future candidate frames or decoherence events in the survival path (the window size over which variance is computed).
- i: The index for summing over the n future frames.
- Ft: The current frame (the agent’s current conscious state or informational configuration).
- Ft+i: The i-th candidate future frame (a possible next state for the agent at time t + i).
- I(Ft → Ft+i): The coherence measure (information similarity, or quantum fidelity) between the current frame and the i-th candidate future frame.
- [1 − I(Ft → Ft+i)]: The degree of informational divergence (or incoherence) between frames; higher values indicate greater differences.
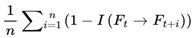 : The average divergence over all n candidate future frames; this gives the overall cognitive variance for the window.
: The average divergence over all n candidate future frames; this gives the overall cognitive variance for the window.
3.4.2. Parameter Fitting and Generalization
- k (integration timescale): This is set to match the system’s minimal processing or integration window on the basis of experimental or computational studies (e.g., the shortest neural or algorithmic cycle, reaction time, or update step).
- B (variance scaling constant): Fit B so that the output variance range (Varcog) covers the full observed spectrum from high coherence (focus) to low coherence (maximal fragmentation) in the target system. Physiological or behavioral data, such as flicker fusion rates, perceptual binding, or task performance limits, are used.
- ϵ (nonlinearity/sensitivity): Tune ϵ to ensure that the frame rate curve matches empirical data across states. For example, choose ϵ so that the model yields maximal frame rates in the correct range for high-alertness states and appropriately low rates for sleep, coma, or high-load digital agents.
3.5. Observer-Relative Survival Paths
- An observer may witness another agent's apparent death.
- However, from the perspective of the dying agent, survival may continue along a distinct, unshared branch.
3.6. Formalization Through Survival Graphs
- V is the set of all frames F,
- E ⊆ V × V is the set of directed edges representing allowable transitions between frames.
- A(F) = 1 if the frame sustains the conscious agent C,
- A(F) = 0 otherwise.
3.6.1. Normalization Condition
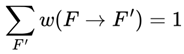

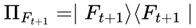 is the projector onto the outcome frame.
is the projector onto the outcome frame.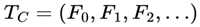
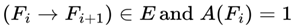
3.7. Binary Interpretation of Survival Paths
- Entropy of Survival Paths: The degree of randomness versus order in survival sequences could reflect underlying structural regularities or instability in the frame network.
- Compression Possibility: If survival sequences are highly compressible, this would suggest patterned, nonrandom dynamics guiding survival threading. High-entropy sequences imply stochastic survival landscapes.
- Error-Correction Analogy: Consciousness navigating survival-compatible frames resembles an information-preserving process, selectively "correcting" for decoherence-induced branching threats.
- Information-Theoretic Interpretation: Subjective continuity itself may be regarded as the maintenance of an informational thread through an environment characterized by probabilistic frame transitions.
3.8. Probabilistic Existence of Survival Paths
- A discrete network of frames F, each assigned a survival value A(F) ∈ {0,1}.
- Transition probabilities P(Ft+1 ∣ Ft) defined over frame pairs, conditioned on A(Ft+1)=1.
- The multiverse structure infinitely branches due to quantum decoherence dynamics, generating an unbounded number of future frame possibilities at each point.
3.8.1. Formal Sketch Using the Infinite Traversal Theorem
3.9. Passive vs Active Navigation of Survival Paths
3.9.1. How Agentic Preference Weight (S) Links to Chunks
3.10. Informational Structure of Transition Distributions
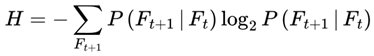
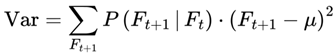
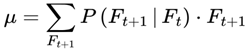
3.11. Informational Coherence and Transition Weighting
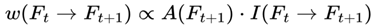
- A(Ft+1) ∈ {0,1} is the survival function, as defined previously.
- I(Ft → Ft+1) is the information coherence function, quantifying the informational, cognitive, or structural consistency between the current frame and the candidate future frame.
- Frames that preserve prior informational content (e.g., memory traces, environmental layout, and cognitive integrity) have higher transition probabilities.
- Frames that break coherence (e.g., hallucination, memory loss, and chaotic shifts) are less likely to be experienced, even if they are survivable.
- This helps explain why lived reality feels smooth and structured, despite the probabilistic and decohering nature of the underlying universe.
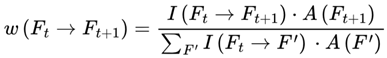
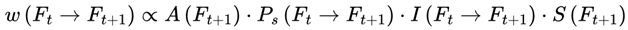
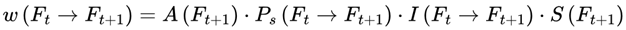
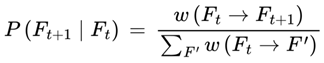
3.12. Frame Continuity Transition Equation
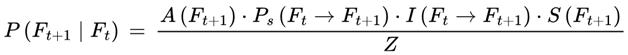
- A(Ft+1) ∈ {0,1} enforces survival compatibility.
- Ps(Ft → Ft+1) ∈ [0,1] captures the likelihood of a decoherence-conditioned transition.
- I(Ft → Ft+1) ∈ [0,1] quantifies the informational coherence between the current and candidate frames.
