Submitted:
19 September 2025
Posted:
22 September 2025
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
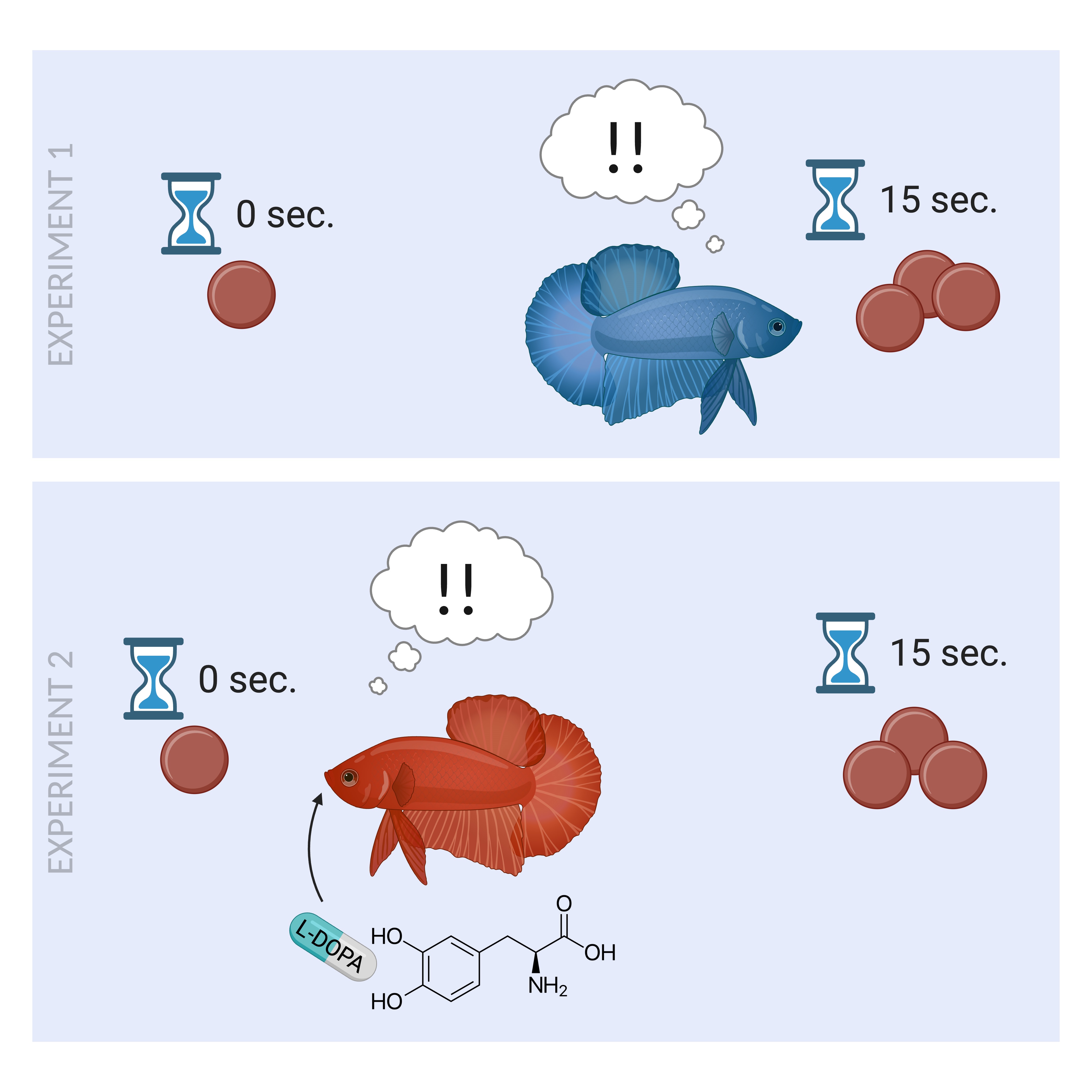
2. Experiment I: Materials and Method
2.1. Animals and Housing
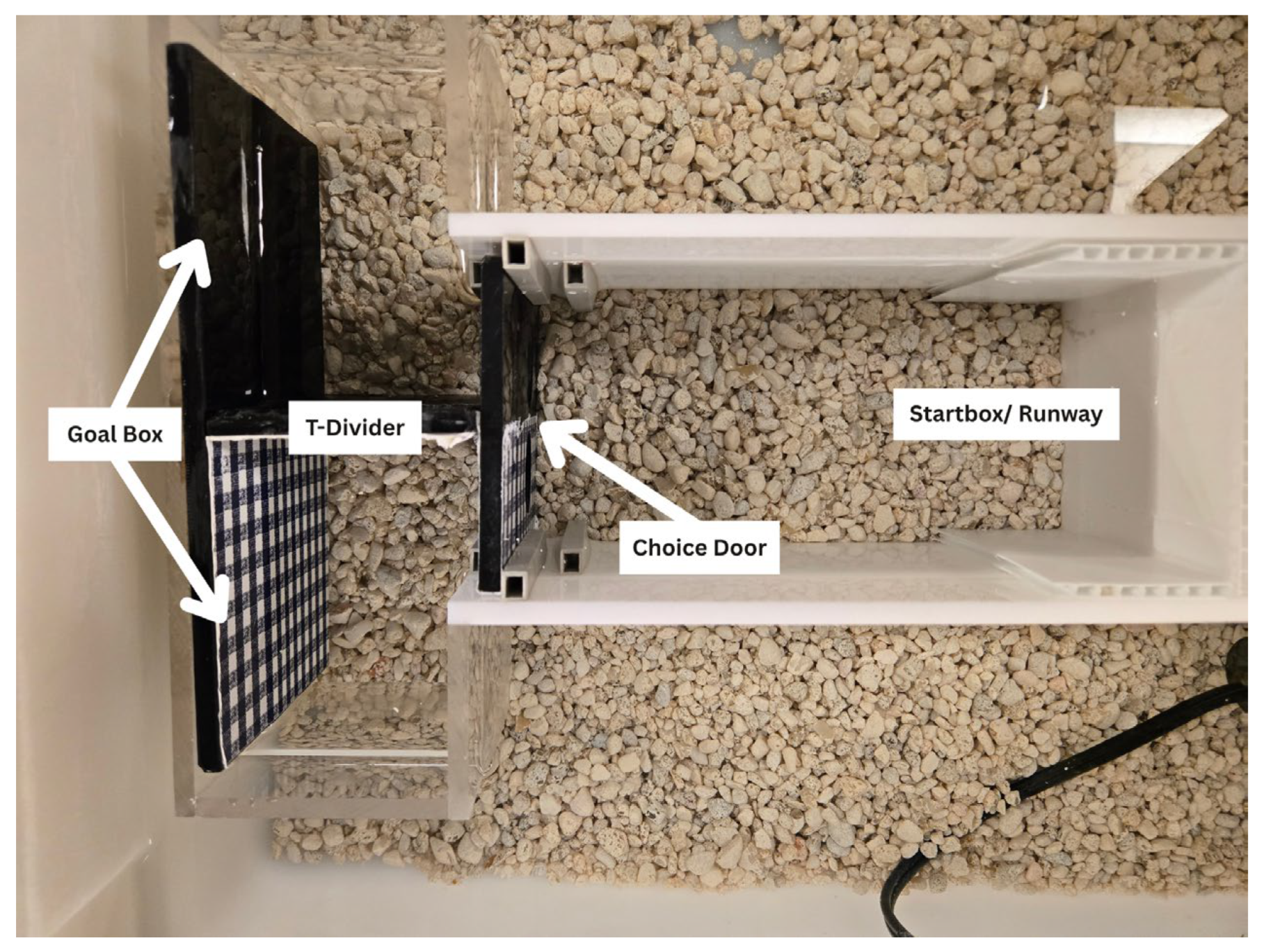
2.2. Materials and Apparatus
2.3. Procedure
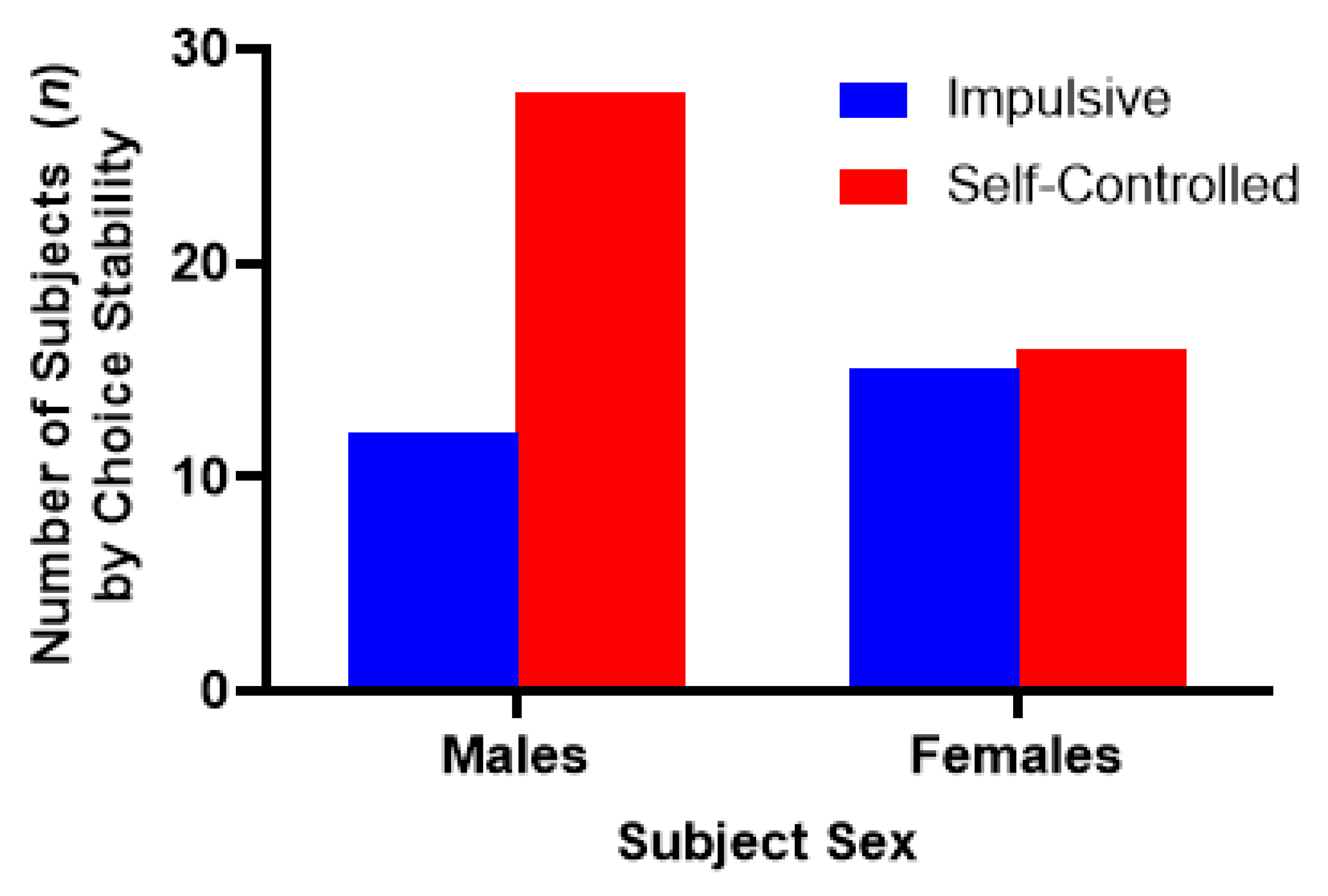
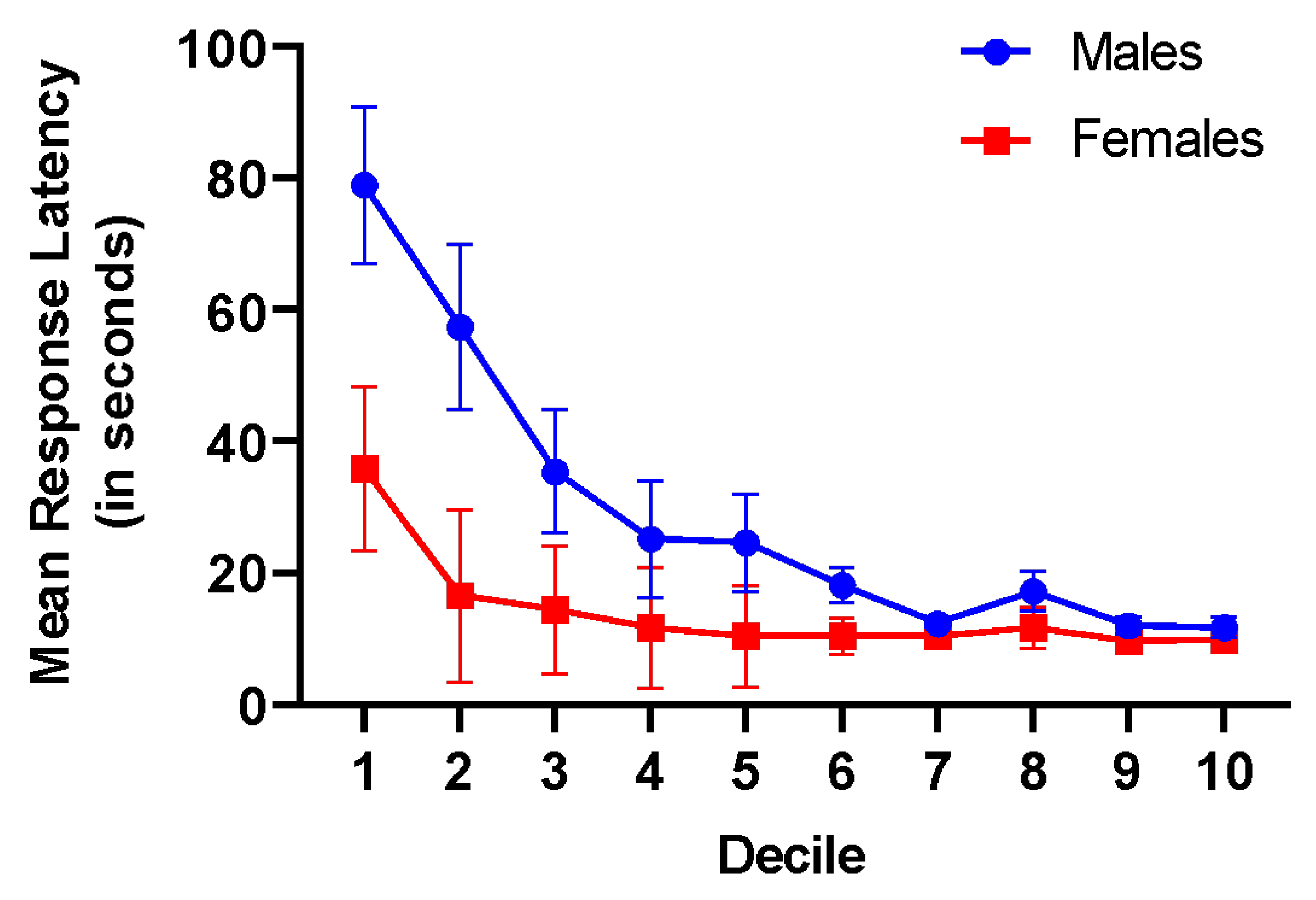
3. Experiment I: Results
4. Experiment I: Discussion
5. Experiment II: Introduction
6. Experiment II: Materials and Method
6.1. Animals and Housing
6.2. Procedure
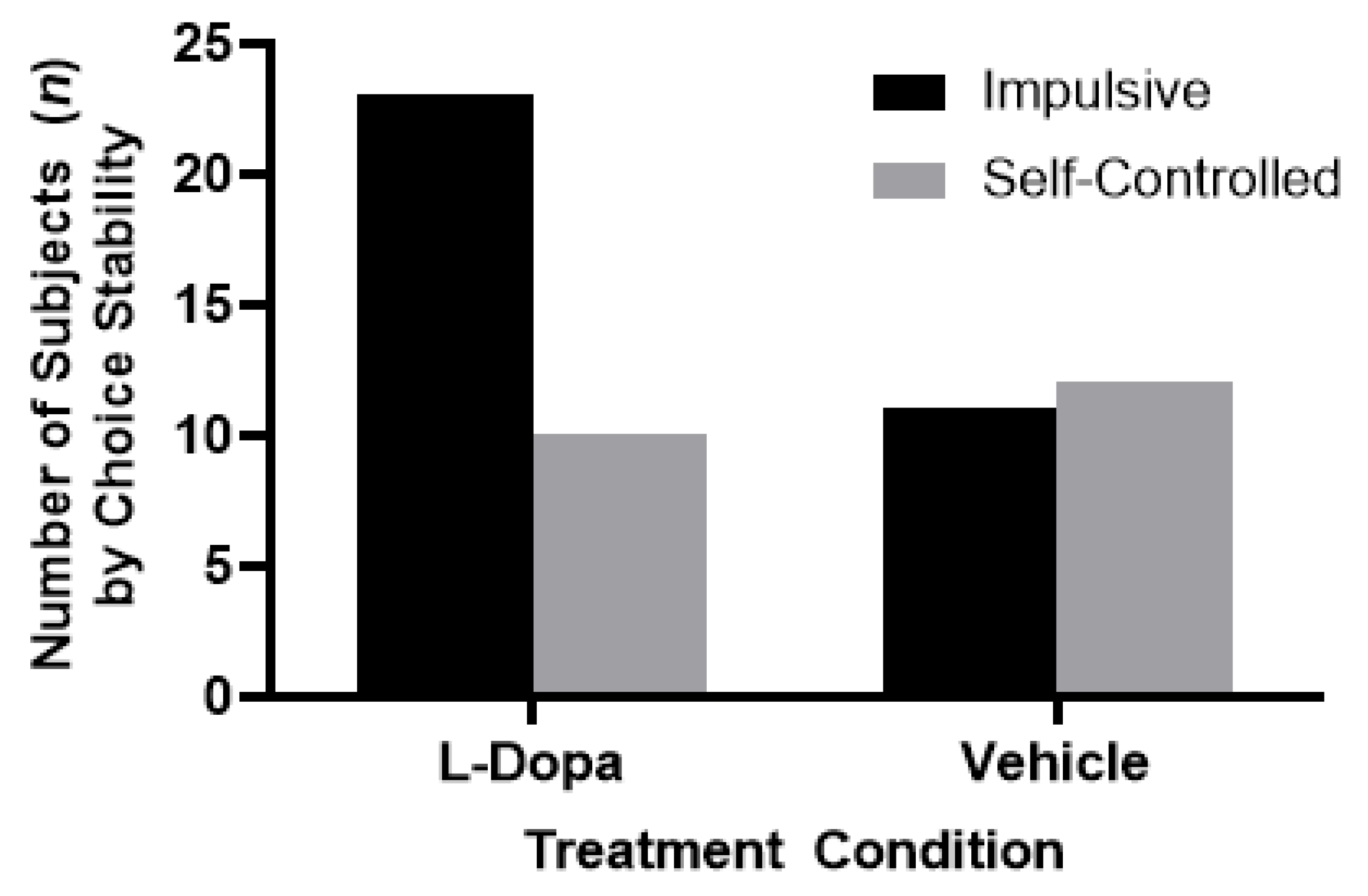
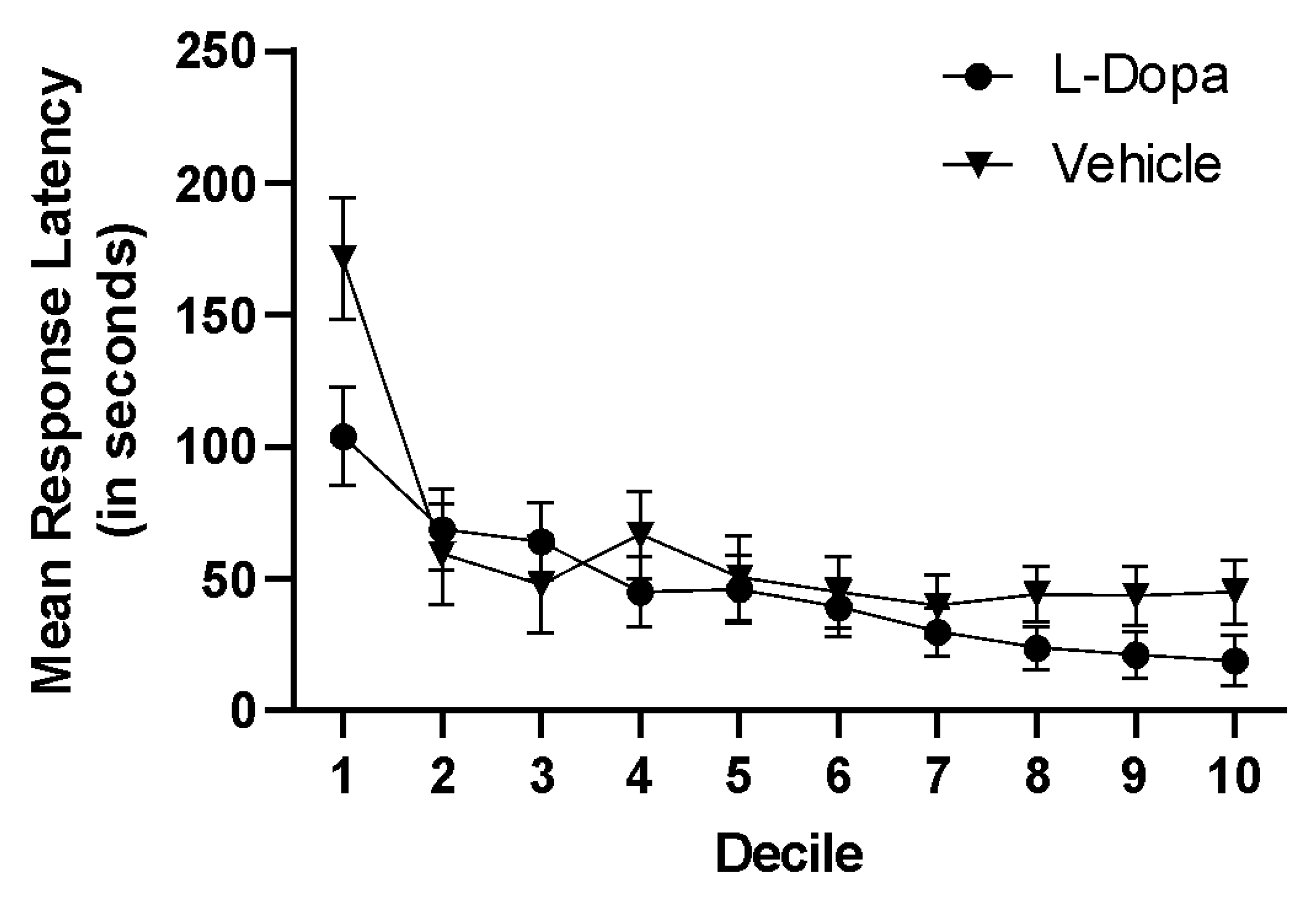
7. Experiment II: Results
8. Experiment II: Discussion
9. General Discussion
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ainslie, G.W. Impulse control in pigeons. J Exp Anal Behav 1974, 21, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Rachlin, H.; Green, L. Commitment, choice and self-control. J Exp Anal Behav 1972, 17, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Logue, A. W. Research on self-control: An integrating framework. Behav Brain Sci 1988, 11, 665–679. [Google Scholar] [CrossRef]
- van Baal, S. T.; Walasek, L.; Verdejo-García, A.; Hohwy, J. Impulsivity and self-control as timeless concepts: A conceptual analysis of intertemporal choice. Decision 2025, 12, 165–189. [Google Scholar] [CrossRef]
