1. Introduction
Aedes aegypti is the primary vector for multiple arboviruses transmitted to humans including dengue virus (DENV), yellow fever virus (YFV), Ross River virus (RRV), chikungunya virus (CHIKV), and Zika virus (ZIKV). With an estimated 300 million cases now being reported annually, DENV is the most prevalent and widespread arbovirus with half of the world population at risk [
1,
2,
3]. In 2019, reported incidents of DENV spread across 129 nations [
2]. Moreover, DENV is rapidly spreading to areas where it was previously eliminated due to exponential population growth rate and changes in climate conditions and urbanization, making new areas suitable habitats for mosquito development including parts of Europe [
4,
5]. Although vaccines exist for some arboviruses, yellow fever vaccine is the only one proven consistently effective. Therefore, vector control strategies to prevent mosquito-borne diseases remain crucial for mitigating disease transmission. Characterizing mosquitoes by age is one way to determine the efficacy of vector control strategies. Determining the age of mosquitoes facilitate our understanding of the capacity of mosquitoes to transmit pathogens [
6]. This is due to the lengthy period required by pathogens to develop inside the mosquito host. To be infectious, mosquitoes must first acquire pathogens from an infected person through blood feeding, support their period of development (the incubation period), and find a susceptible host to transmit the pathogen. For example, at 30°C and 32°C, DENV-2 takes 12 and 7 days, respectively to fully develop within mosquitoes [
7], while CHIKV takes approximately 2-7 days [
8]. Therefore, the estimation of mosquito age and their feeding patterns remain critical for evaluating the ability of vector control strategies to kill infectious or potentially infectious mosquito populations [
9]. However, although knowing the age of mosquitoes is crucial, determining whether a mosquito has previously had a blood meal and how many gonotrophic cycles it has undergone is a better indicator of vector competence of mosquitoes [
9]. This is because a mosquito can only transmit a pathogen on its second or subsequent feed, not the first. Despite the role mosquito blood feeding history plays in vector control, estimating the gonotrophic history of mosquitoes is a difficult undertaking. Traditionally, ovary dissection is used to assess gonotrophic histories of mosquitoes with the Detinova technique being used to identify whether a mosquito has laid eggs previously or not [
10] whereas the more advanced Polovodova’s technique is used to estimate the number of gonotrophic cycles of a mosquito [
11,
12]. While cost-effective, parity dissections require skilled personnel, dissections are technically demanding and time consuming for large sample sizes. These issues are compounded by the presence of varying number of dilatations per ovariole in females believed to have similar physiological age [
13] including observation of additional dilatations than the number of gonotrophic cycles a mosquito has undergone [
14].
The near-infrared spectroscopy (NIRS) technique coupled with machine learning algorithms has been demonstrated to have practical advantages over the parity dissections technique for characterizing entomological parameters. It involves shining a beam of non-destructive near-infrared light on mosquitoes where some of the light is absorbed and some is reflected. The
reflected light is characteristic of chemical and physiological changes happening inside the mosquito. NIRS is rapid, non-destructive, requires minimal sample preparation, and eliminates the need for reagents, allowing large scale analysis of mosquito samples over a short period of time. Only one spectral signature is collected, and that signature can be used to predict multiple parameters following development of training models. Several studies have demonstrated that NIRS can predict the age of major African malaria vectors,
Anopheles gambiae and
An. arabiensis as well as
Ae. aegypti and
Ae. albopictus, the primary and secondary vectors of arboviruses, respectively in both lab, semi field and field conditions [
15,
16,
17,
18,
19,
20]. NIRS has also been used to detect DENV, ZIKV, and CHIKV either in mono and in co-infections [
21,
22,
23] and
Wolbachia in
Ae. aegypti [
24],
Plasmodium in
An. gambiae [
25] and
Trypanosoma cruzi in Triatomine bug species [
26].
However, previous studies have primarily relied on one spectrometer, the Labspec 4i benchtop spectrometer (Malvern Panalytical, Malvern, United Kingdom), which operates across a wavelength of 350 to 2500 nm. Although the Labspec 4i is accurate for predicting the age, species and infection of mosquito, the cost for buying a spectrometer limits its application particularly in resource limited settings. As more affordable, portable, handheld and smartphone operated spectrometers become available on the market, they need to be assessed for predicting entomological parameters alongside the Labspec 4i spectrometer.
This study compared the accuracy of the Labspec 4i spectrometer with with a portable, relatively affordable spectrometer known as NIRvascan spectrometer (Allied Scientific Pro, Canada) with a wavelength of 900-1700nm for predicting the age and parity of Ae. aegypti mosquitoes in the laboratory. We also determined the cost, scanning time, and operational complexity of both spectrometers.
