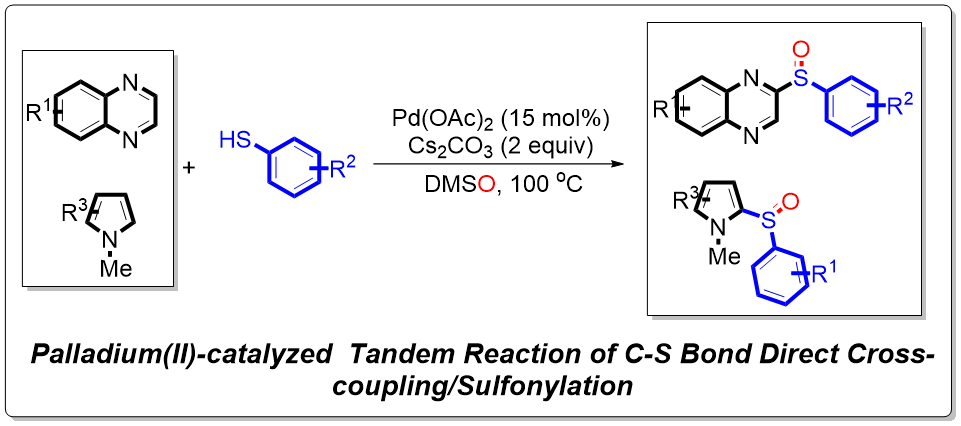
Organic sulfoxides and sulfone compounds have series most important applications in organic synthesis [
1], medicines[
2], functional materials[
3,
4]. For example,
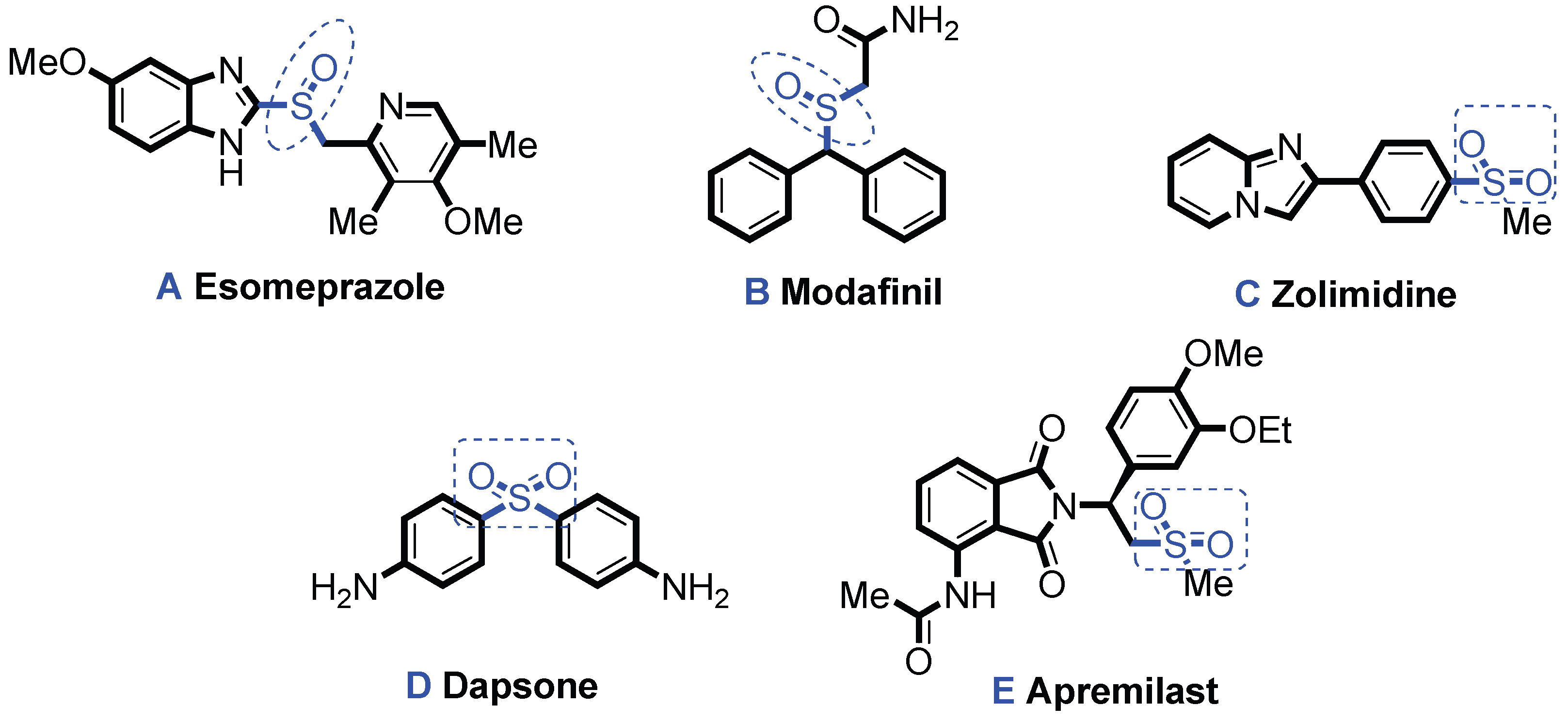
Scheme 1, Esomeprazole (
A) can effectively inhibit gastric acid secretion and it is a most widely used effective drug for treating disease-related diseases such as duodenal ulcer. Since its listing in 1989, the global cumulative sales have exceeded 60 billion dollars [
5]. Modafinil (
B) is an excitatory
α1 receptor agonist, mainly used for the treatment of spontaneous hypersomnia and sleep disorders, and was commercialized in the 1990s [
6]. Zolimidine (
C) is an imidazole heterocyclic derivative drug, mainly used for the treatment of digestive system diseases [
7]. Dapsone (
D) is a prescription drug for external use, used to treat inflammatory and non-inflammatory acne. Apremilast (
E) is the first oral phosphodiesterase-selective inhibitor used to treat active psoriasis and plaque psoriasis [
8].