- Abeyesinghe, S.M.; Nicol, C.J.; Hartnell, S.J.; Wathes, C.M. Can domestic fowl, Gallus gallus domesticus, show self-control? Anim Behav 2005, 70, 1–11. [Google Scholar] [CrossRef]
- Tobin, H.; Logue, A.W. Self-control across species (Columba livia, Homo sapiens, and Rattus norvegicus). J Comp Psychol 1994, 108, 126–133. [Google Scholar] [CrossRef]
- Van Haaren, F.; Van Hest, A.; Van De Poll, N. E. Self-control in male and female rats. J Exp Anal Behav 1988, 49, 201–211. [Google Scholar] [CrossRef]
- MacLean, E. L.; Hare, B.; Nunn, C. L.; Addessi, E.; Amici, F.; Anderson, R. C.; Filippo, A.; Baker, J.M.; Bania, A.E.; Barnard, A.M; et al. The evolution of self-control. Proceedings of the National Academy of Sciences 2014, 111, E2140–E2148. [Google Scholar] [CrossRef]
- Miller, R.; Boeckle, M.; Jelbert, S. A.; Frohnwieser, A.; Wascher, C. A.; Clayton, N. S. Self-control in crows, parrots and nonhuman primates. Wiley Interdiscip. Rev Cogn Sci 2019, 10, e1504. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, E.; Zupan Šemrov, M. Dogs exhibiting high levels of aggressive reactivity show impaired self-control abilities. Front Vet Sci 2022, 9, 869068. [Google Scholar] [CrossRef]
