3.1. NRG1-III Overexpression in Mice Increases Motor Activity and Alters Ventral Root Axon Myelination
We first examined the impact of NRG1-III overexpression in MN, beginning at perinatal stages on motor performance and lifespan. No significant differences in survival were observed between TG mice overexpressing NRG1-III and WT. This was consistent across sexes (WT males: 702.5 ± 21.5 days, n=4; WT females: 699.7± 46.5 days, n = 4; TG males: 687.7 ± 144.8 days, n= 12; TG females: 748.42 ± 90.2, n=12; one-way Anova). Motor behavior was assessed using the Open-field test. Total distance covered and average velocity were measured at different time points from middle-aged to old-aged mice (330-740 days). TG mice showed increased distance covered and higher average velocity compared to WT, with significant differences between 380 and 560 days, consistent with previous data observed at younger stages [
32] (
Figure 1A,B). The CatWalk system revealed reduced paw intensity in TG mice compared to WT across most time points analyzed (
Supplementary Figure S1). However, other parameters examined, such as stride length and the number of rearing and grooming events, showed no significant differences (data not shown). These results suggest that neuronal NRG1-III overexpression initially enhances locomotor activity, but this hyperactivity diminishes in aged stages. At this stage, age-dependent neurodegeneration may counteract the NRG1-dependent hyperactive phenotype.
Figure 1.
NRG1-III overexpression increases motor activity and changes motor axon myelination. A-B) Open-field analysis of motor performance in wild-type (WT) and transgenic (TG) mice. Data show elevations in the distance covered (A) and the average velocity (B) in TG mice. C-I) Examination of L4 ventral nerve root (VR) axons from adult and aged mice using transverse semithin plastic sections. C) Representative micrographs of semithin sections from L4 VRs illustrate increased myelin thickness in adult TG axons and the presence of degenerating axons (arrows) in both adult and old TG mice. D-F) G-ratio measurements indicate increased myelin thickness in adult TG mice and decreased thickness in aged TG mice. G) Quantification of axon numbers in VRs reveals no differences between WT and TG mice in both adult and aged groups. H) A trend toward a higher number of degenerating axons was detected in TG mice in both age groups (adult: p=0.25; old: p= 0.22). I) Relative frequency histogram of axon diameters for adult and old TG L4 VR axons; note the increased proportion of small-caliber axons in old TG, as well as the appearance of a small population of very large axons, probably representing those with age-related swelling and degenerative changes. For motor behavior analyses, sample sizes ranged from n= 8-24 per time point (one WT point at n= 2 and occasionally WT points at n= 4). * p < 0.05, ** p < 0.01 (Student’s t-test for genotype comparisons at each time point). For ventral root axons measurements, sample sizes were as follows: g-ratio n= 204-275 axons from 3 animals; axon number and degenerating axon counts, n= 3 animals per condition (Student’s t-test [for genotype comparisons] and two-way ANOVA, Bonferroni‘s post hoc test). **p < 0.01 and ****p < 0.0001. Scale bar= 5 µm.
Figure 1.
NRG1-III overexpression increases motor activity and changes motor axon myelination. A-B) Open-field analysis of motor performance in wild-type (WT) and transgenic (TG) mice. Data show elevations in the distance covered (A) and the average velocity (B) in TG mice. C-I) Examination of L4 ventral nerve root (VR) axons from adult and aged mice using transverse semithin plastic sections. C) Representative micrographs of semithin sections from L4 VRs illustrate increased myelin thickness in adult TG axons and the presence of degenerating axons (arrows) in both adult and old TG mice. D-F) G-ratio measurements indicate increased myelin thickness in adult TG mice and decreased thickness in aged TG mice. G) Quantification of axon numbers in VRs reveals no differences between WT and TG mice in both adult and aged groups. H) A trend toward a higher number of degenerating axons was detected in TG mice in both age groups (adult: p=0.25; old: p= 0.22). I) Relative frequency histogram of axon diameters for adult and old TG L4 VR axons; note the increased proportion of small-caliber axons in old TG, as well as the appearance of a small population of very large axons, probably representing those with age-related swelling and degenerative changes. For motor behavior analyses, sample sizes ranged from n= 8-24 per time point (one WT point at n= 2 and occasionally WT points at n= 4). * p < 0.05, ** p < 0.01 (Student’s t-test for genotype comparisons at each time point). For ventral root axons measurements, sample sizes were as follows: g-ratio n= 204-275 axons from 3 animals; axon number and degenerating axon counts, n= 3 animals per condition (Student’s t-test [for genotype comparisons] and two-way ANOVA, Bonferroni‘s post hoc test). **p < 0.01 and ****p < 0.0001. Scale bar= 5 µm.
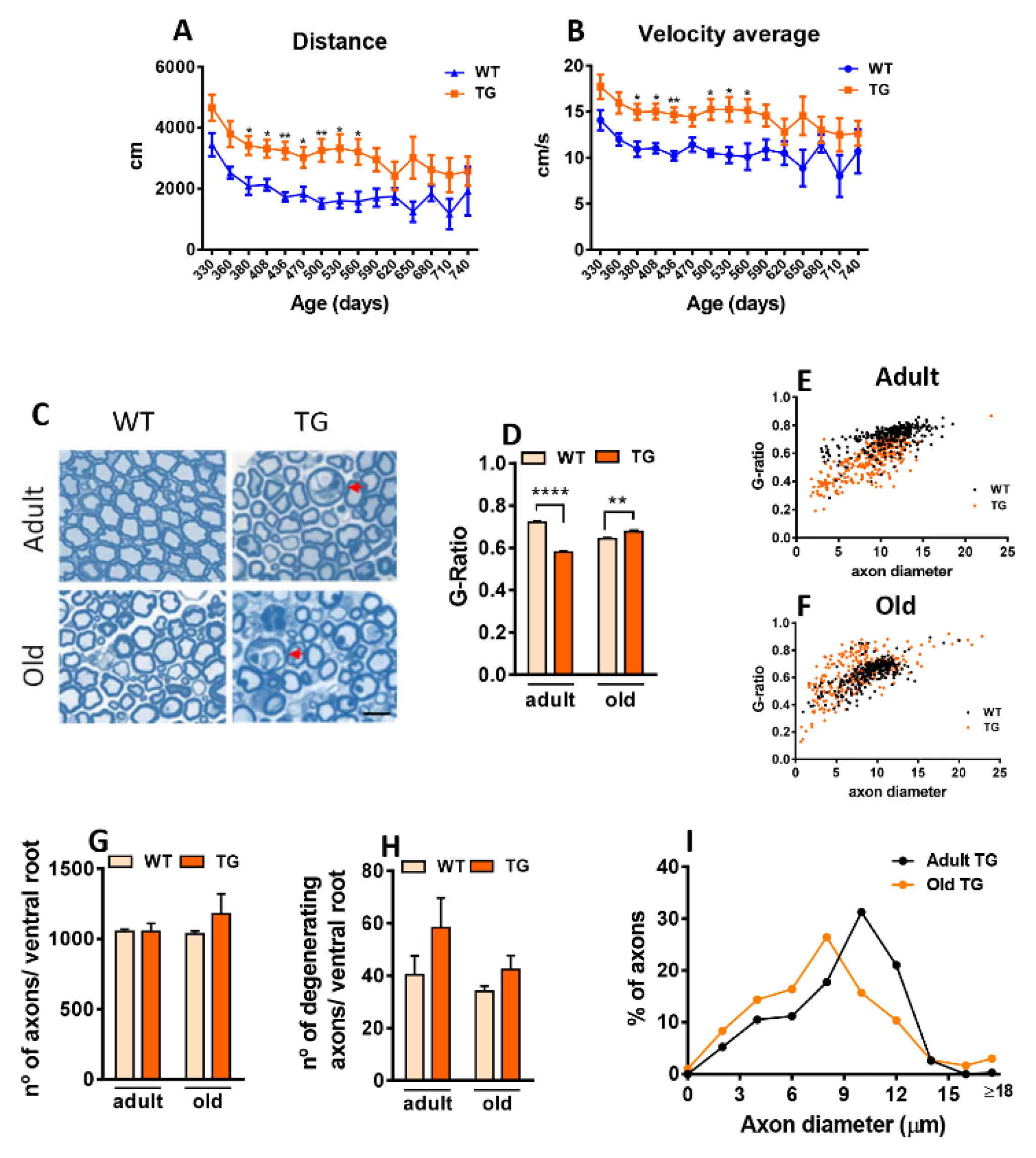
NRG1 is a key regulator of developmental myelination. Overexpression of NRG1-III in MNs of TG mice results in a well-documented phenotype of hypermyelination [
18,
19], which was confirmed here (
Figure 1C). Additionally, we explored whether NRG1-III overexpression retains the capacity to induce long-term hypermyelination in old mice. We determined the G-ratio in L4 VRs, which contain the proximal segments of motor axons that project into the sciatic nerve. Compared to VRs of adult WT mice, those of adult TG animals exhibited a reduced G-ratio, indicating hypermyelination. In old TG mice, however, we observed a decrease in axon myelination in old TG mice (
Figure 1C-F). The total number of axons remained unchanged (
Figure 1G), but we observed a tendency towards higher numbers of degenerating axons in VRs of both adult (p=0.25) and old (p= 0.22) TG mice (
Figure 1C,H). Since we detected no significant axonal loss in old nerve roots, it is plausible that a compensatory mechanism, such as collateral axonal sprouting from healthy axons, occurred. In fact, when comparing histograms of axonal diameter distribution in adult and old TG L4 VR, an increased frequency of small-diameter axons (<8 µm) was observed in old TG mice. In addition, a small increase in the proportion of very large-diameter axons was detected in TG mice, which likely represents a population of swollen, degenerating axons (
Figure 1I).
3.2. Impact of Long-Term NRG1-III Overexpression on MN Synaptic Afferents and C-Bouton Organization
NRG1 is prominently expressed in the soma and proximal dendrites of α-MNs, in which it is concentrated adjacent to VAChT-positive presynaptic terminals in the postsynaptic compartment of cholinergic C-bouton synapses (6, 12). We previously showed that overexpression of NRG1-III in adult TG mice leads to widespread SSC accumulation of NRG1-III in MN somata, which display a set of SSC-specific molecular constituents [
15]. However, the long-term consequences of these changes on MNs have not yet been explored [
6,
15]. Thus, we investigated the impact of NRG1-III overexpression on MN biology from embryonic development to ageing, with a particular focus on plastic changes of C-boutons and other afferent synapses to MNs. Measurements of MN soma area using Nissl staining revealed a similar increase in WT and TG mice from birth to postnatal stages (P10). In adult mice (P60), MN soma size was significantly larger in TG than in WT mice. This was not observed in old animals (P600 [
Figure 2A]). Moreover, no changes were observed in the total number of L4 ventral root axons, suggesting unaltered MN numbers in TG mice, (
Figure 1G).
