Submitted:
18 August 2025
Posted:
19 August 2025
You are already at the latest version
Abstract
Keywords:
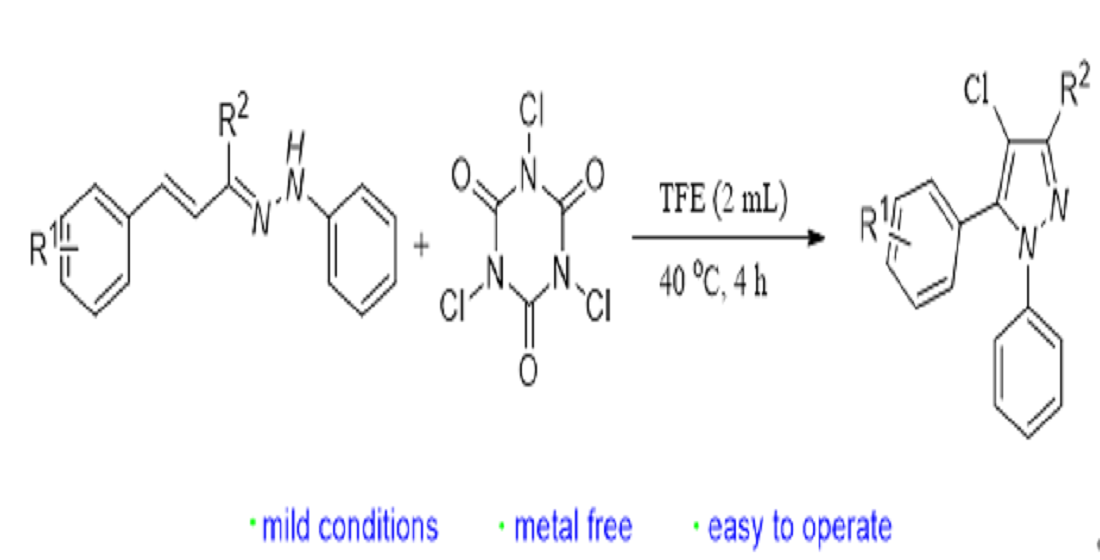
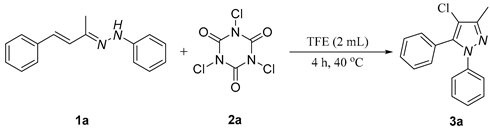
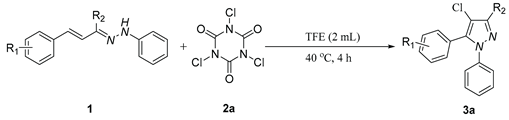
1. Introduction
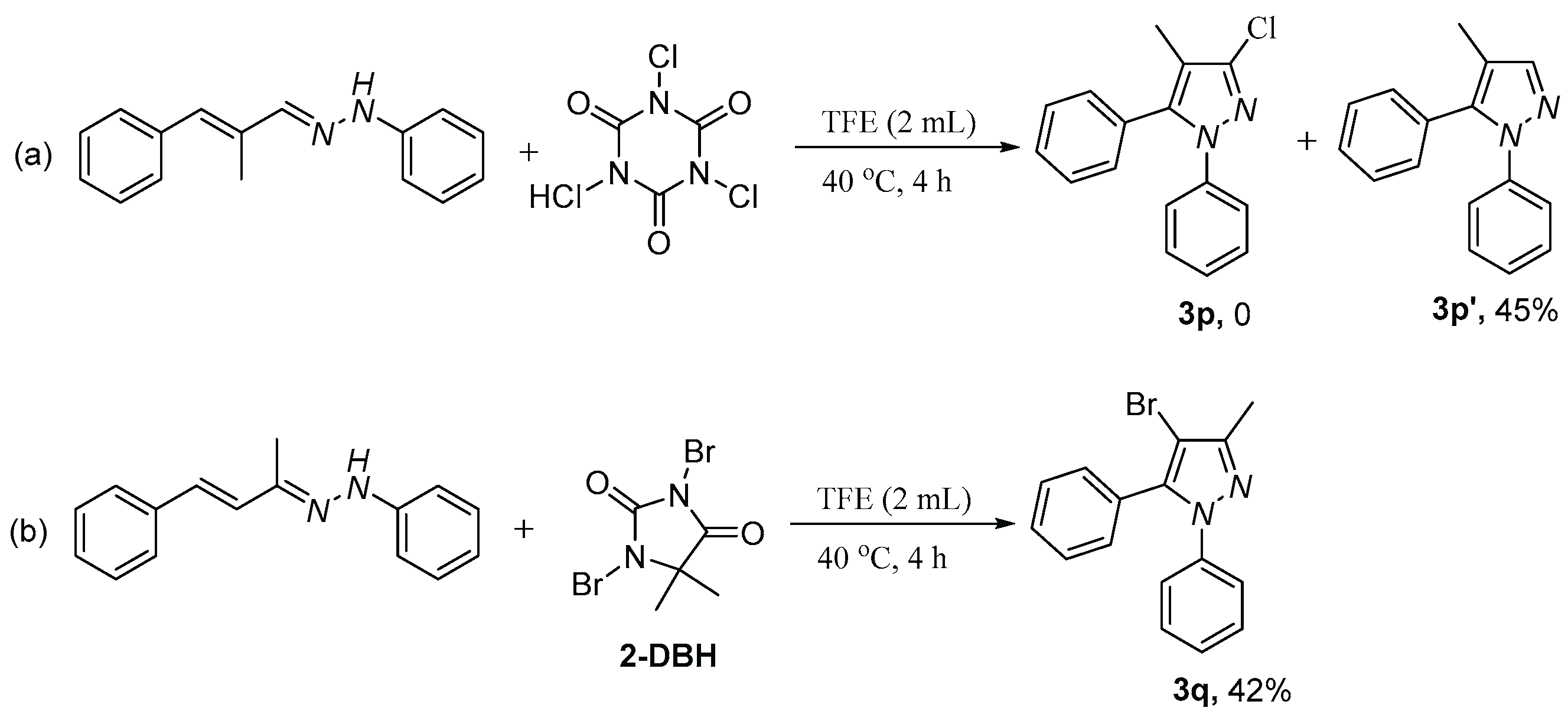
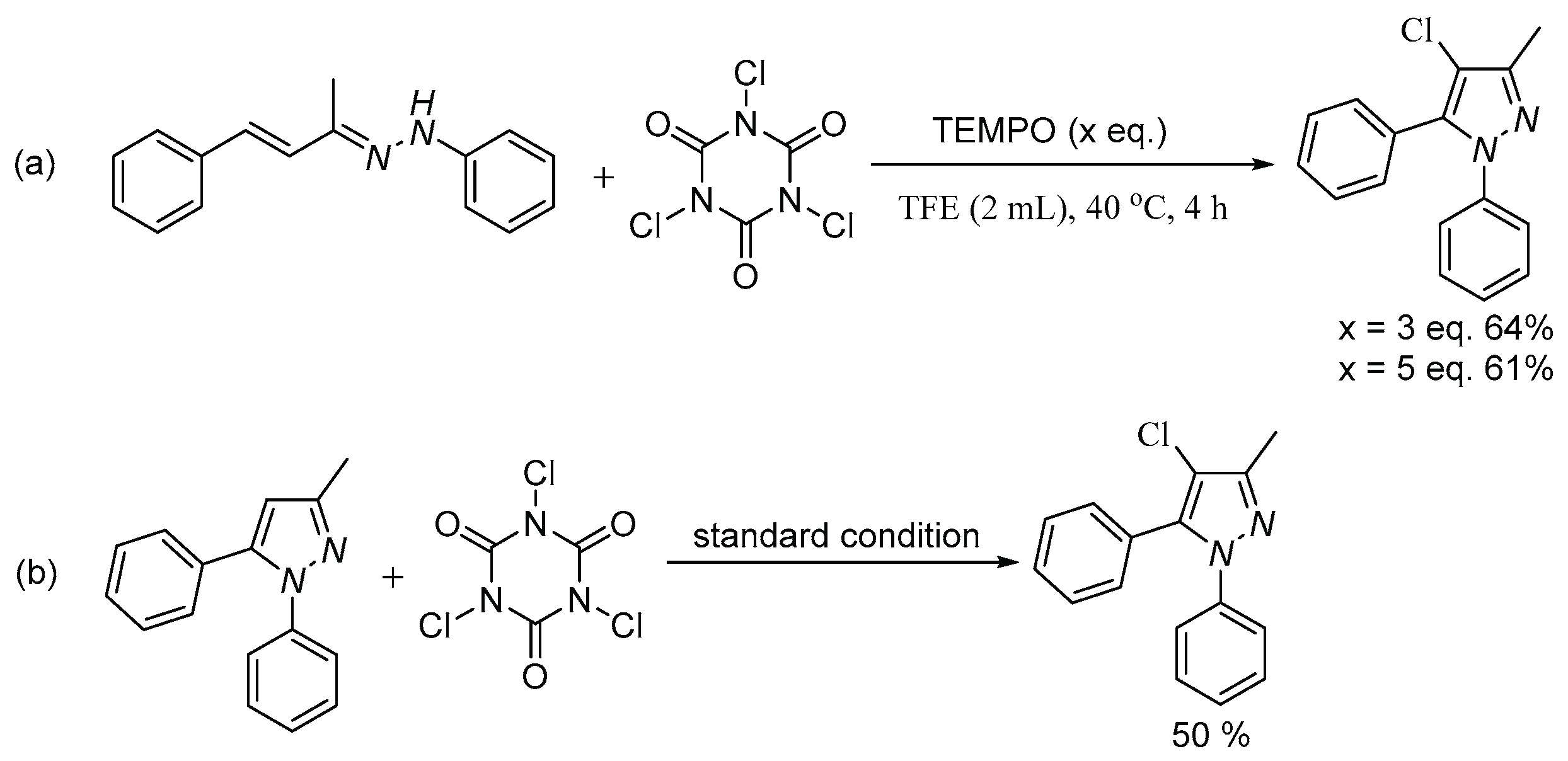
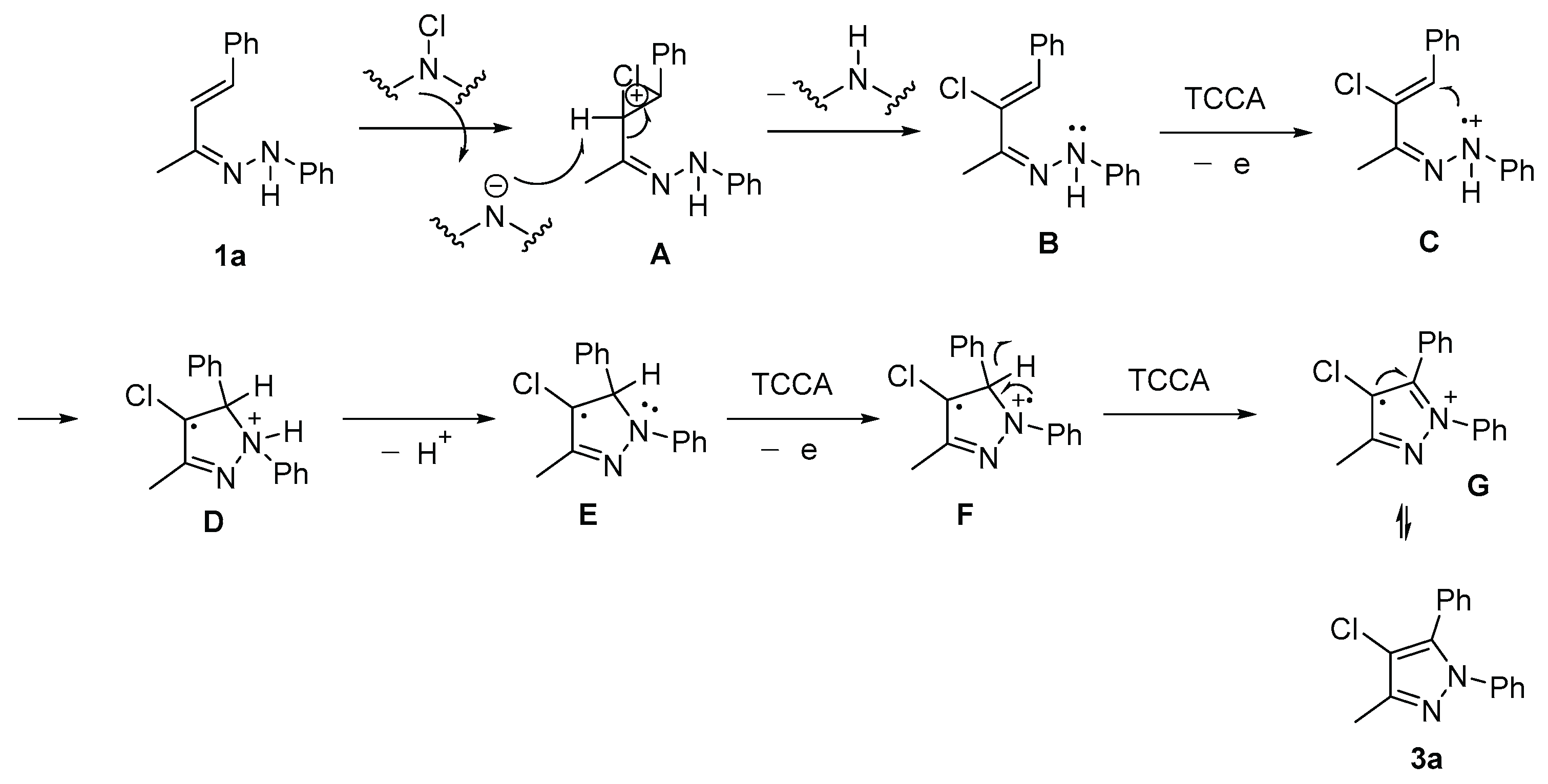
2. Results and discussion
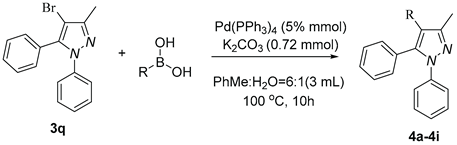 | ||
 |
 |
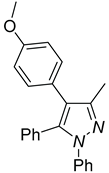 |
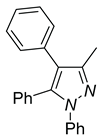
| 4a, 45%b | 4b, 48% | 4c, 40% |
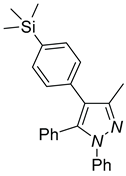 |
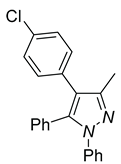 |
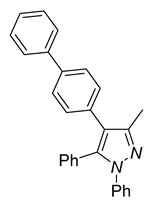 |
| 4d, 86% | 4e, 37% | 4f, 58% |
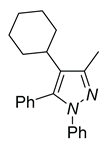 |
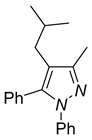 |
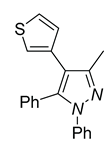 |
| 4g, 0% | 4h, 0% | 4i, 0% |
|
a Reaction conditions: 3q (0.3 mmol), R-B(OH)2 (0.36 mmol) . b Isolated determined. | ||
4. Conclusions
5. Experimental
5.1. General information
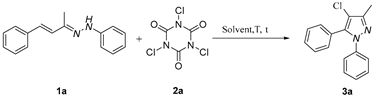
5.2. General procedure for the synthesis of pyrazole derivatives(3)
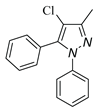
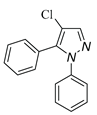
5.3. 4-chloro-3-methyl-1,5-diphenyl-1H-pyrazole (3a)
Funding
References
- Faisal, M.; Saeed, A.; Hussain, S.; Dar, P.; Larik, F. A. Recent developments in synthetic chemistry and biological activities of pyrazole derivatives. Journal of Chemical Sciences. 2019, 131, 1–30. [Google Scholar] [CrossRef]
