Submitted:
25 July 2025
Posted:
28 July 2025
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. Zebrafish Breeding
2.2. Treatment and Sample Collection
2.3. Sample Preparation
2.4. Enzymatic Antioxidant Measurements
2.4.1. SOD Activity
2.4.2. CAT Activity
2.5. Poly(ADP-ribose) Polymerase (PARP) and Poly(ADP-ribose) Glycohydrolase (PARG) Activity Assays
2.6. Thin-Layer Chromatography (TLC) of Synthesised and Digested Poly(ADP-ribose)
2.7. Statistical Analysis
3. Results
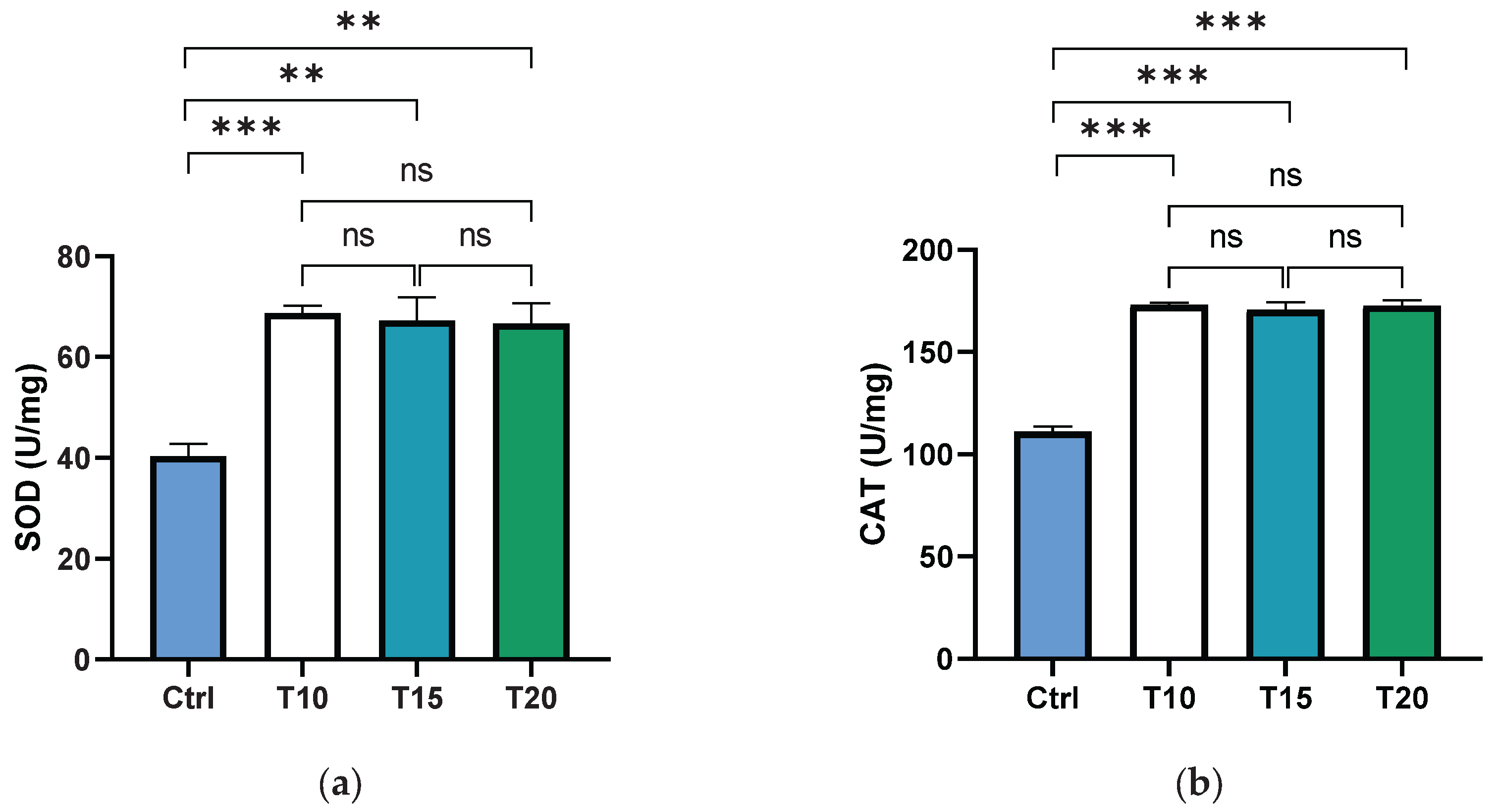
3.1. SOD and CAT Activities
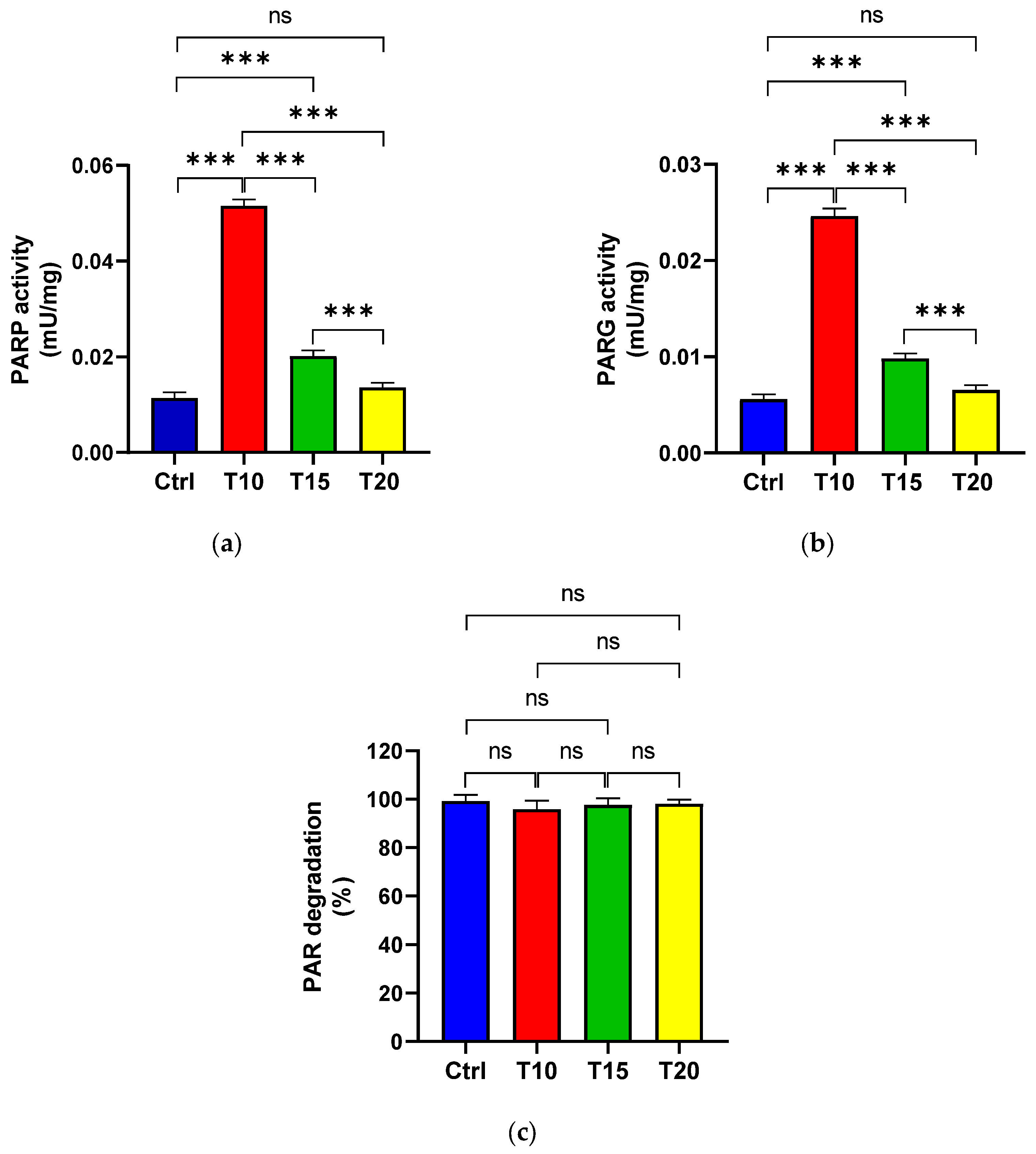
3.2. PARP and PARG Activities
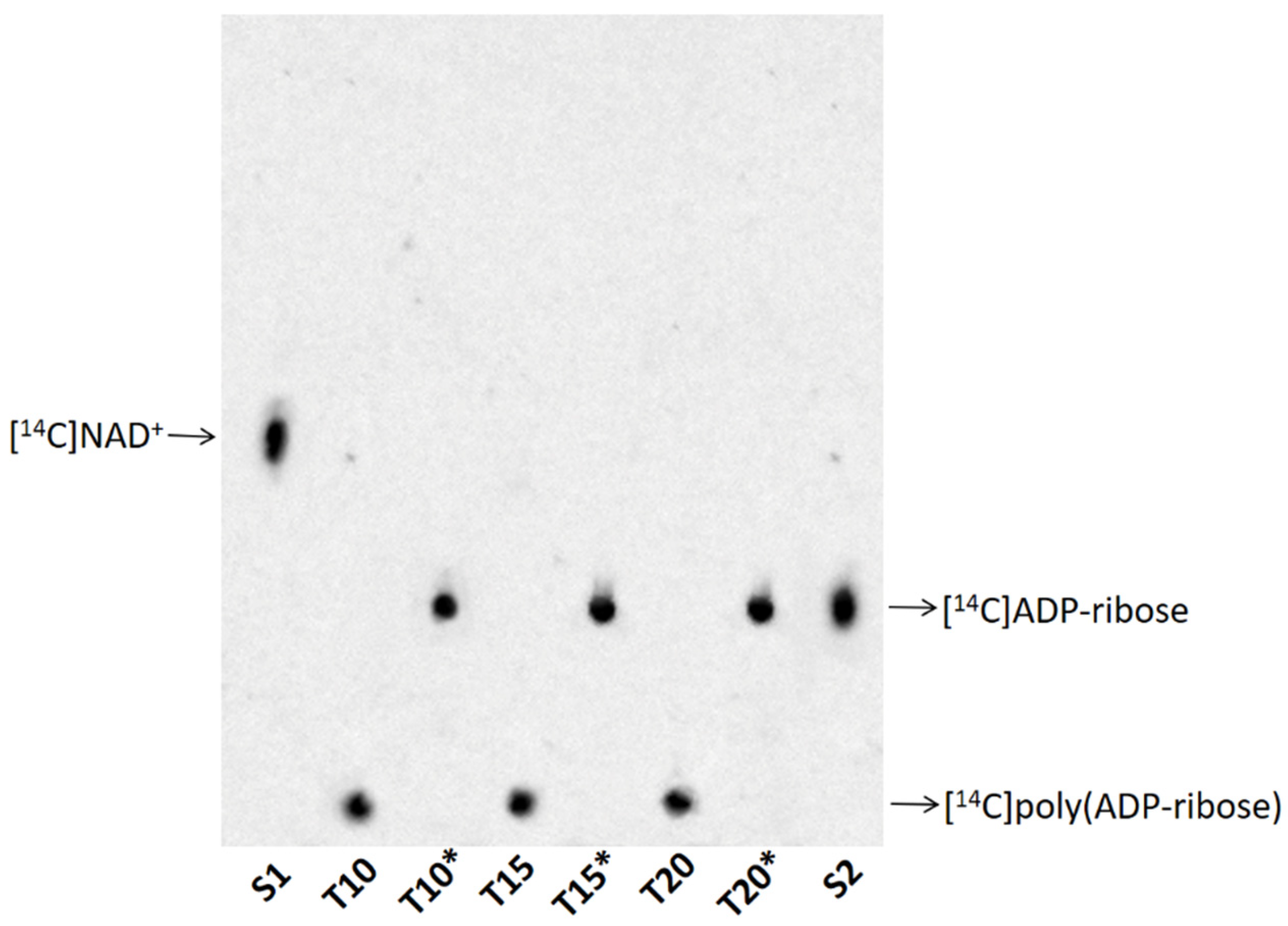
3.3. TLC of Poly(ADP-ribose) and Its Degradation Product
4. Discussion
5. Conclusions
Author Contributions
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woodward, G.; Perkins, D.M.; Brown, L.E. Climate Change and Freshwater Ecosystems: Impacts across Multiple Levels of Organization. Phil. Trans. R. Soc. B 2010, 365, 2093–2106. [Google Scholar] [CrossRef]
- Crozier, L.G.; Hutchings, J.A. Plastic and Evolutionary Responses to Climate Change in Fish. Evol. Appl. 2014, 7, 68–87. [Google Scholar] [CrossRef]