The synthesis of organic sulfoxides and sulfone compounds have attracted extensive attention of synthetic chemists. It is known that transition-metal catalyzed cross coupling reaction is the mostly used methodology for the incorporation of a S atom into aromatic frameworks [
9]. However, prefunctionalization of the substrate is generally requested. Similar methods of C(sp2)-sulfoxide bonds formation have been scarcely described [
10,
11,
12]. Our group interesting are focuses on the tradition-metal catalyzed C-H bond functionizationals [
13]. Herein, a apalladium(II)-catalyzed tandem C-S bond direct cross-coupling/sulfonylation reaction has been developed. Starting from substituted quinoxalines and substituted phenylthiophenols versatile biologically active 2-(phenylsulfinyl)-6,7-dihydroquinoxaline derivatives and 1-methyl-2-(phenylsulfinyl)-1H-pyrrole derivatives were efficiently synthesized. The reaction mechanism was studied by the deuterium isotope experiments. This protocols were under mild reaction conditions, wider substrate scope and provides an economical approach toward C(sp
2)-sulfoxide bond formation.
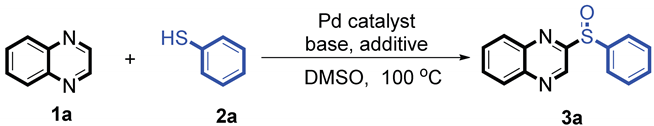
At first, the reaction conditions were screened based on the model reaction of quinoxaline
1a and phenylthiophenol
2a (
Table 1). The palladium catalysts displayed a good catalytic activity (entries 1-7). In addition, Pd(OAc)
2 gave a 70% yield (entry 7), exhibited superior catalytic efficiency over all of the examined palladium catalysts. These results indicated that Cs
