Submitted:
16 July 2025
Posted:
17 July 2025
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
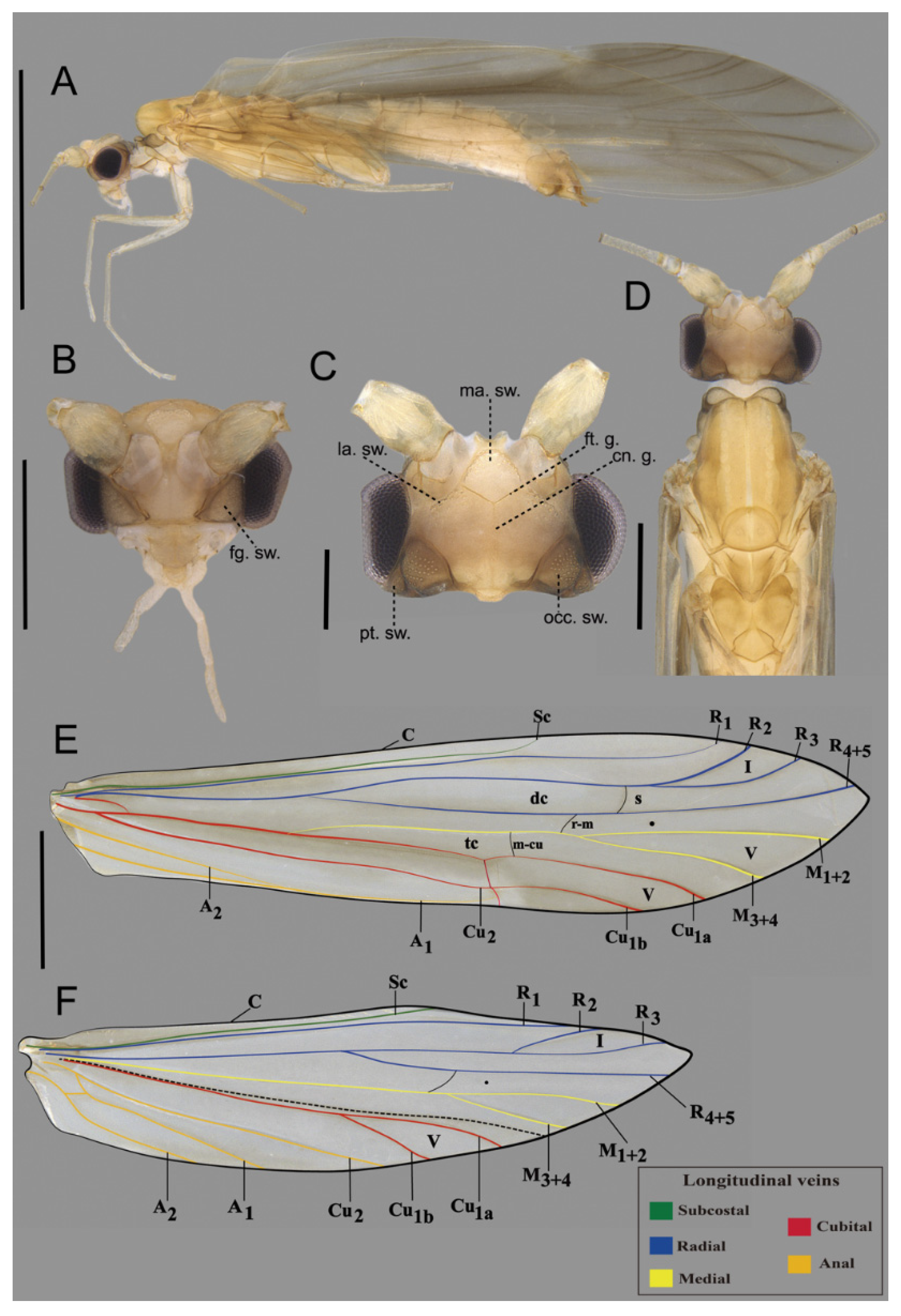
2.1. Study Area, Specimen Collection, Preparation, and Observation
2.2. Illustrations and Map
2.3. Morphological Terminology, Description, and Key
3. Results
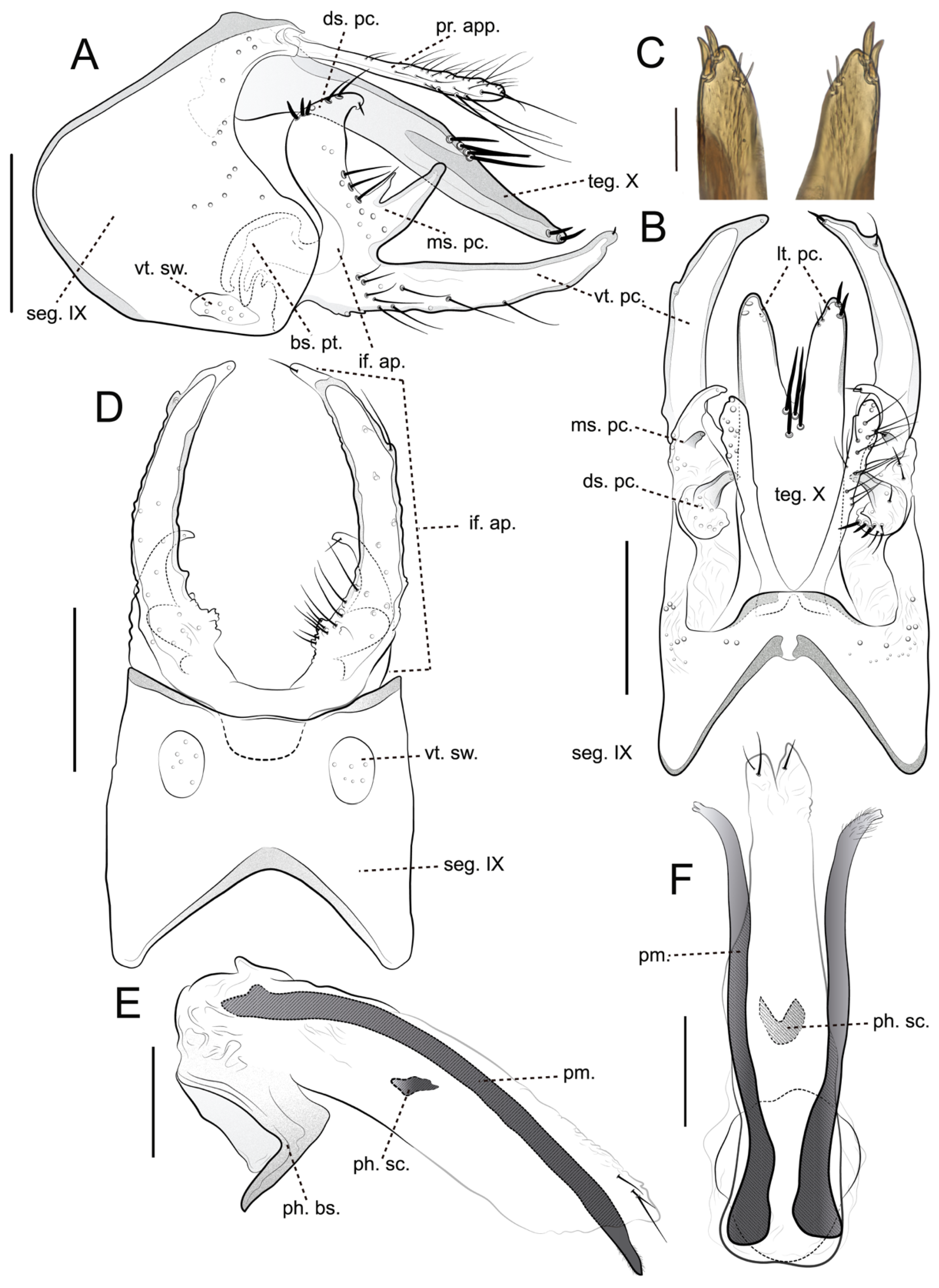
3.1. Species Description
3.2. Key to Males of Brachysetodes s. str.

4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| bs. pt. | basal plate |
| cn. g. | coronal groove |
| dc | discoidal cell |
| ds. pc. | dorsal process |
| fg. sw. | frontogenal wart |
| ft. g. | frontal grooves |
| if. ap. | inferior appendage |
| la. sw. | lateroantennal wart |
| lt. pc. | lateral process |
| ma. sw. | medioantennal wart |
| ms. pc. | mesal process |
| occ. sw. | occipital wart |
| ph. bs. | phallobase |
| ph. sc. | phallotremal sclerite |
| pm. | paramere |
| pt. sw. | postgenal wart |
| tc | thyridial cell |
| vt. pc. | ventral process |
| vt. sw. | ventral setal wart |
References
- Morse, J.C.; Frandsen, P.B.; Graf, W.; Thomas, J.A. Diversity and Ecosystem Services of Trichoptera. Insects 2019, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Malm, T.; Johanson, K.A. A New Classification of the Long-Horned Caddisflies (Trichoptera: Leptoceridae) Based on Molecular Data. BMC Evol. Biol. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.C. A Phylogeny and Classification of Family-Group Taxa of Leptoceridae (Trichoptera). In Proceedings of the Third International Symposium on Trichoptera; Springer Netherlands: Dordrecht, 1981; pp. 257–264. [Google Scholar]
- Henriques-Oliveira, A.L.; Silva, A.L.R.; Nessimian, J.L.; Takiya, D.M. New Long-Horned Caddisfly Genus and Species of Leptoceridae (Insecta: Trichoptera) from Brazil. Zootaxa 2021, 5057, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Schmid, F. Contribution à La Connaissance Des Trichopteres Néotropicaux. Mém. Soc. Vaud. Sci. Nat. 1955, 11, 117–160. [Google Scholar]
- Schmid, F. Contribution a l’etude Des Trichopteres Neotropicaux III. Mitt. Zool. Mus. Berl. 1958, 34(1), 183–217. [Google Scholar] [CrossRef]
- Schmid, F. Contribution a l’etude Des Trichopteres Neotropicaux V. Tijdschr. Entomol. 1964, 107, 307–339. [Google Scholar]
- Flint, O.S. Bredin-Archbold-Smithsonian Biological Survey of Dominica, 9. The Trichoptera (Caddisflies) of the Lesser Antilles. Proc. U.S. Natl. Mus. 1968, 125, 1–86. [Google Scholar] [CrossRef]
- Flint, O.S. Studies of Neotropical caddis flies, IX: New Genera and Species from the Chilean Subregion. Proc. Entomol. Soc. Wash. 1969, 71, 497–514. [Google Scholar]
- Flint, O.S. Studies of Neotropical caddisflies, XIV: on a collection from Northern Argentina. Proc. Biol. Soc. Wash. 1972, 85, 223–248. [Google Scholar]
