Submitted:
22 July 2025
Posted:
23 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Glass Synthesis
2.2. Electrical Resistivity Measurements
2.3. Optical Absorption Spectroscopy
2.4. Density Measurement
2.5. Glass Transition Temperature (Tg)
3. Results and Discussion
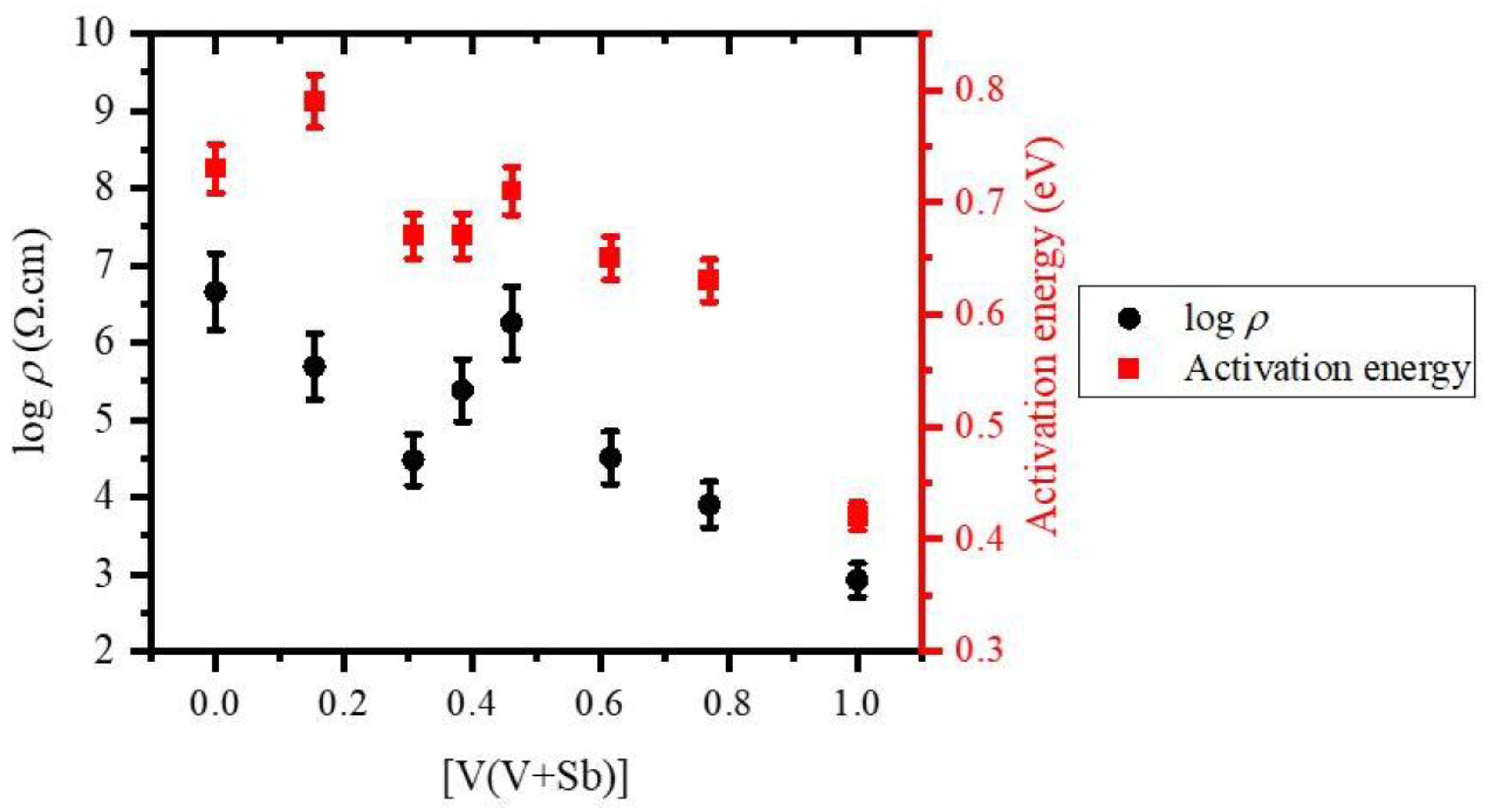
3.1. Electrical Resistivity
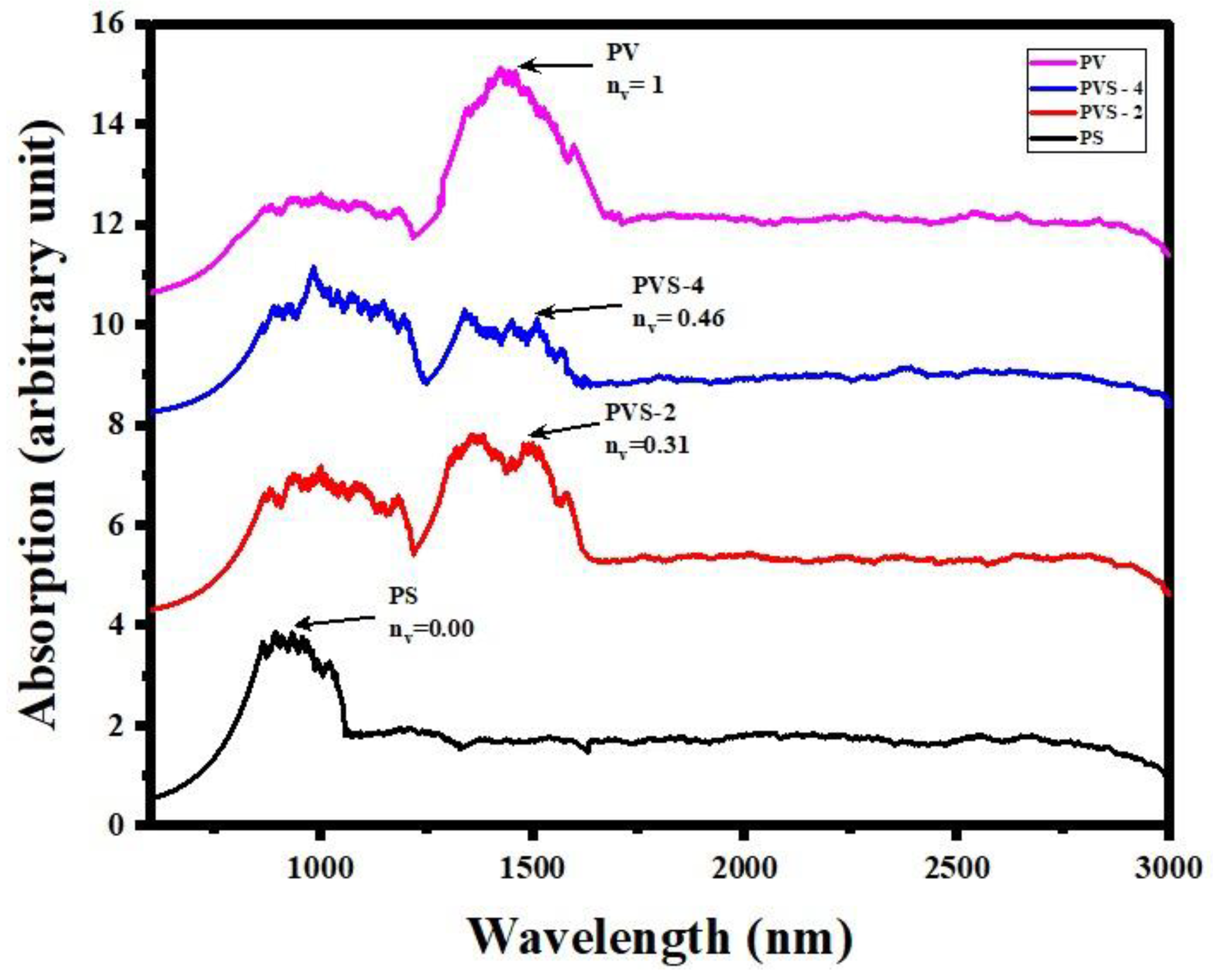
3.3. Optical Absorption Spectroscopy
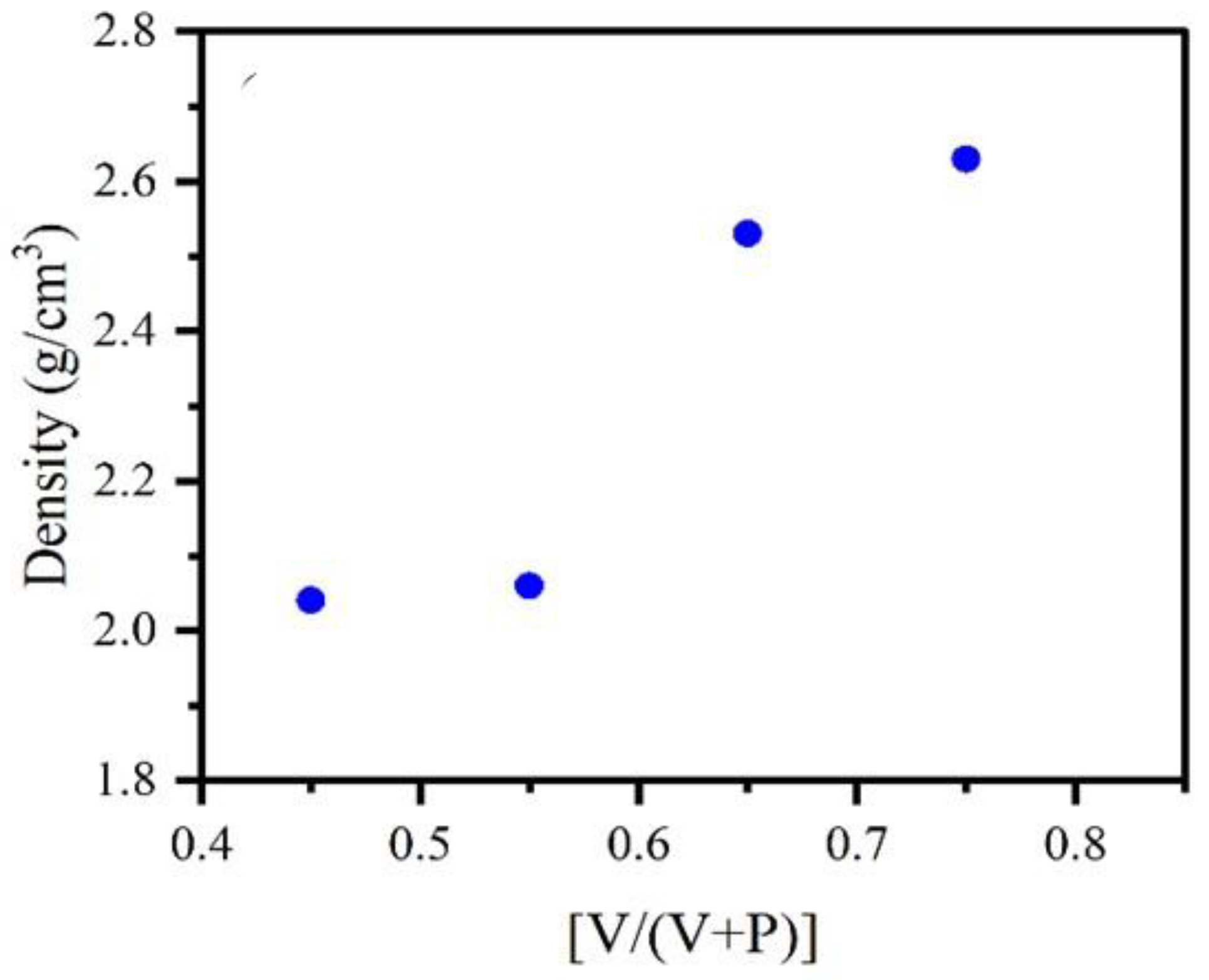
3.4. Density
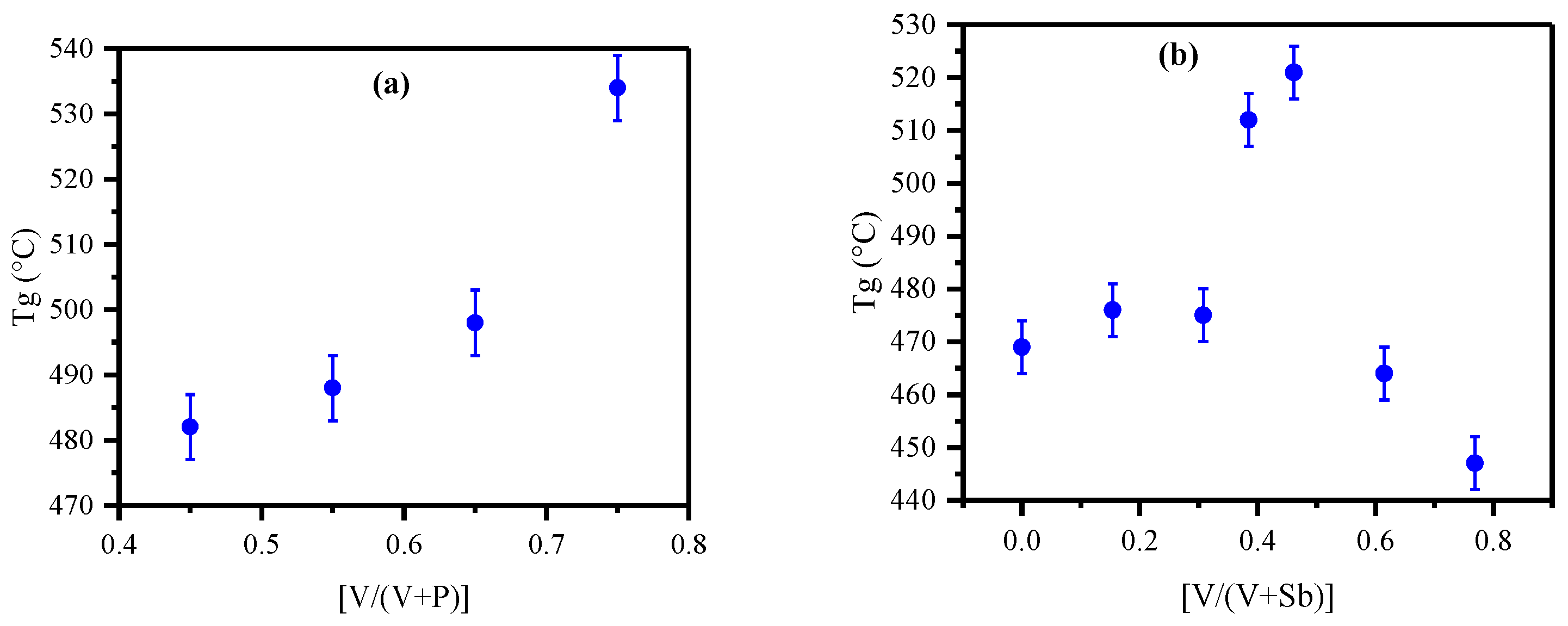
3.5. Glass Transition Temperature (Tg)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Alternating Current |
| DTA | Differential Thermal Analysis |
| DC | Direct Current |
| MAE | Mixed Alkali Effect |
| MIT | Metal–Insulator Transition |
| MTE | Mixed Transition Effect |
| PBT | Polaron–Bipolaron Transition |
| PFM | Phosphate–Fe₂O₃–MnO Glass |
| PS | Phosphate–Sb₂O₃ Glass |
| PV | Phosphate–V₂O₅ Glass |
| PVS | Phosphate–V₂O₅–Sb₂O₃ Glass |
| SP | Small Polaron |
| SBP | Small Bipolaron |
| SPH | Small Polaron Hopping |
| Tg | Glass Transition Temperature |
| TIR | Transition Ion Ratio |
| Tis | Transition Ions |
| TMI | Transition Metal Ion |