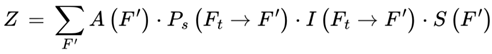
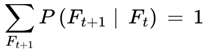
3.12.1. Incorporating Chunk Dynamics
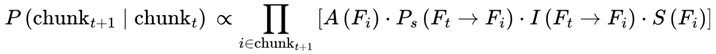
- A(Fi) ∈ {0,1}: binary survival indicator
- Ps(Ft → Fi) ∈ [0,1]: decoherence-conditioned survival probability
- I(Ft → Fi) ∈ [0,1]: informational coherence from the chunk origin to Fi
- S(Fi) ∈ +: agentic preference weighting
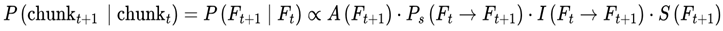
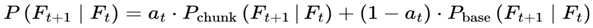
- Pchunk(Ft+1 ∣ Ft): the probability of continuing within a coherent chunk.
- Pbase(Ft+1 ∣ Ft): the original decoherence-based transition.
- αt ∈ [0,1]: dynamically adjusts on the basis of local stability.
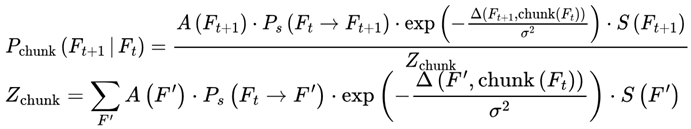
- Ps(Ft → Ft+1) is the decoherence-conditioned survival probability (likelihood of resolving into this frame).
- A(Ft+1) enforces the survival constraint.
- Δ(Ft+1, chunk(Ft)) is the decoherence or informational distance between the new frame and the chunk.
- σ2 controls how tight the chunk’s influence is.
- S(Ft+1) is the agent’s preference (e.g., structural or narrative alignment).
- Zchunk represents normalization, so all chunk probabilities sum to 1.
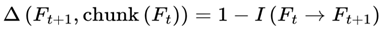
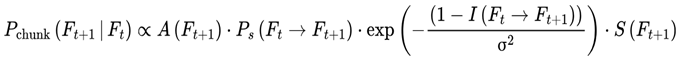
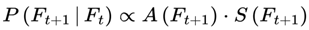
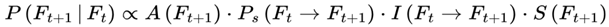
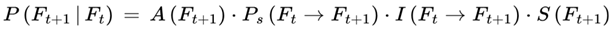
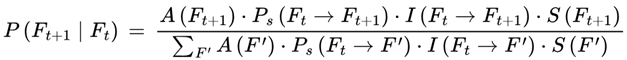
3.12.2. Dynamic Chunk Confidence Coefficient
- at: Dynamic chunk confidence coefficient, recalculated at each time step.
- λ: A sensitivity constant controlling how rapidly it decreases with increasing instability.
- Varwindow: The moving average of cognitive variance, defined above, which quantifies local volatility in the survival trajectory
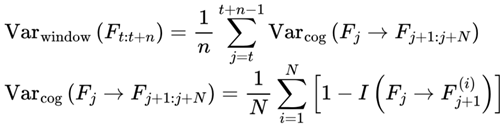
- Varwindow(Ft:t+n): The overall cognitive variance computed across a window of n consecutive frames, representing the local stability or instability of experience within a time chunk.
- Window size (n): The number of recent events (frames) included in the window for the average.
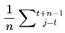 : For each frame Fj in the window (from t to t + n − 1), compute the cognitive variance for its possible next states, and take the average for all frames in the window, providing a summary measure of local cognitive (in)stability over time.
: For each frame Fj in the window (from t to t + n − 1), compute the cognitive variance for its possible next states, and take the average for all frames in the window, providing a summary measure of local cognitive (in)stability over time.- Varcog(Fj → Fj+1:j+N): The cognitive variance at frame Fj, calculated as the average informational divergence between Fj and its N possible next candidate frames.
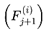 : Each possible frame into which the agent/system can transition from Fj at the next step; N is the total number of such candidates.
: Each possible frame into which the agent/system can transition from Fj at the next step; N is the total number of such candidates.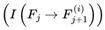 : A measure of how similar (or “coherent”) the present frame is to each candidate next frame; it ranges from 0 (completely different) to 1 (identical).
: A measure of how similar (or “coherent”) the present frame is to each candidate next frame; it ranges from 0 (completely different) to 1 (identical).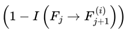 : Quantifies how much the next candidate frame differs from the current frame; higher values indicate greater instability or unpredictability.
: Quantifies how much the next candidate frame differs from the current frame; higher values indicate greater instability or unpredictability.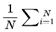 : Compute the mean divergence for all possible next frames at each time step, capturing the expected variability in the immediate future.
: Compute the mean divergence for all possible next frames at each time step, capturing the expected variability in the immediate future.
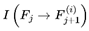 is the information coherence between the current frame and the i-th next-frame candidate. By averaging over both multiple next-frame possibilities and a window of recent events, this measure smooths out transient fluctuations and captures sustained shifts in informational volatility. The result is a robust, dynamically updated index of local cognitive stability, which governs the chunk confidence coefficient and episode segmentation in the model.
is the information coherence between the current frame and the i-th next-frame candidate. By averaging over both multiple next-frame possibilities and a window of recent events, this measure smooths out transient fluctuations and captures sustained shifts in informational volatility. The result is a robust, dynamically updated index of local cognitive stability, which governs the chunk confidence coefficient and episode segmentation in the model.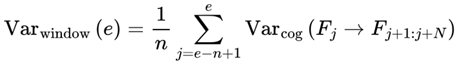
- Varwindow(e):
- Window size (n):
- The number of recent events (frames) included in the window for the average.
