1. Introduction
Preeclampsia is a multisystem pregnancy disorder characterized by new-onset hypertension after 20 weeks of gestation, accompanied by complications such as proteinuria or maternal/uteroplacental end-organ dysfunction [
1]. It remains one of the leading causes of maternal and fetal morbidity and mortality worldwide, with an estimated global incidence of 2–8% of all pregnancies[
2,
3]. The pathophysiology of preeclampsia is highly complex, with several proposed mechanisms, including placental dysfunction[
4], oxidative stress[
5], ferroptosis[
6], and maternal immune maladaptation[
7]. Preeclampsia is a heterogeneous syndrome with distinct pathophysiological mechanisms. Early-onset preeclampsia (EOP), resulting in iatrogenic preterm birth before 34 weeks, is commonly linked to placental dysfunction and fetal growth restriction due to inadequate cytotrophoblast invasion of spiral arteries[
7]. In contrast, late-onset preeclampsia (LOP), particularly at term (>37 weeks), is more often associated with maternal factors such as obesity, inflammation, and advanced maternal age, reflecting a pathogenesis driven by premature placental senescence rather than impaired trophoblast invasion.
A failure of maternal immune tolerance toward trophoblast invasion during early pregnancy is believed to play a central role in the etiology of EOP. A normal maternal immune response to trophoblasts involves a delicate balance among decidual immune cells, including dNK, macrophages, T cells, and dendritic cells[
8]. Proper immune tolerance is essential for successful embryo implantation and placental development. In contrast, immune maladaptation can lead to defective placentation, followed by oxidative stress and placental inflammation, ultimately resulting in the clinical manifestations of EOP[
9].
dNK cells are the dominant immune cell population in the uterine decidua during early pregnancy, playing a crucial role in implantation and the formation of a healthy placenta. dNK cells exhibit low cytotoxicity and instead promote pregnancy by producing ‘type 2’ cytokines and angiogenic factors that facilitate trophoblast invasion and remodeling of the uterine spiral arteries[
10]. The immune interaction between dNK cells and trophoblasts is mediated in part by the expression of non-classical class I Human Leukocyte Antigens (HLA), such as HLA-C, on trophoblasts, which are recognized by Killer Immunoglobulin Receptors (KIRs) on dNK cells[
11]. Disruption in the interaction between dNK cells and trophoblasts, particularly due to the maternal KIR AA genotype lacking activating receptors, may impair dNK-mediated modulation of trophoblast invasion. This imbalance contributes to defective placentation[
12]. In addition to the KIR/HLA interaction, trophoblasts also express immunomodulatory molecules from the B7 family that regulate their interaction with maternal immune cells[
13]. One such molecule is B7-H3 (also known as CD276), an adaptive immune co-stimulatory protein. B7-H3 is consistently expressed in the placenta from the first trimester through term, particularly on EVT cells [
14]. In pregnancy, B7-H3 is involved in regulating adaptive immune responses through the activation or inhibition of lymphocytes, including NK and T cells [
15]. Specifically, B7-H3 has been shown to influence dNK cell function by modulating the secretion of pro-inflammatory cytokines by dNK cells. Emerging evidence suggests that reduced expression of B7-H3 is associated with increased production of pro-inflammatory cytokines by dNK cells, as well as impaired trophoblast migration and invasion[
16]. In cases of recurrent miscarriage, low B7-H3 expression on trophoblasts has been reported to trigger excessive inflammatory cytokine secretion by dNK cells and inhibit trophoblast invasion[
16], potentially resulting in inadequate placentation and in theory, a clinical condition resembling EOP if pregnancy continues.
Despite growing insights into the role of B7-H3 in regulating immune tolerance during pregnancy, studies specifically examining its expression and function in the context of preeclampsia remain scarce. To date, no previous research has directly evaluated the relationship between B7-H3 expression on trophoblasts and the presence or activity of dNK in preeclampsia. Therefore, this study was conducted to investigate the differences in B7-H3 expression on EVT and the number of decidual NK cells between preeclamptic and normal pregnancies and to analyze the relationship between the two. The findings of this study are expected to deepen our understanding of the immunological mechanisms underlying preeclampsia and may provide a foundation for the development of novel therapeutic strategies, such as B7-H3 modulation, for the prevention of preeclampsia in the future.
2. Materials and Methods
Design and Subjects
This study was analytical observational research with a cross-sectional design. A total of 42 placental samples were collected post-delivery and divided into two groups: 21 samples from pregnancies with preeclampsia (based on the criteria of the International Society for the Study of Hypertension in Pregnancy/ISSHP)[
17] and 21 samples from normotensive pregnancies as controls.
Inclusion criteria comprised intrauterine singleton pregnancy, maternal age ≥18 years, informed consent to participate, and fulfillment of the diagnostic criteria for either preeclampsia or normal pregnancy. Exclusion criteria included chronic hypertension, gestational hypertension without preeclampsia, diabetes mellitus, autoimmune diseases, placental abnormalities (e.g., hydatidiform mole), or other severe maternal complications.
