Submitted:
16 July 2025
Posted:
16 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Structure and Synthesis of Pristine CNHs and Their Derivatives Used in Resistive RH Monitoring
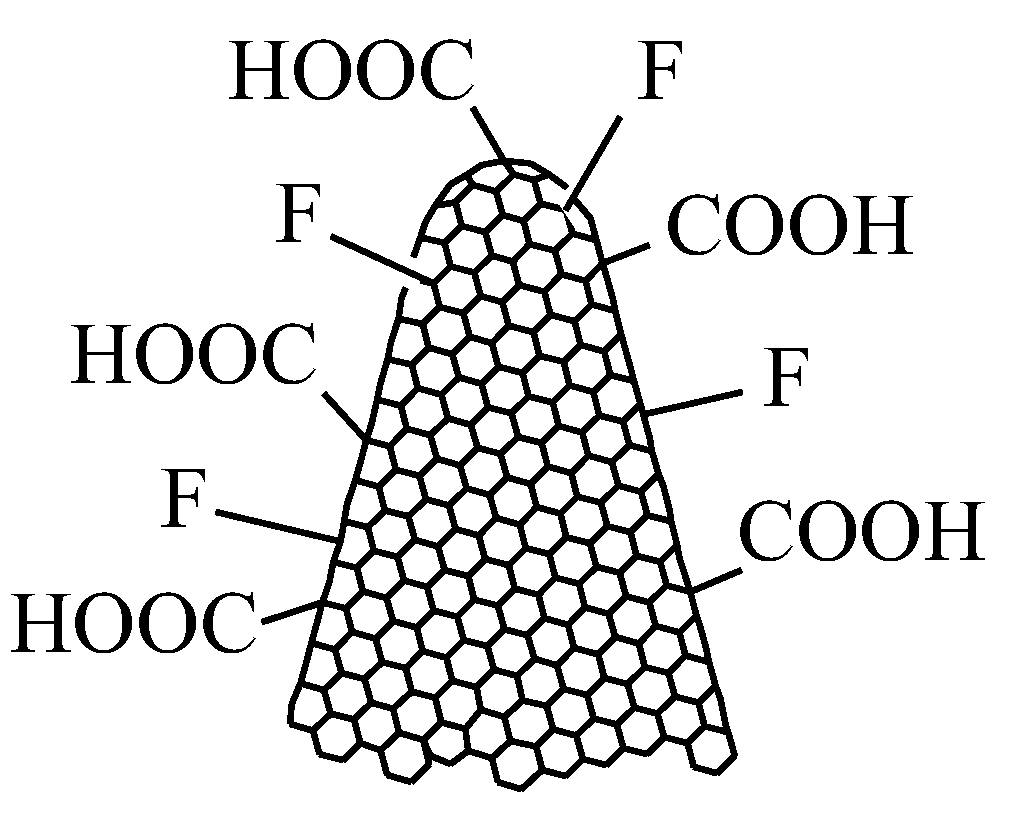
2.1. Structure of CNHs
2.2. Synthesis of Pristine CNHs
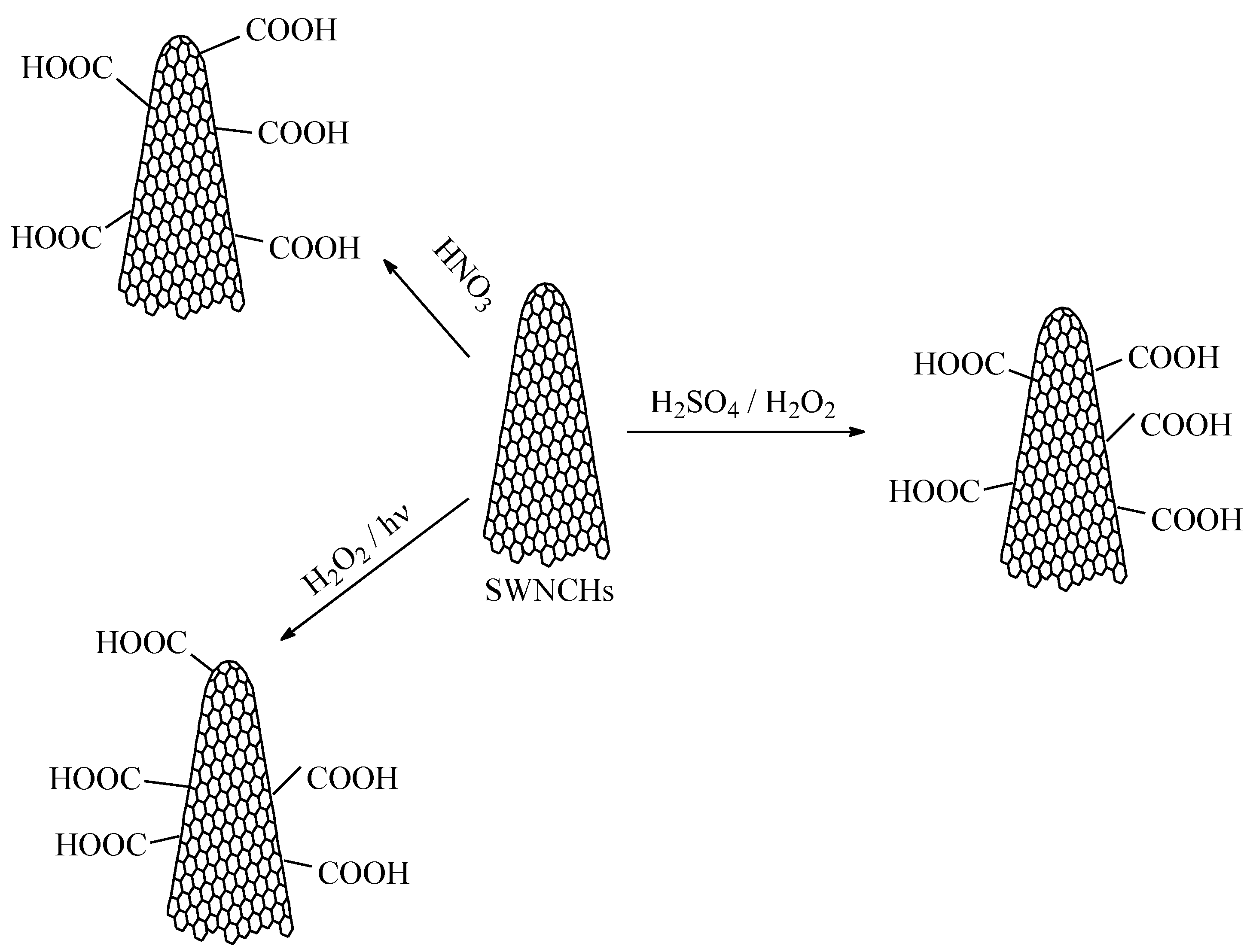
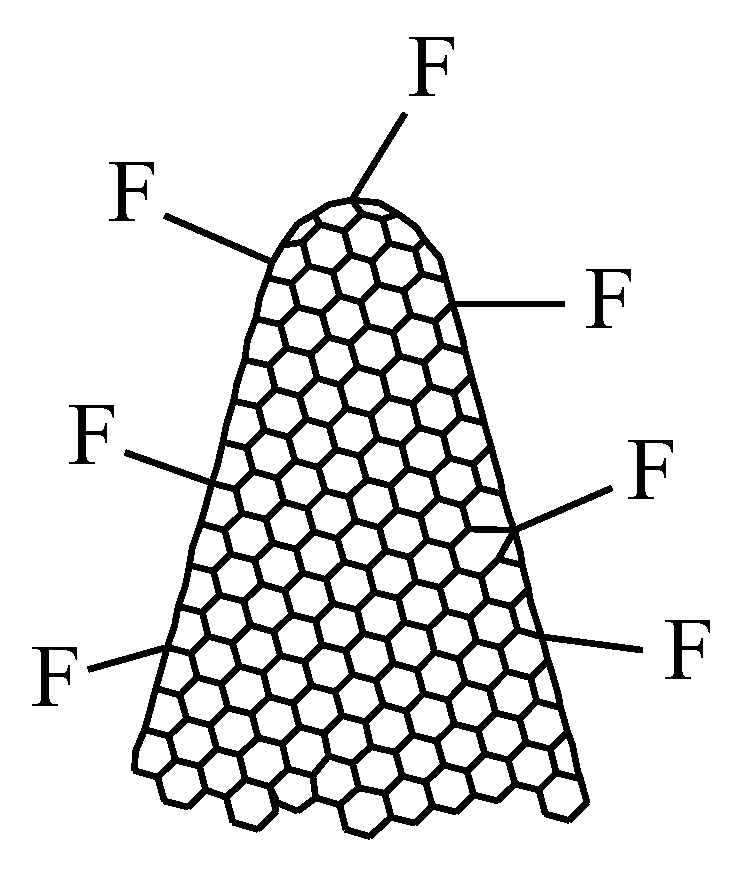
2.3. Synthesis of Functionalized CNHs for Resistive RH Monitoring
3. Properties of CNHs
- High surface area - CNHs have a large specific surface area, which is a key feature for applications such as catalysis and adsorption [68]
- Excellent porosity - The partial oxidation of CNHs gives direct access to internal pores via the generation of nanowindows onto the skeleton of CNHs. Holes can be easily created in pristine CNHs by heat treatment under oxidative and/or acidic conditions [69]
- High adsorption capacity - [70]
- Thermal stability - CNHs generally exhibit good thermal stability, particularly in inert atmospheres. In air, the oxidation of SWNHs starts above 300°C and is completed at 720°C. CNHs can remain stable in a vacuum up to 1800° C [71]
- High purity - CNHs can be synthesized with high purity, often exceeding 95%, and without the need for metal catalysts [72]
- Chemical stability - CNHs generally exhibit good corrosion resistance, particularly when compared to some other nanocarbonic materials [73];
- Low toxicity - Several experiments conducted in recent years show that CNHs have low toxicity [74]
- Catalytic properties - CNHs can act both as catalysts and catalyst supports for metal nanoparticles [75]
- Good electrical conductivity - CNHs generally have lower electrical conductivity compared to CNTs; however, the conductivity of both materials can be influenced by several parameters, such as purity, structure, and type of synthesis. The electrical percolation threshold of carbon nanohorns and their derivatives in several hydrophilic polymers is a key parameter in the evaluation of resistive sensing capabilities of nanocomposites based on CNHs for different gases and RH [76,77]
