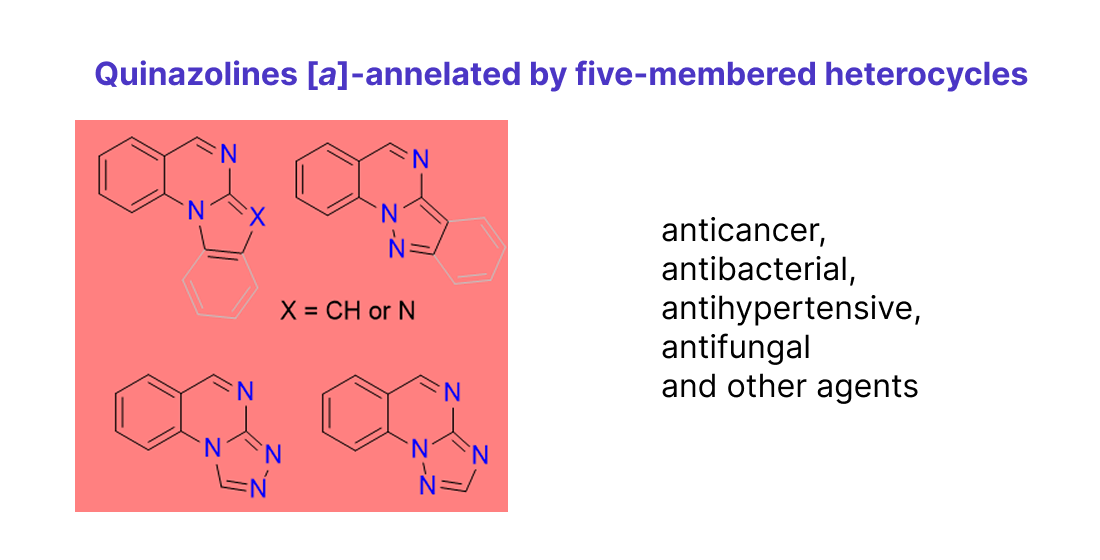
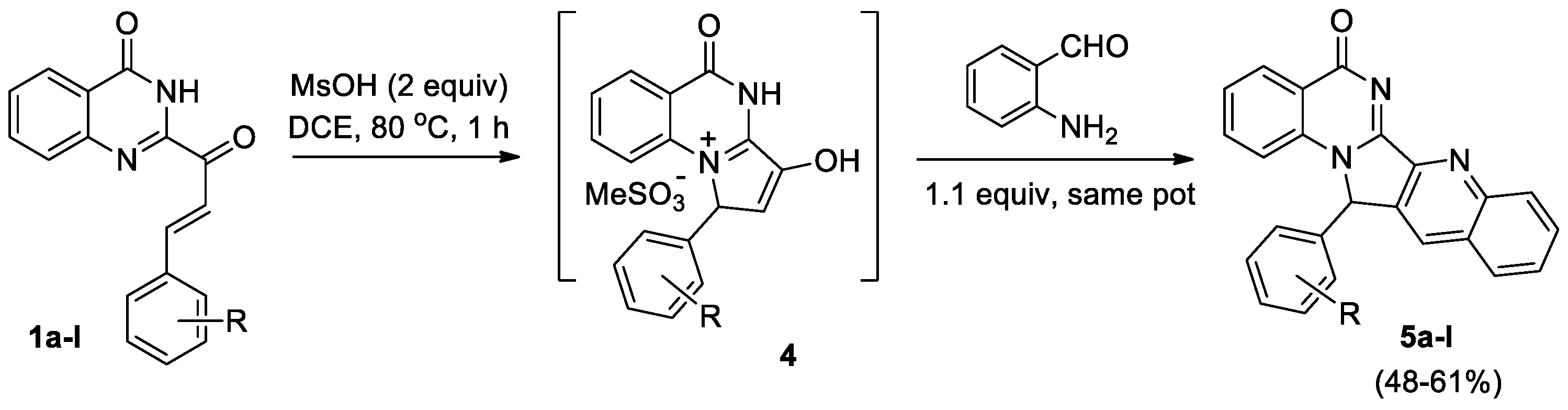
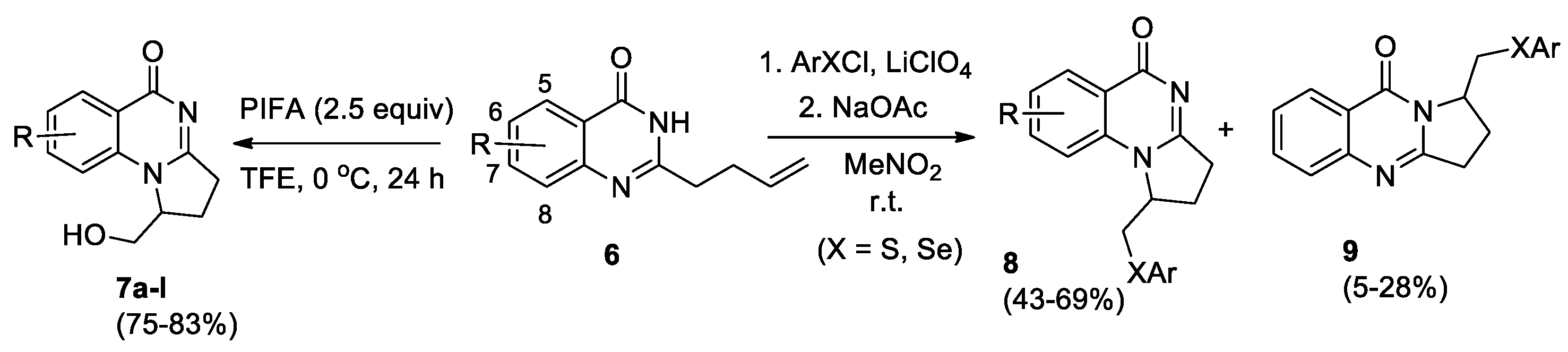
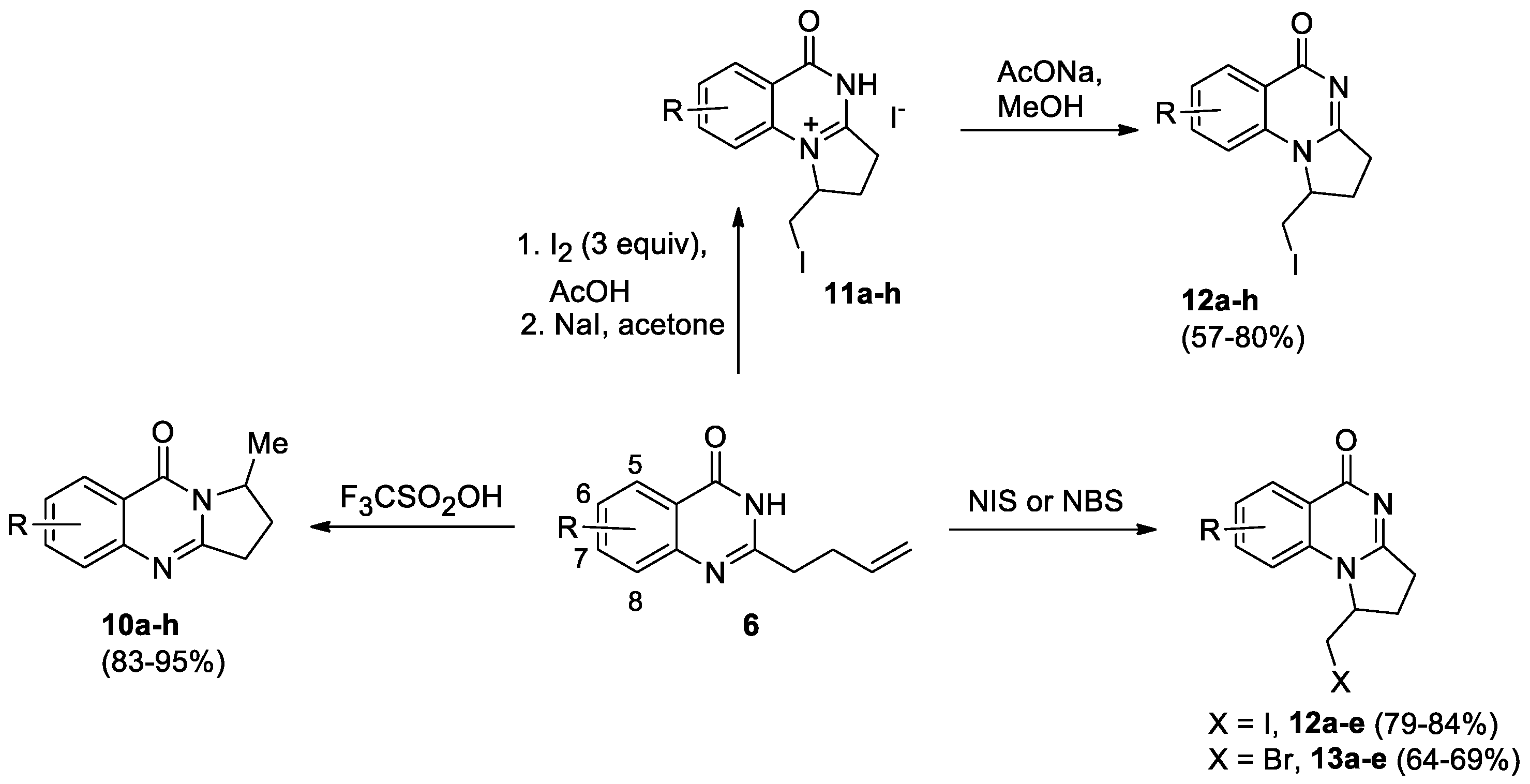
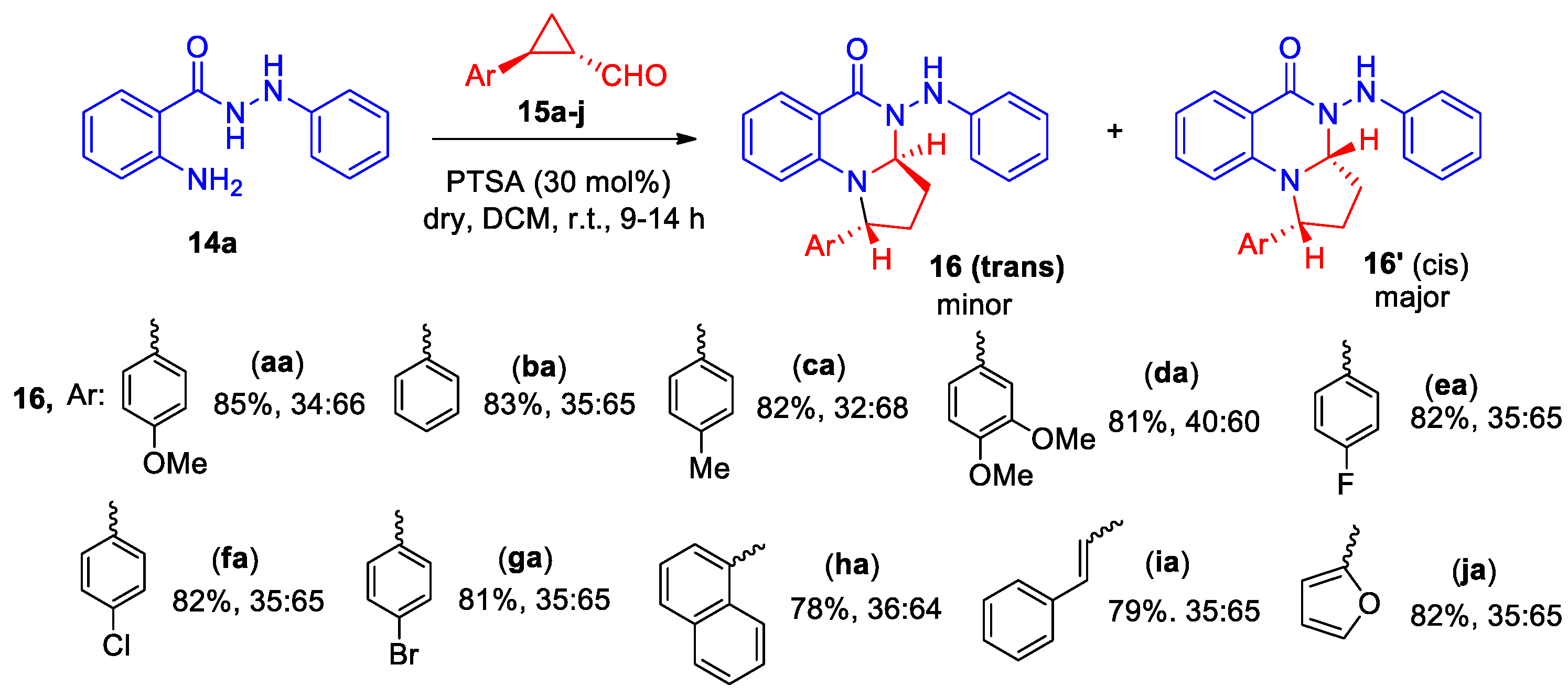
3. Indolo[1,2-a]quinazolinones and isoindolo[2,1-a]quinazolinediones
Indoles are widely found in pharmaceuticals and nature products, the indole-containing quinazoline derivatives were reported as protein kinase CK2 inhibitors and poly(ADP-ribose)polymerase-1 (PARP-1) inhibitors [
24]. Isoindoloquinazolines have shown Tumor Necrosis Factor-alpha (TNF-
α) inhibitory [
25].
Synthetic approaches to bioactive indolo[1,2-
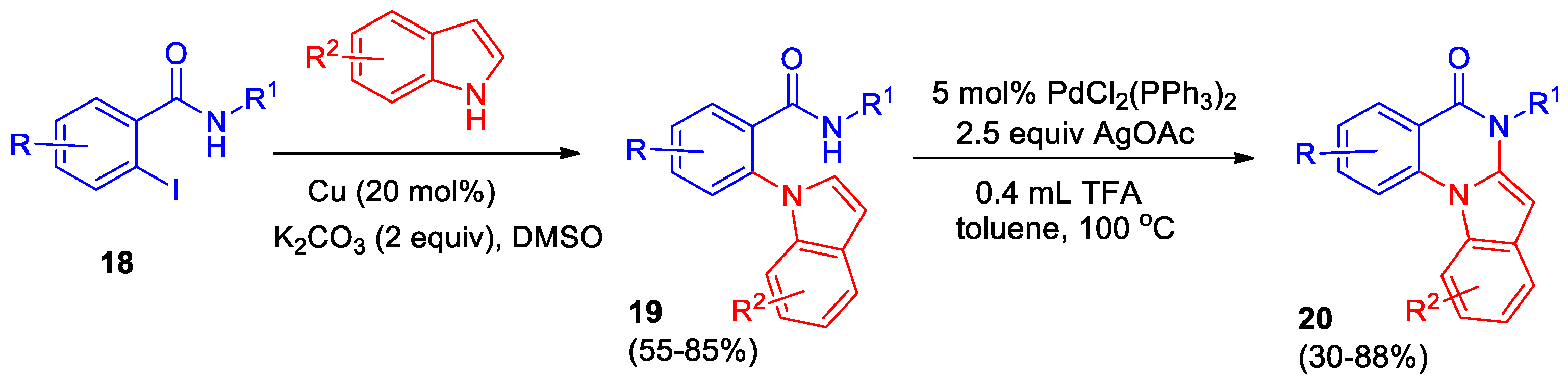
a]quinazolinones are limited. Kotipalli
et al developed an easy and convenient two-step method for the synthesis of indolo[1,2-
a]quinazolinone derivatives, starting from 2-iodobenzamides
18 and indole derivatives [
26]. The first step involves the
N-arylation of indole by 2-iodobenzamide derivatives
via Ullman coupling. Optimal conditions for this stage were found, which allowed obtaining 2-(1
H-indol-1-yl)-
N-substituted benzamide derivatives
19 in up to 85% yield. The second step involves intramolecular C–H amidation using a palladium catalyst and AgOAc as oxidant and proceeds in toluene at 100 °C in the presence of TFA (
Scheme 8). The series of indolo[1,2-
a]quinazolinone derivatives
20 with different substituents R, R
1, R
2 was obtained. It was shown that long alkyl chain in R
1 or the
p-methoxy group in the aryl fragment leads to a decrease in yield to 30−34%, in the case R = NO
2 the cyclization does not occur.
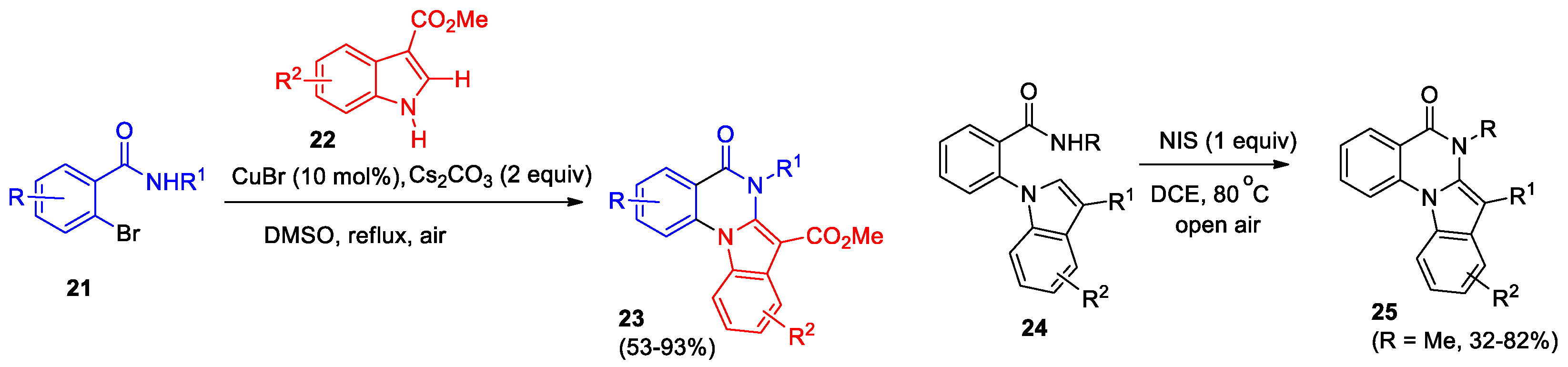
Abe
et al reported a self-relay copper (I)-catalyzed Ullmann
N-arylation/2-amidation cascade to form functionalized indolo[1,2-
a]quinazolinones
23 in one-pot from easily available 2-bromobenzamides
21 with indoles
22 in high yields [
27] (
Scheme 9). It was found that the optimal conditions for the cascade are the following: the presence of CO
2Me group at the position 3 of indole, the use of CuBr as a catalyst, Cs
2CO
3 as a base and DMSO as a solvent, boiling for 16 hours. It was also shown that in the case R = NO
2 or R
1 =
t-Bu indolo[1,2-
a]quinazolinones could not be obtained even under boiling for 72 hours. The authors discuss the proposed process pathways and note that methyl carboxylate could act as activating group in this Ullmann
N-arylation/2-amidation cascade.
Badigenchala
et al developed a transition-metal- and base-free approach to indolo[1,2-
a]quinazolinones [
28]. It was demonstrated that
N-iodosuccinimide (NIS) can be used for intramolecular cross-coupling of C(sp
2)−H and N−H bonds, leading to the formation of indolo[1,2-
a]quinazolinones
25 (
Scheme 9). Optimal cyclization conditions were found for 2-(1
H-indol-1-yl)-N-methylbenzamide, the effect of substituents R, R
1, R
2 on reaction ability was studied. It was shown that the presence of strong electron withdrawing (R
2 = 5-NO
2, 7-azaindole derived) or bulky (R =
t-Bu) group suppressed the reaction completely. Gram scale synthesis has been performed to examine the synthetic utility of the protocol.
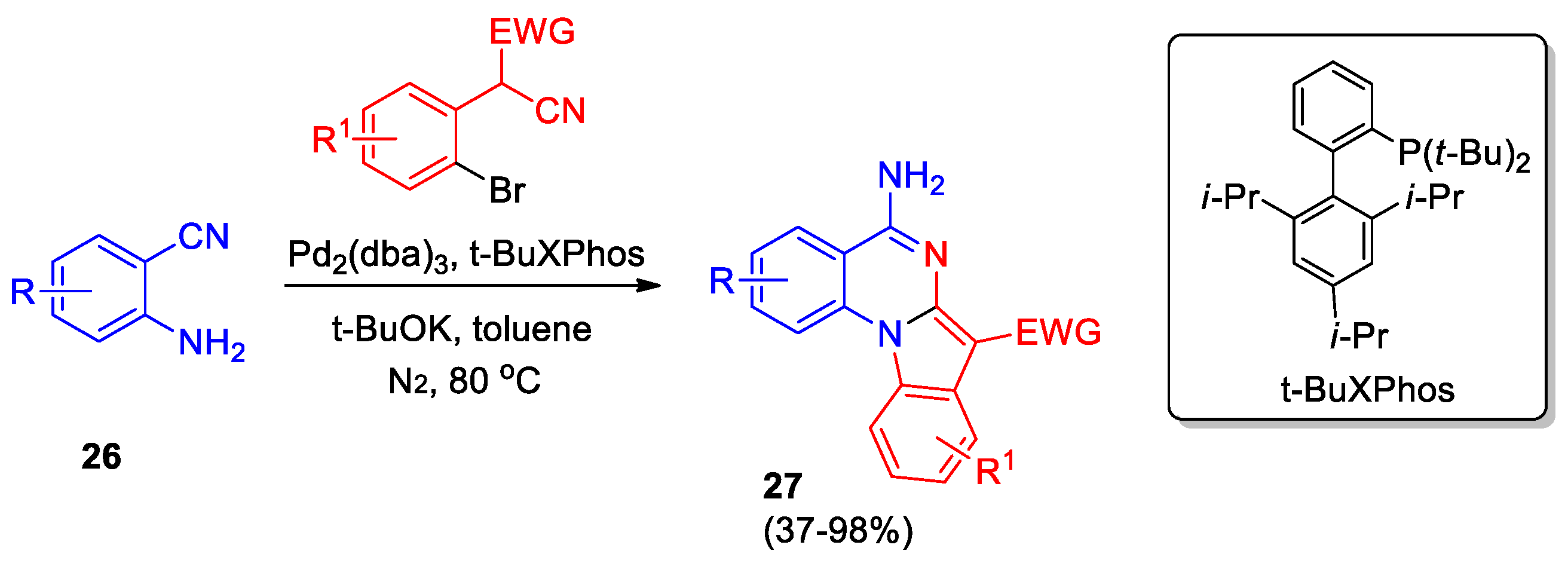
Jiang
et al described Pd-catalyzed domino synthesis of 5-amine-indolo[1,2-
a]quinazolines
27 from readily available 2-aminobenzonitriles
26 and 2-(2-bromophenyl)acetonitriles (
Scheme 10) [
29]. The approach involves a Buchwald-Hartwig type coupling and a base-promoted intramolecular nucleophilic reaction. It was found that in the case of EWG = COOEt, the yield of products
27 is reduced to 55−62%, and in the case of R = CF
3, to 37%.
One approach to the synthesis of isoindoloquinazolinediones is based on a multi-component reaction (MCR) of isatoic anhydride
28, amines and 2-formylbenzoic acid under different conditions (
Scheme 11). The reaction was carried out by heating the reagents in acetic acid at 110 °C, aliphatic and aromatic amines were used, the highest yields of 6,6a-dihydroisoindolo[2,1-
a]quinazoline-5,11-diones
29 were obtained for the more nucleophilic aliphatic amines [
30]. Reddy
et al [
31] described the faster and greener synthesis of the analogues of compounds
29 via a β-с
yclodextrin mediated MCR of the same reagents in water under microwave irradiation.
Esmaeili-Marandi
et al [
32] described novel 1,2,3-triazole-containing isoindolo[2,1-
a]-quinazolines
via a convenient three-step reaction starting from isatoic anhydride
28. Interaction of
28 with 1-aminoprop-2-yne in water at room temperature gives 2-amino-N-(prop-2-yn-1-yl)benzamide, which further undergoes cyclization into isoindolo[2,1-
a]-quinazoline
30 in the presence of TsOH (20 mol%) in EtOH under reflux (
Scheme 11). Product
30 was readily converted to the target compound
31 via click reaction with organic azides, obtained from the corresponding benzyl halogenides and sodium azide.
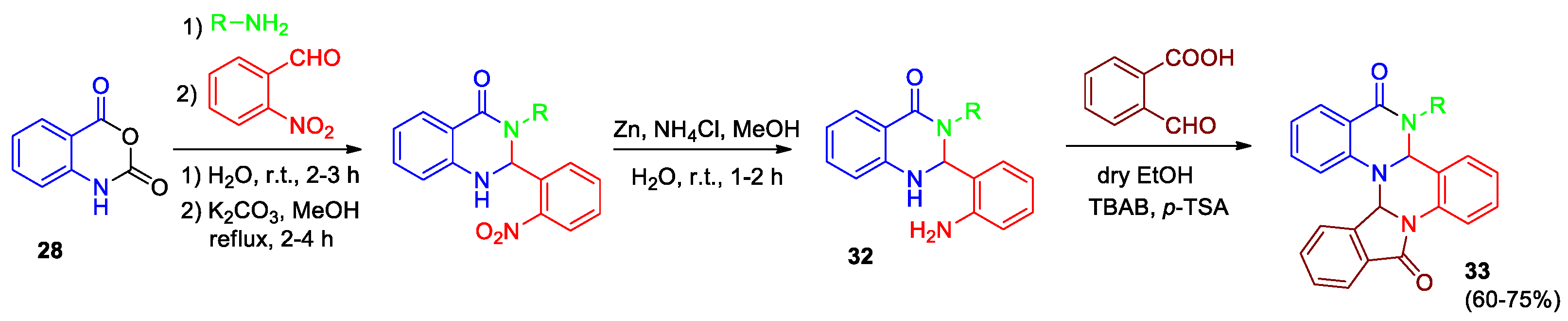
Mahdavi
et al developed a four-step effective and simple
synthetic approach for the synthesis of novel isoinodolo[2,1-a]quinazolino[1,2-
c]quinazolinones
33 (
Scheme 12) [
33], the method can be extended for the preperation of a library of potentially bioactive compounds. Reaction of isatoic anhydride
28 and amines led to various 2-aminobenzamide derivatives, their interaction with
o-nitrobenzaldehyde and subsequent reduction of nitro group resulted in the formation of 2-(2-aminophenyl)-3-R-2,3-dihydroquinazolin-4
(1
H)-ones
32. Optimal conditions for the reaction of compounds
32 with 2-formylbenzoic acid were found: boiling in dry ethanol in the presence of
p-toluenesulfonic acid (
p-TSA) and tetrabutylammonium bromide (TBAB) at a ratio of
p-TSA/TBAB = 0.5 [
33].
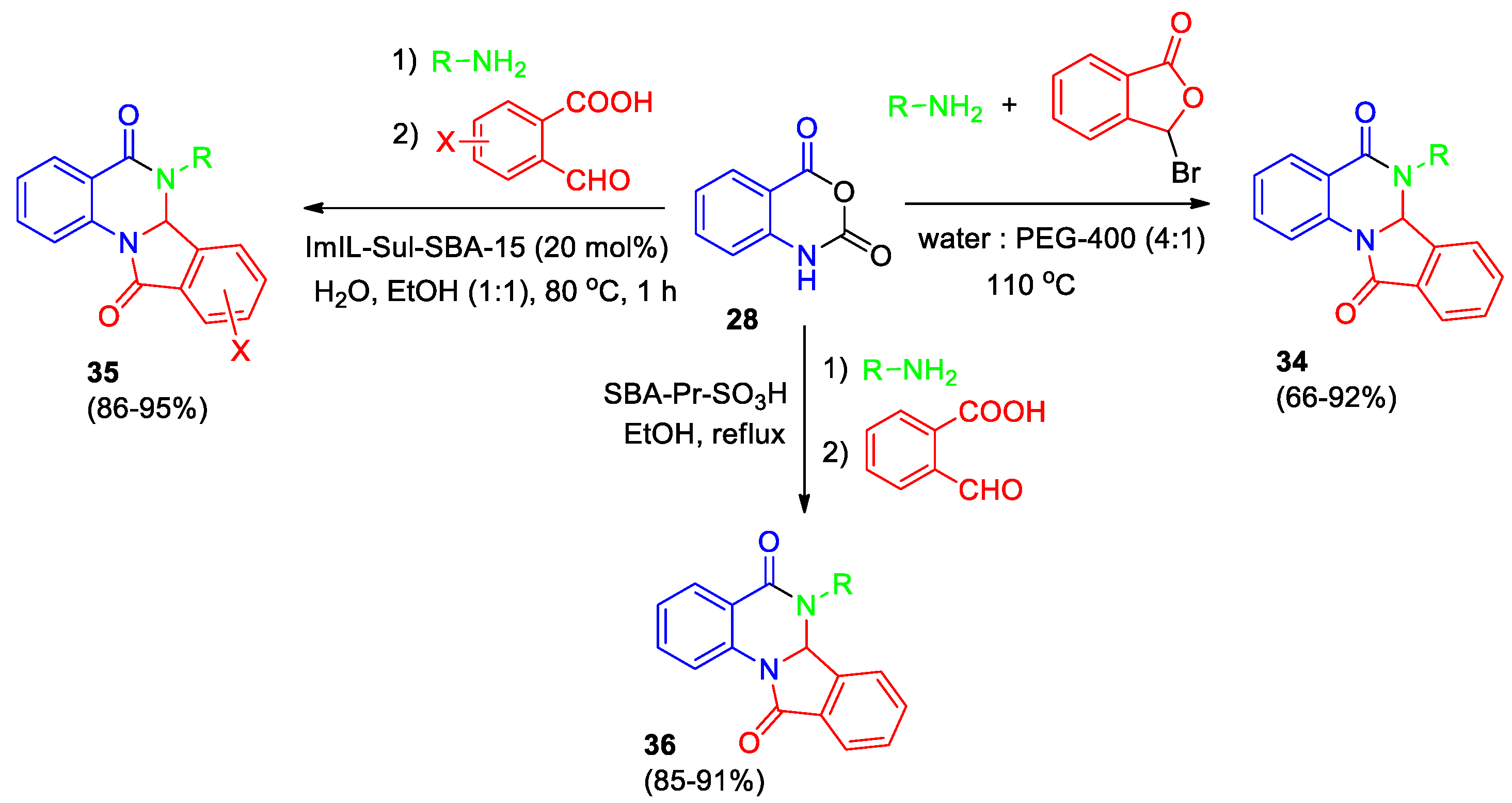
Recently, performing chemical experiments in water with help from plant-based materials has become more popular because it's good for the environment and sustainable. Madhubabu
et al [
34] developed a short and efficient metal free, catalyst-free greener approach for the synthesis of dihydroisoindolo[2,1-
a]quinazoline-5,11-dione derivatives
34 in water (
Scheme 13). Isatoic anhydride, an amine, and 3-bromoisobenzofuran-1(3
H)-one served as starting materials. A mixture of water and polyethylene glycol 400 (PEG-400) in a ratio of 4:1 provided the most efficient solvent system. After completing the reaction, the product underwent filtration, and the recovered solvent could be effectively reused multiple times without noticeable loss of activity compared to fresh solvent. This reliable methodology tolerates both aliphatic and aromatic amines and exhibits exceptional scalability.