| XRF | X-ray Fluorescence |
| XRD | X-ray Diffraction |
References
- Dutta, B.; Fahmy, N.A.; Pegg, I.L. Effect of Mixed Transition-Metal Ions in Glasses. I. The P2O5–V2O5–Fe2O3 System. J. Non. Cryst. Solids 2005, 351, 1958–1966. [Google Scholar] [CrossRef]
- Dutta, B.; Fahmy, N.A.; Pegg, I.L. Effect of Mixing Transition Ions in Glasses. II. The P2O5–Fe2O3–MnO System. J. Non. Cryst. Solids 2005, 351, 2552–2561. [Google Scholar] [CrossRef]
- Dutta, B.; Fahmy, N.A.; Pegg, I.L. Effect of Mixed Transition-Metal Ions in Glasses. Part III: The P2O5–V2O5–MnO System. J. Non. Cryst. Solids 2006, 352, 2100–2108. [Google Scholar] [CrossRef]
- DUTTA, B.; PEGG, I.L. Mixed Transition Ion Effect in Certain Polaronic Semiconductors. J. Ceram. Soc. Japan, Suppl. 2004, 112, S732–S737. [Google Scholar]
- Annamalai, S.; Bhatta, R.P.; Pegg, I.L.; Dutta, B. Mixed Transition-Ion Effect in the Glass System: Fe2O3-MnO-TeO2. J. Non. Cryst. Solids 2012, 358, 1380–1386. [Google Scholar] [CrossRef]
- Doremus, R.H. Mixed-Alkali Effect and Interdiffusion of Na and K Ions in Glass. J. Am. Ceram. Soc. 1974, 57, 478–480. [Google Scholar] [CrossRef]