2CO
3 were the optimal base and additive, which produced the product
3a with a 81% yield (entry 8). It was also noted that the product yield was decreased when the reaction temperature was lower or higher than 100 °C (entries 16 and 17). Furthermore, the results also show that DMSO as an essential solvent is higher than that of other solvents. Thus, the optimum reaction condition was determined as the
1 and
2 ratio of 1:1.5 in the presence of Pd(OAc)
2 (15 mol %), Cs
2CO
3 (2 equiv), at 100 °C (
Table 1, entry 15).
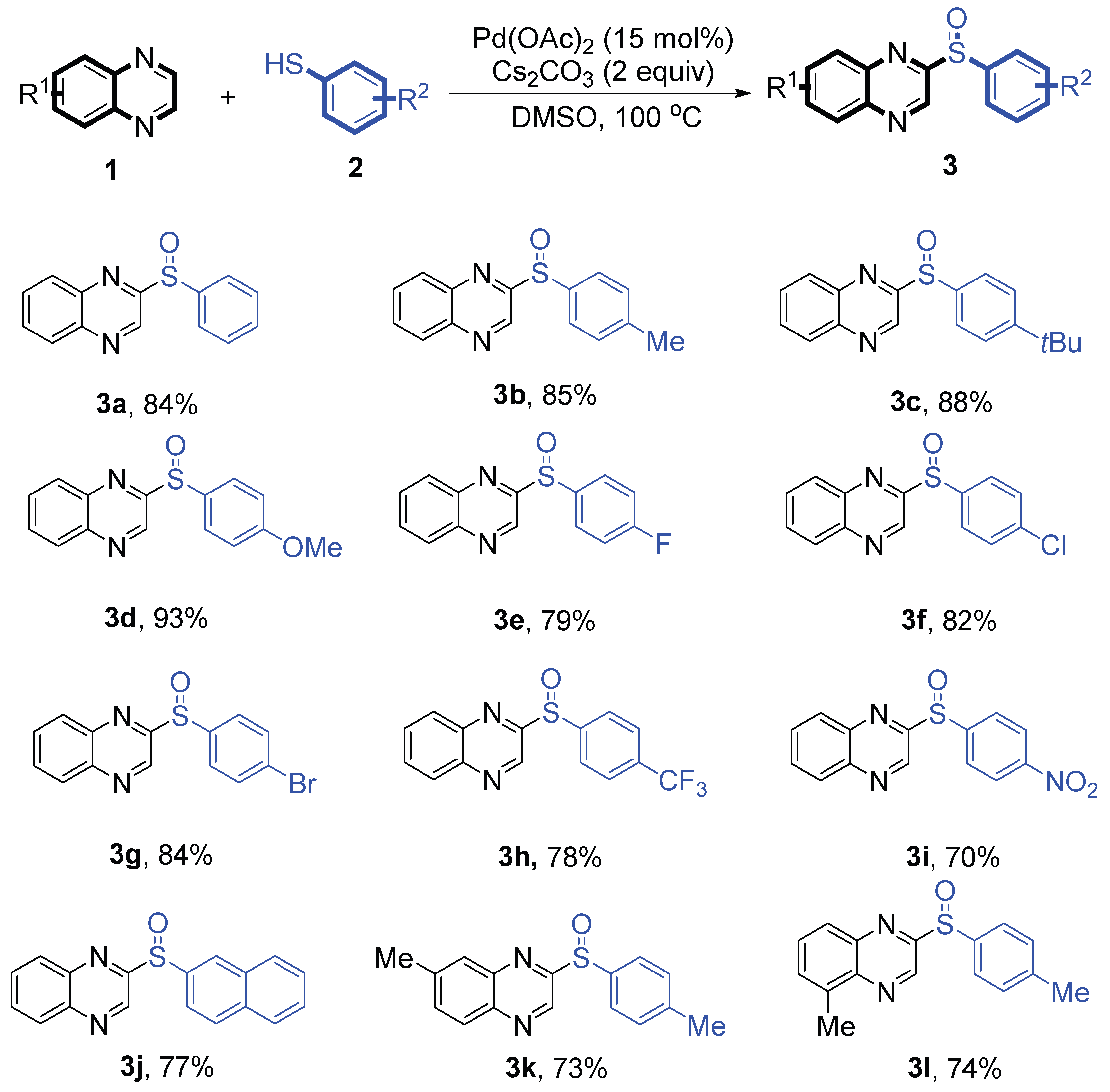
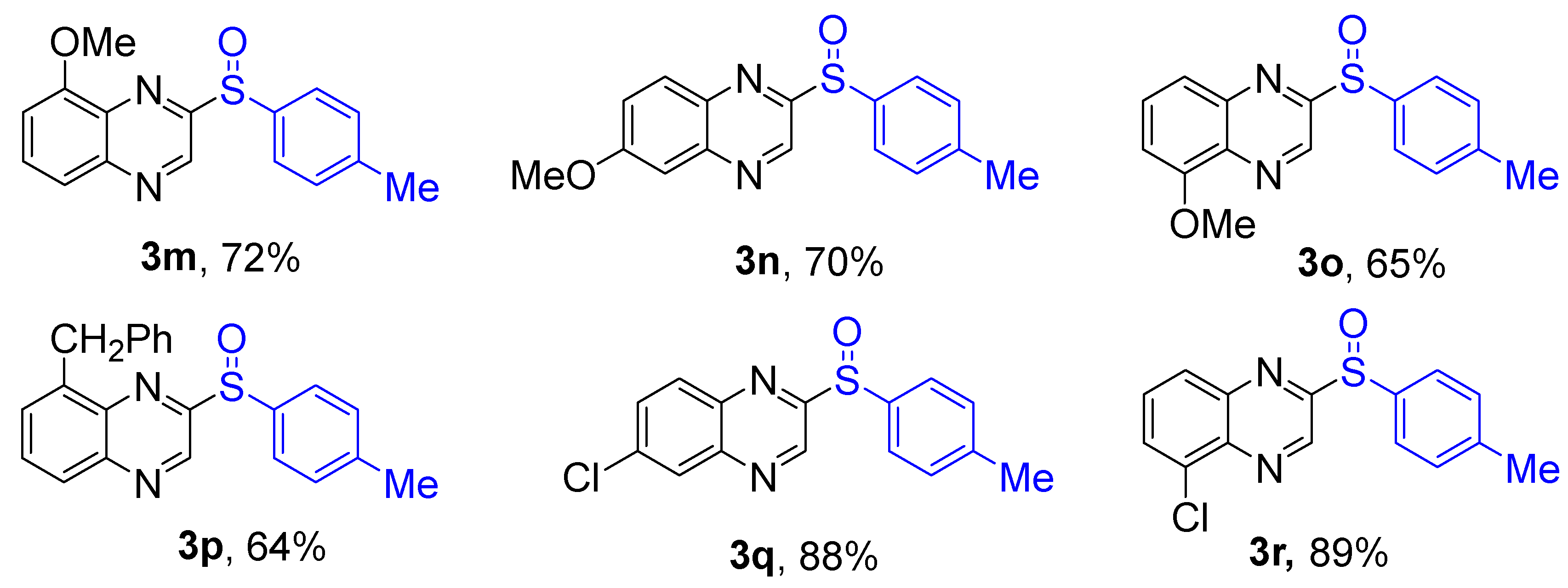
Next, the reaction scope was been screened, a wide array of substituted quinoxalines
1 and substituted phenylthiophenols
2 were subjected to this reaction and given the products 2-(phenylsulfinyl)-6,7-dihydroquinoxalines
3 in good to excellent yields (64-93% yield,
Scheme 2). It was found that both the electron-donating and electron-withdrawing quinoxaline derivatives
1 reacted smoothly with substituted phenylthiophenols
2. Furthermore, quinoxaline derivatives
1 bearing electron-withdrawing groups showed better activity than bearing electron-donating groups. Substituted phenylthiophenols
2 bearing electron-donating groups showed better activity than bearing electron-withdrawing groups. To our delight, despite the electron-withdrawing effect of -NO
2 and -CF
3 group is so strong, the corresponding product
s 3h and
3r were still obtained in 78% and 89% yield.
