Submitted:
11 August 2025
Posted:
13 August 2025
You are already at the latest version
Abstract
Background/Objectives The document comprehensively reviews the clinical applications and future potential of alpha-emitting radionuclides available for targeted alpha-particle therapy (TAT) in cancer treatment. The approval of radium-223 (Ra-223) therapy in 2013 marked a significant advancement in alpha-emitting therapeutic radiopharmaceuticals, which are primarily used in treatment of prostate cancer. The EU SECURE project was introduced as a major initiative to enhance the sustainability and safety of medical alpha-emitting radionuclides production in Europe. Methods: This literature review was conducted by a multidisciplinary team on selected radionuclides, including actinium-225, bismuth-213, astatine-211, lead-212, terbium-149, radium-22323 and thorium-227. These were selected based on their clinical significance, as identified in the EU PRISMAP project and subsequent literature searches. The review process involved searching major databases using specific keywords related to alpha-emitter therapy and was limited to articles in English. For each selected radionuclide, the physical characteristics, the radiochemistry, and the pre-clinical and clinical studies are explored. Results of the review show current and potential clinical applications of new alpha-emitting radionuclides, sharing insights from the SECURE consortium’s experiences and providing recommendations for future clinical trials to establish the therapeutic efficacy of these radionuclides. Conclusion: For each selected radionuclide, conclusion are reported in individual chapters. The results highlight the advantages of alpha particles in targeting cancer cells with minimal radiation to normal tissue, emphasising the need for high specificity and stability in delivery mechanisms, but also suggest that the full clinical potential of alpha particle therapy remains unexplored. Theranostic approach and dosimetric evaluations still represent relevant challenges.
Keywords:
1. Introduction
2. Results
2.1. Actinium-225
2.1.1. Physical Characteristics
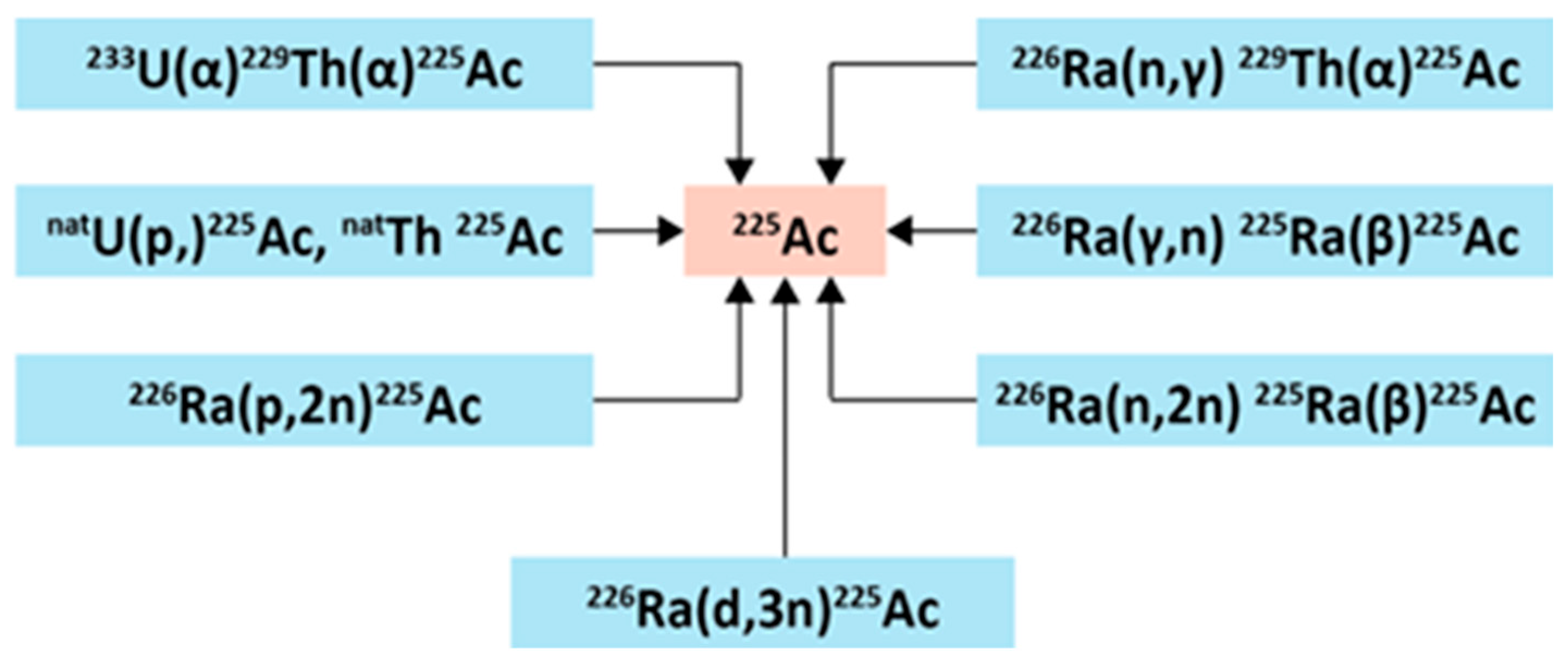
2.1.1.1. Radiochemical Extraction from Thorium-229
2.1.1.2. Accelerator-Based Routes
2.1.2. Radiochemistry
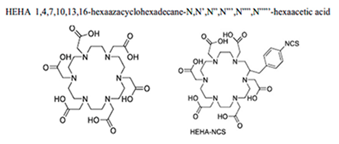
2.1.2.1. Chelating Agents for Actinium-225
2.1.2.2. Actinium-225 Labelled Nanoparticles
2.1.2.3. Assessing the Biodistribution of the Actinium-225 Decay Chain
2.1.3. Preclinical Studies
2.1.4. Clinical Studies
2.1.5. Conclusion
2.2. Bismuth-213
2.2.1. Physical Characteristics
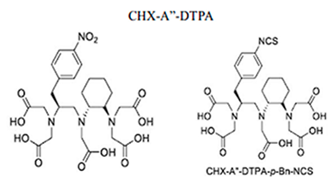
2.2.2. Radiochemistry
2.2.3. Preclinical Studies
2.2.4. Clinical Studies
- Bi-213-radioimmunoconjugates (Bi-213-RICs) were also investigated for therapy of malignant melanoma [69].
- [213Bi]Bi-HuM195 was also successfully attempted for acute myelogenous leukaemia or chronic myelomonocytic leukaemia (CML), involving: 93% of the treated patients had reductions in circulating blasts, and 78% experienced a decline in bone marrow blasts, with no significant extramedullary toxicity reported [54].
- [213Bi]Bi-PSMA-617 for mCRPC, resulted in imaging response and a decrease in prostate-specific antigen levels, and [213Bi]Bi-DOTATOC in neuroendocrine tumours refractory to beta emitter 177Lu/90Y-DOTATOC, which led to a significant reduction in targeting agent uptake, i.e. probable reduction of lesion size [55].
2.2.4.1. Locoregional Administration
2.2.5. Conclusion
2.3. Astatine-211
2.3.1. Physical Characteristics
2.3.2. Radiochemistry
2.3.3. Preclinical Studies
2.3.4. Clinical Studies
2.3.5. Conclusion
2.4. Lead-212
2.4.1. Physical Characteristics
2.4.2. Radiochemistry
2.4.3. Preclinical Studies
2.4.4. Clinical Studies
- [212Pb Pb-VMT-α-NET ([212Pb]Pb-PSC-PEG2-TOC) for somatostatin expressing neuroendocrine tumor (NCT06479811, NCT06427798)
- [212Pb]Pb-VMT01 ([212Pb]Pb- DOTA-PEG2-α-MSH for melanoma tumors expressing the melanocortin sub-type 1 receptor (MC1R) (NCT05655312) [113] .
2.4.5. Conclusion
2.5. Terbium-149
2.5.1. Physical Characteristics
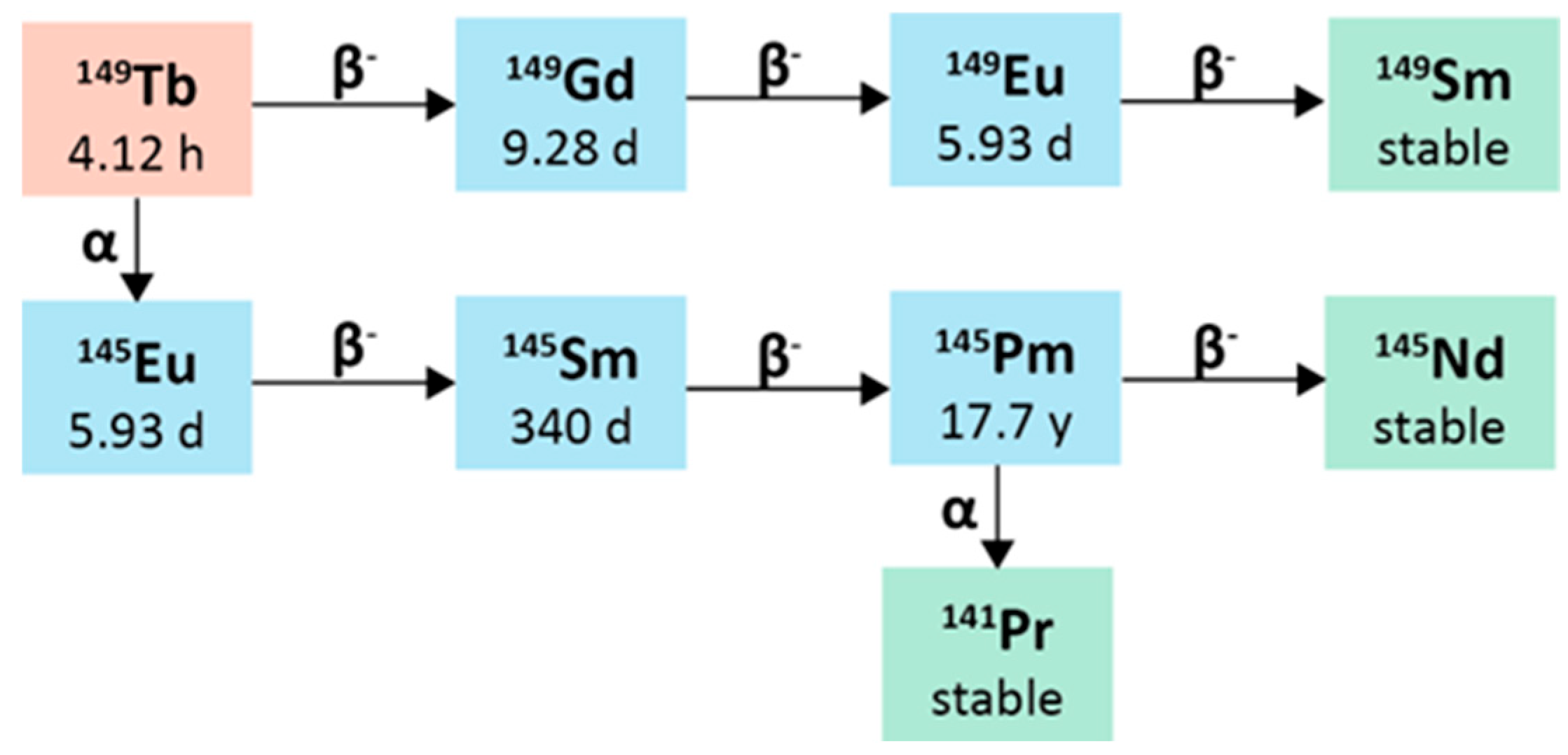
2.5.2. Radiochemistry
2.5.3. Preclinical Studies
2.5.4. Clinical Studies
2.5.5. Conclusion
2.6. Radium-223
2.6.1. Physical Characteristics
2.6.2. Radiochemistry
2.6.3. Preclinical Studies
2.6.4. Clinical Studies
2.6.5. Conclusion
2.7. Thorium-227
2.6.1. Physical Characteristics
2.7.2. Radiochemistry
2.7.3. Preclinical Studies
2.7.4. Clinical Studies
- BAY2287411 (or MSLN-TTC) for solid tumors expressing mesothelin (NCT03507452),
- BAY2701439 (or HER2-TTC) for cancers with HER2 expression as breast cancer or gastric cancer (NCT04147819),
- BAY2315497 (or PSMA-TTC) for mCRPC (NCT03724747). Intermediate results from different studies have already been reported.
- BAY 1862864, which is a [227Th]Th-labelled CD22-targeting antibody, was injected into patients with CD22-positive relapsed/refractory B cell non-Hodgkin lymphoma (R/R-NHL) (NCT02581878), and the therapy resulted in safe and well-tolerated, with an objective response rate of 25% [150].
2.7.5. Conclusion
3. Discussion and General Recommendations
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eychenne, R.; Chérel, M.; Haddad, F.; Guérard, F.; Gestin, J.-F. Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The “Hopeful Eight. ” Pharmaceutics 2021, 13, 906. [Google Scholar] [CrossRef] [PubMed]
- Hatcher-Lamarre, J.L.; Sanders, V.A.; Rahman, M.; Cutler, C.S.; Francesconi, L.C. Alpha Emitting Nuclides for Targeted Therapy. Nucl. Med. Biol. 2021, 92, 228–240. [Google Scholar] [CrossRef]
- Engle, J.W. The Production of Ac-225. Curr. Radiopharm. 2018, 11, 173–179. [Google Scholar] [CrossRef]
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An Overview of Targeted Alpha Therapy with225 Actinium and213 Bismuth. Curr. Radiopharm. 2018, 11, 200–208. [Google Scholar] [CrossRef]
- Pommé, S.; Marouli, M.; Suliman, G.; Dikmen, H.; Van Ammel, R.; Jobbágy, V.; Dirican, A.; Stroh, H.; Paepen, J.; Bruchertseifer, F.; et al. Measurement of the 225Ac Half-Life. Appl. Radiat. Isot. 2012, 70, 2608–2614. [Google Scholar] [CrossRef]
- Suliman, G.; Pommé, S.; Marouli, M.; Van Ammel, R.; Stroh, H.; Jobbágy, V.; Paepen, J.; Dirican, A.; Bruchertseifer, F.; Apostolidis, C.; et al. Half-Lives of 221Fr, 217At, 213Bi, 213Po and 209Pb from the 225Ac Decay Series. Appl. Radiat. Isot. 2013, 77, 32–37. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F. Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics 2020, 13, 49. [Google Scholar] [CrossRef]
- Ahenkorah, S.; Cassells, I.; Deroose, C.M.; Cardinaels, T.; Burgoyne, A.R.; Bormans, G.; Ooms, M.; Cleeren, F. Bismuth-213 for Targeted Radionuclide Therapy: From Atom to Bedside. Pharmaceutics 2021, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Scheinberg, D.; R. McDevitt, M. Actinium-225 in Targeted Alpha-Particle Therapeutic Applications. Curr. Radiopharm. 2011, 4, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R. Managing the Uranium-233 Stockpile of the United States. Sci. Glob. Secur. 2013, 21, 53–69. [Google Scholar] [CrossRef]
- Robertson, A.K.H.; Ramogida, C.F.; Schaffer, P.; Radchenko, V. Development of225 Ac Radiopharmaceuticals: TRIUMF Perspectives and Experiences. Curr. Radiopharm. 2018, 11, 156–172. [Google Scholar] [CrossRef]
- Boll, R.A.; Malkemus, D.; Mirzadeh, S. Production of Actinium-225 for Alpha Particle Mediated Radioimmunotherapy. Appl. Radiat. Isot. 2005, 62, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, C.; Molinet, R.; Rasmussen, G.; Morgenstern, A. Production of Ac-225 from Th-229 for Targeted α Therapy. Anal. Chem. 2005, 77, 6288–6291. [Google Scholar] [CrossRef]
- Kotovskii, A.A.; Nerozin, N.A.; Prokof’ev, I.V.; Shapovalov, V.V.; Yakovshchits, Yu.A.; Bolonkin, A.S.; Dunin, A.V. Isolation of Actinium-225 for Medical Purposes. Radiochemistry 2015, 57, 285–291. [Google Scholar] [CrossRef]
- Ahenkorah, S.; Cassells, I.; Deroose, C.M.; Cardinaels, T.; Burgoyne, A.R.; Bormans, G.; Ooms, M.; Cleeren, F. Bismuth-213 for Targeted Radionuclide Therapy: From Atom to Bedside. Pharmaceutics 2021, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, A.; Apostolidis, C.; Bruchertseifer, F. Supply and Clinical Application of Actinium-225 and Bismuth-213. Semin. Nucl. Med. 2020, 50, 119–123. [Google Scholar] [CrossRef]
- Harvey, J.; Nolen, J.A.; Kroc, T.; Gomes, I.; Horwitz, E. Philip.; Mcalister, D.R. PRODUCTION OF ACTINIUM-225 VIA HIGH ENERGY PROTON INDUCED SPALLATION OF THORIUM-232. In Proceedings of the Applications of High Intensity Proton Accelerators; WORLD SCIENTIFIC: Fermilab, Chicago, June 2010; pp. 321–326. [Google Scholar]
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, F.M.; Fassbender, M.E.; Simon, S.; Birnbaum, E.R.; Atcher, R.W.; John, K.D.; Rixe, O.; et al. Alpha-Emitters and Targeted Alpha Therapy in Oncology: From Basic Science to Clinical Investigations. Target. Oncol. 2018, 13, 189–203. [Google Scholar] [CrossRef]
- Morgenstern, A.; Bruchertseifer, F.; Apostolidis, C. Bismuth-213 and Actinium-225 – Generator Performance and Evolving Therapeutic Applications of Two Generator-Derived Alpha-Emitting Radioisotopes. Curr. Radiopharm. 2012, 5, 221–227. [Google Scholar] [CrossRef]
- Hogle, S.; Boll, R.A.; Murphy, K.; Denton, D.; Owens, A.; Haverlock, T.J.; Garland, M.; Mirzadeh, S. Reactor Production of Thorium-229. Appl. Radiat. Isot. 2016, 114, 19–27. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with225 Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef]
- Englert, M.; Krall, L.; Ewing, R.C. Is Nuclear Fission a Sustainable Source of Energy? MRS Bull. 2012, 37, 417–424. [Google Scholar] [CrossRef]
- Hoehr, C.; Bénard, F.; Buckley, K.; Crawford, J.; Gottberg, A.; Hanemaayer, V.; Kunz, P.; Ladouceur, K.; Radchenko, V.; Ramogida, C.; et al. Medical Isotope Production at TRIUMF – from Imaging to Treatment. Phys. Procedia 2017, 90, 200–208. [Google Scholar] [CrossRef]
- Griswold, J.R.; Medvedev, D.G.; Engle, J.W.; Copping, R.; Fitzsimmons, J.M.; Radchenko, V.; Cooley, J.C.; Fassbender, M.E.; Denton, D.L.; Murphy, K.E.; et al. Large Scale Accelerator Production of 225Ac: Effective Cross Sections for 78–192 MeV Protons Incident on 232Th Targets. Appl. Radiat. Isot. 2016, 118, 366–374. [Google Scholar] [CrossRef]
- Weidner, J.W.; Mashnik, S.G.; John, K.D.; Ballard, B.; Birnbaum, E.R.; Bitteker, L.J.; Couture, A.; Fassbender, M.E.; Goff, G.S.; Gritzo, R.; et al. 225Ac and 223Ra Production via 800MeV Proton Irradiation of Natural Thorium Targets. Appl. Radiat. Isot. 2012, 70, 2590–2595. [Google Scholar] [CrossRef]
- Weidner, J.W.; Mashnik, S.G.; John, K.D.; Hemez, F.; Ballard, B.; Bach, H.; Birnbaum, E.R.; Bitteker, L.J.; Couture, A.; Dry, D.; et al. Proton-Induced Cross Sections Relevant to Production of 225Ac and 223Ra in Natural Thorium Targets below 200MeV. Appl. Radiat. Isot. 2012, 70, 2602–2607. [Google Scholar] [CrossRef]
- Aliev, R.A.; Ermolaev, S.V.; Vasiliev, A.N.; Ostapenko, V.S.; Lapshina, E.V.; Zhuikov, B.L.; Zakharov, N.V.; Pozdeev, V.V.; Kokhanyuk, V.M.; Myasoedov, B.F.; et al. Isolation of Medicine-Applicable Actinium-225 from Thorium Targets Irradiated by Medium-Energy Protons. Solvent Extr. Ion Exch. 2014, 32, 468–477. [Google Scholar] [CrossRef]
- Mastren, T.; Radchenko, V.; Owens, A.; Copping, R.; Boll, R.; Griswold, J.R.; Mirzadeh, S.; Wyant, L.E.; Brugh, M.; Engle, J.W.; et al. Simultaneous Separation of Actinium and Radium Isotopes from a Proton Irradiated Thorium Matrix. Sci. Rep. 2017, 7, 8216. [Google Scholar] [CrossRef]
- Radchenko, V.; Engle, J.W.; Wilson, J.J.; Maassen, J.R.; Nortier, F.M.; Taylor, W.A.; Birnbaum, E.R.; Hudston, L.A.; John, K.D.; Fassbender, M.E. Application of Ion Exchange and Extraction Chromatography to the Separation of Actinium from Proton-Irradiated Thorium Metal for Analytical Purposes. J. Chromatogr. A 2015, 1380, 55–63. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Robertson, A.K.H.; Jermilova, U.; Zhang, C.; Yang, H.; Kunz, P.; Lassen, J.; Bratanovic, I.; Brown, V.; Southcott, L.; et al. Evaluation of Polydentate Picolinic Acid Chelating Ligands and an α-Melanocyte-Stimulating Hormone Derivative for Targeted Alpha Therapy Using ISOL-Produced 225Ac. EJNMMI Radiopharm. Chem. 2019, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.K.H.; McNeil, B.L.; Yang, H.; Gendron, D.; Perron, R.; Radchenko, V.; Zeisler, S.; Causey, P.; Schaffer, P. 232 Th-Spallation-Produced225 Ac with Reduced227 Ac Content. Inorg. Chem. 2020, 59, 12156–12165. [Google Scholar] [CrossRef] [PubMed]
- Nesteruk, K.P.; Ramseyer, L.; Carzaniga, T.S.; Braccini, S. Measurement of the Beam Energy Distribution of a Medical Cyclotron with a Multi-Leaf Faraday Cup. Instruments 2019, 3, 4. [Google Scholar] [CrossRef]
- Higashi, T.; Nagatsu, K.; Tsuji, A.B.; Zhang, M.-R. Research and Development for Cyclotron Production of 225Ac from 226Ra—The Challenges in a Country Lacking Natural Resources for Medical Applications. Processes 2022, 10, 1215. [Google Scholar] [CrossRef]
- Apostolidis, C.; Molinet, R.; McGinley, J.; Abbas, K.; Möllenbeck, J.; Morgenstern, A. Cyclotron Production of Ac-225 for Targeted Alpha therapy11Dedicated to Prof. Dr. Franz Baumgärtner on the Occasion of His 75th Birthday. Appl. Radiat. Isot. 2005, 62, 383–387. [Google Scholar] [CrossRef]
- Morgenstern, A.; Abbas, K.; Bruchertseifer, F.; Apostolidis, C. Production of Alpha Emitters for Targeted Alpha Therapy. Curr. Radiopharm. 2008, 1, 135–143. [Google Scholar] [CrossRef]
- Maslov, O.D.; Sabel’nikov, A.V.; Dmitriev, S.N. Preparation of 225Ac by 226Ra(γ, n) Photonuclear Reaction on an Electron Accelerator, MT-25 Microtron. Radiochemistry 2006, 48, 195–197. [Google Scholar] [CrossRef]
- Melville, G.; Meriarty, H.; Metcalfe, P.; Knittel, T.; Allen, B.J. Production of Ac-225 for Cancer Therapy by Photon-Induced Transmutation of Ra-226. Appl. Radiat. Isot. 2007, 65, 1014–1022. [Google Scholar] [CrossRef]
- Bruchertseifer, F.; Kellerbauer, A.; Malmbeck, R.; Morgenstern, A. Targeted Alpha Therapy with Bismuth-213 and Actinium-225: Meeting Future Demand. J. Label. Compd. Radiopharm. 2019, 62, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Zielińska, B.; Bilewicz, A. The Hydrolysis of Actinium. J. Radioanal. Nucl. Chem. 2004, 261, 195–198. [Google Scholar] [CrossRef]
- López-González, H.; Solache-Ríos, M.; Jiménez-Reyes, M.; Ramírez-García, J.J.; Rojas-Hernández, A. Effect of Chloride Ions on the Hydrolysis of Trivalent Lanthanum, Praseodymium and Lutetium in Aqueous Solutions of 2 M Ionic Strength. J. Solut. Chem. 2005, 34, 427–441. [Google Scholar] [CrossRef]
- Ferrier, M.G.; Stein, B.W.; Batista, E.R.; Berg, J.M.; Birnbaum, E.R.; Engle, J.W.; John, K.D.; Kozimor, S.A.; Lezama Pacheco, J.S.; Redman, L.N. Synthesis and Characterization of the Actinium Aquo Ion. ACS Cent. Sci. 2017, 3, 176–185. [Google Scholar] [CrossRef]
- Davis, I.A.; Glowienka, K.A.; Boll, R.A.; Deal, K.A.; Brechbiel, M.W.; Stabin, M.; Bochsler, P.N.; Mirzadeh, S.; Kennel, S.J. Comparison of 225actinium Chelates: Tissue Distribution and Radiotoxicity. Nucl. Med. Biol. 1999, 26, 581–589. [Google Scholar] [CrossRef]
- Fitzsimmons, J.; Atcher, R.; Cutler, C. Development of a Prelabeling Approach for a Targeted Nanochelator. J. Radioanal. Nucl. Chem. 2015, 305, 161–167. [Google Scholar] [CrossRef]
- Matson, M.L.; Villa, C.H.; Ananta, J.S.; Law, J.J.; Scheinberg, D.A.; Wilson, L.J. Encapsulation of α-Particle–Emitting225 Ac3+ Ions Within Carbon Nanotubes. J. Nucl. Med. 2015, 56, 897–900. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.F.; Woodward, J.; Boll, R.A.; Wall, J.S.; Rondinone, A.J.; Kennel, S.J.; Mirzadeh, S.; Robertson, J.D. Gold Coated Lanthanide Phosphate Nanoparticles for Targeted Alpha Generator Radiotherapy. PLoS ONE 2013, 8, e54531. [Google Scholar] [CrossRef]
- Wang, G.; De Kruijff, R.M.; Rol, A.; Thijssen, L.; Mendes, E.; Morgenstern, A.; Bruchertseifer, F.; Stuart, M.C.A.; Wolterbeek, H.T.; Denkova, A.G. Retention Studies of Recoiling Daughter Nuclides of 225Ac in Polymer Vesicles. Appl. Radiat. Isot. 2014, 85, 45–53. [Google Scholar] [CrossRef]
- Zhu, C.; Bandekar, A.; Sempkowski, M.; Banerjee, S.R.; Pomper, M.G.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Nanoconjugation of PSMA-Targeting Ligands Enhances Perinuclear Localization and Improves Efficacy of Delivered Alpha-Particle Emitters against Tumor Endothelial Analogues. Mol. Cancer Ther. 2016, 15, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Bandekar, A.; Zhu, C.; Jindal, R.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Anti–Prostate-Specific Membrane Antigen Liposomes Loaded with225 Ac for Potential Targeted Antivascular α-Particle Therapy of Cancer. J. Nucl. Med. 2014, 55, 107–114. [Google Scholar] [CrossRef]
- De Kruijff, R.; Wolterbeek, H.; Denkova, A. A Critical Review of Alpha Radionuclide Therapy—How to Deal with Recoiling Daughters? Pharmaceuticals 2015, 8, 321–336. [Google Scholar] [CrossRef]
- De Swart, J.; Chan, H.S.; Goorden, M.C.; Morgenstern, A.; Bruchertseifer, F.; Beekman, F.J.; De Jong, M.; Konijnenberg, M.W. Utilizing High-Energy γ-Photons for High-Resolution213 Bi SPECT in Mice. J. Nucl. Med. 2016, 57, 486–492. [Google Scholar] [CrossRef]
- Robertson, A.K.H.; Ramogida, C.F.; Rodríguez-Rodríguez, C.; Blinder, S.; Kunz, P.; Sossi, V.; Schaffer, P. Multi-Isotope SPECT Imaging of the225 Ac Decay Chain: Feasibility Studies. Phys. Med. Biol. 2017, 62, 4406–4420. [Google Scholar] [CrossRef] [PubMed]
- Cordier, D.; Forrer, F.; Bruchertseifer, F.; Morgenstern, A.; Apostolidis, C.; Good, S.; Müller-Brand, J.; Mäcke, H.; Reubi, J.C.; Merlo, A. Targeted Alpha-Radionuclide Therapy of Functionally Critically Located Gliomas with 213Bi-DOTA-[Thi8,Met(O2)11]-Substance P: A Pilot Trial. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J.G.; Rosenblat, T.L. Targeted Alpha-Particle Immunotherapy for Acute Myeloid Leukemia. Am. Soc. Clin. Oncol. Educ. Book 2014, e126–e131. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC Receptor-Targeted Alpha-Radionuclide Therapy Induces Remission in Neuroendocrine Tumours Refractory to Beta Radiation: A First-in-Human Experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef]
- Woodward, J.; Kennel, S.J.; Stuckey, A.; Osborne, D.; Wall, J.; Rondinone, A.J.; Standaert, R.F.; Mirzadeh, S. LaPO4 Nanoparticles Doped with Actinium-225 That Partially Sequester Daughter Radionuclides. Bioconjug. Chem. 2011, 22, 766–776. [Google Scholar] [CrossRef]
