1. Introduction
As of 2021, approximately 537 million adults aged 20 to 79 worldwide are living with diabetes, representing about 10.5 % of the global adult population. By 2045, projections from the International Diabetes Federation (IDF) indicate that 1 in 8 adults, approximately 783 million people, will be living with diabetes—representing a 46 % increase from current figures. Type 2 diabetes mellitus (T2DM) accounts for the majority of these cases [
1].
The precursor of T2DM, known as prediabetes, is characterised by dysglycemia, in which the markers of glucose metabolism are elevated but still below the clinical threshold for a full T2DM diagnosis. Thus, prediabetes represents an elevated risk that has a significant potential for the future development of T2DM and diabetes-related disorders such diabetic cardiomyopathy [
2]. Several risk factors contribute to prediabetes, including genetics, obesity, physical inactivity, and poor dietary choices [
3]. Implementing lifestyle modifications, such as regular exercise, dietary improvements, better sleep, and weight management, can significantly lower these risks and, in some cases, even reverse prediabetes [
4]. In diabetic cardiomyopathy, diastolic dysfunction presents as one of the earliest signs of impending heart failure. A deeper understanding of the metabolic dysregulation underlying dysglycemia and cardiomyopathy is crucial for developing effective prevention and management strategies.
Calanus oil is a sustainable marine source of long-chain omega-3 polyunsaturated fatty acids (n3 PUFAs), derived from the lipid-rich copepod Calanus finmarchicus, which is harvested from the North Atlantic [
5]. Furthermore, Calanus oil contains antioxidants such as astaxanthin, plant sterols and fatty alcohols. Compared to n3 PUFA sources derived from fish oil, Calanus oil differs fundamentally and contains n3 PUFA in a unique binding form. In Calanus oil 80 % of FAs are bound as wax esters, whereas in fish oil they are primarily bound to triacylglycerols (TAGs) and in krill oil to phospholipids [
6] [
7].
Preliminary evidence suggests that Calanus oil supplementation might antagonize hypertension, fibrosis and lower inflammatory response in obese mice [
8]. In addition, a previous study by Štěpán et al. (2022) examined the effects of exercise training alone or in combination with Calanus oil in older women. Both interventions improved cardiorespiratory performance, but only exercise training combined with Calanus oil supplementation resulted in an increase in stroke volume [
9].
In general, n3 PUFAs, in particular docosahexaenoic acid (DHA, 22:6n3) and eicosapentaenoic acid (EPA, 20:5n3), have been shown to possess cardiometabolic-promoting effects [
10]. Higher EPA and DHA intake and elevated blood levels have been associated with TAG lowering [
11], anti-arrhythmic [
12], and anti-inflammatory effects [
13] as well as improved endothelial function [
14]. Furthermore, EPA and DHA can promote vasodilation, thereby enhancing endothelial function and contributing to overall cardiovascular health [
15].
Available evidence from RCTs indicates that EPA+DHA supplementation reduces systolic blood pressure, while doses of ≥2 g/day also lead to a reduction in diastolic blood pressure [
15]. Furthermore, it is known that EPA+DHA supplementation has a positive effect on left ventricular systolic function in patients with heart failure [
16]. However, it is still unclear how individuals with preserved ejection fraction can benefit from Calanus oil supplementation, which contains EPA+DHA and several other bioactive compounds that may offer additional health benefits in terms of metabolic health and diastolic dysfunction. Given the aforementioned effects of Calanus oil, it is possible that it may reduce diffuse cardiac fibrosis and improve the elastance of the heart, thereby minimising diastolic dysfunction. Accordingly, the aim of this pilot study was to investigate the effects of Calanus oil supplementation on diastolic cardiac function in obese, prediabetic women. The study encompassed a 12 weeks intervention period including a daily intake of 4 g of Calanus oil.
2. Materials and Methods
2.1. Study Design und Study Participants
The present study was conducted as a proof-of-principle intervention trial with one treatment group. This is a sub-study of a randomised, placebo-controlled parallel group intervention study, whose original aim was to examine the effects of 12 weeks of Calanus oil supplementation at varying doses, with or without life style intervention, on parameters of glucose and lipid metabolism, focusing specifically on the HOMA index in obese participants. The study was conducted at the of Food Science and Human Nutrition and at the COR-HELIX laboratory of the Institute of Sport Science, Leibniz University Hannover, Germany according to the guidelines of the Declaration of Helsinki and principles of Good Clinical Practice. The study was registered in the German Clinical Register (DRKS00030256). All participants gave informed consent prior to enrolment. A comprehensive description of the study procedure can be found in Kerlikowsky et al [
17]. Briefly, interested participants were screened for eligibility using a digital screening questionnaire. After the verification of inclusion criteria [age between 30 and 75 years, abdominal obesity characterised by body mass index (BMI) ≥ 28 kg/m2, waist circumference (WC) ≥ 88 cm for women and ≥102 cm for men] and exclusion criteria (e.g., consumption of n3 FA supplements, ≥ 2 portion of fish per week, severe or chronic diseases, pregnancy), participants were invited to the study site for blood testing. After verifying additional blood-test-based inclusion criteria—including HOMA index ≥ 2.5, glucose from 100 mg/dL to 126 mg/dL, or HbA1c from 5.6 % to 6.5%—a total of 266 participants were enrolled in the study. This pilot sub-study was designed as an exploratory mechanistic investigation to assess feasibility and preliminary cardiac effects of the intervention. Due to logistical and resource constraints, echocardiographic data were collected only from a subset of 23 women who consented to participate. Given that the main study already includes a placebo group, this sub-study focused exclusively on detailed echocardiographic assessments in the intervention group. As a result, no placebo group was included in this sub-study. For data collection, two examination days were scheduled at the beginning (t
0) and at the end of the intervention (t
12).