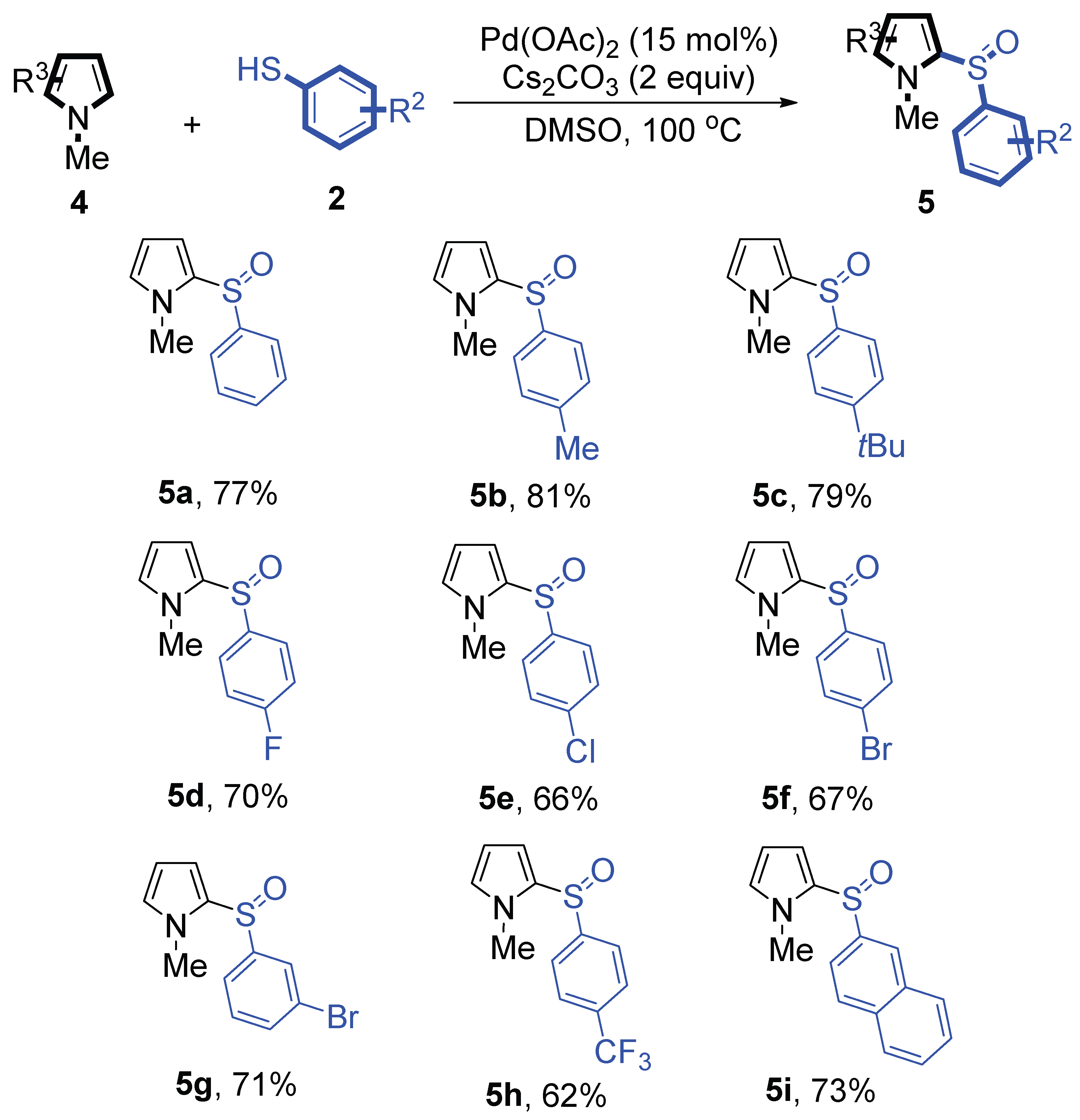
Furthermore, we next focused on evaluating the generality of palladium (II)-catalyzed tandem reaction of C-S bond direct cross-coupling/sulfonylation by using a series of pyrroles
4 (
Scheme 3). To our delight, N-methylpyrrole
4 with phenylthiophenols
2 successfully provided the corresponding products
5 (62-81% yield). For both substrates, this reaction was amenable when electroneutral group, electrondonating group, electron-withdrawing group, Moreover, the trifluoromethyl substituted delivered the product
5h exclusively in 62% yield which bearing of storang strong electron-withdrawing group. Furthermore, reactants with more complex substituents also perform smoothly. Both the results demonstrated the good generality and high functional group tolerance of this method.