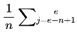 : For each frame Fj in the window ending at event e (i.e., the last n frames from e − n + 1 to e), compute the cognitive variance, then average these values.
: For each frame Fj in the window ending at event e (i.e., the last n frames from e − n + 1 to e), compute the cognitive variance, then average these values.- Varcog(Fj → Fj+1:j+N): The cognitive variance at frame Fj, which is calculated as the average divergence between Fj and its N possible next candidate frames.
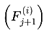 : Each possible frame the system could transition into from Fj at the next step; N is the number of such candidate frames.
: Each possible frame the system could transition into from Fj at the next step; N is the number of such candidate frames.- Information coherence and divergence: For each candidate next frame, compute the information coherence
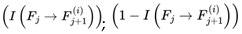 quantifies how different each candidate is from the current frame.
quantifies how different each candidate is from the current frame. - Averaging across windows and candidates: First, the divergence across all N candidates at each step (using Varcog) is averaged across the n recent steps (window), yielding Varwindow(e).
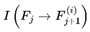 is the information coherence between the current frame and the i-th next-frame candidate. By averaging over both multiple next-frame possibilities and a window of recent events, this moving average captures the local volatility in subjective continuity, ensuring that chunk confidence at is sensitive to both transient fluctuations and sustained periods of instability in cognitive dynamics.
is the information coherence between the current frame and the i-th next-frame candidate. By averaging over both multiple next-frame possibilities and a window of recent events, this moving average captures the local volatility in subjective continuity, ensuring that chunk confidence at is sensitive to both transient fluctuations and sustained periods of instability in cognitive dynamics.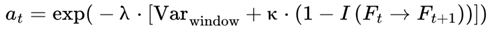
- κ: A nonnegative weighting constant that determines how strongly the informational coherence penalty (1 – I(Ft → Ft+1)) influences the chunk confidence at.
- I(Ft → Ft+1) ∈ [0,1]: A function measuring how well the transition aligns with the chunk structure or informational integrity. High coherence suppresses the penalty; incoherent transitions lower at.
- Interpretation:
- When recent transitions are stable (low variance) and coherence is high, at ≈ 1, the system strongly favors transitions that preserve chunk continuity.
- When variance is high or coherence is low, at → 0, the system deactivates the chunk and reverts to the base survival model, ensuring ongoing conscious continuity even during structural collapse.
- When κ = 0, at is determined solely by the local variance of recent frames (Varwindow). As κ increases, transitions with low informational coherence (I ≈ 0) are penalized more heavily, causing at to drop sharply for incoherent transitions, thereby favoring the persistence of stable chunks. Higher values of κ increase the impact of coherence on at, making the system more sensitive to abrupt or incoherent transitions. The optimal value of κ depends on the sensitivity of the agent or system to abrupt or incoherent transitions and can be calibrated through empirical data (e.g., neural dynamics, behavioral experiments) or chosen for theoretical exploration in simulations.
3.12.3. Chunk Definition (Event Index Form)
4. FSM Implications
4.1. Subjective Immortality
4.2. Relativity of Death Across Survival Paths
4.3. Consciousness Bias
4.4. Private Multiverse Threads
4.5. Subjective Novelty and Continuity
4.6. Altered States, Sleep, and Fragmented Consciousness
4.6.1. Acute Events: Startle, Survival Mode, and “Life-Flashing” Phenomena
4.6.2. Pharmacological, Drug, and Medication Effects on Coherence and Chunking
4.6.3. Mental Illness, Hallucinations, and Fragmented Experience
4.6.4. Emotional States: Unified Modeling in the FSM
4.6.5. Applications to Sensory States:
4.7. Physical Limits to Frame Survival
4.7.1. Entropy and Survival Path Thinning
4.7.1.1. Survival Path Density and Entropy
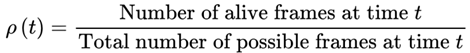
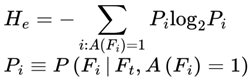
4.8. Survival Path Filtering and Natural Selection: The Entropic Logic of Continuity
4.8.1. Entropy and Survival Path Dynamics
4.8.2. The Analogy of Natural Selection
4.8.3. Adaptive Persistence and the Structure of Experience
4.8.4. Brain–Body Relationship and Mutual Survival Benefit
4.9. Informational Structure of Survival and Emergent Digital Reality
4.10. Duplicate Frames and Divergent Continuity
4.11. Emergence of Consciousness Through Probabilistic Survival
4.12. Time Dilation and Relativistic Subjective Experience
4.12.1. Relativistic Adjustment of k
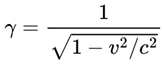
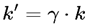
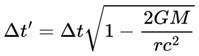
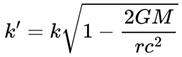
4.12.2. Frame Rate Calculation with Time Dilation
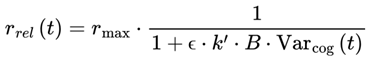
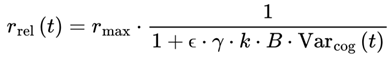
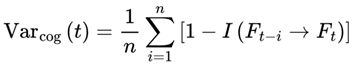
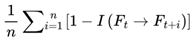 . For real-time frame rate estimation, we adopt the backward-looking form above.
