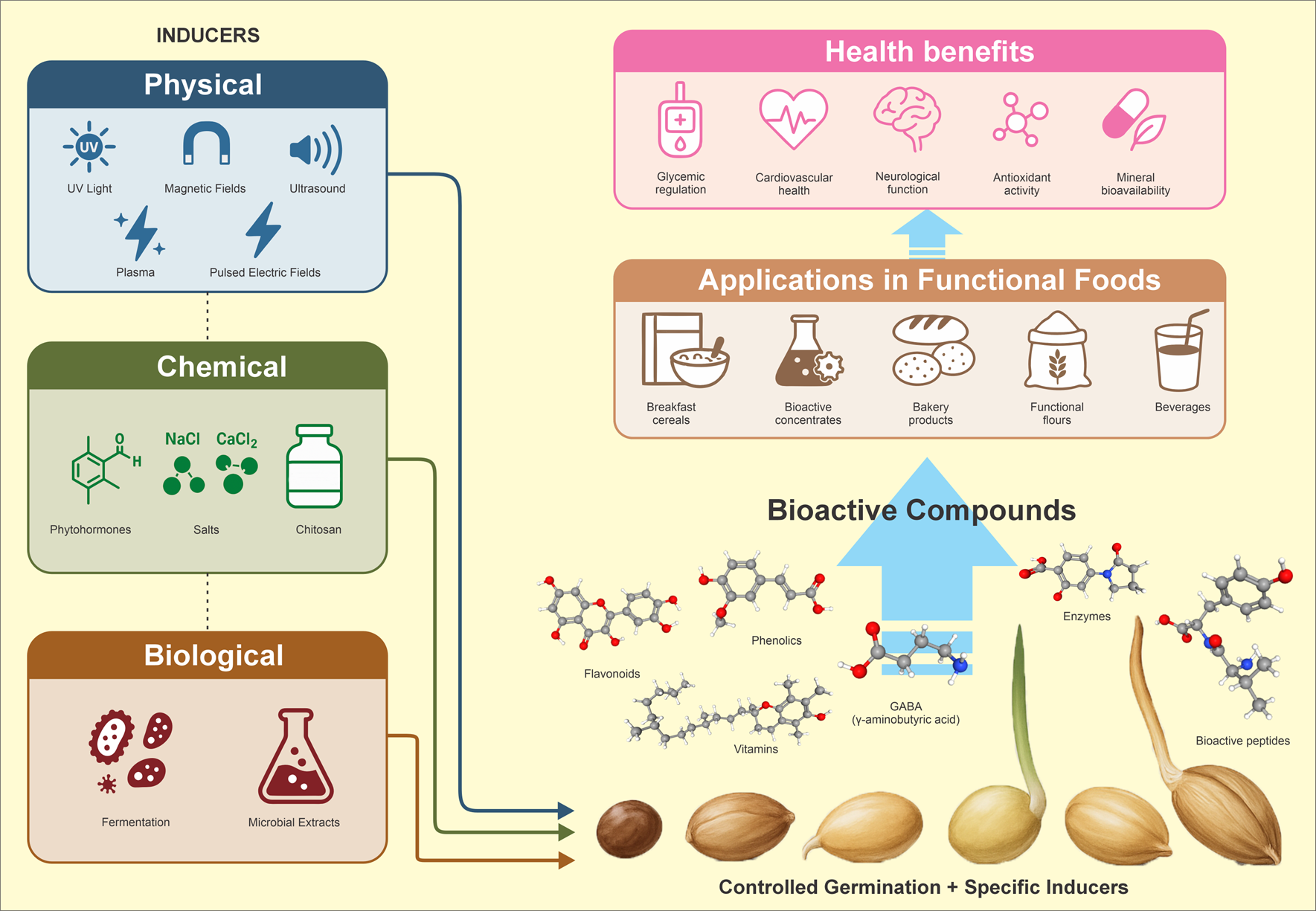
1. Introduction
Cereals and pseudocereals constitute fundamental components in global human nutrition, representing a significant proportion of caloric intake and substantially contributing to protein supply, especially in developing countries [
1,
2]. For decades, these grains undergo various technological processes to improve their nutritional, functional, and sensory properties; however, controlled germination has emerged as a promising biotechnology to enhance their nutritional and functional value [
3].
Germination is a complex physiological process that involves the transition of seeds from a dormant state to a metabolically active state, characterized by profound biochemical transformations, including macronutrient hydrolysis,
de novo synthesis of bioactive compounds, and enzymatic modulation [
4,
5,
6,
7]. This natural process induces significant changes in the phytochemical profile of grains, enhancing the concentration and bioavailability of bioactive compounds with functional properties such as
γ-aminobutyric acid (GABA), phenolic compounds (phenolic acids, flavonoids and non-flavonoids) vitamins, bioavailable minerals, and antioxidant enzymes [
8,
9,
10].
Scientific evidence accumulating over the last decade has established significant correlations between regular consumption of foods derived from germinated cereals and pseudocereals and various health benefits, including reduced risk of cardiovascular diseases, improved glycemic regulation, modulation of inflammatory response, and enhancement of immune function [
11,
12,
13,
14]. These physiological effects are primarily attributed to the synergistic action of bioactive compounds generated or enhanced during the germination process [
11,
15,
16].
Significantly, recent research has demonstrated that the accumulation of these bioactive compounds can be considerably enhanced through the application of specific inducers during germination [
17,
18,
19,
20]. These inducers are classified as physical, chemical, or biological according to their nature and mechanism of action and modulate specific metabolic pathways, activating controlled stress response mechanisms that result in greater synthesis and accumulation of secondary metabolites with biological activity [
21,
22,
23,
24].
Among physical inducers, the application of electromagnetic radiation (UV light, magnetic fields), mechanical treatments (ultrasound), and emerging technologies such as cold plasmas and pulsed electric fields stand out [
25,
26,
27,
28,
29]. Chemical inducers include phytohormones, plant-derived elicitors, specific minerals, and growth regulators [
30,
31,
32,
33], while biological inducers comprise concurrent fermentation processes and application of microbial extracts [
9,
11,
12,
34]. Each category of inducers acts through specific molecular mechanisms to trigger adaptive responses that lead to the accumulation of bioactive compounds [
35,
36,
37,
38].
The technological implementation of these inducers requires a comprehensive understanding of their specific effects, optimal application conditions, potential synergies, and technical limitations, especially considering the variability in response according to species, cultivar, and environmental conditions [
39,
40,
41,
42]. Additionally, the evaluation of bioavailability and biological efficacy of the enhanced bioactive compounds is fundamental to determine their actual nutritional and functional relevance [
13,
14,
30,
43].
The development of functional foods from germinated cereals and pseudocereals under controlled conditions with application of specific inducers has emerged as a promising strategy to address contemporary nutritional challenges, aligning with global trends towards minimally processed foods with demonstrable functional properties [
44,
45,
46,
47]. However, the transition from experimental applications to industrial implementations presents multiple technological, regulatory, and commercial challenges that require systematic research [
35,
37,
48,
49].
This review aims to comprehensively analyze emerging inducers for the germination of cereals and pseudocereals, examining their mechanisms of action, effectiveness in enhancing specific bioactive compounds, technological considerations for industrial implementation, bioavailability and biological efficacy of the enhanced compounds, applications in the development of functional foods, and future research directions. This critical synthesis seeks to establish a conceptual and technical framework that facilitates the translation of knowledge from basic research to industrial and nutritional applications with potentially significant impact on public health.
2. Search Strategies and Brief Bibliometric Analysis
The present study was designed using a systematic bibliometric approach to identify, select, and analyze the most relevant scientific literature on emerging inducers for the germination of cereals and pseudocereals, with a focus on enhancing bioactive compounds.
Search Strategy. The bibliographic search was conducted in three international databases: Scopus, Web of Science and Science Direct, considering publications between 2015 and 2025. Structured combinations of Boolean terms were employed to encompass the various dimensions of the study topic. The search equations included:
(TITLE-ABS-KEY(((“germination” OR “sprouting”) AND (“time” OR “duration”) AND (“temperature”) AND (“cereals” OR “pseudo-cereals”) AND (“bioactive compounds” OR “antioxidants” OR “phenolics” OR “nutritional improvement”)) AND ALL (“temperature” OR “time”)) AND PUBYEAR > 2015 AND PUBYEAR < 2026)
(TITLE-ABS-KEY(((“germination” OR “sprouting”) AND (“time” OR “duration”) AND (“temperature”) AND (“quinoa” OR “amaranto” OR “sarraceno” OR “pallidicaule” OR “cañihua” OR “Chia” OR “amaranth” OR “Buckwheat” OR “Pseudocereals” OR “Ancient grains” OR “Non-cereal grain” OR “Andean grains”) AND (“bioactive compounds” OR “antioxidants” OR “phenolics” OR “nutritional improvement”)) AND ALL(“temperature” OR “time”)) AND PUBYEAR > 2015 AND PUBYEAR < 2026)
Additionally, specific search equations were used for physical treatments and inducers:
TITLE-ABS-KEY((germination OR sprouting) AND (cereal* OR pseudocereal* OR “ancient grains” OR quinoa OR amaranth OR canihua OR chenopodium OR kañiwa OR millet OR sorghum OR teff OR buckwheat) AND (“physical treatment*” OR “physical inductor*” OR “physical elicitor*” OR “germination enhancement” OR “germination treatment*”) AND (“thermal treatment*” OR “heat treatment*” OR “temperature treatment*” OR “light treatment*” OR “light exposure” OR “LED treatment*” OR “electromagnetic field*” OR “ultrasound treatment*” OR “high hydrostatic pressure” OR HHP OR “hydrostatic pressure treatment*”) AND (“bioactive compound*” OR “phenolic compound*” OR “antioxidant capacity” OR “phytochemical content” OR “secondary metabolite*”)).
At this stage, physical inducers were replaced by biological and chemical ones according to previous research.
Selection and Analysis of Studies. The search strategy initially identified 440 articles, which were subjected to a screening process to evaluate their relevance according to the inclusion criteria. Finally, 126 articles were selected for their direct relationship with the research focus and objectives. Bibliographic management was performed using Zotero 7.0.15, which allowed the extraction of metadata in JSON format for subsequent analysis.
Inclusion Criteria. Publication type: Original articles, peer-reviewed, published in indexed scientific journals. Thematic focus: Studies evaluating controlled germination processes in cereals and pseudocereals, including main species such as Chenopodium quinoa, Chenopodium pallidicaule, Amaranthus spp., Panicum miliaceum, Setaria italica, Triticum aestivum, Hordeum vulgare, Zea mays, Oryza sativa and Fagopyrum esculentum. Application of inducers: Studies investigating the application of physical, chemical, or biological inducers during germination, such as UV light, ultrasound, electromagnetic fields, phytohormones, minerals, microbial extracts, or concurrent fermentation. Evaluation of bioactive compounds: Studies reporting quantitative data on the presence or increase of bioactive compounds, such as total phenolic compounds (TPC), flavonoids, GABA, carotenoids, vitamins, bioactive peptides, antioxidant enzymes, among other secondary metabolites with functional potential. Experimental design: Research with clearly defined germination parameters (time, temperature, relative humidity, photoperiod, treatment type). Temporal coverage: Publications between January 2015 and January 2025, with access to the full text. Language: Publications in English.
Exclusion Criteria: Narrative reviews, brief communications, editorials, book chapters, and work without peer review. Studies that did not use germination as a central treatment or that applied inducers in later stages (e.g., drying, cooking, or extrusion). Research focused exclusively on digestibility, starch, proteins, agronomic profile, or plant development, without evaluating bioactive compounds. Studies reporting only qualitative or descriptive results, without verifiable numerical data. Preprints, duplicate articles, or without full text access.
Brief Bibliometric Analysis. For the bibliometric analysis, VOSviewer 1.6.20 was used, which allowed building and visualizing a co-occurrence network of terms. A minimum threshold of 5 occurrences was established for each term, resulting in 76 terms selected for analysis. The network visualization was configured to show the relationships between terms, their prominence, and thematic grouping.
The co-occurrence network analysis was complemented with three types of visualization: Network Visualization, which shows the network of terms and their interrelationships; Overlay Visualization, which incorporates the temporal dimension to identify emerging trends; and Density Visualization, which reflects the intensity of connections between terms.
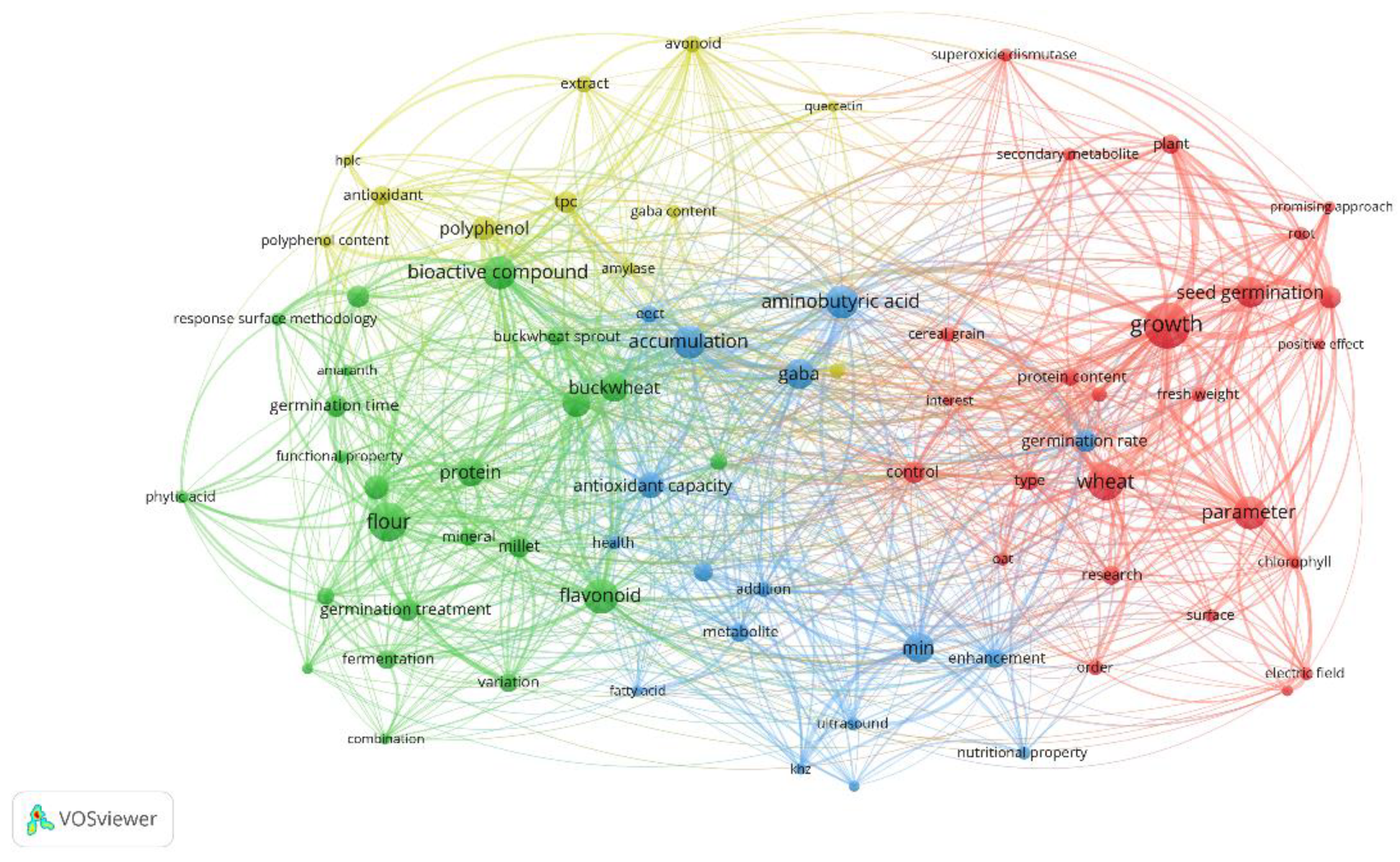
Structure of the Bibliometric Network. The resulting bibliometric network
(Figure 1) revealed a clear organization in four main clusters:
Cluster 1 (25 terms): Centered on aspects of plant growth and seed germination, with predominant terms such as “growth” (146 links), “wheat” (109 links), and “seed germination” (92 links). The weighted average publication year was 2022.
Cluster 2 (23 terms): Focused on specific cereals/pseudocereals and their nutritional properties, highlighting “bioactive compound” (96 links), “flavonoid” (96 links), and “flour” (94 links). The weighted average year was 2022. 13.
Cluster 3 (17 terms): Related to inducers and metabolic processes, with main terms such as “aminobutyric acid” (115 links), “accumulation” (101 links), and “GABA” (97 links). The weighted average year was 2021.
Cluster 4 (11 terms): Concentrated on specific bioactive compounds and antioxidant capacity, highlighting “polyphenol” (73 links), “flavonoid” (61 links), and “TPC” (60 links). The weighted average year was 2020.
Additionally, bridge terms connecting multiple clusters were identified, such as “growth”, “aminobutyric acid”, “wheat”, “accumulation”, and “GABA”, all connected to the four main clusters. These terms indicate points of conceptual convergence in the analyzed literature.