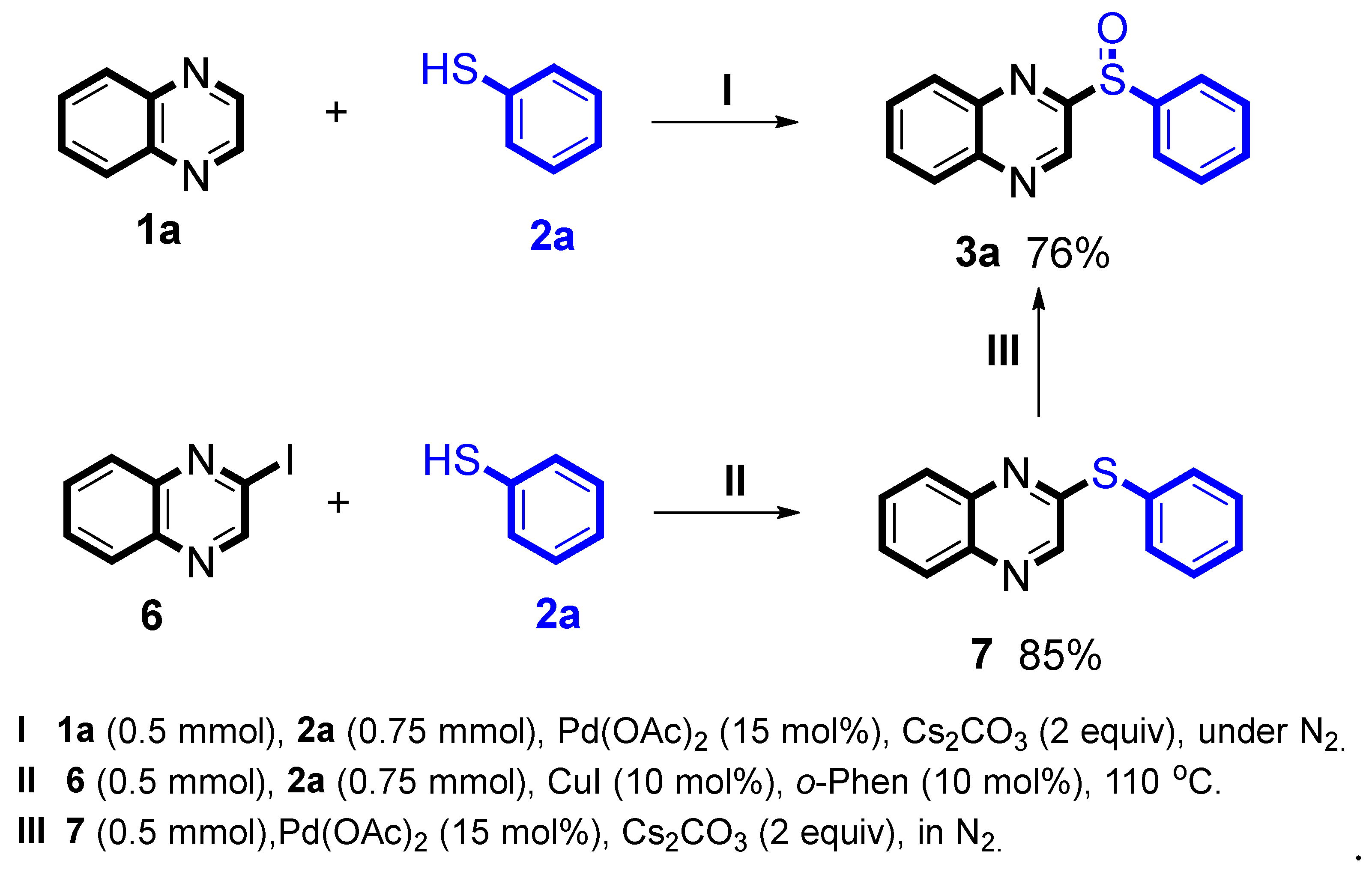
To obtain the preliminary datas of the mechanism, some addition reactions were been done (
Scheme 4). At first, the model reaction (4
I) was conducted in two separate steps: palladium (II)-catalyzed C-S bond direct cross-coupling/sulfonylation of
6 with
2a given a product
7 (4
II, 85% yield) [
13]. Next,
7 was reacted under our standard conditions, the reaction successfully obtained the target product
3a (4
III 76% yield), indicating that the intermediate
7 was involved in the reaction mechanism.
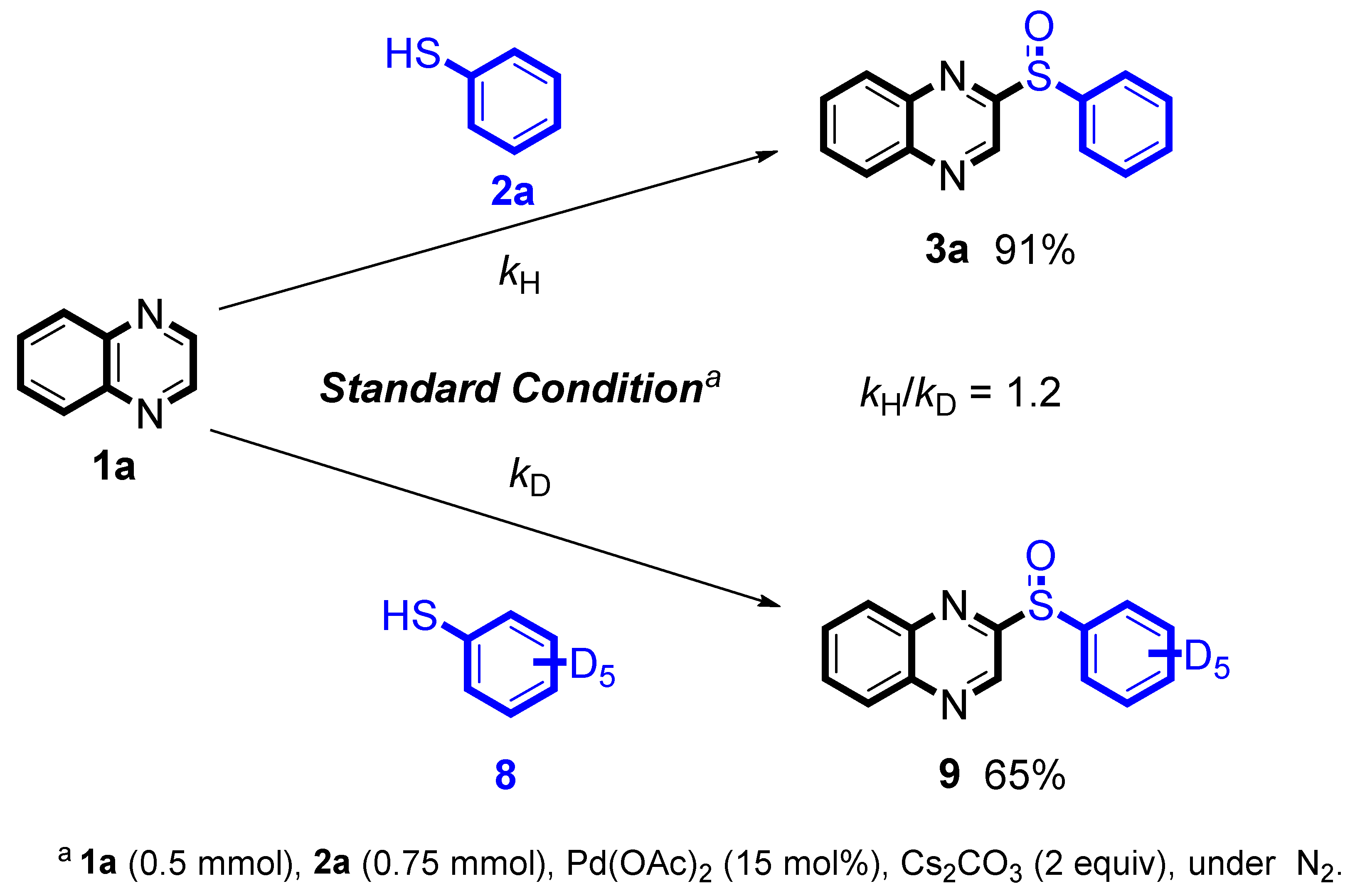
Next, we used isotope experiments to further study the reaction mechanism (
Scheme 5). The kinetic deuterium isotope effects [
