Submitted:
22 July 2025
Posted:
23 July 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Methodology
Experimental Methodology
- i.
-
Liquid Soap – Water mixture:
- i.
- 1000ml of distilled water was poured into a beaker.
- ii.
- 10ml of morning fresh liquid soap was introduced into the distilled water.
- iii.
- The mix was stirred into a homogenous 1% vol–vol mixture using a magnetic stirrer.
- ii.
-
Bitter Leaf Extract – Water mixture:
- i.
- 40ml of Bitter leaf extract was mixed with 10ml of Ethanol.
- ii.
- 10ml of the resulting mix in step (i) was mixed with 1000ml of distilled water.
- iii.
- A magnetic stirrer was used to stir the two fluids into a homogenous 1% vol-vol mixture.
- iii.
-
Other Chemicals – Water mixtures:
- i.
- 10ml of Dry gin; Palm burnt ash; Scent-leaf extract were each mixed with 1000ml of distilled water respectively.
- ii.
- A magnetic stirrer was utilized to further homogenize the 1% vol-vol mixtures.
- iii.
- 10ml of Dry gin was mixed with 5g of Palm burnt ash and 1000ml of distilled water.
Experimental Results
Core Analysis Results
| Sample Name | Empty DB weight (g) | DB + Water weight (g) | DB + Crude oil weight (g) | Relative Crude Density | Crude API Gravity (oAPI) |
|---|---|---|---|---|---|
| Crude oil | 22.89 |
79.42 | 76.83 | 0.9542 | 16.79 |
| Sample Name | Temp ) |