- Cheng, K.; Peña, J.; Porter, M. A.; Irwin, J. D. Self-control in honeybees. Psychon Bull Rev 2002, 9, 259–263. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Starving honeybees lose self-control. Biology letters 2015, 11, 20140820. [Google Scholar] [CrossRef]
- Schnell, A. K.; Boeckle, M.; Rivera, M.; Clayton, N. S.; Hanlon, R. T. Cuttlefish exert self-control in a delay of gratification task. Proceedings of the Royal Society B 2021, 288, 20203161. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Ng, S.; Gray, Q.; Cowie, S. Pigeons’ (Columba livia) intertemporal choice in binary-choice and patch-leaving contexts. J Comp Psychol 2025, 139, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Tobin, H.; Chelonis, J. J.; Logue, A. W. Choice in self-control paradigms using rats. The Psychological Record 1993, 43, 441–453. [Google Scholar]
- Pepperberg, I. M.; Rosenberger, V. A. Delayed gratification: A grey parrot (Psittacus erithacus) will wait for more tokens. J Comp Psychol 2022, 136, 79–89. [Google Scholar] [CrossRef]
- Hackenberg, T. D. Token reinforcement: A review and analysis. J Exp Anal Behav 2009, 91, 257–286. [Google Scholar] [CrossRef]
- Aellen, M.; Dufour, V.; Bshary, R. Cleaner fish and other wrasse match primates in their ability to delay gratification. Anim Behav 2021, 176, 125–143. [Google Scholar] [CrossRef]