14] observed in the control experiments were indicated that the C(sp
2)-H cleavage being the rate-limiting step (
kH/
kD = 1.4, for detail information please see SI).
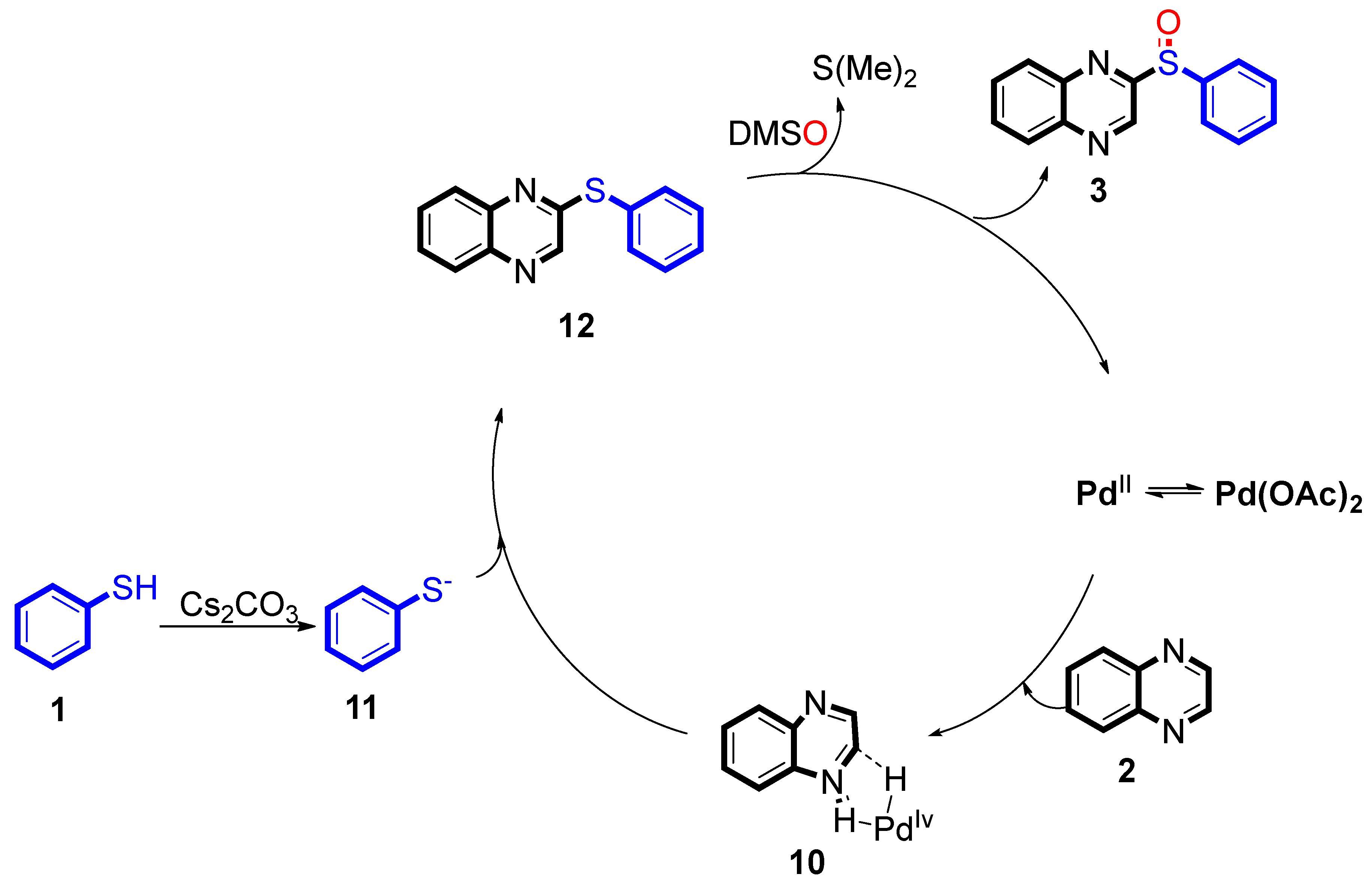
Based on the above results, a possible reaction mechanism was been proposed (
Scheme 6) [
15]. At the beginning, the coordination process of Pd
II and reactant
2 generated a Pd
IV intermediate
10. Then, reactant
1 was converted to intermediate
11 by reacted with Cs
2CO
3. Next, intermediate
12 was provided from intermediate
10 with
11 via C-S bond cross coupling. At last, through the oxidation reaction by DMSO, intermediate
12 generated the desired products
3 and concomitantly formed a Pd
II intermediate
, which re-entered the catalytic cycle.