Figure 2.
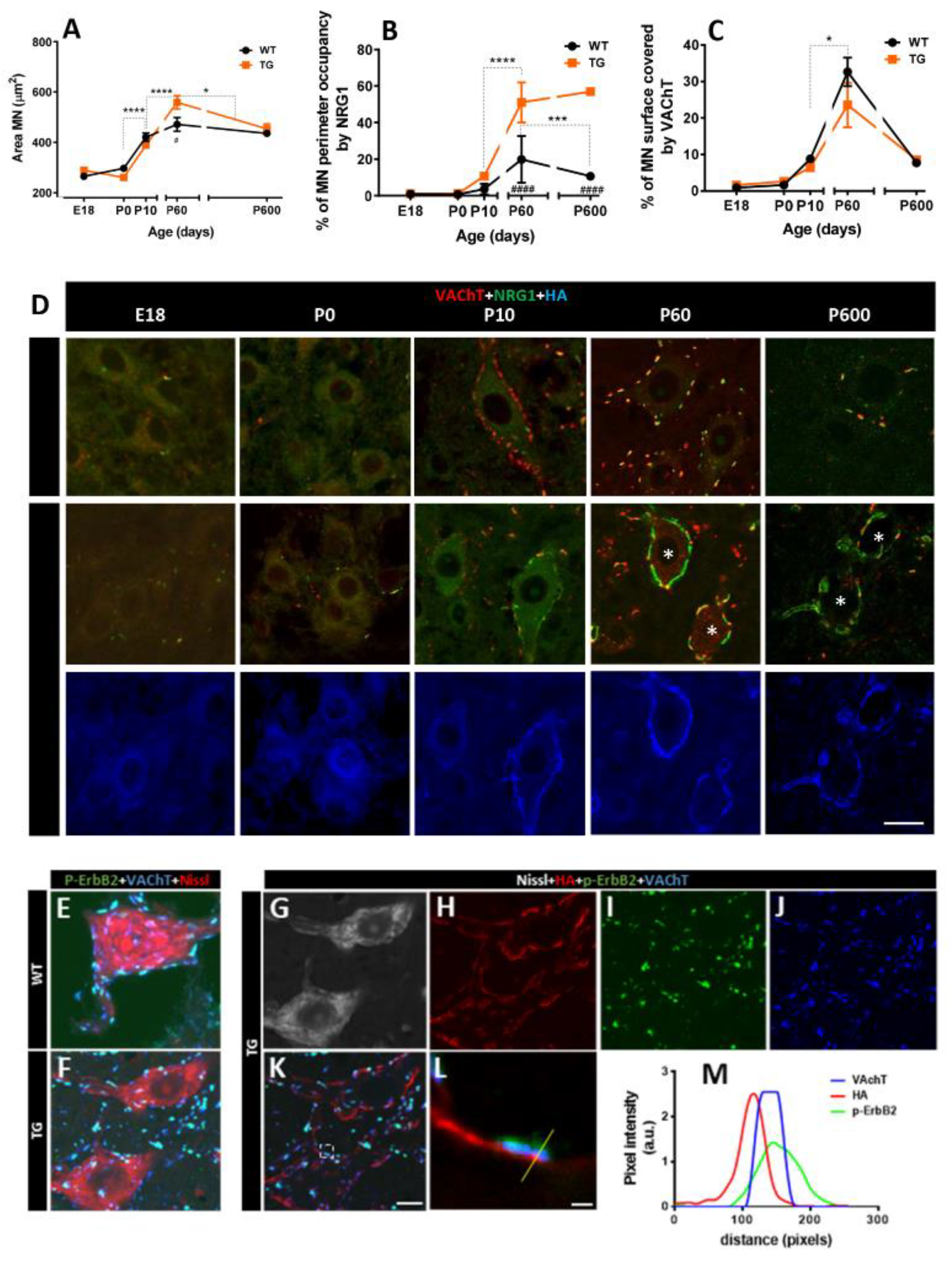
Impact of NRG1-III overexpression on C-bouton organization. Graphs depict the time course of the average motor neuron (MN) area (A), and the MN perimeter occupied by NRG1 (B) and VAChT (C). D) Representative confocal micrographs from each time point show MNs immunolabeled for VAChT (red), NRG1 (green), and HA (blue), illustrating an expansion of NRG1 near the surface of TG MNs at P60 and P600 (*), while VAChT remains unchanged. E- L) MNs labeled for HA (red), p-ErbB2 (green), VAChT (blue) and counterstained with fluorescence Nissl staining (grey), showing the presynaptic association of ErbB2 with VAChT facing HA postsynaptic labeling in both WT and TG MNs. An enlarged detail of the region outlined in panel K is provided in panel L. M) Pixel profile analysis across the line indicated in panel L confirms an overlap between VAChT and p-ErbB2 signals, with no overlap observed for HA. Scale bars: 20 µm in (D), 10 µm in (E–K), and 1 µm in (L).
Figure 2.
Impact of NRG1-III overexpression on C-bouton organization. Graphs depict the time course of the average motor neuron (MN) area (A), and the MN perimeter occupied by NRG1 (B) and VAChT (C). D) Representative confocal micrographs from each time point show MNs immunolabeled for VAChT (red), NRG1 (green), and HA (blue), illustrating an expansion of NRG1 near the surface of TG MNs at P60 and P600 (*), while VAChT remains unchanged. E- L) MNs labeled for HA (red), p-ErbB2 (green), VAChT (blue) and counterstained with fluorescence Nissl staining (grey), showing the presynaptic association of ErbB2 with VAChT facing HA postsynaptic labeling in both WT and TG MNs. An enlarged detail of the region outlined in panel K is provided in panel L. M) Pixel profile analysis across the line indicated in panel L confirms an overlap between VAChT and p-ErbB2 signals, with no overlap observed for HA. Scale bars: 20 µm in (D), 10 µm in (E–K), and 1 µm in (L).
NRG1 immunostaining revealed that NRG1 expression in MNs increased from P10 to adulthood in both WT and TG mice, but this elevation was more pronounced in TG mice. In line with continuous Thy1.2-promoter activity, the expected decline of NRG1 during aging was not observed in TG animals (
Figure 2B,D). Additionally, we analyzed HA-tag immunolabeling to determine whether NRG1-III overexpression in spinal cord MNs of TG mice colocalized with pan-NRG1 immunolabeling. A match between the two markers was observed at the periphery of MN somata (
Figure 2D). Despite postsynaptic NRG1-III overexpression, the developmental profile of VAChT was similar in WT and TG mice (
Figure 2C,D). Moreover, we confirmed co-expression of p-ErbB2 in the presynaptic compartment of C-boutons, overlapping with VAChT [
12]. (
Figure 2E) and found no differences in TG mice (
Figure 2F-M). Ultrastructural analysis revealed that, as expected, in control MNs, SSCs are confined to postsynaptic sites facing C-bouton terminals (
Figure 3A,B). NRG1-III overexpression promotes SSC biogenesis, extending its domain beyond the postsynaptic region (
Figure 3C,D). Notably, SSC enlargement remains stable in old TG mice (
Figure 3E,F). Collectively, these findings are consistent with a specific role of NRG1-III in organizing the postsynaptic, but not the presynaptic compartment of C-boutons [
15].
Figure 3.
Structural changes in MN afferent synapse organization induced by NRG1-III overexpression. A) WT MN in adult mice showing C-boutons (C) and additional synapses (SY) contacting MN surface in adult mice (P100); note that the subsynaptic cistern (SSC) is restricted to C-bouton postsynaptic region (arrows); presynaptic terminals are shaded in green and MN soma is colored in red. B) An enlarged view of the area outlined in (A) detailing the particular organization of C-bouton membrane compartmentation in WT mice in which extracellular space (yellow) and postsynaptic SSC (blue) are delimitated; presynaptic C-bouton terminal with clear spherical vesicles and postsynaptic neuron are shaded in green and red, respectively. C) MN from TG mice (P41) show a prominent enlargement of the SSC adjacent to the plasma membrane (arrows) outpacing the limits of presynaptic C-bouton terminals in which SSC remains circumscribed in WT; additionally, glial processes (G) are observed covering part of MN surface adjacent to SSC but devoid of synapses. D) A further enlargement of the region marked in (C), highlighting the SSC growth induced by NRG1-III overexpression; the color code is the same as A and B with additional shading of glial processes in clear blue. E, F) MN from old TG mice (P725) showing the similar organization of C-boutons and expanded SSC (arrows) as observed in adults. The color code is the same as A-D. Note the accumulation of lipofuscin granules seen in old MNs (*). sv= synaptic vesicles; m= mitochondria; er= endoplasmic reticulum; ex= extracellular space. G-J) Changes in afferent synaptic boutons other than C-boutons on MNs: MN somata labeled for VGluT1, VGluT2, and VGAT (red) and Nissl (green); the distribution of each synaptic type on MN surface is shown (G) and quantified in H-J. Sample sizes in VGluT1 and VGluT2 graphs ranged from n= 15-17 MNs, and VGAT n= 18 MNs per condition from 2 animals. **p < 0.01 (Student’s t-test for genotype comparisons). Scale bars: 1 µm in (A), (C) and (F), 200 nm in (B), 250 nm in (D), 2.5 µm (E) and 10 µm in (G).
Figure 3.
Structural changes in MN afferent synapse organization induced by NRG1-III overexpression. A) WT MN in adult mice showing C-boutons (C) and additional synapses (SY) contacting MN surface in adult mice (P100); note that the subsynaptic cistern (SSC) is restricted to C-bouton postsynaptic region (arrows); presynaptic terminals are shaded in green and MN soma is colored in red. B) An enlarged view of the area outlined in (A) detailing the particular organization of C-bouton membrane compartmentation in WT mice in which extracellular space (yellow) and postsynaptic SSC (blue) are delimitated; presynaptic C-bouton terminal with clear spherical vesicles and postsynaptic neuron are shaded in green and red, respectively. C) MN from TG mice (P41) show a prominent enlargement of the SSC adjacent to the plasma membrane (arrows) outpacing the limits of presynaptic C-bouton terminals in which SSC remains circumscribed in WT; additionally, glial processes (G) are observed covering part of MN surface adjacent to SSC but devoid of synapses. D) A further enlargement of the region marked in (C), highlighting the SSC growth induced by NRG1-III overexpression; the color code is the same as A and B with additional shading of glial processes in clear blue. E, F) MN from old TG mice (P725) showing the similar organization of C-boutons and expanded SSC (arrows) as observed in adults. The color code is the same as A-D. Note the accumulation of lipofuscin granules seen in old MNs (*). sv= synaptic vesicles; m= mitochondria; er= endoplasmic reticulum; ex= extracellular space. G-J) Changes in afferent synaptic boutons other than C-boutons on MNs: MN somata labeled for VGluT1, VGluT2, and VGAT (red) and Nissl (green); the distribution of each synaptic type on MN surface is shown (G) and quantified in H-J. Sample sizes in VGluT1 and VGluT2 graphs ranged from n= 15-17 MNs, and VGAT n= 18 MNs per condition from 2 animals. **p < 0.01 (Student’s t-test for genotype comparisons). Scale bars: 1 µm in (A), (C) and (F), 200 nm in (B), 250 nm in (D), 2.5 µm (E) and 10 µm in (G).