- Menozzi, G.; Merello, L.; Fossa, P.; Schenone, S.; Ranise, A.; Mosti, A.; Bondavalli, F.; Loddo, R.; Murgioni, C.; Mascia, V.; Colla, P. L.; Tamburini, E. Synthesis, antimicrobial activity and molecular modeling studies of halogenated 4-[1H-imidazol-1-yl(phenyl)methyl]-1,5-diphenyl-1H-pyrazoles. Bioorganic & Medicinal Chemistry. 2004, 12, 5465–5483. [Google Scholar] [CrossRef]
- Huang, X.-F.; Lu, X.; Zhang, Y.; Song, G.-Q.; He, Q.-L.; Li, Q.-S.; Yang, X.-H.; Wei, Y.; Zhu, H,-L. Synthesis, biological evaluation, and molecular docking studies of N-((1,3-diphenyl-1H-pyrazol-4-yl)methyl)aniline derivatives as novel anticancer agents. Bioorganic & Medicinal Chemistry. (b) Abdel-Aziz, M.; Abuo-Rahma, G.E.-D.A.; Hassan, A. A. Synthesis of novel pyrazole derivatives and evaluation of their antidepressant and anticonvulsant activities. European Journal of Medicinal Chemistry. 2009, 44, 3480-3487. 2012, 20, 4895–4900. [Google Scholar]
- Fink, B. E.; Mortensen, D. S.; Stuffer, S. R.; Aron, Z. D.; Katzenellenbogen, J. A. Novel structural templates for estrogen-receptor ligands and prospects for combinatorial synthesis of estrogens. Chemistry & Biology. 1999, 6, 205–219. [Google Scholar] [CrossRef]
- Stauffer, S. R.; Coletta, C. J.; Tedesco, R.; Nishiguchi, G.; Carlson, K.; Sun, J.; Katzenellenbogen, B. S.; Katzenellenbogen, J. A. Pyrazole Ligands: Structure-Affinity/Activity Relationships and Estrogen Receptor-α-Selective Agonists. Journal of Medicinal Chemistry. 2000, 43, 4934–4947. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Okuda, F.; Kobayashi, K.; Nozaki, K.; Tanabe, Y.; Ishii, Y.; Haga, M. Syntheses and Phosphorescent Properties of Blue Emissive Iridium Complexes with Tridentate Pyrazolyl Ligands. Inorganic Chemistry. DOI:10.1021/ic800196s. (b) Bernhammer, J. C.; Huynh, H. V. Correlation of spectroscopically determined ligand donor strength and nucleophilicity of substituted pyrazoles. Dalton Transactions. 2012, 41, 8600-8608. DOI:10.1039/c2dt30526g. (c) Mogensen, S. B.; Taylor, M. K.; Lee, J. Homocoupling Reactions of Azoles and Their Applications in Coordination Chemistry. Molecules. 2020, 25, 5950-5979. DOI:10.3390/molecules25245950. (d) Li, X.; Yu, Y.; Tu, Z. Pyrazole Scaffold Synthesis, Functionalization, and Applications in Alzheimer's Disease and Parkinson's Disease Treatment (2011-2020). Molecules (Basel, Switzerland). 2021, 26, 1202-1239. 2008, 47, 7154–7165. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, G.; Merello, L.; Fossa, P.; Schenone, S.; Ranise, A.; Mosti, L.; Bondavalli, F.; Loddo, R.; Murgioni, C.; Mascia, V.; La Colla, P. Tamburini, E. Synthesis, antimicrobial activity and molecular modeling studies of halogenated 4-[1H-imidazol-1-yl(phenyl)methyl]-1,5-diphenyl-1H-pyrazoles. Bioorganic & Medicinal Chemistry. DOI:10.1016/j.bmc.2004.07.029. (b) Ismail, M. A. H.; Lehmann, J.; Abou El Ella, D. A.; Albohy, A. Abouzid, K. A. M. Lonazolac analogues: molecular modeling, synthesis, and in vivo anti-inflammatory activity. Medicinal Chemistry Research. 