Sample Collection Procedure
The decidua basalis tissue of the placenta was immediately fixed in buffered formalin and processed into paraffin blocks. Tissue sections with a thickness of 4 µm were prepared for immunohistochemical staining. Two markers were examined on serial sections: the dNK cell marker using monoclonal anti-CD56 antibody, and the B7-H3 marker on trophoblasts using monoclonal anti-CD276 antibody. The streptavidin-biotin immunohistochemistry method was applied according to standard protocols.
Briefly, after deparaffinization and rehydration, antigen retrieval was performed using heat-induced epitope retrieval, followed by blocking of endogenous peroxidase activity. The sections were then incubated overnight at 4°C. IHC staining was performed using the CD56 antibody (BC56C04, Biocare Medical) and the monoclonal anti-CD276 antibody (Invitrogen MA515693, Fisher Scientific).
The next day, the slides were washed with PBS and incubated with a biotin-labeled secondary antibody, followed by the addition of streptavidin-HRP. The antigen-antibody complexes were visualized using diaminobenzidine, which produced a brown coloration in positively stained cells. Negative controls were performed using identical tissue sections processed without adding primary antibodies.
Expression Assessment
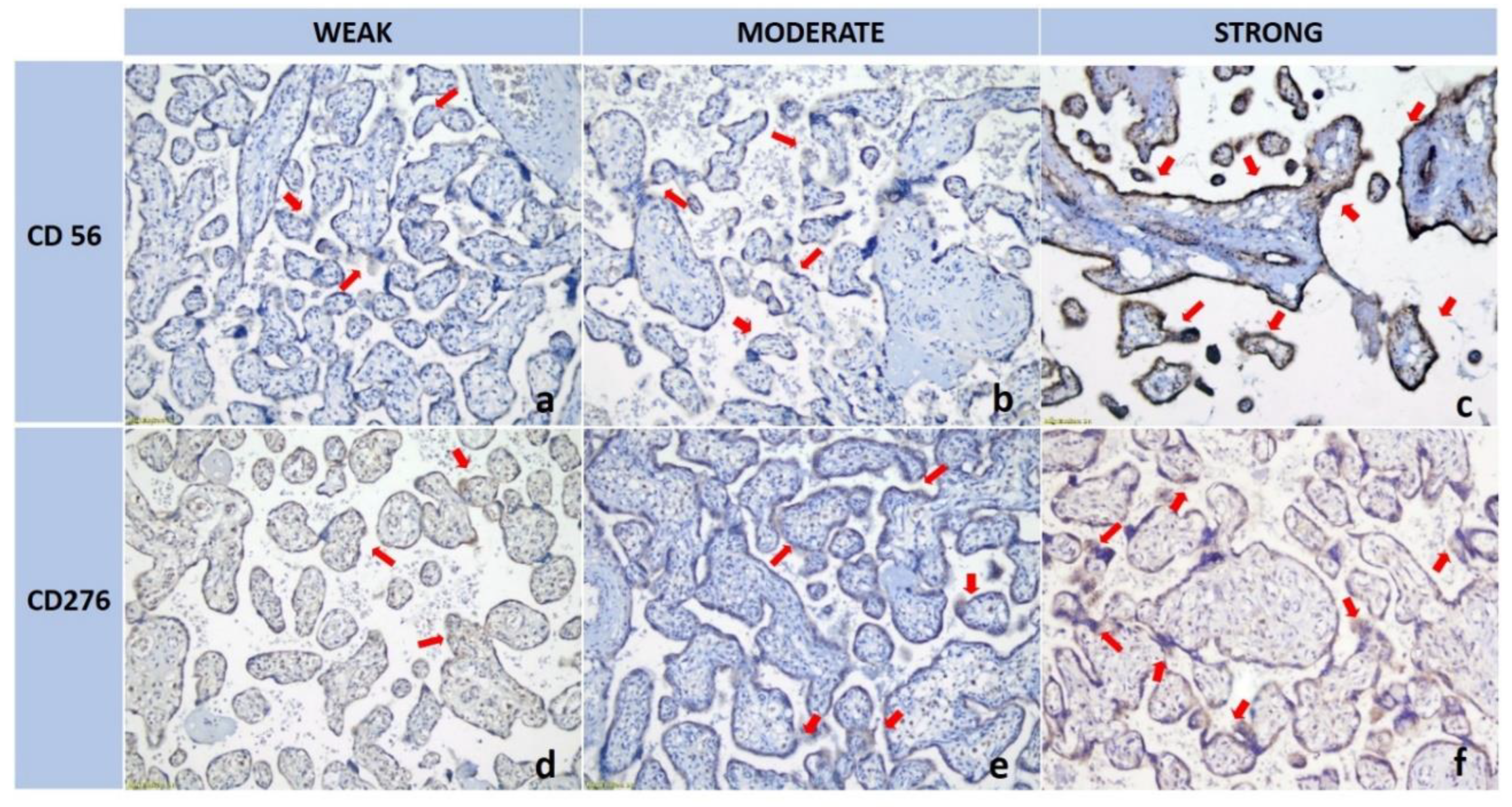
The stained slides were examined under the microscope (Nikon® Eclipse CI light). For each specimen, five random fields within the decidua basalis area containing EVT were selected, confirmed at 40x magnification, and subsequently analyzed at 200x magnification. The expression of dNK cells and B7-H3 was evaluated by identifying brown chromogenic staining in the target cells.