- Ferrandino, I. Zebrafish Models in Toxicology and Disease Studies. Int. J. Mol. Sci. 2024, 25, 8608. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.; Bruder, A.; Matthaei, C.D.; Brodersen, J.; Paterson, R.A. Multiple-stressor Effects on Freshwater Fish: Importance of Taxonomy and Life Stage. Fish Fish. 2018, 19, 974–983. [Google Scholar] [CrossRef]
- Zarrelli, A.; DellaGreca, M.; Parolisi, A.; Iesce, M.R.; Cermola, F.; Temussi, F.; Isidori, M.; Lavorgna, M.; Passananti, M.; Previtera, L. Chemical Fate and Genotoxic Risk Associated with Hypochlorite Treatment of Nicotine. Sci. Total Environ. 2012, 426, 132–138. [Google Scholar] [CrossRef]
- Schulte, P.M. What Is Environmental Stress? Insights from Fish Living in a Variable Environment. J. Exp. Biol. 2014, 217, 23–34. [Google Scholar] [CrossRef]
- Balasch, J.C.; Tort, L. Netting the Stress Responses in Fish. Front. Endocrinol. 2019, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.A. Stress in Fishes: A Diversity of Responses with Particular Reference to Changes in Circulating Corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Killen, S.S.; Marras, S.; Metcalfe, N.B.; McKenzie, D.J.; Domenici, P. Environmental Stressors Alter Relationships between Physiology and Behaviour. Trends