Submitted:
18 July 2025
Posted:
18 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Data Collection and Laboratory Measurements
2.3. Naples Prognostic Score Assessment
2.4. Outcome Definition
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Comparison Between Risk Groups
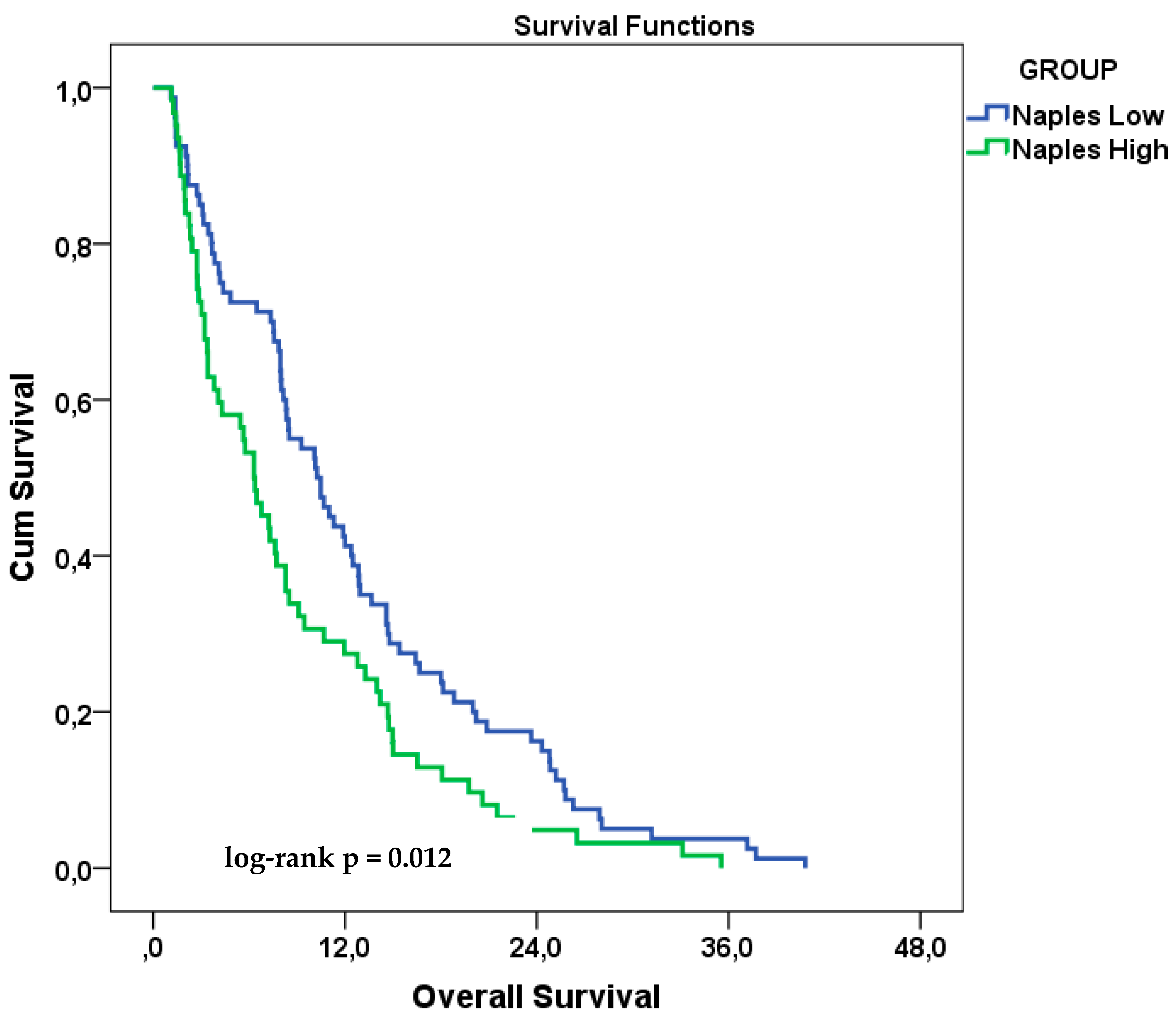
3.3. Overall Survival Analysis
3.4. Univariate Cox Regression Analysis
3.5. Multivariate Cox Regression Analysis
3.5.1. Model 1—Prognostic Impact of the Composite NPS
3.5.2. Model 2—Prognostic Role of Individual NPS Components
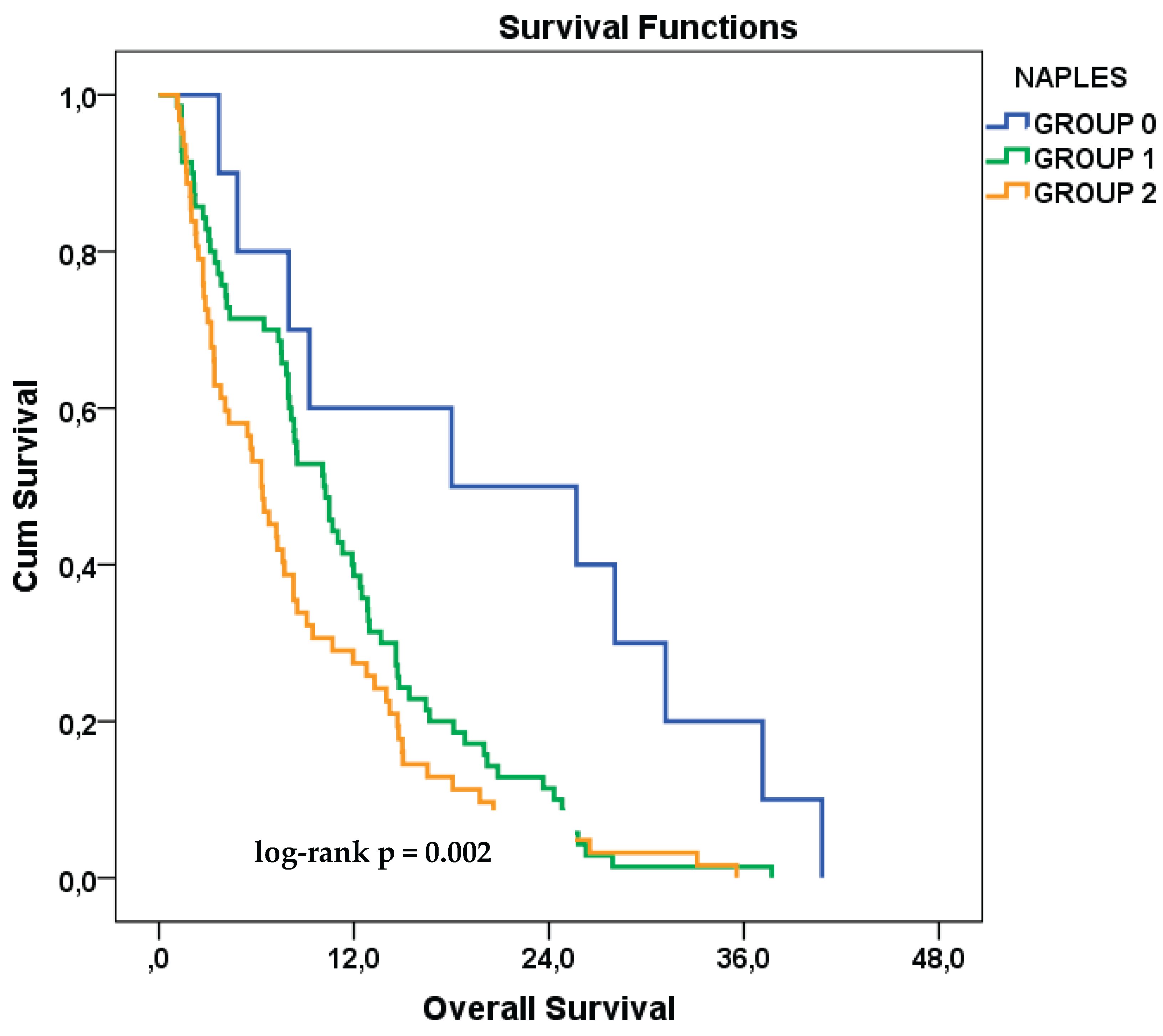
3.6. Exploratory Analysis Based on the Original NPS Classification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCLC | Small Cell Lung Cancer |
| ES-SCLC | Extensive-Stage Small Cell Lung Cancer |
| NSCLC | Non-Small Cell Lung Cancer |
| NPS | Naples Prognostic Score |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| LMR | Lymphocyte-to-Monocyte Ratio |
| ECOG PS | Eastern Cooperative Oncology Group performance status |
| PCI | Prophylactic Cranial Irradiation |
| TME | Tumor Microenvironment |
| MMP | Matrix Metalloproteinase |
| TAM | Tumor-Associated Macrophages |
| CI | Confidence Interval |
| HR | Hazard Ratio |
| IQR | Interquartile Range |
| SPSS | Statistical Package for the Social Sciences |
| ORCID | Open Researcher and Contributor ID |
| SD | Standard Deviation |
| MD | Medical Doctor |
| Ass. Prof. Dr. | Associate Professor Doctor |
| Prof. Dr. | Professor Doctor |
References
- Matera, R.; Chiang, A. What Is New in Small Cell Lung Cancer. Hematol Oncol Clin North Am. 2023, 37, 595–607. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Y.; Yu, Y.; et al. Global variations in lung cancer incidence by histological subtype in 2020: a population-based study. Lancet Oncol. 2023, 24, 1206–1218. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, H.S.; Chiang, A.C. Small Cell Lung Cancer: A Review. JAMA. 2025, 333, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Liu, S.V.; Mansfield, A.S.; et al. Five-year survival in patients with extensive-stage small cell lung cancer treated with atezolizumab in the Phase III IMpower133 study and the Phase III IMbrella A extension study. Lung Cancer. 2024, 196, 107924. [Google Scholar] [CrossRef] [PubMed]
