1. Introduction
Surgical resection remains the cornerstone of curative treatment for stage I–IIIA non-small cell lung cancer (NSCLC) and offers the best chance for long-term survival. Despite advancements in surgical and perioperative care, clinical outcomes remain heterogeneous, with a substantial proportion of patients experiencing early recurrence or a poor prognosis. This variability underscores the need for improved prognostic tools to better stratify risks and guide personalized postoperative management.
The tumor-node-metastasis (TNM) staging system remains the cornerstone for assessing disease extent and guiding treatment decisions in NSCLC. Although overall survival (OS) generally decreases with advancing stage, substantial outcome variability among patients at the same stage highlights the limitations of anatomical staging in fully capturing tumor biology and host-related factors [
1,
2,
3]. Clinical variables such as age, performance status, and smoking history add an important prognostic context, yet they often fail to reflect the underlying biological heterogeneity [
4,
5,
6,
7]. Similarly, pathological features, including histological subtype, tumor size, and pleural invasion, primarily indicate local tumor burden rather than systemic disease behavior [
5,
6,
7,
8,
9,
10,
11,
12,
13]. These limitations have driven a growing interest in integrating systemic biomarkers that reflect the host’s immune, inflammatory, and nutritional status to improve risk stratification and enhance prognostic accuracy in NSCLC.
The prognostic immune and nutritional index (PINI) combines markers of nutritional and immune-inflammatory statuses by integrating serum albumin (ALB) level and absolute monocyte count (AMC). It is calculated using the formula: [ALB (g/dL) × 0.9] − [AMC (/μL) × 0.0007]. The PINI has been validated as an independent prognostic marker in gastrointestinal malignancies [
14,
15,
16,
17]. Jung et al. first reported its potential for predicting survival outcomes in colorectal cancer (CRC) [
14], and subsequent studies confirmed its prognostic utility in patients with stage I–III CRC [
15,
16]. As regards gastric cancer, An et al. demonstrated that adding the PINI to a baseline model (BM), which is based on stage alone, or an intermediate model (IM), which includes age, body mass index (BMI), frailty index, stage, type of gastrectomy, and anemia, significantly enhanced the predictive performance, as evidenced by improvements in both the concordance index (C-index) and integrated area under the curve (iAUC), underscoring its additive value in survival prediction [
17].
Despite emerging evidence, the prognostic significance of the PINI in NSCLC remains to be firmly established. Notably, ALB level is a well-recognized prognostic marker for NSCLC, and it reflects nutritional status and systemic inflammation [
18,
19,
20,
21,
22,
23]. Similarly, elevated AMC has been associated with tumor progression through mechanisms such as immune suppression, angiogenesis, and tumor proliferation [
24,
25,
26,
27,
28,
29,
30]. By combining ALB level and AMC, the PINI serves as a biologically integrated index that may capture both the nutritional and inflammatory landscapes of the patient, potentially enabling a more refined risk stratification in NSCLC [
4,
31,
32,
33].
This study aimed to evaluate the prognostic utility of the PINI as a continuous variable in patients with stage I–IIIA NSCLC undergoing curative-intent resection. Using real-world data and rigorous statistical methods, we investigated whether the inclusion of the PINI enhances survival prediction beyond conventional clinicopathological factors, thereby supporting its use in individualized prognostic modeling for stage I–IIIA NSCLC.
2. Materials and Methods
2.1. Study Population
This retrospective study included consecutive patients with NSCLC who underwent curative-intent surgical resection at Kyung Hee University Hospital at Gangdong between January 2010 and October 2024. Preoperative staging was performed using standard imaging modalities, including chest and abdominopelvic computed tomography (CT) and whole-body positron emission tomography (PET)-CT.
Eligible patients met the following criteria: (i) histologically confirmed primary NSCLC [
34], (ii) stage I–IIIA disease according to the 8th edition of the TNM staging system [
35]; and (iii) complete tumor resection with negative margins [
36]. The exclusion criteria were as follows: (i) previous treatment for NSCLC, (ii) clinical or pathological evidence of stage IIIB or IV disease, (iii) history of other malignancies within the past 5 years, and (iv) active infections or autoimmune diseases requiring treatment.
Adjuvant therapy was administered to eligible patients with stage II or IIIA disease, primarily using platinum-based regimens such as cisplatin with vinorelbine, etoposide, pemetrexed, docetaxel, or gemcitabine [
37]. Follow-up surveillance included chest and abdominopelvic CT scans every 3–6 months for the first 3 years, every 6 months from years 4–5, and annually thereafter. Additional imaging, including PET/CT or brain magnetic resonance imaging, was performed as clinically indicated.
This study was approved by the Institutional Review Board of Kyung Hee University Hospital at Gangdong (IRB No. 2025-07-024). The requirement for informed consent was waived owing to the retrospective design of the study.
2.2. Baseline Clinical Characteristics
A broad range of clinicopathological and laboratory variables was evaluated for their association with OS. Clinical parameters included age; sex; smoking status; alcohol consumption; American Society of Anesthesiologists Physical Status (ASA) classification; BMI; surgical procedure; histological subtype; tumor size; presence of pleural, vascular, lymphatic, or perineural invasion; and stage. Pleural invasion (PL) status was graded from 0 to 3 based on established histopathological criteria [
38]. Alcohol consumption was defined as the intake of alcohol more than once per week, regardless of quantity [
39].
Laboratory variables included routine blood chemistry and complete blood count (CBC) parameters. Biochemical variables included liver function markers such as serum total protein, ALB, total bilirubin, aspartate aminotransferase, alanine aminotransferase, and C-reactive protein (CRP) levels. The hematological indices evaluated included white blood cell (WBC) count, absolute neutrophil count (ANC), absolute lymphocyte count (ALC), AMC, red blood cell (RBC) count, hemoglobin concentration, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), the modified Shine–Lal index (mSLI) [
40], platelet count, and the PINI.
All laboratory assessments were performed within 7 days before surgery. If multiple results were available, the value closest to the date of surgery was used. Blood samples were collected following standardized protocols using ethylenediaminetetraacetic acid-anticoagulated tubes and processed within 1 h of venipuncture. Hematological parameters were measured using a Beckman Coulter LH 1502 impedance analyzer (Beckman Coulter, Miami, FL, USA). Internal and external quality control procedures were routinely implemented to ensure analytical accuracy and reproducibility [
41,
42].
2.3. Statistical Analysis
The primary endpoint was OS, which was defined as the interval from curative-intent surgical resection to death from any cause or the last follow-up. To maintain granularity and generalizability, continuous variables were analyzed in their original, uncategorized form.
Univariate Cox proportional hazards models were used to screen for potential prognostic variables. Covariates with p values < 0.05 and satisfying the proportional hazards assumption, were considered for inclusion in the multivariate models. Multicollinearity was evaluated using variance inflation factors (VIFs). Fractional polynomial (FP) transformations were applied in both univariate and multivariate Cox regression analyses to assess the potential nonlinear associations between the PINI and OS.
Model discrimination was primarily evaluated using the C-index and iAUC. Model differences were tested via 1000 bootstrap resamples. To quantify the incremental prognostic value, we computed the integrated discrimination improvement (IDI) and continuous net reclassification improvement (cNRI) at 3- and 5-year time points. Decision curve analysis (DCA) was also performed at both time points to assess the net clinical benefit across a range of threshold probabilities [
43].
Time-dependent C-indices were computed monthly over a 10-year horizon using bootstrapped cross-validation (1000 resamples). To minimize overfitting, the models were trained on one half of the dataset and validated on the other.
A prognostic nomogram was derived from the full model (FM) and internally validated using 1000 bootstrap replicates. Calibration curves were used to evaluate the agreement between the predicted and observed survival probabilities [
43].
To identify the clinical and laboratory variables most strongly associated with the PINI, we employed least absolute shrinkage and selection operator (LASSO) regression. This technique applies an L1 penalty to enable variable selection and regularization, thus highlighting relevant predictors while reducing overfitting. The optimal penalty parameter (lambda) was selected via 10-fold cross-validation. Model performance was summarized using R-squared, root mean squared error (RMSE), and mean squared error (MSE). Features with non-zero coefficients were interpreted as contributing meaningfully to the PINI. To further enhance interpretability, SHapley Additive exPlanations (SHAP) analysis was conducted. The SHAP values provided model-agnostic estimates of each variable’s contribution to the predicted PINI. Summary plots were used to visualize the overall feature importance, whereas dependence plots illustrated the interaction effects, particularly between ALB level and AMC, the two components of the PINI.
All analyses were performed using R version 4.4.0, and statistical significance was defined as a two-sided p value < 0.05.
3. Results
3.1. Clinicopathological Characteristics of Study Participants
Five hundred and twenty-two patients were included in this study. The majority were of East Asian ethnicity (98.1%, n = 512), with a small proportion of Caucasians (1.9%, n = 10). Regarding disease stage, 377 patients (72.2%) were classified as stage I, 76 (14.6%) as stage II, and 69 (13.2%) as stage IIIA (
Table 1).
3.2. Cox Regression Analysis for Predictors of Overall Survival
The median follow-up duration was 45.7 months. Univariate Cox regression was performed to screen for potential prognostic variables across demographic, clinical, pathological, biochemical, hematological, and composite domains. Candidate variables included age, sex, smoking and alcohol use, ASA, BMI, surgical approach, histological subtype, tumor characteristics (tumor size, PL, and lymphatic/vascular/perineural invasion), stage, serum chemistry (i.e., liver function tests and CRP), hematological parameters (i.e., WBC count, ANC, AMC, ALC, RBC count, hemoglobin level, MCV, MCH, MCHC, mSLI, and platelet count), and the PINI.