Ecol. Evol. 2013, 28, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Cangiano, T.; Dellagreca, M.; Fiorentino, A.; Isidori, M.; Monaco, P.; Zarrelli, A. Effect of Ent-Labdane Diterpenes from Potamogetonaceae on Selenastrum Capricornutum and Other Aquatic Organisms. J. Chem. Ecol. 2002, 28, 1091–1102. [Google Scholar] [CrossRef]
- Whitehead, A.; Clark, B.W.; Reid, N.M.; Hahn, M.E.; Nacci, D. When Evolution Is the Solution to Pollution: Key Principles, and Lessons from Rapid Repeated Adaptation of Killifish (Fundulus Heteroclitus) Populations. Evol. Appl. 2017, 10, 762–783. [Google Scholar] [CrossRef]
- Reid, N.M.; Proestou, D.A.; Clark, B.W.; Warren, W.C.; Colbourne, J.K.; Shaw, J.R.; Karchner, S.I.; Hahn, M.E.; Nacci, D.; Oleksiak, M.F.; et al. The Genomic Landscape of Rapid Repeated Evolutionary Adaptation to Toxic Pollution in Wild Fish. Science 2016, 354, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M. Energy-Limited Tolerance to Stress as a Conceptual Framework to Integrate the Effects of Multiple Stressors. Integr. Comp. Biol. 2013, 53, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy Homeostasis as an Integrative Tool for Assessing Limits of Environmental Stress Tolerance in Aquatic Invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, B.; Xie, J.; Xu, P.; Habte-Tsion, H.