Efflux Time (s) | Viscometer constant | Density (g/cc) | (cSt) | (cP) | pH |
|---|---|---|---|---|---|---|---|
| Crude Oil | 29 |
12606 | 0.0364149 | 0.9542 | 459.046 | 438.022 | 6.5 |
| Tertiary Recovery | ||||||||
|
Sample Name |
BP |
FR (ml/min) |
(psi) |
PV (cc) |
OOIP (cc) |
Oil Recovered (ml) |
RE (%) |
|
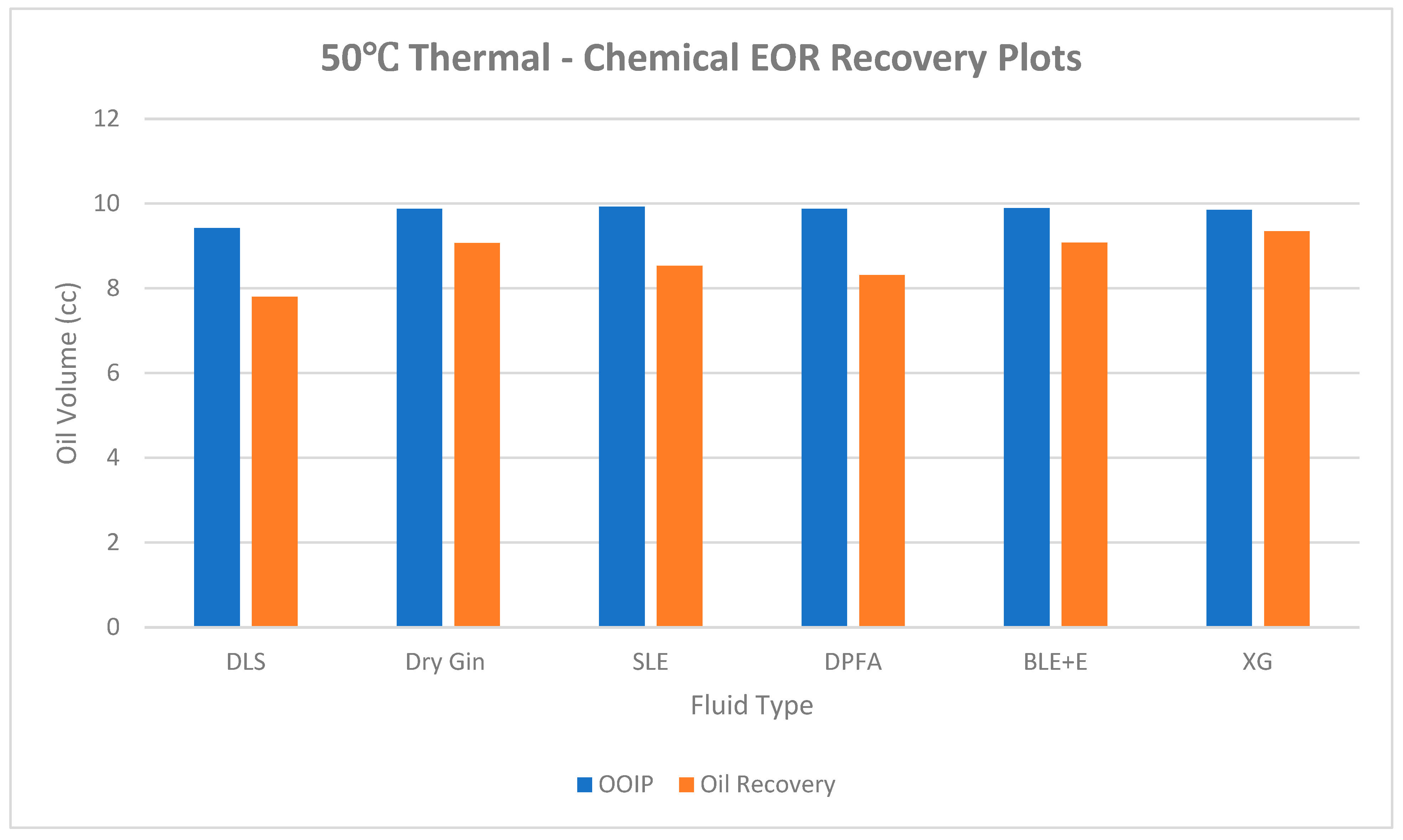
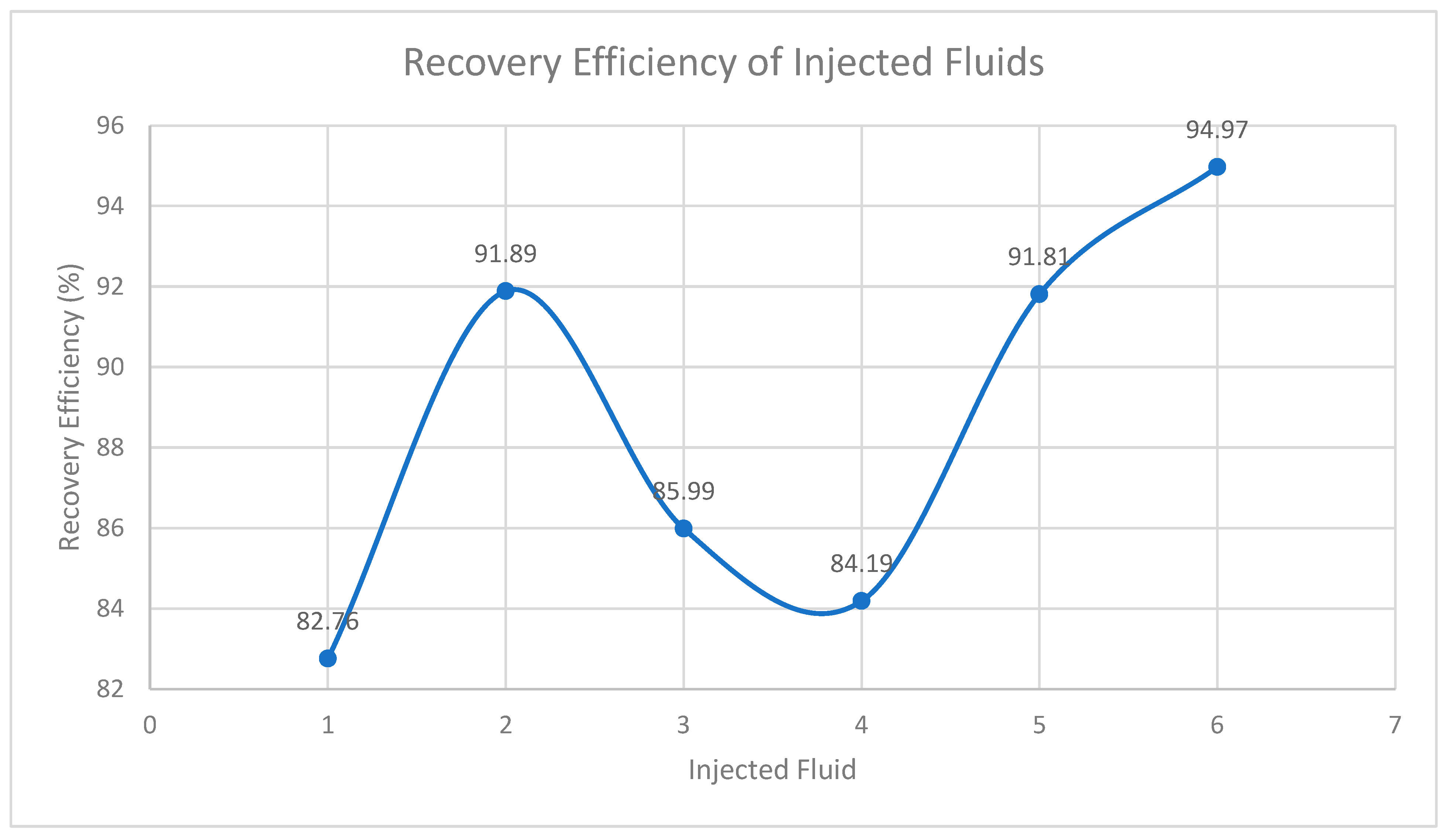
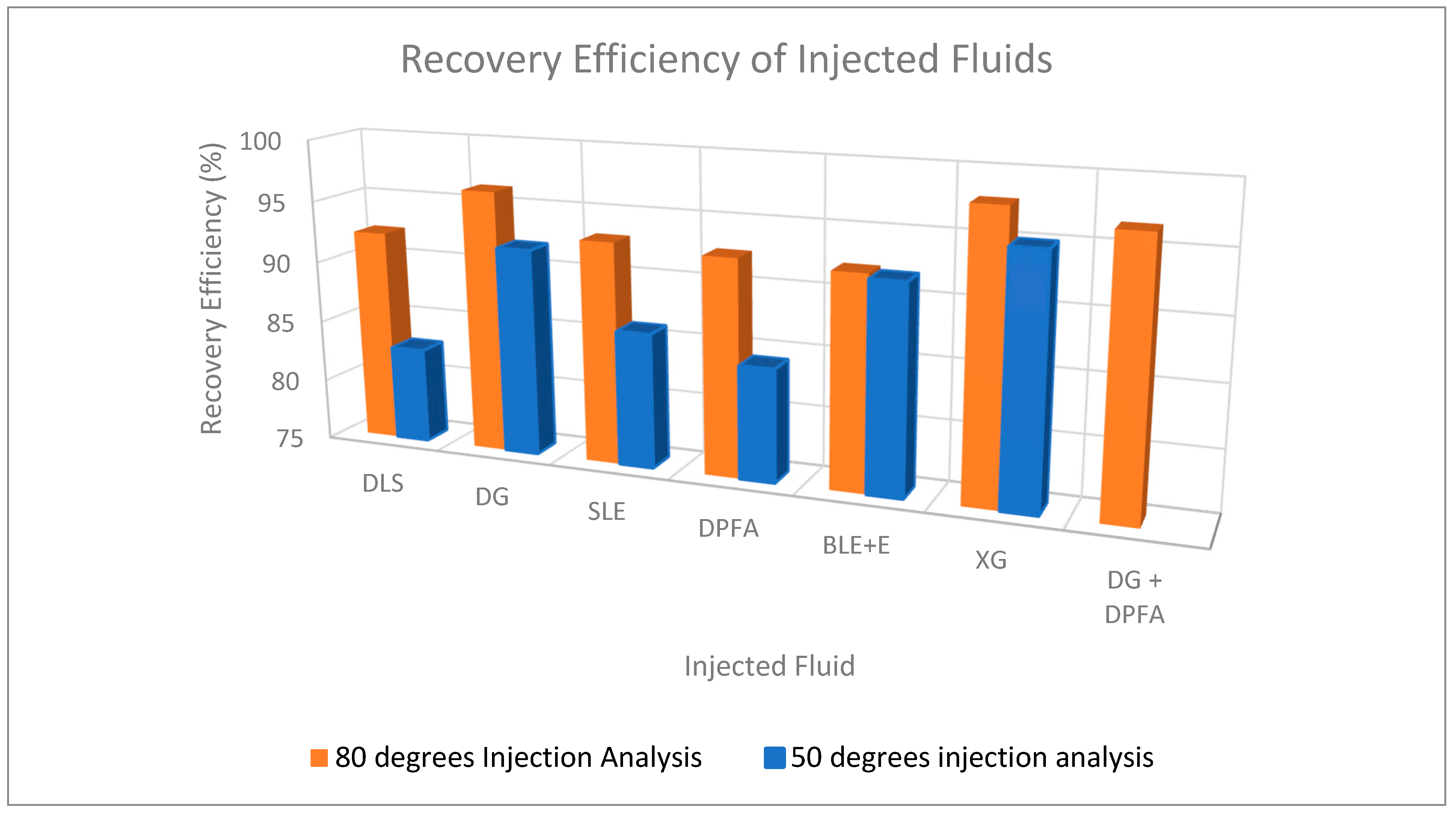
| DLS (core B) |
86 | 75.00 | 0.46 | 12.84 | 9.42 | 3.42 | 7.80 | 82.76 |
| Dry Gin | 84 | 75.70 | 0.43 | 13.92 | 9.87 | 4.05 | 9.07 | 91.89 |
| SLE | 86 | 74.03 | 0.47 | 13.92 | 9.92 | 4.00 | 8.53 | 85.99 |
| DPFA | 86 | 50.50 | 0.51 | 13.92 | 9.87 | 4.05 | 8.31 | 84.19 |
