1. Introduction
Tight junctions (TJs) are specialized intercellular adhesion complexes that form selective barriers in epithelial and endothelial tissues, playing key roles in maintaining tissue integrity, regulating paracellular permeability, and coordinating signaling pathways essential for cell proliferation and immune responses [
1,
2,
3,
4,
5] These structures consist of transmembrane proteins such as claudins, occludin, and JAMs anchored to the actin cytoskeleton via cytoplasmic scaffold proteins [
6,
7]. Among them, ZO-1, encoded by the TJP1 gene, acts as a critical adaptor linking TJ components to the cytoskeleton and various signaling molecules, thereby conferring both structural stability and regulatory plasticity [
8,
9].
ZO-1 not only maintains junctional architecture but also responds to mechanical and biochemical cues in the tumor microenvironment, influencing processes such as epithelial–mesenchymal transition (EMT), proliferation, and immune cell infiltration [
10]. Dysregulation of ZO-1 either through downregulation or cytoplasmic mislocalization has been implicated in diverse malignancies, leading to compromised cell adhesion, increased motility, and enhanced invasiveness [
11,
12]. Such alterations are associated with poor prognosis and metastasis in multiple cancers, including those of the liver, stomach, pancreas, and lung [
13,
14,
15,
16].
In ovarian cancer tissues, ZO-1 expression is frequently downregulated or redistributed from the plasma membrane to the cytoplasm or nucleus. This alteration has been associated with EMT and enhanced tumor invasiveness [
17,
18]. Ovarian cancer is the most lethal gynecologic malignancy, characterized by asymptomatic progression, frequent relapse, and a tendency for peritoneal dissemination [
18,
19]. These metastatic features suggest a role for adhesion molecule dysregulation, including TJ disassembly. While emerging evidence implicates ZO-1 in modulating the tumor microenvironment through inflammatory and stromal interactions [
17], its influence on tumor angiogenesis in ovarian cancer has yet to be elucidated.
