Submitted:
06 August 2025
Posted:
11 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Selection
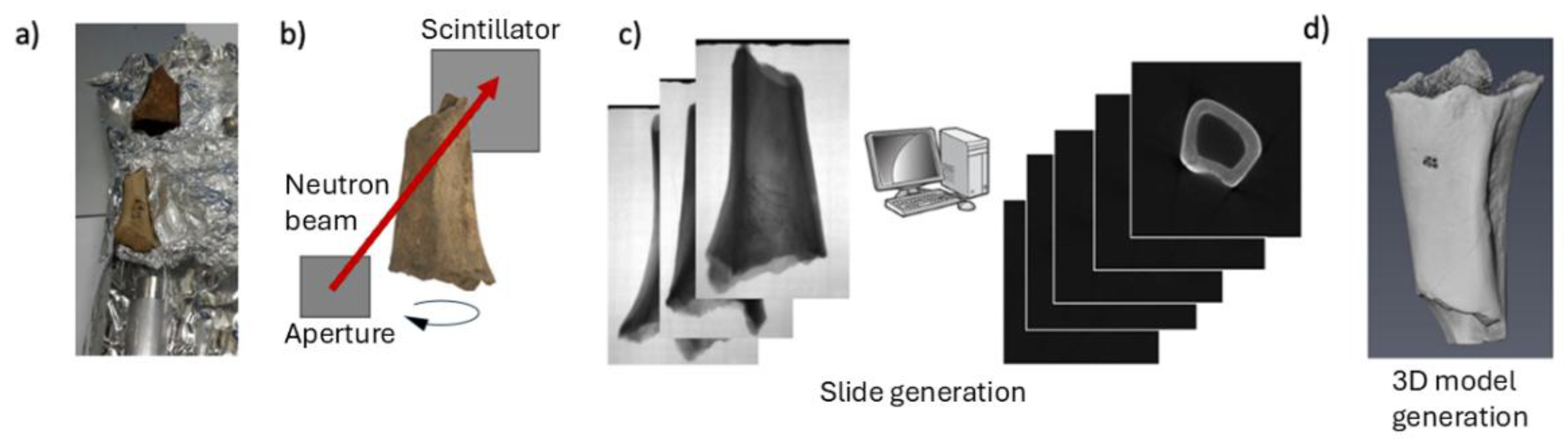
2.2. Neutron and X-ray tomography
2.3. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.4. Portable X-ray Florescence (pXRF)
3. Results
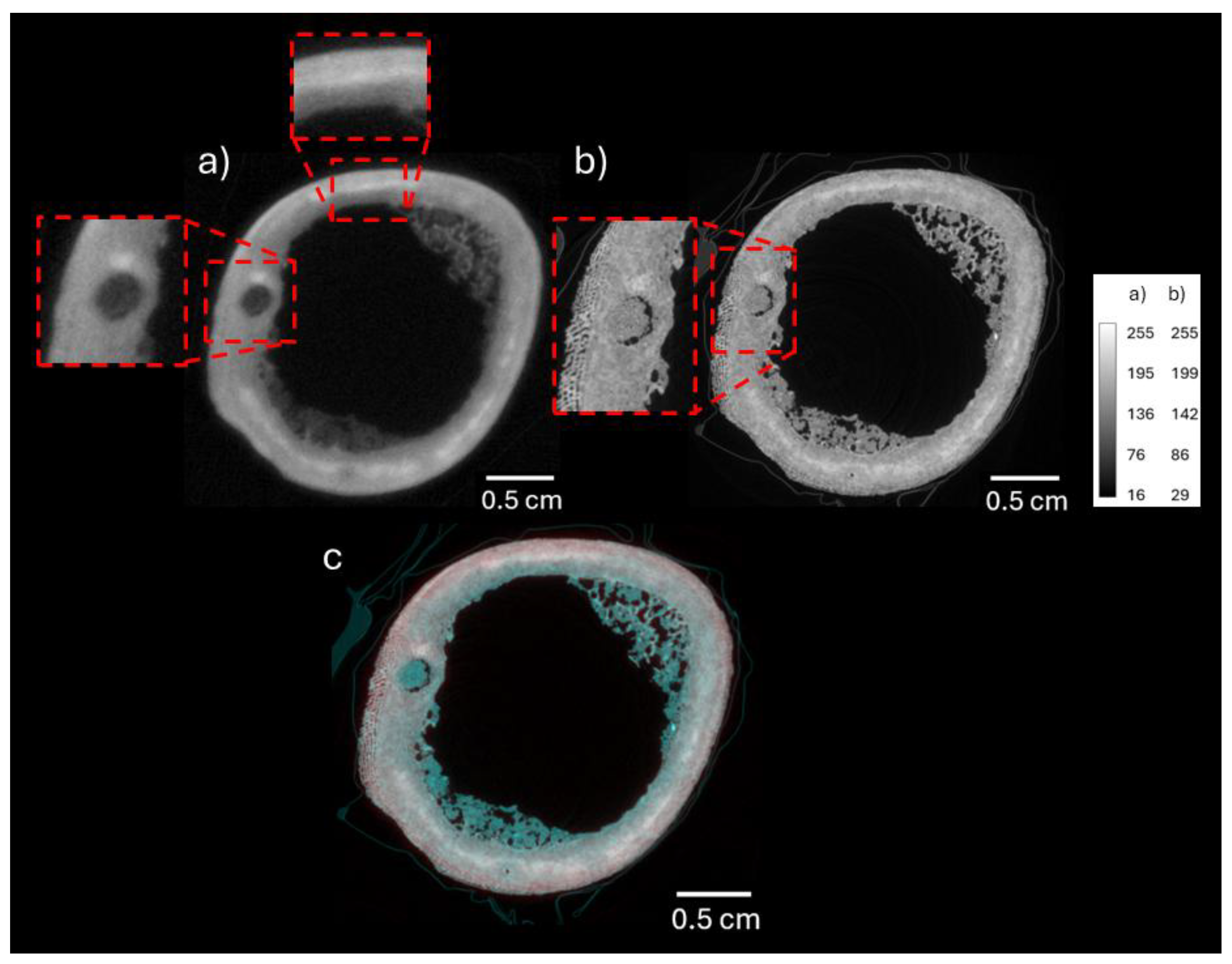
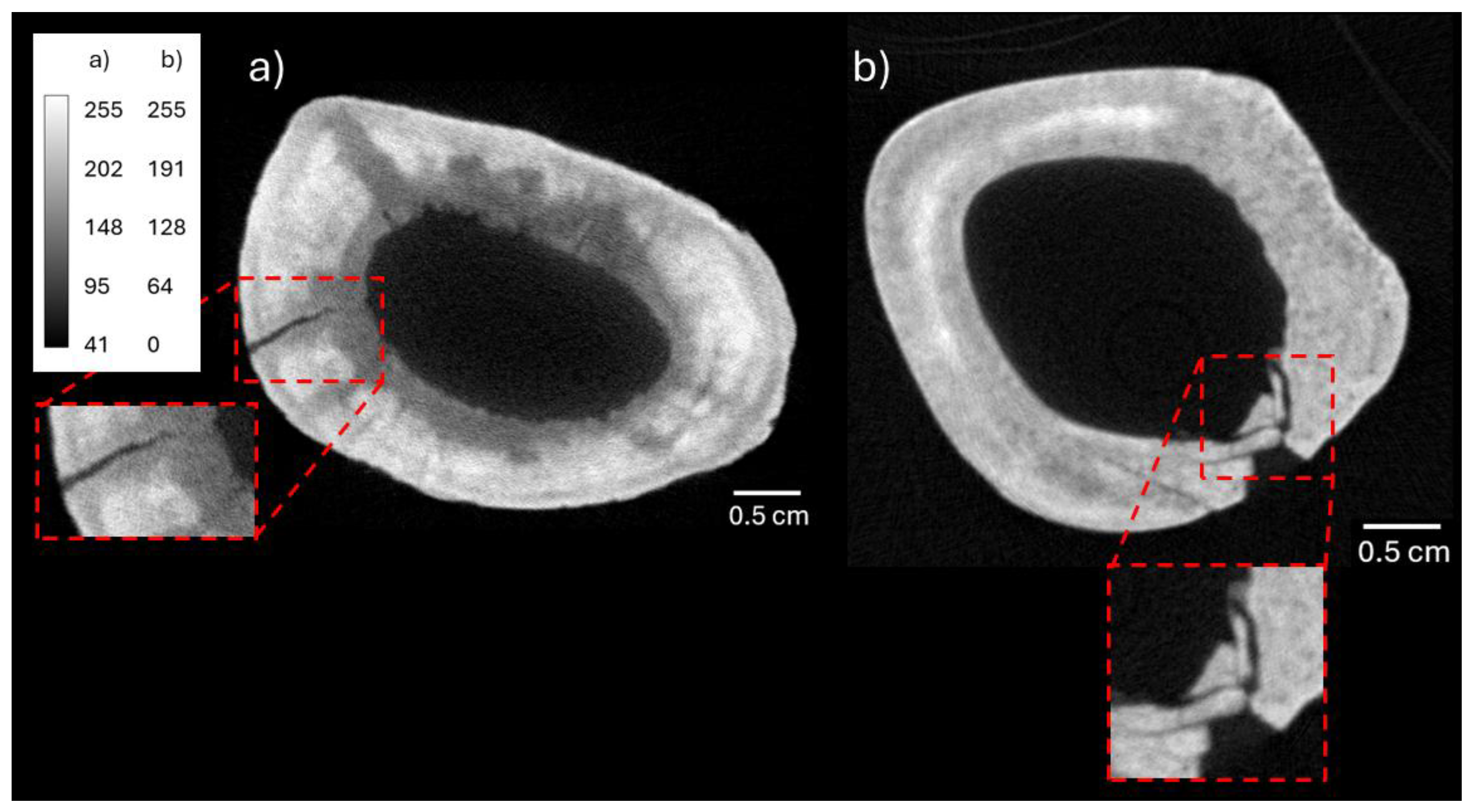
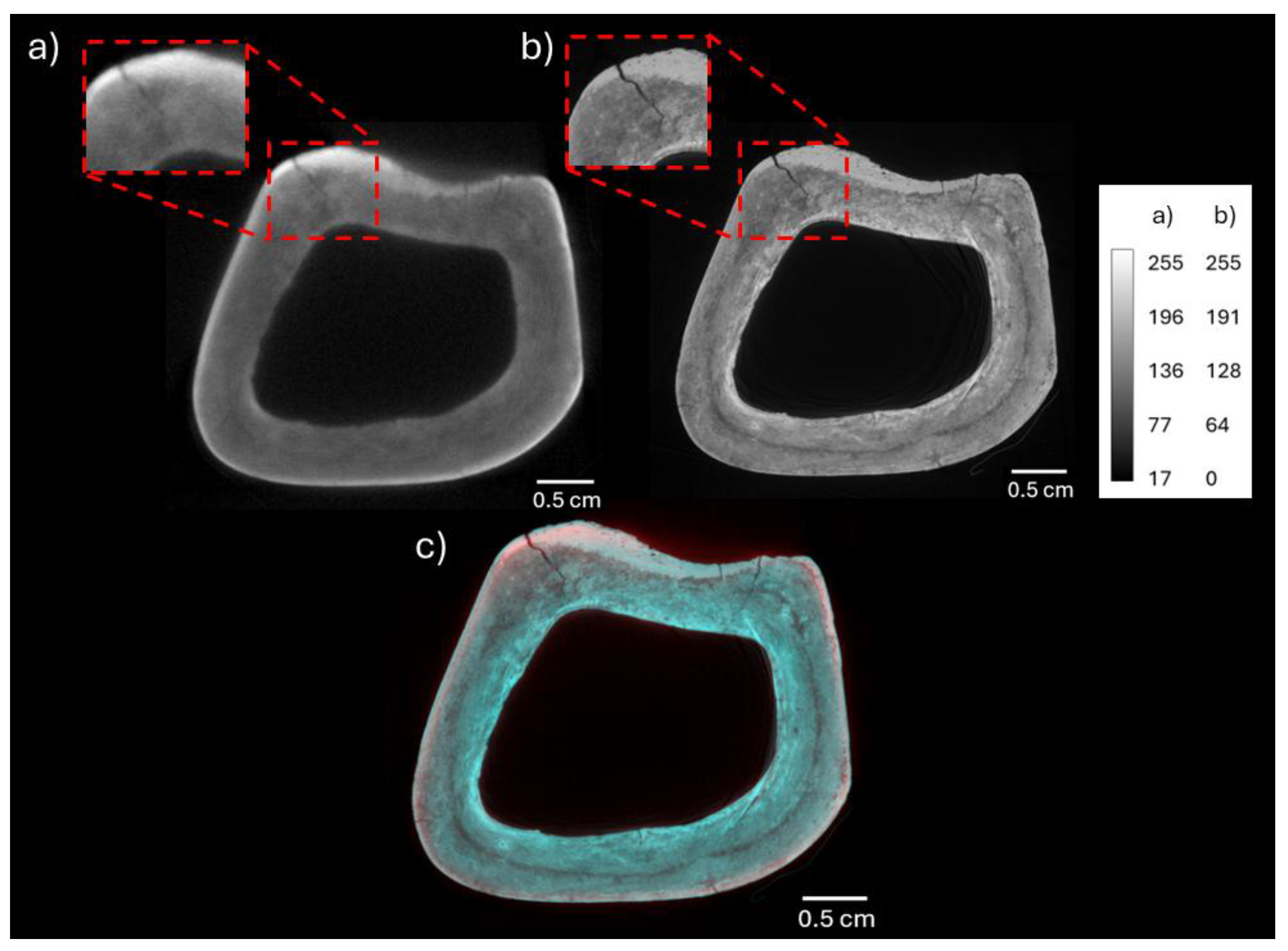
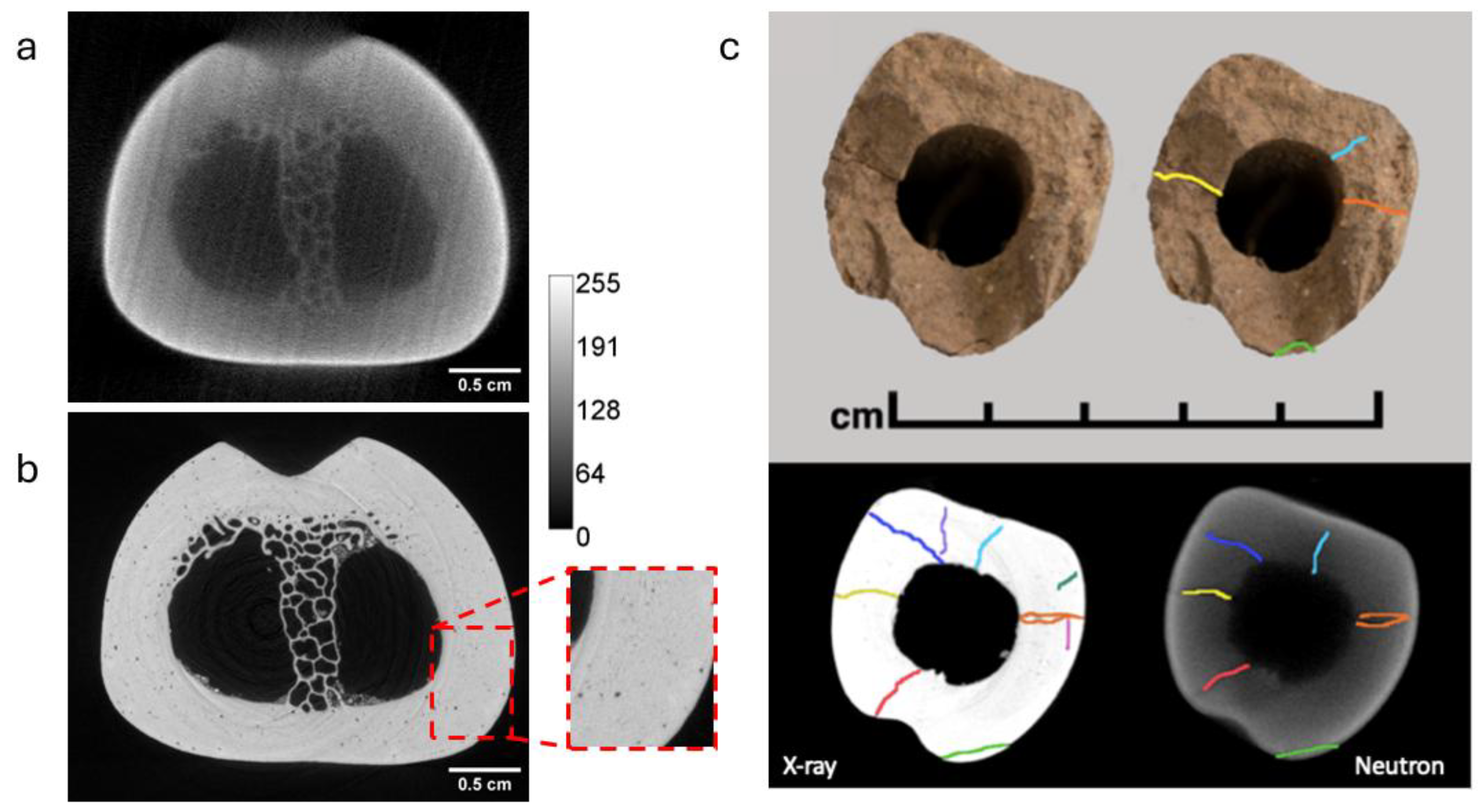
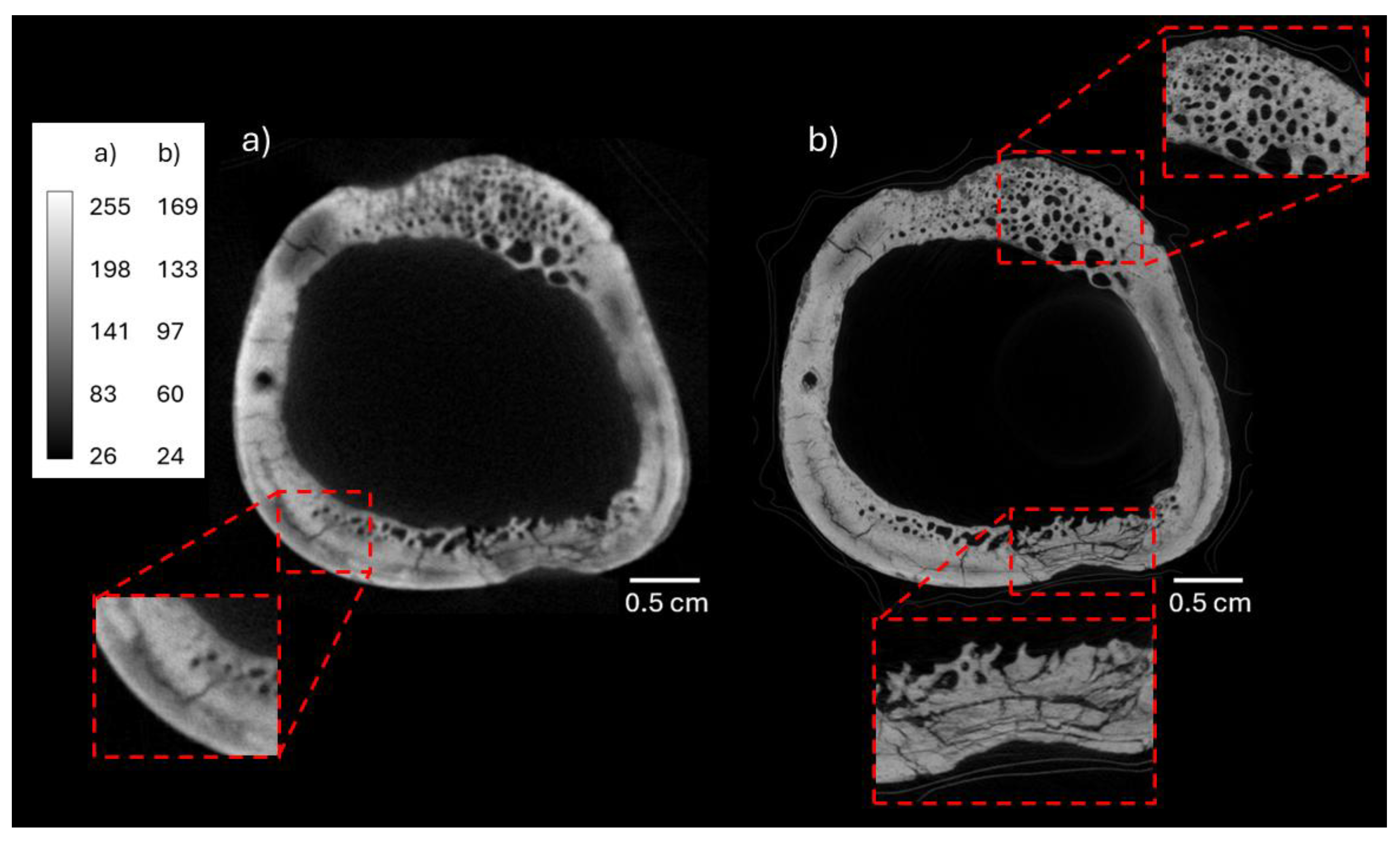
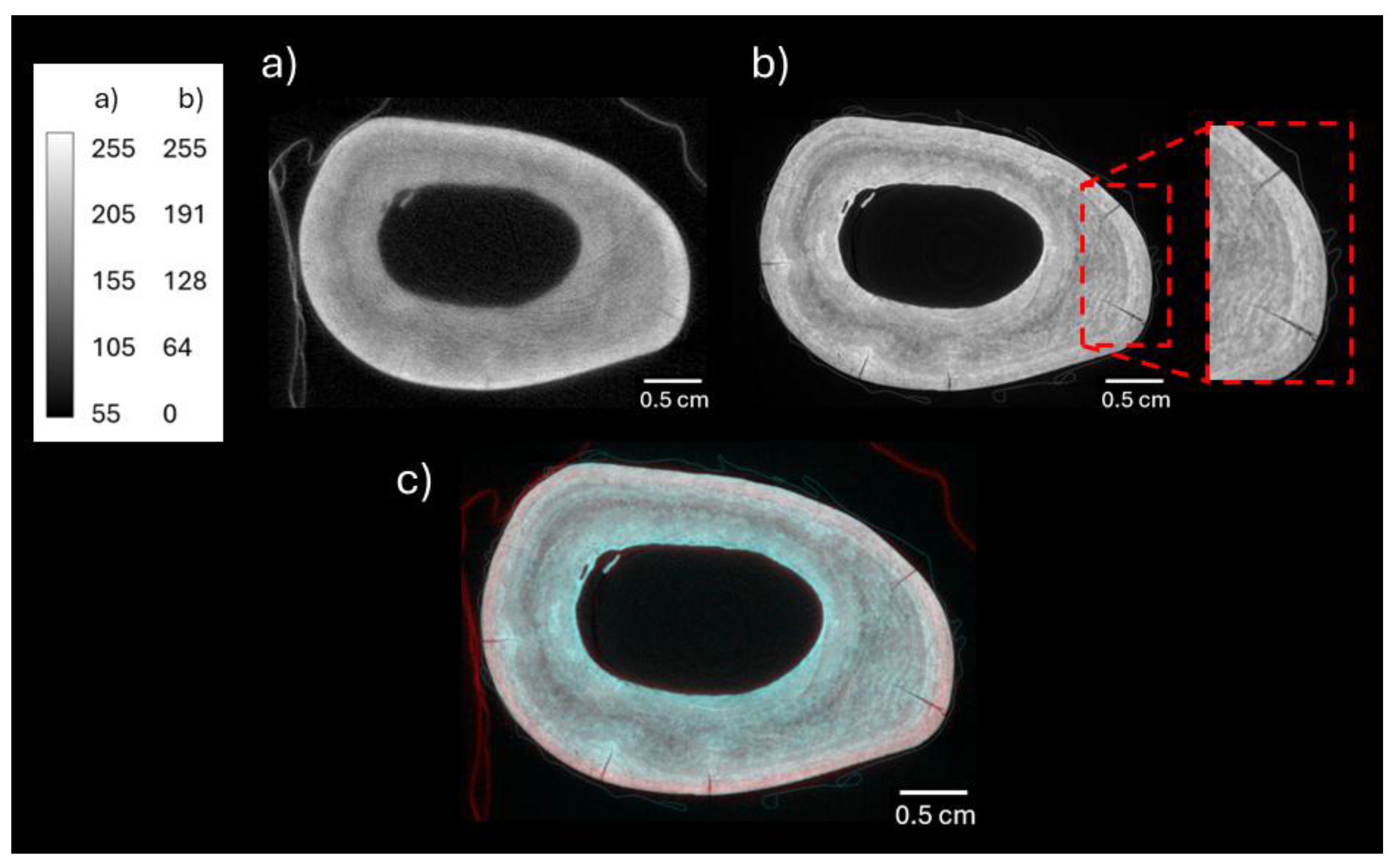
3.1. Neutron and X-Ray Tomography
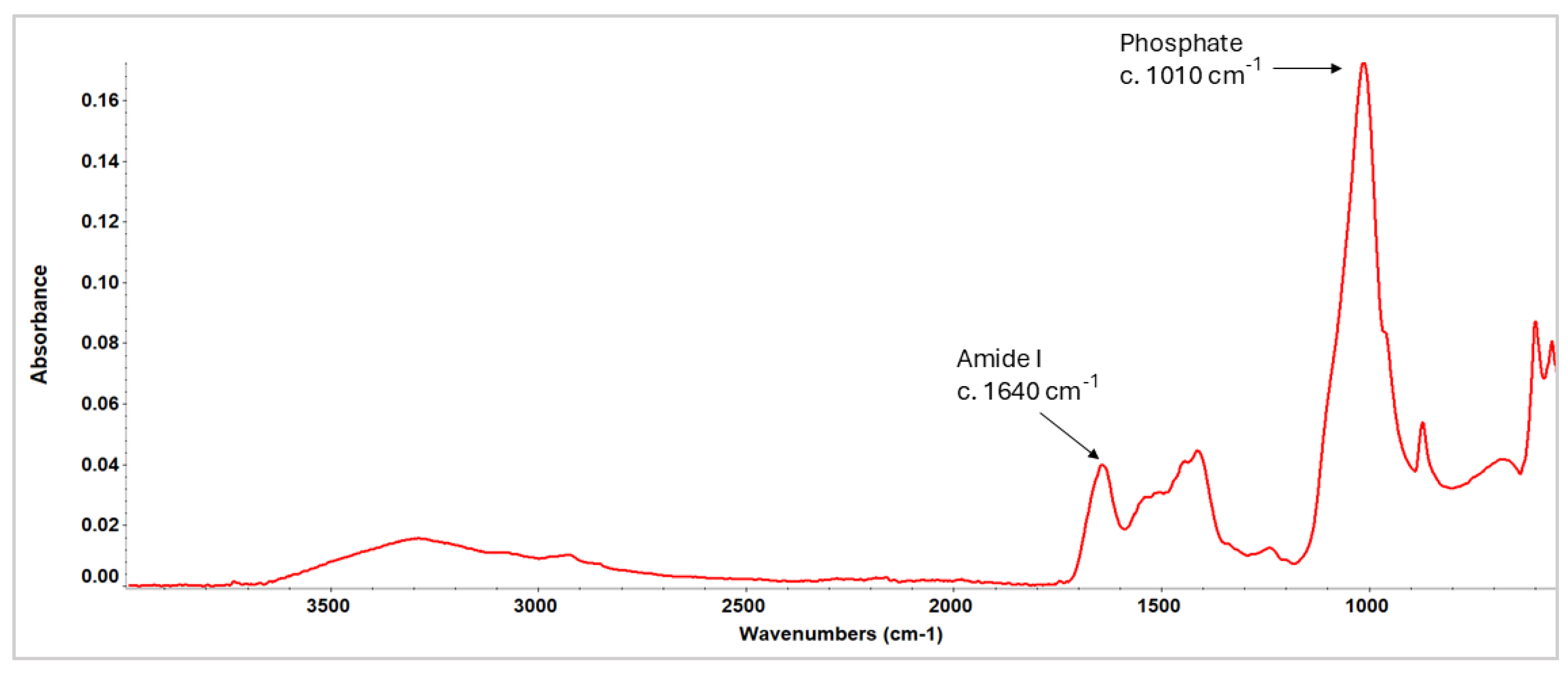
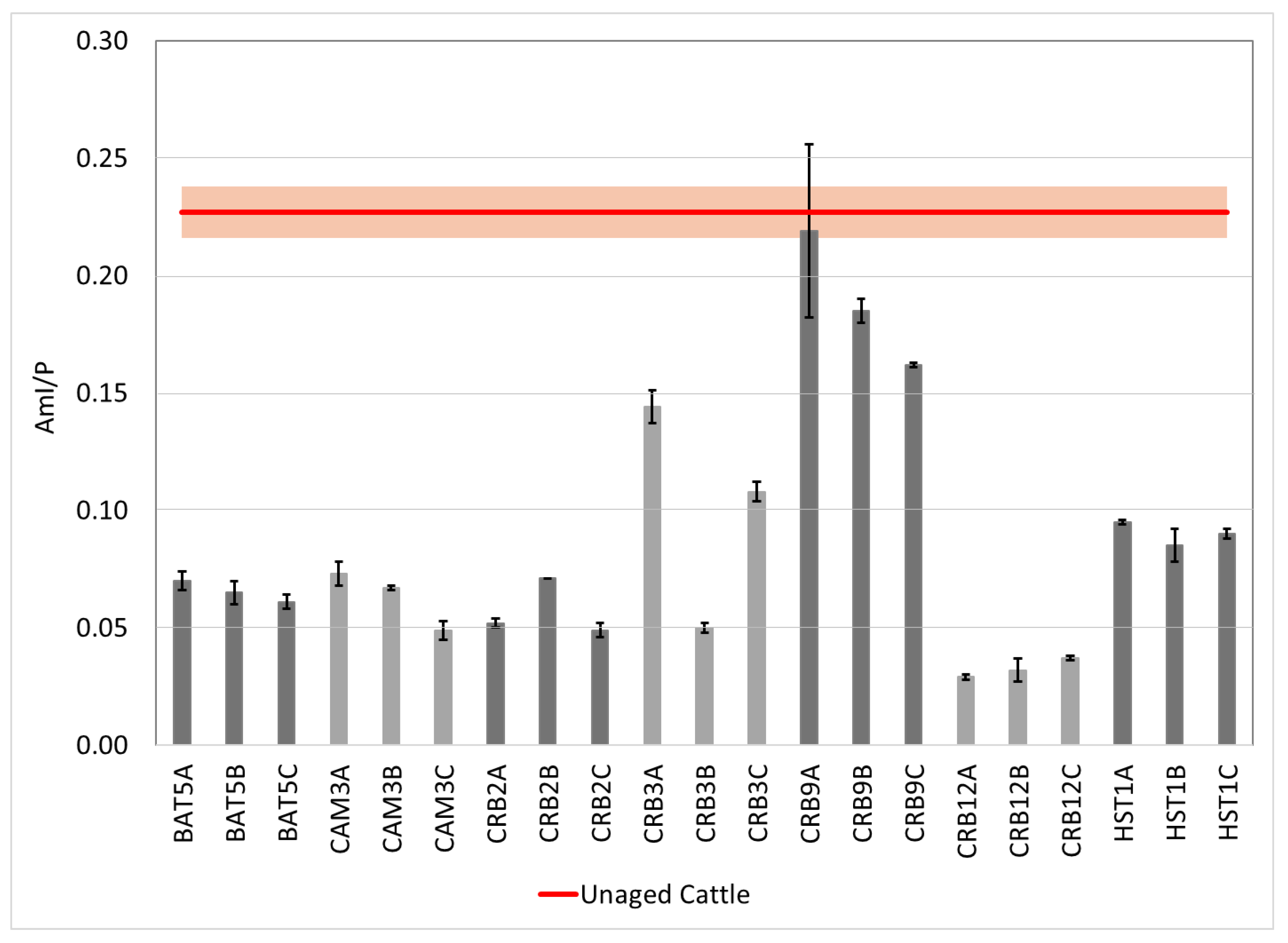