- Stearns, S. C. (1998). The Evolution Of Life Histories. Oxford: Oxford Academic. (Online edn,, 31 Oct. 2023). [CrossRef]
- Veit, W.; Gascoigne, S. J.; Salguero-Gómez, R. Evolution, complexity, and life history theory. Biological Theory 2025, 20, 1–10. [Google Scholar] [CrossRef]
- Jaroensutasinee, M.; Jaroensutasinee, K. Sexual size dimorphism and male contest in wild Siamese fighting fish. J Fish Biol 2001, 59, 1614–1621. [Google Scholar] [CrossRef]
- Forsatkar, M. N.; Nematollahi, M. A.; Brown, C. Social context modulates aggression and courtship in male Siamese fighting fish, Betta splendens. Behav Processes 2016, 133, 79–85. [Google Scholar] [CrossRef]
- Simpson, M. J. A. The display of the Siamese fighting fish, Betta splendens. Anim Behav Monographs 1968, 1, 1–73. [Google Scholar] [CrossRef]
- Lichak, M. R.; Barber, J. R.; Kwon, Y. M.; Francis, K. X.; Bendesky, A. Care and use of Siamese fighting fish (Betta splendens) for research. Comp Med 2022, 72, 169–180. [Google Scholar] [CrossRef]
- Wooster, E.; Whiting, M.; Nimmo, D.; Sayol, F.; Carthey, A.; Stanton, L.; Ashton, B. Predator-prey interactions as drivers of cognitive evolution (preprint). EcoEvoRxiv 2025. [CrossRef]