Abbasian
et al [
35] reported the synthesis of dihydroisoindolo[2,1-
a]quinazoline-5,11-diones
35 using the catalyst on silica-based ordered mesoporous material
(SBA-15) functionalized by imidazolium ionic liquid sulfonic acid (ImIL-Sul-SBA-15) (
Scheme 13). The reaction between isatoic anhydride
28, amines and substituted 2-formylbenzoic acids was conducted in the ethanol-water mixture (1:1) in the presence of ImIL-Sul-SBA-15 (20 mol%) at 50
oС for 1 hour. Once the reaction finished, a mixture containing desired product
35 along with the catalyst (ImIL-Sul-SBA-15) was filtered out. Then this solid was cleaned with ethanol to remove the product, leaving behind just the catalyst. Testing showed that the catalyst could be reused effectively six times before losing any noticeable activity. Benefits include easy recovery of the catalyst, quick reaction time, and high yields of the final product.
Rayatzadeh
et al described the synthesis of a series of 6,6
a-dihydroisoindolo[2,1-
a]quinazoline-5,11-dione derivatives
36 using sulfonic acid functionalized nanoporous silica (SBA-Pr-SO
3H) as a reusable catalyst (
Scheme 13) [
36]. Derivatives
36 were obtained via a two-step process, once the reaction was completed, the catalyst was separated, and the final products were isolated by recrystallization from ethanol. Key advantages are high yields and easy purification process.
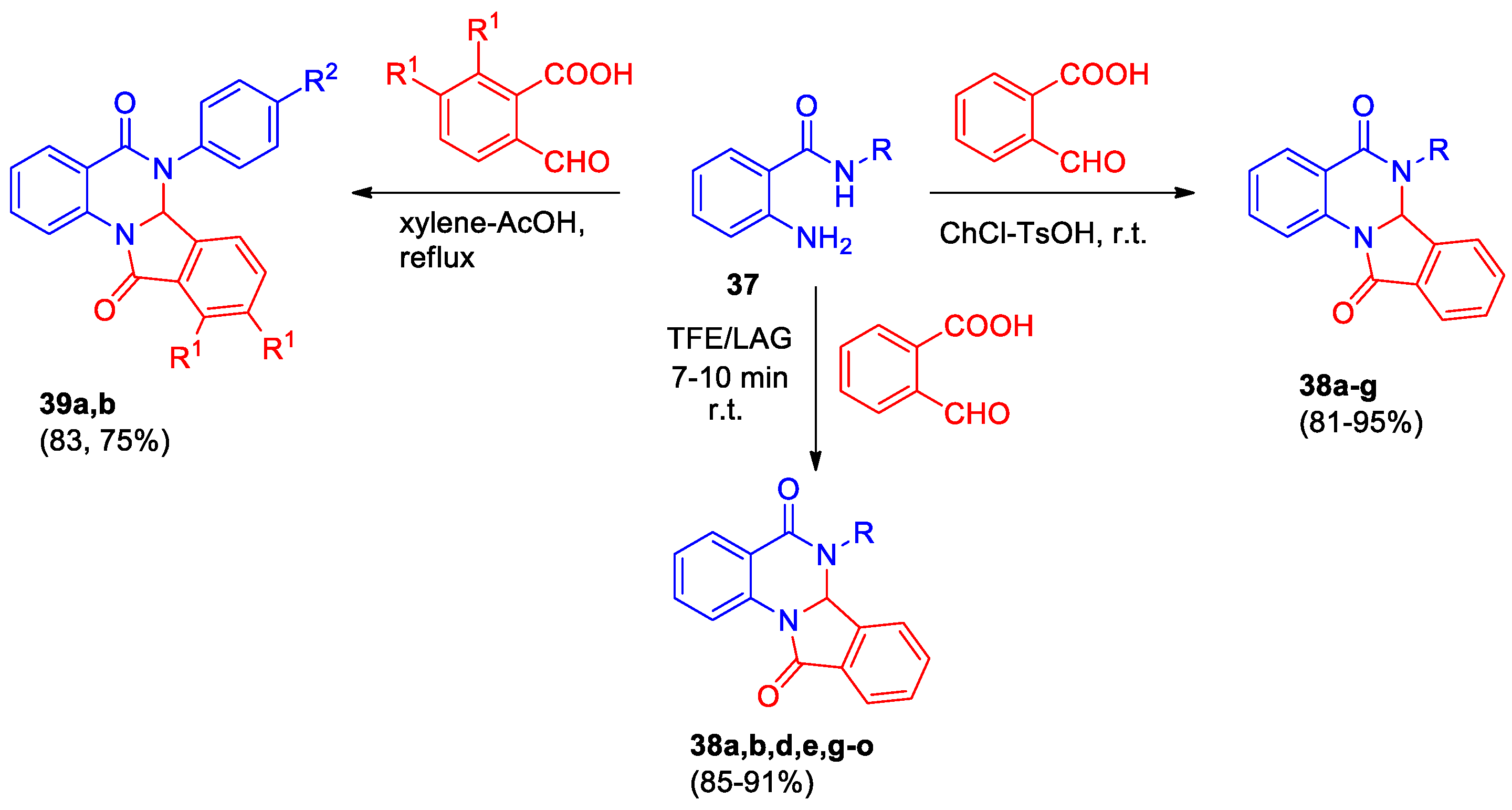
The synthesis of isoindoloquinazolinediones
38 was carried out in a single step starting from 2-aminobenzamides
37 and 2-formylbenzoic acid, conditions for cyclization were selected (
Scheme 14) [
37,
38,
39]. Devi
et al [
37] used a mixture of choline chloride (ChCl) and TsOH as promising green deep eutectic solvent. The reaction proceeds rapidly (15 min) at room temperature leading to dihydroisoindolo[2,1-
a]quinazolin-5,11-diones
38a-g in high yields.
Lohar et al developed an efficient and green mechanochemical method for the synthesis of isoindolo[2,1-
a]quinazolines
38a,b,d,e,g-o via 2,2,2-trifluoroethanol (TFE)-catalyzed liquid-assisted grinding (LAG) (
Scheme 14) [
39]. A mixture of equimolar amounts of reagents and TFE was ground together for 9–11 min at room temperature; the progress of the reaction was monitored by TLC, compounds
38a,b,d,e,g-o were isolated in high yields.
Dihydroisoindolo[2,1-
a]quinazolin-5,11-diones
39 were prepared by cyclization in the xylene/acetic acid mixture at reflux for 3–5 h and isolated as racemates (
Scheme 14). The biological activity of compounds
39 was studied by high-throughput screening (HTS) [
38]. It was found that the 6a
S-enantiomer of derivative
39a inhibits TDP1 and ELG1 protein. Moreover, compound
39a can decrease the intensity of tumor cell proliferation by suppressing the ATG4B protease function. The racemic mixture of the tetracyclic compound
39a was found to be toxic for cardiomyocytes. Compound
39a leads to decomposition of TDP-43 protein. Thus, quinazolinone
39a is promising anticancer agent through inhibition of DNA repair systems and proliferative activity, it also can be useful in neurodegenerative diseases therapy.
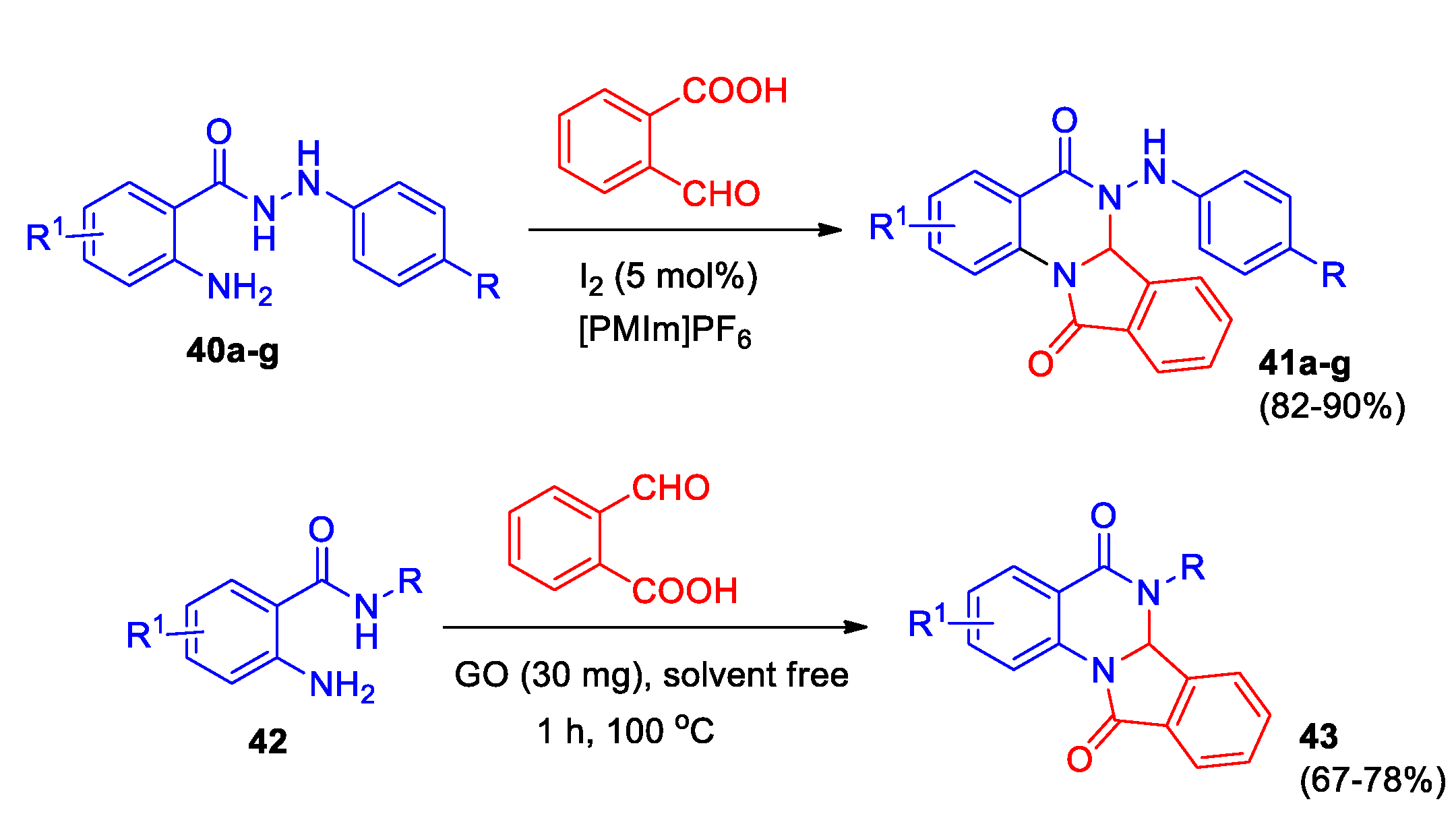
A series of 6-(arylamino)-6,6
a-dihydroisoindolo[2,1-
a]quinazolin-5,11-diones
41a-g was synthesized through iodine-catalyzed reaction of
N’-aryl-2-aminobenzohydrazides
40 and 2-formylbenzoic acid in ionic liquid (
Scheme 15) [
40]. The structure of the compounds was confirmed by X-ray data, for compound
41b as an example. The reaction was shown to be chemoselective, the interaction of unsubstituted 2-aminobenzohydrazide with 2-formylbenzoic acid under the same conditions gave 5
H-phthalazino[1,2-
b]quinazoline.
A fast and eco-friendly technique was created to prepare a series of important, versatile isoindolo[2,1-
a]quinazolines
43 with multiple functions for biology research (
Scheme 15) [
41]. The reaction of 2-aminobenzhydrazide
42 (R = H) with 2-formylbenzoic acid was studied under solvent free conditions using graphene oxide (GO) as mild heterogeneous carbocatalyst. Optimal conditions have been identified, expanding the scope of 2-aminobenzohydrazides. It has been demonstrated that 2-aminobenzamides give high yield of products
43 under the same conditions. The structures of compounds
43 have been validated through X-ray analysis for two compounds
43 (R = NHPh, R
1 = H, and R = CH
2C
6H
4-4-Me, R
1 = 3-Cl). The GO nanosheets were prepared from natural graphite powder. A possible reaction mechanism with GO as catalyst was discussed, it was noted that the acidity of GO plays an important role in the studied transformation.
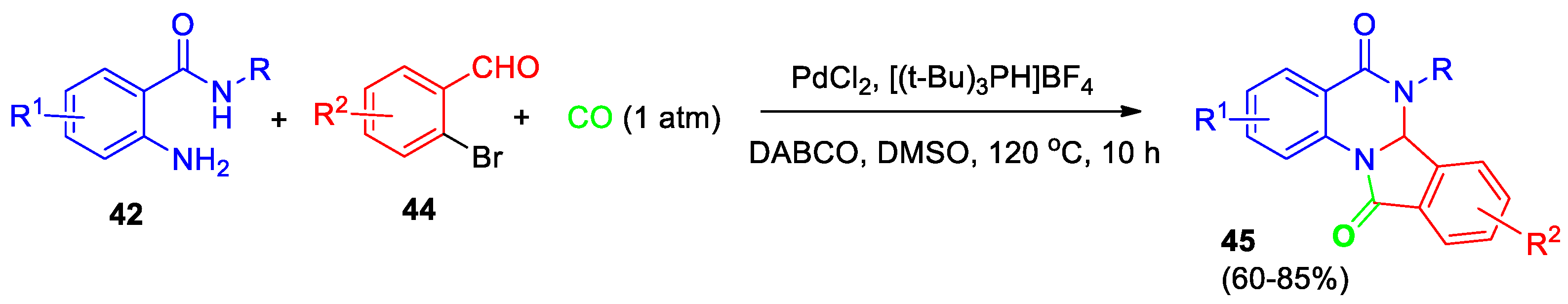
Guo
et al [
42] reported the synthesis of dihydroisoindolo[2,1-
a]quinazolin-5,11-diones
45 without using 2-formylbenzoic acid. The preparation process includes palladium-catalyzed three-component carbonylative cyclization of 2-aminobenzamides
42 with 2-bromobenzaldehydes
44 under an atmospheric pressure of carbon monoxide (
Scheme 16). Optimal synthetic conditions, including selection of catalyst, ligand, base, and solvent, were found, and broad substrate scope was demonstrated. A plausible mechanism involves a palladium-catalyzed cyclocondensation/cyclocarbonylation sequence. According to Guo
et al [
42], this method works well with different types of chemical groups, uses easily available ingredients, demonstrates high regioselectivity, and is straightforward to perform.
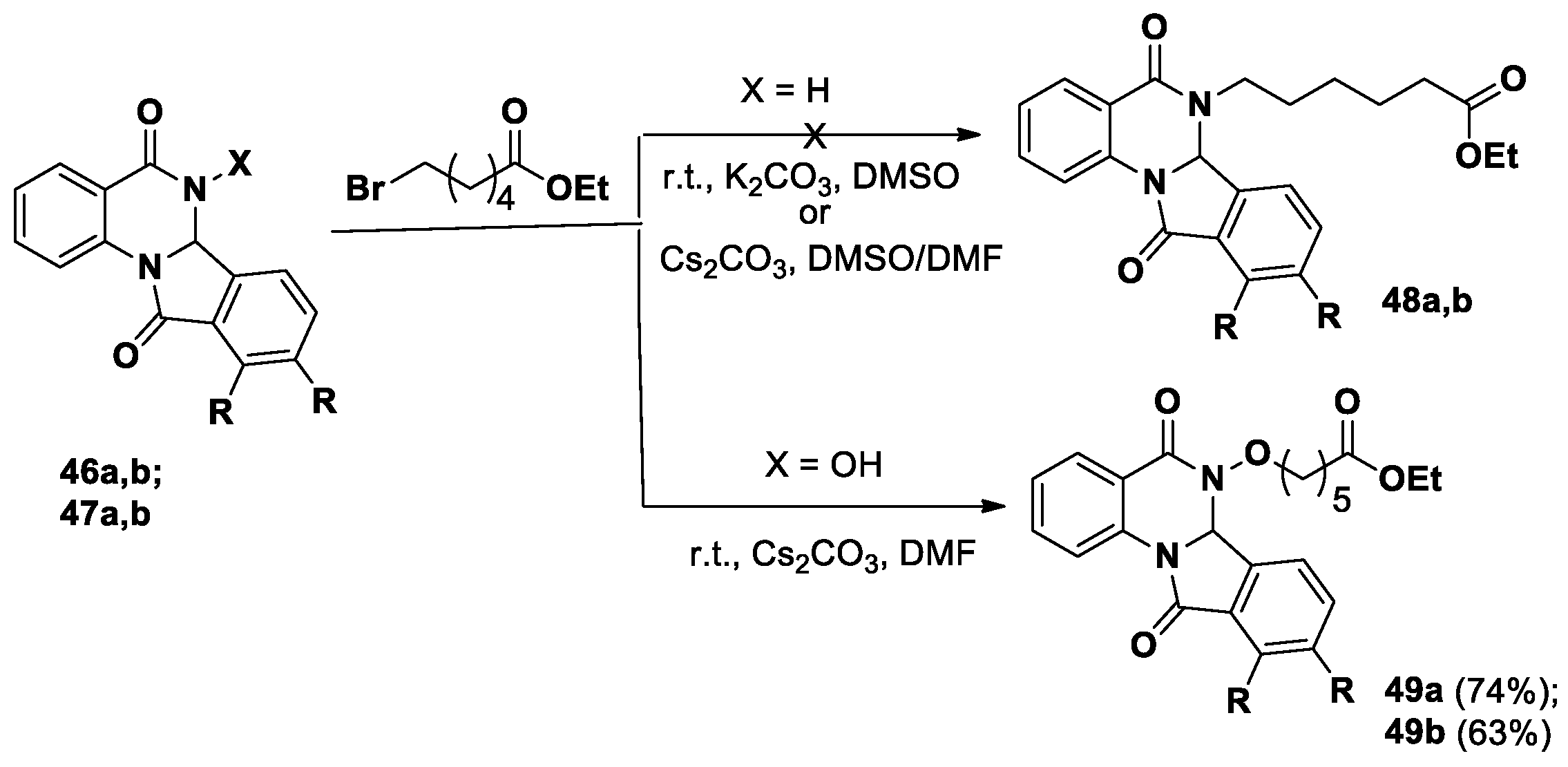
Kolotaev
et al [
43] described synthetic approaches to dihydroisoindolo[2,1-
a]quinazolin-5,11-diones, containing hydroxamic acids, as potential HDAC/VEGFR inhibitors. The first method involved the alkylation of the tetracyclic derivatives
46, 47 obtained by the interaction of substituted anthranilamides and 2-formylbenzoic acids, with ethyl 6-bromohexanoic acid (
Scheme 17). An attempt to obtain tetracyclic quinazolines
48 from NH-derivatives
46a,b was unsuccessful. In the case of hydroxy derivatives
47a,b, the alkylation in the presence of cesium carbonate in DMF resulted in the selective formation of products
49a,b in good yields.
Another approach was selected for the synthesis of dihydroisoindolo[2,1-
a]quinazolin-5,11-diones
48a,b (
Scheme 18). The reaction between isatoic anhydride
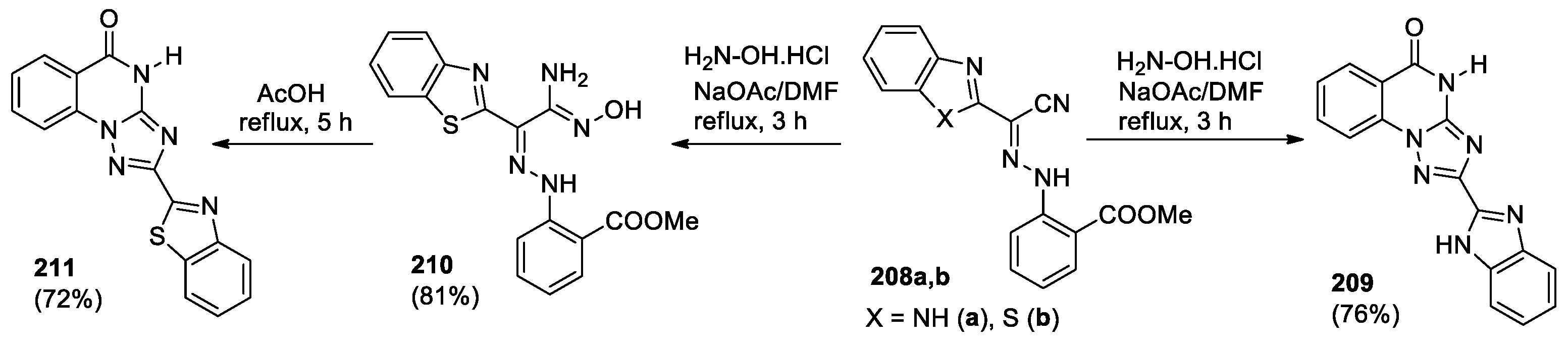
28 and ethyl 6-aminohexanoic acid led to substituted anthranilamide, which further converted into N-substituted tetracycles
48a,b under the action of 2-formylbenzoic acids. The reaction of compounds
48a,b with hydroxylamine allowed someone to obtain the expected derivatives
50a,b, in which hydroxamic acids attached through a linker to tetracyclic quinazolinone-containing fragments.
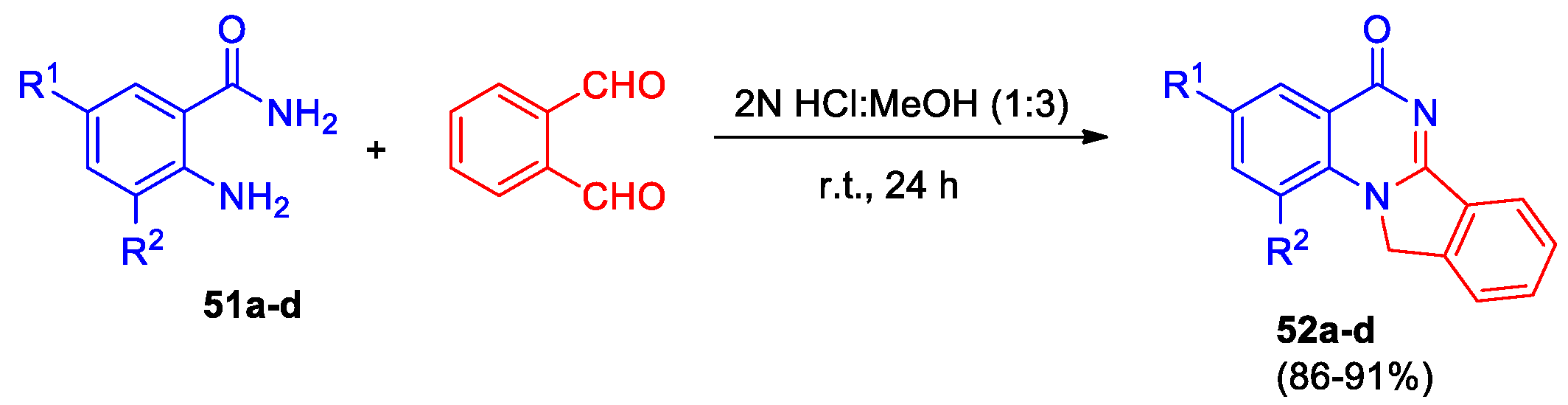
Mondal
et al described one-pot synthesis of isoindole fused quinazolin-4-ones in the presence of an acid catalyst [
44]. Interaction of substituted anthranilamides
51a-d with
o-phthalaldehyde in methanol and 2N HCl in 3:1 ratio at room temperature led to the formation of range of isoindolo[2,1-
a]quinazolin-5(11
H)-ones
52a-d (
Scheme 19). It should be noted that compound
52a was isolated from the reaction mixture as the salt
52a‧HCl, and
52a was obtained only after the treatment with sodium bicarbonate solution. The structure of
52a‧HCl was confirmed by X-ray data, the tetracyclic skeleton is planar, the hydrogen bonding of the amide hydrogen through a water molecule forms a network in the solid. Derivatives
52b-d were isolated from the reaction as major products.
Moreover, Mondal
et al [
44] performed the mechanistic study under deuterated solvent and revealed that the reaction proceeds through intramolecular 1,3-hydride shift. In addition to benzamides
51a-d, various other amides such as 2-aminobenzenesulfonamide and 2-aminotetrahydrobenzothiophenamide were involved in this reaction, leading to corresponding analogs of compounds
52 with similarly high yields. Notably, the nature of substituents within the amide moiety exerts minimal influence on product yield, thus offering substantial flexibility for structural variation of the bicyclic core. Additionally, this method could potentially be applied to many different variations of
o-phthaldialdehyde compounds.
4. Pyrazolo[1,5-a]quinazolines and indazolo[2,3-a]quinazolines
In the review article [
13], some data on the synthetic approaches and bioactivity of pyrazoloquinazolines in which the pyrazole ring is attached to different edges of pyrimidine core are discussed. Regarding the synthesis of pyrazolo[1,5-
a]quinazolines, two strategies were reported: the interaction of 2-hydrazinylbenzoic acid with 3-oxoalkanenitrile in the presence of CH
3COOH [
45] and the three-component reaction of 3(5)-amino-4-phenylpyrazole with aromatic aldehydes and cyclohexanone in acetic acid [
46]. Some pyrazolo[1,5-
a]quinazolines were described as negative allosteric modulators of metabotropic glutamate receptors [
47], other tricyclic derivatives exhibit the topoisomerase-1 inhibitory activity [
48]. Example synthesis of indazoloquinazoline system with
a,
b or
c patterns of annelation of indazole fragment to quinazoline core are provided in the microreview [
49].
A series of new manuscripts on pyrazolo[1,5-
a]- and indazolo[2,3-
a]quinazolines has been published later. Zhang
et al [
50] presented a convenient and simple synthetic procedure for obtaining several pyrazolo[1,5-
a]quinazolin-5(4
H)-ones
54 via copper-catalyzed cascade reactions of 2-bromobenzoates
53 with 1
H-pyrazol-5-amines under ligand-free conditions in water (
Scheme 20). This method is based on commercially available starting materials; water is used as solvent, so the procedure responds to the urgent need for "greener" and "cleaner" chemistry.
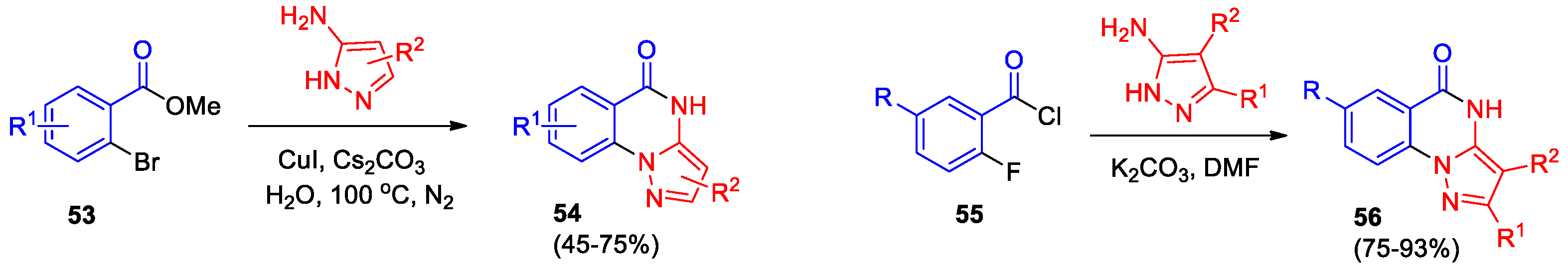
Gnanasekaran
et al [
51] incorporated 2-fluoroaroyl chlorides
55 into reaction with 5-aminopyrazoles (
Scheme 20). The formation of pyrazolo[1,5-
a]quinazolin-5(4
H)-ones
56 is described as two-step sequence: initial acylation of the C5 amino group of the pyrazole was performed in DMF at -10
oC and further heating to 140
oC, then S
NAr ring closure between N1 of the pyrazole and the 2-fluoroarylamide was performed. Products
56 were obtained in high yields regardless of the nature of substituents.