- Pandya, D.N.; Hantgan, R.; Budzevich, M.M.; Kock, N.D.; Morse, D.L.; Batista, I.; Mintz, A.; Li, K.C.; Wadas, T.J. Preliminary Therapy Evaluation of225 Ac-DOTA-c(RGDyK) Demonstrates That Cerenkov Radiation Derived from225 Ac Daughter Decay Can Be Detected by Optical Imaging for In Vivo Tumor Visualization. Theranostics 2016, 6, 698–709. [Google Scholar] [CrossRef]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G. MIRD Pamphlet No. 22 (Abridged): Radiobiology and Dosimetry of α-Particle Emitters for Targeted Radionuclide Therapy. J. Nucl. Med. 2010, 51, 311–328. [Google Scholar] [CrossRef]
- Sgouros, G.; F. Hobbs, R.; Song, H. Modelling and Dosimetry for Alpha-Particle Therapy. Curr. Radiopharm. 2011, 4, 261–265. [Google Scholar] [CrossRef]
- Chouin, N.; Lindegren, S.; Jensen, H.; Albertsson, P.; Bäck, T. Quantification of Activity by Alpha-Camera Imaging and Small-Scale Dosimetry within Ovarian Carcinoma Micrometastases Treated with Targeted Alpha Therapy. Q. J. Nucl. Med. Mol. Imaging Off. Publ. Ital. Assoc. Nucl. Med. AIMN Int. Assoc. Radiopharmacol. IAR Sect. Soc. Of 2012, 56, 487–495. [Google Scholar]
- Miller, B.W.; Gregory, S.J.; Fuller, E.S.; Barrett, H.H.; Bradford Barber, H.; Furenlid, L.R. The iQID Camera: An Ionizing-Radiation Quantum Imaging Detector. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrometers Detect. Assoc. Equip. 2014, 767, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.W.; Frost, S.H.L.; Frayo, S.L.; Kenoyer, A.L.; Santos, E.; Jones, J.C.; Green, D.J.; Hamlin, D.K.; Wilbur, D.S.; Fisher, D.R.; et al. Quantitative Single-particle Digital Autoradiography with α -particle Emitters for Targeted Radionuclide Therapy Using the iQID Camera. Med. Phys. 2015, 42, 4094–4105. [Google Scholar] [CrossRef]
- Singh Jaggi, J.; Kappel, B.J.; McDevitt, M.R.; Sgouros, G.; Flombaum, C.D.; Cabassa, C.; Scheinberg, D.A. Efforts to Control the Errant Products of a Targeted In Vivo Generator. Cancer Res. 2005, 65, 4888–4895. [Google Scholar] [CrossRef] [PubMed]
- Garmestani, K.; Yao, Z.; Zhang, M.; Wong, K.; Park, C.W.; Pastan, I.; Carrasquillo, J.A.; Brechbiel, M.W. Synthesis and Evaluation of a Macrocyclic Bifunctional Chelating Agent for Use with Bismuth Radionuclides. Nucl. Med. Biol. 2001, 28, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Dorso, L.; Bigot-Corbel, E.; Abadie, J.; Diab, M.; Gouard, S.; Bruchertseifer, F.; Morgenstern, A.; Maurel, C.; Chérel, M.; Davodeau, F. Long-Term Toxicity of 213Bi-Labelled BSA in Mice. PLOS ONE 2016, 11, e0151330. [Google Scholar] [CrossRef]
- Šimeček, J.; Hermann, P.; Seidl, C.; Bruchertseifer, F.; Morgenstern, A.; Wester, H.-J.; Notni, J. Efficient Formation of Inert Bi-213 Chelates by Tetraphosphorus Acid Analogues of DOTA: Towards Improved Alpha-Therapeutics. EJNMMI Res. 2018, 8, 78. [Google Scholar] [CrossRef]
- Egorova, B.V.; Matazova, E.V.; Mitrofanov, A.A.; Aleshin, G.Yu.; Trigub, A.L.; Zubenko, A.D.; Fedorova, O.A.; Fedorov, Yu.V.; Kalmykov, S.N. Novel Pyridine-Containing Azacrownethers for the Chelation of Therapeutic Bismuth Radioisotopes: Complexation Study, Radiolabeling, Serum Stability and Biodistribution. Nucl. Med. Biol. 2018, 60, 1–10. [Google Scholar] [CrossRef]
- Rosenblat, T.L.; McDevitt, M.R.; Mulford, D.A.; Pandit-Taskar, N.; Divgi, C.R.; Panageas, K.S.; Heaney, M.L.; Chanel, S.; Morgenstern, A.; Sgouros, G.; et al. Sequential Cytarabine and α-Particle Immunotherapy with Bismuth-213–Lintuzumab (HuM195) for Acute Myeloid Leukemia. Clin. Cancer Res. 2010, 16, 5303–5311. [Google Scholar] [CrossRef]
- Allen, B.J.; Raja, C.; Rizvi, S.; Li, Y.; Tsui, W.; Graham, P.; Thompson, J.; Reisfeld, R.; Kearsley, J.; Morgenstern, A.; et al. Intralesional Targeted Alpha Therapy for Metastatic Melanoma. Cancer Biol. Ther. 2005, 4, 1318–1324. [Google Scholar] [CrossRef]
- Raja, C.; Graham, P.; Rizvi, S.; Song, E.; Goldsmith, H.; Thompson, J.; Bosserhoff, A.; Morgenstern, A.; Apostolidis, C.; Kearsley, J.; et al. Interim Analysis of Toxicity and Response in Phase 1 Trial of Systemic Targeted Alpha Therapy for Metastatic Melanoma. Cancer Biol. Ther. 2007, 6, 846–852. [Google Scholar] [CrossRef]
- Allen, B.J.; Singla, A.A.; Rizvi, S.M.A.; Graham, P.; Bruchertseifer, F.; Apostolidis, C.; Morgenstern, A. Analysis of Patient Survival in a Phase I Trial of Systemic Targeted α-Therapy for Metastatic Melanoma. Immunotherapy 2011, 3, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Autenrieth, M.E.; Seidl, C.; Bruchertseifer, F.; Horn, T.; Kurtz, F.; Feuerecker, B.; D’Alessandria, C.; Pfob, C.; Nekolla, S.; Apostolidis, C.; et al. Treatment of Carcinoma in Situ of the Urinary Bladder with an Alpha-Emitter Immunoconjugate Targeting the Epidermal Growth Factor Receptor: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Królicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Apostolidis, C.; Mirzadeh, S.; Rola, R.; et al. Safety and Efficacy of Targeted Alpha Therapy with 213Bi-DOTA-Substance P in Recurrent Glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 614–622. [Google Scholar] [CrossRef]
- Palm, S.; Humm, J.L.; Rundqvist, R.; Jacobsson, L. Microdosimetry of Astatine-211 Single-Cell Irradiation: Role of Daughter Polonium-211 Diffusion. Med. Phys. 2004, 31, 218–225. [Google Scholar] [CrossRef]
- Krohn, K.A.; Moerlein, S.M.; Link, J.M.; Welch, M.J. Hot Atom Chemistry and Radiopharmaceuticals; Playa del Carmen, Maxico, 2012; pp. 3–15. [Google Scholar]
- Zalutsky, M.R.; Zhao, X.G.; Alston, K.L.; Bigner, D. High-Level Production of Alpha-Particle-Emitting (211)At and Preparation of (211)At-Labeled Antibodies for Clinical Use. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2001, 42, 1508–1515. [Google Scholar]
- Poty, S.; Francesconi, L.C.; McDevitt, M.R.; Morris, M.J.; Lewis, J.S. α-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies—Part 1. J. Nucl. Med. 2018, 59, 878–884. [Google Scholar] [CrossRef]
- Goodhead, D.T.; Munson, R.J.; Thacker, J.; Cox, R. Mutation and Inactivation of Cultured Mammalian Cells Exposed to Beams of Accelerated Heavy Ions IV. Biophysical Interpretation. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1980, 37, 135–167. [Google Scholar] [CrossRef] [PubMed]
- Stepanek, J.; Larsson, B.; Weinreich, R. Auger-Electron Spectra of Radionuclides for Therapy and Diagnostics. Acta Oncol. 1996, 35, 863–868. [Google Scholar] [CrossRef]
- Feng, Y.; Zalutsky, M.R. Production, Purification and Availability of 211At: Near Term Steps towards Global Access. Nucl. Med. Biol. 2021, 100–101, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Molina, B.; Soto, J.R.; Castro, J.J. Halogen-like Properties of the Al13 Cluster Mimicking Astatine. Phys. Chem. Chem. Phys. 2018, 20, 11549–11553. [Google Scholar] [CrossRef]
- Meyer, G. Astatine. J. Label. Compd. Radiopharm. 2018, 61, 154–164. [Google Scholar] [CrossRef]
- Wilbur, D.S. Enigmatic Astatine. Nat. Chem. 2013, 5, 246–246. [Google Scholar] [CrossRef]
- Zalutsky, M.; Vaidyanathan, G. Astatine-211-Labeled Radiotherapeutics An Emerging Approach to Targeted Alpha-Particle Radiotherapy. Curr. Pharm. Des. 2000, 6, 1433–1455. [Google Scholar] [CrossRef]
- Reilly, S.W.; Makvandi, M.; Xu, K.; Mach, R.H. Rapid Cu-Catalyzed [211 At]Astatination and [125 I]Iodination of Boronic Esters at Room Temperature. Org. Lett. 2018, 20, 1752–1755. [Google Scholar] [CrossRef]
- Kaizuka, Y.; Suzuki, H.; Tanaka, H.; Washiya, N.; Tatsuta, M.; Sato, Y.; Watanabe, S.; Ishioka, N.; Shirakami, Y.; Ooe, K.; et al. Metabolic Studies of Astatine- and Radioiodine-Labeled Neopentyl Derivatives. J. Nucl. Med. 2020, 61, 1100. [Google Scholar]
- Guérard, F.; Gestin, J.-F.; Brechbiel, M.W. Production of [211 At]-Astatinated Radiopharmaceuticals and Applications in Targeted α-Particle Therapy. Cancer Biother. Radiopharm. 2013, 28, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zalutsky, M.R.; Narula, A.S. Astatination of Proteins Using an N-Succinimidyl Tri-n-Butylstannyl Benzoate Intermediate. Int. J. Rad. Appl. Instrum. [A] 1988, 39, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Dekempeneer, Y.; Bäck, T.; Aneheim, E.; Jensen, H.; Puttemans, J.; Xavier, C.; Keyaerts, M.; Palm, S.; Albertsson, P.; Lahoutte, T.; et al. Labeling of Anti-HER2 Nanobodies with Astatine-211: Optimization and the Effect of Different Coupling Reagents on Their in Vivo Behavior. Mol. Pharm. 2019, 16, 3524–3533. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, G.; Affleck, D.J.; Bigner, D.D.; Zalutsky, M.R. N-Succinimidyl 3-[211At]Astato-4-Guanidinomethylbenzoate: An Acylation Agent for Labeling Internalizing Antibodies with α-Particle Emitting 211At. Nucl. Med. Biol. 2003, 30, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Vaidyanathan, G.; Koumarianou, E.; Kang, C.M.; Zalutsky, M.R. Astatine-211 Labeled Anti-HER2 5F7 Single Domain Antibody Fragment Conjugates: Radiolabeling and Preliminary Evaluation. Nucl. Med. Biol. 2018, 56, 10–20. [Google Scholar] [CrossRef]
- Dziawer, Ł.; Majkowska-Pilip, A.; Gaweł, D.; Godlewska, M.; Pruszyński, M.; Jastrzębski, J.; Wąs, B.; Bilewicz, A. Trastuzumab-Modified Gold Nanoparticles Labeled with 211At as a Prospective Tool for Local Treatment of HER2-Positive Breast Cancer. Nanomaterials 2019, 9, 632. [Google Scholar] [CrossRef]
- Guérard, F.; Maingueneau, C.; Liu, L.; Eychenne, R.; Gestin, J.-F.; Montavon, G.; Galland, N. Advances in the Chemistry of Astatine and Implications for the Development of Radiopharmaceuticals. Acc. Chem. Res. 2021, 54, 3264–3275. [Google Scholar] [CrossRef]
- Hamilton, J.G.; Durbin, P.W.; Parrott, M.W. Accumulation of Astatine211 by Thyroid Gland in Man. Exp. Biol. Med. 1954, 86, 366–369. [Google Scholar] [CrossRef]
- Andersson, H.; Cederkrantz, E.; Bäck, T.; Divgi, C.; Elgqvist, J.; Himmelman, J.; Horvath, G.; Jacobsson, L.; Jensen, H.; Lindegren, S.; et al. Intraperitoneal α-Particle Radioimmunotherapy of Ovarian Cancer Patients: Pharmacokinetics and Dosimetry of211 At-MX35 F(Ab′)2 —A Phase I Study. J. Nucl. Med. 2009, 50, 1153–1160. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; McLendon, R.E.; Wong, T.Z.; Bigner, D.D. Clinical Experience with Alpha-Particle Emitting 211At: Treatment of Recurrent Brain Tumor Patients with 211At-Labeled Chimeric Antitenascin Monoclonal Antibody 81C6. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2008, 49, 30–38. [Google Scholar] [CrossRef]
- Nakaya, A.; Qiu, H.; Santos, E.B.; Hamlin, D.K.; Wilbur, D.S.; Storb, R.; Sandmaier, B.M. Addition of Astatine-211-Labeled Anti-CD45 Antibody to TBI as Conditioning for DLA-Identical Marrow Transplantation: A Novel Strategy to Overcome Graft Rejection in a Canine Presensitization Model: “Radioimmunotherapy to Overcome Transfusion-Induced Sensitization. ” Transplant. Cell. Ther. 2021, 27, 476.e1–476.e7. [Google Scholar] [CrossRef] [PubMed]
- Ukon, N.; Higashi, T.; Hosono, M.; Kinuya, S.; Yamada, T.; Yanagida, S.; Namba, M.; Nakamura, Y. Manual on the Proper Use of Meta-[211At] Astato-Benzylguanidine ([211At] MABG) Injections in Clinical Trials for Targeted Alpha Therapy (1st Edition). Ann. Nucl. Med. 2022, 36, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Kaneda-Nakashima, K.; Ooe, K.; Liu, Y.; Kurimoto, K.; Murai, T.; Shidahara, Y.; Okuma, K.; Takeuchi, M.; Nishide, M.; et al. Extended Single-Dose Toxicity Study of [211At]NaAt in Mice for the First-in-Human Clinical Trial of Targeted Alpha Therapy for Differentiated Thyroid Cancer. Ann. Nucl. Med. 2021, 35, 702–718. [Google Scholar] [CrossRef]
- Kvassheim, M.; Revheim, M.-E.R.; Stokke, C. Quantitative SPECT/CT Imaging of Lead-212: A Phantom Study. EJNMMI Phys. 2022, 9, 52. [Google Scholar] [CrossRef]
- Smith, D.S.; Stabin, M.G. Exposure Rate Constants and Lead Shielding Values for over 1,100 Radionuclides. Health Phys. 2012, 102, 271–291. [Google Scholar] [CrossRef]
- Napoli, E.; Stenberg, V.Y.; Juzeniene, A.; Hjellum, G.E.; Bruland, Ø.S.; Larsen, R.H. Calibration of Sodium Iodide Detectors and Reentrant Ionization Chambers for 212Pb Activity in Different Geometries by HPGe Activity Determined Samples. Appl. Radiat. Isot. 2020, 166, 109362. [Google Scholar] [CrossRef]
- Radchenko, V.; Morgenstern, A.; Jalilian, A.R.; Ramogida, C.F.; Cutler, C.; Duchemin, C.; Hoehr, C.; Haddad, F.; Bruchertseifer, F.; Gausemel, H.; et al. Production and Supply of α-Particle–Emitting Radionuclides for Targeted α-Therapy. J. Nucl. Med. 2021, 62, 1495–1503. [Google Scholar] [CrossRef]
- McNeil, B.L.; Robertson, A.K.H.; Fu, W.; Yang, H.; Hoehr, C.; Ramogida, C.F.; Schaffer, P. Production, Purification, and Radiolabeling of the 203Pb/212Pb Theranostic Pair. EJNMMI Radiopharm. Chem. 2021, 6, 6. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Wilson, J.; Schultz, M.K.; Andersson, J.D.; Wuest, F. High-Yield Cyclotron Production of 203Pb Using a Sealed 205Tl Solid Target. Nucl. Med. Biol. 2023, 116–117, 108314. [Google Scholar] [CrossRef]
- Yang, H.; Wilson, J.J.; Orvig, C.; Li, Y.; Wilbur, D.S.; Ramogida, C.F.; Radchenko, V.; Schaffer, P. Harnessing α -Emitting Radionuclides for Therapy: Radiolabeling Method Review. J. Nucl. Med. 2022, 63, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, S.; Kumar, K.; Gansow, O.A. The Chemical Fate of212 Bi-DOTA Formed by β- Decay of212 Pb(DOTA)2- ***. ract 1993, 60, 1–10. [Google Scholar] [CrossRef]
- Azure, M.T.; Archer, R.D.; Sastry, K.S.; Rao, D.V.; Howell, R.W. Biological Effect of Lead-212 Localized in the Nucleus of Mammalian Cells: Role of Recoil Energy in the Radiotoxicity of Internal Alpha-Particle Emitters. Radiat. Res. 1994, 140, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Baumhover, N.J.; Liu, D.; Cagle, B.S.; Boschetti, F.; Paulin, G.; Lee, D.; Dai, Z.; Obot, E.R.; Marks, B.M.; et al. Preclinical Evaluation of a Lead Specific Chelator (PSC) Conjugated to Radiopeptides for 203Pb and 212Pb-Based Theranostics. Pharmaceutics 2023, 15, 414. [Google Scholar] [CrossRef]
- Baidoo, K.E.; Milenic, D.E.; Brechbiel, M.W. Methodology for Labeling Proteins and Peptides with Lead-212 (212Pb). Nucl. Med. Biol. 2013, 40, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Meredith, R.; Torgue, J.; Shen, S.; Fisher, D.R.; Banaga, E.; Bunch, P.; Morgan, D.; Fan, J.; Straughn, J.M. Dose Escalation and Dosimetry of First-in-Human α Radioimmunotherapy with212 Pb-TCMC-Trastuzumab. J. Nucl. Med. 2014, 55, 1636–1642. [Google Scholar] [CrossRef]
- Delpassand, E.S.; Tworowska, I.; Esfandiari, R.; Torgue, J.; Hurt, J.; Shafie, A.; Núñez, R. Targeted α -Emitter Therapy with212 Pb-DOTAMTATE for the Treatment of Metastatic SSTR-Expressing Neuroendocrine Tumors: First-in-Humans Dose-Escalation Clinical Trial. J. Nucl. Med. 2022, 63, 1326–1333. [Google Scholar] [CrossRef]
- Li, M.; Liu, D.; Lee, D.; Cheng, Y.; Baumhover, N.J.; Marks, B.M.; Sagastume, E.A.; Ballas, Z.K.; Johnson, F.L.; Morris, Z.S.; et al. Targeted Alpha-Particle Radiotherapy and Immune Checkpoint Inhibitors Induces Cooperative Inhibition on Tumor Growth of Malignant Melanoma. Cancers 2021, 13, 3676. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.J.; Goozee, G.; Sarkar, S.; Beyer, G.; Morel, C.; Byrne, A.P. Production of Terbium-152 by Heavy Ion Reactions and Proton Induced Spallation. Appl. Radiat. Isot. 2001, 54, 53–58. [Google Scholar] [CrossRef]
- Beyer, G.J.; Čomor, J.J.; Daković, M.; Soloviev, D.; Tamburella, C.; Hagebø, E.; Allan, B.; Dmitriev, S.N.; Zaitseva, N.G. Production Routes of the Alpha Emitting149 Tb for Medical Application. Radiochim. Acta 2002, 90, 247–252. [Google Scholar] [CrossRef]
- Müller, C.; Zhernosekov, K.; Köster, U.; Johnston, K.; Dorrer, H.; Hohn, A.; Van Der Walt, N.T.; Türler, A.; Schibli, R. A Unique Matched Quadruplet of Terbium Radioisotopes for PET and SPECT and for α- and β− -Radionuclide Therapy: An In Vivo Proof-of-Concept Study with a New Receptor-Targeted Folate Derivative. J. Nucl. Med. 2012, 53, 1951–1959. [Google Scholar] [CrossRef]
- Imam, S.K. Advancements in Cancer Therapy with Alpha-Emitters: A Review. Int. J. Radiat. Oncol. 2001, 51, 271–278. [Google Scholar] [CrossRef]
- Guerard, F.; Barbet, J.; Chatal, J.F.; Kraeber-Bodere, F.; Cherel, M.; Haddad, F. Which Radionuclide, Carrier Molecule and Clinical Indication for Alpha-Immunotherapy? Q. J. Nucl. Med. Mol. Imaging Off. Publ. Ital. Assoc. Nucl. Med. AIMN Int. Assoc. Radiopharmacol. IAR Sect. Soc. Of 2015, 59, 161–167. [Google Scholar]
- Brechbiel, M.W. Targeted α-Therapy: Past, Present, Future? Dalton Trans. 2007, 4918. [Google Scholar] [CrossRef] [PubMed]
- Stokke, C.; Kvassheim, M.; Blakkisrud, J. Radionuclides for Targeted Therapy: Physical Properties. Mol. Basel Switz. 2022, 27, 5429. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Reber, J.; Haller, S.; Dorrer, H.; Köster, U.; Johnston, K.; Zhernosekov, K.; Türler, A.; Schibli, R. Folate Receptor Targeted Alpha-Therapy Using Terbium-149. Pharmaceuticals 2014, 7, 353–365. [Google Scholar] [CrossRef]
- Steyn, G.F.; Vermeulen, C.; Szelecsényi, F.; Kovács, Z.; Hohn, A.; Van Der Meulen, N.P.; Schibli, R.; Van Der Walt, T.N. Cross Sections of Proton-Induced Reactions on 152Gd, 155Gd and 159Tb with Emphasis on the Production of Selected Tb Radionuclides. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2014, 319, 128–140. [Google Scholar] [CrossRef]
- Zaitseva, N.G.; Dmitriev, S.N.; Maslov, O.D.; Molokanova, L.G.; Starodub, G.Ya.; Shishkin, S.V.; Shishkina, T.V.; Beyer, G.J. Terbium-149 for Nuclear Medicine. The Production of 149Tb via Heavy Ions Induced Nuclear Reactions. Czechoslov. J. Phys. 2003, 53, A455–A458. [Google Scholar] [CrossRef]
- Maiti, M.; Lahiri, S.; Tomar, B.S. Investigation on the Production and Isolation of149,150,151 Tb from12 C Irradiated Natural Praseodymium Target. Radiochim. Acta 2011, 99, 527–534. [Google Scholar] [CrossRef]
- Dmitriev, S.N.; Beyer, G.J.; Zaitseva, N.G.; Maslov, O.D.; Molokanova, L.G.; Starodub, G.Ya.; Shishkin, S.V.; Shishkina, T.V. [No Title Found]. Radiochemistry 2002, 44, 171–173. [Google Scholar] [CrossRef]
- Qaim, S.M.; Scholten, B.; Neumaier, B. New Developments in the Production of Theranostic Pairs of Radionuclides. J. Radioanal. Nucl. Chem. 2018, 318, 1493–1509. [Google Scholar] [CrossRef]
- Maiti, M. New Measurement of Cross Sections of Evaporation Residues from the Nat Pr + 12 C Reaction: A Comparative Study on the Production of 149 Tb. Phys. Rev. C 2011, 84, 044615. [Google Scholar] [CrossRef]
- Beyer, G.-J.; Miederer, M.; Vranješ-Đurić, S.; Čomor, J.J.; Künzi, G.; Hartley, O.; Senekowitsch-Schmidtke, R.; Soloviev, D.; Buchegger, F. ; and the ISOLDE Collaboration Targeted Alpha Therapy in Vivo: Direct Evidence for Single Cancer Cell Kill Using 149Tb-Rituximab. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Guseva, L.I. Radioisotope Generators of Short-Lived α-Emitting Radionuclides Promising for Use in Nuclear Medicine. Radiochemistry 2014, 56, 451–467. [Google Scholar] [CrossRef]
- Müller, C.; Vermeulen, C.; Köster, U.; Johnston, K.; Türler, A.; Schibli, R.; Van Der Meulen, N.P. Alpha-PET with Terbium-149: Evidence and Perspectives for Radiotheragnostics. EJNMMI Radiopharm. Chem. 2017, 1, 5. [Google Scholar] [CrossRef]