- Bateman, A. W.; Vos, M.; Anholt, B. R. When to defend: Antipredator defenses and the predation sequence. Am Nat 2014, 183, 847–855. [Google Scholar] [CrossRef]
- Endler, J. A. Variation in the appearance of guppy color patterns to guppies and their predators under different visual conditions. Vision Research 1991, 31, 587–608. [Google Scholar] [CrossRef]
- Baenninger, R.; Kraus, S. (1981). Some determinants of aggressive and predatory responses in Betta splendens. J Comp Physiol Psychol 1981, 95, 220–227. [Google Scholar] [CrossRef]
- Day, S. W.; Higham, T. E.; Holzman, R.; Van Wassenbergh, S. Morphology, kinematics, and dynamics: the mechanics of suction feeding in fishes. Integr Comp Biol 2015, 55, 21–35. [Google Scholar] [CrossRef]
- Ferry-Graham, L. A.; Lauder, G. V. Aquatic prey capture in ray-finned fishes: A century of progress and new directions. J Morphol 2001, 248, 99–119. [Google Scholar] [CrossRef]
- Wainwright, P.; Carroll, A. M.; Collar, D. C.; Day, S. W.; Higham, T. E.; Holzman, R. A. Suction feeding mechanics, performance, and diversity in fishes. Integr Comp Biol 2007, 47, 96–106. [Google Scholar] [CrossRef]
- Gromova, E. S.; Makhotin, V. V. Diversity of feeding methods in Teleosts (Teleostei) in the context of the morphology of their jaw apparatus. Inland Water Biol 2023, 16, 700–721. [Google Scholar] [CrossRef]