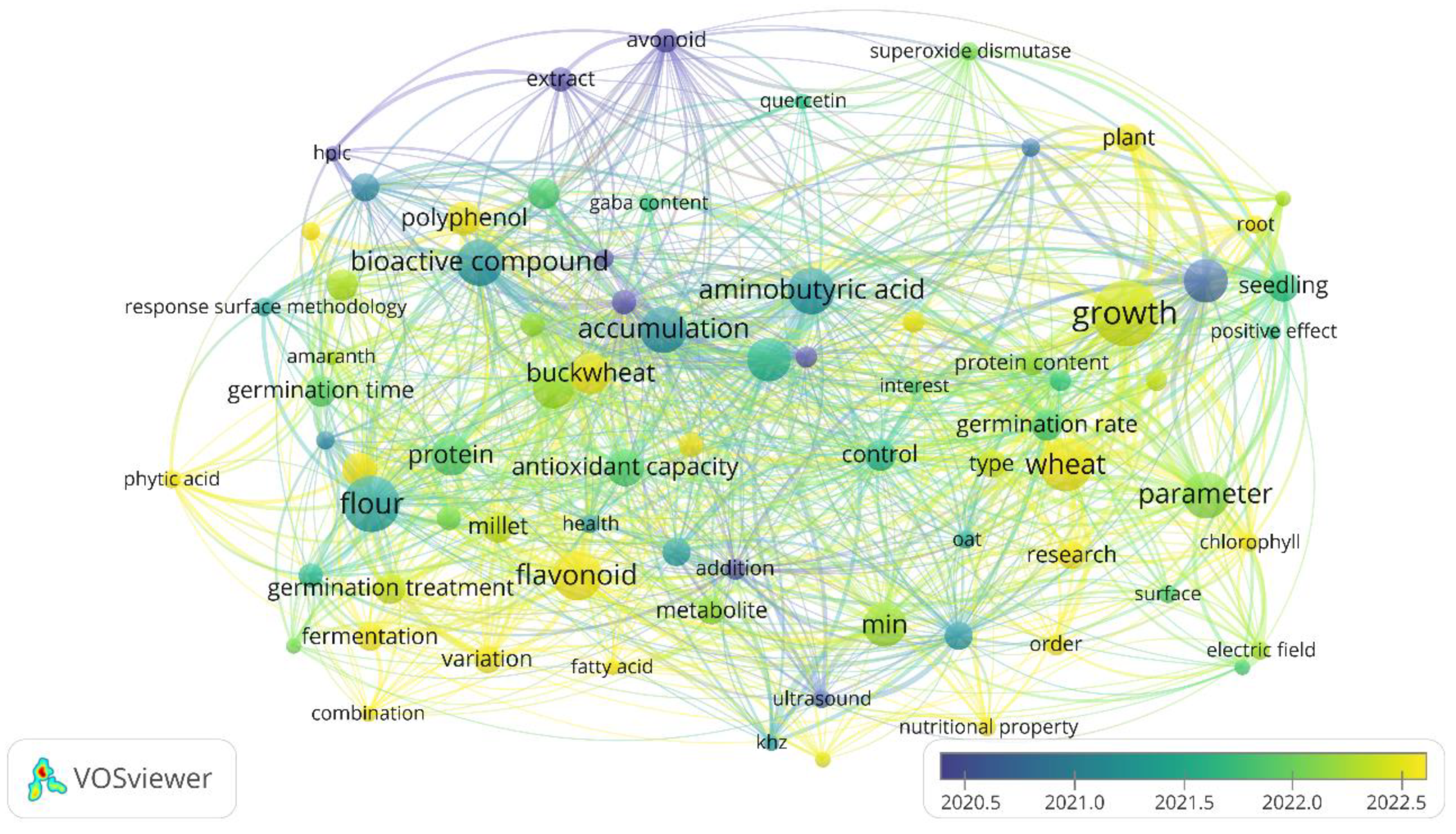
Temporal Trends. The chronological analysis
(Figure 2) revealed an evolution in research approaches. The most recent terms (2022-2023) were concentrated in “combination” (2023.4), “polyphenol content” (2023.33), “flavonoid” (2023.06), and “corn” (2023.00), indicating a growing interest in specific compounds and treatment combinations. The more established terms (2018-2020) included “HPLC” (2018.00), “amylase” (2018.83), and “extract” (2019.63), mainly associated with analytical methodologies.
Specific Terms of Interest. Pseudocereals emerged as prominent study models, with particular attention to “buckwheat” (87 links, year 2022.46), “quinoa” (92 links, year 2022.15), and “amaranth” (34 links, year 2022.20). These terms showed strong associations with bioactive compounds and functional properties.
Among the emerging inducers, “electric field” (33 links, year 2022.17), “ultrasound” (53 links, year 2020.67), “fermentation” (48 links, year 2022.56), and “germination treatment” (58 links, year 2022.30) stood out. Analysis of their connections revealed significant associations with specific bioactive compounds, particularly GABA and flavonoids.
The bioactive compounds with the greatest presence in the network were “aminobutyric acid” (115 links), “GABA” (97 links), “flavonoid” (96 links), and “polyphenol” (73 links). The high frequency of these terms and their multiple connections indicate centrality in research on germination and emerging inducers.
This bibliometric analysis provides a structured overview of current research on emerging inducers for the germination of cereals and pseudocereals, identifying areas of research concentration, emerging trends, and opportunities for future studies. The convergence of terms between clusters suggests an interdisciplinary field with multiple methodological and conceptual approaches to improving the bioactive profile through specific inducers during germination.
3. Fundamentals of Germination and Its Impact on Bioactive Compounds
3.1. Germination Process: Physiological and Biochemical Aspects
The germination process is a complex physiological and biochemical phenomenon that involves a series of key stages [
50]. This process can be divided into three main phases, 1. Imbibition Phase: During this phase, the dry seed rapidly absorbs water, activating cellular metabolism and triggering a series of biochemical changes [
50,
51]. 2. The Metabolic Activation Phase: in this stage, water absorption slows down, but key metabolic processes are activated, such as the synthesis of hydrolytic enzymes that degrade the seed
’s reserve compounds [
51,
52]. 3. The Visible Growth Phase: in this final phase, water absorption resumes, leading to radicle emergence and seedling establishment [
50].
During these three phases, profound changes occur at the physiological and biochemical levels [
50,
51,
52]. Enzymatic pathways are activated, reserve compounds are degraded, new proteins and secondary metabolites are synthesized, and a structural reorganization of seed tissues takes place [
53,
54,
55].
Various factors, such as water stress, salinity, and the presence of heavy metals, can negatively affect the germination process, altering the physiological and biochemical parameters of the seed [
53,
54,
56,
57,
58]. On the other hand, some treatments, such as seed conditioning (priming), can improve plant tolerance to stress and promote more efficient germination [
53,
54,
55]. Understanding these mechanisms is fundamental to optimizing germination and maximizing its composition of bioactive compounds.
3.2. Main Bioactive Compounds in Germinated Seeds
Bioactive compounds are secondary metabolites and physiologically active components present in grains, which acquire special relevance during the germination process. These substances are characterized by exerting specific biological effects that promote human health benefits beyond their basic nutritional value. Germination significantly modifies the phytochemical profile of grains, generating a diverse spectrum of compounds with functional properties. This section describes the main groups of bioactive compounds identified in germinated seeds, cataloged according to their chemical structure and functionality: phenolic compounds, GABA, bioactive peptides, melatonin and indolic compounds, vitamins and bioavailable minerals, antioxidant enzymes, diverse phytochemicals, and dietary fibers
(Table 1). This classification allows understanding the molecular diversity present in germinated grains and establishes the basis for the subsequent analysis of their functional properties and biological action mechanisms.
3.1.1. Phenolic Compounds
Phenolic compounds constitute one of the most important and widely studied groups of phytochemicals in germinated seeds. Total phenols, total flavonoids, and total 3-deoxy-anthocyanidins in germinated red sorghum (
Sorghum bicolor) and pearl millet (
Pennisetum glaucum) were reported [
9]. The presence of polyphenols and total flavonoids in germinated foxtail millet (
Setaria italica L.) were also documented [
34]. Likewise, [
59] characterized a detailed profile of phenolic compounds in germinated buckwheat (
Fagopyrum esculentum) and quinoa (
Chenopodium quinoa), distinguishing between flavonoids (rutin, quercetin, kaempferol, chrysin, hesperidin, catechin, epicatechin) and phenolic acids (
p-hydroxybenzoic, chlorogenic, ellagic, salicylic,
p-coumaric, gentisic, ferulic).
The scientific evidence is considered “moderate” because in vitro studies were presented, but in vivo validation or commercial applications were limited (Table 1).
The presence of phenolic acids is particularly relevant in germinated cereals. Various phenolic acids have been identified and quantified in germinated black rice (
Oryza sativa), including gallic acid, chlorogenic acid, ellagic acid, ferulic acid, hydroxybenzoic acid, isoferulic acid,
p-coumaric acid, protocatechuic acid, sinapic acid, and vanillic acid [
68]. The same authors detected flavonoids (kaempferol, quercetin, rutin) and anthocyanins (cyanidin-3-galactoside, cyanidin-3-glucoside, cyanidin-3,5-diglucoside, cyanidin-3-rutinoside, malvidin-3-galactoside) in this species. The presence of total phenols, total flavonoids and specific phenolic compounds (chlorogenic acid, catechin, 4-hydroxybenzoic acid, sinapic acid, rutin, naringin, quercetin, caffeic acid,
p-coumaric acid, epicatechin) in germinated wheat has been reported [
36].
The profile of phenolic compounds varies considerably between species. Total phenolic compounds and anthocyanins in germinated blue corn (
Zea mays L.) were evaluated, while the identified total phenolic compounds in germinated naked barley [
11]. On the other hand, total flavonoids were reported in soft wheat varieties Zauralochka and Erythrosperium, as well as in barley variety Chelyabinets 1 [
60].
3.1.2. GABA (γ-Aminobutyric Acid)
GABA constitutes one of the widely studied bioactive compounds in germinated grains. GABA has been identified in germinated naked barley [
11]. This compound has been detected in soft wheat varieties Zauralochka and Erythrosperium, as well as in barley variety Chelyabinets [
26]. In one study, the focus was exclusively on the characterization of GABA in germinated brown rice (
Oryza sativa L., variety Nanjing) [
60].
GABA has been identified in various germinated grain species. GABA was detected along with other bioactive compounds in germinated djulis, a native pseudocereal from Taiwan [
12]. Similarly, GABA and total free amino acids were identified in germinated wheat [
28]. GABA was also detected in germinated sorghum [
61], and in germinated kodo millet (
Paspalum scrobiculatum) [
62].
Other researchers have analyzed GABA in combination with other compounds. In germinated coix seed (
Coix lacryma-jobi L.), GABA, soluble proteins, and free amino acids were identified [
64]. GABA, along with total flavonoids, total polyphenols, riboflavin, and
β-glucan were detected in germinated highland barley [
73]. GABA and dietary fiber fractions were also analyzed in different varieties of germinated wheat (
Triticum aestivum) [
4].
3.1.3. Bioactive Peptides
Bioactive peptides constitute a group of compounds of growing interest in germinated grains. Free peptides were identified in germinated djulis, a native pseudocereal from Taiwan [
12], soluble proteins in germinated wheat [
28]. The protein content in sprouts and roots of germinated corn (
Zea mays L., hybrid FH-1036) was analyzed [
63].
Studies in germinated wheat have allowed characterizing soluble proteins and free amino acids [
25]. Free amino acid profile in germinated brown rice grains were analyzed [
29]. These studies have established specific profiles of bioactive nitrogenous compounds during germination.
3.1.4. Melatonin and Indolic Compounds
Melatonin (N-acetyl-5-methoxytryptamine) and other indolic compounds represent a less studied group but of considerable biological importance in germinated grains. A detailed study of bioactive compounds in germinated amaranth (
Amaranthus cruentus) was conducted, identifying tryptophan and indolic derivatives such as caffeoylquinic acid,
p-coumaroylquinic acid, and feruloylquinic acid [
66].
This group of compounds has received less attention in scientific literature on germinated grains, but existing data suggest their significant contribution to bioactive properties, particularly regarding their antioxidant capacity and neuroprotective potential.
3.1.5. Vitamins and Bioavailable Minerals
Germinated grains constitute an important source of vitamins and minerals in bioavailable forms. The bioavailability of essential minerals such as iron and zinc in germinated corn (
Zea mays variety ZM607 - MUTUTU-18A) was studied [
15]. Vitamin C was also detected in germinated wheat [
28].
The vitamin profile of germinated grains includes various vitamins. The 5-methyltetrahydrofolate (5-MTHF),
β-carotene, lutein, vitamin C, and vitamin B2 were detected in various varieties of germinated quinoa (
Chenopodium quinoa Willd.) [
74].
Carotenoids have been identified by various authors. Lutein, zeaxanthin, α-cryptoxanthin,
β-cryptoxanthin,
α-carotene, and
β-carotene were detected in germinated yellow corn (
Zea mays L., cultivar Suyu 29) [
65]. Vitamin E (tocopherols and tocotrienols) and
γ-oryzanols have also been identified in germinated rice, along with anthocyanins and phytosterols [
24].
3.1.6. Antioxidant Enzymes
Antioxidant enzymes constitute an endogenous defense system present in germinated grains that varies between species. The enzymatic antioxidant system commonly includes superoxide dismutase, catalase, peroxidase, and ascorbate peroxidase, which have been identified across various germinated grains [
6,
23]. In germinated wheat, comprehensive antioxidant enzyme profiles have been documented. In the cultivar Belija, superoxide dismutase, catalase, ascorbate peroxidase, guaiacol peroxidase, and pyrogallol peroxidase were identified [
75]. Similarly, in variety Dilkash 2020, peroxidase, superoxide dismutase, and catalase were detected [
69]. Research on germinated barley has demonstrated similar enzymatic profiles, with variety CDC Copeland showing α-amylase,
β-amylase, and
β-glucanase [
40], while variety Bojos exhibited α-amylase and
β-glucanase activity [
37]. Comparable enzyme profiles have been documented in other germinated cereals, including corn, rice, and oats demonstrating the widespread occurrence of these enzymatic systems across cereal species [
23]. In addition to antioxidant enzymes, germination activates important digestive and metabolic enzymes, with α-amylase and
β-amylase being characterized in germinated wheat, while
β-glucanase activity has been particularly noted in barley varieties [
37,
40].
3.1.7. Diverse Phytochemicals
In addition to the main groups of bioactive compounds, germinated grains contain various phytochemicals with important biological properties. Photosynthetic pigments have been widely studied. Total chlorophyll, chlorophyll
a, chlorophyll
b, and carotenoids were detected in germinated wheat, barley, and oats (
Avena sativa L.) [
75]. These same pigments were identified in germinated wheat (
Triticum aestivum L., variety Dilkash 2020) [
69].
The identified carotenoids include specific compounds. Lutein, zeaxanthin,
α-cryptoxanthin,
β-cryptoxanthin,
α-carotene, and
β-carotene were detected in germinated yellow corn (
Zea mays L., cultivar Suyu 29) [
70]. Carotenoids along with chlorophyll
a and chlorophyll
b were identified in germinated wheat [
28].
Other phytochemicals of interest include saponins, phytosterols, and alkaloids. Phytosterols (
β-sitosterol) and triterpenoids (24-methylenecycloartanol) were identified in germinated rice [
24]. Condensed tannins, hydrolyzed tannins, and saponins were also characterized in germinated grains [
49].
Total chlorophyll, carotenoids, and anthocyanins in various germinated grains were studied [
27,
76]. On the other hand, chlorophyll, phenolic content, carotenoids, and proline in germinated grains were analyzed [
77].
3.1.8. Dietary Fiber
The dietary fiber present in germinated grains includes various non-digestible polysaccharides with significant functional properties and health benefits.
β-glucan concentrations have been quantified in several germinated cereals, with notable decreases during germination due to enzymatic degradation. In germinated hulled oats variety Barra,
β-glucan content decreased by 46.8% after 216 hours of germination, while in dehulled oats variety Meeri, concentrations decreased by 55.9% after 156 hours [
71]. Similarly, in germinated highland barley,
β-glucan content showed a reduction of 9.68% after 48 hours of germination [
73]. Despite these reductions, the remaining
β-glucan concentrations are physiologically significant for cholesterol reduction and glycemic control, as dietary intakes of 3-4 g/day have been associated with measurable cardiovascular benefits.
Arabinoxylans represent another crucial component of dietary fiber in cereals, with germination showing positive effects on their accumulation. In germinated wheat, arabinoxylan content increased by 33% after 120 hours of germination. This increase is nutritionally beneficial as arabinoxylans contribute to intestinal health through their prebiotic properties and their ability to modulate glucose absorption. These compounds were comprehensively studied in seven germinated grain species: wheat, oats, barley, rye, sorghum, brown rice, and buckwheat, along with inositol phosphates (InsP4, InsP5, InsP6) which exhibit additional mineral bioavailability enhancement properties [
47].
The comprehensive analysis of dietary fiber fractions reveals the complex nature of these compounds in germinated grains. Soluble dietary fiber, insoluble dietary fiber, and total dietary fiber were characterized in three varieties of germinated wheat: hard red spring wheat, hard white wheat, and soft white wheat [
4]. The functional significance of these fiber fractions lies in their distinct physiological effects: soluble fiber contributes to cholesterol reduction and glucose metabolism regulation through increased intestinal viscosity, while insoluble fiber promotes intestinal transit and serves as substrate for beneficial microbiota fermentation, producing short-chain fatty acids with anti-inflammatory and metabolic regulatory properties [
4,
11,
70,
72].
4. Factors Influencing the Accumulation of Bioactive Compounds
The germination process significantly affects the synthesis and accumulation of bioactive compounds in seeds. Several factors influence this dynamic biochemical transformation, including temperature, moisture availability, light exposure, oxygen levels, and the duration of germination. These conditions modulate enzymatic activity and metabolic pathways, which in turn stimulate the production of phenolic compounds, vitamins, and other antioxidants. Optimizing these parameters can enhance the nutritional and functional properties of germinated seeds, making germination a valuable tool in improving food quality.
4.1. Genetic Factors
The genotype or genetics of cereals significantly influences the production of bioactive compounds [
78]. The amount of phenolic compounds in seed samples is strongly influenced by the genotype (variety/cultivar). This implies that different varieties of the same cereal can produce variable amounts of these bioactive compounds [
7]. It was found that barley cultivars differed in their content of determined phytochemicals, as well as in their antioxidant potential and cholinesterase inhibitory activity, suggesting a genetic basis for these variations [
79]. Eight different cultivars were used, including spring and winter varieties, and differences in the composition of phenolic acids and flavonoids were observed.