2. Materials and Methods
2.1. Mosquito Rearing, Feeding and Sampling
Female
Ae. aegypti mosquitoes were reared at standard insectary conditions, i.e., at 27°C, relative humidity of 70-80% and photoperiod of 12:12 light cycle. Three different cohorts with approximately 2500
Ae. aegypti eggs each were flooded in trays of water at different time points. Approximately 500 larvae were reared per tray and fed with 15 pellets of fish food (Kyorin Food industries, Kyorin, Japan). Approximately 800 pupae were transferred into 4 cages (200 pupae/cage) for emergence. Two of the cages were set aside for sugar feeding and 2 were used exclusively for blood feeding experiments. All adult mosquitoes were provided access to a 10% sugar solution ad
libitum. Prior to blood feeding, female mosquitoes at 5 and 12 days old were starved for 24 hours. Mosquitoes were blood-fed with human blood following the ethics protocol approved by the University of Queensland (Approval number 2020001077). Three different treatment groups of mosquitoes were collected for scanning as follows; Control (unfed), 1, 10, and 17 days old; mosquitoes blood-fed once at 5 days old, then collected at 10 and 17 days old; mosquitoes blood-fed at 5 and 12 days old and collected at 17 days old. Unfed mosquitoes were separated from fed mosquitoes and added to the cage that was not blood-fed. After collection, mosquitoes were stored at 4°C between 5-18 days prior to scanning.
Table 1 shows a summary of the number of mosquitoes sampled per treatment for the three cohorts.
2.2. Mosquito Scanning Using Labspec 4i
To assess the performance of Labspec 4i and NIRvascan spectrometers, each mosquito was scanned first with the Labspec 4i then with the NIRvascan. Scanning with Labspec 4i followed the protocol described by Mayagaya and colleagues [
16]. Briefly, the Labspec 4i spectrometer was connected to a computer and an external fibre optic probe with 6 illumination fibres was attached (Malvern Panalytical). Prior to scanning, the fibre optic probe was positioned 2 mm above the mosquito head and thorax, providing adequate space between the mosquito and the probe. The spectrometer was calibrated (optimized and white-referenced) using a clean spectralon panel and thereafter recalibrated every half an hour during the scanning session. Following calibration, each mosquito was placed on its right side up on the spectralon panel for scanning (
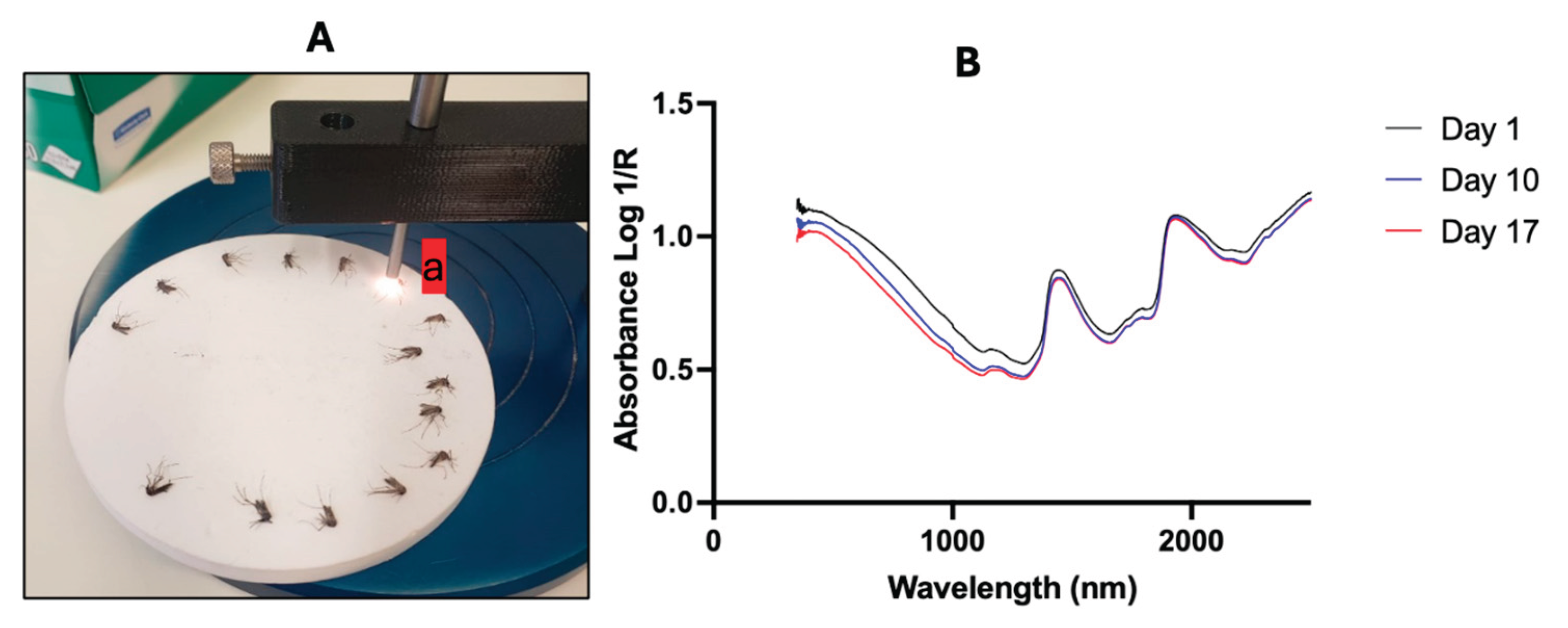
Figure 1). Spectral signatures were collected by pressing the space bar on the laptop connected to the LabSpec.