CD56-positive dNK cells were identified as large granular cells exhibiting brown membranous or cytoplasmic staining within the decidual stroma surrounding the trophoblasts. B7-H3 expression in EVT appeared as brown staining localized to the membranes and cytoplasm (
Figure 1). Quantification was performed semi-quantitatively using the Remmele Immunoreactive Score (IRS). This composite score combines the percentage of immunoreactive cells and the staining intensity (Remmele & Stegner, with methodological modifications) [
18]. The mean IRS for each marker was calculated from five fields per sample, resulting in average expression values for dNK and B7-H3.
CD56 expression is primarily localized to specific cell populations in the decidual stroma, as indicated by red arrows, with variations in distribution patterns observed across the panels (
Figure 1a-c). In the B7-H3 series, a progressive increase in staining intensity is evident from panel d to f, particularly along the membranes and within the cytoplasm of trophoblastic cells (also marked by red arrows), suggesting an upregulation of B7-H3 expression. This comparative analysis underscores the distinct biological roles of CD56 and B7-H3 in modulating immune responses and contributing to trophoblast differentiation within the placental microenvironment.
Data Analysis
Numerical data were expressed as mean ± standard deviation. An unpaired t-test was used to compare the mean expression levels of CD56 (dNK cells) and B7-H3 (CD276) between the preeclampsia and control groups, as both variables were normally distributed within each group according to the Shapiro-Wilk test. In addition, Pearson or Spearman correlation analysis was conducted to assess the relationship between B7-H3 expression and the number of dNK cells in both the preeclampsia and control groups, depending on data distribution. A p-value of < 0.05 was considered statistically significant.
3. Results
A total of 42 placental samples were analyzed (21 from the preeclampsia group and 21 from the control group). There were no significant differences in maternal age, parity, or BMI between the groups (p > 0.05). A significant difference was observed in neonatal birth weight (p < 0.05) (
Table 1).
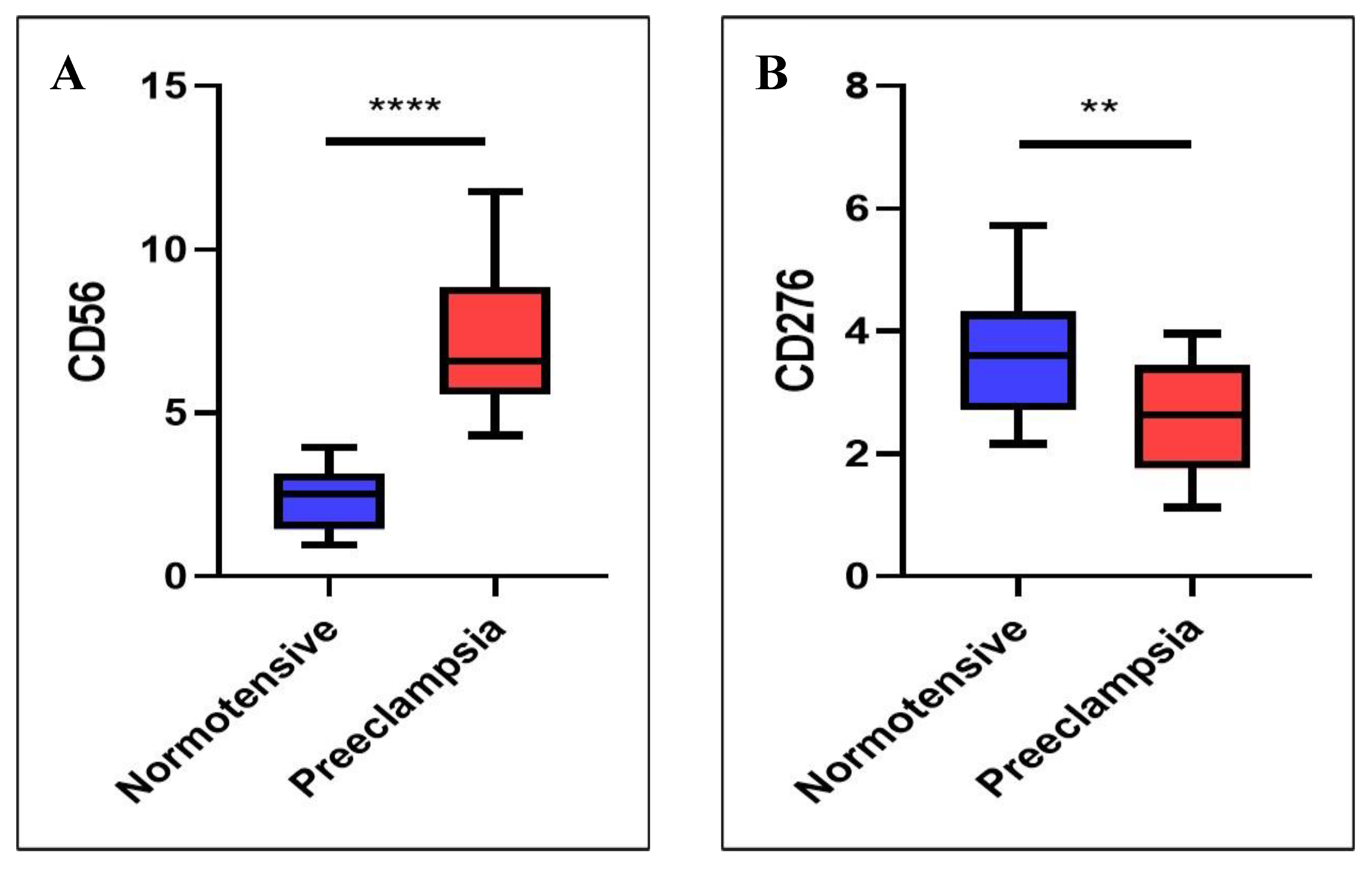
dNK Cell Expression Was Higher in the Preeclamptic Placenta than in the Normotensive Placenta