- Zagryadskii, V.A.; Latushkin, S.T.; Malamut, T.Yu.; Novikov, V.I.; Ogloblin, A.A.; Unezhev, V.N.; Chuvilin, D.Yu. Measurement of Terbium Isotopes Yield in Irradiation of 151Eu Targets by 3He Nuclei. At. Energy 2017, 123, 55–58. [Google Scholar] [CrossRef]
- Moiseeva, A.N.; Aliev, R.A.; Unezhev, V.N.; Zagryadskiy, V.A.; Latushkin, S.T.; Aksenov, N.V.; Gustova, N.S.; Voronuk, M.G.; Starodub, G.Ya.; Ogloblin, A.A. Cross Section Measurements of 151Eu(3He,5n) Reaction: New Opportunities for Medical Alpha Emitter 149Tb Production. Sci. Rep. 2020, 10, 508. [Google Scholar] [CrossRef]
- Ferrier, M.G.; Radchenko, V.; Wilbur, D.S. Radiochemical Aspects of Alpha Emitting Radionuclides for Medical Application. Radiochim. Acta 2019, 107, 1065–1085. [Google Scholar] [CrossRef]
- Chen, X.; Ji, M.; Fisher, D.R.; Wai, C.M. Ionizable Calixarene-Crown Ethers with High Selectivity for Radium over Light Alkaline Earth Metal Ions. Inorg. Chem. 1999, 38, 5449–5452. [Google Scholar] [CrossRef] [PubMed]
- Abou, D.S.; Thiele, N.A.; Gutsche, N.T.; Villmer, A.; Zhang, H.; Woods, J.J.; Baidoo, K.E.; Escorcia, F.E.; Wilson, J.J.; Thorek, D.L.J. Towards the Stable Chelation of Radium for Biomedical Applications with an 18-Membered Macrocyclic Ligand. Chem. Sci. 2021, 12, 3733–3742. [Google Scholar] [CrossRef]
- Majkowska-Pilip, A.; Gawęda, W.; Żelechowska-Matysiak, K.; Wawrowicz, K.; Bilewicz, A. Nanoparticles in Targeted Alpha Therapy. Nanomater. Basel Switz. 2020, 10, 1366. [Google Scholar] [CrossRef]
- Lankoff, A.; Czerwińska, M.; Walczak, R.; Karczmarczyk, U.; Tomczyk, K.; Brzóska, K.; Fracasso, G.; Garnuszek, P.; Mikołajczak, R.; Kruszewski, M. Design and Evaluation of 223Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy-Part II. Toxicity, Pharmacokinetics and Biodistribution. Int. J. Mol. Sci. 2021, 22, 5702. [Google Scholar] [CrossRef]
- Reissig, F.; Hübner, R.; Steinbach, J.; Pietzsch, H.-J.; Mamat, C. Facile Preparation of Radium-Doped, Functionalized Nanoparticles as Carriers for Targeted Alpha Therapy. Inorg. Chem. Front. 2019, 6, 1341–1349. [Google Scholar] [CrossRef]
- Reissig, F.; Zarschler, K.; Hübner, R.; Pietzsch, H.; Kopka, K.; Mamat, C. Sub-10 Nm Radiolabeled Barium Sulfate Nanoparticles as Carriers for Theranostic Applications and Targeted Alpha Therapy. ChemistryOpen 2020, 9, 797–805. [Google Scholar] [CrossRef]
- Gawęda, W.; Pruszyński, M.; Cędrowska, E.; Rodak, M.; Majkowska-Pilip, A.; Gaweł, D.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Trastuzumab Modified Barium Ferrite Magnetic Nanoparticles Labeled with Radium-223: A New Potential Radiobioconjugate for Alpha Radioimmunotherapy. Nanomaterials 2020, 10, 2067. [Google Scholar] [CrossRef]
- Suchánková, P.; Kukleva, E.; Nykl, E.; Nykl, P.; Sakmár, M.; Vlk, M.; Kozempel, J. Hydroxyapatite and Titanium Dioxide Nanoparticles: Radiolabelling and In Vitro Stability of Prospective Theranostic Nanocarriers for 223Ra and 99mTc. Nanomater. Basel Switz. 2020, 10, 1632. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.S.; Simms, M.E.; Bryantsev, V.S.; Benny, P.D.; Griswold, J.R.; Delmau, L.H.; Thiele, N.A. Elucidating the Coordination Chemistry of the Radium Ion for Targeted Alpha Therapy. Chem. Commun. 2022, 58, 9938–9941. [Google Scholar] [CrossRef]
- Morris, M.J.; Corey, E.; Guise, T.A.; Gulley, J.L.; Kevin Kelly, W.; Quinn, D.I.; Scholz, A.; Sgouros, G. Radium-223 Mechanism of Action: Implications for Use in Treatment Combinations. Nat. Rev. Urol. 2019, 16, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Parker, C.; Saad, F.; Miller, K.; Tombal, B.; Ng, Q.S.; Boegemann, M.; Matveev, V.; Piulats, J.M.; Zucca, L.E.; et al. Addition of Radium-223 to Abiraterone Acetate and Prednisone or Prednisolone in Patients with Castration-Resistant Prostate Cancer and Bone Metastases (ERA 223): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2019, 20, 408–419. [Google Scholar] [CrossRef]
- Washiyama, K.; Amano, R.; Sasaki, J.; Kinuya, S.; Tonami, N.; Shiokawa, Y.; Mitsugashira, T. 227Th-EDTMP: A Potential Therapeutic Agent for Bone Metastasis. Nucl. Med. Biol. 2004, 31, 901–908. [Google Scholar] [CrossRef]
- Lindén, O.; Bates, A.T.; Cunningham, D.; Hindorf, C.; Larsson, E.; Cleton, A.; Pinkert, J.; Huang, F.; Bladt, F.; Hennekes, H.; et al. 227Th-Labeled Anti-CD22 Antibody (BAY 1862864) in Relapsed/Refractory CD22-Positive Non-Hodgkin Lymphoma: A First-in-Human, Phase I Study. Cancer Biother. Radiopharm. 2021, 36, 672–681. [Google Scholar] [CrossRef]
- Radzina, M.; Mamis, E.; Saule, L.; Pajuste, E.; Kalnina, M.; Cocolios, T.; Talip, Z.; Stora, T. Deliverable 5.1 - Questionnaire on Industrial and Clinical Key Players and Needs. 2022. [Google Scholar] [CrossRef]
Appendix A. SUPPLEMENTAL DATA
| Chelate (and corresponding tested bifunctional analogues | Donor Set (CN#) | Grade | Radiolabelling Conditions & RCY | Ref. |
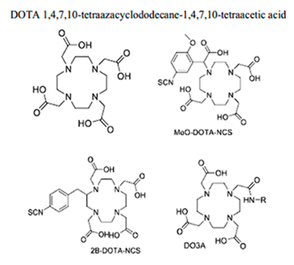 |
N4O4 CN = 8 |
Green -orange | 0.02 M ligand, NH4Ac pH 6, 37 °C, 2 h, RCY = 99% |
[1] |
 |
N4O4 CN = 8 |
Red | 0.02 M ligand, NH4Ac pH 6, 37 °C, 2 h, RCY = 0% |
[2] |
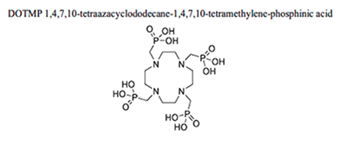 |
N4O4 CN = 8 |
Red | 0.02 M ligand, NH4Ac pH 6, 37 °C, 2 h, RCY = 78% |
[2] |
 |
N4O4 CN = 8 |
Red | 0.02 M ligand, NH4Ac pH 6, 37 °C, 2 h, RCY = 0% |
[2] |
 |
N3O5 CN = 8 |
Red | 0.02 M ligand, NH4Ac pH 6, 37 °C, 2 h, RCY = 0% |
[2] |
 |
N5O5 CN = 10 |
Red | 0.02 M ligand, NH4OAc pH 5.8, 40 °C, 30 min, RCY = 80% |
[3] |
 |
N6O6 CN = 12 |
Orange | 0.01 M ligand, NH4OAc pH 5.8, 40 °C, 30 min, RCY > 95% or > 98% after 2 h |
[3,4] |
 |
N3O5 CN = 8 |
Red | 0.01 M ligand, 0.02 NH4OAc pH 5.8, 0.03 40 °C, 0.04 30 min, 0.05 RCY > 95% |
[3,5] |
 |
N2O4 CN = 6 |
Red | 0.01 M ligand, 0.02 NH4OAc pH 5, 0.03 40 °C, 30 min, 0.04 RCY = 80-90% |
[5] |
| Preclinical model | Radiopharmaceutical | Activity/no of cycles | Main findings | Ref. | |
|---|---|---|---|---|---|
| Ac-225 | AR42J cells | [225Ac]Ac -DOTA-CCK-66 | 37 kBq / 1 cycle |
Substantial increase in mean survival of AR42J tumour-bearing mice upon treat- ment with the minigastrin derivative |
[6] |
| Ac-225 | Human ovarian carcinoma HER2-positive(SKOV-3 cell line) | [225Ac]Ac -H4py4pa |
10.1±0.7 kBq / 1 cycle | Stability study, in vitro and biodistribution |
[7] |
| Ac-225 | Human breast cancer cell lines SUM-225 and MDA-MB-231 | [225Ac]Ac -DOTA-trastuzumab | 0.37 kBq / 0.74 Bq / 1.48 Bq / 1 cycle | In vitro, biodistribution (comp. with In-111-DTPA-trastuzumab), optical imaging and therapy study. |
[8] |
| Ac-225 | Human HER2- positive cell lines SKOV-3 (ovarian cancer) and MDA-MB-231 (breast cancer) | [225Ac]Ac- DOTA-Nb (nanobod) | 30.2 ± 1.4 kBq / 1 cycle | In vitro and biodistribution In vitro, therapy study, dosimetry and toxicity |
[9] |
| Ac-225 | Human HER2- positive cell lines SKOV-3 (ovarian cancer) and MDA-MB-231 (breast cancer) | [225Ac]Ac-DOTA-Nb (nanobod) | 81.67 ± 28.87 kBq / 3 cycles | In vitro and biodistribution In vitro, therapy study, dosimetry and toxicity |
[10] |
| Ac-225 | U87mg human glioblastoma tumour cells |
[225Ac]Ac-DOTA-c(RGDyK) | 10 kBq / 20 kBq / 40 kBq/ 1 cycle | Biodistribution, optical imaging and therapy study | [11] |
| Ac-225 | Human glioblastoma cell line U251 |
[225Ac]Ac-Pep-1L | 40 kBq / 1 cycle | Bioluminescent imaging, therapy study (comparison with Cu-64-PepL1) | [12] |
| Ac-225 | NT2.5 mammary tumour cell line |
[225Ac]Ac-DOTA-anti-PD-L1-BC | 15 KBq / 1 cycle | Biodistribution (comparison with In-111-DTPA anti-PD-L1-BC), imaging, dosimetry |
[13] |
| Ac-225 | Human prostatic carcinoma cells LNCaP | [225Ac,Ac-(macropa)]+ | 26 kBq / 1 cycle | In vitro and biodistribution | [14] |
| Ac-225 | [225Ac,Ac-(macropa)]+ | 37 KBq / 74 KBq / 148 KBq / 1 cycle | In vitro, biodistribution, therapy study and dosimetry | [15] | |
| Ac-225 | Human pancreatic cell line BxPC3 | [225Ac]Ac-DOTA Human antibody 5B1 | 18.5 kBq / 1 cycle | Biodistribution, luminescence imaging, therapy studies (pre-targeting or conventional) and toxicity | [16];[17] |
| Ac-225 | Mammary carcinoma cell lines MFM-223 and BT-474 | [225Ac]Ac-hu11B6H435A | 11.1 kBq / 1 cycle | In vitro, biodistribution and therapeutic study | [18] |
| Ac-225 | Triple-negative breast cancer model SUM149T | [225Ac]Ac-DOTA-cixutumumab |
8.32 kBq / 1 cycle | In vitro, imaging, biodistribution (comp.with In-111-cixutumumab) and efficacy study |
[19] |
| Ac-225 | Malignant melanoma cell line B16F10 | [225Ac,Ac-octapa]-[225Ac,At-(CHXoctapa)]−; [225Ac,Ac(DOTA-CycMSH)] | 12–20 kBq / 1 cycle | Stability study and biodistribution | [20] |
| Ac-225 | Human cutaneous melanoma cells A375 andA375/MC1R and human uveal melanoma cells MEL270 | [225Ac]Ac-DOTA-MC1RL | 148 kBq (±10%) / 1 cycle | In vitro, pharmacokinetic, biodistribution, therapy study and dosimetry |
[21] |
| Ac-225 | Human cutaneous melanoma cells A375 and A375/MC1R | [225Ac]Ac-DOTA-Ahx-MC1RL (225Ac-Ahx); [225Ac]Ac-DOTA-di-d-Glu-MC1RL (225Ac-di-d-Glu) |
94.84 kBq ±7.11% / 56.52 kBq ±8.2 / 1 cycle | Biodistribution, pharmacokinetics, therapy study and toxicity | [22] |
| Ac-225 | Malignant melanoma cell line B16F10 | [225Ac]Ac-DOTA-Anti-VLA-4 | 14.8 kBq / 1 cycle | In vitro, biodistribution, imaging dosimetry and therapeutic efficacy | [23] |
| Ac-225 | Human embryonic kidney epithelial cells HEK-293T and HEK-293T-Hx16 | [225Ac]Ac-DOTA- SC16.56 (radioimmunoconjugate- Humanised site- specific antibodiesN149) |
18.9 – 55.5 kBq / 1 cycle | In vitro, biodistribution and efficacy study (comparison with Lu-177-DOTA-MMA) | [24] |
| Ac-225 | Colorectal cancer (SW1222), breast cancer (BT-474) or neuroblastoma (IMR32) | [225Ac]Ac-Proteus-DOTA (Humanised A33 and C825 (huA33-C825) | 0, 9.25, 18.5, 37, 74, 148, or 296 kBq / 1 cycle | Biodistribution (comparison with 111In-Pr, imaging) therapy study and toxicity(Pretargeted radioimmunotherapy) | [25] |
| Ac-225 | Human pancreatic cell lines PANC-1 and MIA PaCa-2 | [225Ac]Ac-FAPI-04 | 34 kBq / 1 cycle | In vitro, biodistribution and efficacy study | [26] |
| Ac-225 | Human squamous carcinoma A431 cell line | [225Ac]Ac-DOTA-PP-F11N | 45 kBq or 60 kBq / 1 cycle | In vitro, biodistribution and therapy study | [27] |
| Ac-225 | Hepatoblastoma cell line HepG2 and squamous carcinoma A431 (GPC3+) | [225Ac]Ac-Macropa-GC33 | 9.25 kBq or 18.5 kBq / 1 cycle | In vitro, biodistribution, therapy study and toxicity | [28] |
| Bi-213 | Multiple myeloma | [213Bi]Bi-anti CD138 | 3.7 MBq (single dose) | Increased median survival to 80 days, compared with 37 days for the untreated control group |
[29];[30] |
| Bi-213 | Bladder carcinoma | [213Bi]Bi-anti-EGFR-mAb | 0.94 MBq (fractioned dose) |
Overall survival of 141.5 days on average, in contrast with 65.4 and 57.6 days for the two control groups | [31] |
| Bi-213 | AR42J tumour- bearing mice; H69 human small-cell lung carcinoma; CA20948 rat pancreatic tumour | [213Bi]Bi-DOTATATE | 2–4 MBq/0.3 nmol/ 200 μL |
Significant tumour burden reduction and improved overall survival | [32];[33] |
| At-211 | syngeneic immunocompetent rat model | [211At]At-BR96 | 2.5 or 5 MBq | Possibility of treating small, solid colon carcinoma tumours with tolerable toxicity | [34];[35] |
| At-211 | U87MG cells Nude mice bearing xenograft tumours |
[211At]At-iRGD- C6-lys-C6-DA7R | 180, 370 and 740 kBq | Inhibition of cell viability, induced cell apoptosis, arrested the cell cycle in the G2/M phase, and increased intracellular ROS levels in a dose-dependent manner; inhibition of tumour growth and prolongation of the survival of mice | [36] |
| At-211 | T98G glioma cell line | [211At]At-Rh[16aneS4]- SP5–11 | 75–1200 kBq/mL | Cytotoxic effect on glioma cells | [37];[32] |
| At-211 | DBTRG-05MG glioma cell line, female BDIX rats with intracranial glioblastomas |
2-[211At]At-Phenylalanine 4- [211At]At-Phenylalanine |
1000 kBq (1 or 2 cycles) | Enhanced survival time of rats with intracranial glioblastomas | [38]; [39] |
| At-211 | Athymic mice bearing subcutaneous D-54 MG human glioma xenografts | [211At]At-ch81C6 | 74 kBq | Calculation of human radiation dose for i.v. and intrathecal administration | [40] |
| At-211 | HNSCC-Bearing female nude mice (balb/c nu/nu) | [211At]At-U36 (Chimeric mAb) |
200 kBq | Specific binding to the glycoprotein and efficient therapeutic response | [41] |
| At-211 | HL-60 and CI-1 cells | [211At]At -rituximab; [211At]At -gemtuzumab; [211At]At gemtuzumab ozogamicin. |
0.03 to 9.29 kBq (to 106 cells) | The affinity and specificity of the respective epitopes are not compromised | [42] |
| At-211 | leukemic SJL/J mice | [211At]At-30F11 (anti-murine CD45; mAb) |
444, 740 and 888 kBq | Improvements in overall survival when combined with bone marrow transplantation in a disseminated model of murine leukaemia with minimal renal toxicity | [43] |
| At-211 | Female BALB/c mice | [211At]At-30F11- ADTM | 74, 370, 740 and 1850 kBq |
more effective at myelosuppression than 213Bi, no significant non hematopoietic toxicity | [44] |
| At-211 | Human ML xenograft model in male hymic BALB/c nude mice | [211At]At -CXCR4 (mAb) | 320 kBq | clearance from blood and the tumour uptake matched the physical half-life of 211At; tumour uptake was relatively low | [45] |
| At-211 | Female and male NOD-Rag1null IL2rɣnull/J (NRG) mice | [211At]At-B10 (conjugated anti-CD123; mAb) |
185, 370, 740 or 1480 kBq |
decreased tumour burden and significantly prolonged dose-dependent survival | [46] |
| At-211 | Female athymic nude mice (s.c. injected Ramos cells) | [211At]At-1F5- B10 | Up to 1776 kBq | highly efficacious in minimal residual disease, no significant renal or hepatic toxicity | [47] |
| At-211 | Normal Kunming (KM) mice, BALB/c nude mice (s.c. injected A549 cells) | [211At]At -SPC-octreotide | 2294 kBq | more lethal effect than control groups (PBS, octreotide and free 211At), a possible treatment option for NSCLC | [48] |
| At-211 | Human melanoma- xenografted nude mice | [211At]At-MTB (methylene blue) |
3,5 MBq | highly effective, no adverse effects of TAT | [49] |
| At-211 | Female and male NOD.Cg Rag1tm1Mom Il2rgtm1Wjl/SzJ (NRG) mice | [211At]At-OKT10- B10 | 277 to 1665 kBq | potential to eliminate residual MM cell clones in low-disease-burden settings with minimal toxicity | [50] |
| At-211 | KaLwRij C57/BL6 mice (i.v. injected 5T33 cells) | [211At]At-9E7.4 | 370, 555, 740 or 1110 kBq |
the activity of 740 kBq showed 65% overall survival 150 days after the treatment with no evident sign of toxicity in MDR of multiple myeloma. | [51] |
| At-211 | NB-EBC1x tumour- bearing mouse model (female SCID CB17 mice) | [211At]At-parthanatine (PTT) | 185 kBq | maximum tolerated dose (MTD 36 MBq/kg/fraction x4), complete tumour response was observed in 81.8% with reversible haematological and marrow toxicity | [52] |
| At-211 | Male ICR mice (6 weeks old) | [211At]At-MABG (astatobenzylguanidine) |
185 kBq (biodistribution) 1.1, 2.2, 3.3, 4.4 MBq (body weigth studies) |
the MTD was 3.3 MBq for ICR mice. | [53];[54] |
| At-211 | female BALB/c nude mice s.c. inoculated with NIH: OVCAR-3 cells |
[211At]At-farletuzumab | 700 kBq | the tumour-free fraction (TFF) was shown to be 91% for i.p. administered 211At-farletuzumab | [55] |
| At-211 | nude Balb/c nu/nu mice (i.p. inoculated with OVCAR-3 cells) | [211At]At-MX35 (mAb) |
800 kBq and 3× ∼267 kBq ∼400 kBq and 3× ∼133 kBq ∼50 kBq or 3× ∼17 kBq |
no advantage in the therapeutic efficacy of a fractionated regimen compared with a single administration and lower side effects | [56] |
| At-211 | nude Balb/c nu/nu mice (i.p. inoculated with OVCAR-3 cells) | [211At]At-MX35 (mAb) |
350 - 540 kBq | micrometastatic growth of an ovarian cancer cell line was reduced with no considerable signs of toxicity | [57] |
| At-211 | nude Balb/c nu/nu mice (i.p. inoculated with SKOV-3 cells) | [211At]At-trastuzumab (mAb) |
100 – 800 kBq | statistically significant dose-response relationship for a single i.p. injection, a combination of 500 μg trastuzumab and 400 kBq 211At-trastuzumab had the greatest effect | [58] |
| At-211 | s.c. and PMGC (peritoneal metastasis of gastric cancer) xenograft mice | [211At]At-trastuzumab (mAb) |
100 and 1000 kBq | locoregionally administered [211At]At-trastuzumab significantly prolonged the survival time |
[59] |
| At-211 | Female nude BALB/c (nu/nu) mice (s.c. inoculated with SKOV-3 cells) | N-succinimidyl- 3-[211At]At-5- guanidinomethyl benzoate |
700 kBq | fast and high accumulation in a HER2+ tumour mouse model with a low non- target organ uptake | [60] |
| At-211 | female athymic mice (s.c. inoculation of 9BT474 xenografts) | Iso-[211At]At SAGMB-5F7 Iso-[211At]At SAGMB- VHH_2001 |
130 - 175 kBq | significant tumour growth delay and survival prolongation in a murine model of HER2-expressing breast cancer with no apparent normal- tissue toxicities | [61] |
| At-211 | C.B17/Icr-scid mice (s.c. implantation of MDA-361/DYT2 cells) |
[211At]At-SAPS C6.5 (diabody); [211At]At-SAPS T84.66 (diabody; [211At]At-SAPS (anti-MISIIR GM17 diabody) |
740, 1110 or 1665 kBq | single i.v. treatment resulted in dose- dependent delays in tumour growth | [62] |
| At-211 | Athymicmice bearing PSMA+ PC3, PIP and PSMA- PC3 flu flank xenografts |
(2S)-2-(3-(1-carboxy-5-(4-[211At]At astatobenzamido)pentyl)ureido)-pentanedioic acid | 200 kBq, 740 kBq | specific PC cell kill in vitro and in vivo after systemic administration and late nephrotoxicity | [63] |
| At-211 | LNCaP xenograft mice, normal ICR mice | [211At]At-PSMA1; [211At]At-PSMA5; [211At]At-PSMA6 |
110 – 400 kBq | [211At]At-PSMA5 exhibited excellent tumour growth suppression in xenograft models of prostate cancer, with minimal side effects. | [64] |
| At-211 | Male nude BALB/c nu/nu mice (s.c. inoculated with PC3- PSCA tumour cells) | [211At]At-A11 (anti-PSCA mini body) |
260 ± 20 kBq, 800 kBq and 1500 kBq |
growth inhibition on both macro tumours and intratibial micro tumours and multiple fractions resulted in radiotoxicity | [65] |
| At-211 | Male nude BALB/c nu/nu mice (s.c. inoculated with PC-3 cells) | [211At]At-AB-3 | 85 kBq | poor in vivo stability | [66] |
| At-211 | NIS-6 cells | [211At]At-astatide | 50-100 kBq | uptake is shown to be NIS-dependent | [67];[68] |
| At-211 | NMRI-nu/nu nude mice (s.c. inoculated with xenografts of a human papillary thyroid carcinoma cell line, K1) | [211At]At-astatide | 100, 500 and 1000 kBq | high tumouricidal potential in NIS gene–transfected tumours without major side effects | [69] |
| At-211 | Healthy male Balb/C nu/nu mice | [211At]At-AuNP (gold nanoparticles) |
900 kBq | high in vitro and in vivo stability | [70] |
| At-211 | Male nude BALB/c- nu-nu (s.c. inoculated PANC-1 cells) |
[211At]At-FAPI-1; [211At]At- FAPI-5 |