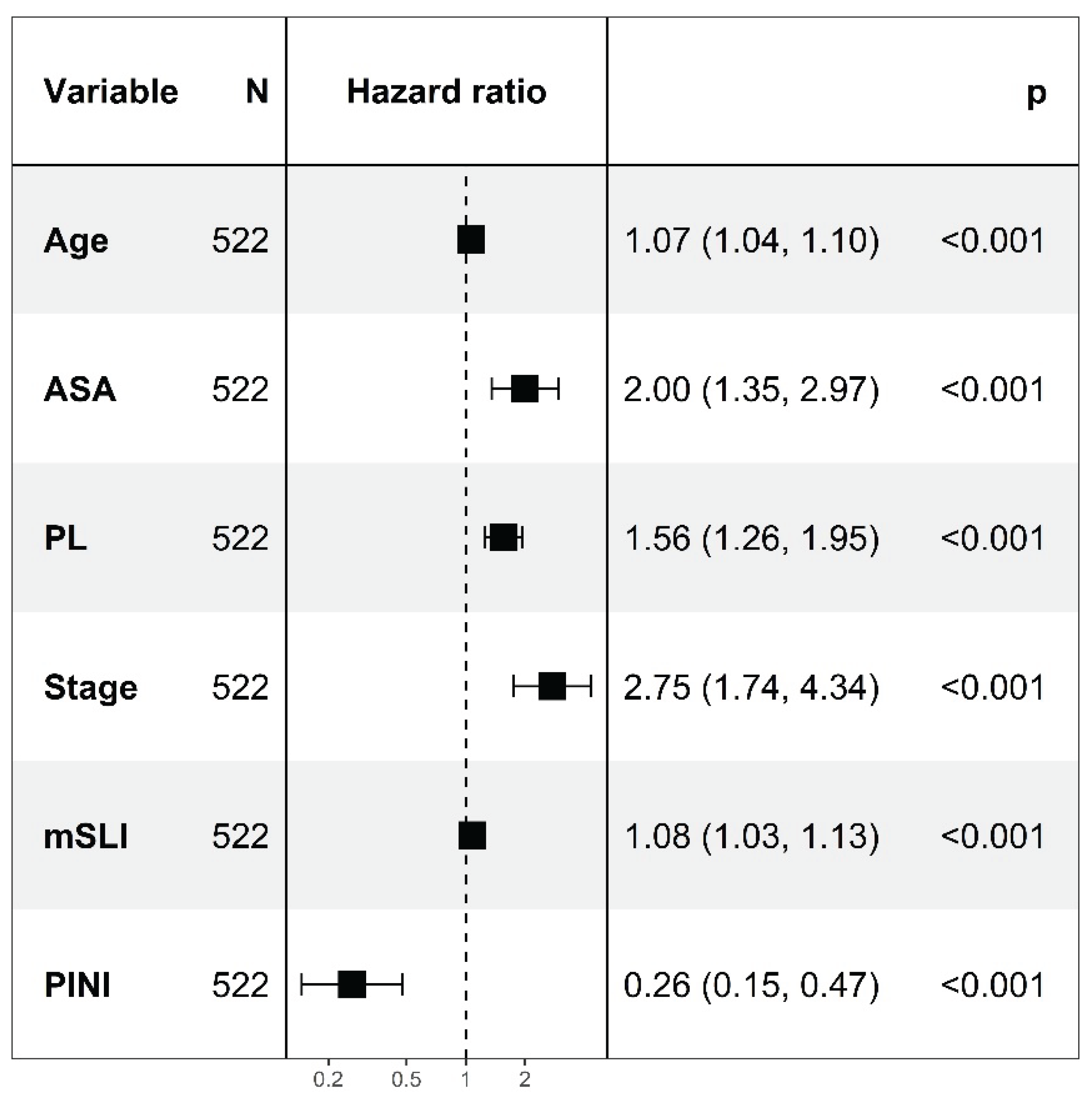
In multivariate modeling, age (HR, 1.07;
p < 0.001), ASA (HR, 2.00;
p < 0.001), PL (HR, 1.56;
p < 0.001), stage (HR, 2.75;
p < 0.001), mSLI (HR, 1.08;
p < 0.001), and the PINI (HR, 0.26;
p < 0.001) were significant with a C-index of 0.841, constituting the FM. The VIFs for the variables were as follows: age, 1.06; ASA, 1.05; stage, 1.12; PL, 1.11; mSLI, 1.05; and PINI, 1.11, indicating low multicollinearity among all covariates (
Figure 1).
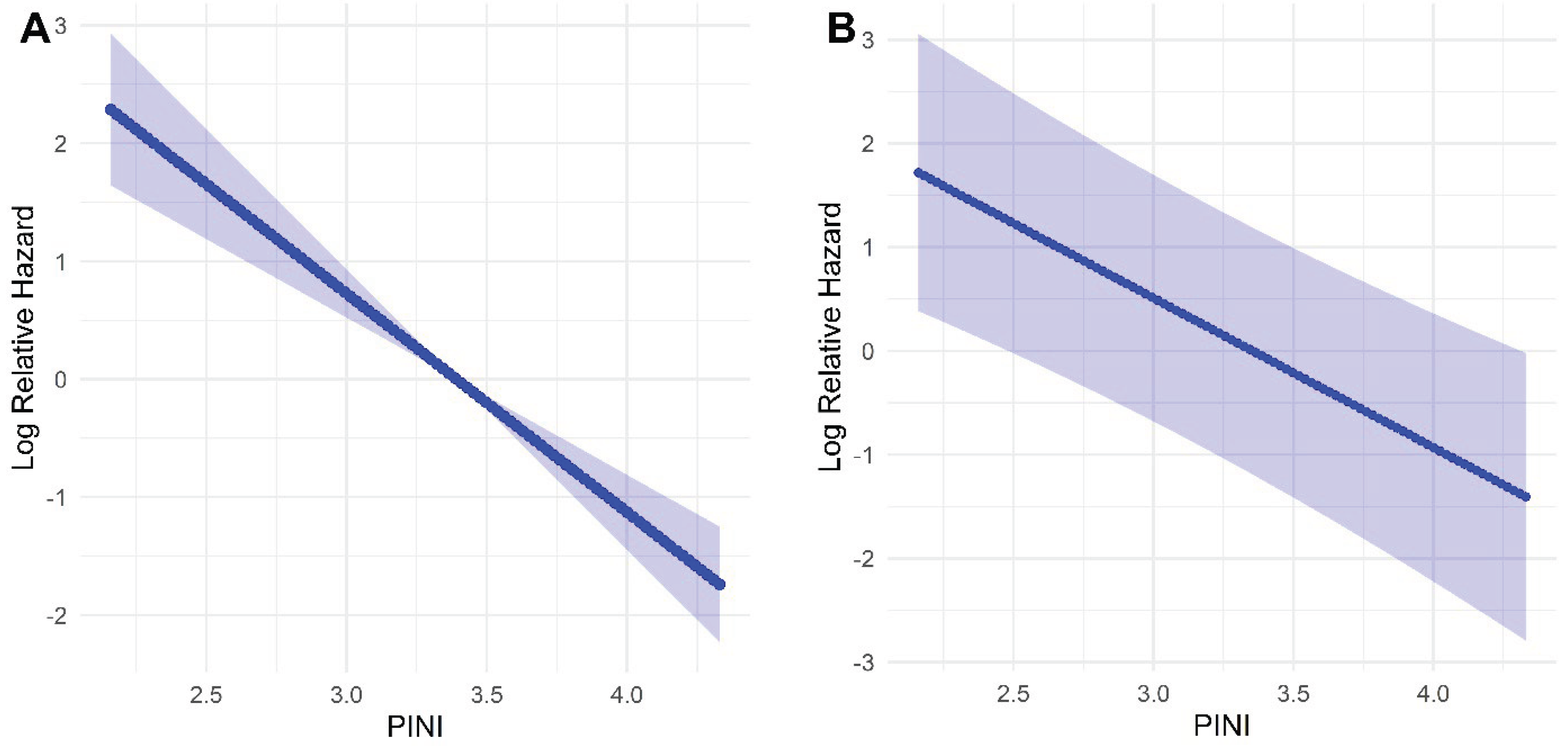
In the univariate model, FP analysis demonstrated a linear inverse association between the PINI and log-relative hazard, indicating that higher PINI values were associated with improved survival. This linear relationship remained consistent after multivariable adjustment for key clinical covariates, including age, ASA, PL, stage, and the mSLI. These results support the role of the PINI as a continuous and independent prognostic factor for OS in patients with NSCLC (
Figure 2).