4.2. Environmental Conditions During Growth
Phenolic compound levels in seeds are strongly affected by environmental conditions during growth. Soil composition, climate, and harvest maturity influence metabolic pathways involved in biosynthesis. These factors impact both the accumulation and profile of bioactive compounds, making environmental management crucial for enhancing the nutritional and functional quality of seed-derived products [
7].
4.3. Germination Process Parameters
Germination is a key factor that can significantly increase the content of bioactive compounds [
8]. The specific parameters that influence are germination temperature, which affects the activity of enzymes involved in the biosynthesis and release of bioactive compounds [
27,
42]. Moderate temperatures may be more effective for certain compounds [
27]. Humidity, adequate relative humidity is essential for enzymatic activation and metabolism during germination, which indirectly influences the accumulation of bioactives [
36,
42]. Lighting, the duration and intensity of light, including UV-B light, can stimulate the production of compounds such as phenols and flavonoids in sprouts [
27,
42]. Optimal intensity is crucial [
73]. Germination time, the content of bioactive compounds varies significantly with the duration of germination. There is an optimal time to maximize the accumulation of specific compounds such as polyphenols and GABA [
8,
42]. The pH of the soaking and germination medium can influence enzymatic activity and the solubility of bioactive compounds [
30].
4.4. Other Processing Treatments
In addition to germination, other processes can affect the accumulation or release of bioactive compounds. Fermentation can increase the solubility and extractability of flavonoids and other phenolic compounds due to microbial enzymatic activity [
9,
62]. Soaking can reduce some antinutritional compounds, but release water-soluble bioactive compounds [
62]. Cooking (autoclaving, baking, steaming) can have variable effects, from degradation to the release of certain bioactive compounds, depending on temperature and time [
30,
80]. Irradiation (microwave, controlled UV-B radiation, plasma) can stimulate the synthesis of bioactive compounds or improve their extraction by altering cellular structures [
22,
42,
81]. For example, UV-B irradiation can increase the content of flavonoids and polyphenols [
65]. Plasma treatment can also influence growth and accumulation of phytochemicals [
22].
4.5. Abiotic Stress
Exposure to mild to moderate stress conditions, such as salinity or suboptimal temperature, can activate defense mechanisms in the plant that result in greater production of secondary metabolites with antioxidant activity [
23,
27]. Understanding these factors has allowed the development of strategies to maximize the synthesis of specific bioactive compounds, leading to the concept of “directed germination” or “inducer-assisted germination”.
5. Physical Inducers of Germination
5.1. Controlled Germination
Temperature and germination time are critical parameters that significantly influence the germination of cereals and pseudocereals, as well as the enhancement of their bioactive compounds and antioxidant capacity [
8,
46,
47,
82,
83,
84,
85,
86]
(Table 2). Optimal temperatures vary according to species: for example, finger and pearl millet show better growth at 30°C, while buckwheat prefers lower temperatures (22°C) [
85] or 25°C [
27]. In quinoa, 20°C for 42 hours maximizes total phenolic content (TPC) with an 80% increase and antioxidant activity with a 30% increase [
87]. However, elevated temperatures (60°C) during drying can decrease TPC in red quinoa sprouts [
86]. Germination time is also crucial; in quinoa, the most pronounced increases in bioactive metabolites occur between the third and fifth day [
82], and GABA content doubles at 48 hours [
8]. Nevertheless, prolonged times can reduce phenols and antioxidant activity in some millets [
85]. Initial soaking is essential to activate enzymes and solubilize antinutrients [
8,
88]; however, prolonged soaking in water can worsen some nutritional parameters in quinoa [
8]. The light/dark regime also plays a role; an extended photoperiod (20/4 h) can stimulate pigment biosynthesis in buckwheat microgreens [
27]. Germination in darkness was commonly used in studies [
84,
86]. Other parameters such as ultrasonication and microwave treatment, often combined with germination, can enhance the extraction of phytochemicals and antioxidant activity in millets [
83,
88]. The use of priming with GABA increased germination and antioxidant activity in aged wheat and triticale (
×Triticosecale Wittm.) [
89]. The general mechanism of action involves the activation of hydrolytic enzymes that release phenolic compounds and other bioactives, de novo synthesis of secondary metabolites, improvement in nutrient bioavailability, and activation of enzymatic and non-enzymatic antioxidant defense systems [
8,
46,
47,
86,
88,
90].
Systematic optimization of germination conditions constitutes a fundamental approach for enhancing bioactive compound profiles across diverse cereal and pseudocereal species (
Table 3), while quinoa-specific optimization protocols establish benchmarks for controlled germination methodologies (
Table 2).
5.2. Plasma Activated Water (PAW) Treatments
The application of plasma activated water (PAW) has emerged as an inducer with significant impacts on germination and enhancement of bioactive compounds. Studies reveal that PAW not only promotes germination in cereals such as wheat and barley but also enhances the accumulation of key bioactive compounds and antioxidant capacity [
28,
40]. In particular, it was demonstrated that the optimal PAW treatment for 3 minutes (PAW-3) in wheat increased germination by up to 100%, concomitant with notable increases in chlorophyll
a (89.46%), chlorophyll
b (112.46%), carotenoids (91.58%), total phenolic content (10.46%), and superoxide dismutase activity (47.12%), translating into a robust antioxidant capacity (up to 35.34% by ORAC) [
28]. Improvements in germination and an increase in
β-amylase activity in barley treated with PAW were also observed [
40]. The underlying mechanism for this enhancement of bioactive compounds is attributed to a synergistic combination of factors, including improved nitrogen supply from nitrates and nitrites present in the PAW [
28], induction of mild abiotic stress that activates metabolic defense pathways, and the signaling action of reactive oxygen and nitrogen species (RONS), which, at controlled concentrations, modulate gene expression and enzymatic activity involved in the biosynthesis of these valuable compounds [
28,
40]. These results establish PAW as a promising tool for producing functional foods by improving nutritional value.
5.3. High Hydrostatic Pressure (HHP) Treatments
High hydrostatic pressure treatments demonstrate variable effects on cereals depending on the specific technology and conditions employed. Traditional HHP combined with soaking significantly enhanced bioactive compound content, with buckwheat flour showing a 16.1% increase in total phenolic content when treated with one cycle of HHP (600 MPa, 30 min) following soaking pretreatment [
104]. The mechanism involves improved cell permeabilization and enhanced extractability of phenolic compounds through pressure-induced mass transport facilitation.
In contrast, high pressure carbon dioxide (HPCD) treatments showed predominantly negative effects on germination capacity. HPCD significantly reduced oat germination from 58% to 0% and completely inhibited barley germination under treatment conditions [
105]. The mechanism involves dissolved CO₂ penetrating seeds, modifying cellular pH, and forming bicarbonate complexes that affect key enzymes such as α-amylase, with water activity being a critical determining factor.
These findings indicate that while traditional HHP with soaking proves effective for enhancing bioactive compound extractability, CO₂-based pressure treatments require careful optimization to avoid detrimental effects on seed viability, suggesting that pressure treatment outcomes are highly dependent on the specific methodology and target cereal species [
104,
105].
5.4. Pulsed Electric Fields (PEF)
The application of Pulsed Electric Fields (PEF) emerges as a technology with notable effects on the physiology and biochemistry of seeds and seedlings of cereals such as wheat and barley.
Regarding germination, PEF treatment can exert both stimulating and inhibitory effects, depending on the treatment parameters and pre-existing conditions of the seed. It was observed that the application of PEF at 6 kV·cm
-1 with 50 pulses increased wheat seed germination, an effect that is directly associated with increased water uptake induced by cell membrane permeabilization [
25]. Similarly, it was reported that PEF treatments of lower intensity (3 kV·cm
-1, 9.9-19.8 kJ·kg
˗1) applied before the first hydration cycle in wheat improved germination parameters [
37]. However, it is noted that prolonged pre-soaking before PEF treatment can be detrimental to germinative energy in barley, suggesting an optimal window of PEF application to favor germination without compromising embryo viability [
106] .
Regarding the enhancement of bioactive compounds, research indicates a positive impact of PEF on the accumulation of valuable metabolites. Significant increases in carotenoids were found in the juice of wheat seedlings treated with PEF, a 34% increase with treatment at 6 kV·cm
-1 and 50 pulses [
25]. Significant increases in total phenolic compound content (18.56%) and chlorophylls (373%) were also reported.
Notable increases in
α-amylase activity (up to 104%) and
β-amylase (up to 25%) with PEF treatments (3 kV·cm
-1, 9.9-19.8 kJ·kg
-1) have been reported in wheat malting, highlighting the ability of PEF to modulate the production of key enzymes with industrial applications [
37].
Antioxidant capacity, evaluated by the DPPH assay, was also significantly increased (5.78%) in the juice of wheat seedlings treated with PEF, which correlates with the increase in phenolic compound content and other metabolites with antioxidant properties [
25].
The underlying mechanism of action for these various effects of PEF focuses on cell membrane permeabilization [
25,
37,
106]. The application of high voltage, short duration pulses induce an increase in transmembrane potential, which can lead to the formation of micropores in the cell membrane, facilitating mass transport and water absorption [
37]. This process can be reversible or irreversible depending on the treatment intensity [
37]. Permeabilization can also trigger stress responses in the plant, including the production of reactive oxygen species (ROS), which in turn can activate defense mechanisms that lead to the accumulation of antioxidant compounds and other secondary metabolites, such as carotenoids, total phenolic compounds, and chlorophylls [
25]. Additionally, PEF can directly influence enzyme activity and synthesis by altering protein structure or facilitating cofactor availability [
25,
37].
Finally, PEF technology represents a promising tool for modulating germination, enriching key bioactive compounds (including enzymes and antioxidants) in cereals and pseudocereals. Optimization of PEF parameters, considering factors such as electric field intensity, number and duration of pulses, as well as seed pre-treatment conditions (e.g., hydration level), is crucial to direct PEF effects toward the desired outcomes in various food industry applications.
5.5. High Voltage Electric Fields (HVEFs)
Treatment with high voltage electric fields (HVEFs) has demonstrated potential for enhancing germination parameters in cereals, though research on bioactive compound enhancement remains limited. Pre-sowing stimulation using constant, alternating, and pulsed high voltage electric fields improved germination speed and uniformity in winter triticale and barley, with optimal constant high voltage electric field (CHVEF) treatment at 3 kV.cm
-1 for 60s achieving 96.7% germination energy and 98.7% uniformity. While significant improvements in growth parameters were observed, including 28.7% increase in root system length and 31.0% increase in grains per spike in triticale, no specific bioactive compounds were analyzed in these studies [
107].
The stimulating mechanism involves redistribution of electrical charges within the seed
’s internal structure, altering physicochemical processes and intensifying biological activities. Although HVEFs showed greater resistance to drought stress, which could be indirectly related to oxidative stress mechanisms, antioxidant activity and specific bioactive compound accumulation were not quantified [
107]. Further research is needed to evaluate the potential of HVEF treatments for enhancing bioactive compound content in germinated cereals, as current studies focus primarily on germination and growth performance rather than phytochemical enrichment.
5.6. Magnetic Fields
Studies reveal significant effects of magnetic fields on germination and the accumulation of bioactive compounds [
17,
26]. In triticale seeds, the application of magnetic fields in the range of 2.23-3.72 mT accelerated germination, evidenced by the decrease in time required to reach 50% germination and the achievement of final germination rates above 90% [
17]. The magnetic time model explains this phenomenon. In contrast, in germinated brown rice treated with a 10 mT magnetic field in the presence of exogenous GABA, no significant promotion of root growth was observed [
26]. A notable finding is the substantial increase in GABA content in magnetically treated GBR with exogenous GABA supplementation, reaching increases in levels from 56% to 207%. Regarding antioxidant capacity, magnetic treatment had a modest effect on the activity of antioxidant enzymes in GABA-enriched GBR, suggesting that the increase in GABA content is the dominant factor in modulating the antioxidant response [
26]. The proposed mechanism of action for germination acceleration involves an interaction with temporal processes within the seed [
17]. On the other hand, it has been elucidated that the increase in GABA is mainly due to an improvement in cell membrane permeability, facilitating the absorption of exogenous GABA, with a possible minor contribution from the inhibition of the GABA-aminotransferase enzyme [
26].
Considering the evidence presented, magnetic fields demonstrate a positive effect on accelerating triticale germination and significant potential for increasing GABA content in germinated brown rice in the presence of exogenous GABA, primarily through improved cell membrane permeability.
5.7. High Pressure Carbon Dioxide (HPCD)
The findings presented suggest that HPCD demonstrates limited potential as a germination enhancer, showing predominantly inhibitory effects on seed viability across cereal types, with treatment conditions, especially hydration, exacerbating the negative impact on germination rates [
105]. Further research is needed to optimize HPCD parameters that could potentially balance antimicrobial efficacy with preservation of germination capacity.
5.8. Microwave Irradiation
Microwave irradiation proved to be an effective strategy to enhance the accumulation of bioactive compounds in cereals and pseudocereals during germination. In tartary buckwheat, microwave treatment (300 W/50 s) significantly increased the total flavonoid content in sprouts by 31.78% compared to the control [
81]. This increase correlated with higher activity of key enzymes in flavonoid biosynthesis, such as phenylalanine ammonia-lyase (PAL), chalcone isomerase (CHI), and flavonol synthase (FLS) [
81]. Similarly, exposure to microwaves (600 W/30 s) stimulated the total flavone content in tartary buckwheat (
Fagopyrum tataricum) sprouts [
108]. In barley seedlings, microwave treatment increased the total amount of phenolic substances with antioxidant properties [
109].
The proposed mechanism of action involves the ability of microwaves to penetrate seed tissues, altering macromolecular structure and affecting physicochemical characteristics. Additionally, it is suggested that microwave irradiation induces the accumulation of stress-related transcription factors and the expression of key genes for flavonoid biosynthetic enzymes. Finally, an increase in tyrosinase and acetylcholinesterase inhibitory activities was observed, which could also be related to the higher concentration of phenolic compounds and flavonoids [
81,
108].
In summary, the controlled application of microwave irradiation during germination emerges as a promising technique to enrich cereals and pseudocereals with key bioactive compounds, mainly flavonoids and other phenols, through the modulation of enzymatic biosynthetic pathways, suggesting added value for functional food production.
5.9. Light Intensity
Controlled visible light modulation during germination represents a precise biotechnological strategy for enhancing bioactive compound profiles in cereals and pseudocereals. This approach demonstrates significant potential for developing superior functional foods through targeted secondary metabolite accumulation.
In common buckwheat (
Fagopyrum esculentum), photoperiod manipulation emerges as a critical factor. Extended photoperiod conditions (20/4 h light/dark) increased total chlorophyll content by 35.40% and total carotenoids by 21.34%, while maximizing total flavonoid production and antioxidant activity [
27]. Similarly, tartary buckwheat germination under optimized light intensities (6,000-10,000 lux) enhanced rutin, flavonoids, and total polyphenol accumulation, with maximum antioxidant capacity achieved at 10,000 lux [
42].
Blue corn germination under controlled light/dark cycles using white fluorescent tubes (16 W/2,700 K) resulted in a 9.9% increase in total anthocyanin content, demonstrating the importance of visible light in pigment biosynthesis during seedling development [
21,
101]. These findings establish that precise control of light intensity and duration constitutes a powerful tool for optimizing the functional and nutritional quality of cereal and pseudocereal sprouts through quantifiable bioactive compound accumulation.
5.10. Pulsed Light (PL)
Pulsed light (PL) emerges as an effective technology to positively influence cereal germination, as demonstrated by studies on germinated brown rice [
110] and germinated corn [
111]. In brown rice, pulsed light treatment (PLT) significantly increased sprout length between 12.7% and 26.9% in eight varieties [
110]. Likewise, in corn, PL promoted germination and accelerated macromolecule hydrolysis [
111]. Regarding the enhancement of bioactive compounds, GABA stands out as the main enriched compound. Germinated brown rice showed an increase in GABA over 100% in the eight varieties analyzed, being more significant in the Koshihikari variety [
110]. In germinated corn, a 27.20% increase in GABA content was observed after pulsed light treatment [
111]. The mechanism of action of pulsed light varies according to the cereal. In brown rice, it is proposed that PLT activates metabolic pathways related to phenylalanine biosynthesis, carbohydrate and energy metabolism, as well as the GABA shunt pathway and polyamine degradation, with the OsbZIP56 transcription factor playing a key regulatory role [
110]. In germinated corn, the mechanism involves the activation of the glutamate decarboxylase (GAD) enzyme, crucial for GABA synthesis from glutamic acid, and the inhibition of
γ-aminobutyric transaminase (GABA-T), which degrades GABA, leading to its accumulation. Metabolomic analysis in corn revealed the activation of metabolic pathways associated with amino acid and carbohydrate metabolism, which influence GABA production [
111].
Considering the evidence presented, pulsed light is an effective tool for promoting germination and significantly enriching GABA content in cereals such as brown rice and corn, by modulating specific metabolic pathways and activity.
Considering the evidence presented, pulsed light is an effective tool for promoting germination and significantly enriching GABA content in cereals such as brown rice and corn, by modulating specific metabolic pathways and activity. The electromagnetic and pressure technologies discussed in the preceding sections, including their optimal parameters, mechanisms of action, and quantitative results for bioactive compound enhancement, are comprehensively detailed in
Table 4.
