1. Introduction
Chikungunya virus (CHIKV) remains a major global public health challenge, particularly in regions where competent vectors and susceptible populations coexist. Transmitted primarily by
Aedes aegypti and secondarily by
Aedes albopictus, the virus has demonstrated a remarkable ability to adapt to different ecological settings.[
1,
2] While most patients experience a self-limiting condition, a significant proportion can develop severe or even fatal forms of disease.[
3,
4] These outcomes have been well documented in various outbreaks, and several pathophysiological mechanisms have already been explored,[
5] challenging the long-standing notion that chikungunya is a benign infection.[
6,
7,
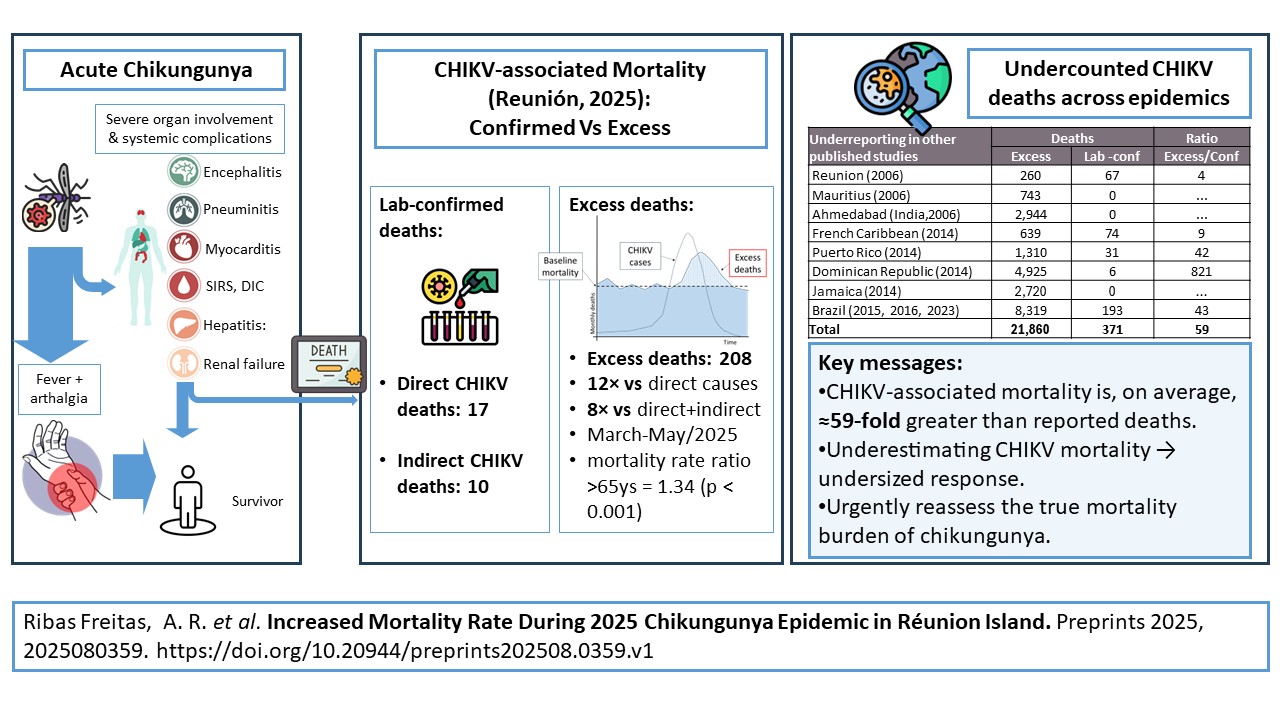
8] The resurgence of chikungunya virus (CHIKV) transmission in Réunion Island in mid-2024 and into 2025 has renewed concerns about the disease’s mortality burden. By the end of June 2025, a total of 54,250 biologically confirmed cases had been reported. During the same period, 27 deaths were officially attributed to chikungunya by the national causality assessment committee—17 classified as directly related and 10 as indirectly related—while 26 additional deaths remained under investigation.[
9]
In the first decade of this century, large outbreaks occurred in areas with exclusive presence of
A. albopictus, most notably in Réunion Island, while autochthonous transmission was also documented in mainland Europe, though without large-scale outbreaks. These events were facilitated by a viral lineage carrying the A226V mutation in the E1 glycoprotein, which enhanced transmission efficiency by this vector.[
1,
2] This same mutation, along with other genetic changes, has been identified in the lineage currently circulating in Réunion Island (2024–2025).[
10] Climatic factors—particularly the exceptionally hot summer this year[
11] —combined with the widespread presence of
Aedes albopictus and the circulation of this adapted viral lineage may be contributing to the unusually early and simultaneous transmission observed in multiple parts of Europe and in China in 2025. .[
12,
13]
The present study aims to quantify all-cause excess mortality during the 2025 chikungunya epidemic in Réunion Island, using robust statistical modeling aligned with EuroMOMO methodology (adopted by Santé Publique France), consistent with European Centre for Disease Prevention and Control (ECDC) protocols, and World Health Organization recommendations for analyzing mortality associated with influenza, COVID-19, and natural disasters.[
11,
14,
15,
16] By detecting unusual mortality patterns beyond expected seasonal fluctuations, this study contributes to the growing body of evidence indicating that chikungunya can cause substantial mortality during large-scale outbreaks and reinforces the importance of strengthened surveillance, better clinical recognition of severe cases, and reconsideration of chikungunya as a potentially life-threatening arboviral disease in global health agendas.
2. Materials and Methods
2.1. Source and Preparation of Mortality and Population Data
All data were obtained from publicly available official sources. We used secondary mortality data obtained from the Institut National de la Statistique et des Études Économiques (INSEE)[
17], the official source for national mortality statistics in France. With comprehensive and up-to-date mortality data, the INSEE system is an effective tool for tracking fluctuations in overall mortality in both epidemics and extreme heat waves.[
11] Chikungunya case and death notifications were sourced from Santé Publique France [
https://www.santepubliquefrance.fr].[
9]
Mortality data covered the period from January 2010 to May 2025 and included all-cause mortality in La Réunion, disaggregated by month and age group. For the purposes of this analysis, mortality data were stratified into three age groups: <25 years, 25–64 years, and ≥65 years. Monthly population estimates were derived by geometric interpolation based on annual population figures provided by INSEE[
17].
2.2. Estimation of Expected Deaths Based in Last 2 years
Considering that the COVID-19 pandemic ended in August 2022, monthly baseline mortality rates were defined using observed mortality data from September 2022 to May 2024. For each month
m and age group, the baseline mortality rate (
λₘ) was calculated as:
where
Dₘ,ᵧ represents the number of deaths and
Pₘ,ᵧ the population for month
m in year
y.