| BLE + E | 83 | 62.30 | 0.46 | 13.92 | 9.89 | 4.03 | 9.08 | 91.81 |
| XG | 85 | 61.07 | 0.57 | 13.92 | 9.85 | 4.08 | 9.35 | 94.97 |
| Tertiary Recovery | ||||||||
|
Sample Name |
BP |
FR (ml/min) |
(psi) |
PV (cc) |
OOIP (cc) |
Oil Recovered (ml) |
RE (%) |
|
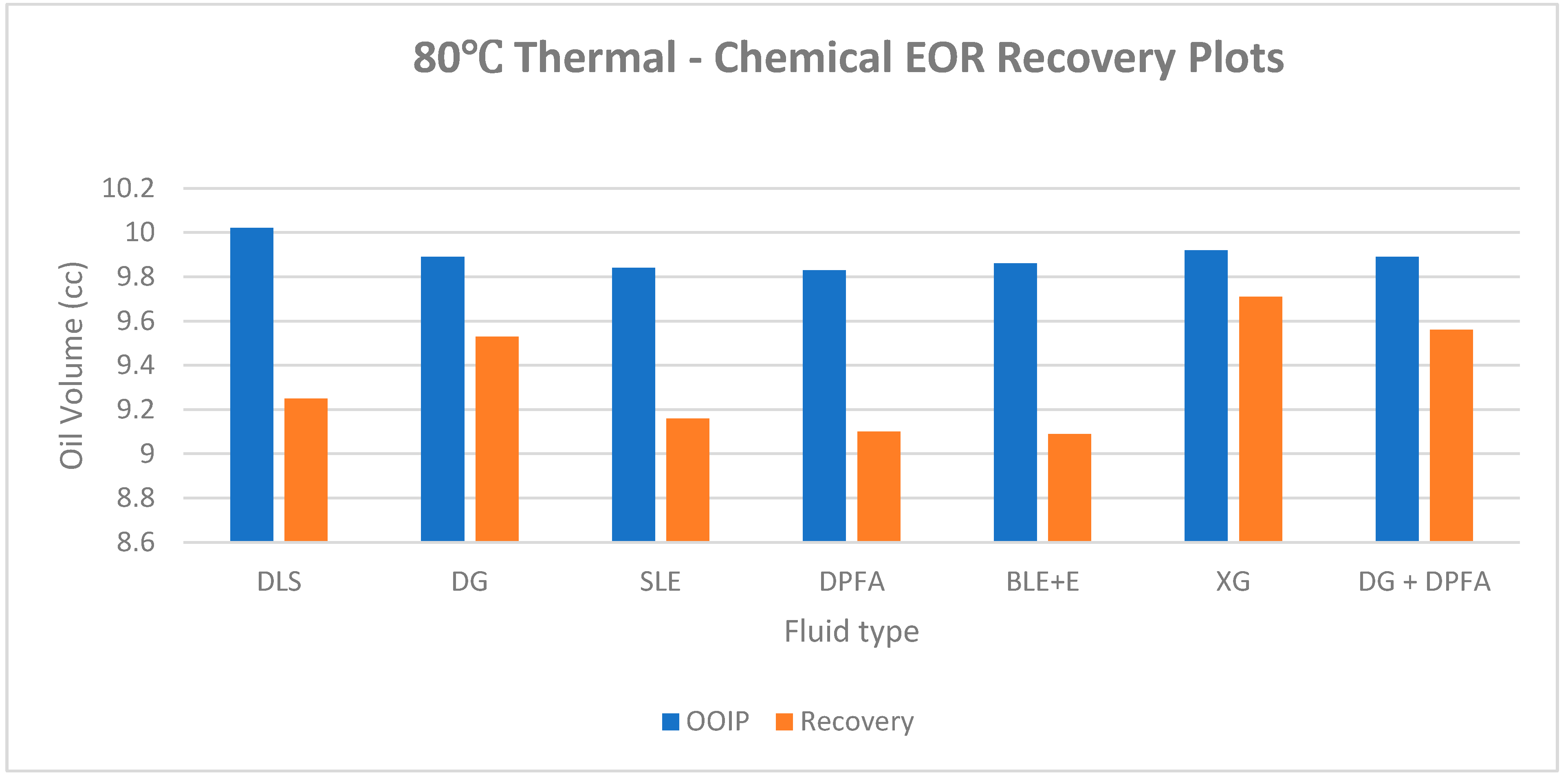
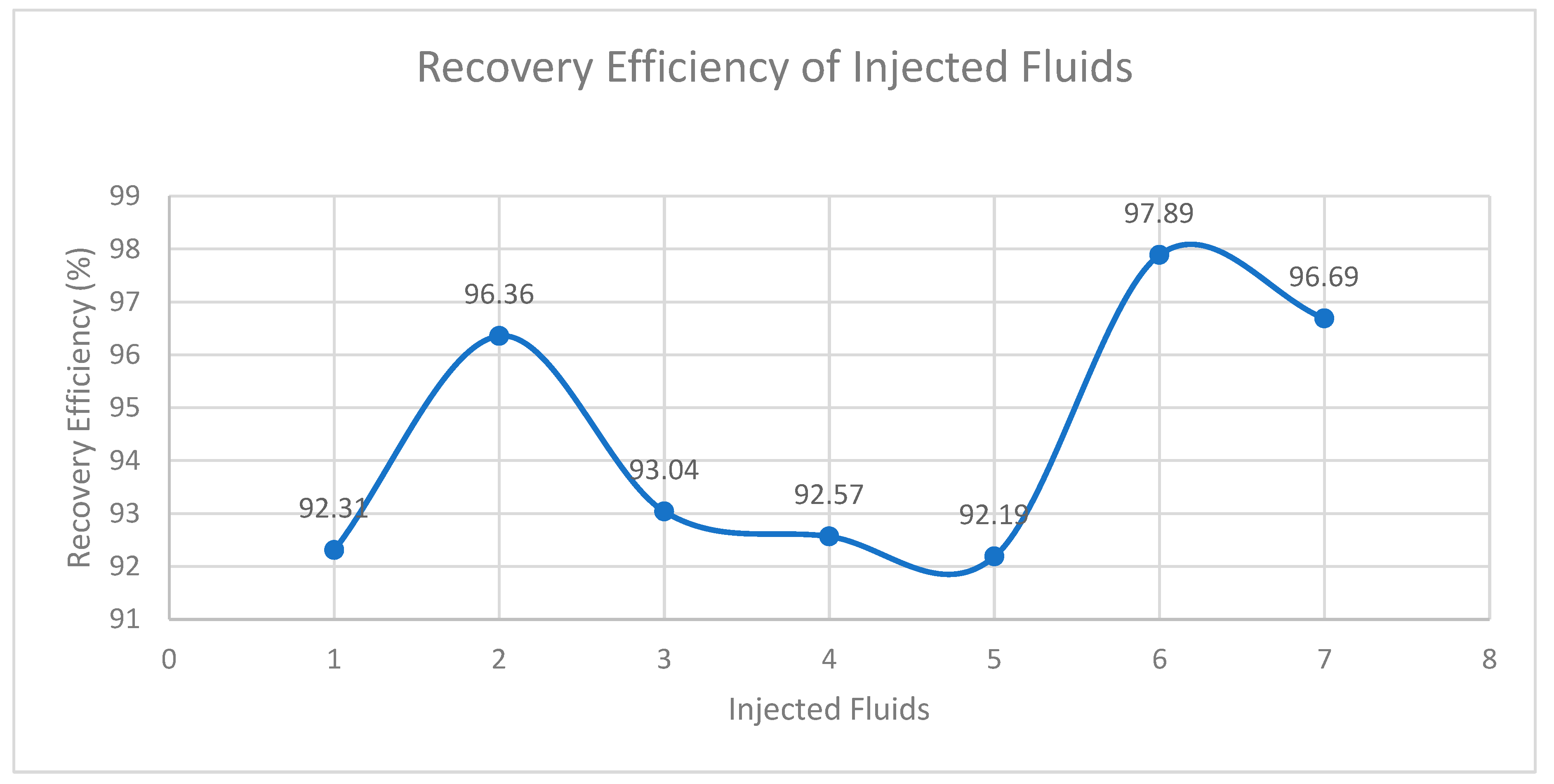
| DLS (core B) |
86 | 95.00 | 0.56 | 13.92 | 10.02 | 3.90 | 9.25 | 92.31 |
| DG | 84 | 95.69 | 0.55 | 13.92 | 9.89 | 4.03 | 9.53 | 96.36 |
| SLE | 86 | 96.24 | 0.57 | 13.92 | 9.84 | 4.08 | 9.16 | 93.04 |
| DPFA | 86 | 61.60 | 0.60 | 13.92 | 9.83 | 4.09 | 9.10 | 92.57 |
| BLE + E | 83 | 83.00 | 0.56 | 13.92 | 9.86 | 4.06 | 9.09 | 92.19 |