- Misawa, K.; Miura, K.; Nakajima, M.; et al. Durvalumab plus platinum-etoposide chemotherapy for extensive-stage small cell lung cancer: a retrospective real-world study. Transl Lung Cancer Res. 2024, 13, 1585–1594. [Google Scholar] [CrossRef]
- Hamakawa, Y.; Kamimaki, I.; Tanaka, M.; et al. Prognostic value of systemic immune-inflammation index in patients with small-cell lung cancer treated with immune checkpoint inhibitors. BMC Cancer. 2025, 25, 17. [Google Scholar] [CrossRef]
- Farris, M.K.; Mitra, N.; Sun, Y.; et al. Prognostic Factors in Limited-Stage Small Cell Lung Cancer: A Secondary Analysis of CALGB 30610-RTOG 0538. JAMA Netw Open. 2024, 7, e2440673. [Google Scholar] [CrossRef]
- Kang, L.; Li, M.; Tang, X.; et al. Association of Neutrophil-to-Lymphocyte Ratio with Nutrition in Patients with Various Types of Malignant Tumors: A Multicenter Cross-Sectional Study. J Inflamm Res. 2023, 16, 1419–1429. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawada, K.; Obama, K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int J Mol Sci. 2021, 22, 8182. [Google Scholar] [CrossRef]
- Tan, S.; Wei, Y.; Huang, J.; et al. Prognostic value of inflammatory markers NLR, PLR, and LMR in gastric cancer patients treated with immune checkpoint inhibitors: a meta-analysis and systematic review. Front Immunol. 2024, 15, 1408700. [Google Scholar] [CrossRef]
- Proctor, M.J.; Talwar, D.; Balmar, S.M.; et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011, 47, 2633–2641. [Google Scholar] [CrossRef]
- Galizia, G.; Lieto, E.; Auricchio, A.; et al. Naples Prognostic Score, Based on Nutritional and Inflammatory Status, is an Independent Predictor of Long-term Outcome in Patients Undergoing Surgery for Colorectal Cancer. Dis Colon Rectum. 2017, 60, 1273–1284. [Google Scholar] [CrossRef]