2009, 18, 725-744. DOI:10.1007/s00044-009-9163-2. (c) Khan, M. F.; Alam, M. M.; Verma, G.; Akhtar, W.; Akhter, M. Shaquiquzzaman, M. The therapeutic voyage of pyrazole and its analogs: A review. European Journal of Medicinal Chemistry. 2016, 120, 170-201. DOI:10.1016/j.ejmech.2016.04.077. (d) Santos, N. E.; Carreira, A. R. F.; Silva, V. L. M.; Braga, S. S. Natural and Biomimetic Antitumor Pyrazoles, A Perspective. Molecules. 2020, 25, 1364-1375. 2004, 12, 5465–5483. [Google Scholar] [CrossRef]
- Lamberth, C. Pyrazole chemistry in crop protection. Heterocycles. 2007, 71, 1467-1502. (b) Giornal, F.; Pazenok, S.; Rodefeld, L.; Lui, N.; Vors, J.; Leroux, F. R. Synthesis of diversely fluorinated pyrazoles as novel active agrochemical ingredients. Journal of Fluorine Chemistry. 2013, 152, 2–11. [Google Scholar] [CrossRef]
- Fioravanti, R.; Bolasco, A.; Manna, F.; Rossi, F.; Orallo, F.; Yáñez, M.; Vitali, A.; Ortuso, F.; Alcaro, S. Synthesis and molecular modelling studies of prenylated pyrazolines as MAO-B inhibitors. Bioorganic & Medicinal Chemistry Letters. DOI:10.1016/j.bmcl.2010.09.061. (b) Dumeunier, R.; Lamberth, C.; Trah, S. Synthesis of Tetrasubstituted Pyrazoles through Different Cyclization Strategies; Isosteres of Imidazole Fungicides. Synlett. 2013, 24, 1150-1154. DOI:10.1055/s-0033-1338433. (c) Huang, X.; Lu, X.; Zhang, Y.; Song, G.; He, Q.; Li, Q.; Yang, X.; Wei, Y. Zhu, H. Synthesis, biological evaluation, and molecular docking studies of N-((1,3-diphenyl-1H-pyrazol-4-yl)methyl)aniline derivatives as novel anticancer agents. Bioorganic & Medicinal Chemistry. 