540 – 970 kBq | higher tumour retention of [211At]At- FAPI(s) compared with [131I]I -FAPI(s) | [71] |
| Pb-212 | Model A - Female naïve CD-1Elite mice; Model B – Female Athymic mice bearing AR42J tumour Xenografts |
[212Pb]Pb-PSC-PEG-T | Model A- Single injection of 74 kBq; Model B- Single injection of 3.7 MBq |
Model A - fast clearance from blood circulation, cleared through the kidneys. Model B - prolonged accumulation in tumour and minimal retention in kidneys (0.9%ID in tumour; 1%ID in kidneys) |
[72] |
| Pb-212 | Female athymic-NCR- nude mice with SK-OV-3 tumour xenografts: Model A - tumour volume 15 mm3 Model B – tumour volume 146 mm3 |
[212Pb]Pb-DOTA-AE1 | Model A - Single injection of 740 kBq; Model B – Single injection of 925 kBq |
Model A – the rate of tumour growth was inhibited in the period after the [212Pb]Pb-DOTA-AE1 therapy; Model B - [212Pb]Pb-DOTA-AE1 did not provide effective therapy for large established tumours. |
[73] |
| Pb-212 | Male non-obese, diabetic/Shi-scid/IL- 2rgnull (NSG) mice: Model A - bearing PSMA(+) PC3 PIP tumour xenografts. Tumour volume 60–100 mm3. Model B - PSMA(+) micrometastatic model, mice were injected intravenously with 1 x 106 PC3-ML-Luc-PSMA cells |
[212Pb]Pb-L2 |
Model A - Single dose of 3.7 MBq Model B - 0, 0.7, 1.5, or 3.7 MBq |
Model A - A single administration of 1.5 or 3.7 MBq showed significant tumour growth delay only in PSMA(+) Model B - the median survival time for the mice administered [212Pb]Pb-L2 (3.7 MBq) was 58 days, demonstrating moderate but significant improvement. |
[74] |
| Pb-212 | Athymic Nude-Foxn1nu mice bearing C4-2 tumour xenografts. Tumour volume 250-1000 mm3 |
[212Pb]Pb-NG001; [212Pb]Pb-PSMA-617 |
Single dose of 10-56 kBq of [212Pb]Pb-NG001; A single dose of 79 kBq of [212Pb]Pb-PSMA-617 |
The uptake values (%ID/g) for tumour and kidneys at 2-hour post-injection were 17.61±6.76 and 21.07±10.33 for [212Pb]Pb-NG001 and 17.93±2.90 and 52.82±26.62 for [212Pb]Pb-PSMA-617 |
[75] |
| Pb-212 | SCID mice bearing PC3 tumour xenografts |
[212Pb]Pb-RM2 |
Single dose of 1.85 MBq or 3.7 MBq |
Both [212Pb]Pb-RM2 treatment groups (1.85 MBq or 3.7MBq) demonstrated initial tumour control for 4-5 weeks post-treatment. 18 days pi, tumour regression was observed in the 3.7 MBq group (maximum per cent change of -49.3%) 40 days pi, tumour regrowth was observed in the 3.7 MBq group (+91.6% change from predose) |
[76] |
| Tb-149 | SCID mouse model of leukaemia | [149Tb]Tb-rituximab |
5.5MBq labelled antibody conjugate (1.11GBq/mg) 2 days after an intravenous graft of 5106 Daudi cells | Tumour-free survival for >120 days in 89% of treated animals | [77] |
| Tb-149 | Tumour-bearing mice | [149Tb]Tb-cm09 (DOTA-folate conjugate) |
Group A: saline only Group B: 2.2 MBq; Group C: 3.0 MBq; |
A significant tumour growth delay was found in treated animals resulting in an increased average survival time of mice which received 149Tb-cm09 (B: 30.5 d; C: 43 d) compared to untreated controls (A: 21 d). | [78] |
| Ra-223 | Balb/c | [223Ra]RaCl2 |
450 kBq/kg of 223Ra | High activity concentration in bone; High retention in the kidney and spleen among OARs |
[79] |
| Ra-223 |
Balb/c |
[223Ra]RaCl2 |
1250, 2500, 3750 kBq/kg | Minimal to moderate depletion of osteocytes and osteoblasts | [80] |
| Ra-223 | Intratibial LNCaP or LuCaP 58 | [223Ra]RaCl2 | 300 kBq/kg – 2 cycles |
Inhibition of tumour cellular growth | [81] |
| Th-227 | Human lymphoma Raji | [22tTh]Th -Rituximab | 50, 200, 1000 kBq/kg | Complete regression in 60% of mice treated with 200 kBq/kg | [82] |
| Th-227 | HER2-overexpressing subcutaneous SKOV-3 or SKBR-3 | [22tTh]Th-trastuzumab | 1000 kBq/kg - 1 cycle; 250 kBq/kg - 4 cycles |
Survival with a tumour diameter of less than 16 mm was prolonged | [83] |
| Th-227 | subcutaneous xenograft mouse model using HL- 60 cells at a single dose regimen | [22tTh]Th-CD33-TTC | 50, 150, or 300 kBq/kg – 1 cycle a second injection of 150 kBq/kg for some animals | Dose- dependent significant survival benefit | [84] |
| Th-227 | NCI-H716, SNU- 16, and MFM- 223 |
[22tTh]Th-FGFR2-TTC | 500 kBq/kg | significant inhibition of tumour growth at a dose of 500 kBq/kg | [85] |
| NCT Number | Radio | Radiopharmaceutical | Study Title | Study Status | Conditions | Sponsor | Phases |
| NCT06939036 | Ac-225 | [225Ac]Ac-SSO110 | Study of [225Ac]Ac-SSO110 in Subjects With ES-SCLC or MCC (SANTANA-225 ) | Ongoing, estimated completion 2026-12 | Small Cell Lung Cancer Extensive Stage|Merkel Cell Carcinoma | Ariceum Therapeutics GmbH | Phase I/II |
| NCT06888323 | Ac-225 | [225Ac]Ac-lintuzumab | Testing an Anti-cancer Radio-Active Immunotherapy Called [225Ac]Ac-lintuzumab in Patients With High-Risk Myelodysplastic Syndrome That Has Not Responded to Other Treatment | Not yet recruting | Refractory Myelodysplastic Syndrome | National Cancer Institute (NCI) | Phase I |
| NCT06881823 | Ac-225 | [225Ac]Ac-PSMA-R2 (AAA802); [177Lu]Lu-PSMA-R2 (AAA602) |
Study to Assess [177Lu]Lu-PSMA-R2 (AAA602) and [225Ac]Ac-PSMA-R2 (AAA802) in Participants With PSMA-positive HRLPC | Not yet recruting | Prostate Cancer | Novartis Pharmaceuticals | Phase I/II |
| NCT06879041 | Ac-225 | [225Ac]Ac-AZD2284 | A Phase I Study of [225Ac]Ac-AZD2284 in Patients With Metastatic Castration-Resistant Prostate Cancer | Ongoing, estimated completion 2029-04 | Metastatic Castration-Resistant Prostate Cancer | AstraZeneca | Phase I |
| NCT06802523 | Ac-225 | [225Ac]Ac-lintuzumab | Testing the Combination of Targeted Radiotherapy With Anti-Cancer Drugs, Venetoclax and ASTX-727, to Improve Outcomes for Adults With Newly Diagnosed Acute Myeloid Leukemia | Not yet recruting | Acute Myeloid Leukemia | National Cancer Institute (NCI) | Phase I |
| NCT06736418 | Ac-225 | [225Ac]Ac-ABD147 | Study of [225Ac]Ac-ABD147to Establish Optimal Dose in Patients With SCLC and LCNEC of the Lung That Previously Received Platinum-based Chemotherapy | Ongoing, estimated completion 2027-01 | Small-Cell Lung Cancer (SCLC)|Large Cell Neuroendocrine Carcinoma of the Lung | Abdera Therapeutics Inc. | Phase I |
| NCT06726161 | Ac-225 | [225Ac]Ac-RYZ811; [225Ac]Ac-RYZ801 | Study of the Theranostic Pair RYZ811 (Diagnostic) and RYZ801 (Therapeutic) to Identify and Treat Subjects With GPC3+ Unresectable HCC | Ongoing, estimated completion 2031-01 | HCC | RayzeBio, Inc. | Phase I |
| NCT06590857 | Ac-225 | [225Ac]Ac-DOTATATE (RYZ101) | Trial of [225Ac]Ac-DOTATATE (RYZ101) in Subjects with ER+, HER2-negative Unresectable or Metastatic Breast Cancer Expressing SSTRs. | Ongoing, estimated completion 2033-01 | Metastatic Breast Cancer HER2-negative ER+ | RayzeBio, Inc. | Phase I/II |
| NCT06287944 | Ac-225 | [225Ac]Ac-DOTA- Daratumumab |
[225Ac]Ac-DOTA -Anti-CD38 Daratumumab Monoclonal Antibody With Fludarabine, Melphalan and Total Marrow and Lymphoid Irradiation as Conditioning Treatment for Donor Stem Cell Transplant in Patients With High-Risk Acute Myeloid Leukemia, Acute Lymphoblastic Leukemia and Myelodysplastic Syndrome | Ongoing, estimated completion 2028-05 | Acute Lymphoblastic Leukemia; Acute Myeloid Leukemia; Myelodysplastic Syndrome | City of Hope Medical Center | Phase I |
| NCT06229366 | Ac-225 | [225Ac]Ac-PSMA-62 | [225Ac]Ac-PSMA-62 Trial in Oligometastatic Hormone Sensitive and Metastatic Castration Resistant Prostate Cancer | Ongoing, estimated completion 2027-09 | Prostate Cancer | Eli Lilly and Company | Phase I |
| NCT05983198 | Ac-225 | [225Ac]Ac-PSMA-R2 | Phase I/II Study of [225Ac]Ac-PSMA-R2 in PSMA-positive Prostate Cancer, With/Without Prior [177Lu]Lu-PSMA RLT (SatisfACtion) | Ongoing, estimated completion 2029-11 | mCRPC treated with prior ARPI in post- 177Lu and pre-177Lu settings | Novartis Pharmaceuticals | Phase I/II |
| NCT05605522 | Ac-225 | [225Ac]Ac-FPI-2059 | A Study of [225Ac]Ac-FPI-2059 in Adult Participants With Solid Tumours | Active not recruting, estimated completion 2025-09 | NTSR1-positive solid tumours refractory to standard therapies | Fusion Pharmaceuticals Inc. | Phase I |
| NCT05595460 | Ac-225 | [225Ac]Ac-DOTATATE (RYZ101) | Study of RYZ101 in Combination With SoC in Subjects With SSTR+ ES-SCLC | Ongoing, estimated completion 2029-03 | SSTR2-positive extensive-stage small- cell lung cancer | RayzeBio, Inc. | Phase I |
| NCT05567770 | Ac-225 | [225Ac]Ac-J591 | Actinium-J591 Radionuclide Therapy in PSMA-Detected Metastatic HOrmone-Sensitive Recurrent Prostate CaNcer | WITHDRAWN | Prostate Cancer Metastatic | Weill Medical College of Cornell University | Phase I |
| NCT05477576 | Ac-225 | [225Ac]Ac-DOTATATE (RYZ101) | Study of RYZ101 Compared With SOC in Pts w Inoperable SSTR+ Well-differentiated GEP-NET That Has Progressed Following 177Lu-SSA Therapy | Ongoing, estimated completion 2028- 07 |
SSTR2-positive gastroenteropancreatic neuroendocrine tumours with prior 177Lu therapy |
RayzeBio, Inc. | Phase III |
| NCT05363111 | Ac-225 | [225Ac]Ac-DOTA- daratumuab |
Radioimmunotherapy [111I]I/[225Ac]Ac-DOTA -daratumumab) for the Treatment of Relapsed/Refractory Multiple Myeloma | Ongoing, estimated completion 2025-06 | Relapsed or refractory multiple myeloma after at least 2 lines of prior therapy | City of Hope Medical Center | Phase I |
| NCT05219500 | Ac-225 | [225Ac]Ac-FPI-2265 (PSMA-I&T) |
Targeted Alpha Therapy With [225Ac]Ac-FPI-2265-Prostate Specific Membrane Antigen (PSMA)-I&T of Castration-resISTant Prostate Cancer (TATCIST) | Active, not recruting, estimated completion 2025-07 |
mCRPC with prior ARPI | Fusion Pharmaceuticals | Phase II |
| NCT05204147 | Ac-225 | [225Ac]Ac-DOTA-M5A | Actinium 225 Labeled Anti-CEA Antibody ([225Ac]Ac-DOTA-M5A) for the Treatment of CEA Producing Advanced or Metastatic Cancers | Ongoing, estimated completion 2025-08 | Metastatic solid tumours expressing CEA | City of Hope Medical Center | Phase I |
| NCT04946370 | Ac-225 | [225Ac]Ac-J591 | Phase I/II Trial of Pembrolizumab and Androgen-receptor Pathway Inhibitor With or Without [225Ac]Ac-J591for Progressive Metastatic Castration Resistant Prostate Cancer | Ongoing, estimated completion 2028- 06 |
mCRPC treated with prior ARPI | Weill Medical College of Cornell University | Phase I/II |
| NCT04886986 | Ac-225 | [225Ac]Ac-J591 with [177Lu]Lu-PSMA-I&T | Phase I/II [225Ac]Ac-J591 Plus [177Lu]Lu-PSMA-I&T for Progressive Metastatic Castration Resistant Prostate Cancer | Suspended, estimated completion 2027-12 | mCRPC treated with prior ARPI | Weill Medical College of Cornell University | Phase I/II |
| NCT04644770 | Ac-225 | [225Ac]Ac DOTA-h11B6 (JNJ-69086420) |
A Study of JNJ-69086420, an Actinium-225-Labeled Antibody Targeting Human Kallikrein-2 (hK2) for Advanced Prostate Cancer | Ongoing, estimated completion 2025-12 |
mCRPC with prior ARPI | Janssen Research & Development, LLC | Phase I |
| NCT04597411 | Ac-225 | [225Ac]Ac-PSMA-617 | Study of [225Ac]Ac-PSMA-617 in Men With PSMA-positive Prostate Cancer | Ongoing, estimated completion 2027-01 |
mCRPC | Endocyte | Phase I |
| NCT04576871 | Ac-225 | [225Ac]Ac-J591 | Re-treatment [225Ac]Ac-J591for mCRPC | Active non recruting, estimated completion 2026-12 |
mCRPC treated with prior ARPI | Weill Medical College of Cornell University | Phase I |
| NCT04506567 | Ac-225 | [225Ac]Ac-J591 | Fractionated and Multiple Dose [225Ac]Ac-J591for Progressive mCRPC | Active non recruting, estimated completion 2027-06 | mCRPC treated with prior ARPI | Weill Medical College of Cornell University | Phase I/II |
| NCT03932318 | Ac-225 | [225Ac]Ac-Lintuzumab | Venetoclax, Azacitidine, and [225Ac]Ac-Lintuzumab in AML Patients | WITHDRAWN | Acute Myeloid LeukemiaRelapsed Adult AML | Actinium Pharmaceuticals | Phase I/II |
| NCT03867682 | Ac-225 | [225Ac]Ac-Lintuzumab | Venetoclax and [225Ac]Ac-Lintuzumab in AML Patients | Unknown status | Relapsed/refractory AML | Actinium Pharmaceuticals | Phase I/II |
| NCT03746431 | Ac-225 | [225Ac]Ac-FPI-1434 | A Phase 1/2 Study of [225Ac]AcFPI-1434 Injection | Ongoing, estimated completion 2026- 06 |
IGF-1R-positive solid tumours refractory to standard therapies | Fusion Pharmaceuticals | Phase I/II |
| NCT03705858 | Ac-225 | [225Ac]Ac-Lintuzumab | [225Ac]Ac -Lintuzumab in Patients With Acute Myeloid Leukemia | WITHDRAWN | Acute Myeloid Leukemia | Joseph Jurcic, Columbia University | Phase I |
| NCT03441048 | Ac-225 | [225Ac]Ac-Lintuzumab | [225Ac]Ac-Lintuzumab in Combination with Cladribine + Cytarabine + Filgastrim + Mitoxantrone (CLAG-M) for Relapsed/Refractory Acute Myeloid Leukemia | Completed; 2024-05 | Acute Myeloid Leukemia | Medical College of Wisconsin | Phase I |
| NCT03276572 | Ac-225 | [225Ac]Ac-J591 | Phase I Trial of [225Ac]Ac-J591 in Patients With mCRPC | Completed with results, 2023- 09 |
mCRPC treated with prior ARPI | Weill Medical College of Cornell University | Phase I |
| NCT02998047 | Ac-225 | [225Ac]Ac-Lintuzumab | A Phase I Study of [225Ac]Ac-Lintuzumab in Patients With Refractory Multiple Myeloma | Terminated, 2020-05 | Refractory Multiple Myeloma | Actinium Pharmaceuticals | Phase I |
| NCT00672165 | Ac-225 | [225Ac]Ac-Lintuzumab | Targeted Atomic Nano-Generators (Actinium-225-Labeled Humanised Anti-CD33 Monoclonal Antibody HuM195) in Patients With Advanced Myeloid Malignancies | Completed, 2015-02 | Leukemia, Myelodisplastic syndrome | Memorial Sloan Kettering Cancer Center | Phase I |
| NCT00014495 | Bi-213 | [213Bi]Bi-Lintuzumab-(Bi213 MOAB M195 ) | Chemotherapy and Monoclonal Antibody Therapy in Treating Patients With Advanced Myeloid Cancer | Completed, 2009-12 | LeukemiaMyelodysplastic SyndromesMyelodysplastic/Myeloproliferative Neoplasms | Memorial Sloan Kettering Cancer Center | Phase I/II |
| NCT06441994 | At-211 | PSW-1025 ([211At]At-PSMA-5) | Clinical Trial of Targeted Alpha Therapy Using [211At]At-PSMA-5] for Prostate Cancer | Ongoing, estimated completion 2027-03 | Prostate Cancer | Osaka University | Phase I |
| NCT05275946 | At-211 | TAH-1005 ([211At] NaAt) | Targeted Alpha Therapy Using Astatine-211 Against Differentiated Thyroid Cancer | Completed, 2025-03 | Thyroid Cancer | Osaka University | Phase I |
| NCT04579523 | At-211 | [211At]At -OKT10-B10 | [211At]At -OKT10-B10and Fludarabine Alone or in Combination With Cyclophosphamide and Low-Dose TBI Before Donor Stem Cell Transplant for the Treatment of Newly Diagnosed, Recurrent, or Refractory High-Risk Multiple Myeloma | Not yet recruting, estimated completion 2028-12 | Multiple Myeloma|Recurrent Multiple Myeloma|Refractory Multiple Myeloma | Fred Hutchinson Cancer Center | Phase I |
| NCT04466475 | At-211 | [211At]At-OKT10-B10 | Radioimmunotherapy [211At]At -OKT10-B10 and Chemotherapy (Melphalan) Before Stem Cell Transplantation for the Treatment of Multiple Myeloma | WITHDRAWN | Plasma Cell Myeloma | Fred Hutchinson Cancer Center | Phase I |
| NCT04461457 | At-211 | [211At]At-MX35 F(ab’)2 | Targeted Radiation Therapy for Ovarian Cancer: Intraperitoneal Treatment With [211At]At-MX35 F(ab’)2 | Completed, 2012-01 | Ovarian Cancer | Vastra Gotaland Region | Early Phase I |
| NCT04083183 | At-211 | [211At]At-BC8-B10 Monoclonal Antibody | Total Body Irradiation and [211At]At-BC8-B10 Monoclonal Antibody for the Treatment of Nonmalignant Diseases | Ongoing, estimated completion 2028-01 | Non-Malignant Neoplasm | Fred Hutchinson Cancer Center | Phase I/II |
| NCT03670966 | At-211 | [211At]At-BC8-B10 | [211At]At-BC8-B10 Followed by Donor Stem Cell Transplant in Treating Patients With Relapsed or Refractory High-Risk Acute Leukemia or Myelodysplastic Syndrome | Ongoing, estimated completion 2029-03 | hematology plan | Fred Hutchinson Cancer Center | Phase I/II |
| NCT00003461 | At-211 | [211At]At-monoclonal antibody 81C6 | Radiolabeled Monoclonal Antibody Therapy in Treating Patients With Primary or Metastatic Brain Tumours | Completed, 2005-02 | Brain and Central Nervous System TumoursMetastatic CancerNeuroblastoma | Duke University | Phase I/II |
| NCT06710756 | Pb-212 | [212Pb]Pb-At PSV359 | [212Pb]Pb-At PSV359 Therapy for Patients With Solid Tumours | Ongoing, estimated completion 2032-05 | Pancreatic Ductal AdenocarcinomaGastric CancerEsophageal CancerColorectal CancerOvarian CancerHead and Neck Cancer | Perspective Therapeutics | Phase I/II |
| NCT06479811 | Pb-212 | [203Pb]Pb-VMT-alpha-NET; [212Pb]Pb-VMT-alpha-NET | [212Pb]Pb-VMT-Alpha-NET in Metastatic or Inoperable Somatostatin-Receptor Positive Gastrointestinal Neuroendocrine Tumours, Pheochromocytoma/Paragangliomas, Small Cell Lung, Renal Cell, and Head and Neck Cancers | Not yet recruting, estimated completion 2032-01 | Head and Neck TumoursKidney CancersSmall Cell Lung CancersPheochromocytoma/ParagangliomasGastrointestinal Neuroendocrine TumoursSomatostatin Receptor Positive | National Cancer Institute (NCI) | Phase I |
| NCT06427798 | Pb-212 | [203Pb]Pb-VMT-alpha-NET; [212Pb]Pb]VMT-alpha-NET | Somatostatin-Receptors (SSTR)-Agonist [212Pb]Pb-VMT-alpha-NET in Metastatic or Inoperable SSTR+ Gastrointestinal Neuroendocrine Tumour and Pheochromocytoma/Paraganglioma Previously Treated With Systemic Targeted Radioligand Therapy | Ongoing, estimated completion 2039-07 | Somatostatin Receptor PositiveGastrointestinal Neuroendocrine TumoursPheochromocytomaParagangliomas | National Cancer Institute (NCI) | Phase I/II |
| NCT06148636 | Pb-212 | [212Pb]Pb-VMT-alpha-NET; [212Pb]Pb-VMT-alpha-NET | A Safety Study of [212Pb]Pb-VMT-alpha-NET in Patients With Neuroendocrine Tumours | Active not recruting, estimated completion 2027-11 | Neuroendocrine Tumours | David Bushnell | Early Phase I |
| NCT05725070 | Pb-212 | [212Pb]Pb -NG001 | Phase 0/1 Study of [212Pb]Pb -NG001 in mCRPC | Completed, 2023-07 | Metastatic Castration-resistant Prostate Cancer | ARTBIO Inc. | Early Phase I |
| NCT05720130 | Pb-212 | [212Pb]Pb-ADVC001 | Phase Ib/IIa Dose Escalation and Expansion Study of [²¹²Pb]Pb-ADVC001 in Metastatic Castration Resistant Prostate Cancer (TheraPb - Phase I/II Study). | Ongoing, estimated completion 2029-12 | mCRPC with prior ARPI and no prior exposure to 177Lu |
AdvanCell Pty Limited | Phase I/II |
| NCT05655312 | Pb-212 | [203Pb]Pb-VMT01; [212Pb]Pb-VMT01 | MC1R-targeted Alpha-particle Monotherapy and Combination Therapy Trial With Nivolumab in Adults With Advanced Melanoma | Ongoing, estimated completion 2029-12 | Melanoma | Perspective Therapeutics | Phase I/II |
| NCT05636618 | Pb-212 | [212Pb]VMT-α-NET; [212Pb]VMT-α-NET | Targeted Alpha-Particle Therapy for Advanced SSTR2 Positive Neuroendocrine Tumours | Ongoing, estimated completion 2029-12 | Metastatic Castration-resistant Prostate Cancer | Perspective Therapeutics | Phase I/II |
| NCT05557708 | Pb-212 | [203Pb]Pb-Pentixather; [212Pb]Pb-Pentixather | A Safety Study of [212Pb]Pb-Pentixather Radioligand Therapy | Not yet recruting, estimated completion 2030-06 | Carcinoid Tumour LungNeuroendocrine Tumour of the LungCarcinoma, Small-Cell Lung | Yusuf Menda | Early Phase I |
