1. Introduction
In primary care practice, the growing number of patients with dementia syndromes presents an increasing challenge. According to WHO data, by 2030, the population of patients with dementia is expected to rise by approximately 50%, and by 2050, it could increase by as much as 250%. Dementia syndromes also pose an economic problem, contributing annually to global losses at the level of two trillion US dollars [
1]. Unfortunately, existing studies indicate that family doctors often overlook the assessment of cognitive function in their older patients. When cognitive function is assessed, the accuracy of diagnosing dementia is lower than 60%, and even lower for MCI [
2].
Mild cognitive impairment is a heterogeneous syndrome characterized by impairment in usually one cognitive domain. Researchers estimate that it affects around 10-30% of patients over the age of 60, with 10-20% progressing to Alzheimer's disease, frontotemporal dementia, or dementia with Lewy bodies [
3]. Patients commonly complain of forgetfulness regarding appointments, frequent misplacement of objects, problems with spatial orientation, or finding the right words; however, these are subjective complaints that do not impact daily behavior or independence. MCI can be diagnosed using objective methods, such as standardized tests – MMSE (Mini-Mental State Examination), ACE-III (Addenbrooke's Cognitive Examination-III), or GPCOG (The General Practitioner Assessment of Cognition) when the test result is lower by 1.5 standard deviations from the expected value, and the criteria for neurocognitive disorders are not met [
4].
According to neurologists and neuropsychologists, specialized diagnostic testing for neurocognitive disorders should be performed at the end of the diagnostic process, after excluding causes such as poisoning, alcohol or benzodiazepine abuse, delirium syndromes, mood disorders, or exacerbation of chronic disease. This is a significant challenge for primary care physicians due to limited consultation time and the lack of many diagnostic tools [
5]. Screening for dementia would be facilitated by an easily accessible biomarker or predictor, which numerous research teams have been searching for over the years.
The study may serve as an important guideline in planning preventive and proactive measures. The conclusions from the study may help family doctors select the high-risk group for neurocognitive disorders and accelerate further diagnostics.
2. Materials and Methods
The research was conducted by Dr. Agnieszka Gostyńska as part of her doctoral dissertation preparation. The study group (n=137) consisted of patients from the Family Medicine Clinic at the Department of Family Medicine, Karol Marcinkowski Medical University in Poznań. The project received approval from the Bioethics Committee at the Medical University of Poznań, resolution number 1061/16.
The study included men and women aged 50-76 years. Exclusion criteria included a history of: unstable coronary artery disease, heart failure with an ejection fraction lower than 40%, cardiomyopathy, arrhythmias (not effectively treated with a pacemaker), heart defects, secondary hypertension, alcohol, drug or medication dependence, and stage 3 hypertension according to ESH guidelines. Other exclusion criteria included the presence of malignant tumors, hematological diseases, cirrhosis, kidney failure, neurological diseases, severe mental disorders, previously diagnosed dementia, and visual or auditory impairments preventing participation in the psychological assessments.
The study was divided into three stages. The first stage involved conducting an initial interview, obtaining written consent to participate, and gathering information regarding past illnesses, risk factors, and demographic and socioeconomic data. With patient consent, data from their medical records were collected, including current laboratory tests such as lipid profile and fasting glucose levels, and BMI was calculated based on weight and height. The second stage involved administering psychological tests: MMSE and ACE-III. The third stage consisted of measuring ABI and PWV (subclinical organ damage parameters) using a BOSSO device.
The dependent variables included the following groups of variables: results of psychological tests (
Table 1) and data from the interview regarding risk factors for cardiovascular disease (CVD) and neurocognitive disorders (NCD). These data included: past or current chronic diseases, family history of CVD, family history of NCD, smoking, alcohol abuse, socioeconomic factors, history of CVD episodes, and medications taken. It is important to emphasize that risk factors for both cardiovascular diseases and cognitive disorders are largely shared.
The independent variables included data from the BOSSO device: systolic and diastolic blood pressure, ABI, and PWV. Additionally, medical records were used, including weight, height, and BMI calculated from these values, fasting glucose levels, and lipid profile (total cholesterol, triglycerides, HDL, LDL).