2.2. Composition of Supplement
Participants were advised to take 4 g/day (8 capsules) of Calanus oil (Zooca®Lipids, Tromsø, Norway), divided throughout the day and taken with at least 200 ml of water and a meal, for 12 weeks, to ensure adequate fat digestion and absorption. The composition of Calanus oil is shown in
Table 1. To assess compliance, participants received a defined number of capsules, which were counted at the end of the intervention at the t
12 examination. A subject was judged as compliant if at least 85 % of the capsules were taken.
2.3. Anthropometry
Height was measured using a stadiometer (Seca GmbH & Co., KG, Hamburg, Ger-many). WC was measured between the lowest rib and the highest hip bone at the narrowest part of the midsection using a tape measure. Body weight was measured digitally (Seca GmbH & Co., KG, Hamburg, Germany) to the nearest 0.1 kg (lightly dressed, without shoes). The body composition markers relative to fat mass, phase angle, and visceral fat mass were analysed using an 8-point bioelectrical impedance analyzer (BIA, mBCA525, Seca Company, Hamburg, Germany). Prior to this measurement, participants were instructed to urinate and remove all jewelry. Participants were then instructed to lie down on a stretcher and rest for about 5 min to ensure a balanced distribution of body fluids. Pulse wave velocity (PWV) and augmentation index (Alx) were determined using the boso ABI-system 100 PWV, BOSCH + SOHN Jungingen, Germany, 2019 according to the manufacturer’s recommendations. All measurements were taken by a trained nutritionist.
2.4. Medication and Physical Activity
The amount of regular physical activity during the intervention was recorded at t
0 and t
12 using the Freiburg Physical Activity Questionnaire described by Frey et al [
18]. Participants were instructed not to change their dietary habits (especially regarding the intake of n3 PUFA-rich foods) or physical activity during the intervention period. Participants were advised to report any changes in medication during the intervention phase.
2.5. Blood Markers, Blood Pressure and Aggregated Scores
Blood samples were taken from the participants after an overnight fast of at least 12 hours, between 6:00 am and 10:00 am, ideally at the same time on both examination days. Venipuncture was performed on an arm vein using EDTA tubes, serum tubes, and Gluco Exact tubes (Sarstedt AG & Co., KG, Nümbrecht, Germany). The samples were stored at 5 °C and transported on the same day to an accredited and certified laboratory (Laboratory Group Dr. Kramer and Colleagues). Glucose levels were assessed using a photometric method (Beckman Coulter GmbH, Krefeld, Germany). Insulin levels were measured using an electrochemiluminescence immunoassay (ECLIA) with the cobas 801e system (Roche Di-agnostics GmbH, Mannheim, Germany), while HbA1c was determined using high-pressure liquid chromatography (HPLC) (Tosoh Bioscience, Griesheim, Germany). TAG, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were analysed from serum tubes using a photometric method (Beckman Coulter GmbH, Germany). Plasma concentrations of CRP were determined using a human Magnetic Luminex Assay (Bio-Techne, Abingdon, Oxon, UK) and a Magpix Luminex instrument (Luminex Corp, Austin, Texas, US) according to the manufacturer's instructions. Blood pressure measurements were conducted at the Institute of Sport Science. Participants were strictly required to not perform any physical activity prior to the examination. The blood pressure assessment encompassed both brachial and central (aortic) blood pressure, along with a PWV analysis (Mobil-O-Graph, IEM, Aachen, Germany). The browser-based American Metabolic Syndrome (Met-S) Severity Calculator (
https://metscalc.org/metscalc/) was used to calculate Met-S severity scores for each subject. The calculated Met-S severity score was first described by Gurka and De Boer et al. and takes into account the following cardiovascular disease risk parameters: systolic blood pressure, TAG, HDL-C, fasting glucose, as well as information on sex, age, race/ethnicity, and weight [
19]. As a result, a single value based on WC was calculated for each woman. To evaluate insulin resistance (IR), the Homeostasis Model Assessment (HOMA) index was used, based on the formula: HOMA index= fasting glucose [mg/dl] × Insulin [μU/ml]/405 (Matthews et al., 1985) [
20] and the Triacylglycerols-glucose-waist height ratio (TyG-WHtR) was calculated according to the following formula: (1) TyG = ln(TAG [mg/dl] × glucose [mg/dl]); (2) WHtR= WC [cm]/body height [cm]; (3) TyG-WHtR=TyG x WHtR [
21].
