Submitted:
18 August 2025
Posted:
19 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Multilayer Film Characterization
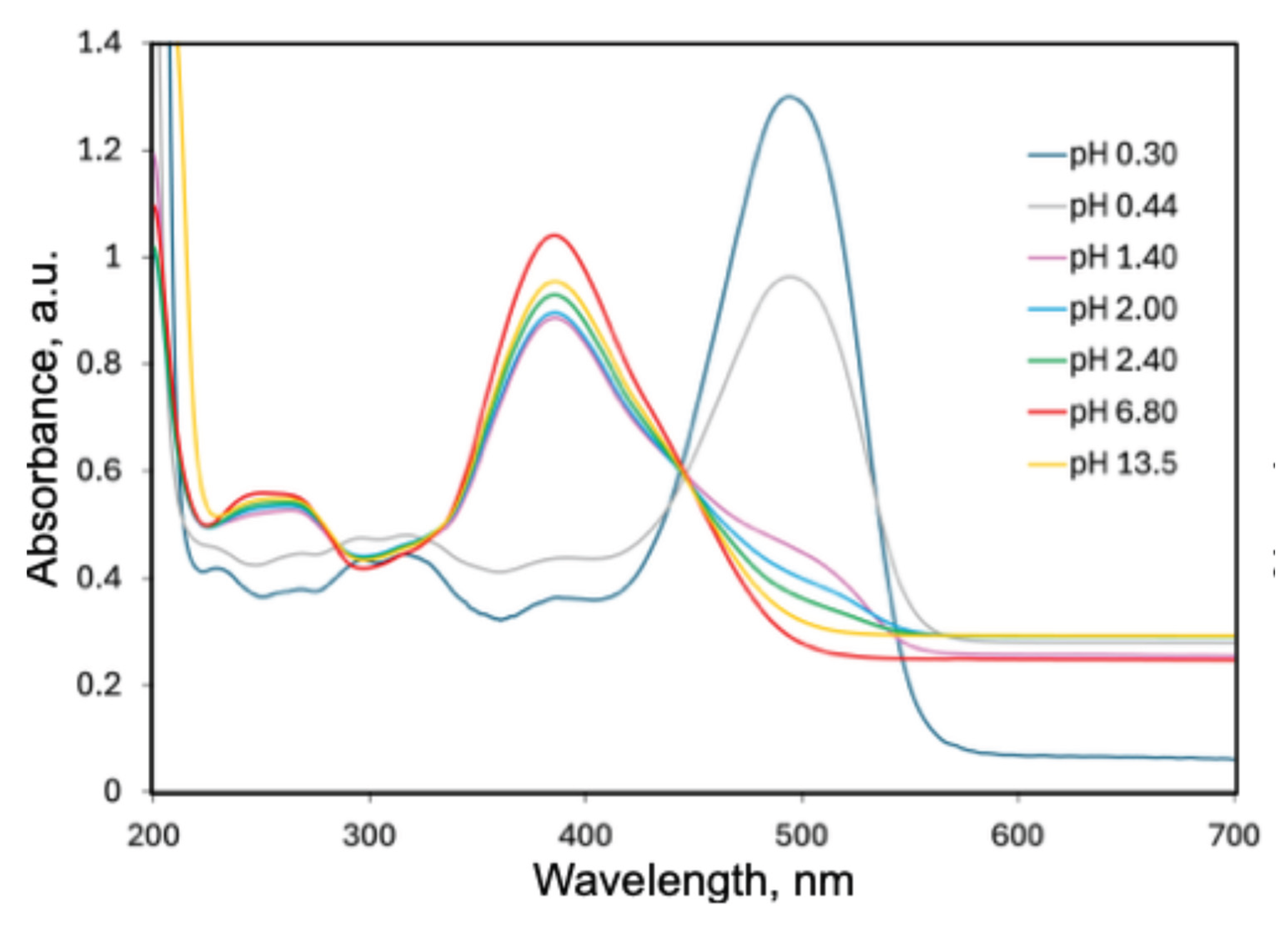
2.2. UV-Vis Spectra of AY9 Solutions and Multilayer Films
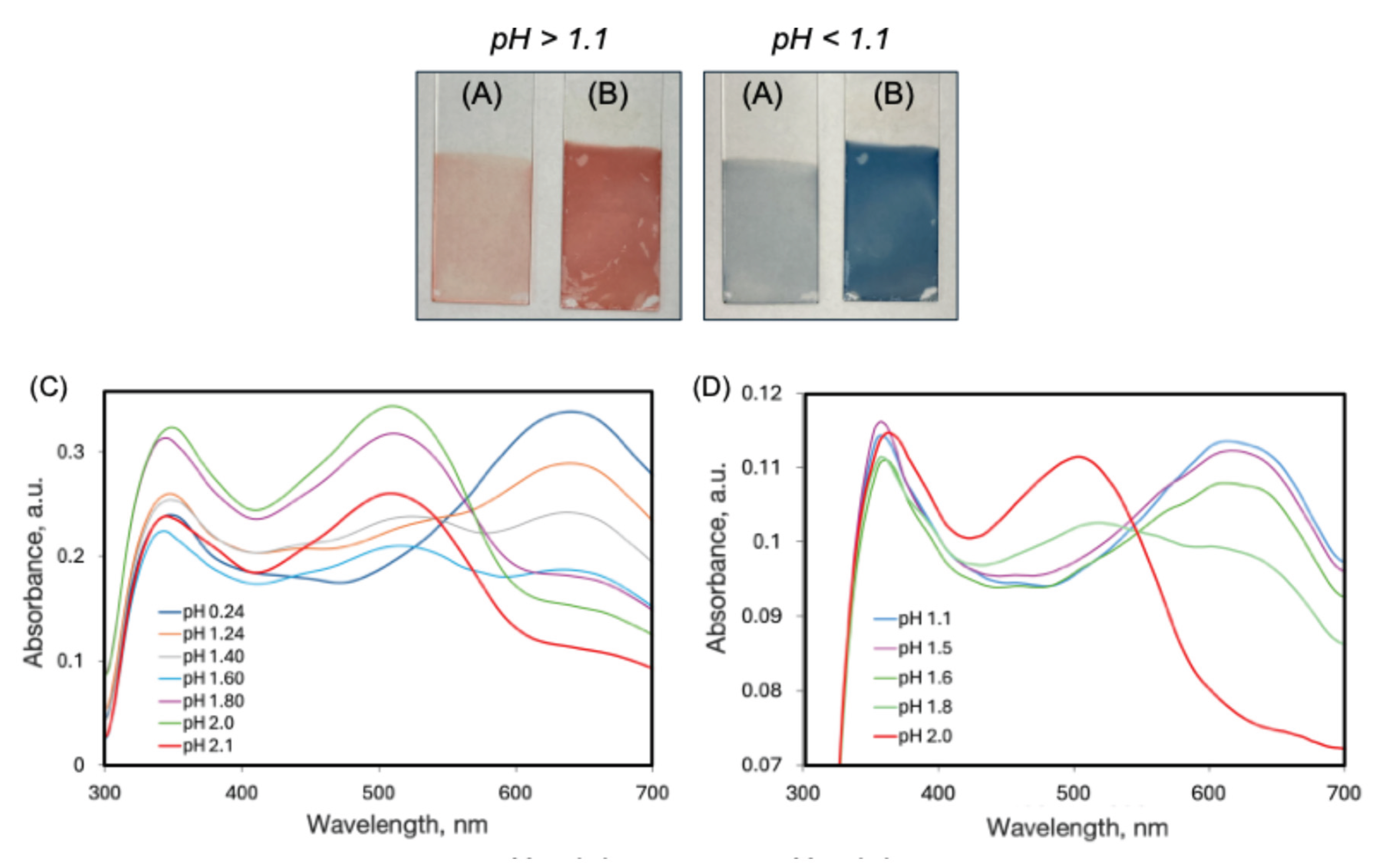
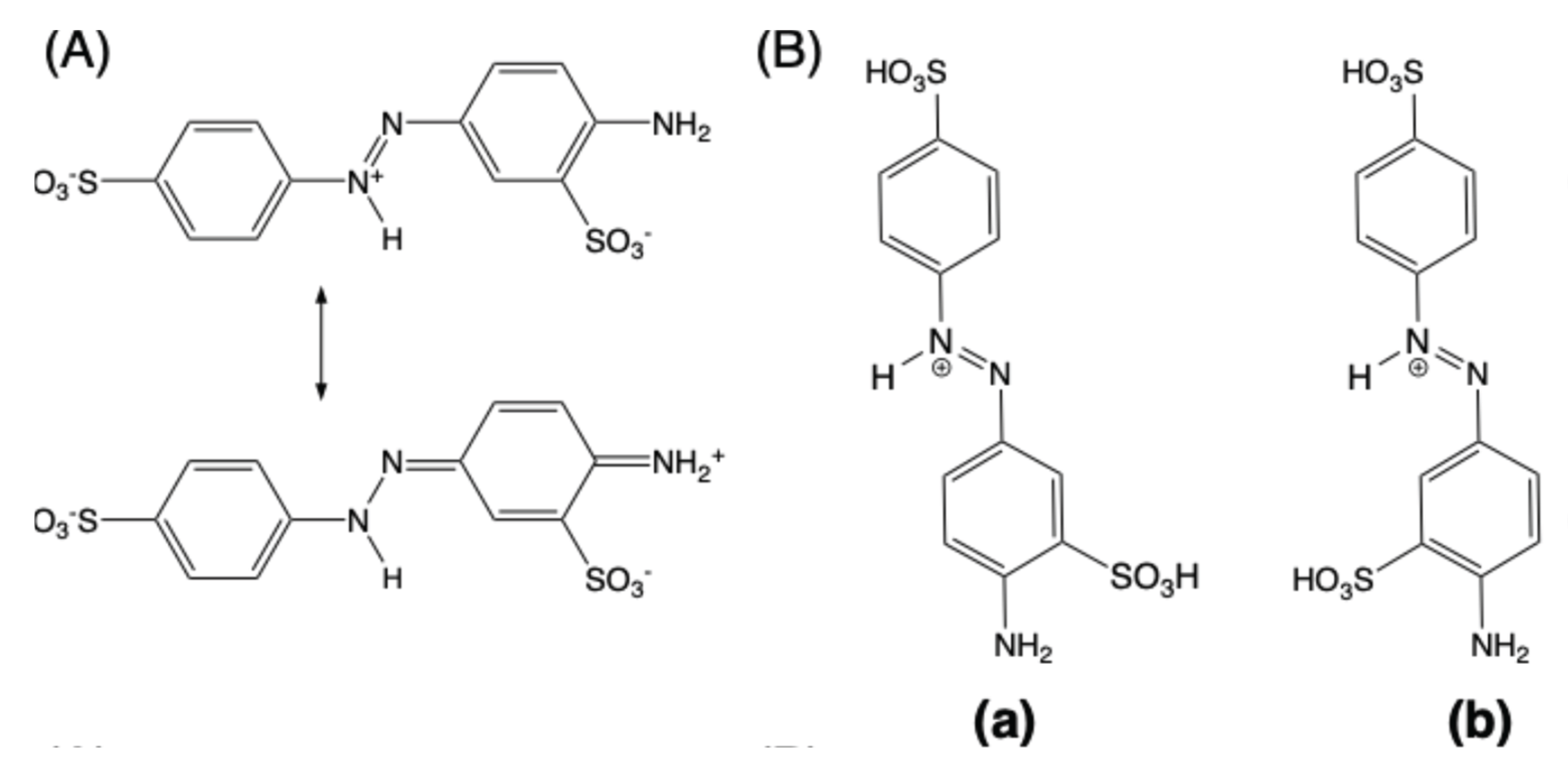
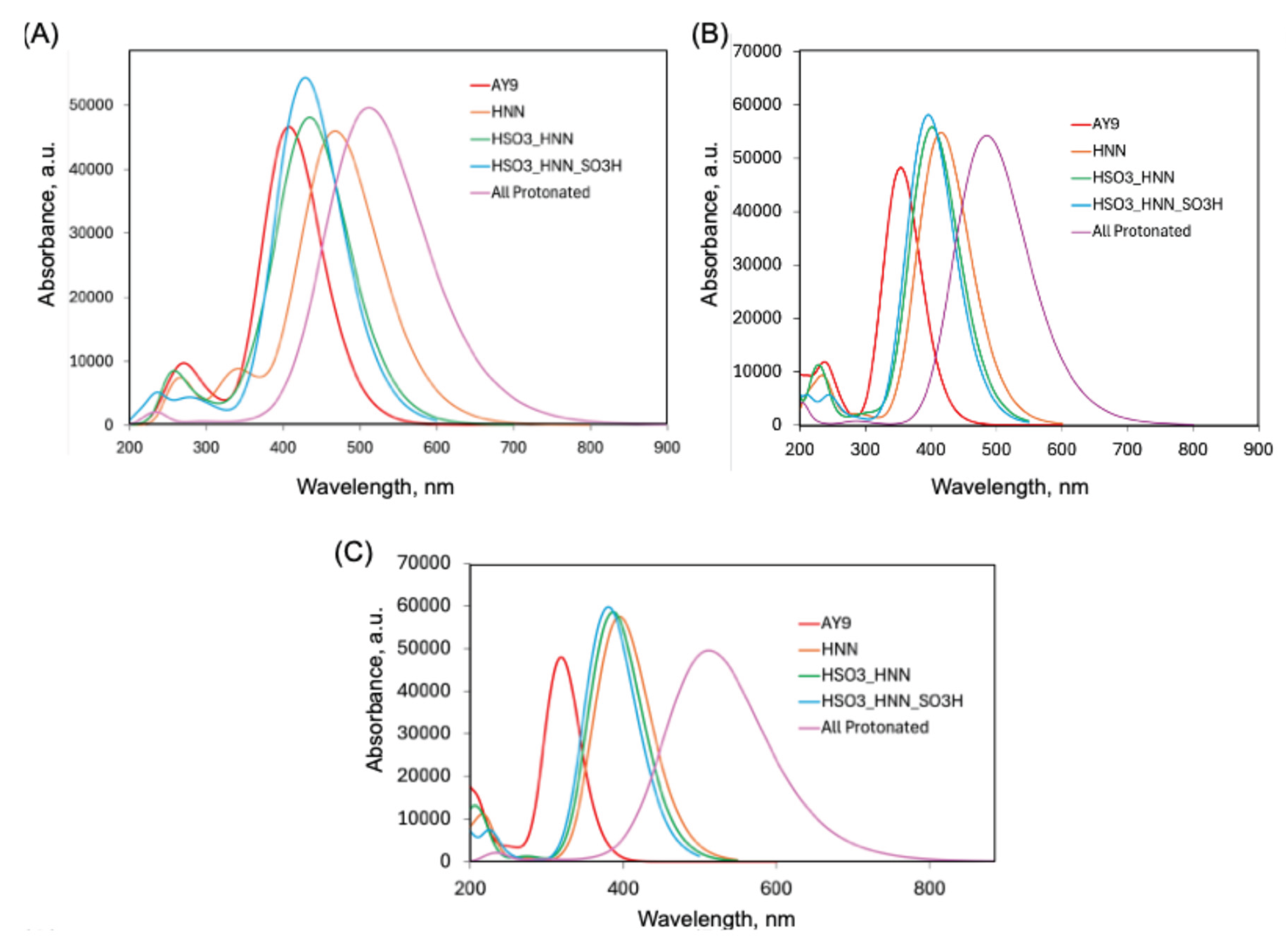
2.3. DFT and TD-DFT calculations
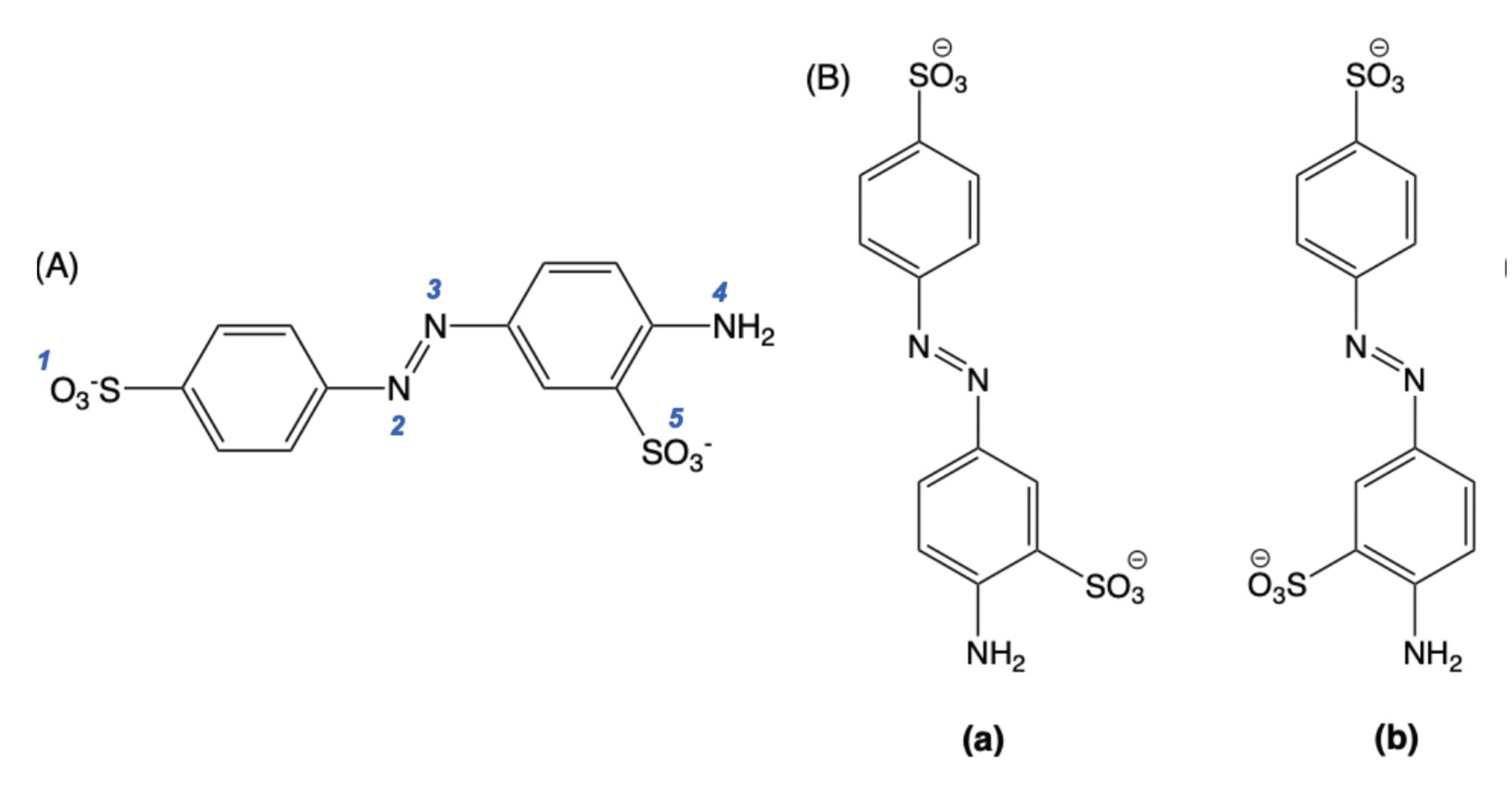
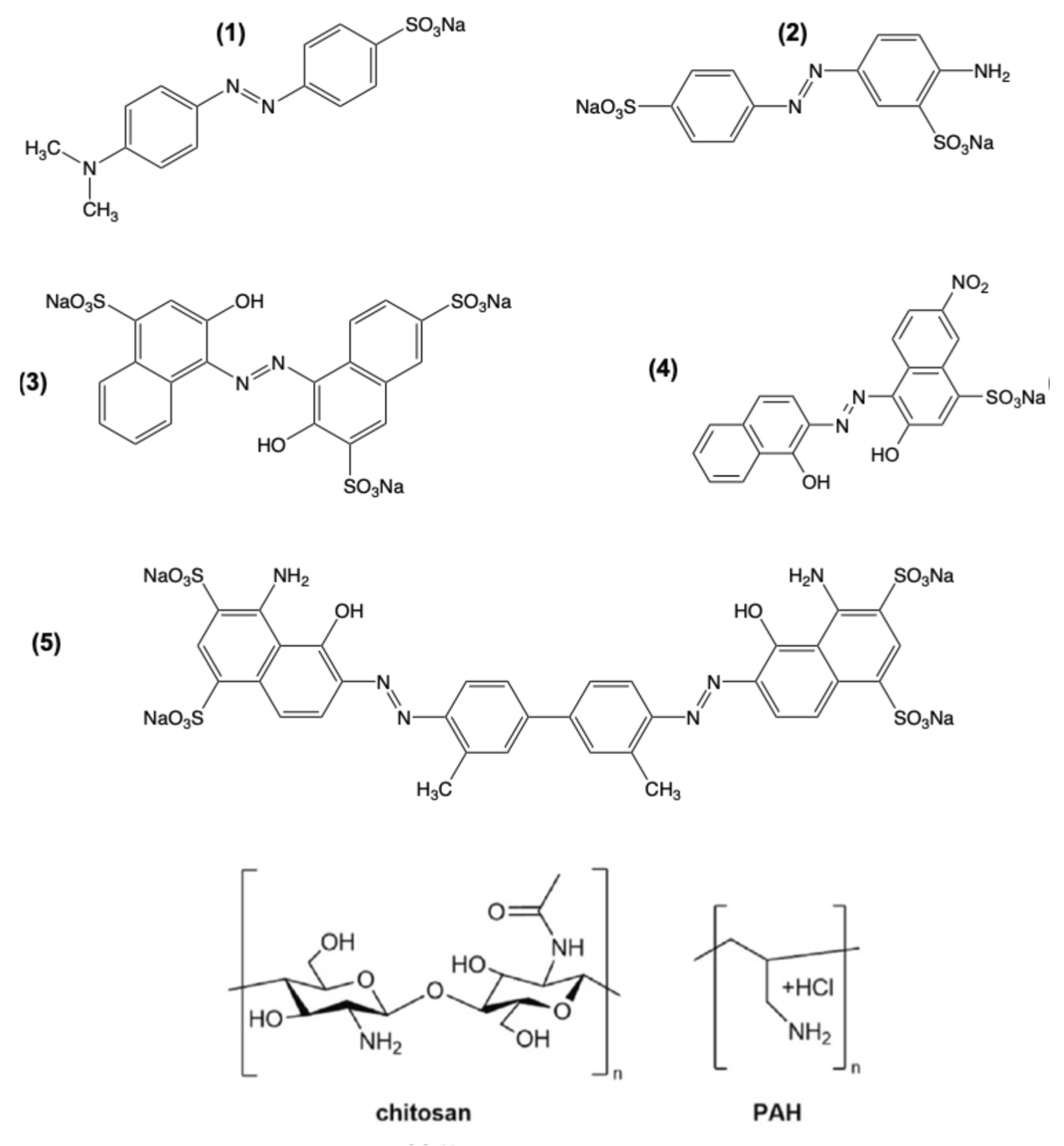
2.4. Effect of the Counter-Polymer
3. Discussion
4. Materials and Methods
4.1. LbL Assembly of Thin Films
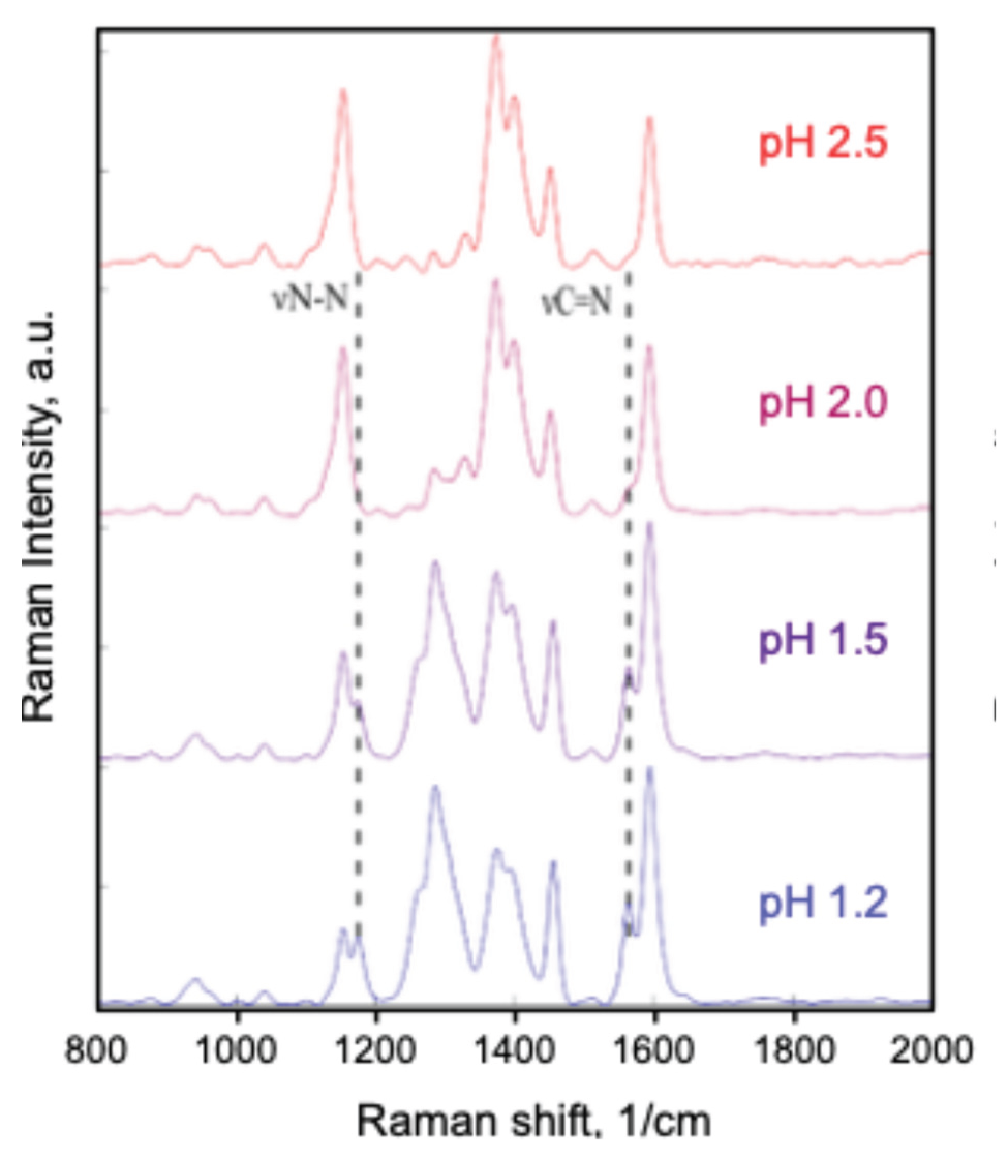
4.2. Raman Spectroscopy
4.3. UV-Visible (UV-Vis) Spectroscopy
4.4. pH Measurements
4.5. Photo-Response of the Multilayer Films
4.6. Computational Chemistry
4.7. Data Processing and Visualization, Computational Chemistry Software
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PAH | Poly(allylamine hydrochloride) |
