Submitted:
15 August 2025
Posted:
18 August 2025
You are already at the latest version
Abstract
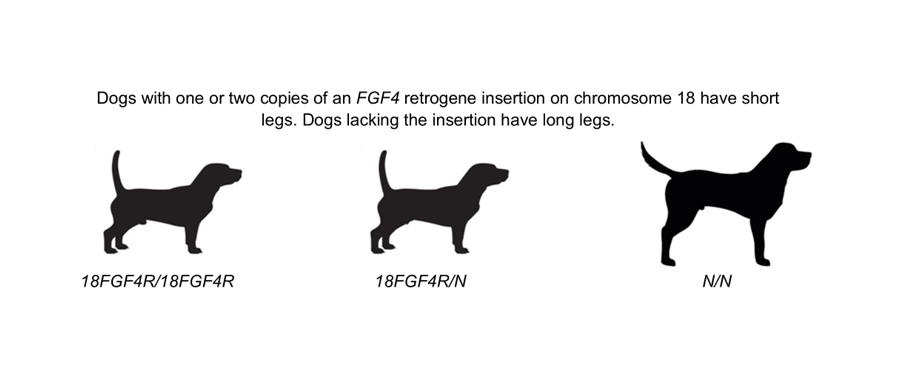
Keywords:
1. Introduction
2. Materials and Methods
2.1. Subject Dogs
2.2. Molecular Genetics

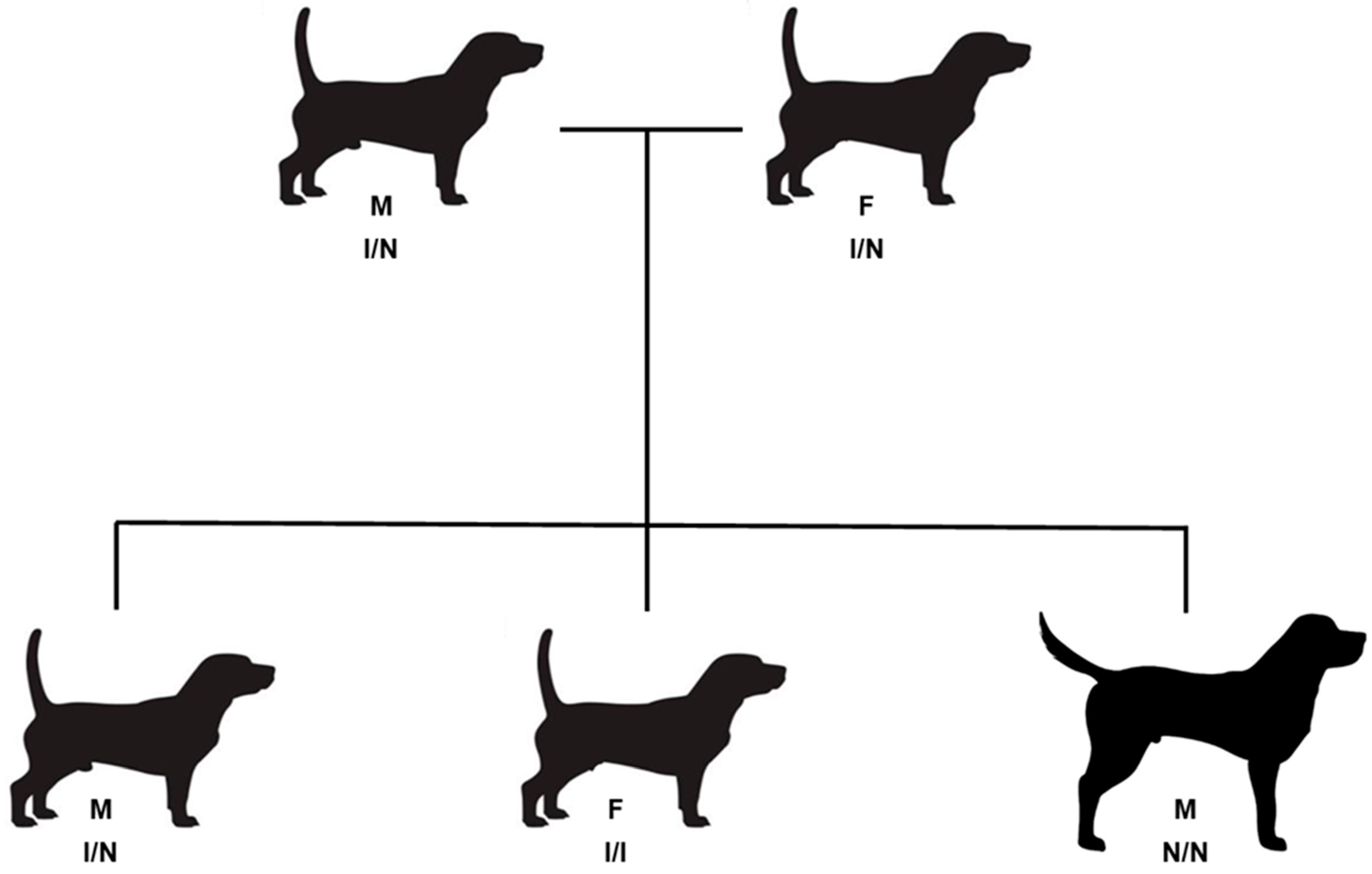
2.3. Statistical Analysis
3. Results
| 18-FGF4R Insertion | Short | Tall |
|---|---|---|
| Insertion/Insertion | 5 | 0 |
| Insertion/No Insertion | 13 | 0 |
| No Insertion/No Insertion | 0 | 4 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GBGV | Grand Basset Griffon Vendéen |
| PBGV | Petit Basset Griffon Vendéen |
| EDTA | Ethylene diamine tetra acetic acid |
| PCR | Polymerase chain reaction |
| I | Insertion |
| N | Non-insertion |
| FGF4 | fibroblast growth factor 4 |
References
- Parker, H.G.; VonHoldt, B.M.; Quignon, P.; Margulies, E.H.; Shao, S.; Mosher, D.S.; Spady, T.C.; Elkahloun, A.; Cargill, M.; Jones, P.G.; et al. An Expressed Fgf4 Retrogene Is Associated with Breed-Defining Chondrodysplasia in Domestic Dogs. Science 2009, 325, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Helmink, S.K.; Shanks, R.D.; Leighton, E.A. Investigation of Breeding Strategies to Increase the Probability That German Shepherd Dog and Labrador Retriever Dog Guides Would Attain Optimum Size. J Anim Sci 2003, 81, 2950–2958. [Google Scholar] [CrossRef]
- Chen, F.L.; Zimmermann, M.; Hekman, J.P.; Lord, K.A.; Logan, B.; Russenberger, J.; Leighton, E.A.; Karlsson, E.K. Advancing Genetic Selection and Behavioral Genomics of Working Dogs Through Collaborative Science. Front Vet Sci 2021, 8, 662429. [Google Scholar] [CrossRef]
- Hecht, E.E.; Smaers, J.B.; Dunn, W.D.; Kent, M.; Preuss, T.M.; Gutman, D.A. Significant Neuroanatomical Variation Among Domestic Dog Breeds. J Neurosci 2019, 39, 7748–7758. [Google Scholar] [CrossRef]
- Bannasch, D.; Batcher, K.; Leuthard, F.; Bannasch, M.; Hug, P.; Marcellin-Little, D.J.; Dickinson, P.J.; Drogemuller, M.; Drogemuller, C.; Leeb, T. The Effects of <ovid:I>FGF4</Ovid:I> Retrogenes on Canine Morphology. Genes (Basel) 2022, 13. [Google Scholar] [CrossRef]