- Henderson, M.; Bhatta, R.P.; Eufrasio, A.M.; Pegg, I.L.; Dutta, B. Quantum Phase Transition in Phosphate Glasses Containing Multiple Transition Metal Oxides. J. Electron. Mater. 2019, 48, 3105–3114. [Google Scholar] [CrossRef]
- Mott, N.F. Conduction in Non-Crystalline Materials; Oxford University Press, 1993. [Google Scholar]
- Emin, D. Lattice Relaxation and Small-Polaron Hopping Motion in Disordered Materials. J. Non. Cryst. Solids 1972, 8–10, 511–515. [Google Scholar] [CrossRef]
- Edwards, P.P.; Sienko, M.J. Universality Aspects of the Metal-Nonmetal Transition in Condensed Media. Phys. Rev. B 1978, 17, 2575–2581. [Google Scholar] [CrossRef]
- Mott, N.F. The Transition to the Metallic State. Philos. Mag. A J. Theor. Exp. Appl. Phys. 1961, 6, 287–309. [Google Scholar] [CrossRef]
- Hubbard, J.; Flowers, B.H. Electron Correlations in Narrow Energy Bands. Proc. R. Soc. London. Ser. A. Math. Phys. Sci. 1963, 276, 238–257. [Google Scholar] [CrossRef]
- Holstein, T. Studies of Polaron Motion: Part II. The “Small” Polaron. Ann. Phys. (N. Y). 1959, 8, 343–389. [Google Scholar] [CrossRef]
- Alexandrov, A.S.; Mott, N.F. Polarons and Bipolarons; WORLD SCIENTIFIC, 1996. [Google Scholar]