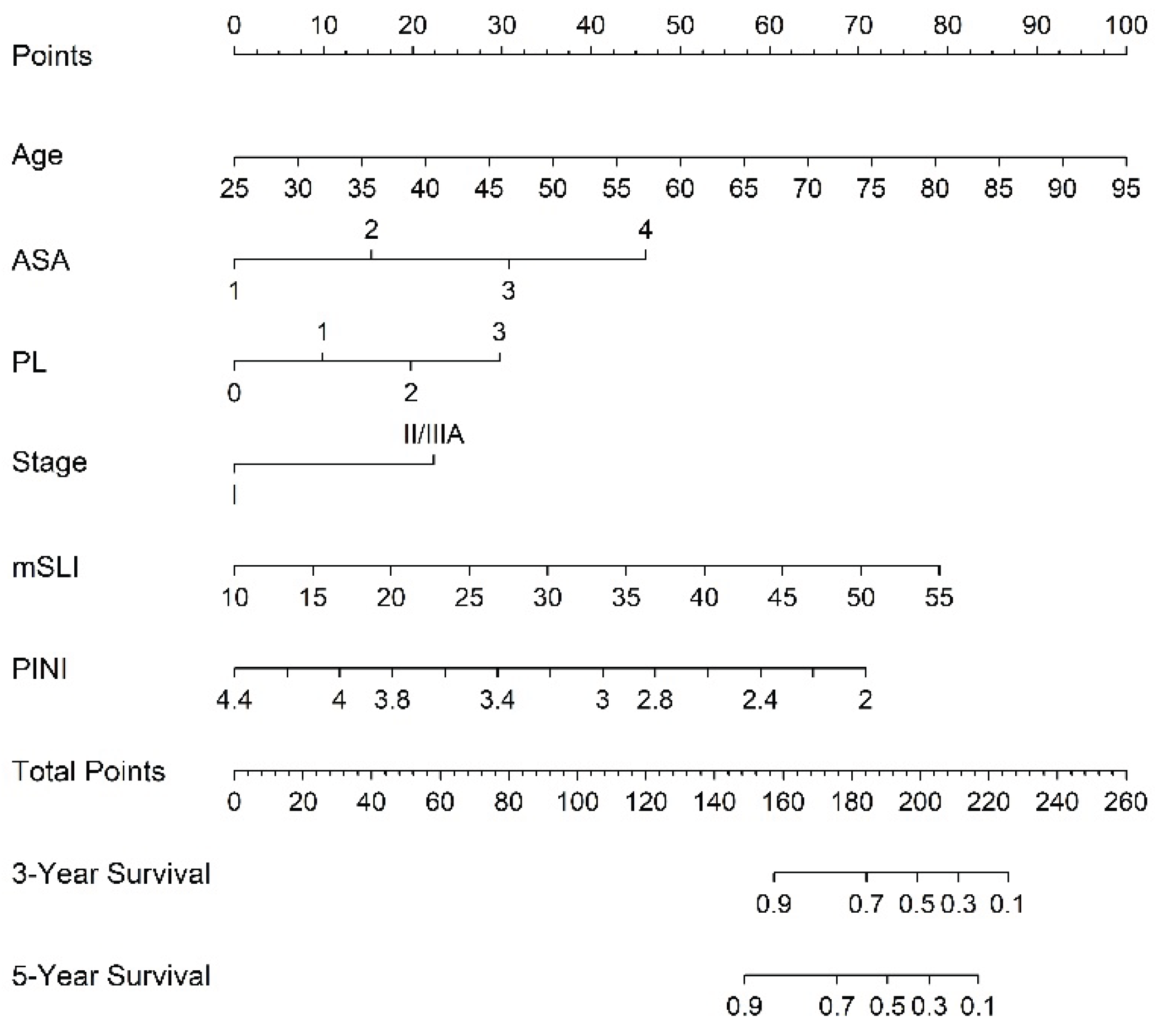
3.3. Nomogram Derived from the FM to Predict 3- and 5-Year Survival
A nomogram derived from the FM was constructed to generate individualized estimates of the 3- and 5-year OS rates. Incorporating key prognostic variables, such as age, ASA, PL, stage, mSLI, and PINI, is a practical and user-friendly tool to enhance personalized risk assessment and guide postoperative management (
Figure 3).
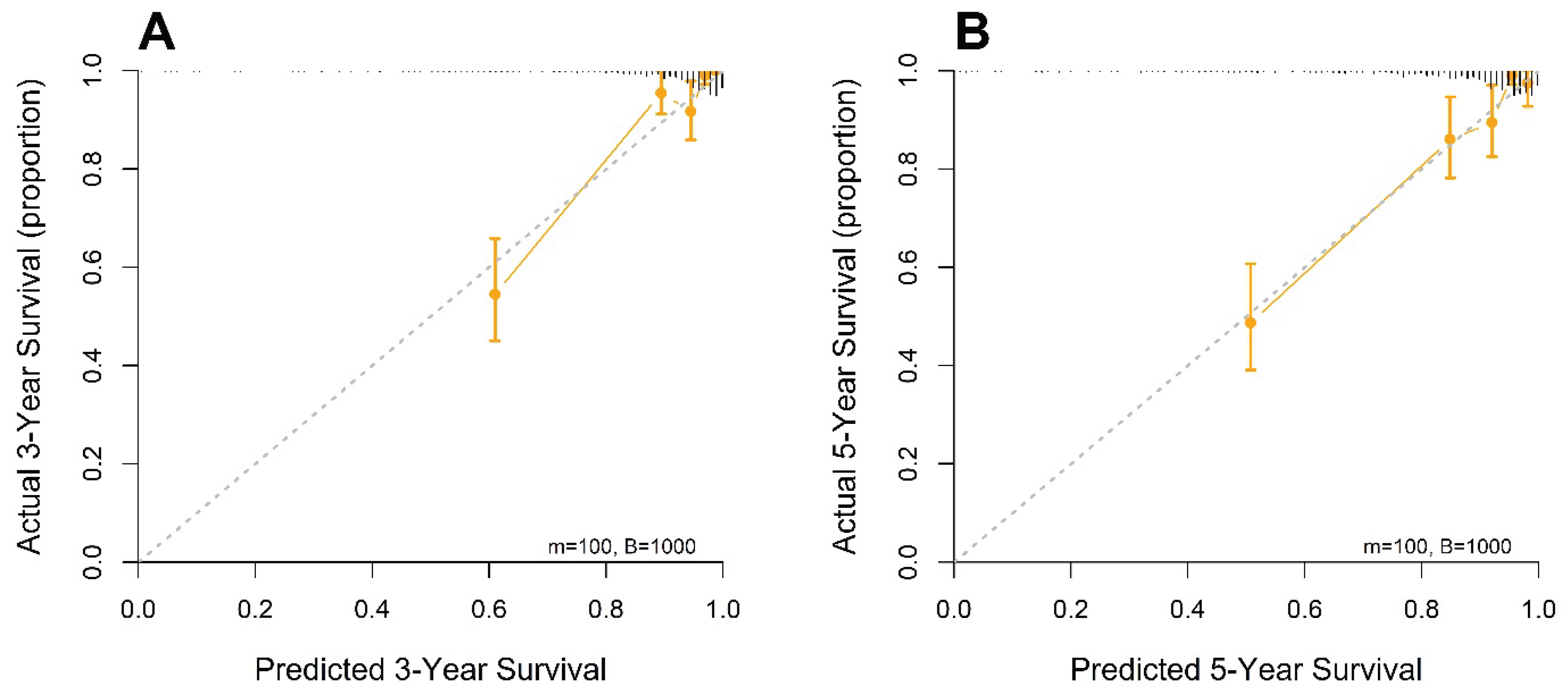
Calibration plots at 3 and 5 years demonstrated close concordance between the predicted survival probabilities and actual outcomes, indicating excellent agreement and minimal deviation from the ideal 45-degree reference line. The predictive accuracy of the model was further supported by bootstrap resampling (1000 iterations), which yielded calibration curves with negligible bias and consistent performance across the risk spectrum. These results affirm the reliability and generalizability of the nomogram for individualized survival prediction in patients with resected stage I–IIIA NSCLC (
Figure 4).
3.4. Model Comparison for Survival Prediction: FM vs. IM vs. BM
To evaluate the incremental prognostic value of the FM incorporating the PINI, we compared it with two reference models: a BM including only stage and an IM that included all the FM variables except the PINI.
The C-index and their standard errors (SEs) were 0.841 (SE = 0.022), 0.820 (SE = 0.025), and 0.692 (SE = 0.028) for the FM, IM, and BM, respectively. Similarly, the iAUC values and their SEs were 0.804 (SE = 0.019), 0.793 (SE = 0.020), and 0.663 (SE = 0.027) for the FM, IM, and BM, respectively.
The FM significantly outperformed the BM across all performance metrics. It demonstrated a higher C-index (p < 0.001) and superior iAUC (p < 0.001), indicating stronger overall discrimination. These results highlight the superior prognostic accuracy of the FM and its value in refining risk stratification beyond traditional staging. To evaluate the incremental contribution of the PINI, the FM was compared with the IM. The FM showed a modest but statistically significant improvement in the C-index (p = 0.012) and iAUC (p = 0.001).
At the 3-year time point, the FM demonstrated significantly improved discrimination compared to the BM, with an IDI of 0.252 (p < 0.001), indicating a 25.2% absolute increase in predictive accuracy. The cNRI was also substantial, at 0.502 (p < 0.001), reflecting the markedly enhanced ability of the FM to reclassify patient risk. When the FM and IM were compared, statistically significant improvements were still observed, although they were more modest in magnitude. The FM showed an IDI of 0.054 (p = 0.008) and a cNRI of 0.320 (p = 0.020), supporting the additive prognostic value of the PINI, even after accounting for other clinical variables.
At the 5-year time point, the improvements remained significant, although they were slightly attenuated. Compared to the BM, the FM achieved an IDI of 0.230 (
p < 0.001) and a cNRI of 0.418 (
p < 0.001). In comparison with the IM, the FM again showed statistically significant gains, with an IDI of 0.039 (
p = 0.018) and a cNRI of 0.238 (
p = 0.032), reaffirming the long-term prognostic contribution of the PINI in stage I–IIIA NSCLC (
Table 2).