Data from 2022–2023 were used for September–December 2024, and from 2023–2024 for January–May 2025. February 2024 counts were standardized to 28 days to account for the leap year. Expected deaths (
Eₘ) for months in 2024–2025 were then estimated as:
The incidence rate ratio (
IRRₘ) was computed as:
where
Oₘ denotes the observed deaths in month
m.
Ninety-five percent confidence intervals (CIs) for
IRRₘ were derived under a Poisson assumption using both exact methods and Byar's approximation. Statistical significance was assessed under the null hypothesis that:
Two-tailed p-values were calculated to detect deviations in mortality (increase or decrease), and one-tailed p-values to specifically detect increases in mortality. Months were considered to exhibit significant excess mortality only when the lower bound of the 95% CI for
IRRₘ exceeded 1.00 and the one-tailed p-value was below 0.05. Excess mortality was computed as:
Analyses were performed in Python (version 3.13, 64-bit) using the libraries numpy, pandas, scipy.stats, and statsmodels.
2.3. Estimation of Expected Deaths Using Poisson Regression Model
To construct a robust baseline for mortality estimation during the chikungunya epidemic, we employed Poisson regression models that account for long-term trends (2010–2025), seasonal variability, and population growth. The years 2020 to 2022 were excluded from the baseline estimation due to the impact of the COVID-19 pandemic on mortality patterns.[
18] This baseline intentionally incorporates background excess mortality potentially associated with influenza and other infectious or environmental drivers of seasonal fluctuations, ensuring that any unusual mortality patterns during the chikungunya epidemic are detected against a conservative, real-world reference level.
Two modeling strategies were used: (1) a Serfling-type seasonal regression model incorporating Fourier harmonics to capture periodic fluctuations in mortality, and (2) an alternative approach using month as a nominal categorical variable to flexibly model intra-annual variation without assuming a specific seasonal pattern. In both models, a log-transformed population offset was included to adjust for differences in population size over time.
-
a.
Serfling-type Regression
To construct the Serfling model, we standardized monthly death counts to a 30-day month to ensure comparability across months and over time. We used monthly terms instead of weekly ones, as commonly done in systems like EuroMOMO, because the INSEE database did not provide the exact date of death (only month and year) for the period 2010 to 2017.[
14,
16,
17] We applied a generalized linear model with a Poisson distribution and a log-link function. The model incorporated a linear time trend (month number) and seasonal components via harmonic terms to capture both annual and sub-annual periodicities, which are particularly important in tropical and subtropical regions.[
18,
19,
20,
21,
22] Following the principle of parsimony, we sequentially tested models including one to four seasonal harmonics Fourier terms. The final model included the linear trend and all seasonal harmonics, which provided an adequate fit to the observed seasonal variation. For the ≥65 years age group, the full model was not only the best-fitting specification according to multiple criteria (deviance, AIC, and BIC), but all harmonic terms were statistically significant, highlighting the importance of capturing detailed seasonal variation in this subgroup. To ensure consistency across age groups, we adopted the same model structure for all strata, including the linear time trend and sine and cosine terms up to the fourth harmonic to ensure comparability of trends across groups along. The final model equation was specified as:
Where:
E(Yₜ): expected number of deaths at month t;
t: time in months;
β₀: intercept;
β₁: linear time trend coefficient;
β₂ₖ, β₂ₖ₊₁: seasonal harmonic coefficients;
Pₜ: population at risk (offset).
-
b.
Sensitivity Analysis (Poisson Model Using Month as a Categorical Term)
As a sensitivity analysis, we also tested an alternative and well-established approach using a Poisson regression model in which the month was treated as a categorical (nominal) variable.[
14,
15,
23] This method captures seasonal effects by assigning a distinct baseline to each calendar month, without assuming a sinusoidal pattern. This allows for greater flexibility in detecting deviations from expected mortality trends, particularly when mortality fluctuations do not follow a strictly harmonic structure.
The final model equation, with month as a nominal variable, was specified as:
Where:
E(Yt) is the expected number of deaths at time t,
β0 is the intercept,
β1 is the coefficient for the linear time trend,
βm are the coefficients for each month (Mm) treated as a nominal variable, with m=1,2,…,12, representing each month of the year.
To estimate excess mortality, we applied the fitted Poisson regression models to the full time series, including the epidemic period (February–May 2025), generating expected mortality values under non-epidemic conditions. Excess mortality was then calculated by comparing observed deaths to these model-based predictions.
To stabilize the variance and approximate normality in mortality counts, we applied a Box–Cox power transformation with exponent 2/3, as recommended by Farrington et al. (1996)[
24] and adopted by the EuroMOMO project[
25]. This transformation was applied to both observed and expected deaths, and computed the residuals in the transformed scale:
Where
Ot is the observed and
Et the expected death count for month
t.
To estimate the standard deviation (SD) of these residuals, we used only the residuals from
non-epidemic months, thereby avoiding inflation of baseline variability. Z-scores were then calculated as:
Consistent with the EuroMOMO methodology[
25], a Z-score > 2 was considered indicative of statistically significant excess mortality. Periods with sustained Z-scores above this threshold were interpreted as excess mortality events potentially related to the chikungunya epidemic.
Both Poisson regression analyses were conducted using IBM SPSS Statistics, version 24 (IBM Corp.).