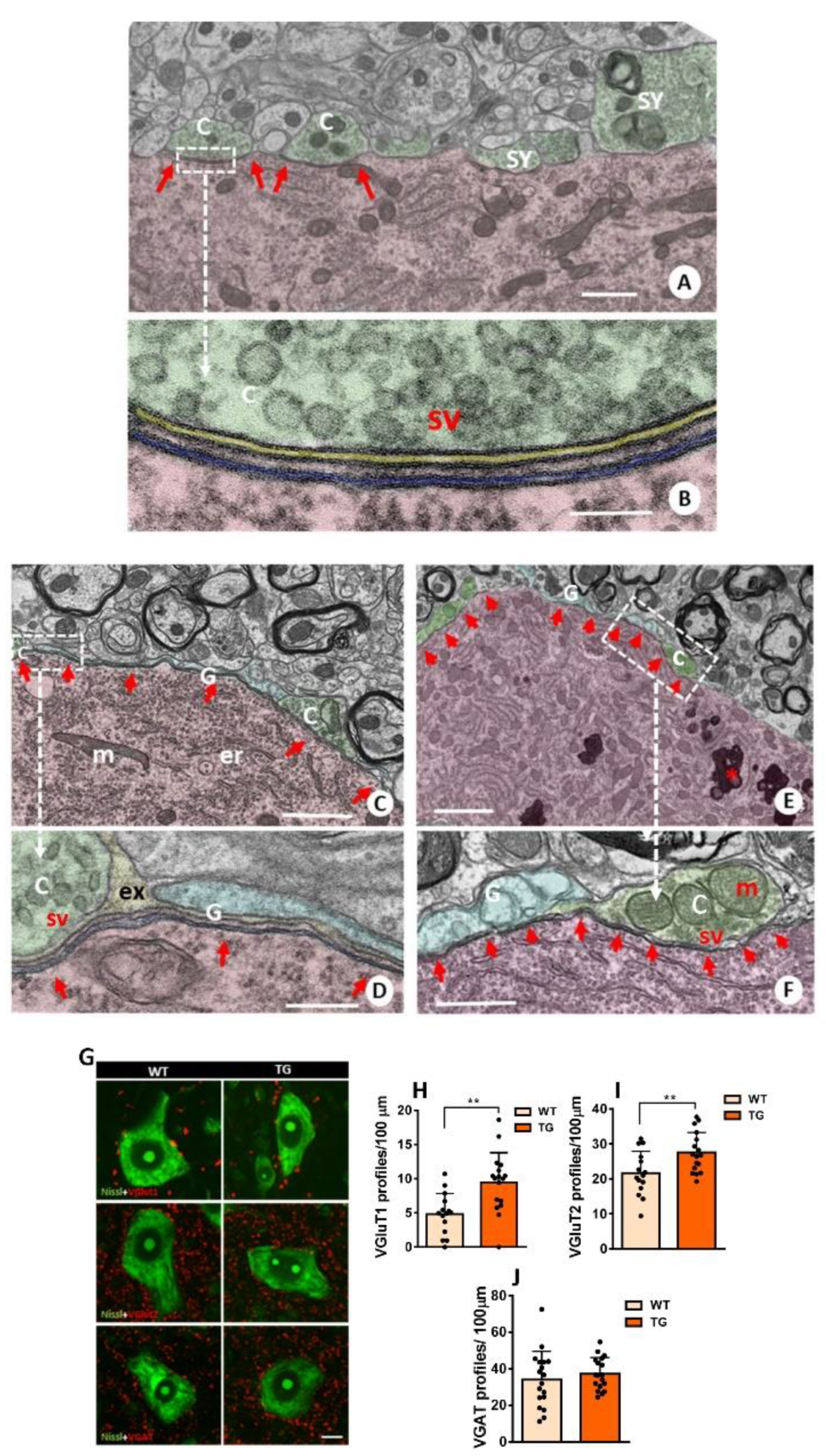
Finally, we investigated the impact of NRG1-III overexpression on MN synaptic connectivity beyond cholinergic inputs. Quantification of excitatory synapses using antibodies against VGluT1 and VGluT2 revealed a higher density of positive spots on the MN surface of TG mice (
Figure 3G-I). In contrast, quantification of inhibitory synapses using antibodies against VGAT showed no significant differences in synaptic density between TG and WT MNs. (
Figure 3G,J). These observations suggest that NRG1-III overexpression in either MNs or sensory neurons specifically results in increased glutamatergic connectivity in MNs.
3.3. Sustained NRG1-III Overexpression Promotes MN Plasticity and the Development of a Fast-Fatigable MN Phenotype
CGRP, a member of the calcitonin family of peptides [
33], is produced in peripheral and central neurons and is associated with axonal growth and synaptic plasticity, particularly in NMJs [
34,
35,
36]. CGRP immunoreactivity was increased in MNs from adult TG mice compared to WT (
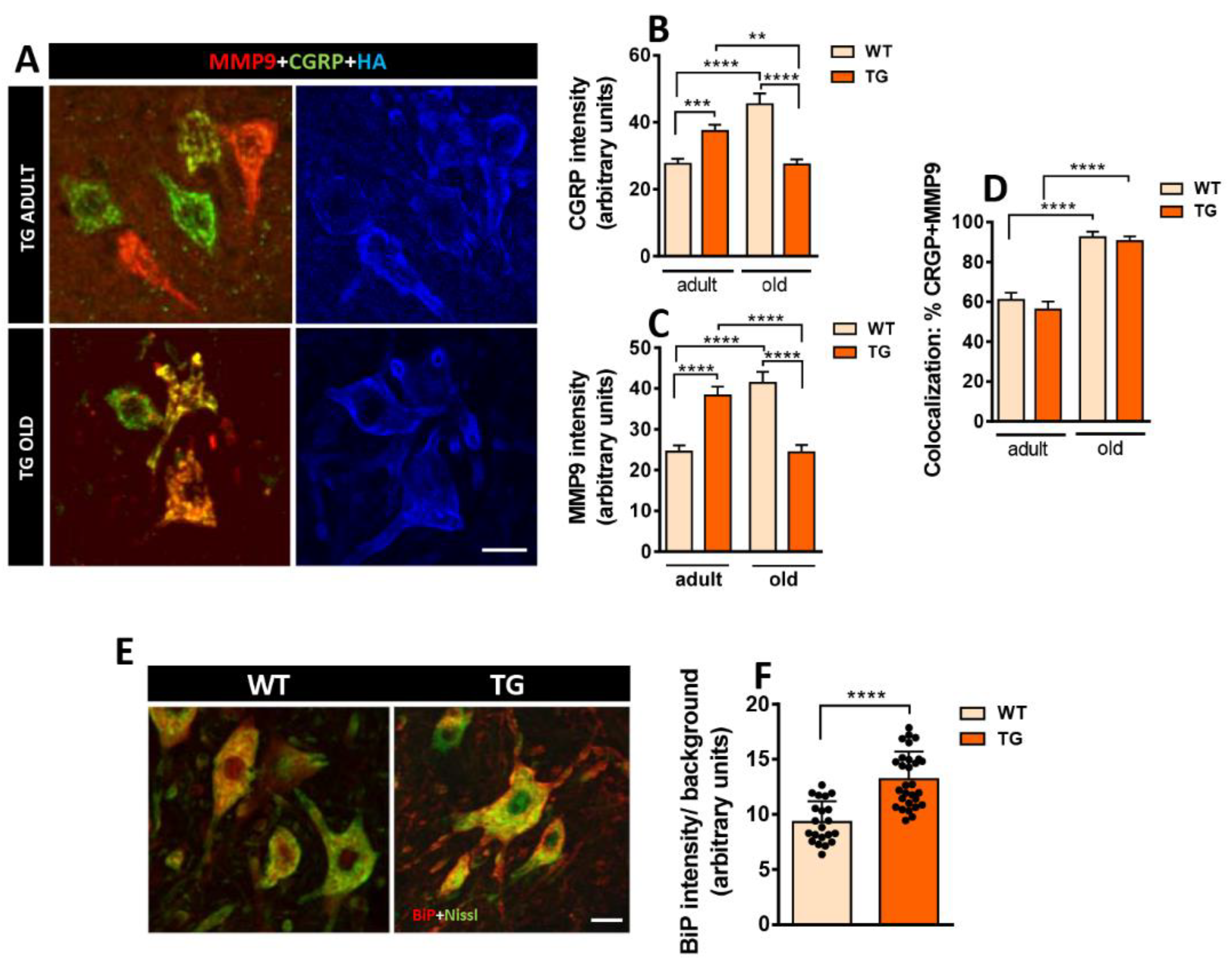
Figure 4A,B). Notably, during aging, the CGRP expression was further increased in WT. Conversely, CGRP immunoreactivity was reduced in MNs of old TG mice when compared to both old WT and adult TG MNs. These findings suggest that MNs of old TG mice may be less responsive to the reactive plastic changes mediated by CGRP upregulation, which occurs in old WT MNs [
37]. A similar pattern was observed for MMP9 immunoreactivity, with an increase in adult TG MNs followed by a subsequent decrease in old TG MNs (
Figure 4A,C). MMP9 is specifically expressed by fast MNs, which are selectively vulnerable in MN diseases [
38]. Moreover, old mice exhibited a higher degree of CGRP-MMP9 colocalization compared to adult mice, although no differences were observed between MNs from old WT and TG mice (
Figure 4A,D). Furthermore, there were no differences in the degree of MMP9+ MNs, HA+ MNs, or double/triple-labeled MNs (CGRP+/HA+, MMP9+/HA+, and CGRP+/MMP9+/HA+) between adult and old TG mice (data not shown).
Figure 4.