Sociodemographic variables were treated as secondary variables and included: sex, age, education level, possession of a high school diploma, years of education, type of employment (manual or intellectual), and current and past occupational activity.
The study included 137 participants, comprising 73 women and 64 men, aged 50-76 years (M = 63.46 years, SD = 5.532). Nearly all participants (135 individuals) reported that they were currently not professionally active. Half of the participants had previously worked in manual labor (69 individuals), while the other half had worked in intellectual professions (68 individuals). Twenty-one participants had primary education, 37 had vocational education, 33 had secondary education, and 46 had higher education.
The study group was characterized using standard statistical descriptive methods: mean, standard deviation, percentage, minimum and maximum values, and their range. Statistical analysis was performed using SPSS 24 software. The variables were processed using the Mann-Whitney U test for non-parametric variables, Dunnett's T3 test for variance analysis, and regression analysis, and the strength of associations between variables were described using Pearson's correlation coefficient (r).
3. Results
Based on the formulated questionnaire, the socioeconomic conditions of the study group were assessed. The questions addressed both well-known risk factors for the development of dementia (such as depression, past trauma, bereavement, or loneliness) and the cognitive abilities of the participants. Only 27 participants reported living alone, the same number reported the loss of a close friend in the past year, and 20 individuals reported a lack of a close person. The vast majority (94.89%) stated that they did not have serious problems in their relationship with their spouse; three-quarters of the participants reported being able to share their emotions with loved ones; and 41.61% indicated that they had been exposed to traumatic events in the past. Among the participants, 25.55% reported a sense of hopelessness, 13.14% derived no pleasure from life, 16.79% experienced anxiety and distress, and 18.98% excessively worried. Dysphoric symptoms were slightly more frequent among the respondents than feelings of anxiety or depression. Almost 30% reported frequent anger outbursts over trivial matters, and 35% expressed excessive irritation due to the habits of others. 57.66% of participants were satisfied with their income, but only 17.52% assessed their economic status as poor. Only half of the participants engaged in physical activity at least once a week. 86.13% of respondents reported coping well with work demands. Additionally, 62.04% of participants used a home computer, which could be considered evidence of maintaining cognitive function or as a neuroprotective factor.
In the study group, 21.90% had experienced or were currently suffering from any mental disorder, 5.84% from any neurological disease, and 10.22% had a positive family history of dementia. Among the medications used in psychiatry and neurology, the largest group of respondents reported using sedative and hypnotic drugs (26.28%), followed by pro-cognitive medicines (16.06%) and antidepressants (13.87%). A smaller group reported using neuroleptics (10.95%) and anticonvulsants (5.84%).
Using the BOSSO device, subclinical organ damage parameters were assessed: the ankle-brachial index and pulse wave velocity. The mean ABI in the study group was M = 1.064 (SD = 0.138), and the mean PWV was M = 9.320 m/s (SD = 1.908) (
Figure 1a,b).
The cognitive abilities of the participants were assessed using standardized psychological tests. The average score on the MMSE was M=28.073 (SD=5.11), indicating that most of the participants (n=103) did not show cognitive impairments (
Table 2). A score above 26 points on the MMSE indicates no cognitive impairment, a score between 24-26 points suggests mild cognitive impairment and a score below 24 points suggests probable dementia.
The average score on the ACE-III was M=86.037 (SD=11.04), while the score on the abbreviated version, M-ACE, was M=24.199 (SD=4.788). The ACE-III test is more comprehensive than the MMSE, providing information on the entire profile of individual cognitive functions (
Table 3) and demonstrating greater sensitivity in detecting dementia [
6]. The maximum score on the test is 100; a score below 88 points suggests cognitive impairment with very high sensitivity, while a score below 82 points indicates cognitive impairment with very high specificity [
7].