- Facile covalent and noncovalent functionalization - [78]
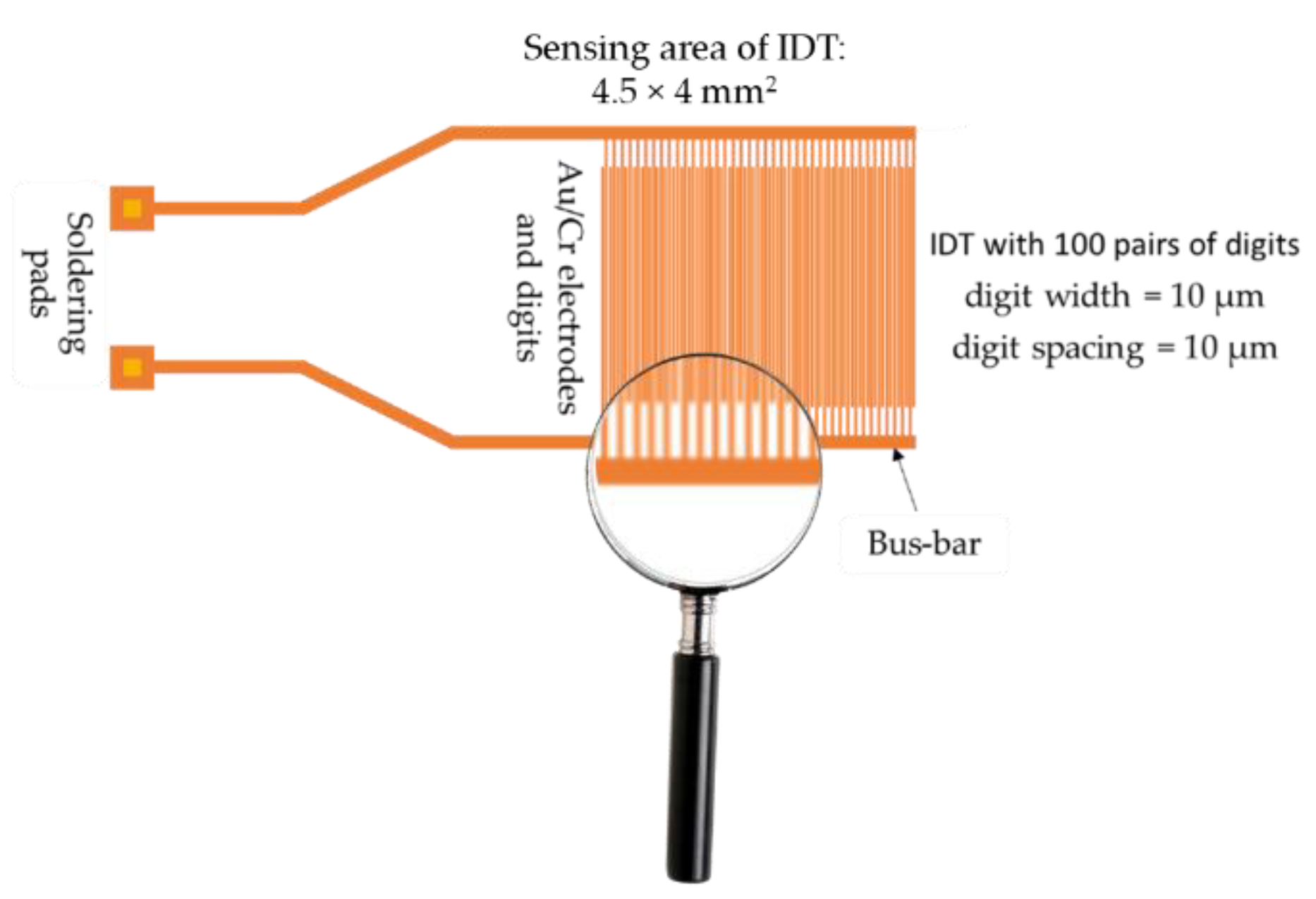
4. Structure of CNHs-Based Resistive RH Sensors
5. RH Resistive Sensors Based on CNHS and Their Nanocomposites/Nanohybrids
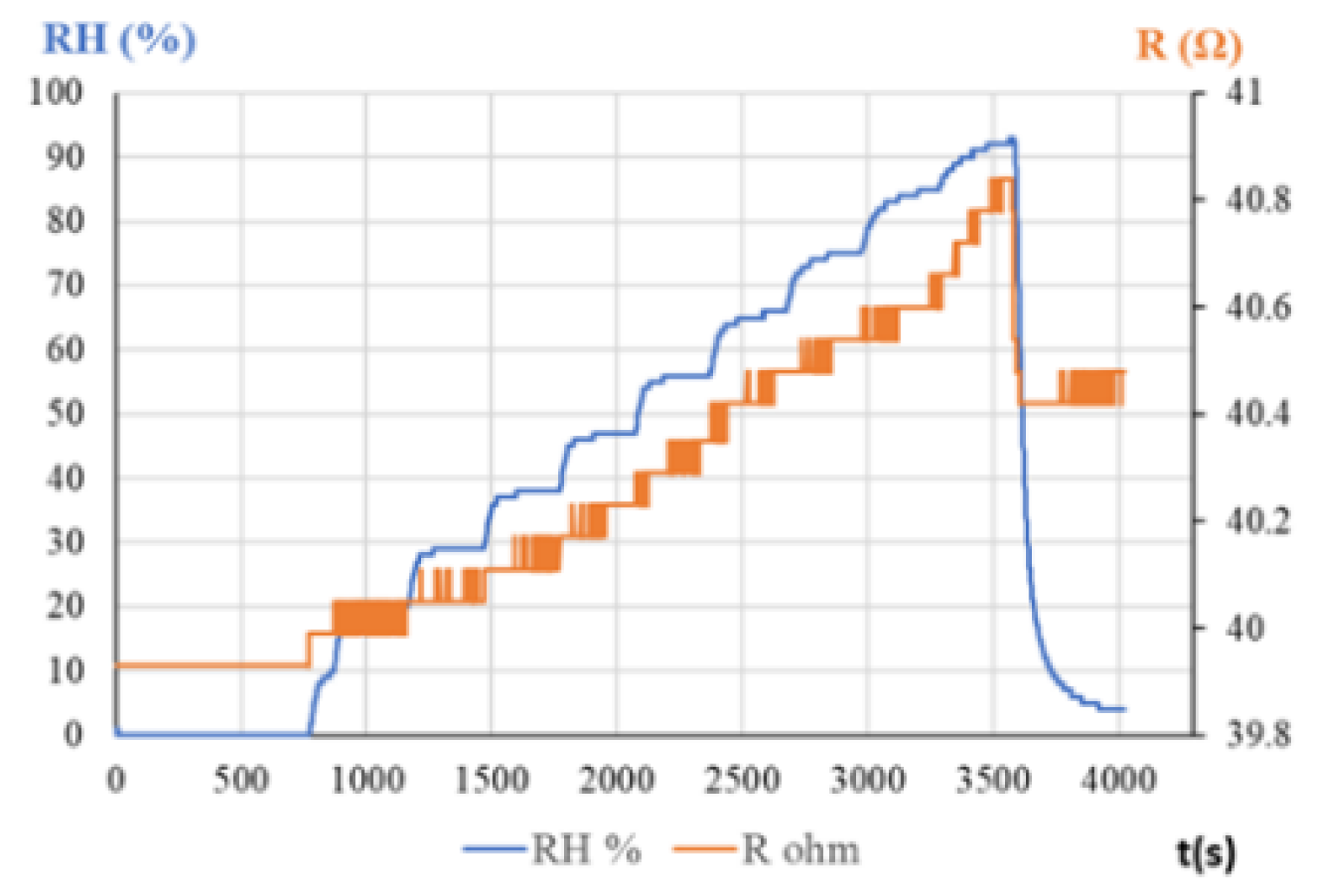
5.1 Oxidized CNHs as Sensing Layers in RH Resistive Sensors
5.2. Nanocomposite-Based CNHs as Sensing Layers in RH Resistive Sensors
5.2.1. Pristine CNHs–Hydroxyethylcellulose as Sensing Layer in RH Resistive Layers
5.2.2 CNHox–PVP as Sensing Layer in RH Resistive Layers
5.2.3. CNHox - poly (ethylene glycol)-blockpoly(propylene glycol)-block-poly (ethylene glycol) (PEG-PPG-PEG) as Sensing Layer in RH Resistive Layers
5.2.4. GO-CNHox–PVP as Sensing Layer in RH Resistive Layers
5.2.5. Pristine CNHs-PVP as Sensing Layer in RH Resistive Layers
5.3. Organic-Inorganic Nanohybrids Comprising CNHs Used as Sensing Layers in RH Resistive Sensors
5.3.1. Organic-Inorganic CNHox/KCl/PVP Nanohybrids Used as Sensing Layers in RH Resistive Sensors
5.3.2. Organic-Inorganic CNHox/TiO2/PVP Nanohybrids Used as Sensing Layers in RH Resistive Sensors
5.3.3. Organic-Inorganic CNHox/ZnO/PVP Nanohybrids Used as Sensing Layers in RH Resistive Sensors
5.3.4. Organic-Inorganic CNHox/SnO2/ZnO/PVP Nanohybrids Used as Sensing Layers in RH Resistive Sensors
5.3.5. Organic-Inorganic CNHox/GO/SnO2/PVP Nanohybrid Used as Sensing Layers in RH Resistive Sensors
6. Sensing Mechanisms for RH Resistive Monitoring Using CNHs and Their Nanocomposites/Nanohybrids
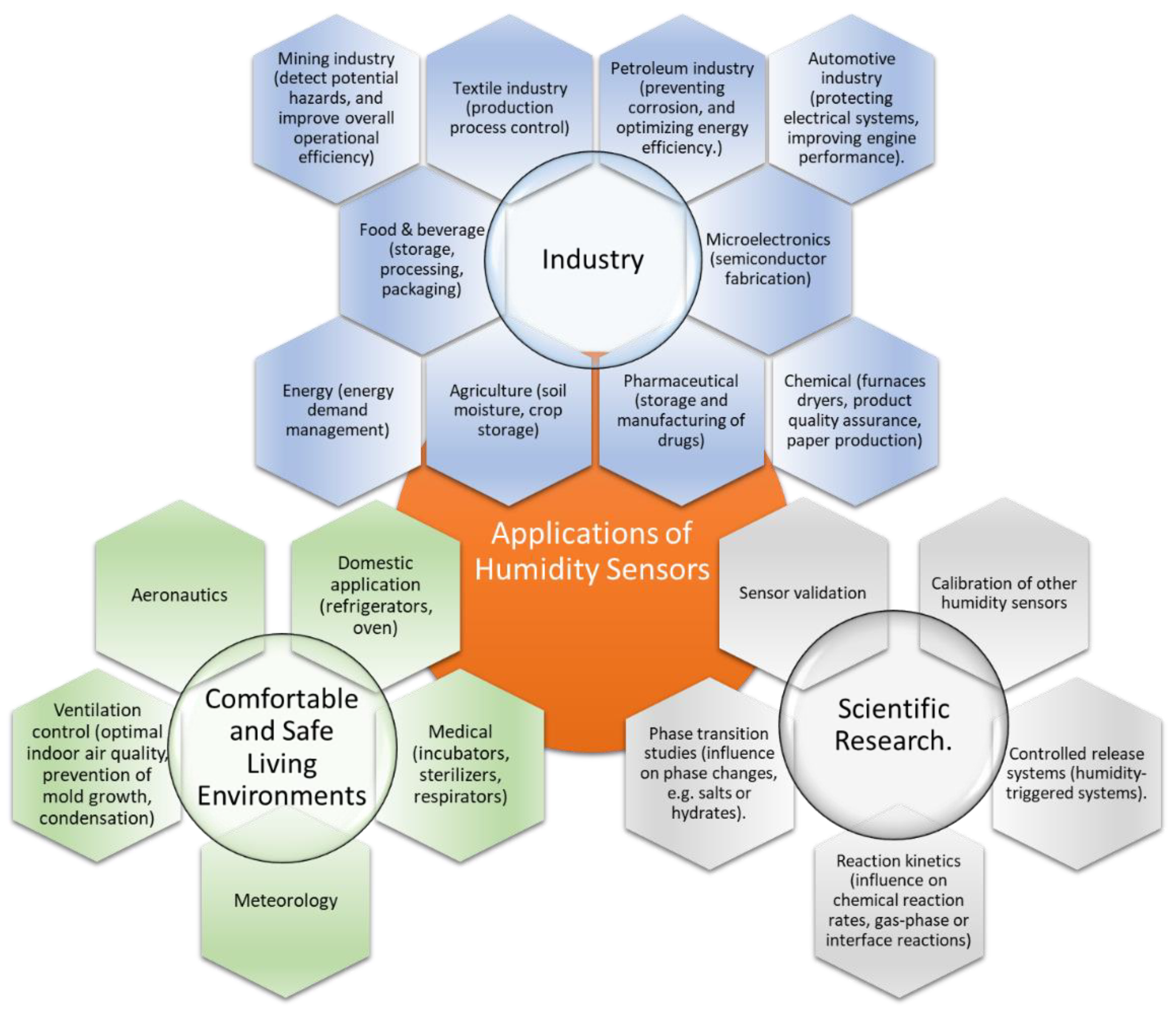
7. Why Are CNHs Used Less Frequently than CNTs and Graphene Derivatives for Resistive RH Sensing? Possible Opportunities and Future Research Directions
- The absence of metallic particles as impurities- Synthesis of the CNHs is conducted in the absence of metal catalyst [54].