3. Results
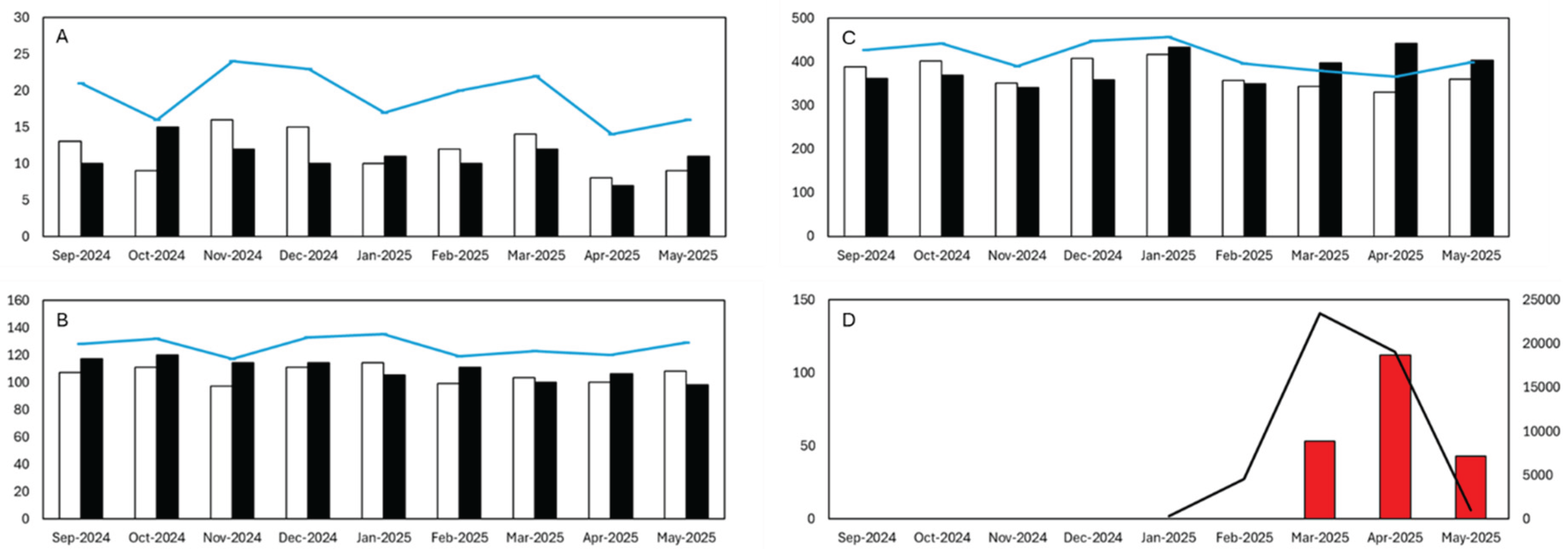
The analysis revealed a clear temporal association between the chikungunya epidemic and increased mortality in older adults on Réunion Island in 2025. Overall, mortality patterns remained stable for individuals under 65 years, while a marked surge in deaths was observed among those aged ≥65 years between March and May 2025.
Table 1 presents the observed and expected monthly deaths for three age groups (<25, 25–64, and ≥65 years) from September 2024 to May 2025, along with corresponding confidence intervals (CIs), excess deaths, incidence rate ratios (IRRs), and p-values. Among individuals under 65 years old, no statistically significant excess mortality was observed in any month. In contrast, for individuals aged 65 years and older, a marked increase in mortality was detected during the chikungunya epidemic period from March to May 2025.
Specifically, significant excess deaths occurred in March (53 excess deaths, IRR = 1.16, p < 0.01), April (112 excess deaths, IRR = 1.34, p < 0.000001), and May (43 excess deaths, IRR = 1.12, p < 0.05). These results demonstrate a strong temporal and spatial association between the epidemic period and increased mortality in older adults, while mortality remained within expected limits for younger age groups.
The overall estimated excess mortality for individuals aged ≥65 years during this three-month period was 208 deaths. The highest impact was observed in April 2025, with a mortality rate ratio of 1.34 (95% CI: 1.22–1.47; p < 0.000001) in this age group (
Figure 1;
Table 1).
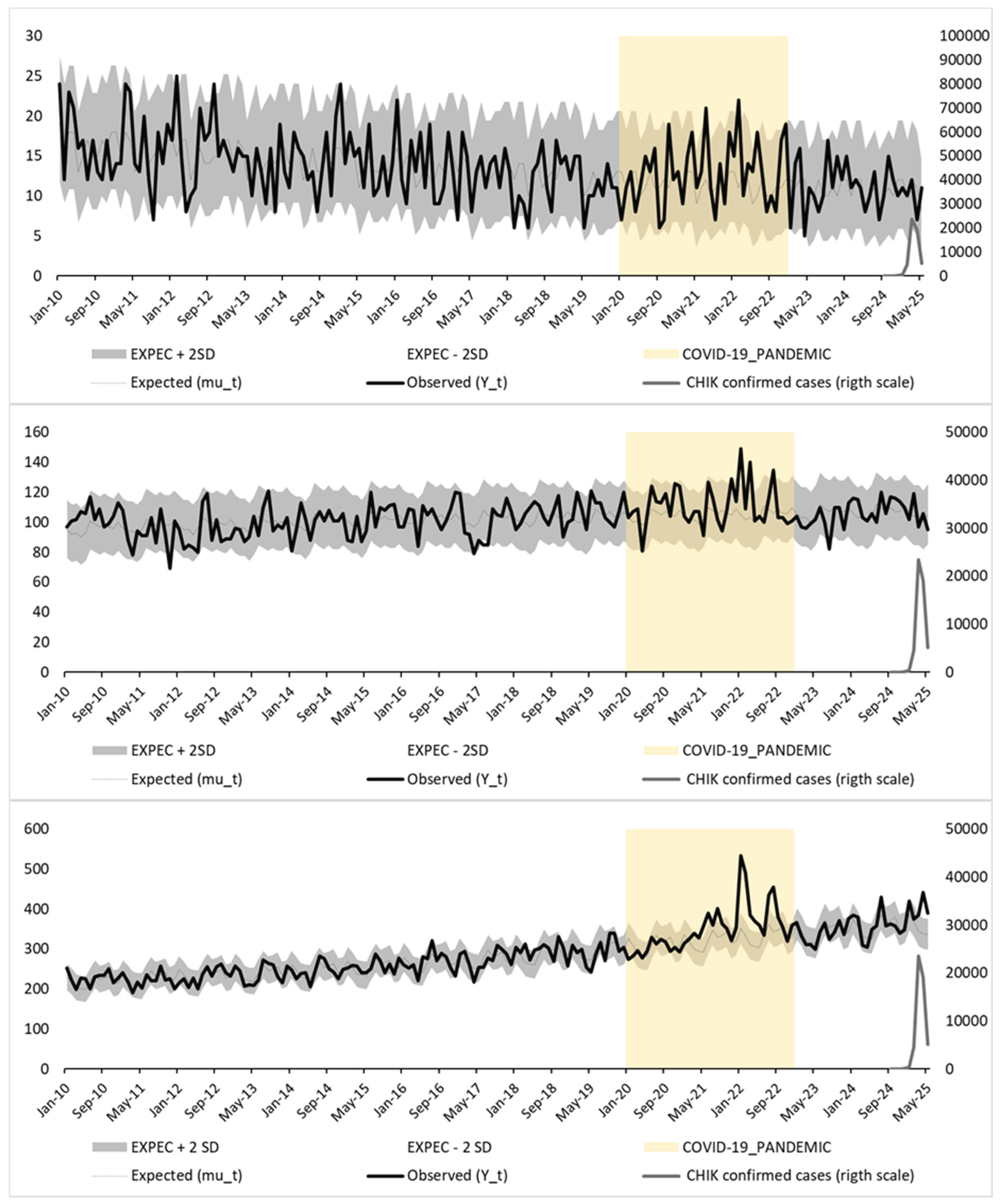
Figure 2 shows the results of a Poisson regression model incorporating seasonal Fourier terms, following a Serfling-type approach. This method assumes a sinusoidal seasonal pattern to estimate baseline mortality, fitted to pre-epidemic data.