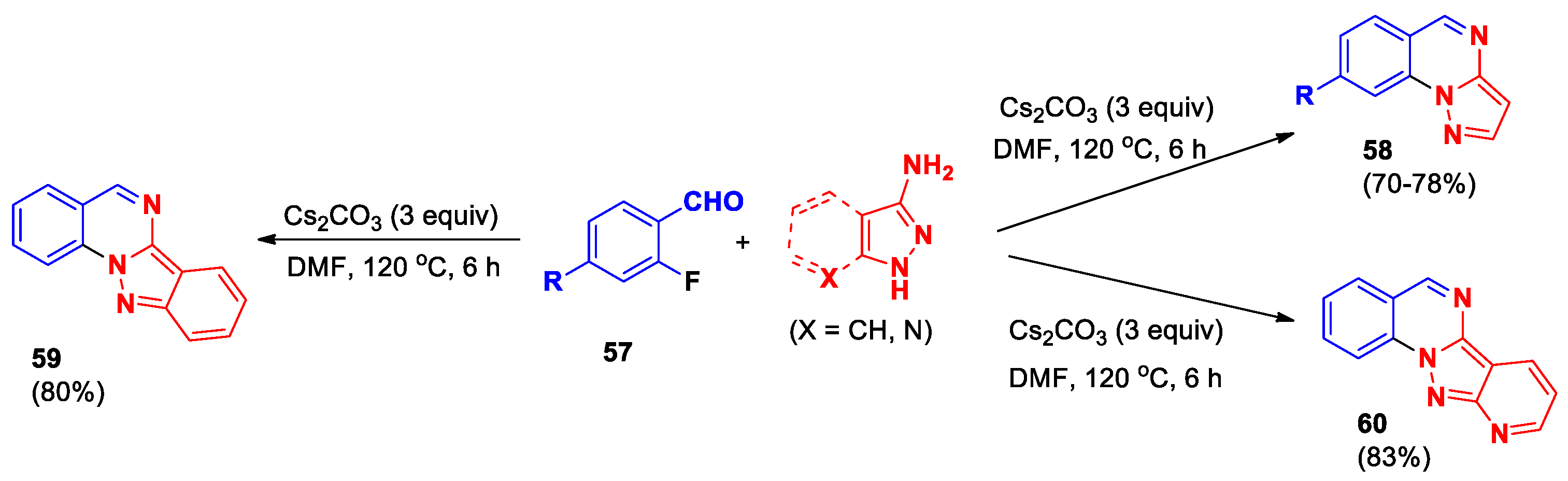
An efficient method for the synthesis of pyrazolo[1,5-
a]quinazolines
58, indazolo[2,3-
a]quinazoline
59, and 10-azaindazolo[2,3-
a]quinazoline
60 was described using various 2-fluorobenzaldehydes
57 interacting with 1
H-pyrazol-3-amines or 1
H-indazol-3-amine (and its 7-aza derivative) under metal-free conditions (
Scheme 21) [
52]. The process involves an intermolecular condensation step followed by a metal-free base-promoted intramolecular C–N coupling reaction. This methodology opens wide opportunities for preparation of various polycyclic quinazoline derivatives. Importantly, the bromine atom stays unchanged during the reaction, so it can later be used for further chemical changes or transformations.
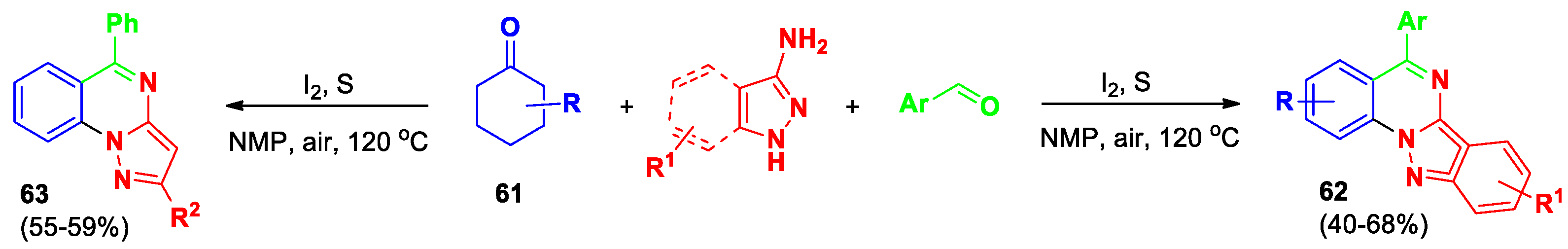
Gao
et al [
53] developed regioselective synthesis of indazolo[2,3-
a]quinazolines
62 via a sequential annulation and dehydrogenative aromatization of cyclohexanones
61 (
Scheme 22). The reaction is carried out in 1-methyl-pyrrolidin-2-one (NMP) as a solvent in the presence of iodine and sulfur at 120 °C for 20 hours. A control experiment verified the sequential nature of the process steps. This reaction works well with many kinds of aminoindazole compounds, including ones with aromatic and heterocyclic aldehydes, as well as differently modified cyclohexanones. Additionally, this method was also used to prepare pyrazolo[1,5-
a]quinazolines
63.
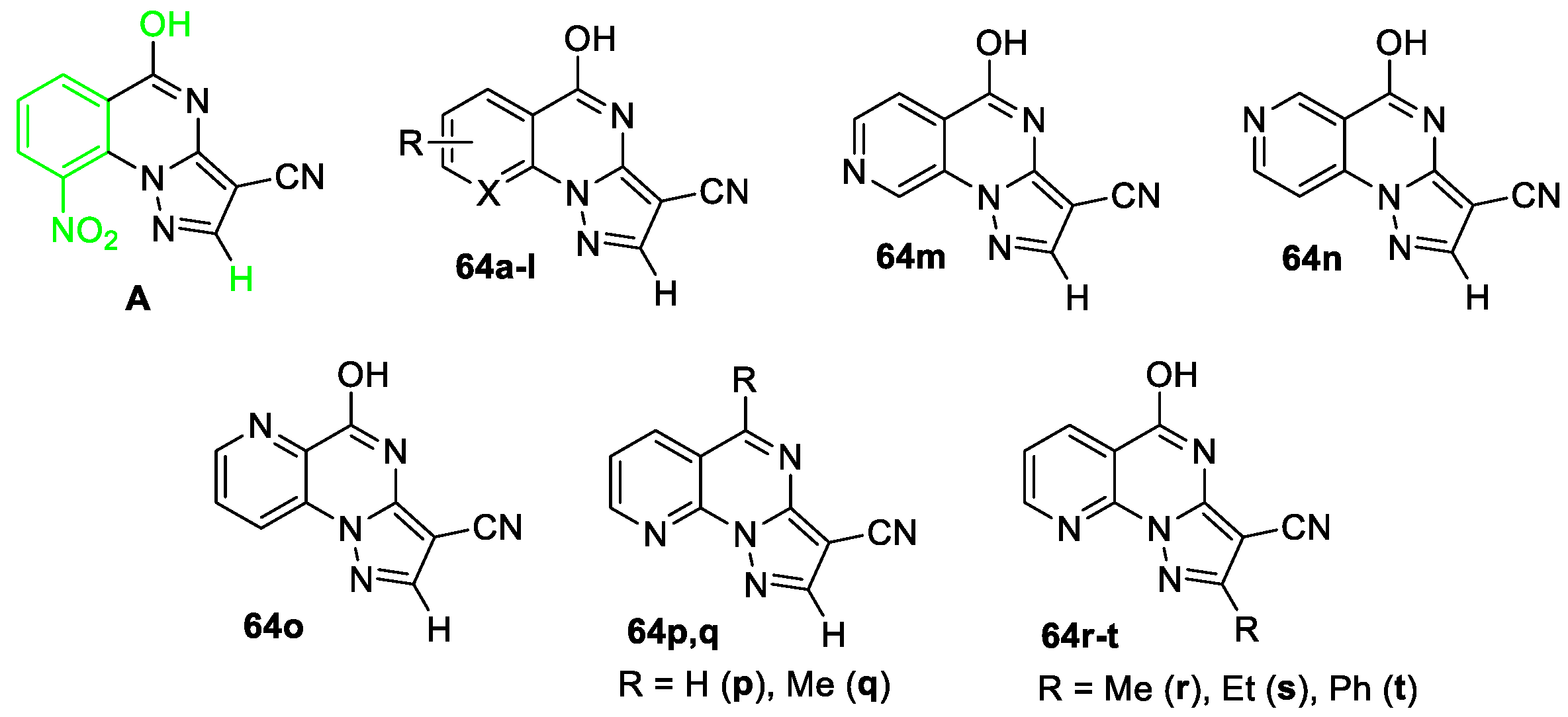
A series of pyrazolo[1,5-
a]quinazolines and their aza-analogues were synthesized and studied as inhibitors of histone lysine demethylase 4D (KDM4D) [
54]. Initially, Fang
et al performed molecular docking for 30 compounds, two tricyclic derivatives showed an inhibition rate greater than 50% against KDM4D at a concentration of 10 µM; one of leading compounds was pyrazolo[1,5-
a]quinazoline derivative (
A), its activity was measured towards three additional KDM enzymes (KDM2B, KDM3B, and KDM5A), excellent selectivity toward KDM4D was demonstrated. Structural modifications focusing on three fragments of compound
A (highlighted in green color) led to the synthesis of a series of derivatives
64a–t. These derivatives were prepared by reacting 2-chloroacetyl chlorides, derived from either benzoic acid or picolinic acid, with corresponding 3-amino-
1H-pyrazole-4-carbonitriles (
Figure 3).
All target compounds were initially tested for their inhibitory activity against KDM4D at a concentration of 10 μM. Only three compounds (
64p,
64r, and
64s) exhibited higher potencies compared to
A. For these three compounds, IC
50 values were determined, derivative
64r was chosen as lead compound (
Table 1). Moreover, quinazoline
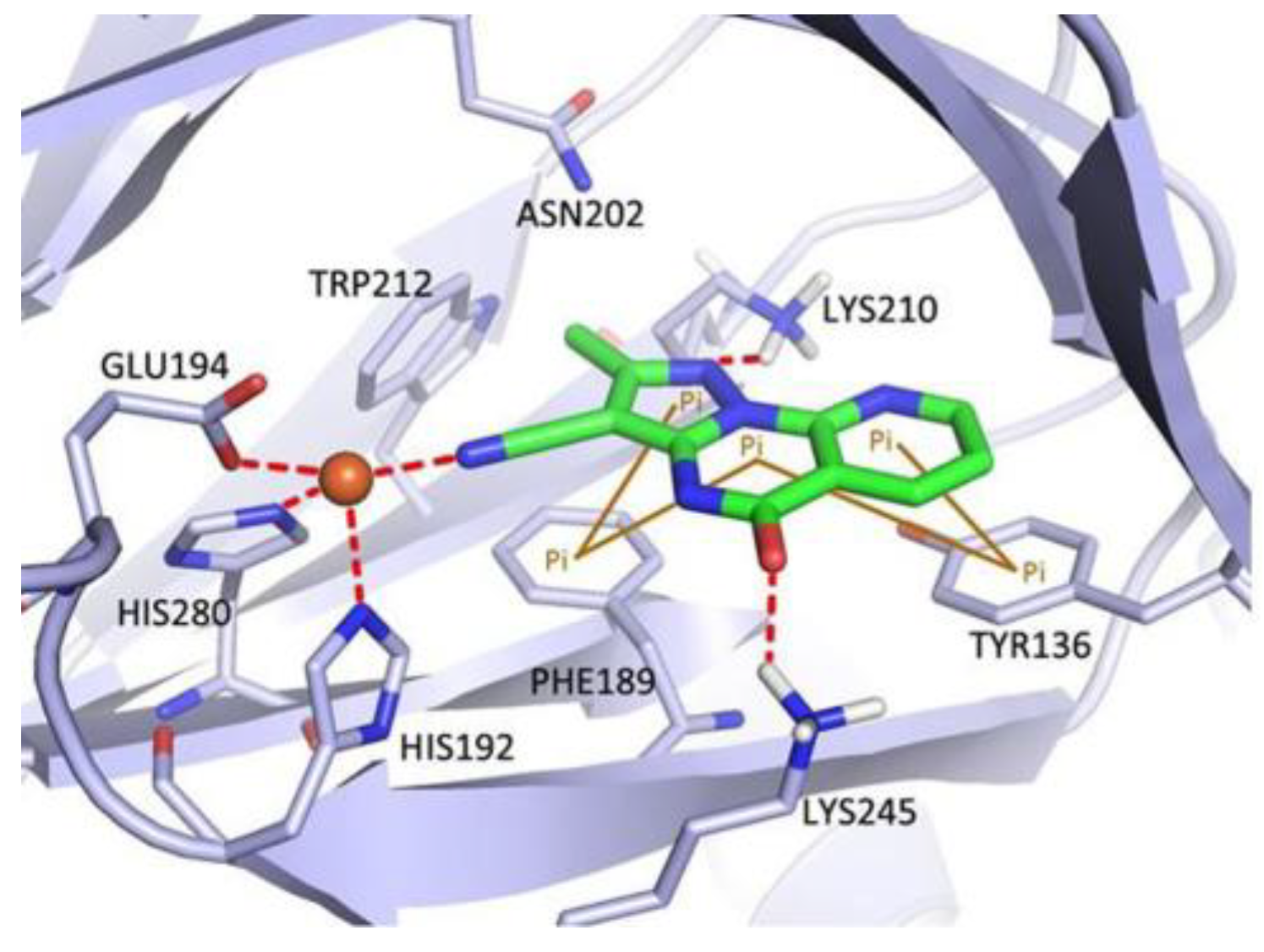
64r exhibits good selectivity for KDM4D; molecular docking was used to predict the binding model of compound
64r in the active pocket of KDM4D (
Figure 4).
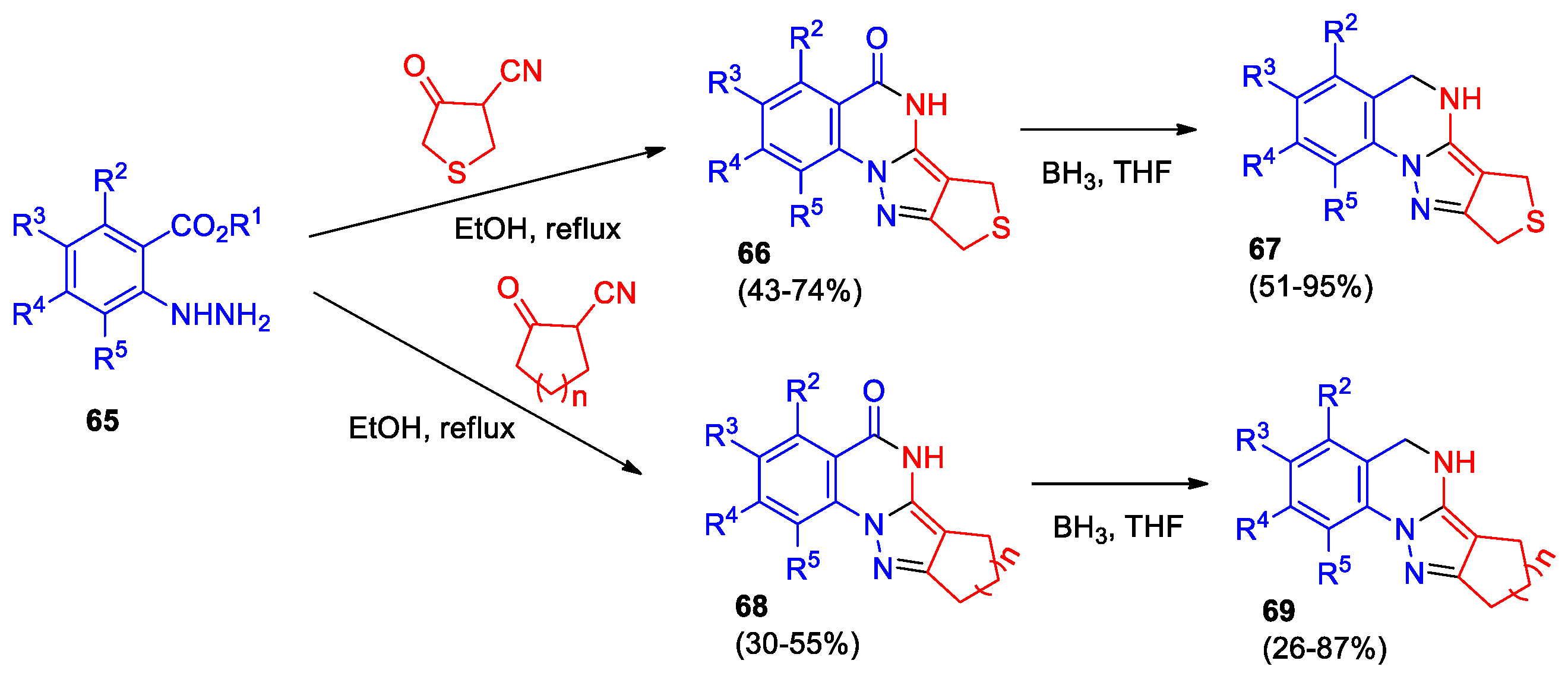
Kovács
et al [
55] reported the synthesis of pyrazolo[1,5-
a]quinazoline derivatives through the reaction of 2-hydrazinobenzenecarboxylic acids or their esters (compounds
65) with α-oxo-cyanides (
Scheme 23). Refluxing a mixture of compound
65 and 4-oxotetrahydrothiophene-3-carbonitrile in ethanol led to the formation of 6,7-dihydro-
5H,9H-thieno[3’,4’:3,4]pyrazolo[1,5-
a]quinazolin-5-ones
66, which were subsequently converted into amines
67.
Interaction of 2-oxocyclopentanecarbonitrile (n = 1) or 2-oxocyclohexanecarbonitrile (n = 2) with arylhydrazines 65 in ethanol afforded a series of novel tetracyclic pyrazolo[1,5-a]quinazoline derivatives 68, the reduction of the latter leads to formation of amines 69. Notably, when R2 = Me, Br or R5 = Me, the yield of pyrazolo[1,5-a]quinazoline derivatives 66 and 68 significantly decreased, and their corresponding amines 69 were not detected.
5-Substituted pyrazolo[1,5-
a]quinazolines were synthesized and evaluated as ligands of GABA
A receptor [
56], only one compound showed receptor recognition in the nanomolar range. To find derivatives with higher activity, novel 3- and/or 8-substituted pyrazolo[1,5-
a]quinazolines were synthesized [
57]. Using a copper-catalyzed tandem reaction, appropriate 2-bromo-4-R-benzaldehyde and 5-amino-
1H-pyrazole-4-carbonitrile, 3-cyanoderivatives
70a-c were isolated in low yields (
Scheme 24). Later [
58], derivative
70b was obtained in 55% yield by fusion of 5-amino-
1H-pyrazole-4-carbonitrile with 2-chlorobenzaldehyde at 160 °C for 6 hours. Interaction of ethyl 1-(3-methoxyphenyl)-5-aminopyrazole-3-carboxylate
71 with formaldehyde or paraformaldehyde in an acidic medium afforded 8-methoxy-pyrazolo[1,5-
a]quinazoline
72a, however, separating it from its 6-methoxy isomer was difficult. The key intermediate
72a was synthesized in higher yield from 2-carboxy-5-methoxyphenylhydrazine via intermediate 8-methoxy-pyrazolo[1,5-
a]quinazolines
73,
74, and
75 (
Scheme 24). Derivative
72a and ethyl pyrazolo[1,5-
a]quinazoline-3-carboxylate
72b were hydrolyzed to give the respective 3-carboxylic acids
76a,
b, which were further transformed into 3-ester derivatives
77a-e and
78a-c. Finally, compounds
77a,
b,
d were converted into the corresponding 4,5-dihydroderivatives
79a,
b,
d.
The in vitro study of the BZ site/GABAA-R binding affinity of synthesized pyrazolo[1,5-a]quinazolines derivatives showed that the new compounds have binding recognition in the nanomolar range (3.16 < Ki (nM) < 529.3) at fixed concentrations of 10 μM, raising the subnanomolar affinity value of 0.27 nM for compound 77b. The pharmacological tests evidenced for compound 77b a profile of positive allosteric modulator with anxiolytic and antihyperalgesic activity, lacking toxicity when tested in human neuronal-like cells and in vivo models.
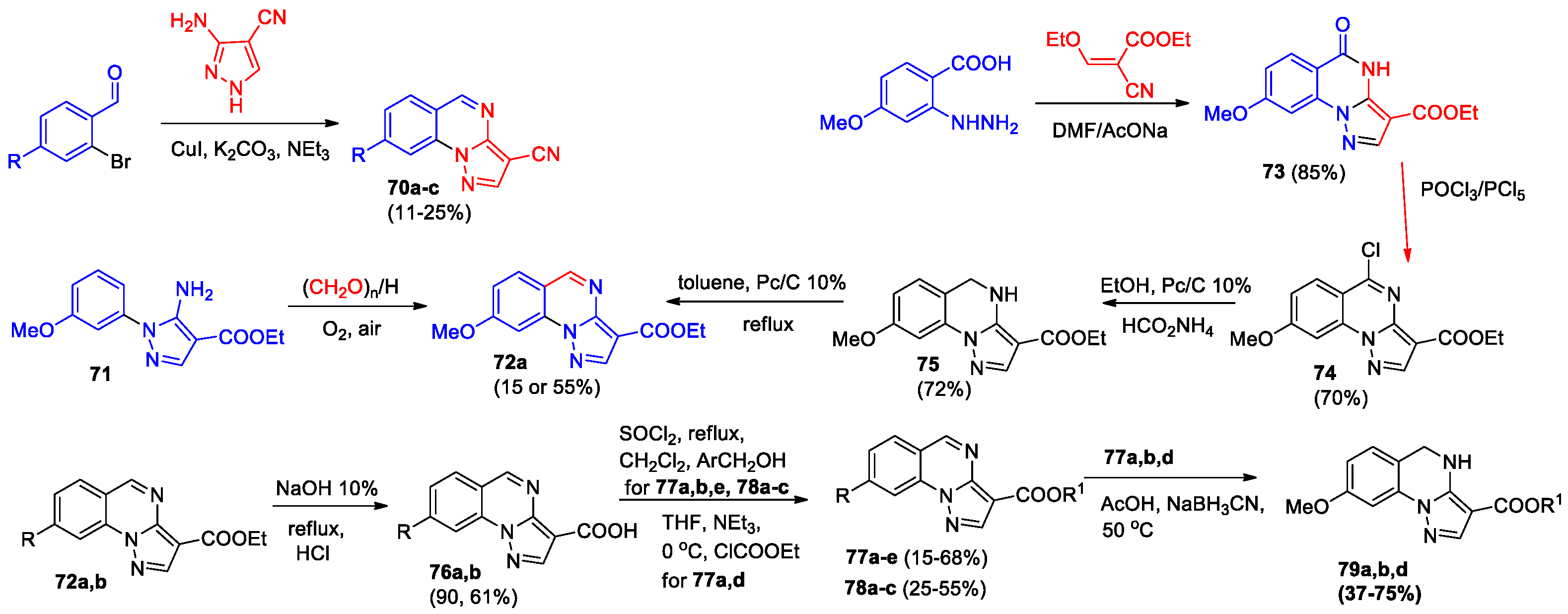
In the next work of the same team [
59], modification of pyrazolo[1,5-
a]quinazolines
77 was performed by shortening or removing the linker between aryl(hetaryl) ring and the pyrazolo[1,5-
a]quinazoline core, 3-aryl(heteryl)-pyrazolo[1,5-
a]quinazolines
82 and 3-(hetero)aroyl-pyrazolo[1,5-
a]quinazolines
83 were developed. Cyclization of hydrazinobenzoic acid with appropriate propanenitrile resulted in the formation of pyrazolo[1,5-
a]quinazolines bearing at position 3 (hetero)aryl group (compounds
80a-e) or (hetero)aroyl group (compounds
80f-h) (
Scheme 25). Treatment of compounds
80a-e with LiAlH
4 in anhydrous THF followed by oxidation led to 3-(hetero)aryl derivatives
82a-e. Compounds
80f-h were transformed into 3-(hetero)aroylpyrazolo[1,5-
a]quinazolines
83a-c via intermediate chloroquinazolines
81.
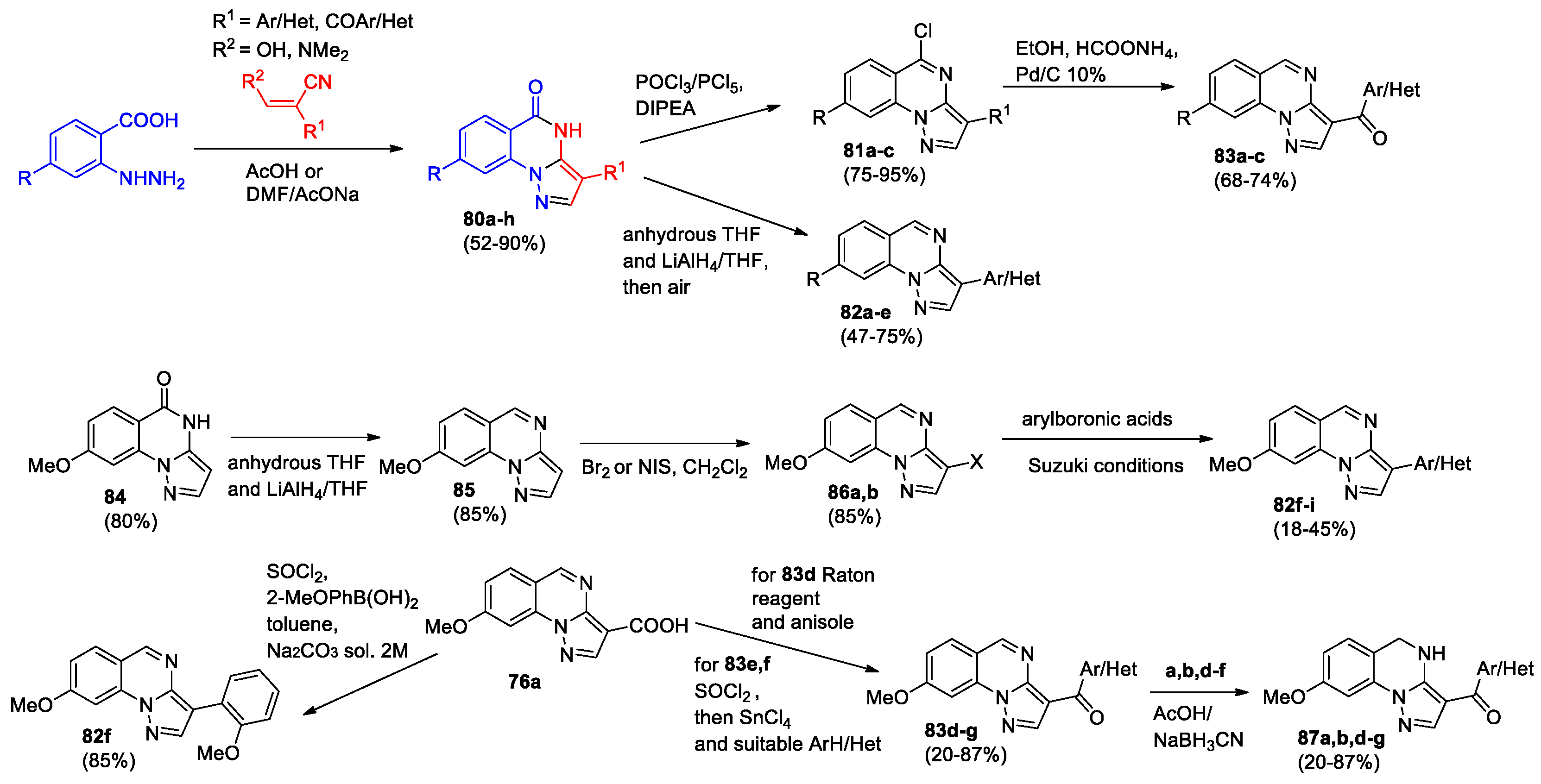
The synthesis of compounds
82f-i was accomplished using the Suzuki cross-coupling based on halogenated derivatives
86a,b, which were obtained from 8-methoxypyrazolo[1,5-
a]quinazoline
85 (
Scheme 25). The starting quinazolinone
84 was synthesized by decarboxylation of derivative
73. Derivative
76a used for the preparation of quinazolines
82f and
83d-g (
Scheme 25). Treating 3-(hetero)aroyl-pyrazolo[1,5-
a]quinazolines
83a,b,d-g with sodium borohydride complex (NaBH
3CN) in AcOH allowed obtaining their dihydro analogs
87a,b,d-g.
All target compounds were previously evaluated for their ability to displace [3H]flumazenil (Ro-151788) from its specific binding to Bz receptors in bovine membrane samples. Electrophysiological studies on recombinant α1ꞵ2ɤ2L GABAA receptors were carried out for more promising compounds 82d–f, 83a–d, 6f,g, 87a,b,d. Quinazolines 82 bearing Ar/Het group could not modulate the GABAA function, but they were found to act as null modulators or antagonists. Among aroyl/Het derivatives 83a and 83b can modulate the GABAA receptor in an opposite manner: 83b enhances and 83a reduces the variation of the chlorine current. The most potent derivative 87d reached a maximal activity at 1 μM (+54%) and enhanced the chlorine current at ≥ 0.01 μM. Moreover, compound 83g demonstrated the ability to antagonize the full agonist diazepam.