2012, 20, 4895-4900. DOI:10.1016/j.bmc.2012.06.056. (d) Merimi, I.; Touzani, R.; Aouniti, A.; Chetouani, A.; Hammouti, A. Pyrazole derivatives efficient organic inhibitors for corrosion in aggressive media: A comprehensive review. Int. J. Corros. Scale Inhib. 2020, 4, 1237-1260. 2010, 20, 6479–6482. [Google Scholar]
- Huang, X.; Lu, X.; Zhang, Y.; Song, G.; He, Q.; Li, Q.; Yang, X.; Wei, Y. Zhu, H. Synthesis, biological evaluation, and molecular docking studies of N-((1,3-diphenyl-1H-pyrazol-4-yl)methyl)aniline derivatives as novel anticancer agents. Bioorganic & Medicinal Chemistry. 2012, 20, 4895–4900. [Google Scholar]
- Menozzi, G.; Merello, L.; Fossa, P.; Schenone, S.; Ranise, A.; Mosti, L.; Bondavalli, F.; Loddo, R.; Murgioni, C.; Mascia, V.; La Colla, P.; Tamburini, E. Synthesis, antimicrobial activity and molecular modeling studies of halogenated 4-[1H-imidazol-1-yl(phenyl)methyl]-1,5-diphenyl-1H-pyrazoles. Bioorganic & Medicinal Chemistry. 2004, 12, 5465–5483. [Google Scholar]
- Boyd, S. T.; Fremming, B. A. Rimonabant-a selective CB1 antagonist. Annals Pharmacother. 2005, 39, 684–690. [Google Scholar] [CrossRef] [PubMed]
- [13] Ismail, M. A. H.; Lehmann, J.; Abou El Ella, D. A.; Albohy, A. Abouzid, K. A. M. Lonazolac analogues: molecular modeling, synthesis, and in vivo anti-inflammatory activity. Medicinal Chemistry Research. 2009, 18, 725–744. [Google Scholar]