Conclusions
In summary, in this paper a palladium (II)-catalyzed tandem reaction of C-S bond direct cross-coupling/sulfonylation has been developed. Starting from substituted quinoxalines and substituted phenylthiophenols versatile biologically active 2-(phenylsulfinyl)-6,7-dihydroquinoxaline and 1-methyl-2-(phenylsulfinyl)-1H-pyrrole derivatives were efficiently synthesized. The reaction mechanism was studied by the deuterium isotope experiments. This protocol features were under mild reaction conditions, wider substrate scope and provides an economical approach toward C(sp2)-sulfoxide bond formation.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Acknowledgments
Financial support provided by the Natural Science Foundation of China (No. 21702186), the Scientific Research Fund of Zhejiang Provincial Education Department (No. Y202454916) and the Huzhou Science and Technology Plan Project. In addition, authors Runsheng Xu and Jin Xu are both first authors.
References
- (a) J. Trenner, C. Depken, T. Weber and A. Breder, Angew. Chem., Int. Ed. 52 (2013) 8952-8956; (b) L. W. Huang, X. D. Xun, M. Zhao, J. Z. Xue, G. F. Li and L. Hong, J. Org. Chem. 84 (2019) 11885-11890; (c) R. B. Wei, H. G. Xiong, C. Q. Ye, Y. J. Li, and H. L. Bao, Org. Lett. 22 (2020) 3195-3199.
- (a) L. Engman, D. Stern, H. Frisell, K. Vessman, M. Berglund, B. Ek and C.-M. Andersson, Bioorg. Med. Chem. 3 (1995) 1255-1262; (b) T. Wirth, Angew. Chem., Int. Ed. 54 (2015) 10074-10076.
- S. Panda, A. Panda, and S. S. Zade, Coordin. Chem. Rev. 300 (2015) 86-100.
- S. Somasundaram, C. R. Chenthamarakshan, N. R. de Tacconi, Y. Ming, and K. Rajeshwar, Chem. Mater. 16 (2004) 3846-3852.
- K. S. Jain, A. K. Shah, J. Bariwal, S. M. Shelke, A. P. Kale, J. R. Jagtap and A. V. Bhosale, Bioorgan. Med. Chem., 15(2007) 1181-205.
- S. Tanganelli, K. Fuxe, L. Ferraro, A. M. Janson and C. Bianchi, N-S Arch. Pharmacol., 345(1992) 461-465.
- C. He, J. Hao, H. Xu, Y. P. Mo, H. Y. Liu, J. J. Han and A. W. Lei, Chem. Commun., 48 (2012) 11073-11075.
- H. W. Man, P. Schafer, L. M. Wong, R. T. Patterson, L. G. Corral, H. Raymon, K. Blease, J. Leisten, M. Shirley, A. Y. Tang, D. M. Babusis, R. Chen, D. Stirling and G. W. Muller, J. Med. Chem., 52(2009) 1522-1524.
- R. Qiu, V. P. Reddy, T. Iwasaki and N. Kambe, J. Org. Chem., 80 (2015) 367-374.
- S. Yu, B. Wan and X. Li, Org. Lett. 17 (2015) 58-61.
- W. Xie, B. Li and B. Wang, J. Org. Chem. 81 (2016) 396-403.
- G. He, Y. Zhao and S. Zhang, J. Am. Chem. Soc. 134 (2011) 3-6; (b) P. Xie, Y. Xie and B. Qian, J. Am. Chem. Soc. 134, (2012) 9902-9905; (c) J. He, S. Li, and Y. Deng, Science 343 (2014) 1216-1220.
- R. S. Xu, J. P. Wan, H. Mao and Y. J. Pan, J. Am. Chem. Soc. 132 (2010) 15531-15533; (b) F. F. Duan, S. Q. Song and R. S. Xu, Chem. Commun. 53 (2017) 2737-2739; (c) R. R. Cai, Z. D. Zhou, Q. Q. Chai, Y. E. Zhu and R. S. Xu, RSC Adv. 8 (2018) 26828-26836; (d) S. L. Guan, Y. Chen, H. J. Wu, R. R. Xu, Y. E. Zhu, F. X. Xing and S. L. Tong, Catalysts, 9(2019) 1-8; (e) R. R. Cai, Q. C. Wei and R. S. Xu, RSC Adv. 10(2020) 26414-26417; (f)R. R. Cai, Z.D. Zhou, Q. Q. Chai, Y. E. Zhu and R. S. Xu, RSC Adv.8 (2020) 26828-26836; (e) X. Y. Zhou, Y. Q. Xue, Y. Y. Cheng and R.S. Xu, Arkivoc 4 (2021) 119-129.