| AY9 | Acid Yellow 9 |
| LbL | Layer-by-layer |
| UV-Vis | Ultraviolet-visible |
| DFT | Density functional theory |
| TD-DFT | Time-dependent density functional theory |
| DW | Distilled water |
| PAA | Poly(acrylic acid) |
References
- E. Mitscherlich Ueber Die Zusammensetzung Des Nitrobenzids Und Sulfobenzids. Annalen der Pharmacie 1834, 12, 305–311. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in Different Classes of Azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Kim, M.; Hillel, C.; Edwards, K.; Borchers, T.H.; Ozzy Mermut; Pietro, W.J.; Barrett, C.J. Azo Dye Polyelectrolyte Multilayer Films Reversibly Re-Soluble with Visible Light. Frontiers in Materials 2024, 11, 1334863. [Google Scholar] [CrossRef]
- Edwards, K.E.K.; Kim, M.; Borchers, T.H.; Barrett, C.J. Controlled Disassembly of Azobenzene Cellulose-Based Thin Films Using Visible Light. Materials advances 2022, 3, 6222–6230. [Google Scholar] [CrossRef]
- Kortekaas, L.; Simke, J.; Arndt, N.B.; Böckmann, M.; Doltsinis, N.L. ; Bart Jan Ravoo Acid-Catalysed Liquid-To-Solid Transitioning of Arylazoisoxazole Photoswitches. Chemical Science 2021, 12, 11338–11346. [Google Scholar] [CrossRef] [PubMed]
- Rickhoff, J.; Arndt, N.B.; Böckmann, M.; Doltsinis, N.L.; Ravoo, B.J.; Kortekaas, L. Reversible, Red-Shifted Photoisomerization in Protonated Azobenzenes. The Journal of Organic Chemistry 2022, 87, 10605–10612. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Amorós, J.; Sánchez-Ferrer, A.; Massad, W.A.; Nonell, S.; Velasco, D. Kinetic Study of the Fast Thermal Cis-To-Trans Isomerisation of Para-, Ortho- and Polyhydroxyazobenzenes. Physical Chemistry Chemical Physics 2010, 12, 13238. [Google Scholar] [CrossRef]
- Merino, E.; Ribagorda, M. Control over Molecular Motion Using the Cis–Trans Photoisomerization of the Azo Group. Beilstein Journal of Organic Chemistry 2012, 8, 1071–1090. [Google Scholar] [CrossRef]