Angiogenesis is a hallmark of cancer that enables tumor growth and metastasis by providing oxygen, nutrients, and routes for dissemination [
20,
21]. It is controlled by a balance between pro- and anti-angiogenic signals, and its disruption leads to abnormal tumor vasculature [
22]. Recent studies suggest that junctional proteins like ZO-1 may intersect with angiogenic signaling, either directly through gene regulation or indirectly via microenvironmental modulation [
23,
24,
25]. However, the mechanistic relationship between ZO-1 and angiogenesis remains largely unknown in ovarian cancer.
In this study, we aim to elucidate the role of TJP1 (ZO-1) in regulating tumor angiogenesis in ovarian cancer. Using SKOV3 and OVCAR3 cell lines as models, we generated CRISPR-Cas9-mediated TJP1 knockout cells and assessed angiogenic behavior through both in vitro tube formation assays and in vivo Matrigel plug experiments. To further investigate the molecular pathways affected by ZO-1 loss, we examined the expression of key angiogenic mediators, including IL-8 and KLF5. This study seeks to provide new insights into the functional relevance of ZO-1 in ovarian cancer pathogenesis and highlight its potential as a target for anti-angiogenic therapeutic strategies.
2. Results
2.1. Establishment of ZO-1-Deficient Ovarian Cancer Cell Lines
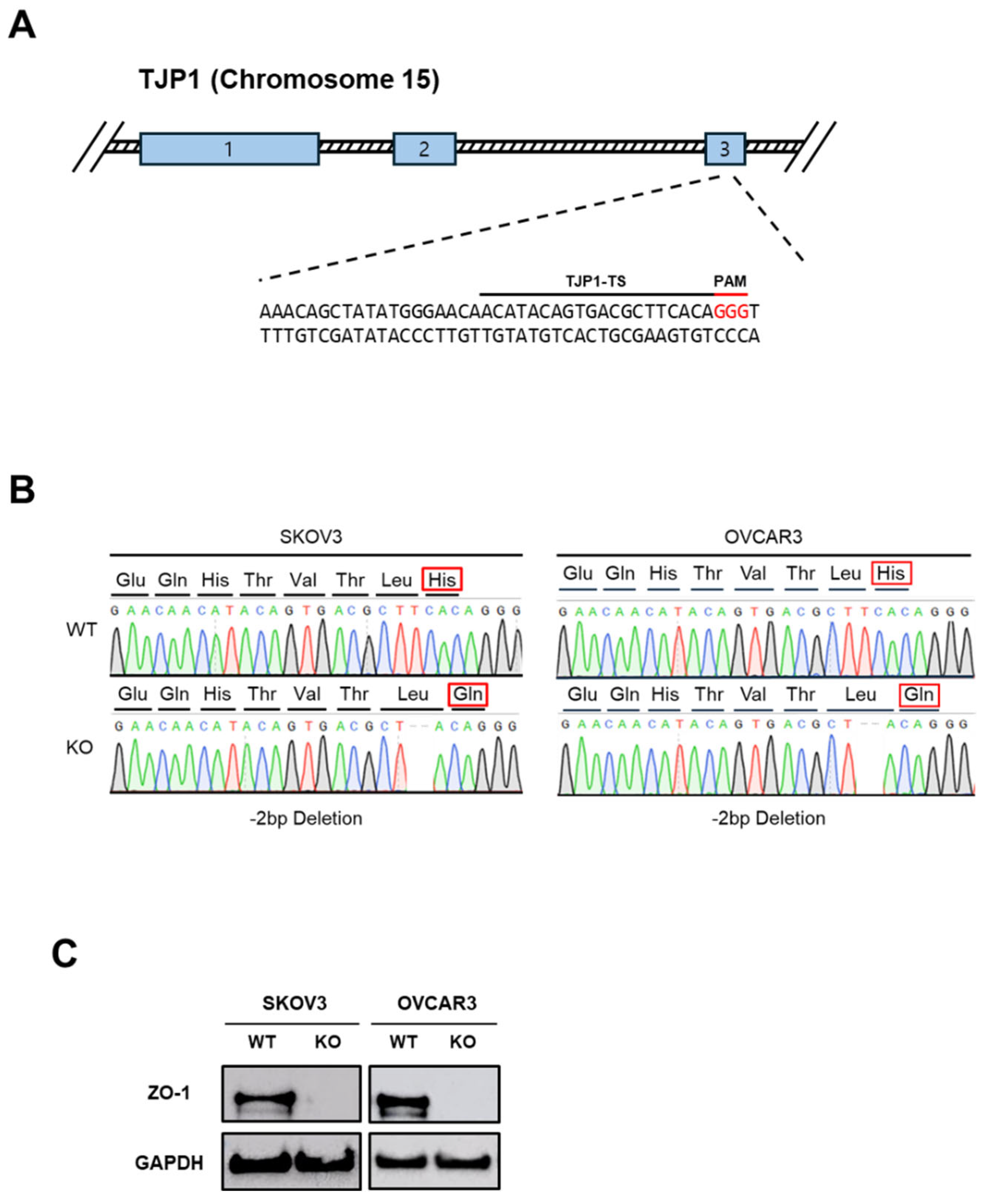
To investigate the functional role of ZO-1 in ovarian cancer, we generated ZO-1 KO cell lines using the CRISPR/Cas9 genome editing system. Two epithelial ovarian cancer cell lines, SKOV3 and OVCAR3, were transfected with plasmids encoding Cas9 and guide RNAs (gRNAs) specifically targeting the ZO-1 gene (TJP1). The gRNA sequences used for targeting are listed in
Figure 1A. To confirm successful genome editing, the targeted genomic regions were amplified and subjected to Sanger sequencing. As shown in