2.6. Echocardiography
Diastolic function of the left ventricle (LV) was examined using a commercially available ultrasound system (Vivid E95, GE HealthCare, Trondheim, Norway) equipped with a 4Vc probe. Left ventricular end-diastolic volume (LVEDV), Left ventricular end-systolic volume (LVESV), Left ventricular stroke volume (LVSV), Left ventricular ejection fraction (LVEF) and longitudinal strain (LS) were analysed by 2-D B-mode analysis as well as speckle tracking and automated function imaging (AFI) of the four-chamber view. If speckle tracking was not possible due to insufficient image quality, the autoEF function was used. The same method was applied for the two measurement time points. Importantly, frame rates were kept the same between visits and all parameters were obtained by averaging three consecutive cardiac cycles. The transmitral E/A ratio (E/A), Deceleration time (DT) and isovolumic relaxation time (IVRT) were obtained from a pulsed wave Doppler signal in between the tips of the mitral valve leaflets. In addition, E/e’ was obtained by adding the pulsed wave tissue doppler imaging (TDI) results from the septal mitral annulus. Diastolic dysfunction was classified according to the 2016 ASE/ EACVI (American Society of Echocardiography/ European Association of Cardiovascular Imaging) guidelines for evaluation of LV diastolic function in participants with normal LVEF [
22]. Diastolic dysfunction was ategorizedd by evaluating two key indicators: average E/e’ (>14) and septal e’ velocity (<7 cm/s), which go beyond the set cut-off values. Diastolic function was ategorized as normal if both variables fell within the normal range. If only one of them exceeded the cut-off value, diastolic function was deemed indeterminate. Conversely, if both variables exceeded the cut-off the presence of diastolic dysfunction was assumed. All offline analysis was performed using the EchoPAC Software (Version 204, GE HealthCare, Trondheim, Norway).
2.7. Statistical Analyses
Continuous variables are shown as mean ± standard deviation (SD), while qualitative variables are presented either as absolute or relative frequencies, or only in relative figures. All analyses were performed per protocol using GraphPad Prism statistical software (GraphPad Prism 5, California, USA). The Shapiro–Wilk test assessed normality of the data. Additionally, quantile-quantile plots provided a visual inspection of the distribution. Differences in normally distributed variables were evaluated using the paired t-test. A p-value <0.05 was considered statistically significant. Linear regression models were used to examine associations between echocardiographic parameters and markers of metabolic health. One blood pressure measurement with the Mobil-O-Graph was incomplete and excluded from statistical analysis. For the evaluation of heart volumes, only 12 participants were included in the final analysis due to insufficient image quality in this population.
3. Results
3.1. Baseline Characteristics
Of the 23 recruited participants, 20 completed the study (
Table 2). The three dropouts were caused by absence from the final examination (t
12). The mean age of the participants was 59 ± 10 years and mean BMI was 34 ± 4 kg/m
2. The cohort comprised predominantly of non-smokers (95 %) while 20 % reported no medication use. Use of antihypertensive medication was reported by 55 % of the total cohort. No participant reported a change in the medication during the study period.
3.2. Body Composition, Blood Markers, Blood Pressure, Physical Activity
After 12 weeks of Calanus oil intake, a decrease in WC and absolute fat mass were observed (
Table 3: p=0.049 and p=0.003, respectively). Vice versa, total body water (TBW) and extracellular water (ECW) increased (p=0.001; p=0.040). Post intervention, fasting glucose and TAG concentrations were lower (p=0.010 and p=0.001, respectively), with no change in other parameters of glucose and lipid metabolism. Central diastolic blood pressure (DBP) and heart rate (HR) decreased (p=0.048 and p=0.047, respectively). Overall, we observed a significant improvement in metabolic health status. This is summarised by a significant decrease in the Met-S score based on WC, reflecting lower severity of the metabolic syndrome, as well as a significant decrease in the TyG-WHtR, reflecting improvement in insulin sensitivity (both p<0.001). However, no significant differences were observed in CRP, central systolic BP, Alx or PWV. No changes in physical activity levels were observed (
Table S1).
3.3. Cardiac Function
Data on cardiac function are presented in
Table 4. At baseline examination (t
0) no subject had diastolic dysfunction according to our dual factor criteria (E/e’ [>14] and/or septal e' velocity [<7 cm/s]). After the intervention no differences in LV volumes was noted. Similarly, no alterations were detected in DecT or IVRT, and e' velocity or E/e' throughout the intervention period. However, E/A was significantly improved and shifted from 0.97 to 1.03 (p=0.023).