. For real-time frame rate estimation, we adopt the backward-looking form above.4.12.3. Comparison with a Stationary Observer
- Stationary observer:
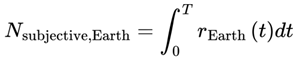
- Relativistically moving observer:
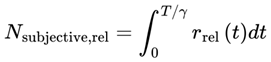
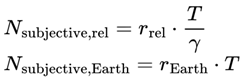
4.13. Implications for Cosmic Life and Consciousness Emergence
4.13.1. Implications for Alien Intelligence and the Fermi Paradox
4.13.2. Implications for Digital Life and Artificial Consciousness
4.13.2.1. Digital Minds and Survival Paths
4.13.2.2. AI Design and Consciousness Engineering
4.13.2.3. Digital Evolution and the Emergence of Consciousness
4.13.2.4. Ethics, Rights, and AI Safety
4.13.2.5. Digital Immortality and Personal Continuity
4.13.3. Outlook on Animal Consciousness and Survival
4.14. Paradox Implications of the FSM
4.14.1. Fermi Paradox and Synchronization Paradox (SETI/Astrobiology)
4.14.2. The Grandfather Paradox (Time Travel Paradox)
4.14.3. Quantum Immortality Paradox
4.14.4. Subjective Death Paradox
4.14.5. The Measure Problem (Many-Worlds Probability)
4.14.6. Duplicate Frame/Merging Identity Paradox
4.14.7. Arrow of Time Paradox
4.14.8. Sleeping Beauty and Anthropic Paradoxes
4.14.9. Observer-Relativity of Death
4.14.10. Private Multiverse Threading/Observer-Relative Worlds
4.14.11. Subjective Novelty Paradox
4.14.12. Death Relativity/“Local Death” Paradox
4.14.13. Black Hole and Time Dilation Paradoxes
4.14.14. Digital Mind Pause/Death Paradox
4.14.15. Closed Timelike Curves (CTCs) and Causality Paradoxes
4.14.16. Boltzmann Brain Paradox
4.14.16.1. Further Paradoxes and Generalization
5. Implications for Future Research
5.1. Potential Experimental Implications
5.1.1. Thought Experiment: Decoherence Modulation in Neural Systems
5.2. Simulation of Survival Path Dynamics
- Nodes: Each node represents a decoherence-resolved frame F, assigned a survival status A(F) ∈ {0,1}.
- Edges: Directed edges represent allowable transitions between frames. An edge from Ft to Ft+1 exists only if A(Ft+1)= 1.
-
Survival Probability Assignment:
- ○
- Upon generation, each frame is assigned a survival status on the basis of a survival probability Ps.
- ○
- For initial simulations, I set Ps ≈ 0.99, reflecting a high baseline chance of survival between frames under normal decoherence conditions.
-
Random Decay Dynamics:
- ○
- Frame-by-frame variability in survival probability is introduced through stochastic noise.
- ○
- For example, model survival decays using a Gaussian random walk, where the survival probability slightly drifts at each step:
-
Ps(t + 1) = Ps(t) + N(0,σ2)
- ○
- where σ controls environmental volatility.
- ○
- This allows survival conditions to deteriorate realistically over long subjective timescales, simulating entropy-driven survival thinning.
- Begin with an initial frame F0 such that A(F0) = 1.
-
At each step:
- ○
- Sample possible next frames on the basis of survival probabilities.
- ○
- Traverse to a randomly selected frame Ft+1 such that A(Ft+1) = 1.
- If no alive frames are reachable (i.e., no outgoing edges to A(F) = 1), subjective continuity terminates.
- Survival Path Lengths: Average number of consecutive alive frames traversed before termination.
- Survival Path Entropy: Degree of randomness versus order across different runs.
- Critical Thresholds: Identify the environmental volatility levels (σ) at which survival paths collapse rapidly.
5.3. Statistical Models of Extreme Survival
5.4. Directions for Future Research on the FSM
5.4.1. Dynamics of Interaction Between Agentic Preference and Coherence
5.4.2. Explicit Functional Dependencies: Modeling Interactions between Variables
5.4.3. Empirical Identification of Φcritical and Key Model Parameters
- Correlating integrated information measures (Φ) with markers of conscious and unconscious states (e.g., comparing awake, asleep, anesthetized, or comatose subjects).
- Identifying a transition point or threshold—where loss of consciousness occurs across species or substrates—to suggest a universal or context-dependent Φcritical.
- Simulating or constructing artificial agents (digital or AI systems) and tracking when, if ever, they surpass the threshold for supporting subjective experience, as operationalized in the FSM.
- Using perturbational or information-theoretic methods (e.g., brain stimulation, system knockouts) to test the stability of subjective continuity and frame viability under controlled changes to Φ or related variables.
5.4.4. Adaptive Chunking based on Entropy and Environmental Volatility
5.4.5. Modeling the Content of Consciousness
5.4.6. Precise Cognitive and Physical Decoherence Timescales
5.4.7. Initial Parameter Ranges and Simulation Guidelines
5.4.7.1. Core Parameters for Future Simulations
- Range: 0.01–0.8
-
Typical values:
- ○
- High focus/flow: 0.01–0.1
- ○
- Everyday alertness: 0.1–0.3
- ○
- Sleep/fatigue: 0.3–0.5
- ○
- Coma, extreme fragmentation: 0.5–0.6+
-
Interpretation:Low values = high coherence/stability;high values = fragmentation/instability.Coherence Threshold (Imin)
- Default: 0.5
- Range: ≈ 0.2–0.7
-
Interpretation:Minimum frame-to-frame similarity for inclusion in the survival path;adjust the chunking sensitivity.Agentic Preference Weight (S)
-
Default (passive agent): 1.0 for all framesRange: +–3(1 = minimal/no preference; >1 = strong preference)
-
Interpretation:Increase for goal-directed or strongly motivated agents;keep at 1 for neutral/passive agents.Integrated Information Threshold (Φcritical)
- Empirical value: Currently unknown; set as a relative threshold in simulation (e.g., “top 1–5% of possible frames” or via normalization).