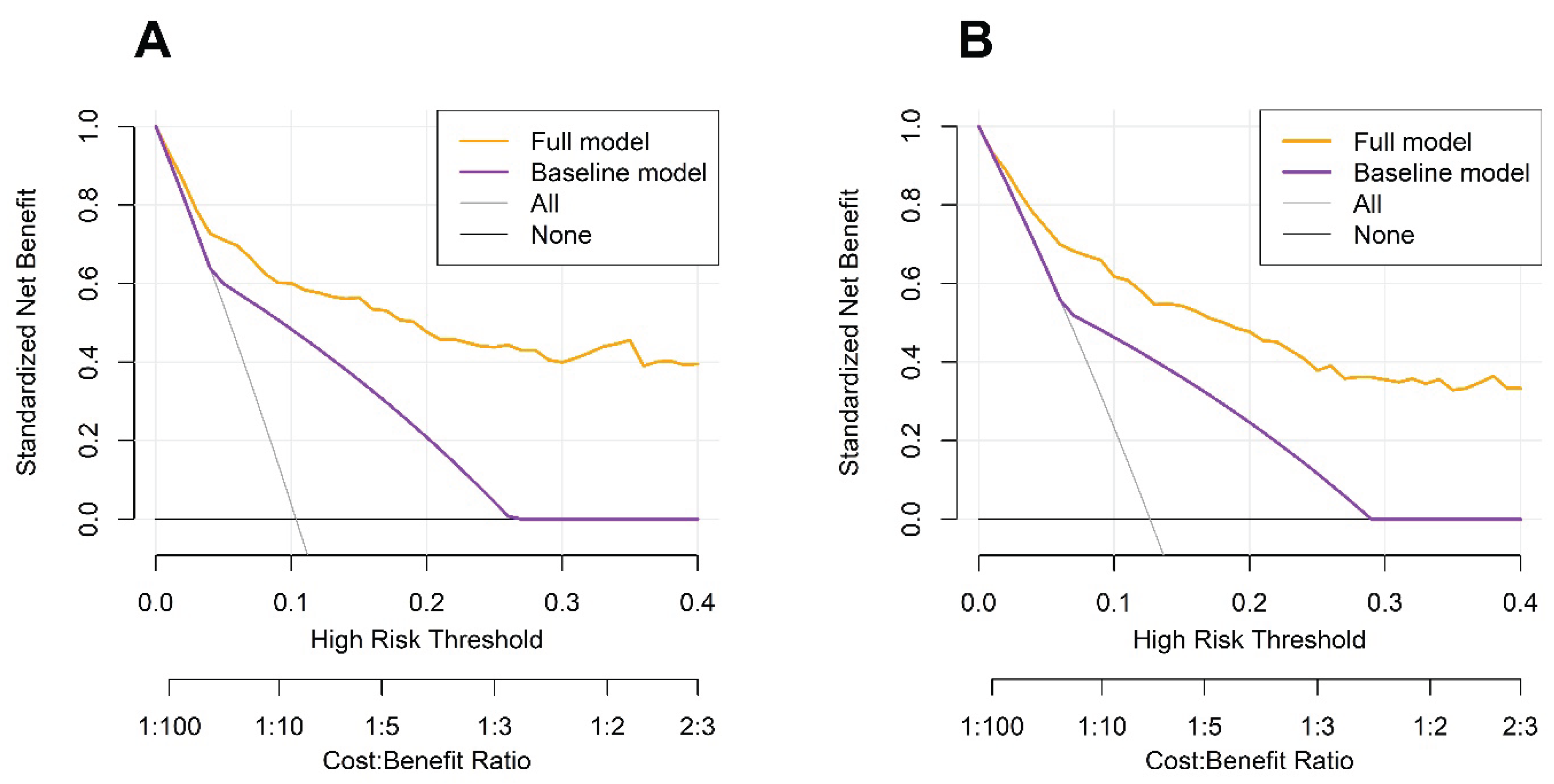
DCA further demonstrated that the FM consistently offered a greater net clinical benefit than the BM across a broad range of threshold probabilities for predicting both 3-year and 5-year OS. These findings suggest that incorporating additional clinical and hematological variables, particularly the PINI, enhances the ability of the model to stratify risk at the individual level. By outperforming stage-based prediction alone, the FM reduced the potential for overtreatment or undertreatment, thereby facilitating more precise and personalized postoperative management (
Figure 5).
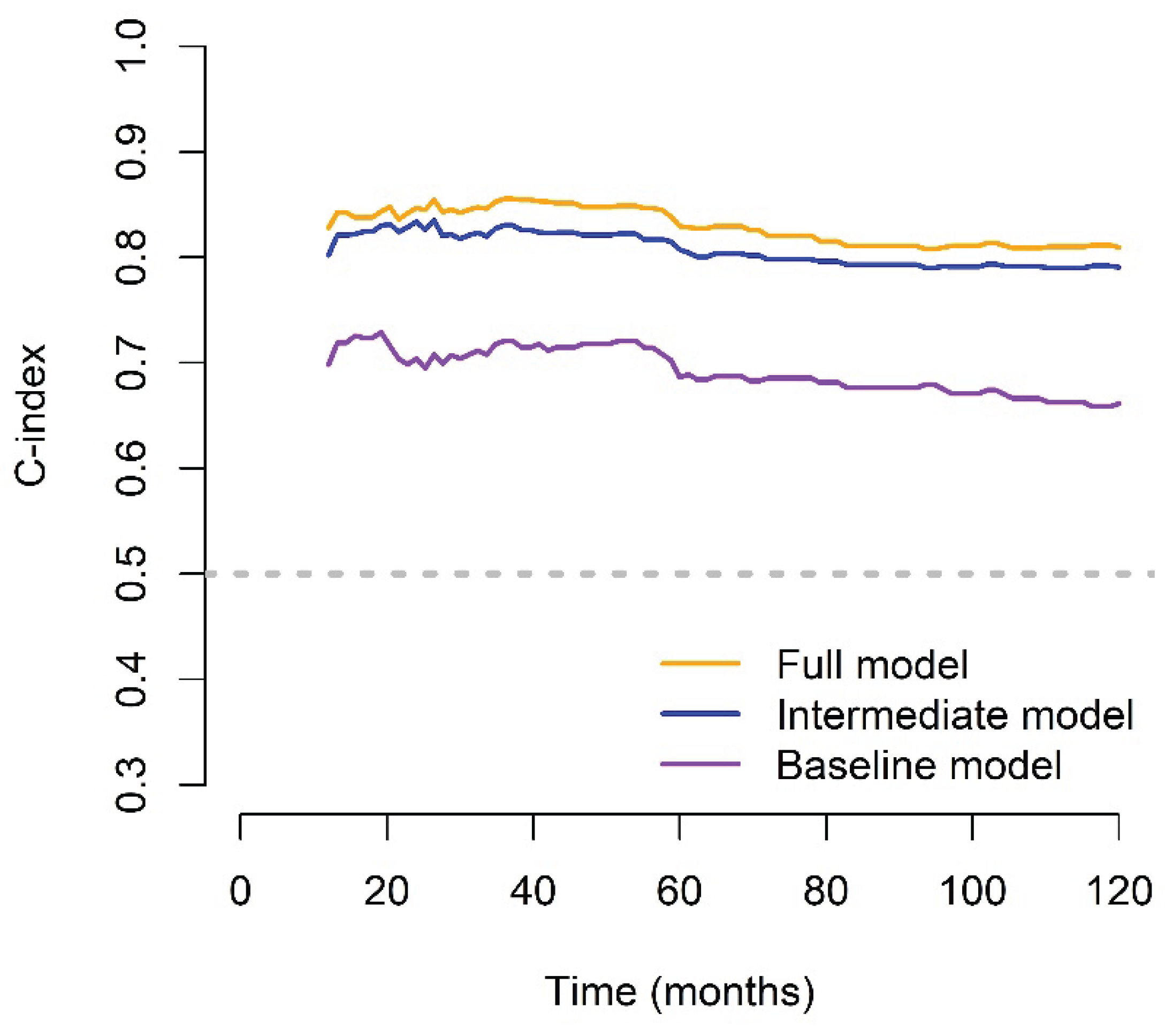
The FM demonstrated substantial and sustained improvements over both the BM and IM across the 10-year follow-up period, as reflected by consistently higher time-dependent C-index values. Notably, the BM had the lowest C-index values throughout, underscoring its limited prognostic utility when used in isolation (
Figure 6).
