Submitted:
16 July 2025
Posted:
21 July 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
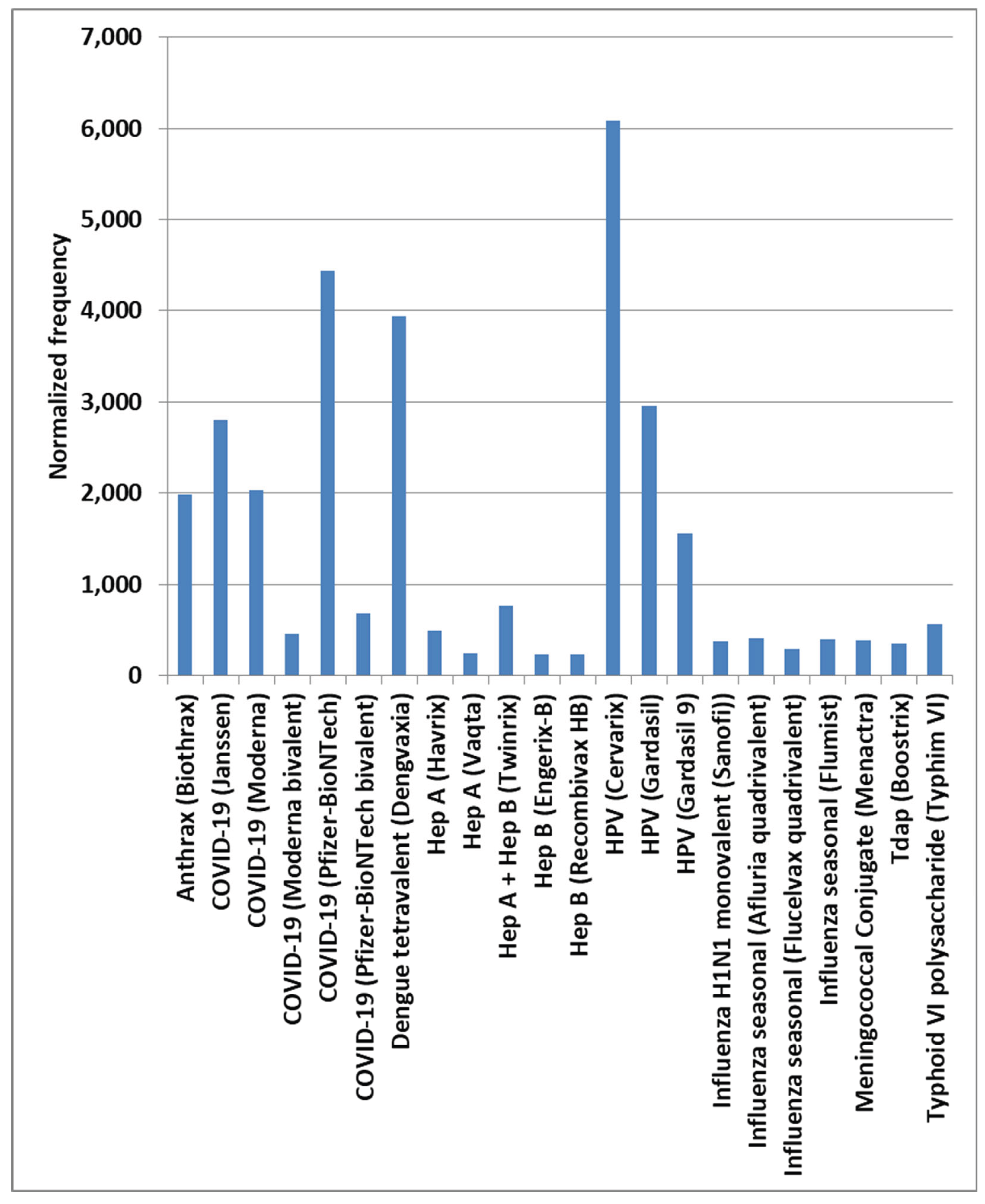
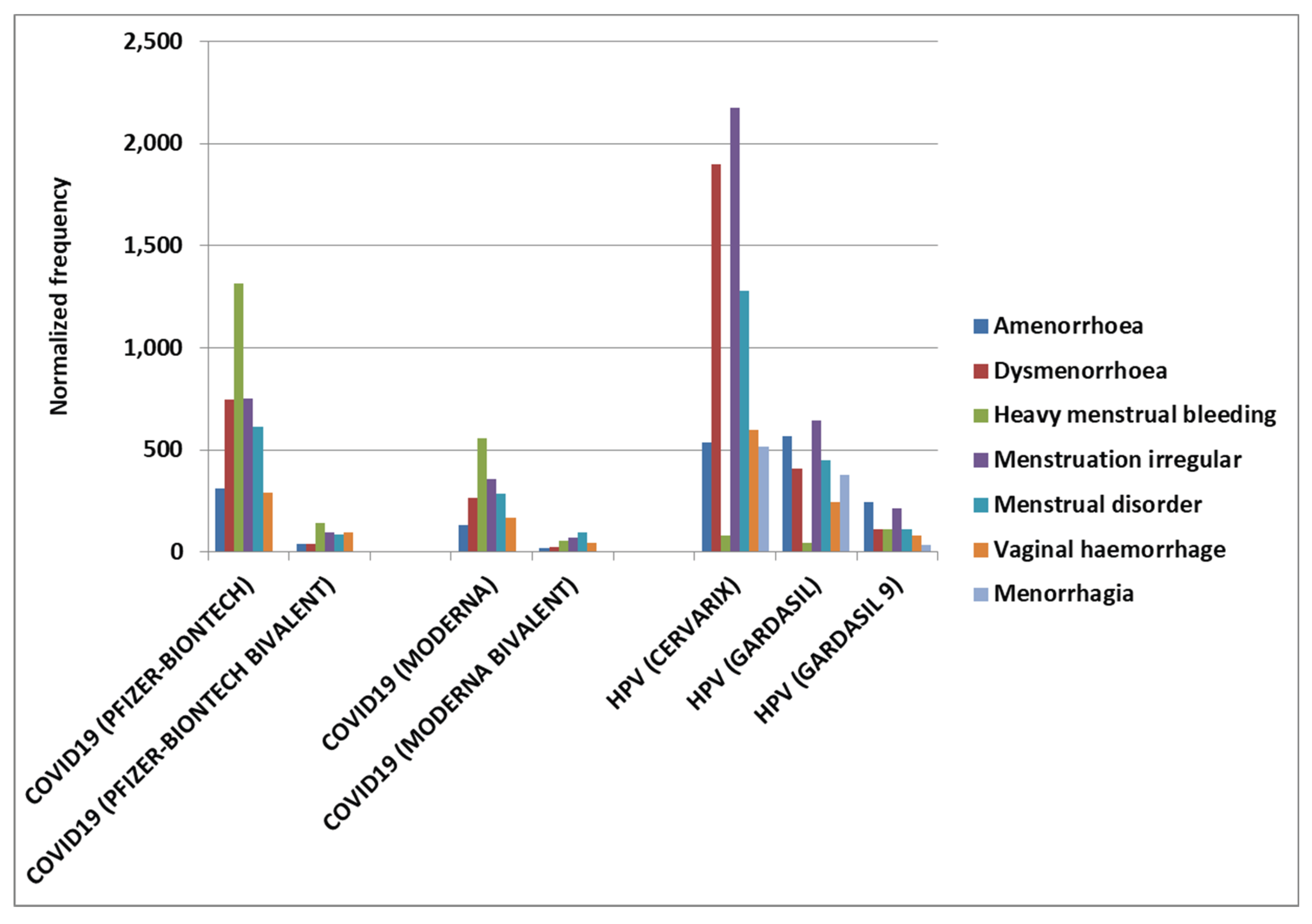
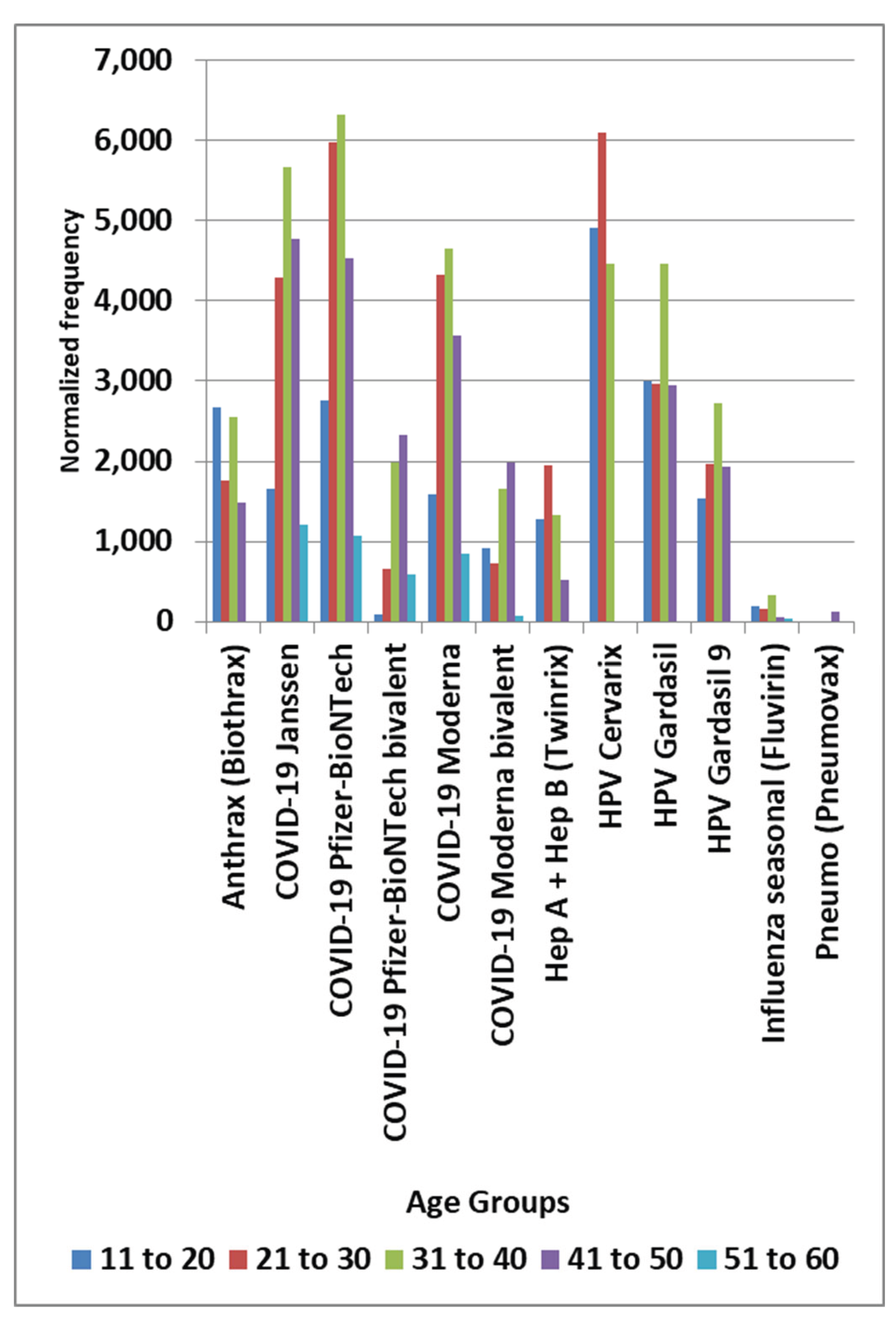
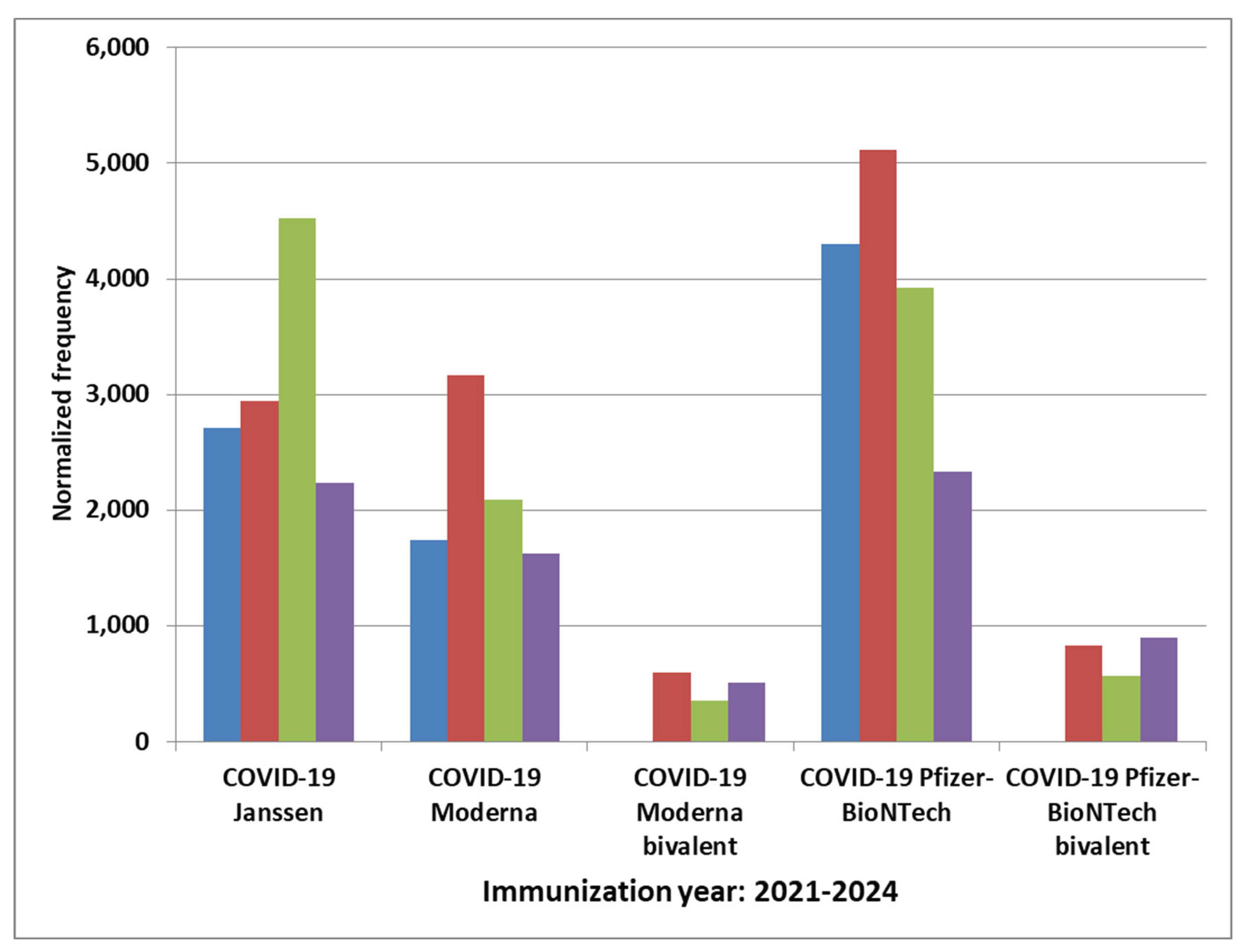
Results
Discussion
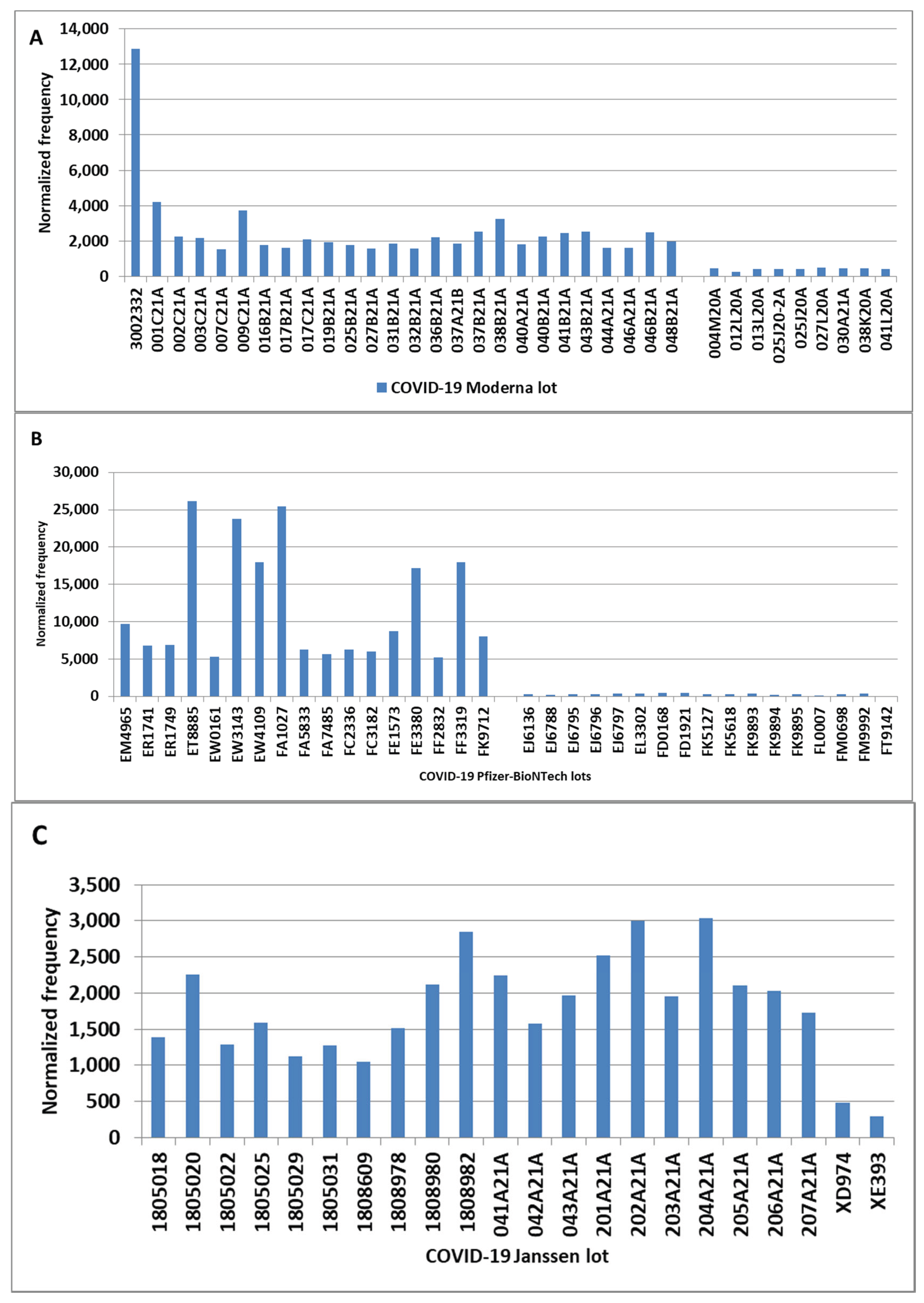
COVID-19 Vaccines
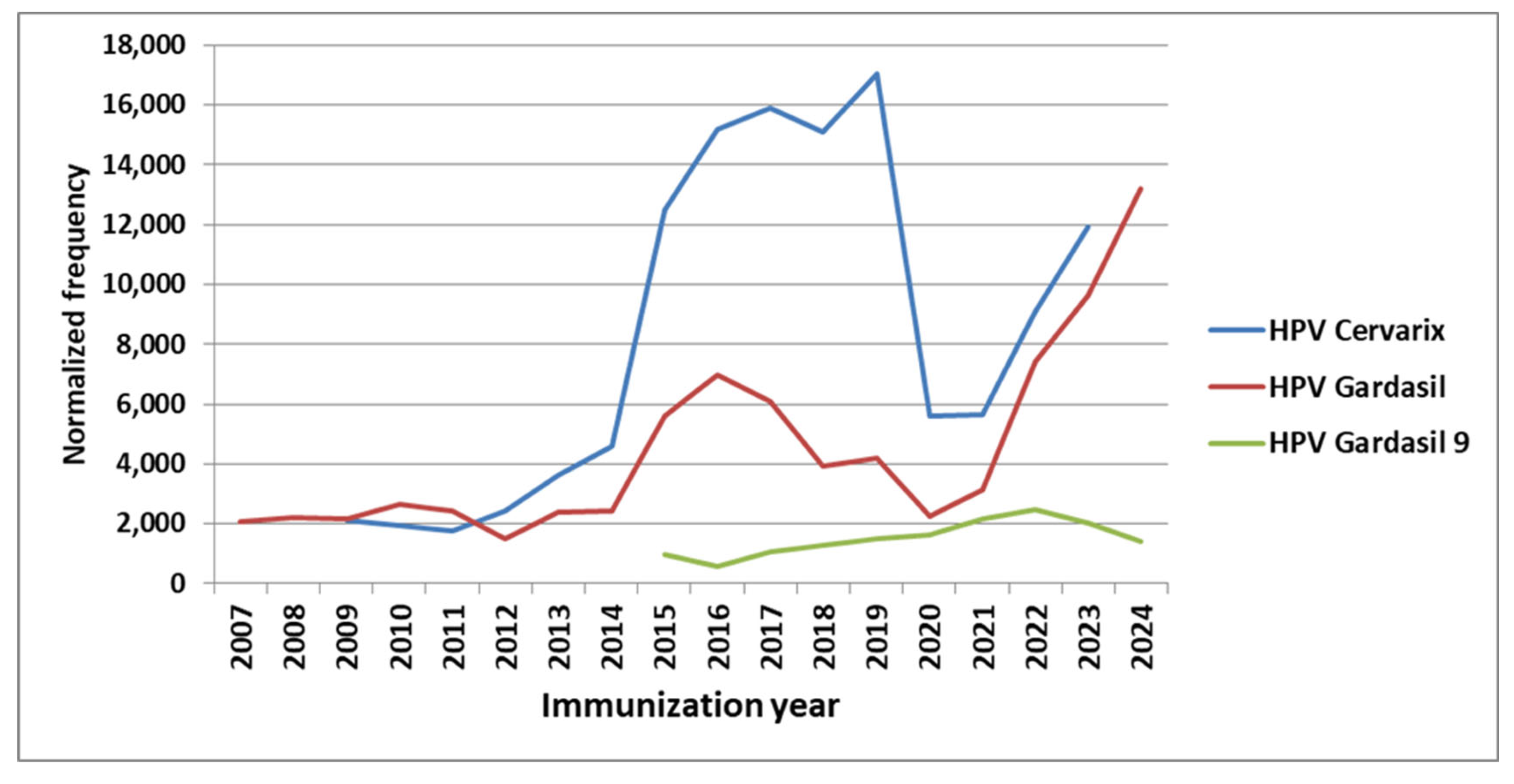
HPV Vaccines
All Vaccines
Conclusions
Funding
Author contributions
Availability of Data
Conflicts of interest