- High Surface Area- CNHox possesses a large specific surface area (1,300–1,400 m²/g BET), providing more sites for the adsorption of water, which increases its RH sensitivity [79].
- Tunable Surface Chemistry– The versatile hydrophilization of CNHs (through oxidation in solution or plasma treatment) allows for the optimization of the response of the sensor to different RH levels [54].
- Good Electrical Conductivity - CNHox retains good electrical conductivity even after hydrophilization, which is a key feature for resistive RH sensors. The interaction of water molecules with the CNHox surface can modify the electrical resistance of the sensing film, allowing for accurate RH monitoring [63].
- Excellent Linearity and Stability - CNHox-based RH sensors have demonstrated excellent linearity in their response across a wide range of RH levels, and they have also shown good stability over time [83].
- Rapid Response and Recovery Times - The large surface area and tunable surface chemistry of CNHox contribute to obtaining fast response and recovery time [84].
- Compatibility with other materials- CNHox can be easily incorporated into nanocomposites or nanohybrids with different materials, like polymers (e.g. PVP, PEG-PPG-PEG), carbonic materials (GO), metal oxides (e.g., TiO2, ZnO, SnO2), further enhancing their sensing properties and enabling the development of RH sensors [51]
- Potential for Flexible and Wearable Sensors- The ability to create thin films and dispersions makes CNHox suitable for integration into flexible and wearable sensors, expanding the possible applications of RH sensors [54].
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CNHs | Carbon nanohorns |
| CNHs-F | Fluorinated carbon nanohorns |
| CNHox | Oxidized carbon nanohorns |
| CNHox-F | Oxi fluorinated carbon nanohorns |
| CNCs | Carbon nano coils |
| CNCs | Carbon nano tubes |
| GO | Graphene oxide |
| IDT | Interdigitated |
| LCP | Liquid crystal polymer |
| PDAC | Poly(diallyldimethylammonium chloride) |
| PEG | Poly(ethylene glycol) |
| PEG-PPG-PEG | Poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) |
| PET | Polyethylene terephthalate |
| PPG | propylene glycol |
| PVA | Polyvinyl Alcohol |
| PVP | Polyvinylpyrrolidone |
| PTFE | Polytetrafluoroethylene |
| MWCNTs | Multi-walled carbon nanotubes |
| RH | Relative humidity |
| SWCNTs | Single-walled carbon nanotubes |
| SWNHs | Single-walled nanohorns |
| SDC | Shellac-derived carbon |
References
- Kuzubasoglu, B.A. Recent studies on the humidity sensor: A mini review. ACS Appl. Electron. Mater. 2022, 4, 4797–4807. [Google Scholar] [CrossRef]
- Arundel, A.V.; Sterling, E.M.; Biggin, J.H.; Sterling, T.D. Indirect health effects of relative humidity in indoor environments. Environ. Health Perspect. 1986, 65, 351–361. [Google Scholar]
- Davis, R.E.; McGregor, G.R.; Enfield, K.B. Humidity: A review and primer on atmospheric moisture and human health. Environ. Res. 2016, 144, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Wolkoff, P. Indoor air humidity, air quality, and health–An overview. Int. J. Hyg. Environ. Health 2018, 221, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Barmpakos, D.; Kaltsas, G. A review on humidity, temperature, and strain printed sensors—Current trends and future perspectives. Sensors 2021, 21, 739. [Google Scholar] [CrossRef]
- Alam, N.; Abid; Islam, S. S. Advancements in trace and low humidity sensors technologies using nanomaterials: A review. ACS Appl. Nano Mater. 2024, 7, 13836–13864. [Google Scholar] [CrossRef]
- Huang, C.; Jiang, M.; Liu, F. Recent progress on environmentally friendly humidity sensor: A mini review. ACS Appl. Electron. Mater. 2023, 5, 4067–4079. [Google Scholar] [CrossRef]
- Sajid, M.; Khattak, Z.J.; Rahman, K.; Hassan, G.; Choi, K.H. Progress and future of relative humidity sensors: A review from materials perspective. Bull. Mater. Sci. 2022, 45, 238. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Simonenko, N.P.; Simonenko, E.P.; Sysoev, V.V.; Brinzari, V. Based humidity sensors as promising flexible devices, state of the art, part 2: Humidity-sensor performances. Nanomaterials 2023, 13, 1381. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lee, G.B. Humidity sensors: A review. Sens. Lett. 2005, 3, 1–15. [Google Scholar] [CrossRef]
- Okcan, B.; Akin, T. A thermal conductivity-based humidity sensor in a standard CMOS process. In Proceedings of the 17th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Maastricht, The Netherlands, 25–29 January 2004; IEEE: New York, NY, USA, 2004; pp. 552–555. [Google Scholar]
- Atalay, S.; Izgi, T.; Kolat, V.S.; Erdemoglu, S.; Inan, O.O. Magnetoelastic humidity sensors with TiO2 nanotube sensing layers. Sensors 2020, 20, 425. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, Z.; Zhang, B.; Yuan, Z.; Zhao, Q.; Jiang, Y.; Tai, H. Electrochemical humidity sensor enabled self-powered wireless humidity detection system. Nano Energy 2023, 115, 108745. [Google Scholar] [CrossRef]
- Wu, T.T.; Chen, Y.Y.; Chou, T.H. A high sensitivity nanomaterial-based SAW humidity sensor. J. Phys. D Appl. Phys. 2008, 41, 085101. [Google Scholar] [CrossRef]
- Ashley, G.M.; Kirby, P.B.; Butler, T.P.; Whatmore, R.; Luo, J.K. Chemically sensitized thin-film bulk acoustic wave resonators as humidity sensors. J. Electrochem. Soc. 2010, 157, J419. [Google Scholar] [CrossRef]
- Ascorbe, J.; Corres, J.M.; Arregui, F.J.; Matias, I.R. Recent developments in fiber optics humidity sensors. Sensors 2017, 17, 893. [Google Scholar] [CrossRef] [PubMed]
- Ragazzini, I.; Castagnoli, R.; Gualandi, I.; Cassani, M.C.; Nanni, D.; Gambassi, F.; Ballarin, B. A resistive sensor for humidity detection based on cellulose/polyaniline. RSC Adv. 2022, 12, 28217–28226. [Google Scholar] [CrossRef]
- Hussain, M.; Hasnain, S.; Khan, N.A.; Bano, S.; Zuhra, F.; Ali, M.; Ali, A. Design and fabrication of a fast response resistive-type humidity sensor using polypyrrole (ppy) polymer thin film structures. Polymers 2021, 13, 3019. [Google Scholar] [CrossRef]
- Packirisamy, M.; Stiharu, I.; Li, X.; Rinaldi, G. A polyimide based resistive humidity sensor. Sens. Rev. 2005, 25, 271–276. [Google Scholar] [CrossRef]
- Demir, R.; Okur, S.; Seker, M. Electrical characterization of CdS nanoparticles for humidity sensing applications. Ind. Eng. Chem. Res. 2012, 51, 3309–3313. [Google Scholar] [CrossRef]
- Chang, S.P.; Chang, S.J.; Lu, C.Y.; Li, M.J.; Hsu, C.L.; Chiou, Y.Z.; Chen, I.C. A ZnO nanowire-based humidity sensor. Superlattices Microstruct. 2010, 47, 772–778. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, P.; Kumar, M.S.; Gupta, G.; Shivagan, D.D.; Bapna, K. SnO2 nanostructured thin film as humidity sensor and its application in breath monitoring. Ceram. Int. 2023, 49, 24911–24921. [Google Scholar] [CrossRef]
- Dai, J.; Zhao, H.; Lin, X.; Liu, S.; Liu, Y.; Liu, X.; Zhang, T. Ultrafast response polyelectrolyte humidity sensor for respiration monitoring. ACS Appl. Mater. Interfaces 2019, 11, 6483–6490. [Google Scholar] [CrossRef] [PubMed]
- Tambwe, K.; Ross, N.; Baker, P.; Bui, T.T.; Goubard, F. Humidity sensing applications of lead-free halide perovskite nanomaterials. Materials 2022, 15, 4146. [Google Scholar] [CrossRef] [PubMed]
- Tulliani, J.M.; Inserra, B.; Ziegler, D. Carbon-based materials for humidity sensing: A short review. Micromachines 2019, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Elgammal, K.; Niklaus, F.; Delin, A.; Fischer, A.C.; Vaziri, S.; Lemme, M.C. Resistive graphene humidity sensors with rapid and direct electrical readout. Nanoscale 2015, 7, 19099–19109. [Google Scholar] [CrossRef]
- Saqib, M.; Ali Khan, S.; Mutee Ur Rehman, H.M.; Yang, Y.; Kim, S.; Rehman, M.M.; Young Kim, W. High-performance humidity sensor based on the graphene flower/zinc oxide composite. Nanomaterials 2021, 11, 242. [Google Scholar] [CrossRef]
- Popov, V.I.; Kotin, I.A.; Nebogatikova, N.A.; Smagulova, S.A.; Antonova, I.V. Graphene-PEDOT:PSS humidity sensors for high sensitive, low-cost, highly-reliable, flexible, and printed electronics. Materials 2019, 12, 3477. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, R.; Guha, P.K.; Bhattacharyya, T.K. Humidity sensor based on high proton conductivity of graphene oxide. IEEE Trans. Nanotechnol. 2015, 14, 931–937. [Google Scholar] [CrossRef]