- Gao, M.; Kwaria, D.; Yasuo Norikane; Yue, Y. Visible-Light-Switchable Azobenzenes: Molecular Design, Supramolecular Systems, and Applications. Natural sciences 2022, 3. [Google Scholar]
- Cruickshank, D.L.; Hendon, C.H.; Verbeek, M.J.R.; Walsh, A.; Wilson, C.C. Polymorphism of the Azobenzene Dye Compound Methyl Yellow. CrystEngComm 2016, 18, 3456–3461. [Google Scholar] [CrossRef]
- Gardner, H.C.; Kennedy, A.R.; McCarney, K.M.; Staunton, E.; Stewart, H.; Teat, S.J. Structures of Five Salt Forms of Disulfonated Monoazo Dyes. Acta Crystallographica Section C Structural Chemistry 2020, 76, 972–981. [Google Scholar] [CrossRef]
- Honda, A.; Shunta Kakihara; Kawai, M.; Takahashi, T.; Miyamura, K. Cold Crystallization and Polymorphism Triggered by the Mobility of the Phenyl Group in Alkyl Azo Dye Molecules. Crystal Growth & Design 2021, 21, 6223–6229. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, H.; Napolitano, S.; Zuo, B. Crystallization in Thin Films of Polymer Glasses: The Role of Free Surfaces, Solid Interfaces and Their Competition. Progress in Polymer Science 2023, 144, 101725–101725. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, X.; He, Y.; Guo, B.; Xu, J. In Situ Monitoring and Tuning Multilayer Stacking of Polymer Lamellar Crystals in Solution with Aggregation-Induced Emission. Nano Letters 2024, 24, 4717–4724. [Google Scholar] [CrossRef]
- Carr, J.M.; Langhe, D.S.; Ponting, M.T.; Hiltner, A.; Baer, E. Confined Crystallization in Polymer Nanolayered Films: A Review. Journal of Materials Research 2012, 27, 1326–1350. [Google Scholar] [CrossRef]
- Begum, S.; Cianci, M.; Durbeej, B.; Falklöf, O.; Hädener, A.; Helliwell, J.R.; Helliwell, M.; Regan, A.C.; Watt, C.I.F. On the Origin and Variation of Colors in Lobster Carapace. Physical Chemistry Chemical Physics 2015, 17, 16723–16732. [Google Scholar] [CrossRef]
- Hunt, D.M.; Wilkie, S.E.; Bowmaker, J.K.; Poopalasundaram, S. Vision in the Ultraviolet. Cellular and Molecular Life Sciences 2001, 58, 1583–1598. [Google Scholar] [CrossRef]
- Hazel, I. van; Amir Sabouhanian; Day, L.; Endler, J.A.; Chang, B.S. Functional Characterization of Spectral Tuning Mechanisms in the Great Bowerbird Short-Wavelength Sensitive Visual Pigment (SWS1), and the Origins of UV/Violet Vision in Passerines and Parrots. BMC Evolutionary Biology 2013, 13, 250. [Google Scholar]
- Stockman, A.; Sharpe, L.T.; Merbs, S.L.; Nathans, J. [42] Spectral Sensitivities of Human Cone Visual Pigments Determined in Vivo and in Vitro. Methods in Enzymology, Academic Press 2000, 316, 626–650. [Google Scholar]
- Matthias Broser Far-Red Absorbing Rhodopsins, Insights from Heterodimeric Rhodopsin-Cyclases. Frontiers in Molecular Biosciences 2022, 8, 806–922.