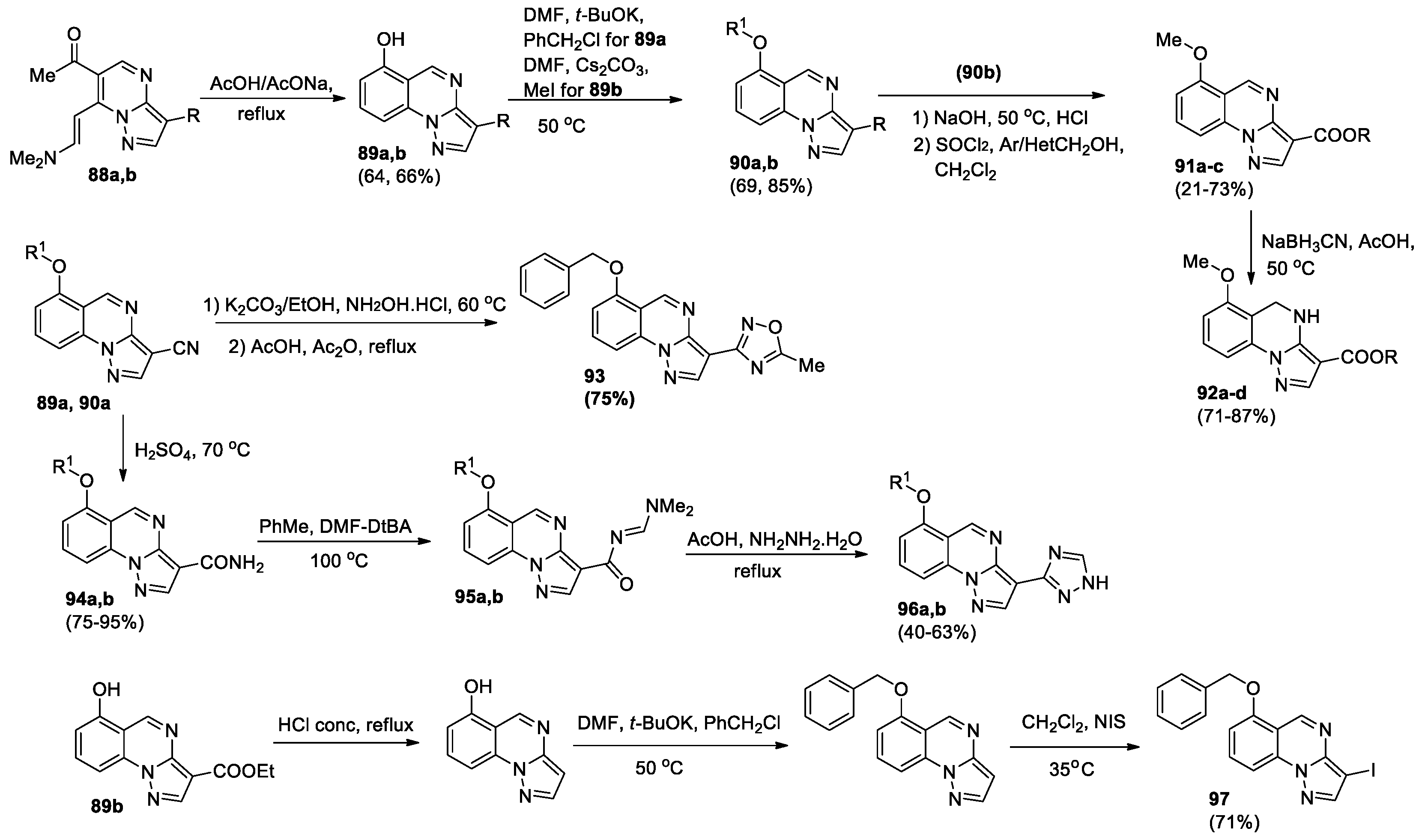
Guerrini
et al [
60] evaluated the effect of the shift of the methoxy group from position 8 to position 6, with the same (hetero)aryl ester groups at position 3. 6-Acetyl-7-(2-dimethylaminovinyl)pyrazolo[1,5-
a]pyrimidine 3-cyano or 3-ethoxycarbonyl
88a,b, obtained according to the described method [
61], were used for the synthesis of 3,6-disubstituted pyrazolo[1,5-
a]quinazolines. Boiling of these compounds in an acetic buffer solution led to the formation of 6-hydroxypyrazolo[1,5-
a]quinazolines
89a,b, whose alkylation yielded derivatives
90a,b (
Scheme 26). Compound
90b was hydrolyzed to produce the corresponding 3-carboxylic acid, which was esterified to ethers
91a-c, and then reduced to 4,5-dihydroderivatives
92a-d. Compound
90a under the treating with hydroxylamine hydrochloride in ethanol and potassium carbonate, followed by cyclization was transformed into 3-(5-methyl-1,2,4-oxadiazol-3-yl)-derivative
93. To prepare derivatives
96a,b, compounds
89a and
90a were converted into 3-carboxyamide derivatives
94a,b, which upon reaction with dimethylformamide-di-
tert-butylacetate (DMF-DtBA) in toluene formed acylamidines
95a,b. Boiling these acylamidines with hydrazine hydrate yielded compounds
96a,b. 3-Iodo-6-benzyloxypyrazolo[1,5-
a]quinazoline
97 was obtained from compound
89b after decarboxylation, alkylation, and iodination (
Scheme 26).
Novel 3,6-disubstituted pyrazolo[1,5-a]quinazolines 89–94, 96, 97were studied as ligands to GABAA receptor. The GABAA-binding affinity of compounds was evaluated for their ability to displace [3H]flumazenil (Ro-151788) from its specific binding in a bovine membrane. From the obtained results, it follows that the compounds demonstrated the percent of inhibition of specific [3H]Ro15-1788 binding at 10 μM concentration from 1% to 43.7%, compound 97 showed the highest inhibition. Based on experimental data and molecular modeling study on compound 91b authors concluded that the movement of substituents from position 8 to position 6 is essential for binding.
New 8-methoxypyrazolo[1,5-
a]quinazolines
98a-i and their dihydro derivatives
99a-i bearing the amide fragment at the position 3 were synthesized [
62] as analogues of 8-methoxypyrazolo[1,5-
a]quinazoline 3-ester [
56,
57], 3-(hetero)aroyl and 3-(hetero)aryl derivatives [
59] identified as modulators of GABA
A receptors. 8-Methoxypyrazolo[1,5-
a]quinazoline-3-carboxylic acid
78a after the treatment with thionyl chloride or trichloroacetonitrile/PPh
3 in CCl
4 led to the formation of Het-C(O)Cl intermediate, which without isolation interacted with the appropriate amine, giving compounds
98a-i, which were transformed into the corresponding 4,5-dihydroderivatives
99a-i by the treatment with sodium cyanoborohydride (NaBH
3CN) in acetic acid (
Figure 5).
All the new compounds 98a-i and 99a-i have been evaluated in vitro for their ability to modulate the chlorine current on recombinant GABAA receptors of the α1β2γ2L type (expressed in frog oocytes of the Xenopus laevis species). Two groups of compounds were identified from electrophysiological test: positive modulators agonists (98e,h,i and 99e,h) and null modulators antagonist (98a,b,d,f,g and 99a-d,f,g).
In the next work [
63], new 8-chloropyrazolo[1,5-
a]quinazoline derivatives
100–107 (
Figure 5) were presented as GABA
A receptor modulators. Their synthesis are like ones demonstrated in
Scheme 24 and
Scheme 25. Compounds
100–107 underwent molecular dynamics simulations performed on an isolated segment of the GABA
A receptor protein located between α and γ chains, where the benzodiazepine-binding site is identified. Using the 'Proximity Frequencies' model (PF), Crocetti
et al [
63] predicted that compounds
100a,
103a, and
106b belong to the agonist class with 93.1% probability. On the contrary, derivatives
101c,
103c, and
107 fall into the antagonist class, with 62–73% prediction. Thus, two types of compounds occupy different areas in the binding site. The virtual prediction for
106b and
107 as agonist and antagonist, respectively, was confirmed through electrophysiological assays.
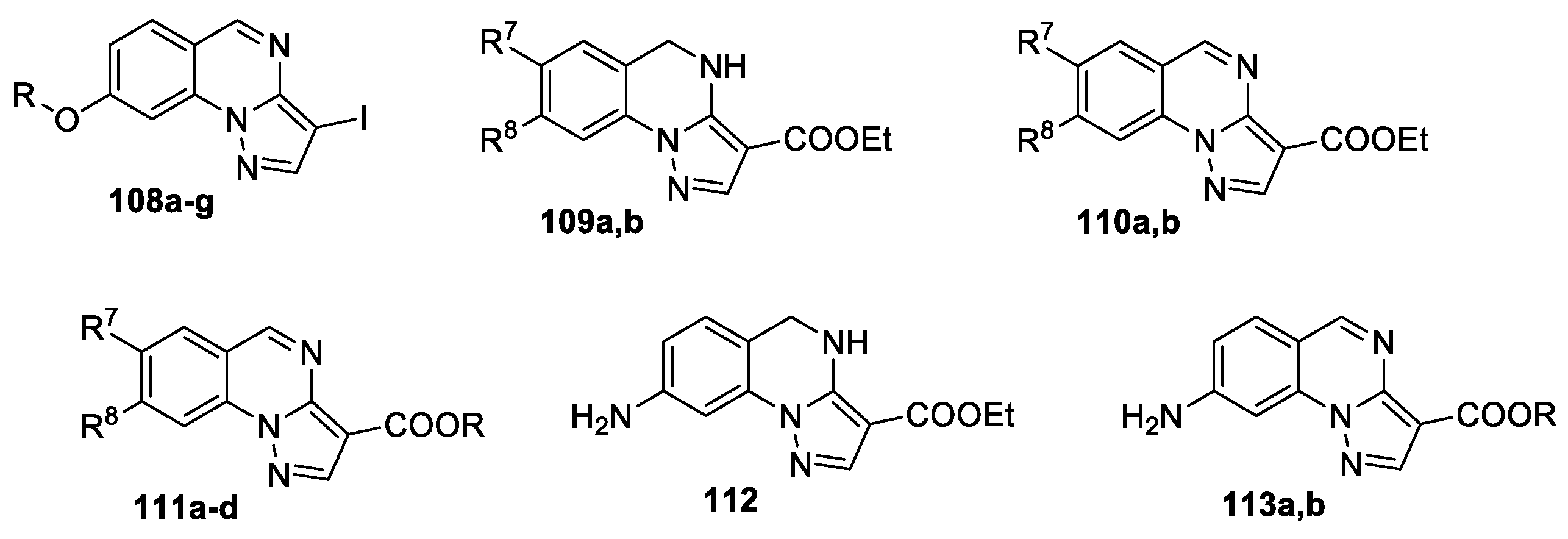
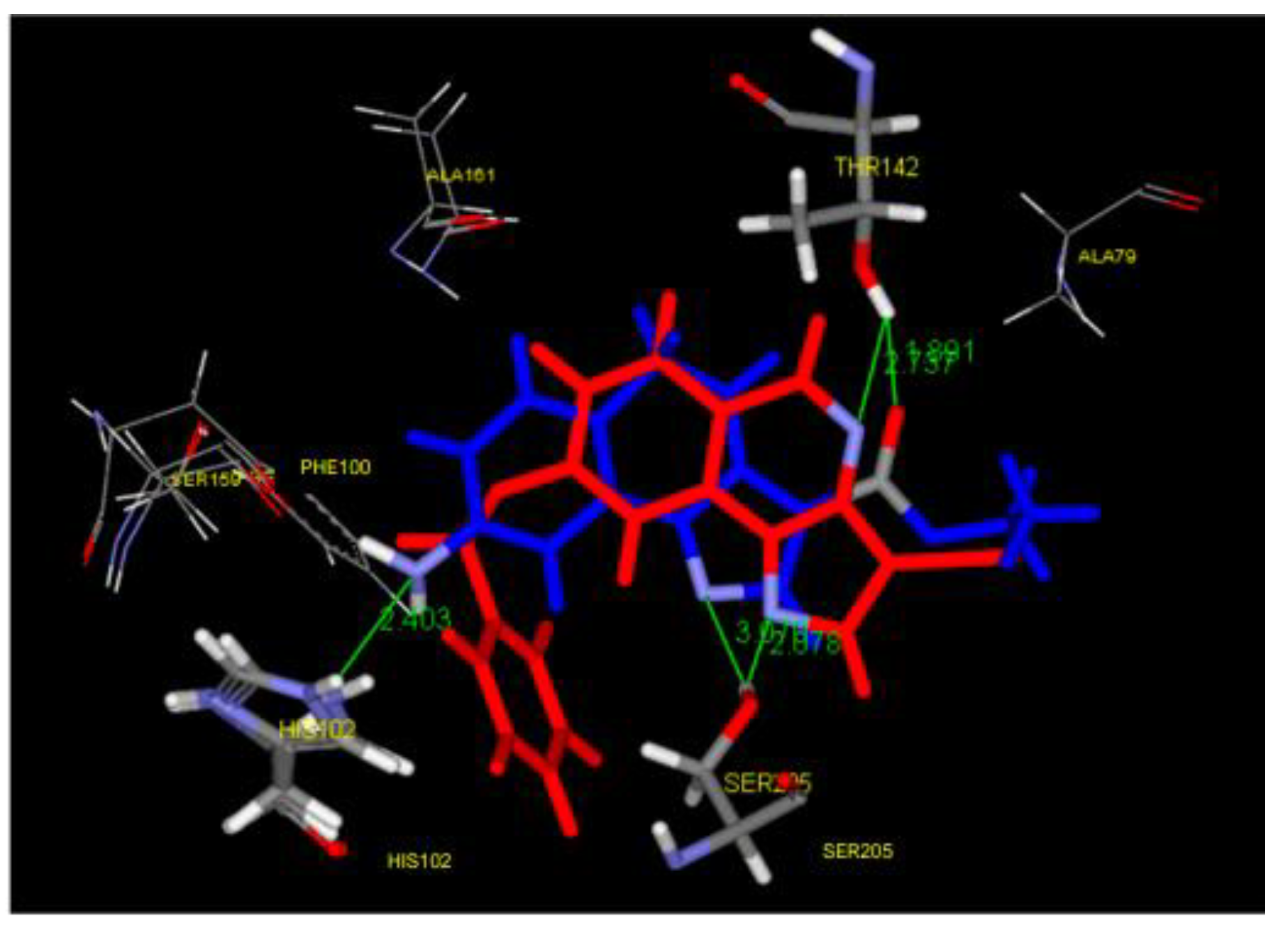
3,8-Disubstituted pyrazolo[1,5-
a]quinazoline
108–113 (
Figure 6), bearing oxygen or nitrogen function at position 8, were synthesized and studied as GABA
A receptor modulators [
64]. The synthesis is based on approaches presented in
Scheme 24 and
Scheme 25.
Compounds
108–113 were screened through electrophysiological techniques on recombinant α1β2γ2L-GABA
A receptors expressed in
Xenopus laevis oocytes, some compounds exhibited certain ability to bind the receptor. The most promising electrophysiological results were obtained for compounds
108d,
109a,
109b, and
112. Among the 3-iododerivatives, compound
108d, which does not modulate the chlorine current, was evaluated for its ability to antagonize the full agonist lorazepam (1 μM). Compounds
109a and
112 were found to exhibit agonist profile while quinazolines
109b and
108d act as antagonists. Molecular modelling studies and Hierarchical Cluster Analysis (HCA) data have collocated these ligands in the class corresponding to their pharmacological profile (
Figure 7).
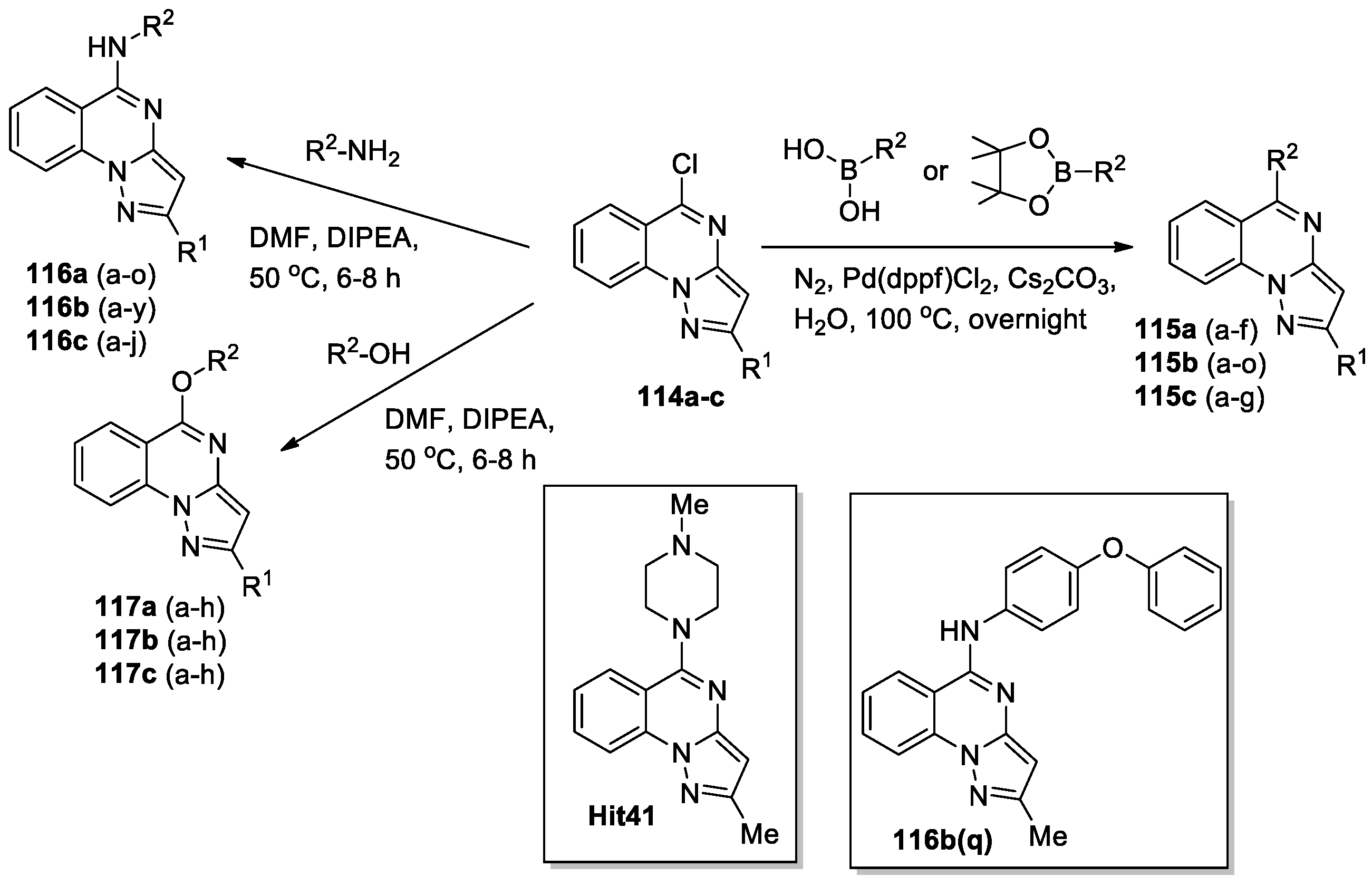
A large series (102 compounds) of pyrazolo[1,5-
a]quinazoline derivatives were designed and synthesized to investigate their activity as SIRT6 activators [
65]. SIRT6 is a particularly important member of Sirtuins and has emerged as a novel therapeutic target for various diseases. Previously [
66], a 2-methyl-5-(4-methylpiperazin-1-yl)pyrazolo[1,5-
a]quinazoline
(Hit41) had been found as a new SIRT6 de-fatty-acylation activator. Zhang
at al [
65] discovered highly active SIRT6 de-fatty-acylation activators by structural modifications of Hit41, focusing on expanding substituents at positions 2 (R
1) and 5 (R
2) of the pyrazolo[1,5-
a]quinazoline scaffold. Three series of derivatives were synthesized (
Scheme 27):
115a–117a (R
1 = H);
115b–117b (R
1 = Me);
115c–117c (R
1 =
tBu). In each series, R
2 represented a broad set of Ar or Het substituents (number indicated in parentheses). Chlorinated precursors
114a-c, obtained using one of the approaches shown in
Scheme 24, were used as starting materials.
Fluor de Lys (FDL) assays were performed for compounds
115–117, for some compounds additional calculations were made to determine the concentration of the compound (μM) at which the compound can increase the enzymatic activity by 50% values (EC
1.5), structure-activity relationship (SAR) was studied. A set of novel SIRT6 activators was obtained (13 compounds with EC
1.5<50.79 μM); among them, 2-methyl-
N-(4-phenoxyphenyl)pyrazolo[1,5-
a]quinazolin-5-amine
116b(q) is the most potent one, which exhibited excellent defatty-acylation activation activity against SIRT6 (EC
1.5 1.85±0.41 μM and EC
50 11.15±0.33 μM). Notably, that the calculated EC
1.5 value for
Hit41 is only 49.30±0.74 μM. The bioactivity of
116b(q) was further verified by differential scanning fluorimetry (DSF) and surface plasmon resonance (SPR) assays. Molecular docking showed that the pyrazolo[1,5-
a]quinazoline
116b(q) formed a hydrogen bond with Val115 and four π–π interactions with Phe64, Phe82 and Phe86;
116b(q) can significantly improve the thermal stability of SIRT6 protein and inhibit the PI3K/Akt signaling pathway in mouse embryonic fibroblasts (MEFs), thereby inhibiting the proliferation of MEFs. As a result, Zhang
at al [
65] discovered a new potent SIRT6 activator, which can be taken as a lead compound for later studies.
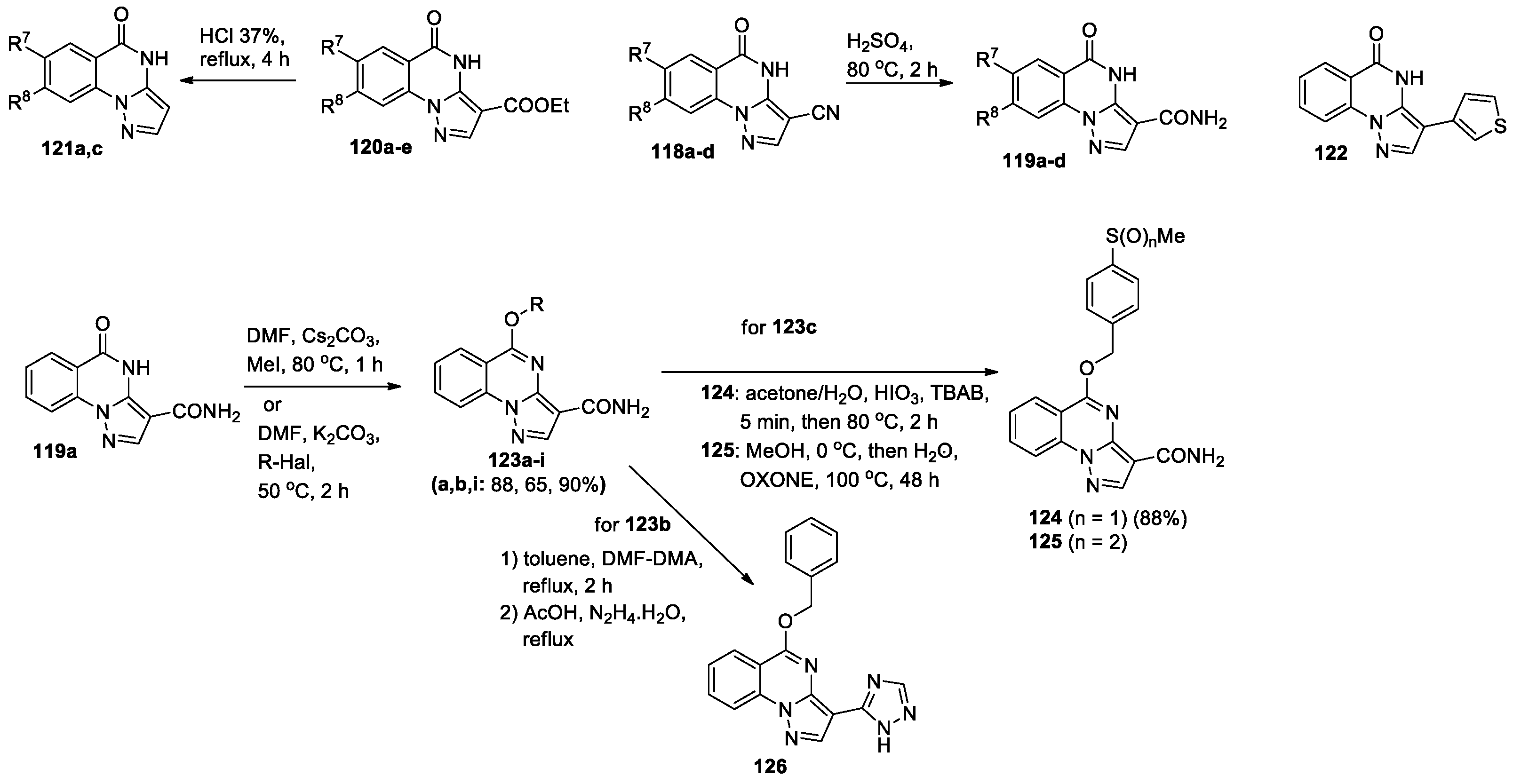
A library of new pyrazolo[1,5-
a]quinazoline compounds substituted at positions 3, 7 or 8 was synthesized and their anti-inflammatory activity was studied [
67]. The key intermediates
118a-d,
120a-e, and
122 were obtained by the condensation of disubstituted 2-hydrazinobenzoic acid with ethoxymethylene-malononitrile, ethyl-2-cyano-3-ethoxyacrylate, and 3-oxo-2-(3-thienyl)proponitrile, respectively (
Scheme 28). Acid hydrolysis of nitriles
118 yielded amides
119, while carboethoxy derivatives
120 were converted into unsubstituted counterparts
121. Pyrazolo[1,5-
a]quinazolines
118–122 were subjected to further transformations, and 45 compounds were synthesized for screening. For example, from derivative
119a, a series of 5-alkoxy-substituted pyrazolo[1,5-
a]quinazolines
123a-i was prepared, the compound
123c was oxidized to yield sulfoxide
124 and then sulfone
125, whereas
123b was transformed into 3-(1,2,4-triazole) derivative
126 (
Scheme 28).
Compounds
123–126 were tested for their ability to inhibit lipopolysaccharide (LPS)-induced nuclear factor κB (NF-κB) transcriptional activity in human THP-1Blue monocytic cells. Only 13 compounds were able to inhibit NF-κB activity with IC
50<50 μM, two of them showed the highest activity:
123i (5-[(4-sulfamoylbenzyl)oxy]pyrazolo[1,5-
a]quinazoline-3-carboxamide) and
124 (5-[(4-(methylsulfinyl)benzyloxy]pyrazolo[1,5-
a]quinazoline-3-carboxamide). Pharmacophore mapping of potential targets and molecular modeling allowed to conclude that these compounds could effectively bind to extracellular signal-regulated kinase 2 (ERK2), p38α, and c-Jun N-terminal kinase 3 (JNK3) with the highest complementarity to JNK3. Moreover, compounds
123i and
124 exhibited micromolar binding affinities for JNK1, JNK2, and JNK3. Obtained results [
67] demonstrate the potential for developing lead anti-inflammatory drugs of pyrazolo[1,5-
a]quinazoline nature.
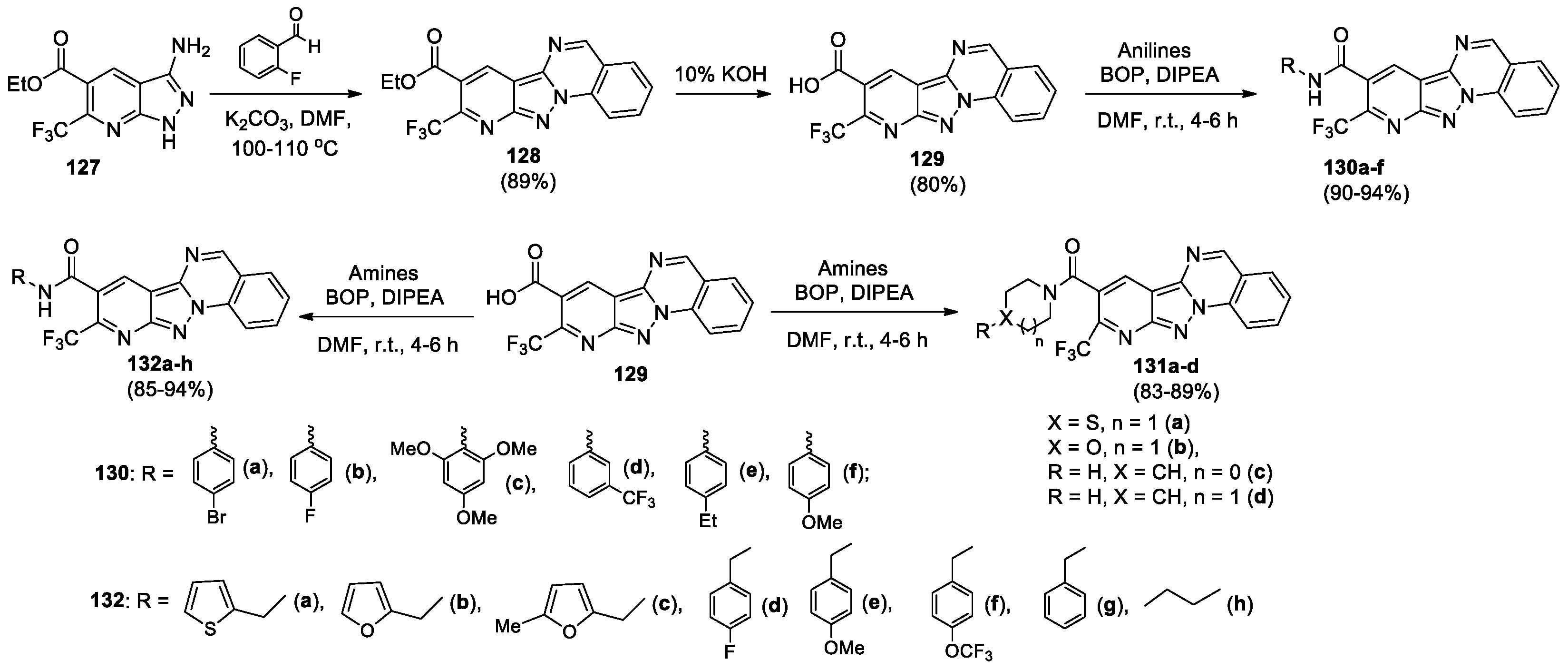
Kumar
et al presented the synthesis of tetracyclic hybrid structures
127–132, combining pyrazolo[3,4-
b]pyridine and pyrazolo[1,5-
a]quinazoline fragments, and data of their biological activity [
68]. Reaction of 3-amino-pyrazolo[3,4-
b]pyridine-5-carboxylate
127 with 2-fluorobenzaldehyde resulted in the formation of ethyl pyrido[2’,3’:3,4]pyrazolo[1,5-
a]quinazoline-8-carboxylate
128 in 89% yield. Hydrolysis of compound
128 under alkaline conditions produced carboxylic acid derivative
129, which upon reaction with various substituted anilines, cyclic secondary amines, and primary aliphatic amines led to the corresponding amide-substituted pyridopyrazolo-quinazolines
130a-f,
131a-d, and
132a-h (
Scheme 29). In all cases, the reaction between
129 and amines was carried out in the presence of benzotriazol-1-yloxy-tris(dimethylamino)phosphonium hexafluorophosphate (BOP).