- Noh, W.; Go, Y.; An, H. Reduced graphene oxide/polyelectrolyte multilayers for fast resistive humidity sensing. Sensors 2023, 23, 1977. [Google Scholar] [CrossRef]
- Zhang, X.; Maddipatla, D.; Bose, A.K.; Hajian, S.; Narakathu, B.B.; Williams, J.D.; Atashbar, M.Z. Printed carbon nanotubes-based flexible resistive humidity sensor. IEEE Sens. J. 2020, 20, 12592–12601. [Google Scholar] [CrossRef]
- Pan, X.; Xue, Q.; Zhang, J.; Guo, Q.; Jin, Y.; Lu, W.; Ling, C. Effective enhancement of humidity sensing characteristics of novel thermally treated MWCNTs/Polyvinylpyrrolidone film caused by interfacial effect. Adv. Mater. Interfaces 2016, 3, 1600153. [Google Scholar] [CrossRef]
- Serban, B.C.; Dumbravescu, N.; Buiu, O.; Bumbac, M.; Brezeanu, M.; Pachiu, C.; Tucureanu, V. Carbon Nano-Onions-Based Matrix Nanocomposite as Sensing Film for Resistive Humidity Sensor. Rom. J. Inf. Sci. Technol. 2025, 28, 77–88. [Google Scholar] [CrossRef]
- Serban, B.C.; Dumbravescu, N.; Buiu, O.; Bumbac, M.; Dumbravescu, C.; Brezeanu, M.; Brincoveanu, O. Carbon Nano-Onions–Polyvinyl Alcohol Nanocomposite for Resistive Monitoring of Relative Humidity. Sensors 2025, 25, 3047. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.P.; Lim, L.T.; Min, N.K.; Lee, M.J.; Lee, C.J.; Park, C.W. Novel resistive-type humidity sensor based on multiwall carbon nanotube/polyimide composite films. Sens. Actuators B Chem. 2010, 145, 120–125. [Google Scholar] [CrossRef]
- Epeloa, J.; Repetto, C.E.; Gómez, B.J.; Nachez, L.; Dobry, A. Resistivity humidity sensors based on hydrogenated amorphous carbon films. Mater. Res. Express 2018, 6, 025604. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Y.; Lin, S.; Xiao, H.; Huang, C.; Yu, W.; Xia, C. A thin film resistive humidity sensor based on polymer and carbon black nanoparticle composites. Meas. Sci. Technol. 2023, 35, 025140. [Google Scholar] [CrossRef]
- Joshi, S.R.; Kim, B.; Kim, S.K.; Song, W.; Park, K.; Kim, G.H.; Shin, H. Low-cost and fast-response resistive humidity sensor comprising biopolymer-derived carbon thin film and carbon microelectrodes. J. Electrochem. Soc. 2020, 167, 147511. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.M.; Wu, Z.; Li, X.; Wang, N.; Tao, K.; Wang, G.P. Carbon nanocoil-based fast-response and flexible humidity sensor for multifunctional applications. ACS Appl. Mater. Interfaces 2019, 11, 4242–4251. [Google Scholar] [CrossRef]
- Meng, J.; Liu, T.; Meng, C.; Lu, Z.; Li, J. Porous carbon nanofibres with humidity sensing potential. Microporous Mesoporous Mater. 2023, 359, 112663. [Google Scholar] [CrossRef]
- Chu, J.; Peng, X.; Feng, P.; Sheng, Y.; Zhang, J. Study of humidity sensors based on nanostructured carbon films produced by physical vapor deposition. Sens. Actuators B Chem. 2013, 178, 508–513. [Google Scholar] [CrossRef]
- Zhang, X.; Ming, H.; Liu, R.; Han, X.; Kang, Z.; Liu, Y.; Zhang, Y. Highly sensitive humidity sensing properties of carbon quantum dots films. Mater. Res. Bull. 2013, 48, 790–794. [Google Scholar] [CrossRef]
- Afify, A.S.; Ahmad, S.; Khushnood, R.A.; Jagdale, P.; Tulliani, J.-M. Elaboration and characterization of novel humidity sensor based on micro-carbonized bamboo particles. Sens. Actuators B Chem. 2017, 239, 1251–1256. [Google Scholar] [CrossRef]
- Ziegler, D.; Palmero, P.; Giorcelli, M.; Tagliaferro, A.; Tulliani, J.M. Biochars as innovative humidity sensing materials. Chemosensors 2017, 5, 35. [Google Scholar] [CrossRef]
- Ruiz, V.; Fernández, I.; Carrasco, P.; Cabañero, G.; Grande, H.J.; Herrán, J. Graphene quantum dots as a novel sensing material for low-cost resistive and fast-response humidity sensors. Sens. Actuators B Chem. 2015, 218, 73–77. [Google Scholar] [CrossRef]
- Ionete, E.I.; Spiridon, S.I.; Monea, B.F.; Ebrasu-Ion, D.; Vaseashta, A. SWCNT-Pt-P2O5-Based Sensor for Humidity Measurements. IEEE Sens. J. 2016, 16, 7593–7599. [Google Scholar] [CrossRef]
- Zhou, G.; Byun, J.H.; Oh, Y.; Jung, B.M.; Cha, H.J.; Seong, D.G.; Chou, T.W. Highly sensitive wearable textile-based humidity sensor made of high-strength, single-walled carbon nanotube/poly(vinyl alcohol) filaments. ACS Appl. Mater. Interfaces 2017, 9, 4788–4797. [Google Scholar] [CrossRef]
- Dai, H.; Feng, N.; Li, J.; Zhang, J.; Li, W. Chemiresistive humidity sensor based on chitosan/zinc oxide/single-walled carbon nanotube composite film. Sens. Actuators B Chem. 2019, 283, 786–792. [Google Scholar] [CrossRef]
- Qin, J.; Yang, X.; Shen, C.; Chang, Y.; Deng, Y.; Zhang, Z.; Shan, C. Carbon nanodot-based humidity sensor for self-powered respiratory monitoring. Nano Energy 2022, 101, 107549. [Google Scholar] [CrossRef]
- Liu, X.; Ying, Y.; Ping, J. Structure, synthesis, and sensing applications of single-walled carbon nanohorns. Biosens. Bioelectron. 2020, 167, 112495. [Google Scholar] [CrossRef]
- Serban, B.C.; Bumbac, M.; Buiu, O.; Cobianu, C.; Brezeanu, M.; Nicolescu, C. Carbon nanohorns and their nanocomposites: Synthesis, properties, and applications. A concise review. Ann. Acad. Rom. Sci. Ser. Math. Appl. 2018, 11, 5–18. [Google Scholar]
- Zhu, S.; Xu, G. Single-walled carbon nanohorns and their applications. Nanoscale 2010, 2, 2538–2549. [Google Scholar] [CrossRef]
- Liu, X.; Ying, Y.; Ping, J. Structure, synthesis, and sensing applications of single-walled carbon nanohorns. Biosens. Bioelectron. 2020, 167, 112495. [Google Scholar] [CrossRef]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, properties, functionalization, and applications of carbon nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Z.; Zhao, K.; Shi, Z.; Gu, Z.; Xu, S. Synthesis of single-wall carbon nanohorns by arc-discharge in air and their formation mechanism. Carbon 2010, 48, 1580–1585. [Google Scholar] [CrossRef]
- Gattia, D.M.; Antisari, M.V.; Marazzi, R. AC arc discharge synthesis of single-walled nanohorns and highly convoluted graphene sheets. Nanotechnology 2007, 18, 255604. [Google Scholar] [CrossRef]
- Berkmans, J.; Jagannatham, M.; Reddy, R.; Haridoss, P. Synthesis of thin bundled single-walled carbon nanotubes and nanohorn hybrids by arc discharge technique in open air atmosphere. Diam. Relat. Mater. 2015, 55, 12–15. [Google Scholar]
- Wang, H.; Chhowalla, M.; Sano, N.; Jia, S.; Amaratunga, G.A.J. Large-scale synthesis of single-walled carbon nanohorns by submerged arc. Nanotechnology 2004, 15, 546. [Google Scholar] [CrossRef]
- Yuge, R.; Yudasaka, M.; Toyama, K.; Yamaguchi, T.; Iijima, S.; Manako, T. Buffer gas optimization in CO2 laser ablation for structure control of single-wall carbon nanohorn aggregates. Carbon 2012, 50, 1925–1933. [Google Scholar] [CrossRef]
- Schiavon, M. Device and Method for Production of Carbon Nanotubes, Fullerene and their Derivatives. U.S. Patent 7,125,525; EP 1428794, Filed 2003, Granted 2006. [Google Scholar]
- Casteignau, F.; Aissou, T.; Allard, C.; Ricolleau, C.; Veilleux, J.; Martel, R.; Braidy, N. Synthesis of carbon nanohorns by inductively coupled plasma. Plasma Chem. Plasma Process. 2022, 42, 465–481. [Google Scholar] [CrossRef]
- Cioffi, C.; Campidelli, S.; Sooambar, C.; Marcaccio, M.; Marcolongo, G.; Meneghetti, M.; Prato, M. Synthesis, characterization, and photoinduced electron transfer in functionalized single-wall carbon nanohorns. J. Am. Chem. Soc. 2007, 129, 3938–3945. [Google Scholar] [CrossRef]
- Arti, N.; Alam, N.; Ansari, J.R. Nanostructures and Fascinating Properties of Carbon Nanohorns. In Handbook of Functionalized Carbon Nanostructures: From Synthesis Methods to Applications; Springer International Publishing: Cham, Switzerland, 2024; pp. 351–389. [Google Scholar]
- Serban, B.C.; Buiu, O.; Marinescu, M.R. Resistive humidity sensor based on fluorinated nanocarbon materials. RO13 7256A2, 2023.
- Serban, B.C.; Buiu, O.; Marinescu, M.R. Oxy-fluoro-nanocarbon sensitive layers for resistive detection of humidity. RO13 7257A2, 2023.
- Kumar, P.S.; Kumar, K.S.; Geethan, K.A.; Santhosh, D. Properties and Potential Applications of Carbon Nano Horns Over Carbon Nano Tubes as a Nano Fluid—A Review. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: 2021; Volume 1130, p. 012008.
