Submitted:
18 August 2025
Posted:
21 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
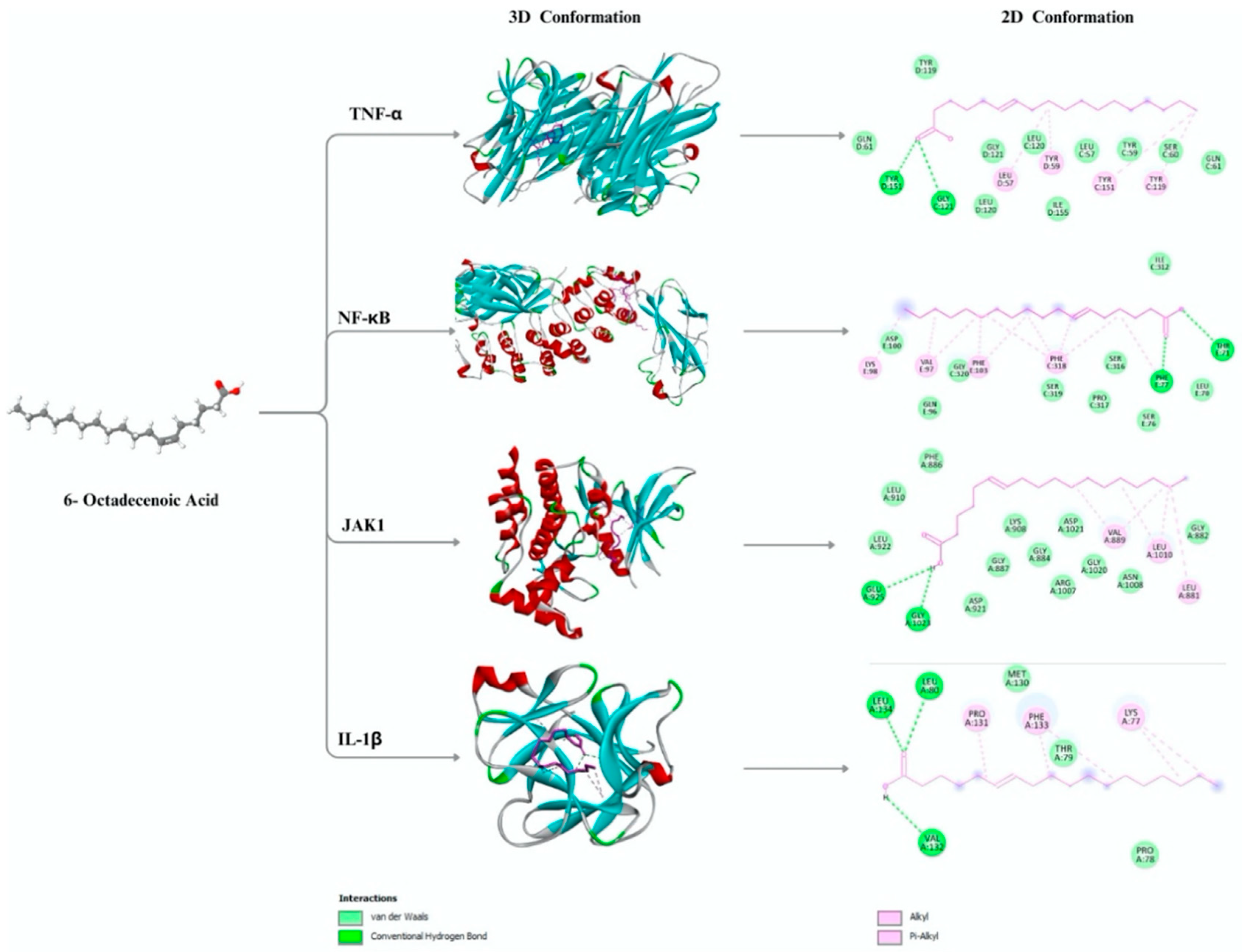
2.1. In Silico Binding Profile of (6Z)-Octadecenoic Acid Against Key Inflammatory Targets
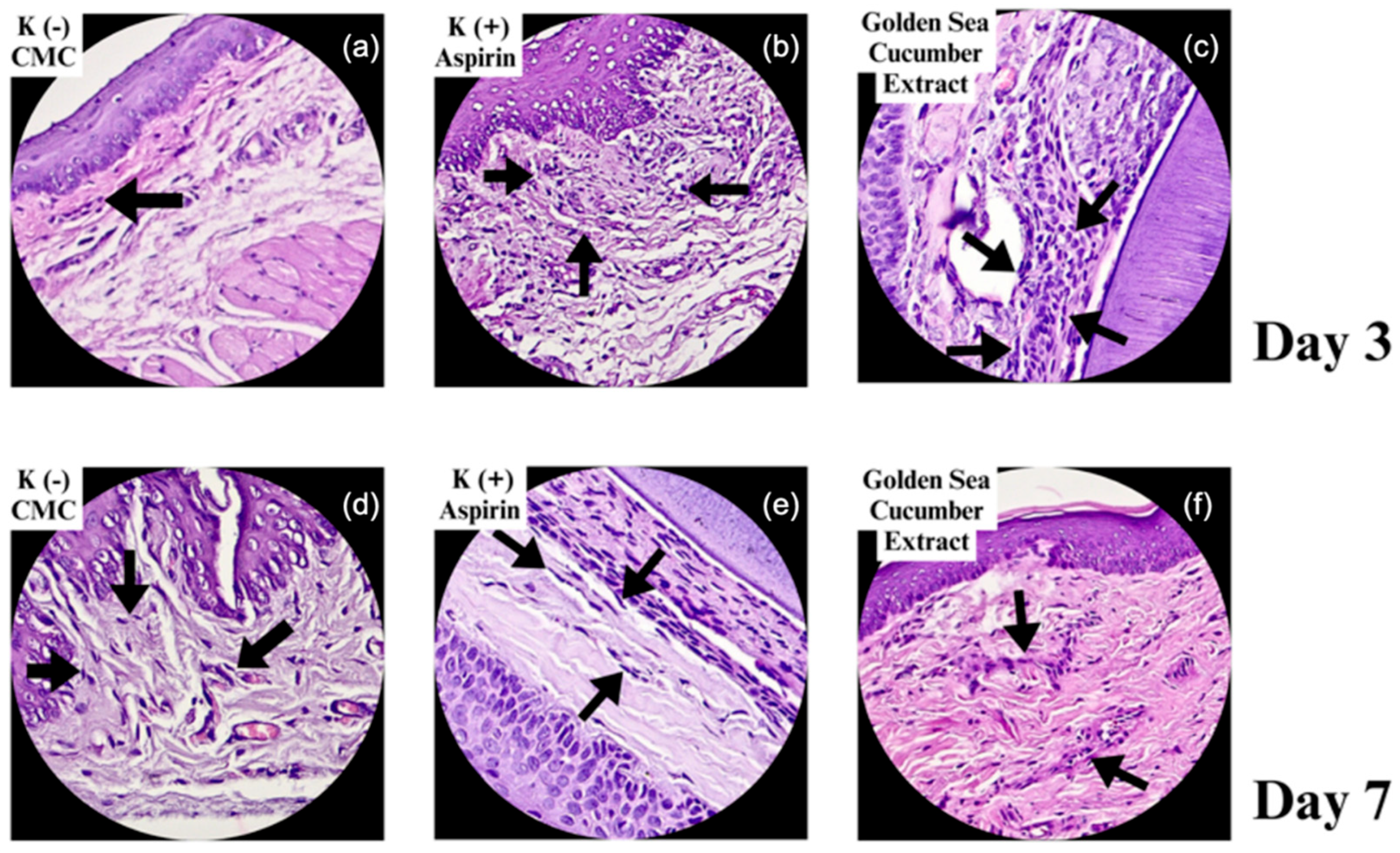
2.2. Histological Features of Fibroblast Proliferation in Gingival Wound Healing
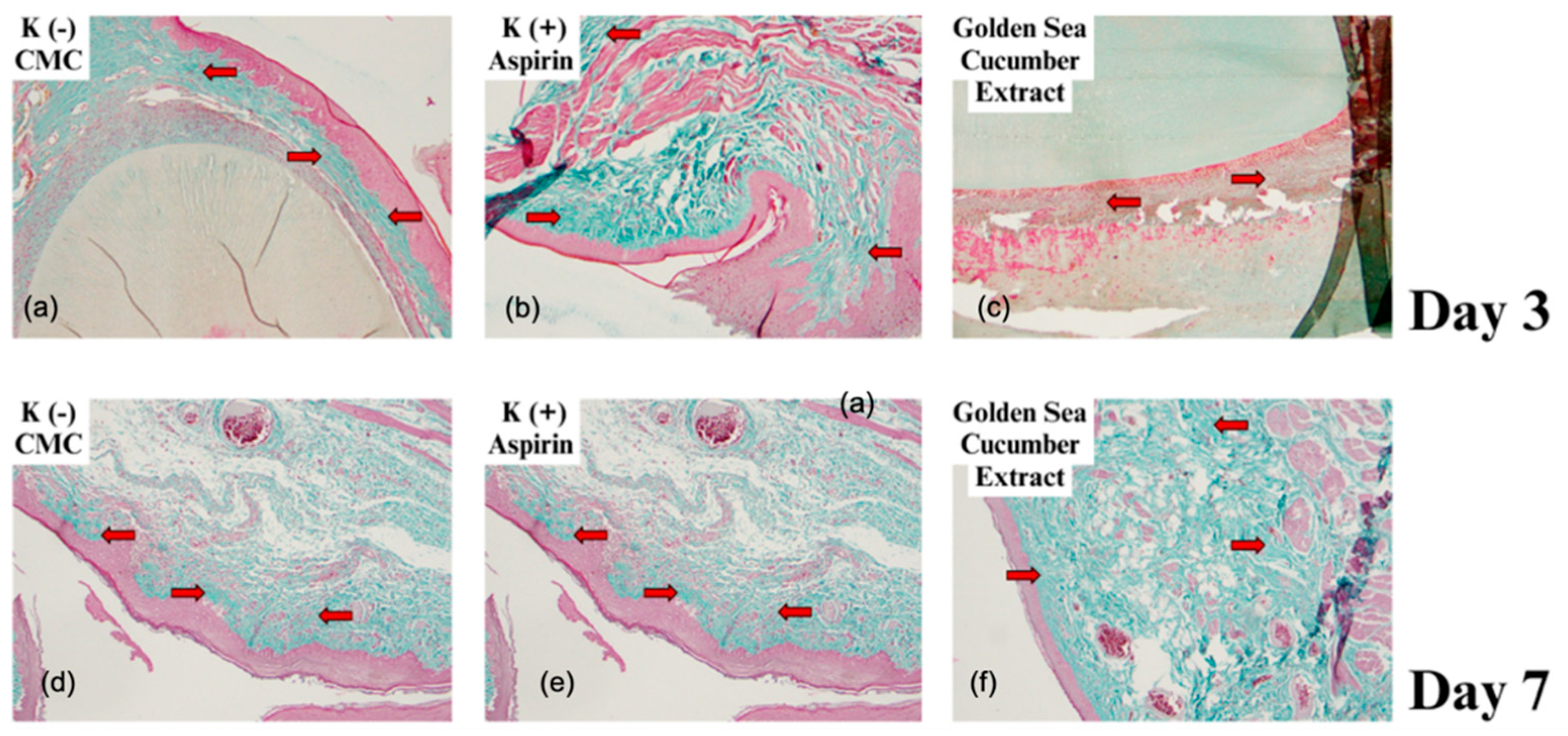
2.3. Collagen Fiber Visualization Using Masson's Trichrome Staining
2.4. Quantitative Evaluation of Fibroblast Density and Collagen Fiber Thickness
2.5. ADME-Tox Profiling of (6Z)-Octadecenoic Acid
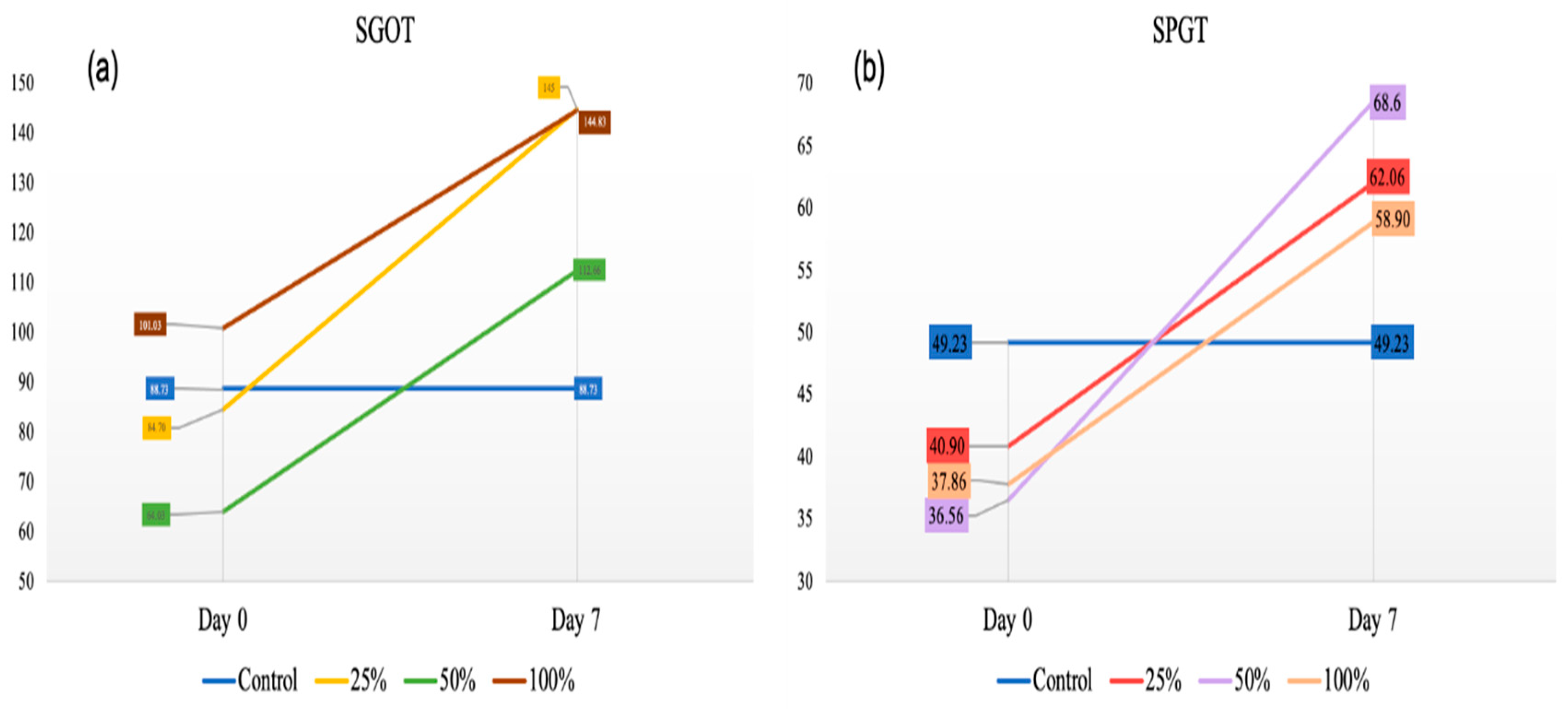
2.6. Serum Liver Enzyme Levels: SGOT and SGPT
3. Discussion
Limitation Study
4. Materials and Methods
4.1. In-Silico Studies
4.1.1. Quantitative Structure-Activity Relationship Analysis
4.1.2. Pharmacokinetics and Toxicity Prediction
4.2. Preparation of Stichopus hermanii Extract
4.2.1. Preparation Controlled Variables
4.3. Preparation of Animal Studies (Rattus Norvegicus)
4.3.1. Blood Collection for Toxicity Testing
4.3.2. Fibroblast Test and Collagen Test
4.3.3. Tissue Collection and Histological Examination
4.4. Ethical Clearance