3.2. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.3. Portable X-ray Florescence (pXRF)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chadefaux, C. , et al., Curve-fitting micro-ATR-FTIR studies of the amide I and II bands of type I collagen in archaeological bone materials. E-Preservation Science 2009, 6, 129–137. [Google Scholar]
- Lopes, D. , et al., Bone physiology as inspiration for tissue regenerative therapies. Biomaterials 2018, 185, 240–275. [Google Scholar] [CrossRef] [PubMed]
- Nielsen-Marsh, C.M. , et al., Bone diagenesis in the European Holocene II: taphonomic and environmental considerations. Journal of Archaeological Science 2007, 34, 1523–1531. [Google Scholar] [CrossRef]
- Kendall, C. , et al., Diagenesis of archaeological bone and tooth. Palaeogeography, Palaeoclimatology, Palaeoecology 2018, 491, 21–37. [Google Scholar] [CrossRef]
- Kontopoulos, I. , et al., Bone diagenesis in a Mycenaean secondary burial (Kastrouli, Greece). Archaeological and Anthropological Sciences 2019, 11, 5213–5230. [Google Scholar] [CrossRef]
- Alvarez-Lloret, P. , et al., Quantative analysis of bone mineral using FTIR. MACLA 2006, 6, 45–47. [Google Scholar]
- Kontopoulos, I. , et al., Screening archaeological bone for palaeogenetic and palaeoproteomic studies. PLOS ONE 2020, 15, e0235146. [Google Scholar] [CrossRef]
- Brock, F., T. Higham, and C.B. Ramsey, Pre-screening techniques for identification of samples suitable for radiocarbon dating of poorly preserved bones. Journal of Archaeological Science 2010, 37, 855–865. [Google Scholar] [CrossRef]