Ethical approval
Consent for Publication
References
- Matar SG, Nourelden AZ, Assar A, et al (2023) Effect of COVID-19 vaccine on menstrual experience among females in six Arab countries: A cross sectional study. Influenza Other Respir Viruses 17:e13088. [CrossRef]
- Baena-García L, Aparicio VA, Molina-López A, Aranda P, Cámara-Roca L, Ocón-Hernández O (2022) Premenstrual and menstrual changes reported after COVID-19 vaccination: The EVA project. Womens Health 18:17455057221112237. [CrossRef]
- Rastegar T, Feryduni L, Fakhraei M (2023) COVID-19 vaccine side effects on menstrual disturbances among Iranian women. New Microbes New Infect 53:101114. [CrossRef]
- Dabbousi AA, El Masri J, El Ayoubi LM, Ismail O, Zreika B, Salameh P (2023) Menstrual abnormalities post-COVID vaccination: a cross-sectional study on adult Lebanese women. Ir J Med Sci 1971 - 192:1163– 1170.
- Lee KM, Junkins EJ, Luo C, Fatima UA, Cox ML, Clancy KB (2022) Investigating trends in those who experience menstrual bleeding changes after SARS-CoV-2 vaccination. medRxiv 2021.10.11.21264863.
- Blix K, Laake I, Juvet L, Robertson AH, Caspersen IH, Mjaaland S, Skodvin SN, Magnus P, Feiring B, Trogstad L Unexpected vaginal bleeding and COVID-19 vaccination in nonmenstruating women. Sci Adv 9:eadg1391.
- Botton J, Bertrand M, Jabagi M-J, et al (2024) Risk of heavy menstrual bleeding following COVID-19 vaccination: A nationwide case-control study. Vaccine 42:126252.
- Muhaidat N, Alshrouf MA, Azzam MI, Karam AM, Al-Nazer MW, Al-Ani A (2022) Menstrual Symptoms After COVID-19 Vaccine: A Cross-Sectional Investigation in the MENA Region. Int J Womens Health 14:395–404.
- VAERS (2025) Vaccine Adverse Event Reporting System. U.S. Department of Health & Human Services.
- Ricke DO (2025) VAERS-Tools. https://github.com/doricke/VAERS-Tools Accessed July 1, 2025.
- Social Science Statistics (2025) Chi Square Calculator for 2x2. https://www.socscistatistics.com/tests/chisquare/ Accessed July 1, 2025.
- Bidne KL, Dickson MJ, Ross JW, Baumgard LH, Keating AF (2018) Disruption of female reproductive function by endotoxins. Reproduction 155:R169–R181.
- Wastila L, Fu Y-H, Tung CC, Qato DM (2025) Association Between Vaccination for Human Papillomavirus (HPV) and Autonomic Dysfunction and Menstrual Irregularities: A Self-Controlled Case Series Analysis. Drugs - Real World Outcomes. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