- Flint, O.S. Studies of Neotropical Caddisflies, XXXIII: New Species from Austral South America (Trichoptera). Smithson. Contrib. Zool. 1983, 1–100. [Google Scholar] [CrossRef]
- Holzenthal, R.W. Studies in Neotropical Leptoceridae (Trichoptera) I: Achoropsyche, a new genus. In Proceedings of the 4th International Symposium on Trichoptera, Clemson, USA, 11-16 July 1983. [Google Scholar]
- Holzenthal, R.W. Studies in Neotropical Leptoceridae (Trichoptera) II: Amphoropsyche, a new genus and species of Leptocerinae from northern South America. Int. J. Entomol. 1985, 27, 255–269. [Google Scholar]
- Holzenthal, R.W. Studies in Neotropical Leptoceridae (Trichoptera), IV: A Revision of Brachysetodes Schmid. Trans. Am. Entomol. Soc. 1986, 111, 407–440. [Google Scholar]
- Morrone, J.J. Biogeographical Regionalisation of the Andean Region. Zootaxa 2015, 3936. [Google Scholar] [CrossRef] [PubMed]
- Holzenthal, R.W.; Calor, A.R. Catalog of the Neotropical Trichoptera (Caddisflies). ZooKeys 2017, 654, 1–566. [Google Scholar] [CrossRef] [PubMed]
- Gressitt, J.L.; Gressitt, M.K. An improved Malaise trap. Pac. Insects. 1962, 4, 87–90. [Google Scholar]
- González Lagos, N. Centro de Ecoturismo Parque Tricahue : Oportunidad de Un Nuevo Pacto Entre Arquitectura y Naturaleza. Tesis, Universidad de Chile - Facultad de Arquitectura y Urbanismo: Santiago, Chile, 2021.
