Submitted:
08 August 2025
Posted:
08 August 2025
You are already at the latest version
Abstract
Keywords:
Introduction
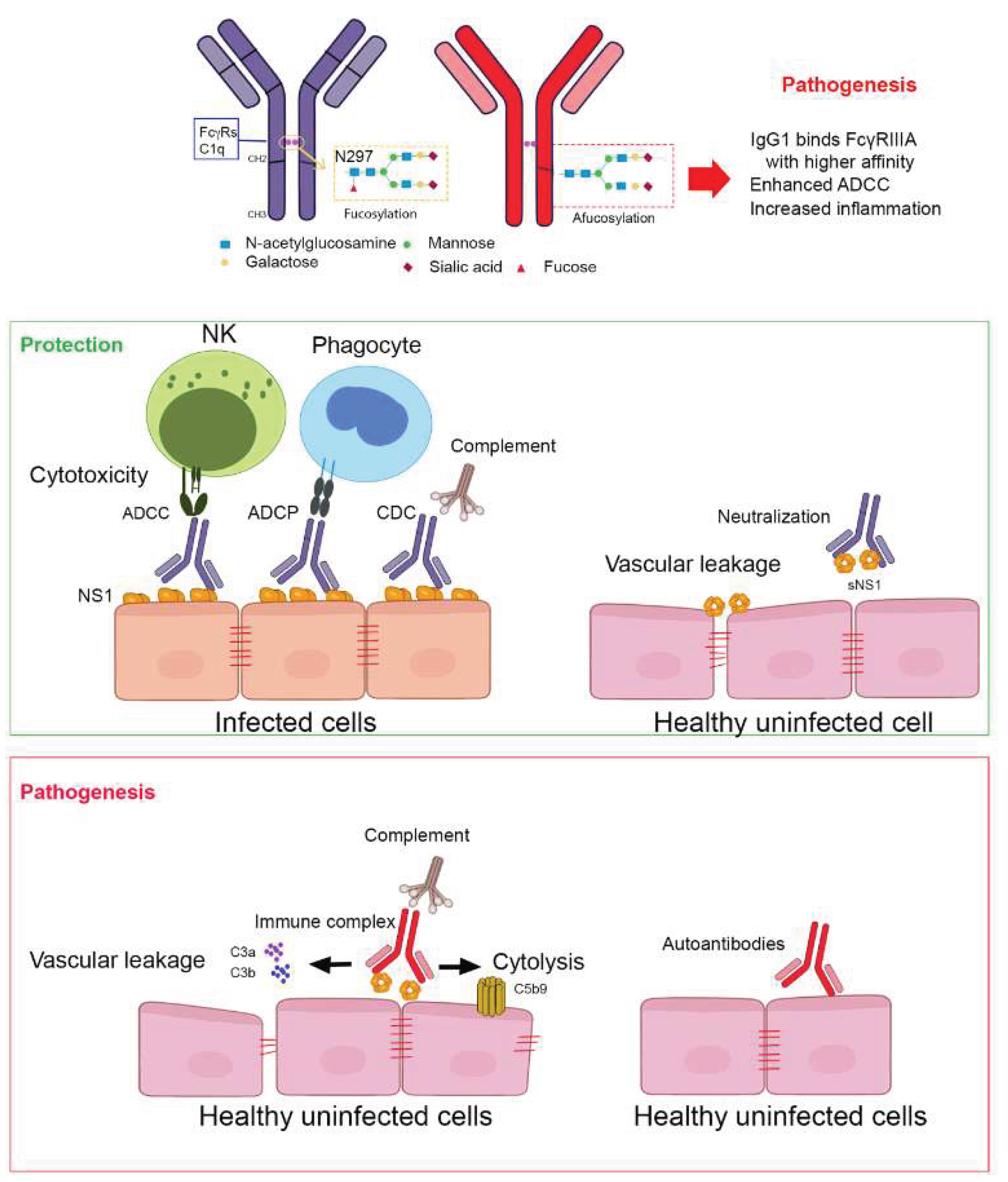
Roles of Anti-NS1 Antibodies
IgG Subclasses and Functions
Fc glycosylation and disease severity
Clinical Relevance and Translational Potential
Conclusion and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzman, M.G.; et al. Dengue infection. Nat Rev Dis Primers 2016, 2, 16055. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–7. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, G. , WHO recommends additional tests for Sanofi's dengue vaccine after safety concerns. Bmj 2018, 361, k1765. [Google Scholar] [CrossRef]
- Cracknell Daniels, B., N. A.-O. Ferguson, and I.A.-O. Dorigatti, Efficacy, public health impact and optimal use of the Takeda dengue vaccine. LID - 10.1038/s41591-025-03771-y [doi]. (1546-170X (Electronic)).