- V. K. Akkilagunta and R. R. Kakulapati, J. Org. Chem. 76 (2011) 6819-6824; (b) O. Vyhivskyi, D. N. Laikov, A. V. Finko, D. A. Skvortsov, I. V. Zhirkina, V. A. Tafeenko, N. Vasil'evich Zyk, A. G. Majouga and E. K. Beloglazkina, J. Org. Chem. 85 (2020) 3160-3173.
- B. Jiang, Z. W. Zhan, Q. Shi, Y. H. Liao, Y. R. Zou, Y. K. Tian and P. A. Peng, Anal. Chem. 91 (2019) 2209-2215.
Scheme 1.
The important clinical drugs of organic sulfoxides and sulfone compounds.
Scheme 1.
The important clinical drugs of organic sulfoxides and sulfone compounds.
Scheme 2.
Palladium (II)-catalyzed C-S bond direct cross-coupling/sulfonylation of quinoxalines with phenylthiophenols. a. a Unless noted, reaction conditions were 1 (0.5 mmol), 2 (0.75 mmol), Pd(OAc)2 (15 mol%), Cs2CO3 (2 equiv), under a N2 atmosphere, DMSO (15 mL), 100 °C for 12 h. b Isolated yield.
Scheme 2.
Palladium (II)-catalyzed C-S bond direct cross-coupling/sulfonylation of quinoxalines with phenylthiophenols. a. a Unless noted, reaction conditions were 1 (0.5 mmol), 2 (0.75 mmol), Pd(OAc)2 (15 mol%), Cs2CO3 (2 equiv), under a N2 atmosphere, DMSO (15 mL), 100 °C for 12 h. b Isolated yield.
Scheme 3.
Palladium (II)-catalyzed C-S bond direct cross-coupling/sulfonylation of N-methylpyrroles with phenylthiophenols. a a Unless noted, reaction conditions were 1 (0.5 mmol), 2 (0.75 mmol), Pd(OAc)2 (15 mol%), Cs2CO3 (2 equiv), under a N2 atmosphere, DMSO (15 mL), 100 °C for 12 h. b Isolated yield.
Scheme 3.
Palladium (II)-catalyzed C-S bond direct cross-coupling/sulfonylation of N-methylpyrroles with phenylthiophenols. a a Unless noted, reaction conditions were 1 (0.5 mmol), 2 (0.75 mmol), Pd(OAc)2 (15 mol%), Cs2CO3 (2 equiv), under a N2 atmosphere, DMSO (15 mL), 100 °C for 12 h. b Isolated yield.
Scheme 4.
Preliminary datas of the palladium (II)-catalyzed C-S bond direct cross-coupling/sulfonylation reaction mechanism.
Scheme 4.
Preliminary datas of the palladium (II)-catalyzed C-S bond direct cross-coupling/sulfonylation reaction mechanism.
Scheme 5.
The kinetic deuterium isotope effects of palladium (II)-catalyzed C-S bond direct cross-coupling/sulfonylation reaction.
Scheme 5.
The kinetic deuterium isotope effects of palladium (II)-catalyzed C-S bond direct cross-coupling/sulfonylation reaction.
Scheme 6.
Proposed palladium (II)-catalyzed C-S bond direct cross-coupling/sulfonylation reaction mechanism.
Scheme 6.
Proposed palladium (II)-catalyzed C-S bond direct cross-coupling/sulfonylation reaction mechanism.
Table 1.
Optimization of the reaction conditions.a.
Table 1.
Optimization of the reaction conditions.a.
 |
| Entry |
Palladium catalyst |
Base |
Solvent |
1a: 2a
|
Yield (%)b |
| 1 |
Pd(CO)4
|
Na2CO3
|
CH3CN |
1:1 |
0 |
| 2 |
PdCl2(PPh3)2
|
Na2CO3
|
DMSO |
1:1 |
13 |
| 3 |
[PdCl(C3H5)]2
|
Na2CO3
|
DMSO |
1:1 |
24 |
| 4 |
PdCl2
|
Na2CO3
|
DMSO |
1:1 |
29 |
| 5 |
PdBr2
|
Na2CO3
|
DMSO |
1:1 |
55 |
| 6 |
PdSO4
|
Na2CO3
|
DMSO |
1:1 |
39 |
| 7 |
Pd(OAc)2
|
Na2CO3
|
DMSO |
1:1 |
70 |
| 8 |
Pd(OAc)2
|
Cs2CO3
|
DMSO |
1:1 |
81 |
| 9 |
Pd(OAc)2
|
NaOH |
DMSO |
1:1 |
51 |
| 10 |
Pd(OAc)2
|
Na2SO4
|
DMSO |
1:1 |
44 |
| 11 |
Pd(OAc)2
|
NaOEt |
DMSO |
1:1 |
60 |
| 12 |
Pd(OAc)2
|
Cs2CO3
|
DMSO |
1:1 |
0 |
| 13 |
Pd(OAc)2
|
Cs2CO3
|
DMSO |
1:1 |
43 |
| 14 |
Pd(OAc)2
|
Cs2CO3
|
DMSO |
1:1 |
48 |
| 15 |
Pd(OAc)2
|
Cs2CO3
|
DMSO |
1:1.5 |
84 |
| 16 |
Pd(OAc)2
|
Cs2CO3
|
DMSO |
1:1.5 |
71c
|
| 17 |
Pd(OAc)2
|
Cs2CO3
|
DMSO |
1:1.5 |
79d
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).