- Child, A.M. , Microbial Taphonomy of Archaeological Bone. Studies in Conservation 1995, 40, 19–30. [Google Scholar] [CrossRef]
- Smith, C.I. , et al. , Bone diagenesis in the European Holocene I: patterns and mechanisms. 2007, 34, 1485–1493. [Google Scholar]
- Cherns, L. , et al., Correlative tomography of an exceptionally preserved Jurassic ammonite implies hyponome-propelled swimming. Geology 2022, 50, 397–401. [Google Scholar] [CrossRef]
- Fernandez, V. , et al., Evidence of Egg Diversity in Squamate Evolution from Cretaceous Anguimorph Embryos. PLOS ONE 2015, 10, e0128610. [Google Scholar] [CrossRef] [PubMed]
- Laaß, M. , et al., New insights into the respiration and metabolic physiology of Lystrosaurus. Acta Zoologica 2011, 92, 363–371. [Google Scholar] [CrossRef]
- Burca, G. , Combined Neutron Imaging and Diffraction: Instrumentation and Experimentation, in Faculty of Mathematics, Computing and Technology (MCT). 2013, The Open University: Ann Arbor.
- Bishop, M.C. and J.N. Dore, Corbridge: Excavations of the Roman fort and town, 1947–1980. Archaeological Report. 1988, London: Historic Buildings & Monuments Commission for England.
- Hare, J.N. , Battle Abbey: The Eastern Range and the Excavations of 197-80. Vol. Archaeological Report no.2. 1985, London: English Heritage.
- Connell, B. and S. Davis, The Animal Bones, in Henry VIII’s Coastal Artillery Fort at Camber Castle, Rye, East Sussex, M. Biddle, et al., Editors. 2001, Oxford Archaeological Unit for English Heritage: Oxford. p. 301-341.
- Rushmore, A. , Housesteads Roman Fort - The Grandest Station: The Material Assemblages. Archaeological Reports. Vol. 2. 2009, Swindon: English Heritage.
- Burca, G. , et al., Exploring the potential of neutron imaging for life sciences on IMAT. Journal of Micropscopy 2018, 272, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Burca, G. , et al. Modelling of an imaging beamline at the ISIS pulsed neutron source. Journal of Instrumentation 2013, 8, P10001. [Google Scholar] [CrossRef]
- Dierick, M., B. Masschaele, and L. Van Hoorebeke, Octopus, a fast and user-friendly tomographic reconstruction package developed in LabView®. Measurement Science and Technology 2004, 15(1366).
- Avizo 9.0.1 FEI (2015) User’s guide Avizo ® 9. Thermofisher. Waltham, Massachusetts.