- Peng, S.M.; Zhang, J.J.; Wang, Z.H.; et al. The prognostic value of the Naples prognostic score for patients with non-small-cell lung cancer. Sci Rep. 2022, 12, 5782. [Google Scholar] [CrossRef]
- Gulturk, I.; Kaplan, M.A.; Genc, A.B.; et al. Naples prognostic score may predict overall survival in metastatic pancreatic cancer. J Cancer Res Ther. 2024, 20, 249–254. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.; Yang, H.; et al. Naples Prognostic Score as a Novel Prognostic Prediction Tool for Resectable Locally Advanced Esophageal Squamous Cell Carcinoma After Neoadjuvant Therapy. J Inflamm Res. 2025, 18, 4843–4856. [Google Scholar] [CrossRef]
- Gitmez, M.; Ekingen, E.; Zaman, S. Predictive Value of the Naples Prognostic Score for One-Year Mortality in NSTEMI Patients Undergoing Selective PCI. Diagnostics 2025, 15, 608. [Google Scholar] [CrossRef]
- Chen, S.; Liu, X.; Wang, L.; et al. Naples Prognostic Score is an Independent Prognostic Factor in Patients with Small Cell Lung Cancer and Nomogram Predictive Model Established. J Inflamm Res. 2022, 15, 3719–3731. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016, 16, 431–446. [Google Scholar] [CrossRef]
- Yang, Q.; Ye, Z.; Zhang, L.; et al. Prognostic value of pre-treatment Naples prognostic score (NPS) in patients with osteosarcoma. World J Surg Oncol. 2020, 18, 24. [Google Scholar] [CrossRef]
- Bekes, E.M.; Schweighofer, B.; Kupriyanova, T.A.; et al. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011, 179, 1455–1470. [Google Scholar] [CrossRef]
- Kumari, N.; Choi, S.H. Tumor-associated macrophages in cancer: recent advancements in cancer nanoimmunotherapies. J Exp Clin Cancer Res. 2022, 41, 68. [Google Scholar] [CrossRef]
- Almasaudi, A.S.; McSorley, S.T.; Edwards, C.A.; et al. Hypoalbuminemia Reflects Nutritional Risk, Body Composition and Systemic Inflammation and Is Independently Associated with Survival in Patients with Colorectal Cancer. Cancers 2020, 12, 1868. [Google Scholar] [CrossRef]