- Interpretation: Only frames with Φ > Φcritical are considered conscious/alive.
-
Recommendation:Test a range of values to explore model sensitivity.Sensitivity Parameters (λ, κ)Typical range:
- λ: ~ +–3
- κ: 0–3.0
5.4.7.2. Interpretation
- For fragile/unstable states, a higher λ is used. For resilient/focused states, a lower λ is used.
- Default (human-like agents): 10–30
- Range: 1–50
- Interpretation:
- 5.0 for human brains (empirically fits frame rates 50–1 Hz)
- Lower (3.0–5.5) for substrates with slower processing
- Higher (10.0–15.0) for digital or engineered systems with very fast or slow subjective timescales
- Range: +–40 (empirically tunable)
-
Typical values:
- ○
- Low sensitivity: +–10
- ○
- Medium sensitivity (humans): 15–30 (empirical fit: 27)
- ○
- High sensitivity: 30–40+
- Interpretation: Controls how sharply the subjective frame rate slows as cognitive variance increases; higher values indicate more rapid slowdown and fragmentation with instability.
6. Methods
7. Conclusion
- Formalizing survival using binary outcome logic,
- Grounding frame transitions in decoherence dynamics relevant to cognitive systems,
- Introducing private multiverse threading for each conscious agent,
- And quantifying consciousness bias toward survival outcomes.
Author Contributions
Funding
Conflict of Interest
Author Biography:
References
- Tegmark, M.: The interpretation of quantum mechanics: many worlds or many words? Fortschr. Phys. 46, 855–862 (1998). [CrossRef]
- Albert, D.Z., Loewer, B.: Interpreting the many-worlds interpretation. Synthese 77(2), 195–213 (1988). [CrossRef]
- Albert, D.Z., Loewer, B.: Tails of Schrödinger’s cat. In: Clifton, R. (ed.) Perspectives on Quantum Reality, pp. 81–92. Springer, Dordrecht (1996). [CrossRef]
- Zurek, W.H.: Decoherence, einselection, and the quantum origins of the classical. Rev. Mod. Phys. 75, 715–775 (2003). [CrossRef]
- Seth, A.K., Barrett, A.B., Barnett, L.: Causal density and integrated information as measures of conscious level. Philos. Trans. R. Soc. A 369, 3748–3767 (2011). [CrossRef]
- Tononi, G.: Consciousness as integrated information: a provisional manifesto. Biol. Bull. 215, 216–242 (2008). [CrossRef]
- Tononi, G., Boly, M., Massimini, M., Koch, C.: Integrated information theory: from consciousness to its physical substrate. Nat. Rev. Neurosci. 17, 450–461 (2016). [CrossRef]
- Born, M.: Zur quantenmechanik der stoßvorgänge. Z. Phys. 37, 863–867 (1926). [CrossRef]
- Wallace, D.: The Emergent Multiverse: Quantum Theory according to the Everett Interpretation. Oxford University Press, Oxford (2012).
- Pöppel, E.: A hierarchical model of temporal perception. Trends Cogn. Sci. 1, 56–61 (1997). [CrossRef]
- VanRullen, R., Koch, C.: Is perception discrete or continuous? Trends Cogn. Sci. 7, 207–213 (2003). [CrossRef]
- Schlosshauer, M.: Decoherence and the Quantum-to-Classical Transition. Springer, Berlin (2007). [CrossRef]
- Schrödinger, E.: Quantisierung als eigenwertproblem (erste mitteilung). Ann. Phys. 384, 361–376 (1926). [CrossRef]
- Tononi, G., Koch, C.: Consciousness: here, there and everywhere? Philos. Trans. R. Soc. B 370, 20140167 (2015). [CrossRef]
- Panksepp, J.: Affective neuroscience of the emotional BrainMind: evolutionary perspectives and implications for understanding depression. Dialogues Clin. Neurosci. 12, 533–545 (2010). [CrossRef]
- Rottenberg, J.: Mood and emotion in major depression. Curr. Dir. Psychol. Sci. 14, 167–170 (2005). [CrossRef]
- Wittmann, M.: Moments in time. Front. Integr. Neurosci. 5, 66 (2011). [CrossRef]
- Foa, E.B., Keane, T.M., Friedman, M.J., Cohen, J.A.: Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies. Guilford Press, New York (2006).
- LeDoux, J.: Rethinking the emotional brain. Neuron 73, 653–676 (2012). [CrossRef]
- Mobbs, D., Petrovic, P., Marchant, J.L., Hassabis, D., Weiskopf, N., Seymour, B., Dolan, R.J., Frith, C.D.: When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science 317, 1079–1083 (2007). [CrossRef]
- Van der Kolk, B.A.: The Body Keeps the Score: Brain, Mind, and Body in the Healing of Trauma. Viking, New York (2014). ISBN 978-0-670-78593-3.
- Greyson, B.: Near-death experiences. In: Cardeña, E., Lynn, S.J., Krippner, S.C. (eds.) Varieties of Anomalous Experience: Examining the Scientific Evidence, pp. 315–352. American Psychological Association, Washington, DC (2000).