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J Dent Res 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, G. Stages of wound healing and their clinical relevance. Vet Clin North Am Small Anim Pract 2006, 36, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.C.; Cáceres, M.; Martínez, C.; Oyarzún, A.; Martínez, J. Gingival wound healing: an essential response disturbed by aging? J Dent Res 2015, 94, 395–402. [Google Scholar] [CrossRef]
- Kaner, D.; Soudan, M.; Zhao, H.; Gaßmann, G.; Schönhauser, A.; Friedmann, A. Early Healing Events after Periodontal Surgery: Observations on Soft Tissue Healing, Microcirculation, and Wound Fluid Cytokine Levels. International Journal of Molecular Sciences 2017, 18, 283. [Google Scholar] [CrossRef]
- Visentin, D.; Gobin, I.; Maglica, Ž. Periodontal Pathogens and Their Links to Neuroinflammation and Neurodegeneration. Microorganisms 2023, 11. [Google Scholar] [CrossRef]
- Cavaillon, J.M.; Adib-Conquy, M. The Pro-Inflammatory Cytokine Cascade. In Immune Response in the Critically Ill; Marshall, J.C., Cohen, J., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2002; pp. 37–66. [Google Scholar]
- Esche, C.; Stellato, C.; Beck, L.A. Chemokines: Key Players in Innate and Adaptive Immunity. Journal of Investigative Dermatology 2005, 125, 615–628. [Google Scholar] [CrossRef]
- Wautier, J.L.; Wautier, M.P. Pro- and Anti-Inflammatory Prostaglandins and Cytokines in Humans: A Mini Review. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Galvão, I.; Sugimoto, M.; Vago, J.P.; Gomes Machado, M.; Sousa, L. Mediators of Inflammation. 2018; 3–32. [Google Scholar]
- Batool, F.; Özçelik, H.; Stutz, C.; Gegout, P.Y.; Benkirane-Jessel, N.; Petit, C.; Huck, O. Modulation of immune-inflammatory responses through surface modifications of biomaterials to promote bone healing and regeneration. J Tissue Eng 2021, 12, 20417314211041428. [Google Scholar] [CrossRef]
- Morand, D.N.; Davideau, J.L.; Clauss, F.; Jessel, N.; Tenenbaum, H.; Huck, O. Cytokines during periodontal wound healing: potential application for new therapeutic approach. Oral Dis 2017, 23, 300–311. [Google Scholar] [CrossRef]
- Martu, M.A.; Maftei, G.A.; Luchian, I.; Stefanescu, O.M.; Scutariu, M.M.; Solomon, S.M. The Effect of Acknowledged and Novel Anti-Rheumatic Therapies on Periodontal Tissues-A Narrative Review. Pharmaceuticals (Basel) 2021, 14. [Google Scholar] [CrossRef]
- Hasturk, H.; Kantarci, A. Activation and resolution of periodontal inflammation and its systemic impact. Periodontol 2000 2015, 69, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Soh, Y.; Heo, S.M. Recent Advances of Therapeutic Targets for the Treatment of Periodontal Disease. Biomol Ther (Seoul) 2021, 29, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Łasica, A.; Golec, P.; Laskus, A.; Zalewska, M.; Gędaj, M.; Popowska, M. Periodontitis: etiology, conventional treatments, and emerging bacteriophage and predatory bacteria therapies. Front Microbiol 2024, 15, 1469414. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Barman, M.; Hota, S. Present and Future Treatment Modalities for the Mitigation and Cure of Periodontal Diseases. Online Journal of Dentistry & Oral Health 2023, 7, 1–13. [Google Scholar] [CrossRef]
- Arampatzis, A.S.; Karra, A.; Kyrilas, E.; Kampasakali, E.; Tsalikis, L.; Barmpalexis, P.; Christofilos, D.; Assimopoulou, A. Bioactive 3D printed scaffolds for the treatment of periodontal diseases, 2022; Volume 88.