Figure 1.
Positioning of mosquitoes on a white spectralon plate and pointing the light down to the mosquitoes using an external probe (a) connected to a labspec 4i spectrometer. Panel B shows the average raw spectra of day 1, 10 and 17 days old mosquitoes.
Figure 1.
Positioning of mosquitoes on a white spectralon plate and pointing the light down to the mosquitoes using an external probe (a) connected to a labspec 4i spectrometer. Panel B shows the average raw spectra of day 1, 10 and 17 days old mosquitoes.
2.3. Mosquito Scanning Using NIRvascan
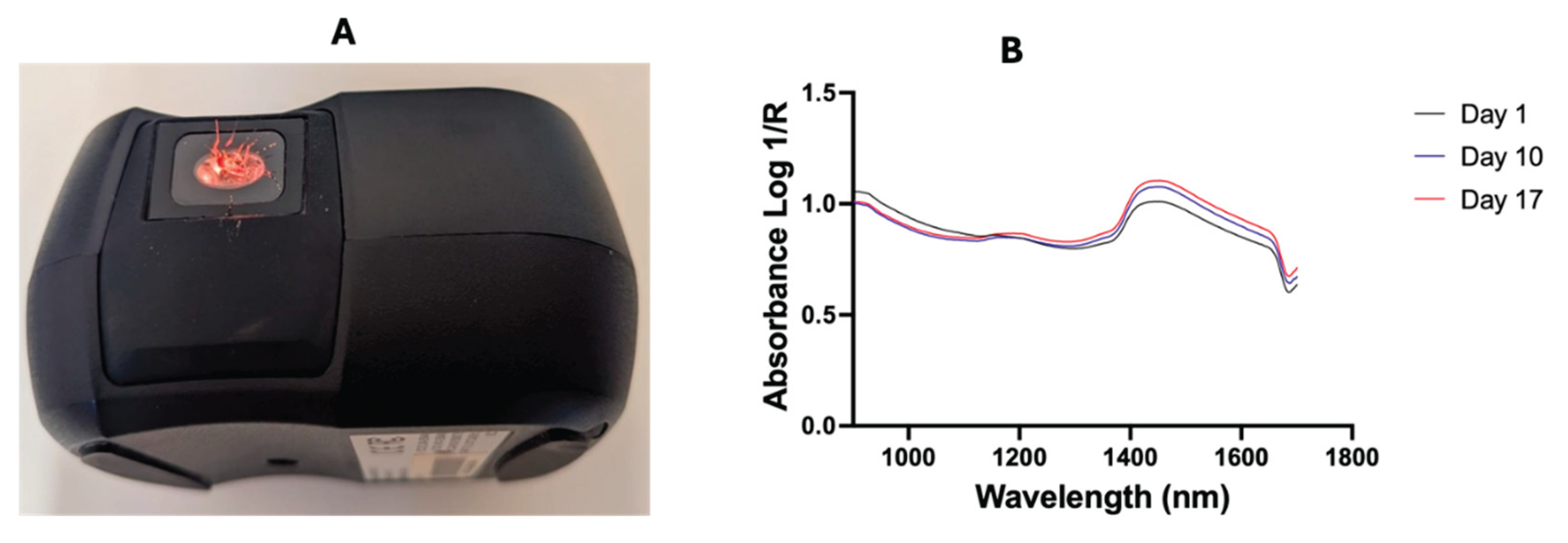
Prior to scanning, NIRvascan spectrometer was connected to a smartphone via Bluetooth using the ISC NIRScan app (InnoSpectra Corporation, Singapore). A whole mosquito was placed on its right side up on the detector, allowing the whole mosquito to be scanned from underneath the light (
Figure 2). Unlike Labspec 4i where light is shone on mosquitoes from above, mosquitoes are scanned using NIRvascan with light from below (
Figure 2).