- Tricou, V.; et al. Long-term efficacy and safety of a tetravalent dengue vaccine (TAK-003): 4·5-year results from a phase 3, randomised, double-blind, placebo-controlled trial. (2214-109X (Electronic)).
- Nogueira, M.L.; et al. Efficacy and safety of Butantan-DV in participants aged 2-59 years through an extended follow-up: results from a double-blind, randomised, placebo-controlled, phase 3, multicentre trial in Brazil. (1474-4457 (Electronic)).
- Nanaware, N.; et al. Dengue Virus Infection: A Tale of Viral Exploitations and Host Responses. LID - 10.3390/v13101967 [doi] LID - 1967. (1999-4915 (Electronic)).
- Urcuqui-Inchima, S.; et al. Recent developments in understanding dengue virus replication. (1557-8399 (Electronic)).
- Praneechit, H.; et al. Whole-blood model reveals granulocytes as key sites of dengue virus propagation, expanding understanding of disease pathogenesis. (2150-7511 (Electronic)).
- Dejnirattisai, W.; et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010, 328, 745–8. [Google Scholar] [CrossRef]
- Jayathilaka, D.; et al. Role of NS1 antibodies in the pathogenesis of acute secondary dengue infection. Nat Commun 2018, 9, 5242. [Google Scholar] [CrossRef]
- Hertz, T.A.-O.; et al. Antibody Epitopes Identified in Critical Regions of Dengue Virus Nonstructural 1 Protein in Mouse Vaccination and Natural Human Infections. (1550-6606 (Electronic)).
- Katzelnick, L.C.; et al. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; et al. Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine. Front Cell Infect Microbiol 2020, 10, 572681. [Google Scholar] [CrossRef]
- Westaway, E.G. and M.R. Goodman, Variation in distribution of the three flavivirus-specified glycoproteins detected by immunofluorescence in infected Vero cells. Arch Virol, 1987. 94(3-4): p. 215-28.
- Chambers, T.J.; et al. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol 1990, 44, 649–88. [Google Scholar] [CrossRef]
- Winkler, G.; et al. Evidence that the mature form of the flavivirus nonstructural protein NS1 is a dimer. Virology 1988, 162, 187–96. [Google Scholar] [CrossRef]
- Flamand, M.; et al. Dengue virus type 1 nonstructural glycoprotein NS1 is secreted from mammalian cells as a soluble hexamer in a glycosylation-dependent fashion. J Virol 1999, 73, 6104–10. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; et al. CryoEM structures of the multimeric secreted NS1, a major factor for dengue hemorrhagic fever. Nat Commun 2022, 13, 6756. [Google Scholar] [CrossRef]
- Gutsche, I.; et al. Secreted dengue virus nonstructural protein NS1 is an atypical barrel-shaped high-density lipoprotein. Proc Natl Acad Sci U S A 2011, 108, 8003–8. [Google Scholar] [CrossRef]
- Yap, S.S.L.; et al. Dengue Virus Glycosylation: What Do We Know? Front Microbiol 2017, 8, 1415. [Google Scholar] [CrossRef]
- Winkler, G.; et al. Newly synthesized dengue-2 virus nonstructural protein NS1 is a soluble protein but becomes partially hydrophobic and membrane-associated after dimerization. Virology 1989, 171, 302–5. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.M., M. K. Jones, and P.R. Young, Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology 1996, 220, 232-40.
- Lindenbach, B.D. and C.M. Rice, trans-Complementation of yellow fever virus NS1 reveals a role in early RNA replication. J Virol 1997, 71, 9608–17. [CrossRef]
- Pryor, M.J. and P.J. Wright, Glycosylation mutants of dengue virus NS1 protein. J Gen Virol, 1994. 75 ( Pt 5): p. 1183-7.