CGRP, MMP9, and BiP immunoreactivity in MNs of WT and TG mice. A) Immunostaining for MMP9 (red), CGRP (green), and HA (blue) in spinal cord ventral horn of adult and aged TG mice; note the colocalization of MMP9 and CGRP in some MNs. B, C) Age-related changes in the intensity of MMP9 and CGRP immunoreaction in WT and TG MNs. D) Quantification of the proportion of MNs double-labeled for MMP9 and CGRP. E, F) Immunolabeling and quantification of BiP (red) in MNs delimited by fluorescent Nissl staining (green). For CRGP intensity quantification, sample sizes ranged from n= 96-165 MNs from 3 animals; for MMP9 n= 165-242 MNs from 3 animals; for the percentage of CGRP-MMP9 colocalization, n= 18-31 images; for BiP n= 21-29 MNs from 2 animals (one-way ANOVA and Bonferroni‘s post hoc test, and Student’s t-test). **p < 0.01, ***p < 0.001 and ****p < 0.0001. Scale bars: 25 µm in (A) and 20 µm in (E).
Figure 4.
CGRP, MMP9, and BiP immunoreactivity in MNs of WT and TG mice. A) Immunostaining for MMP9 (red), CGRP (green), and HA (blue) in spinal cord ventral horn of adult and aged TG mice; note the colocalization of MMP9 and CGRP in some MNs. B, C) Age-related changes in the intensity of MMP9 and CGRP immunoreaction in WT and TG MNs. D) Quantification of the proportion of MNs double-labeled for MMP9 and CGRP. E, F) Immunolabeling and quantification of BiP (red) in MNs delimited by fluorescent Nissl staining (green). For CRGP intensity quantification, sample sizes ranged from n= 96-165 MNs from 3 animals; for MMP9 n= 165-242 MNs from 3 animals; for the percentage of CGRP-MMP9 colocalization, n= 18-31 images; for BiP n= 21-29 MNs from 2 animals (one-way ANOVA and Bonferroni‘s post hoc test, and Student’s t-test). **p < 0.01, ***p < 0.001 and ****p < 0.0001. Scale bars: 25 µm in (A) and 20 µm in (E).
Immunoglobulin heavy chain binding protein (BiP), an ER stress sensor associated with MN degeneration, was also examined [
39,
40,
41]. A significant increase in BiP intensity was observed in MN from adult TG mice compared to adult WT animals. This suggests that NRG1 overexpression may lead to increased ER stress, potentially linked to excessive SSC-like ER biogenesis (
Figure 4E,F).
3.4. Axotomized MNs in TG Mice Exhibit Disrupted NRG1 Compartmentalization and Exacerbated Microglial Recruitment
Peripheral nerve transection alters the soma of MNs and surrounding glial cells, a process aimed at restoring MN connectivity with their targets. [
15,
42,
43]. We previously reported that increased microglial recruitment to axotomized MNs involves selective tropism for contacting afferent synaptic terminals, particularly those with glutamatergic and cholinergic phenotype (C-boutons) [
12]. Thus, C-bouton-associated proteins may exert a chemoattractive signal for microglia. Moreover, NRG1-III overexpression-linked SSC expansion in TG animals is accompanied by a corresponding enrichment of additional C-bouton-associated proteins [
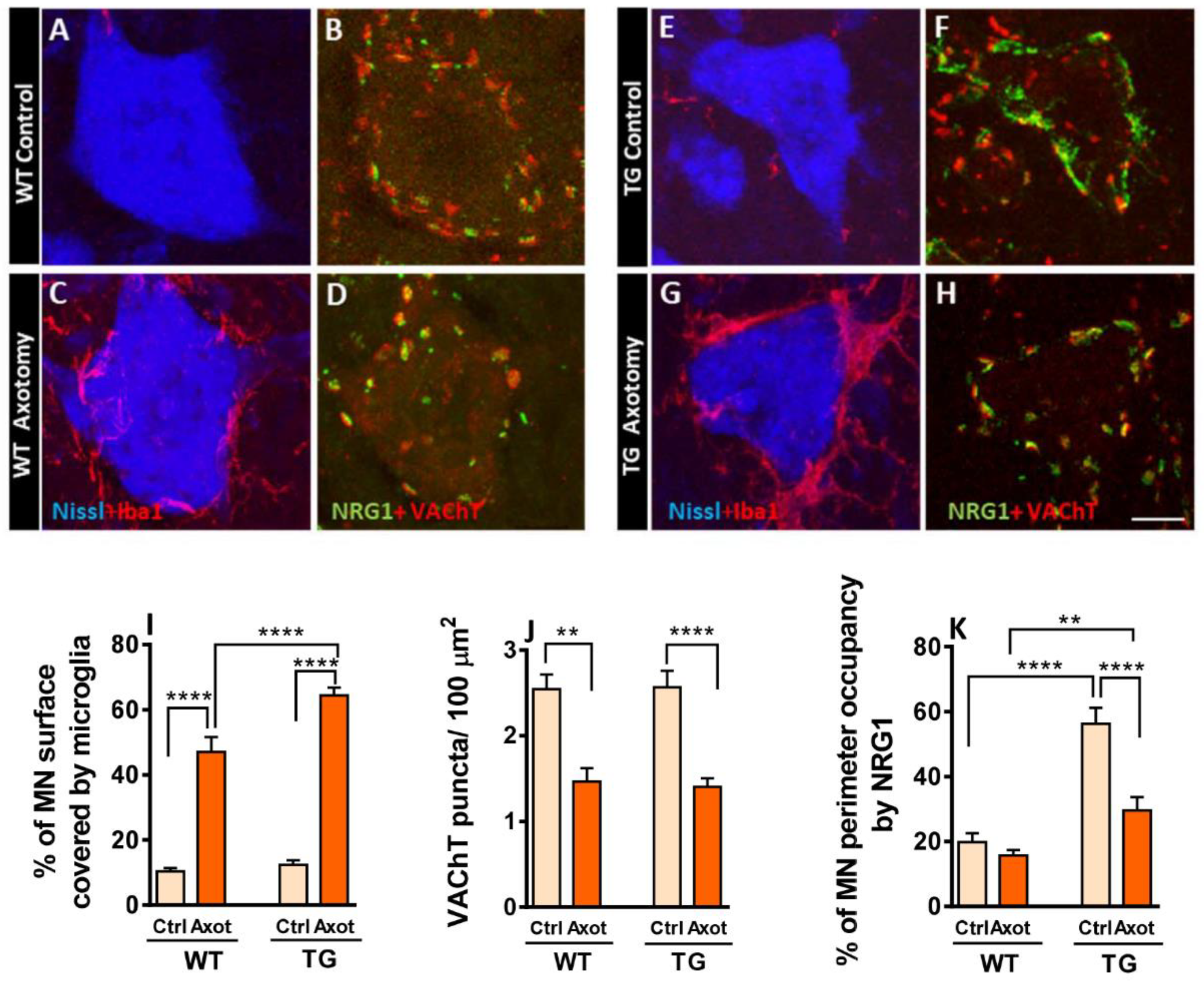
15]. Therefore, we explored how altered molecular organization of the MN surface affects microglial response post-axotomy. When we measured the MN surface covered by Iba1-labeled microglia in adult mice 7 days post-axotomy, we found an exacerbation of microgliosis in TG mice compared to WT (
Figure 5A,C,E,G,I). However, loss of cholinergic afferent boutons following axotomy (measured by MN surface area covered by VAChT-positive synaptic profiles) was similar in TG and WT mice (
Figure 5B,D,F,H,J). Next, we measured the extension of NRG1 profiles closely associated with MN surface. Axotomized MNs of WT mice showed a tendency (p value= 0.78) to a reduction in NRG1 immunolabelling compared to uninjured controls. In contrast, axotomized MNs of TG mice exhibited a significant and pronounced decrease in NRG1 expression relative to TG controls (
Figure 5B,D,F,H,K). These results demonstrate that NRG1-III overexpression has no effect on axotomy-induced C-bouton loss. However, in contrast to WT animals, axonal injury in TG mice leads to a prominent reduction of surface-associated NRG1 clusters, suggesting a higher susceptibility of TG NRG1 clusters to disruption in response to injury.
Figure 5.
Impact of NRG1-III overproduction on the MN cell body response and central glial reaction to peripheral nerve injury. In A, C, E, and G, WT and TG MNs stained with Nissl (blue) show microglia (red) contacting MN surface, highlighting an exacerbated microglial response in TG MNs at P60, 7 days post-axotomy; in B, D, F, and H, double labeling for NRG1 (green) and VAChT (red), showing the reduction in C-bouton synapses after axotomy in both WT and TG MNs and the notable decrease in peripherally associated NRG1 in the soma of axotomized TG MNs. Graphs depict measurements of the perisomatic-microglial covering of MN (I), the density of C-boutons revealing that the reduction following axotomy is not altered in TG MNs (J), and the proportion of the MN periphery exhibiting NRG1 labeling (K). Scale bar: 10 µm (A-H).
Figure 5.
Impact of NRG1-III overproduction on the MN cell body response and central glial reaction to peripheral nerve injury. In A, C, E, and G, WT and TG MNs stained with Nissl (blue) show microglia (red) contacting MN surface, highlighting an exacerbated microglial response in TG MNs at P60, 7 days post-axotomy; in B, D, F, and H, double labeling for NRG1 (green) and VAChT (red), showing the reduction in C-bouton synapses after axotomy in both WT and TG MNs and the notable decrease in peripherally associated NRG1 in the soma of axotomized TG MNs. Graphs depict measurements of the perisomatic-microglial covering of MN (I), the density of C-boutons revealing that the reduction following axotomy is not altered in TG MNs (J), and the proportion of the MN periphery exhibiting NRG1 labeling (K). Scale bar: 10 µm (A-H).