Figure 2 shows that the average raw spectra of 1-, 10- and 17-days mosquitoes are inverse to the average raw spectra of spectra collected by the Labspec 4i spectrometer due to differences in scanning methods used.
Figure 1.
Positioning of mosquitoes on the detector of a NIRvascan and scanning mosquiteos from underneath using an internal light source. Panel B shows the average raw spectra of day 1, 10 and 17 days old mosquitoes.
Figure 1.
Positioning of mosquitoes on the detector of a NIRvascan and scanning mosquiteos from underneath using an internal light source. Panel B shows the average raw spectra of day 1, 10 and 17 days old mosquitoes.
2.4. Data Analysis
JMP Pro (SAS Institute Inc., North Carolina, US) was used to train machine learning algorithms for predicting mosquito age and blood-feeding status for both spectrometers. Separate models were developed for spectral data collected using Labspec 4i and the NIRvascan spectrometers. Artificial neural networks (ANN) and K-fold (K=5) cross validation were used to analyze raw spectral data with wavelength being treated as an independent factor. Mosquito age and blood feeding status were treated as dependent factors in the model creating two separate models for predicting the age and blood feeding history for the two datasets. The wavelength that resulted in the most accurate results for age grading and blood feeding history prediction for each spectrometer is shown in
Table 2. In summary age grading and blood feeding history prediction models were developed using mosquitoes from one cohort but representative samples from the remaining two cohorts were added to improve the robustness of the model (
Table 2). For age prediction models, mosquitoes were assigned a value that represented their actual age. Similarly, to predict blood feeding status a value of “0” was assigned to the spectra of mosquitoes that were unfed (Control), and a value of “1” was assigned to the spectra of mosquitoes that were blood fed once and a value of “2” for mosquitoes that blood fed twice. The resulting ANN model was then applied to a pre-validation set of mosquitoes that were included in the model but were not used in training the model and to a validation set of mosquitoes from other cohorts as shown in
Table 2. The accuracy of the models for each spectrometer developed to predict the age and blood meal history was tested on mosquitoes at 1, 10, and 17 days old that were either blood fed or unfed. ANOVA was used to compare the difference between age and blood meal predictive accuracies of the two spectrometers.
3. Results
3.1. Prediction Within the Training Set
Following prediction, results were grouped into two groups for age (< or ≥ 10 days old) representing young mosquitoes (1day old) and old and potentially infectious mosquitoes ≥10 days old mosquitoes. The most accurate training algorithm for predicting the age of mosquitoes that were scanned with the Labspec 4i and NIRvascan spectrometers was 92.9% and 91.3%, respectively, with 10 days old mosquitoes less accurately predicted by both devices. We used the 10-days as a cut off point for age prediction hence it was expected that this age group would be the least accurately predicted. The accuracy for predicting very young mosquitoes (1 day old) and 17 days old mosquitoes was 100% for both spectrometers.
In terms of blood feeding, the training model using data collected by NIRvascan was generally more accurate (97%) than the model developed using Labspec 4i data (94%). NIRvascan was 97.8% accurate for predicting older mosquitoes in the 17-18 days age group as having previously received or not received a blood meal, whereas Labspec 4i predicted mosquitoes in the same age group as having received or not received a blood meal with 86% accuracy.
Table 3.
Results from the training set indicating the performance of the two spectrometers for predicting the age and blood meal history for samples that were used in the training set only.
Table 3.
Results from the training set indicating the performance of the two spectrometers for predicting the age and blood meal history for samples that were used in the training set only.
| Age in days |
Total per age group [N] |
Feeding condition |
Total per feeding condition [N] |
|
| Labspec 4i [N] |
NIRvascan[N] |
|
Labspec 4i[N] |
NIRvascan[N] |
| 1d (< 10d) |
100 [40] |
100 [62] |
Unfed (0) |
96.3 [134] |
96.8 [158] |
| 10d (≥10d) |
81.3 [80] |
69.5 [82] |
Fed Once* |
97.4 [77] |
98.2 [111] |
| 17d (≥10d) |
100 [90] |
100 [142] |
Fed Twice* |
86 [57] |
97.8 [47] |
| Average |
92.9 [210] |
91.3 [286] |
Average |
94.4 [268] |
97.4 [316] |
3.2. Validation Set
3.2.1. Prediction of Age
Generally, age prediction results for the validation set were consistent with the results from the training set. Labspec 4i predicted the age of mosquitoes with an overall accuracy of 94% (N=366) while the NIRvascan predicted the age of mosquitoes with an overall accuracy of 90% (N=290). Generally, Labspec 4i performance was not any better in predicting 10–11 days and 17–18 days age groups where accuracies of 87.3% and 97.3%, respectively were achieved, compared to NIRvascan’s 83.6% and 91.8% for the same age groups. Both spectrometers predicted the average age of 10- and 17-days mosquitoes as 13 and 14 days old, respectively and this prediction was not significantly different with two-way ANOVA for mosquitoes that were 10 days old (P=0.5871) or 17 days old (P=0.5586). For mosquitoes within the 1–2 days age group, NIRvascan outperformed Labspec 4i, achieving 100% accuracy compared to Labspec’s 96.3% accuracy. The mean predicted age of 1-day old mosquitoes was 4 days when Labspec 4i was used and 0.7 days old when NIRvascan was used and this prediction was significant between the two groups (P<0.00001). Generally, both spectrometers predicted the age of younger and older mosquitoes more accurately than they did for 10 days old mosquitoes. This is due to the fact that 10 days was selected as a cut off for correct or incorrect prediction for both spectrometers.