- Chen, P.; Zhang, C.; He, P.; Pan, S.; Zhong, W.; Wang, Y.; Xiao, Q.; Wang, X.; Yu, W.; He, Z.; et al. A Biomimetic Smart Nanoplatform as "Inflammation Scavenger" for Regenerative Therapy of Periodontal Tissue. Int J Nanomedicine 2022, 17, 5165–5186. [Google Scholar] [CrossRef]
- Attiq, A.; Jalil, J.; Husain, K.; Ahmad, W. Raging the War Against Inflammation With Natural Products. Front Pharmacol 2018, 9, 976. [Google Scholar] [CrossRef]
- World Health, O. WHO global report on traditional and complementary medicine 2019; World Health Organization: Geneva, 2019. [Google Scholar]
- Agrawal, S.; Acharya, D.; Adholeya, A.; Barrow, C.J.; Deshmukh, S.K. Nonribosomal Peptides from Marine Microbes and Their Antimicrobial and Anticancer Potential. Front Pharmacol 2017, 8, 828. [Google Scholar] [CrossRef]
- Almaliti, J.; Gerwick, W.H. Methods in marine natural product drug discovery: what's new? Expert Opin Drug Discov 2023, 18, 687–691. [Google Scholar] [CrossRef]
- Ahmed, I.; Asgher, M.; Sher, F.; Hussain, S.M.; Nazish, N.; Joshi, N.; Sharma, A.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M.N. Exploring Marine as a Rich Source of Bioactive Peptides: Challenges and Opportunities from Marine Pharmacology. Mar Drugs 2022, 20. [Google Scholar] [CrossRef]
- Adam, M.; Achmad, H.; Tanumihardja, M.; Ramadhan, S.R.J.; Masyta, N. The benefits of golden sea cucumber (Stichopus hermanni) as an alternative antimicrobial material in oral health. Journal of International Dental and Medical Research 2022, 15, 1806–1815. [Google Scholar]
- Mercier, A.; Purcell, S.W.; Montgomery, E.M.; Kinch, J.; Byrne, M.; Hamel, J.F. Revered and Reviled: The Plight of the Vanishing Sea Cucumbers. Ann Rev Mar Sci 2025, 17, 115–142. [Google Scholar] [CrossRef]
- Pangestuti, R.; Arifin, Z. Medicinal and health benefit effects of functional sea cucumbers. Journal of Traditional and Complementary Medicine 2018, 8, 341–351. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A.; Smirnov, A.V. Sea Cucumbers Triterpene Glycosides, the Recent Progress in Structural Elucidation and Chemotaxonomy. Phytochemistry Reviews 2005, 4, 221–236. [Google Scholar] [CrossRef]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants (Basel) 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Hu, C.; Fan, S. Chemical composition and nutritional quality of sea cucumbers. J Sci Food Agric 2010, 90, 2469–2474. [Google Scholar] [CrossRef] [PubMed]
- Shaik, M.I.; Kadir, A.N.A.; Sarbon, N.M. Physicochemical and thermal properties of pepsin- and acid-soluble collagen isolated from the body wall of sea cucumbers (Stichopus hermanni). J Food Sci 2024, 89, 320–329. [Google Scholar] [CrossRef]
- Sari, R.P.; Larashati, D.I.D.; Aldiana, C.; Nafi'ah, N.; Damaiyanti, D.W.; Kurniawati, A. Application of Stichopus hermanni Nanoparticle Gel in the Healing of Traumatic Ulcers. Eur J Dent 2023, 17, 330–336. [Google Scholar] [CrossRef]
- Li, X.; Luo, L.; Cai, Y.; Yang, W.; Lin, L.; Li, Z.; Gao, N.; Purcell, S.W.; Wu, M.; Zhao, J. Structural Elucidation and Biological Activity of a Highly Regular Fucosylated Glycosaminoglycan from the Edible Sea Cucumber Stichopus herrmanni. J Agric Food Chem 2017, 65, 9315–9323. [Google Scholar] [CrossRef]
- Bahrami, Y.; Franco, C.M. Acetylated Triterpene Glycosides and Their Biological Activity from Holothuroidea Reported in the Past Six Decades. Mar Drugs 2016, 14. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Masoud, R.; McClements, D.J. Bioactive functional ingredients from aquatic origin: a review of recent progress in marine-derived nutraceuticals. Critical Reviews in Food Science and Nutrition 2022, 62, 1242–1269. [Google Scholar] [CrossRef]