Pyridopyrazolo-quinazolines 128–132 were evaluated for antibacterial activity against Gram-positive and Gram-negative bacterial strains, compounds 129, 130a, 130c, 130f, 131a, 132c, 132f and 132h exhibited promising antibacterial activity against various bacterial strains. Compound 129 showed high antibacterial (MIC 3.9 μg/mL) and broad-spectrum anti-biofilm activity. Compounds 129 and 132b also showed good antifungal activity against various Candida strains. Compound 129 was found to be promising as a broad-spectrum biofilm inhibitor both against bacterial pathogens and against C. albicans MTCC 3017. Further, compound 129 reduced the ergosterol content in three Candida strains (C. albicans MTCC 227, C. albicans MTCC 1637 and C. albicans MTCC 3017). Both compound 129 and Miconazole showed the same amount of inhibition of ergosterol content in C. albicans MTCC 227, modelling studies were also performed for validation. Compounds 131d, 132a, 132b and 132d exhibited inhibition >90% against MCF7 (breast) cancer cell line; compounds 130d, 131a, 132a, 132b, 132f and 132g exhibited inhibition >90% against SKOV3 (ovarian) cancer cell line, Doxorubicin was used as a standard.
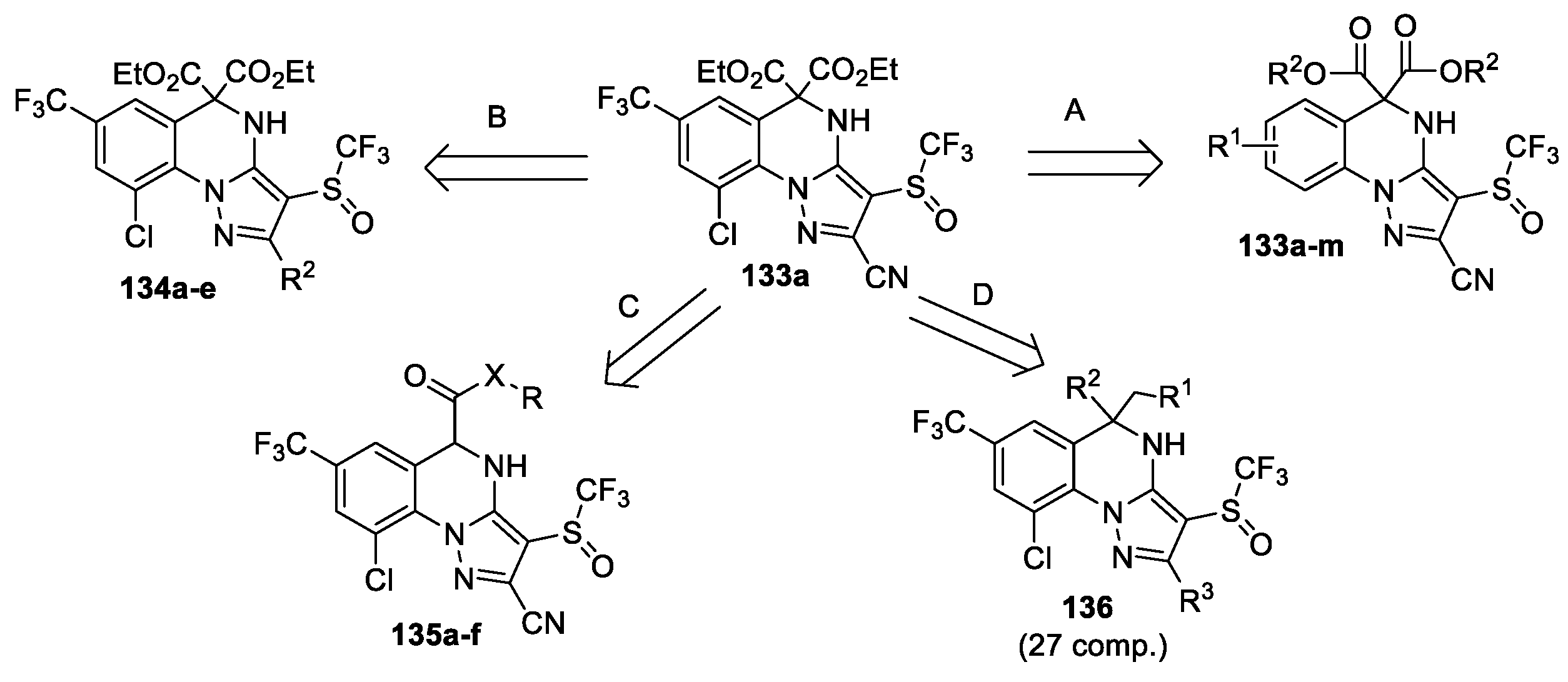
Synthesis and insecticidal activities of polysubstituted 4,5-dihydropyrazolo[1,5-
a]quinazolines
133–136 (
Scheme 30) were described [
69,
70,
71]. 5,5-Disubstituted 4,5-dihydropyrazolo[1,5-
a]quinazoline derivative
133a, obtained by interaction of 5-amino-1
H-phenylpyrazole with dialkyl bromomalonate, exhibited promising insecticidal activity against
P. xylostella [
69]. Later the same team [
70] performed some modifications of compound
133a: substituents in benzene ring (direction A), position 3 of the pyrazole ring (direction B), position 5 of the quinazoline ring (direction C), synthesized three series of novel 4,5-dihydropyrazolo[1,5-
a]quinazoline derivatives (
133–135). Afterwords, an expanded series of analogues
136 was obtained [
71].
Insecticidal activities of compounds 133–135 against insect pests P. xylostella, S. frugiperda and S. invicta were evaluated. The most compounds exhibited good (>50% mortality) to excellent (100% mortality) insecticidal effect against P. xylostella; 15 compounds from the series showed significant mortality rate >50% at 100 mg/L, and 9 compounds were able to show mortality rate >50% at 25 mg/L, the LC50 values were found to be 3.87−24.35 mg/L. Compound 133 (R1 = CF3, Cl, R2 = Me) exhibited the best insecticidal activity, its LC50 value against P. xylostella (3.87 mg/L) was comparable to that of indoxacarb (4.82 mg/L), which is one of the major commercial pesticides to control P. xylostella. Insecticidal activities of compounds 133a and 135 (X = NH, 2-Cl-5-Me-thiazole) against S. frugiperda (mortality rate 79.63% and 72.12%) were comparable to that of fipronil (mortality rate 68.44%). The compounds 133 (R1 = CF3, Cl, R2 = Me) and 135 (X = O, R = Me) showed high insecticidal activities against S. invicta (mortality rate 96.67% and 95.56%) comparable to that of fipronil (mortality rate 100%) after 5 days of treatment at 1.0 mg/L. Moreover, electrophysiological studies indicated that compound 136 (R1 = CO2Me, R2 = H, R3 = CN) could act as a potent GABA receptor antagonist (2 μΜ, inhibition 68.25%).
5. Imidazo[1,2-a]quinazolines, benzimidazo[1,2-a]quinazolines
Synthetic approaches to imidazoquinazolines, in which imidazole cycle is attached to
с,
b or
g edges of quinazoline core, as well as to benzo[
4,
5]imidazo[1,2-
c]- and benzo[
4,
5]imidazo[1,2-
a]quinazolines are described in book chapter [
12]. Two main approaches to [
a]-annelated derivatives are used: condensation of 2-aminobenzimidazole with various aromatic aldehydes bearing Hal or NO
2; and intramolecular C ̶ N bond formation. This section of the current manuscript contains data on the synthesis and biological activity of imidazo- and benzimidazo[
a]quinazolines, which were not included in the book chapter [
12] or appeared later.
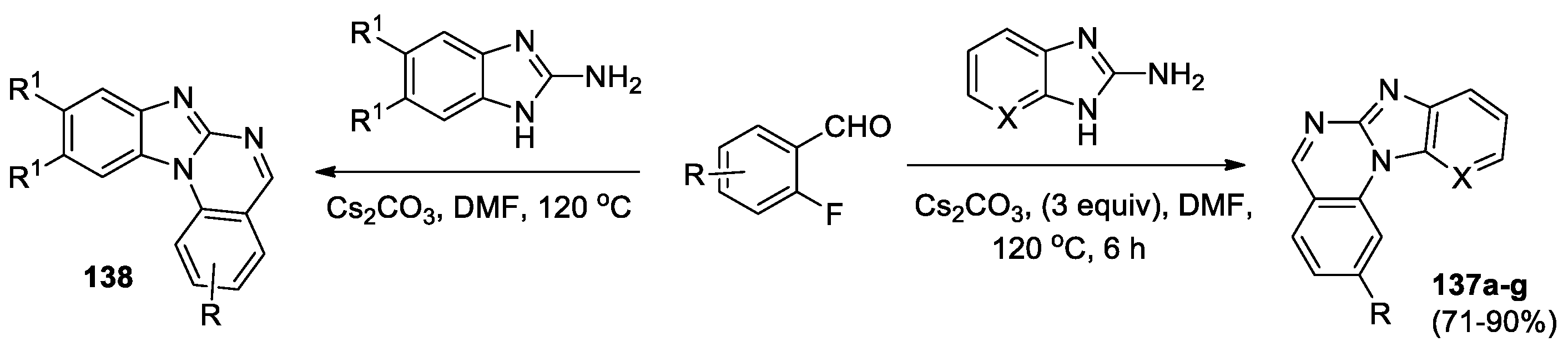
Annareddygari
et al [
52] developed an efficient method for the synthesis of benzo[
4,
5]imidazo[1,2-
a]quinazolines
137 from different 2-fluoro-benzaldehydes with 2-aminobenzimidazole (its 7-aza derivative) under metal-free conditions in high yields (
Scheme 31). The process includes an intermolecular condensation followed by metal-free base-promoted intramolecular C–N coupling reaction.
Using this approach, a series of trisubstituted benzo[
4,
5]imidazo[1,2-
a]quinazolines
138 was obtained to study their anti-fungal activities against six plant pathogenic fungi and analyze SAR [
72]. It was found that compound
138 (R = R
1 = H) demonstrated broad-spectrum antifungal activities with EC
50 = 4.43 μg/mL.
Microwave-assisted synthesis of 1,2,3,4-tetrahydrobenzo[
4,
5]imidazo[1,2-
a]quinazolines
140 was presented
[73]. The reaction of 2-bromocyclohex-1-ene carbaldehydes (
139a-c) and 2-aminobenzimidazoles in the presence of both base and magnesium sulfate under microwave irradiation led to the formation of derivatives
140 in 58–70% yields (
Scheme 32).
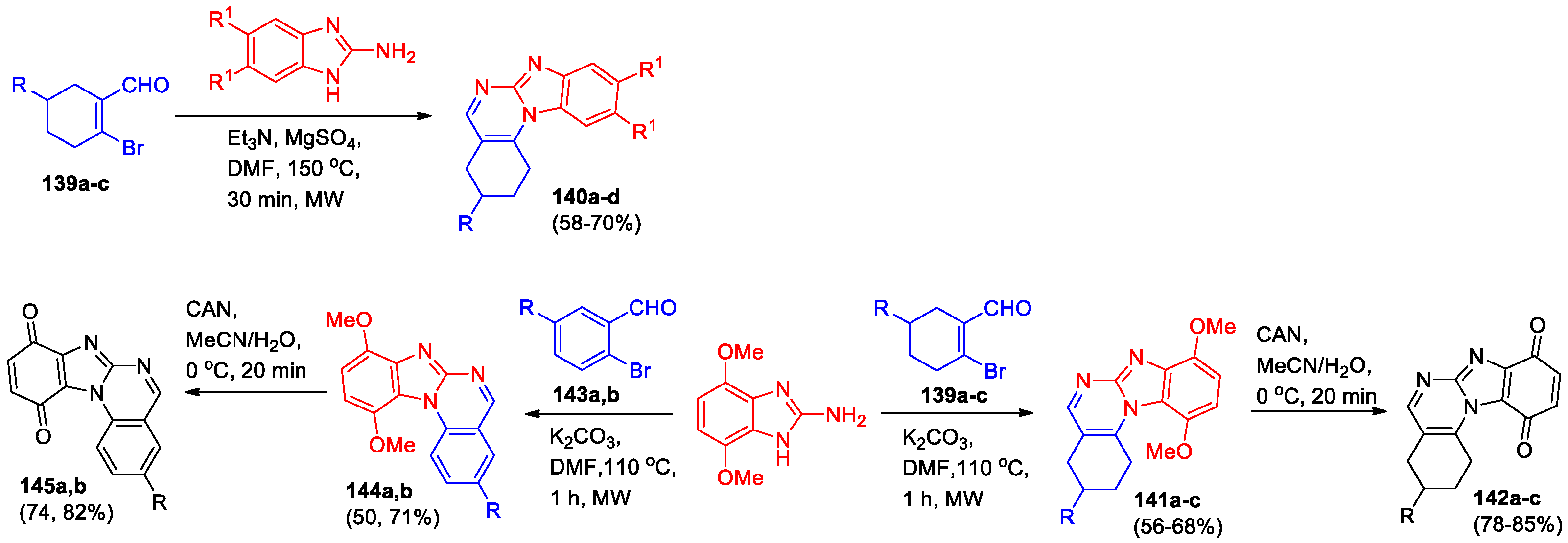
In the next work [
74] 2-bromocyclohex-1-enecarbaldehydes
139a-c were incorporated into the reaction with 4,7-dimethoxy-
1H-benzimidazole-2-amine under optimized conditions (microwave irradiation) for the obtaining of tetrahydrobenzo[
4,
5]imidazo[1,2-
a]quinazolines
141 and subsequent oxidation (
Scheme 32). It was demonstrated that 4,7-dimethoxy-1
H-benzimidazole-2-amine also interacts effectively with 2-bromobenzaldehydes
143 with the formation of benzimidazo[1,2-
a]quinazolines
144. Oxidation of quinazoline-fused dimethoxybenzimidazoles
141 and
144 was conducted using ceric ammonium nitrate (СAN) in the MeCN/H
2O medium. The corresponding benzo[
4,
5]imidazo[1,2-
a]quinazoline-8,11-diones
142 and
145 were obtained in good yields.
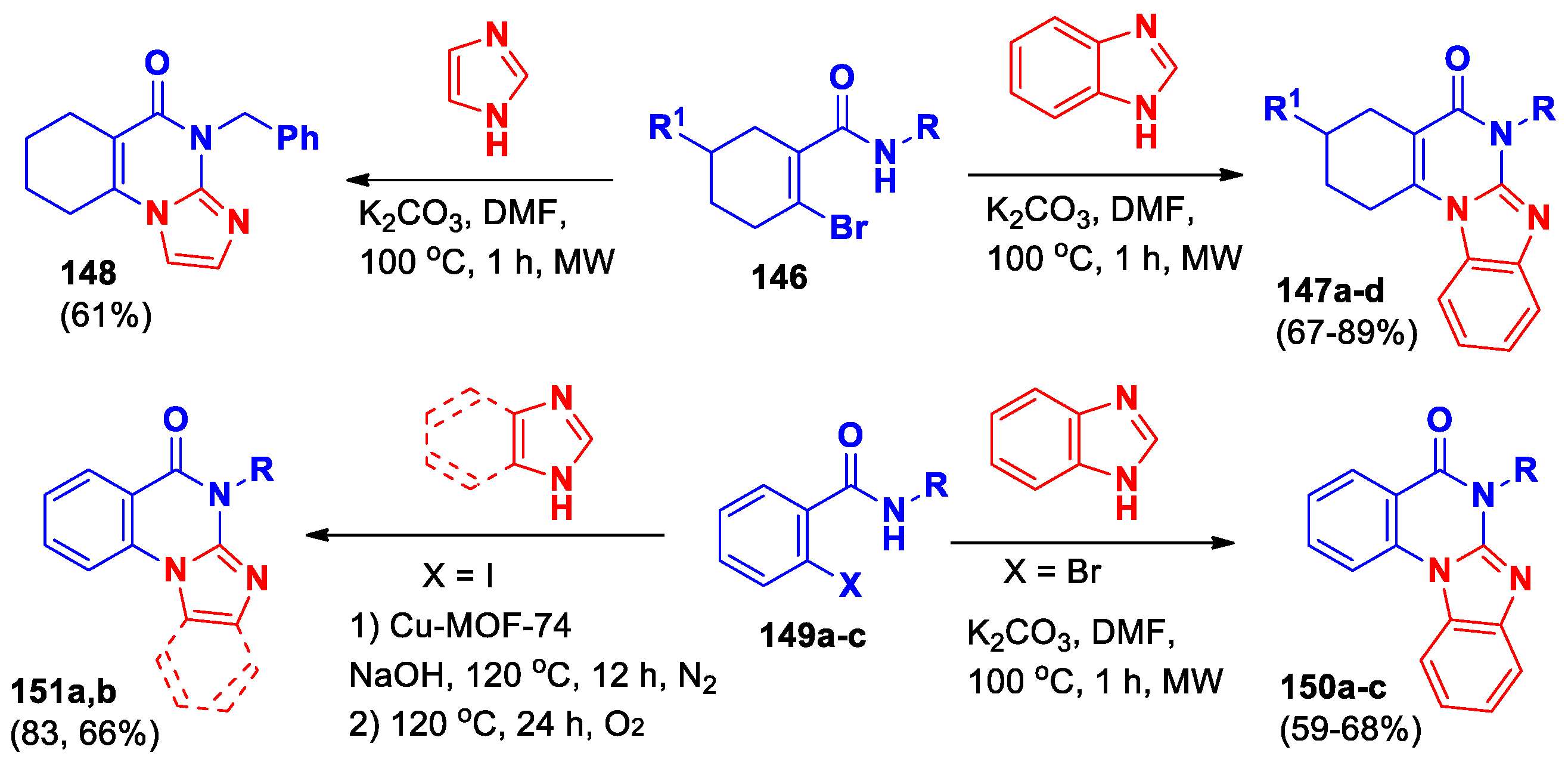
Dao
et al [
75] reported the synthesis of 1,2,3,4-tetrahydrobenzo[
4,
5]imidazo[1,2-
a]quinazolin-5-ones
147 from ꞵ-bromo-α,ꞵ-unsaturated amides and benzimidazole
via copper powder catalyzed C
–N coupling and subsequent C
–N bond formative cyclization by C
–H activation under microwave irradiation conditions (
Scheme 33). Interaction of amide
146 with imidazole under the same conditions led to the formation of 1,2,3,4-tetrahydroimidazo[1,2-
a]quinazolin-5-one
148. It was also demonstrated that benzamides
149 under similar conditions allowed to obtain benzimidazo[1,2-
a]quinazolin-5-ones
150a-c (
Scheme 33). This reaction provides the first known way to make these unique hybrid nitrogen-containing structures using common starting materials.
Heterogeneous amorphous Cu
–MOF-74 catalyst was developed for C–N сoupling reaction and applied for the synthesis of benzimidazo/imidazo[1,2-
a]quinazolin-5-ones
151a,b [
76] (
Scheme 33). Ma
et al demonstrated that crystalline Cu–MOF-74 can be used as a catalyst precursor to synthesize aCu-MOF-74 under the action of alkali and high temperature in the reaction solution, and then it acts as a true heterogeneous catalyst to catalyze С–N coupling between 2-iodobenzamides
149 and benzimidazole/imidazole, which gives higher isolated yields. The catalyst can be simply removed after the reaction and reused up to six times without losing much effectiveness.
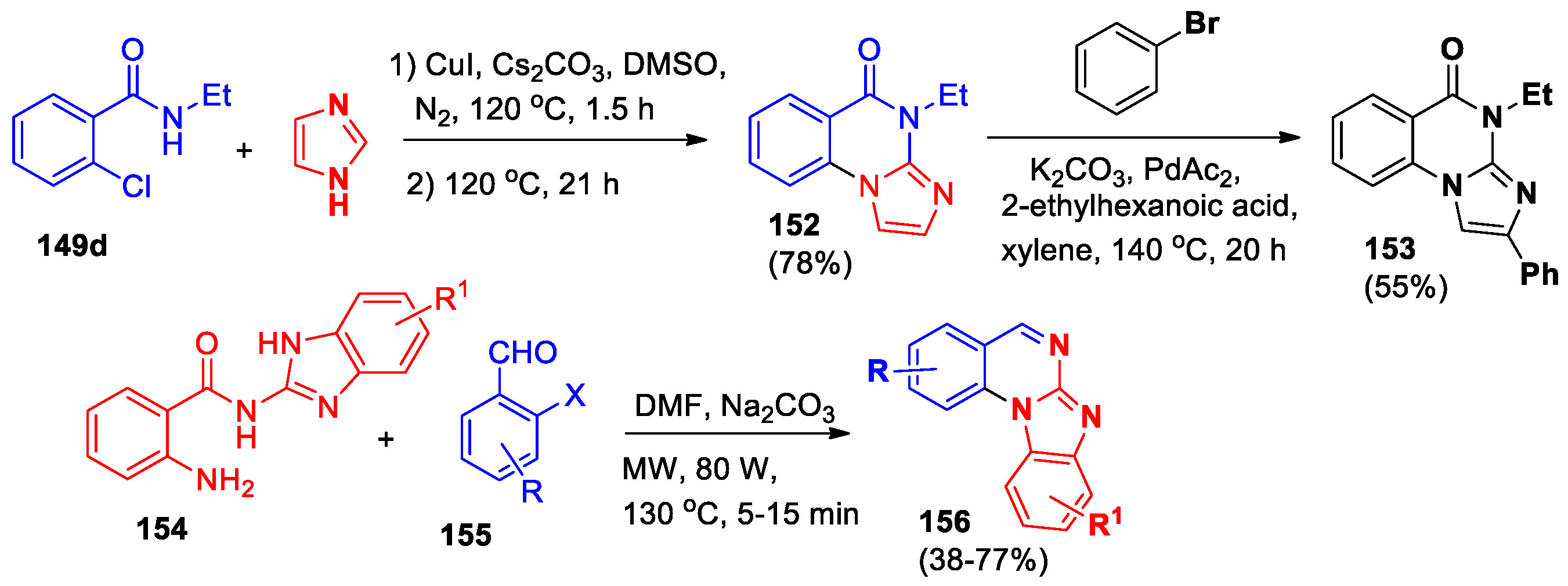
An approach based on the Ullman coupling of 2-chlorobenzamide
149d with imidazole followed by oxidative ring formation was used for the synthesis of imidazo[1,2-
a]quinazolin-5-one
152 [
77] (
Scheme 34). When 4-phenylimidazole was applied, the yield of compound
153 was only 23%, while functionalization of derivative
152 by palladium-catalyzed direct C–H arylation with bromobenzene led to the formation of 2-phenyl-4-ethyl-imidazo[1,2-
a]quinazolin-5-one
153 in 55% yield.
Rapid and efficient microwave-assisted metal-free base-mediated synthetic approach to the series of benzimidazo[1,2-
a]quinazolines
156 (15 compounds) (
Scheme 34) from readily available building blocks was developed [
12,
78]. In the presence of groups X = NO
2 or R = 5-Br in the aldehyde
155, the yields of compounds
156 decreased to 38% and 44%, respectively. This method uses easy-to-get starting materials, works with many different substrates, is simple to carry out, and allows making various benzimidazo[1,2-
a]quinazoline compounds without needing metals.
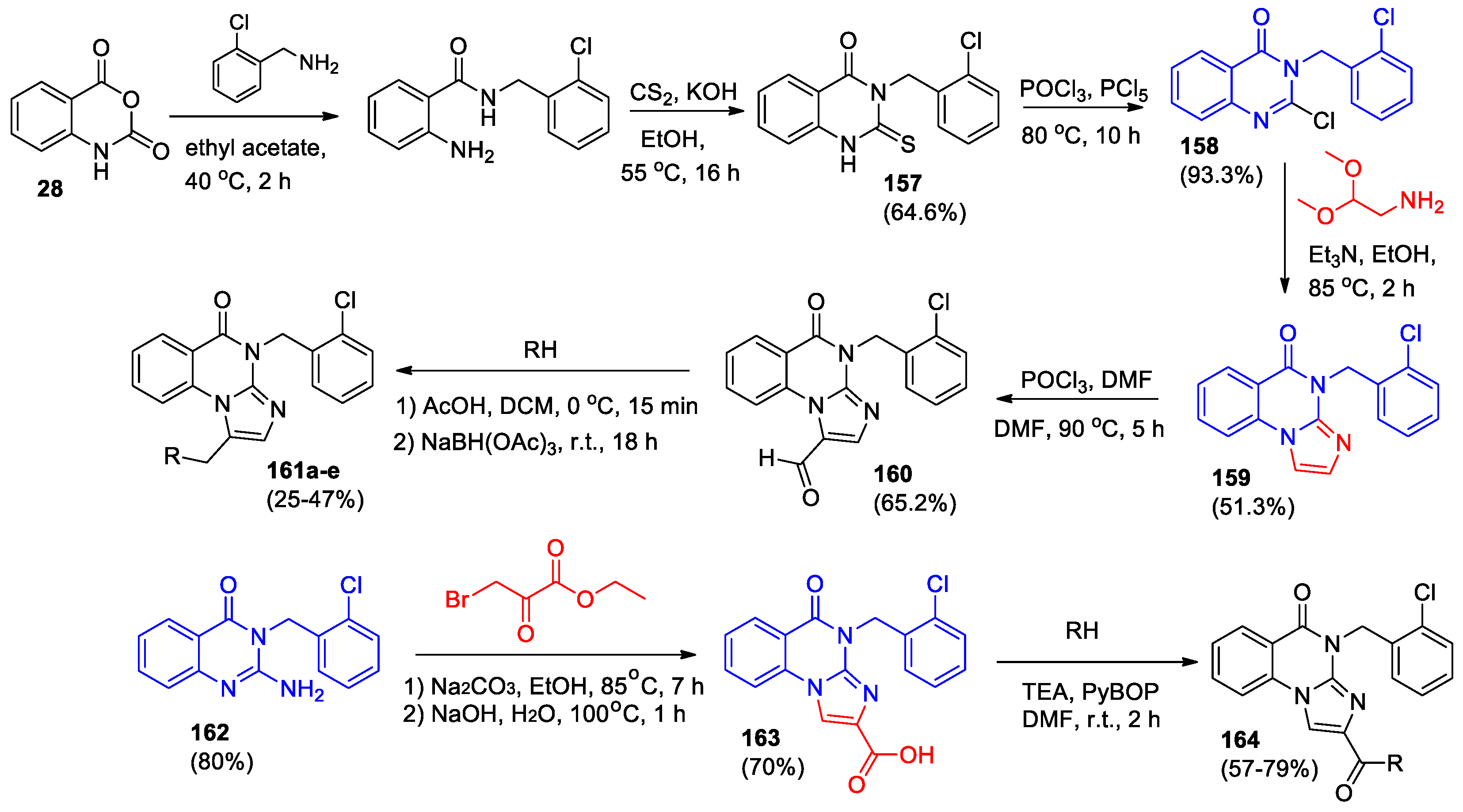
One more strategy for the synthesis of imidazole[1,2-
a]quinazolin-5(4
H)-ones was described [
79]. Interaction of isatoic anhydride
28 with
o-chlorobenzyl amine gave 2-amino-N-(2-chlorobenzyl)benzamide, which under the reaction with CS
2 in the alkali medium was converted into 3-(2-chlorobenzyl)-2-thioxo-2,3-dihydroquinazolin-4(1
H)-one
157 (
Scheme 35). Heating this compound with a mixture of POCl
3 and PCl
5 yielded the corresponding 2-chloro derivative
158, which upon reaction with 2,2-dimethoxyethylamine in the presence of NEt
3 afforded 4-(2-chlorobenzyl)imidazo[1,2-
a]quinazolin-5(
4H)-one
159. The structure of compound
159 was confirmed by various spectroscopic methods including X-ray crystallography.
In the next work [
80] 4-(2-chlorobenzyl)imidazolo[1,2-
a]quinazolin-5(4
H)-one
159 was functionalized at imidazole cycle by the Vilsmeier-Hacck reaction with DMF and POCl
3 and then reductive amination with a secondary amine reagent to obtain compounds
161a-e (
Scheme 35). Second series of 4-(2-chlorobenzyl)imidazole[1,2-
a]quinazolin-5(4
H)-ones (
164, 27 compounds) was synthesized based on 2-amino-3-(2-chlorobenzyl)quinazolin-4(3
H)-one
162, obtained from chloro derivative
158 in the reaction with
p-methoxy-benzylamine. Removing the benzyl group, cyclizing
162 with ethyl bromopyruvate and condensing with amine compounds led to the formation of compounds
164 in good yields (
Scheme 35).