5.11. Ultraviolet (UV) Radiation
The application of ultraviolet (UV) radiation can influence the germination process of cereals and pseudocereals, enhancing the accumulation of bioactive compounds and antioxidant capacity [
48]. In some cases, UV-C radiation did not affect germination yield and even decreased the time needed to reach commercial height in chia (
Salvia hispanica L.) [
48]. Regarding the enhancement of bioactive compounds, in blue corn, germination combined with UV-B elicitation significantly increased the content of total phenolic compounds by 587.2%, total anthocyanins by 29.9%, and GABA by 199.9% [
21]. In amaranth sprouts treated with UV-C, a 17.7% increase in
p-coumaroylquinic acid was observed [
66]. In buckwheat, optimized UV-B treatment increased total flavonoid content by 97% [
113]. In germinated highland barley, UV-B radiation elevated polyphenol levels by up to 49.40% under specific conditions [
73]. Regarding antioxidant capacity, in blue corn germinated and elicited with UV-B, antioxidant activity measured by ABTS increased by 133.9% and by DPPH by 173.4% [
21]. In chia, UV-C radiation positively influenced the antioxidant properties of the sprouts, with significant increases in DPPH activity [
48]. In buckwheat, UV-B treatment also improved antioxidant capacity [
113]. The main mechanism of action implies that UV radiation induces stress in seeds and seedlings, which activates metabolic defense pathways, including the phenylpropanoid pathway, crucial for the synthesis of phenolic compounds and flavonoids that act as protectors against UV damage [
73,
113]. Additionally, UV radiation can generate reactive oxygen species (ROS), which in turn stimulates the activity and expression of antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX), strengthening the plant
’s antioxidant defense system [
113].
In summary, UV radiation applied in a controlled manner during germination emerges as an effective strategy to enrich germinated cereals and pseudocereals with key bioactive compounds and enhance their antioxidant capacity, although it is crucial to optimize exposure conditions to avoid negative effects on germination and growth.
5.12. Cold Atmospheric Plasma
Cold atmospheric plasma (CAP) treatment emerges as a technology with diverse effects on the germination of cereals and pseudocereals. It has been demonstrated that dielectric barrier discharge (DBD) plasma improves rice germination, with a maximum increase of 9.0% in germination rate with a 60 s exposure, in addition to increasing vigor index and germination speed [
114]. Similarly, it was found that an optimal 6 min plasma exposure increased the fresh weight of barley seedlings by 137.5% compared to the control [
22]. However, it was observed that, although in vitro germination of buckwheat was not affected, seedling emergence in the field decreased between 11% and 20%, suggesting that effects under laboratory conditions do not always translate to the field [
115]. Likewise, short treatments (8.7 s) with Ar-O₂ and Ar-air plasma post-discharge can improve the root system of wheat, barley, and rye, increasing their mass by up to 16.2% in barley [
117]. On the other hand, Cold Atmospheric Pressure Plasma (CAPP) can have a negative effect on barley germination with increasing doses and prolonged exposure times, even completely inhibiting it with nitrogen plasma at 60 s or more [
118].
Regarding the enhancement of bioactive compounds, a single 6-min plasma exposure significantly increased soluble sugars, free amino acids, and key secondary metabolites in barley sprouts, such as saponarin (50%), GABA (90%), and policosanols (90%) [
22]. Alterations in the biochemical composition of harvested buckwheat were also noted, including changes in Fe, Zn, and quercetin content [
115]. It has been observed that cold plasma accelerated the time to reach the maximum content of
γ-oryzanols in germinated rice and could increase the total vitamin E content in certain cultivars [
24].
Regarding antioxidant capacity, no significant differences were found in antioxidant activity between plasma-treated and untreated germinated rice, although the activity was higher in both groups compared to brown rice [
24]. Cultivar-dependent changes in free radical scavenging activity in buckwheat treated with plasma and electromagnetic field were evidenced [
115].
The mechanism of action of cold plasma appears to involve modification of the seed surface, as observed by scanning electron microscopy (SEM) in rice [
24,
114,
117]. This modification can increase hydrophilicity and water absorption, which in turn accelerates germination [
43,
114]. Additionally, plasma can stimulate the activity of enzymes related to germination [
118] and secondary metabolism [
22]. The generation of reactive oxygen and nitrogen species (RONS) also plays a crucial role, acting as signaling molecules that modulate germination pathways and oxidative stress [
118,
119], although excessive exposure can be detrimental [
118].
In retrospect, cold plasma has the potential to positively influence germination and the content of bioactive compounds in cereals and pseudocereals through seed surface modification and activation of metabolic processes, although effects vary significantly according to species, cultivar, and treatment parameters.
5.13. Ultrasonication
The application of ultrasonication has been investigated as a technique to stimulate the germination of cereals and pseudocereals, as well as to enhance the accumulation of bioactive compounds and antioxidant capacity. In terms of germination, ultrasonication can accelerate the process and increase germination rates in wheat, brown rice, corn, and oats [
29,
38,
120]. Regarding bioactive compounds, a significant increase in GABA content has been observed in several ultrasonically treated germinated cereals, such as wheat (up to 30.7% more in buckwheat) [
4,
5], red rice (
Oryza sativa L.) [
5,
29], brown rice [
38,
120] and corn (30.55% more) [
38]. In germinated oats, ultrasonication also enhanced the accumulation of avenanthramides [
5]. Additionally, ultrasound treatment can increase the total phenolic compound content in germinated oats (11.24% more at 24 h) and brown rice [
5,
29], as well as proline in brown rice [
29]. Regarding antioxidant capacity, ultrasonication can improve DPPH free radical scavenging activity in germinated oats and brown rice (72.45% more at 24 h in brown rice) [
5,
29], as well as ferric reducing antioxidant power (FRAP) in brown rice (non-significant increase reported) [
29]. The proposed mechanism of action suggests that ultrasonic waves induce mechanical stress and cavitation effects, which can alter cell membrane permeability, facilitate water entry, increase the activity of endogenous enzymes such as glutamic acid decarboxylase (GAD) [
4,
5,
29,
38], stimulate metabolic pathways such as the GABA-shunt pathway, and activate antioxidant defense mechanisms in seeds [
5,
38]. Additionally, ultrasonication could affect the microstructure of the grain, increasing the availability of substrates for enzymatic hydrolysis [
29,
38].
Considering the evidence presented, ultrasonication emerges as a promising strategy to improve germination and nutritional value of cereals and pseudocereals by increasing key bioactive compounds and antioxidant capacity, through multifactorial mechanisms related to physical stress and metabolic activation.
The radiation, plasma, and ultrasound technologies discussed above, including their optimal parameters, mechanisms of action, and quantitative effects on bioactive compound enhancement, are comprehensively summarized in
Table 5.
6. Chemical Inducers of Germination
6.1. Plant-Derived Inducers
Plant-derived elicitors demonstrate remarkable efficacy in modulating secondary metabolite biosynthesis during cereal and pseudocereal germination. Chitosan treatment (0.1%) induced a 23% enhancement in total phenolic content in buckwheat, while jasmonic acid (150
μM) elicited a more pronounced 148% increase in phenol accumulation [
19]. The enhanced metabolite profile encompassed gallic acid, rutin, catechin, chlorogenic acid, and (-)-epicatechin, suggesting coordinated upregulation of phenylpropanoid pathway enzymes. Both elicitors activate secondary metabolite accumulation through defense response induction and biosynthetic enzyme stimulation, whereas salicylic acid demonstrated no measurable effect on phenolic biosynthesis [
19].
Vegetable ashes (immature banana peel ash) employed as a plant-derived mineral source in corn malting significantly enhanced antioxidant properties and phenolic/flavonoid accumulation in the Coca-sr variety through enzymatic cofactor provision, antinutrient-protein complex disruption, and phytase activation. This multi-target mechanism facilitates metabolic optimization by eliminating inhibitory factors while simultaneously enhancing enzymatic efficiency [
49].
These findings establish plant-derived inducers as potent biotechnological tools for targeted enhancement of phenolic compounds in germinated grains, operating through distinct yet complementary molecular mechanisms that optimize secondary metabolite accumulation and antioxidant capacity [
19,
49].
6.2. Minerals and Trace Elements
The application of minerals and trace elements during the germination of cereals and pseudocereals exerts significant effects on the accumulation of bioactive compounds and antioxidant capacity. Salt stress induced by NaCl in yellow corn (
Zea mays) and quinoa has proven to be a key factor in enhancing secondary metabolites with antioxidant activity [
18,
23]. In yellow corn, although it negatively affected germination and growth, treatment with NaCl (300 mM) increased antioxidant capacity measured by DPPH and ORAC [
18]. Similarly, in quinoa, salt stress (300 mM NaCl) induced a substantial increase in total polyphenol content (approximately 152%) and antioxidant activity, with a notable increase of 3700% in DPPH radical scavenging activity [
23]. This increase is attributed to a defense mechanism where the plant increases the production of phenolic compounds to counteract the oxidative stress generated by salinity [
23]. In wheat , NaCl stress during germination also resulted in a significant increase in total phenolic content (up to 243% at 48 h), associated with higher antioxidant activity, suggesting that salt stress can stimulate metabolic pathways leading to the synthesis of these compounds [
121].
Supplementation with other minerals also influences biochemical composition. In yellow corn, the addition of CaCl₂ (5 mM), besides mitigating the negative effects of NaCl on germination, also increased lutein content (up to 37%) and improved antioxidant capacity, through the regulation of genes involved in carotenoid biosynthesis [
18]. Similarly, in buckwheat sprouts, the application of sodium silicate (SIL) and iron chelate (SIL-Fe) was shown to modulate the phenolic compound profile, with an increase in certain flavonoids (iso-rhamnetin, vitexin) and phenolic acids (ferulic, chlorogenic, sinapic). This effect is explained by elicitation, a method that induces the accumulation of secondary metabolites in plants [
33].
In summary, the manipulation of mineral and trace element availability during germination emerges as an effective strategy to modulate bioactive compound content and antioxidant capacity in cereals and pseudocereals, through the activation of stress response mechanisms and specific metabolic pathways.
6.3. Plant Growth Regulators
Plant growth regulators, phytohormones, and vitamin B6 (pyridoxal phosphate) significantly modulate germination and bioactive properties in cereals and pseudocereals. Gibberellic acid (GA₃) promotes germination in wheat by accelerating α-amylase activity and endosperm degradation, while abscisic acid (ABA) exerts inhibitory effects [
32]. The exogenous application of indoleacetic acid (IAA), salicylic acid (SA), and GA₃ at low concentrations stimulates growth and enhances antioxidant capacity in wheat sprouts. The combination of IAA (0.01 mg/mL), GA (0.001 mg/mL), and SA (0.001 mg/mL) synergistically increases antioxidant activity (FRAP 108%, DPPH 106%) and phenolic compounds (128% total phenols, 182% flavonoids) by activating enzymatic and non-enzymatic antioxidant defenses [
31].
Pyridoxal phosphate (PLP) plays a crucial role as a cofactor for glutamate decarboxylase (GAD) in the synthesis of GABA. In germinated buckwheat, PLP treatment increases GABA content up to 867% compared to non-germinated, significantly enhancing antioxidant capacity (DPPH +15.52%, ABTS +31.47%) and antihypertensive capacity through angiotensin-converting enzyme inhibition, as well as increasing polyphenol content (+10.72%) [
14].
In summary, these regulators constitute effective tools for modulating germination and enhancing bioactive compounds in cereals and pseudocereals through the regulation of key enzymes and specific metabolic pathways.
6.4. Synthetic Chemical Inducers
Synthetic chemical inducers, for the purposes of this review, comprise compounds or solutions prepared in the laboratory, not directly derived from biological sources, that are applied during germination to stimulate specific metabolic responses in cereals and pseudocereals [
10,
16,
30,
122]. These agents, such as hydrogen-rich water, sucrose solutions with CaCl₂, acidic media, and electrolyzed water, cause moderate oxidative stress that activates secondary biochemical pathways, resulting in greater accumulation of bioactive compounds and improved antioxidant capacity [
10,
16,
23,
122].
In research on the germination of cereals and pseudocereals, the application of various synthetic chemical inducers has proven to be an effective strategy for modulating the accumulation of bioactive compounds and antioxidant capacity.
Hydrogen-rich water (HRW) favored the accumulation of chlorophyll and soluble protein, crucial for growth and stress tolerance. The mechanism of action could be related to hydrogen’s ability to modulate oxidative stress in plants [
122].
In buckwheat sprouts, the combined application of sucrose (3%) and CaCl₂ (7.5 mM) significantly increases the content of polyphenols and total flavonoids. This treatment notably improves DPPH and ABTS radical scavenging, reducing power, and inhibition of lipid peroxidation by activating key enzymes in the phenylpropanoid pathway, such as phenylalanine ammonia-lyase (PAL) and tyrosine ammonia-lyase (TAL) [
16].
The use of an acidic medium during germination has also been shown to enhance bioactive compounds. It has been reported that germination of brown rice under acidic conditions (pH 2.0-2.7) reduces phytic acid content and increases phytase activity, enhancing the bioaccessibility of minerals such as calcium, iron, and zinc [
30]. Pretreatment with citric and lactic acid in adlay (
Coix lacryma-jobi L.) germination has shown that citric acid was particularly effective in increasing total polyphenol content (TPC) by 119.6%, total flavonoid content (TFC) by 209.7%, and antioxidant capacity (ORAC) by 646.8% compared to raw adlay. The suggested mechanism is the stimulation of the phenolic biosynthetic pathway by citric acid [
123].
Slightly acidic electrolyzed water (SAEW) is particularly effective in brown rice, significantly increasing antioxidant capacity (DPPH 839.7%, ABTS 792.2%, FRAP 934.2%), total phenols (746.1%), and total flavonoids (579.7%) compared to raw grain. This treatment also increases the levels of GABA, ferulic and
p-coumaric acids, quercetin, and ascorbic acid, possibly through the activation of latent enzymes in response to mild oxidative stress [
10].
Gaseous ozone (O₃) has been investigated as an inducer in cereals, mainly for its fungicidal effect and its influence on germination [
124]. The effect of different durations of O₃ exposure (50 ppm, 1 L·min⁻¹, 1-5 hours) in wheat and other seeds has been evaluated [
39]. Their findings revealed that O₃ treatment exerted a predominantly adverse impact on total phenolic compound content (TPC), with an average decrease of 39.4% in treated wheat grains compared to controls. A similar reduction was observed in wheat sprouts after ozone exposure. Regarding antioxidant activity (AA), although germination generally increases AA in sprouts compared to dry seeds, prolonged exposure to O₃ (4-5 h) resulted in a significant decrease (
P < 0.05) of AA in wheat sprouts [
39]. The proposed mechanism suggests that O₃, due to its potent oxidative capacity, induces the production of reactive oxygen species (ROS) that could initially stimulate antioxidant synthesis; however, prolonged exposures or elevated concentrations cause over-oxidation, degrading phenolic compounds and other antioxidants [
39,
124]. The effects of O₃ show clear dose-dependence: low concentrations preserve germination viability while high doses significantly reduce it [
124]. It is crucial to more thoroughly investigate the optimal exposure conditions of O₃ for cereals, determining specific parameters that avoid negative impacts on their biochemical profile and antioxidant capacity.
The findings presented suggest that synthetic chemical inducers constitute a promising strategy for significantly increasing bioactive compounds and antioxidant capacity in germinated cereals and pseudocereals, by modulating specific metabolic pathways and stress response. Optimization of concentration and exposure times is crucial to maximizing these benefits without compromising germination.
6.5. Nanomaterials
Nanomaterials represent an emerging technological approach for enhancing cereal and pseudocereal germination and bioactive compound accumulation. Zinc oxide nanoparticles (ZnO NPs) have demonstrated significant stimulating effects on germination of cereals such as pearl millet and corn. In pearl millet, seed priming with 150 ppm ZnO NPs improved germination by 20% and vigor index by 51% under laboratory conditions [
125]. Similarly, in corn, ZnO NPs significantly improved germination, recording 92% compared to 68% for the control [
77]. This effect is attributed to the nanoparticles
’ ability to stimulate pre-germinative metabolism and improve tolerance to various abiotic stresses [
125]. Regarding bioactive compound potentiation, an increase in chlorophyll content was observed in both species. In pearl millet treated with 150 ppm ZnO NPs, chlorophyll a and b levels increased by 12.13% and 11.22%, respectively, compared to the control [
125]. Likewise, significant increases in chlorophyll content, sugar, proline, and phenolic compounds were reported in corn seedlings treated with ZnO NPs, suggesting improvement in various secondary metabolites [
77]. Regarding antioxidant capacity, pearl millet extracts treated with ZnO NPs at 150 ppm exhibited DPPH radical inhibition of 78.11%, representing an 8.74% increase compared to control (71.83%) [
125].
Silver nanoparticles (AgNPs) synthesized from cyanobacterial extracts constitute another promising nanomaterial approach. These biologically synthesized AgNPs positively influenced germination of cereals such as barley (cvs. Giza-123, Giza-2000) and wheat (cvs. Benisweif-7, Misr-3), evidencing relative increases in germination percentages, germination rate index (GRI%), and germination velocity coefficient (GVC%), along with slight reductions in mean germination times (MGT). Treatment with AgNPs based on cyanobacterial extracts showed superior promoting effects on germination attributes compared to extracts alone. While specific bioactive compound enhancement data for AgNPs were not detailed, their dual antimicrobial and germination-promoting properties suggest potential for developing multifunctional seed treatments [
67].
The proposed mechanisms of action suggest that both ZnO and AgNPs, due to their small size and large surface area, enhance absorption of essential micronutrients for plant growth and metabolism, including chlorophyll synthesis and activation of antioxidant enzymes [
77,
125]. Additionally, they can influence gene expression related to germination and stress responses, while AgNPs may act as protective particles or carriers of bioactive substances [
67,
125]. These findings establish nanomaterials, particularly ZnO and biologically synthesized AgNPs, as promising strategies to improve germination and enhance key bioactive compounds in cereals, offering significant benefits for sustainable agriculture and improved nutritional quality of crops.
The chemical and biochemical inducers discussed above, including their optimal processing parameters, mechanisms of action, and quantitative effects on bioactive compound accumulation, are systematically summarized in
Table 6.
7. Biological Inducers of Germination
7.1. Fermentation Concurrent with Germination
The combination of germination and fermentation emerges as a synergistic biotechnological strategy to enrich the bioactive compound profile and enhance antioxidant capacity in various cereals and pseudocereals [
11,
12,
34]. In naked barley, germination followed by fermentation significantly increased GABA (
γ-aminobutyric acid) content up to 0.0103 %, while maintaining high levels of
β-glucan (5.66%) and improving antioxidant properties and total phenolic compound content (TPC) [
11]. Similarly, in djulis sprouts, bioreactor fermentation (BF) on a large scale preceded by four days of germination demonstrated a notable increase in free peptide content and hydrolytic enzyme activity (amylase, glucosidase, and proteinase), suggesting the release of bioactive compounds and the generation of new metabolites. This process also elevated the levels of phenolic compounds, carotenoids, chlorophyll a, chlorophyll b, and anthocyanins [
12].