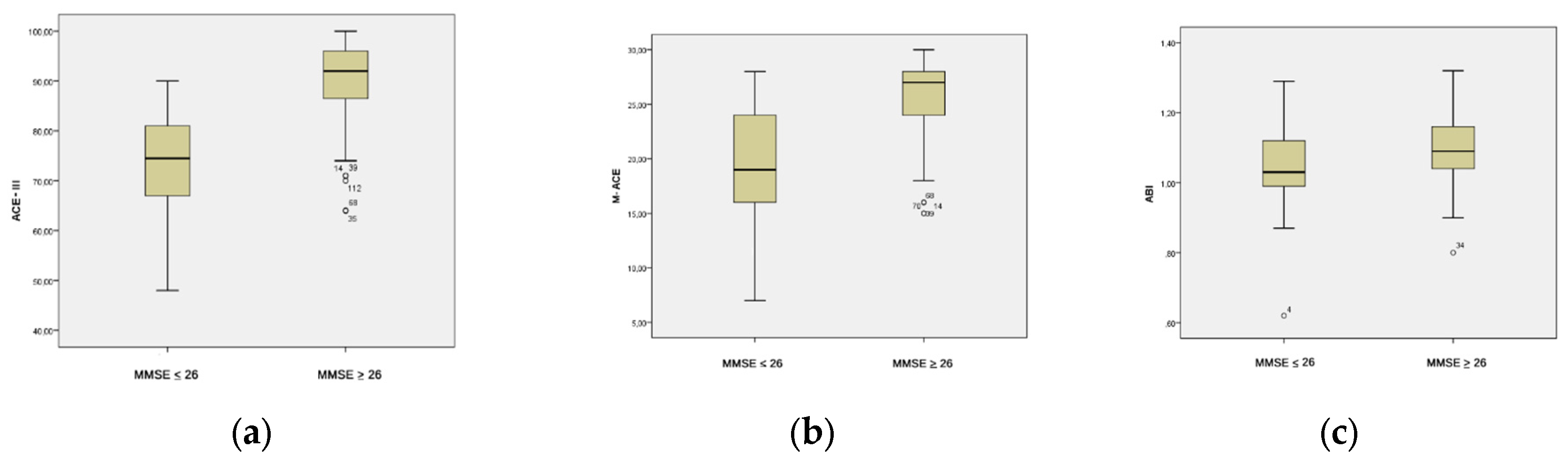
The Mann-Whitney U test for non-parametric variables revealed statistically significant differences between the group with cognitive impairments (MMSE ≤ 26) and the group without cognitive impairments (MMSE > 26) in terms of ABI values (U = 1037.000; p = 0.005) and results in psychological tests ACE-III (U = 285.000; p = 25.882) and M-ACE (U = 579.000; p = 34.529). Participants with cognitive impairments identified in the MMSE test achieved lower scores in the ACE-III (
Figure 2b) and M-ACE (
Figure 2c) tests, as well as lower ABI values (
Figure 2a) (
Table 4).
The Pearson correlation coefficient (r) was calculated between the ACE-III (and M-ACE) test scores and subclinical organ damage measured by the ABI index, which was found to be statistically significant. Lower ACE-III and M-ACE scores were associated with lower ABI values (
Table 5).
The relationship between cognitive functioning, assessed using the MMSE (
Table 6) and ACE-III (
Table 7) tests, and socioeconomic factors such as computer usage, physical activity, traumatic experiences, and feelings of hopelessness were examined using the Mann-Whitney U test. Individuals who achieved better results on the psychological tests reported that they regularly use a computer, engage in physical activity, and do not experience feelings of hopelessness. Interestingly, respondents with higher psychological test scores affirmed having experienced a traumatic event.
Factors such as receiving compensation proportional to effort, good work performance, not experiencing the loss of a close friend in the past year, and avoiding sharing personal thoughts with others were statistically significantly and positively correlated with high MMSE scores but did not affect ACE-III results. On the other hand, frequent irritation due to the habits of others showed a statistically significant positive correlation with high ACE-III scores but did not influence MMSE results.