- Blahnik, R.J.; Holzenthal, R.W. Collection and Curation of Trichoptera, with an Emphasis on Pinned Material. Nectopsyche, Neotropical Trichoptera Newsletter 2004, 1, 8–20. [Google Scholar]
- Desiderio, G.R.; Santana, V.; Pereira, E.S.; Pes, A.M.; Hamada, N. On the Identity of Smicridea (Smicridea) Aequalis Banks, 1920 (Trichoptera: Hydropsychidae): Morphology of Adults and Immature Stages, Bionomics, Distribution, and Male Color Dimorphism. Neotrop. Entomol. 2021, 50, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Kawada, R.; Buffington, M.L. A Scalable and Modular Dome Illumination System for Scientific Microphotography on a Budget. PLOS ONE 2016, 11, e0153426. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.M. A High Resolution Shapefile of the Andean Biogeographical Region. Data Brief 2017, 13, 230–232. [Google Scholar] [CrossRef] [PubMed]
- GBIF.Org. GBIF Occurrence Download. [CrossRef]
- Oláh, J.; Johanson, K.A. Trinominal Terminology for Cephalic Setose Warts in Trichoptera (Insecta). Braueria (Lunz am See, Austria) 2007, 34, 43–50. [Google Scholar]
- Nielsen, A. A Comparative Study of the Genital Segments and Their Appendages in Male Trichoptera. 1957, 8, 1–159.
- Mosely, M.E.; Kimmins, D.E. The Trichoptera (Caddis-Flies) of Australia and New Zealand; British Museum (Natural History): London, England, 1953. [Google Scholar]
- Moreira-Munoz, A. Plant Geography of Chile; Plant and Vegetation; Springer Netherlands: Dordrecht, 2011; ISBN 978-90-481-8747-8. [Google Scholar]
- Pádua, D.G.; Moreira-Muñoz, A.; Morales-Fierro, V.; Araujo, R.O. Chilean Darwin Wasps (Ichneumonidae): Biogeographic Relationships and Distribution Patterns. Insects 2024, 15, 415. [Google Scholar] [CrossRef] [PubMed]
- Morrone, J.J. Evolutionary Biogeography of the Andean Region; 1st ed.; CRC Press: Boca Raton: Taylor & Francis, 2018. | Series: CRC biogeography series, 2018; ISBN 978-0-429-48608-1.
- Rázuri-Gonzales, E.; Holzenthal, R.W.; Ríos-Touma, B. Two New Species of the Rare Neotropical Caddisfly Genus Amphoropsyche Holzenthal (Trichoptera, Leptoceridae). ZooKeys 2017, 707, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Blahnik, R.; Holzenthal, R. Review and Redescription of Species in the Oecetis Avara Group, with the Description of 15 New Species (Trichoptera, Leptoceridae). ZooKeys 2014, 376, 1–83. [Google Scholar] [CrossRef] [PubMed]
- Quinteiro, F.B.; Holzenthal, R.W. Fourteen New Species of Oecetis McLachlan, 1877 (Trichoptera: Leptoceridae) from the Neotropical Region. PeerJ 2017, 5, e3753. [Google Scholar] [CrossRef] [PubMed]
- Holzenthal, R.W.; Andersen, T. The Caddisfly Genus Triaenodes in the Neotropics (Trichoptera: Leptoceridae). Zootaxa 2004, 511. [Google Scholar] [CrossRef]
- Sganga, J.V.; Brand, C.; Santos, A.P.M.; Rueda Martín, P.A. Trichoptera. In Biodiversidad de Artrópodos Argentinos; Claps, L.E., Roig-Juñent, S., Morrone, J.J., Eds.; Editorial INSUE UNT: San Miguel de Tucumán, Argentina, 2023; Volume 5, pp. 213–228. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