- Noyes, R., Kletti, R.: Depersonalization in the face of life-threatening danger: a description. Psychiatry 39, 19–27 (1976). [CrossRef]
- Singer, W.: Consciousness and the binding problem. Ann. N. Y. Acad. Sci. 929, 123–146 (2001). [CrossRef]
- Nielsen, M.A., Chuang, I.L.: Quantum Computation and Quantum Information (10th Anniversary Edition). Cambridge University Press, Cambridge (2010).
- Csikszentmihalyi, M.: Flow: The Psychology of Optimal Experience. Harper & Row, New York (1990).
- Palva, S., Palva, J.M.: New vistas for alpha-frequency band oscillations. Trends Neurosci. 30, 150–158 (2007). [CrossRef]
- Tononi, G., Sporns, O., Edelman, G.M.: A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc. Natl. Acad. Sci. 91, 5033–5037 (1994). [CrossRef]
- Baldassano, C., Chen, J., Zadbood, A., Pillow, J.W., Hasson, U., Norman, K.A.: Discovering event structure in continuous narrative perception and memory. Neuron 95, 709–721.e5 (2017). [CrossRef]
- Carhart-Harris, R.L., Leech, R., Hellyer, P.J., Shanahan, M., Feilding, A., Tagliazucchi, E., Chialvo, D.R., Nutt, D.: The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front. Hum. Neurosci. 8, 20 (2014). [CrossRef]
- Hudetz, A.G.: General anesthesia and human brain connectivity. Brain Connect. 2, 291–302 (2012). [CrossRef]
- Massimini, M., Ferrarelli, F., Huber, R., Esser, S.K., Singh, H., Tononi, G.: Breakdown of cortical effective connectivity during sleep. Science 309, 2228–2232 (2005). [CrossRef]
- Brown, E.N., Lydic, R., Schiff, N.D.: General anesthesia, sleep, and coma. N. Engl. J. Med. 363, 2638–2650 (2010). [CrossRef]
- Minzenberg, M.J., Carter, C.S.: Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology 33, 1477–1502 (2008). [CrossRef]
- Everett, H.: "Relative state" formulation of quantum mechanics. Rev. Mod. Phys. 29, 454–462 (1957). [CrossRef]
- Tegmark, M.: Is “the theory of everything” merely the ultimate ensemble theory? Ann. Phys. 270, 1–51 (1998). [CrossRef]
- Tegmark, M.: Importance of quantum decoherence in brain processes. Phys. Rev. E 61, 4194–4206 (2000). [CrossRef]
- Lim, J., Dinges, D.F.: Sleep deprivation and vigilant attention. Ann. N. Y. Acad. Sci. 1129, 305–322 (2008). [CrossRef]
- Durrett, R.: Probability: Theory and Examples (5th ed.). Cambridge University Press, Cambridge (2019).
- Grimmett, G.: Percolation (2nd ed.). Springer, Berlin (1999).
- Lyons, R., Peres, Y.: Probability on Trees and Networks. Cambridge University Press, Cambridge (2016).
- Dennett, D.C.: Consciousness Explained. Little, Brown and Company, New York (1991).
- O’Reilly, R.C., Munakata, Y., Frank, M.J., Hazy, T.E., Contributors: Computational Cognitive Neuroscience. Wiki Book (2012). ISBN 978-0982477273.
- Friston, K.: The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. 11, 127–138 (2010). [CrossRef]
- Seth, A.: Explanatory correlates of consciousness: theoretical and computational challenges. Cogn. Comput. 1, 50–63 (2009). [CrossRef]
- Robbins, T.W., Arnsten, A.F.T.: The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu. Rev. Neurosci. 32, 267–287 (2009). [CrossRef]
- Pezzulo, G., Rigoli, F., Friston, K.: Hierarchical Active Inference: A Theory of Motivated Control. Trends in Cognitive Sciences 22(4), 294–306 (2018). [CrossRef]
- Baars, B.J.: A Cognitive Theory of Consciousness. Cambridge University Press, New York (1988).