In amaranth, although the study focused on optimization of germination, an increase in proteins, antioxidants, and dietary fiber was observed, along with a reduction in antinutritional factors such as phytic acid and tannins, and an improvement in fatty acids such as oleic and linoleic [
44]. On the other hand, in red sorghum and pearl millet, the combination of germination and spontaneous fermentation resulted in a considerable reduction of phytates and an improvement in mineral status; however, antioxidant activity measured by DPPH was diminished [
9]. Nevertheless, in contrast, in the case of foxtail millet, the combined application of germination and fermentation showed the most pronounced increase in antioxidant activity evaluated through different assays: DPPH (81.54%), FRAP (33.46%), and reducing power (184.52%) expressed as mg of ascorbic acid equivalent (AAE) per 100 g of dry flour [
34].
The fundamental mechanism of action underlying these beneficial effects lies in the activation of endogenous enzymes during germination and the production of microbial enzymes during the fermentation process [
11,
12,
34]. These enzymes catalyze the hydrolysis of complex macromolecules, releasing bioactive compounds that were previously bound or inaccessible, as is the case with the increase in GABA through the activation of glutamate decarboxylase (GAD) [
11]. Fermentation also contributes to the degradation of antinutritional factors such as phytates and tannins, which indirectly can improve the bioavailability of other nutrients and release endogenous enzymes that participate in the modification of cellular components and the generation of compounds with greater antioxidant activity [
34].
As can be deduced from the analysis, the strategic combination of germination and fermentation represents an effective methodology to optimize the bioactive profile and antioxidant capacity of cereals and pseudocereals through enzymatic activation and metabolic modification of their components.
Table 7 provides a comprehensive overview of biological inducers, detailing fermentation-based treatments and microbial derivatives, their optimal application parameters, mechanistic pathways, and quantitative results for bioactive compound accumulation during cereal and pseudocereal germination.
8. Combination of Inducers and Integrated Approaches
8.1. Synergies Between Physical and Biological Inducers
The combination of germination with ultrasonic treatment and fermentation in cereals, specifically wheat and barley, has been shown to have significant effects on the quality of the food ingredient obtained [
60]. Regarding the enhancement of bioactive compounds, notable increases in the content of flavonoids and GABA were observed. Flavonoid content increased between 35% and 68%, being more pronounced in fermented wheat varieties. Similarly, GABA content increased significantly, between 300% and 400% in fermented ingredients compared to controls. Total antioxidant activity also experienced an increase, ranging between 31% and 51% [
60]. The mechanism of action behind these improvements is attributed to the activation of enzymes during germination and fermentation, including proteolytic and amylolytic enzymes that improve digestibility [
11,
34]. Fermentation with a complex starter of microorganisms, such as
Streptococcus thermophilus,
Lactobacillus spp., and
Bifidobacterium spp., contributes to the production of GABA from glutamic acid and increases the solubility and extractability of flavonoids [
11]. Additionally, ultrasonic treatment prior to germination intensifies these processes, favoring the synthesis of bioactive compounds [
4,
5,
60]. Therefore, the combination of germination with ultrasound and fermentation represents an effective strategy to obtain cereal food ingredients with higher bioactive compound content and antioxidant capacity, in addition to improving their uniformity and digestibility [
60].
8.2. Synergies Between Physical and Chemical Inducers
The combination of physical and chemical inducers significantly modulates germination and enriches the bioactive profile of cereals and pseudocereals. In buckwheat and quinoa, the application of germination along with pretreatments such as ultrasound, soaking, or the use of alkali proved to be an effective strategy to increase the content of phenolic compounds and antioxidant activity [
59]. Specifically, a substantial increase in antioxidant activity (AOA) was observed in quinoa after 72 h of germination with ultrasound (64%) and alkali (53%), which was attributed to a greater accumulation of flavonoids and phenolic acids. Ultrasound treatment also favored the accumulation of important bioactive compounds such as rutin, quercetin, and gentisic acid in germinated quinoa, while soaking notably increased hesperidin content (58%). Similarly, in corn grains, the combination of UV-B radiation and CaCl₂ significantly elevated the concentration of carotenoids such as lutein and zeaxanthin compared to control samples. This increase was related to the regulation of key gene expression in the carotenoid biosynthesis pathway [
65]. In selenium-enriched black rice, the use of ultrasound prior to hot air drying improved the extraction of phenolic compounds, with gallic acid being one of the most abundant, in addition to positively influencing the profile of volatile compounds, which could contribute to better preservation of grain quality [
68]. Treatment with GA₃ and KBC (potassium-enriched biochar) in wheat under osmotic stress increased chlorophyll a (up to 34.35%) and b (up to 9.09%) levels [
69]. The general mechanism of action underlying these combined effects involves the activation of crucial metabolic pathways, such as the phenylpropanoid pathway, which is fundamental for the synthesis of phenolic compounds, flavonoids, and other bioactive metabolites [
59]. Controlled germination, especially when combined with physical pretreatments such as ultrasound or chemicals, can induce moderate stress in seeds, which in turn activates endogenous defense systems, resulting in a greater accumulation of compounds with potent antioxidant activity [
75].
Consequently, the combined and strategic application of physical and chemical inducers during germination represents an effective methodology for the selective enhancement of key bioactive compounds and the significant increase of antioxidant capacity in cereals and pseudocereals, highlighting their valuable potential as functional ingredients in the food industry.
8.3. Synergies Between Physical Inducers
The strategic combination of multiple physical inducers demonstrates enhanced effects beyond individual applications, evidencing true synergistic potential for bioactive compound optimization. The combined application of ultrasound (US) and pulsed electric field (PEF) in wheat seedling juice resulted in the highest values of bioactive compounds and antioxidant activity compared to individual treatments, with total phenolic content increasing by 8.59%, total flavonoids by 14.06%, and chlorophyll by 12.06%, while antioxidant capacity measured by DPPH and ORAC increased by 8.58% and 2.34%, respectively [
76]. This synergy suggests that PEF complements ultrasound action by enhancing cellular permeabilization and facilitating extraction of intracellular components beyond what each treatment achieves individually.
The sequential combination of ultrasound and microwave (MW) as pretreatments in sorghum has demonstrated substantial bioactive compound accumulation, with ultrasound treatment (15 minutes) generating the highest GABA accumulation (87.14
μg/g) and achieving superior sprouting percentage (97.33%), while also elevating total phenolic content and antioxidant activity (DPPH inhibition reaching 84.53%) significantly higher than untreated controls [
126]. Additionally, the combination of UV-B radiation with CaCl₂ supplementation in yellow corn exhibited synergistic effects for carotenoid enhancement, with combined treatment significantly elevating lutein (+77.38%), zeaxanthin (+121.07%), α-cryptoxanthin (+75.19%),
β-cryptoxanthin (+65.52%), α-carotene (+79.17%), and
β-carotene (+86.49%) concentrations compared to individual treatments [
65].
The underlying mechanisms involve complementary cellular modifications: ultrasound creates membrane alterations through cavitation that facilitate release and extraction of bioactive compounds while improving grain hydration through enhanced capillary flow [
76,
126], PEF induces electroporation enhancing cellular permeability [
76],while UV-B radiation stimulates biosynthetic pathways that are further optimized by mineral supplementation [
65]. These synergistic combinations demonstrate that integrated physical approaches can achieve superior bioactive compound enhancement through multiple, complementary mechanisms of action, establishing a promising strategy to improve germination, enrich key bioactive compounds, and enhance antioxidant capacity in cereals and pseudocereals.
The integrated approaches discussed in the preceding sections, encompassing synergistic combinations between physical, chemical, and biological inducers, along with their processing conditions, reported synergies, and quantitative results for bioactive compound optimization, are comprehensively detailed in
Table 8.
9. Applications in the Food Industry and Technological Considerations
The development of functional foods from germinated cereals and pseudocereals represents a promising biotechnological strategy for the development of functional foods with improved nutritional and bioactive properties. This natural process allows the transformation of food matrix components, increasing the bioavailability of nutrients and the synthesis of specific bioactive compounds (Table 9).
9.1. Functional Flours
Flours derived from germinated cereals and pseudocereals represent functional ingredients with versatile applications. Flours from germinated foxtail millet suitable for gluten-free foods and improved nutritional formulations have been developed [
34]. It has been possible to obtain flour from germinated amaranth with higher protein and dietary fiber contents for products with increased nutritional value [
44]. Flours from germinated millets with antidiabetic properties for formulations aimed at glycemic control have been obtained [
103]. From germinated corn malted flours for gluten-free foods, natural sweeteners and enriched bakery products have been developed [
49]. Buckwheat flour treated with high hydrostatic pressure for gluten-free bakery products with improved bioactive properties has been proposed [
104]. Finally, flours from germinated quinoa and millets for food products with improved protein digestibility have been obtained [
94].
9.2. Functional Bakery Products
Germinated cereals and pseudocereals offer excellent opportunities to develop functional bakery products. Wheat malt can be used as a “clean label” ingredient in baking, providing anti-aging capacity [
37]. Germinated wheat ingredients have been developed to improve the nutritional quality of baked products [
4]. Cookies with sorghum sprouts with antioxidants and anti-inflammatory properties stable after baking have been made [
13]. Similarly, germinated barley ingredients with prebiotic potential for bakery products have been created [
46]. And finally, germinated rice malt for baked products with improved functional properties has been proposed [
45].
9.3. Functional Breakfast Cereals and Snacks
Breakfast cereals incorporating germinated grains provide an effective vehicle for delivering bioactive compounds through daily dietary consumption. Cereals formulated from germinated wheat, barley, and sorghum have been developed specifically to reduce chronic disease risk through enhanced phenolic content and antioxidant capacity [
47]. Amaranth sprouts demonstrate significant potential as functional ingredients in breakfast formulations due to their superior antioxidant properties [
66]. Germinated blue corn flour has been successfully applied in breakfast cereal development, exhibiting pronounced hypoglycemic properties that support glycemic management [
101]. Additionally, breakfast cereals enriched with GABA from germinated barley and rye (
Secale cereale L.) offer therapeutic benefits for individuals with diabetes or elevated colon cancer risk [
72].
Snack products derived from germinated cereals and pseudocereals represent innovative functional food alternatives with targeted health benefits. Specialized snacks formulated from germinated amaranth, quinoa, and buckwheat have been developed to address the nutritional needs of individuals with celiac disease while providing enhanced bioactive compound profiles [
96]. Germinated buckwheat-based snacks demonstrate cytoprotective efficacy against oxidative cellular damage, offering potential applications in preventive nutrition [
16]. Functional snack formulations utilizing germinated buckwheat and quinoa as primary sources of phenolic compounds have shown promise for antioxidant-enhanced convenience foods [
59]. Furthermore, amaranth sprout-based snacks provide superior bioactive compound concentrations, establishing their potential as premium health-oriented snack alternatives [
66].
The potential applications of cereals and pseudocereals processed using enhanced germination techniques with emerging inducers, along with their recommended inductors, ideal matrices, development status, and commercial prospects, are systematically presented in
Table 9.
9.4. Functional Beverages
Beverages derived from germinated cereals and pseudocereals represent an innovative delivery system for bioactive compound consumption with enhanced bioavailability and functional properties. Wheat seedling juices have been developed with extended shelf-life characteristics, positioning them as viable supplements and functional beverages for commercial applications [
76]. Non-dairy probiotic beverage formulations incorporating germinated grains combined with plant-based milk alternatives have been specifically designed to address the nutritional needs of lactose-intolerant consumers while delivering enhanced bioactive profiles [
99].
Functional juice preparations from germinated wheat demonstrate diverse therapeutic properties, including anti-inflammatory and immunomodulatory activities that support immune system regulation [
28]. Additionally, wheat-derived beverages exhibit antihypertensive and neuroprotective properties, offering potential applications in cardiovascular health management and cognitive function support [
4]. These developments establish germinated cereal-based beverages as promising functional food platforms that combine convenience with targeted health benefits through optimized bioactive compound delivery systems.
9.5. Fermented Foods
Fermented foods based on germinated cereals and pseudocereals offer amplified functional benefits. Fermented products from germinated corn with prebiotic effect to address nutritional deficiencies in vulnerable populations have been developed [
15]. The elaboration of fermented products from germinated sorghum and millet with probiotic properties for functional foods and infant nutritional formulations is reported [
9]. From fermentation with
Rhizopus oligosporus in germinated djulis, food supplements and nutraceutical ingredients have been produced [
12]. Germinated and fermented barley products with high GABA and
β-glucan content as functional components have been formulated [
11]. Fermented products from germinated blue corn have been developed for foods with nutraceutical properties [
44], and germinated barley malt has been applied to produce fermented beverages with prebiotic properties [
106].
9.6. Bioactive Concentrates
Bioactive concentrates derived from germinated cereals and pseudocereals offer applications as functional ingredients. Concentrates from germinated barley rich in saponarin, GABA, and policosanols have been developed as ingredients with antioxidant and neuroprotective properties [
22]. Concentrates from germinated buckwheat with rutin, quercetin, and epicatechin have been obtained for the food and cosmetic industry [
19]. Germinated wild rice (
Zizania latifolia) concentrates have been used as natural nutrient enhancers [
61]. From germinated bitter buckwheat, concentrates with high GABA content have been developed for foods with antihypertensive properties [
14].
9.7. Functional Foods for Glycemic Control
Various researchers have developed foods for glycemic control from germinated cereals and pseudocereals. Products with germinated millets with digestive enzyme inhibition for the management of postprandial hyperglycemia have been formulated [
103]. Foods from germinated quinoa with lower glycemic index for people with obesity and type 2 diabetes have been created [
93]. Products with germinated djulis with dipeptidyl peptidase IV (DP
P-IV) inhibitory properties have been developed [
12]. Likewise, germinated quinoa products with α-amylase and α-glucosidase inhibition have been proposed [
43]. Finally, germinated blue corn foods with hypoglycemic properties have been elaborated [
101].
9.8. Infant Foods
Germinated cereals and pseudocereals offer ideal characteristics for improved infant foods. Germinated amaranth flours for infant foods with better essential amino acid profile have been developed [
44]. Formulations of germinated kodo millet for weaning foods and porridges with low viscosity and higher GABA content have been proposed [
62]. Preparations of germinated and fermented corn with better bioavailability of iron and zinc for infants have also been obtained [
15]. It is also possible to formulate porridges and complementary foods with improved digestibility [
49], developing infant nutritional formulations with germinated and fermented sorghum and millet [
9].
9.9. Foods with Improved Bioavailability
Foods from germinated cereals and pseudocereals present improved bioavailability of essential nutrients. For this purpose, germinated corn products with greater bioavailability of iron and zinc through reduction of phytates can be created [
15]. From brown rice germinated in acidic medium, products with improved bioavailability of calcium and zinc can be developed [
30]. Foods with ultrasound-treated germinated brown rice with greater bioavailability of iron and calcium have been formulated [
29]. Iron-enriched germinated buckwheat products for populations with deficiencies of this mineral have been made [
33]. Germinated and fermented sorghum and millet foods with better mineral bioavailability have been proposed [
9].
9.10. Functional Malted Products
Several studies specifically address malted products with functional properties. Barley malt with improved prebiotic properties has been developed [
106]. Malt with reduced germination times, but with preserved prebiotic potential has been elaborated [
46]. Rice malt with improved antioxidant properties has been developed [
45]. Malts of various cereals for products with greater stability during processing have been reported [
47].
Germinated cereals and pseudocereals represent an extraordinary raw material for developing a wide range of functional foods, from flour, bakery products, breakfast cereals, beverages, and snacks, to bioactive concentrates and products formulated for specific nutritional needs. The diversity of functional properties allows these products to be oriented towards specific health needs, constituting a promising field for the food industry and the development of preventive nutritional strategies.
10. Challenges and Technological Considerations
The industrial implementation of functional foods derived from germinated cereals and pseudocereals presents multiple technical challenges that require systematic approach to achieve commercially viable products.
The optimization of germination conditions constitutes a fundamental challenge. The need for precise control in the intensity and duration of exposure to physical inducers to prevent inhibitory effects on germination and seedling development has been documented [
35]. In accordance, it has been demonstrated that rigorous control of exposure time to UV-B radiation is critical to maximize the synthesis of bioactive compounds without compromising vegetative development [
21]. The complexity of balancing the induction of bioactive compounds through salt stress against the inevitable inhibition of growth was identified, quantifying a 60% reduction in sprout length at 300 mM NaCl concentrations [
23].
Industrial scaling represents a significant technological barrier. Technical incompatibilities when transferring optimal laboratory conditions to commercial production systems have been documented, where the available light intensity (maximum 200 lux) was substantially lower than that experimentally determined as optimal (6,000 lux) [
42]. The need for specific parametric optimization according to the cereal matrix has been established, with critical dependence on initial humidity and electrical conductivity for the effectiveness of pulsed electric fields at industrial scale [
37]. The criticality of temporal control in ozone exposure has been identified, given that prolonged periods (>6 h) induce significant degradation of bioactive compounds [
39].
The stability of compounds during processing and storage constitutes a determining technological limitation. It has been evidenced that certain bioactive compounds reach maximum concentration at specific temporal points during germination to subsequently decrease, indicating the need for precise determination of the optimal harvest time [
8]. Instability in anthocyanins and aromatic compounds such as 2-acetyl-1-pyrroline during extended germination (3-4 days) has been identified, underlining the importance of strict control of process times [
24]. It is reported that UV-B radiation significantly inhibits sprout length and germination percentage, although this inhibition is partially attenuated through supplementation with CaCl₂ [
18,
65].