-M.; Zhang, Y. Effect of Heat Stress and Recovery on Viability, Oxidative Damage, and Heat Shock Protein Expression in Hepatic Cells of Grass Carp (Ctenopharyngodon Idellus). Fish Physiol. Biochem. 2014, 40, 721–729. [Google Scholar] [CrossRef]
- Malek, R.L.; Sajadi, H.; Abraham, J.; Grundy, M.A.; Gerhard, G.S. The Effects of Temperature Reduction on Gene Expression and Oxidative Stress in Skeletal Muscle from Adult Zebrafish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 138, 363–373. [Google Scholar] [CrossRef]
- Slaninova, A.; Smutna, M.; Modra, H.; Svobodova, Z. A Review: Oxidative Stress in Fish Induced by Pesticides. Neuro Endocrinol. Lett. 2009, 30 Suppl 1, 2–12. [Google Scholar]
- Lushchak, V.I. Environmentally Induced Oxidative Stress in Aquatic Animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding Mechanisms of Antioxidant Action in Health and Disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Singh, P.; Kesharwani, R.K.; Keservani, R.K. Antioxidants and Vitamins. In Sustained Energy for Enhanced Human Functions and Activity; Elsevier, 2017; pp. 385–407 ISBN 978-0-12-805413-0.