- Pecci, F.; Scotto, G.; Bacigalupo, B.; et al. Prognostic Impact of Blood Lipid Profile in Patients With Advanced Solid Tumors Treated With Immune Checkpoint Inhibitors: A Multicenter Cohort Study. Oncologist. 2024, 29, e372–e81. [Google Scholar] [CrossRef]
- Xie, Y.M.; Zhang, W.; Wang, H.X.; et al. Naples Prognostic Score is an Independent Prognostic Factor in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2023, 10, 1423–1433. [Google Scholar] [CrossRef]
- Gu, J.; Wang, Z.; Tian, Y.; et al. Modified Naples prognostic score for evaluating the prognosis of patients with obstructive colorectal cancer. BMC Cancer. 2023, 23, 941. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Y.; Tang, L.; et al. The value of the preoperative Naples prognostic score in predicting prognosis in gallbladder cancer surgery patients. World J Surg Oncol. 2023, 21, 303. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Wang, Q.; et al. Prognostic value of Naples Prognostic Score in locally advanced cervical cancer patients undergoing concurrent chemoradiotherapy. Biomol Biomed. 2025, 25, 986–999. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, L.; Liang, R.; et al. Assessment of Naples prognostic score in predicting survival for small cell lung cancer patients treated with chemoradiotherapy. Ann Med. 2023, 55, 2242254. [Google Scholar] [CrossRef]
- Zou, Z.; Shen, L.; Huang, Y.; et al. Naples Prognostic Score as an Independent Predictor of Survival Outcomes for Resected Locally Advanced Non-Small Cell Lung Cancer Patients After Neoadjuvant Treatment. J Inflamm Res. 2023, 16, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Wu, Q.; Wang, H.; et al. Clinical Significance of Preoperative Naples Prognostic Score in Patients With Non-Small Cell Lung Cancer. Technol Cancer Res Treat. 2022, 21, 15330338221129447. [Google Scholar] [CrossRef]
- Wang, Y.S.; Li, Y.; Zhang, W.; et al. Naples prognostic score as a predictor of outcomes in lung cancer: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2023, 27, 8144–8153. [Google Scholar]