| XG | 85 | 60.38 | 0.66 | 13.92 | 9.92 | 4.00 | 9.71 | 97.89 |
| DG + DPFA | 85 | 82.17 | 0.59 | 13.92 | 9.89 | 4.03 | 9.56 | 96.69 |
| Steam Injection Pressure (psi) | Steam Injection Temp. (oC) |
Steam Injection rate (ml/min) |
Steam Quality (%) |
Heat of Steam (btu/lb) |
Heat Injection Rate (btu/hr) |
| 100 | 120 | 135 | 60 | 850 | 14320.80 |
|
Fluid Name |
Temp (oC) |
FR (ml/min) |
(psi) |
PV (cc) |
OOIP (cc) |
Oil Recovered (ml) |
RE (%) |
|
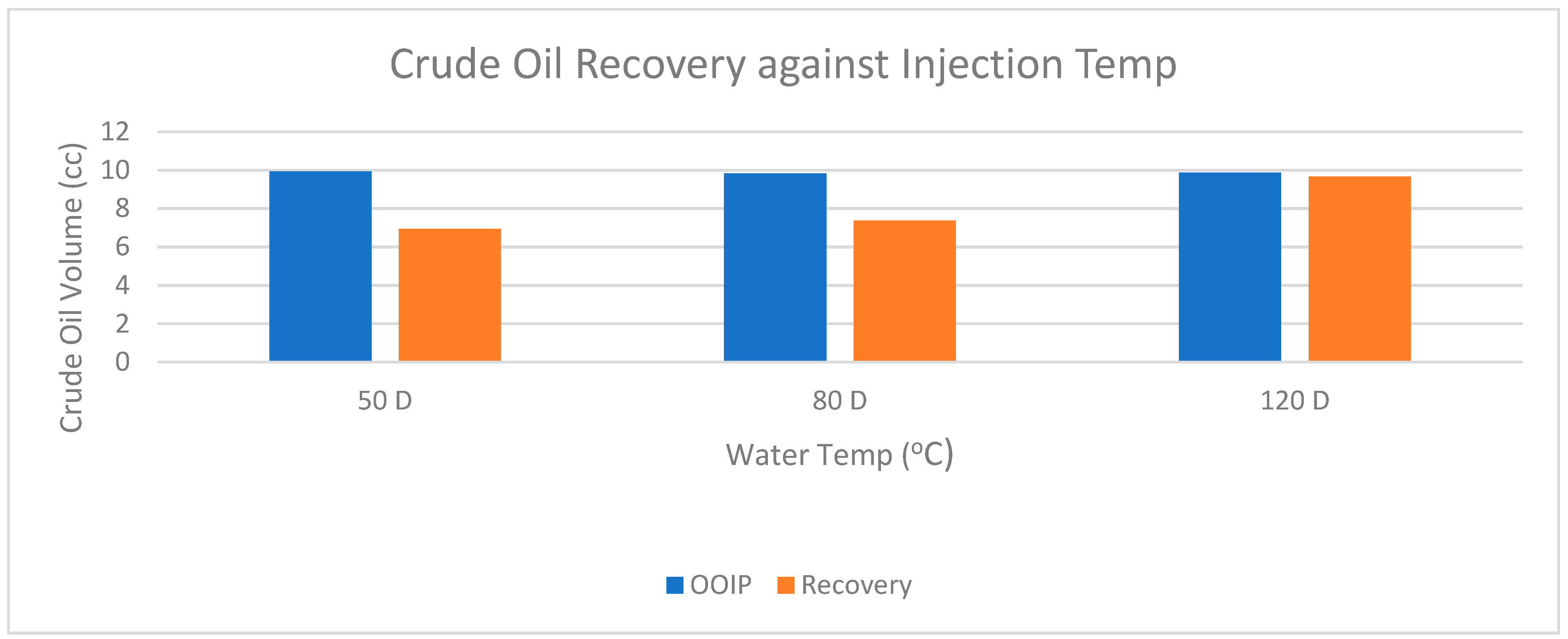
| Hot Water | 50 | 79.00 | 0.49 | 13.92 | 9.92 | 4.00 | 6.94 | 69.96 |
| Hot Water | 80 | 115.00 | 0.51 | 13.92 | 9.83 | 4.09 | 7.37 | 74.97 |
| Steam | 120 | 135.00 | 0.56 | 13.92 | 9.86 | 4.06 | 9.66 | 97.97 |
- i.
- Net Present Value (NPV)
- ii.
- Net Present Value (NPV)
- iii.
- Net Present Value (NPV)
- iv.
- Net Present Value (NPV)
- i.
- 1 barrel of oil = $50
- ii.
- The theoretical Heavy Oil field, has an OOIP of 5,000,000bbl.
- iii.
- 1BTU of energy = $0.0075154.
- iv.
- $1 = 891.04 NGN.
- v.
- A strong linear correlation between experimental prototypes production rate and on-field production rates
- vi.
- A production duration of 1 year.
- vii.
- Field scale steam flow rate (M) of 2917 kg/hr.
- viii.
- Energy content of a 150L (85% Butane, 15% Propane) LPG (E) = 4,064,440 BTU.
- iv.
- 150 Liters of LPG (85% Butane, 15% Propane) = 75kg.