Figure 1B, sequence analysis revealed the presence of deletions at the target loci, resulting in frameshift mutations and subsequent alterations in the encoded amino acid sequence. These findings confirm that the CRISPR/Cas9 system efficiently introduced disruptive mutations into the TJP1 gene. Subsequently, we assessed ZO-1 protein expression in the edited cells by Western blot analysis. As presented in
Figure 1C, ZO-1 protein was undetectable in both SKOV3 and OVCAR3 KO cell lines, indicating a complete loss of ZO-1 protein expression. Together, these results verify the successful establishment of ZO-1-deficient ovarian cancer cell lines, which serve as a robust model to explore the biological role of ZO-1 in tumor progression.
2.2. Enhanced In Vitro Tumor Angiogenesis Following ZO-1 Knockout
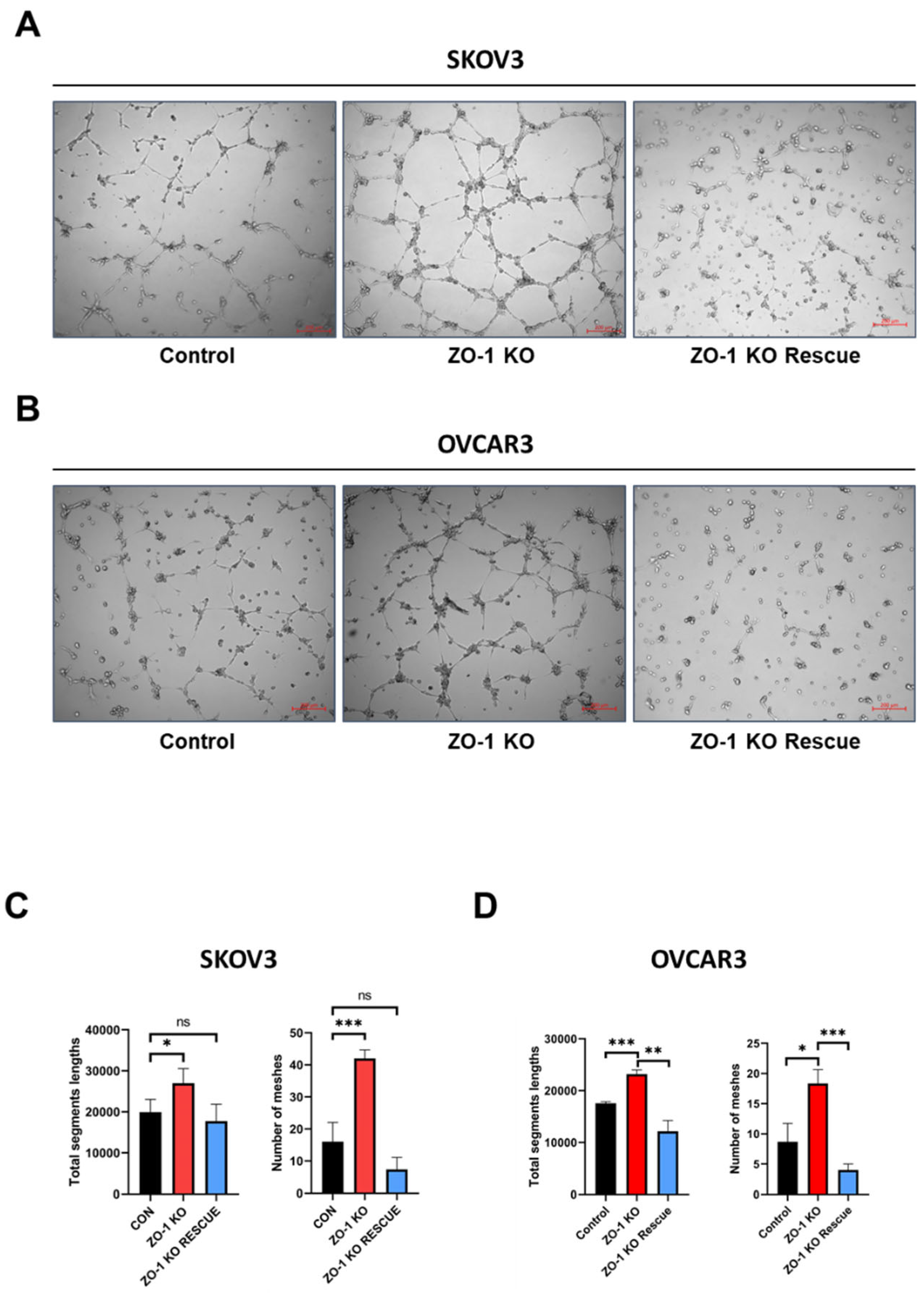
To evaluate the impact of ZO-1 loss on tumor angiogenesis, we performed an in vitro tube formation assay using conditioned media (CM) derived from ZO-1 KO ovarian cancer cells. CM was collected from SKOV3 and OVCAR3 cells following CRISPR/Cas9-mediated ZO-1 depletion. In parallel, CM was also obtained from ZO-1-rescued cell lines to determine whether re-expression of ZO-1 could reverse the pro-angiogenic phenotype(
Supplementary Figure S1). Human umbilical vein endothelial cells (HUVECs) were seeded onto Matrigel-coated 96-well plates and treated with the collected CM for six hours. As shown in
Figure 2A,B, CM from ZO-1 KO SKOV3 and OVCAR3 cells significantly increased endothelial tube formation compared to CM from control cells. Notably, the pro-angiogenic effect was attenuated when ZO-1 expression was restored in the KO cells, indicating that ZO-1 negatively regulates angiogenic potential in ovarian cancer cells. To quantitatively assess angiogenesis, tube network parameters such as mesh length and segment length were measured and are presented in
Figure 2C,D. These analyses further confirmed that ZO-1 depletion leads to enhanced vascular network formation, which is reversed upon ZO-1 re-expression. Collectively, these findings demonstrate that ZO-1 plays a suppressive role in the regulation of angiogenesis in ovarian cancer, and its loss promotes a tumor microenvironment conducive to vascularization.