- Cagle, M. D. Sexual dimorphism and potential hormonal modulation of feeding mechanics in Siamese fighting fish, Betta splendens (Unpublished Master’s thesis, Northern Arizona University) 2014. Retrieved from https://cnu.idm.oclc.org/login?url=https://www.proquest.com/dissertations-theses/sexual-dimorphism-potential-hormonal-modulation/docview/1611782153/se-2?accountid=10100 on 20 June, 2025.
- Goldstein, R.J. (2015). The Betta Handbook. Barron’s Educational Series: Hauppauge, NY, 2015; ISBN 978-0764127281.
- Monvises, A.; Nuangsaeng, B.; Sriwattanarothai, N.; Panijpan, B. The Siamese fighting fish: well-known generally but little-known scientifically. ScienceAsia 2009, 35, 8–16. [Google Scholar] [CrossRef]
- Jaroensutasinee, M.; Jaroensutansinee, K. Bubble nest habitat characteristics of wild Siamese fighting fish. J Fish Biol 2001, 58, 1311–1319. [Google Scholar] [CrossRef]
- Craft, B.B.; Velkey, A.J.; Szalda-Petree, A. Instrumental conditioning of choice behavior in male Siamese fighting fish (Betta splendens). Behav Processes 2003, 63, 171–175. [Google Scholar] [CrossRef]
- National Research Council (2011). Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA. ISBN 978-0-309-15400-0.