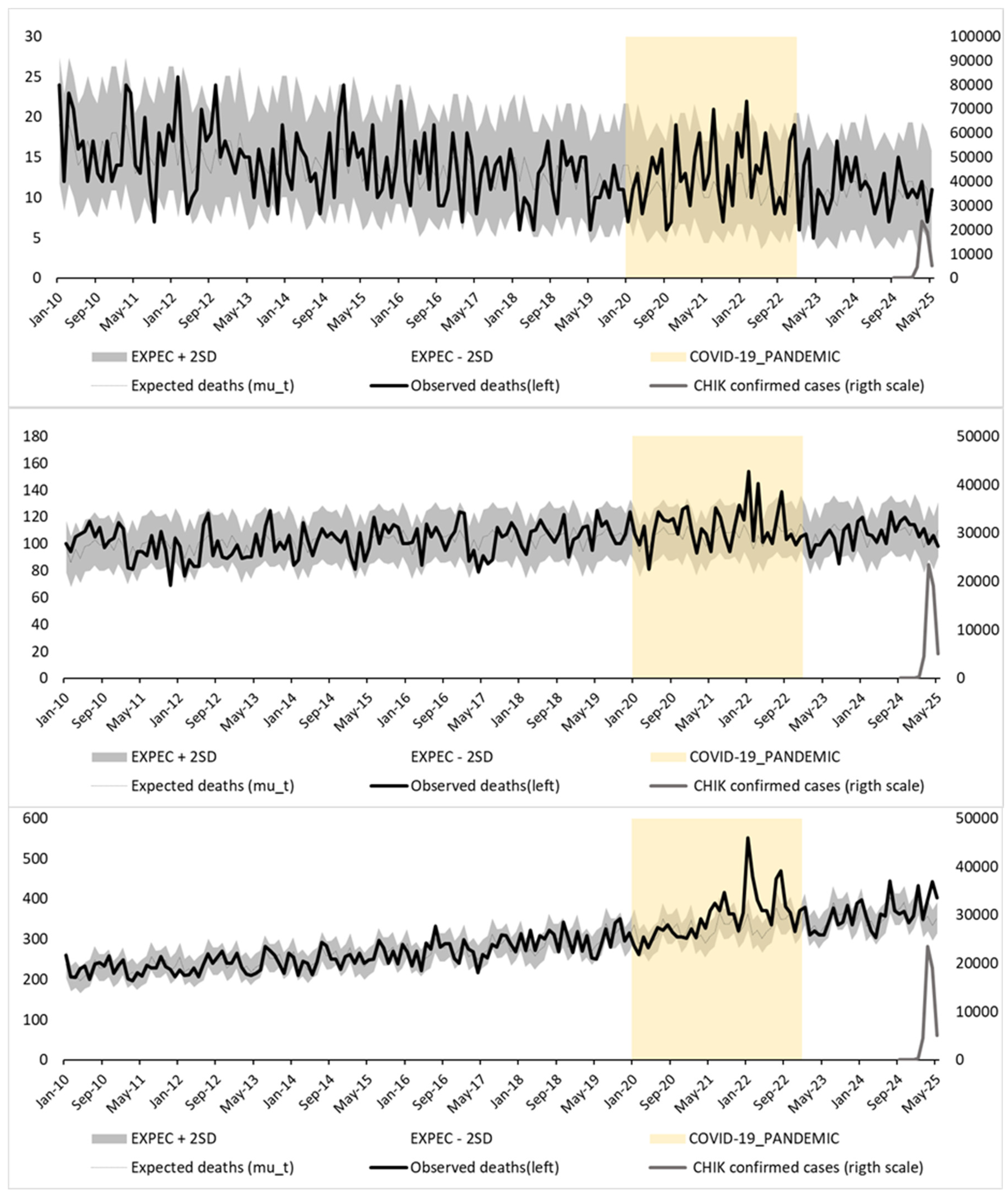
Figure 3 presents the same data modeled using a Poisson regression with categorical month terms as fixed effects. This alternative method captures seasonal variation without imposing a predefined functional form, allowing each calendar month to contribute independently to the expected mortality pattern.
In both figures, observed mortality (black line) is plotted against expected mortality (gray line), with 95% prediction intervals (±2 standard deviations; shaded area). Confirmed monthly chikungunya cases (gray line, right Y-axis) and the COVID-19 pandemic period (yellow shaded area) are also shown for context. A marked mortality peak is observed in early 2025—particularly among individuals aged ≥65 years—consistently exceeding the upper prediction bounds in both models.
Table 2 presents monthly observed and expected deaths, 95% confidence intervals, and Z-scores for the three age groups in Réunion Island from September 2024 to May 2025 under both modeling strategies. Substantial and sustained excess mortality was observed only among individuals aged ≥65 years between March and May 2025, with Z-scores consistently exceeding 2. The estimated excess deaths in this group reached 202 under the Serfling-type model and 198 under the nominal-month model, confirming the robustness of the findings.
4. Discussion
Our findings demonstrate a clear temporal and spatial association between the chikungunya epidemic in Réunion Island in 2025. The overall estimated excess mortality for individuals aged ≥65 years during this three-month period was 208 deaths. A marked increase in mortality among older adults, with minimal impact observed in younger age groups. The highest impact was observed in April 2025, with a mortality rate ratio of 1.34 (95% CI: 1.22–1.47; p < 0.000001) in this age group (
Figure 1;
Table 1). The observed excess deaths occurred in a narrow three-month window that coincided with the peak of the chikungunya epidemic, suggesting a direct or indirect link between viral transmission and increased mortality risk in vulnerable populations. Results obtained using a simple approach based on mean same months age-specific mortality rates of 2 past years produced highly concordant results to two modeling strategies, strengthens the validity of the findings.