3.5. In Vitro Expression of C-Bouton-Associated Molecules in MNs from NRG1-III Overexpressing Mice
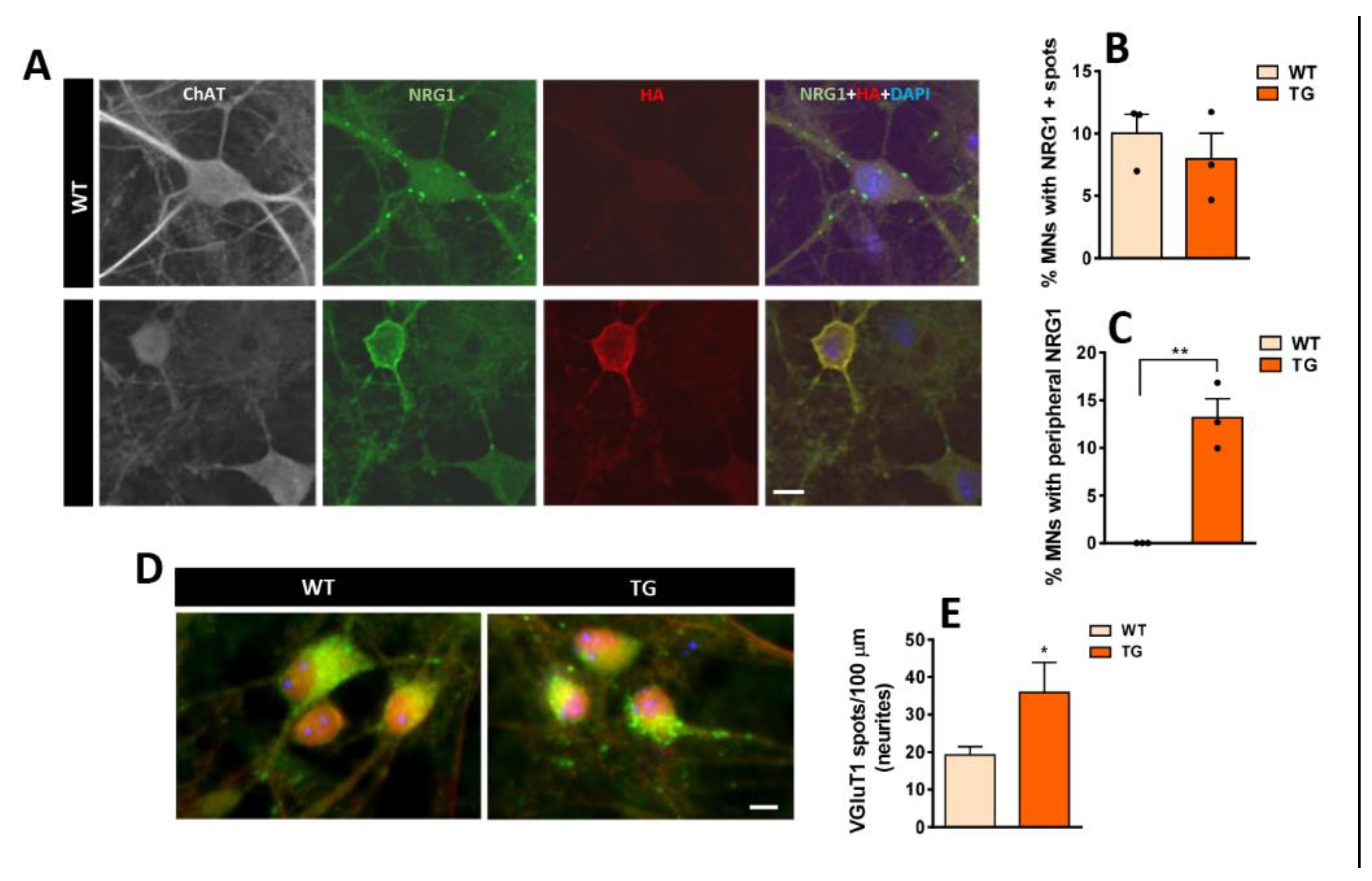
Cultures from whole-dissociated spinal cords of WT and NRG1-III TG mice were maintained from 14 to 23 DIV, during which astrocytes formed an almost continuous layer that supported the growth of various types of neurons, including MNs. MNs were identified based on their size, shape, and ChAT immunolabeling. We then explored the distribution of NRG1 immunolabeling in MNs within these cultures. NRG1 clusters were scattered across the MN surface in both WT and TG MNs, resembling the pattern observed in vivo in WT animals (
Figure 6A,B). Additionally, the soma of some TG MNs exhibited an extended peripheral accumulation of NRG1, which colocalized with HA and mirrored the pattern found in TG mice in vivo (
Figure 6A,C). Next, we explored the in vitro expression of other well-characterized C-bouton proteins, such as Kv2.1, M2 , and VAChT [
9,
11] (
Supplementary Figure S2). In TG MNs, increased peripheral Kv2.1 immunolabeling was observed alongside NRG1, although the overlap was not complete (
Supplementary Figure S2A); M2 exhibited a similar distribution pattern to NRG1, appearing as scattered spots near the MN surface in some cells and as enlarged peripheral labeling in others (
Supplementary Figure S2B). While multiple synapses on MN soma and neurites were labeled with SYN, no colocalization was observed between either NRG1 or M2 and SYN, suggesting that these proteins accumulate in MNs outside of synaptic sites (
Supplementary Figure S2B,C). Remarkably, VAChT-positive synaptic boutons were scarce in vitro. In some cases, VAChT-positive puncta were heterogeneously distributed along neurites and on the MN surface; however, in these cases, VAChT immunolabeling did not usually colocalize with NRG1 (see
Supplementary Figure S2D). These findings suggest that NRG1 clusters in vitro do not form fully differentiated C-boutons comparable to those observed in vivo.
Figure 6.
Immunolocalization of NRG1 expression along with VGluT1 to mark excitatory afferent synapses developed in cultured spinal cord MNs from WT and TG mice (19 DIV). A) MNs showing NRG1 (green), HA (red), ChAT (grey), and DAPI (blue). In WT MNs, NRG1 appears as scattered spots near the cell surface, whereas in TG MNs expressing HA, a pronounced peripheral accumulation is observed. Graphs illustrate the percentage of MNs exhibiting either a spotty pattern (B) or peripheral accumulation (C) of NRG1 distribution. D) Immunostaining for VGluT1 in MNs from WT and TG cultures, synaptic spots on neurites are quantified in E. For the percentage of MNs with NRG1 positive spots and peripheral accumulation of NRG1 n= 3 cultures; for VGluT1 density n= 23-29 neurites from 3 cultures. * p < 0.05, **p < 0.01 (Student’s t-test for genotype comparisons). Scale bars: 10 µm.
Figure 6.
Immunolocalization of NRG1 expression along with VGluT1 to mark excitatory afferent synapses developed in cultured spinal cord MNs from WT and TG mice (19 DIV). A) MNs showing NRG1 (green), HA (red), ChAT (grey), and DAPI (blue). In WT MNs, NRG1 appears as scattered spots near the cell surface, whereas in TG MNs expressing HA, a pronounced peripheral accumulation is observed. Graphs illustrate the percentage of MNs exhibiting either a spotty pattern (B) or peripheral accumulation (C) of NRG1 distribution. D) Immunostaining for VGluT1 in MNs from WT and TG cultures, synaptic spots on neurites are quantified in E. For the percentage of MNs with NRG1 positive spots and peripheral accumulation of NRG1 n= 3 cultures; for VGluT1 density n= 23-29 neurites from 3 cultures. * p < 0.05, **p < 0.01 (Student’s t-test for genotype comparisons). Scale bars: 10 µm.
3.6. In Vitro Development of Excitatory Inputs to MNs from NRG1-III-Overexpressing Mice and Changes in Glutamate Receptor-Mediated Vulnerability
Given the observed excessive number of excitatory synaptic inputs on spinal cord MNs in vivo, we sought to determine whether similar alterations would occur in vitro. We assessed the density of VGluT1-immunolabeled spots on neurites and observed higher density in TG cultures, consistent with in vivo findings (
Figure 6D,E). In contrast, the more general synaptic marker SYN did not show differences between WT and TG cultures, indicating that synapses are not globally affected by NRG1-III overexpression (data not shown). In conclusion, findings in cultured MN (in the absence of sensory neurons with NRG1-III overexpression) suggest a MN-intrinsic regulation of glutamatergic synapses.
NRG signaling has been involved in the regulation of NMDA receptors in cortical interneurons. Specifically, activation of NMDA receptors triggers the shedding of NRG2 extracellular domain, which promotes ErbB4 association with NMDA receptors. This association leads to the rapid internalization of surface receptors and potent downregulation of NMDA receptor currents, but does not affect AMPA receptor currents [
44]. We explored whether a similar interplay could also occur in spinal cord cultures overexpressing NRG1-III. We first investigated whether NRG1-III overexpression affects MN survival. Quantification of MN numbers revealed a decrease in MN survival in TG cultures (
Supplementary Figure S3 A,B), suggesting that NRG1-III overexpression has detrimental effects on MNs. Next, we assessed MN death following acute NMDA application for 30 min. We observed a trend (p value= 0,0562) towards decreased MN death in TG cultures compared to WT (
Supplementary Figure S3 A,B), suggesting changes in NMDA receptor expression in TG cultures. Thus, we examined NMDA receptor levels in WT and TG cultures by western blotting. A trend toward reduced levels of the NMDA receptor subunits GluN2A (p= 0.2), GluN2B (p= 0.3), and GluN1 (p= 0.4) were observed in TG cultures (
Supplementary Figure S3C). Moreover, immunocytochemistry against the glutamate receptor subunit GluN2B and the AMPA receptor subunit GluA2 revealed a punctate pattern in the soma of WT and TG MNs, with some MNs displaying a more robust surface labeling (
Supplementary Figure S3D). Quantification revealed lower expression of GluN2B but no change in GluA2 levels in TG cultures (
Supplementary Figure S3E,F). Taken together, our data suggest that NRG1-III overexpressing MNs are less susceptible to excitotoxicity during early stages, potentially due to reduced NMDA receptor expression, suggesting that mechanisms other than glutamate- mediated excitotoxicity are involved in the unhealthy state of MNs seen after NRG1 overexpression.Ca
2+ signaling plays a key role in regulating neuronal excitability. In MNs
3.7. Dysregulation of Calcium Homeostasis in MNs from NRG1-III Overexpressing Mice
Ca
2+ signaling plays a key role in regulating neuronal excitability. In MNs, C-boutons are important regulators of excitability by modulating K
+ current-mediated afterhyperpolarization [
45]. Furthermore, intracellular Ca
2+ dynamics at SSC influence the activity of Ca
2+-dependent K
+ channels, which are also spatially enriched at C-boutons [
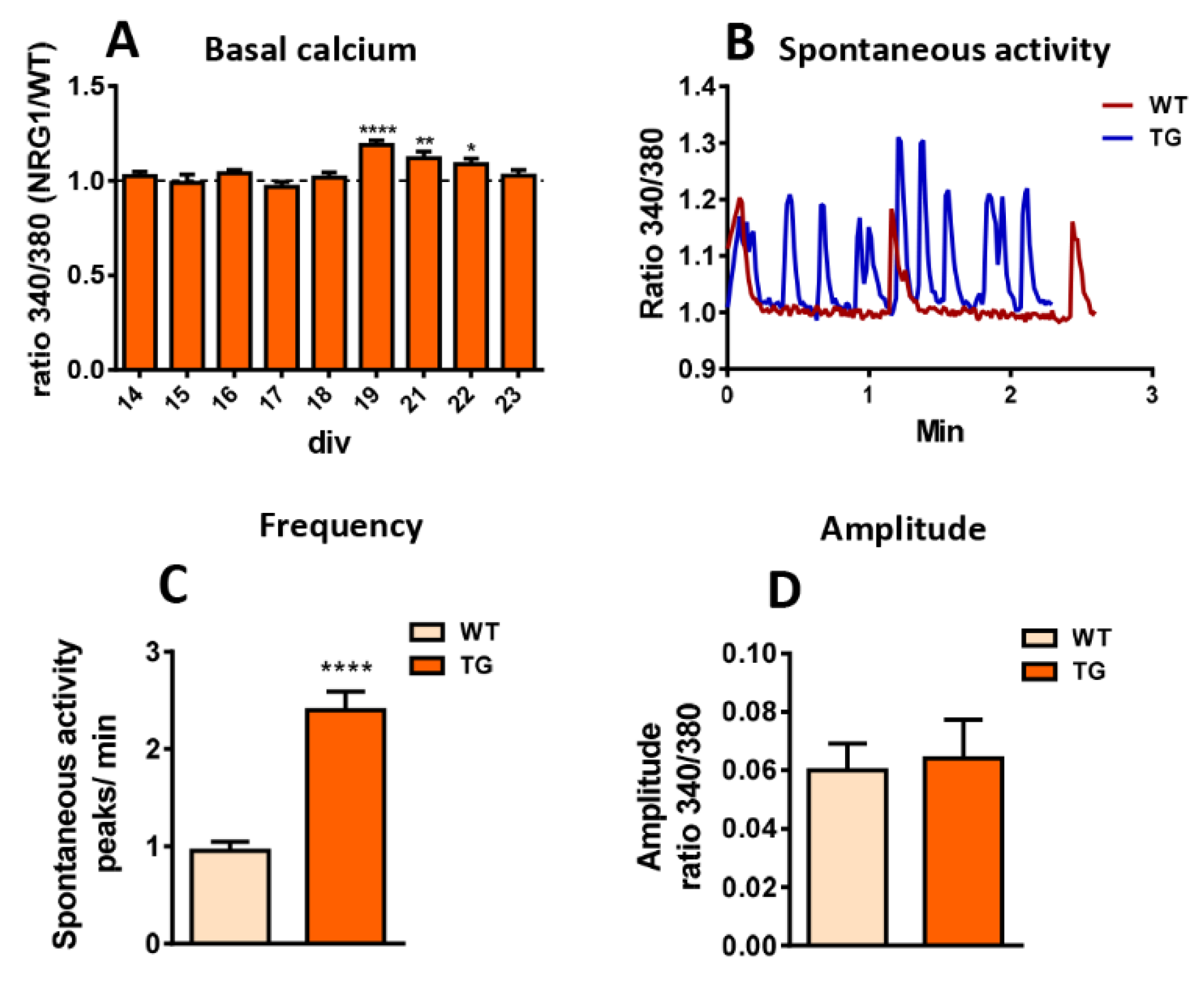
8]. While we were unable to observe full development of C-bouton-like synapses on MNs in vitro, clusters of molecules typically found at the postsynapse of C-boutons were present in these cultures and were altered by NRG1-III overexpression. Hence, we used this in vitro system to explore the impact of NRG1-III overexpression on Ca
2+ homeostasis using fura-2 imaging in cultured MNs. Measurements of basal Ca
2+ levels revealed higher levels in TG MNs from 19 to 22 DIV (
Figure 7A). Notably, we observed spontaneous MN activity in both WT and TG cultures, but TG exhibited more frequent rhythmic patterns compared to WT MNs (
Figure 7B,C). No differences in the amplitude of Ca²⁺ transients were detected between the two groups (
Figure 7B,D).