- Bols, R.J.; Hogan, J.A. Runway behavior of Siamese fighting fish (Betta splendens) for aggressive display and food reinforcement. Anim Learn Behav 1979, 7, 537–542. [Google Scholar] [CrossRef]
- Craft, B.B.; Szalda-Petree, A.D. Effect of various discriminative stimuli on choice behavior in male Siamese Fighting Fish (Betta splendens). Percept Mot Skills 2007, 104, 575–580. [Google Scholar] [CrossRef]
- Hogan, J.A.; Bols, R.J. Priming of aggressive motivation in Betta splendens. Anim Behav 1980, 28, 1189–1196. [Google Scholar] [CrossRef]
- Pepperberg, I. M.; Hartsfield, L. A. A study of executive function in grey parrots (Psittacus erithacus): Experience can affect delay of gratification. J Comp Psychol 2024, 138, 8–19. [Google Scholar] [CrossRef]
- Prétôt, L.; Bshary, R.; Brosnan, S. F. Factors influencing the different performance of fish and primates on a dichotomous choice task. Anim Behav 2016, 119, 189–199. [Google Scholar] [CrossRef]
- Alcaro, A.; Huber, R.; Panksepp, J. Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev 2007, 56, 283–321. [Google Scholar] [CrossRef]
- Diotel, N.; Lübke, L.; Strähle, U.; Rastegar, S. Common and distinct features of adult neurogenesis and regeneration in the telencephalon of zebrafish and mammals. Front Neurosci 2020, 14, 568930. [Google Scholar] [CrossRef]
- Dupeyron, S.; Wallace, K. J. Quantifying the neural and behavioral correlates of repeated social competition in the fighting fish Betta splendens. Fishes 2023, 8, 384. [Google Scholar] [CrossRef]
- Yamamoto, K.; Vernier, P. (2011). The evolution of dopamine systems in chordates. Front Neuroanat 2023, 5, 21. [Google Scholar] [CrossRef]
- Pérez-Fernández, J.; Barandela, M.; Jiménez-López, C. The dopaminergic control of movement-evolutionary considerations. Int J Mol Sci 2021, 22, 11284. [Google Scholar] [CrossRef]
- Costa, K. M.; Schoenbaum, G. Dopamine. Curr Biol 2022, 32, R817–R824. [Google Scholar] [CrossRef]
- Yamamoto, K.; Fontaine, R.; Pasqualini, C.; Vernier, P. Classification of dopamine receptor genes in vertebrates: Nine subtypes in osteichthyes. Brain Behav Evol 2015, 86, 164–175. [Google Scholar] [CrossRef]
- Schultz, W. A dopamine mechanism for reward maximization. Proceedings of the National Academy of Sciences 2024, 121, e2316658121. [Google Scholar] [CrossRef]
- Kobayashi, S.; Schultz, W. Reward contexts extend dopamine signals to unrewarded stimuli. Curr Biol 2014, 24, 56–62. [Google Scholar] [CrossRef]
- Lak, A.; Stauffer, W. R.; Schultz, W. Dopamine prediction error responses integrate subjective value from different reward dimensions. Proceedings of the National Academy of Sciences 2014, 111, 2343–2348. [Google Scholar] [CrossRef]
- Jin, F.; Yang, L.; Yang, L.; Li, J.; Li, M.; Shang, Z. Dynamics learning rate bias in pigeons: insights from reinforcement learning and neural correlates. Animals 2024, 14, 489. [Google Scholar] [CrossRef]
- Soares, M.C.; Cardoso, S.C.; Malato, J.T.; Messias, J.P. Can cleanerfish overcome temptation? A selective role for dopamine influence on cooperative-based decision making. Physiol Behav 2017, 169, 124–129. [Google Scholar] [CrossRef]
- Mersereau, E.J.; Boyle, C.A.; Poitra, S.; Espinoza, A.; Seiler, J.; Longie, R.; Delvo, L.; Szarkowski, M.; Maliske, J.; Chalmers, S.; Darland, D.C. Longitudinal effects of embryonic exposure to cocaine on morphology, cardiovascular physiology, and behavior in zebrafish. Int J Mol Sci 2016, 17, 847. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M., Jamwal, A., Chivers, D.P., Niyogi, S. Modulatory effects of dopamine receptors on associative learning performance in zebrafish (Danio rerio). Behav Brain Res 2016, 303, 109-119. [CrossRef]
- Höglund, E.; Silva, P.I.M.; Vindas, M.A.; Øverli, Ø. “Contrasting coping styles meet the wall: a dopamine driven dichotomy in behavior and cognition”. Front Neurosci 2017, 17, 383. [Google Scholar] [CrossRef]
- Vindas, M.A.; Sørensen, C.; Johansen, I.B.; Folkedal, O.; Höglund, E.; Khan, U.W.; Stien, L.H.; Kristiansen, T.S.; Braastad, B.O.; Øverli, Ø. Coping with unpredictability: dopaminergic and neurotrophic responses to omission of expected reward in Atlantic salmon (Salmo salar L.). PloS one 2014, 9, p.e85543. [Google Scholar] [CrossRef]
- Collins, A. G.; Frank, M. J. Opponent actor learning (OpAL): Modeling interactive effects of striatal dopamine on reinforcement learning and choice incentive. Psychol Rev 2014, 121, 337–366. [Google Scholar] [CrossRef]
- Rink, E.; Wullimann, M. F. (2002). Development of the catecholaminergic system in the early zebrafish brain: An immunohistochemical study. Developmental brain research 2002, 137, 89-100. [Google Scholar] [CrossRef]