IHC analysis demonstrated the presence of dNK cells dispersed within the decidual stroma surrounding the trophoblasts. Quantitatively, dNK cell expression (reflecting the number of dNK cells) differed significantly between the preeclampsia and control groups. The mean dNK cell IRS in preeclamptic placentas was 7.19 ± 2.16. significantly higher than in normotensive pregnancies. which was 2.42 ± 0.96 (p < 0.001) (
Table 2;
Figure 2A). These findings indicate an increased number of dNK cells in preeclamptic conditions.
B7H3 Expression Was Lower in the Preeclamptic Placenta than in the Normotensive Placenta
B7-H3 expression was observed on the membrane/cytoplasm of EVT within decidual tissue. The B7-H3 expression average in the preeclampsia group was 2.63 ± 0.90, significantly lower than that in the control group, which was 3.62 ± 0.99 (p = 0.002) (
Table 2;
Figure 2B). These findings suggest that trophoblasts in preeclamptic pregnancies exhibit reduced expression of the B7-H3 molecule compared to those in normal pregnancies.
The Relationship Between B7-H3 Expression and dNK Cells
In normotensive pregnancies, the expression of dNK cells differs significantly from that of B7-H3, with it showing markedly higher expression levels (
Figure 3A). In contrast, in preeclamptic pregnancies, dNK cell expression is considerably higher than B7-H3 expression (p < 0.001) (
Figure 3B).
Correlation analysis demonstrated a significant positive association between B7-H3 expression in trophoblasts and the number of dNK cells in both normotensive and preeclampsia. In the normotensive group, this relationship was strong (r = 0.605; p < 0.01), suggesting that higher B7-H3 expression is closely linked to an increased number of dNK cells under physiological conditions. Interestingly, a moderate yet significant correlation was also observed in preeclamptic cases (r = 0.465; p < 0.05), indicating that despite lower absolute B7-H3 levels and higher dNK counts, the linear association between these two variables remains consistent. This implies that within preeclamptic samples, those with relatively higher B7-H3 expression also tend to have more dNK cells.
Table 3.
Correlation between B7-H3 and dNK cells in both groups.
Table 3.
Correlation between B7-H3 and dNK cells in both groups.