| NCT05283330 | Pb-212 | [212Pb]Pb-DOTAM-GRPR1 | Safety and Tolerability of [212Pb]Pb-DOTAM-GRPR1 in Adult Subjects With Recurrent or Metastatic GRPR-expressing Tumours | Ongoing, estimated completion 2027- 08 |
GRPR1-positive solid tumours refractory to standard therapies |
Orano Med LLC | Phase I |
| NCT05153772 | Pb-212 | [212Pb]Pb-DOTAMTATE | Targeted Alpha-emitter Therapy of PRRT Naïve and Previous PRRT Neuroendocrine Tumour Patients | Active not recruting, estimated completion 2028-10 | Neuroendocrine Tumours | Orano Med LLC | Phase II |
| NCT03466216 | Pb-212 | [212Pb]Pb-DOTAMTATE | Phase 1 Study of AlphaMedix™ in Adult Subjects With SSTR (+) NET | Terminated, 2023-04 | SSTR2-positive neuroendocrine tumours refractory to standard therapies |
Radiomedix and Orano Med | Phase I |
| NCT01384253 | Pb-212 | [212Pb]Pb-TCMC-Trastuzumab | Safety Study of [212PbPb -TCMC-Trastuzumab Radio Immunotherapy | Completed, 2016-07 | Breast NeoplasmsPeritoneal NeoplasmsOvarian NeoplasmsPancreatic NeoplasmsStomach Neoplasms | Orano Med LLC | Phase I |
| NCT05924672 | Ra-223 | [223Ra]RaCl2 |
Efficacy of Radium-223 in PSMA PET Optimally Selected Patients | Ongoing, estimated completion 2028-05 | Castration-Resistant Prostate Carcinoma|Metastatic Malignant Neoplasm in the Bone|Stage IVB Prostate Cancer AJCC v8 | University of California, San Francisco | Phase II |
| NCT05301062 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
A Research Called CREDIT Studies How Safe the Study Treatment Radium-223 is and How Well it Works in Chinese Men With Advanced Prostate Cancer That Has Spread to the Bones and Does Not Respond to Treatments for Lowering Testosterone Levels | Terminated, 2023-06 | Metastatic Castration-resistant Prostate Cancer; Bone Metastases | Bayer | observational |
| NCT05133440 | Ra-223 | [223Ra]RaCl2 |
A Study of Stereotactic Body Radiation Therapy and [223Ra]RaCl2 in Prostate Cancer That Has Spread to the Bones | Active not recruting, estimated completion 2027-11 | Prostate Cancer | Memorial Sloan Kettering Cancer Center | Phase II |
| NCT04681144 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
A Study to Learn More About How Radium-223 Affects the Quality of Life of Colombian Patients With Prostate Cancer That Has Not Responded to Testosterone Lowering Treatment and Has Spread to the Bones, and to Better Understand Its Safety | Completed, 2022-11 | Prostate Cancer | Bayer | observational |
| NCT04597125 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Investigation of [223Ra]RaCl2 (Xofigo), a Treatment That Gives Off Radiation That Helps Kill Cancer Cells, Compared to a Treatment That Inactivates Hormones (New Antihormonal Therapy, NAH) in Patients With Prostate Cancer That Has Spread to the Bone Getting Worse on or After Earlier NAH | Active not recruting, estimated completion 2026-10 | Metastatic Castrate Resistant Prostate Cancer (mCRPC) | Bayer | Phase IV |
| NCT04587427 | Ra-223 | [223Ra]RaCl2 |
A Study to Learn More About How Radium-223 is Being Used With Other Treatments in European Patients Who Have Not Received Radium-223 Before | Completed, 2023-05 | Bone Metastatic Castration-resistant Prostate Cancer | Bayer | observational |
| NCT04521361 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
A Study to Assess How Radium-223 Distributes in the Body of Patients With Prostate Cancer Which Spread to the Bones | Active not recruting, estimated completion 2025-09 | Bone Metastatic Castration-resistant Prostate Cancer | Bayer | Phase I |
| NCT04516161 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
EPIX, a Study to Gather More Information About Characteristics of Patients and Other Factors Which May Contribute to Survival Over a Long Period of Time in Patients With Metastatic Castration-resistant Prostate Cancer (mCRPC) Treated With Radium-223 (Xofigo) | Completed, 2021-03 | Metastatic Castration Resistant Prostate Cancer (mCRPC) | Bayer | observational |
| NCT04489719 | Ra-223 | [223Ra]RaCl2 |
Impact of DNA Repair Pathway Alterations on Sensitivity to Radium-223 in Bone Metastatic Castration-resistant Prostate Cancer | Ongoing, estimated completion 2029-08 | Castration-Resistant Prostate Carcinoma; Metastatic Malignant Neoplasm in the Bone | University of Washington | observational |
| NCT04281147 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Study to Gather Information About the Use of Healthcare Services and the Way the Disease is Cared for in Canadian Patients With Prostate Gland Cancer Which Spread Throughout the Body | Completed, 2021-06 | Prostate Cancer | Bayer | observational |
| NCT04256993 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
PRECISE, a Study to Gather More Information About Bone Fractures and Survival in Castration-resistant Prostate Cancer (CRPC) patients Treated With Radium-223 in Routine Clinical practIce in SwedEn | Completed, 2021-06 | Metastatic Castration-Resistant Prostate Cancer | Bayer | observational |
| NCT04237584 | Ra-223 | [223Ra]RaCl2 |
A Study Comparing ARB With Radium-223 vs ARB Therapy With Placebo and the Effect Upon Survival for mCRPC Patients | Terminated, 2022-03 | Metastatic Castration-resistant Prostate Cancer | MANA RBM | Phase III |
| NCT04232761 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Study to Gather Information on the Safety and How [223Ra]RaCl2, an Alpha Particle-emitting Radioactive Agent, Works Under Routine Clinical Practice in Taiwan in Patients With Castration-resistant Prostate Cancer (CRPC) Which Has Spread to the Bone | Completed, 2024-04 | Castration-resistant Prostate Cancer | Bayer | observational |
| NCT04110782 | Ra-223 | [223Ra]RaCl2 |
Radical Prostatectomy and External Beam Radiotherapy in mCRPC With [223Ra]RaCl2 (RaProRad) | UNKNOWN | Prostate Cancer | Azienda Policlinico Umberto I | observational |
| NCT04090398 | Ra-223 | [223Ra]RaCl2 |
Testing the Addition of Radium Therapy ([223Ra]RaCl2) to the Usual Chemotherapy Treatment (Paclitaxel) for Advanced Breast Cancer That Has Spread to the Bones | Active not recruting, estimated completion 2026-06 | Anatomic Stage IV Breast Cancer; Metastatic HER2-Negative Breast Carcinoma; Metastatic Malignant Neoplasm in the Bone | National Cancer Institute (NCI) | Phase II |
| NCT04071236 | Ra-223 | [223Ra]RaCl2 |
Radiation Medication ([223Ra]RaCl2) Versus [223Ra]RaCl2 Plus Radiation Enhancing Medication (M3814) Versus [223Ra]RaCl2 M3814 Plus Avelumab (a Type of Immunotherapy) for Advanced Prostate Cancer Not Responsive to Hormonal Therapy | Ongoing, estimated completion 2026-04 | Metastatic Castration-Resistant Prostate Carcinoma; Metastatic Malignant Neoplasm in the Bone; Metastatic Malignant Neoplasm in the Lymph Nodes; Stage IVB Prostate Cancer | National Cancer Institute (NCI) | Phase I/II |
| NCT04071223 | Ra-223 | [223Ra]RaCl2 |
Testing the Addition of a New Anti-cancer Drug, [223Ra]RaCl2, to the Usual Treatment (Cabozantinib) for Advanced Renal Cell Cancer That Has Spread to the Bone, RadiCaL Study | Ongoing, estimated completion 2025-10 | Advanced Renal Cell Carcinoma; Chromophobe Renal Cell Carcinoma; Clear Cell Renal Cell Carcinoma; Collecting Duct Carcinoma; Kidney Medullary Carcinoma; Metastatic Malignant Neoplasm in the Bone; Papillary Renal Cell Carcinoma|Stage IV Renal Cell Cancer; Unclassified Renal Cell Carcinoma | National Cancer Institute (NCI) | Phase II |
| NCT03996473 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Study to Test the Safety and How [223Ra]RaCl2 an Alpha Particle-emitting Radioactive Agent Works in Combination With Pembrolizumab an Immune Checkpoint Inhibitor in Patients With Stage IV Non-small Cell Lung Cancer With Bone Metastases | Terminated, 2023-01 | Carcinoma, Non-Small-Cell Lung | Bayer | Phase I |
| NCT03903835 | Ra-223 | [223Ra]RaCl2 |
ProBio: A Biomarker Driven Study in Patients With Metastatic Prostate Cancer | Ongoing, estimated completion 2026-12 | Metastatic Castration-resistant Prostate Cancer (mCRPC); Metastatic Hormone-Sensitive Prostate Cancer (mHSPC) | Karolinska Institutet | Phase III |
| NCT03896984 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Descriptive Analysis of Clinical Outcomes in Patients With Prostate Gland Cancer, Which Spreads to Other Parts of the Body, Who Were Treated First With Novel Anti-hormone Therapy Followed by a Second Line Treatment With Novel Anti-Hormone Therapy or RadIum-223 (Xofigo). | Completed, 2020-12 | Metastatic Castration-resistant Prostate Cancer (mCRPC) | Bayer | observational |
| NCT03737370 | Ra-223 | [223Ra]RaCl2 |
Fractionated Docetaxel and Radium-223 in Metastatic Castration-Resistant Prostate Cancer | Active not recruting, estimated completion 2026-12 | Metastatic Castrate Resistant Prostate Cancer | Tufts Medical Center | Phase I |
| NCT03563014 | Ra-223 | [223Ra]RaCl2 (Xofigo, Bay88-8223) |
A Local Retrospective Observational Study to Evaluate the Treatment Patterns of mCRPC Patients in Belgium Treated With Radium-223 | Completed, 2019-01 | Prostatic Neoplasms, Castration-Resistant | Bayer | observational |
| NCT03458559 | Ra-223 | [223Ra]RaCl2 |
Rhenium-188-HEDP vs. [223Ra]RaCl2 in Patients With Advanced Prostate Cancer Refractory to Hormonal Therapy | UNKNOWN | Prostate Cancer Metastatic to Bone | Amsterdam UMC, location VUmc | Phase III |
| NCT03419442 | Ra-223 | [223Ra]RaCl2 |
Multi-academic Center Study of Xofigo Patients | Completed, 2019-10 | Prostate Cancer, Castration Resistant | Bayer | observational |
| NCT03368989 | Ra-223 | [223Ra]RaCl2 |
The Effects of [223Ra]RaCl2 Therapy on Radionuclide Bone Scan Lesions. | Completed, 2017-02 | Bony Metastases From Castrate Refractory Prostate Cancer | The University of Texas Health Science Center, Houston | observational |
| NCT03361735 | Ra-223 | [223Ra]RaCl2 |
Radium [223Ra]RaCl2, Hormone Therapy and Stereotactic Body Radiation Therapy in Treating Patients With Metastatic Prostate Cancer | Active not recruting, estimated completion 2026-02 | Prostate Adenocarcinoma | City of Hope Medical Center | Phase II |
| NCT03344211 | Ra-223 | [223Ra]RaCl2 |
Enzalutamide With or Without [223Ra]RaCl2 in Patients With Metastatic, Castration-Resistant Prostate Cancer | Active not recruting, estimated completion 2025-11 | Bone Metastatic Castration-resistant Prostate Cancer | University of Southern California | Phase II |
| NCT03325127 | Ra-223 | [223Ra]RaCl2 |
Outcomes of mCRPC Patients Treated With Radium-223 Concomitant With Abiraterone or Enzalutamide- A Chart Review Study | WITHDRAWN | Prostatic Neoplasms, Castration-Resistant | Bayer | observational |
| NCT03317392 | Ra-223 | [223Ra]RaCl2 |
Testing the Safety of Different Doses of Olaparib Given Radium-223 for Men With Advanced Prostate Cancer With Bone Metastasis | Active not recruting, estimated completion 2026-04 | Castration-Resistant Prostate Carcinoma; Metastatic Prostate Adenocarcinoma | National Cancer Institute (NCI) | Phase I/II |
| NCT03315260 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Treatment Satisfaction With Radium-223 in Japan | Completed, 2023-03 | Prostatic Neoplasms | Bayer | observational |
| NCT03304418 | Ra-223 | [223Ra]RaCl2 |
Radium-223 and Radiotherapy in Hormone-Naïve Men With Oligometastatic Prostate Cancer to Bone | Completed, 2023-08 | Prostate Cancer Metastatic to Bone | University of Utah | Phase II |
| NCT03223597 | Ra-223 | [223Ra]RaCl2 |
Registry of Treatment Outcomes of Symptomatic Metastasized Castration Resistant Prostate Cancer Treated With Radium-223 | Completed, 2018-03 | Prostate Cancer Metastatic; Bone Metastases | The Netherlands Cancer Institute | observational |
| NCT03093428 | Ra-223 | [223Ra]RaCl2 |
Study Evaluating the Addition of Pembrolizumab to Radium-223 in mCRPC | Completed, 2025-02 | Prostate Cancer | Dana-Farber Cancer Institute | Phase II |
| NCT03076203 | Ra-223 | [223Ra]RaCl2 |
Phase IB Trial of Radium-223 and Niraparib in Patients With Castrate Resistant Prostate Cancer (NiraRad) | Completed, 2022-11 | Bone-only Metastatic Castration-Resistant Prostate Cancer (CRPC) | Sidney Kimmel Cancer Center at Thomas Jefferson University | Phase I |
| NCT03062254 | Ra-223 | [223Ra]RaCl2 |
Metabolic Change in Prostate Cancer Bone Metastases on [68Ga]Ga-HBED-CC-PSMA PET/CT Following Radium-223 Therapy | Completed, 2021-07 | Prostate Cancer | Sir Mortimer B. Davis - Jewish General Hospital | Phase II |
| NCT02928029 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Study Testing [223Ra]RaCl2 in Relapsed Multiple Myeloma | Terminated, 2019-03 | Multiple Myeloma | Bayer | Phase I/II |
| NCT02925702 | Ra-223 | [223Ra]RaCl2 55mBq/Kg every 4 weeks intravenously |
PRORADIUM: Prospective Multi-centre Study of Prognostic Factors in mCRPC Patients Treated With Radium-223. | UNKNOWN | Advanced Prostate Cancer|Castration Resistant | Centro Nacional de Investigaciones Oncologicas CARLOS III | observational |
| NCT02903160 | Ra-223 | [223Ra]RaCl2 |
Prostate Cancer Intensive, Non-Cross Reactive Therapy (PRINT) for Castration Resistant Prostate Cancer (CRPC) | Completed, 2021-11 | Prostate Cancer | Icahn School of Medicine at Mount Sinai | Phase II |
| NCT02899104 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Navigant Study- Treatment Patterns in mCRPC (Metastatic Castrate Resistant Prostate Cancer ) | Completed, 2019-03 | Prostatic Neoplasms, Castration-Resistant | Bayer | observational |
| NCT02880943 | Ra-223 | [223Ra]RaCl2 |
Dose-finding, Safety and Efficacy Study of [223Ra]RaCl2 (XOFIGO) in RCC Patients With Bone Metastases. (EIFFEL) | UNKNOWN | Clear-cell Metastatic Renal Cell Carcinoma; Bone Metastases | Association Pour La Recherche des Thérapeutiques Innovantes en Cancérologie | Phase I/II |
| NCT02814669 | Ra-223 | [223Ra]RaCl2 |
Safety and Tolerability of Atezolizumab (ATZ) in Combination With [223Ra]RaCl2 (R-223-D) in Metastatic Castrate-Resistant Prostate Cancer (CRPC) Progressed Following Treatment With an Androgen Pathway Inhibitor | Completed, 2019-07 | Castrate-Resistant Prostate Cancer | Hoffmann-La Roche | Phase I |
| NCT02803437 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Drug Use Investigation of Xofigo, Castration Resistant Prostate Cancer With Bone Metastases | Completed, 2024-12 | Prostatic Neoplasms, Castration-Resistant | Bayer | observational |
| NCT02729103 | Ra-223 | [223Ra]RaCl2 |
Treatment Patterns in Metastatic Prostate Cancer | Completed, 2017-01 | Prostatic Neoplasm | Bayer | observational |
| NCT02656563 | Ra-223 | [223Ra]RaCl2 |
Radium-223 Following Intermittent ADT | WITHDRAWN | Prostate Cancer | Canadian Urology Research Consortium | Phase II |
| NCT02605356 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Phase 1b/2 Study Testing [223Ra]RaCl2/Bortezomib/Dexamethasone Combination in Relapsed Multiple Myeloma | WITHDRAWN | Multiple Myeloma | Bayer | Phase I/II |
| NCT02582749 | Ra-223 | [223Ra]RaCl2 |
Androgen Deprivation Therapy +/- [223Ra]RaCl2 in Metastatic Prostate Cancer With Bone Metastases | Terminated, 2017-09 | Prostate Cancer|Bone Metastases|Prostate Neoplasms | Ajjai Alva, MD | Phase II |
| NCT02518698 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Treatment Patterns in Castrate Resistant Prostate Cancer Patients With Bone Metastases in a Medicare Population | Completed, 2017-09 | Prostate Cancer | Bayer | observational |
| NCT02507570 | Ra-223 | [223Ra]RaCl2 |
Open Label Phase Two Study of Enzalutamide With Concurrent Administration of [223Ra]RaCl2 in Castration-Resistant (Hormone-Refractory) Prostate Cancer Subjects With Symptomatic Bone Metastasis | Completed, 2019-01 | Prostate Carcinoma Metastatic to the Bone | Carolina Research Professionals, LLC | Phase II |
| NCT02484339 | Ra-223 | [223Ra]RaCl2 |
Treatment of Advanced Castration Resistant Prostate Carcinoma With Limited Bone Metastases (α-RT) | UNKNOWN | Prostate Carcinoma | University Hospital Freiburg | Phase II |
| NCT02463799 | Ra-223 | [223Ra]RaCl2 |
Study of Sipuleucel-T W/ or W/O Radium-223 in Men With Asymptomatic or Minimally Symptomatic Bone-MCRPC | Completed, 2019-12 | Prostate Cancer | Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins | Phase II |
| NCT02450812 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Non-interventional Study With Ra-223 Dichloride Assessing Overall Survival and Effectiveness Predictors for mCRPC Patients in a Real Life Setting in Germany | Completed, 2020-09 | Prostatic Neoplasms, Castration-Resistant | Bayer | observational |
| NCT02442063 | Ra-223 | [223Ra]RaCl2 |
Phase Ib Study of Radium Ra 223 Dichloride in Combination With Paclitaxel in Cancer Subjects With Bone Lesions | Completed, 2016-10 | Neoplasms;Bone Diseases | Bayer | Phase I |
| NCT02406521 | Ra-223 | [223Ra]RaCl2 |
Exploratory Study of Radium-223 and VEGF-Targeted Therapy in Patients With Metastatic Renal Cell Carcinoma and Bone Mets | Completed, 2019-12 | Metastatic Renal Cell Carcinoma | Dana-Farber Cancer Institute | Phase I |
| NCT02398526 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Pain Evaluation in Radium-223 Treated Castration Resistant Prostate Cancer Patients With Bone Metastases | Completed, 2020-07 | Castration-Resistant Prostatic Cancer | Bayer | observational |
| NCT02396368 | Ra-223 | [223Ra]RaCl2 |
A Study of Radium-223 in Combination With Tasquinimod in Bone-only Metastatic Castration-Resistant Prostate Cancer | WITHDRAWN | Bone-only Metastatic Castration-Resistant Prostate Cancer (CRPC) | Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins | Phase I |
| NCT02390934 | Ra-223 | [223Ra]RaCl2 |
Efficacy of Radium 223 in Radioactive Iodine Refractory Bone Metastases From Differentiated Thyroid Cancer | Completed, 2019-04 | Thyroid Cancer | Gustave Roussy, Cancer Campus, Grand Paris | Phase II |
| NCT02366130 | Ra-223 | [223Ra]RaCl2 |
Trial of [223Ra]RaCl2 in Combination With Hormonal Therapy and Denosumab in the Treatment of Patients With Hormone-Positive Bone-Dominant Metastatic Breast Cancer | Completed, 2020-12 | Breast Cancer | M.D. Anderson Cancer Center | Phase II |
| NCT02346526 | Ra-223 | [223Ra]RaCl2 |
A Biomarker Study of Standard-of-care [223Ra]RaCl2 for Metastatic Castration-resistant Prostate Cancer | Completed, 2020-12 | Prostate Cancer; Castration-resistant Prostate Cancer; Castration-resistant Prostate Cancer Metastatic to Bone | Massachusetts General Hospital | Phase II |
| NCT02331303 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
A Drug Utilization Study of Radium-223 in Sweden | Completed, 2017-12 | Neoplasms | Bayer | observational |
| NCT02283749 | Ra-223 | [223Ra]RaCl2 |
BrUOG L301 With Non-Small Cell Lung Cancer and Bone Metastases | Completed, 2018-11 | Non Small Cell Lung Cancer With Bone Metastatses | Angela Taber MD | Phase II |