2.3. ZO-1 Loss Induces Angiogenesis in the Matrigel Plug Assay
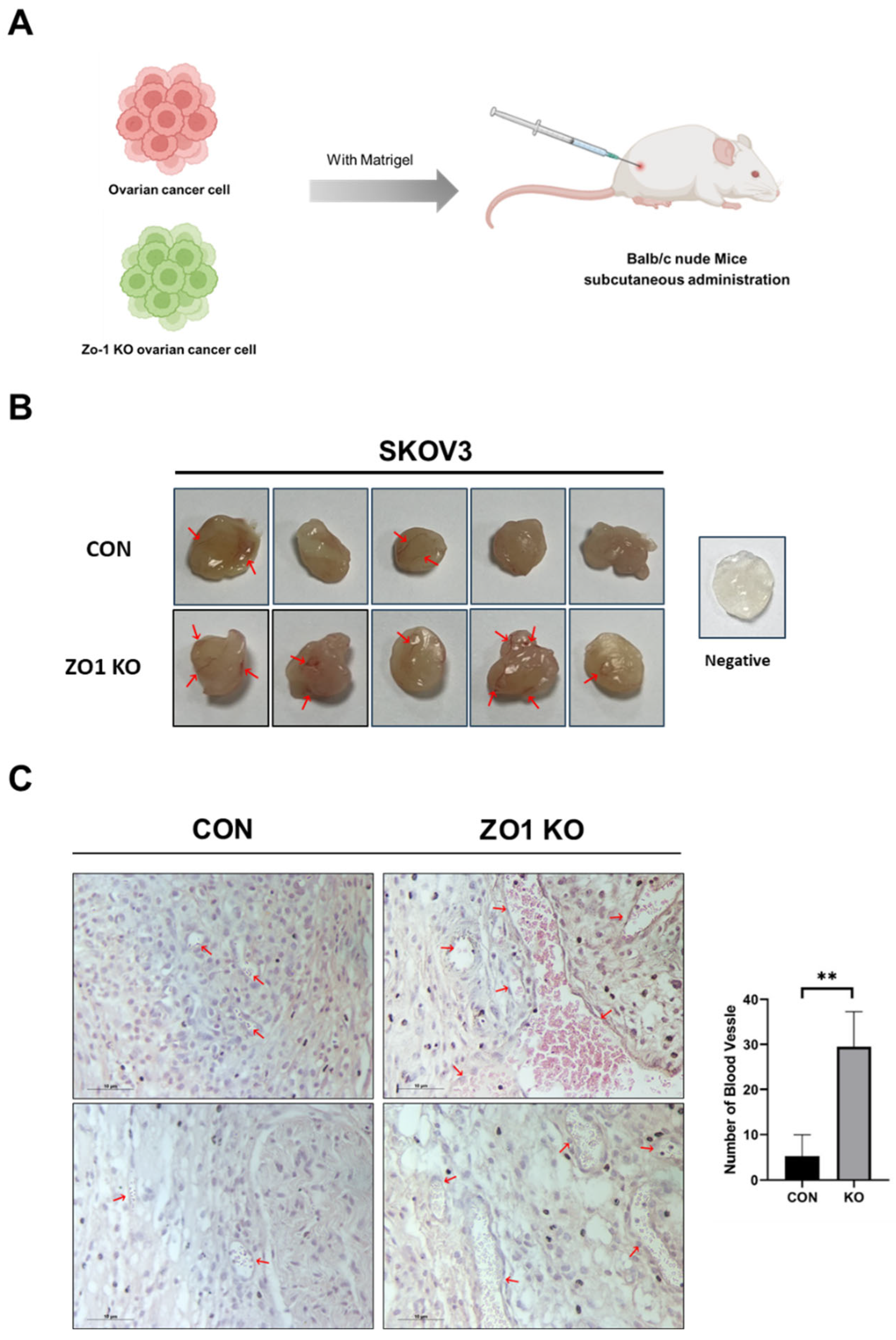
To evaluate the role of ZO-1 in tumor-induced angiogenesis, a Matrigel plug assay was performed in BALB/c nude mice. SKOV3 ovarian cancer cells were suspended in Matrigel and subcutaneously injected into the flanks of the mice (
Supplementary Figure S2). Two groups were established: a control (CON) group using wild-type SKOV3 cells and a ZO-1 KO group using ZO-1-deficient SKOV3 cells (
Figure 3A). The mice were maintained for two weeks to allow tumor cells to interact with the surrounding microenvironment and induce vascularization within the plugs. After two weeks, the Matrigel plugs were harvested and assessed for gross morphology. Compared to the CON group, plugs from the ZO-1 KO group appeared larger and exhibited more prominent surface vascularization, as indicated by visible blood vessels (
Figure 3B, red arrows). To assess vascularization at the histological level, the plugs were fixed, embedded in paraffin, sectioned, and subjected to hematoxylin and eosin (H&E) staining. Histological analysis revealed a significantly higher number of blood vessels in the ZO-1 KO group compared to the CON group (
Figure 3C, red arrows). Quantification of vascular density demonstrated a marked increase in blood vessel formation in the ZO-1 KO plugs relative to controls (
Figure 3C, bar graph; p < 0.01). These results suggest that loss of ZO-1 promotes angiogenesis, potentially by modulating the tumor microenvironment to favor neovascularization.
2.4. Regulation of ZO-1–Dependent Angiogenesis by IL-8 and KLF5
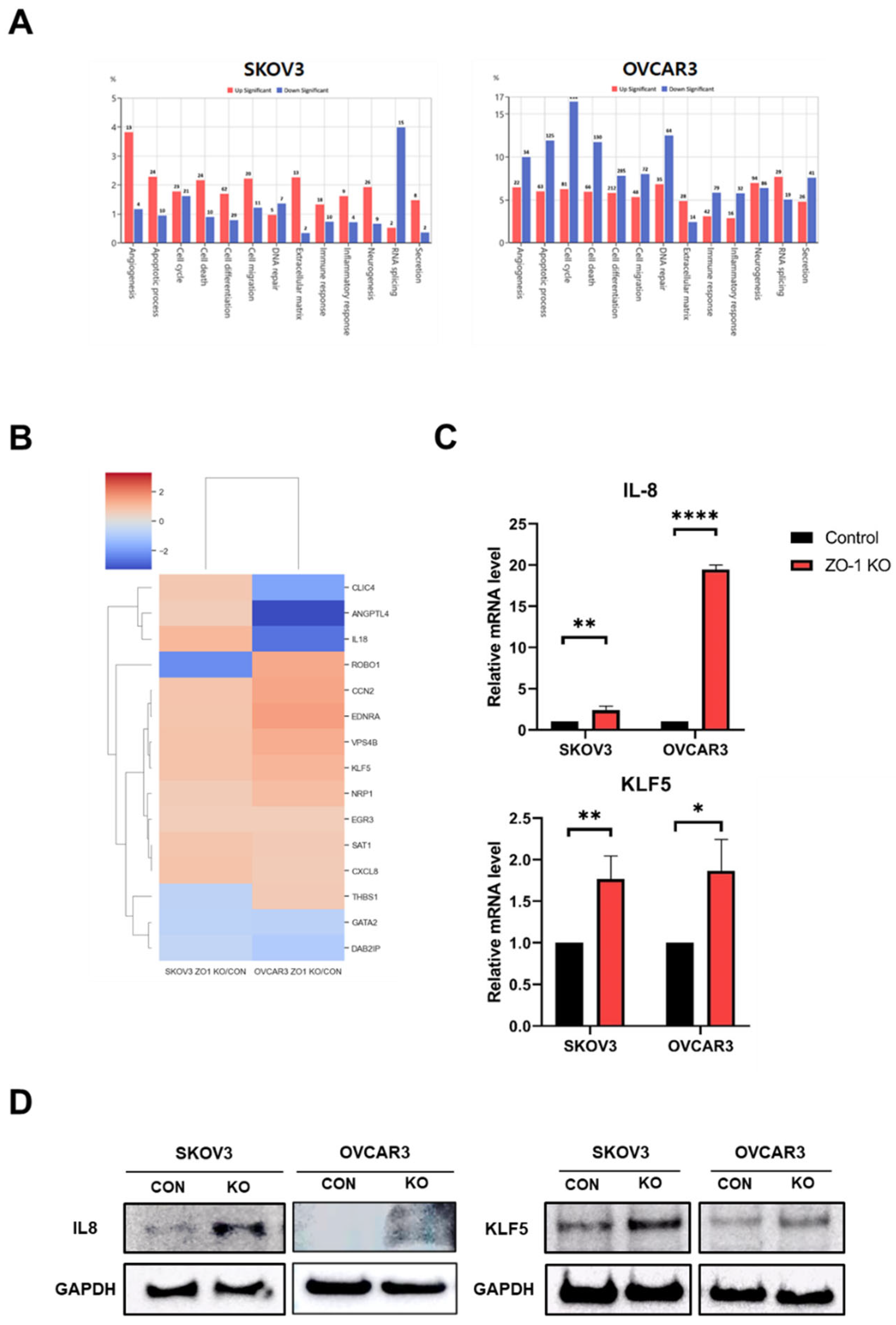
Based on the results described above, ZO-1 appears to play a regulatory role in angiogenesis in ovarian cancer cells. To further elucidate the underlying molecular mechanisms, transcriptome analysis was performed using RNA sequencing. Gene expression changes associated with angiogenesis were analyzed in both SKOV3 and OVCAR3 ovarian cancer cell lines.