Microbiological control during germination represents a critical concern for safety. Technical limitations in the treatment of large volumes of seeds with plasma have been documented, as well as variable responses according to the cereal species [
41]. There is evidence that plasma treatment for reduction of mycotoxins such as deoxynivalenol does not achieve complete elimination (maximum 58.4% reduction) due to insufficient penetration of reactive species into the inner layers of the grain where mycotoxins persist [
40]. Restrictions in the efficacy of microbial inactivation through UV-C in seeds with irregular surfaces have been identified, characterizing a “shadow effect” that compromises the effectiveness of the treatment [
48].
Sensory and organoleptic properties present significant challenges for commercial acceptability. The potential degradation of key aromatic compounds (2-acetyl-1-pyrroline) during treatment with plasma activated water has been documented [
15]. The need for specific validation according to geographic context and rigorous evaluation of sensory acceptability of fermented products for different cultural environments has been established [
28]. Methodological deficiencies in the comprehensive evaluation of sensory attributes such as flavor, texture, and aroma that decisively determine consumer acceptability have also been noted [
49].
Additionally, various investigations identify specific limitations according to the inducer applied. It has been observed that prolonged durations of ultrasonic treatment (>15 min) reduce the efficacy in GABA accumulation and can cause excessive biomass loss [
88]. It has been determined that after 48 h, germination with slightly acidic electrolyzed water manifested adverse effects on the germinative potential of brown rice [
73]. Similarly, exposure to high NaCl concentrations significantly reduces sprout length (21% reduction) and germination percentage (from 85% to 55%) [
18].
The industrialization of functional foods derived from germinated cereals and pseudocereals requires systematically addressing multiple technological challenges related to optimization of processing parameters, industrial scaling, stability of bioactive compounds, microbiological control, and sensory properties. Resolving these limitations is critical for the successful development of commercially viable products with preserved functional properties throughout their shelf life.
The bioavailability and efficacy of bioactive compounds present in germinated cereals and pseudocereals constitute critical parameters for determining their functional value as ingredients in food matrices. Contemporary research ranges from in vitro digestibility models to evaluations of specific biological activities, providing substantial evidence on the potential of these compounds to confer specific physiological benefits.
Multiple investigations have systematically evaluated mineral bioavailability through in vitro digestion models. It has been demonstrated that the sequential integration of soaking, germination, and fermentation with Lactobacillus plantarum in corn matrices significantly reduces phytate concentration (85.6%), optimizing the bioavailability of iron and zinc by decreasing the molar phytate ratios (81%, from 40.76 to 7.77) and phytate (85%, from 41.42 to 6.24) [
15]. Mineral bioaccessibility in brown rice germinated under acidic conditions has been quantified, documenting substantial increases in bioavailability of calcium (32.9%, from 18.84% to 25.04%) and zinc (44.4%, from 19.56% to 28.24%) [
30]. Synergistic effects between germination and spontaneous fermentation have been observed, achieving significant reductions of phytates (90.1% in sorghum and 85.1% in millet), with the consequent improvement in bioavailability of iron, zinc, and calcium [
9].
The stability and bioaccessibility of phenolic compounds have been the subject of exhaustive characterization. The bioaccessibility of phenolic compounds in sorghum sprouts treated with UV-A radiation incorporated in cookie matrices has been evaluated, verifying that these compounds maintain stability during thermal processing and preserve their bioaccessibility after simulated gastrointestinal digestion [
13]. The influence of germination protocols on bioaccessibility of phenolic compounds in quinoa has been analyzed, documenting significant increases in release and transport rate during in vitro digestion [
43]. The bioaccessibility of 47 specific phenolic compounds in germinated quinoa has also been meticulously characterized, evidencing significant increases in the bioavailable fraction of determined phenolic acids and flavonoids [
8].
Regarding biological efficacy, various studies have quantitatively evaluated antioxidant activity through complementary methodologies. The antioxidant capacity of brown rice germinated in slightly acidic electrolyzed water has been quantified through DPPH, ABTS, and FRAP assays, recording increases of 839%, 792%, and 934%, respectively [
10]. An extraordinary increase (3700%) in antioxidant activity determined by DPPH in quinoa sprouts subjected to controlled salt stress has been evidenced [
23]. The cytoprotective effect against oxidative damage has been evaluated, demonstrating that extracts of buckwheat sprouts treated with sucrose and calcium conferred significant protection to human liver cells (HepG2) and dermal fibroblasts (Hs68) against experimentally induced oxidative stress [
16].
Enzymatic inhibition related to carbohydrate metabolism has been rigorously characterized. It has been determined that secondary metabolites produced during germination and fermentation of djulis exhibit significant inhibitory activity on dipeptidyl peptidase-IV (DP
P-IV) and angiotensin-converting enzyme (ACE), critical biomarkers for glycemic control and blood pressure regulation, respectively [
12]. The inhibitory activity of germinated millet extracts on α-amylase and α-glucosidase has been quantified, identifying potent inhibitory effects relevant for the attenuation of postprandial hyperglycemia [
103]. It has been documented that α-glucosidase inhibitory activity in germinated sorghum increased by 25% (from 16% to 20%), while α-amylase inhibition in germinated barley increased by 650% (from 3% to 35%) [
72].
Antihypertensive capacity has been the subject of specific characterization. The inhibitory activity on angiotensin-converting enzyme (ACE) in bitter buckwheat sprouts treated with pyridoxal phosphate has been quantified, evidencing an increase of 135% (from 32.86% to 77.26%) in this activity, suggesting potential application in blood pressure regulation [
14]. In vivo models with rats subjected to oxidative stress have been implemented, demonstrating that extracts of germinated quinoa sprout significantly improved oxidative stress biomarkers, including malondialdehyde (MDA), glutathione (GSH), and superoxide dismutase (SOD) [
95].
Intestinal microbiota modulation represents an additional functional parameter evaluated. The prebiotic effect of germinated quinoa has been characterized, evidencing its capacity to enhance the production of short-chain fatty acids and favorably modulate colonic microbiota composition [
43]. The prebiotic potential of germinated barley malt has been specifically evaluated, documenting selective stimulation of the growth of probiotic bifidobacteria without promoting the proliferation of potentially pathogenic microorganisms [
46].
Complementarily, various studies have addressed bioavailability through characterization of morphostructural modifications in food components. In vitro digestibility studies have been integrated with scanning electron microscopy analysis, demonstrating that ultrasound treatment significantly alters the supramolecular structure of starch in germinated brown rice, increasing its susceptibility to enzymatic hydrolysis and optimizing the bioavailability of various nutrients [
29]. It has been evidenced that germination preferentially affects the molecular structure of amylose, while amylopectin conformation remains relatively stable, generating specific modifications in starch digestion kinetics with direct implications for postprandial glycemic response [
93,
121].
The available scientific evidence conclusively demonstrates that germination processes, particularly when integrated with specific inducers, significantly optimize the bioavailability and biological efficacy of various bioactive compounds present in cereals and pseudocereals. The underlying mechanisms include reduction of antinutritional factors, structural modifications that favor compound release, and biochemical transformations that enhance specific biological activity. These findings support the potential of germinated cereals and pseudocereals as functional ingredients with demonstrable physiological benefits, although validation through controlled clinical studies in humans is required to fully confirm these effects under habitual consumption conditions.
11. Conclusions and Future Perspectives
The comprehensive review of emerging inducers for cereal and pseudocereal germination has established a theoretical-practical framework on effective strategies for enhancing the content, bioavailability, and efficacy of bioactive compounds. The accumulated evidence demonstrates that physical, chemical, and biological inducers, applied during controlled germination, can significantly increase the concentration of specific functional compounds through defined and reproducible mechanisms of action.
Physical inducers, particularly ultraviolet radiation, electromagnetic fields, ultrasound, and cold plasmas have demonstrated efficacy for increasing bioactive compounds such as GABA, phenolics, flavonoids, and carotenoids. The optimization of parameters such as intensity, exposure duration, and application timing is critical for balancing the maximization of bioactive compounds with viable vegetative development. The response to these inducers shows specificity according to species and variety, which underlines the importance of detailed characterizations for each plant matrix.
Chemical inducers, including phytohormones, plant-derived elicitors, specific minerals, and growth regulators, act primarily through the simulation of moderate stress conditions that stimulate secondary metabolic pathways. Compounds such as jasmonic acid, chitosan, pyridoxal phosphate, and slightly acidic electrolyzed water have demonstrated the capacity to significantly increase phenolics, flavonoids, and GABA. The strategic application of salt stress and mineral supplementation emerges as a particularly promising approach for selective modulation of specific phytochemical profiles.
Biological inducers, mainly concurrent fermentative processes and application of microbial extracts, offer distinctive advantages by combining the enhancement of bioactive compounds with the reduction of antinutritional factors and improvement in nutrient bioavailability. Fermentation with specific cultures has demonstrated synergistic effects with germination, particularly for increasing GABA, total phenolics, and bioactive peptides, in addition to significantly improving mineral bioavailability.
Synergistic combinations between inducers of different categories represent a particularly promising approach, evidencing enhancing effects that surpass the individual application of each inducer. These synergies allow simultaneous modulation of different metabolic pathways and mechanisms of action, resulting in bioactive compound profiles optimized for specific applications.
The translation of knowledge from fundamental research to industrial applications faces multiple technological challenges. The optimization of germination conditions, industrial scaling, compound stability during processing and storage, microbiological control, and sensory acceptability represent critical considerations for the successful development of commercially viable functional foods.
The bioavailability and biological efficacy of bioactive compounds enhanced through specific inducers constitute fundamental parameters for determining their nutritional and functional relevance. In vitro and in vivo studies have confirmed significant improvements in antioxidant capacity, enzymatic inhibitory activity, and anti-hypertensive properties, although the need for clinical validation in humans under habitual consumption conditions persists.
The field of research on inducers for cereal and pseudocereal germination presents multiple strategic directions for future research. The optimization of processes at industrial scale, clinical validation of bioactive compound efficacy, development of processing technologies that preserve these compounds, exploration of synergies between different treatments and inducers, selection and improvement of specific plant varieties, study of underlying molecular mechanisms, comprehensive evaluation of production process sustainability, and research on sensory acceptability represent priority areas that require multidisciplinary approaches.
The convergence between basic science, food technology, clinical nutrition, and market studies will be fundamental for developing ingredients and products with optimized functional properties, improved bioavailability, and consumer acceptance. Germination enhanced through specific inducers, thus emerging as a promising strategy for the development of foods with verifiable functional properties, representing a technological frontier with significant potential for addressing contemporary nutritional challenges from a preventive and integral perspective.
Author Contributions
Conceptualization, H.H.M.-V., L.M.P.-M. and M.S.; methodology, H.H.M.-V.; formal analysis, H.H.M.-V., M.S. A.-S.B., J.V.-R. and L.M.P.-M.; investigation, H.H.M.-V. and M.S.; resources, M.S.; data curation, H.H.M.-V.; writing—original draft preparation, H.H.M.-V.; writing—review and editing, A.-S.B., J.V.-R., L.M.P.-M. and M.S.; visualization, H.H.M.-V., A.-S.B., J.V.-R., L.M.P.-M. and M.S.; supervision, L.M.P.-M. and M.S.; project administration, L.M.P.-M.; funding acquisition, L.M.P.-M. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONCYTEC & PROCIENCIA, under the grant “E033-2023-01-BM Fase 2”, Contract N° PE501084298-2023-PROCIENCIA and the grant “E077-2023-01-BM”, Contract N° PE501089261-2024-PROCIENCIA.
Data Availability Statement
Not applicable.
Acknowledgments
This research was conducted with the institutional support of the Universidad Nacional del Santa (UNS) and the Federal University of Jequitinhonha and Mucuri Valleys (UFVJM). Academic leave granted by the Universidad Nacional de Jaén enabled the pursuit of doctoral studies, contributing to this work. We also thank the National Council for Scientific and Technological Development (CNPq) for the productivity grant to M. S. (312759/2025-8) and the CONCYTEC & PROCIENCIA for financial support.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.; Van Den Broeck, H.C.; Brouns, F.J.P.H.; De Brier, N.; et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Comp Rev Food Sci Food Safe 2019, 18, 305–328. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Hussain, M.B.; Waheed, M.; Ahmad, K.; Kassymov, S.; Shariati, M.A.; Akram, M.; Mishra, A.P.; Egbuna, C. Effect of Germination Processing on Bioactive Compounds of Cereals and Legumes. In Functional Foods and Nutraceuticals; Egbuna, C., Dable Tupas, G., Eds.; Springer International Publishing: Cham, 2020; ISBN 978-3-030-42318-6. [Google Scholar]