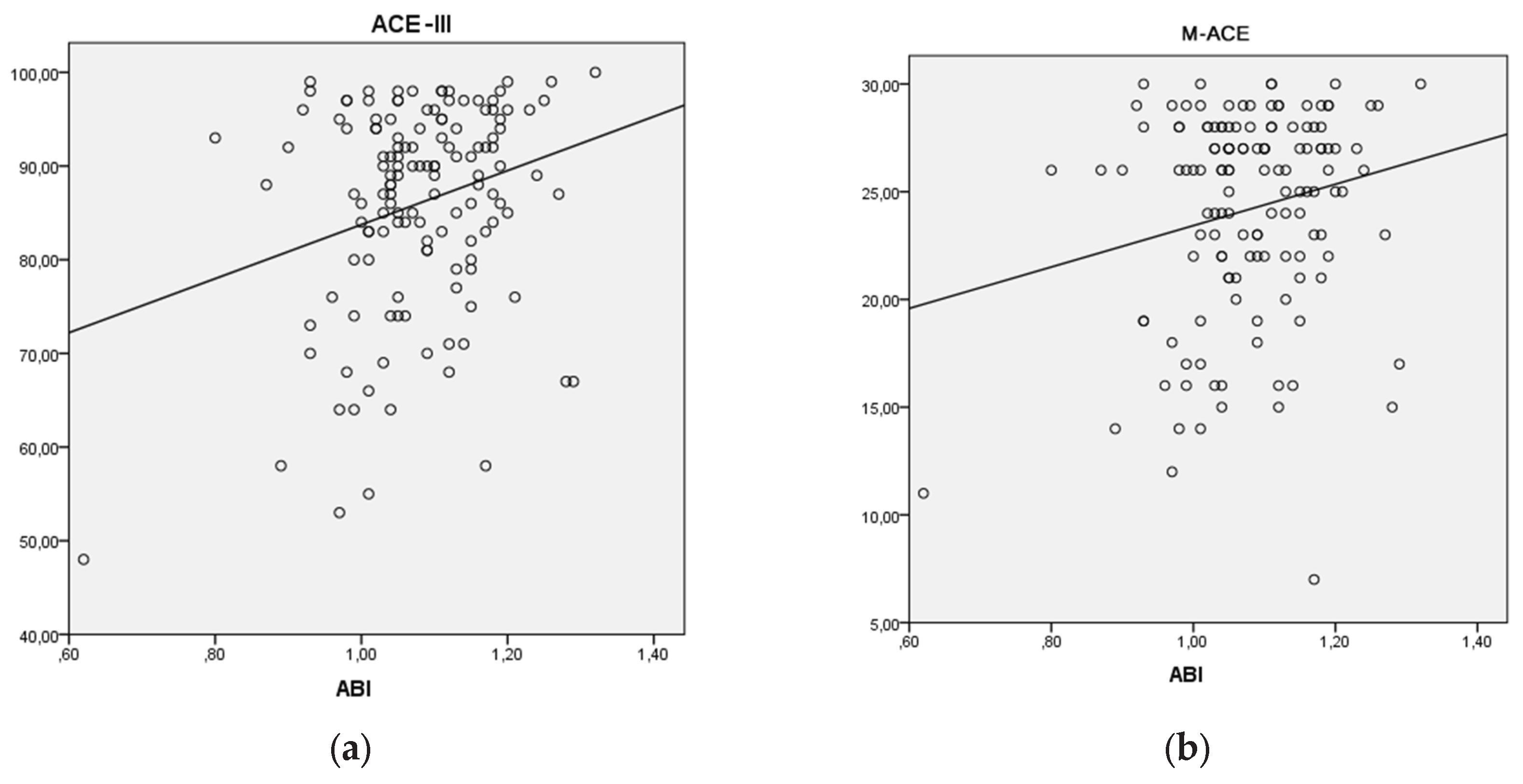
To predict the occurrence of cognitive impairment based on the ABI predictor, a univariate regression analysis was conducted. Linear models were generated for the relationship between the ACE-III score and ABI (
Figure 3a), as well as for the relationship between the M-ACE score and ABI (
Figure 3b).