Figure 4A shows that 17 angiogenesis-related genes were differentially expressed in SKOV3 cells following ZO-1 KO, with 13 genes upregulated and 4 downregulated. In OVCAR3 cells, 56 genes exhibited significant differential expression, including 22 upregulated and 34 downregulated genes. Additional candidate genes potentially involved in angiogenesis were identified through heatmap analysis of differentially expressed angiogenesis-related genes (
Figure 4B). Comparison of these transcriptomic results with tube formation assay data revealed commonly upregulated genes in both cell lines, notably Krüppel-like factor 5 (KLF5) and C-X-C motif chemokine ligand 8 (CXCL8/IL-8). IL-8 has previously been implicated in promoting angiogenesis in pancreatic cancer, while KLF5 has been reported to enhance angiogenic activity in bladder cancer [
26,
27]. To validate these findings, quantitative real-time PCR (qRT-PCR) was conducted and confirmed that IL-8 and KLF5 mRNA levels were significantly elevated in ZO-1 KO cells compared to controls (
Figure 4C). Consistently, Western blot analysis further demonstrated increased protein expression levels of IL-8 and KLF5 in both SKOV3 and OVCAR3 cells following ZO-1 deletion (
Figure 4D). Collectively, these results suggest that IL-8 and KLF5 are key mediators of ZO-1–regulated angiogenesis in ovarian cancer cells.
3. Discussion
In this study, we demonstrated that CRISPR-Cas9-mediated knockout of ZO-1 in human ovarian cancer cell lines (SKOV3 and OVCAR3) enhances angiogenic potential. Functional assays revealed that conditioned media from ZO-1-deficient cells promoted tube formation in HUVECs, indicating that ZO-1 may regulate the secretion of pro-angiogenic factors. This phenotype was reversed by re-expression of ZO-1, suggesting a ZO-1–dependent mechanism that modulates endothelial behavior. Given that tumor cells do not form blood vessels themselves [
28,
29], these results support a role for ZO-1 in regulating the tumor microenvironment by influencing the behavior of neighboring endothelial cells rather than acting solely within tumor cells.
Consistent with in vitro findings, Matrigel plug assays in vivo showed significantly increased vascularization in plugs containing ZO-1 knockout cells, reinforcing the anti-angiogenic role of ZO-1.
Transcriptomic and qRT-PCR analyses identified IL-8 and KLF5 as key pro-angiogenic genes upregulated in ZO-1–deficient cells. IL-8 is a chemokine known to promote endothelial proliferation, migration, and vascular remodeling [
30,
31,
32], while KLF5 is a zinc finger transcription factor involved in angiogenesis and tumor progression through regulation of multiple downstream targets [
26,
33,
34]. These findings suggest that loss of ZO-1 enhances angiogenesis by upregulating IL-8 and KLF5.
Interestingly, previous studies have proposed that IL-8 overexpression can suppress ZO-1 expression, indicating a reciprocal regulatory loop between ZO-1 and angiogenic signaling pathways [
35,
36]. Moreover, ZO-1 has been proposed to function as a tumor suppressor through regulation of the tumor microenvironment and angiogenic signaling [
37,
38,
39].
While our data strongly support a role for ZO-1 as a negative regulator of tumor angiogenesis, further studies are warranted to determine whether inhibition of IL-8 or KLF5 can reverse the angiogenic phenotype in ZO-1–deficient cells, and whether ZO-1 loss contributes to tumor progression and metastasis in vivo.
In conclusion, our findings reveal that ZO-1 suppresses angiogenesis in ovarian cancer by negatively regulating pro-angiogenic mediators such as IL-8 and KLF5. This highlights a novel role for ZO-1 in modulating the tumor microenvironment and suggests that restoring ZO-1 function or targeting its downstream effectors may represent promising strategies for limiting angiogenesis and disease progression in ovarian cancer.
4. Materials and Methods
3.1. Cell Culture
Human ovarian cancer cell lines SKOV3 and OVCAR3 were obtained from the Korean Cell Line Bank (KCLB, Seoul, Republic of Korea), and HUVECs were purchased from ATCC (Manassas, VA, USA). SKOV3 and OVCAR3 cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, and L-glutamine. HUVECs were cultured in M199 medium containing 20% FBS, 1% Antibiotic-Antimycotic (Gibco), and 1% endothelial cell growth supplement (ECGS). All cells were maintained at 37 °C in a humidified incubator with 5% CO₂.