- Yuge, R.; Nihey, F.; Toyama, K.; Yudasaka, M. Preparation, electrical properties, and supercapacitor applications of fibrous aggregates of single-walled carbon nanohorns. Carbon 2018, 138, 379–383. [Google Scholar] [CrossRef]
- Jung, H.J.; Kim, Y.J.; Han, J.H.; Yudasaka, M.; Iijima, S.; Kanoh, H.; Yang, C.M. Thermal-treatment-induced enhancement in effective surface area of single-walled carbon nanohorns for supercapacitor application. J. Phys. Chem. C 2013, 117, 25877–25883. [Google Scholar] [CrossRef]
- Utsumi, S.; Miyawaki, J.; Tanaka, H.; Hattori, Y.; Itoi, T.; Ichikuni, N.; Kaneko, K. Opening mechanism of internal nanoporosity of single-wall carbon nanohorn. J. Phys. Chem. B 2005, 109, 14319–14324. [Google Scholar] [CrossRef] [PubMed]
- Zambano, A.J.; Talapatra, S.; Lafdi, K.; Aziz, M.T.; McMillin, W.; Shaughnessy, G.; Takahashi, K. Adsorbate binding energy and adsorption capacity of xenon on carbon nanohorns. Nanotechnology 2002, 13, 201. [Google Scholar] [CrossRef]
- Nisha, J.A.; Yudasaka, M.; Bandow, S.; Kokai, F.; Takahashi, K.; Iijima, S. Adsorption and catalytic properties of single-wall carbon nanohorns. Chem. Phys. Lett. 2000, 328, 381–386. [Google Scholar] [CrossRef]
- Hou, S.; Xie, Z.; Zhang, D.; Yang, B.; Lei, Y.; Liang, F. High-purity graphene and carbon nanohorns prepared by base-acid treated waste tires carbon via direct current arc plasma. Environ. Res. 2023, 238, 117071. [Google Scholar] [CrossRef]
- Peng, H.; Xie, Z.; Lu, S.; Zhang, D.; Yang, B.; Liang, F. Dual-functionality composites of polyaniline-coated oxidized carbon nanohorns: Efficient wave absorption and enhanced corrosion resistance. Chin. Chem. Lett. 2025, 110818. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, G. Carbon nanohorns and their biomedical applications. Nanotechnologies for the Life Sciences 2012, 9. [Google Scholar]
- Pandit, J.; Alam, M.S.; Javed, M.N.; Waziri, A.; Imam, S.S. Emerging roles of carbon nanohorns as sustainable nanomaterials in sensor, catalyst, and biomedical applications. In Handbook of Green and Sustainable Nanotechnology: Fundamentals, Developments and Applications; Springer International Publishing: Cham, Switzerland, 2023; pp. 1721–1747. [Google Scholar]
- Serban, B.C.; Cobianu, C.; Dumbravescu, N.; Buiu, O.; Bumbac, M.; Nicolescu, C.M.; Serbanescu, M. Electrical percolation threshold and size effects in polyvinylpyrrolidone-oxidized single-wall carbon nanohorn nanocomposite: The impact for relative humidity resistive sensors design. Sensors 2021, 21, 1435. [Google Scholar] [CrossRef]
- Serban, B.C.; Cobianu, C.; Dumbrăvescu, N.; Buiu, O.; Avramescu, V.; Bumbac, M.; Brezeanu, M. Electrical percolation threshold in oxidized single-wall carbon nanohorn-polyvinylpyrrolidone nanocomposite: A possible application for high sensitivity resistive humidity sensor. In 2020 International Semiconductor Conference (CAS), Sinaia, Romania, 7–9 October 2020; IEEE: New York, NY, USA, 2020; pp. 239–242. [Google Scholar]
- Pagona, G.; Sandanayaka, A.S.; Araki, Y.; Fan, J.; Tagmatarchis, N.; Charalambidis, G.; Ito, O. Covalent functionalization of carbon nanohorns with porphyrins: Nanohybrid formation and photoinduced electron and energy transfer. Adv. Funct. Mater. 2007, 17, 1705–1711. [Google Scholar] [CrossRef]
- Serban, B.C.; Buiu, O.; Dumbravescu, N.; Cobianu, C.; Avramescu, V.; Brezeanu, M.; Nicolescu, C.M. Oxidized carbon nanohorns as novel sensing layer for resistive humidity sensor. Acta Chim. Slov. 2020, 67, 2. [Google Scholar] [CrossRef]
- Selvam, K.P.; Nakagawa, T.; Marui, T.; Inoue, H.; Nishikawa, T.; Hayashi, Y. Synthesis of solvent-free conductive and flexible cellulose–carbon nanohorn sheets and their application as a water vapor sensor. Mater. Res. Express 2020, 7, 056402. [Google Scholar] [CrossRef]
- Șerban, B.C.; Buiu, O.; Cobianu, C.; Avramescu, V.; Pachiu, C.; Ionescu, O.; Marinescu, M.R. Chemiresistive humidity sensor based on carbon nanocomposites. RO 134261 A2, 29 Priority 2018. [Google Scholar]
- Șerban, B.C.; Cobianu, C.; Dumbrăvescu, N.; Buiu, O. ; Oxidized Carbon Nanohorns and Their Nanocompozites as Sensing Layers for Chemiresistive Relative Humidity Sensor, Spring Scientific Conference of the Romanian Academy of Scientists, -6, 2019, Bucharest, pg.34. 4 April.
- Serban, B.C.; Cobianu, C.; Dumbrăvescu, N.; Buiu, O.; Avramescu, V.; Brezeanu, M.; Nicolescu, C.M.; Serbanescu, M. Oxidized carbon nanohorn-hydrophilic polymer nanocomposite as the resistive sensing layer for relative humidity. Anal. Lett. 2021, 54, 527–540. [Google Scholar] [CrossRef]
- Serban, B.C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Pachiu, C.; Brezeanu, M.; Cobianu, C. Ternary nanocomposites based on oxidized carbon nanohorns as sensing layers for room temperature resistive humidity sensing. Materials 2021, 14, 2705. [Google Scholar] [CrossRef] [PubMed]
- Șerban, B.C.; Buiu, O.; Cobianu, C.; Avramescu, V.; Dumbrăvescu, N.; Brezeanu, M.; Marinescu, M.R. Ternary carbon-based nanocomposite as sensing layer for resistive humidity sensor. In Proceedings of the 29th International Semiconductor Conference, Sinaia, Romania, 7–9 October 2019; MDPI: Basel, Switzerland, 2019; Volume 1, p. 114. [Google Scholar]
- Serban, B.C.; Dumbrăvescu, N.; Buiu, O.; Bumbac, M.; Pachiu, C.; Brezeanu, M.; Cobianu, C. Carbon Nanohorns-PVP Nanocomposite as Sensing Layer for Resistive Humidity Monitoring: Preliminary Results. In 2024 International Semiconductor Conference (CAS), Sinaia, Romania, 9–11 October 2024; IEEE: New York, NY, USA, 2024; pp. 39–42. [Google Scholar]
- Bogdan-Catalin Serban; Octavian Buiu; Nicolae Dumbrăvescu; Mihai Brezeanu; Marius Bumbac; Cristina Mihaela Nicolescu; Vlad Diaconescu. Pristine carbon nanohorns-polyvinyl pyrrolidone as sensing film for relative humidity detection. 7th International Conference on Emerging Technologies in Materials Engineering, 30–31 October.
- Shakeel, A.; Rizwan, K.; Farooq, U.; Iqbal, S.; Altaf, A.A. Advanced polymeric/inorganic nanohybrids: An integrated platform for gas sensing applications. Chemosphere 2022, 294, 133772. [Google Scholar] [CrossRef]
- Verma, A.; Yadav, D.; Natesan, S.; Gupta, M.; Yadav, B.C.; Mishra, Y.K. Advancements in nanohybrid material-based acetone gas sensors relevant to diabetes diagnosis: A comprehensive review. Microchem. J. 2024, 110713. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Z.; Li, Z.; Wang, W.; Xu, X.; Liu, X.; Wang, C. Synergic effect within n-type inorganic–p-type organic nano-hybrids in gas sensors. J. Mater. Chem. C 2013, 1, 3017–3025. [Google Scholar] [CrossRef]
- Zegebreal, L.T.; Tegegne, N.A.; Hone, F.G. Recent progress in hybrid conducting polymers and metal oxide nanocomposite for room-temperature gas sensor applications: A review. Sens. Actuators A Phys. 2023, 359, 114472. [Google Scholar] [CrossRef]
- Serban, B.C.; Dumbrăvescu, N.; Buiu, O.; Bumbac, M.; Brezeanu, M.; Cobianu, C.; Nicolescu, C.M. Oxidized carbon nanohorns/KCl/PVP nanohybrid as sensing layer for chemiresistive humidity sensor. In 2023 International Semiconductor Conference (CAS), Sinaia, Romania, 11–13 October 2023; IEEE: New York, NY, USA, 2023; pp. 75–78. [Google Scholar]
- Serban, B.C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Pachiu, C.; Brezeanu, M.; Cobianu, C. Ternary Holey Carbon Nanohorn/Potassium Chloride/Polyvinylpyrrolidone Nanohybrid as Sensing Film for Resistive Humidity Sensor. Coatings 2024, 14, 517. [Google Scholar] [CrossRef]
- Bogdan-Catalin Șerban; Octavian Buiu; Nicolae Dumbrăvescu; Viorel Avramescu; Mihai Brezeanu; Maria-Roxana Marinescu; Marius Bumbac; Cristina Nicolescu. Hydrophilic oxidized carbon nanohorns/PVP/KCl nanohybrid for chemiresistive humidity sensor. 14th International Conference on Physics of Advanced Materials (ICPAM-14), 8–15 September.