Imidazoquinazolinone derivatives
161 and
164 were studied as allosteric inhibitors of SHP2 phosphatase,
in vitro enzymatic assays were conducted. The inhibitory activities of
161 and
164 against SHP2 protease at 100 μM and 200 μM were evaluated; results demonstrated that most of the compounds have certain inhibitory activity against SHP2 protease. Imidazoquinazolinones
161a (22.14% inhibition) and
164 (R = 4-HO
-piperidinyl) (29.81% inhibition) showed better inhibition of
SHP2 protein activity than
SHP244 as a positive control, (14.95% inhibition) at 100 mM. The
in vitro cytotoxicity of the compounds
161,
164 100 μM on the melanoma cell line A375 was evaluated, according to results, compared with
SHP244 and
sorafenib (inhibition 13.81% and 14.79%), most of the compounds showed significant cytotoxicity to A375. Among them
161a (76.15% inhibition) and
164 (R =
m-CF
3-aniline) (27.93% inhibition) showed effective activity. The IC
50 values were also measured for the series of compounds and generally it was shown that compared with
SHP244, the tumor cell activity of
161 and
164 compounds is significantly better than enzyme activity. The docking studies revealed that C=O groups of imidazoquinazolinones form hydrogen bond interactions with Lys274 and His84, respectively (
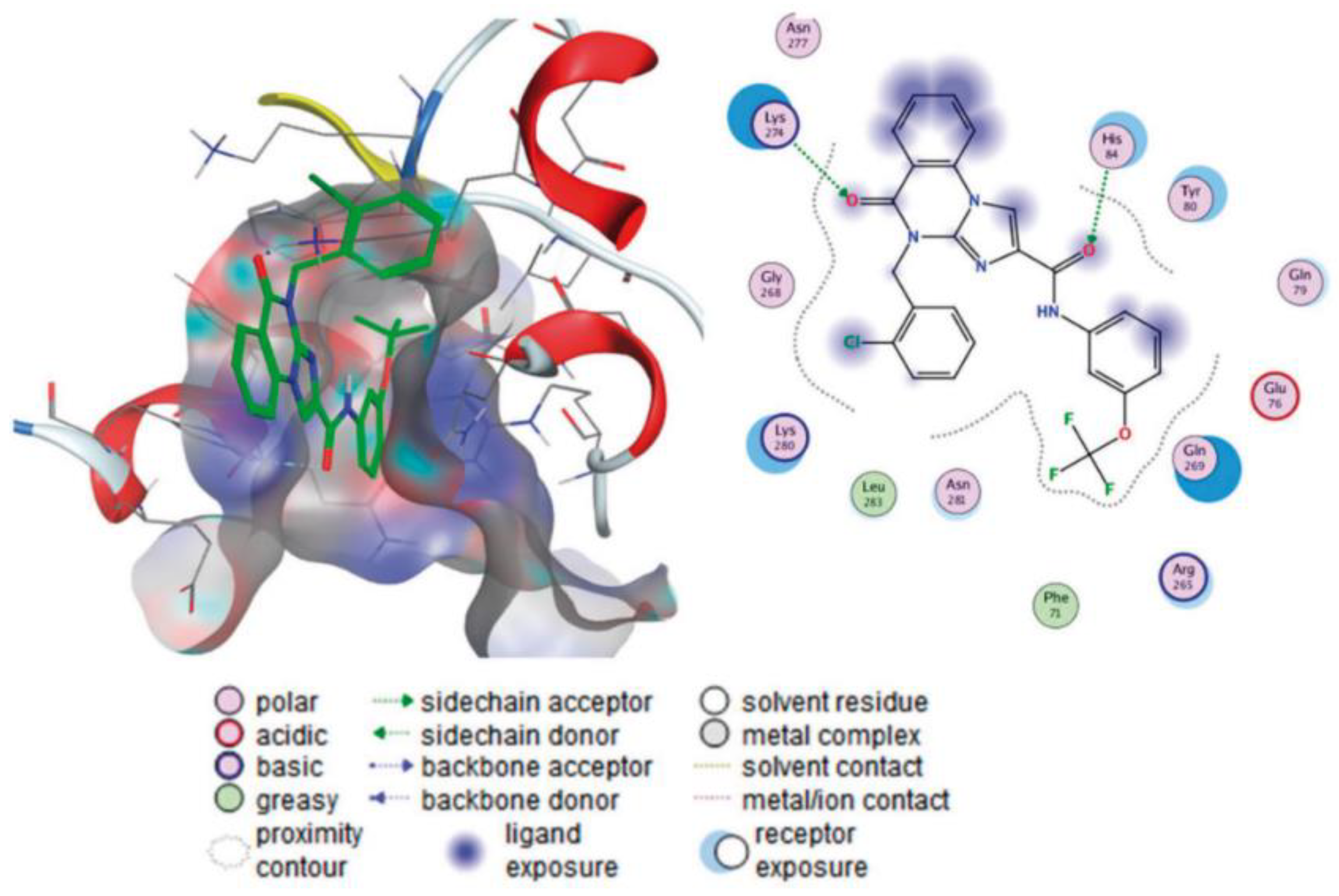
Figure 8).
Ghashghaei
et al [
81] presented selective multiple multicomponent Groebke–Blackburn–Bienaymé reaction (GBBR), which allows to obtain imidazoazines by acid-catalyzed interaction of α-aminoazines, aldehydes and isocyanides. Thus, diaminoquinazoline
165 underwent a regioselective GBBR providing mono-adduct
166 at components ratio 1:1:1 (
Scheme 36). In a different reaction, substrate
165 afforded the symmetrical bis-adduct
167 (components ratio 1:2:2). Moreover, a second GBBR, performed upon
166, gave the non-symmetrical compound
168 (components ratio 1:1:1). This method can be widely applied and create complicated molecules selectively, adjustably, and directly.
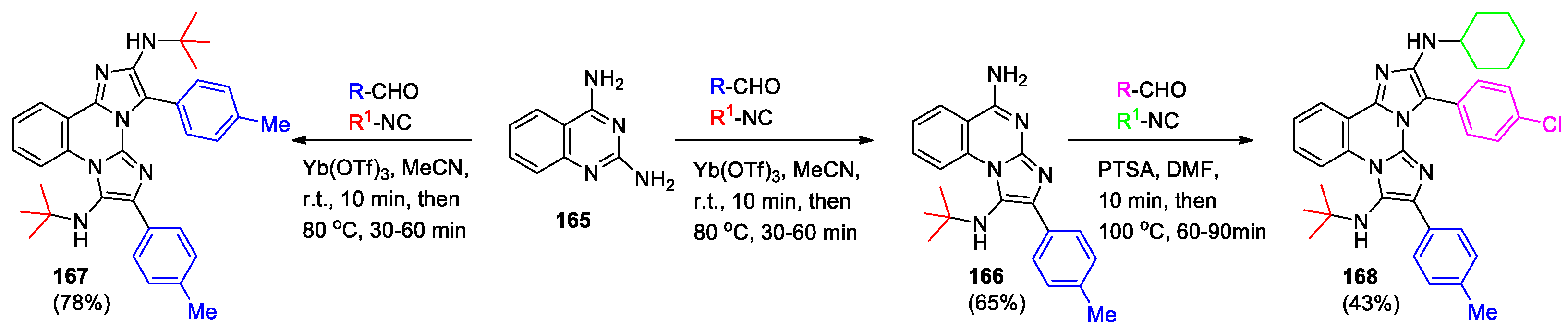
Annareddygari
et al [
52] developed the general approach to azoloquinazolines. Thus, the reaction of 2-aminobenzothiazole with 2-fluorobenzaldehyde led to the formation of benzo[
4,
5]thiazolo[3,2-
a]quinazoline (
169) in 73% yield under the optimized conditions (
Scheme 37).
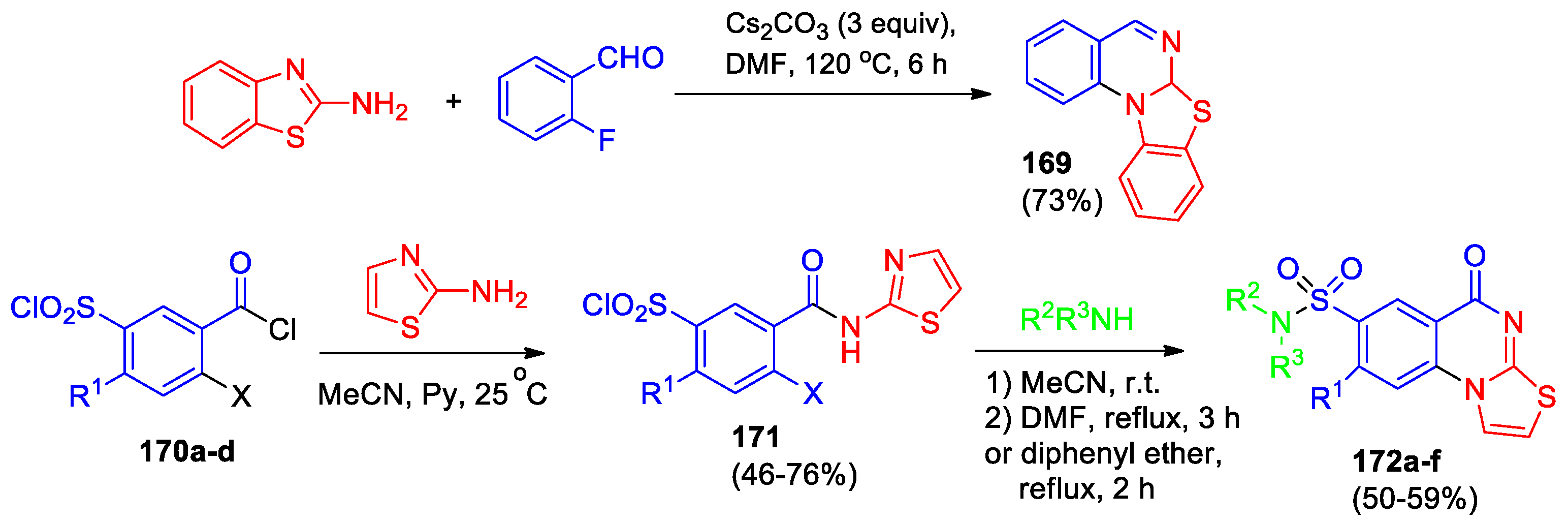
The series of thiazolo[3,2-
a]quinazolin-5-one derivatives
172a-f containing a sulfonamide group on C7 was described [
82]. Interaction of chlorosulfonyl-substituted benzoyl halogenides
170a-d with aminothiazoles led to the formation of amides
171 (
Scheme 37). The treatment them with amine under mild conditions and subsequent cyclization under reflux in DMF or diphenyl ether allowed to obtain thiazolo[3,2-
a]quinazolin-5-ones
172a-f. The influence of substituent R
1 and the nature of halogen on the yield of target products has been analyzed.
170, 171: X, R1 = F, H (a); Cl, H (b); Cl, Cl (c); Br, H (d); 172: R1 = H (a-c), Cl (d-f); R2R3N = morpholin-4-yl (a, d); R2 = H, R3 = Ph (b, e); R2R3N = piperidin-4-yl (c, f).
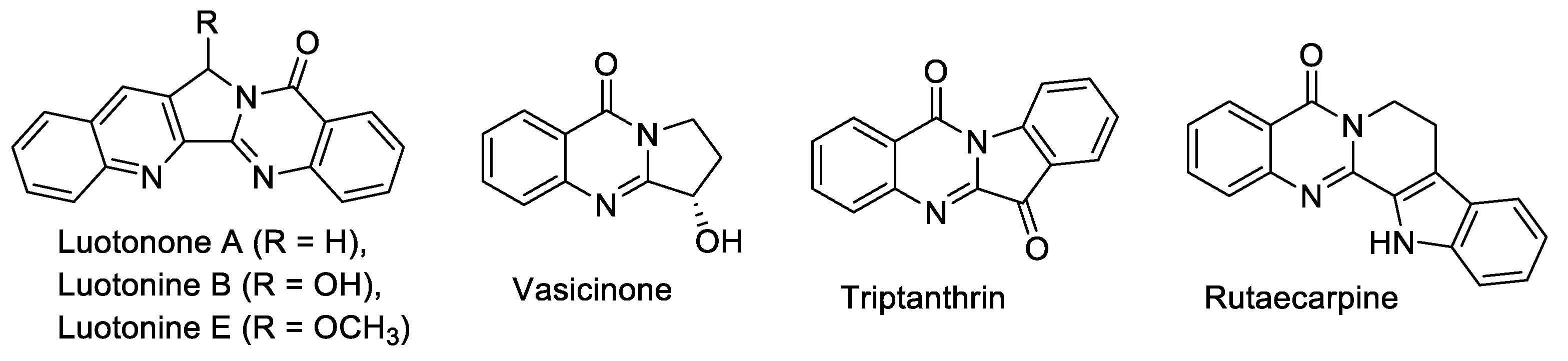
Figure 1.
Polycyclic quinazolines of nature origin, which can be regarded as prototypes of biologically important [a]-annelated quinazolines.
Figure 1.
Polycyclic quinazolines of nature origin, which can be regarded as prototypes of biologically important [a]-annelated quinazolines.
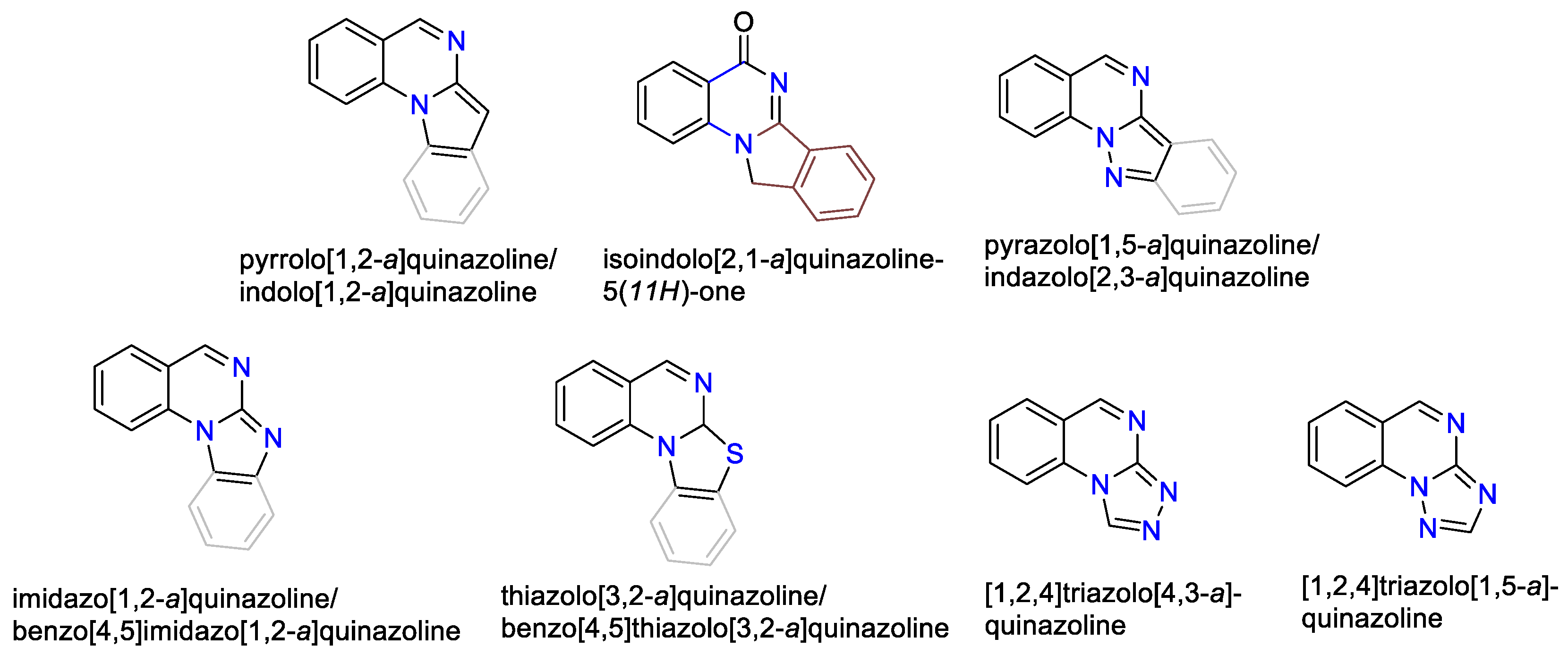
Figure 2.
Types of polycyclic quinazolines considered in the current review.
Figure 2.
Types of polycyclic quinazolines considered in the current review.
Scheme 1.
Synthetic approach to [a]- or [b]-annelated quinazolinones 2 and 3.
Scheme 1.
Synthetic approach to [a]- or [b]-annelated quinazolinones 2 and 3.
Scheme 2.
Synthesis of angular luotonins 5a-l. 1, 4, 5: R = H (a), 4-OMe (b), 4-Cl (c), 3-OMe (d), 3,4,5-(OMe)3 (e), 4-Br (f), 4-F (g), 4-CF3 (h), 3-Br (i), 4-SMe (j), 2-Me (k), 4-Me (l).
Scheme 2.
Synthesis of angular luotonins 5a-l. 1, 4, 5: R = H (a), 4-OMe (b), 4-Cl (c), 3-OMe (d), 3,4,5-(OMe)3 (e), 4-Br (f), 4-F (g), 4-CF3 (h), 3-Br (i), 4-SMe (j), 2-Me (k), 4-Me (l).
Scheme 3.
Synthesis of 2,3-dihydropyrrolo[1,2-a]quinazolin-5(1H)-ones 7, 8, 9. 7: R = H (a), 5-NO2 (b), 5-F (c), 6-Me (d), 6-OMe (e), 6-Cl (f), 6-NO2 (g), 6,7-(OMe)2 (h), 7-Cl (i), 8-Me (j), 8-Br (k), 8-F (l).
Scheme 3.
Synthesis of 2,3-dihydropyrrolo[1,2-a]quinazolin-5(1H)-ones 7, 8, 9. 7: R = H (a), 5-NO2 (b), 5-F (c), 6-Me (d), 6-OMe (e), 6-Cl (f), 6-NO2 (g), 6,7-(OMe)2 (h), 7-Cl (i), 8-Me (j), 8-Br (k), 8-F (l).
Scheme 4.
Synthetic approach to pyrroloquinazolinones 10, 12, 13. R = H (a), 5-F (b), 6-Cl (c), 7-Cl (d), 8-Me (e), 6-OMe (f), 6-NO2 (g), 6,7-(OMe)2 (h).
Scheme 4.
Synthetic approach to pyrroloquinazolinones 10, 12, 13. R = H (a), 5-F (b), 6-Cl (c), 7-Cl (d), 8-Me (e), 6-OMe (f), 6-NO2 (g), 6,7-(OMe)2 (h).
Scheme 5.
Synthesis of tetrahydropyrrolo[1,2-a]quinazolin-5(1H)-ones 16aa-ja.
Scheme 5.
Synthesis of tetrahydropyrrolo[1,2-a]quinazolin-5(1H)-ones 16aa-ja.
Scheme 6.
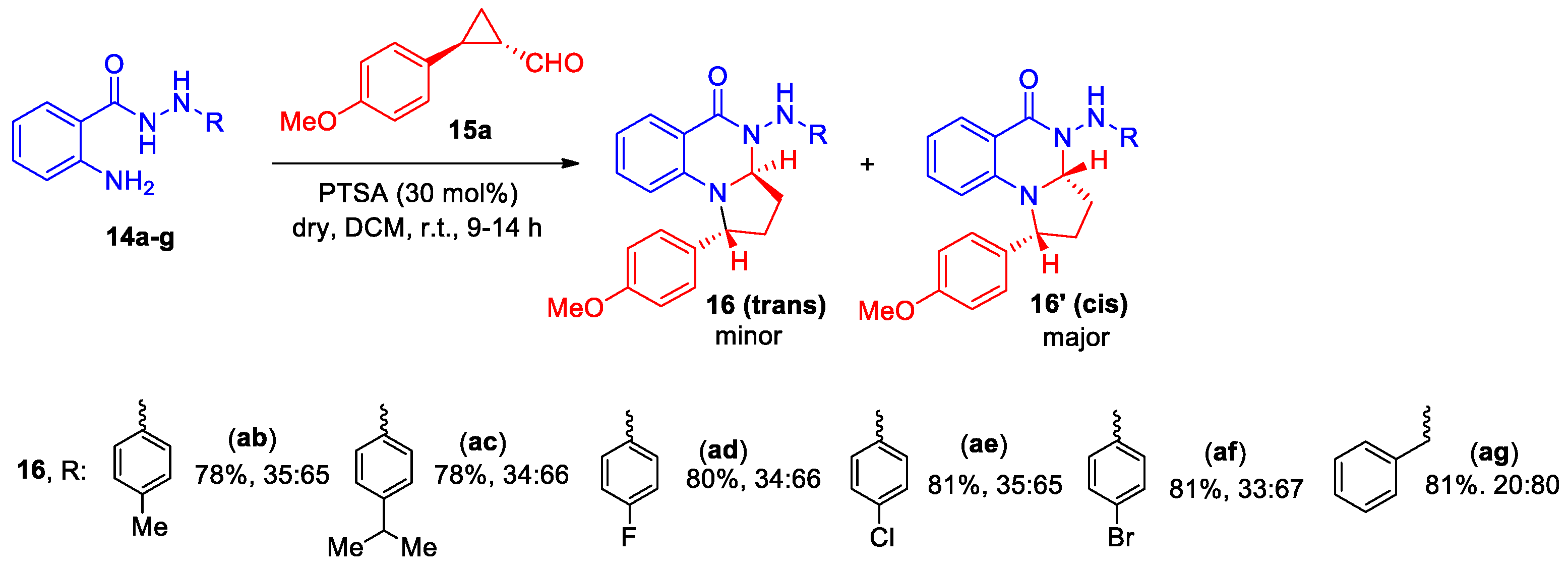
Synthesis of tetrahydropyrrolo[1,2-a]quinazolin-5(1H)-ones 16ab-ag.
Scheme 6.
Synthesis of tetrahydropyrrolo[1,2-a]quinazolin-5(1H)-ones 16ab-ag.
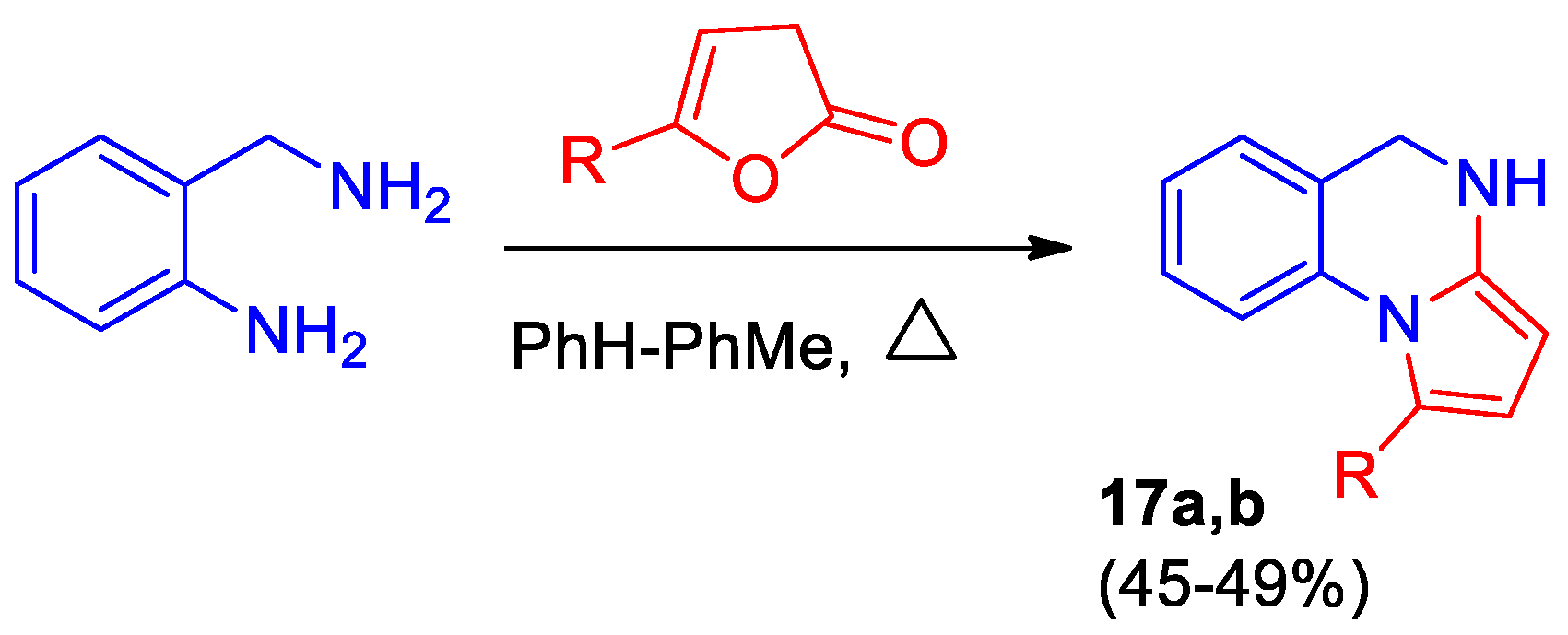
Scheme 7.
This is a figure. Schemes follow the same formatting. Synthesis of 1-R-4,5-dihydropyrrolo[1,2-a]quinazolines 19. R = Ph (a), 4-MeC6H4 (b).
Scheme 7.
This is a figure. Schemes follow the same formatting. Synthesis of 1-R-4,5-dihydropyrrolo[1,2-a]quinazolines 19. R = Ph (a), 4-MeC6H4 (b).
Scheme 8.
Synthetic approach to indolo[1,2-a]quinazolinone derivatives 20. R = H, Cl, (MeO)2; R1 = Me, Et, iso-Pr, Bn, Ph, Ar; R2 = H, OMe, OBn, Cl, Br.
Scheme 8.
Synthetic approach to indolo[1,2-a]quinazolinone derivatives 20. R = H, Cl, (MeO)2; R1 = Me, Et, iso-Pr, Bn, Ph, Ar; R2 = H, OMe, OBn, Cl, Br.
Scheme 9.
Synthesis of indolo[1,2-a]quinazolinones 23 and 25. 23: R = H, MeO, Cl, Br; R1 = H, Me, Bn, Ph; R2 = H, Me, Cl; 25: R = H, Me, Et, Bn, Ar; R1 = H, Me; R2 = H, Me, Et, MeO, Cl, Br.
Scheme 9.
Synthesis of indolo[1,2-a]quinazolinones 23 and 25. 23: R = H, MeO, Cl, Br; R1 = H, Me, Bn, Ph; R2 = H, Me, Cl; 25: R = H, Me, Et, Bn, Ar; R1 = H, Me; R2 = H, Me, Et, MeO, Cl, Br.
Scheme 10.
Synthesis of 5-amine-indolo[1,2-a]quinazolines 27. R = H, Me, F, CF3; R1 = H, F, MeO; EWG = CN, COOEt.
Scheme 10.
Synthesis of 5-amine-indolo[1,2-a]quinazolines 27. R = H, Me, F, CF3; R1 = H, F, MeO; EWG = CN, COOEt.
Scheme 11.
Synthetic approach to 6,6a-dihydroisoindolo[2,1-a]quinazoline-5,11-dione derivatives 29, 31. 29: R = H, Me, Et, n-Pr, (CH2)2-OH, (CH2)3-OH, (CH2)3-COOH; 31: Ar = Ph, 2-Me-C6H4, 4-F-C6H4, 2-Cl-C6H4, 2-Br-C6H4, 2-NO2-C6H4, 2,3-Cl2-C6H3, 3,4-Cl2-C6H3.
Scheme 11.
Synthetic approach to 6,6a-dihydroisoindolo[2,1-a]quinazoline-5,11-dione derivatives 29, 31. 29: R = H, Me, Et, n-Pr, (CH2)2-OH, (CH2)3-OH, (CH2)3-COOH; 31: Ar = Ph, 2-Me-C6H4, 4-F-C6H4, 2-Cl-C6H4, 2-Br-C6H4, 2-NO2-C6H4, 2,3-Cl2-C6H3, 3,4-Cl2-C6H3.
Scheme 12.
Synthetic approach to isoinodolo[2,1-a]quinazolino[1,2-c]quinazolineones 33. R = (CH2)2Ph, cyclopentyl, cyclopropyl, allyl, hexyl, butyl, propyl, ethyl.
Scheme 12.
Synthetic approach to isoinodolo[2,1-a]quinazolino[1,2-c]quinazolineones 33. R = (CH2)2Ph, cyclopentyl, cyclopropyl, allyl, hexyl, butyl, propyl, ethyl.
Scheme 13.
Synthesis of dihydroisoindolo[2,1-a]quinazoline-5,11-dione derivatives 34, 35, 36. 34: R = Me, Bu, prop-2-yne, cyclopropyl, (CH2)2Ph, 1-Nf, Bn-4-Cl, Bn-4-OMe, Bn-2,4-Cl2, 4-Me-C6H4, 4-CN-C6H4, 2-MeO-C6H4, 2,3-(Me)2-C6H3, 2-Cl,3-Me-C6H3, 4-COOMe-C6H4, 4-t-Bu-C6H4; 35: R = propen-2-yl-methyl, Bn, Bn-2-Cl, Bn-4-Cl, furan-2-yl-methyl, Py-2-yl-methyl; X = H, 2,3-(MeO)2; 36: R = propen-2-yl, n-Pr, n-Bu, cyclopropyl, Bn, Bn-4-Me, Bn-2-OMe, Ph, 4-Me-C6H4, 4-MeO-C6H4.
Scheme 13.
Synthesis of dihydroisoindolo[2,1-a]quinazoline-5,11-dione derivatives 34, 35, 36. 34: R = Me, Bu, prop-2-yne, cyclopropyl, (CH2)2Ph, 1-Nf, Bn-4-Cl, Bn-4-OMe, Bn-2,4-Cl2, 4-Me-C6H4, 4-CN-C6H4, 2-MeO-C6H4, 2,3-(Me)2-C6H3, 2-Cl,3-Me-C6H3, 4-COOMe-C6H4, 4-t-Bu-C6H4; 35: R = propen-2-yl-methyl, Bn, Bn-2-Cl, Bn-4-Cl, furan-2-yl-methyl, Py-2-yl-methyl; X = H, 2,3-(MeO)2; 36: R = propen-2-yl, n-Pr, n-Bu, cyclopropyl, Bn, Bn-4-Me, Bn-2-OMe, Ph, 4-Me-C6H4, 4-MeO-C6H4.
Scheme 14.
Synthetic approach to dihydroisoindolo[2,1-a]quinazolin-5,11-diones 38, 39. 37: R = H, Alk, Bn, Ar; 38: R = H (a), Bn (b), Me (c), Ph (d), 4-Me-C6H4 (e), 3-Me-C6H4 (f), 4-MeO-C6H4 (g), 4-ClC6H4 (h), 4-BrC6H4 (i), 4-NO2C6H4 (k), 3-NO2C6H4 (l), 2-Nf (m), cyclohexyl (n), Bu (o); 39: R2 = R1 = H (a), R2 = R1 = OMe (b).
Scheme 14.