3.2. Western Blot Analysis
Proteins were extracted using PRO-PREP™ (iNtRON Biotechnology, Korea), separated by SDS-PAGE on a Bolt™ 4–12% Bis-Tris gel (Invitrogen), and transferred onto nitrocellulose membranes. Membranes were blocked in TBS-T containing 5% skim milk for 2 h at room temperature and incubated overnight at 4 °C with primary antibodies: ZO-1 (1:1000, Invitrogen), IL-8 (1:1000, Abcam), KLF5 (1:1000, Abclonal), and GAPDH (1:5000, Invitrogen). After washing, membranes were incubated with HRP-conjugated secondary antibodies, and signals were detected using an ECL detection kit.
3.3. Generation of ZO-1 Knockdown Cells
CRISPR-Cas9-mediated knockout of ZO-1 was performed using gRNA (5′-ACATACAGTGACGCTTCACA-3′) designed via the online tool of Bioneer(Daejeon, Republic of Korea). The gRNA was cloned into pRGEN-Cas9-CMV/T7-Hygro-EGFP and CRISPR/Cas9-Puro plasmids (ToolGen, Seoul, Republic of Korea). SKOV3 and OVCAR3 cells were transfected and selected with 100 µg/mL hygromycin for 48 h. After two weeks, individual clones were isolated using cloning cylinders and validated by RT-PCR, qRT-PCR, and Western blotting.
3.4. Tube Formation Assay
Matrigel (Corning, NY, USA) was added to 96-well plates and polymerized at 37 °C for 1 h. HUVECs were suspended in 100 µL of conditioned media (CM) and seeded onto the Matrigel. After 6–8 h incubation, tube structures were imaged under a fluorescence microscope, and total tube numbers, meshes, and segments were quantified.
3.5. Matrigel Plug Assay
Matrigel was thawed overnight at 4 °C before use. SKOV3 and ZO-1 KO SKOV3 cells (1 × 10⁶) were mixed with 250 µL of Matrigel and 50 µL of medium (total 300 µL) and then injected subcutaneously into the flanks of 6–8-week-old BALB/c nude mice (n = 5 per group). After 14 days, mice were sacrificed, and plugs were fixed in 4% paraformaldehyde, paraffin-embedded, and sectioned for histological analysis.
3.6. Collection of Conditioned Media
SKOV3, OVCAR3, and their respective ZO-1 KO cells were cultured in 6-well plates. Upon confluency, cells were incubated in serum-free medium for 4–6 h, followed by replacement with medium containing 10% FBS. Conditioned media were collected 24 h later, centrifuged to remove debris, and used for downstream assays.
3.7. Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted using TRIzol Reagent (Invitrogen), and cDNA was synthesized using a reverse transcription kit (Bioneer, Daejeon, Republic of Korea). qRT-PCR was conducted using TB Green Premix Taq (Takara, Japan) on a QuantStudio 3 system (Thermo Fisher Scientific). Relative expression was calculated by the 2^−ΔΔCt method and normalized to GAPDH. The primer sequences for CXCL8 genes are as follows: CXCL8 sense, 5′-CAG TTT TGC CAA GGA GTG CT-3′; antisense, 5′- ACT TCT CCA CAA CCC TCT GC -3′; KLF5 sense, 5′- ATT TAA AAG CTC ACC TGA GGA C -3′; antisense, 5′-CTG GTG CCT CTT CAT ATG C-3. GAPDH was used as a control (sense primer, 5′- CAA TGA CCC CTT CAT TGA CC -3′; antisense primer, 5′- GAC AAG CTT CCC GTT CTC AG -3′).
3.8. Hematoxylin and Eosin (H&E) Staining
Paraffin-embedded sections were deparaffinized in xylene, rehydrated through graded ethanol, and stained with hematoxylin (3–5 min) and eosin (1 min). Slides were dehydrated, cleared in xylene, and mounted using synthetic mounting medium before microscopic evaluation.
3.9. mRNA Sequencing and Analysis
Transcriptomic profiling was performed by Ebiogen Inc. (Seoul, Republic of Korea). Total RNA from SKOV3, ZO-1 KO SKOV3, OVCAR3, and ZO-1 KO OVCAR3 cells was subjected to next-generation sequencing. Differentially expressed genes (DEGs) were identified, and cluster analysis and visualization were conducted using ExDEGA software (v5.1.1.4, Ebiogen).