| Subject |
n |
p Value |
r (Correlation Coefficient) |
| Normotensive |
21 |
0.004 |
0.605 |
| Preeclampsia |
21 |
0.034 |
0.465 |
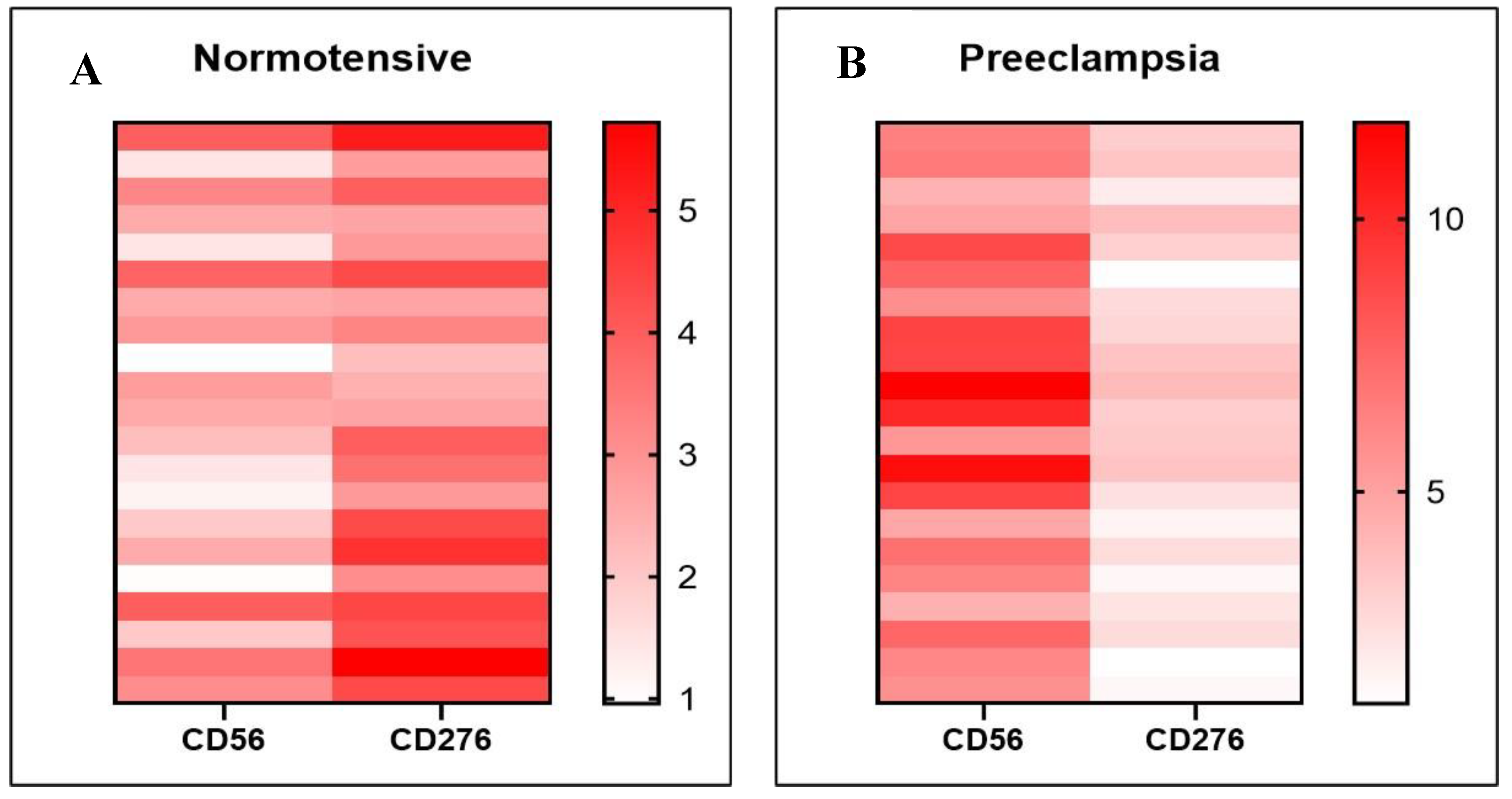
A detailed examination within each group reveals notable inter-sample variability in expression patterns. Through heatmap visualization (
Figure 4A,B), distinct differences in the expression levels of dNK cells and B7-H3 across individual samples become evident. These findings provide further support for a strong inverse association between B7-H3 expression and dNK cell abundance, whereby lower B7-H3 levels are consistently associated with elevated dNK cell expression and vice versa.
4. Discussion
This pilot case-control study demonstrates significant alterations in maternal-fetal immune components in preeclampsia, characterized by decreased expression of B7-H3 on EVT and an increased number of dNK cells. B7-H3, a member of the B7 family of immune checkpoint molecules, plays a complex and context-dependent role in immunoregulation during pregnancy. B7-H3 was initially identified as a co-stimulatory molecule that enhances CD4⁺/CD8⁺ T cell proliferation and IFN-γ production. However. subsequent studies have reported opposing findings. suggesting that B7-H3 may also function as a negative immune checkpoint. Thus. its role in T cell regulation appears to be context-dependent and remains a subject of ongoing controversy[
19]. B7-H3 mRNA expression is markedly elevated in placental tissue compared to other normal tissues[
20], strongly suggesting its role as a “tolerogenic molecule” that may contribute to the maintenance of maternal immune tolerance toward the fetus.
In normal gestation, maternal immune tolerance is essential for maintaining fetal survival, and this involves intricate interactions between decidual immune cells and trophoblasts. B7-H3 is expressed at the maternal-fetal interface, particularly by EVT and stromal cells, where it is believed to contribute to immune tolerance by modulating the activity of dNK cells and other maternal immune cells[
14,
21]. Its immunosuppressive properties may help prevent an excessive maternal immune response against the semi-allogeneic fetus, facilitating successful implantation, placental development, and fetal growth.