The analyzed linear model (
Figure 3a) was found to be a good fit for the data (F(1,135) = 9.668; p < 0.05). Based on the ABI index value, 6.7% of the variance in the dependent variable can be predicted. The relationship is weak and positive (beta = 0.259). This means that a decrease of 0.1 in the ABI value corresponds to a decrease of 2.88 points in the ACE-III score (B = 28.842).
The analyzed linear model (
Figure 3b) was found to be a good fit for the data (F(1,135) = 5.531; p < 0.05). Based on the ABI index value, 3.9% of the variance in the dependent variable can be predicted. The relationship is weak and positive (beta = 0.198). This means that a decrease of 0.1 in the ABI index corresponds to a decrease of 0.96 points in the M-ACE score (B = 9.601).
4. Discussion
The purpose of the study was to assess the relationship between the occurrence of mild cognitive impairment (MCI) and the coexistence of subclinical organ damage, measured by ABI and PWV.
ABI is a tool widely used in vascular surgery as a non-invasive method for assessing the degree of chronic ischemia in the lower limbs. It represents the ratio of the pressure at the ankle to the higher of the two brachial artery pressures. The calculated index value should fall within the range of 0.9–1.15. A lower value suggests narrowing of the lower limb arteries and a risk of ischemia, while a higher value indicates excessive vessel stiffness [
8]. Many research teams have examined the usefulness of ABI in monitoring chronic diseases other than chronic limb ischemia, such as coronary artery disease [
9], diabetes [
10], chronic kidney disease [
11], and stroke [
12].
The utility of ABI in the context of cognitive disorders remains a topic requiring further exploration. In the APAC (Asymptomatic Polyvascular Abnormalities Community Study) involving over 3,000 individuals, a correlation was found between impaired cognitive performance and a low ABI score [
13]. Additionally, in the EPIDEMICA study, which involved a population of 1,662 individuals over 65 years of age in Central Africa, the prevalence of cognitive disorders was 13.6%. This frequency was higher among individuals with ABI ≤ 0.90 and ABI ≥ 1.40 compared to those with ABI values within the range of 0.90 < ABI < 1.40 (20.1% and 17% vs 12%, P = 0.0024) [
14]. This study is particularly significant because approximately two-thirds of individuals with cognitive disorders live in developing countries, where access to advanced diagnostic tools is limited. Thus, ABI as an inexpensive and simple diagnostic tool, may serve as a valuable and effective solution for assessing the risk of cognitive disorders in this population.
In 2021, a study similar to the present one was conducted in Shanghai, where a research group with neurocognitive disorder symptoms (n=217) and a control group without such symptoms (n=259) were recruited. Regression analysis showed that low ABI was an effective predictor of cognitive decline (p < 0.05). Pearson’s correlation analysis revealed that a low ABI (< 0.9) had a significant impact on memory and spatial-visual functions within the cognitive domain (p < 0.05) [
15]. In a systematic review by M. Guerchet et al. (2011), 12 publications were analyzed, 6 of which conducted cross-sectional analyses, and 6 performed longitudinal analyses. All but one of the studies found a significant association between low ankle-brachial index and cognitive impairment, dementia, or Alzheimer's disease [
16].
In this study, a correlation was found between cognitive performance measured by the ACE-III test and subclinical organ damage measured by ABI (
Figure 3a). Regression analysis showed that ABI values could predict the occurrence of cognitive disorders measured by ACE-III and M-ACE (
Figure 3a,b). Therefore, it can be inferred that a low ABI value could serve as a predictor for the onset of cognitive disorders. The mean ACE-III score in the study group was M=86.03; SD=11.041 (
Table 3).