- Avirutnan, P.; et al. Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E. PLoS Pathog 2007, 3, e183. [Google Scholar] [CrossRef] [PubMed]
- Libraty, D.H.; et al. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 2002, 186, 1165–8. [Google Scholar] [CrossRef]
- Youn, S.; et al. Evidence for a genetic and physical interaction between nonstructural proteins NS1 and NS4B that modulates replication of West Nile virus. J Virol 2012, 86, 7360–71. [Google Scholar] [CrossRef]
- Akey, D.L.; et al. Structure-guided insights on the role of NS1 in flavivirus infection. Bioessays 2015, 37, 489–94. [Google Scholar] [CrossRef]
- Akey, D.L.; et al. Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science 2014, 343, 881–5. [Google Scholar] [CrossRef] [PubMed]
- Biering, S.B.; et al. Structural basis for antibody inhibition of flavivirus NS1-triggered endothelial dysfunction. Science 2021, 371, 194–200. [Google Scholar] [CrossRef]
- Beatty, P.R.; et al. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med 2015, 7, 304ra141. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; et al. Dengue virus or NS1 protein induces trans-endothelial cell permeability associated with VE-Cadherin and RhoA phosphorylation in HMEC-1 cells preventable by Angiopoietin-1. (1465-2099 (Electronic)).
- Modhiran, N.; et al. A broadly protective antibody that targets the flavivirus NS1 protein. Science 2021, 371, 190–194. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; et al. Flavivirus NS1 Triggers Tissue-Specific Disassembly of Intercellular Junctions Leading to Barrier Dysfunction and Vascular Leak in a GSK-3β-Dependent Manner. Pathogens, 2022. 11.
- Wang, C.; et al. Endocytosis of flavivirus NS1 is required for NS1-mediated endothelial hyperpermeability and is abolished by a single N-glycosylation site mutation. PLoS Pathog 2019, 15, e1007938. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Guardo, H., D. R. Glasner, and E. Harris, Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLOS Pathogens 2016, 12, e1005738.
- Glasner, D.R.; et al. Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components. PLoS Pathog 2017, 13, e1006673. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; et al. DENV NS1 and MMP-9 cooperate to induce vascular leakage by altering endothelial cell adhesion and tight junction. PLoS Pathog 2021, 17, e1008603. [Google Scholar] [CrossRef]
- Chen, H.R.; et al. Dengue Virus Nonstructural Protein 1 Induces Vascular Leakage through Macrophage Migration Inhibitory Factor and Autophagy. PLoS Negl Trop Dis 2016, 10, e0004828. [Google Scholar] [CrossRef]
- Modhiran, N.; et al. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med 2015, 7, 304ra142. [Google Scholar] [CrossRef]
- Modhiran, N.; et al. Dengue virus NS1 protein activates immune cells via TLR4 but not TLR2 or TLR6. Immunol Cell Biol 2017, 95, 491–495. [Google Scholar] [CrossRef]
- Avirutnan, P.; et al. Antagonism of the complement component C4 by flavivirus nonstructural protein NS1. J Exp Med 2010, 207, 793–806. [Google Scholar] [CrossRef]
- Avirutnan, P.; et al. Binding of flavivirus nonstructural protein NS1 to C4b binding protein modulates complement activation. J Immunol 2011, 187, 424–33. [Google Scholar] [CrossRef]
- Thiemmeca, S.; et al. Secreted NS1 Protects Dengue Virus from Mannose-Binding Lectin-Mediated Neutralization. J Immunol 2016, 197, 4053–4065. [Google Scholar] [CrossRef]
- Conde, J.N.; et al. Inhibition of the Membrane Attack Complex by Dengue Virus NS1 through Interaction with Vitronectin and Terminal Complement Proteins. J Virol 2016, 90, 9570–9581. [Google Scholar] [CrossRef]
- Bailey, M.J.; et al. Human antibodies targeting Zika virus NS1 provide protection against disease in a mouse model. Nat Commun 2018, 9, 4560. [Google Scholar] [CrossRef] [PubMed]
- Wessel, A.W.; et al. Antibodies targeting epitopes on the cell-surface form of NS1 protect against Zika virus infection during pregnancy. Nat Commun 2020, 11, 5278. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.W.; et al. Therapeutic Effects of Monoclonal Antibody against Dengue Virus NS1 in a STAT1 Knockout Mouse Model of Dengue Infection. J Immunol 2017, 199, 2834–2844. [Google Scholar] [CrossRef]
- Lai, Y.C.; et al. Antibodies Against Modified NS1 Wing Domain Peptide Protect Against Dengue Virus Infection. Sci Rep, 2017. 7.
- Chung, K.M.; et al. Antibody recognition of cell surface-associated NS1 triggers Fc-gamma receptor-mediated phagocytosis and clearance of West Nile Virus-infected cells. J Virol 2007, 81, 9551–5. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.M.; et al. Antibodies against West Nile Virus nonstructural protein NS1 prevent lethal infection through Fc gamma receptor-dependent and -independent mechanisms. J Virol 2006, 80, 1340–51. [Google Scholar] [CrossRef]
- Tien, S.M.; et al. Therapeutic efficacy of humanized monoclonal antibodies targeting dengue virus nonstructural protein 1 in the mouse model. (1553-7374 (Electronic)).