- Greaves, G.N. Small Polaron Conduction in V2O5-P2O5 Glasses. J. Non. Cryst. Solids 1973, 11, 427–446. [Google Scholar] [CrossRef]
- Austin, I.; Mott, N.F. Polarons in crystalline and non-crystalline materials. Advances in physics 1969, 18, 41–102. [Google Scholar] [CrossRef]
- Al-Shahrani, A.; Al-Hajry, A.; El-Desoky, M.M. Non-Adiabatic Small Polaron Hopping Conduction in Sodium Borate Tungstate Glasses. Phys. status solidi 2003, 200, 378–387. [Google Scholar] [CrossRef]
- Anderson, P.W. Absence of Diffusion in Certain Random Lattices. Phys. Rev. 1958, 109, 1492–1505. [Google Scholar] [CrossRef]
- Mott, N.F.; Davis, E.A. Electronic Processes in Non-Crystalline Materials; Electronic Processes in Non-crystalline Materials; OUP Oxford, 1979. [Google Scholar]
- Kumar, D.; Chakravorty, D. Electrical Properties of Vanadium Phosphate Glasses Containing Antimony and Arsenide Oxide. J. Phys. D. Appl. Phys. 1982, 15, 305. [Google Scholar] [CrossRef]
- Datta, A.; Giri, A.K.; Chakravorty, D. Ac Conductivity of Sb2O3-P2O5 Glasses. Phys. Rev. B 1993, 47, 16242–16246. [Google Scholar] [CrossRef] [PubMed]