Table 4.
Age prediction accuracy for Labspec 4i 4 and NIRvascan for mosquitoes that were used to validate the models.
Table 4.
Age prediction accuracy for Labspec 4i 4 and NIRvascan for mosquitoes that were used to validate the models.
| Age |
Labspec 4i |
NIRvascan |
| < or ≥ 10 days age group |
Average predicted age in days [N] |
< or ≥ 10 days
Age group |
Average predicted age in days |
| 1 d |
96.3 [81] |
4.0 [81] |
100 [58] |
0.7 [58] |
| 10 d |
87.3 [118] |
13.3 [118] |
83.6 [116] |
13.6 [116] |
| 17d |
97.6 [167] |
14.5 [167] |
91.8 [116] |
14.6 [116] |
| Average |
94 [366] |
11.4 [366>] |
90 [290] |
11.8 [290] |
3.2.2. Prediction of Blood-Meal History
A prediction was considered correct if an unfed mosquito was classified as unfed or if a blood-fed mosquito (whether fed once or twice) was classified as blood-fed. Overall, the Labspec 4i predicted feeding history of mosquitoes with an overall accuracy of 82.8% (n=308) compared to NIRvascan’s 71.3% (n=300). Regardless of the mosquito age, the Labspec 4i spectrometer predicted the feeding status of unfed (0), fed once (1), and fed twice (2) groups into the fed and unfed groups with an accuracy of 77.9%, 88.5%, and 95.8%, respectively, compared to NIRvascan’s 72.4%, 70.5%, and 68.9%, for the same age groups. Regardless of the age of the mosquito analyzed, Labspec 4i was more accurate in predicting whether a mosquito had received a blood meal than when it was unfed. Comparatively, NIRvascan was more accurate in predicting unfed mosquitoes than those previously fed. However, both spectrometers were not able to predict more than one feeding cycle of mosquitoes (
Table 5).
3.3. Effect of Blood Meal on Age Prediction by Both Spectrometers
We determined using ANOVA the effect of blood feeding on age prediction of mosquitoes (
Table 6). Mosquitoes that received a blood meal and laid eggs were generally predicted slightly more accurately by both spectrometers than those that did not blood feed. There was a difference between the mean predicted age of mosquitoes that received a blood meal from those that did not receive a blood meal for mosquitoes that were 10d old (P=0.049) but not for mosquitoes that were 17 days old (P=0.678) for NIRvascan. For the Labspec 4i, blood fed mosquitoes at 10 days were predicted as 12.3 days old compared to the unfed ones which were predicted as 14.3 days old. This mean predicted age was significantly different (P<0.001) whereas blood fed mosquitoes that were 17 days old were predicted as 14.1days old and this prediction significantly differed from that of unfed mosquitoes at the same age group (P=0.005). This indicates the physiological changes that within blood fed and unfed mosquitoes have an overall effect on the ageing process of the mosquito ultimately affecting their age prediction.
3.4. Comparative Analysis of Labspec 4i and NIRvascan in Terms of Time, Cost and Operational Complexity
Lastly, we compared the Labspec 4i and NIRvascan spectrometers in terms of outlay cost, spectral range, resolution and average sampling time.
Table 5 provides a summarised overview of these comparisons
Table 7.
Comparative analysis of the Labspec 4i and NIRvascan spectrometers in terms of time, cost and operation.
Table 7.