- Serban, B.C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Brezeanu, M.; Comanescu, F. Ternary Holey Carbon Nanohorns/TiO2/PVP nanohybrids as sensing films for resistive humidity sensors. Coatings 2021, 11, 1065. [Google Scholar] [CrossRef]
- Serban, B.C.; Buiu, O.; Bumbac, M.; Marinescu, R.; Dumbravescu, N.; Avramescu, V.; Comanescu, F. Ternary oxidized carbon nanohorns/TiO2/PVP nanohybrid as sensitive layer for chemoresistive humidity sensor. Chem. Proc. 2021, 5, 12. [Google Scholar]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Choi, H.C.; Jung, Y.M.; Kim, S.B. Size effects in the Raman spectra of TiO2 nanoparticles. Vib. Spectrosc. 2005, 37, 33–38. [Google Scholar] [CrossRef]
- Mathpal, M.C.; Tripathi, A.K.; Singh, M.K.; Gairola, S.P.; Pandey, S.N.; Agarwal, A. Effect of annealing temperature on Raman spectra of TiO2 nanoparticles. Chem. Phys. Lett. 2013, 555, 182–186. [Google Scholar] [CrossRef]
- Serban, B.C.; Dumbrăvescu, N.; Buiu, O.; Bumbac, M.; Brezeanu, M.; Cobianu, C.; Nicolescu, C.M. Ternary holey carbon-based nanohybrid for resistive relative humidity sensor. In 2023 International Semiconductor Conference (CAS), Sinaia, Romania, 11–13 October 2023; IEEE: New York, NY, USA, 2023; pp. 25–28. [Google Scholar]
- Serban, B.C.; Cobianu, C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Comanescu, F.C. Quaternary holey carbon nanohorns/SnO2/ZnO/PVP nano-hybrid as sensing element for resistive-type humidity sensor. Coatings 2021, 11, 1307. [Google Scholar] [CrossRef]