- Mott, N.F. Conduction in Glasses Containing Transition Metal Ions. J. Non. Cryst. Solids 1968, 1, 1–17. [Google Scholar] [CrossRef]
- Ghosh, A. Electrical Properties of Semiconducting Amorphous Copper-Tellurite Glasses. J. Phys. Condens. Matter 1989, 1, 7819. [Google Scholar] [CrossRef]
- Ghosh, A.; Chakravorty, D. Electrical Conduction in Some Sol-Gel Silicate Glasses. Phys. Rev. B 1993, 48, 5167–5171. [Google Scholar] [CrossRef] [PubMed]
- Lanzara, A.; Saini, N.L.; Brunelli, M.; Natali, F.; Bianconi, A.; Radaelli, P.G.; Cheong, S.-W. Crossover from Large to Small Polarons across the Metal-Insulator Transition in Manganites. Phys. Rev. Lett. 1998, 81, 878–881. [Google Scholar] [CrossRef]
- Capone, M.; Ciuchi, S. Polaron Crossover and Bipolaronic Metal-Insulator Transition in the Half-Filled Holstein Model. Phys. Rev. Lett. 2003, 91, 186405. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.R. A Theory of a.c. Conduction in Chalcogenide Glasses. Philos. Mag. A J. Theor. Exp. Appl. Phys. 1977, 36, 1291–1304. [Google Scholar] [CrossRef]
- Emin, D. Optical Properties of Large and Small Polarons and Bipolarons. Phys. Rev. B 1993, 48, 13691–13702. [Google Scholar] [CrossRef] [PubMed]