3.10. Statistical Analysis
All in vitro experiments were performed in triplicate. Statistical significance was evaluated using Student’s t-test in GraphPad Prism 8.0.2 (GraphPad Software, USA). Data are presented as mean ± SD, and p-values were considered significant as follows: p < 0.05 , p < 0.01, and p < 0.001.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, S.C. and H.-J.C.; data curation, S.C., K.-H.K., D.-Y.K., M.-H.K. and H.A.; formal analysis, S.C. ; funding acquisition, H.K. and H.-J.C.; investigation, S.C., K.-H.K., D.-Y.K., M.-H.K. and H.A.; project administration, S.C., K.-H.K., H.K. and H.-J.C.; supervision, W.-K.E, J.-Y.L, H.K., H.K. and H.-J.C.; writing—original draft, S.C. and H.-J.C.; writing—review and editing, W.-K.E, J.-Y.L, H.K., H.K. and H.-J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korean government (NRF-2021R1A4A1031380).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional r Ethics Committee of Kosin University College of Medicine Institutional Animal Care and Use Committee (KUCMIACUC; KMAP-24-09).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are contained in this article and there are no repository data.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| CM |
conditioned media |
| DEGs |
differentially expressed genes |
| EMT |
epithelial-mesenchymal transition |
| JAMs |
Junctional adhesion molecules |
| KO |
Knockout |
| Tjp |
Tight junction protein |
| ZO |
zonula occludens |
References
- Ko, E.J.; Kim, D.Y.; Kim, M.H.; An, H.; Kim, J.; Jeong, J.Y.; Song, K.S.; Cha, H.J. Functional Analysis of Membrane-Associated Scaffolding Tight Junction (TJ) Proteins in Tumorigenic Characteristics of B16-F10 Mouse Melanoma Cells. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Otani, T.; Furuse, M. Tight Junction Structure and Function Revisited. Trends Cell Biol 2020, 30, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Nehme, Z.; Roehlen, N.; Dhawan, P.; Baumert, T.F. Tight Junction Protein Signaling and Cancer Biology. Cells 2023, 12. [Google Scholar] [CrossRef]
- Shin, K.; Fogg, V.C.; Margolis, B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol 2006, 22, 207–235. [Google Scholar] [CrossRef] [PubMed]
- Citi, S.; Fromm, M.; Furuse, M.; González-Mariscal, L.; Nusrat, A.; Tsukita, S.; Turner, J.R. , A short guide to the tight junction. J Cell Sci 2024, 137. [Google Scholar] [CrossRef]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Guillemot, L.; Paschoud, S.; Pulimeno, P.; Foglia, A.; Citi, S. The cytoplasmic plaque of tight junctions: a scaffolding and signalling center. Biochim Biophys Acta 2008, 1778, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Ram, A.K.; Vairappan, B. Role of zonula occludens in gastrointestinal and liver cancers. World J Clin Cases 2022, 10, 3647–3661. [Google Scholar] [CrossRef]
- Paris, L.; Tonutti, L.; Vannini, C.; Bazzoni, G. Structural organization of the tight junctions. Biochim Biophys Acta 2008, 1778, 646–659. [Google Scholar] [CrossRef]
- Neyrinck-Leglantier, D.; Lesage, J.; Blacher, S.; Bonnomet, A.; Hunziker, W.; Noël, A.; Dormoy, V.; Nawrocki-Raby, B.; Gilles, C.; Polette, M. ZO-1 Intracellular Localization Organizes Immune Response in Non-Small Cell Lung Cancer. Front Cell Dev Biol 2021, 9, 749364. [Google Scholar] [CrossRef]
- Kleeff, J.; Shi, X.; Bode, H.P.; Hoover, K.; Shrikhande, S.; Bryant, P.J.; Korc, M.; Büchler, M.W.; Friess, H. Altered expression and localization of the tight junction protein ZO-1 in primary and metastatic pancreatic cancer. Pancreas 2001, 23, 259–265. [Google Scholar] [CrossRef]
- Yu, S.; He, J.; Xie, K. Zonula Occludens Proteins Signaling in Inflammation and Tumorigenesis. Int J Biol Sci 2023, 19, 3804–3815. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Zhang, H.; Tu, F.; Qiang, Y.; Nie, C. Decreased expression of ZO-1 is associated with tumor metastases in liver cancer. Oncol Lett 2019, 17, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, S.; Terashima, M.; Satoh, J.; Soeta, N.; Saze, Z.; Kashimura, S.; Ohsuka, F.; Hoshino, Y.; Kogure, M.; Gotoh, M. Expression of tight-junction-associated proteins in human gastric cancer: downregulation of claudin-4 correlates with tumor aggressiveness and survival. Gastric Cancer 2009, 12, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, J.; Zhang, Y.; Zhou, Z.; Cui, X.; Zhang, L.; Fung, K.M.; Zheng, W.; Allard, F.D.; Yee, E.U.; Ding, K.; Wu, H.; Liang, Z.; Zheng, L.; Fernandez-Zapico, M.E.; Li, Y.P.; Bronze, M.S.; Morris, K.T.; Postier, R.G.; Houchen, C.W.; Yang, J.; Li, M. ZIP4 Promotes Pancreatic Cancer Progression by Repressing ZO-1 and Claudin-1 through a ZEB1-Dependent Transcriptional Mechanism. Clin Cancer Res 2018, 24, 3186–3196. [Google Scholar] [CrossRef]
- Orbán, E.; Szabó, E.; Lotz, G.; Kupcsulik, P.; Páska, C.; Schaff, Z.; Kiss, A. Different expression of occludin and ZO-1 in primary and metastatic liver tumors. Pathol Oncol Res 2008, 14, 299–306. [Google Scholar] [CrossRef]