| NCT02278055 | Ra-223 | [223Ra]RaCl2 |
Non-Randomized Trial Assessing Pain Efficacy With Radium-223 in Symptomatic Metastatic Castration-Resistant Prostate Cancer | Completed, 2022-02 | Metastatic Prostate Cancer|Pain | Memorial Sloan Kettering Cancer Center | Phase II |
| NCT02258464 | Ra-223 | [223Ra]RaCl2 |
Study of [223Ra]RaCl2 Versus Placebo and Hormonal Treatment as Background Therapy in Subjects With Bone Predominant HER2 (Human Epidermal Growth Factor Receptor 2) Negative Hormone Receptor Positive Metastatic Breast Cancer | Terminated, 2019-08 | Breast Neoplasms | Bayer | Phase II |
| NCT02258451 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Study of [223Ra]RaCl2 in Combination With Exemestane and Everolimus Versus Placebo in Combination With Exemestane and Everolimus in Subjects With Bone Predominant HER2 Negative Hormone Receptor Positive Metastatic Breast Cancer | Completed, 2022-10 | Breast Neoplasms | Bayer | Phase II |
| NCT02199197 | Ra-223 | [223Ra]RaCl2 |
Radium-223 With Enzalutamide Compared to Enzalutamide Alone in Men With Metastatic Castration Refractory Prostate Cancer | Completed, 2019-10 | Prostate Cancer | University of Utah | Phase II |
| NCT02194842 | Ra-223 | [223Ra]RaCl2 |
Phase III Radium-223 mCRPC-PEACE III | Active not recruting, estimated completion 2028-12 | Prostate Cancer | European Organisation for Research and Treatment of Cancer - EORTC | Phase III |
| NCT02141438 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Observational Study for the Evaluation of Long-term Safety of Radium-223 Used for the Treatment of Metastatic Castration Resistant Prostate Cancer | Completed, 2024-10 | Metastatic Castration-resistant Prostate Cancer | Bayer | observational |
| NCT02135484 | Ra-223 | [223Ra]RaCl2 Alpharadin |
Radium-223 in Castrate Resistant Prostate Cancer Bone Metastases | Completed, 2020-12 | Prostate Cancer | M.D. Anderson Cancer Center | NA |
| NCT02097303 | Ra-223 | [223Ra]RaCl2 |
Open Label Phase Two Trial of [223Ra]RaCl2 With Concurrent Administration of Abiraterone Acetate Plus Prednisone in Symptomatic Castration-Resistant (Hormone-Refractory) Prostate Cancer Subjects With Bone Metastasis | Completed, 2015-12 | Prostate Cancer | Carolina Research Professionals, LLC | Phase II |
| NCT02043678 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
[223Ra]RaCl2 and Abiraterone Acetate Compared to Placebo and Abiraterone Acetate for Men With Cancer of the Prostate When Medical or Surgical Castration Does Not Work and When the Cancer Has Spread to the Bone, Has Not Been Treated With Chemotherapy and is Causing no or Only Mild Symptoms | Completed, 2024-02 | Prostatic Neoplasms | Bayer | Phase III |
| NCT02034552 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
A Randomized Phase IIa Efficacy and Safety Study of [223Ra]RaCl2 With Abiraterone Acetate or Enzalutamide in Metastatic Castration-resistant Prostate Cancer (CRPC) | Completed, 2018-06 | Prostatic Neoplasms | Bayer | Phase II |
| NCT02023697 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Standard Dose Versus High Dose and Versus Extended Standard Dose [223Ra]RaCl2 in Castration-resistant Prostate Cancer Metastatic to the Bone | Completed, 2018-08 | Prostatic Neoplasms | Bayer | Phase II |
| NCT01934790 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Re-treatment Safety of [223Ra]RaCl2 in Castration-resistant Prostate Cancer With Bone Metastases | Completed, 2017-04 | Prostatic Neoplasms | Bayer | Phase I/II |
| NCT01929655 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Japanese BAY88-8223 Monotherapy Phase II Study | Completed, 2017-05 | Prostatic Neoplasms | Bayer | Phase II |
| NCT01810770 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
[223Ra]RaCl2 Asian Population Study in the Treatment of CRPC Patients With Bone Metastasis | Completed, 2017-09 | Prostatic Neoplasms | Bayer | Phase III |
| NCT01798108 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Dose Escalation Study of [223Ra]RaCl2 in Patients With Advanced Skeletal Metastases | Completed, 2003-06 | Neoplasm Metastasis | Bayer | Phase I |
| NCT01618370 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
[223Ra]RaCl2 (Alpharadin) in Castration-Resistant (Hormone-Refractory) Prostate Cancer Patients With Bone Metastases | Completed, 2016-02 | Prostatic Neoplasms | Bayer | Phase III |
| NCT01565746 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Safety, Biodistribution, Radiation Dosimetry and Pharmacokinetics Study of BAY88-8223 in Japanese Patients | Completed, 2016-04 | Prostatic Neoplasms | Bayer | Phase I |
| NCT01106352 | Ra-223 | [223Ra]RaCl2 (Xofigo, BAY88-8223)|DRUG: Docetaxel |
A Study of Alpharadin With Docetaxel in Patients With Bone Metastasis From Castration-Resistant Prostate Cancer (CRPC) | Completed, 2015-06 | Bone Metastases|Castration-Resistant Prostate Cancer | Bayer | Phase I/II |
| NCT01070485 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
BAY88-8223, Alpharadin, Breast Cancer Patients With Bone Dominant Disease | Completed, 2012-01 | Breast Cancer|Bone Metastases | Bayer | Phase II |
| NCT00748046 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
Alpharadin™ ([223Ra]RaCl2) Safety and Dosimetry With HRPC That Has Metastasized to the Skeleton | Completed, 2009-10 | Prostate Cancer|Metastases|Pharmacokinetics | Bayer | Phase I |
| NCT00699751 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
A Phase III Study of [223Ra]RaCl2 in Patients With Symptomatic Hormone Refractory Prostate Cancer With Skeletal Metastases | Completed, 2014-02 | Hormone Refractory Prostate Cancer|Bone Metastases | Bayer | Phase III |
| NCT00667537 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
PK in Pts With HRPC & Skeletal Metastes | Completed, 2008-12 | Prostatic Neoplasms | Bayer | Phase I |
| NCT00667199 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
BAY88-8223, Does Response Study in HRPC Patients | Completed, 2009-10 | Hormone Refractory Prostate Cancer; Bone Metastases | Bayer | Phase II |
| NCT00459654 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
A Placebo-controlled Phase II Study of Bone-targeted Radium-223 in Symptomatic Hormone-refractory Prostate Cancer | Completed, 2007-05 | Prostate Cancer|Neoplasm Metastasis | Bayer | Phase II |
| NCT00337155 | Ra-223 | [223Ra]RaCl2 (BAY88-8223) |
BAY88-8223, Dose Finding Study in Patients With HRPC | Completed, 2009-12 | Prostate Cancer|Neoplasm Metastasis | Bayer | Phase II |
| NCT04147819 | Th-227 | BAY2701439 | A First in Human Study of BAY2701439 to Look at Safety, How the Body Absorbs, Distributes and Excretes the Drug, and How Well the Drug Works in Participants With Advanced Cancer Expressing the HER2 Protein | Completed, 2023-09 | Cancers With HER2 Expression | Bayer | Phase I |
| NCT03724747 | Th-227 | BAY2315497 | Study to Evaluate the Safety, Tolerability,Pharmacokinetics, and Antitumour Activity of a Thorium-227 Labeled Antibody-chelator Conjugate Alone and in Combination With Darolutamide, in Patients With Metastatic Castration Resistant Prostate Cancer | Completed, 2024-10 | Metastatic Castration Resistant Prostate Cancer (mCRPC) | Bayer | Phase I |
| NCT03507452 | Th-227 | BAY2287411 | First-in-human Study of BAY2287411 Injection, a Thorium-227 Labeled Antibody-chelator Conjugate, in Patients With Tumours Known to Express Mesothelin | Completed, 2022-03 | Advanced Recurrent Malignant Pleural Epithelioid Mesothelioma; Advanced Recurrent Malignant Peritoneal Epithelioid Mesothelioma; Advanced Recurrent Serous Ovarian Cancer; Advanced Pancreatic Ductal Adenocarcinoma | Bayer | Phase I |
| NCT02581878 | Th-227 | BAY1862864 | Safety and Tolerability of BAY1862864 Injection in Subjects With Relapsed or Refractory CD22-positive Non-Hodgkin’s Lymphoma | Completed, 2019-11 | Lymphoma, Non-Hodgkin | Bayer | Phase I |
- McDevitt, M.R.; Ma, D.; Simon, J.; Frank, R.K.; Scheinberg, D.A. Design and Synthesis of 225Ac Radioimmunopharmaceuticals. Appl. Radiat. Isot. 2002, 57, 841–847, doi:10.1016/S0969-8043(02)00167-7.
- McDevitt, M.R.; Ma, D.; Simon, J.; Frank, R.K.; Scheinberg, D.A. Design and Synthesis of 225Ac Radioimmunopharmaceuticals. Appl. Radiat. Isot. 2002, 57, 841–847, doi:10.1016/S0969-8043(02)00167-7.
- Deal, K.A.; Davis, I.A.; Mirzadeh, S.; Kennel, S.J.; Brechbiel, M.W. Improved in Vivo Stability of Actinium-225 Macrocyclic Complexes. J. Med. Chem. 1999, 42, 2988–2992, doi:10.1021/jm990141f.
- Chappell, L.L.; Deal, K.A.; Dadachova, E.; Brechbiel, M.W. Synthesis, Conjugation, and Radiolabeling of a Novel Bifunctional Chelating Agent for 225 Ac Radioimmunotherapy Applications. Bioconjug. Chem. 2000, 11, 510–519, doi:10.1021/bc990153f.
- Davis, I.A.; Glowienka, K.A.; Boll, R.A.; Deal, K.A.; Brechbiel, M.W.; Stabin, M.; Bochsler, P.N.; Mirzadeh, S.; Kennel, S.J. Comparison of 225actinium Chelates: Tissue Distribution and Radiotoxicity. Nucl. Med. Biol. 1999, 26, 581–589, doi:10.1016/s0969-8051(99)00024-4.
- Holzleitner, N.; Vilangattil, M.; Swaidan, A.; Garcia-Prada, C.D.; Taddio, M.F.; Jeanjean, P.; Mona, C.E.; Lapa, C.; Casini, A.; Günther, T.; et al. Preclinical Evaluation of 225Ac-Labeled Minigastrin Analog DOTA-CCK-66 for Targeted Alpha Therapy. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 458–468, doi:10.1007/s00259-024-06927-z.
- Li, L.; Rousseau, J.; Jaraquemada-Peláez, M.D.G.; Wang, X.; Robertson, A.; Radchenko, V.; Schaffer, P.; Lin, K.-S.; Bénard, F.; Orvig, C. 225 Ac-H 4 Py4pa for Targeted Alpha Therapy. Bioconjug. Chem. 2021, 32, 1348–1363, doi:10.1021/acs.bioconjchem.0c00171.
- Yoshida, T.; Jin, K.; Song, H.; Park, S.; Huso, D.L.; Zhang, Z.; Liangfeng, H.; Zhu, C.; Bruchertseifer, F.; Morgenstern, A.; et al. Effective Treatment of Ductal Carcinoma in Situ with a HER-2-Targeted Alpha-Particle Emitting Radionuclide in a Preclinical Model of Human Breast Cancer. Oncotarget 2016, 7, 33306–33315, doi:10.18632/oncotarget.8949.
- Pruszynski, M.; D’Huyvetter, M.; Bruchertseifer, F.; Morgenstern, A.; Lahoutte, T. Evaluation of an Anti-HER2 Nanobody Labeled with 225 Ac for Targeted α-Particle Therapy of Cancer. Mol. Pharm. 2018, 15, 1457–1466, doi:10.1021/acs.molpharmaceut.7b00985.
- Puttemans, J.; Dekempeneer, Y.; Eersels, J.L.; Hanssens, H.; Debie, P.; Keyaerts, M.; Windhorst, A.D.; Van Der Aa, F.; Lecocq, Q.; Breckpot, K.; et al. Preclinical Targeted α- and Β−-Radionuclide Therapy in HER2-Positive Brain Metastasis Using Camelid Single-Domain Antibodies. Cancers 2020, 12, 1017, doi:10.3390/cancers12041017.
- Pandya, D.N.; Hantgan, R.; Budzevich, M.M.; Kock, N.D.; Morse, D.L.; Batista, I.; Mintz, A.; Li, K.C.; Wadas, T.J. Preliminary Therapy Evaluation of 225 Ac-DOTA-c(RGDyK) Demonstrates That Cerenkov Radiation Derived from 225 Ac Daughter Decay Can Be Detected by Optical Imaging for In Vivo Tumour Visualization. Theranostics 2016, 6, 698–709, doi:10.7150/thno.14338.
- Sattiraju, A.; Sai, K.K.S.; Xuan, A.; Pandya, D.N.; Almaguel, F.G.; Wadas, T.J.; Herpai, D.M.; Debinski, W.; Mintz, A. IL13RA2 Targeted Alpha Particle Therapy against Glioblastomas. Oncotarget 2017, 8, 42997–43007, doi:10.18632/oncotarget.17792.
- Nedrow, J.R.; Josefsson, A.; Park, S.; Bäck, T.; Hobbs, R.F.; Brayton, C.; Bruchertseifer, F.; Morgenstern, A.; Sgouros, G. Pharmacokinetics, Microscale Distribution, and Dosimetry of Alpha-Emitter-Labeled Anti-PD-L1 Antibodies in an Immune Competent Transgenic Breast Cancer Model. EJNMMI Res. 2017, 7, 57, doi:10.1186/s13550-017-0303-2.
- Thiele, N.A.; Brown, V.; Kelly, J.M.; Amor-Coarasa, A.; Jermilova, U.; MacMillan, S.N.; Nikolopoulou, A.; Ponnala, S.; Ramogida, C.F.; Robertson, A.K.H.; et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem. Int. Ed. 2017, 56, 14712–14717, doi:10.1002/anie.201709532.
- Kelly, J.M.; Amor-Coarasa, A.; Ponnala, S.; Nikolopoulou, A.; Williams, C.; Thiele, N.A.; Schlyer, D.; Wilson, J.J.; DiMagno, S.G.; Babich, J.W. A Single Dose of 225 Ac-RPS-074 Induces a Complete Tumour Response in an LNCaP Xenograft Model. J. Nucl. Med. 2019, 60, 649–655, doi:10.2967/jnumed.118.219592.
- Poty, S.; Membreno, R.; Glaser, J.M.; Ragupathi, A.; Scholz, W.W.; Zeglis, B.M.; Lewis, J.S. The Inverse Electron-Demand Diels–Alder Reaction as a New Methodology for the Synthesis of225 Ac-Labelled Radioimmunoconjugates. Chem. Commun. 2018, 54, 2599–2602, doi:10.1039/C7CC09129J.
- Poty, S.; Carter, L.M.; Mandleywala, K.; Membreno, R.; Abdel-Atti, D.; Ragupathi, A.; Scholz, W.W.; Zeglis, B.M.; Lewis, J.S. Leveraging Bioorthogonal Click Chemistry to Improve 225Ac-Radioimmunotherapy of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2019, 25, 868–880, doi:10.1158/1078-0432.CCR-18-1650.
- Thorek, D.L.J.; Ku, A.T.; Mitsiades, N.; Veach, D.; Watson, P.A.; Metha, D.; Strand, S.-E.; Sharma, S.K.; Lewis, J.S.; Abou, D.S.; et al. Harnessing Androgen Receptor Pathway Activation for Targeted Alpha Particle Radioimmunotherapy of Breast Cancer. Clin. Cancer Res. 2019, 25, 881–891, doi:10.1158/1078-0432.CCR-18-1521.
- Solomon, V.R.; Alizadeh, E.; Bernhard, W.; Hartimath, S.V.; Hill, W.; Chekol, R.; Barreto, K.M.; Geyer, C.R.; Fonge, H. 111 In- and 225 Ac-Labeled Cixutumumab for Imaging and α-Particle Radiotherapy of IGF-1R Positive Triple-Negative Breast Cancer. Mol. Pharm. 2019, 16, 4807–4816, doi:10.1021/acs.molpharmaceut.9b00542.
- Ramogida, C.F.; Robertson, A.K.H.; Jermilova, U.; Zhang, C.; Yang, H.; Kunz, P.; Lassen, J.; Bratanovic, I.; Brown, V.; Southcott, L.; et al. Evaluation of Polydentate Picolinic Acid Chelating Ligands and an α-Melanocyte-Stimulating Hormone Derivative for Targeted Alpha Therapy Using ISOL-Produced 225Ac. EJNMMI Radiopharm. Chem. 2019, 4, 21, doi:10.1186/s41181-019-0072-5.
- Tafreshi, N.K.; Tichacek, C.J.; Pandya, D.N.; Doligalski, M.L.; Budzevich, M.M.; Kil, H.; Bhatt, N.B.; Kock, N.D.; Messina, J.L.; Ruiz, E.E.; et al. Melanocortin 1 Receptor–Targeted α-Particle Therapy for Metastatic Uveal Melanoma. J. Nucl. Med. 2019, 60, 1124–1133, doi:10.2967/jnumed.118.217240.
- Tichacek, C.J.; Tafreshi, N.K.; Kil, H.; Engelman, R.W.; Doligalski, M.L.; Budzevich, M.M.; Gage, K.L.; McLaughlin, M.L.; Wadas, T.J.; Silva, A.; et al. Biodistribution and Multicompartment Pharmacokinetic Analysis of a Targeted α Particle Therapy. Mol. Pharm. 2020, 17, 4180–4188, doi:10.1021/acs.molpharmaceut.0c00640.
- Cortez, A.; Josefsson, A.; McCarty, G.; Shtekler, A.E.; Rao, A.; Austin, Z.; Nedrow, J.R. Evaluation of [225Ac]Ac-DOTA-Anti-VLA-4 for Targeted Alpha Therapy of Metastatic Melanoma. Nucl. Med. Biol. 2020, 88–89, 62–72, doi:10.1016/j.nucmedbio.2020.07.006.
- Lakes, A.L.; An, D.D.; Gauny, S.S.; Ansoborlo, C.; Liang, B.H.; Rees, J.A.; McKnight, K.D.; Karsunky, H.; Abergel, R.J. Evaluating 225 Ac and 177 Lu Radioimmunoconjugates against Antibody–Drug Conjugates for Small-Cell Lung Cancer. Mol. Pharm. 2020, 17, 4270–4279, doi:10.1021/acs.molpharmaceut.0c00703.
- Cheal, S.M.; McDevitt, M.R.; Santich, B.H.; Patel, M.; Yang, G.; Fung, E.K.; Veach, D.R.; Bell, M.; Ahad, A.; Vargas, D.B.; et al. Alpha Radioimmunotherapy Using 225 Ac-Proteus-DOTA for Solid Tumours - Safety at Curative Doses. Theranostics 2020, 10, 11359–11375, doi:10.7150/thno.48810.
- Watabe, T.; Liu, Y.; Kaneda-Nakashima, K.; Shirakami, Y.; Lindner, T.; Ooe, K.; Toyoshima, A.; Nagata, K.; Shimosegawa, E.; Haberkorn, U.; et al. Theranostics Targeting Fibroblast Activation Protein in the Tumour Stroma: 64 Cu- and 225 Ac-Labeled FAPI-04 in Pancreatic Cancer Xenograft Mouse Models. J. Nucl. Med. 2020, 61, 563–569, doi:10.2967/jnumed.119.233122.
- Qin, Y.; Imobersteg, S.; Blanc, A.; Frank, S.; Schibli, R.; Béhé, M.P.; Grzmil, M. Evaluation of Actinium-225 Labeled Minigastrin Analogue [225Ac]Ac-DOTA-PP-F11N for Targeted Alpha Particle Therapy. Pharmaceutics 2020, 12, 1088, doi:10.3390/pharmaceutics12111088.
- Bell, M.M.; Gutsche, N.T.; King, A.P.; Baidoo, K.E.; Kelada, O.J.; Choyke, P.L.; Escorcia, F.E. Glypican-3-Targeted Alpha Particle Therapy for Hepatocellular Carcinoma. Molecules 2020, 26, 4, doi:10.3390/molecules26010004.
- Fichou, N.; Gouard, S.; Maurel, C.; Barbet, J.; Ferrer, L.; Morgenstern, A.; Bruchertseifer, F.; Faivre-Chauvet, A.; Bigot-Corbel, E.; Davodeau, F.; et al. Single-Dose Anti-CD138 Radioimmunotherapy: Bismuth-213 Is More Efficient than Lutetium-177 for Treatment of Multiple Myeloma in a Preclinical Model. Front. Med. 2015, 2, doi:10.3389/fmed.2015.00076.
- Teiluf, K.; Seidl, C.; Blechert, B.; Gaertner, F.C.; Gilbertz, K.-P.; Fernandez, V.; Bassermann, F.; Endell, J.; Boxhammer, R.; Leclair, S.; et al. α-Radioimmunotherapy with 213Bi-Anti-CD38 Immunoconjugates Is Effective in a Mouse Model of Human Multiple Myeloma. Oncotarget 2015, 6, 4692–4703, doi:10.18632/oncotarget.2986.
- Fazel, J.; Rötzer, S.; Seidl, C.; Feuerecker, B.; Autenrieth, M.; Weirich, G.; Bruchertseifer, F.; Morgenstern, A.; Senekowitsch-Schmidtke, R. Fractionated Intravesical Radioimmunotherapy with (213)Bi-Anti-EGFR-MAb Is Effective without Toxic Side-Effects in a Nude Mouse Model of Advanced Human Bladder Carcinoma. Cancer Biol. Ther. 2015, 16, 1526–1534, doi:10.1080/15384047.2015.1071735.
- Kunikowska, J.; Królicki, L. Targeted α-Emitter Therapy of Neuroendocrine Tumours. Semin. Nucl. Med. 2020, 50, 171–176, doi:10.1053/j.semnuclmed.2019.11.003.
- Chan, H.S.; Konijnenberg, M.W.; de Blois, E.; Koelewijn, S.; Baum, R.P.; Morgenstern, A.; Bruchertseifer, F.; Breeman, W.A.; de Jong, M. Influence of Tumour Size on the Efficacy of Targeted Alpha Therapy with (213)Bi-[DOTA(0),Tyr(3)]-Octreotate. EJNMMI Res. 2016, 6, 6, doi:10.1186/s13550-016-0162-2.
- Eriksson, S.E.; Bäck, T.; Elgström, E.; Jensen, H.; Nilsson, R.; Lindegren, S.; Tennvall, J. Successful Radioimmunotherapy of Established Syngeneic Rat Colon Carcinoma with 211At-mAb. EJNMMI Res. 2013, 3, 23, doi:10.1186/2191-219X-3-23.
- Eriksson, S.E.; Elgström, E.; Bäck, T.; Ohlsson, T.; Jensen, H.; Nilsson, R.; Lindegren, S.; Tennvall, J. Sequential Radioimmunotherapy with177 Lu- and211 At-Labeled Monoclonal Antibody BR96 in a Syngeneic Rat Colon Carcinoma Model. Cancer Biother. Radiopharm. 2014, 29, 238–246, doi:10.1089/cbr.2014.1625.
- Liu, W.; Ma, H.; Liang, R.; Chen, X.; Li, H.; Lan, T.; Yang, J.; Liao, J.; Qin, Z.; Yang, Y.; et al. Targeted Alpha Therapy of Glioma Using 211At-Labeled Heterodimeric Peptide Targeting Both VEGFR and Integrins. Mol. Pharm. 2022, 19, 3206–3216, doi:10.1021/acs.molpharmaceut.2c00349.
- Lyczko, M.; Pruszynski, M.; Majkowska-Pilip, A.; Lyczko, K.; Was, B.; Meczynska-Wielgosz, S.; Kruszewski, M.; Szkliniarz, K.; Jastrzebski, J.; Stolarz, A.; et al. 211At Labeled Substance P (5-11) as Potential Radiopharmaceutical for Glioma Treatment. Nucl. Med. Biol. 2017, 53, 1–8, doi:10.1016/j.nucmedbio.2017.05.008.