Excess mortality associated with chikungunya epidemics is not a new observation. The phenomenon was first documented during the earliest laboratory-confirmed chikungunya epidemic in Calcutta (1964–1965)[
26]. Between August and November 1964, 218 deaths from unspecified febrile illnesses were recorded in the study area, compared with an expected 60 deaths based on previous years.[
27] The mortality curve closely mirrored the epidemic curve of laboratory-confirmed chikungunya cases, confirmed by viral culture and seroconversion using HI and CF tests. Among 302 hospitalized suspected cases, 64 were virologically confirmed by viral culture, including 62 non-fatal and 2 fatal cases.[
26,
27] Overall, 10 deaths occurred among hospitalized patients. Gelfand noted that “During this period of time, no other epidemic of infectious disease was known to be occurring in Calcutta. The month-wise distribution of deaths coincides with the monsoon rains, with a presumed increase in the abundance of the Aedes aegypti.”[
27] This early evidence already pointed to a higher-than-expected lethal potential for chikungunya outbreaks—a risk that has since been repeatedly underestimated. In multiple countries, large discrepancies between excess mortality estimates and officially reported deaths have been observed: in the Dominican Republic (2014), more than 800 times more deaths were estimated than reported; in Puerto Rico (2014), 1,310 excess deaths contrasted with only 31 laboratory-confirmed fatalities; and in several Brazilian states (Bahia 2015–2016, Pernambuco 2016, Rio Grande do Norte 2016, Minas Gerais 2023), substantial excess mortality was documented despite very few confirmed deaths.[
23,
28,
29] During a severe epidemic in India (2006), thousands of excess deaths and several fatal laboratory-confirmed cases were published in peer-reviewed journals, yet no deaths were officially reported.[
30,
31] Similar underreporting occurred in Jamaica (2014) and Mauritius (2006), where thousands of excess deaths were observed, but none were captured by official surveillance systems.[
32,
33]
Excess mortality analysis has long been recognized as fundamental for accurately estimating the true impact of epidemic diseases. As early as the 19th century, Jacques Bertillon’s seminal investigation of the 1890 influenza pandemic in Paris demonstrated that deaths officially attributed to influenza vastly underestimated the outbreak’s toll.[
34] By comparing observed mortality during epidemic peaks to a pre-pandemic baseline, he estimated at least 5,000 influenza-attributable deaths—yet only 243 of these were certified as such by examining physicians. Similar discrepancies were documented in other European capitals, including London and Berlin.[
34] Since the 1970s, the World Health Organization has recommended using excess-death estimates to capture the true burden of influenza and to guide decisions on appropriately scaled control measures; these methods have since been extended to RSV, the COVID-19 pandemic, environmental disasters, and other high-impact events.[
15,
35,
36] Over the last two decades, Santé publique France has systematically monitored heatwaves and rapidly estimated excess deaths to support risk communication and guide preventive actions.[
37] During the June–July 2025 heatwave, authorities reported 480 excess deaths—predominantly among older adults—underscoring their expertise and transparency in mortality surveillance [
18]. However, despite compelling evidence of excess mortality during the 2005–2006 chikungunya epidemic in Réunion, as documented by Josseran and colleagues[
38,
39], this analytical approach has yet to be formally integrated into routine arboviral surveillance, highlighting the persistent challenges in recognizing the link between chikungunya transmission and fatal outcomes.[
40]
The experience of French Overseas Territories during chikungunya epidemics—including Réunion (2005–2006 and 2025) and the French Caribbean in 2014—demonstrates that, despite substantial investments in healthcare and research, resulting in numerous peer-reviewed publications, officially confirmed deaths accounted for only a fraction of total excess mortality.[
39,
41,
42] This underreporting likely reflects both clinical challenges in recognizing fatal cases—many resulting from underdiagnosed complications such as myocarditis, encephalitis, or multi-organ failure, or indirectly from decompensation of pre-existing conditions[
3,
5,
8,
43,
44,
45,
46] —and a persistent, misleading perception of chikungunya as a non-lethal disease, often reinforced by public health communications. [
47,
48,
49] Evidence from active epidemiological surveillance, including both classic autopsies and minimally invasive tissue sampling, further supports the conclusion that many chikungunya-related fatalities go undetected during clinical care.[
45] Incorporating excess mortality analysis as a complementary tool could strengthen risk assessment and public health responses by capturing the true impact of arboviral epidemics, as well as outbreaks of newly emerging viruses.
Limitations of this study include the use of preliminary mortality data from INSEE, which may be subject to later revisions. The analysis did not account for the potential mitigating effect of the vaccination campaign conducted during the outbreak, which may have influenced mortality patterns. Additionally, other circulating pathogens or extreme natural events were not incorporated into the models and could have contributed to mortality fluctuations. The relatively small population size of Réunion Island and the wide age group classifications may have limited the ability to detect subtle increases in mortality across specific strata, potentially leading to underdetection of age-specific excess deaths. Finally, as with all ecological studies, these analyses rely on aggregate data, which precludes individual-level causal inference and leaves room for residual confounding.
This recent epidemic in European territory underscores the growing and evolving risk of chikungunya transmission across the continent. The widespread presence of
Aedes albopictus populations, the circulation of a highly adapted ECSA-2 viral lineage carrying the E1-A226V, E2-I211T, and E2-L210Q mutations, and the record-breaking heat experienced in several European countries in June 2025 have likely acted synergistically to create favorable conditions for early and widespread transmission.[
10,
11,
13] Notably, autochthonous transmission was documented at latitudes as high as 48°N in Jun—an atypical occurrence for chikungunya virus and the earliest recorded in mainland Europe. By mid-July, 13 localities in Italy and France had reported 31 confirmed cases, already surpassing the cumulative number of affected areas documented between 2007 and 2024.[
13] Guangdong Province, China, faced its worst documented chikungunya epidemic in July 2025, with thousands of locally transmitted cases reported.[
12] This unprecedented global scenario—with simultaneous outbreaks in multiple European localities at northernmost latitudes ever recorded for chikungunya and the most severe epidemic ever observed in East Asia—in the global expansion of chikungunya virus.
The rapid geographic spread, coupled with the historical under-recognition of the mortality burden, reinforces the urgent need to integrate excess mortality surveillance into arboviral monitoring frameworks and to consider preventive vaccination strategies in at-risk regions. These concurrent events underscore that climate anomalies, widespread vector presence, and viral adaptations can jointly amplify transmission risk far beyond historically observed patterns. Such evolving risk scenarios strongly reinforce the need to adopt a comprehensive preparedness approach that combines surveillance innovations, vaccination programs, and improved clinical care for vulnerable populations.
A recent clinical consensus statement by the European Society of Cardiology,
"Vaccination as a new form of cardiovascular prevention", [
50] illustrates the importance of explicit recognition by leading medical societies that indirect mortality—such as cardiovascular deaths triggered or exacerbated by infections—must be properly acknowledged. Such recognition is essential to ensure that medical professionals, other health workers, patients, and policymakers can deliver proportionate and timely responses.
The same logic applies to chikungunya: without formal acknowledgment of both its direct and indirect fatal burden, surveillance systems will continue to underestimate its true impact, ultimately delaying appropriate clinical and public health interventions.
5. Final Comments: Immediate Priorities for Action
The persistent under-recognition of chikungunya-associated mortality continues to hinder effective epidemic response. There is an urgent need to reassess the outdated narrative of chikungunya as a non-lethal disease, prioritizing research, improved diagnostics, expanded postmortem investigations, and the development of surveillance methods capable of capturing both direct and indirect fatal outcomes.
The recently launched WHO Integrated Guidelines on the Clinical Management of Arboviral Diseases[
51] represent an important step forward, recommending the adoption of syndromic surveillance and management approaches to improve case detection in settings where dengue, chikungunya, Zika, and yellow fever may circulate simultaneously and diagnostic capacity is limited. This approach can enhance sensitivity for early identification of severe cases and improve patient outcomes, particularly in vulnerable populations.