3.5. PINI vs. Established Biomarkers: Model Discrimination for Survival Outcomes
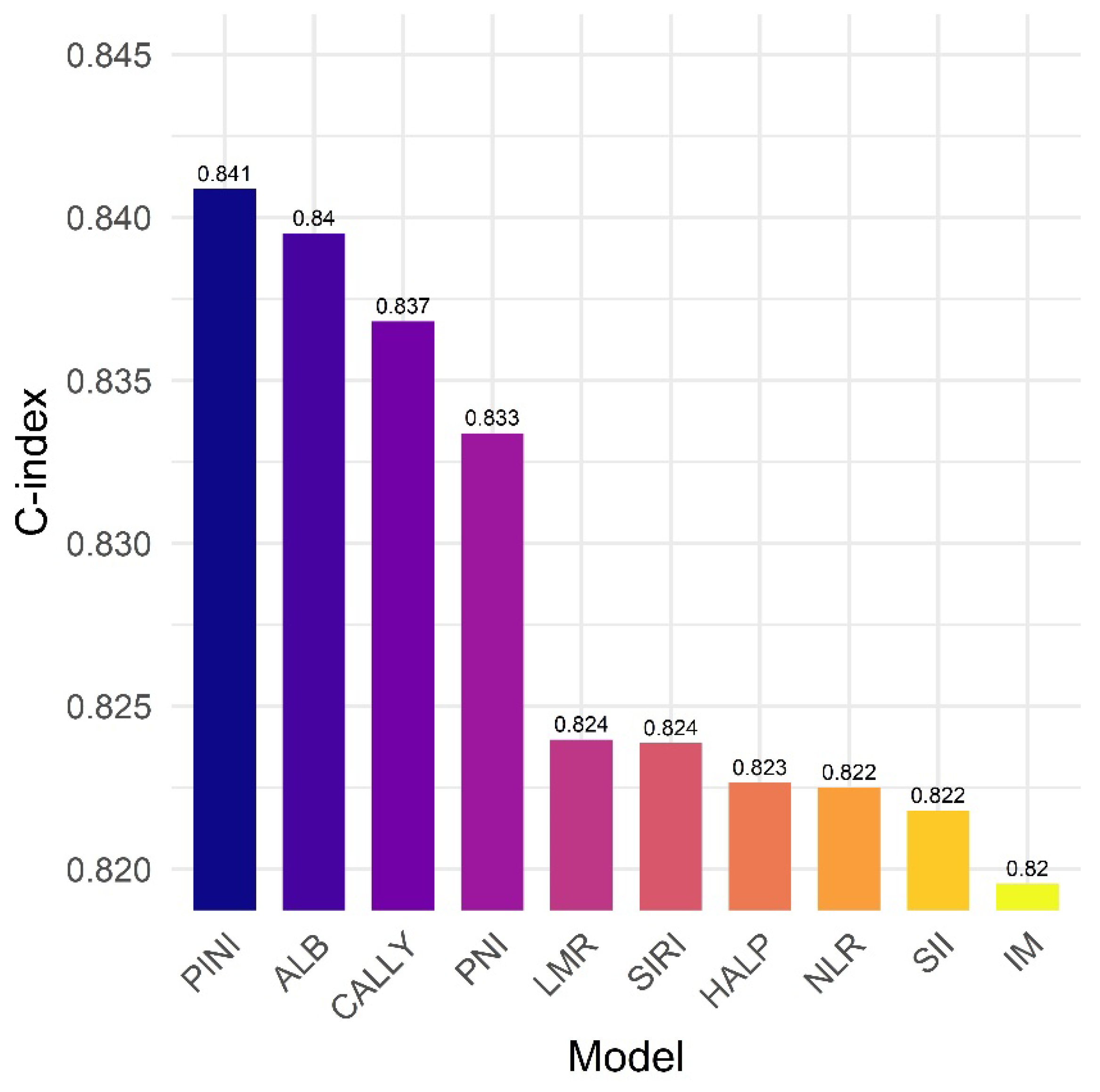
To assess the prognostic utility of the PINI in comparison with ALB level and other established composite biomarkers, we constructed separate multivariate Cox models by individually adding each biomarker—ALB, neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR), prognostic nutritional index (PNI), systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), hemoglobin–ALB–lymphocyte–platelet (HALP) score, and CRP–ALB–lymphocyte (CALLY) index—to the IM framework, which included age, ASA, PL, stage, and mSLI [4,44–53].
Among all models, the model incorporating the PINI achieved the highest C-index (0.841), indicating superior discriminative performance. Models including the ALB level (C-index = 0.840), CALLY (C-index = 0.837), and PNI (C-index = 0.833) also performed well, whereas those with the LMR, SIRI, HALP, NLR, and SII showed more modest improvements (C-index range: 0.822–0.824). These results suggest that although several inflammation- and nutrition-based biomarkers contribute to survival prediction, the PINI provided the most robust prognostic value in this cohort (
Figure 7).
3.6. Factors Affecting the PINI
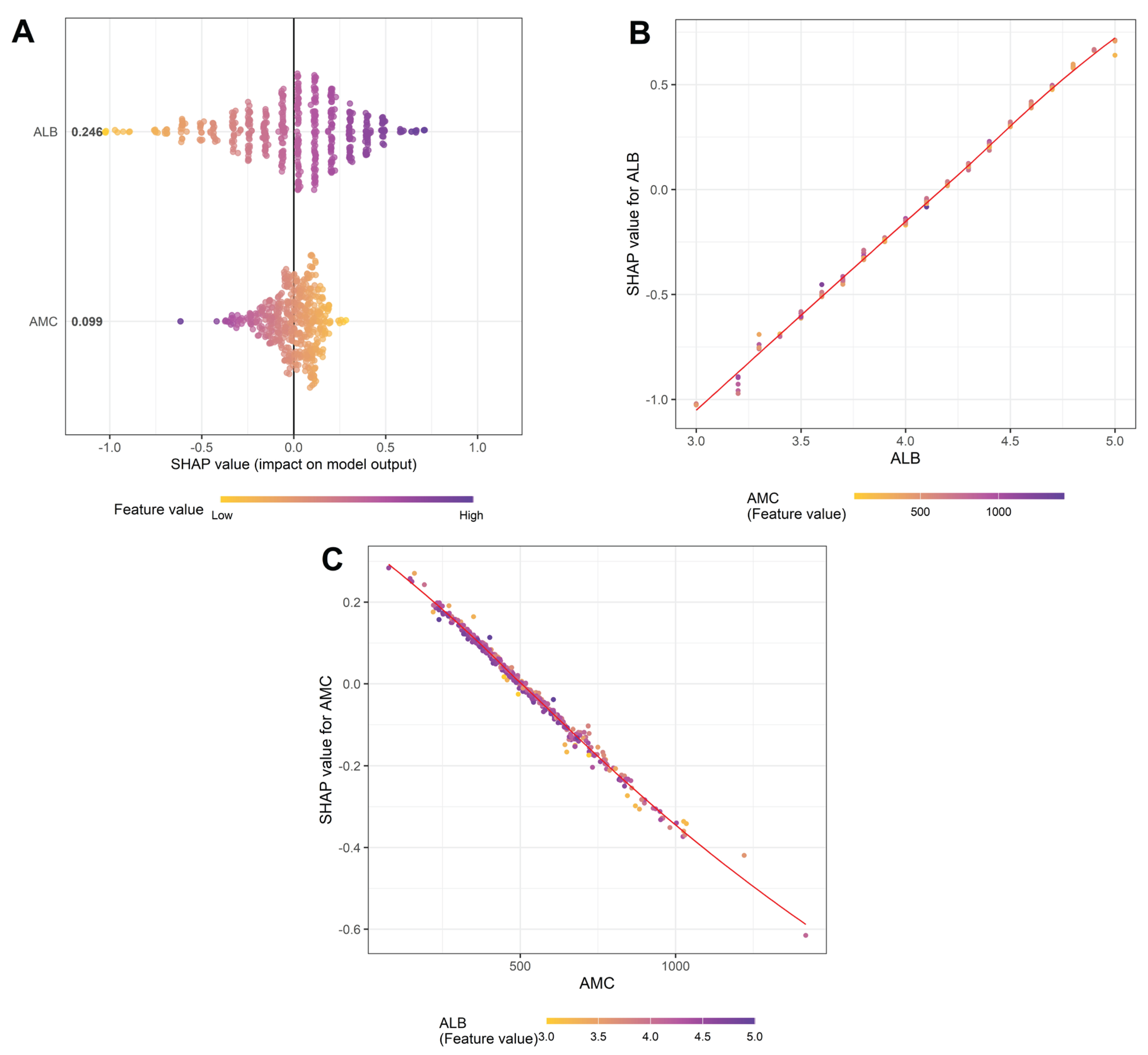
LASSO regression and SHAP analysis were performed to elucidate factors influencing the PINI. Among 30 candidate variables, only ALB level (coefficient: 0.8799) and AMC (coefficient: –0.0007) retained nonzero coefficients in the LASSO model, reinforcing their exclusive roles in defining the PINI. The predictive performance of the model was excellent, with an R-squared of 0.999, RMSE of 0.0117, and MSE of 0.00014.
SHAP analysis further revealed that ALB level contributed more strongly to the PINI than AMC (mean SHAP value: 0.246 vs. 0.099); however, both variables had substantial and opposing effects: ALB level was positively associated, while AMC was negatively associated with SHAP values of the PINI (
Figure 8A). Notably, SHAP dependence plots indicated a strong interactive effect between ALB level and AMC. In the ALB dependence plot (
Figure 8B), the SHAP values for ALB level (i.e., how much ALB contributed to the PINI score) increased almost linearly with increasing ALB concentration, and this effect was amplified in the context of low AMC values. Conversely, the AMC dependence plot (
Figure 8C) demonstrated that the negative contribution of AMC to the PINI score became more pronounced at higher ALB levels, indicating a potential interaction between AMC and ALB level. These findings suggest that the prognostic strength of the PINI arises not only from the additive effects of its components but also from a synergistic interplay between AMC and ALB level. This interaction likely contributes to the superior prognostic utility of the PINI compared to its individual components.
4. Discussion
The FM, which incorporated the PINI along with key clinical and hematologic variables, demonstrated significantly better predictive performance than the BM that included only stage. This was evidenced by higher C-index and iAUC values, as well as substantial improvements in risk discrimination and reclassification, as measured by the IDI and cNRI. When compared to the IM, which included the same clinical variables except the PINI, the FM continued to show statistically significant gains across all performance metrics. These findings underscore the added prognostic value of the PINI in enhancing survival prediction among patients with stage I–IIIA NSCLC undergoing curative-intent resection.
Furthermore, the FM consistently outperformed both the BM and IM in time-dependent C-index analyses over a 10-year follow-up period. The most substantial performance gains were observed within the first 5 years and remained stable thereafter. Although the FM maintained a pronounced advantage over the BM throughout the study period, it also demonstrated consistently superior performance compared to the IM, albeit with a more modest but sustained margin. The progressive decline in the C-index of the BM over time underscores the limitations of staging as a standalone prognostic tool. Although staging remains essential for baseline risk stratification, it does not account for dynamic patient-specific factors such as systemic inflammation, nutritional decline, treatment response, or emerging comorbidities. These evolving influences likely have an increasing impact on long-term survival, thereby diminishing the predictive utility of static anatomical staging alone. Taken together, these findings highlight the value of integrative prognostic models that combine conventional staging with dynamic biomarker inputs to enable more accurate and durable risk stratification.