Comparative analysis of the Labspec 4i and NIRvascan spectrometers in terms of time, cost and operation.
| Feature |
NIRvascan* |
Labspec 4i |
| General configuration |
 |
 |
| Size |
82.2 x 66 x 45 mm, highly portable, lightweight (136g) |
127 x 356 x 292 mm, Portable but larger than NIRvascan (5600g) |
| Spectral range |
900 – 1700 nm |
350 – 2500 nm |
| Resolution |
10 nm |
- 3 @ 700nm (Visible)
- 10 @ 1400nm (SWIR1)
- 10 @ 2100nm (SWIR2) |
| Current applications |
- Agricultural monitoring
- Food quality inspection
- Pharmaceutical analysis
- Recycling and material identification |
- Mineral identification
- Environmental analysis
- Biological and agricultural research
- Mosquito analysis |
| Average sampling time |
30-45sec/sample |
5-10sec/per sample |
| Average training time |
10 mins |
30 mins |
| Cost |
USD 2,695 |
USD 60,000 |
| Advantages |
- Easy to use
- Portable
- Ideal for in-field, rapid analysis
- More affordable
- Can be operated with a smartphone |
- Broad spectral range
- High signal to noise ratio
- Versatile analysis modes
- Sample scanning and prediction can be automated |
| Limitations |
- Limited spectral range
- Low signal to noise ratio
- scanning and prediction cannot be automated |
- Less portable
- Costly
-Requires a laptop computer to operate |
4. Discussion
Entomological surveillance is a crucial undertaking in vector control programs as it determines the efficacy of existing interventions. Determining mosquito age structure is one way to assess how well interventions are performing. Traditionally, this is achieved through parity dissections, a time-consuming and unlikely scalable way for large programs. Other techniques such as the assessment of the abundance of transcriptional profiles [
27] and changes in cuticular hydrocarbons [
28] were developed in the last decade. However, due to their operational complexity and high operational costs, their uptake has been slow. NIRS technique has shown promise for age grading both
Anopheles and
Aedes mosquitoes in the lab [
16], semi-field system [
17] and in the field [
29,
30] using the Labspec 4i. However, affordable spectrometers that have been developed recently are yet to be assessed for this application. This study compared the performance of a portable handheld spectrometer known as NIRvascan and a benchtop spectrometer known as Labsepc 4i for analyzing lab reared
Ae. aegypti mosquitoes to determine their chronological age and blood feeding history. To our knowledge this is the first study to demonstrate that a handheld NIR instrument could be a viable alternative to the traditional Labspec 4i spectrometer for rapid characterization of mosquito traits to improve entomological surveillance.
The chronological status of mosquitoes was defined using an age threshold of 10 days representing mosquitoes that are less likely to be infectious (< 10 days) and more likely to be infectious (≥ 10 days old), with predictions classified as either younger than 10 days or ≥10 days. In a past study, the Labspec 4i spectrometer accurately differentiated
Ae. aegypti and
Ae. albopictus into two age categories with a predictive accuracy of 91% for
Ae. aegypti [
20] and 94% for
Ae. albopictus [
19]. These predictive accuracies are consistent with the accuracy of 94% and 91% achieved by the Labspec 4i and NIRvascan, respectively, under this study. Both spectrometers predicted the age of younger (1 day old) and older (17 days old) mosquitoes more accurately than the middle age group (10 days old) and this was also consistent with the previous studies [
19,
20]. NIRvascan predicted the age of 1 day old mosquitoes more accurately than the Labspec 4i. Day 1 old mosquitoes were predicted on average as 0.7 days compared to the prediction of 4 days achieved by the Labspec 4i. The wavelength that was used to predict the age of mosquitoes was within 1050-2350nm for the Labspec 4i and 950-1650nm for the NIRvascan. The high age prediction accuracy achieved by both spectrometers indicate age related changes are predominantly happening within the 900-1650nm and that the additional wavelengths offered by the Labspec 4i have very little effect on the overall prediction of chronological age prediction of
Ae. aegypti mosquitoes. Importantly, NIR is comprised of repeated overtones and combinational bands which means some of the information within the combination region and 1st overtone (1800-2350nm) of the Labspec 4i spectrometer is present within the 2nd (11-1600nm) or 3rd overtone (900-1400) of the NIRvascan spectrometer. This is good news as it means, more affordable handheld instruments can be used as an alternative to the Labspec 4i for age prediction.
For physiological age prediction, the Labspec 4i spectrometer generally performed better than the NIRvascan with 82% (N=302) of mosquitoes correctly predicted as either having never blood fed or received at least one blood meal. Additionally, 96% (N=48) of mosquitoes that received more than one blood meal were correctly predicted in comparison with mosquitoes that fed only once (88.5%, N=68). Comparatively, NIRvascan differentiated unfed from fed mosquitoes with 70% (N=300) with the unfed, fed once and fed twice groups predicted with a similar accuracy. The wavelength that was used to predict blood feeding history of mosquitoes was within 500-2350nm for the Labspec 4i and 950-1650nm for the NIRvascan. It is plausible that hematophagy induces significant structural and biochemical alterations in mosquitoes, manifesting as detectable spectral signatures within the visible range (500–750 nm) and the 1800–2350 nm region. The absence of coverage in these wavelengths by the NIRvascan system may account for its diminished accuracy in detecting such physiological changes. Nonetheless, if used in the field the 70% accuracy achieved by the NIRvascan would be sufficient to estimate changes in population age structure and the degree of exposure of mosquitoes to hosts.