- Serban, B.C.; Cobianu, C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Comanescu, F. Quaternary Oxidized Carbon Nanohorns—Based Nanohybrid as Sensing Coating for Room Temperature Resistive Humidity Monitoring. Coatings 2021, 11, 530. [Google Scholar] [CrossRef]
- Zieleniewska, A.; Lodermeyer, F.; Prato, M.; Rumbles, G.; Guldi, D.M.; Blackburn, J.L. Elucidating the electronic properties of single-wall carbon nanohorns. J. Mater. Chem. C 2022, 10, 5783–5786. [Google Scholar] [CrossRef]
- Uceta, H.; Cabrera-Espinoza, A.; Barrejón, M.; Sánchez, J.G.; Gutierrez-Fernandez, E.; Kosta, I.; Delgado, J.L. p-Type Functionalized Carbon Nanohorns and Nanotubes in Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2023, 15, 45212–45228. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, J.; Xia, B. Humidity-sensing properties of chemically reduced graphene oxide/polymer nanocomposite film sensor based on layer-by-layer nano self-assembly. Sens. Actuators B Chem. 2014, 197, 66–72. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 1968, 45, 581. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part II: Underlying theories. J. Chem. Educ. 1968, 45, 643. [Google Scholar] [CrossRef]
- Pearson, R.G. The HSAB principle—more quantitative aspects. Inorg. Chim. Acta 1995, 240, 93–98. [Google Scholar] [CrossRef]
- Ayers, P.W.; Parr, R.G.; Pearson, R.G. Elucidating the hard/soft acid/base principle: A perspective based on half-reactions. J. Chem. Phys. 2006, 124, 194107. [Google Scholar] [CrossRef] [PubMed]