Figure 7.
Calcium homeostasis in MNs from mice overexpressing NRG1-III. A) Ca2+ recordings from cultured MNs (14- 23 days in vitro [DIV]) reveal increased basal Ca2+ levels in TG compared to WT. B) Representative profile of intracellular Ca2+ dynamics in basal conditions of WT and TG cultures illustrating spontaneous activity. C, D) Quantitative analysis of Ca2+ transient frequency (C) and amplitude (D) in cultures between 15 and 19 DIV. Sample sizes for basal Ca2+ measurements ranged from n= 21-230 MNs and for spontaneous activity frequency and amplitude from n= 41- 49 cultures, 176-235 MNs. * p < 0.05, **p < 0.01, ****p < 0.0001 (Student’s t-test for genotype comparisons).
Figure 7.
Calcium homeostasis in MNs from mice overexpressing NRG1-III. A) Ca2+ recordings from cultured MNs (14- 23 days in vitro [DIV]) reveal increased basal Ca2+ levels in TG compared to WT. B) Representative profile of intracellular Ca2+ dynamics in basal conditions of WT and TG cultures illustrating spontaneous activity. C, D) Quantitative analysis of Ca2+ transient frequency (C) and amplitude (D) in cultures between 15 and 19 DIV. Sample sizes for basal Ca2+ measurements ranged from n= 21-230 MNs and for spontaneous activity frequency and amplitude from n= 41- 49 cultures, 176-235 MNs. * p < 0.05, **p < 0.01, ****p < 0.0001 (Student’s t-test for genotype comparisons).
To investigate cytoplasmic-ER Ca
2+ signaling after ER replenishment, we stimulated cells with caffeine, a ryanodine receptor agonist and potent Ca
2+ mobilizer from ER stores [
46]. After washing, thapsigargin was applied; to selectively inhibit the ER-associated Ca
2+ pump that induces irreversible depletion of ER Ca
2+ stores [
47]. Measurements of cytoplasmic Ca
2+ signals revealed no impact of NRG1-III overexpression on ER Ca
2+ dynamics in this paradigm (
Supplementary Figure S3G). We also assessed Ca²⁺ influx into MNs following application of the glutamate receptor agonists NMDA and KA but observed no differences between WT and TG cultures (
Supplementary Figure S3G). Additionally, following KCl-induced depolarization, Ca
2+ transients remained unchanged in TG cultures (
Supplementary Figure S3G,H). In summary, the Ca
2+ imaging experiments revealed subtle alterations in Ca
2+ signaling, including slightly elevated basal Ca
2+ levels and more rapid spontaneous transients. These findings suggest that NRG1-III overexpression may induce dysregulation of Ca
2+ homeostasis, potentially contributing to changes in MN activity. Overall, our results from Ca
2+ dynamics indicate that PM instead of ER-SSC accounts for the increased spontaneous activity observed in TG MNs.
3.8. Overexpression of NRG1 in MNs Leads to Plastic Changes in NMJs
Morphological alterations in NMJs induced by NRG1-III overexpression were examined in two different hindlimb muscles (TA and SOL) from adult and old mice, thereby extending previous findings in NRG1-III TG mice obtained at postnatal and young adult ages [
20]. These muscles were chosen for their distinct fiber composition: the SOL muscle predominantly contains slow-twitch fibers, whereas the TA muscle has a higher proportion of fast-twitch fibers. We performed immunostaining using NF68 and SV2 to visualize nerve terminals and combined it with α-Bgtx to label AChRs at the postsynaptic NMJ membrane. No significant changes were observed in the total size of motor endplates in TG compared to WT muscles. However, NMJs in adult SOL muscles were smaller compared to those in adult TA muscles and the NMJs from old TA muscles were smaller than those in adult (
Figure 8A). Additionally, adult TG muscles displayed a significant increase in NMJ perimeter (
Figure 8B). Next, we measured the postsynaptic α-Bgtx-labeled area. TG TA muscles exhibited smaller areas compared to WT TA muscles in both adult and old mice, but no differences were observed in the size of SOL muscles between TG and WT (
Figure 8C). Small clusters of extrasynaptic AChRs scattered along muscle fibers, characteristic of denervated fibers, have been previously observed in muscles of old mice [
37,
48]. Consistent with the presence of denervated fibers, TA muscles of old TG mice exhibited a higher number of AChR-dispersed spots compared to WT, while no differences were found in SOL muscles between both groups (
Figure 8D). Furthermore, changes in NMJ morphology from a well-defined pretzel-like shape to a more fragmented appearance, have been reported in old muscles and NRG1-III overexpressing adult muscles [
20,
37,
49]. Consistent with these reports, we observed an increase in the number of NMJ fragments in TG muscles of adult mice, and this increase was maintained in old TA muscles, but not in old SOL muscles (
Figure 8E). The number of fragmented NMJs also significantly increased in TG adult TA muscles, while it only tendentially increased in adult TG SOL muscles (p value = 0.16) (
Figure 8F). To explore whether NRG1-III overexpression induces changes in NMJ innervation patterns, we analyzed the colocalization of postsynaptic α-Bgtx with presynaptic nerve terminal labeling. TG mice exhibited increased partial denervation in TA muscles, suggesting a greater loss of nerve terminal branches at these NMJs (
Figure 8G). In muscles from old animals, this denervation was accompanied by some NMJs displaying morphologies indicative of degeneration (
Figure 8M,O). Notably, we observed an increase in endplate degeneration of TG TA muscles. Endplate degeneration of TG SOL muscles was also tendentially higher in TG mice (p value = 0.22) (
Figure 8H). These results suggest that persistent NRG1-III overexpression in MNs triggers regressive changes at the NMJs, which may ultimately lead to their degeneration in old mice.
Figure 8.
NMJs are altered in mice overexpressing NRG1-III. Changes in neuromuscular junction (NMJ) whole size (A), perimeter (B), and postsynaptic area delimited by α-Bungarotoxin (α-Bgtx) (C) in muscles from adult and old WT and TG mice. D) Density of extrasynaptic α-Bgtx spots in soleus (SOL) and tibialis anterior (TA) muscles from aged mice. The number of NMJ fragments is shown in (E), and the percentage of fragmented NMJs in (F). The amount of partial denervated NMJs in adult and old muscles is displayed in (G) and the incidence of NMJs with degenerative morphology in WT and aged TG muscles in (H). I) Changes in terminal Schwann cell (tSC) numbers at NMJs in adult and old mice (WT and TG). J-O) Representative images of adult and aged NMJs from WT and TG mice stained for α-Bgtx (red) illustrating various structural changes such as fragmentation (K, M, O); extrasynaptic acetylcholine receptors (AchR) spots indicated by arrows (L, M) and degeneration in TG (O) evidenced by a disrupted morphology when compared with WT (N). P) An NMJ immunostained for α-Bgtx (red) and NF68 plus SV2 (blue), showing postsynaptic domains lacking presynaptic labeling (arrows), indicative of partial denervation in the TG. Q-R) NMJs labeled for S100 (gray) as a t-SC marker in conjunction with α-Bgtx (red) and DAPI (blue); arrows point to S100-positive cells at the NMJ. For quantification, simple sizes are: NMJ area and perimeter, n= 24-137 NMJs from 3-7 mice; α-Bgtx area n= 21-33 NMJs from 3 mice; extrasynaptic α-Bgtx spots, n= 24-43 NMJs from 3-4 mice; number of NMJ fragments, n= 24-45 NMJs from 3-4 mice; percentage of fragmented NMJs, n= 3-4 mice (24-45 NMJs per muscle); percentage of partial denervation, n= 3-4 mice (NMJs = 33-44 per muscle); number of tSC per NMJ, n= 14-23 NMJs from 2-3 mice (one-way or two-way ANOVA and Bonferroni‘s post hoc test, and Student’s t-test). * p < 0.05, ** p < 0.01, *** p < 0.001 and ****p < 0.0001. Scale bars: 10 µm.