Aligned with these recommendations, active surveillance of severe cases and deaths is urgently needed, supported by classical autopsies and minimally invasive tissue sampling to clarify causes of death that often go unrecognized in routine systems. Furthermore, real-time excess mortality analyses should be fully integrated into arboviral surveillance frameworks, providing actionable evidence for decision-makers and enabling timely, proportionate interventions to reduce preventable deaths during future epidemics.
6. Ethical Statements and Considerations
All data used in this analysis were obtained from publicly available official sources provided by French governmental institutions. We would like to express our appreciation for the transparency, timeliness, and public accessibility of these data, which reflect the French government’s commitment to open data and evidence-based public health action. The study did not involve individual-level data or human subjects, and therefore did not require ethical approval.
Our research group has been systematically investigating chikungunya-associated excess mortality across different countries and regions, using similar methodologies based on officially reported aggregate data. This approach is not intended to identify or expose shortcomings in local surveillance systems, but rather to explore broader mortality impacts that may not be fully captured by case-based surveillance—particularly given the inherent complexity of attributing deaths that occur after variable delays following infection.
Mortality attribution related to chikungunya remains a global challenge, as delayed or indirect deaths may occur days, weeks, or even months after the initial illness, often resulting from diverse clinical complications affecting multiple organ systems. This complexity hinders individual-level attribution.
Routine surveillance systems in Réunion Island are notably comprehensive, integrating multiple data sources and ranking among the most advanced worldwide. Nonetheless, like all surveillance systems, they may face intrinsic limitations in capturing the full spectrum of chikungunya-related mortality. This study aims to contribute to the scientific understanding of this public health challenge, without criticism of local surveillance efforts or public health authorities.
We hope that these materials contribute to a transparent, reproducible, and evidence-based understanding of chikungunya-related mortality and support improved surveillance and epidemic preparedness strategies globally.
7. Transparency and Reproducibility
The SPSS syntax files used in the analyses, together with Python scripts (version 3.13, 64-bit, using numpy, pandas, scipy.stats, and statsmodels), as well as all source data, model outputs, and analytical spreadsheets, are publicly available in our GitHub repository[
52], [
https://github.com/aribasfreitas/chikungunya].
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. A detailed comparative analysis provided in Supplementary Material demonstrates substantial discrepancies between excess mortality and officially reported chikungunya deaths across multiple epidemics worldwide. Even in high-income territories with structured surveillance systems (e.g., Réunion Island 2006 and 2024–2025, French Caribbean 2014), most deaths were only mentioned on death certificates and rarely confirmed by laboratory testing. In low- and middle-income settings, the gap between excess and reported deaths was far larger, with several epidemics showing thousands of unexplained excess deaths and no officially acknowledged fatalities. These findings underscore the limitations of traditional surveillance in quantifying chikungunya mortality and highlight the value of excess mortality analyses as a critical complementary tool for epidemic risk assessment.
Author Contributions
Conceptualization, A.R.R.F.; Methodology, A.R.R.F. and L.H.F.; Data curation, A.S.L.N., L.P.G.C., and P.M.A.-E.; Formal analysis, A.R.R.F. and L.H.F.; Investigation, A.R.R.F., L.H.F. A.S.L.N., L.P.G.C., and P.M.A.-E.; Software, L.H.F.; Supervision, A.R.R.F.; Writing—original draft, A.R.R.F.; Writing—review and editing, A.R.R.F., L.H.F., A.S.L.N., L.P.G.C., and P.M.A.-E. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, R.; Puri, V.; Fedorova, N.; Lin, D.; Hari, K.L.; Jain, R.; Rodas, J.D.; Das, S.R.; Shabman, R.S.; Weaver, S.C. Comprehensive Genome Scale Phylogenetic Study Provides New Insights on the Global Expansion of Chikungunya Virus. Journal of Virology 2016, 90, 10600–10611. [CrossRef]
- Tsetsarkin, K.A.; Chen, R.; Sherman, M.B.; Weaver, S.C. Chikungunya Virus: Evolution and Genetic Determinants of Emergence. Current opinion in virology 2011, 1, 310–317. [CrossRef]
- de Lima, S.T.S.; de Souza, W.M.; Cavalcante, J.W.; da Silva Candido, D.; Fumagalli, M.J.; Carrera, J.-P.; Simões Mello, L.M.; De Carvalho Araújo, F.M.; Cavalcante Ramalho, I.L.; de Almeida Barreto, F.K.; et al. Fatal Outcome of Chikungunya Virus Infection in Brazil. Clinical Infectious Diseases 2021, 73, e2436–e2443. [CrossRef]
- de Morais Alves Barbosa Oliveira, R.; Kalline de Almeida Barreto, F.; Praça Pinto, G.; Timbó Queiroz, I.; Montenegro de Carvalho Araújo, F.; Wanderley Lopes, K.; Lúcia Sousa do Vale, R.; Rocha Queiroz Lemos, D.; Washington Cavalcante, J.; Machado Siqueira, A.; et al. Chikungunya Death Risk Factors in Brazil, in 2017: A Case-Control Study. PLOS ONE 2022, 17, e0260939. [CrossRef]
- Souza, W.M. de; Fumagalli, M.J.; Lima, S.T.S. de; Parise, P.L.; Carvalho, D.C.M.; Hernandez, C.; Jesus, R. de; Delafiori, J.; Candido, D.S.; Carregari, V.C.; et al. Pathophysiology of Chikungunya Virus Infection Associated with Fatal Outcomes. Cell Host & Microbe 2024, 32, 606-622.e8. [CrossRef]
- Freitas, A.R.R.; Cavalcanti, L.P. de G.; Gérardin, P. Chikungunya: Time to Change the Paradigm of a Non-Fatal Disease. InterAmerican Journal of Medicine and Health 2020, 3, 3–3. [CrossRef]
- Cavalcanti, L.P.G.; Freitas, A.R.R.; Brasil, P.; da Cunha, R.V. Surveillance of Deaths Caused by Arboviruses in Brazil: From Dengue to Chikungunya. Memorias do Instituto Oswaldo Cruz 2017, 112. [CrossRef]
- De Góes Cavalcanti, L.P.; Gadelha Farias, L.A.B.; De Almeida Barreto, F.K.; Siqueira, A.M.; Ribeiro, G.S.; Freitas, A.R.R.; Weaver, S.C.; Kitron, U.; Brito, C.A.A. Chikungunya Case Classification after the Experience with Dengue Classification: How Much Time Will We Lose? American Journal of Tropical Medicine and Hygiene 2020, 102. [CrossRef]
- Santé Publique France Surveillance sanitaire à La Réunion. Bulletin du 26 juin 2025. Available online: https://www.santepubliquefrance.fr/regions/ocean-indien/documents/bulletin-regional/2025/surveillance-sanitaire-a-la-reunion.-bulletin-du-26-juin-2025 (accessed on 23 July 2025).