- Doweidar, H.; El-Damrawi, G.M.; Moustafa, Y.M. Transport Properties of Semiconducting Fe2O3-PbO-B2O3 Glasses. J. Phys. Condens. Matter 1994, 6, 8829. [Google Scholar] [CrossRef]
- Sidebottom, D.L. Connecting Glass-Forming Fragility to Network Topology. Front. Mater. 2019, 6, 114. [Google Scholar] [CrossRef]
- Gohar, I.A.; Moustafa, A.; Megahed, A. Electrical Properties of semicondcuting barium vanadate glasses containing iron oxide. Phys. and Chem. Of Glasses, 1998, 39, 56–60. [Google Scholar]
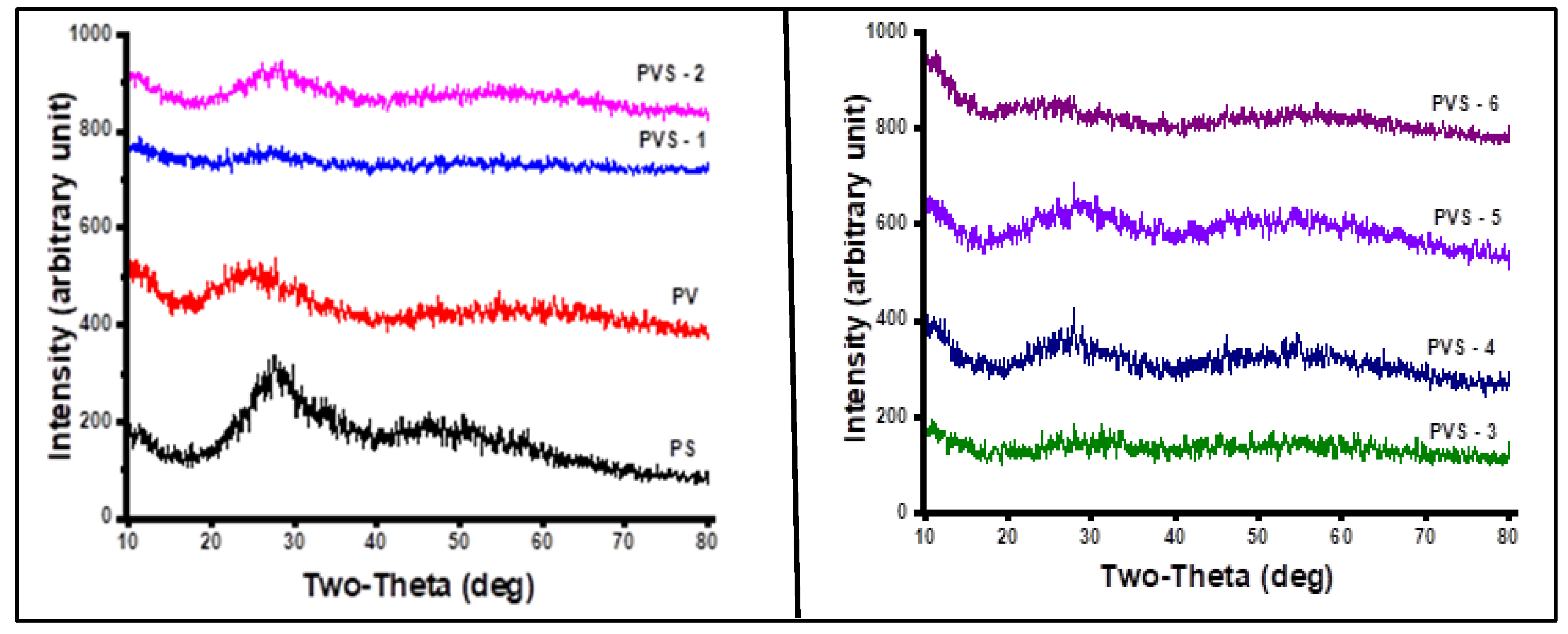
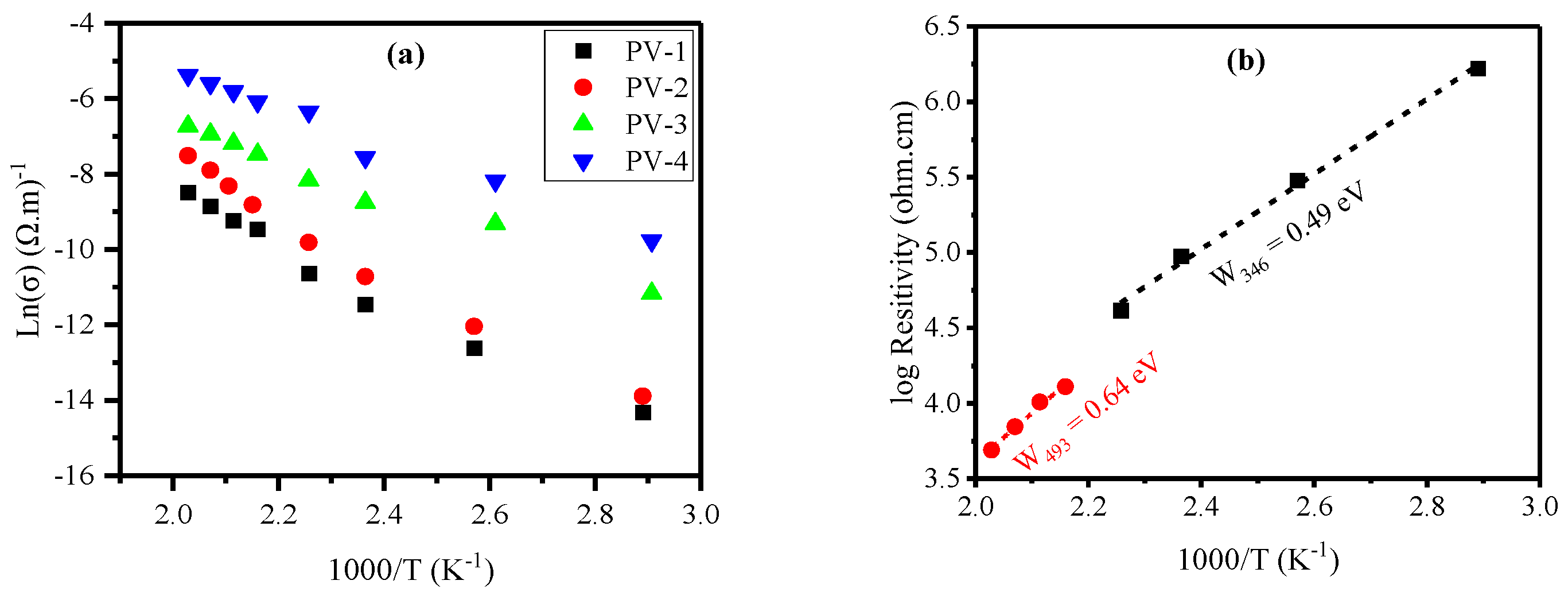
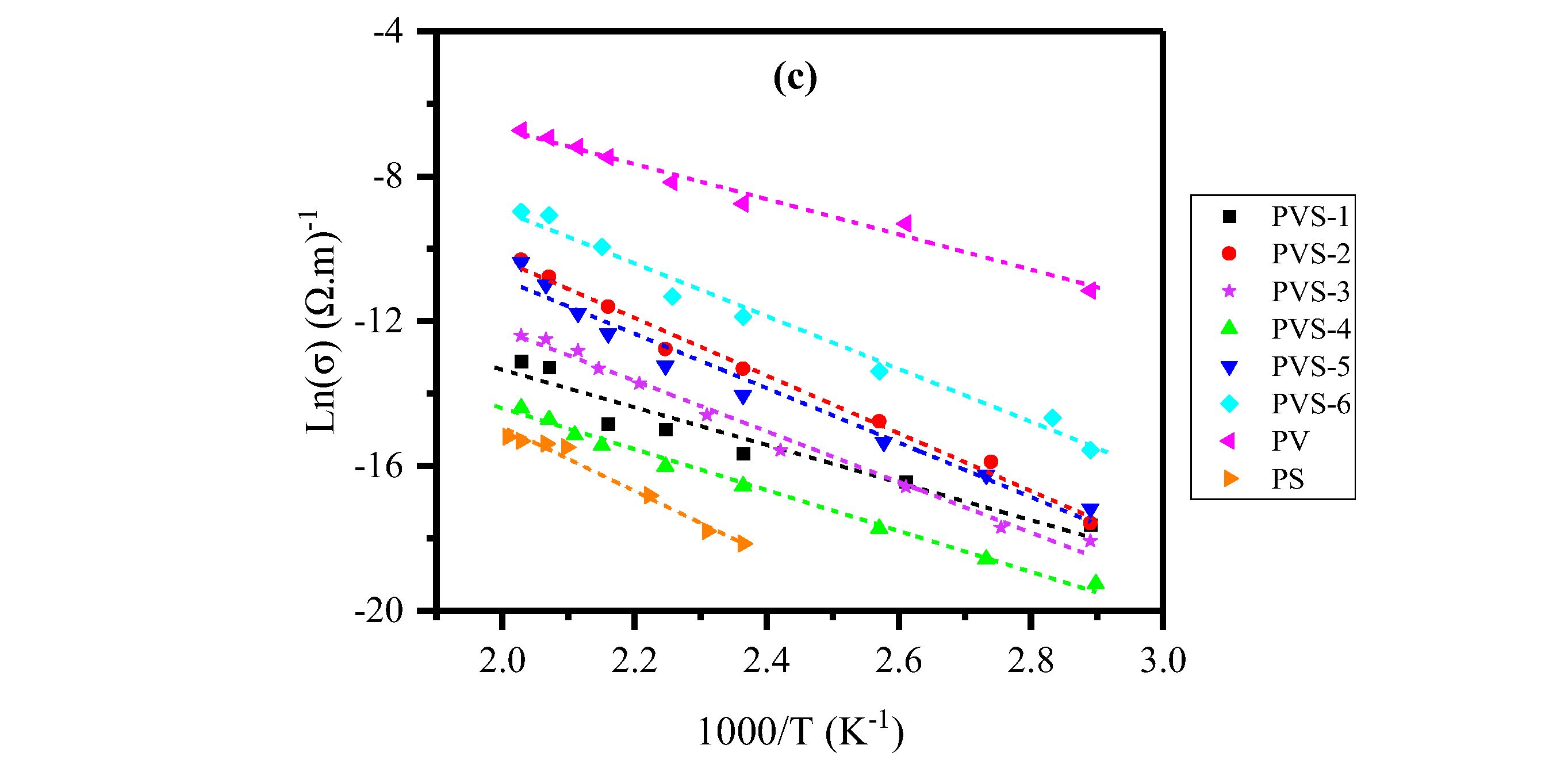
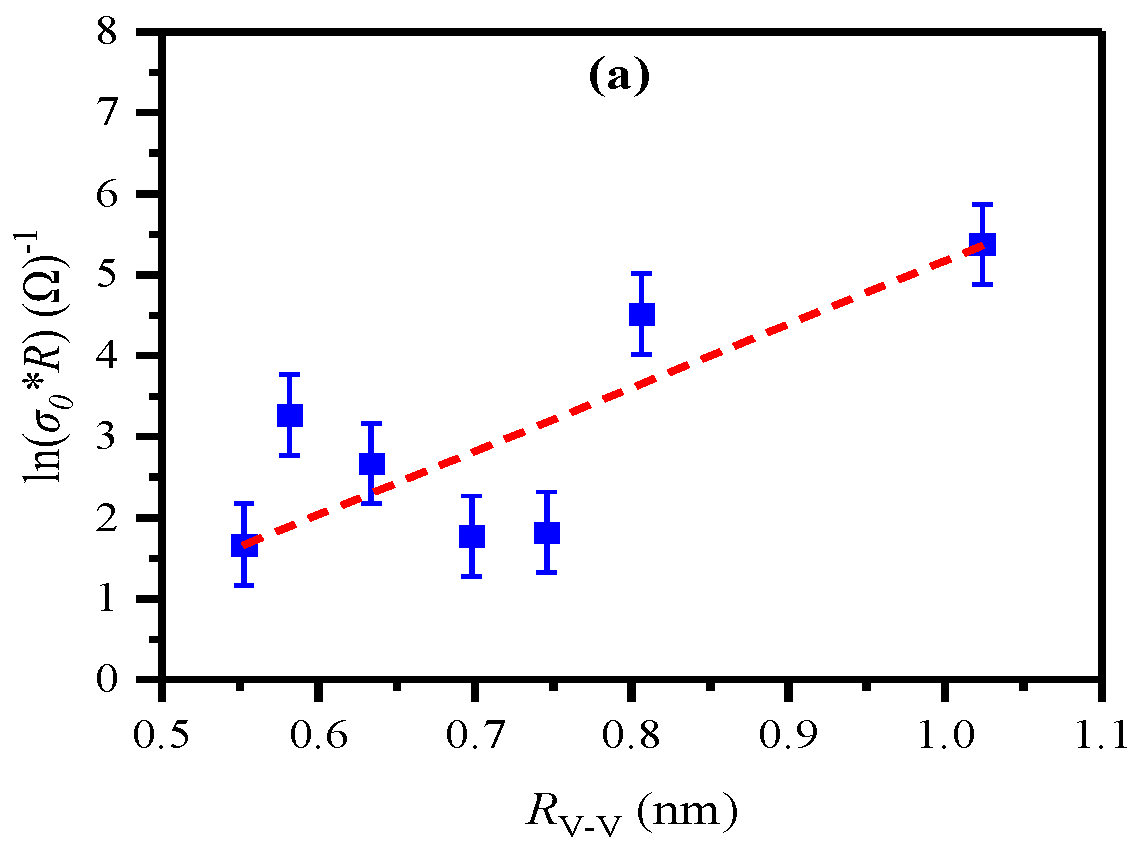
| Glass ID |
[V/(V+P)] |
P2O5 (mole %) | V2O5 (mole %) | Glass transition temperature(Tg) (°C) | ||
| Nominal | XRF normalize | Nominal | XRF normalize | |||
| PV-1 | 0.45 | 55 | 48.95 | 45 | 51.05 | 482 |
| PV-2 | 0.55 | 45 | 41.42 | 55 | 58.58 | 488 |
| PV-3 | 0.65 | 35 | 30.32 | 65 | 69.68 | 498 |
| PV-4 | 0.75 | 25 | 23.49 | 75 | 76.51 | 534 |
| Glass ID | [V/ (V + Sb)] | P2O5 (mole %) | V2O5 (mole %) | Sb2O3 (mole %) | Glass transition temperature(Tg) (°C) | |||
| Nominal | XRF normalize | Nominal | XRF normalize | Nominal | XRF normalize | |||