- Peker, P.; Alacacıoğlu, A.; Karcı, E.; et al. Prognostic Power of the Naples Score in Non-Small Cell Lung Cancer: Can Inflammation and Nutrition Predict Survival? J Clin Med. 2025, 14, 3715. [Google Scholar] [CrossRef] [PubMed]
| Clinical Variable | Category | Total (n, %) | Low-Risk Group | High-Risk Group | p-value |
|---|---|---|---|---|---|
| Age | <65 years | 95 (66.9%) | 56 (70.0%) | 39 (62.9%) | 0.472 |
| ≥65 years | 47 (33.1%) | 24 (30.0%) | 23 (37.1%) | ||
| ECOG PS | 0 | 91 (64.1%) | 58 (72.5%) | 33 (53.2%) | 0.018 |
| ≥1 | 51 (35.9%) | 22 (27.5%) | 29 (46.8%) | ||
| Sex | Female | 11 (7.7%) | 5 (6.3%) | 6 (9.7%) | 0.449 |
| Male | 131 (92.3%) | 75 (93.8%) | 56 (90.3%) | ||
| Smoking Status | Non-current smoker | 34 (23.9%) | 17 (21.3%) | 17 (27.4%) | 0.393 |
| Current smoker | 108 (76.1%) | 63 (78.8%) | 45 (72.6%) | ||
| Comorbidity | Absent | 72 (50.7%) | 41 (51.2%) | 31 (50.0%) | 0.883 |
| Present | 70 (49.3%) | 39 (48.8%) | 31 (50.0%) | ||
| Stage at Diagnosis | Limited disease | 39 (27.5%) | 23 (28.7%) | 16 (25.8%) | 0.697 |
| Extensive disease | 103 (72.5%) | 57 (71.3%) | 46 (74.2%) | ||
| PCI | Not administered | 107 (75.4%) | 56 (70.0%) | 51 (82.3%) | 0.093 |
| Administered | 35 (24.6%) | 24 (30.0%) | 11 (17.7%) | ||
| Number of Metastatic Sites | Single site | 45 (31.7%) | 25 (31.3%) | 20 (32.3%) | 0.898 |
| ≥2 sites | 97 (68.3%) | 55 (68.8%) | 42 (67.7%) | ||
| Brain Metastasis | Absent | 102 (71.8%) | 57 (71.3%) | 45 (72.6%) | 0.861 |
| Present | 40 (28.2%) | 23 (28.7%) | 17 (27.4%) | ||
| Bone Metastasis | Absent | 71 (50.0%) | 44 (55.0%) | 27 (43.5%) | 0.176 |
| Present | 71 (50.0%) | 36 (45.0%) | 35 (56.5%) | ||
| Liver Metastasis | Absent | 105 (73.9%) | 61 (76.3%) | 44 (71.0%) | 0.477 |
| Present | 37 (26.1%) | 19 (23.8%) | 18 (29.0%) | ||
| Lung Metastasis | Absent | 117 (82.4%) | 67 (83.8%) | 50 (80.6%) | 0.630 |
| Present | 25 (17.6%) | 13 (16.3%) | 12 (19.4%) | ||
| Adrenal Metastasis | Absent | 109 (76.8%) | 61 (76.3%) | 48 (77.4%) | 0.870 |
| Present | 33 (23.2%) | 19 (23.8%) | 14 (22.6%) |
| Clinical Variable | Category | HR (95% CI) | p-value | Reference Category |