- Schindelin, J. , et al., Fiji: an open-source platform for biological-image analysis. Nature Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Drakopoulos, M. , et al., I12, the Joint Engineering, Environment and Processing (JEEP) beamline at Diamond Light Source. Journal of Synchrotron Radiation 2015, 22, 828–838. [Google Scholar] [CrossRef]
- Wasaeson, N. and M. Basham, Savu: A Python-based, MPI Framework for Simultaneous Processing of Multiple, N-dimensional Large Tomography Datasets. arXiv arXiv:1610.08015, 2016.
- Nicolet iS5 User Guide (2018). Thermofisher. Waltham, Massachusetts.
- GRAMS AI 8.0. Thermofisher. Waltham, Mssachusetts.
- Van Loon, L.L. , et al., Peakaboo: Advanced software for the interpretation of X-ray fluorescence spectra from synchrotrons and other intense X-ray sources. Software Impacts 2019, 2, 100010. [Google Scholar] [CrossRef]
- Hillier, M. , L and L. Bell, Differentiating Human Bone from Animal Bone: A Review of Histological Methods. Journal of Forensic Sciences 2007, 52, 249–263. [Google Scholar] [CrossRef]
- Valtierra, N. , et al., Cleaning archaeological bones: Influence of water, ethanol, and acetone on microhardness. International Journal of Osteoarchaeology 2023, 33, 967–972. [Google Scholar] [CrossRef]
- Pearce, C. , Preservation Assessment of Archaeological Animal Bones using Combined Analytical and Advanced Imaging Techniques. PhD. Birkbeck, University of London. 2024.
- Paschalis, E. P. , et al. FTIR Microspectroscopic Analysis of Normal Human Cortical and Trabecular Bone. Calcified Tissue International 1997, 61, 480–486. [Google Scholar] [CrossRef]
- Trueman, C. N. G. , et al. Mineralogical and compositional changes in bones exposed on soil surfaces in Amboseli National Park, Kenya: diagenetic mechanisms and the role of sediment pore fluids. Journal of Archaeological Science 2004, 31, 721–739. [Google Scholar] [CrossRef]
- Currey, J. , Incompatible mechanical properties in compact bone. Journal of Theoretical Biology 2004, 231, 569–580. [Google Scholar] [CrossRef]