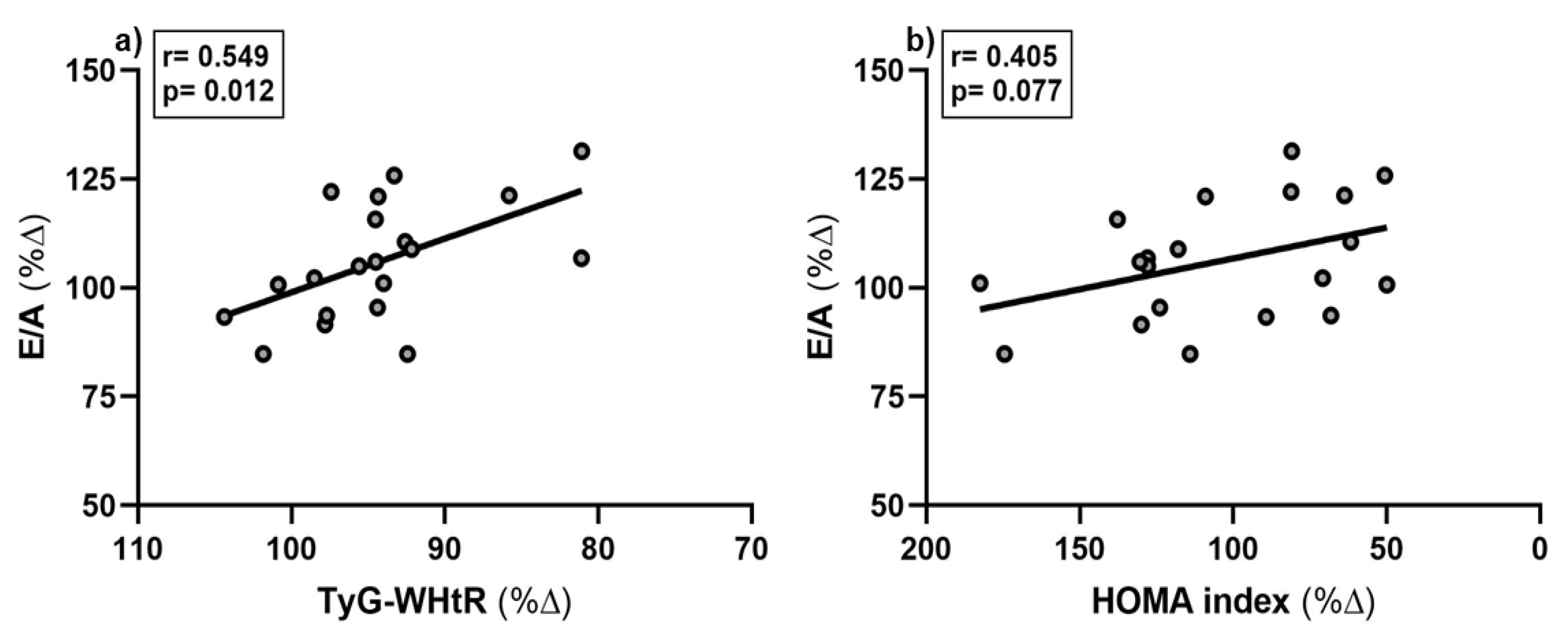
3.4. Relationships Between Cardiac Function and Other Variables
At t
0, E/A was significantly associated with TyG-WHtR, lower absolute fat mass, lower age and lower PWV (all p-values < 0.05; data not shown). The percentage increase in E/A was inversely associated with the percentage decrease in TyG-WHtR (
Figure 1 a), p=0.012) but not with percentage changes in other metabolic health parameters (
Table 5). However, a trend towards a decrease in the HOMA index and an increase in E/A was observed (
Figure 1 b), p=0.077).
4. Discussion
This proof-of-principle trial demonstrated that 12 weeks of Calanus oil supplementation might lead to favorable changes in E/A, central DBP and heart rate in prediabetic women. In addition, positive effects on parameters of metabolic health were observed.
The relationship between impaired glucose metabolism and left ventricular diastolic dysfunction is well established [
23]. Hyperglycaemia promotes myocardial fibrosis and increased myocardial stiffness, leading to impaired diastolic relaxation [
24]. Stahrenberg et al (2010) found an elevated prevalence of subclinical diastolic dysfunction in prediabetic individuals [
25]. Similarly, Di Pino et al. (2017) reported significant lower E/A in prediabetic participants (1.10 ± 0.24 (compared to healthy controls (1.18 ± 0.23) [
26]. The participants in the present study started with an E/A of 0.97 ± 0.30, which is lower than that reported by Di Pino et al. for prediabetic participants and close to the lower limit of the normal range (0.8-2.0), suggesting a slightly impaired but not pathological diastolic relaxation pattern. The significant increase in E/A to 1.03 ± 0.30 after the intervention indicates an improvement in early diastolic filling. The improvement in E/A appears to be primarily driven by an increase in E-wave velocity, while A-wave velocity remained stable or slightly increased. This pattern typically reflects enhanced passive ventricular filling in early diastole, a possible marker of improved myocardial relaxation and compliance. These changes may, in part, be due to the observed reduction in central DBP (table 3), which reduces ventricular afterload and facilitates early filling.