- Revol-Cavalier, J.; Quaranta, A.; Newman, J.W.; Brash, A.R.; Hamberg, M.; Wheelock, C.E. The Octadecanoids: Synthesis and Bioactivity of 18-Carbon Oxygenated Fatty Acids in Mammals, Bacteria, and Fungi. Chem Rev 2025, 125, 1–90. [Google Scholar] [CrossRef]
- Quaranta, A.; Revol-Cavalier, J.; Wheelock, C.E. The octadecanoids: an emerging class of lipid mediators. Biochem Soc Trans 2022, 50, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, C.; Jia, Q.; Zhao, C.; Taylor, D.C.; Li, D.; Zhang, M. Transcriptome Analysis Reveals Candidate Genes for Petroselinic Acid Biosynthesis in Fruits of Coriandrum sativum L. J Agric Food Chem 2020, 68, 5507–5520. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.; Richter, K.D.; Schulte, E.; Mukherjee, K.D. Petroselinic acid from dietary triacylglycerols reduces the concentration of arachidonic acid in tissue lipids of rats. J Nutr 1995, 125, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Ham, Y.M.; Heo, S.J.; Yoon, S.A.; Cho, S.H.; Kwon, S.H.; Jeong, M.S.; Jeon, Y.J.; Sanjeewa, K.; Yoon, W.J.; et al. Anti-inflammation effects of 8-oxo-9-octadecenoic acid isolated from Undaria peterseniana in lipopolysaccharide-stimulated macrophage cells. Excli j 2018, 17, 775–783. [Google Scholar] [CrossRef]
- Fatwati, K.; Amin, A.; Indriani, L.; Ladju, R.B.; Akbar, F.H.; Hamrun, N. GC–MS analysis and in silico approaches to Stichopus hermanii as anti-inflammatory through PKC-β inhibition. Results in Chemistry 2025, 14, 102086. [Google Scholar] [CrossRef]
- Brierly, G.; Celentano, A.; Breik, O.; Moslemivayeghan, E.; Patini, R.; McCullough, M.; Yap, T. Tumour Necrosis Factor Alpha (TNF-α) and Oral Squamous Cell Carcinoma. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Pathak, J.L.; Fang, Y.; Chen, Y.; Ye, Z.; Guo, X.; Yan, Y.; Zha, J.; Liang, D.; Ke, X.; Yang, L.; et al. Downregulation of Macrophage-Specific Act-1 Intensifies Periodontitis and Alveolar Bone Loss Possibly via TNF/NF-κB Signaling. Frontiers in Cell and Developmental Biology 2021, 9. [Google Scholar] [CrossRef]
- Lee, C.H.; Chang, J.S.; Syu, S.H.; Wong, T.S.; Chan, J.Y.; Tang, Y.C.; Yang, Z.P.; Yang, W.C.; Chen, C.T.; Lu, S.C.; et al. IL-1β promotes malignant transformation and tumor aggressiveness in oral cancer. J Cell Physiol 2015, 230, 875–884. [Google Scholar] [CrossRef]
- Spinelli, F.R.; Colbert, R.A.; Gadina, M. JAK1: Number one in the family; number one in inflammation? Rheumatology (Oxford) 2021, 60, ii3–ii10. [Google Scholar] [CrossRef]
- Plemmenos, G.; Evangeliou, E.; Polizogopoulos, N.; Chalazias, A.; Deligianni, M.; Piperi, C. Central Regulatory Role of Cytokines in Periodontitis and Targeting Options. Curr Med Chem 2021, 28, 3032–3058. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.A.; Rossa, C., Jr.; Garlet, G.P.; Nogueira, A.V.; Cirelli, J.A. Modulation of host cell signaling pathways as a therapeutic approach in periodontal disease. J Appl Oral Sci 2012, 20, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, N.; Ghorbani, M.; Shariatifar, H.; Farasat, A. Effects of unsaturated fatty acids (Arachidonic/Oleic Acids) on stability and structural properties of Calprotectin using molecular docking and molecular dynamics simulation approach. PLoS One 2020, 15, e0230780. [Google Scholar] [CrossRef] [PubMed]
- Vetrivel, U.; Ravichandran, S.B.; Kuppan, K.; Mohanlal, J.; Das, U.N.; Narayanasamy, A. Agonistic effect of polyunsaturated fatty acids (PUFAs) and its metabolites on brain-derived neurotrophic factor (BDNF) through molecular docking simulation. Lipids in Health and Disease 2012, 11, 109. [Google Scholar] [CrossRef]