- Ding, J.; Hou, G.G.; Nemzer, B.V.; Xiong, S.; Dubat, A.; Feng, H. Effects of Controlled Germination on Selected Physicochemical and Functional Properties of Whole-Wheat Flour and Enhanced γ-Aminobutyric Acid Accumulation by Ultrasonication. Food Chemistry 2018, 243, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Johnson, J.; Chu, Y.F.; Feng, H. Enhancement of γ-Aminobutyric Acid, Avenanthramides, and Other Health-Promoting Metabolites in Germinating Oats (Avena Sativa L.) Treated with and without Power Ultrasound. Food Chemistry 2019, 283, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Yang, J.; Zhang, J.; Fang, W.; Yin, Y. Physiology and Metabolism Alterations in Flavonoid Accumulation During Buckwheat (Fagopyrum Esculentum Moench.) Sprouting. Plants 2024, 13, 3342. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Saxena, D.C.; Singh, S. Effect of Germination on Nutritional, Functional, Pasting, and Microstructural Properties of Chenopodium (Chenopodium Album) Flour: Gluten Free Flour and Germination Effect on Various Properties. Journal of Food Processing and Preservation 2017, 41, e12959. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, X.; Wang, L.; Zhang, W.; Song, Y.; Zhao, S.; Yang, X.; Liu, X. Change of Physiochemical Characteristics, Nutritional Quality, and Volatile Compounds of Chenopodium Quinoa Willd. during Germination. Food Chemistry 2024, 445, 138693. [Google Scholar] [CrossRef] [PubMed]
- Mawouma, S.; Cotârleț, M.; Lazăr, N.N.; Stănciuc, N.; Râpeanu, G. Effect of Combined Germination and Spontaneous Fermentation on the Bioactive, Mineral, and Microbial Profile of Red Sorghum and Pearl Millet Flours. AUDJG - Food Technology 2023, 47, 207–220. [Google Scholar] [CrossRef]
- Tyagi, A.; Chen, X.; Shabbir, U.; Chelliah, R.; Oh, D.H. Effect of Slightly Acidic Electrolyzed Water on Amino Acid and Phenolic Profiling of Germinated Brown Rice Sprouts and Their Antioxidant Potential. LWT 2022, 157, 113119. [Google Scholar] [CrossRef]
- AL-Ansi, W.; Mahdi, A.A.; Al-Maqtari, Q.A.; Mushtaq, B.S.; Ahmed, A.; Karrar, E.; Mohammed, J.K.; Fan, M.; Li, Y.; Qian, H.; et al. The Potential Improvements of Naked Barley Pretreatments on GABA, β-Glucan, and Antioxidant Properties. LWT 2020, 130, 109698. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Yu, S.-H.; Cheng, K.-W.; Liou, Y.-W.; Hsu, C.-C.; Hsieh, C.-W.; Kuo, C.-H.; Cheng, K.-C. Production and Analysis of Metabolites from Solid-State Fermentation of Chenopodium Formosanum (Djulis) Sprouts in a Bioreactor. Food Research International 2023, 168, 112707. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hernández, A.A.; Rouzaud-Sández, O.; Frias, J.; Ayala-Zavala, F.; Astiazarán-García, H.; Salazar–López, N.J.; López-Saiz, C.M.; De La Reé-Rodríguez, S.C.; Sánchez, M.R. Antioxidant and Anti-Inflammatory Potential of a Food Produced from Irradiated (UV-A LED) Sorghum Sprouts Subjected to in Vitro Gastrointestinal Simulation. Journal of Functional Foods 2023, 110, 105857. [Google Scholar] [CrossRef]
- Yan, H.; Chen, H.; Liu, J.; Yao, T.; Xia, M.; Liao, Q.; Huang, L.; Li, W.; Song, Y.; Peng, L.; et al. Pyridoxal Phosphate Promotes the γ-Aminobutyric Acid Accumulation, Antioxidant and Anti-Hypertensive Activity of Germinated Tartary Buckwheat. Journal of Cereal Science 2024, 120, 104024. [Google Scholar] [CrossRef]
- Nsabimana, S.; Ismail, T.; Lazarte, C.E. Enhancing Iron and Zinc Bioavailability in Corn (Zea Mays) through Phytate Reduction: The Impact of Fermentation Alone and in Combination with Soaking and Germination. Front. Nutr. 2024, 11, 1478155. [Google Scholar] [CrossRef] [PubMed]
- Sim, U.; Sung, J.; Lee, H.; Heo, H.; Jeong, H.S.; Lee, J. Effect of Calcium Chloride and Sucrose on the Composition of Bioactive Compounds and Antioxidant Activities in Buckwheat Sprouts. Food Chemistry 2020, 312, 126075. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.; Martinez, E.; Carbonell, V.; Florez, M. Magnetic-Time Model for Triticale Seeds Germination. Romanian Journal of Physics 2019, 64, 10. [Google Scholar]
- He, W.; Wang, Y.; Luo, H.; Li, D.; Liu, C.; Song, J.; Zhang, Z.; Liu, C.; Niu, L. Effect of NaCl Stress and Supplemental CaCl2 on Carotenoid Accumulation in Germinated Yellow Corn Kernels. Food Chemistry 2020, 309, 125779. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yeo, H.J.; Park, Y.E.; Chun, S.W.; Chung, Y.S.; Lee, S.Y.; Park, S.U. Influence of Chitosan, Salicylic Acid and Jasmonic Acid on Phenylpropanoid Accumulation in Germinated Buckwheat (Fagopyrum Esculentum Moench). Foods 2019, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, H.; Wang, S. Application of Ultrasound, Microwaves, and Magnetic Fields Techniques in the Germination of Cereals. Food Science and Technology Research 2019, 25, 489–497. [Google Scholar] [CrossRef]
- Chavarín-Martínez, C.D.; Reyes-Moreno, C.; Milán-Carrillo, J.; Perales-Sánchez, J.X.K.; Canizalez-Román, V.A.; Cuevas-Rodriguez, E.-O.; López-Valenzuela, J.A.; Gutiérrez-Dorado, R. Effect of Germination and UV-B Elicitation on Chemical Compositions, Antioxidant Activities, and Phytochemical Contents of Underutilised Mexican Blue Corn Seeds. IFRJ 2022, 29, 300–310. [Google Scholar] [CrossRef]
- Song, J.-S.; Lee, M.J.; Ra, J.E.; Lee, K.S.; Eom, S.; Ham, H.M.; Kim, H.Y.; Kim, S.B.; Lim, J. Growth and Bioactive Phytochemicals in Barley Sprouts Affected by Atmospheric Pressure Plasma during Seed Germination. J. Phys. D: Appl. Phys. 2020, 53, 314002. [Google Scholar] [CrossRef]
- Souid, A.; Bellani, L.; Tassi, E.L.; Ben Hamed, K.; Longo, V.; Giorgetti, L. Early Physiological, Cytological and Antioxidative Responses of the Edible Halophyte Chenopodium Quinoa Exposed to Salt Stress. Antioxidants 2023, 12, 1060. [Google Scholar] [CrossRef] [PubMed]
- Yodpitak, S.; Mahatheeranont, S.; Boonyawan, D.; Sookwong, P.; Roytrakul, S.; Norkaew, O. Cold Plasma Treatment to Improve Germination and Enhance the Bioactive Phytochemical Content of Germinated Brown Rice. Food Chemistry 2019, 289, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Manzoor, M.F.; Ahmad, N.; Zeng, X.; Din, Z.U.; Roobab, U.; Qayum, A.; Siddique, R.; Siddeeg, A.; Rahaman, A. Impact of Pulsed Electric Field Treatments on the Growth Parameters of Wheat Seeds and Nutritional Properties of Their Wheat Plantlets Juice. Food Science & Nutrition 2020, 8, 2490–2500. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tao, Y.; Han, Y.; Ding, Y.; Jian, X.; Li, D. Preparation of Germinated Brown Rice with High γ-Aminobutyric Acid Content and Short Root by Magnetic Field Treatment. Journal of Cereal Science 2023, 112, 103720. [Google Scholar] [CrossRef]
- Johnson, M.A.; Kumar, M.; Thakur, S. Effect of Variation in Temperature and Light Duration on Morpho-Physiology and Phytochemical Content in Sprouts and Microgreens of Common Buckwheat (Fagopyrum Esculentum Moench). Plant Foods Hum Nutr 2024, 79, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, J.-H.; Sun, D.-W. Enhancement of Wheat Seed Germination, Seedling Growth and Nutritional Properties of Wheat Plantlet Juice by Plasma Activated Water. J Plant Growth Regul 2023, 42, 2006–2022. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Tao, H.; Li, Y.; Pan, D.; Cao, J.; Liu, L.; Zhou, X.; Barba, F.J. Characterizing Physicochemical, Nutritional and Quality Attributes of Wholegrain Oryza Sativa L. Subjected to High Intensity Ultrasound-Stimulated Pre-Germination. Food Control 2020, 108, 106827. [Google Scholar] [CrossRef]
- Lee, H.-H.; Yiu, E.; Zheng, A.-L.-T.; Bong, J.-C.-F.; Loh, S.-P.; Yiu, P.H. Optimisation of Phytate Degradation in Whole Grain Rice During Germination Processing Using Response Surface Methodology. BJRST 2023, 13, 132–141. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, S. Effect of Exogenous Phytohormone Treatment on Antioxidant Activity, Enzyme Activity and Phenolic Content in Wheat Sprouts and Identification of Metabolites of Control and Treated Samples by UHPLC-MS Analysis. Food Research International 2023, 169, 112811. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, L.L.; Chen, X.Y.; Yang, Y.; Wang, Z.; Xiong, F. Effects of Exogenous Gibberellic Acid and Abscisic Acid on Germination, Amylases, and Endosperm Structure of Germinating Wheat Seeds. Seed Sci. Technol. 2016, 44, 64–76. [Google Scholar] [CrossRef]
- Dębski, H.; Wiczkowski, W.; Szawara-Nowak, D.; Horbowicz, M. Elicitation with Sodium Silicate and Iron Chelate Affects the Contents of Phenolic Compounds and Minerals in Buckwheat Sprouts. Pol. J. Food Nutr. Sci. 2021, 21–28. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, S. Anti-Nutrient & Bioactive Profile, in Vitro Nutrient Digestibility, Techno-Functionality, Molecular and Structural Interactions of Foxtail Millet (Setaria Italica L.) as Influenced by Biological Processing Techniques. Food Chemistry 2022, 368, 130815. [Google Scholar] [CrossRef] [PubMed]
- Evrendilek, G.A.; Atmaca, B.; Bulut, N.; Uzuner, S. Development of Pulsed Electric Fields Treatment Unit to Treat Wheat Grains: Improvement of Seed Vigour and Stress Tolerance. Computers and Electronics in Agriculture 2021, 185, 106129. [Google Scholar] [CrossRef]
- Kanjevac, M.; Bojović, B.; Jakovljević, D. Improvement of Physiological Performance of Selected Cereals by Modulating Pregerminative Metabolic Activity in Seeds. CEREAL RESEARCH COMMUNICATIONS 2021, 50, 831–839. [Google Scholar] [CrossRef]
- Polachini, T.C.; Norwood, E.-A.; Le-Bail, P.; Le-Bail, A.; Cárcel, J.A. Pulsed Electric Field (PEF) Application on Wheat Malting Process: Effect on Hydration Kinetics, Germination and Amylase Expression. Innovative Food Science & Emerging Technologies 2023, 86, 103375. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, N.; Li, W.; Zhang, B.; Shi, T.; Xie, M.; Yu, M. Effect of Ultrasonic Induction on the Main Physiological and Biochemical Indicators and γ–Aminobutyric Acid Content of Corn during Germination. Foods 2022, 11, 1358. [Google Scholar] [CrossRef]
- Bernate, I.; Kince, T.; Radenkovs, V.; Juhnevica-Radenkova, K.; Cinkmanis, I.; Bruveris, J.; Sabovics, M. Impact of Ozone Exposure on the Biochemical Composition of Wheat, Broccoli, Alfalfa, and Radish Seeds During Germination. Agronomy 2024, 14, 2571. [Google Scholar] [CrossRef]
- Feizollahi, E.; Jeganathan, B.; Reiz, B.; Vasanthan, T.; Roopesh, M.S. Reduction of Deoxynivalenol during Barley Steeping in Malting Using Plasma Activated Water and the Determination of Major Degradation Products. Journal of Food Engineering 2023, 352, 111525. [Google Scholar] [CrossRef]
- Lazukin, A.V.; Grabel’nykh, O.I.; Serdyukov, Yu.A.; Pobezhimova, T.P.; Nurminskii, V.N.; Korsukova, A.V.; Krivov, S.A. The Effect of Surface Barrier Discharge Plasma Products on the Germination of Cereals. Tech. Phys. Lett. 2019, 45, 16–19. [Google Scholar] [CrossRef]
- Shin, J.; Yang, J.; Yang, J.-Y. Germination of Tartary Buckwheat at Various Light Strengths to Enhance Flavonoid Content and Scale-up of the Process Using Smart-Farm Systems. Journal of Cereal Science 2023, 112, 103727. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Gao, Z.; Wu, M.; Ren, T.; Wu, C.; Wang, J.; Geng, Y.; Lv, W.; Zhou, Q.; et al. Metabolomic Insights into the Profile, Bioaccessibility, and Transepithelial Transport of Polyphenols from Germinated Quinoa during in Vitro Gastrointestinal Digestion/Caco-2 Cell Transport, and Their Prebiotic Effects during Colonic Fermentation. Food Research International 2024, 186, 114339. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Kumar, K.; Ahmed, N.; Pal Singh, T.; Thakur, P.; Hyder Rizvi, Q.-U.-E.; Yadav, A.N.; Dhaliwal, H.S. Effect of Processing Treatments on the Nutritional, Anti-Nutritional, and Bioactive Composition of Blue Corn (Zea Mays L.). Curr Res Nutr Food Sci 2022, 10, 171–182. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, C.H. Effects of Processing Conditions on Change of Amino Acids, Reducing Sugar Andtotal Polyphenols of Caramelized Malt Produced from IR50404 Rice Variety. Food Res. 2024, 8, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Van De Velde, F.; Méndez-Galarraga, M.P.; Albarracín, M.; Garzón, A.G.; Aquino, M.; Cian, R.E.; Vinderola, G.; Drago, S.R. Exploring the Effects of Barley Germination on Chemical Composition, Phytic Acid, and Potential Malt Prebiotic Properties. Journal of Food Composition and Analysis 2025, 138, 107016. [Google Scholar] [CrossRef]
- Vingrys, K.; Mathai, M.; Ashton, J.F.; Stojanovska, L.; Vasiljevic, T.; McAinch, A.J.; Donkor, O.N. The Effect of Malting on Phenolic Compounds and Radical Scavenging Activity in Grains and Breakfast Cereals. Journal of Food Science 2022, 87, 4188–4202. [Google Scholar] [CrossRef] [PubMed]
- García-Santiesteban, A.E.; Ramírez-Corona, N.; López-Malo, A.; Palou, E.; Jiménez-Munguía, M.T. UVC Light Influence on the Sanitization of Alfalfa (Medicago Sativa), Wheat (Triticum Aestivum) and Chia (Salvia Hispanica) Seeds, Sprout Germination and Antioxidant Properties. Postharvest Biology and Technology 2024, 214, 112958. [Google Scholar] [CrossRef]
- Tene, S.T.; Ndinteh, D.T.; Dongmo, J.R.; Adebo, O.A.; Kewuyemi, Y.O.; Kengne Kamdem, M.H.; Obilana, A.O.; Klang, J.M.; Njobeh, P.B.; Womeni, H.M. Optimization Using Response Surface Methodology of Amylolytic Capacity of Corn Atp-Y and Coca-Sr Varieties: In Vitro Digestibility Capacity, Physico-Chemical and Functional Properties of Optimal Sample. Journal of Agriculture and Food Research 2022, 9, 100342. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ma, W.; Shen, S.; Gu, A. Underlying Biochemical and Molecular Mechanisms for Seed Germination. IJMS 2022, 23, 8502. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Lu, H.; Shu, Q.; Zhang, Y.; Chen, Q. New Perspectives on Physiological, Biochemical and Bioactive Components during Germination of Edible Seeds: A Review. Trends in Food Science & Technology 2022, 123, 187–197. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Gu, Z.; Sun, M.; Yang, R. Effects of Germination on Physio-Biochemical Metabolism and Phenolic Acids of Soybean Seeds. Journal of Food Composition and Analysis 2022, 112, 104717. [Google Scholar] [CrossRef]
- Bhandari, U.; Gajurel, A.; Khadka, B.; Thapa, I.; Chand, I.; Bhatta, D.; Poudel, A.; Pandey, M.; Shrestha, S.; Shrestha, J. Morpho-Physiological and Biochemical Response of Rice to Drought Stress: A Review. Heliyon 2023, 9, e13744. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xu, X.; Guo, L.; Yuzuak, S.; Lu, Y. Physiological and Biochemical Effects of Polystyrene Micro/Nano Plastics on Arabidopsis Thaliana. Journal of Hazardous Materials 2024, 469, 133861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Gao, G.; Ali, I.; Wu, X.; Tang, M.; Chen, L.; Jiang, L.; Liang, T. Effects of Various Seed Priming on Morphological, Physiological, and Biochemical Traits of Rice under Chilling Stress. Front. Plant Sci. 2023, 14, 1146285. [Google Scholar] [CrossRef] [PubMed]
- Bellache, M.; Moltó, N.; Benfekih, L.A.; Torres-Pagan, N.; Mir, R.; Verdeguer, M.; Boscaiu, M.; Vicente, O. Physiological and Biochemical Responses to Water Stress and Salinity of the Invasive Moth Plant, Araujia Sericifera Brot., during Seed Germination and Vegetative Growth. Agronomy 2022, 12, 361. [Google Scholar] [CrossRef]
- Gao, Z.-W.; Mu, Y.-G.; Ding, J.-J.; Ding, K.-J.; Li, J.-T.; Li, X.-N.; He, L.-J.; Wang, Z.-J.; Mu, C.-S.; Alharbi, S.A.; et al. The Effects of Salinity Stress on Amorpha Fruticosa Linn. Seed Germination, Physiological and Biochemical Mechanisms. Not Bot Horti Agrobo 2024, 52, 13552. [Google Scholar] [CrossRef]
- Hafeez, A.; Rasheed, R.; Ashraf, M.A.; Qureshi, F.F.; Hussain, I.; Iqbal, M. Effect of Heavy Metals on Growth, Physiological and Biochemical Responses of Plants. In Plants and Their Interaction to Environmental Pollution; Elsevier, 2023; pp. 139–159 ISBN 978-0-323-99978-6.