- Costa, S.M.; et al. Protection against dengue type 2 virus induced in mice immunized with a DNA plasmid encoding the non-structural 1 (NS1) gene fused to the tissue plasminogen activator signal sequence. Vaccine 2006, 24, 195–205. [Google Scholar] [CrossRef]
- Wan, S.W.; et al. Protection against dengue virus infection in mice by administration of antibodies against modified nonstructural protein 1. PLoS One 2014, 9, e92495. [Google Scholar] [CrossRef]
- Falgout, B.; et al. Immunization of mice with recombinant vaccinia virus expressing authentic dengue virus nonstructural protein NS1 protects against lethal dengue virus encephalitis. J Virol 1990, 64, 4356–63. [Google Scholar] [CrossRef]
- Dias, A.G.; et al. Anti-dengue virus antibodies that elicit complement-mediated lysis of Zika virion correlate with protection from severe dengue disease. (2211-1247 (Electronic)).
- Sanchez-Vargas, L.A.; et al. Protective Role of NS1-Specific Antibodies in the Immune Response to Dengue Virus through Antibody-Dependent Cellular Cytotoxicity. J Infect Dis, 2024.
- Sharma, M.; et al. Magnitude and Functionality of the NS1-Specific Antibody Response Elicited by a Live-Attenuated Tetravalent Dengue Vaccine Candidate. (1537-6613 (Electronic)).
- Avirutnan, P.; et al. Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis 2006, 193, 1078–88. [Google Scholar] [CrossRef]
- Lee, P.X.; et al. Relative contribution of nonstructural protein 1 in dengue pathogenesis. LID - 10.1084/jem.20191548 [doi] LID - e20191548. (1540-9538 (Electronic)).
- Ramu, S.T.; et al. Antibody and memory B cell responses to the dengue virus NS1 antigen in individuals with varying severity of past infection. Immunology 2023, 170, 47–59. [Google Scholar] [CrossRef]
- Muller, D.A.; et al. Kinetics of NS1 and anti-NS1 IgG following dengue infection reveals likely early formation of immune complexes in secondary infected patients. Sci Rep 2025, 15, 6684. [Google Scholar] [CrossRef] [PubMed]
- Premazzi Papa, M.; et al. Dengue NS1 Antibodies Are Associated With Clearance of Viral Nonstructural Protein-1. (1537-6613 (Electronic)).
- Bailey, M.J.; et al. Antibodies Elicited by an NS1-Based Vaccine Protect Mice against Zika Virus. mBio, 2019. 10.
- Sun, D.S.; et al. Antiplatelet autoantibodies elicited by dengue virus non-structural protein 1 cause thrombocytopenia and mortality in mice. J Thromb Haemost 2007, 5, 2291–9. [Google Scholar] [CrossRef]
- Vidarsson, G., G. Dekkers, and T. Rispens, IgG subclasses and allotypes: from structure to effector functions. (1664-3224 (Print)).
- Posadas-Mondragón, A.; et al. Indices of anti-dengue immunoglobulin G subclasses in adult Mexican patients with febrile and hemorrhagic dengue in the acute phase. (1348-0421 (Electronic)).
- Dias, A.G., Jr.; et al. Antibody Fc characteristics and effector functions correlate with protection from symptomatic dengue virus type 3 infection. Sci Transl Med 2022, 14, eabm3151. [Google Scholar] [CrossRef]
- Bruhns, P.; et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009, 113, 3716–25. [Google Scholar] [CrossRef] [PubMed]
- de Taeye, S.W.; et al. FcγR Binding and ADCC Activity of Human IgG Allotypes. Front Immunol 2020, 11, 740. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G., G. Dekkers, and T. Rispens, IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014, 5, 520.
- Duncan, A.R. and G. Winter, The binding site for C1q on IgG. Nature 1988, 332, 738–40. [Google Scholar] [CrossRef]
- Mehlhop, E.; et al. Complement protein C1q inhibits antibody-dependent enhancement of flavivirus infection in an IgG subclass-specific manner. Cell Host Microbe 2007, 2, 417–26. [Google Scholar] [CrossRef]
- Garred, P., T. E. Michaelsen, and A. Aase, The IgG subclass pattern of complement activation depends on epitope density and antibody and complement concentration. Scand J Immunol 1989, 30, 379-82.