The second marker of subclinical organ damage analyzed in the study was pulse wave velocity (PWV). The value of PWV depends on the stiffness, elasticity, and compliance of the arterial system [
17]. In healthy vessels, which are elastic, the pulse wave propagates relatively slowly. As vessels become stiffer, the pulse wave propagates faster. High PWV thus suggests a higher risk for conditions such as hypertension, stroke, myocardial infarction, and presumably also dementia. In the Sydney Memory and Ageing Study, after applying Bonferroni correction, no significant relationship between PWV and cognitive functions was found [
18]. However, a separate analysis of this relationship in male and female groups showed that higher PWV values in men were associated with lower levels of overall cognitive function and memory. Nevertheless, no significant differences were observed in the relationship between PWV and cognitive functions between men and women. In the study by E. Nilsson et al. (2017), PWV was measured in 3056 participants, followed by screening tests for neurocognitive performance. Patients who scored low were further diagnosed with dementia. A total of 159 cases of dementia were identified, including 57 chronic cases and 102 new cases. Logistic regression analysis showed that PWV was not associated with chronic dementia of any etiology [
19].
In the present study, no association between PWV and lower cognitive performance was found; the relationship was not statistically significant in either the Mann-Whitney U test, Pearson correlation test, or regression analysis. This appears to be consistent with the results of studies conducted by other research teams.
Dean and Ayesha Sherzai developed their proprietary NEUROplan (Nutrition, Exercise, Unwind, Restore, Optimize), aimed at reducing modifiable risk factors for dementia [
20]. Expanding on the acronym, the authors recommend following a Mediterranean or DASH diet, ensuring physical activity, training concentration and memory, introducing stress management techniques, promoting body regeneration, and optimizing cognitive reserve. The development, implementation, application, and evaluation of an individual plan for changing habits poses a significant challenge for both the patient and their primary care physician. However, the authors provide low-cost and appealing sets of cognitive and physical exercises, along with ready-to-use meal plans. The potential benefits of such preventive measures not only improve the health of the primary care physician's patient population but also minimize the costs incurred by the state in the fight against dementia.
The study demonstrated a relationship between cognitive functioning, assessed both through the MMSE and ACE-III tests and physical activity participation. The socioeconomic interview conducted during the experiment included a question regarding whether the respondent engages in physical activity at least once a week for at least 30 minutes. Unfortunately, more than half of the participants (52.6%) answered negatively, even though 30 minutes of activity per week is considered significantly insufficient compared to the World Health Organization (WHO) recommendations, which suggest a minimum of 150 minutes of physical activity per week for older adults.
There is an increasing body of evidence that aerobic exercise improves cognitive functions [
21]. In studies conducted by DeFina et al., involving nearly 20,000 patients from a preventive medicine clinic in Texas, it was shown that higher levels of physical activity in middle age reduce the risk of developing dementia later in life [
22]. This relationship was observed both in individuals with and without a prior stroke, providing evidence that physical activity protects against dementia regardless of its cause, not just vascular dementia. The analysis by F. Sofi et al. (2010), which included 15 prospective studies, demonstrated the neuroprotective role of physical activity, regardless of its intensity. The study sample consisted of 33,816 individuals without a dementia diagnosis, monitored throughout 1 to 12 years [
23]. During the observation period, a decline in cognitive functions was observed in 3,210 participants. Statistical analysis, using a random effects model, showed that those engaging in intense physical activity were significantly protected against cognitive decline (-38%). Additionally, analysis of low and moderate levels of physical activity also revealed substantial protection against cognitive impairment (-35%). Healthcare professionals should encourage their patients to incorporate physical activity into their daily routine, not only due to its obvious benefits for cardiovascular health but also for its neuroprotective effects.
One of the most significant limitations of the study was the specific characteristics of the study group, which consisted of patients from a pre-clinical primary healthcare clinic. Due to the exploratory nature of the study, no control group was included, which limits the ability to compare the results with a healthy population. Each participant reported suffering from at least one chronic disease, which, on the one hand, introduces additional variables into the analysis, but on the other hand, is justified by the criteria for participant selection: the participants were patients who regularly visited their general practitioners. This group showed some homogeneity in terms of the types of past or existing illnesses, which can be attributed to the high prevalence of certain chronic diseases, such as hypertension or rheumatic diseases, among patients in such clinics.
Moreover, over 90% of the participants were regularly taking medications, yet the study did not account for either the potential side effects of these medications or their long-term impact on cognitive function. This is particularly relevant for medications such as benzodiazepines and statins, which are thought to have potential anti-cognitive effects. The study also did not consider the possible pro-cognitive and neuroprotective properties of nicotine, which represents another significant variable that could have influenced the study results.