- Cui, T.; Schopfer, F.J.; Zhang, J.; Chen, K.; Ichikawa, T.; Baker, P.R.; Batthyany, C.; Chacko, B.K.; Feng, X.; Patel, R.P.; et al. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J Biol Chem 2006, 281, 35686–35698. [Google Scholar] [CrossRef]
- Khoo, N.K.H.; Li, L.; Salvatore, S.R.; Schopfer, F.J.; Freeman, B.A. Electrophilic fatty acid nitroalkenes regulate Nrf2 and NF-κB signaling:A medicinal chemistry investigation of structure-function relationships. Sci Rep 2018, 8, 2295. [Google Scholar] [CrossRef]
- Wang, P.; Killeen, M.E.; Sumpter, T.L.; Ferris, L.K.; Falo, L.D., Jr.; Freeman, B.A.; Schopfer, F.J.; Mathers, A.R. Electrophilic nitro-fatty acids suppress psoriasiform dermatitis: STAT3 inhibition as a contributory mechanism. Redox Biol 2021, 43, 101987. [Google Scholar] [CrossRef]
- Ontoria-Oviedo, I.; Amaro-Prellezo, E.; Castellano, D.; Venegas-Venegas, E.; González-Santos, F.; Ruiz-Saurí, A.; Pelacho, B.; Prósper, F.; Pérez Del Caz, M.D.; Sepúlveda, P. Topical Administration of a Marine Oil Rich in Pro-Resolving Lipid Mediators Accelerates Wound Healing in Diabetic db/db Mice through Angiogenesis and Macrophage Polarization. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- McDaniel, J.C.; Belury, M.; Ahijevych, K.; Blakely, W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen 2008, 16, 337–345. [Google Scholar] [CrossRef]
- Rizzo-Valente, V.S.; Fusco, M.A.; Cruz, R.M.M.L.; Santos, R.A.; Silva, L.S.; Escaleira, R.C.; Schulz, D.F.; Barroso, S.P.C.; Miranda, B.L.; Santos, D.Z.; et al. Effects of Dermatan Sulfate from Marine Invertebrate Styela plicata in the Wound Healing Pathway: A Natural Resource Applied to Regenerative Therapy. Marine Drugs 2022, 20, 676. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Y.; Sun, X.; Meng, H.; Hao, W.; Yin, J.; Ma, F.; Guo, X.; Du, L.; Sun, L.; et al. Krill Oil Turns Off TGF-β1 Profibrotic Signaling in the Prevention of Diabetic Nephropathy. J Agric Food Chem 2022, 70, 9865–9876. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.; Bichel, J.; Euvrard, S.; Guidi, B.; Proby, C.M.; van de Kerkhof, P.C.; Amerio, P.; Rønnevig, J.; Slade, H.B.; Stockfleth, E. Topical immunomodulation under systemic immunosuppression: results of a multicentre, randomized, placebo-controlled safety and efficacy study of imiquimod 5% cream for the treatment of actinic keratoses in kidney, heart, and liver transplant patients. Br J Dermatol 2007, 157 Suppl 2, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Brewer, A.R.; McCarberg, B.; Argoff, C.E. Update on the use of topical NSAIDs for the treatment of soft tissue and musculoskeletal pain: a review of recent data and current treatment options. Phys Sportsmed 2010, 38, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Bhati, A.; Fageeh, H.; Ibraheem, W.; Fageeh, H.; Chopra, H.; Panda, S. Role of hyaluronic acid in periodontal therapy (Review). Biomed Rep 2022, 17, 91. [Google Scholar] [CrossRef]
- Polizzi, A.; Leanza, Y.; Belmonte, A.; Grippaudo, C.; Leonardi, R.; Isola, G. Impact of Hyaluronic Acid and Other Re-Epithelializing Agents in Periodontal Regeneration: A Molecular Perspective. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Sprott, H.; Fleck, C. Hyaluronic Acid in Rheumatology. Pharmaceutics 2023, 15. [Google Scholar] [CrossRef]
- Asbi, T.; Hussein, H.A.; Horwitz, J.; Gabay, E.; Machtei, E.E.; Giladi, H.Z. A single application of chlorhexidine gel reduces gingival inflammation and interleukin 1-β following one-stage implant placement: A randomized controlled study. Clin Implant Dent Relat Res 2021, 23, 726–734. [Google Scholar] [CrossRef]