This is the first clinical trial that investigated the effects of Calanus oil supplementation on early-stage diabetic cardiomyopathy. However, preliminary evidence has shown that Calanus oil supplementation has beneficial effects on BP, inflammation, and fibrosis in obese mice [
27]. Moreover, a number of animal-based studies have also revealed the beneficial effects of n3 PUFAs on diabetic cardiomyopathy in rats [28-30]. Mechanistically, EPA and DHA have been shown to support myocardial relaxation by reducing inflammation, improving endothelial function, inhibiting mitochondrial oxidative damage, increasing nitric oxide (NO) bioavailability or through the autophagic pathway [
31].
These effects might contribute to improved diastolic function in early stages of metabolic dysfunction, even in the absence of overt diastolic dysfunction. However, in this trial, CRP levels remained unchanged following Calanus oil supplementation, which may be attributable to the absence of elevated levels at baseline (< 3 mg/dl). Nevertheless, we observed a significant improvement in TyG-WHtR, a reliable surrogate indicator of IR [
32]. This improvement was significantly associated with an improvement in the E/A, suggesting that it may reflect not only improved systemic glucose metabolism but also a shift in myocardial substrate utilization — specifically, from predominant fatty acid oxidation toward a more balanced or glucose-oriented energy metabolism. This warrants a brief explanation: In the healthy heart, energy is mainly produced via mitochondrial oxidative phosphorylation, with flexible substrate use. In insulin-resistant states, this flexibility is lost: fatty acid oxidation predominates, while glucose utilization declines due to impaired insulin signaling [
33,
34]. As fatty acid oxidation is less oxygen-efficient and promotes lipid intermediate accumulation (e.g., ceramides, diacylglycerols), it contributes to lipotoxicity, mitochondrial dysfunction, and altered calcium handling [
35,
36]. These changes are linked to impaired relaxation and increased myocardial stiffness, promoting diastolic dysfunction. In contrast, a more efficient energy supply through enhanced glucose metabolism could support active myocardial relaxation during early diastole, thereby improving ventricular compliance and E/A, even in the absence of overt clinical dysfunction. Additionally, we observed significant improvements in other parameters of metabolic health, including the aggregated marker Met-S score based on WC, heart rate and central DBP.
The observed reductions in central DBP and heart rate further support vascular and autonomic benefits of Calanus oil supplementation. Burhop et al. (2022) found no improvement in BP after 12 weeks of 2 g Calanus oil supplementation [
37]. In this study, participants supplemented 4 g of Calanus oil which may explain stronger and significant effects on central DBP. Several meta-analyses have already shown that n3 PUFA supplementation from fish oil is associated with improvements in BP [
38,
39]. A meta-analysis by Zhang et al. (2022) concluded that 2–3 g EPA+DHA per day is optimal to lower BP in healthy individuals, with stronger effects (>5 g/day) in those with dyslipidemia [
40]. In the current study, the daily amount of supplemented EPA and DHA was significantly lower at 276 mg and 256 mg, respectively. Furthermore, there are also chemical differences between the n3 PUFA present in fish oil and Calanus oil. In Calanus oil more than 80 % of FAs are bound as wax esters, whereas in fish oil, they are primarily bound to TAGs. In addition, Calanus oil contains several other bioactive compounds, such as astaxanthin and policosanol. These compounds reduce oxidative stress and may therefore have beneficial effects on NO bioavailability, BP, and fibrosis [
41].
Limitations
This pilot study has some limitations that warrant recognition. First, the study was not placebo controlled, which makes it more difficult to directly attribute the observed effects to Calanus oil supplementation. In addition, we did not assess the omega-3index (O3I) to evaluate the supply status of n3 PUFA at baseline and after 12 weeks of intervention. A significant association between an increase in EPA+DHA blood status and a change in E/A would contribute significantly to a better understanding of the variation in E/A and can also be used to monitor compliance. However, we screened all participants for low fish intake (less than 1x per week), which is a common approach to selecting participants with initial low O3I, as fish intake is the main dietary source of long-chain n3 PUFA. In addition, compliance was assessed by counting capsules at t12. A significant reduction in WC, absolute fat mass, and visceral fat mass was observed as secondary outcomes. While these changes are physiologically relevant, they may confound the interpretation of changes in E/A. However, without a placebo group, no adjusted models were conducted, as confounding and mediation effects cannot be distinguished due to the known influence of n3 PUFAs on WC and fat mass. Besides the E/A, multiple pre-specified outcomes related to metabolic and cardiac function were assessed. No formal correction for multiple testing was applied, which increases the risk of type I error. However, given the exploratory nature of the study and the mechanistic interest across several physiological domains, results should be interpreted with appropriate caution. As a final point, the image quality of the echocardiography data did not consistently allow a complete analysis. This issue arose due to the inherent physiological differences across individual participants. These limitations collectively underscore the need for cautious interpretation of the study's outcomes and suggest avenues for refinement in future investigations.