- Burke, S.E.; Barrett, C.J. Acid−Base Equilibria of Weak Polyelectrolytes in Multilayer Thin Films. Langmuir 2003, 19, 3297–3303. [Google Scholar] [CrossRef]
- You, K.; Kwon, O.; Kim, D. Effects of the Protonation and the Polar Solvation on the Molecular Properties of Methyl Orange: A Density Functional Theory Study. Bulletin of the Korean Chemical Society 2023, 44, 523–527. [Google Scholar] [CrossRef]
- ZHENG, D.; YUAN, X.-A.; MA, J. Rationalization of PH-Dependent Absorption Spectrum of O-Methyl Red in Aqueous Solutions: TD-DFT Calculation and Experiment Study. Acta Physico-Chimica Sinica 2016, 32, 290–300. [Google Scholar] [CrossRef]
- Pirone, D.; Bandeira, N.A.G.; Tylkowski, B.; Boswell, E.; Labeque, R.; Garcia Valls, R.; Giamberini, M. Contrasting Photo-Switching Rates in Azobenzene Derivatives: How the Nature of the Substituent Plays a Role. Polymers 2020, 12, E1019. [Google Scholar] [CrossRef]
- Gelabert, R.; Moreno, M.; Lluch, J.M. Predicting the Electronic Absorption Band Shape of Azobenzene Photoswitches. International Journal of Molecular Sciences 2023, 24, 25. [Google Scholar] [CrossRef]
- Peach, M.J.G.; Benfield, P.; Helgaker, T.; Tozer, D.J. Excitation Energies in Density Functional Theory: An Evaluation and a Diagnostic Test. The Journal of Chemical Physics 2008, 128, 044118. [Google Scholar] [CrossRef]
- Tomasi, J.; Menucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. ChemInform 2005, 36, 628. [Google Scholar] [CrossRef]
- Sharma, A.; Bekir, M.; Lomadze, N.; Santer, S. Photo-Isomerization Kinetics of Azobenzene Containing Surfactant Conjugated with Polyelectrolyte. Molecules 2020, 26, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-M.; Jin, X.-Y.; Zhang, Z.-X.; Wang, J.; Bai, F.-Q. Theoretical Study on the Reaction Mechanism of the Thermal Cis–Trans Isomerization of Fluorine-Substituted Azobenzene Derivatives. RSC Advances 2018, 8, 11580–11588. [Google Scholar] [CrossRef]
- Lee, H.; Machida, K.; A. Kuwae; Saito, Y. Resonance Raman Spectra and Structure of P-Aminoazobenzene Dyes in Diprotonated Form. Journal of Molecular Structure 1980, 68, 51–57. [Google Scholar] [CrossRef]
- Trotter, P.J. Azo Dye Tautomeric Structures Determined by Laser-Raman Spectroscopy. Applied Spectroscopy 1977, 31, 30–35. [Google Scholar] [CrossRef]
- Cembran, A.; Bernardi, F.; Garavelli, M.; Gagliardi, L.; Orlandi, G. On the Mechanism of the Cis−Trans Isomerization in the Lowest Electronic States of Azobenzene: S0, S1, and T1. Journal of the American Chemical Society 2004, 126, 3234–3243. [Google Scholar] [CrossRef]
- Md Sahanawaz; Manik Lal Maity; Goswami, K.G.; Sar, P.; De, P.; Bandyopadhyay, S. Sequence Effects on the Thermal Cis–Trans Isomerization of Side-Chain Stearate-Containing Azobenzene Polymers. Journal of Physical Organic Chemistry 2024, 37.
- Camila Anchau Wegermann; Cesar, J.; Sueli Maria Drechsel; Fábio Souza Nunes Semi-Empirical ZINDO/S Description of the Electronic Structure and the Spectral Features of Methyl Orange and Its Products of Oxidation. A Study of Relationship between Molecular Geometry and Spectroscopic Properties. Dyes and Pigments 2013, 99, 839–849.
- Jacquemin, D.; Aurélien Planchat; Adamo, C.; Benedetta Mennucci TD-DFT Assessment of Functionals for Optical 0–0 Transitions in Solvated Dyes. Journal of Chemical Theory and Computation 2012, 8, 2359–2372.
- Casida, M.E. Time-Dependent Density-Functional Theory for Molecules and Molecular Solids; Journal of Molecular Structure: THEOCHEM, 2009, 914, 318.
- Marques, M.A.L.; Maitra, N.T.; Nogueira, F.; Gross, E.K.U.; Rubio, A. Fundamentals of Time-Dependent Density Functional Theory; Springer-Verlag: Heidelberg; New York, 2012.
- Appel, H. Oscillator Strengths from Time-Dependent Density Functional Theory; New Brunswick, New Jersey, 1999.
- “How Do I Generate Natural Transition Orbitals?” Available online: https://gaussian.com/faq4, 2016.
- Spange, S.; Mayerhöfer, T.G. The Negative Solvatochromism of Reichardt‘s Dye B30 – a Complementary Study. ChemPhysChem 2022, 23.
- Kuntze, K.; Viljakka, J.; Matti Virkki; Huang, C.-Y.D.; Hecht, S.; Arri Priimagi Red-Light Photoswitching of Indigos in Polymer Thin Films. Chemical Science 2023, 14, 2482–2488.
- Tang, Z.; Chang, C.; Bao, F.; Tian, L.; Liu, H.; Wang, M.; Zhu, C.; Xu, J. Feasibility of Predicting Static Dielectric Constants of Polymer Materials: A Density Functional Theory Method. Polymers 2021, 13, 284. [Google Scholar] [CrossRef] [PubMed]
- Hensel, R.C.; Pereira-da-Silva, M.A.; Riul, A.; Rodrigues, V. Dielectric Permittivity and Surface Charge Density in Layer-By-Layer Poly(Diallyldimethylammonium Chloride)/Poly(Styrenesulfonate) Nanostructured Films: Implications for Biosensing. ACS applied nano materials 2020, 3, 1749–1754. [Google Scholar] [CrossRef]
- Durstock, M.F.; Rubner, M.F. Dielectric Properties of Polyelectrolyte Multilayers. Langmuir 2001, 17, 7865–7872. [Google Scholar] [CrossRef]
- Yin, L.; He, Y.; Guo, W.; Wang, S.; He, J.; Wang, T. Low Dielectric Constant Ultrathin Polyimide Films Alternated by Poly(Amic Acid) Salt/Poly(Allylamine Hydrochloride)/Imogolite through Layer by Layer Deposition. Advanced Composites and Hybrid Materials/Advanced composites and hybrid materials 2023, 6, 223. [Google Scholar] [CrossRef]
- Smallwood, I.M. Handbook of Organic Solvent Properties; Arnold ; New York ; Toronto: London ; Sydney ; Auckland, 1996.