Conversely, dysregulated expression of B7-H3 has been implicated in the pathogenesis of disorders characterized by impaired placentation. Emerging evidence suggests that alterations in B7-H3 expression are involved in the underlying pathophysiology of unexplained recurrent pregnancy loss[
16]. Reduced B7-H3 expression is associated with heightened production of pro-inflammatory cytokines by dNK cells, which may exacerbate immune dysregulation and compromise trophoblast invasion and vascular remodeling during early pregnancy, ultimately contributing to pregnancy failure.
The preeclampsia cases in this study demonstrate decreased expression of B7-H3 on EVT and an increased number of dNK cells. In normal pregnancy, high B7-H3 expression on trophoblasts appears to correlate positively with a well-regulated. This reflects a state of immune tolerance, wherein trophoblasts and maternal immune cells interact harmoniously. B7-H3 functions as an immunoregulatory molecule that modulates the maternal immune response to the fetus, allowing for the presence of a substantial number of dNK cells with a tolerogenic, non-cytotoxic phenotype.
A positive correlation between B7-H3 expression and dNK cell abundance suggests that a strongly immunotolerant decidual environment permits a greater presence of dNK cells without triggering maternal immune rejection of the fetus. In healthy pregnancy, dNK cell populations undergo dynamic changes across gestation. They are highly abundant during the first trimester, comprising roughly 50–70% of decidual lymphoid cells [
22,
23]. As pregnancy progresses into the second and third trimesters, the number and proportion of dNK cells in the decidua gradually decline[
10].
In contrast, preeclampsia is characterized by significantly reduced B7-H3 expression accompanied by an increased accumulation of dNK cells. This downregulation of B7-H3 suggests a partial loss of immunoinhibitory signaling in the preeclamptic placenta, potentially leading to dysregulation of maternal immune cell activity. The elevated number of dNK cells could reflect an exaggerated immune response or a compensatory reaction to underlying placental stress. It is well recognized that preeclampsia involves substantial placental stress even at term. Similarly, in term preeclampsia, a dysfunctional syncytiotrophoblast (STB) releases pro-inflammatory and anti-angiogenic factors that drive the maternal syndrome[
9]. One consequence of this stress is activation of the complement system in the placental microenvironment. Complement overactivation triggers an inflammatory cascade, generating anaphylatoxins that recruit and activate immune cells [
24,
25]. These processes create a pro-inflammatory milieu in the preeclamptic placenta and provide a mechanistic context for the observed increase in dNK cell accumulation when B7-H3 is downregulated, linking placental stress with innate immune cell recruitment in preeclampsia.
Activated dNK cells can secrete pro-inflammatory cytokines, which may hinder trophoblast invasion and disrupt uterine artery remodelling. Thus, our findings support the hypothesis of immune maladaptation in preeclampsia, where diminished expression of tolerogenic molecules, such as B7-H3, leads to uncontrolled effector immune responses, ultimately contributing to shallow placentation and maternal endothelial dysfunction.
In our study, only 2 out of 21 preeclamptic pregnancies were complicated by fetal growth restriction (FGR). This is notable because preeclampsia with FGR is often linked to shallow EVT invasion of the uterine wall, resulting in inadequate spiral artery remodeling and consequent uteroplacental insufficiency[
26]. The presence of FGR in these placentas likely indicates deficient trophoblast invasion, reinforcing our interpretation that reduced B7-H3 expression and subsequent immune dysregulation contribute to shallow EVT invasion and poor placental perfusion[
26]. Overall, our findings align with established mechanisms of shallow placentation in preeclampsia, and the observed interplay of STB stress, complement-driven inflammation (with decidual NK cell recruitment), and FGR further supports the role of shallow trophoblast invasion in such cases.
This study is also in tone with previous reports highlighting the critical role of immune checkpoint molecules in the placenta[
16]. Reduced B7-H3 expression on trophoblasts has been associated with recurrent pregnancy loss, potentially through mechanisms involving increased cytokine secretion by NK cells that impair trophoblast invasion. Although the clinical contexts differ (miscarriage versus preeclampsia), both conditions reflect a common underlying scenario: the deficiency of immunoregulatory signals leads to a more pro-inflammatory decidual environment that is less conducive to successful placentation.
Our findings provide additional evidence that immune regulation at the maternal-fetal interface involves not only the HLA system but also the B7-H3/CD276 pathway. It is important to note that the increased number of dNK cells observed in preeclamptic placentas does not necessarily indicate normal functional activity. It is plausible that the dNK cells in preeclampsia represent a subpopulation with a more cytotoxic or pro-inflammatory phenotype compared to those found in normal pregnancy.
The increased number of dNK cells may also result from the recruitment of immune cells in response to placental hypoxia or tissue damage, suggesting that their accumulation could be a consequence of preeclampsia development[
27,
28]. Although the exact cause-and-effect relationship is unclear, the moderate but significant correlation in the preeclamptic group suggests that higher B7-H3 expression is generally linked to a greater number of dNK cells, even under disease conditions.