- Botvinick, M.M.: Hierarchical models of behavior and prefrontal function. Trends Cogn. Sci. 12, 201–208 (2008). [CrossRef]
- Brunec, I.K., Bellana, B., Ozubko, J.D., Man, V., Robin, J., Liu, Z.-X., Grady, C., Rosenbaum, R.S., Winocur, G., Barense, M.D., Moscovitch, M.: Multiple scales of representation along the hippocampal anteroposterior axis in humans. Curr. Biol. 28, 2129–2135.e6 (2018). [CrossRef]
- Shannon, C.E.: A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423 (1948). [CrossRef]
- Kurby, C.A., Zacks, J.M.: Segmentation in the perception and memory of events. Trends Cogn. Sci. 12, 72–79 (2008). [CrossRef]
- Zacks, J.M., Speer, N.K., Swallow, K.M., Braver, T.S., Reynolds, J.R.: Event perception: a mind-brain perspective. Psychol. Bull. 133, 273–293 (2007). [CrossRef]
- Kent, L.S., Van Wassenhove, V., Sarasso, S.: Sleep and subjective time perception. Front. Hum. Neurosci. 12, 410 (2018). [CrossRef]
- Sarasso, S., Boly, M., Napolitani, M., Gosseries, O., Charland-Verville, V., Casarotto, S., Rosanova, M., Casali, Adenauer G., Brichant, J.-F., Boveroux, P., Rex, S., Tononi, G., Laureys, S., Massimini, M.: Consciousness and complexity during unresponsiveness induced by propofol, xenon, and ketamine. Curr. Biol. 25, 3099–3105 (2015). [CrossRef]
- Schartner, M., Seth, A., Noirhomme, Q., Boly, M., Bruno, M.-A., Laureys, S., Barrett, A.: Complexity of multi-dimensional spontaneous EEG decreases during propofol induced general anaesthesia. PLoS One 10, e0133532 (2015). [CrossRef]
- Nir, Y., Tononi, G.: Dreaming and the brain: from phenomenology to neurophysiology. Trends Cogn. Sci. 14, 88–100 (2010). [CrossRef]
- Hobson, A., Voss, U.: A mind to go out of: reflections on primary and secondary consciousness. Conscious. Cogn. 20, 993–997 (2011). [CrossRef]
- Siclari, F., Baird, B., Perogamvros, L., Bernardi, G., LaRocque, J.J., Riedner, B., Boly, M., Postle, B.R., Tononi, G.: The neural correlates of dreaming. Nat. Neurosci. 20, 872–878 (2017). [CrossRef]
- Voss, U., Holzmann, R., Tuin, I., Hobson, A.J.: Lucid dreaming: a state of consciousness with features of both waking and non-lucid dreaming. Sleep 32, 1191–1200 (2009). [CrossRef]
- Kleim, B., Wysokowsky, J., Schmid, N., Seifritz, E., Rasch, B.: Effects of stress on memory formation and retrieval. Neurobiol. Learn. Mem. 98, 146–153 (2012). [CrossRef]
- Stetson, C., Fiesta, M.P., Eagleman, D.M.: Does time really slow down during a frightening event? PLoS One 2, e1295 (2007). [CrossRef]
- Mashour, G.A.: Cognitive unbinding: a neuroscientific paradigm of general anesthesia and related states of unconsciousness. Neurosci. Biobehav. Rev. 37, 2751–2759 (2013). [CrossRef]
- Franks, N.P.: General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 9, 370–386 (2008). [CrossRef]
- Barrett, F.S., Doss, M.K., Sepeda, N.D., Pekar, J.J., Griffiths, R.R.: Emotions and brain function are altered up to one month after a single high dose of psilocybin. Sci. Rep. 10, 2214 (2020). [CrossRef]
- Friston, K.J.: Transients, metastability, and neuronal dynamics. NeuroImage 5, 164–171 (1997). [CrossRef]
- Northoff, G., Qin, P., Nakao, T.: Rest-stimulus interaction in the brain: a review. Trends Neurosci. 33, 277–284 (2010). [CrossRef]
- Ford, J.M., Roach, B.J., Faustman, W.O., Mathalon, D.H.: Out-of-synch and out-of-sorts: dysfunction of motor-sensory communication in schizophrenia. Biol. Psychiatry 71, 807–815 (2012). [CrossRef]
- Whitfield-Gabrieli, S., Thermenos, H.W., Milanovic, S., Tsuang, M.T., Faraone, S.V., McCarley, R.W., Shenton, M.E., Green, A.I., Nieto-Castanon, A., LaViolette, P., Wojcik, J., Gabrieli, J.D.E., Seidman, L.J.: Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 106, 1279–1284 (2009). [CrossRef]
- Sierra, M., Berrios, G.E.: Depersonalization: neurobiological perspectives. Biol. Psychiatry 44, 898–908 (1998). [CrossRef]
- Simeon, D., Abugel, J.: Feeling Unreal: Depersonalization Disorder and the Loss of the Self. Oxford University Press, Oxford (2006).
- Barrett, L.F.: How Emotions are Made: The Secret Life of the Brain. Houghton Mifflin Harcourt, Boston, MA (2017).
- Frijda, N.H.: The Emotions. Cambridge University Press, Cambridge (1986).
- Davidson, R.J., Jackson, D.C., Kalin, N.H.: Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol. Bull. 126, 890–909 (2000). [CrossRef]
- Fredrickson, B.L.: The role of positive emotions in positive psychology: the broaden-and-build theory of positive emotions. Am. Psychol. 56, 218–226 (2001). [CrossRef]
- Grupe, D.W., Nitschke, J.B.: Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat. Rev. Neurosci. 14, 488–501 (2013). [CrossRef]
- Bartels, A., Zeki, S.: The neural basis of romantic love. NeuroReport 11, 3829–3834 (2000). [CrossRef]
- Zeki, S.: The neurobiology of love. FEBS Lett. 581, 2575–2579 (2007). [CrossRef]
- Rolls, E.T.: Emotion and Decision Making Explained. Oxford University Press, Oxford (2014).
- Tracey, I., Mantyh, P.W.: The cerebral signature for pain perception and its modulation. Neuron 55(3), 377–391 (2007). [CrossRef]
- Apkarian, A.V., Bushnell, M.C., Treede, R.D., Zubieta, J.K.: Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 9(4), 463–484 (2005). [CrossRef]
- Berthoud, H.R.: Multiple neural systems controlling food intake and body weight. Neurosci. Biobehav. Rev. 26(4), 393–428 (2002). [CrossRef]
- Zimmerman, C.A., Leib, D.E., Knight, Z.A.: Neural circuits underlying thirst and fluid homeostasis. Nat. Rev. Neurosci. 18(8), 459–469 (2017). [CrossRef]
- Proske, U., Gandevia, S.C.: The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 92(4), 1651–1697 (2012). [CrossRef]
- Abraira, V.E., Ginty, D.D.: The sensory neurons of touch. Neuron 79(4), 618–639 (2013). [CrossRef]
- Saunders, S., Barrett, J., Kent, A., Wallace, D.: Many Worlds? Everett, Quantum Theory, & Reality. Oxford University Press, Oxford (2010).