- Di Domenico, M.; Feola, A.; Ambrosio, P.; Pinto, F.; Galasso, G.; Zarrelli, A.; Di Fabio, G.; Porcelli, M.; Scacco, S.; Inchingolo, F.; et al. Antioxidant Effect of Beer Polyphenols and Their Bioavailability in Dental-Derived Stem Cells (D-dSCs) and Human Intestinal Epithelial Lines (Caco-2) Cells. Stem Cells Int. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Santos, R.; Joyeux, A.; Besnard, A.; Blanchard, C.; Halkett, C.; Bony, S.; Sanchez, W.; Devaux, A. An Integrative Approach to Assess Ecological Risks of Surface Water Contamination for Fish Populations. Environ. Pollut. 2017, 220, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Cachot, J.; Geffard, O.; Augagneur, S.; Lacroix, S.; Le Menach, K.; Peluhet, L.; Couteau, J.; Denier, X.; Devier, M.H.; Pottier, D.; et al. Evidence of Genotoxicity Related to High PAH Content of Sediments in the Upper Part of the Seine Estuary (Normandy, France). Aquat. Toxicol. 2006, 79, 257–267. [Google Scholar] [CrossRef]
- Polard, T.; Jean, S.; Gauthier, L.; Laplanche, C.; Merlina, G.; Sánchez-Pérez, J.M.; Pinelli, E. Mutagenic Impact on Fish of Runoff Events in Agricultural Areas in South-West France. Aquat. Toxicol. 2011, 101, 126–134. [Google Scholar] [CrossRef]
- Costa, P.M.; Caeiro, S.; Lobo, J.; Martins, M.; Ferreira, A.M.; Caetano, M.; Vale, C.; DelValls, T.Á.; Costa, M.H. Estuarine Ecological Risk Based on Hepatic Histopathological Indices from Laboratory and in Situ Tested Fish. Mar. Pollut. Bull. 2011, 62, 55–65. [Google Scholar] [CrossRef]
- Xing, H.; Li, S.; Wang, Z.; Gao, X.; Xu, S.; Wang, X. Histopathological Changes and Antioxidant Response in Brain and Kidney of Common Carp Exposed to Atrazine and Chlorpyrifos. Chemosphere 2012, 88, 377–383. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A Comparative and Evolutionary Approach to Oxidative Stress in Fish: A Review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, L.; Yu, Y.; Yang, G.; Xu, Z.; Wang, Q.; Cai, L. Single and Joint Toxic Effects of Five Selected Pesticides on the Early Life Stages of Zebrafish (Denio Rerio). Chemosphere 2017, 170, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, M.; Moss, J. Functional Role of ADP-Ribosyl-Acceptor Hydrolase 3 in Poly(ADPRibose) Polymerase-1 Response to Oxidative Stress. Curr. Protein Pept. Sci. 2016, 17, 633–640. [Google Scholar] [CrossRef]
- Harrision, D.; Gravells, P.; Thompson, R.; Bryant, H.E. Poly(ADP-Ribose) Glycohydrolase (PARG) vs. Poly(ADP-Ribose) Polymerase (PARP) – Function in Genome Maintenance and Relevance of Inhibitors for Anti-Cancer Therapy. Front. Mol. Biosci. 2020, 7, 191. [Google Scholar] [CrossRef]
- Brochu, G.; Duchaine, C.; Thibeault, L.; Lagueux, J.; Shah, G.M.; Poirier, G.G. Mode of Action of Poly(ADP-Ribose) Glycohydrolase. Biochim. Biophys. Acta 1994, 1219, 342–350. [Google Scholar] [CrossRef]
- Barkauskaite, E.; Brassington, A.; Tan, E.S.; Warwicker, J.; Dunstan, M.S.; Banos, B.; Lafite, P.; Ahel, M.; Mitchison, T.J.; Ahel, I.; et al. Visualization of Poly(ADP-Ribose) Bound to PARG Reveals Inherent Balance between Exo- and Endo-Glycohydrolase Activities. Nat. Commun. 2013, 4, 2164. [Google Scholar] [CrossRef]
- Meyer, R.G.; Meyer-Ficca, M.L.; Jacobson, E.L.; Jacobson, M.K. Human Poly(ADP-Ribose) Glycohydrolase (PARG) Gene and the Common Promoter Sequence It Shares with Inner Mitochondrial Membrane Translocase 23 (TIM23). Gene 2003, 314, 181–190. [Google Scholar] [CrossRef]
- Meyer-Ficca, M.L.; Meyer, R.G.; Coyle, D.L.; Jacobson, E.L.; Jacobson, M.K. Human Poly(ADP-Ribose) Glycohydrolase Is Expressed in Alternative Splice Variants Yielding Isoforms That Localize to Different Cell Compartments. Exp. Cell Res. 2004, 297, 521–532. [Google Scholar] [CrossRef]