| PS | 0.00 | 35 | 35.20 | 0 | 0.00 | 65 | 64.80 | 469 |
| PVS-1 | 0.15 | 35 | 46.6 | 10 | 13.3 | 55 | 40.03 | 476 |
| PVS-2 | 0.31 | 35 | 32.55 | 20 | 27.93 | 45 | 39.52 | 475 |
| PVS-3 | 0.38 | 35 | 38.55 | 25 | 29.04 | 40 | 32.41 | 512 |
| PVS-4 | 0.46 | 35 | 34.11 | 30 | 31.54 | 35 | 34.36 | 521 |
| PVS-5 | 0.62 | 35 | 38.28 | 40 | 43.33 | 25 | 18.39 | 464 |
| PVS-6 | 0.77 | 35 | 38.84 | 50 | 51.23 | 15 | 9.93 | 447 |
| PV | 1.00 | 35 | 30.32 | 65 | 69.68 | 0.00 | 0.00 | 498 |
| Glass ID |
[V/(V+P)] Nominal |
Density (g/cm3) | Activation energy (W) (eV) | Resistivity (ρ) at 493K (Ωcm) |
Concentration of Vanadium ions (cm)-3 (NV) |
| PV-1 | 0.45 | 2.04 | 0.60 | 4.90E+03 | 3.45E+21 |
| PV-2 | 0.55 | 2.06 | 0.59 | 1.83E+03 | 4.16E+21 |
| PV-3 | 0.65 | 2.53 | 0.42 | 8.37E+02 | 5.92E+21 |
| PV-4 | 0.75 | 2.63 | 0.40 | 2.164E+02 | 6.90E+21 |
| Glass ID | [V/(V+Sb)] |
Activation energy (W, eV) |
Resistivity at 493K ρ (Ωcm) | Pre-exponential | Wavelength* (nm) |
| PS | 0.00 | 0.73 | 4.50E+6 | 10.96 | 849 |
| PVS-1 | 0.15 | 0.79 | 4.92E+5 | 190.55 | 774 |
| PVS-2 | 0.31 | 0.67 | 3.00E+4 | 269.15 | 898 |