- Altıkardeş, E.; Güzel, N. Impact of Germination Pre-Treatments on Buckwheat and Quinoa: Mitigation of Anti-Nutrient Content and Enhancement of Antioxidant Properties. Food Chemistry: X 2024, 21, 101182. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, N.; Fatkullin, R.; Popova, N.; Ruskina, A.; Kalinina, I.; Morozov, R.; Avdin, V.V.; Antonova, A.; Vasileva, E. Effect of a Combination of Ultrasonic Germination and Fermentation Processes on the Antioxidant Activity and γ-Aminobutyric Acid Content of Food Ingredients. Fermentation 2023, 9, 246. [Google Scholar] [CrossRef]
- Chu, C.; Yan, N.; Du, Y.; Liu, X.; Chu, M.; Shi, J.; Zhang, H.; Liu, Y.; Zhang, Z. iTRAQ-Based Proteomic Analysis Reveals the Accumulation of Bioactive Compounds in Chinese Wild Rice (Zizania Latifolia) during Germination. Food Chemistry 2019, 289, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Jan, R.; Riar, C.S.; Bansal, V. Analyzing the Effect of Germination on the Pasting, Rheological, Morphological and in- Vitro Antioxidant Characteristics of Kodo Millet Flour and Extracts. Food Chemistry 2021, 361, 130073. [Google Scholar] [CrossRef] [PubMed]
- Anwar, T.; Qureshi, H.; Akhtar, M.S.; Siddiqi, E.H.; Fatimah, H.; Zaman, W.; Alhammad, B.A.; Seleiman, M.F. Enhancing Corn Growth and Resilience to Environmental Stress with Biochar, Gibberellic Acid and Rhizobacteria. Front. Plant Sci. 2024, 15, 1396594. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Yang, Y.; Zhao, Y.; Liu, Z.; Li, C.; He, L.; Liu, L. Effect of Different Conditions on the Germination of Coix Seed and Its Characteristics Analysis. Food Chemistry: X 2024, 22, 101332. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Y.; Dai, Z.; Liu, C.; Xiao, Y.; Wei, Q.; Song, J.; Li, D. Effect of UV-B Radiation and a Supplement of CaCl2 on Carotenoid Biosynthesis in Germinated Corn Kernels. Food Chemistry 2019, 278, 509–514. [Google Scholar] [CrossRef] [PubMed]
- García-Mosqueda, C.; Cerón-García, A.; León-Galván, M.F.; Ozuna, C.; López-Malo, A.; Sosa-Morales, M.E. Changes in Phenolics and Flavonoids in Amaranth and Soybean Sprouts after UV-C Treatment. J Food Sci 2023, 88, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Aly, B.E.; Mona, B.H.; Higazy, A.M. Green Synthesis of Silver Nanoparticles by Cyanobacterial Extracts: An Approach Guarantees Potential Bioactivity and Proper Cereal Seed Germination. Egyptian Pharmaceutical Journal 2023, 22, 613–631. [Google Scholar] [CrossRef]
- Rashid, M.T.; Liu, K.; Wei, D.-Z.; Jatoi, M.A.; Li, Q.; Sarpong, F. Drying Kinetics and Quality Dynamics of Ultrasound-Assisted Dried Selenium-Enriched Germinated Black Rice. Ultrasonics Sonochemistry 2023, 98, 106468. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, G.; Anwar, T.; Malik, M.; Rehman, H.U.; Danish, S.; Alahmadi, T.A.; Ansari, M.J. Evaluation of Potassium-Enriched Biochar and GA3 Effectiveness for Improving Wheat Growth under Drought Stress. BMC Plant Biol 2023, 23, 615. [Google Scholar] [CrossRef] [PubMed]
- Lalaleo, L.; Hidalgo, D.; Valle, M.; Calero-Cáceres, W.; Lamuela-Raventós, R.M.; Becerra-Martínez, E. Differentiating, Evaluating, and Classifying Three Quinoa Ecotypes by Washing, Cooking and Germination Treatments, Using 1H NMR-Based Metabolomic Approach. Food Chemistry 2020, 331, 127351. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-García, N.; Martínez-Villaluenga, C.; Frias, J.; Peñas, E. Changes in Protein Profile, Bioactive Potential and Enzymatic Activities of Gluten-Free Flours Obtained from Hulled and Dehulled Oat Varieties as Affected by Germination Conditions. LWT 2020, 134, 109955. [Google Scholar] [CrossRef]
- Donkor, O.N.; Stojanovska, L.; Ginn, P.; Ashton, J.; Vasiljevic, T. Germinated Grains – Sources of Bioactive Compounds. Food Chemistry 2012, 135, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, H.; Liao, C.; Liu, X. Influences of UV-B Treatment and Cooking Methods on Bioactive Components in Germinated Highland Barley. LWT 2023, 186, 115194. [Google Scholar] [CrossRef]
- He, Y.; Song, S.; Li, C.; Zhang, X.; Liu, H. Effect of Germination on the Main Chemical Compounds and 5-Methyltetrahydrofolate Metabolism of Different Quinoa Varieties. Food Research International 2022, 159, 111601. [Google Scholar] [CrossRef] [PubMed]
- Kanjevac, M.; Bojović, B.; Ćirić, A.; Stanković, M.; Jakovljević, D. Seed Priming Improves Biochemical and Physiological Performance of Wheat Seedlings under Low-Temperature Conditions. Agriculture 2022, 13, 2. [Google Scholar] [CrossRef]
- Ahmed, Z.; Faisal Manzoor, M.; Hussain, A.; Hanif, M.; Zia-ud-Din; Zeng, X. -A. Study the Impact of Ultra-Sonication and Pulsed Electric Field on the Quality of Wheat Plantlet Juice through FTIR and SERS. Ultrasonics Sonochemistry 2021, 76, 105648. [Google Scholar] [CrossRef] [PubMed]
- Naseer, I.; Javed, S.; Shah, A.A.; Tariq, A.; Ahmad, A. Influence of Phyto-Mediated Zinc Oxide Nanoparticles on Growth of (Zea Mays L.). PAK. J. BOT. 2024, 56. [Google Scholar] [CrossRef] [PubMed]
- Masure, H.G.; Fierens, E.; Delcour, J.A. Current and Forward Looking Experimental Approaches in Gluten-Free Bread Making Research. Journal of Cereal Science 2016, 67, 92–111. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szulc, P.; Szczepaniak, O.; Dziedziński, M.; Szymanowska, D.; Szymandera-Buszka, K.; Goryńska-Goldmann, E.; Gazdecki, M.; Telichowska, A.; Ligaj, M. Variability of Hordeum Vulgare L. Cultivars in Yield, Antioxidant Potential, and Cholinesterase Inhibitory Activity. Sustainability 2020, 12, 1938. [Google Scholar] [CrossRef]
- Popoola, O.O. Phenolic Compounds Composition and in Vitro Antioxidant Activity of Nigerian Amaranthus Viridis Seed as Affected by Autoclaving and Germination. Measurement: Food 2022, 6, 100028. [Google Scholar] [CrossRef]
- Ma, H.; Xu, X.; Wang, S.; Wang, J.; Wang, S. Effects of Microwave Irradiation of Fagopyrum Tataricum Seeds on the Physicochemical and Functional Attributes of Sprouts. LWT 2022, 165, 113738. [Google Scholar] [CrossRef]
- Dostalíková, L.; Hlásná Čepková, P.; Janovská, D.; Jágr, M.; Svoboda, P.; Dvořáček, V.; Viehmannová, I. The Impact of Germination and Thermal Treatments on Bioactive Compounds of Quinoa (Chenopodium Quinoa Willd.) Seeds. Eur Food Res Technol 2024, 250, 1457–1471. [Google Scholar] [CrossRef]
- Jena, K.; Vairakannu, P.; Singha, S. Microwave Drying of Sprouted Little Millet and Assessment of Their Kinetic and Physiochemical Properties. Journal of Cereal Science 2025, 122, 104122. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J.; Peñas, E. Response Surface Optimisation of Germination Conditions to Improve the Accumulation of Bioactive Compounds and the Antioxidant Activity in Quinoa. International Journal of Food Science & Technology 2018, 53, 516–524. [Google Scholar] [CrossRef]
- Sneha, K.; Kumar, M.; Kaushik, D.; Kumar, A.; Bansal, V.; Oz, F.; Proestos, C. To Study the Germination Effect on Finger Millet, Pearl Millet and Buckwheat: It’s Impact on Phytochemical Properties and Their Prebiotic Effect. J Food Chem Nanotechnol 2023, 9. [Google Scholar] [CrossRef]
- Złotek, U.; Gawlik-Dziki, U.; Dziki, D.; Świeca, M.; Nowak, R.; Martinez, E. Influence of Drying Temperature on Phenolic Acids Composition and Antioxidant Activity of Sprouts and Leaves of White and Red Quinoa. Journal of Chemistry 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J.; Peñas, E. Response Surface Optimisation of Germination Conditions to Improve the Accumulation of Bioactive Compounds and the Antioxidant Activity in Quinoa. Int J of Food Sci Tech 2018, 53, 516–524. [Google Scholar] [CrossRef]
- Dey, S.; Saxena, A.; Kumar, Y.; Maity, T.; Tarafdar, A. Synergistic Effects of Germination and Ultrasonication on Nutritional and Structural Characteristics of Kodo ( Paspalum Scrobiculatum ) and Little ( Panicum Sumatrense ) Millet. Journal of Food Quality 2024, 2024, 4951196. [Google Scholar] [CrossRef]
- Shakhov, I.V.; Kokorev, A.I.; Yuriev Institute of Plant Production, National Academy of Agrarian Sciences of Ukraine, 142 Heroiv Kharkova Ave. , Kharkiv 61060, Ukraine; Yastreb, T.O.; Yuriev Institute of Plant Production, National Academy of Agrarian Sciences of Ukraine, 142 Heroiv Kharkova Ave., Kharkiv 61060, Ukraine; Dmitriev, A.P.; Institute of Cell Biology and Genetic Engineering, National Academy of Sciences of Ukraine, 148 Akademika Zabolotnogo Str., Kyiv 03143, Ukraine; Kolupaev, Yu.E.; Yuriev Institute of Plant Production, National Academy of Agrarian Sciences of Ukraine, 142 Heroiv Kharkova Ave., Kharkiv 61060, Ukraine Increasing germination and antioxidant activity of aged wheat and triticale grains by priming with gamma-aminobutyric acid. Ukr. Bot. J. 2024, 81, 290–304. [Google Scholar] [CrossRef]
- Ge, X.; Saleh, A.S.M.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Li, W. Germination and Drying Induced Changes in the Composition and Content of Phenolic Compounds in Naked Barley. Journal of Food Composition and Analysis 2021, 95, 103594. [Google Scholar] [CrossRef]
- Xing, B.; Teng, C.; Sun, M.; Zhang, Q.; Zhou, B.; Cui, H.; Ren, G.; Yang, X.; Qin, P. Effect of Germination Treatment on the Structural and Physicochemical Properties of Quinoa Starch. Food Hydrocolloids 2021, 115, 106604. [Google Scholar] [CrossRef]
- Ramos-Pacheco, B.S.; Choque-Quispe, D.; Ligarda-Samanez, C.A.; Solano-Reynoso, A.M.; Palomino-Rincón, H.; Choque-Quispe, Y.; Peralta-Guevara, D.E.; Moscoso-Moscoso, E.; Aiquipa-Pillaca, Á.S. Effect of Germination on the Physicochemical Properties, Functional Groups, Content of Bioactive Compounds, and Antioxidant Capacity of Different Varieties of Quinoa (Chenopodium Quinoa Willd.) Grown in the High Andean Zone of Peru. Foods 2024, 13, 417. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Guan, X.; Gong, B.; Li, C. Chemical Components and Chain-Length Distributions Affecting Quinoa Starch Digestibility and Gel Viscoelasticity after Germination Treatment. Food Funct. 2021, 12, 4060–4071. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Sahu, J.K. Effect of Soaking and Germination on Grain Matrix and Glycaemic Potential: A Comparative Study on White Quinoa, Proso and Foxtail Millet Flours. Food Bioscience 2023, 56, 103105. [Google Scholar] [CrossRef]
- Al-Qabba, M.M.; El-Mowafy, M.A.; Althwab, S.A.; Alfheeaid, H.A.; Aljutaily, T.; Barakat, H. Phenolic Profile, Antioxidant Activity, and Ameliorating Efficacy of Chenopodium Quinoa Sprouts against CCl4-Induced Oxidative Stress in Rats. Nutrients 2020, 12, 2904. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Kumar, K.; Ahmed, N.; Chauhan, D.; Eain Hyder Rizvi, Q.U.; Jan, S.; Singh, T.P.; Dhaliwal, H.S. Effect of Soaking and Germination Treatments on Nutritional, Anti-Nutritional, and Bioactive Properties of Amaranth (Amaranthus Hypochondriacus L.), Quinoa (Chenopodium Quinoa L.), and Buckwheat (Fagopyrum Esculentum L.). Current Research in Food Science 2021, 4, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Gunathunga, C.; Senanayake, S.; Jayasinghe, M.A.; Brennan, C.S.; Truong, T.; Marapana, U.; Chandrapala, J. Germination Effects on Nutritional Quality: A Comprehensive Review of Selected Cereals and Pulses Changes. Journal of Food Composition and Analysis 2024, 128, 106024. [Google Scholar] [CrossRef]
- Jágr, M.; Hofinger-Horvath, A.; Ergang, P.; Čepková, P.H.; Schönlechner, R.; Pichler, E.C.; D́Amico, S.; Grausgruber, H.; Vagnerová, K.; Dvořáček, V. Comprehensive Study of the Effect of Oat Grain Germination on the Content of Avenanthramides. Food Chemistry 2024, 437, 137807. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, S.; Han, N.; Bian, H.; Song, D. Effects of Germinated and Ungerminated Grains on the Production of Non-Dairy Probiotic-Fermented Beverages. Qual. Assur. Saf. Crops Foods 2022, 14, 32–39. [Google Scholar] [CrossRef]
- Bassey, S.O.; Chinma, C.E.; Ezeocha, V.C.; Adedeji, O.E.; Jolayemi, O.S.; Alozie-Uwa, U.C.; Adie, I.E.; Ofem, S.I.; Adebo, J.A.; Adebo, O.A. Nutritional and Physicochemical Changes in Two Varieties of Fonio (Digitaria Exilis and Digitaria Iburua) during Germination. Heliyon 2023, 9, e17452. [Google Scholar] [CrossRef] [PubMed]
- Chavarín-Martínez, C.D.; Gutiérrez-Dorado, R.; Perales-Sánchez, J.X.K.; Cuevas-Rodríguez, E.O.; Milán-Carrillo, J.; Reyes-Moreno, C. Germination in Optimal Conditions as Effective Strategy to Improve Nutritional and Nutraceutical Value of Underutilized Mexican Blue Corn Seeds. Plant Foods Hum Nutr 2019, 74, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Al-Taher, F.; Nemzer, B. Effect of Germination on Fatty Acid Composition in Cereal Grains. Foods 2023, 12, 3306. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, P.M.; Sreerama, Y.N. Impact of Processing on the Phenolic Profiles of Small Millets: Evaluation of Their Antioxidant and Enzyme Inhibitory Properties Associated with Hyperglycemia. Food Chemistry 2015, 169, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, Á.L.; Rico, D.; Ronda, F.; Martín-Diana, A.B.; Caballero, P.A. Development of a Gluten-Free Whole Grain Flour by Combining Soaking and High Hydrostatic Pressure Treatments for Enhancing Functional, Nutritional and Bioactive Properties. Journal of Cereal Science 2022, 105, 103458. [Google Scholar] [CrossRef]
- Fang, Y.; Franke, C.; Manthei, A.; McMullen, L.; Temelli, F.; Gänzle, M.G. Effects of High-Pressure Carbon Dioxide on Microbial Quality and Germination of Cereal Grains and Beans. The Journal of Supercritical Fluids 2021, 175, 105272. [Google Scholar] [CrossRef]
- Karabín, M.; Jelínek, L.; Průšová, N.; Ovesná, J.; Stránská, M. Pulsed Electric Field Treatment Applied to Barley before Malting Reduces Fusarium Pathogens without Compromising the Quality of the Final Malt. LWT 2024, 206, 116575. [Google Scholar] [CrossRef]
- Yudaev, I.V.; Daus, Y.V.; Eviev, V.A.; Soumyanova, E.V.; Goldvarg, T.B. Pre-Sowing Stimulation of Cereal Seeds in High Voltage Electric Field. BIO Web Conf. 2024, 103, 00063. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Guo, Y. Microwave Irradiation Enhances the Germination Rate of Tartary Buckwheat and Content of Some Compounds in Its Sprouts. Pol. J. Food Nutr. Sci. 2018, 68, 195–205. [Google Scholar] [CrossRef]
- Kondratenko, E.P.; Soboleva, O.M.; Konstantinova, O.B. Synthesis of Phenolic Compounds in Barley Seedlings Under the Influence of the Microwave Electromagnetic Field of Ultrahigh Frequency. JAC 2023, 141–147. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, J.; Wang, Y.; Hua, D.; Zhang, H.; He, Y.; Liu, Y.; Tang, A.; Liu, H.; Sun, J. A Multi-Omics Study Revealed the Effect of Pulsed Light Treatment on Germinated Brown Rice: Promotion of Sprouting Efficiency and Gamma-Aminobutyric Acid Enrichment. Food Bioscience 2024, 61, 104196. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Xu, L.; Xie, M.; Yu, M. Non-Targeted Metabolomics Analysis of γ–Aminobutyric Acid Enrichment in Germinated Corn Induced by Pulsed Light. Foods 2024, 13, 2675. [Google Scholar] [CrossRef]
- Wani, H.M.; Sharma, P.; Wani, I.A.; Kothari, S.L.; Wani, A.A. Influence of Γ-irradiation on Antioxidant, Thermal and Rheological Properties of Native and Irradiated Whole Grain Millet Flours. Int J of Food Sci Tech 2021, 56, 3752–3762. [Google Scholar] [CrossRef]
- Xue, J.; Hu, M.; Yang, J.; Fang, W.; Yin, Y. Optimization of Ultraviolet-B Treatment for Enrichment of Total Flavonoids in Buckwheat Sprouts Using Response Surface Methodology and Study on Its Metabolic Mechanism. Foods 2024, 13, 3928. [Google Scholar] [CrossRef] [PubMed]
- Amnuaysin, N.; Korakotchakorn, H.; Chittapun, S.; Poolyarat, N. Seed Germination and Seedling Growth of Rice in Response to Atmospheric Air Dielectric-Barrier Discharge Plasma. Songklanakarin Journal of Science & Technology 2018, 40, 819–823. [Google Scholar]
- Ivankov, A.; Naučienė, Z.; Degutytė-Fomins, L.; Žūkienė, R.; Januškaitienė, I.; Malakauskienė, A.; Jakštas, V.; Ivanauskas, L.; Romanovskaja, D.; Šlepetienė, A.; et al. Changes in Agricultural Performance of Common Buckwheat Induced by Seed Treatment with Cold Plasma and Electromagnetic Field. Applied Sciences 2021, 11, 4391. [Google Scholar] [CrossRef]
- Kriz, P.; Petr, B.; Zbynek, H.; Jaromir, K.; Pavel, O.; Petr, S.; Miroslav, D. Influence of Plasma Treatment in Open Air on Mycotoxin Content and Grain Nutriments. Plasma Med 2015, 5, 145–158. [Google Scholar] [CrossRef]
- Martinovs, A.; Martinovs, A.; Rēvalde, G.; Dombrovska, D.; Koļčs, G.; Tretjakova, R.; Zaicevs, E. Effect of Short-term Treatment of Some Cereal Grains with Atmospheric Pressure Ar–O2 and Ar–Air Plasma. Plasma Processes & Polymers 2024, 21, e2400093. [Google Scholar] [CrossRef]
- Peťková, M.; Švubová, R.; Kyzek, S.; Medvecká, V.; Slováková, Ľ.; Ševčovičová, A.; Gálová, E. The Effects of Cold Atmospheric Pressure Plasma on Germination Parameters, Enzyme Activities and Induction of DNA Damage in Barley. IJMS 2021, 22, 2833. [Google Scholar] [CrossRef] [PubMed]
- Szőke, C.; Nagy, Z.; Gierczik, K.; Székely, A.; Spitkól, T.; Zsuboril, Z.T.; Galiba, G.; Marton, C.L.; Kutasi, K. Effect of the Afterglows of Low Pressure Ar/N2 -O2 Surface-wave Microwave Discharges on Barley and Corn Seeds. Plasma Processes & Polymers 2018, 15, 1700138. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, J.; Wang, Y.; Sun, X.; Huang, S.; Huang, L.; Liu, Y.; Liu, H.; Sun, J. Combined Transcriptome and Metabolome Analyses Reveal the Mechanisms of Ultrasonication Improvement of Brown Rice Germination. Ultrasonics Sonochemistry 2022, 91, 106239. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Pan, Y.; Yang, D.; Zheng, X.; Liu, Z.; Shang, J. Effect of NaCl Stress Germination on Microstructure and Physicochemical Properties of Wheat Starch. International Journal of Biological Macromolecules 2025, 297, 139924. [Google Scholar] [CrossRef] [PubMed]
- Islam, Md.A.; Shorna, Most. N.A.; Islam, S.; Biswas, S.; Biswas, J.; Islam, S.; Dutta, A.K.; Uddin, Md.S.; Zaman, S.; Akhtar-E-Ekram, Md.; et al. Hydrogen-Rich Water: A Key Player in Boosting Wheat Seedling Growth and Drought Resilience. Sci Rep 2023, 13, 22521. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, N.; Wu, F.; Jin, Z.; Xu, X. Effect of Acid Pretreatment on the Physicochemical and Antioxidant Properties of Germinated Adlay ( Coix Lachryma-jobi L.). J Food Process Preserv 2018, 42, e13511. [Google Scholar] [CrossRef]
- Sanchez, D.R.; Jespersen, B.M.; Rasmussen, L.H.; Andersen, M.L. Fungicidal Effect of Gaseous Ozone in Malting Barley: Implications for Fusarium Infections and Grain Germination. Journal of Cereal Science 2024, 118, 103973. [Google Scholar] [CrossRef]
- Kumar, R.; Dadhich, A.; Dhiman, M.; Sharma, L.; Sharma, M.M. Stimulatory Effect of ZnO Nanoparticles as a Nanofertilizer in Seed Priming of Pearl Millet (Pennisetum Glaucum) and Their Bioactivity Studies. South African Journal of Botany 2024, 165, 30–38. [Google Scholar] [CrossRef]
- Deore, A.; Athmaselvi, K.A.; Venkatachalapathy, N. Effect of Ultrasound and Microwave Pretreatment on Sprouting, GABA, Bioactive Compounds, and Other Physicochemical Properties of Sorghum. Grain & Oil Science and Technology 2023, 6, 91–99. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).