However, the underlying mechanisms by which the PINI predicts OS in patients with NSCLC remain unclear. In a study by An et al. involving patients with gastric cancer, LASSO regression retained only ALB level and AMC as predictors of the PINI. Their model exhibited excellent performance with an R² of 0.9992 and an RMSE of 0.0112 on the test set, indicating an exceptionally strong fit. These findings support the notion that ALB level and AMC are the principal determinants of PINI expression and underscore their robustness as biologically grounded biomarkers [
17]. Consistent with these findings, our LASSO regression analysis identified ALB level and AMC as the sole contributors among candidate variables. The model demonstrated near-perfect performance (R² = 0.9990, RMSE = 0.0117, MSE = 0.00014), underscoring the dominant influence of these two parameters in determining the PINI. Further interpretability was achieved through SHAP analysis. Dependence plots revealed that the SHAP values of ALB increased almost linearly with higher ALB levels, particularly when the AMC was low. Conversely, AMC exhibited the most negative impact on the PINI when ALB levels were high. These interactions suggest that the prognostic utility of the PINI is not merely due to the additive effects of its components but also stems from their dynamic interplay, which captures the interaction between systemic inflammation (i.e., AMC) and protein nutritional reserve (i.e., ALB level). This interaction explains why the PINI may outperform ALB level or AMC alone in predicting outcomes; it dynamically integrates both effects. Together, these findings reinforce the biological rationale for using the PINI as a robust, interpretable, and parsimonious index.
In addition to the PINI, variables such as age, ASA, PL, stage, and the mSLI emerged as significant predictors of OS and were incorporated into the FM. Age is a widely recognized prognostic factor associated with worse outcomes, primarily owing to the presence of comorbidities and diminished physiological reserve [
5,
6,
7,
44]. Similarly, elevated ASA scores, which reflect a poorer preoperative health status, are linked to greater perioperative risk and reduced survival [
44]. PL signifies more advanced disease and elevated recurrence risk [
5,
6]. Staging remains the cornerstone of NSCLC prognosis, with advanced stages reflecting lower survival rates owing to limited surgical options [
4,
5,
6,
7,
13]. Although less extensively studied, RBC-derived markers are gaining recognition as prognostic indicators for NSCLC [
45,
46]. Among these, the mSLI, a composite metric calculated as (MCV² × MCH) × 0.0001 and originally developed for the diagnosis of thalassemia, has recently emerged as a promising prognostic marker [
40]. The present study reinforces the prognostic significance of RBC-derived indices, including the mSLI, in patients with stage I‒IIIA NSCLC.
In comparative analyses, the PINI outperformed other well-established biomarkers, including the ALB level, NLR, LMR, PNI, SII, SIRI, HALP, and CALLY index, when each was evaluated within a consistent multivariate framework. Specifically, the model incorporating the PINI achieved the highest C-index (0.841), whereas the other models yielded slightly lower values, ranging from 0.822 to 0.840. These findings underscore the strong prognostic value of the PINI relative to other inflammation- and nutrition-based indices in patients with stage I–IIIA NSCLC. The reason for the limited incremental benefit observed with biomarkers other than PINI could be explained by the potential overlap in the captured prognostic information, highlighting the importance of selecting parsimonious and biologically relevant variables through rigorous multivariable modeling. In addition, the variability in previous findings across studies may stem from differences in patient populations, disease stages, or modeling approaches, particularly whether variables were treated as continuous or categorized. Overall, our results support integrating the PINI into clinical prognostic models and emphasize the need for careful biomarker selection to enhance predictive accuracy without redundancy.
The principal strength of this study lies in its focused evaluation of the PINI as a prognostic biomarker in patients with stage I–IIIA NSCLC undergoing curative-intent surgical resection. Based on the results of this study, incorporating the PINI into multivariable prognostic models may enhance both the predictive performance and risk stratification capacity. The FM demonstrated superior discrimination across various statistical metrics and provided more clinically actionable predictions through DCA and an individualized nomogram. These results suggest that the FM can help identify high-risk patients who may benefit from adjuvant therapies, closer surveillance, or tailored follow-up regimens, while simultaneously sparing low-risk individuals from overtreatment. Given its affordability, objectivity, and universal availability in routine CBC, the PINI is uniquely positioned as a pragmatic biomarker in real-world clinical settings. It avoids the limitations of expensive or technically complex assays and is not influenced by interobserver variability, offering immediate clinical applicability without increased healthcare costs. These findings underscore the potential of the PINI to complement existing staging systems and support individualized care pathways in patients with stage I–IIIA NSCLC. Although the precise biological underpinnings of the PINI remain to be fully elucidated, its robust and independent prognostic value, coupled with its accessibility, makes it a compelling candidate for broader inclusion in clinical decision-making tools and future prospective trials.
Nonetheless, this study had several limitations. As this was a retrospective, single-center study, the analysis was inherently subject to selection bias and residual confounding, which may limit causal inferences and generalizability. The cohort’s ethnic homogeneity (i.e., comprising 98.1% East Asian patients) further restricts its applicability to broader populations, considering the potential racial and genetic differences in tumor biology and hematologic parameters. In addition, the use of baseline cross-sectional laboratory data precluded the evaluation of longitudinal changes in the PINI and their relationship with disease progression or treatment response. Postoperative PINI assessment was also challenging because adjuvant therapies, often involving chemotherapy and/or radiation, can significantly alter ALB level and AMC, leading to unstable and difficult-to-interpret values. Although internal validation via 1000 bootstrap iterations supported the robustness of the model, the absence of external validation in independent cohorts limits its broader clinical applicability. These limitations underscore the need for future prospective multicenter studies incorporating ethnically diverse populations, serial biomarker measurements, and external validation to fully establish the prognostic utility of the PINI in NSCLC [
47,
48].
5. Conclusions
This study established the PINI as an independent and clinically meaningful biomarker for predicting OS in patients with stage I–IIIA NSCLC undergoing curative-intent surgical resection. Incorporating the PINI into the FM significantly enhanced its prognostic performance compared with that of the IM and BM, thereby improving risk stratification and supporting its potential role in guiding personalized postoperative management. Owing to its simplicity, low cost, and accessibility through routine preoperative blood tests, the PINI is a practical tool for clinical application. However, external validation in independent multi-ethnic cohorts is essential to confirm the robustness and generalizability of these findings.
Author Contributions
Conceptualization, S.A., W.E., S.L., and S.K.; methodology, S.A., W.E., S.L., and S.K.; resources, S.A., W.E., S.L., and S.K.; formal analysis, S.A., W.E., S.L., and S.K.; investigation, S.A., W.E., S.L., and S.K..; resources, S.A., W.E., S.L., and S.K.; data curation, S.A., W.E., S.L., and S.K.; writing—original draft preparation, S.A., W.E., S.L., and S.K.; writing—original draft preparation, S.A., W.E., S.L., and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Kyung Hee University Hospital at Gangdong, Seoul, Korea (IRB approval number: 2025-07-024).
Informed Consent Statement
Informed patient consent was waived owing to the retrospective nature of the study.
Data Availability Statement
The datasets presented in this study are available upon request from the corresponding author owing to ethical reasons.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the study design, collection, analyses, interpretation of data, writing of the manuscript, or decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ALB |
Albumin |
| ALC |
Absolute lymphocyte
count |
| ALT |
Alanine
aminotransferase |
| AMC |
Absolute monocyte
count |
| ANC |
Absolute neutrophil
count |
| ASA |
American society of
anesthesiologists physical status |
| AST |
Aspartate
aminotransferase |
| AUC |
Area under the curve |
| BMI |
Body mass index |
| CALLY |
CRP-albumin-lymphocyte |
| C-index |
Concordance index |
| cNRI |
Continuous net
reclassification improvement |
| CRP |
C-reactive protein |
| DCA |
Decision curve
analysis |
| DFS |
Disease-free survival |
| FP |
Fractional polynomial |
| HALP |
Hemoglobin-albumin-lymphocyte-platelet |
| HR |
Hazard ratio |
| iAUC |
Integrated AUC |
| IDI |
Integrated
discrimination improvement |
| IQR |
Interquartile range |
| LASSO |
Least absolute
shrinkage and selection operator |
| LMR |
Monocyte-to-lymphocyte
ratio |
| MCH |
Mean corpuscular
hemoglobin |
| MCHC |
Mean corpuscular
hemoglobin concentration |
| MCV |
Mean corpuscular
volume |
| mSLI |
Modified Shine–Lal
index |
| NLR |
Neutrophil-to-lymphocyte
ratio |
| NSCLC |
Non-small cell lung
cancer |
| OS |
Overall survival |
| PINI |
Prognostic immune and
nutritional index |
| PL |
Pleural invasion |
| PLR |
Platelet-to-lymphocyte
ratio |
| PNI |
Prognostic
nutritional index |
| RBC |
Red blood cell |
| SHAP |
SHapley Additive
exPlanations |
References
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: more than meets the eye. The Lancet. Oncology 2015, 16, e173–180. [Google Scholar] [CrossRef]
- Oñate-Ocaña, L.F.; Aiello-Crocifoglio, V.; Gallardo-Rincón, D.; Herrera-Goepfert, R.; Brom-Valladares, R.; Carrillo, J.F.; Cervera, E.; Mohar-Betancourt, A. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Annals of surgical oncology 2007, 14, 381–389. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, X.; Zhang, Q.; Ruan, G.T.; Liu, T.; Xie, H.L.; Ge, Y.Z.; Song, M.M.; Deng, L.; Shi, H.P. The value of CRP-albumin-lymphocyte index (CALLY index) as a prognostic biomarker in patients with non-small cell lung cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 2023, 31, 533. [Google Scholar] [CrossRef]