Limitations of the Study
This study has several limitations. First, the cross-sectional design at the time of delivery may not fully capture the dynamic changes of the placenta during early pregnancy. However, the consistent expression of B7-H3 on EVT in late gestation is in line with previous studies. Then, the relatively small sample size limits the statistical power and generalizability of the results. This pilot study likely reflects population-level trends, but the findings may vary when stratified by preeclampsia phenotypes. Notably, the classical early-onset preeclampsia (EOP) phenotype associated with fetal growth restriction (FGR) was underrepresented in our cohort. Even when applying a threshold of the 25th percentile for birthweight, only 4 out of 21 preeclamptic cases met this criterion. This scarcity of classical EOP with FGR should be acknowledged as a key limitation of our study. Future research involving larger, phenotype-specific cohorts is necessary to enable a more robust comparison between different preeclampsia subtypes, including normotensive FGR, and to validate the immunological patterns observed in this preliminary investigation.
Future Perspective
From a clinical perspective, our findings highlight the potential of B7-H3 and dNK cells as future biomarkers and therapeutic targets. If reduced B7-H3 expression can be reliably detected early in pregnancy, this molecule could be developed as part of a predictive risk assessment tool for preeclampsia, complementing existing screening models. Similarly, interventions aimed at enhancing immunotolerant signalling at the placental interface merit further exploration. Although still in development, future treatments in obstetrics may include immunotherapy, such as agents that increase B7-H3 levels or control overactive dNK cells to help prevent preeclampsia in women at high risk.
5. Conclusions
This study provides compelling evidence of a disrupted immunological environment in preeclamptic placentas, characterized by reduced expression of the immune checkpoint molecule B7-H3 and a concomitant increase in dNK cell numbers. The strong correlation between B7-H3 and dNK cells underscores the critical role of immune regulation at the maternal-fetal interface. These findings support the hypothesis that impaired immune tolerance contributes to preeclampsia pathogenesis. B7-H3 holds promise as a novel biomarker and potential therapeutic target in preeclampsia. Future longitudinal studies are warranted to explore the predictive value of B7-H3 in early pregnancy and to evaluate strategies aimed at restoring immune balance in high-risk pregnancies.
Author Contributions
Conceptualization, K.E.G. and A.I.H.; methodology, K.E.G. and A.I.H.; software, K.E.G. and A.I.H.; validation, K.E.G. and A.I.H.; formal analysis, K.E.G., A.I.H., and B.U.; investigation, A.I.H. and G.A.; resources, K.E.G., A.I.H., and M.P.W.; data curation, K.E.G. and A.I.H.; writing—original draft preparation, K.E.G. and A.I.H.; writing—review and editing, K.E.G., A.I.H., M.P.W., E.E., A.S., B.U., G.A., M.T., E.G.D., and G.D.; visualization, K.E.G., and M.T..; supervision, K.E.G. and E.G.D.; project administration, K.E.G., A.I.H., and M.P.W.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study subjects were pregnant women who delivered at Dr. Soetomo General Academic Hospital (ethical clearance No. 1014/KEPK/VI/2024) and Universitas Airlangga Hospital (ethical approval No. 063/KEP/2024), Surabaya, Indonesia
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to express their sincere gratitude to the officers and administrative staff of Dr. Soetomo General Academic Hospital, Surabaya, Indonesia, and Universitas Airlangga Hospital, Surabaya, Indonesia, for their invaluable support and assistance during this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; et al. Pre-eclampsia. Nat Rev Dis Primers 2023, 9, 8. [Google Scholar] [CrossRef]
- Gumilar, K.E.; Rauf, K.B.A.; Akbar, M.I.A.; Imanadha, N.C.; Atmojo, S.; Putri, A.Y.; et al. Connecting the Dots: Exploring the Interplay Between Preeclampsia and Peripartum Cardiomyopathy. J Pregnancy 2024, 2024, 7713590. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet 2019, 145 Suppl 1, 1–33. [Google Scholar] [CrossRef]
- Jung, E.; Romero, R.; Yeo, L.; Gomez-Lopez, N.; Chaemsaithong, P.; Jaovisidha, A.; et al. The etiology of preeclampsia. Am J Obstet Gynecol 2022, 226, S844–S66. [Google Scholar] [CrossRef]
- Aldika Akbar, M.I.; Rosaudyn, R.; Gumilar, K.E.; Shanmugalingam, R.; Dekker, G. Secondary prevention of preeclampsia. Front Cell Dev Biol 2025, 13, 1520218. [Google Scholar] [CrossRef]