|---|---|---|---|---|
| Age | ≥65 years | 1.35 (0.94–1.92) | 0.101 | <65 years |
| ECOG PS | ECOG ≥1 | 1.43 (1.01–2.03) | 0.047 | ECOG 0 |
| Sex | Male | 0.98 (0.53–1.83) | 0.957 | Female |
| Smoking Status | Current smoker | 1.05 (0.71–1.55) | 0.809 | Non-current smoker |
| Comorbidity | Present | 1.26 (0.91–1.76) | 0.168 | Absent |
| Stage at Diagnosis | De novo | 0.93 (0.65–1.34) | 0.681 | Relapsed |
| PCI | Yes | 0.98 (0.67–1.45) | 0.935 | No PCI |
| Number of Metastatic Sites | ≥2 sites | 1.59 (1.11–2.29) | 0.012 | Single site |
| Brain Metastasis | Present | 1.38 (0.95–2.00) | 0.088 | Absent |
| Bone Metastasis | Present | 1.41 (1.01–1.98) | 0.044 | Absent |
| Liver Metastasis | Present | 1.08 (0.74–1.58) | 0.691 | Absent |
| Lung Metastasis | Present | 1.52 (0.98–2.33) | 0.061 | Absent |
| Adrenal Metastasis | Present | 0.97 (0.65–1.44) | 0.868 | Absent |
| NLR | High (≥ cutoff) | 1.34 (0.95–1.89) | 0.098 | Low (< cutoff) |
| LMR | High (≥ cutoff) | 1.62 (1.06–2.47) | 0.027 | Low (< cutoff) |
| Serum Cholesterol | High (≥ cutoff) | 0.86 (0.57–1.30) | 0.475 | Low (< cutoff) |
| Serum Albumin | High (≥ cutoff) | 1.44 (1.02–2.03) | 0.040 | Low (< cutoff) |
| NPS | High (3–4) | 1.54 (1.10–2.15) | 0.013 | Low (0–2) |
| Variable | HR (95% CI) | p-value |
|---|---|---|
| High NPS (3–4) | 1.45 (1.02–2.06) | 0.041 |
| Bone metastasis | 1.27 (0.88–1.82) | 0.203 |
| Brain metastasis | 1.23 (0.84–1.78) | 0.290 |
| Lung metastasis | 0.68 (0.43–1.08) | 0.100 |
| ECOG PS ≥1 | 1.19 (0.82–1.73) | 0.367 |
| Age ≥65 | 1.20 (0.83–1.73) | 0.329 |
| Variable | HR (95% CI) | p-value |
|---|---|---|
| LMR (High) | 1.65 (1.04–2.61) | 0.034 |
| Serum albumin (High) | 1.48 (1.03–2.11) | 0.033 |
| NLR (High) | 1.01 (0.69–1.49) | 0.954 |
| Bone metastasis | 1.30 (0.89–1.88) | 0.164 |
| Brain metastasis | 1.18 (0.80–1.73) | 0.408 |
| Lung metastasis | 0.71 (0.45–1.12) | 0.139 |
| ECOG PS ≥1 | 1.43 (0.97–2.11) | 0.068 |
| Age ≥65 | 1.11 (0.77–1.60) | 0.591 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