5. Conclusions
In summary, 12 weeks of Calanus oil supplementation may improve diastolic function by increasing E/A in prediabetic women. In addition, the supplementation of Calanus oil may have had a positive effect on metabolic health. While these explorative results suggest metabolic and hemodynamic benefits, the underlying mechanisms and their clinical relevance warrant closer examination. Future studies should investigate the combined use of Calanus oil with lifestyle interventions in larger, more diverse and placebo-controlled trials, including direct measures of myocardial metabolism by positron emission tomography.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
FK: Validation, Formal Analysis, Data Curation, Writing - Original Draft Preparation, Visualization,; FS: Validation, Formal Analysis, Investigation, Data Curation, Writing - Original Draft Preparation; EJS: Conceptualization, Methodology, Validation, Writing - Review & Editing, Supervision; SJ: Methodology, Investigation, Visualization; WJ: Conceptualization, Methodology, Investigation; EF: Methodology, Investigation, Data Curation; JPS: Conceptualization, Methodology, Validation, Writing - Review & Editing AH: Conceptualization, Methodology, Validation, Writing - Review & Editing, Supervision; All authors have read and agreed to the submitted version of the manuscript.
Funding
This research was partially funded by Zooca®Lipids, Tromsø, Norway, grant number 60422758
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Ethic Committee of the Medical Chamber of Lower Saxony (Hannover, Germany) with the protocol code and date of approval: Bo/35/2022, 8 January 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon request from the corresponding author.
Conflicts of Interest
This research was partially funded by Zooca®Lipids, Tromsø, Norway, grant number 60422758. The sponsors had no role in the design, execution, interpretation, or writing of the study. The authors declare no conflicts of interest. All authors have read and agreed to the submitted version of the manuscript.
Abbreviations
The following abbreviations are used in this manuscript:
| Alx |
Augmentation index |
| AFI |
Automated function imaging |
| BMI |
Body mass index |
| DBP |
Central diastolic blood pressure |
| BP |
Central blood pressure |
| DT |
Deceleration time |
| DHA |
Docosahexaenoic acid |
| E/A |
Ratio of the early (E) to late (A) ventricular filling velocities |
| EPA |
Eicosapentaenoic acid |
| ECW |
Extracellular water |
| HOMA |
The Homeostasis Model Assessment |
| HR |
Heart rate |
| HDL-C |
High-density lipoprotein cholesterol |
| HPLC |
High-pressure liquid chromatography |
| IR |
Insulin resistance |
| IVRT |
Isovolumic relaxation time |
| LDL-C |
Low-density lipoprotein cholesterol |
| LV |
Left ventricle |
| LVEF |
Left ventricular ejection fraction |
| LVEDV |
Left ventricular end-diastolic volume |
| LVESV |
Left ventricular end-systolic volume |
| LVSV |
Left ventricular stroke volume |
| LS |
Longitudinal strain |
| Met-S score |
Metabolic syndrome severity score |
| n3 PUFAs |
Long-chain omega-3 polyunsaturated fatty acids |
| NO |
Nitric oxide |
| O3I |
Omega-3 index |
| PWV |
Pulse wave velocity |
References
- IDF Diabetes Atlas, (11th (Ed.) IDF Diabetes Atlas (11th Ed.). Available online: https://diabetesatlas.org/ (accessed on 23 May 2025).