- Meyer, G.J.; Walte, A.; Sriyapureddy, S.R.; Grote, M.; Krull, D.; Korkmaz, Z.; Knapp, W.H. Synthesis and Analysis of 2-[211At]-L-Phenylalanine and 4-[211At]-L-Phenylalanine and Their Uptake in Human Glioma Cell Cultures in-Vitro. Appl. Radiat. Isot. Data Instrum. Methods Use Agric. Ind. Med. 2010, 68, 1060–1065, doi:10.1016/j.apradiso.2009.12.043.
- Borrmann, N.; Friedrich, S.; Schwabe, K.; Hedrich, H.J.; Krauss, J.K.; Knapp, W.H.; Nakamura, M.; Meyer, G.-J.; Walte, A. Systemic Treatment with 4-211Atphenylalanine Enhances Survival of Rats with Intracranial Glioblastoma. Nukl. Nucl. Med. 2013, 52, 212–221, doi:10.3413/Nukmed-0580-13-05.
- Zalutsky, M.R.; Stabin, M.G.; Larsen, R.H.; Bigner, D.D. Tissue Distribution and Radiation Dosimetry of Astatine-211-Labeled Chimeric 81C6, an Alpha-Particle-Emitting Immunoconjugate. Nucl. Med. Biol. 1997, 24, 255–261, doi:10.1016/s0969-8051(97)00060-7.
- Cheng, J.; Ekberg, T.; Engström, M.; Nestor, M.; Jensen, H.J.; Tolmachev, V.; Anniko, M. Radioimmunotherapy With Astatine-211 Using Chimeric Monoclonal Antibody U36 in Head and Neck Squamous Cell Carcinoma. The Laryngoscope 2007, 117, 1013–1018, doi:10.1097/MLG.0b013e31804b1a6d.
- Walte, A.; Sriyapureddy, S.S.R.; Korkmaz, Z.; Krull, D.; Bolte, O.; Hofmann, M.; Meyer, G.-J.; Knapp, W.H. Preparation and Evaluation of 211At Labelled Antineoplastic Antibodies. J. Pharm. Pharm. Sci. Publ. Can. Soc. Pharm. Sci. Soc. Can. Sci. Pharm. 2007, 10, 277s–285s.
- Orozco, J.J.; Bäck, T.; Kenoyer, A.; Balkin, E.R.; Hamlin, D.K.; Wilbur, D.S.; Fisher, D.R.; Frayo, S.L.; Hylarides, M.D.; Green, D.J.; et al. Anti-CD45 Radioimmunotherapy Using (211)At with Bone Marrow Transplantation Prolongs Survival in a Disseminated Murine Leukemia Model. Blood 2013, 121, 3759–3767, doi:10.1182/blood-2012-11-467035.
- Nakamae, H.; Wilbur, D.S.; Hamlin, D.K.; Thakar, M.S.; Santos, E.B.; Fisher, D.R.; Kenoyer, A.L.; Pagel, J.M.; Press, O.W.; Storb, R.; et al. Biodistributions, Myelosuppression, and Toxicities in Mice Treated with an Anti-CD45 Antibody Labeled with the Alpha-Emitting Radionuclides Bismuth-213 or Astatine-211. Cancer Res. 2009, 69, 2408–2415, doi:10.1158/0008-5472.CAN-08-4363.
- Oriuchi, N.; Aoki, M.; Ukon, N.; Washiyama, K.; Tan, C.; Shimoyama, S.; Nishijima, K.; Takahashi, K.; Ito, H.; Ikezoe, T.; et al. Possibility of Cancer-Stem-Cell-Targeted Radioimmunotherapy for Acute Myelogenous Leukemia Using 211At-CXCR4 Monoclonal Antibody. Sci. Rep. 2020, 10, 6810, doi:10.1038/s41598-020-63557-9.
- Laszlo, G.S.; Orozco, J.J.; Kehret, A.R.; Lunn, M.C.; Huo, J.; Hamlin, D.K.; Scott Wilbur, D.; Dexter, S.L.; Comstock, M.L.; O’Steen, S.; et al. Development of [211At]Astatine-Based Anti-CD123 Radioimmunotherapy for Acute Leukemias and Other CD123+ Malignancies. Leukemia 2022, 36, 1485–1491, doi:10.1038/s41375-022-01580-7.
- Green, D.J.; Shadman, M.; Jones, J.C.; Frayo, S.L.; Kenoyer, A.L.; Hylarides, M.D.; Hamlin, D.K.; Wilbur, D.S.; Balkan, E.R.; Lin, Y.; et al. Astatine-211 Conjugated to an Anti-CD20 Monoclonal Antibody Eradicates Disseminated B-Cell Lymphoma in a Mouse Model. Blood 2015, 125, 2111–2119, doi:10.1182/blood-2014-11-612770.
- Zhao, B.; Qin, S.; Chai, L.; Lu, G.; Yang, Y.; Cai, H.; Yuan, X.; Fan, S.; Huang, Q.; Yu, F. Evaluation of Astatine-211-Labeled Octreotide as a Potential Radiotherapeutic Agent for NSCLC Treatment. Bioorg. Med. Chem. 2018, 26, 1086–1091, doi:10.1016/j.bmc.2018.01.023.
- Link, E.M.; Costa, D.C.; Lui, D.; Ell, P.J.; Blower, P.J.; Spittle, M.F. Targeting Disseminated Melanoma with Radiolabelled Methylene Blue: Comparative Bio-Distribution Studies in Man and Animals. Acta Oncol. 1996, 35, 331–341, doi:10.3109/02841869609101650.
- O’Steen, S.; Comstock, M.L.; Orozco, J.J.; Hamlin, D.K.; Wilbur, D.S.; Jones, J.C.; Kenoyer, A.; Nartea, M.E.; Lin, Y.; Miller, B.W.; et al. The α-Emitter Astatine-211 Targeted to CD38 Can Eradicate Multiple Myeloma in a Disseminated Disease Model. Blood 2019, 134, 1247–1256, doi:10.1182/blood.2019001250.
- Gouard, S.; Maurel, C.; Marionneau-Lambot, S.; Dansette, D.; Bailly, C.; Guérard, F.; Chouin, N.; Haddad, F.; Alliot, C.; Gaschet, J.; et al. Targeted-Alpha-Therapy Combining Astatine-211 and Anti-CD138 Antibody in A Preclinical Syngeneic Mouse Model of Multiple Myeloma Minimal Residual Disease. Cancers 2020, 12, 2721, doi:10.3390/cancers12092721.
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, F.M.; Fassbender, M.E.; Simon, S.; Birnbaum, E.R.; Atcher, R.W.; John, K.D.; Rixe, O.; et al. Alpha-Emitters and Targeted Alpha Therapy in Oncology: From Basic Science to Clinical Investigations. Target. Oncol. 2018, 13, 189–203, doi:10.1007/s11523-018-0550-9.
- Vaidyanathan, G.; Friedman, H.S.; Keir, S.T.; Zalutsky, M.R. Evaluation of Meta-[211At]Astatobenzylguanidine in an Athymic Mouse Human Neuroblastoma Xenograft Model. Nucl. Med. Biol. 1996, 23, 851–856, doi:10.1016/0969-8051(96)00115-1.
- Sudo, H.; Tsuji, A.B.; Sugyo, A.; Nagatsu, K.; Minegishi, K.; Ishioka, N.S.; Ito, H.; Yoshinaga, K.; Higashi, T. Preclinical Evaluation of the Acute Radiotoxicity of the α-Emitting Molecular-Targeted Therapeutic Agent 211At-MABG for the Treatment of Malignant Pheochromocytoma in Normal Mice. Transl. Oncol. 2019, 12, 879–888, doi:10.1016/j.tranon.2019.04.008.
- Palm, S.; Bäck, T.; Aneheim, E.; Hallqvist, A.; Hultborn, R.; Jacobsson, L.; Jensen, H.; Lindegren, S.; Albertsson, P. Evaluation of Therapeutic Efficacy of 211At-Labeled Farletuzumab in an Intraperitoneal Mouse Model of Disseminated Ovarian Cancer. Transl. Oncol. 2021, 14, 100873, doi:10.1016/j.tranon.2020.100873.
- Elgqvist, J.; Andersson, H.; Bäck, T.; Claesson, I.; Hultborn, R.; Jensen, H.; Lindegren, S.; Olsson, M.; Palm, S.; Warnhammar, E.; et al. Fractionated Radioimmunotherapy of Intraperitoneally Growing Ovarian Cancer in Nude Mice with 211At-MX35 F(Ab′)2: Therapeutic Efficacy and Myelotoxicity. Nucl. Med. Biol. 2006, 33, 1065–1072, doi:10.1016/j.nucmedbio.2006.07.009.
- Gustafsson, A.M.E.; Bäck, T.; Elgqvist, J.; Jacobsson, L.; Hultborn, R.; Albertsson, P.; Morgenstern, A.; Bruchertseifer, F.; Jensen, H.; Lindegren, S. Comparison of Therapeutic Efficacy and Biodistribution of 213Bi- and 211At-Labeled Monoclonal Antibody MX35 in an Ovarian Cancer Model. Nucl. Med. Biol. 2012, 39, 15–22, doi:10.1016/j.nucmedbio.2011.07.003.
- Palm, S.; Bäck, T.; Claesson, I.; Danielsson, A.; Elgqvist, J.; Frost, S.; Hultborn, R.; Jensen, H.; Lindegren, S.; Jacobsson, L. Therapeutic Efficacy of Astatine-211–Labeled Trastuzumab on Radioresistant SKOV-3 Tumours in Nude Mice. Int. J. Radiat. Oncol. 2007, 69, 572–579, doi:10.1016/j.ijrobp.2007.06.023.
- Li, H.K.; Morokoshi, Y.; Nagatsu, K.; Kamada, T.; Hasegawa, S. Locoregional Therapy with α-Emitting Trastuzumab against Peritoneal Metastasis of Human Epidermal Growth Factor Receptor 2-Positive Gastric Cancer in Mice. Cancer Sci. 2017, 108, 1648–1656, doi:10.1111/cas.13282.
- Dekempeneer, Y.; Bäck, T.; Aneheim, E.; Jensen, H.; Puttemans, J.; Xavier, C.; Keyaerts, M.; Palm, S.; Albertsson, P.; Lahoutte, T.; et al. Labeling of Anti-HER2 Nanobodies with Astatine-211: Optimization and the Effect of Different Coupling Reagents on Their in Vivo Behavior. Mol. Pharm. 2019, 16, 3524–3533, doi:10.1021/acs.molpharmaceut.9b00354.
- Feng, Y.; Meshaw, R.; Zhao, X.-G.; Jannetti, S.; Vaidyanathan, G.; Zalutsky, M.R. Effective Treatment of Human Breast Carcinoma Xenografts with Single-Dose 211At-Labeled Anti-HER2 Single-Domain Antibody Fragment. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2023, 64, 124–130, doi:10.2967/jnumed.122.264071.
- Robinson, M.K.; Shaller, C.; Garmestani, K.; Plascjak, P.S.; Hodge, K.M.; Yuan, Q.-A.; Marks, J.D.; Waldmann, T.A.; Brechbiel, M.W.; Adams, G.P. Effective Treatment of Established Human Breast Tumour Xenografts in Immunodeficient Mice with a Single Dose of the Alpha-Emitting Radioisotope Astatine-211 Conjugated to Anti-HER2/Neu Diabodies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 875–882, doi:10.1158/1078-0432.CCR-07-1250.
- Kiess, A.P.; Minn, I.; Vaidyanathan, G.; Hobbs, R.F.; Josefsson, A.; Shen, C.; Brummet, M.; Chen, Y.; Choi, J.; Koumarianou, E.; et al. (2 S )-2-(3-(1-Carboxy-5-(4-211 At-Astatobenzamido)Pentyl)Ureido)-Pentanedioic Acid for PSMA-Targeted α-Particle Radiopharmaceutical Therapy. J. Nucl. Med. 2016, 57, 1569–1575, doi:10.2967/jnumed.116.174300.
- Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Liu, Y.; Ooe, K.; Teramoto, T.; Toyoshima, A.; Shimosegawa, E.; Nakano, T.; Kanai, Y.; et al. Targeted Alpha Therapy Using Astatine (211At)-Labeled Phenylalanine: A Preclinical Study in Glioma Bearing Mice. Oncotarget 2020, 11, 1388–1398, doi:10.18632/oncotarget.27552.
- Bäck, T.A.; Jennbacken, K.; Hagberg Thulin, M.; Lindegren, S.; Jensen, H.; Olafsen, T.; Yazaki, P.J.; Palm, S.; Albertsson, P.; Damber, J.-E.; et al. Targeted Alpha Therapy with Astatine-211-Labeled Anti-PSCA A11 Minibody Shows Antitumour Efficacy in Prostate Cancer Xenografts and Bone Microtumours. EJNMMI Res. 2020, 10, 10, doi:10.1186/s13550-020-0600-z.
- Aoki, M.; Zhao, S.; Takahashi, K.; Washiyama, K.; Ukon, N.; Tan, C.; Shimoyama, S.; Nishijima, K.-I.; Ogawa, K. Preliminary Evaluation of Astatine-211-Labeled Bombesin Derivatives for Targeted Alpha Therapy. Chem. Pharm. Bull. (Tokyo) 2020, 68, 538–545, doi:10.1248/cpb.c20-00077.
- Carlin, S.; Mairs, R.J.; Welsh, P.; Zalutsky, M.R. Sodium-Iodide Symporter (NIS)-Mediated Accumulation of [(211)At]Astatide in NIS-Transfected Human Cancer Cells. Nucl. Med. Biol. 2002, 29, 729–739, doi:10.1016/s0969-8051(02)00332-3.
- Petrich, T.; Helmeke, H.-J.; Meyer, G.J.; Knapp, W.H.; Pötter, E. Establishment of Radioactive Astatine and Iodine Uptake in Cancer Cell Lines Expressing the Human Sodium/Iodide Symporter. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 842–854, doi:10.1007/s00259-002-0784-7.
- Petrich, T.; Quintanilla-Fend, L.; Knapp, W.; Pötter, E. Effective Cancer Therapy by the α-Particle Emitter [At-211]-Astatine in a Mouse Model of Genetically Modified NIS-Expressing Tumours. Exp. Clin. Endocrinol. Diabetes 2005, 113, s-2005-862947, doi:10.1055/s-2005-862947.
- Sporer, E.; Poulie, C.B.M.; Lindegren, S.; Aneheim, E.; Jensen, H.; Bäck, T.; Kempen, P.J.; Kjaer, A.; Herth, M.M.; Jensen, A.I. Surface Adsorption of the Alpha-Emitter Astatine-211 to Gold Nanoparticles Is Stable In Vivo and Potentially Useful in Radionuclide Therapy. J. Nanotheranostics 2021, 2, 196–207, doi:10.3390/jnt2040012.
- Aso, A.; Nabetani, H.; Matsuura, Y.; Kadonaga, Y.; Shirakami, Y.; Watabe, T.; Yoshiya, T.; Mochizuki, M.; Ooe, K.; Kawakami, A.; et al. Evaluation of Astatine-211-Labeled Fibroblast Activation Protein Inhibitor (FAPI): Comparison of Different Linkers with Polyethylene Glycol and Piperazine. Int. J. Mol. Sci. 2023, 24, 8701, doi:10.3390/ijms24108701.
- Li, M.; Baumhover, N.J.; Liu, D.; Cagle, B.S.; Boschetti, F.; Paulin, G.; Lee, D.; Dai, Z.; Obot, E.R.; Marks, B.M.; et al. Preclinical Evaluation of a Lead Specific Chelator (PSC) Conjugated to Radiopeptides for 203Pb and 212Pb-Based Theranostics. Pharmaceutics 2023, 15, 414, doi:10.3390/pharmaceutics15020414.
- Horak, E.; Hartmann, F.; Garmestani, K.; Wu, C.; Brechbiel, M.; Gansow, O.A.; Landolfi, N.F.; Waldmann, T.A. Radioimmunotherapy Targeting of HER2/Neu Oncoprotein on Ovarian Tumour Using Lead-212-DOTA-AE1. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1997, 38, 1944–1950.
- Banerjee, S.R.; Minn, I.; Kumar, V.; Josefsson, A.; Lisok, A.; Brummet, M.; Chen, J.; Kiess, A.P.; Baidoo, K.; Brayton, C.; et al. Preclinical Evaluation of203/212 Pb-Labeled Low-Molecular-Weight Compounds for Targeted Radiopharmaceutical Therapy of Prostate Cancer. J. Nucl. Med. 2020, 61, 80–88, doi:10.2967/jnumed.119.229393.
- Stenberg, V.Y.; Juzeniene, A.; Chen, Q.; Yang, X.; Bruland, Ø.S.; Larsen, R.H. Preparation of the Alpha-emitting Prostate-specific Membrane Antigen Targeted Radioligand [212 Pb]Pb-NG001 for Prostate Cancer. J. Label. Compd. Radiopharm. 2020, 63, 129–143, doi:10.1002/jlcr.3825.
- Rold, T.L.; Devanny, E.A.; Okoye, N.C.; Quinn, T.P.; Hoffman, T.J. Abstract 5347: Preliminary Evaluation of BB2r TAT Using 212Pb-RM2 in a PC3 Human Prostate Cancer Xenograft Model. Cancer Res. 2020, 80, 5347–5347, doi:10.1158/1538-7445.AM2020-5347.
- Beyer, G.-J.; Miederer, M.; Vranješ-Đurić, S.; Čomor, J.J.; Künzi, G.; Hartley, O.; Senekowitsch-Schmidtke, R.; Soloviev, D.; Buchegger, F.; and the ISOLDE Collaboration Targeted Alpha Therapy in Vivo: Direct Evidence for Single Cancer Cell Kill Using 149Tb-Rituximab. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 547–554, doi:10.1007/s00259-003-1413-9.
- Müller, C.; Reber, J.; Haller, S.; Dorrer, H.; Köster, U.; Johnston, K.; Zhernosekov, K.; Türler, A.; Schibli, R. Folate Receptor Targeted Alpha-Therapy Using Terbium-149. Pharmaceuticals 2014, 7, 353–365, doi:10.3390/ph7030353.
- Henriksen, G.; Fisher, D.R.; Roeske, J.C.; Bruland, Ø.S.; Larsen, R.H. Targeting of Osseous Sites with Alpha-Emitting 223Ra: Comparison with the Beta-Emitter 89Sr in Mice. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2003, 44, 252–259.
- Larsen, R.H.; Saxtorph, H.; Skydsgaard, M.; Borrebaek, J.; Jonasdottir, T.J.; Bruland, O.S.; Klastrup, S.; Harling, R.; Ramdahl, T. Radiotoxicity of the Alpha-Emitting Bone-Seeker 223Ra Injected Intravenously into Mice: Histology, Clinical Chemistry and Hematology. Vivo Athens Greece 2006, 20, 325–331.
- Suominen, M.I.; Fagerlund, K.M.; Rissanen, J.P.; Konkol, Y.M.; Morko, J.P.; Peng, Z.; Alhoniemi, E.J.; Laine, S.K.; Corey, E.; Mumberg, D.; et al. Radium-223 Inhibits Osseous Prostate Cancer Growth by Dual Targeting of Cancer Cells and Bone Microenvironment in Mouse Models. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 4335–4346, doi:10.1158/1078-0432.CCR-16-2955.
- Dahle, J.; Jonasdottir, T.J.; Heyerdahl, H.; Nesland, J.M.; Borrebaek, J.; Hjelmerud, A.K.; Larsen, R.H. Assessment of Long-Term Radiotoxicity after Treatment with the Low-Dose-Rate Alpha-Particle-Emitting Radioimmunoconjugate (227)Th-Rituximab. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 93–102, doi:10.1007/s00259-009-1197-7.
- Heyerdahl, H.; Abbas, N.; Brevik, E.M.; Mollatt, C.; Dahle, J. Fractionated Therapy of HER2-Expressing Breast and Ovarian Cancer Xenografts in Mice with Targeted Alpha Emitting 227Th-DOTA-p-Benzyl-Trastuzumab. PloS One 2012, 7, e42345, doi:10.1371/journal.pone.0042345.
- Hagemann, U.B.; Wickstroem, K.; Wang, E.; Shea, A.O.; Sponheim, K.; Karlsson, J.; Bjerke, R.M.; Ryan, O.B.; Cuthbertson, A.S. In Vitro and In Vivo Efficacy of a Novel CD33-Targeted Thorium-227 Conjugate for the Treatment of Acute Myeloid Leukemia. Mol. Cancer Ther. 2016, 15, 2422–2431, doi:10.1158/1535-7163.MCT-16-0251.
- Wickstroem, K.; Hagemann, U.B.; Kristian, A.; Ellingsen, C.; Sommer, A.; Ellinger-Ziegelbauer, H.; Wirnitzer, U.; Hagelin, E.-M.; Larsen, A.; Smeets, R.; et al. Preclinical Combination Studies of an FGFR2 Targeted Thorium-227 Conjugate and the ATR Inhibitor BAY 1895344. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 410–422, doi:10.1016/j.ijrobp.2019.06.2508.