Figure 8.
NMJs are altered in mice overexpressing NRG1-III. Changes in neuromuscular junction (NMJ) whole size (A), perimeter (B), and postsynaptic area delimited by α-Bungarotoxin (α-Bgtx) (C) in muscles from adult and old WT and TG mice. D) Density of extrasynaptic α-Bgtx spots in soleus (SOL) and tibialis anterior (TA) muscles from aged mice. The number of NMJ fragments is shown in (E), and the percentage of fragmented NMJs in (F). The amount of partial denervated NMJs in adult and old muscles is displayed in (G) and the incidence of NMJs with degenerative morphology in WT and aged TG muscles in (H). I) Changes in terminal Schwann cell (tSC) numbers at NMJs in adult and old mice (WT and TG). J-O) Representative images of adult and aged NMJs from WT and TG mice stained for α-Bgtx (red) illustrating various structural changes such as fragmentation (K, M, O); extrasynaptic acetylcholine receptors (AchR) spots indicated by arrows (L, M) and degeneration in TG (O) evidenced by a disrupted morphology when compared with WT (N). P) An NMJ immunostained for α-Bgtx (red) and NF68 plus SV2 (blue), showing postsynaptic domains lacking presynaptic labeling (arrows), indicative of partial denervation in the TG. Q-R) NMJs labeled for S100 (gray) as a t-SC marker in conjunction with α-Bgtx (red) and DAPI (blue); arrows point to S100-positive cells at the NMJ. For quantification, simple sizes are: NMJ area and perimeter, n= 24-137 NMJs from 3-7 mice; α-Bgtx area n= 21-33 NMJs from 3 mice; extrasynaptic α-Bgtx spots, n= 24-43 NMJs from 3-4 mice; number of NMJ fragments, n= 24-45 NMJs from 3-4 mice; percentage of fragmented NMJs, n= 3-4 mice (24-45 NMJs per muscle); percentage of partial denervation, n= 3-4 mice (NMJs = 33-44 per muscle); number of tSC per NMJ, n= 14-23 NMJs from 2-3 mice (one-way or two-way ANOVA and Bonferroni‘s post hoc test, and Student’s t-test). * p < 0.05, ** p < 0.01, *** p < 0.001 and ****p < 0.0001. Scale bars: 10 µm.
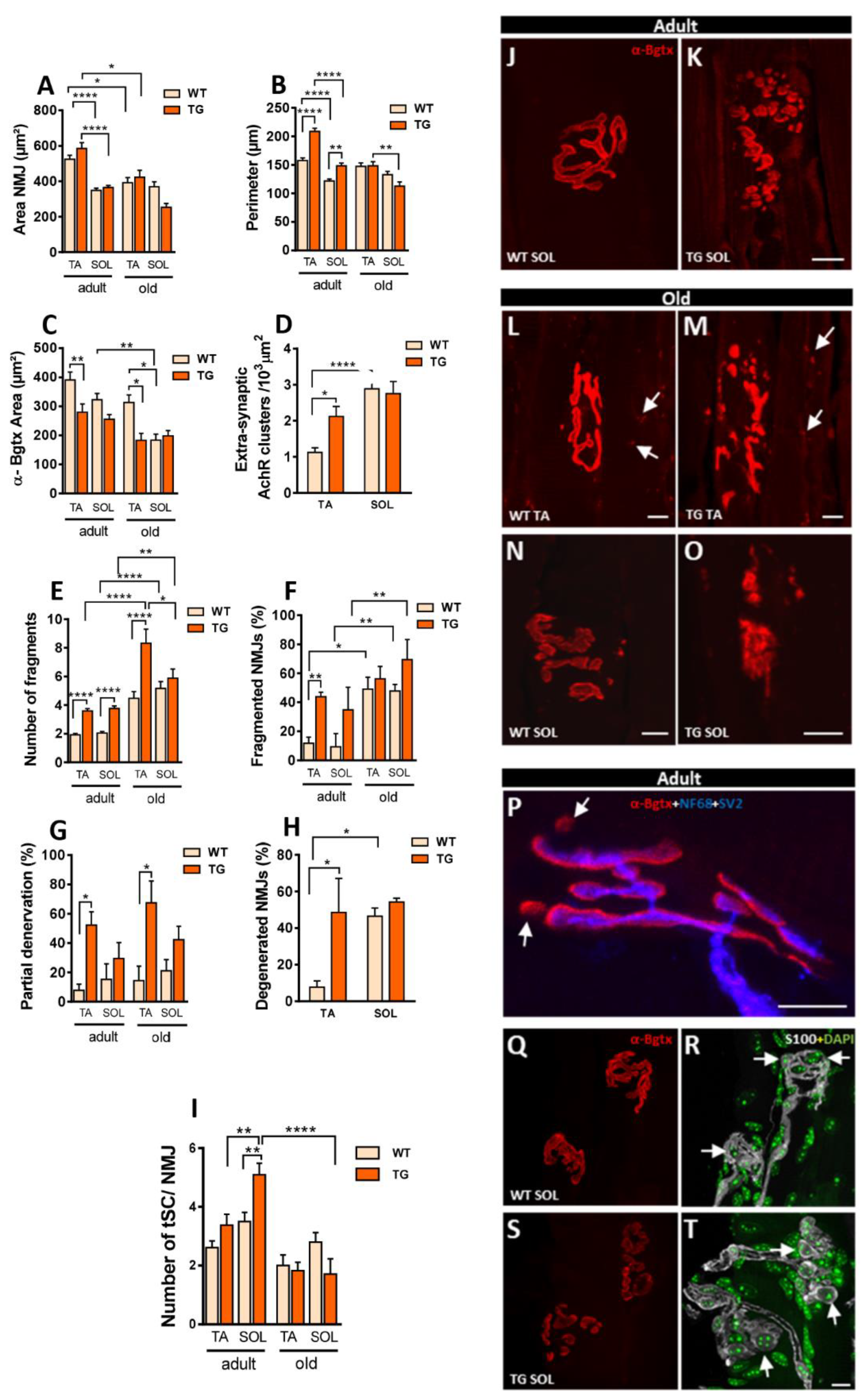
As NRG1 promotes Schwann cell proliferation, including tSCs at NMJs [
20], we explored whether NRG1-III overexpression causes persistent changes in tSCs in adult and old mice. We performed tSC immunolabeling with S-100 antibody combined with DAPI for nuclear labeling. In adult TG mice, a higher number of tSCs was observed in SOL NMJs compared to WT animals, as previously described [
20]. However, no differences were detected in old mice. These results suggest that the capacity of NRG1-III to induce tSCs proliferation is limited in old mice and associated to other major alterations at the NMJs (
Figure 8I, Q-T).
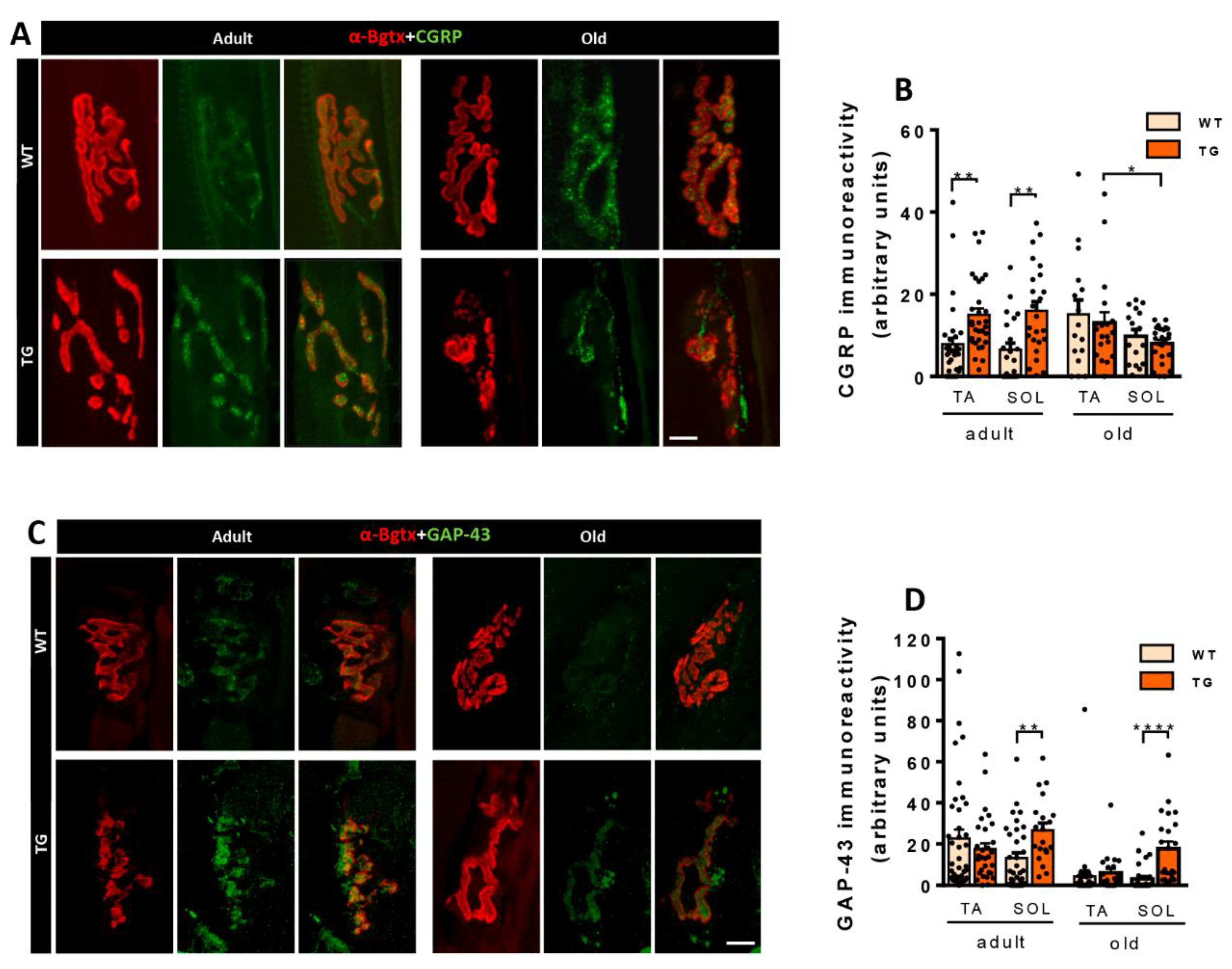
CGRP is expressed in MNs and is axonally transported to the NMJ. CGRP levels increase during NMJ development and decrease in mature NMJs. However, when axonal growth and plasticity are induced in nerve terminals, CGRP levels are again elevated [
34,
35,
36]. Consistent with the plastic changes observed in NMJs of NRG1-III overexpressing mice, adult TG mice exhibited high levels of CGRP. In contrast, NMJs from old TG mice displayed CGRP levels similar to those found in WT mice (
Figure 9A,B). In addition to CGRP, GAP-43 expression is closely linked to axonal growth and NMJ plasticity [
50]. To explore whether alterations in NMJ morphology were accompanied by changes in GAP-43 levels, we determined the expression of this protein in adult and old mice. SOL muscles of adult and old TG mice showed higher levels of GAP-43, while no differences were observed in TA muscles (
Figure 9C,D). These results suggest that axonal NRG1-III overexpression in adult SOL NMJs, likely through increased NRG1-III-tSC signaling, induces nerve terminal plasticity regulated by CGRP and GAP-43. Overall, these data demonstrate a differential reaction of slow- vs. fast-twitch muscles in TG mice.