- Viguet-Carrin, S., P. Garnero, and P.D. Delmas, The Role of Collagen in Bone Strength. Osteoporos Int 2006, 17, 319–336. [Google Scholar] [CrossRef]
- Boaks, A., D. Siwek, and F. Mortazavi, The temporal degradation of bone collagen: A histochemical approach. Forensic Science International 2014, 240, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, G.P. , et al., Portable FTIR for on-site screening of archaeological bone intended for ZooMS collagen fingerprint analysis. Journal of Archaeological Science: Reports 2019, 26, 101862. [Google Scholar]
- Lebon, M. , et al., Rapid Quantification of Bone Collagen Content by ATR-FTIR Spectroscopy. Radiocarbon 2016, 58, 131–145. [Google Scholar] [CrossRef]
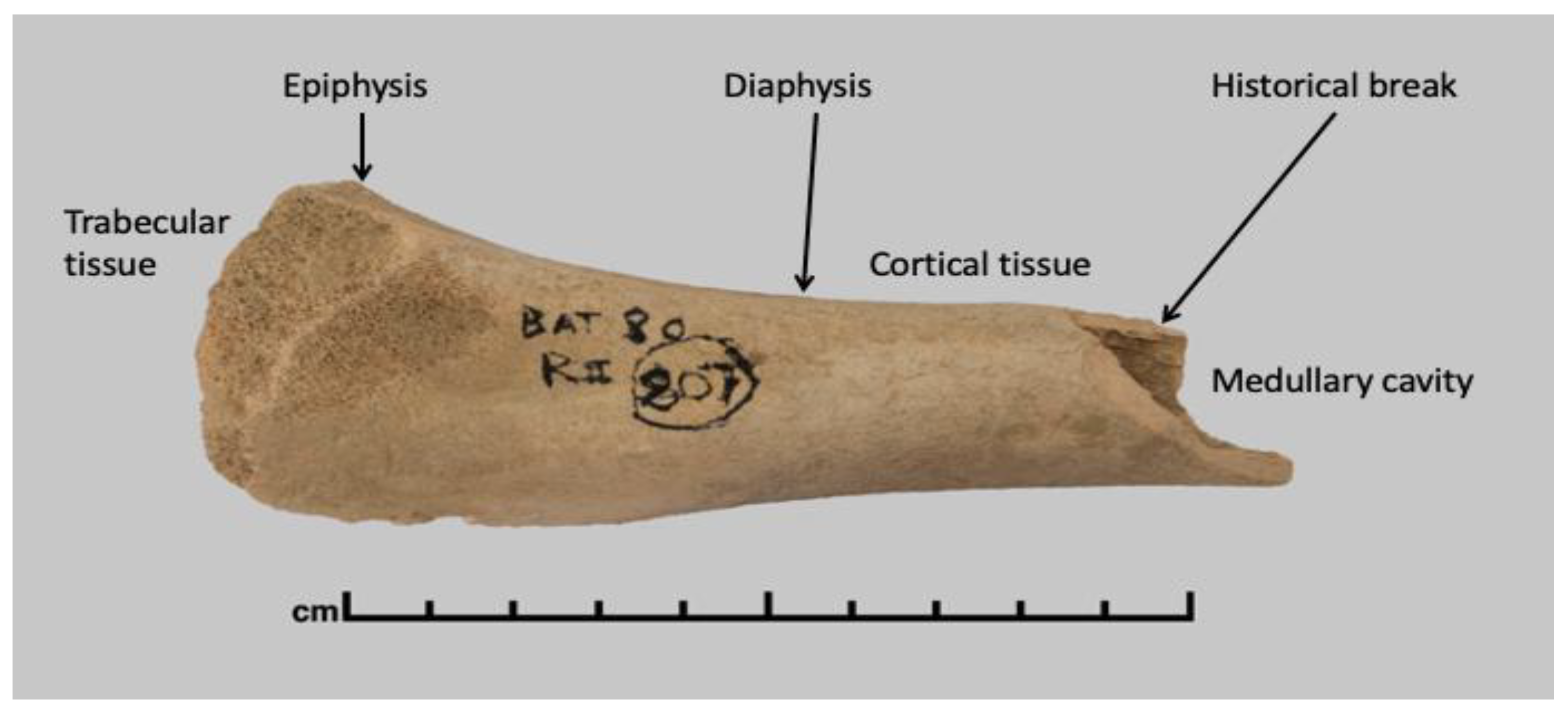
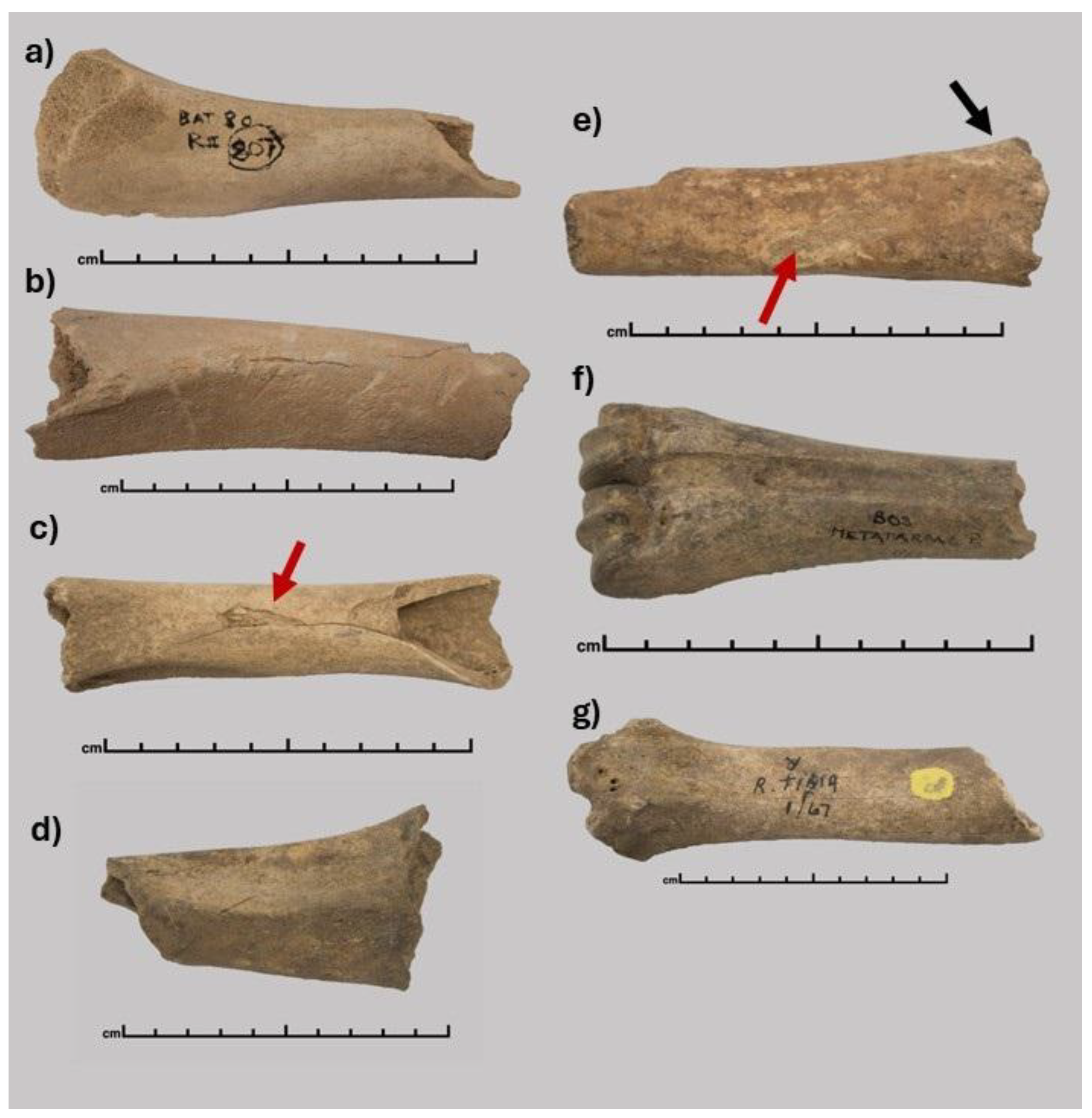
| Sample | Site | Period | Excavation | Species | Age |
|---|---|---|---|---|---|
| BAT5 | Battle Abbey | Medieval | 1978 – 80 | Cattle | Juvenile |
| CAM3 | Camber Castle | Tudor | 1982 – 83 | Cattle | Adult |
| CRB2 | Corbridge | Roman | 1966 – 67 | Cattle | Adult |
| CRB3 | Corbridge | Roman | 1966 – 67 | Large mammal | Adult |
| CRB9 | Corbridge | Roman | 1966 – 67 | Cattle | Adult |
| CRB12 | Corbridge | Roman | 1966 – 67 | Cattle | Adult |
| HST1 | Housesteads | Roman | 1984 | Cattle | Sud-adult |
| Sample | Location | P Kα | Ca Kα | Mn Kα | Fe Kα | Cu Kα | Zn Kα | Pb Lα |
|---|---|---|---|---|---|---|---|---|
| BAT5 | X1 | m | M | t | m | t | m | m |
| BAT5 | X2 | t | M | t | m | t | t | |
| CAM3 | X1 | m | M | m | t | t | m | |
| CAM3 | X2 | m | M | t | m | t | t | m |
| CRB2 | X1 | t | M | t | M | t | ||
| CRB2 | X2 | m | M | m | M | t | t | |
| CRB3 | X1 | m | M | t | m | t | m | m |
| CRB3 | X2 | m | M | m | M | t | m | m |
| CRB9 | X1 | m | M | m | M | t | m | t |
| CRB9 | X2 | m | M | m | M | t | m | t |
| CRB12 | X1 | m | M | m | m | t | m | |
| CRB12 | X2 | m | M | m | M | t | m | |
| HST1 | X1 | m | M | t | m | t | t | |
| HST1 | X2 | t | M | t | m | t | t | |
| HST1 | X3 | t | M | t | M | t | t |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