- Bianchi, A.R.; La Pietra, A.; Guerretti, V.; De Maio, A.; Capriello, T.; Ferrandino, I. Synthesis and Degradation of Poly(ADP-Ribose) in Zebrafish Brain Exposed to Aluminum. Int. J. Mol. Sci. 2023, 24, 8766. [Google Scholar] [CrossRef] [PubMed]
- Ruf, A.; Mennissier De Murcia, J.; De Murcia, G.; Schulz, G.E. Structure of the Catalytic Fragment of Poly(AD-Ribose) Polymerase from Chicken. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 7481–7485. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.W. Crystal Structure of the Catalytic Fragment of Murine Poly(ADP-Ribose) Polymerase-2. Nucleic Acids Res. 2004, 32, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Li, L.; Fattah, F.J.; Dong, Y.; Bey, E.A.; Patel, M.; Gao, J.; Boothman, D.A. Review of Poly (ADP-Ribose) Polymerase (PARP) Mechanisms of Action and Rationale for Targeting in Cancer and Other Diseases. Crit. Rev. Eukaryot. Gene Expr. 2014, 24, 15–28. [Google Scholar] [CrossRef]
- Amé, J.; Spenlehauer, C.; De Murcia, G. The PARP Superfamily. BioEssays 2004, 26, 882–893. [Google Scholar] [CrossRef]
- Hottiger, M.O.; Hassa, P.O.; Lüscher, B.; Schüler, H.; Koch-Nolte, F. Toward a Unified Nomenclature for Mammalian ADP-Ribosyltransferases. Trends Biochem. Sci. 2010, 35, 208–219. [Google Scholar] [CrossRef]
- Duma, L.; Ahel, I. The Function and Regulation of ADP-Ribosylation in the DNA Damage Response. Biochem. Soc. Trans. 2023, 51, 995–1008. [Google Scholar] [CrossRef]
- Kim, M.Y.; Zhang, T.; Kraus, W.L. Poly(ADP-Ribosyl)Ation by PARP-1: `PAR-Laying’ NAD+ into a Nuclear Signal. Genes Dev. 2005, 19, 1951–1967. [Google Scholar] [CrossRef]
- Schreiber, V.; Dantzer, F.; Ame, J.-C.; De Murcia, G. Poly(ADP-Ribose): Novel Functions for an Old Molecule. Nat. Rev. Mol. Cell Biol. 2006, 7, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Hassa, P., O. The Diverse Biological Roles of Mammalian PARPS, a Small but Powerful Family of Poly-ADP-Ribose Polymerases. Front. Biosci. 2008, 13, 3046. [Google Scholar] [CrossRef]
- Hassa, P.O.; Haenni, S.S.; Elser, M.; Hottiger, M.O. Nuclear ADP-Ribosylation Reactions in Mammalian Cells: Where Are We Today and Where Are We Going? Microbiol. Mol. Biol. Rev. 2006, 70, 789–829. [Google Scholar] [CrossRef]
- Murata, M.M.; Kong, X.; Moncada, E.; Chen, Y.; Imamura, H.; Wang, P.; Berns, M.W.; Yokomori, K.; Digman, M.A. NAD+ Consumption by PARP1 in Response to DNA Damage Triggers Metabolic Shift Critical for Damaged Cell Survival. Mol. Biol. Cell. 2019, 30, 2584–2597. [Google Scholar] [CrossRef]
- Hay Mele, B.; Bianchi, A.R.; Guerretti, V.; Pugliese, M.; De Maio, A.; Arena, C. Antioxidant Defenses and Poly(ADP-Ribose) Polymerase (PARP) Activity Provide “Radioresilience” Against Ionizing Radiation-Induced Stress in Dwarf Bean Plants. Antioxidants 2025, 14, 261. [Google Scholar] [CrossRef]
- Vitale, L.; Vitale, E.; Bianchi, A.R.; De Maio, A.; Arena, C. Role of Poly(ADP-Ribose) Polymerase (PARP) Enzyme in the Systemic Acquired Acclimation Induced by Light Stress in Phaseolus Vulgaris L. Plants. Plants 2022, 11, 1870. [Google Scholar] [CrossRef]
- Vitale, L.; Vitale, E.; Costanzo, G.; De Maio, A.; Arena, C. Photo-Protective Mechanisms and the Role of Poly (ADP-Ribose) Polymerase Activity in a Facultative CAM Plant Exposed to Long-Term Water Deprivation. Plants 2020, 9, 1192. [Google Scholar] [CrossRef] [PubMed]
- Giorio, P.; Guida, G.; Mistretta, C.; Sellami, M.H.; Oliva, M.; Punzo, P.; Iovieno, P.; Arena, C.; De Maio, A.; Grillo, S.; et al. Physiological, Biochemical and Molecular Responses to Water Stress and Rehydration in Mediterranean Adapted Tomato Landraces. Plant Biol. J. 2018, 20, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.R.; Di Capua, I.; Guerretti, V.; Guagliardi, A.; Carotenuto, Y.; De Maio, A. The Poly(ADP-Ribosyl)Ation System in the Crustacean Copepod Temora Stylifera (Dana, 1853–1855) from a Coastal Area of the Mediterranean Sea: A New Biomarker of the Health Status. Euro-Mediterr. J. Environ. Integr. 2025, 10, 501–513. [Google Scholar] [CrossRef]