- Sato, S.; Sezaki, R.; Shinohara, H. Significance of preoperative evaluation of modified advanced lung cancer inflammation index for patients with resectable non-small cell lung cancer. Gen Thorac Cardiovasc Surg 2024. [CrossRef]
- Takada, K.; Takamori, S.; Matsubara, T.; Haratake, N.; Akamine, T.; Kinoshita, F.; Ono, Y.; Wakasu, S.; Tanaka, K.; Oku, Y.; et al. Clinical significance of preoperative inflammatory markers in non-small cell lung cancer patients: A multicenter retrospective study. PloS one 2020, 15, e0241580. [Google Scholar] [CrossRef]
- Taylor, M.; Evison, M.; Michael, S.; Obale, E.; Fritsch, N.C.; Abah, U.; Smith, M.; Martin, G.P.; Shackcloth, M.; Granato, F.; et al. Pre-Operative Measures of Systemic Inflammation Predict Survival After Surgery for Primary Lung Cancer. Clin Lung Cancer 2024, 25, 460–467.e467. [Google Scholar] [CrossRef]
- Pellini, B.; Chaudhuri, A.A. Circulating Tumor DNA Minimal Residual Disease Detection of Non-Small-Cell Lung Cancer Treated With Curative Intent. J Clin Oncol 2022, 40, 567–575. [Google Scholar] [CrossRef]
- Balata, H.; Foden, P.; Edwards, T.; Chaturvedi, A.; Elshafi, M.; Tempowski, A.; Teng, B.; Whittemore, P.; Blyth, K.G.; Kidd, A.; et al. Predicting survival following surgical resection of lung cancer using clinical and pathological variables: The development and validation of the LNC-PATH score. Lung cancer (Amsterdam, Netherlands) 2018, 125, 29–34. [Google Scholar] [CrossRef]
- Onaitis, M.W.; Furnary, A.P.; Kosinski, A.S.; Kim, S.; Boffa, D.; Tong, B.C.; Cowper, P.; Jacobs, J.P.; Wright, C.D.; Putnam, J.B., Jr.; et al. Prediction of Long-Term Survival After Lung Cancer Surgery for Elderly Patients in The Society of Thoracic Surgeons General Thoracic Surgery Database. The Annals of thoracic surgery 2018, 105, 309–316. [Google Scholar] [CrossRef]
- Hara, M.; Matsuzaki, Y.; Shimuzu, T.; Tomita, M.; Ayabe, T.; Enomoto, Y.; Onitsuka, T. Preoperative serum C-reactive protein level in non-small cell lung cancer. Anticancer research 2007, 27, 3001–3004. [Google Scholar]
- Motono, N.; Mizoguchi, T.; Ishikawa, M.; Iwai, S.; Iijima, Y.; Uramoto, H. Prognostic Impact of Cancer Inflammation Prognostic Index for Non-small Cell Lung Cancer. Lung 2023, 201, 603–610. [Google Scholar] [CrossRef]
- Tomita, M.; Shimizu, T.; Hara, M.; Ayabe, T.; Onitsuka, T. Preoperative leukocytosis, anemia and thrombocytosis are associated with poor survival in non-small cell lung cancer. Anticancer research 2009, 29, 2687–2690. [Google Scholar]
- Jung, S.H.; Hao, J.; Shivakumar, M.; Nam, Y.; Kim, J.; Kim, M.J.; Ryoo, S.B.; Choe, E.K.; Jeong, S.Y.; Park, K.J.; et al. Development and validation of a novel strong prognostic index for colon cancer through a robust combination of laboratory features for systemic inflammation: a prognostic immune nutritional index. British journal of cancer 2022, 126, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wei, L.; Liu, M.; Liang, Y.; Yuan, G.; Gao, S.; Wang, Q.; Lin, X.; Tang, S.; Gan, J. Prognostic significance of preoperative prognostic immune and nutritional index in patients with stage I-III colorectal cancer. BMC cancer 2022, 22, 1316. [Google Scholar] [CrossRef]
- Shibutani, M.; Kashiwagi, S.; Fukuoka, T.; Iseki, Y.; Kasashima, H.; Maeda, K. Significance of the Prognostic Immune and Nutritional Index in Patients With Stage I-III Colorectal Cancer. Cancer Diagn Progn 2023, 3, 354–359. [Google Scholar] [CrossRef]
- An, S.; Eo, W.; Lee, S. Prognostic Immune and Nutritional Index as a Predictor of Survival in Patients Undergoing Curative-Intent Resection for Gastric Cancer. Medicina (Kaunas) 2025, 61. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Yonei, A.; Ayabe, T.; Tomita, M.; Nakamura, K.; Onitsuka, T. Postoperative serum C-reactive protein levels in non-small cell lung cancer patients. Annals of thoracic and cardiovascular surgery : official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia 2010, 16, 85–90. [Google Scholar] [PubMed]
- Alifano, M.; Falcoz, P.E.; Seegers, V.; Roche, N.; Schussler, O.; Younes, M.; Antonacci, F.; Forgez, P.; Dechartres, A.; Massard, G.; et al. Preresection serum C-reactive protein measurement and survival among patients with resectable non-small cell lung cancer. The Journal of thoracic and cardiovascular surgery 2011, 142, 1161–1167. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutrition journal 2010, 9, 69. [Google Scholar] [CrossRef]
- Miura, K.; Hamanaka, K.; Koizumi, T.; Kitaguchi, Y.; Terada, Y.; Nakamura, D.; Kumeda, H.; Agatsuma, H.; Hyogotani, A.; Kawakami, S.; et al. Clinical significance of preoperative serum albumin level for prognosis in surgically resected patients with non-small cell lung cancer: Comparative study of normal lung, emphysema, and pulmonary fibrosis. Lung cancer (Amsterdam, Netherlands) 2017, 111, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, O.; Avci, N.; Oktay, E.; Kalemci, S.; Pilanci, K.N.; Cokmert, S.; Menekse, S.; Kocar, M.; Sen, C.A.; Akman, T.; et al. Pretreatment Serum Albumin Level is an Independent Prognostic Factor in Patients with Stage IIIB Non-Small Cell Lung Cancer: A Study of the Turkish Descriptive Oncological Researches Group. Asian Pac J Cancer Prev 2015, 16, 5971–5976. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Yoshioka, H.; Ikeo, S.; Morita, M.; Sone, N.; Niwa, T.; Nishiyama, A.; Yokoyama, T.; Sekine, A.; Ogura, T.; et al. Serum albumin level as a potential marker for deciding chemotherapy or best supportive care in elderly, advanced non-small cell lung cancer patients with poor performance status. BMC cancer 2017, 17, 797. [Google Scholar] [CrossRef] [PubMed]
- Kiss, M.; Caro, A.A.; Raes, G.; Laoui, D. Systemic Reprogramming of Monocytes in Cancer. Front Oncol 2020, 10, 1399. [Google Scholar] [CrossRef]
- Huang, B.; Lei, Z.; Zhao, J.; Gong, W.; Liu, J.; Chen, Z.; Liu, Y.; Li, D.; Yuan, Y.; Zhang, G.M.; et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett 2007, 252, 86–92. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov 2018, 17, 887–904. [Google Scholar] [CrossRef]
- Schiefer, S.; Wirsik, N.M.; Kalkum, E.; Seide, S.E.; Nienhüser, H.; Müller, B.; Billeter, A.; Büchler, M.W.; Schmidt, T.; Probst, P. Systematic Review of Prognostic Role of Blood Cell Ratios in Patients with Gastric Cancer Undergoing Surgery. Diagnostics (Basel) 2022, 12. [Google Scholar] [CrossRef]
- An, S.; Eo, W.; Lee, S.; Lee, Y.J. Monocyte-to-lymphocyte ratio as a determinant of survival in patients with gastric cancer undergoing gastrectomy: A cohort study. Medicine 2023, 102, e33930. [Google Scholar] [CrossRef]
- Eo, W.K.; Jeong, D.W.; Chang, H.J.; Won, K.Y.; Choi, S.I.; Kim, S.H.; Chun, S.W.; Oh, Y.L.; Lee, T.H.; Kim, Y.O.; et al. Absolute monocyte and lymphocyte count prognostic score for patients with gastric cancer. World journal of gastroenterology 2015, 21, 2668–2676. [Google Scholar] [CrossRef]
- Yazici, H.; Yegen, S.C. Is Systemic Inflammatory Response Index (SIRI) a Reliable Tool for Prognosis of Gastric Cancer Patients Without Neoadjuvant Therapy? Cureus 2023, 15, e36597. [Google Scholar] [CrossRef]
- An, S.; Eo, W.; Kim, D.H.; Lee, S. Prognostic Value of the Noble and Underwood Score in Patients with Non-Small Cell Lung Cancer Undergoing Surgical Resection. Journal of Cancer 2024, 15, 6185–6195. [Google Scholar] [CrossRef]
- Hwang, J.J.; Hur, J.Y.; Eo, W.; An, S.; Kim, D.H.; Lee, S. Clinical significance of C-Reactive Protein to Lymphocyte Count Ratio as a prognostic factor for Survival in Non-small Cell Lung Cancer Patients undergoing Curative Surgical Resection. Journal of Cancer 2021, 12, 4497–4504. [Google Scholar] [CrossRef]
- Eo, W.K.; Chang, H.J.; Suh, J.; Ahn, J.; Shin, J.; Hur, J.Y.; Kim, G.Y.; Lee, S.; Park, S.; Lee, S. The Prognostic Nutritional Index Predicts Survival and Identifies Aggressiveness of Gastric Cancer. Nutrition and cancer 2015, 67, 1260–1267. [Google Scholar] [CrossRef]
- Travis, W.D. The 2015 WHO classification of lung tumors. Der Pathologe 2014, 35 Suppl 2, 188. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Hermanek, P.; Wittekind, C. The pathologist and the residual tumor (R) classification. Pathology, research and practice 1994, 190, 115–123. [Google Scholar] [CrossRef]
- Arriagada, R.; Bergman, B.; Dunant, A.; Le Chevalier, T.; Pignon, J.P.; Vansteenkiste, J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. The New England journal of medicine 2004, 350, 351–360. [Google Scholar] [CrossRef]
- Kawase, A.; Yoshida, J.; Miyaoka, E.; Asamura, H.; Fujii, Y.; Nakanishi, Y.; Eguchi, K.; Mori, M.; Sawabata, N.; Okumura, M.; et al. Visceral pleural invasion classification in non-small-cell lung cancer in the 7th edition of the tumor, node, metastasis classification for lung cancer: validation analysis based on a large-scale nationwide database. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2013, 8, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Seishima, R.; Matsui, S.; Shigeta, K.; Okabayashi, K.; Kitagawa, Y. The prognostic impact of preoperative mean corpuscular volume in colorectal cancer. Japanese journal of clinical oncology 2022, 52, 562–570. [Google Scholar] [CrossRef]
- An, S.; Eo, W.; Lee, S. Prognostic Significance of Modified Shine and Lal Index in Patients with Non-Small Cell Lung Cancer Undergoing Surgical Resection. Biomedicines 2025, 13. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Goodall, A.H. Studies on Mean Platelet Volume (MPV) - New Editorial Policy. Platelets 2016, 27, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Noris, P.; Melazzini, F.; Balduini, C.L. New roles for mean platelet volume measurement in the clinical practice? Platelets 2016, 27, 607–612. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology (Cambridge, Mass.) 2010, 21, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kyriss, T.; Dippon, J.; Hansen, M.; Boedeker, E.; Friedel, G. American Society of Anesthesiologists physical status facilitates risk stratification of elderly patients undergoing thoracoscopic lobectomy. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery 2018, 53, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Zhang, T.; Ma, H.; Sui, P.; Du, J. Lower mean corpuscular hemoglobin concentration is associated with unfavorable prognosis of resected lung cancer. Future oncology (London, England) 2014, 10, 2149–2159. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J. Clinical significance of mean corpuscular volume as a prognostic indicator of radiotherapy for locally advanced lung cancer: a retrospective cohort study. Journal of thoracic disease 2022, 14, 4916–4924. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Chen, W.; Niu, N.; Zhao, J.; Qi, W.; Xu, Y. Development and validation of a prognostic nomogram for early stage non-small cell lung cancer: a study based on the SEER database and a Chinese cohort. BMC cancer 2022, 22, 980. [Google Scholar] [CrossRef]
- Kassir, N.; Chan, P.; Dang, S.; Bruno, R. External validation of a tumor growth inhibition-overall survival model in non-small-cell lung cancer based on atezolizumab studies using alectinib data. Cancer Chemother Pharmacol 2023, 92, 205–210. [Google Scholar] [CrossRef]
Figure 1.