In addition to assessing their predictive accuracy for these two entomological indicators, we also conducted a comparative analysis of both spectrometers in terms of their portability, cost, resolution and sampling time with the aim of evaluating the feasibility of using NIRvascan as a field-deployable alternative to the Labspec 4i spectrometer. The Labspec 4i spectrometer has a wavelength ranging from visible light region (350 to 700nm) compared to NIRvascan’s wavelength of 900 to 1700 nm with a 3 nm resolution in the visible spectrum and 10 nm in the SWIR filters. The signal to noise ratio for the Labspec 4i is much higher (1 nm) compared to NIRvascan’s 10 nm resolution. In terms of the sampling speed and depending on the operator’s experience, Labspec 4i was 5 times faster than NIRvascan. It took 5-15 seconds on average to scan a sample with the Labspec 4i spectrometer and 30sec-1min with the NIRvascan. NIRvascan’s speed is largely due to logistics involved in positioning samples on the detector, and acquiring a spectrum through a mobile phone connected to the spectrometer via Bluetooth. Comparatively, for the Labspec 4i 15-20 samples can be pre-positioned on a spectralon plate directly under an external probe (
Figure 1) followed by 5 sec spectral acquisition using the space bar on a computer. Despite these limitations, NIRvascan offers attractive advantages, including being highly portable as it only weighs 136g (roughly the weight of a smartphone), compared to Labspec 4i, which weighs 5.6kg (excluding the laptop, 1-metre-long probe, a spectralon plate/platform and charging cables). It took 10 mins to train students to use NIRvascan and half an hr to use the Labspec 4i. Perhaps, the most significant advantage of NIRvascan is its cost, it is approximately 23 times (USD3000) cheaper than the Labspec 4i (USD60000).
Our study is subject to several limitations. First, our findings are derived exclusively from a laboratory-reared colony of Ae. aegypti, rather than field-collected populations, which may exhibit divergent physiological and behavioral traits. Similarly, experimental conditions were highly controlled which restricts the future application of the tool to the conditions tested in this study and limits applications in natural field environments. Consequently, extrapolating our lab-based observations to field scenarios should be approached with caution, as direct correlations may not hold. Additionally, the comparative analysis between the NIRvascan and Labspec 4i spectrometers was constrained by their non-overlapping spectral ranges, restricting direct comparisons to shared absorption features. Nevertheless, our evaluation extended beyond mere trait detection, encompassing a comprehensive assessment of multiple spectroscopic attributes to ensure a robust analytical framework.
5. Conclusions
In summary, both spectrometers predicted the age of lab reared mosquitoes into the two groups with a similar accuracy and although a higher predictive accuracy was achieved by the Labspec 4i for predicting blood feeding history, the predictive accuracy achieved by NIRvascan is sufficient for determining changes occurring at a population level if such findings were replicated in the field. We therefore recommend further assessments to evaluate the performance of the NIRvascan spectrometer in the field for physiological age prediction validated with the traditional parity dissections. We conclude that the use of NIRvascan for age prediction of Ae. aegypti is consistent with accuracies of Labspec 4i thereby extending the range of NIR spectrometers available for use for entomological surveillance.
Author Contributions
Study conceptualization: MTS-L, RMF; Conducted experiments: AT, EA, TK; conducted analysis: AT, EA, MTS-L; Drafting-original draft manuscript; AT, EA, Writing-review and editing: MTS-L and RMF, Resources; MTS-L, Supervision; TK, MTS-L
Funding
Partial financial support was received from the University of Queensland to support AT and EA in their postgraduate research program
Data Availability Statement
Dataset is available on request. The raw data supporting the conclusions of this article will be made available by the authors on request
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhatt, S., et al., The global distribution and burden of dengue. Nature, 2013. 496(7446): p. 504-7.
- Hasan, S., et al., Dengue virus: A global human threat: Review of literature. Journal of international society of preventive and community dentistry, 2016. 6(1): p. 1-6.
- Guzman, M.G., et al., Dengue: a continuing global threat. Nature reviews microbiology, 2010. 8(Suppl 12): p. S7-S16.
- Brem, J., et al., Dengue “homegrown” in Europe (2022 to 2023). New Microbes and New Infections, 2023. 56: p. 101205.
- Buchs, A., et al. The threat of dengue in Europe. New Microbes and New Infections 2022, 49, 101061. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G. 1957: London: Oxford University Press.