Synthetic approach to dihydroisoindolo[2,1-a]quinazolin-5,11-diones 38, 39. 37: R = H, Alk, Bn, Ar; 38: R = H (a), Bn (b), Me (c), Ph (d), 4-Me-C6H4 (e), 3-Me-C6H4 (f), 4-MeO-C6H4 (g), 4-ClC6H4 (h), 4-BrC6H4 (i), 4-NO2C6H4 (k), 3-NO2C6H4 (l), 2-Nf (m), cyclohexyl (n), Bu (o); 39: R2 = R1 = H (a), R2 = R1 = OMe (b).
Scheme 15.
Synthetic approach to dihydroisoindolo[2,1-a]quinazoline-5,11-diones 41, 43. 41: R, R1 = Me, 5-Br (a); H, 5-Me (b); Me, H (c); Cl, 5-Br (d); H, H (e); H, 5-Cl (f); Me, 4-Cl (g); 43: R = NHAr, CH2Ar, allyl, cyclopropyl, etc.; R1 = H, Br, Cl.
Scheme 15.
Synthetic approach to dihydroisoindolo[2,1-a]quinazoline-5,11-diones 41, 43. 41: R, R1 = Me, 5-Br (a); H, 5-Me (b); Me, H (c); Cl, 5-Br (d); H, H (e); H, 5-Cl (f); Me, 4-Cl (g); 43: R = NHAr, CH2Ar, allyl, cyclopropyl, etc.; R1 = H, Br, Cl.
Scheme 16.
Synthesis of dihydroisoindolo[2,1-a]quinazoline-5,11-dione derivatives 45. 42, 45: R = H, Bn, NHPh; R1 = Me, MeO, Cl; 44, 45: R2 = Me, OMe, F, Cl, CF3.
Scheme 16.
Synthesis of dihydroisoindolo[2,1-a]quinazoline-5,11-dione derivatives 45. 42, 45: R = H, Bn, NHPh; R1 = Me, MeO, Cl; 44, 45: R2 = Me, OMe, F, Cl, CF3.
Scheme 17.
Synthetic approach to dihydroisoindolo[2,1-a]quinazoline-5,11-diones 49a,b. 46: X = H, R = H (a), OMe (b); 47: X = OH, R = H (a), OMe (b); 49: R = H (a), OMe (b).
Scheme 17.
Synthetic approach to dihydroisoindolo[2,1-a]quinazoline-5,11-diones 49a,b. 46: X = H, R = H (a), OMe (b); 47: X = OH, R = H (a), OMe (b); 49: R = H (a), OMe (b).
Scheme 18.
Synthetic approach to dihydroisoindolo[2,1-a]quinazolin-5,11-diones 50a,b. 48, 50: R = H (a), OMe (b).
Scheme 18.
Synthetic approach to dihydroisoindolo[2,1-a]quinazolin-5,11-diones 50a,b. 48, 50: R = H (a), OMe (b).
Scheme 19.
Synthesis of isoindolo[2,1-a]quinazolin-5(11H)-ones 52a-d. 51, 52: R1, R2 = H, H (a); 3-NO2, H (b); 3-Br, H (c); Br, Br (d).
Scheme 19.
Synthesis of isoindolo[2,1-a]quinazolin-5(11H)-ones 52a-d. 51, 52: R1, R2 = H, H (a); 3-NO2, H (b); 3-Br, H (c); Br, Br (d).
Scheme 20.
Synthetic approach to pyrazolo[1,5-a]quinazolines 54 and 56. 54: R1 = H, Cl, OMe, NO2; R2 = H, Me, CN, Ph, cyclopropyl, 2-thienyl; 56: R = H, NO2; R1 = H, Me, COOEt, cyclopropyl, 4-MeC6H4, 2-thienyl; R2 = H, CN.
Scheme 20.
Synthetic approach to pyrazolo[1,5-a]quinazolines 54 and 56. 54: R1 = H, Cl, OMe, NO2; R2 = H, Me, CN, Ph, cyclopropyl, 2-thienyl; 56: R = H, NO2; R1 = H, Me, COOEt, cyclopropyl, 4-MeC6H4, 2-thienyl; R2 = H, CN.
Scheme 21.
Synthesis of pyrazolo[1,5-a]quinazolines 58, indazolo[2,3-a]quinazoline 59, 10-azaindazolo[2,3-a]quinazoline 60. 57, 58: R = H, Br, CF3.
Scheme 21.
Synthesis of pyrazolo[1,5-a]quinazolines 58, indazolo[2,3-a]quinazoline 59, 10-azaindazolo[2,3-a]quinazoline 60. 57, 58: R = H, Br, CF3.
Scheme 22.
Synthesis of indazolo[2,3-a]- and pyrazolo[1,5-a]quinazolines 62, 63. 62: R = H, F, OH, Cl, Br, Me, CN; R1 = H, Me, Et, n-Pr, CF3, OMe, COOEt, Ph, cyclohexyl-4-Pr; Ar = Ph, substituted Ar, naphthalene-2-yl, antracen-9-yl, hetaryl; 63: R2 = H, CONH2, CN, COOEt.
Scheme 22.
Synthesis of indazolo[2,3-a]- and pyrazolo[1,5-a]quinazolines 62, 63. 62: R = H, F, OH, Cl, Br, Me, CN; R1 = H, Me, Et, n-Pr, CF3, OMe, COOEt, Ph, cyclohexyl-4-Pr; Ar = Ph, substituted Ar, naphthalene-2-yl, antracen-9-yl, hetaryl; 63: R2 = H, CONH2, CN, COOEt.
Figure 3.
Chemical structures of the compounds A and 64a-t. 64a-l: X = N, R = 8-Cl (a), 7-NO2 (b), 8-CF3 (c), H (d), 8-Me (e); X = CH, R = H (f), 8-MeSO2 (g), 8-CF3 (h), 9-CF3 (i), 8-NO2 (j), 9-NH2 (k), 8-NH2 (l).
Figure 3.
Chemical structures of the compounds A and 64a-t. 64a-l: X = N, R = 8-Cl (a), 7-NO2 (b), 8-CF3 (c), H (d), 8-Me (e); X = CH, R = H (f), 8-MeSO2 (g), 8-CF3 (h), 9-CF3 (i), 8-NO2 (j), 9-NH2 (k), 8-NH2 (l).
Figure 4.
3D docking model of compound
64r in the active pocket of KDM4D.
Reproduced with permission of Elsevier [
54].
Figure 4.
3D docking model of compound
64r in the active pocket of KDM4D.
Reproduced with permission of Elsevier [
54].
Scheme 23.
Synthesis of pyrazolo[1,5-a]quinazoline derivatives 66–69. 65–69: R1 = H, Me; R2 = H, Me, Br; R3 = H, Me, OMe; R4 = H, Me; R5 = H, Me; 68, 69: n = 1, 2.
Scheme 23.
Synthesis of pyrazolo[1,5-a]quinazoline derivatives 66–69. 65–69: R1 = H, Me; R2 = H, Me, Br; R3 = H, Me, OMe; R4 = H, Me; R5 = H, Me; 68, 69: n = 1, 2.
Scheme 24.
Synthetic approach to pyrazolo[1,5-a]quinazolines 70, 72, 73-75, 76, 77, 78, 79. 70: R = OMe (a), H (b), O(CH2)2OMe (c); 72: R = OMe (a), H (b); 76: R = OMe (a), H (b); 77: R = OMe, R1 = CH2Ph (a), CH2-(2-OMe)Ph (b), CH2-thienyl (c), CH2-2-furyl (d), CHMe2 (e); 78: R = H, R1 = CH2Ph (a), CH2-(2-OMe)Ph (b), CH2-thienyl (c); 79: R1 = CH2Ph (a), CH2-(2-OMe)Ph (b), CH2-2-furyl (d).
Scheme 24.
Synthetic approach to pyrazolo[1,5-a]quinazolines 70, 72, 73-75, 76, 77, 78, 79. 70: R = OMe (a), H (b), O(CH2)2OMe (c); 72: R = OMe (a), H (b); 76: R = OMe (a), H (b); 77: R = OMe, R1 = CH2Ph (a), CH2-(2-OMe)Ph (b), CH2-thienyl (c), CH2-2-furyl (d), CHMe2 (e); 78: R = H, R1 = CH2Ph (a), CH2-(2-OMe)Ph (b), CH2-thienyl (c); 79: R1 = CH2Ph (a), CH2-(2-OMe)Ph (b), CH2-2-furyl (d).
Scheme 25.
Synthetic approach to pyrazolo[1,5-a]quinazolines 80, 82, 83, 87. 80: R = H, R1 = Ph (a), 2-thienyl (b), 3-thienyl (c); R = OMe, R1 = 2-thienyl (d), 3-thienyl (e), CO-(2-OMe)-Ph (f), 2-CO-2-thienyl (g), CO-3-thienyl (h); 82: R = H, Ar/Het = Ph (a), 2-thienyl (b), 3-thienyl (c); R = OMe, Ar/Het = 2-thienyl (d), 3-thienyl (e), 2-MeOPh (f), 3-furyl (g), 1-Boc-2-pyrrolyl (h), 1H-2-pyrrolyl (i); 83: R = OMe, Ar/Het = (2-MeO)Ph (a), 2-thienyl (b), 3-thienyl (c), (4-MeO)Ph (d), 2-furyl (e), 2(1H)-pyrrolyl (f), 2-(1-methyl)pyrrolyl (g); 86: X = Br (a), I (b); 87: Ar/Het = 2-MeOPh (a), 2-thienyl (b), 4-MeOPh (d), 2-furyl (e), 2(1H)-pyrrolyl (f), 2-(1-methyl)pyrrolyl (g).
Scheme 25.
Synthetic approach to pyrazolo[1,5-a]quinazolines 80, 82, 83, 87. 80: R = H, R1 = Ph (a), 2-thienyl (b), 3-thienyl (c); R = OMe, R1 = 2-thienyl (d), 3-thienyl (e), CO-(2-OMe)-Ph (f), 2-CO-2-thienyl (g), CO-3-thienyl (h); 82: R = H, Ar/Het = Ph (a), 2-thienyl (b), 3-thienyl (c); R = OMe, Ar/Het = 2-thienyl (d), 3-thienyl (e), 2-MeOPh (f), 3-furyl (g), 1-Boc-2-pyrrolyl (h), 1H-2-pyrrolyl (i); 83: R = OMe, Ar/Het = (2-MeO)Ph (a), 2-thienyl (b), 3-thienyl (c), (4-MeO)Ph (d), 2-furyl (e), 2(1H)-pyrrolyl (f), 2-(1-methyl)pyrrolyl (g); 86: X = Br (a), I (b); 87: Ar/Het = 2-MeOPh (a), 2-thienyl (b), 4-MeOPh (d), 2-furyl (e), 2(1H)-pyrrolyl (f), 2-(1-methyl)pyrrolyl (g).
Scheme 26.
Synthetic approach to pyrazolo[1,5-a]quinazolines 89–94, 96, 97. 89: R = CN (a), COOEt (b); 90: R, R1 = CN, CH2Ph (a), COOEt, Me (b); 91: R = CH2Ph (a), CH2(2-OMe-Ph) (b), CH2-2-furyl (c); 92: R = CH2Ph (a), CH2(2-OMe-Ph) (b), CH2-2-furyl (c), Et (d); 94: R = H (a), CH2Ph (b); 96: R = H (a), CH2Ph (b).
Scheme 26.
Synthetic approach to pyrazolo[1,5-a]quinazolines 89–94, 96, 97. 89: R = CN (a), COOEt (b); 90: R, R1 = CN, CH2Ph (a), COOEt, Me (b); 91: R = CH2Ph (a), CH2(2-OMe-Ph) (b), CH2-2-furyl (c); 92: R = CH2Ph (a), CH2(2-OMe-Ph) (b), CH2-2-furyl (c), Et (d); 94: R = H (a), CH2Ph (b); 96: R = H (a), CH2Ph (b).
Figure 5.
Chemical structures of the compounds 98, 99, 100-107. 98, 99: R = H (a), CHMe2 (b), cyclohexyl (c), CH2cyclohexyl (d), Ph (e), CH2Ph (f), CH2(2-OMe)Ph (g), CH2(2-furyl) (h), CH2(2-thienyl) (i); 100: R = CH2(2-OMe)Ph (a), CH2(2-thienyl) (b), t-Bu (c); 101: R = CH2(2-OMe)Ph (a), CH2(2-thienyl) (b), Et (c); 102: R = CH2(2-OMe)Ph (a), CH2(2-thienyl) (b), t-Bu (c); 103: R = CH2(2-OMe)Ph (a), CH2(2-thienyl) (b), Et (c); 104: R1 = H (a), Me (b); 106: R1 = H (a), Me (b).
Figure 5.
Chemical structures of the compounds 98, 99, 100-107. 98, 99: R = H (a), CHMe2 (b), cyclohexyl (c), CH2cyclohexyl (d), Ph (e), CH2Ph (f), CH2(2-OMe)Ph (g), CH2(2-furyl) (h), CH2(2-thienyl) (i); 100: R = CH2(2-OMe)Ph (a), CH2(2-thienyl) (b), t-Bu (c); 101: R = CH2(2-OMe)Ph (a), CH2(2-thienyl) (b), Et (c); 102: R = CH2(2-OMe)Ph (a), CH2(2-thienyl) (b), t-Bu (c); 103: R = CH2(2-OMe)Ph (a), CH2(2-thienyl) (b), Et (c); 104: R1 = H (a), Me (b); 106: R1 = H (a), Me (b).
Figure 6.
Chemical structures of the compounds 108–113. 108: R = Me (a), CH2cPr (b), propyne (c), CH2Ph (d), CH2(2-MePh) (e), CH2(2-MeOPh) (f), CH2(pyridin-4-yl) (g); 109: R7 = H, R8 = NO2 (a), R7 = NO2, R8 = H (b); 110: R7 = H, R8 = NO2 (a), R7=NO2, R8=H (b); 111: R7 = H, R8 = NO2, R = CH2(2-MeOPh) (a), R7 = H, R8 = NO2, R = CH2-thien-2-yl (b), R7 = NO2, R8 = H, R = CH2(2-MeOPh) (c), R7 = NO2, R8 = H, R = CH2-thien-2-yl (d); 113: R = CH2(2-MeOPh) (a), CH2-thien-2-yl (b).
Figure 6.
Chemical structures of the compounds 108–113. 108: R = Me (a), CH2cPr (b), propyne (c), CH2Ph (d), CH2(2-MePh) (e), CH2(2-MeOPh) (f), CH2(pyridin-4-yl) (g); 109: R7 = H, R8 = NO2 (a), R7 = NO2, R8 = H (b); 110: R7 = H, R8 = NO2 (a), R7=NO2, R8=H (b); 111: R7 = H, R8 = NO2, R = CH2(2-MeOPh) (a), R7 = H, R8 = NO2, R = CH2-thien-2-yl (b), R7 = NO2, R8 = H, R = CH2(2-MeOPh) (c), R7 = NO2, R8 = H, R = CH2-thien-2-yl (d); 113: R = CH2(2-MeOPh) (a), CH2-thien-2-yl (b).
Figure 7.
Hydrogen bonds among the agonist
112 (blue), the antagonist
108d (red), and γThr142, αHis102, and αSer205 in the binding site.
Reproduced with permission of MDPI [
64].
Figure 7.
Hydrogen bonds among the agonist
112 (blue), the antagonist
108d (red), and γThr142, αHis102, and αSer205 in the binding site.
Reproduced with permission of MDPI [
64].
Scheme 27.
Synthesis of pyrazolo[1,5-a]quinazoline derivatives 115a (a-f), 115b (a-o),. 115c (a-g); 116a (a-o), 116b (a-y), 116c (a-j); 117a (a-h), 117b (a-h), 117ca (a-h). R1 = H (a), Me (b), tBu (c); R2 = Ar, Ar-Ar, Ar-O-Ar, thiazolyl, benzothiazolyl, benzooxazolyl, pyrazinyl etc.
Scheme 27.
Synthesis of pyrazolo[1,5-a]quinazoline derivatives 115a (a-f), 115b (a-o),. 115c (a-g); 116a (a-o), 116b (a-y), 116c (a-j); 117a (a-h), 117b (a-h), 117ca (a-h). R1 = H (a), Me (b), tBu (c); R2 = Ar, Ar-Ar, Ar-O-Ar, thiazolyl, benzothiazolyl, benzooxazolyl, pyrazinyl etc.
Scheme 28.
Synthesis of pyrazolo[1,5-a]quinazoline derivatives 118a-d, 119a-d, 120a-e, 121a-c, 122, 123a-e, 124, 125, 126. 118–121: R7, R8 = H, H (a); H, Cl (b), H, NO2 (c); NO2, H (d); H, OMe (e); 123: R = Me (a), CH2-Ph (b), CH2-4-SMe-Ph (c), CH2-2-Cl-Ph (d), CH2-3-Cl-Ph (e), CH2-4-Br-Ph (f), CH2-2-Me-Ph (g), CH2-3-OMe-Ph (h), CH2-4-SO2NH2-Ph (i).
Scheme 28.
Synthesis of pyrazolo[1,5-a]quinazoline derivatives 118a-d, 119a-d, 120a-e, 121a-c, 122, 123a-e, 124, 125, 126. 118–121: R7, R8 = H, H (a); H, Cl (b), H, NO2 (c); NO2, H (d); H, OMe (e); 123: R = Me (a), CH2-Ph (b), CH2-4-SMe-Ph (c), CH2-2-Cl-Ph (d), CH2-3-Cl-Ph (e), CH2-4-Br-Ph (f), CH2-2-Me-Ph (g), CH2-3-OMe-Ph (h), CH2-4-SO2NH2-Ph (i).
Scheme 29.
Synthetic approach to 9-(trifluoromethyl)-pyrido[2’,3’:3,4]pyrazolo[1,5-a]quinazoline derivatives 128–132.
Scheme 29.
Synthetic approach to 9-(trifluoromethyl)-pyrido[2’,3’:3,4]pyrazolo[1,5-a]quinazoline derivatives 128–132.
Scheme 30.
Synthetic approach to 4,5-dihydropyrazolo[1,5-a]quinazolines 133–136. 133: R1 = CF3, NO2, CN, Cl, Br, F, OCF3; R2=Et, Me; 134: R2 = Me, Et, n-Pr, cyclobutylmethyl, 4-chlorobenzyl; 135: X = O, NH; R = Me, Et, CH2CF3, 2,4-Me2Ph, 2-Cl-5-Me-thiazole, 2-Cl-5-Me-Py; 136: R1 = esters, amides, acylsulfur; R2 = H, esters; R3 = SOCF3 or SO2CF3.
Scheme 30.
Synthetic approach to 4,5-dihydropyrazolo[1,5-a]quinazolines 133–136. 133: R1 = CF3, NO2, CN, Cl, Br, F, OCF3; R2=Et, Me; 134: R2 = Me, Et, n-Pr, cyclobutylmethyl, 4-chlorobenzyl; 135: X = O, NH; R = Me, Et, CH2CF3, 2,4-Me2Ph, 2-Cl-5-Me-thiazole, 2-Cl-5-Me-Py; 136: R1 = esters, amides, acylsulfur; R2 = H, esters; R3 = SOCF3 or SO2CF3.
Scheme 31.
Synthetic approach to benzimidazo[1,2-a]quinazolines 137, 138. 137: X = CH, R = H (a), Br (b), OMe (c), CF3 (d), NO2 (e); X = N, R = H (f), Br (g). 138: R = F, Cl, Br, CF3, Me, OMe; R1 = H, Cl, Me.
Scheme 31.
Synthetic approach to benzimidazo[1,2-a]quinazolines 137, 138. 137: X = CH, R = H (a), Br (b), OMe (c), CF3 (d), NO2 (e); X = N, R = H (f), Br (g). 138: R = F, Cl, Br, CF3, Me, OMe; R1 = H, Cl, Me.
Scheme 32.
Synthetic approach to tetrahydrobenzo [
4,
5]imidazo[1,2-
a]quinazolines
140,
141, benzimidazo[1,2-
a]quinazolines
144 and benzimidazole-4,7-dione derivatives
142a-c,
145.
140: R, R
1 = H, H (
a), H, Me (
b), Me, H (
c), Ph, H (
d);
141,
142: R = H (
a), Me (
b), Ph (
c);
143-145: R = H (
a), F (
b).
Scheme 32.
Synthetic approach to tetrahydrobenzo [
4,
5]imidazo[1,2-
a]quinazolines
140,
141, benzimidazo[1,2-
a]quinazolines
144 and benzimidazole-4,7-dione derivatives
142a-c,
145.
140: R, R
1 = H, H (
a), H, Me (
b), Me, H (
c), Ph, H (
d);
141,
142: R = H (
a), Me (
b), Ph (
c);
143-145: R = H (
a), F (
b).
Scheme 33.
Synthetic approach to tetrahydrobenzo[
4,
5]imidazo[1,2-
a]quinazolines
147, tetrahydroimidazo[1,2-
a]quinazolin-5-one
148, benzimidazo[1,2-
a]quinazolin-5-ones
150a-c,
151a, imidazo[1,2-
a]quinazolin-5-one
151b.
147: R, R
1 = Bz, H (
a); Bu, H (
b); Bz, Me (
c); Bz, Ph (
d);
150: R = Bz (
a), Bu (
b), Ph (
c);
151a: R =
n-Pr, benzimidazole;
151b: R =
n-Bu, imidazole.
Scheme 33.
Synthetic approach to tetrahydrobenzo[
4,
5]imidazo[1,2-
a]quinazolines
147, tetrahydroimidazo[1,2-
a]quinazolin-5-one
148, benzimidazo[1,2-
a]quinazolin-5-ones
150a-c,
151a, imidazo[1,2-
a]quinazolin-5-one
151b.
147: R, R
1 = Bz, H (
a); Bu, H (
b); Bz, Me (
c); Bz, Ph (
d);
150: R = Bz (
a), Bu (
b), Ph (
c);
151a: R =
n-Pr, benzimidazole;
151b: R =
n-Bu, imidazole.
Scheme 34.
Synthesis of imidazo[1,2-a]quinazolin-5-one153 and benzimidazo[1,2-a]quinazolines 156. 154: R1 = H, 5,6-Cl; 155: X = F, Cl, Br, NO2; R = H, 4-F, 5-F, 4-Cl, 3-OMe, 5-Br, 4-Br, 3,6-Br.
Scheme 34.
Synthesis of imidazo[1,2-a]quinazolin-5-one153 and benzimidazo[1,2-a]quinazolines 156. 154: R1 = H, 5,6-Cl; 155: X = F, Cl, Br, NO2; R = H, 4-F, 5-F, 4-Cl, 3-OMe, 5-Br, 4-Br, 3,6-Br.
Scheme 35.
Synthetic approach to 4-(2-chlorobenzyl)imidazole[1,2-a]quinazolin-5(4H)-ones 159, 161a-e, 164. 161: R = 4-Me-piperazino (a), pyrrolidinyl (b), piperidinyl (c), morpholino (d), thiomorpholino (e); 164: R = cycloimines, cycloamines, arylamines.
Scheme 35.
Synthetic approach to 4-(2-chlorobenzyl)imidazole[1,2-a]quinazolin-5(4H)-ones 159, 161a-e, 164. 161: R = 4-Me-piperazino (a), pyrrolidinyl (b), piperidinyl (c), morpholino (d), thiomorpholino (e); 164: R = cycloimines, cycloamines, arylamines.
Figure 8.
Interaction of compound
164 (R = 3-(OCF
3)C
6H
4NH) with SHP2 protein.
Reproduced with permission of Taylor & Francis [
80].
Figure 8.
Interaction of compound
164 (R = 3-(OCF
3)C
6H
4NH) with SHP2 protein.
Reproduced with permission of Taylor & Francis [
80].
Scheme 36.
Synthesis of imidazo[1,2-a]quinazoline derivative 166, diimidazo[1,2-a:1',2'-c]quinazoline derivatives 167, 168.
Scheme 36.
Synthesis of imidazo[1,2-a]quinazoline derivative 166, diimidazo[1,2-a:1',2'-c]quinazoline derivatives 167, 168.
Scheme 37.
Synthesis of benzo[
4,
5]thiazolo[3,2-
a]quinazoline
169, thiazolo[3,2-
a]quinazolin-5-ones
172.
Scheme 37.
Synthesis of benzo[
4,
5]thiazolo[3,2-
a]quinazoline
169, thiazolo[3,2-
a]quinazolin-5-ones
172.
Scheme 38.
Synthesis of 4-aryl-[
1,
2,
4]triazolo[4,3-
a]quinazolin-5(
4H)-ones
175 (24 compounds) and
176–180. 174, 175: R = H,
o(
p)-OMe,
p-OC
3H
7,
p-OC
4H
9,
p-OC
5H
11,
p-OC
6H
13,
p-OC
8H
17,
p-OC
10H
21,
p-OC
12H
25,
p-OC
14H
29,
o(
m,
p)-F,
o(
m,
p)-Cl,
m(
p)-Br,
o(
m,
p)-Me,
p-CF
3,
o,
p-Cl
2.
Scheme 38.
Synthesis of 4-aryl-[
1,
2,
4]triazolo[4,3-
a]quinazolin-5(
4H)-ones
175 (24 compounds) and
176–180. 174, 175: R = H,
o(
p)-OMe,
p-OC
3H
7,
p-OC
4H
9,
p-OC
5H
11,
p-OC
6H
13,
p-OC
8H
17,
p-OC
10H
21,
p-OC
12H
25,
p-OC
14H
29,
o(
m,
p)-F,
o(
m,
p)-Cl,
m(
p)-Br,
o(
m,
p)-Me,
p-CF
3,
o,
p-Cl
2.
Scheme 39.
Synthesis of 1-substituted-[
1,
2,
4]triazolo[4,3-
a]quinazolin-5(4
H)-ones
182,
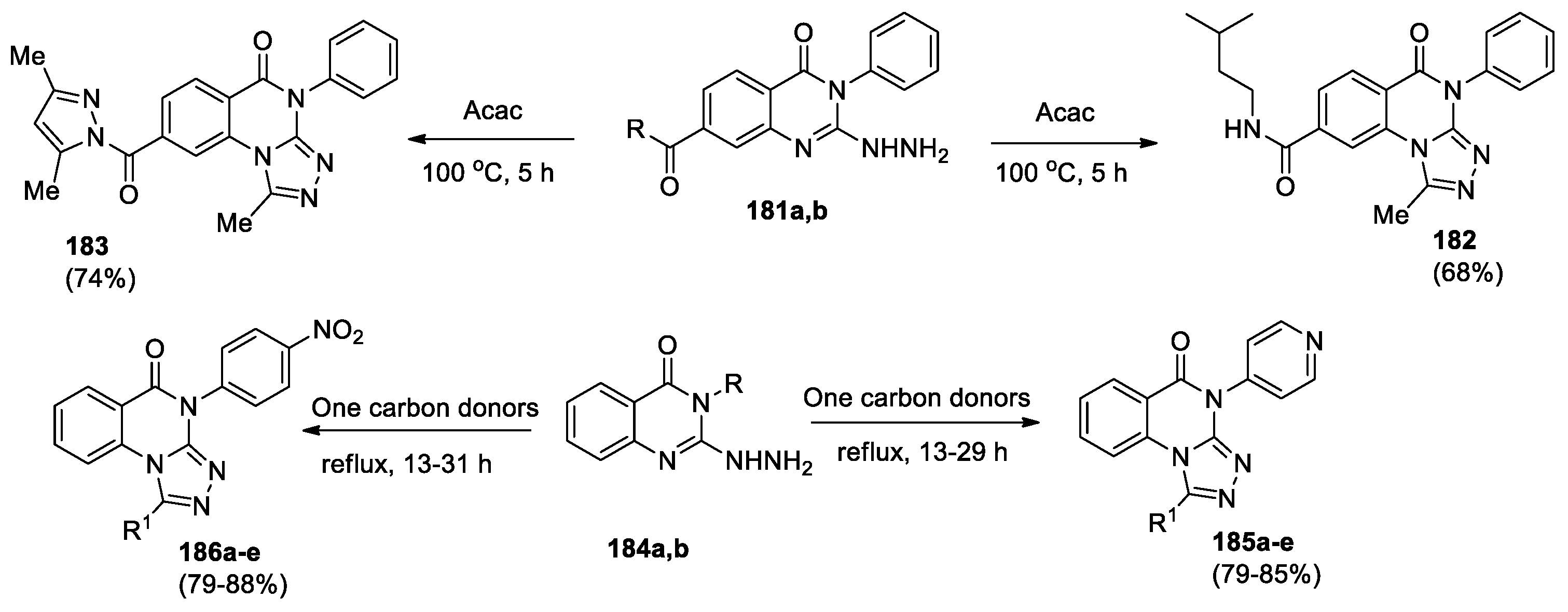
183, 185a-e, 186a-e. 181: R = NHNH
2 (
a), NH-
iC
5H
11 (
b);
184: R = pyridine-4-yl (
a), 4-nitrophenyl (
b);
185, 186: R
1 = H (
a), Me (
b), Et (
c),
n-Pr (
d), CH
2Cl (
e).
Scheme 39.
Synthesis of 1-substituted-[
1,
2,
4]triazolo[4,3-
a]quinazolin-5(4
H)-ones
182,
183, 185a-e, 186a-e. 181: R = NHNH
2 (
a), NH-
iC
5H
11 (
b);
184: R = pyridine-4-yl (
a), 4-nitrophenyl (
b);
185, 186: R
1 = H (
a), Me (
b), Et (
c),
n-Pr (
d), CH
2Cl (
e).
Scheme 40.