- El Bakkouri, Y.; Chidiac, R.; Delisle, C.; Corriveau, J.; Cagnone, G.; Gaonac'h-Lovejoy, V.; Chin, A.; Lécuyer, É.; Angers, S.; Joyal, J.S.; Topisirovic, I.; Hulea, L.; Dubrac, A.; Gratton, J.P. ZO-1 interacts with YB-1 in endothelial cells to regulate stress granule formation during angiogenesis. Nat Commun 2024, 15, 4405. [Google Scholar] [CrossRef]
- Choe, S.; Jeon, M.; Yoon, H. Advanced Therapeutic Approaches for Metastatic Ovarian Cancer. Cancers (Basel) 2025, 17. [Google Scholar] [CrossRef] [PubMed]
- Sambasivan, S. Epithelial ovarian cancer: Review article. Cancer Treat Res Commun 2022, 33, 100629. [Google Scholar] [CrossRef]
- Batlle, R.; Andrés, E.; Gonzalez, L.; Llonch, E.; Igea, A.; Gutierrez-Prat, N.; Berenguer-Llergo, A.; Nebreda, A.R. Regulation of tumor angiogenesis and mesenchymal-endothelial transition by p38α through TGF-β and JNK signaling. Nat Commun 2019, 10, 3071. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; Liao, Q.; Xiang, B.; Zhou, M.; Guo, C.; Zeng, Z.; Li, G.; Li, X.; Xiong, W. The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res 2020, 39, 204. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Yang, F.; Liu, X.Q.; He, J.Z.; Xian, S.P.; Yang, P.F.; Mai, Z.Y.; Li, M.; Liu, Y.; Zhang, X.D. Occludin facilitates tumour angiogenesis in bladder cancer by regulating IL8/STAT3 through STAT4. J Cell Mol Med 2022, 26, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Tornavaca, O.; Chia, M.; Dufton, N.; Almagro, L.O.; Conway, D.E.; Randi, A.M.; Schwartz, M.A.; Matter, K.; Balda, M.S. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol 2015, 208, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wu, H.; Ren, H.; Cao, J.; Shao, Y.; Liu, G.; Lu, P. Inhibition of Experimental Corneal Neovascularization by the Tight Junction Protein ZO-1. J Ocul Pharmacol Ther 2024, 40, 379–388. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, K.; Chen, Y.; Zhou, J.; Du, C.; Shi, Q.; Xu, S.; Jia, J.; Tang, X.; Li, F.; Hui, K.; He, D.; Guo, P. Beyond proliferation: KLF5 promotes angiogenesis of bladder cancer through directly regulating VEGFA transcription. Oncotarget 2015, 6, 43791–43805. [Google Scholar] [CrossRef]
- Matsuo, Y.; Ochi, N.; Sawai, H.; Yasuda, A.; Takahashi, H.; Funahashi, H.; Takeyama, H.; Tong, Z.; Guha, S. CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote invasiveness and angiogenesis in pancreatic cancer. Int J Cancer 2009, 124, 853–861. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther 2023, 8, 198. [Google Scholar] [CrossRef]
- Lopes-Bastos, B.M.; Jiang, W.G.; Cai, J. Tumour-Endothelial Cell Communications: Important and Indispensable Mediators of Tumour Angiogenesis. Anticancer Res 2016, 36, 1119–1126. [Google Scholar]
- Martin, D.; Galisteo, R.; Gutkind, J.S. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem 2009, 284, 6038–6042. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin Cancer Res 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Shahzad, M.M.; Arevalo, J.M.; Armaiz-Pena, G.N.; Lu, C.; Stone, R.L.; Moreno-Smith, M.; Nishimura, M.; Lee, J.W.; Jennings, N.B.; Bottsford-Miller, J.; Vivas-Mejia, P.; Lutgendorf, S.K.; Lopez-Berestein, G.; Bar-Eli, M.; Cole, S.W.; Sood, A.K. Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J Biol Chem 2010, 285, 35462–35470. [Google Scholar] [CrossRef]
- Li, Y.; Kong, R.; Chen, H.; Zhao, Z.; Li, L.; Li, J.; Hu, J.; Zhang, G.; Pan, S.; Wang, Y.; Wang, G.; Chen, H.; Sun, B. Overexpression of KLF5 is associated with poor survival and G1/S progression in pancreatic cancer. Aging (Albany NY) 2019, 11, 5035–5057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, C.; Ju, Z.; Jiao, D.; Hu, D.; Qi, L.; Liu, S.; Wu, X.; Zhao, C. , Krüppel-like factors in tumors: Key regulators and therapeutic avenues. Front Oncol 2023, 13, 1080720. [Google Scholar] [CrossRef]
- Si, W.; Liu, J.; Wang, Y.; Mao, Y.; Zhang, Y.; Xu, S.; Guo, K.; Zhang, Y.; Hu, Y.; Zhang, F. IL-8 promotes lens capsular residual cells migration by down-regulates expression of E-cadherin and ZO-1 via the CXCR1/2-NF-κB-RhoA signal pathway. Int Immunopharmacol 2024, 142 (Pt A), 113074. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, X.; Ma, Y.; Gao, M.; Wang, O.; Gao, T.; Shen, Y.; Liu, X. Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions. Int J Biol Sci 2013, 9, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Lesage, J.; Suarez-Carmona, M.; Neyrinck-Leglantier, D.; Grelet, S.; Blacher, S.; Hunziker, W.; Birembaut, P.; Noël, A.; Nawrocki-Raby, B.; Gilles, C.; Polette, M. Zonula occludens-1/NF-κB/CXCL8: a new regulatory axis for tumor angiogenesis. Faseb j 2017, 31, 1678–1688. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, S.I.; Choi, Y.R.; Kim, J.; Eun, J.W.; Song, K.S.; Jeong, J.Y. GNAQ-Regulated ZO-1 and ZO-2 Act as Tumor Suppressors by Modulating EMT Potential and Tumor-Repressive Microenvironment in Lung Cancer. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Salvador, E.; Burek, M.; Förster, C.Y. Tight Junctions and the Tumor Microenvironment. Curr Pathobiol Rep 2016, 4, 135–145. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).