- Gruss, S.M.; Nhim, K.; Gregg, E.; Bell, M.; Luman, E.; Albright, A. Public Health Approaches to Type 2 Diabetes Prevention: The US National Diabetes Prevention Program and Beyond. Curr Diab Rep 2019, 9, 78–19. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. An Atlas on Risk Factors for Type 2 Diabetes: A Wide-Angled Mendelian Randomisation Study. Diabetologia 2020, 63, 2359–2371. [Google Scholar] [CrossRef]
- Fritsche, A.; Wagner, R.; Heni, M.; Kantartzis, K.; Machann, J.; Schick, F.; Lehmann, R.; Peter, A.; Dannecker, C.; Fritsche, L.; et al. Different Effects of Lifestyle Intervention in High-and Low-Risk Prediabetes: Results of the Randomized Controlled Prediabetes Lifestyle Intervention Study (PLIS). Diabetes 2021, 70, 2785–2795. [Google Scholar] [CrossRef] [PubMed]
- Payton, L.; Noirot, C.; Last, K.S.; Grigor, J.; Hüppe, L.; Conway, D.V.P.; Dannemeyer, M.; Suin, A.; Meyer, B. Annual Transcriptome of a Key Zooplankton Species, the Copepod Calanus Finmarchicus. Ecol Evol 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.F.; Hagen, W.; Kattner, G. Lipid Storage in Marine Zooplankton. Mar Ecol Prog Ser 2006, 307, 273–306. [Google Scholar] [CrossRef]
- Vosskötter, F.; Burhop, M.; Hahn, A.; Schuchardt, J.P. Equal Bioavailability of Omega-3 PUFA from Calanus Oil, Fish Oil and Krill Oil: A 12-Week Randomized Parallel Study. Lipids 2023, 58, 129–138. [Google Scholar] [CrossRef]
- Salma, W.; Franekova, V.; Lund, T.; Höper, A.; Ludvigsen, S.; Lund, J.; Aasum, E.; Ytrehus, K.; Belke, D.D.; Larsen, T.S. Dietary Calanus Oil Antagonizes Angiotensin II-Induced Hypertension and Tissue Wasting in Diet-Induced Obese Mice. Prostaglandins Leukot Essent Fatty Acids 2016, 108, 13–21. [Google Scholar] [CrossRef]
- Štěpán, M.; Dad’ová, K.; Matouš, M.; Krauzová, E.; Sontáková, L.; Koc, M.; Larsen, T.; Kuda, O.; Štich, V.; Rossmeislová, L.; et al. Exercise Training Combined with Calanus Oil Supplementation Improves the Central Cardiodynamic Function in Older Women. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Bernasconi, A.A.; Wiest, M.M.; Lavie, C.J.; Milani, R. V.; Laukkanen, J.A. Effect of Omega-3 Dosage on Cardiovascular Outcomes: An Updated Meta-Analysis and Meta-Regression of Interventional Trials. Mayo Clin Proc 2021, 96, 304–313. [Google Scholar] [CrossRef]
- Harris, W.S.; Miller, M.; Tighe, A.P.; Davidson, M.H.; Schaefer, E.J. Omega-3 Fatty Acids and Coronary Heart Disease Risk: Clinical and Mechanistic Perspectives. Atherosclerosis 2008, 197, 12–24. [Google Scholar] [CrossRef]
- Mason D., Marcus; Mark, S. Link Omega-3 Fatty Acids and Arrhythmias. Circulation 2024, 150. [Google Scholar]
- Calder, P.C. Marine Omega-3 Fatty Acids and Inflammatory Processes: Effects, Mechanisms and Clinical Relevance. Biochim Biophys Acta Mol Cell Biol Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Zehr, K.R.; Walker, M.K. Omega-3 Polyunsaturated Fatty Acids Improve Endothelial Function in Humans at Risk for Atherosclerosis: A Review. Prostaglandins Other Lipid Mediat 2018, 134, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.E.; Van Elswyk, M.; Alexander, D.D. Long-Chain Omega-3 Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid and Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Am J Hypertens 2014, 27, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Barbarawi, M.; Lakshman, H.; Barbarawi, O.; Alabdouh, A.; Al Kasasbeh, M.; Djousse, L.; Manson, J.A.E. Omega-3 Supplementation and Heart Failure: A Meta-Analysis of 12 Trials Including 81,364 Participants. Contemp Clin Trials 2021, 107. [Google Scholar] [CrossRef]
- Kerlikowsky, F.; Bartsch, M.; Jonas, W.; Hahn, A.; Schuchardt, J.P. Calanus Oil and Lifestyle Interventions Improve Glucose Homeostasis in Obese Subjects with Insulin Resistance. Mar Drugs 2025, 23. [Google Scholar] [CrossRef]
- Frey I; Berg A; Grathwohl D; Keul J Freiburg Questionnaire of Physical Activity--Development, Evaluation and Application. Soz Praventivmed 1999, 44, 55–64. [CrossRef]
- DeBoer, M.D.; Filipp, S.L.; Gurka, M.J. Use of a Metabolic Syndrome Severity z Score to Track Risk during Treatment of Prediabetes: An Analysis of the Diabetes Prevention Program. In Proceedings of the Diabetes Care; American Diabetes Association Inc. 2018, 41, 2421–2430. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.R.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Fl-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. 1985, 28. [CrossRef]