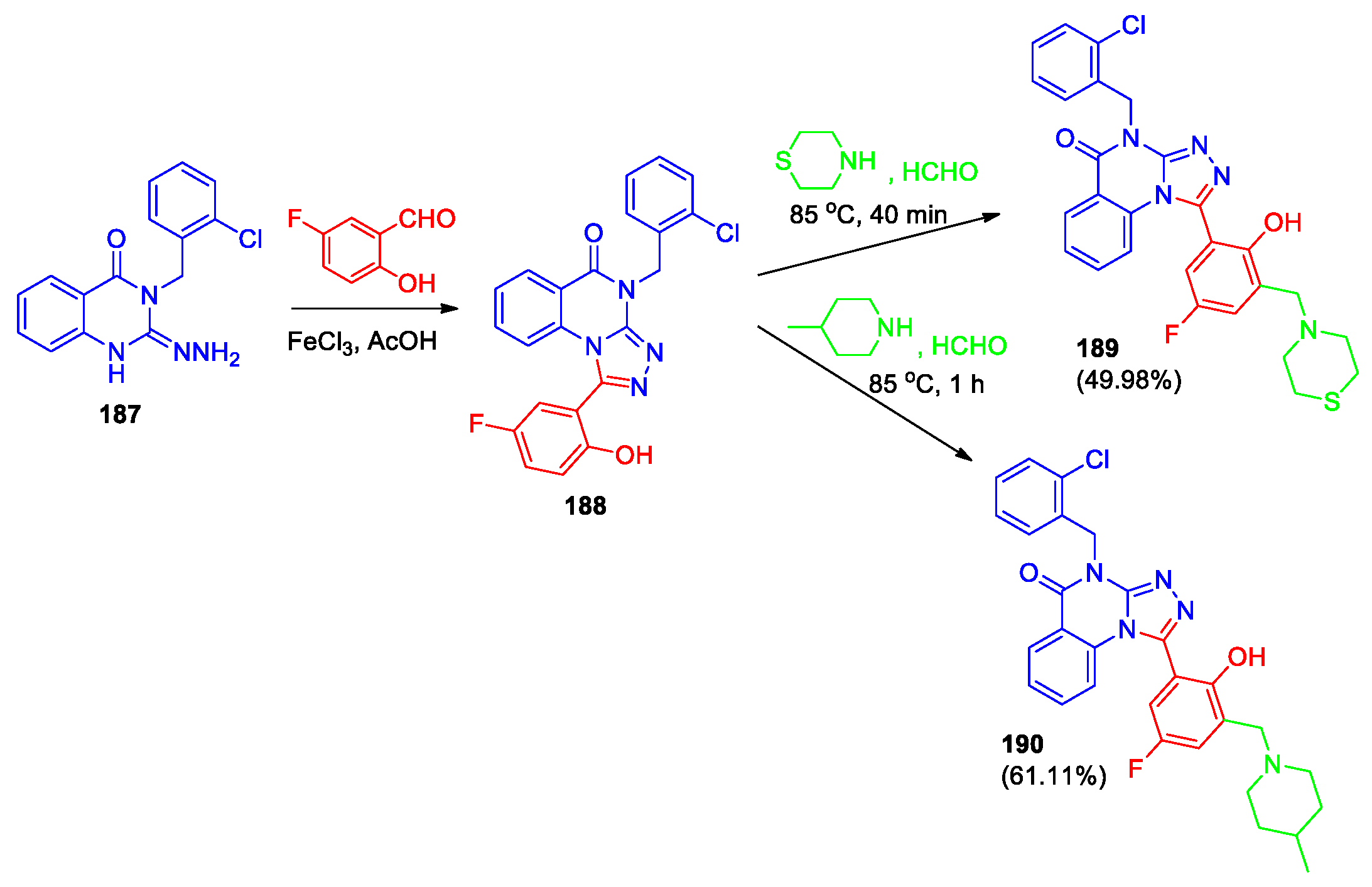
Synthesis of 4-(2-chlorobenzyl)containing [
1,
2,
4]triazolo[4,3-
a]quinazolin-5(4
H)-ones
189 and
190.
Scheme 40.
Synthesis of 4-(2-chlorobenzyl)containing [
1,
2,
4]triazolo[4,3-
a]quinazolin-5(4
H)-ones
189 and
190.
Scheme 41.
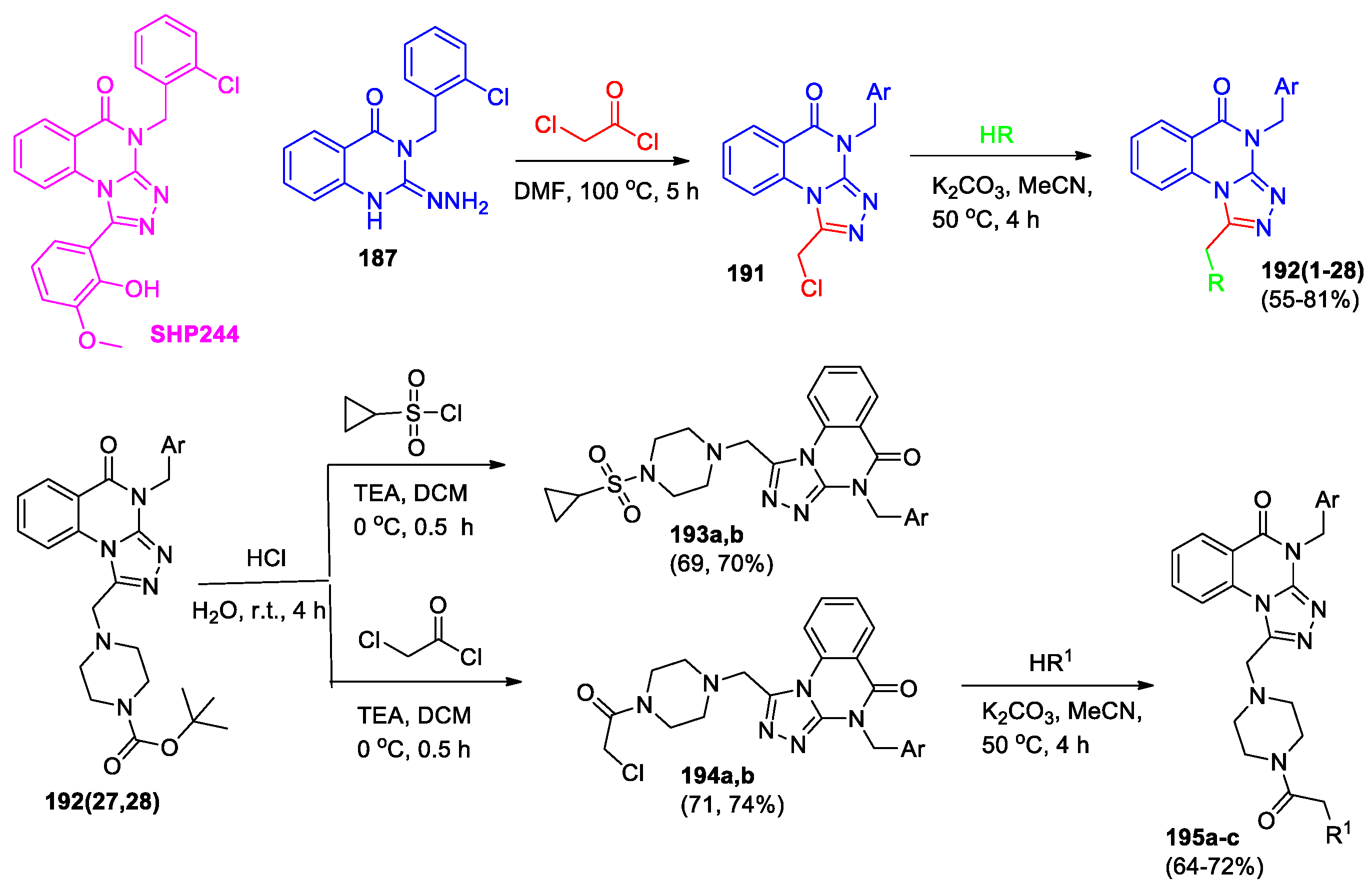
Synthesis of [
1,
2,
4]triazolo[4,3-
a]quinazolin-5(4
H)-one derivatives
192,
193,
195.
192: Ar = furan-2-ylmethyl (
1-6), thiophen-2-ylmethyl (
7-10), 2-chlorobenzyl (
11), 4-fluorobenzyl (
12-16,
27), 4-methoxybenzyl (
17-26,
28);
193, 194: Ar = 4-fluorobenzyl (
a), 4-methoxybenzyl (
b);
195: Ar, R
1 = 4-fluorobenzyl, 2-morpholinoacetyl (
a), 4-methoxybenzyl, 2-morpholinoacetyl (
b), 4-methoxybenzyl, 2-(pyrrolidin-1-yl)acetyl (
c).
Scheme 41.
Synthesis of [
1,
2,
4]triazolo[4,3-
a]quinazolin-5(4
H)-one derivatives
192,
193,
195.
192: Ar = furan-2-ylmethyl (
1-6), thiophen-2-ylmethyl (
7-10), 2-chlorobenzyl (
11), 4-fluorobenzyl (
12-16,
27), 4-methoxybenzyl (
17-26,
28);
193, 194: Ar = 4-fluorobenzyl (
a), 4-methoxybenzyl (
b);
195: Ar, R
1 = 4-fluorobenzyl, 2-morpholinoacetyl (
a), 4-methoxybenzyl, 2-morpholinoacetyl (
b), 4-methoxybenzyl, 2-(pyrrolidin-1-yl)acetyl (
c).
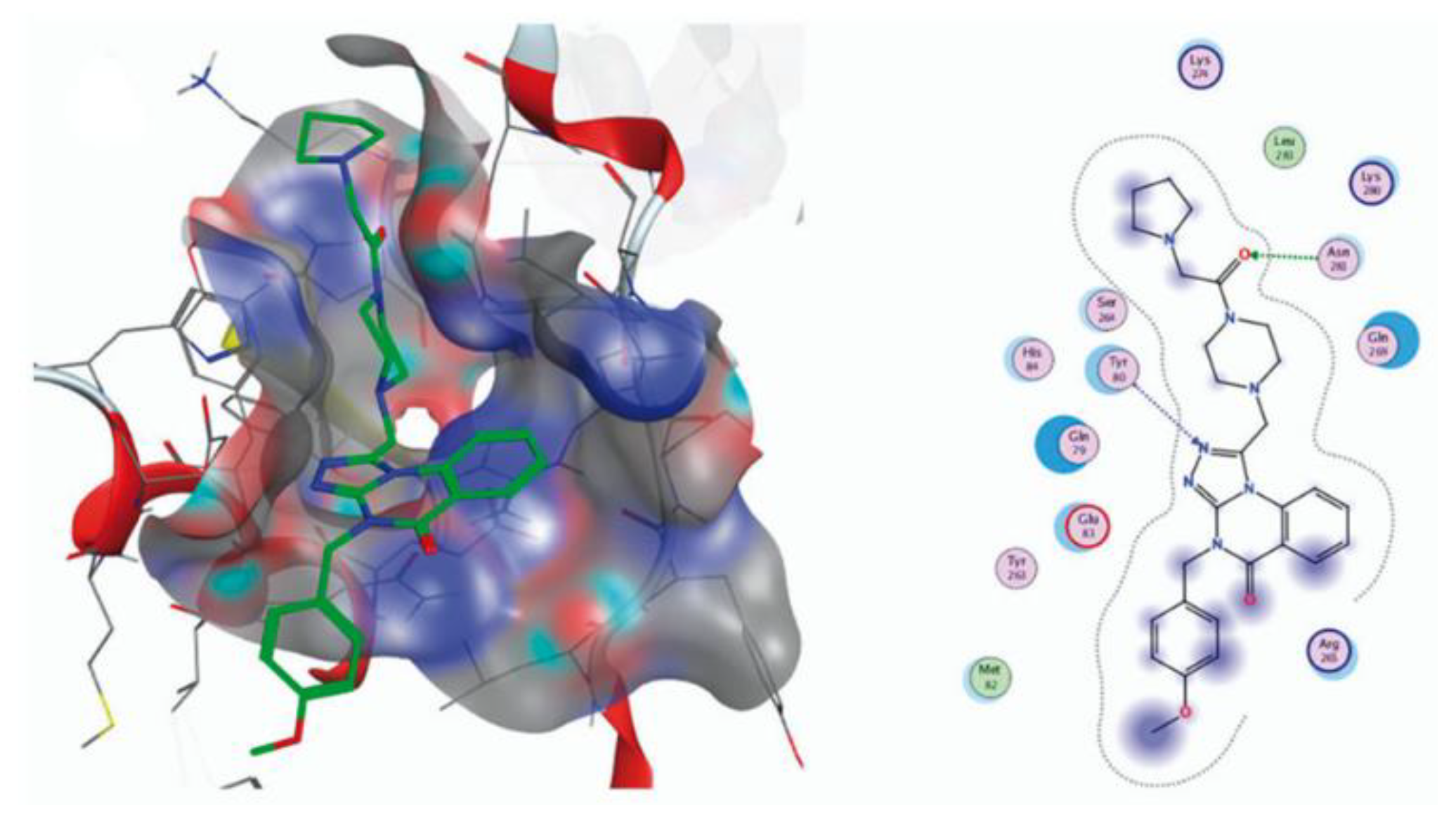
Figure 9.
Interaction of compound
195c with SHP2 protein.
Reproduced with permission of Taylor & Francis [
80].
Figure 9.
Interaction of compound
195c with SHP2 protein.
Reproduced with permission of Taylor & Francis [
80].
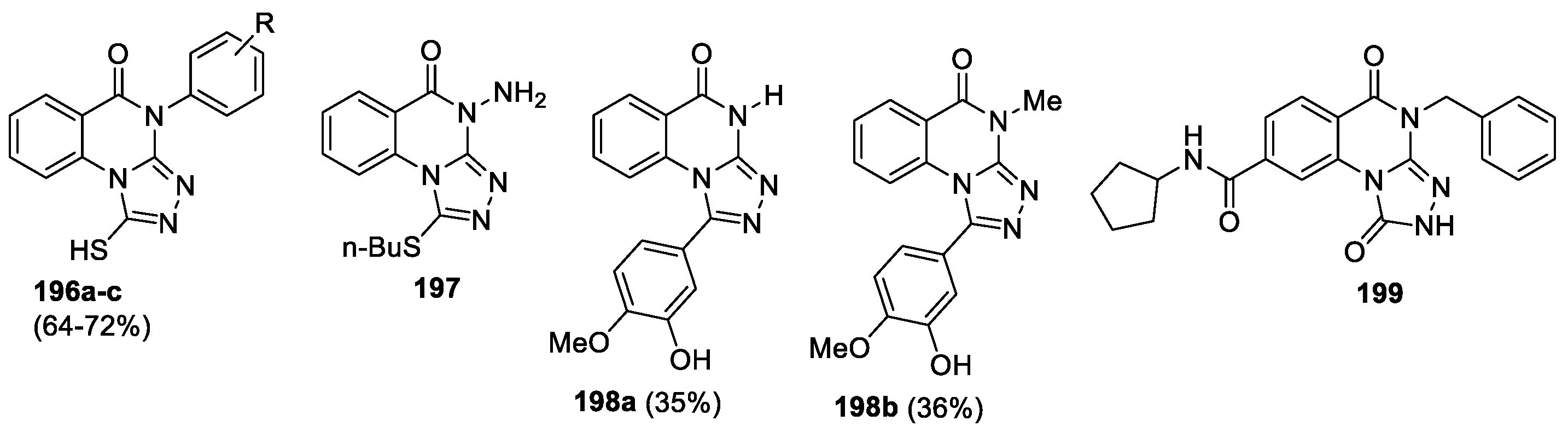
Figure 10.
Chemical structures of the compounds 196, 197, 198a,b, 199. 196: R = H (a), 4-OEt (b), 4-Me (c).
Figure 10.
Chemical structures of the compounds 196, 197, 198a,b, 199. 196: R = H (a), 4-OEt (b), 4-Me (c).
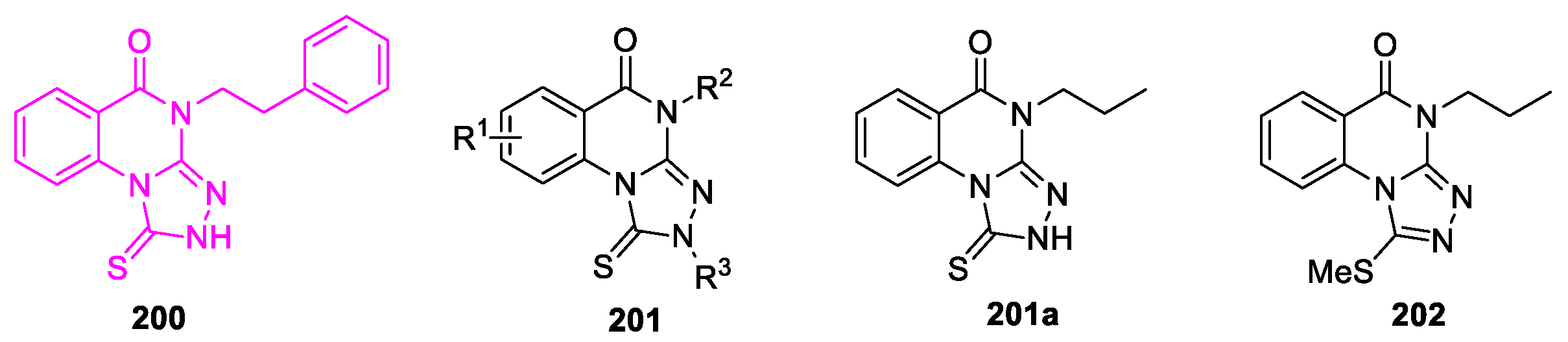
Figure 11.
Chemical structures of the compounds 200, 201, 202.
Figure 11.
Chemical structures of the compounds 200, 201, 202.
Scheme 42.
Synthesis of [
1,
2,
4]triazolo[1,5-
a]quinazolin-5-ones
204 and their aza-analogs 205, [
1,
2,
4]triazolo[1,5-
a]quinazolines
206, 207. 204,
205: R
1 = H (
a), Ph (
b);
206: R = H (
a), Br (
b), Me (
c). Efficient method was developed for the synthesis of [
1,
2,
4]triazolo[1,5-
a]quinazolines
206 from 2-fluoro benzaldehydes
57 with 1
H-1,2,4-triazol-3-amine under metal-free conditions in high yields (
Scheme 42) [
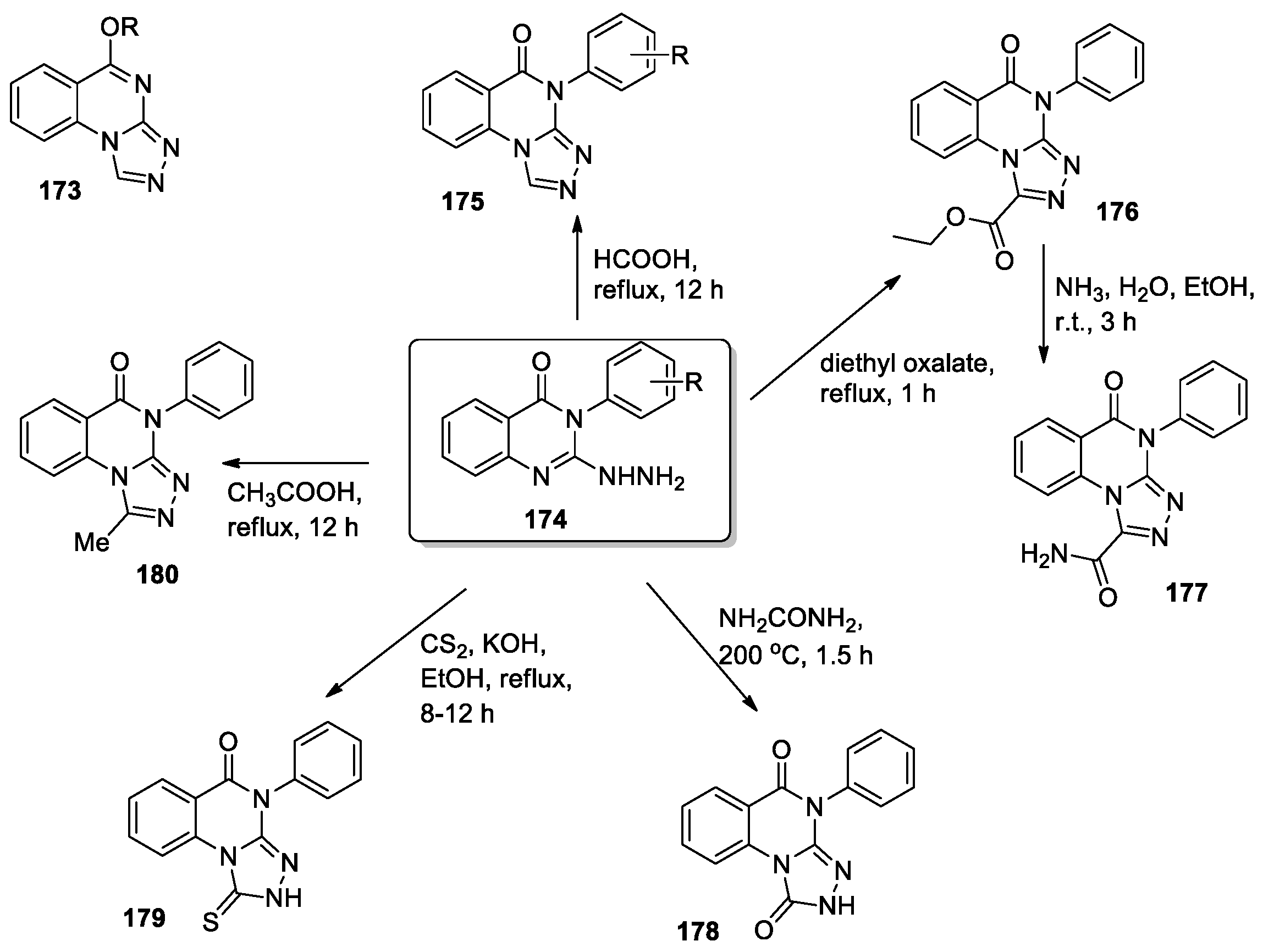
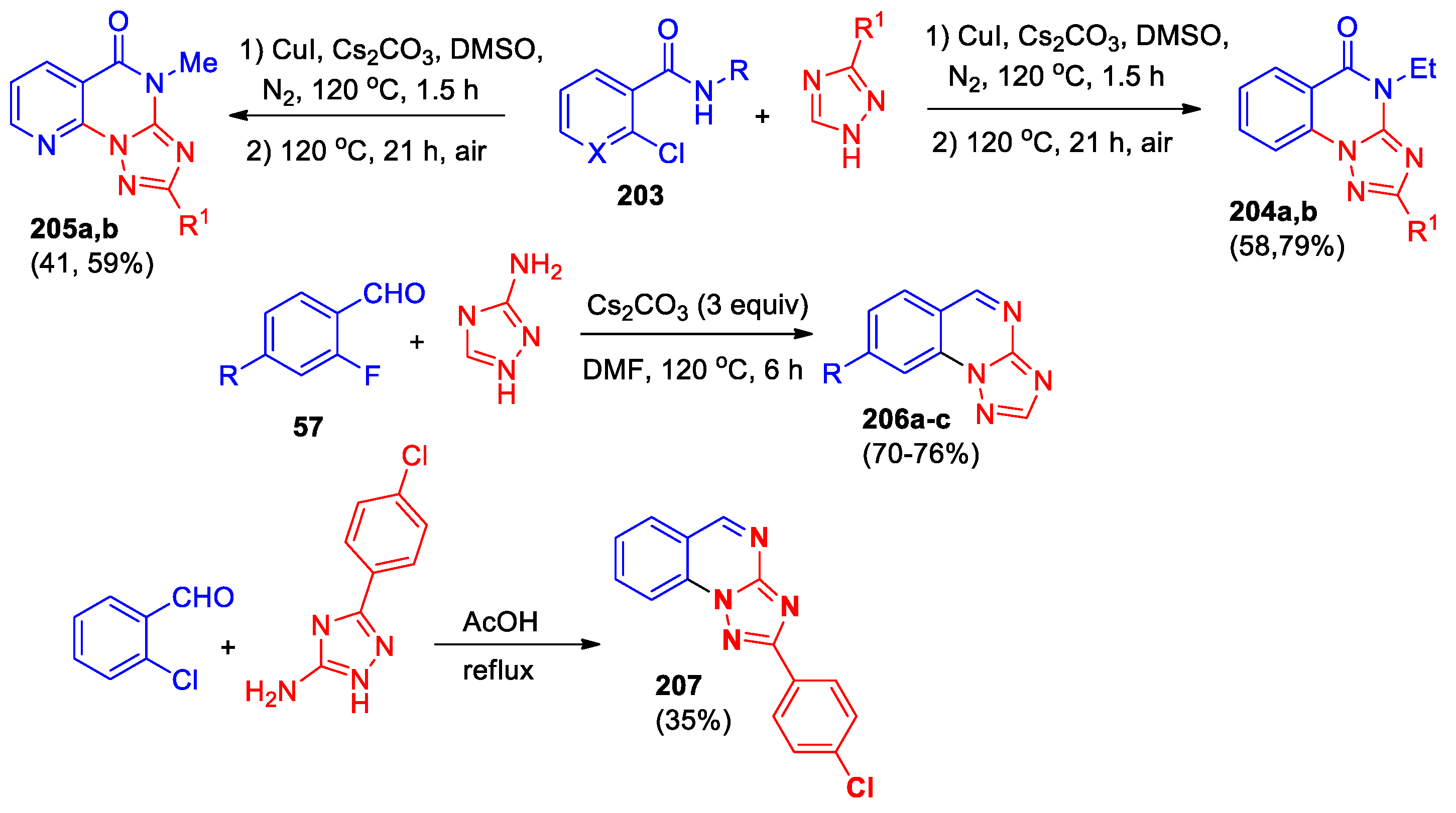
52]. The process includes an intermolecular condensation followed by metal-free base-promoted intramolecular C–N coupling reaction. It's worth mentioning that the bromine atom stays intact during the reaction and can later be modified or used in additional steps.
Scheme 42.
Synthesis of [
1,
2,
4]triazolo[1,5-
a]quinazolin-5-ones
204 and their aza-analogs 205, [
1,
2,
4]triazolo[1,5-
a]quinazolines
206, 207. 204,
205: R
1 = H (
a), Ph (
b);
206: R = H (
a), Br (
b), Me (
c). Efficient method was developed for the synthesis of [
1,
2,
4]triazolo[1,5-
a]quinazolines
206 from 2-fluoro benzaldehydes
57 with 1
H-1,2,4-triazol-3-amine under metal-free conditions in high yields (
Scheme 42) [
52]. The process includes an intermolecular condensation followed by metal-free base-promoted intramolecular C–N coupling reaction. It's worth mentioning that the bromine atom stays intact during the reaction and can later be modified or used in additional steps.
Scheme 43.
Synthesis of [
1,
2,
4]triazolo[1,5-
a]quinazolin-5-ones
209,
211.
Scheme 43.
Synthesis of [
1,
2,
4]triazolo[1,5-
a]quinazolin-5-ones
209,
211.
Scheme 44.
Synthesis of 2-phenoxybenzo[
g][
1,
2,
4]triazolo[1,5-
a]quinazolin-5(4
H)-ones
212–215.
213: R = Et, allyl, 2(3)-Me-benzyl, 3-OMe-benzyl, 3(4)-CN-benzyl, 4-Cl-benzyl, 4-NO
2-benzyl, 2-(piperidin-1-yl)ethyl, 2-morpholinoethyl, 2-(phtalimido-2-yl)propyl.
Scheme 44.
Synthesis of 2-phenoxybenzo[
g][
1,
2,
4]triazolo[1,5-
a]quinazolin-5(4
H)-ones
212–215.
213: R = Et, allyl, 2(3)-Me-benzyl, 3-OMe-benzyl, 3(4)-CN-benzyl, 4-Cl-benzyl, 4-NO
2-benzyl, 2-(piperidin-1-yl)ethyl, 2-morpholinoethyl, 2-(phtalimido-2-yl)propyl.
Figure 12.
Chemical structures of the compounds 217–220. 217: R, R1 = H, OPh (a); H, SMe (b); Me, OPh (c); H, SO2Me (d); 218: R, R1 = OPh, 4-NO2-Bn (a); OPh, Et (b); SO2Me, allyl (c); SO2Me, 4-NO2-Bn (d); 220: R = Et (a), n-Pr (b).
Figure 12.
Chemical structures of the compounds 217–220. 217: R, R1 = H, OPh (a); H, SMe (b); Me, OPh (c); H, SO2Me (d); 218: R, R1 = OPh, 4-NO2-Bn (a); OPh, Et (b); SO2Me, allyl (c); SO2Me, 4-NO2-Bn (d); 220: R = Et (a), n-Pr (b).
Figure 13.
Chemical structures of the compounds 221, 222, 223a-d, 224, 225a,b, 226a,b. 223: R, R1 = H, allyl (a); Me, Bn (b); H, Bn (c); H, 4-NO2-Bn (d); 225: R1, R2 = H, 3-Py (a); Me, 4-NO2-Ph (b); 226: R1 = allyl (a), cyclohexyl (b).
Figure 13.
Chemical structures of the compounds 221, 222, 223a-d, 224, 225a,b, 226a,b. 223: R, R1 = H, allyl (a); Me, Bn (b); H, Bn (c); H, 4-NO2-Bn (d); 225: R1, R2 = H, 3-Py (a); Me, 4-NO2-Ph (b); 226: R1 = allyl (a), cyclohexyl (b).
Figure 14.
Chemical structures of the compounds 227, 228, 229a,b, 230, 231. 229: R1 = Et (a), allyl (b).
Figure 14.
Chemical structures of the compounds 227, 228, 229a,b, 230, 231. 229: R1 = Et (a), allyl (b).
Table 1.
Inhibitory activities of compounds A, 64p, 64r, 64s against KDM4D.
Table 1.
Inhibitory activities of compounds A, 64p, 64r, 64s against KDM4D.
| Compound |
Inhibition rate at 10 μM |
IC50 (μM) |
| A |
73.33 ± 19.14 |
3.14 ± 0.18 |
| 64p |
85.06 ± 3.54 |
4.03 ± 1.02 |
| 64r |
88.82 ± 9.58 |
0.41 ± 0.03 |
| 64s |
78.06 ± 6.27 |
1.85 ± 0.14 |