Figure 9.
Proteins involved in the plasticity of NMJs, such as CGRP and GAP-43, are upregulated in mice overexpressing NRG1-III. A) Representative images showing CGRP immunoreactivity (green) at NMJs labeled with α-Bgtx (AChR, red) in tibialis anterior (TA) muscles of adult and aged mice (WT and TG). Note that CGRP immunoreactivity is higher in NMJs of adult TG mice compared to WT, while no difference is observed in old mice. B) Quantification of CGRP immunoreactivity at NMJs in TA and soleus (SOL) muscles of adult and old mice (WT and TG). C) Representative images of GAP-43 immunoreactivity (green) at NMJs labeled with α-Bgtx (AChR) (red) in SOL muscles of adult and aged mice (WT and TG) showing increased GAP-43 positivity in both adult and aged SOL muscles of TG mice. D) The graph depicts measurements of GAP-43 immunolabeling at NMJs in TA and SOL muscles of adult and old mice (WT and TG). Simple size for CGRP intensity analysis ranges from n= 16-33 NMJs (3-4 animals), and for GAP-43 from n= 20-42 NMJs (3-4 animals). *p < 0.05, **p < 0.01, ****p < 0.0001 (Student’s t-test for genotype comparisons). Scale bars: 10 µm.
Figure 9.
Proteins involved in the plasticity of NMJs, such as CGRP and GAP-43, are upregulated in mice overexpressing NRG1-III. A) Representative images showing CGRP immunoreactivity (green) at NMJs labeled with α-Bgtx (AChR, red) in tibialis anterior (TA) muscles of adult and aged mice (WT and TG). Note that CGRP immunoreactivity is higher in NMJs of adult TG mice compared to WT, while no difference is observed in old mice. B) Quantification of CGRP immunoreactivity at NMJs in TA and soleus (SOL) muscles of adult and old mice (WT and TG). C) Representative images of GAP-43 immunoreactivity (green) at NMJs labeled with α-Bgtx (AChR) (red) in SOL muscles of adult and aged mice (WT and TG) showing increased GAP-43 positivity in both adult and aged SOL muscles of TG mice. D) The graph depicts measurements of GAP-43 immunolabeling at NMJs in TA and SOL muscles of adult and old mice (WT and TG). Simple size for CGRP intensity analysis ranges from n= 16-33 NMJs (3-4 animals), and for GAP-43 from n= 20-42 NMJs (3-4 animals). *p < 0.05, **p < 0.01, ****p < 0.0001 (Student’s t-test for genotype comparisons). Scale bars: 10 µm.
Ultrastructural examination of old TG NMJs revealed significant alterations consistent with the degenerative changes observed in confocal images. While some NMJs retained normally appearing nerve terminals (
Figure 10A,C), others showed an accumulation of multilamellar and electron-dense structures, most likely representing endosomal/autophagic/lysosome-like vacuolar elements and multivesicular bodies (
Figure 10B,D). These features indicate ongoing degradation of nerve terminals involving enhanced autophagic activity. Additionally, in some areas of the muscle surface, we identified remnants of former NMJs, characterized by remaining postsynaptic folds in the muscle membrane covered by tSCs, but unmatched by presynaptic elements. These findings suggest nerve terminal retraction and muscle denervation (
Figure 10E). The increase in tSC processes reported during TG NMJ development [
20] seems to be a transient phenomenon, as this was not detected at more advanced ages.
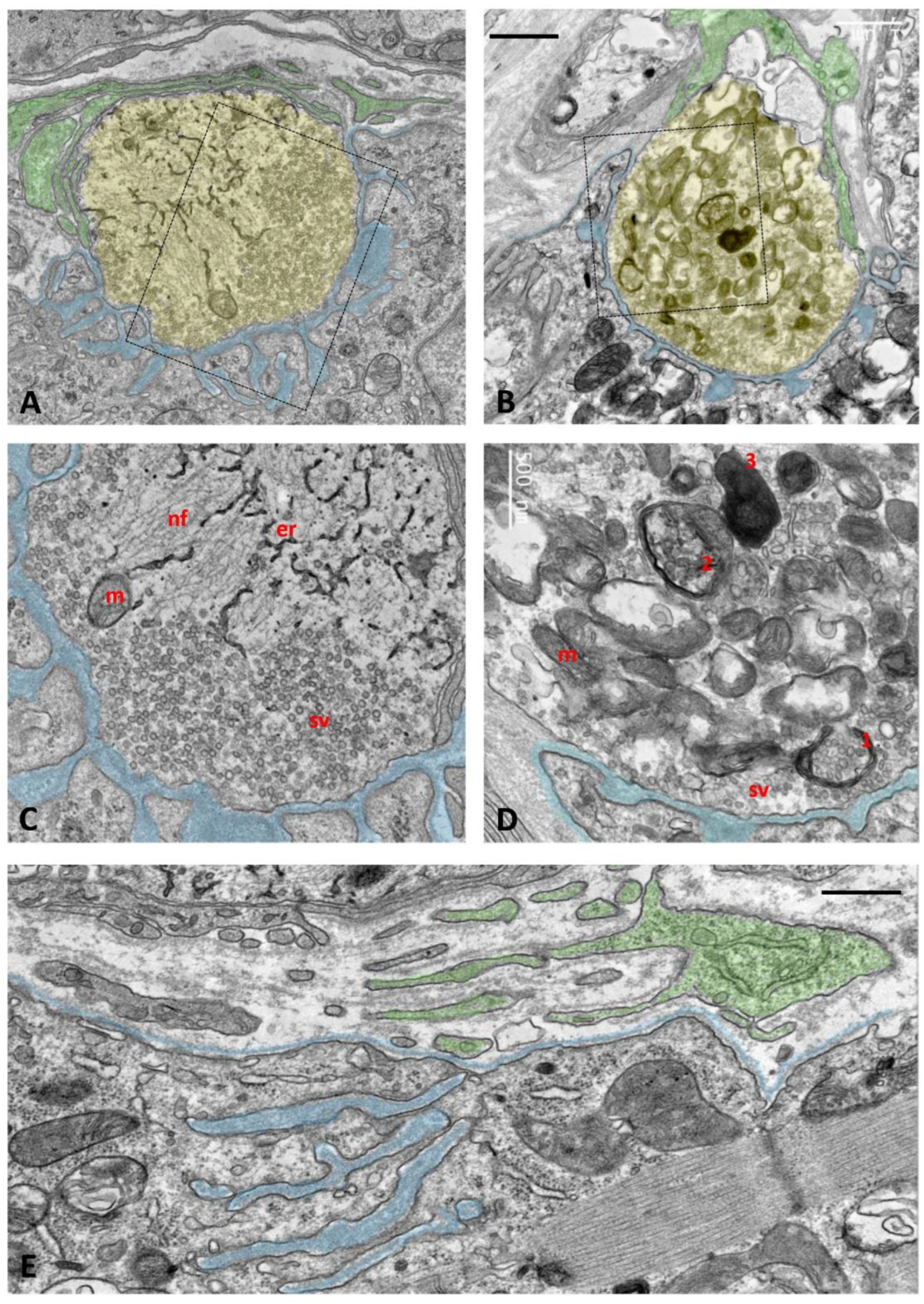
Figure 10.
Ultrastructural analysis of NMJs of aged TG mice. Some nerve terminals appear morphologically normal, while others exhibit various degrees of degeneration. A-D) Representative images of a normal and a degenerating nerve terminal, taken from the SOL and TA muscles of aged mice (P533), are shown in panels A and B, respectively. Presynaptic terminals are shaded in yellow, postsynaptic membrane foldings are indicated in blue, and tSC profiles capping the nerve terminals are shaded in green. Enlarged views of the indicated regions are presented in panels C and D; note the clustered synaptic vesicles (sv) near the presynaptic membrane, mitochondria (m), endoplasmic reticulum (er), and neurofilament bundles (nf). In panel D, the degenerating nerve terminal displays an accumulation of autophagosomal-lysosomal-like structures at presumptive different stages of formation: (1) phagophore-like membranes engulfing clusters of synaptic vesicles, (2) autophagosomes containing synaptic vesicle remnants, and (3) electron-dense lysosomes. Several vacuolated mitochondria (vm) are observed adjacent to morphologically normal mitochondria (m). E) A fully denervated NMJ is also shown: note the muscle fiber basal lamina and subneural folds (blue) lacking a facing presynaptic terminal; the whole structure is partially covered by tSC profiles (green). Scale bars: 1 µm in (A, B); 500nm in (C-E).
Figure 10.
Ultrastructural analysis of NMJs of aged TG mice. Some nerve terminals appear morphologically normal, while others exhibit various degrees of degeneration. A-D) Representative images of a normal and a degenerating nerve terminal, taken from the SOL and TA muscles of aged mice (P533), are shown in panels A and B, respectively. Presynaptic terminals are shaded in yellow, postsynaptic membrane foldings are indicated in blue, and tSC profiles capping the nerve terminals are shaded in green. Enlarged views of the indicated regions are presented in panels C and D; note the clustered synaptic vesicles (sv) near the presynaptic membrane, mitochondria (m), endoplasmic reticulum (er), and neurofilament bundles (nf). In panel D, the degenerating nerve terminal displays an accumulation of autophagosomal-lysosomal-like structures at presumptive different stages of formation: (1) phagophore-like membranes engulfing clusters of synaptic vesicles, (2) autophagosomes containing synaptic vesicle remnants, and (3) electron-dense lysosomes. Several vacuolated mitochondria (vm) are observed adjacent to morphologically normal mitochondria (m). E) A fully denervated NMJ is also shown: note the muscle fiber basal lamina and subneural folds (blue) lacking a facing presynaptic terminal; the whole structure is partially covered by tSC profiles (green). Scale bars: 1 µm in (A, B); 500nm in (C-E).