- Emtiazi, H.; Amrollahi, M. A.; Mirjalili, B. B. F. Nano-silica sulfuric acid as an efficient catalyst for the synthesis of substituted pyrazoles. Arabian Journal of Chemistry. DOI: org/10.1016/j.arabjc.2013.06.008. (b) Li, M.; Zhao, B. X. Progress of the synthesis of condensed pyrazole derivatives (from 2010 to mid-2013). European Journal of Medicinal Chemistry 2014, 85, 311-340. 2015, 8, 793–797. [Google Scholar] [CrossRef]
- Santos, F.; María, S.-R.; Pablo, B.; Antonio, S.-F. From 2000 to Mid-2010: A Fruitful Decade for the Synthesis of Pyrazoles. Chemical Reviews. 2011, 111, 6984–7034. [Google Scholar] [CrossRef]
- Hu, J.; Cheng, Y.; Yang, Y.; Rao, Y. A general and efficient approach to 2H-indazoles and 1H-pyrazoles through copper-catalyzed intramolecular N–N bond formation under mild conditions. Chemical Communications. DOI:10.1039/c1cc13908h. (b) Yang, Y.; Kuang, C.; Jin, H.; Yang, Q.; Zhang, Z. Efficient synthesis of 1,3-diaryl-4-halo-1H-pyrazoles from 3-arylsydnones and 2-aryl-1,1-dihalo-1-alkenes. Beilstein J Org. Chem. 2011, 7, 1656-1662. DOI:10.3762/bjoc.7.195. (c) Li, X.; He, L.; Chen, H.; Wu, W.; Jiang, H. Copper-Catalyzed Aerobic C(sp2)–H Functionalization for C–N Bond Formation: Synthesis of Pyrazoles and Indazoles. The Journal of Organic Chemistry. 2013, 78, 3636-3646. DOI:10.1021/jo400162d. (d) Sar, D.; Bag, R.; Yashmeen, A.; Bag, S. S.; Punniyamurthy, T. Synthesis of Functionalized Pyrazoles via Vanadium-Catalyzed C–N Dehydrogenative Cross-Coupling and Fluorescence Switch-On Sensing of BSA Protein. Organic Letters. 2015, 17, 5308-5311. 2011, 47, 10133–10135. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Zhu, Q. A Facile Synthesis of Pyrazoles through Metal-Free Oxidative C(sp2)-H Cycloamination of Vinyl Hydrazo. Asian Journal of Organic Chemistry. DOI:10.1002/ajoc.201402254. (b) Zhang, X.; Kang, J.; Niu, P.; Wu, J.; Yu, W.; Chang, J. I2-Mediated Oxidative C–N Bond Formation for Metal-Free One-Pot Synthesis of Di-, Tri-, and Tetrasubstituted Pyrazoles from α,β-Unsaturated Aldehydes/Ketones and Hydrazines. The Journal of Organic Chemistry. 2014, 79, 10170-10178. 2015, 4, 42–45. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, R.; Marrot, J.; Coeffard, V.; Xiong, Y. Hypervalent iodine-mediated synthesis of benzoxazoles and benzimidazoles via an oxidative rearrangement. Tetrahydron. DOI: 10.1016/j.tet.2014.11.066. (b) Liu, Q.; Zhang, X.; He, Y.; Hussain, M. I.; Hu, W.; Xiong, Y.; Zhu, X. Oxidative rearrangement strategy for synthesis of 2,4,5-trisubstituted oxazoles utilizing hypervalent iodine reagent. Tetrahydron. 2016, 72, 5749-5753. DOI: 10.1016/j.tet.2016.07.082. (c) Hu, L.; Pan, J.; Zhang, X.; Hu W.; Xiong,Y.; Zhu, X. Synthesis of N, O π-conjugated boron complexes and the reactivity of Suzuki cross-coupling. Tetrahydron. 2017, 73, 223-229. DOI: 10.1016/j.tet.2016.11.068. (d) Shen H.; Deng Q.; Liu, R.; Feng, Y.; Zheng C.; Xiong Y. Intramolecular aminocyanation of alkenes promoted by hypervalent iodine. Organic Chemistry Frongtiers. 2017, 4, 1806-1811. 2015, 71, 700–708. [Google Scholar] [CrossRef]
- Zhang T, Bao W. Synthesis of 1H-Indazoles and 1H-Pyrazoles Via Febr3 /O2 Mediated Intramolecular C-H Amination. J. Org. Chem., 2013, 78 (3): 1317-1322.