- Frumence, E.; Piorkowski, G.; Traversier, N.; Amaral, R.; Vincent, M.; Mercier, A.; Ayhan, N.; Souply, L.; Pezzi, L.; Lier, C.; et al. Genomic Insights into the Re-Emergence of Chikungunya Virus on Réunion Island, France, 2024 to 2025. Eurosurveillance 2025, 30. [CrossRef]
- Santé Publique France Canicule et santé : excès de mortalité. Bulletin du 23 juillet 2025. Available online: https://www.santepubliquefrance.fr/determinants-de-sante/climat/fortes-chaleurs-canicule/documents/bulletin-national/canicule-et-sante-exces-de-mortalite.-bulletin-du-23-juillet-2025 (accessed on 29 July 2025).
- Li, Y.; Jiang, S.; Zhang, M.; Li, Y.; He, J.; Yang, Z.; Huang, X.; Guan, Q.; Li, Z.; Xie, J.; et al. An Outbreak of Chikungunya Fever in China — Foshan City, Guangdong Province, China, July 2025. CCDCW 2025, 7, 1–2. [CrossRef]
- European Centre for Disease Prevention and Control (ECDC) Seasonal Surveillance of Chikungunya Virus Disease in the EU/EEA Available online: https://www.ecdc.europa.eu/en/chikungunya-virus-disease/surveillance-and-updates/seasonal-surveillance (accessed on 4 August 2025).
- European Centre for Disease Prevention and Control.; EpiConcept. Trend Analysis Guidance for Surveillance Data.; Publications Office: LU, 2024;
- 2023; 15. World Health Organisation Methods for Estimating the Excess Mortality Associated with the COVID-19 Pandemic; 2023;
- European mortality monitoring activity network EUROMOMO Available online: https://euromomo.eu/ (accessed on 26 July 2025).
- Institut National de la Statistique et des Études Économiques L’INSEE Available online: https://www.insee.fr/fr/statistiques?theme=0 (accessed on 2 July 2025).
- M’nemosyme, N.; Frumence, E.; Souply, L.; Heaugwane, D.; Traversier, N.; Mercier, A.; Daoudi, J.; Casalegno, J.-S.; Valette, M.; Moiton, M.-P.; et al. Shifts in Respiratory Virus Epidemiology on Reunion Island From 2017 to 2023: Impact of COVID-19 Pandemic and Non-Pharmaceutical Interventions. Influenza and Other Respiratory Viruses 2025, 19, e70075. [CrossRef]
- Liu, X.-X.; Li, Y.; Zhu, Y.; Zhang, J.; Li, X.; Zhang, J.; Zhao, K.; Hu, M.; Qin, G.; Wang, X.-L. Seasonal Pattern of Influenza Activity in a Subtropical City, China, 2010–2015. Sci Rep 2017, 7, 17534. [CrossRef]
- Alonso, W.J.W.J.; Viboud, C.; Simonsen, L.; Hirano, E.W.E.W.; Daufenbach, L.Z.L.; Miller, M. a M.A. Seasonality of Influenza in Brazil: A Traveling Wave from the Amazon to the Subtropics. American Journal of Epidemiology 2007, 165, 1434–1442. [CrossRef]
- Hirve, S.; Newman, L.P.; Paget, J.; Azziz-Baumgartner, E.; Fitzner, J.; Bhat, N.; Vandemaele, K.; Zhang, W. Influenza Seasonality in the Tropics and Subtropics – When to Vaccinate? PLoS ONE 2016, 11, e0153003. [CrossRef]
- Young, B.E.; Chen, M. Influenza in Temperate and Tropical Asia: A Review of Epidemiology and Vaccinology. Hum Vaccin Immunother 2020, 16, 1659–1667. [CrossRef]
- Ribas Freitas, A.R.; Lima Neto, A.S.; Rodrigues, R.; Alves de Oliveira, E.; Andrade, J.S.; Cavalcanti, L.P.G. Excess Mortality Associated with Chikungunya Epidemic in Southeast Brazil, 2023. Front. Trop. Dis. 2024, 5, 1466207. [CrossRef]
- Farrington, C.P.; Andrews, N.J.; Beale, A.D.; Catchpole, M.A. A Statistical Algorithm for the Early Detection of Outbreaks of Infectious Disease. Journal of the Royal Statistical Society. Series A (Statistics in Society) 1996, 159, 547–563. [CrossRef]
- STATENS SERUM INSTITUT A European Algorithm for a Common Monitoring of Mortality across Europe.; 2011;
- Sarkar, J.K.; Chatterjee, S.N.; Chakravarty, S.K. Three-Year Study of Mosquito-Borne Haemorrhagic Fever in Calcutta*. Transactions of The Royal Society of Tropical Medicine and Hygiene 1967, 61, 725–735. [CrossRef]
- Gelfand, H; Bose, PN; Sehgal, PN; Mukherjee, RN Epidemiological Observations on the Outbreak of Acute Haemorrhagic Fever in Calcutta in 1963. In Report of the WHO Seminar on Mosquito-borne Haemorrhagic Fevers in South-East Asia and Western Pacific Regions, Bangkok, 19-26 October 1964; World Health Organization. Regional Office for South-East Asia and Regional Office for the Western Pacific: Bangkok, Thailand, 1964; p. IR/Haem.Fever/Sem.1/WP/33.
- Freitas, A.R.R.; Donalisio, M.R.; Alarcón-Elbal, P.M. Excess Mortality and Causes Associated with Chikungunya, Puerto Rico, 2014–2015. Emerging Infectious Diseases 2018, 24, 2352–2355. [CrossRef]
- Freitas, A.R.R.; Cavalcanti, L.; Zuben, A.P.V.; Donalisio, M.R. Excess Mortality Related To Chikungunya Epidemics In The Context Of Co-Circulation Of Other Arboviruses In Brazil. PLOS Currents Outbreaks 2017, 140491. [CrossRef]
- Mavalankar, D.; Shastri, P.; Bandyopadhyay, T.; Parmar, J.; Ramani, K.V. Increased Mortality Rate Associated with Chikungunya Epidemic, Ahmedabad, India. Emerging Infectious Diseases 2008, 14, 412–415.