| Steam Line | Valves | Thermometer | Pressure Gauge | Vessel | Furnace | Total | |
| CAPEX ($) | 15.21 | 8.34 | 17.20 | 11.22 | 50.13 | 35.02 | 137.12 |
|
T (oC) |
OOIP (bbl) |
OP (bbl) |
RR Bopd |
GR ($) |
LSG CAPEX ($) |
COE ($) |
REV ($) |
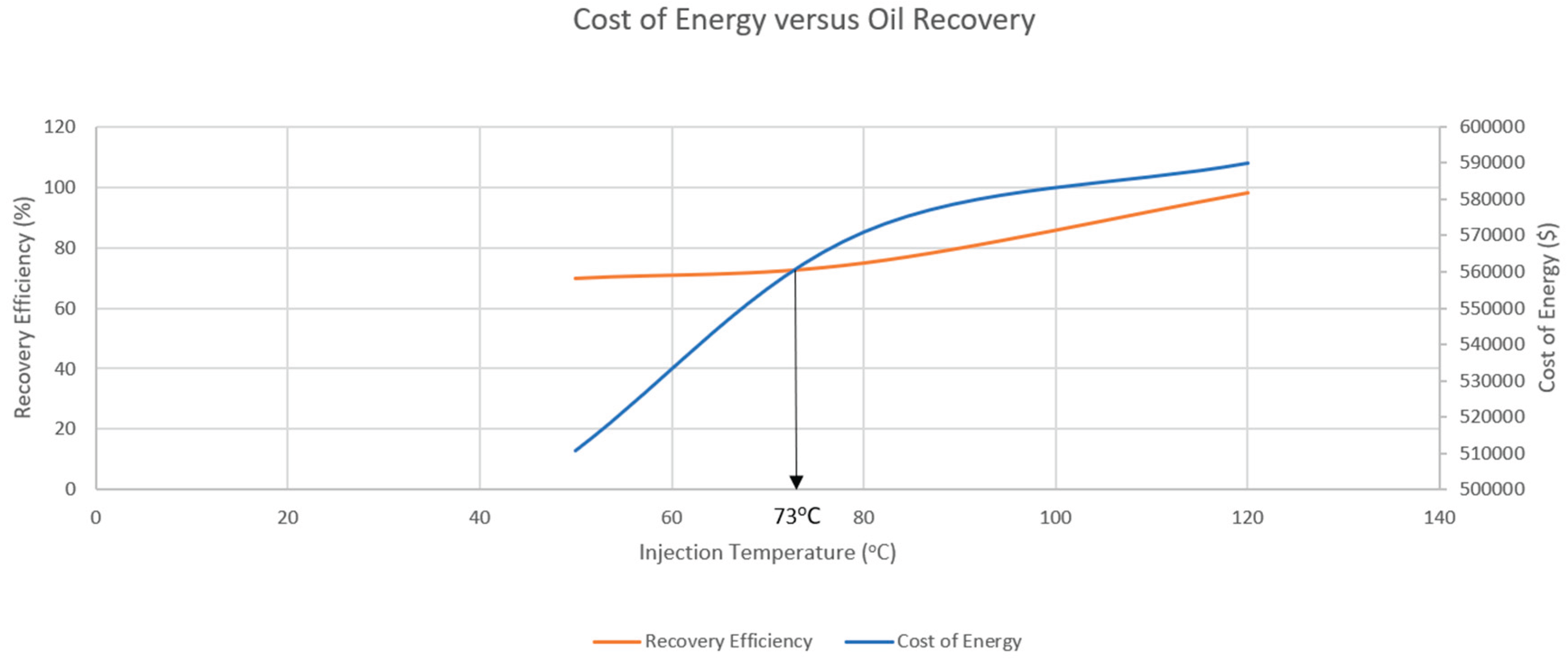
| 50 | 5 million | 762,277 | 2087 | 38,113,850 | 399,979 | 110,746 | 37,603,125 |
| 80 | 5 million | 1,108,169 | 3034 | 55,408,450 | 399,979 | 170,865 | 54,837,606 |
| 120 | 5 million | 1,302,116 | 3565 | 65,105,800 | 399,979 | 189,850 | 64,515,971 |
| Temp (oC) | Oil Flowrate (bpd) |
Revenue ($) |
2% ($) |
50% ($) |
100% ($) |
| 73 | 2817.82 | 51,460,438 | 50,451,410 | 34,306,959 | 25,730,219 |
- i.
- 1 barrel of oil = $50.
- ii.
- The theoretical Heavy Oil field, has an OOIP of 5,000,000bbl.
- iii.
- 1BTU of energy = $0.0075154.
- iv.
- $1 = 891.04 NGN.
- v.
- Local market prices of agricultural feedstock as at march 2024.
- vi.
- A strong linear correlation between experimental prototypes production rate and on-field production rates.
- vii.
- A production duration of 1 year.
- viii.
- Field scale magnification of 2917.
- ix.
- Energy content of a 150L (85% Butane, 15% Propane) LPG (E) = 4,064,440 BTU.
- x.
- 150 Liters of LPG (85% Butane, 15% Propane) = 75kg.
- xi.
- Cost of a 1% vol – vol DG + DPFA (surfactant – alkali) solution in distilled water (1L) = $0.1.
- xii.
- Cost of a 75kg LPG = $126.23 ($1.68/kg)
Nomenclature
References
- Orodu, O.D.; et al. Enhanced oil recovery using locally sourced materials in Nigeria. Journal of Petroleum Science and Engineering 2021, 204, 108745. [Google Scholar]
- Thomas, S. Enhanced oil recovery—an overview. Oil & Gas Science and Technology–Revue d’IFP Energies nouvelles 2008, 63, 9–19. [Google Scholar]
- Manrique, E.J.; et al. EOR: Current status and opportunities. SPE Reservoir Evaluation & Engineering 2007, 10, 669–686. [Google Scholar]
- Sheng, J.J. Enhanced Oil Recovery Field Case Studies. Gulf Professional Publishing, 2013.
- Green, D.W. , and Willhite, G.P. Enhanced Oil Recovery, 1998. [Google Scholar]
- Kumar, M.; et al. “Economic evaluation of steam injection projects.” Journal of Canadian Petroleum Technology 41.11 (2002).