- Alizadeh, K.; Rezaei, B.; Maddah, B. Spectrophotometric Determination of Aqueous Acidity Constants of Three Azo Dyes. Open Chemistry 2010, 8, 392–395. [Google Scholar] [CrossRef]
- Ferreira, Q.; Gomes, S.; Ribeiro, P.Q.; Jones, N.C.; Søren Vrønning Hoffmann; Mason, N.J.; Oliveira, O.N.; Oliveira, O.N. Determination of Degree of Ionization of Poly(Allylamine Hydrochloride) (PAH) and Poly [1-[4-(3-Carboxy-4 Hydroxyphenylazo)Benzene Sulfonamido]-1,2-Ethanediyl, Sodium Salt] (PAZO) in Layer-By-Layer Films Using Vacuum Photoabsorption Spectroscopy. Langmuir 2012, 29, 448–455.
- Pliego Jr, J.R.; Riveros, J.M. Gibbs Energy of Solvation of Organic Ions in Aqueous and Dimethyl Sulfoxide Solutions. Physical Chemistry Chemical Physics 2002, 4, 1622–1627. [Google Scholar] [CrossRef]
- Dong, M.; Babalhavaeji, A.; Samanta, S.; Beharry, A.A.; Woolley, G.A. Red-Shifting Azobenzene Photoswitches for in Vivo Use. Accounts of Chemical Research 2015, 48, 2662–2670. [Google Scholar] [CrossRef]
- Dantas, D.; Ribeiro, A.I.; Carvalho, F.; Gil-Martins, E.; Silva, R.; Remião, F.; Zille, A.; Cerqueira, F.; Pinto, E.; Dias, A.M. Red-Shifted and PH-Responsive Imidazole-Based Azo Dyes with Potent Antimicrobial Activity. Chemical Communications 2023, 19, 2791–2794. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, W.; Lin, Y.; Xie, Y.; Liu, S.H.; Yin, J. Visible and Near-Infrared Light Activated Azo Dyes. Chinese Chemical Letters 2021, 32, 2359–2368. [Google Scholar] [CrossRef]
- Swanson, N.; Sabat, R.G. Inscription and Analysis of Non-Uniform Diffraction Gratings in Azobenzene Molecular Glass Thin Films. Optics Express 2018, 26, 7876–7876. [Google Scholar] [CrossRef] [PubMed]
| Protonated state | Total charge | Functional | ||
|---|---|---|---|---|
| B3LYP | CAM-B3LYP | LC-ωHPBE | ||
| SO3_NN_NH2_SO3 (a) | −2 | 0 | 0 | 0 |
| SO3_NN_NH2_SO3 (b) | 0.0001 | 0.0001 | 0.0004 | |
| SO3_HNN_NH2_SO3 (a) | −1 | −0.4412 | −0435 | −0.4331 |
| SO3_HNN_NH2_SO3 (b) | −0.4417 | −0.4349 | −0.4325 | |
| HSO3_ HNN _NH2_SO3 (a) | 0 | −0.8743 | −0.8648 | −0.8636 |
| HSO3_ HNN _NH2_SO3 (b) | −0.8737 | −0.8648 | −0.8642 | |
| HSO3_HNN_NH2_SO3H (a) | +1 | −1.2935 | −1.2817 | −1.2825 |
| HSO3_HNN_NH2_SO3H (b) | −1.2915 | −1.2800 | −1.2807 | |
| HSO3_HNNH_NH2H_SO3H (a) | +3 | −2.0223 | −1.9997 | −1.9990 |
| HSO3_HNNH_NH2H_SO3H (b) | −2.0229 | −2.0010 | −1.9995 | |
| Protonated state | Excited state | Functional | ||
|---|---|---|---|---|
| B3LYP | CAM-B3LYP | LC-ωHPBE | ||
| SO3_NN_NH2_SO3 | S1 | 2.66 (0.0000) | 2.85 (0.0001) | 2.92 (0.0001) |
| S2 | 3.04 (1.1516) | 3.51 (0.0210) | 3.89 (1.1832) | |
| Sn | 4.53 (0.1680)/S8 | 5.28 (0.1996)/S7 | 7.27 (0.3175)/S18 | |
| SO3_HNN_NH2_SO3 | S1 | 2.54 (0.0004) | 2.98 (1.3539) | 3.14 (1.4213) |
| S2 | 2.65 (1.1334) | 4.09 (0.0242) | 4.46 (0.0029) | |
| Sn | 3.65 (0.1447)/S8 | 5.20 (0.1869)/S9 | 6.95 (0.3695)/S17 | |
| HSO3_ HNN _NH2_SO3 | S1 | 2.60 (0.0000) | 3.09 (1.3784) | 3.21 (1.4477) |
| S2 | 2.83 (1.1281) | 3.98 (0.0009) | 4.44 (0.0053) | |
| Sn | 3.28 (0.1963)/S3 | 6.68 (0.2263)/S20 | 7.14 (0.4329)/S16 | |
| HSO3_HNN_NH2_SO3H | S1 | 2.89 (1.3353) | 3.13 (1.4363) | 3.26 (1.4763) |
| S2 | 3.42 (0.0290) | 4.32 (0.0224) | 4.42 (0.0025) | |
| Sn | 5.26 (0.0746)/S14 | 6.80 (0.2640)/S17 | 7.20 (0.3345)/S13 | |
| HSO3_HNNH_NH2H_SO3H | S1 | 2.03 (0.0072) | 2.43 (0.0408) | 2.67 (1.2842) |
| S2 | 2.36 (0.2236) | 2.56 (1.3002) | 2.84 (0.0638) | |
| Sn | 2.38 (0.5720)/S3 | 6.06 (0.0388)/S17 | 6.98 (0.1485)/S20 | |
| Polymer, conditions of LbL fabrication | Peprotonated (red colour) |
Protonated (blue colour) |
||
|---|---|---|---|---|
| Absorption peaks | Shift | Absorption peaks | Shift | |
| PAH, DW | 512 348 |
125 86 |
642 350 |
255 88 |
| PAH, NaCl | 500 364 |
113 102 |
630 358 |
243 96 |
| CS, DW | 505 364 |
118 102 |
620 357 |
233 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