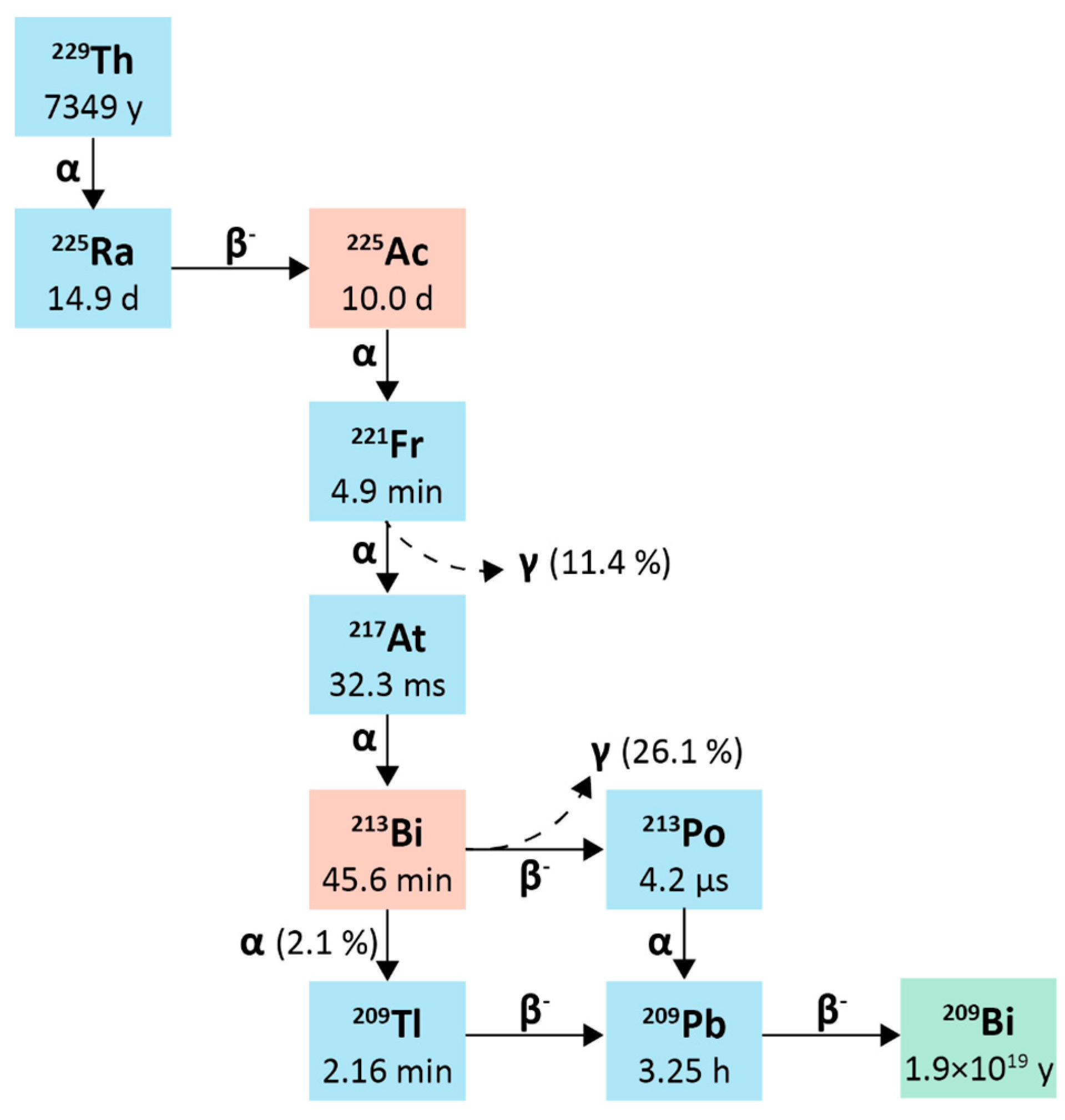
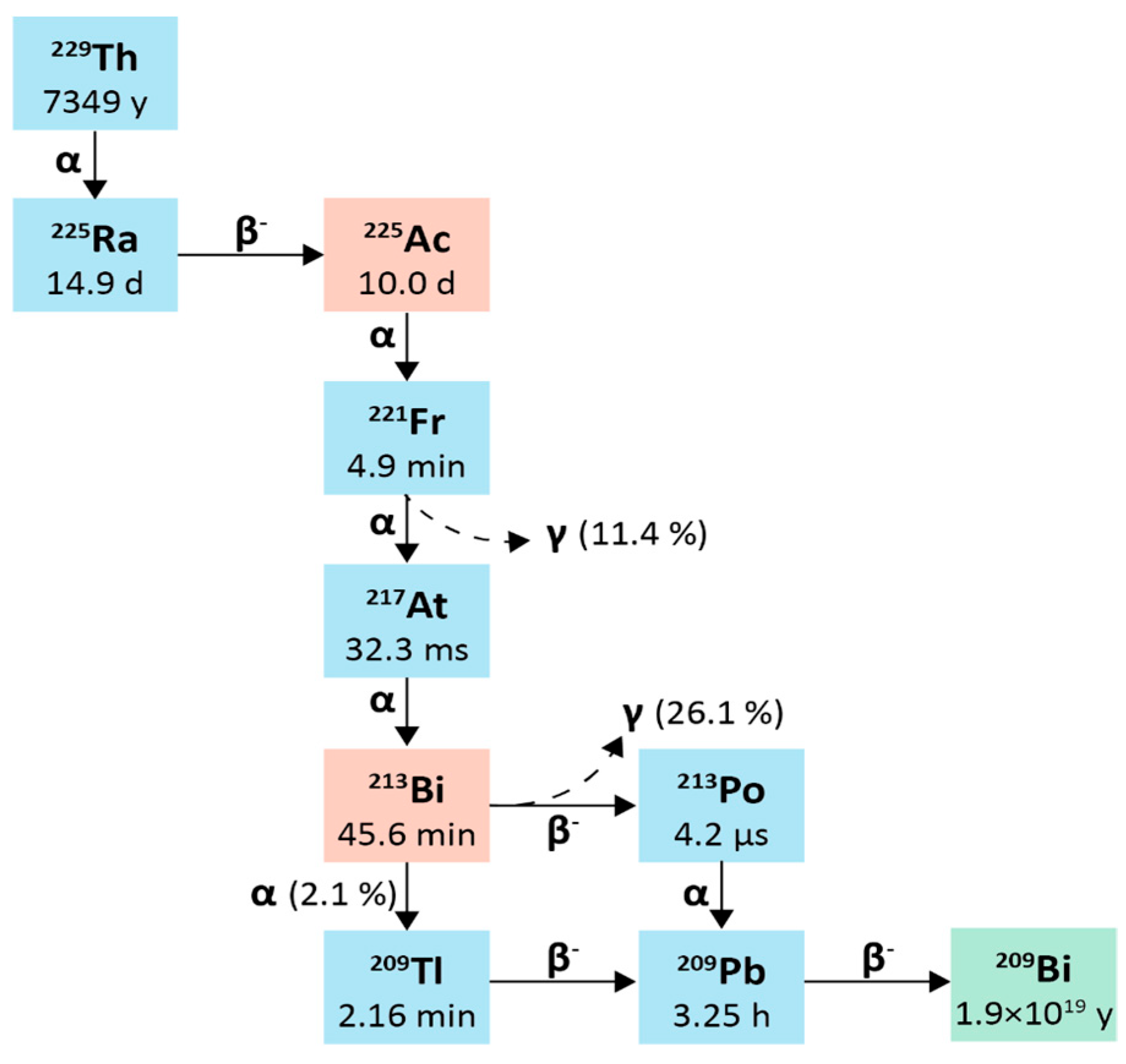
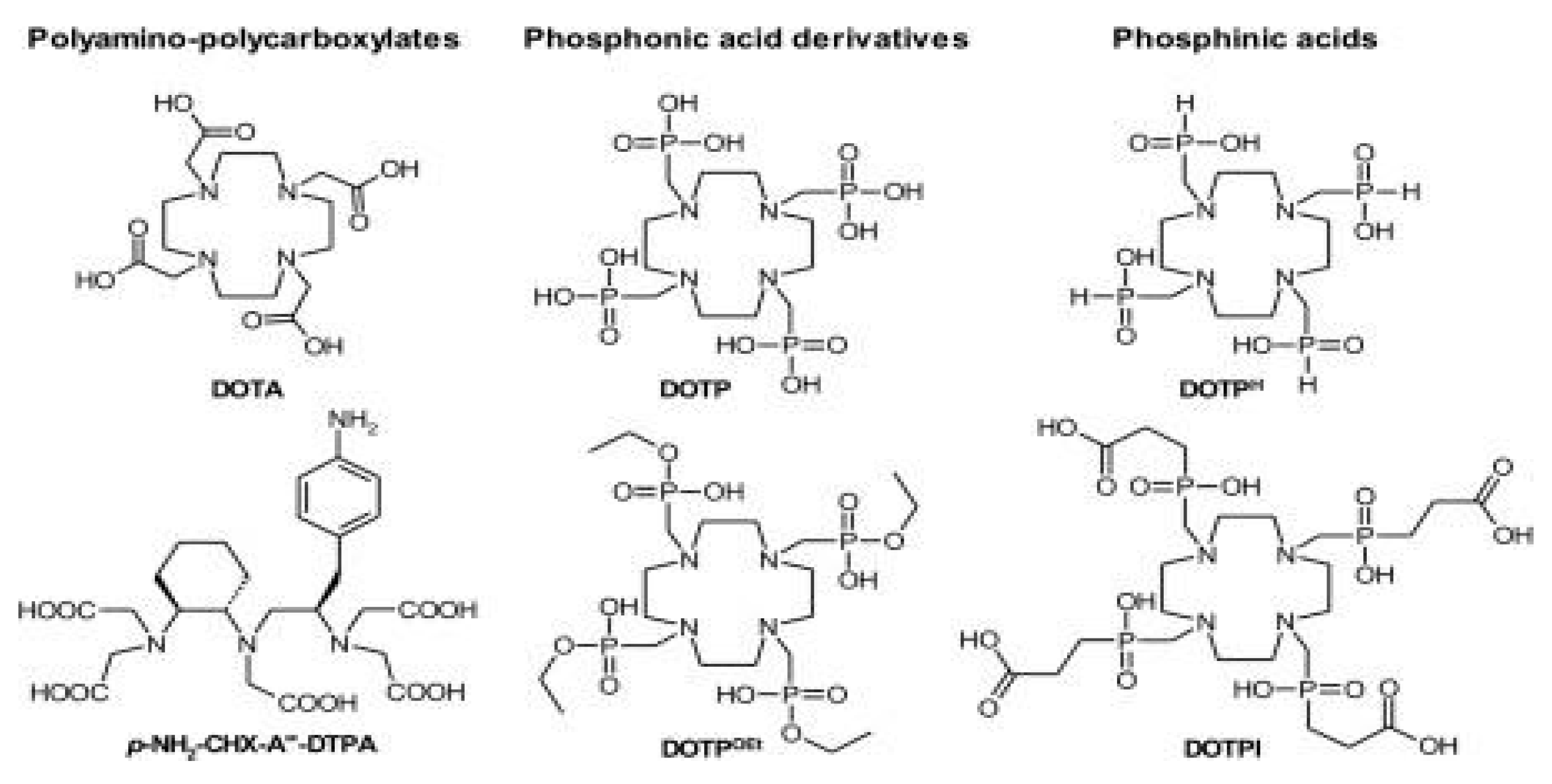
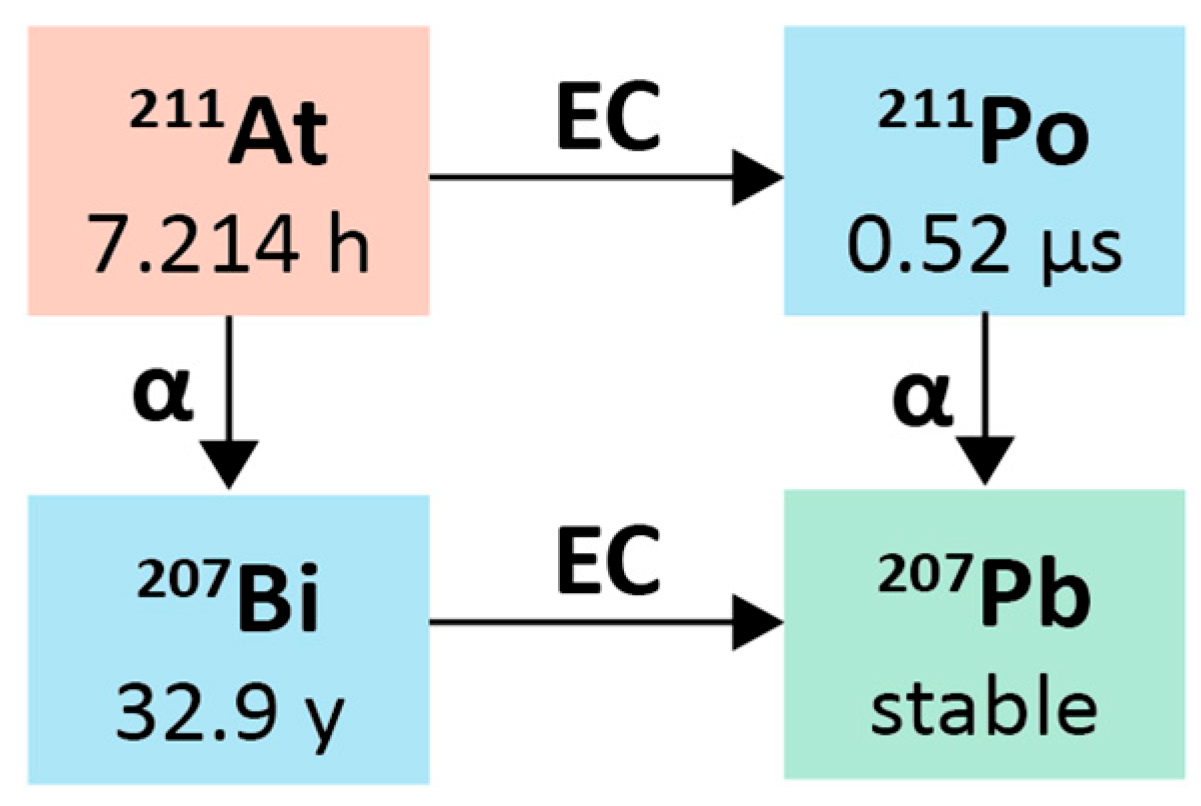
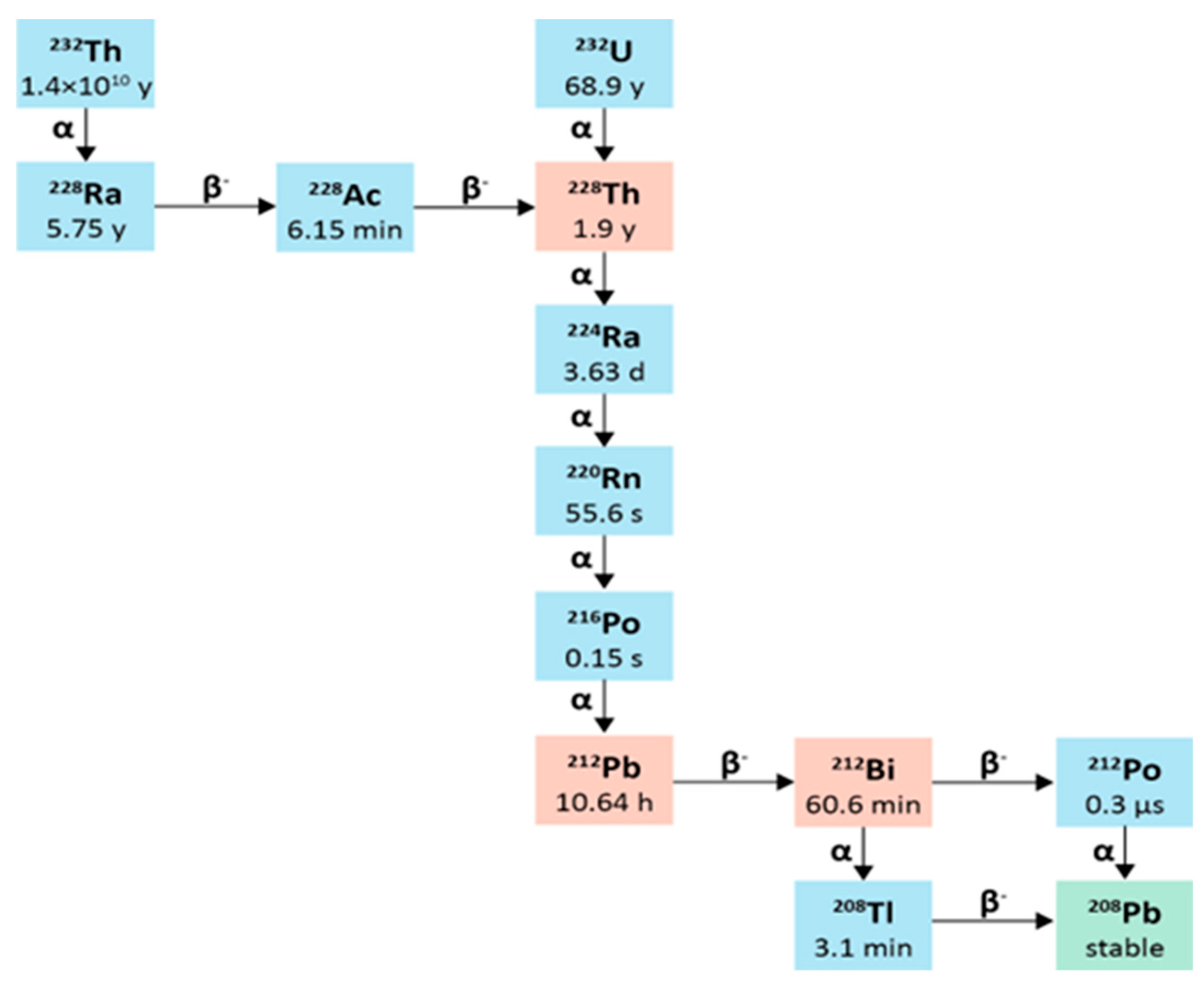
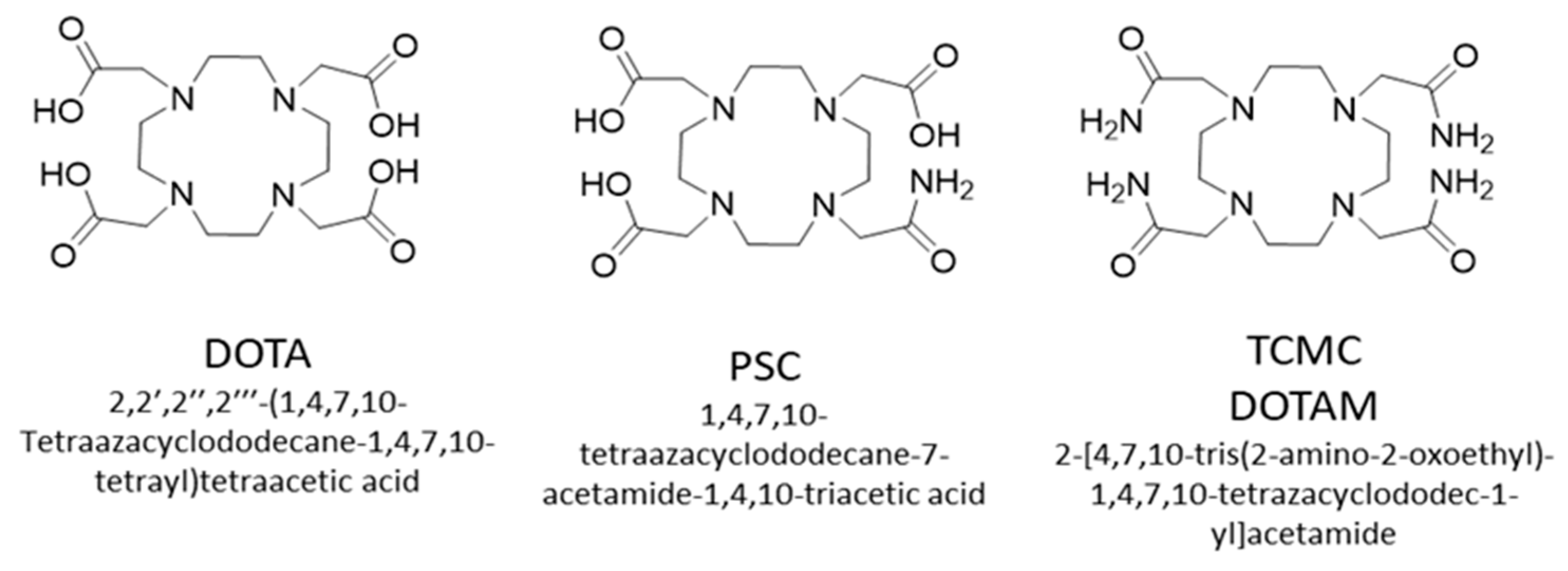
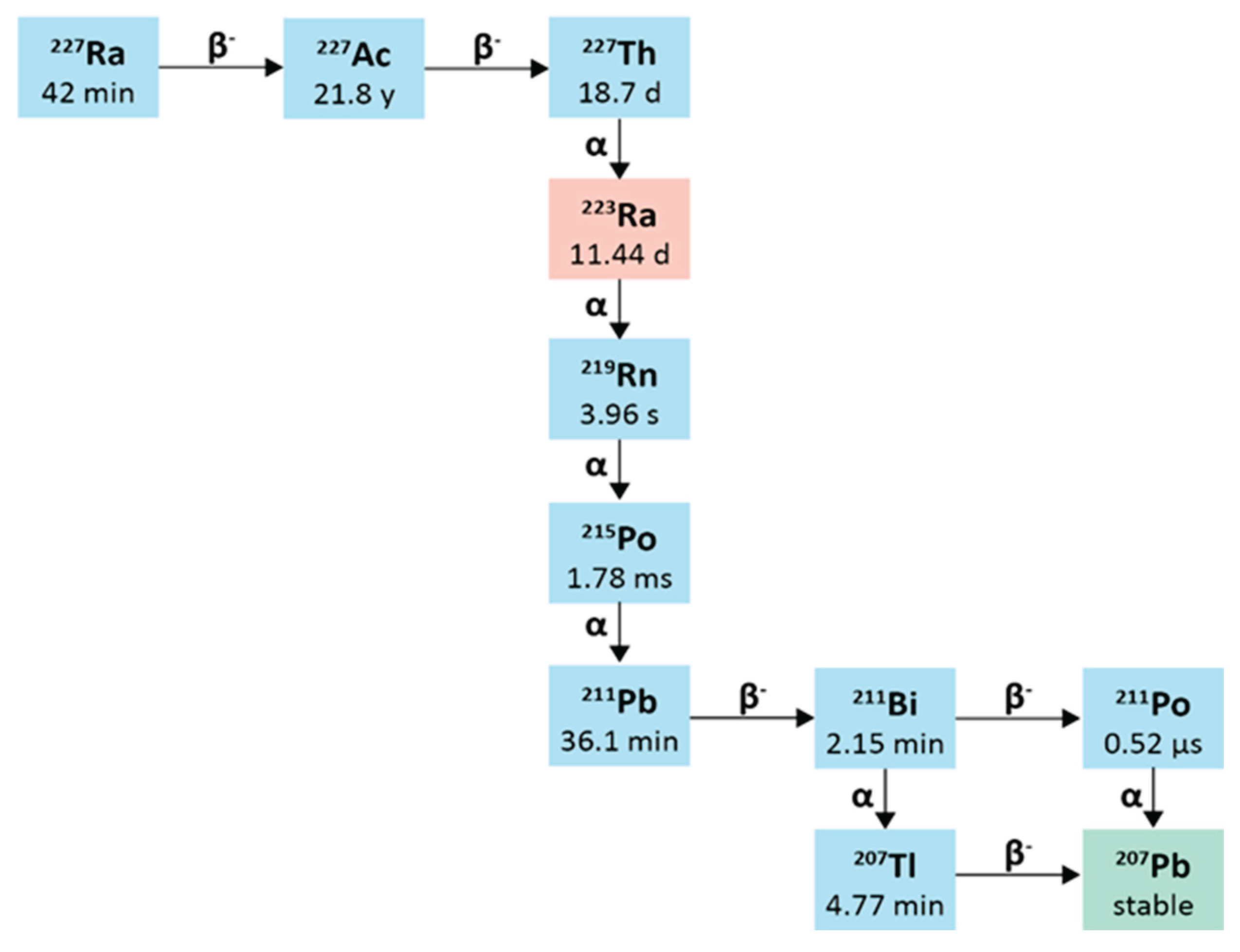
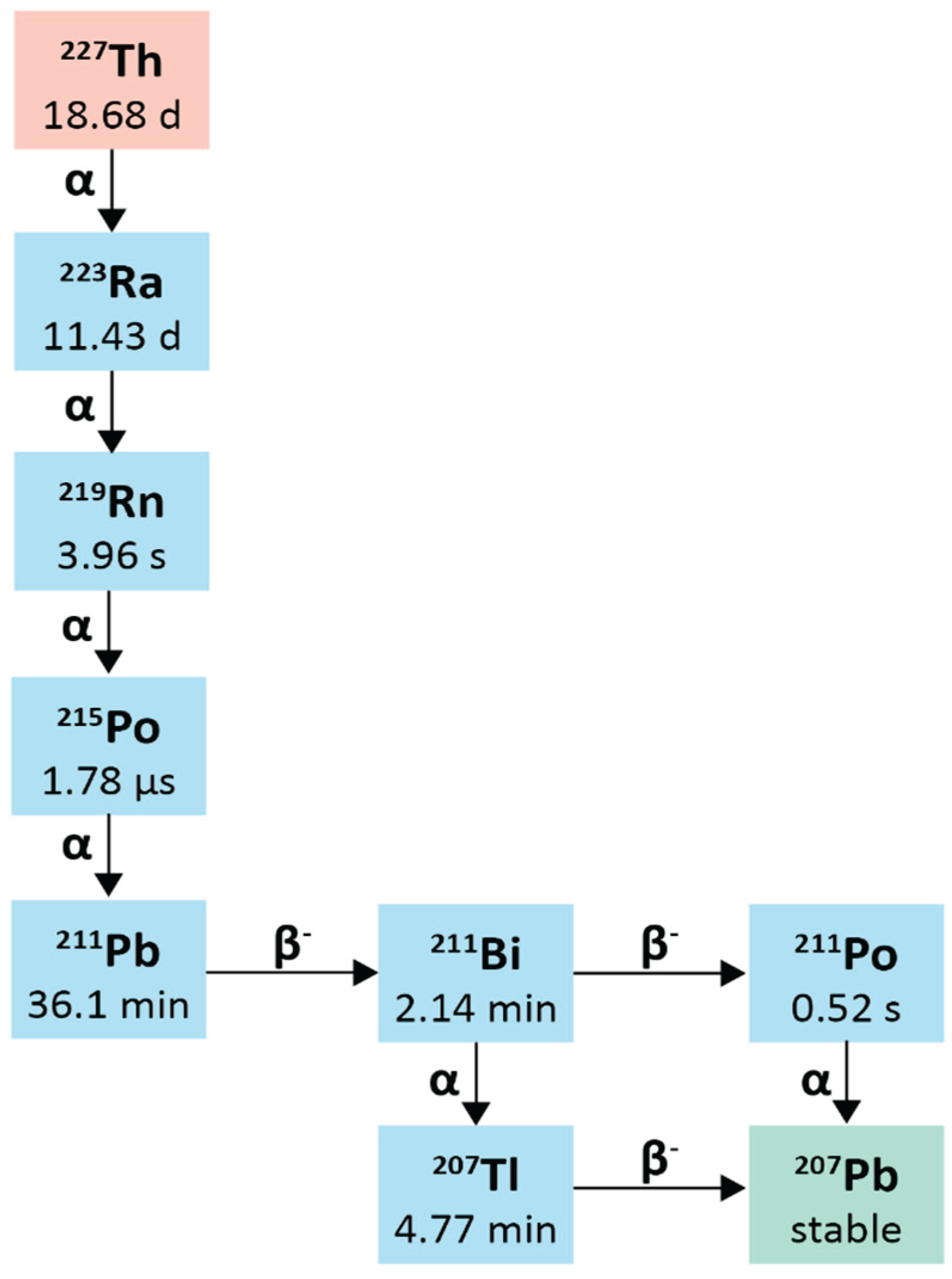
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