- Kokal, S.; Al-Kaabi, A. Enhanced oil recovery: Challenges & opportunities. World Petroleum Council Journal 2010, 62, 64–70. [Google Scholar]
- Sheng, J.J. Status of surfactant EOR technology. Petroleum 2018, 4, 1–11. [Google Scholar] [CrossRef]
- Gupta, R. , and Trivedi, J.J. A comprehensive review on chemical enhanced oil recovery. Journal of Petroleum Exploration and Production Technology 2018, 8, 1241–1250. [Google Scholar]
- Kumar, M.; et al. Polymer flooding: Performance of various polymers under harsh reservoir conditions. SPE Journal 2009, 14, 478–484. [Google Scholar]
- Hirasaki, G.J.; Zhang, D.L. Surface chemistry of oil recovery from fractured, oil-wet, carbonate formations. SPE Journal 2004, 9, 151–162. [Google Scholar] [CrossRef]
- Manrique, E.J.; et al. EOR: Current status and opportunities. SPE Reservoir Evaluation & Engineering 2007, 10, 669–686. [Google Scholar]
- Levitt, D.B.; et al. The rheological properties of polymer solutions used for chemical flooding. SPE Journal 2009, 14, 282–294. [Google Scholar]
- Donato, A.R.; et al. Economic evaluation of enhanced oil recovery projects. SPE Reservoir Evaluation & Engineering 2008, 11, 149–157. [Google Scholar]
- Alvarado, V. , and Manrique, E. Enhanced oil recovery: An update review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Orodu, O.D.; et al. Enhanced oil recovery using locally sourced materials in Nigeria. Journal of Petroleum Science and Engineering 2021, 204, 108745. [Google Scholar]
- Akpan, E.U.; Udoh, F.D. Local materials for enhanced oil recovery: A case study in Nigeria. Journal of Petroleum Exploration and Production Technology 2020, 10, 1205–1216. [Google Scholar]
- Al-Jawad, M.S.; et al. The impact of using local materials on the economics of enhanced oil recovery projects. Petroleum Science and Technology 2015, 33, 212–220. [Google Scholar]
- Lake, L.W.; Walsh, M.P. Enhanced Oil Recovery. Prentice Hall, 2010.
- Thomas, S. Enhanced oil recovery: An overview. Oil & Gas Science and Technology 2008, 63, 9–19. [Google Scholar]
- Awan, A.R.; et al. “Recent developments in chemical EOR: An overview. ” Journal of Petroleum Science and Engineering 2007, 58, 105–114. [Google Scholar]


| Core ID | Core Length (cm) | Core Diameter (cm) | Bulk Volume (cm3) | Dry Weight (g) | Core Density (g/cc) | Rock Type |
|---|---|---|---|---|---|---|
| A | 5.751 | 3.753 | 63.51 | 132.64 | 2.09 | Sandy-Shale |
| B | 5.585 | 3.812 | 63.74 | 115.39 | 1.81 | Sandy-Shale |
| Core ID | Saturated Weight (g) | Pore Volume (cm3) | Porosity (%) | Core Permeability (mD) | |
|---|---|---|---|---|---|
| A |
145.74 | 12.84 | 20.22 | 60.28 | 1.50 |
| B |
129.59 | 13.92 | 21.83 | 79.93 | 1.53 |
| Sample Name | Empty DB weight (g) | DB + Water weight (g) | DB + Fluid weight (g) | Relative Fluid Density | Fluid Density (kg/m3) |
|---|---|---|---|---|---|
| DLS | 22.89 |
79.42 | 79.44 | 1.0003 | 1000.30 |
| BLE + E | 22.89 | 79.42 |
79.35 | 0.9988 | 999.80 |
| SLE | 22.89 | 79.42 |
79.34 | 0.9990 | 999.90 |
| DPFA | 22.89 | 79.42 |
87.36 | 1.100 | 1100.00 |
| Dry Gin |
22.89 | 79.42 | 79.44 | 1.0003 | 1000.30 |
| XG | 22.89 | 79.42 |
119.13 | 1.5000 | 1500.00 |
| Sample Name |
Temp ) |
Efflux Time (s) | Viscometer constant | Specific Gravity | (cSt) | (cP) | pH |
| DLS |
29 | 27.14 | 0.0364149 | 1.0003 | 0.9883 | 0.9886 | 6.7 |
| BLE + E | 29 | 28.09 | 0.0364149 | 0.9988 | 1.0228 | 1.0216 | 6.8 |
| SLE |
29 | 27.95 | 0.0364149 | 0.9990 | 1.0177 | 1.0167 | 6.7 |
| DPFA |
29 | 26.60 | 0.0364149 | 1.1000 | 0.9685 | 1.0653 | 8.7 |
| DG |
29 | 28.16 | 0.0364149 | 1.0003 | 1.0254 | 1.0257 | 5.9 |
| XG |
29 | 427.02 | 0.0364149 | 1.5000 | 15.5500 | 23.3200 | 6.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