- Chan, K.R.; et al. Ligation of Fc gamma receptor IIB inhibits antibody-dependent enhancement of dengue virus infection. Proc Natl Acad Sci U S A 2011, 108, 12479–84. [Google Scholar] [CrossRef]
- Bournazos, S.; et al. Antibody fucosylation predicts disease severity in secondary dengue infection. Science 2021, 372, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; et al. IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science 2017, 355, 395–398. [Google Scholar] [CrossRef]
- Teo, A.; et al. Understanding antibody-dependent enhancement in dengue: Are afucosylated IgG1s a concern? PLoS Pathog 2023, 19, e1011223. [Google Scholar]
- Golay, J., A. E. Andrea, and I. Cattaneo, Role of Fc Core Fucosylation in the Effector Function of IgG1 Antibodies. Front Immunol 2022, 13, 929895.
- Oosterhoff, J.J.; et al. Afucosylated IgG responses in humans - structural clues to the regulation of humoral immunity. Trends Immunol 2022, 43, 800–814. [Google Scholar] [CrossRef]
- Larsen, M.D.; et al. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science, 2021. 371.
- Gupta, A.; et al. Mechanism of glycoform specificity and in vivo protection by an anti-afucosylated IgG nanobody. Nat Commun 2023, 14, 2853. [Google Scholar] [CrossRef]
- Thulin, N.K.; et al. Maternal Anti-Dengue IgG Fucosylation Predicts Susceptibility to Dengue Disease in Infants. (2211-1247 (Electronic)).
- Kraivong, R.; et al. Cross-reactive antibodies targeting surface-exposed non-structural protein 1 (NS1) of dengue virus-infected cells recognize epitopes on the spaghetti loop of the β-ladder domain. PLoS One 2022, 17, e0266136. [Google Scholar] [CrossRef]
- Carpio, K.L. and A.D.T. Barrett, Flavivirus NS1 and Its Potential in Vaccine Development. Vaccines (Basel), 2021. 9.
- Falconar, A.K. , The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis. Arch Virol 1997, 142, 897–916. [Google Scholar] [CrossRef] [PubMed]
- Luangaram, P.; et al. Differential critical residues on the overlapped region of the non-structural protein-1 recognized by flavivirus and dengue virus cross-reactive monoclonal antibodies. Sci Rep 2022, 12, 21548. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.J.; et al. Molecular mimicry of human endothelial cell antigen by autoantibodies to nonstructural protein 1 of dengue virus. J Biol Chem 2011, 286, 9726–36. [Google Scholar] [CrossRef]
- Visciano, M.L.; et al. Effects of adjuvants on IgG subclasses elicited by virus-like particles. J Transl Med 2012, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Kayesh, M.E.H., M. Kohara, and K. Tsukiyama-Kohara, TLR agonists as vaccine adjuvants in the prevention of viral infections: an overview. Front Microbiol 2023, 14, 1249718.
- Ramadhany, R.; et al. Antibody with an engineered Fc region as a therapeutic agent against dengue virus infection. Antiviral Res 2015, 124, 61–8. [Google Scholar] [CrossRef] [PubMed]
- Khandia, R.; et al. Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection. Front Immunol 2018, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Haslund-Gourley, B.S., B. Wigdahl, and M.A. Comunale, IgG N-glycan Signatures as Potential Diagnostic and Prognostic Biomarkers. Diagnostics (Basel), 2023. 13.
- Wilder-Smith, A. , Controlled human infection study underpins efficacy of the tetravalent live-attenuated dengue vaccine TV005. LID - 10.1172/JCI177610 [doi] LID - e177610. (1558-8238 (Electronic)).
- Pierce Kk Fau - Whitehead, S.S.; et al. Evaluation of a new dengue 3 controlled human infection model for use in the evaluation of candidate dengue vaccines. LID - 2023.06.07.23291100 [pii] LID - 10.1101/2023.06.07.23291100 [doi].
| Aspect | Protective Roles | Pathogenic Roles |
|---|---|---|
| Mechanism of Action |
|
|
| Experimental Evidence |
|
|
| Clinical Correlation |
|
| IgG1 | IgG3 | IgG4 | |
|---|---|---|---|
| Complement Activation Efficiency | High (especially under high antigen concentration) | Strongest (especially under low antigen density) | Minimal to none |
| Association with Dengue Outcome | Elevated in DHF; associated with enhanced inflammation and severity [11,62] | More common in DF; potentially protective via FcγRIIB engagement [11,62] | Elevated in children protected from symptomatic dengue; may dampen IgG1 effector functions [69] |
| Proposed Role | Contributes to inflammation and complement activation | Suppresses excessive immune activation | Competes with IgG1; limits immune-mediated pathology |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