Risk factors for various chronic diseases are often identical or tend to co-occur, making individuals suffering from one chronic condition particularly vulnerable to the development of other diseases. From a psychological and motivational perspective, the study participants were specific. First, participation in the experiment required participants to set aside free time, meaning that those with more free time were more likely to volunteer, including individuals not currently employed (72.3% of the participants). Second, some of the participants expressed concerns about their cognitive function, noticing subtle changes in their memory or having family histories of mental disorders, which might have influenced their motivation to participate in the study.
Data regarding the occurrence of neurodegenerative diseases in the participants' families were obtained directly from the participants themselves. However, it should be noted that many of them lacked precise knowledge about the health of their parents or grandparents. It is important to emphasize that the participants' parents and grandparents lived in the early 20th century when knowledge about dementia and cognitive disorders was limited. Furthermore, a significant portion of the participants' relatives died prematurely due to World War II, infectious diseases, or famine, without exhibiting cognitive disorders typical of old age.
Information on the participants' socioeconomic conditions was obtained through interviews. Since all participants were aware that they were taking part in a study on risk factors for neurodegenerative diseases, there was a possibility of manipulating their responses to present themselves in a more favorable light. It is also worth noting that the study did not use standardized psychological questionnaires to assess depressive or dysphoric symptoms. Therefore, the actual mental state of the participants may have been difficult to assess objectively.
5. Conclusions
The assessment of subclinical organ damage using the ABI allows for the prediction of the occurrence of neurocognitive disorders. Patients who obtained lower scores in psychological cognitive function tests also had subclinical vascular organ damage.
Participants who engaged in regular physical activity achieved better results in cognitive function tests.
Participants who used computers more frequently, experienced fewer depressive symptoms, and had a past trauma experience, had a lower risk of developing neurocognitive disorders.
Screening for subclinical organ damage in the population of family medicine patients can be a useful tool for the early diagnosis and prevention of neurocognitive disorders.
Author Contributions
Initial conception of the study: Anna Posadzy-Małaczyńska, Agnieszka Gostyńska; Acquisition of data: Agnieszka Gostyńska, Agata Puszcz, Nadia Kruszyńska, Marzena Bielas, Lucyna Woźnicka-Leśkiewicz; Analysis and interpretation of data: Agnieszka Gostyńska; Writing and revising the paper: Agnieszka Gostyńska, Agata Puszcz; Critical review of the paper: Nadia Kruszyńska, Anna Posadzy-Małaczyńska. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Funding comes from the resources of the Department of Family Medicine.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Poznań University of Medical Sciences (protocol code: 1061/16).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Acknowledgments
The authors would like to express their gratitude to all the participants who took part in this study. The appreciation also goes to the faculty and staff in the Department of Family Medicine, Poznań University of Medical Sciences, whose resources and assistance have been invaluable. The authors also extend their appreciation to our supervisor, Anna Posadzy-Małańczyńska, for her guidance, support, and mentorship throughout this research.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABI |
Ankle-Brachial Index |
| NCD |
Neurocognitive Disorders |
| PWV |
Pulse Wave Velocity |
| CVD |
Cardiovascular Disease |
| MCI |
Mild Cognitive Impairment |
References
- Alzheimer’s Disease International. World Alzheimer Report 2021: Journey through the diagnosis of dementia. London: Alzheimer’s Disease International; 2021.
- Gostyńska A, Ostrowska B. Diagnosing cognitive function disorders in elderly patients in general practice. Neuropsychiatry and Neuropsychology. 2018;13(3):114-119. [CrossRef]
- Petersen RC. Mild Cognitive Impairment. Continuum (Minneap Minn). 2016 Apr;22(2 Dementia):404-18. [CrossRef]
- Petersen RC, Caraciciolo B, Brayne C, et al. Mild cognitive impairment: A concept in evolution. J Intern Med. 2014;275(3):214-28. [CrossRef]
- Barczak A. Jak rozpoznać otępienie? Wskazówki neuropsychologa. [How to diagnose dementia? A neuropsychologist’s view]. Medycyna po dyplomie. Zeszyt edukacyjny. 2013;2 (49), 4-7.