| PVS-3 | 0.38 | 0.67 | 2.43E+5 | 11.48 | 984 |
| PVS-4 | 0.46 | 0.71 | 1.79E+6 | 12.59 | 849 |
| PVS-5 | 0.62 | 0.65 | 3.20E+4 | 76.61 | 911 |
| PVS-6 | 0.77 | 0.63 | 7.93E+3 | 275.42 | 984 |
| PV | 1.00 | 0.42 | 8.37E+2 | 20.42 | 1430 |
| Glass ID | [V/(V+Sb)] | Density (g/cm3) | N Concentration of ions (cm)-3 | V-V distance R (nm) |
rp (nm) |
|
| NV | NSb | |||||
| PS | 0.00 | 3.74 | 0.00E+00 | 6.14E+21 | ||
| PVS-1 | 0.15 | 3.53 | 9.35E+20 | 5.13E+21 | 1.02 | 0.41 |
| PVS-2 | 0.31 | 3.44 | 1.91E+21 | 4.29E+21 | 0.81 | 0.32 |
| PVS-3 | 0.38 | 3.38 | 2.41E+21 | 3.86E+21 | 0.75 | 0.30 |
| PVS-4 | 0.46 | 3.36 | 2.95E+21 | 3.44E+21 | 0.70 | 0.28 |
| PVS-5 | 0.62 | 3.18 | 3.93E+21 | 2.45E+21 | 0.63 | 0.26 |
| PVS-6 | 0.77 | 3.11 | 5.09E+21 | 1.53E+21 | 0.58 | 0.23 |
| PV | 1.00 | 2.53 | 5.93E+21 | 0.00E+00 | 0.55 | 0.22 |
| Glass ID | PVS-6 | PV-1 | PV | PS |
| V2O5 | 51.23 | 51.05 | 69.68 | 0.00 |
| P2O5 | 38.84 | 48.95 | 30.32 | 35.20 |
| Sb2O3 | 9.39 | 0.31 | 0.00 | 64.80 |
| Resistivity (ρ) at 493K (Ω cm) | 7.93E+3 | 4.90E+03 | 8.37E+2 | 4.50E+6 |
| Activation energy (W) (eV) | 0.63 | 0.60 | 0.42 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