- Sullivan, S.; Szeremeta, K.J.; Kutzler, M. Case Report: FGF4L1 Retrogene Insertion Is Lacking in the Tall Dachshund Phenotype. Front Vet Sci 2024, 11, 1522745. [Google Scholar] [CrossRef]
- Brown, E.A.; Dickinson, P.J.; Mansour, T.; Sturges, B.K.; Aguilar, M.; Young, A.E.; Korff, C.; Lind, J.; Ettinger, C.L.; Varon, S.; et al. FGF4 Retrogene on CFA12 Is Responsible for Chondrodystrophy and Intervertebral Disc Disease in Dogs. Proc Natl Acad Sci U S A 2017, 114, 11476–11481. [Google Scholar] [CrossRef]
- Katz, M.L.; Khan, S.; Awano, T.; Shahid, S.A.; Siakotos, A.N.; Johnson, G.S. A Mutation in the CLN8 Gene in English Setter Dogs with Neuronal Ceroid-Lipofuscinosis. Biochem Biophys Res Commun 2005, 327. [Google Scholar] [CrossRef]
- Zeng, R.; Coates, J.R.; Johnson, G.C.; Hansen, L.; Awano, T.; Kolicheski, A.; Ivansson, E.; Perloski, M.; Lindblad-Toh, K.; O’Brien, D.P.; et al. Breed Distribution of SOD1 Alleles Previously Associated with Canine Degenerative Myelopathy. J Vet Intern Med 2014, 28. [Google Scholar] [CrossRef]
- Thalmann, O.; Shapiro, B.; Cui, P.; Schuenemann, V.J.; Sawyer, S.K.; Greenfield, D.L.; Germonpre, M.B.; Sablin, M.V.; Lopez-Giraldez, F.; Domingo-Roura, X.; et al. Complete Mitochondrial Genomes of Ancient Canids Suggest a European Origin of Domestic Dogs. Science 2013, 342, 871–874. [Google Scholar] [CrossRef]
- Ni Leathlobhair, M.; Perri, A.R.; Irving-Pease, E.K.; Witt, K.E.; Linderholm, A.; Haile, J.; Lebrasseur, O.; Ameen, C.; Blick, J.; Boyko, A.R.; et al. The Evolutionary History of Dogs in the Americas. Science 2018, 361, 81–85. [Google Scholar] [CrossRef]
- Sinding, M.-H.S.; Gopalakrishnan, S.; Ramos-Madrigal, J.; de Manuel, M.; Pitulko, V.V.; Kuderna, L.; Feuerborn, T.R.; Frantz, L.A.F.; Vieira, F.G.; Niemann, J.; et al. Arctic-Adapted Dogs Emerged at the Pleistocene-Holocene Transition. Science 2020, 368, 1495–1499. [Google Scholar] [CrossRef]