- Larner AJ, Hancock P. ACE-R or MMSE? A weighted comparison. International Journal of Geriatric Psychiatry. 2014. [CrossRef]
- Hsieh S, Schubert S, Hoon C, et al.: Validation of the Addenbrooke’s Cognitive Examination III in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2013;36(3-4):242-50. [CrossRef]
- Casey S, Lanting S, Oldmeadow C, et al. The reliability of the ankle-brachial index: a systematic review. J Foot Ankle Res. 2019 Aug 2;12:39. [CrossRef]
- Papamichael CM, Lekakis JP, Stamatelopoulos KS, et al. Ankle-brachial index as a predictor of the extent of coronary atherosclerosis and cardiovascular events in patients with coronary artery disease, The American Journal of Cardiology. 2000 Sep 15;86(6):615-8. [CrossRef]
- Alves-Cabratosa L, Comas-Cufí M, Ponjoan A, et al. Levels of ankle-brachial index and the risk of diabetes mellitus complications: BMJ Open Diabetes Research & Care 2020;8:e000977. [CrossRef]
- Dorans KS, He H, Chen J, et al: Change in ankle-brachial index and mortality among individuals with chronic kidney disease: findings from the Chronic Renal Insufficiency. Cohort Study. Nephrol Dial Transplant. 2021 Dec 2;36(12):2224-2231. [CrossRef]
- Hong JB, Leonards CO, Endres M, et al. Ankle-brachial index and recurrent stroke risk: meta-analysis. Stroke, 2016, 47.2: 317-322. [CrossRef]
- Wang A, Jiang R, Su Z, et al. A low ankle-brachial index is associated with cognitive impairment: The APAC study. Atherosclerosis. 2016 Dec;255:90-95. [CrossRef]
- Desormais I, Aboyans V, Guerchet M, et al. Ankle-Brachial Index: An Ubiquitous Marker of Cognitive Impairment-The EPIDEMCA Study. Angiology. 2018 Jul;69(6):497-506. [CrossRef]
- Guo HF, Wu Y, Fu GX, et al. Correlation between ankle-brachial index and subtle cognitive decline. Brain Behav. 2023 Jun;13(6):e3019. [CrossRef]
- Guerchet M, Aboyans V, Nubukpo P, et al. Ankle-brachial index as a marker of cognitive impairment and dementia in the general population. A systematic review. Atherosclerosis. 2011 Jun;216(2):251-7. [CrossRef]
- Molisz A, Faściszewska M, Wożakowska-Kapłon B, Siebert J. Prędkość fali tętna - wartości referencyjne i zastosowanie. Folia Cardiologica 2015;10(4):268-274. [CrossRef]
- Singer J, Trollor JN, Crawford J, et al. The association between pulse wave velocity and cognitive function: the Sydney Memory and Ageing Study. PLoS One. 2013 Apr 30;8(4):e61855. [CrossRef]
- Nilsson ED, Elmståhl S, Minthon L, et al. No independent association between pulse wave velocity and dementia. J Hypertens. 2017 Dec;35(12):2462-2467. [CrossRef]
- Sherzai D, Sherzai A. The Alzheimer’s Solution. San Francisco (CA): HarperOne; 2017.
- Rybakowski F, Drews K. The influence of physical activity on cognitive functions in patients suffering from schizophrenia. Neuropsychiatry and Neuropsychology 2017; 12, 4: 170–17. [CrossRef]
- DeFina LF, Willis BL, Radford NB, Gao A, Leonard D, Haskell WL, et al. The association between midlife cardiorespiratory fitness levels and later-life dementia: A cohort study. Ann Intern Med. 2013 Feb 5;158(3):162–168. [CrossRef]
- Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, Casini A, i in. Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J Intern Med. 2011 Jan;269(1):107-17. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).