- Marinaro, C.; Marino, A.; Bianchi, A.R.; Berman, B.; Trifuoggi, M.; Marano, A.; Palumbo, G.; Chianese, T.; Scudiero, R.; Rosati, L.; et al. Molecular and Toxicological Mechanisms behind the Effects of Chromium (VI) on the Male Reproductive System of Mytilus Galloprovincialis: First Evidence for Poly-ADP-Ribosylation of Protamine-like II. Chem. Biol. Interact. 2024, 401, 111186. [Google Scholar] [CrossRef]
- Marinaro, C.; Lettieri, G.; Chianese, T.; Bianchi, A.R.; Zarrelli, A.; Palatucci, D.; Scudiero, R.; Rosati, L.; De Maio, A.; Piscopo, M. Exploring the Molecular and Toxicological Mechanism Associated with Interactions between Heavy Metals and the Reproductive System of Mytilus Galloprovincialis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 275, 109778. [Google Scholar] [CrossRef]
- Carbone, G.; Lettieri, G.; Marinaro, C.; Costabile, M.; Notariale, R.; Bianchi, A.R.; De Maio, A.; Piscopo, M. A Molecular Mechanism to Explain the Nickel-Induced Changes in Protamine-like Proteins and Their DNA Binding Affecting Sperm Chromatin in Mytilus Galloprovincialis: An In Vitro Study. Biomolecules 2023, 13, 520. [Google Scholar] [CrossRef]
- Pacher, P.; Szabo, C. Role of the Peroxynitrite-Poly(ADP-Ribose) Polymerase Pathway in Human Disease. Am. J. Pathol. 2008, 173, 2–13. [Google Scholar] [CrossRef]
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; Mortimer, P.; Swaisland, H.; Lau, A.; O’Connor, M.J.; et al. Inhibition of Poly(ADP-Ribose) Polymerase in Tumors from BRCA Mutation Carriers. N. Engl. J. Med. 2009, 361, 123–134. [Google Scholar] [CrossRef]
- Exley, C. Aluminium in the Brain and Heart of the Rainbow Trout. J. Fish Biol. 1996, 48, 706–713. [Google Scholar] [CrossRef]
- Chen, X.-F.; Lin, Z.-C.; Qi, Z.; Cai, Z.; Chen, Z.-F. Effects of Pollutant Toxicity on the Eyes of Aquatic Life Monitored by Visual Dysfunction in Zebrafish: A Review. Environ. Chem. Lett. 2023, 21, 1177–1201. [Google Scholar] [CrossRef]
- Burreau, S.; Broman, D.; Örn, U. Tissue Distribution of 2,2 ′,4,4 ′ -Tetrabromo[ 14 C]Diphenyl Ether ([ 14 C]-PBDE 47) in Pike ( Esox Lucius ) after Dietary Exposure – a Time Series Study Using Whole Body Autoradiography. Chemosphere 2000, 40, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.S.M.; Noltie, D.B.; Tillitt, D.E. Ontogenetic Improvement of Visual Function in the Medaka Oryzias Latipes Based on an Optomotor Testing System for Larval and Adult Fish. Anim. Behav. 2002, 64, 1–10. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Wei, P.; Tian, H.; Wang, W.; Ru, S. Long-Term Exposure to Bisphenol S Damages the Visual System and Reduces the Tracking Capability of Male Zebrafish (Danio Rerio). J. Appl. Toxicol. 2018, 38, 248–258. [Google Scholar] [CrossRef] [PubMed]
- La Pietra, A.; Bianchi, A.R.; Capriello, T.; Mobilio, T.; Guagliardi, A.; De Maio, A.; Ferrandino, I. Regeneration of Zebrafish Retina Following Toxic Injury. Environ. Toxicol. Pharmacol. 2024, 112, 104582. [Google Scholar] [CrossRef] [PubMed]
- Capriello, T.; Di Meglio, G.; De Maio, A.; Scudiero, R.; Bianchi, A.R.; Trifuoggi, M.; Toscanesi, M.; Giarra, A.; Ferrandino, I. Aluminium Exposure Leads to Neurodegeneration and Alters the Expression of Marker Genes Involved to Parkinsonism in Zebrafish Brain. Chemosphere 2022, 307, 135752. [Google Scholar] [CrossRef]
- Kuder, R.S.; Philip, G.H. Antioxidant Enzymatic Activities and Lipid Peroxidation in Liver and Ovary of Zebrafish (Danio Rerio) Exposed to Deltamethrin. Chem. Ecol. 2017, 33, 739–749. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A Simple Method for Clinical Assay of Superoxide Dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- De Maio, A.; Porzio, E.; D’Angelo, R.; Rotondo, S.; Bianchi, A.; Confalone, E.; Raucci, R.; Natale, E.; Faraone-Mennella, M. A Glycosyltransferase from Sulfolobus Solfataricus MT-4 Exhibits Poly(ADP-Ribose) Glycohydrolase Activity. Curr. Proteom. 2015, 12, 253–263. [Google Scholar] [CrossRef]