 | |||||
| Entry | Solvent(mL) | t/h | T/℃ | TCCA(eq.) | Yield b (%) |
| 1 | TFE | 4 | 30 | 1.0 | 69 |
| 2 | MeOH | 4 | 30 | 1.0 | 8 |
| 3 | EtOH | 4 | 30 | 1.0 | 38 |
| 4 | HFIP | 4 | 30 | 1.0 | 40 |
| 5 | DMF | 4 | 30 | 1.0 | 32 |
| 6 | HOAc | 4 | 30 | 1.0 | 25 |
| 7 | 1,4-Dioxane | 4 | 30 | 1.0 | 14 |
| 8 | DCM | 4 | 30 | 1.0 | 6 |
| 9 | TFE | 4 | 0 | 1.0 | 64 |
| 10 | TFE | 4 | 40 | 1.0 | 75, (65) c |
| 11 | TFE | 4 | 60 | 1.0 | 70 |
| 12 | TFE | 2 | 40 | 1.0 | 70 |
| 13 | TFE | 6 | 40 | 1.0 | 68 |
| 14 | TFE | 4 | 40 | 0.8 | 68 |
| 15 | TFE | 4 | 40 | 1.2 | 58 |
 | ||
| Entry | additive (5wt%) | Yield b (%) |
| 1 | 4Å MS | 47 |
| 2 | CH3COOH | 57 |
| 3 | Pyridine | 63 |
| 4 | K2CO3 | 49 |
| 5 | Zn(OAc)2·2H2O | 63 |
| 6 | Cu(OAc)2·2H2O | 64 |
| 7 | Fe(OAc)2 | 72 |
 | |||
 |
 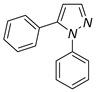
|
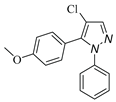 |
|
| 3a, 75%b | 3b, 0 | 3b′, 25% | 3c, 45% |
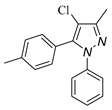 |
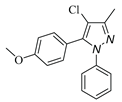 |
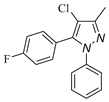 |
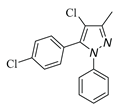 |
| 3d, 59% | 3e, 40% | 3f, 40% | 3g, 43% |
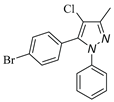 |
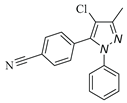 |
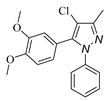 |
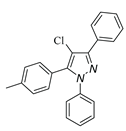 |
| 3h, 45% | 3i, 92% | 3j, 72% | 3k, 15% |
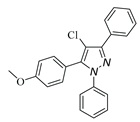 |
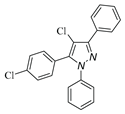 |
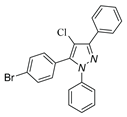 |
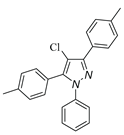 |
| 3l, 18% | 3m, 18% | 3n,25% | 3o,15% |
|
a Reaction conditions: 1a (0.5 mmol), 2a (0.5 mmol) in TFE (2 mL) at 40 ℃ for 4 h. b Isolated determined. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