- Tandale, B.V.; Sathe, P.S.; Arankalle, V. a; Wadia, R.S.; Kulkarni, R.; Shah, S.V.; Shah, S.K.; Sheth, J.K.; Sudeep, a B.; Tripathy, A.S.; et al. Systemic Involvements and Fatalities during Chikungunya Epidemic in India, 2006. Journal of Clinical Virology 2009, 46, 145–149. [CrossRef]
- Beesoon, S.; Funkhouser, E.; Kotea, N.; Spielman, A.; Robich, R.M. Chikungunya Fever, Mauritius, 2006. Emerging Infectious Disease 2008, 14, 337–338.
- Freitas, A.R.R.; Gérardin, P.; Kassar, L.; Donalisio, M.R. Excess Deaths Associated with the 2014 Chikungunya Epidemic in Jamaica. Pathogens and Global Health 2019, 00, 1–5. [CrossRef]
- Bertillon, J. La grippe à Paris et dans quelques autres villes de France et de l’étranger en 1889-1890; Paris : Imprimerie municipale, 1892;
- Assaad, F.; Cockburn, W.C.; Sundaresan, T.K. Use of Excess Mortality from Respiratory Diseases in the Study of Influenza. Bull World Health Organ 1973, 49, 219–233.
- 2023; 36. World Health Organization WHO Guidance on Research Methods for Health Emergency and Disaster Risk Management; 2023;
- Pirard, P.; Vandentorren, S.; Pascal, M.; Laaidi, K.; Le Tertre, A.; Cassadou, S.; Ledrans, M. Summary of the Mortality Impact Assessment of the 2003 Heat Wave in France. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 2005, 10, 153–156.
- Josseran, L.; Paquet, C.; Zehgnoun, A.; Caillere, N.; Tertre, A.L.; Solet, J.-L.; Ledrans, M. Chikungunya Disease Outbreak , Reunion Island. Emerging Infectious Diseases 2006, 12, 1994–1995.
- Santé Publique France Bulletin Epidémiologique Hebdomadaire, 21 octobre 2008, n°38-39-40 Qu’avons-nous appris de l’épidémie de chikungunya dans l’Océan Indien en 2005-2006 ? Available online: https://www.santepubliquefrance.fr/import/bulletin-epidemiologique-hebdomadaire-21-octobre-2008-n-38-39-40-qu-avons-nous-appris-de-l-epidemie-de-chikungunya-dans-l-ocean-indien-en-2005-20 (accessed on 24 July 2025).
- Baghdadi, Y.; Gallay, A.; Caserio-Schönemann, C.; Fouillet, A. Evaluation of the French Reactive Mortality Surveillance System Supporting Decision Making. Eur J Public Health 2019, 29, 601–607. [CrossRef]
- Dorleans, F.; Hoen, B.; Najioullah, F.; Herrmann-Storck, E.; Maria Schepers, K.; Abel, S.; Lamaury, I.; Fagour, L.; Guyomard, S.; Troudard, R.; et al. Outbreak of Chikungunya in the French Caribbean Islands of Martinique and Guadeloupe: Findings from a Hospital-Based Surveillance System (2013-2015). Am. J. Trop. Med. Hyg 2018, 98, 1819–1825. [CrossRef]
- Ribas Freitas, A. R.; Alarcón-Elbal, P.M.; Donalisio, M.R. Excess Mortality in Guadeloupe and Martinique, Islands of the French West Indies, during the Chikungunya Epidemic of 2014. Epidemiology and Infection 2018, 146, 2059–2065.
- Economopoulou, A.; Dominguez, M.; Helynck, B.; Sissoko, D.; Wichmann, O.; Quenel, P.; Germonneau, P.; Quatresous, I. Atypical Chikungunya Virus Infections: Clinical Manifestations, Mortality and Risk Factors for Severe Disease during the 2005-2006 Outbreak on Réunion. Epidemiology and infection 2009, 137, 534–541. [CrossRef]
- Sharp, T.M.; Keating, M.K.; Shieh, W.-J.; Bhatnagar, J.; Bollweg, B.C.; Levine, R.; Blau, D.M.; Torres, J.V.; Rivera, A.; Perez-Padilla, J.; et al. Clinical Characteristics, Histopathology, and Tissue Immunolocalization of Chikungunya Virus Antigen in Fatal Cases. Clinical Infectious Diseases 2021, 73, e345–e354. [CrossRef]
- Almeida, L.M.D.; Melo, D.N.D.; Silva, M.M.D.; Souza, P.M.M.D.; Silva, F.K.D.S.; Coelho, T.M.S.; Lima, S.T.S.D.; Mota, A.G.D.M.; Monteiro, R.A.D.A.; Saldiva, P.H.N.; et al. Usefulness of Minimally Invasive Autopsy in the Diagnosis of Arboviruses to Increase the Sensitivity of the Epidemiological Surveillance System in Ceará, Brazil. Epidemiol. Serv. Saúde 2024, 33, e2024008. [CrossRef]
- Freitas, A.R.R.; Pinheiro Chagas, A.A.; Siqueira, A.M.; Pamplona de Góes Cavalcanti, L. How Much of the Current Serious Arbovirus Epidemic in Brazil Is Dengue and How Much Is Chikungunya? The Lancet Regional Health – Americas 2024, 34. [CrossRef]
- European Centre for Disease Prevention and Control (ECDC) Factsheet for Health Professionals about Chikungunya Virus Disease Available online: https://www.ecdc.europa.eu/en/chikungunya/facts/factsheet (accessed on 4 August 2025).
- Guangdong Provincial Center for Disease Control and Prevention Chikungunya Fever Diagnosis and Treatment Plan (2025 Edition) Available online: https://cdcp.gd.gov.cn/ywdt/zdzt/yfjkkyr/yfjkkyrzs/content/post_4752702.html (accessed on 4 August 2025).
- World Health Organization Regional Office for South-East Asia Guidelines on Clinical Management of Chikungunya Fever; SEARO | WHO South-East Asia Region: New Delhi, India, 2008;
- Heidecker, B.; Libby, P.; Vassiliou, V.S.; Roubille, F.; Vardeny, O.; Hassager, C.; Gatzoulis, M.A.; Mamas, M.A.; Cooper, L.T.; Schoenrath, F.; et al. Vaccination as a New Form of Cardiovascular Prevention: A European Society of Cardiology Clinical Consensus Statement: With the Contribution of the European Association of Preventive Cardiology (EAPC), the Association for Acute CardioVascular Care (ACVC), and the Heart Failure Association (HFA) of the ESC. European Heart Journal 2025, ehaf384. [CrossRef]
- World Health Organization WHO Guidelines for Clinical Management of Arboviral Diseases: Dengue, Chikungunya, Zika and Yellow Fever; World Health Organization: Geneva, 2025; ISBN 978-92-4-011111-0.
- Ribas Freitas, A.R.; Hughes Freitas, Luana Chikungunya Data and Analysis (GitHub) Available online: https://github.com/aribasfreitas/chikungunya (accessed on 21 July 2025).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).