- Dang, K.; Wang, X.; Hu, J.; Zhang, Y.; Cheng, L.; Qi, X.; Liu, L.; Ming, Z.; Tao, X.; Li, Y. The Association between Triglyceride-Glucose Index and Its Combination with Obesity Indicators and Cardiovascular Disease: NHANES 2003–2018. Cardiovasc Diabetol 2024, 23. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Aneja, A.; Tang, W.H.W.; Bansilal, S.; Garcia, M.J.; Farkouh, M.E. Diabetic Cardiomyopathy: Insights into Pathogenesis, Diagnostic Challenges, and Therapeutic Options. American Journal of Medicine 2008, 121, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Maack, C. Metabolic Remodelling in Heart Failure. 2018. [CrossRef]
- Stahrenberg, R.; Edelmann, F.; Mende, M.; Kockskämper, A.; Düngen, H.D.; Scherer, M.; Kochen, M.M.; Binder, L.; Herrmann-Lingen, C.; Gelbrich, G.; et al. Association of Glucose Metabolism with Diastolic Function along the Diabetic Continuum. Diabetologia 2010, 53, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, A.; Mangiafico, S.; Urbano, F.; Scicali, R.; Scandura, S.; D’Agate, V.; Piro, S.; Tamburino, C.; Purrello, F.; Rabuazzo, A.M. HbA1c Identifies Subjects with Prediabetes and Subclinical Left Ventricular Diastolic Dysfunction. Journal of Clinical Endocrinology and Metabolism 2017, 102, 3756–3764. [Google Scholar] [CrossRef]
- Salma, W.; Franekova, V.; Lund, T.; Höper, A.; Ludvigsen, S.; Lund, J.; Aasum, E.; Ytrehus, K.; Belke, D.D.; Larsen, T.S. Dietary Calanus Oil Antagonizes Angiotensin II-Induced Hypertension and Tissue Wasting in Diet-Induced Obese Mice. Prostaglandins Leukot Essent Fatty Acids 2016, 108, 13–21. [Google Scholar] [CrossRef]
- Gui, T.; Li, Y.; Zhang, S.; Zhang, N.; Sun, Y.; Liu, F.; Chen, Q.; Gai, Z. Docosahexaenoic Acid Protects against Palmitate-Induced Mitochondrial Dysfunction in Diabetic Cardiomyopathy. Biomedicine and Pharmacotherapy 2020, 128. [Google Scholar] [CrossRef]
- Eraky, S.M.; Ramadan, N.M. Effects of Omega-3 Fatty Acids and Metformin Combination on Diabetic Cardiomyopathy in Rats through Autophagic Pathway. Journal of Nutritional Biochemistry 2021, 97. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, M.; Xu, H. Ginger Extract and Omega-3 Fatty Acids Supplementation: A Promising Strategy to Improve Diabetic Cardiomyopathy. Physiol Res 2024, 73, 351–367. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 Fatty Acids and Cardiovascular Disease: Effects on Risk Factors, Molecular Pathways, and Clinical Events. J Am Coll Cardiol 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Chen, X.; Qin, C.; Hu, J.; Zeng, X.; Luo, H.; Yang, P.; Luo, H.; Yuan, C.; et al. Higher Triglyceride Glucose-Waist Height Ratio Index Is Associated with Higher Prevalence of Gallstone: A Population-Based Study. Front Med (Lausanne) 2024, 11. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ Res 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Kenny, H.C.; Abel, E.D. Heart Failure in Type 2 Diabetes Mellitus: Impact of Glucose-Lowering Agents, Heart Failure Therapies, and Novel Therapeutic Strategies. Circ Res 2019, 124, 121–141. [Google Scholar] [CrossRef]
- Peterson, L.R.; Herrero, P.; Schechtman, K.B.; Racette, S.B.; Waggoner, A.D.; Kisrieva-Ware, Z.; Dence, C.; Klein, S.; Marsala, J.A.; Meyer, T.; et al. Effect of Obesity and Insulin Resistance on Myocardial Substrate Metabolism and Efficiency in Young Women. Circulation 2004, 109, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; DeMarco, V.G.; Sowers, J.R. Insulin Resistance and Hyperinsulinaemia in Diabetic Cardiomyopathy. Nat Rev Endocrinol 2016, 12, 144–153. [Google Scholar] [CrossRef]
- Burhop, M.; Schuchardt, J.P.; Nebl, J.; Müller, M.; Lichtinghagen, R.; Hahn, A. Marine Oil from C. Finmarchicus Enhances Glucose Homeostasis and Liver Insulin Resistance in Obese Prediabetic Individuals. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Geelen, A.; Brouwer, I.A.; Geleijnse, J.M.; Zock, P.L.; Katan, M.B. Effect of Fish Oil on Heart Rate in Humans: A Meta-Analysis of Randomized Controlled Trials. Circulation 2005, 112, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Geleijnse, J.M.; Giltay, E.J.; Grobbee, E.; Donders, A.R.T.; Kok, F.J. Blood Pressure Response to Fish Oil Supplementation: Metaregression Analysis of Randomized Trials; Lippincott Williams & Wilkins 2002, 20. [CrossRef]
- Zhang, X.; Ritonja, J.A.; Zhou, N.; Chen, B.E.; Li, X. Omega-3 Polyunsaturated Fatty Acids Intake and Blood Pressure: A Dose-Response Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc 2022, 11. [Google Scholar] [CrossRef]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic Cardiomyopathy: A Hyperglycaemia- and Insulin-Resistance-Induced Heart Disease. Diabetologia 2018, 61, 21–28. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).