- Izzotti, A.; Bagnis, A.; Sacca, S. The Role of Oxidative Stress in Glaucoma. Mut. Res. 2006, 612, 105–114. [Google Scholar] [CrossRef]
- Grzybowski, A.; Nita, M. Smoking and Eye Pathologies. A Systemic Review. Part I. Anterior Eye Segment Pathologies. Curr. Pharm. Des. 2017, 23, 629–638. [Google Scholar] [CrossRef]
- Coleman, J.G.; Johnson, D.R.; Stanley, J.K.; Bednar, A.J.; Weiss, C.A.; Boyd, R.E.; Steevens, J.A. Assessing the Fate and Effects of Nano Aluminum Oxide in the Terrestrial Earthworm, Eisenia Fetida. Environ. Toxicol. Chem. 2010, 29, 1575–1580. [Google Scholar] [CrossRef]
- Allin, C. Effects of Pre-Acclimation to Aluminium on the Physiology and Swimming Behaviour of Juvenile Rainbow Trout (Oncorhynchus Mykiss) during a Pulsed Exposure. Aquat. Toxicol. 2000, 51, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Poléo, A.B.S.; Hytterød, S. The Effect of Aluminium in Atlantic Salmon (Salmo Salar) with Special Emphasis on Alkaline Water. J. Inorg. Biochem. 2003, 97, 89–96. [Google Scholar] [CrossRef]
- Keinänen, M.; Tigerstedt, C.; Kålax, P.; Vuorinen, P.J. Fertilization and Embryonic Development of Whitefish (Coregonus Lavaretus Lavaretus) in Acidic Low-Ionic-Strength Water with Aluminum. Ecotoxicol. Environ. Saf. 2003, 55, 314–329. [Google Scholar] [CrossRef]
- Vuorinen, P.J.; Keinänen, M.; Peuranen, S.; Tigerstedt, C. Reproduction, Blood and Plasma Parameters and Gill Histology of Vendace (Coregonus Albula L.) in Long-Term Exposure to Acidity and Aluminum. Ecotoxicol. Environ. Saf. 2003, 54, 255–276. [Google Scholar] [CrossRef]
- Teien, H.-C.; Salbu, B.; Heier, L.S.; Kroglund, F.; Olav Rosseland, B. Fish Mortality during Sea Salt Episodes—Catchment Liming as a Countermeasure. J. Environ. Monit. 2005, 7, 989. [Google Scholar] [CrossRef]
- Capriello, T.; Monteiro, S.M.; Félix, L.M.; Donizetti, A.; Aliperti, V.; Ferrandino, I. Apoptosis, Oxidative Stress and Genotoxicity in Developing Zebrafish after Aluminium Exposure. Aquat. Toxicol. 2021, 236, 105872. [Google Scholar] [CrossRef] [PubMed]
- Ferrandino, I.; Capriello, T.; Félix, L.M.; Di Meglio, G.; Santos, D.; Monteiro, S.M. Histological Alterations and Oxidative Stress in Adult Zebrafish Muscle after Aluminium Exposure. Environ. Toxicol. Pharmacol. 2022, 94, 103934. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Capriello, T.; Venditti, P.; Fasciolo, G.; La Pietra, A.; Trifuoggi, M.; Giarra, A.; Agnisola, C.; Ferrandino, I. Aluminum Induces a Stress Response in Zebrafish Gills by Influencing Metabolic Parameters, Morphology, and Redox Homeostasis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 271, 109633. [Google Scholar] [CrossRef] [PubMed]
- Capriello, T.; Félix, L.M.; Monteiro, S.M.; Santos, D.; Cofone, R.; Ferrandino, I. Exposure to Aluminium Causes Behavioural Alterations and Oxidative Stress in the Brain of Adult Zebrafish. Environ. Toxicol. Pharmacol. 2021, 85, 103636. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kam, T.-I.; Dawson, T.M.; Dawson, V.L. Poly (ADP-Ribose) (PAR)-Dependent Cell Death in Neurodegenerative Diseases. In International Review of Cell and Molecular Biology; Elsevier, 2020; Vol. 353, pp. 1–29 ISBN 978-0-12-820135-0.
- Eisemann, T.; Pascal, J.M. Poly(ADP-Ribose) Polymerase Enzymes and the Maintenance of Genome Integrity. Cell. Mol. Life Sci. 2020, 77, 19–33. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Dawson, T.M.; Dawson, V.L. Mitochondrial and Nuclear Cross Talk in Cell Death: Parthanatos. Ann. New York Acad. Sci. 2008, 1147, 233–241. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