Cox regression analysis for predictors of overall survival. mSLI, modified Shine-Lal index; PINI, prognostic immune and nutritional index; PL, pleural invasion.
Figure 1.
Cox regression analysis for predictors of overall survival. mSLI, modified Shine-Lal index; PINI, prognostic immune and nutritional index; PL, pleural invasion.
Figure 2.
Association between the prognostic immune and nutritional index and the log-relative hazard of death using fractional polynomial Cox regression. (A) Univariate model; (B) Multivariate model adjusted for clinical covariates. Shaded areas represent 95% confidence intervals.
Figure 2.
Association between the prognostic immune and nutritional index and the log-relative hazard of death using fractional polynomial Cox regression. (A) Univariate model; (B) Multivariate model adjusted for clinical covariates. Shaded areas represent 95% confidence intervals.
Figure 3.
Predictive nomogram for overall survival based on the full model. mSLI, modified Shine-Lal index; PINI, prognostic immune and nutritional index; PL, pleural invasion.
Figure 3.
Predictive nomogram for overall survival based on the full model. mSLI, modified Shine-Lal index; PINI, prognostic immune and nutritional index; PL, pleural invasion.
Figure 4.
Calibration curve analysis for predicting 3-year (A) and 5-year (B) overall survival based on the full model.
Figure 4.
Calibration curve analysis for predicting 3-year (A) and 5-year (B) overall survival based on the full model.
Figure 5.
Decision curve analysis for 3-year (A) and 5-year (B) overall survival.
Figure 5.
Decision curve analysis for 3-year (A) and 5-year (B) overall survival.
Figure 6.
Time-dependent C-index over a 10-year period for the full, intermediate, and baseline models.
Figure 6.
Time-dependent C-index over a 10-year period for the full, intermediate, and baseline models.
Figure 7.
The prognostic immune and nutritional index vs. established biomarkers: Model discrimination for survival outcomes. ALB, albumin; CALLY, CRP–ALB–lymphocyte index; HALP, hemoglobin–ALB–lymphocyte–platelet; IM, intermediate model; LMR, lymphocyte-to-monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PINI, prognostic immune and nutritional index; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index.
Figure 7.
The prognostic immune and nutritional index vs. established biomarkers: Model discrimination for survival outcomes. ALB, albumin; CALLY, CRP–ALB–lymphocyte index; HALP, hemoglobin–ALB–lymphocyte–platelet; IM, intermediate model; LMR, lymphocyte-to-monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PINI, prognostic immune and nutritional index; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index.
Figure 8.
SHapley Additive exPlanations (SHAP) analysis of top features affecting the prognostic immune and nutritional index. (A) SHAP summary plot showing the overall impact and direction of key predictors. (B) Dependence plot for albumin (ALB), colored by absolute monocyte count (AMC). (C) Dependence plot for AMC, colored by ALB level. Red diagonal lines demonstrate the line of best fit.
Figure 8.
SHapley Additive exPlanations (SHAP) analysis of top features affecting the prognostic immune and nutritional index. (A) SHAP summary plot showing the overall impact and direction of key predictors. (B) Dependence plot for albumin (ALB), colored by absolute monocyte count (AMC). (C) Dependence plot for AMC, colored by ALB level. Red diagonal lines demonstrate the line of best fit.
Table 1.
Clinicopathological characteristics of study participants.
Table 1.
Clinicopathological characteristics of study participants.
| Variables |
N (%) or median (IQR) |
Variables |
N (%) or median (IQR) |
| Age, years |
69.0 (62.0–74.0) |
Vascular invasion |
|
| Sex |
|
|
Yes |
28 (5.4%) |
| |
Men |
303 (58.0%) |
|
No |
494 (94.6%) |
| |
Women |
219 (42.0%) |
Perineural invasion |
|
| Smoking |
|
|
Yes |
8 (1.5%) |
| |
Current/Past |
210 (40.2%) |
|
No |
514 (98.5%) |
| |
Never |
312 (59.8%) |
TNM stage |
|
| Alcohol consumption |
|
|
IA/IB |
377 (72.2%) |
| |
Yes |
132 (25.3%) |
|
IIA/IIB/IIIA |
145 (27.8%) |
| |
No |
390 (74.7%) |
Protein, g/dL |
7.1 (6.8–7.5) |
| ASA-PS |
|
Albumin, g/dL |
4.2 (4.0–4.4) |
| |
1/2 |
428 (82.0%) |
Total bilirubin, mg/dL |
0.5 (0.4–0.6) |
| |
3/4 |
94 (18.0%) |
AST, U/L |
22.0 (19.0–27.0) |
| BMI, kg/m2 |
23.9 (21.9–26.2) |
ALT, U/L |
17.0 (12.0–23.0) |
| Resection |
|
CRP, mg/dL |
0.1 (0.1–0.3) |
| |
Sublobar resection |
194 (37.2%) |
WBC, per μL |
6315.0 (5330.0–7460.0) |
| |
Lobectomy |
315 (60.3%) |
ANC, per μL |
3622.0 (2920.0–4634.0) |
| |
Bilobectomy |
6 (1.1%) |
AMC, per μL |
479.0 (382.0–610.0) |
| |
Pneumonectomy |
7 (1.3%) |
ALC, per μL |
1831.5 (1532.0–2240.0) |
| Histology |
|
RBC, × 106 per μL |
4.3 (3.9–4.6) |
| |
Squamous |
115 (22.0%) |
Hemoglobin, g/dL |
13.3 (12.1–14.2) |
| |
Non-squamous |
407 (78.0%) |
MCV, fL |
91.8 (89.2–94.8) |
| Tumor size, cm |
2.5 (1.7–3.5) |
MCH, g/dL |
30.9 (30.0–32.1) |
| Pleural invasion (PL) |
|
MCHC, g/dL |
33.7 (33.1–34.3) |
| |
0 |
417 (79.9%) |
mSLI |
26.0 (24.1–28.8) |
| |
≥1 |
105 (20.1%) |
Platelet, × 103 per μL |
236.0 (202.0–278.0) |
| Lymphatic invasion |
|
PINI |
3.4 (3.2–3.6) |
| |
Yes |
64 (12.3%) |
|
|
| |
No |
458 (87.7%) |
|
|
Table 2.
Model comparison for survival prediction.
Table 2.
Model comparison for survival prediction.
| Metrics |
Improvement
(FM vs. BM) |
p value
(FM vs. BM) |
Improvement (FM vs. IM) |
p value
(FM vs. IM) |
| C-index |
0.153 (0.023) |
<0.001 |
0.022 (0.012) |
0.012 |
| iAUC |
0.141 (0.009) |
<0.001 |
0.013 (0.004) |
0.001 |
| IDI at 3 years |
0.252 (0.044) |
<0.001 |
0.054 (0.024) |
0.008 |
| cNRI at 3 years |
0.502 (0.069) |
<0.001 |
0.320 (0.088) |
0.020 |
| IDI at 5 years |
0.230 (0.042) |
<0.001 |
0.039 (0.021) |
0.018 |
| cNRI at 5 years |
0.418 (0.068) |
<0.001 |
0.238 (0.086) |
0.032 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).