- Batcher, K.; Dickinson, P.; Giuffrida, M.; Sturges, B.; Vernau, K.; Knipe, M.; Rasouliha, S.H.; Drögemüller, C.; Leeb, T.; Maciejczyk, K.; et al. Phenotypic Effects of FGF4 Retrogenes on Intervertebral Disc Disease in Dogs. Genes (Basel) 2019, 10. [Google Scholar] [CrossRef]
- Reunanen, V.L.J.; Jokinen, T.S.; Lilja-Maula, L.; Hytonen, M.K.; Lappalainen, A.K. Allelic Frequency of 12-FGF4RG and the Association between the Genotype with Number of Calcified Intervertebral Discs Visible on Radiographs in Coton de Tulear and French Bulldog Breeds. BMC Vet Res 2025, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.K.; Blacksmith, M.S.; Kidd, J.M. Duplications and Retrogenes Are Numerous and Widespread in Modern Canine Genomic Assemblies. Genome Biol Evol 2024, 16. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Adetula, A.A.; Wu, Y.; Chen, H. Analysis of New Retrogenes Provides Insight into Dog Adaptive Evolution. Ecol Evol 2019, 9, 11185–11197. [Google Scholar] [CrossRef]
- Ciomborowska-Basheer, J.; Staszak, K.; Kubiak, M.R.; Makalowska, I. Not So Dead Genes-Retrocopies as Regulators of Their Disease-Related Progenitors and Hosts. Cells 2021, 10. [Google Scholar] [CrossRef]
- Vinckenbosch, N.; Dupanloup, I.; Kaessmann, H. Evolutionary Fate of Retroposed Gene Copies in the Human Genome. Proc Natl Acad Sci U S A 2006, 103, 3220–3225. [Google Scholar] [CrossRef]
- Boulet, A.M.; Capecchi, M.R. Signaling by FGF4 and FGF8 Is Required for Axial Elongation of the Mouse Embryo. Dev Biol 2012, 371, 235–245. [Google Scholar] [CrossRef]
- Batcher, K.; Varney, S.; Affolter, V.K.; Friedenberg, S.G.; Bannasch, D. An SNN Retrocopy Insertion Upstream of GPR22 Is Associated with Dark Red Coat Color in Poodles. G3 (Bethesda) 2022, 12. [Google Scholar] [CrossRef]
- Machado, J.P.; Antunes, A. The Genomic Context of Retrocopies Increases Their Chance of Functional Relevancy in Mammals. Genomics 2020, 112, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