- Gumilar, K.E.; Priangga, B.; Lu, C.H.; Dachlan, E.G.; Tan, M. Iron metabolism and ferroptosis: A pathway for understanding preeclampsia. Biomed Pharmacother 2023, 167, 115565. [Google Scholar] [CrossRef]
- Robillard, P.-Y.; Dekker, G.; Scioscia, M.; Saito, S. Progress in the understanding of the pathophysiology of immunologic maladaptation related to early-onset preeclampsia and metabolic syndrome related to late-onset preeclampsia. American Journal of Obstetrics & Gynecology 2022, 226, S867–S75. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Zhao, Y.; Wang, H.; Pan, Q.; Shao, Q. Decidual Natural Killer Cells: A Good Nanny at the Maternal-Fetal Interface During Early Pregnancy. Front Immunol 2021, 12, 663660. [Google Scholar] [CrossRef]
- Redman, C.W.; Staff, A.C. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am J Obstet Gynecol 2015, 213, S9.e1–S9. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H. Role of Decidual Natural Killer Cells in Human Pregnancy and Related Pregnancy Complications. Front Immunol 2021, 12, 728291. [Google Scholar] [CrossRef] [PubMed]
- Rauf, K.A.; Dekker, G.A.; Sulistyono, A.; Aditiawarman, A.; Harsono, A.; Gumilar, K.E.; et al. 22. Decidual killer immunoglobulin-like receptor (kir)2dl1 expression and the onset of preeclampsia, birth weight and placental weight in early and late onset preeclampsia. Pregnancy Hypertension 2018, 13, S56. [Google Scholar] [CrossRef]
- Hiby, S.E.; Regan, L.; Lo, W.; Farrell, L.; Carrington, M.; Moffett, A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum Reprod 2008, 23, 972–976. [Google Scholar] [CrossRef]
- Petroff, M.G.; Kharatyan, E.; Torry, D.S.; Holets, L. The immunomodulatory proteins B7-DC, B7-H2, and B7-H3 are differentially expressed across gestation in the human placenta. Am J Pathol 2005, 167, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Petroff, M.G.; Perchellet, A. B7 family molecules as regulators of the maternal immune system in pregnancy. Am J Reprod Immunol 2010, 63, 506–519. [Google Scholar] [CrossRef]
- Collins, M.; Ling, V.; Carreno, B.M. The B7 family of immune-regulatory ligands. Genome Biology 2005, 6, 223. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, Y.; Ding, C.; Zhang, R.; Duan, T.; Zhou, Q. Decreased B7-H3 promotes unexplained recurrent miscarriage via RhoA/ROCK2 signaling pathway and regulates the secretion of decidual NK cellsdagger. Biol Reprod 2023, 108, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens 2022, 27, 148–169. [Google Scholar] [CrossRef]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue - a review. Diagn Pathol 2014, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Marozio, L.; Nuzzo, A.M.; Gullo, E.; Moretti, L.; Canuto, E.M.; Tancredi, A.; et al. Immune Checkpoints in Recurrent Pregnancy Loss: New Insights into a Detrimental and Elusive Disorder. Int J Mol Sci 2023, 13071. [Google Scholar] [CrossRef]
- Getu, A.A.; Tigabu, A.; Zhou, M.; Lu, J.; Fodstad, O.; Tan, M. New frontiers in immune checkpoint B7-H3 (CD276) research and drug development. Mol Cancer 2023, 22, 43. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Q.; Jin, L. The Role of B7 Family Molecules in Maternal-Fetal Immunity. Front Immunol 2020, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Chiossone, L.; Vacca, P.; Orecchia, P.; Croxatto, D.; Damonte, P.; Astigiano, S.; et al. In vivo generation of decidual natural killer cells from resident hematopoietic progenitors. Haematologica 2014, 99, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, M.; Lee, S.K. Role of endometrial immune cells in implantation. Clin Exp Reprod Med 2011, 38, 119–125. [Google Scholar] [CrossRef]
- Deer, E.; Herrock, O.; Campbell, N.; Cornelius, D.; Fitzgerald, S.; Amaral, L.M.; et al. The role of immune cells and mediators in preeclampsia. Nature Reviews Nephrology 2023, 19, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Meng, J. Role of Decidual Natural Killer Cells in the Pathogenesis of Preeclampsia. Am J Reprod Immunol 2025, 93, e70033. [Google Scholar] [CrossRef]
- Geldenhuys, J.; Rossouw, T.M.; Lombaard, H.A.; Ehlers, M.M.; Kock, M.M. Disruption in the Regulation of Immune Responses in the Placental Subtype of Preeclampsia. Front Immunol 2018, 9, 1659. [Google Scholar] [CrossRef]
- Deer, E.; Herrock, O.; Campbell, N.; Cornelius, D.; Fitzgerald, S.; Amaral, L.M.; et al. The role of immune cells and mediators in preeclampsia. Nat Rev Nephrol 2023, 19, 257–270. [Google Scholar] [CrossRef]
- Wallace, A.E.; Fraser, R.; Cartwright, J.E. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update 2012, 18, 458–471. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).