- Darwin, C.: On the Origin of Species by Means of Natural Selection. John Murray, London (1859).
- Deacon, T.W.: Incomplete Nature: How Mind Emerged from Matter. W. W. Norton & Company, New York (2012).
- Smith, J.M.: Evolution and the Theory of Games. Cambridge University Press, Cambridge (1982).
- Barlow, H.: Conditions for versatile learning, Helmholtz's unconscious inference, and the task of perception. Vis. Res. 30, 1561–1571 (1990). [CrossRef]
- Damasio, A.: The Feeling of what Happens: Body and Emotion in the Making of Consciousness. Harcourt, New York (1999).
- Tononi, G.: Consciousness and complexity. Science 282, 1846–1851 (1998). [CrossRef]
- Wolfram, S.: A New Kind of Science. Wolfram Media, Champaign, IL (2002).
- Einstein, A.: On the electrodynamics of moving bodies. Ann. Phys. 17, 891–921 (1905). [CrossRef]
- Einstein, A.: The foundation of the general theory of relativity. Ann. Phys. 49, 769–822 (1916). [CrossRef]
- Greene, B.: The Elegant Universe: Superstrings, Hidden Dimensions, and the Quest for the Ultimate Theory. W. W. Norton & Company, New York (1999).
- Kent, A.: Quantum jumps and relativistic quantum theory. Found. Phys. 40, 1629–1645 (2010). [CrossRef]
- Taylor, E.F., Wheeler, J.A.: Spacetime Physics: Introduction to Special Relativity (2nd ed.). W. H. Freeman, New York (1992).
- Carter, B.: Five- or six-step scenario for evolution? In: Seckbach, J. (ed.) Life as We Know It, pp. 231–241. Springer, Dordrecht (2008).
- Cleland, C.E.: The Quest for a Universal Theory of Life: Searching for life as we don’t know it. Cambridge University Press, Cambridge (2019).
- Davies, P.C.W.: The Fifth Miracle: The Search for the Origin of Life. Simon & Schuster, New York (1999).
- Lineweaver, C.H., Davis, T.M.: Does the rapid appearance of life on earth suggest that life is common in the universe? Astrobiology 2, 293–304 (2002). [CrossRef]
- Fermi, L.: Where is everybody? Phys. Today 13, 43–48 (1950).
- Webb, S.: If the Universe is Teeming with Aliens... Where is Everybody? Seventy-Five Solutions to the Fermi Paradox and the Problem of Extraterrestrial Life (3rd ed.). Springer, Switzerland (2022).
- Brin, G.D.: The great silence—The controversy concerning extraterrestrial intelligent life. Q. J. R. Astron. Soc. 24, 283–309 (1983).
- Davies, P.C.W.: The Eerie Silence: Renewing our search for alien intelligence. Houghton Mifflin Harcourt, Boston, MA (2011).
- Tarter, J.: The search for extraterrestrial intelligence (SETI). Annu. Rev. Astron. Astrophys. 39, 511–548 (2001). [CrossRef]
- Ward, P.D., Brownlee, D.: Rare Earth: Why Complex Life is Uncommon in the Universe. Copernicus, New York (2000).
- Bostrom, N.: Are we living in a computer simulation? Philos. Q. 53, 243–255 (2003). [CrossRef]
- Schneider, S.: Artificial You: AI and the Future of your Mind. Princeton University Press, Princeton (2019).
- Goertzel, B.: Artificial general intelligence: concept, state of the art, and future prospects. J. Artif. Gen. Intell. 5, 1–48 (2014). [CrossRef]
- Tegmark, M.: Our Mathematical Universe: My Quest for the Ultimate Nature of Reality. Knopf, New York (2014).
- Bostrom, N.: Superintelligence: Paths, Dangers, Strategies. Oxford University Press, Oxford (2014).
- Dawkins, R.: The Selfish Gene. Oxford University Press, Oxford (1976).
- Cirković, M.M.: Fermi's paradox - the last challenge for copernicanism? Serb. Astron. J., 1–20 (2009). [CrossRef]
- Nahin, P.J.: Time Machines: Time Travel in Physics, Metaphysics, and Science Fiction. Springer, New York (1999).
- Novikov, I.D.: Evolution of the Universe. Cambridge University Press, Cambridge (1992).
- Saunders, S.: Time, quantum mechanics, and probability. Synthese 114, 373–404 (1998). [CrossRef]
- Schrödinger, E.: Die gegenwärtige situation in der quantenmechanik. Naturwissenschaften 23, 807–812, 823–828, 844–849 (1935). [CrossRef]
- Deutsch, D.: Quantum mechanics near closed timelike lines. Phys. Rev. D 44, 3197–3217 (1991). [CrossRef]
- Earman, J., Smeenk, C., Wüthrich, C.: Do the laws of physics forbid the operation of time machines? Synthese 169, 91–124 (2009). [CrossRef]
- Albrecht, A., Sorbo, L.: Can the universe afford inflation? Phys. Rev. D 70, 063528 (2004). [CrossRef]
- Boltzmann, L.: Entgegnung auf die wärmetheoretischen Betrachtungen des Hrn. E. Zermelo. Ann. Phys. 293, 773–784 (1896). [CrossRef]
- Carroll, S.: The Big Picture: On the Origins of Life, Meaning, and the Universe Itself. Dutton, New York (2017). ISBN 978-1101984253.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