- O’Connell, L. A.; Hofmann, H. A. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol 2011, 519, 3599–3639. [Google Scholar] [CrossRef]
- Mueller, T. What is the thalamus in zebrafish? Front Neurosci 2012, 6, 64. [Google Scholar] [CrossRef]
- Salas, C.; Broglio, C.; Rodríguez, F. Evolution of forebrain and spatial cognition in vertebrates: conservation across diversity. Brain Behav Evol 2003, 62, 72–82. [Google Scholar] [CrossRef]
- Rutledge, R. B.; Skandali, N.; Dayan, P.; Dolan, R. J. Dopaminergic modulation of decision making and subjective well-being. J Neurosci 2015, 35, 9811–9822. [Google Scholar] [CrossRef]
- Pine, A.; Shiner, T.; Seymour, B.; Dolan, R. J. Dopamine, time, and impulsivity in humans. J Neurosci 2010, 30, 8888–8896. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Barone, P.; Trojano, L.; Vitale, C. Pathological gambling in Parkinson’s disease. A comprehensive review. Park Relat Disord 2013, 19, 645–653. [Google Scholar] [CrossRef]
- Culicetto, L.; Impellizzeri, F.; Lo Buono, V.; Marafioti, G.; Di Lorenzo, G.; Sorbera, C.; Brigandì, A.; Quartarone, A.; Marino, S. Gambling disorder in Parkinson’s disease: A scoping review on the challenge of rehabilitation strategies. J Clin Med 2025, 14, 737. [Google Scholar] [CrossRef]
- Voon, V.; Hassan, K.; Zurowski, M.; Duff-Canning, S.; De Souza, M.; Fox, S.; Lang, A.; Miyasaki, J. Prospective prevalence of pathologic gambling and medication association in Parkinson disease. Neurology 2006, 66, 1750–1752. [Google Scholar] [CrossRef]
- Pirritano, D.; Plastino, M.; Bosco, D.; Gallelli, L.; Siniscalchi, A.; De Sarro, G. Gambling disorder during dopamine replacement treatment in Parkinson’s disease: A comprehensive review. BioMed Res Int 2014, 2014, 728038. [Google Scholar] [CrossRef]
- Pietras, C.J.; Cherek, D.R.; Lane, S.D.; Tcheremissine, O.V.; Steinberg, J.L. Effects of methylphenidate on impulsive choice in adult humans. Psychopharmacology 2003, 170. [Google Scholar] [CrossRef]
- Arkell, T.R.; Bradshaw, K.; Downey, L.A.; Hayley, A.C. Acute effects of amphetamine and related psychostimulants on impulsivity: A systematic review of clinical trials. Addict Biol 2022, 27, p.e13128. [Google Scholar] [CrossRef]
- Quintero, J.; Gutiérrez-Casares, J.R.; Álamo, C. Molecular characterisation of the mechanism of action of stimulant drugs lisdexamfetamine and methylphenidate on ADHD neurobiology: A review. Neurol Ther 2022, 11, 1489–1517. [Google Scholar] [CrossRef]
- Pearce, R.; Heikkilä, M.; Lindén, I. B.; Jenner, P. L-dopa induces dyskinesia in normal monkeys: Behavioural and pharmacokinetic observations. Psychopharmacology 2001, 156, 402–409. [Google Scholar] [CrossRef]
- Contin, M.; Martinelli, P. Pharmacokinetics of levodopa. J Neurol 2010, 257 (Suppl 2), S253–S261. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.M.; Szalda-Petree, A.D. Fins of fury or fainéant: Fluoxetine impacts the aggressive behavior of fighting fish (Betta splendens). Behav Processes 2022, 194, 104544. [Google Scholar] [CrossRef]
- Eisenreich, B.R.; Greene, S.; Szalda-Petree, A. Of fish and mirrors: Fluoxetine disrupts aggression and learning for social rewards. Physiol Behav 2017, 173, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S; Burch, R.L. The Principles of Humane Experimental Technique. Methuen: London, England. 1959. Chapter 4.
- Yuan, M.; Fang, Q.; Lu, W.; Wang, X.; Hao, T.; Chong, C.M.; Chen, S. Stress in fish: Neuroendocrine and neurotransmitter responses. Fishes, 2025, 10, p.307. [CrossRef]
- Fizet, J.; Cassel, J. C.; Kelche, C.; Meunier, H. A review of the 5-Choice Serial Reaction Time (5-CSRT) task in different vertebrate models. Neurosci Biobehav Rev 2016, 71, 135–153. [Google Scholar] [CrossRef]
| Mammalian Brain Region | Function | Fish Homolog | Evidence |
|---|---|---|---|
| Ventral Tegmental Area (VTA) | Source of dopaminergic projections to forebrain (reward, motivation) |
Posterior tuberculum (TPp) | Dopaminergic neurons in TPp project to telencephalic targets, functionally similar to VTA [61] |
| Nucleus Accumbens (NAcc) | Integrates dopaminergic signals (reward processing) | Ventral part of the ventral telencephalon (Vv/Vd) | Gene expression (e.g., dopamine receptors, neuropeptides), connectivity, behavioral roles [62] |
| Amygdala | Emotion, social behavior, fear processing |
Medial and dorsal parts of the ventral telencephalon (Dm) | Dm is involved in fear, aggression, and social learning in fish [63] |
| Hippocampus | Learning and memory | Dorsolateral telencephalon (Dl) | Homologous by gene expression (e.g., zic1, emx), lesion studies, and spatial tasks [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