- Fiorillo, L.; D'Amico, C.; Mehta, V.; Cicciù, M.; Cervino, G. Chlorhexidine cytotoxicity on oral Behaviors: Last 20 Years systematic review. Oral Oncology Reports 2024, 9, 100245. [Google Scholar] [CrossRef]
- Pucher, J.J.; Daniel, J.C. The effects of chlorhexidine digluconate on human fibroblasts in vitro. J Periodontol 1992, 63, 526–532. [Google Scholar] [CrossRef]
- Adam, M.; Thahir, H.; Supiaty; Achmad, H.; Putri, S.W.; Azizah; Satya, D.E. The Potential of Golden Sea Cucumber (Stichopus Hermanii) in the Regeneration of Periodontal Tissues: A Literature Review. Annals of the Romanian Society for Cell Biology 2021, 25, 4407–4418. [Google Scholar]
- Ujianti, I.; Lakhsmi, B.S.; Nurusshofa, Z.; Sukarya, W.S. Evaluation of the potential of Stichopus Herrmanni extract in inhibiting cervical cancer cell proliferation. Phytomedicine Plus 2024, 4, 100577. [Google Scholar] [CrossRef]
- Prasetyaningrum, N.; Priana, S. Star fruit leaves (Averrhoa bilimbi) extract and shrimp shell chitosan gel improves neovascularization in gingival wound healing in vivo. Indonesian Journal of Dental Medicine 2024, 7, 25–29. [Google Scholar] [CrossRef]
- Amin, A.; Thalib, B.; Natsir, N.; Thalib, A.; Hasyim, R. The increase of fibroblast cells number in rat (rattus norvegicus) gingival wound after the application of moringa (moringa oleifera lam) fruit oil. Journal of Dentomaxillofacial Science 2020, 5, 173. [Google Scholar] [CrossRef]
| Receptor | PDB ID | Resolution | 3D Structure |
|---|---|---|---|
| TNF-α | 6RMJ | 2.65 Å | |
| NF-κB | 1NFI | 2.7 Å | |
| JAK-1 | 6N79 | 2.27 Å | |
| IL-1β | 7JWQ | 2 Å |
| Compound | Protein | Binding affinity (Kcal/mol) | Interacting Residu in the Binding Pocket | |
|---|---|---|---|---|
| Hydrophobic interactions | Hydrogen Bond | |||
| (6Z)-Octadecenoic acid | TNF-α | -5.3 | TYR119, TYR151, TYR59, LEU57 | TYR151, GLY121 |
| NF-κB | -5 | VAL97, PHE318, PHE77, PHE103 | TYR71, PHE77 | |
| JAK-1 | -5.4 | VAL889, LEU1010, LEU881 | GLU925, GLY1023 | |
| IL-1β | -4.4 | LYS77, PRO131, PHE133 | VAL132, LEU134, LEU 80 | |
| Group | n | Day 3 | Day 7 | ||
|---|---|---|---|---|---|
| Fibroblasts (Mean ± SD) |
Collagenization | Fibroblasts (Mean ± SD) |
Collagenization | ||
| Na-CMC | 2 | 119 ± 5.38 | 112.00 μm | 166 ± 13.8 | 175.75 μm |
| Aspirin | 2 | 177 ± 14.62 | 238.50 μm | 350 ± 24.54 | 452.75 μm |
| Stichopus hermanii | 2 | 213 ± 13.2 | 177.25 μm | 251 ± 21.7 | 717.25 μm |
| P value | 0.001* | 0.002* | 0.001* | 0.000* | |
| *One-way ANOVA test (p<0.05) | |||||
| Compound | ADME-Tox | |
|---|---|---|
| Properties | Value | |
| (6Z)-Octadecenoic acid | Caco-2 permeability (nm.sec-1) | 1.563 |
| Human Intestinal Absorpstion (%) | 91.82 | |
| VDss (log L/kg) | 0.558 | |
| CYP2D6 Inhibitor | No | |
| Total Clearance | 1.884 | |
| AMES Toxicity | No | |
| Hepatotoxicity | No | |
| Oral Rate Acute Toxicity (LD50) (mol/kg) | 1.417 | |
| Oral Rate Chronic Toxicity (LOAEL) (log mg/kg_bw/day) | 3.259 | |
| Compound | Molecular Formula | SMILES | Lipinski’s Rule of Five | |
|---|---|---|---|---|
| Properties | Value | |||
| (6Z)-Octadecenoic acid | C18H34O2 | CCCCCCCCCCC/C=C\CCCCC(=O)O | WM | 282.5 |
| HBA | 2 | |||
| HBD | 1 | |||
| Log P | 6.11 | |||
| Rm | 87.08 | |||
| Violation | 1 | |||
| *Violation of criteia, maximum violation of criteria is two. MW: Weight of mass (<500 Da); HBA: Hydrogen Bond Acceptors (<10); HBD : Hydrogen Bond Donor (<5); LogP: partition coefficient (5); Rm: Refractivity of Molar (40-130 cm3/mol). | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