- Watts, D.M., et al., Effect of temperature on the vector efficiency of Ae. aegypti for dengue 2 virus. 1986.
- Dubrulle, M., et al. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PloS one 2009, 4, e5895. [Google Scholar] [CrossRef] [PubMed]
- Garrett jones, C. and G.R. Shidrawi, Malaria Vectorial Capacity of a Population of Anopheles Gambiae - an Exercise in Epidemiological Entomology. Bulletin of the World Health Organization, 1969. 40(4): p. 531-+.
- Detinova, T. , Age-grouping methods in Diptera of medical importance, with special reference to some vectors of malaria. Monogr Ser World Health Organ, 1962. 47: p. 13 - 191.
- Polovodova, V. , Age changes in ovaries of Anopheles and methods of determination of age composition in mosquito population. Med Parazit (Mosk), 1941. 10: p. 387.
- Polovodova, V.P. , The determination of the physiological age of female Anopheles by number of gonotrophic cycles completed. Med Parazitol Parazitar Bolezni, 1949. 18: p. 352-355.
- Kay, B. , Age structure of populations of Culex annulirostris (Diptera: Culicidae) at Kowanyama and Charleville, Queensland. Journal of Medical Entomology, 1979. 16(4): p. 309-316.
- Bellamy, R.E. and P.S. Corbet, Occurrence of ovariolar dilatations in nulliparous mosquitoes. 1974.
- Lambert, B. , et al., Monitoring the Age of Mosquito Populations Using Near-Infrared Spectroscopy. Sci Rep, 2018. 8(1): p. 5274.
- Mayagaya, V. , et al., Non-destructive determination of age and species of Anopheles gambiae s.l. using Near-infrared spectroscopy. Am J Trop Med Hyg, 2009. 81: p. 622 - 630.
- Sikulu, M. , et al., Near-infrared spectroscopy as a complementary age grading and species identification tool for African malaria vectors. Parasit Vectors, 2010. 3: p. 49.
- Sikulu, M.T. , et al., Using a Near-Infrared Spectrometer to Estimate the Age of Anopheles Mosquitoes Exposed to Pyrethroids. PLoS ONE, 2014. 9(3): p. e90657.
- Sikulu-Lord, M.T. , et al., First report on the application of near-infrared spectroscopy to predict the age of Aedes albopictus Skuse. Scientific Reports, 2018. 8(1): p. 9590.
- Sikulu-Lord, M.T. , et al., Near-infrared spectroscopy, a rapid method for predicting the age of male and female wild-type and Wolbachia infected Ae. aegypti. PLoS Negl Trop Dis, 2016. 10(10): p. e0005040.
- Fernandes, J.N. , et al., Rapid, noninvasive detection of Zika virus in Ae. aegypti mosquitoes by near-infrared spectroscopy. Science advances, 2018. 4(5): p. eaat0496.
- Garcia, G. , et al., First report of rapid, non-invasive, and reagent-free detection of malaria through the skin of patients with a beam of infrared light. 2022.
- Santos, L. , et al., High throughput estimates of Wolbachia, Zika and chikungunya infection in Ae. aegypti by near-infrared spectroscopy to improve arbovirus surveillance. Communications biology, 2021. 4(1): p. 1-9.
- Sikulu-Lord, M.T., et al. Rapid and Non-destructive Detection and Identification of Two Strains of Wolbachia Ae. aegypti by Near-Infrared Spectroscopy. PLoS Negl Trop Dis 2016, 10, e0004759. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.F. , et al., Detection of Plasmodium falciparum infected Anopheles gambiae using near-infrared spectroscopy. Malaria journal, 2019. 18(1): p. 85.
- Tátila-Ferreira, A. , et al., Near infrared spectroscopy accurately detects Trypanosoma cruzi non-destructively in midguts, rectum and excreta samples of Triatoma infestans. Scientific reports, 2021. 11(1): p. 1-10.
- Cook, P.E. , et al., The use of transcriptional profiles to predict adult mosquito age under field conditions. Proc Nat Acad Sci, 2006. 103(48): p. 18060-18065.
- Hugo, L.E. , et al., Investigation of cuticular hydrocarbons for determining the age and survivorship of Australian mosquitoes. Am J Trop Med Hyg, 2006. 74(3): p. 462-474.
- Milali, M.P. , et al., An autoencoder and artificial neural network-based method to estimate parity status of wild mosquitoes from near-infrared spectra. PLoS One, 2020. 15(6): p. e0234557.
- Joy, T. , et al., Assessing near-infrared spectroscopy (NIRS) for evaluation of Ae. aegypti population age structure. Insects, 2022. 13(4): p. 360.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).