- Serban, B.; Kumar, A.S.; Costea, S.; Mihaila, M.; Buiu, O.; Brezeanu, M.; Cobianu, C. Polymer-amino carbon nanotube nanocomposites for surface acoustic wave CO2 detection. Rom. J. Inf. Sci. Technol. 2009, 12, 376–384. [Google Scholar]
- Serban, B.C.; Brezeanu, M.; Cobianu, C.; Costea, S.; Buiu, O.; Stratulat, A.; Varachiu, N. Materials selection for gas sensing: An HSAB perspective. In 2014 International Semiconductor Conference (CAS), Sinaia, Romania, 13–15 October 2014; IEEE: New York, NY, USA, 2014; pp. 21–30. [Google Scholar]
- Serban, B.; Kumar, A.S.; Cobianu, C.; Buiu, O.; Costea, S.; Bostan, C.; Varachiu, N. Selection of sensing materials using the Hard Soft Acid Base theory; application to Surface Acoustic Wave CO2 detection. In CAS 2010 Proceedings (International Semiconductor Conference), Sinaia, Romania, 11–13 October 2010; IEEE: New York, NY, USA, 2010; Volume 1, pp. 247–250. [Google Scholar]
- Serban, B.C.; Buiu, O.; Dumbrăvescu, N.; Brezeanu, M.; Cobianu, C.; Bumbac, M.; Nicolescu, M. Some considerations about the sensing mechanisms and electrical response of carbon nanohorns–based gas sensors. Sci. Technol. 2024, 27, 137–150. [Google Scholar] [CrossRef]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
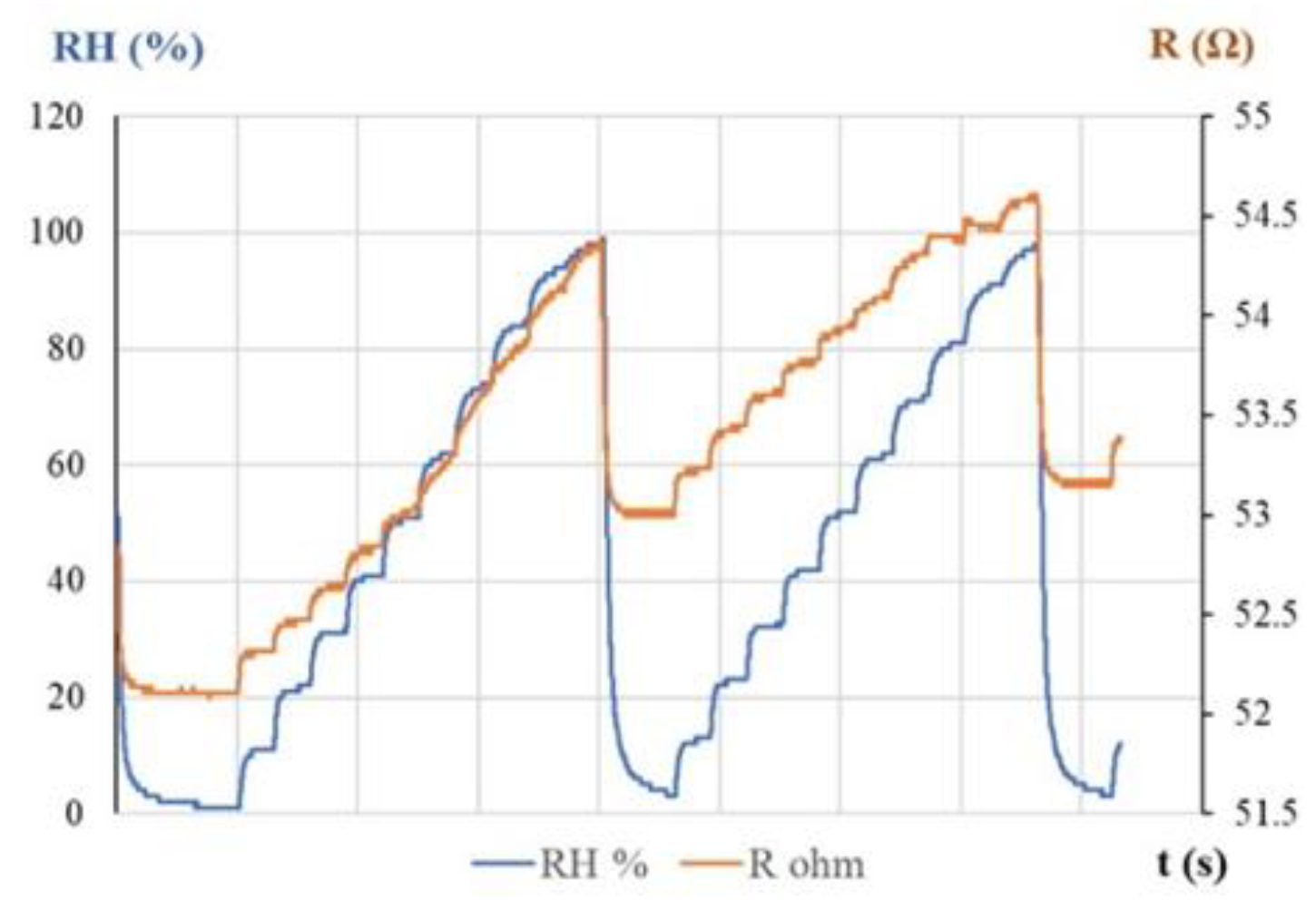
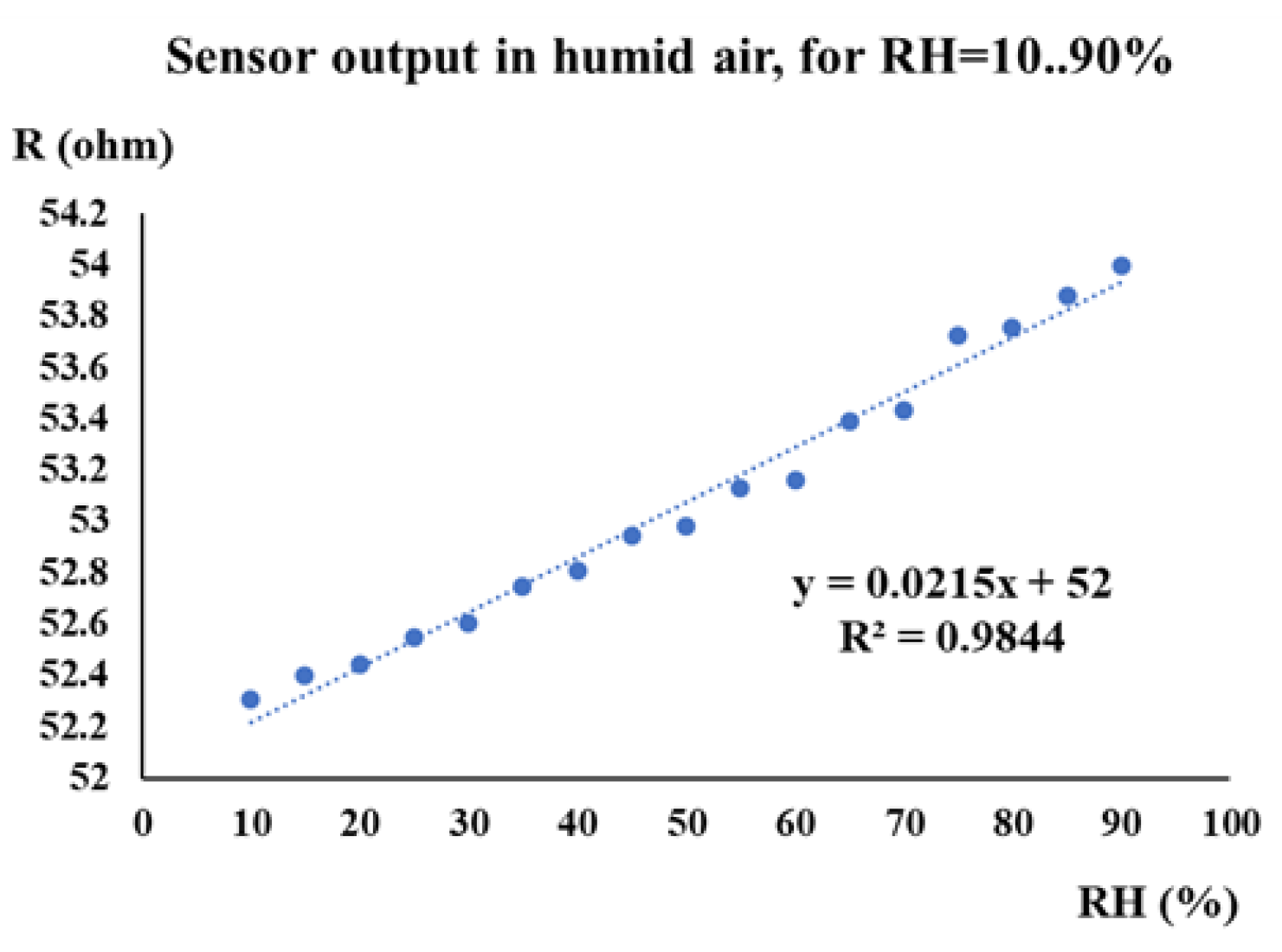
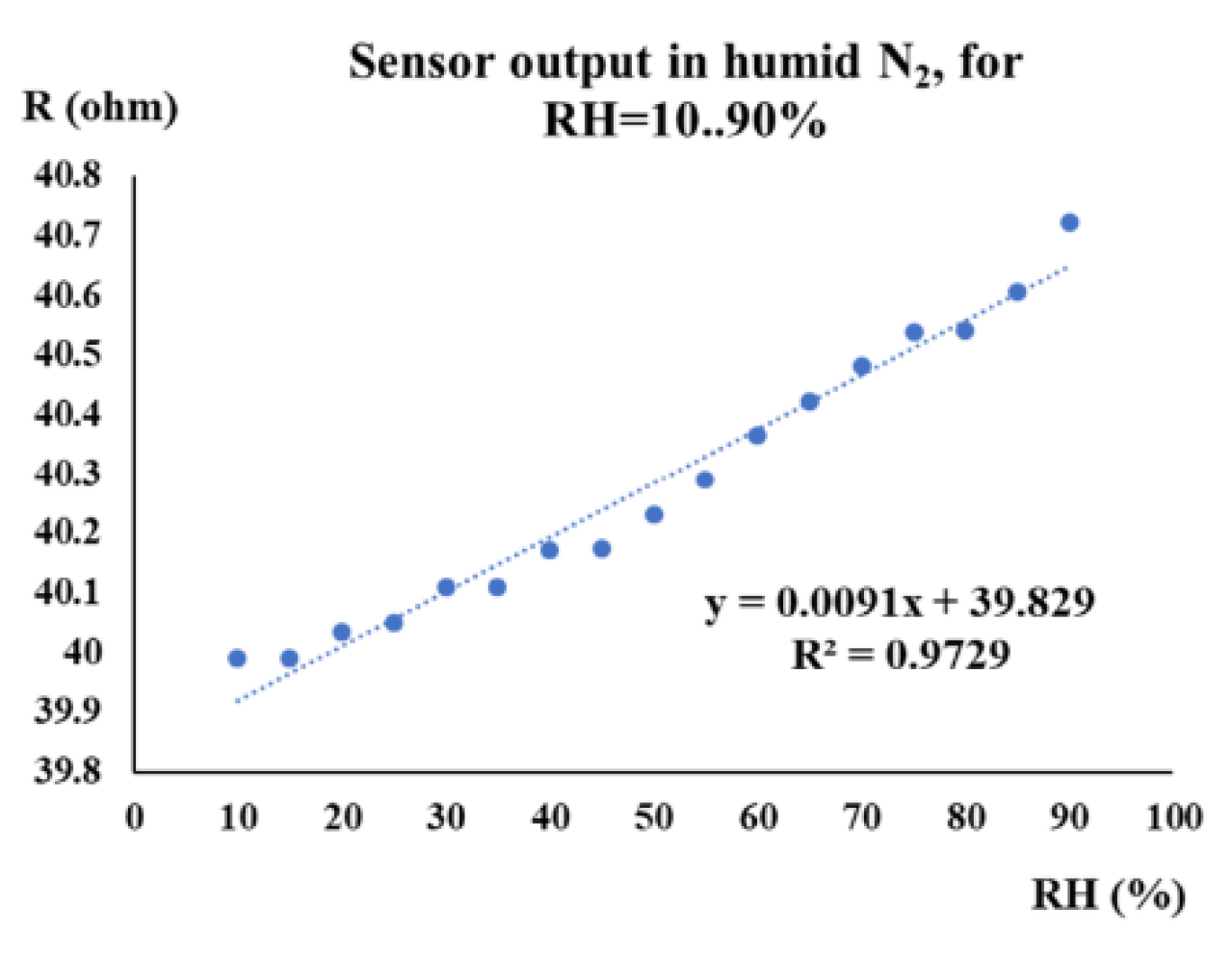
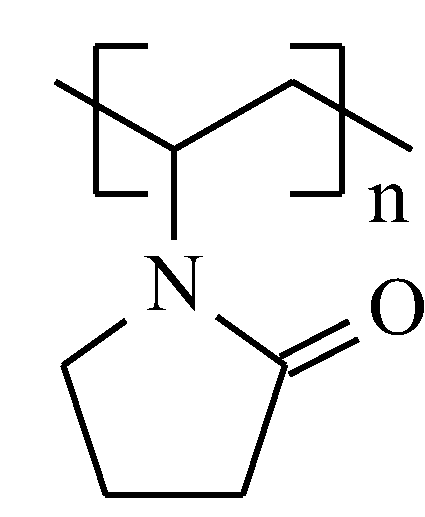
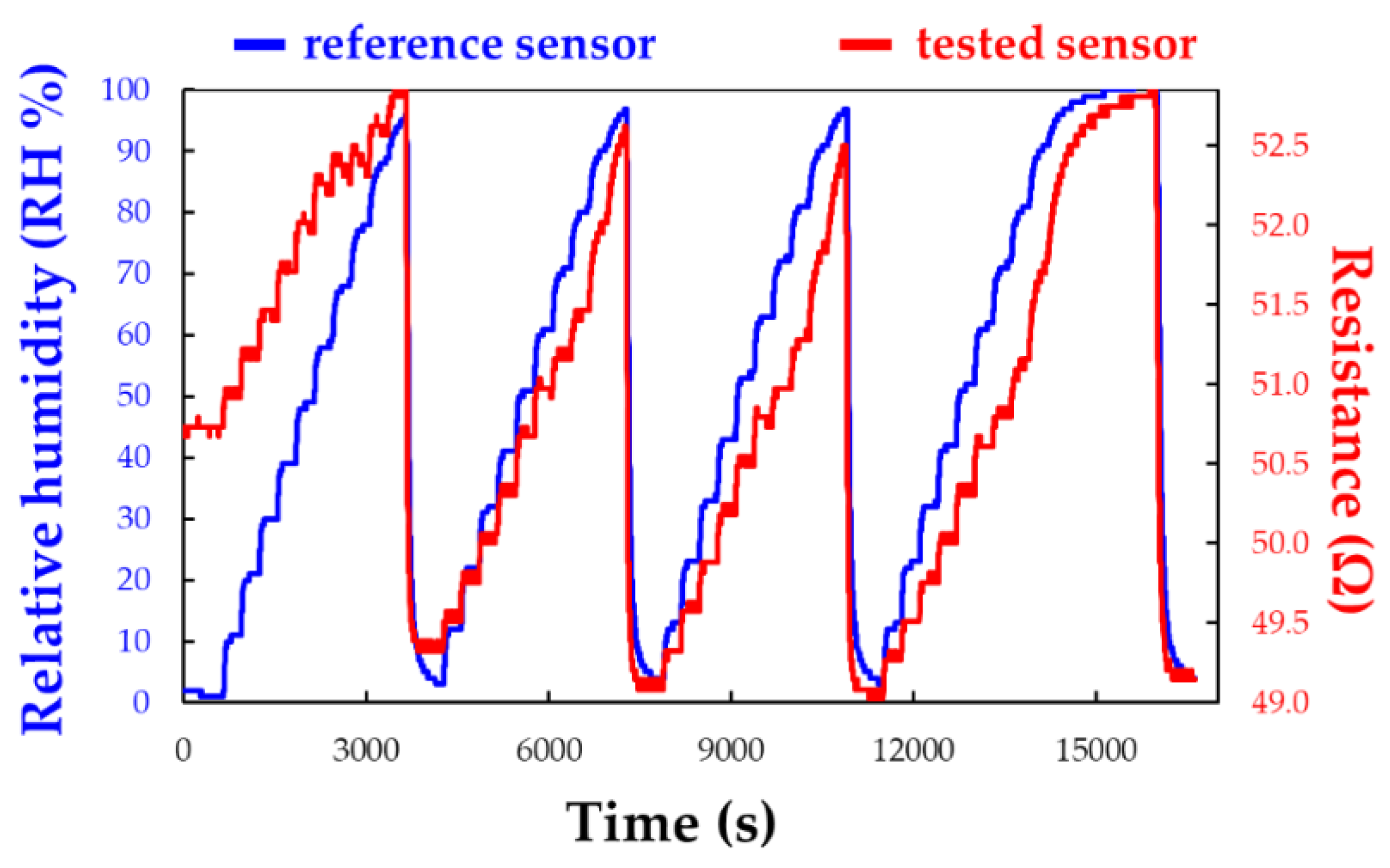
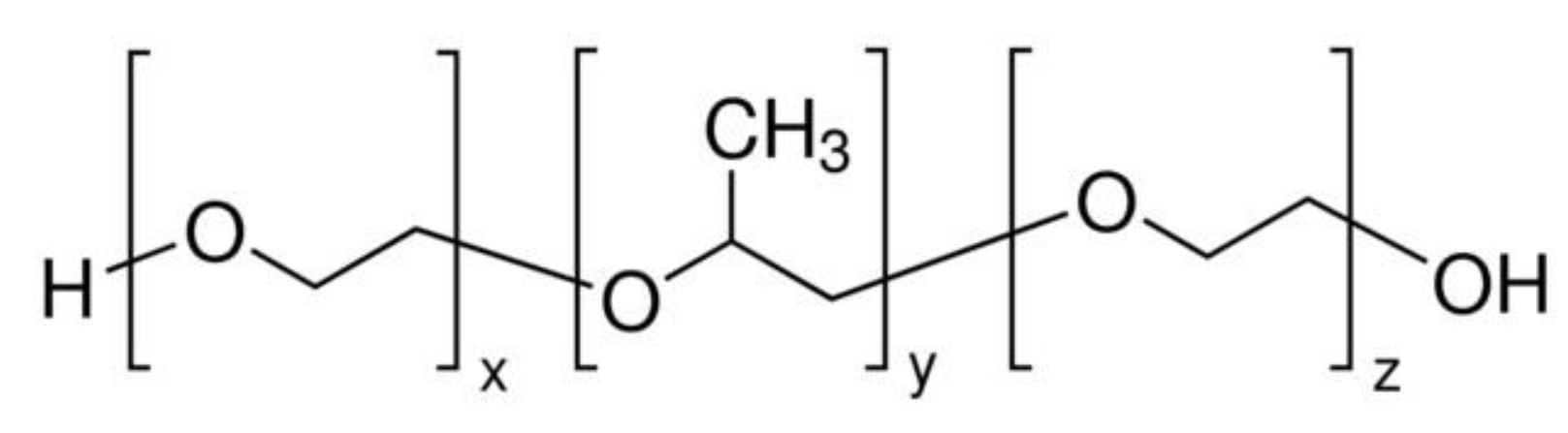
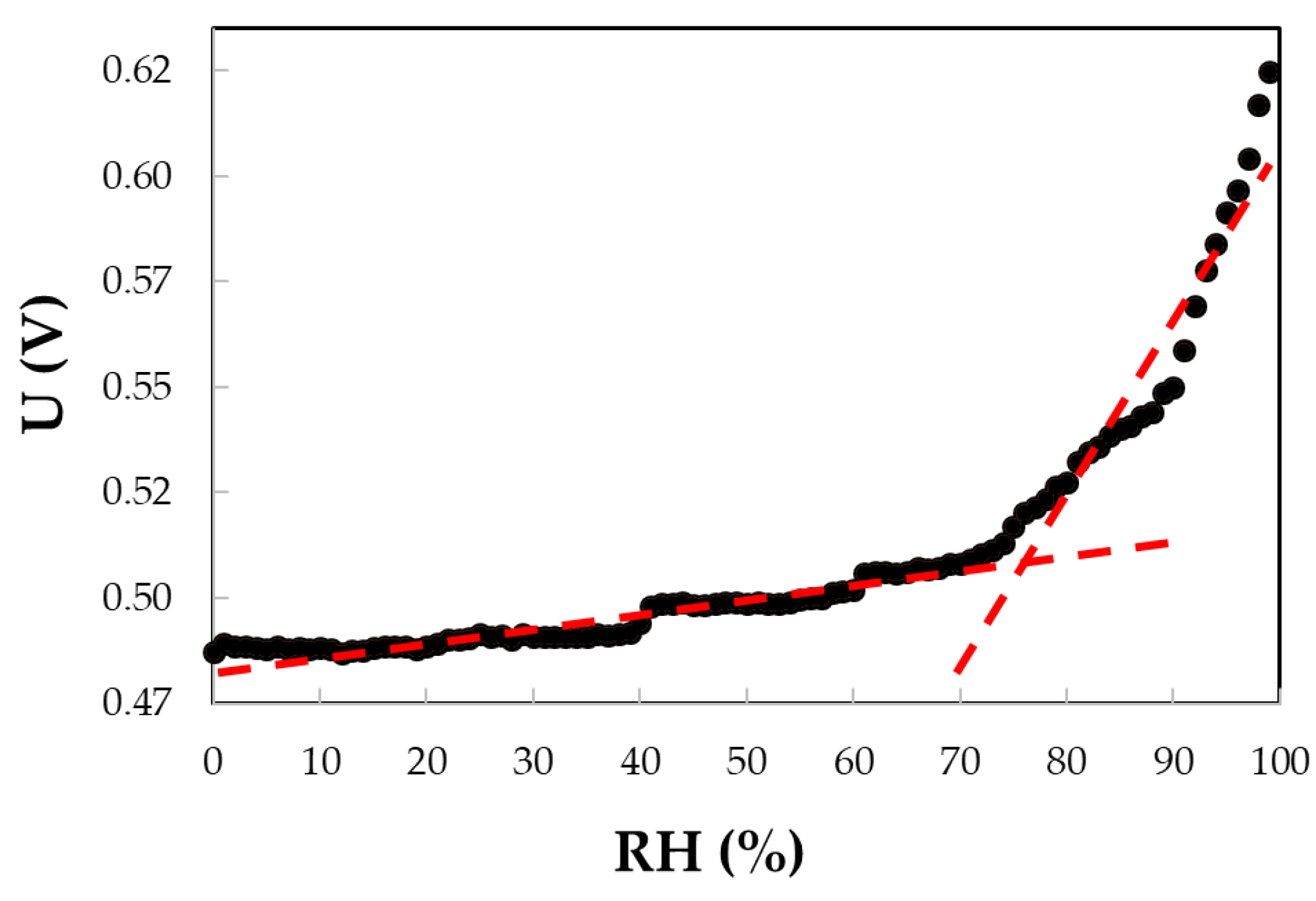
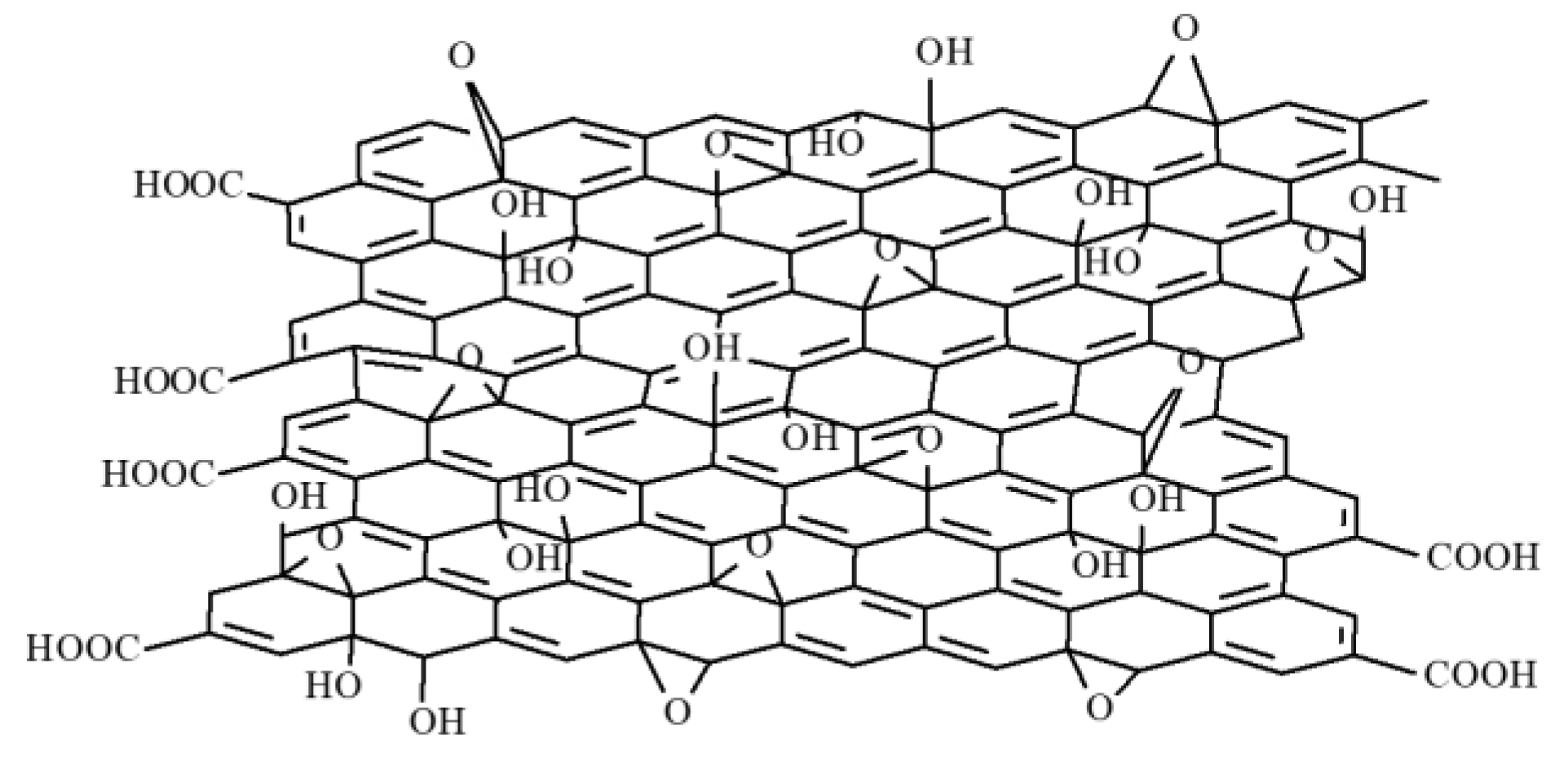
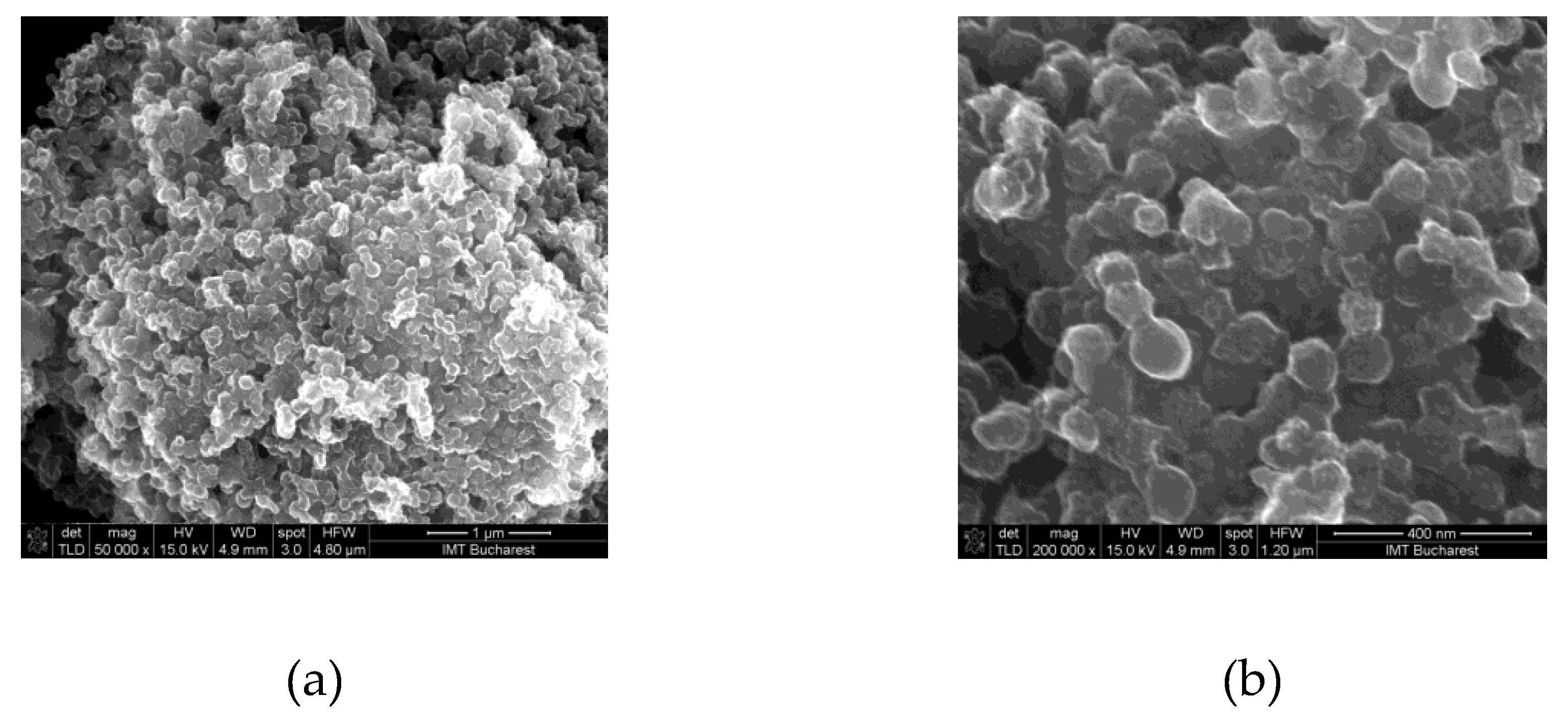
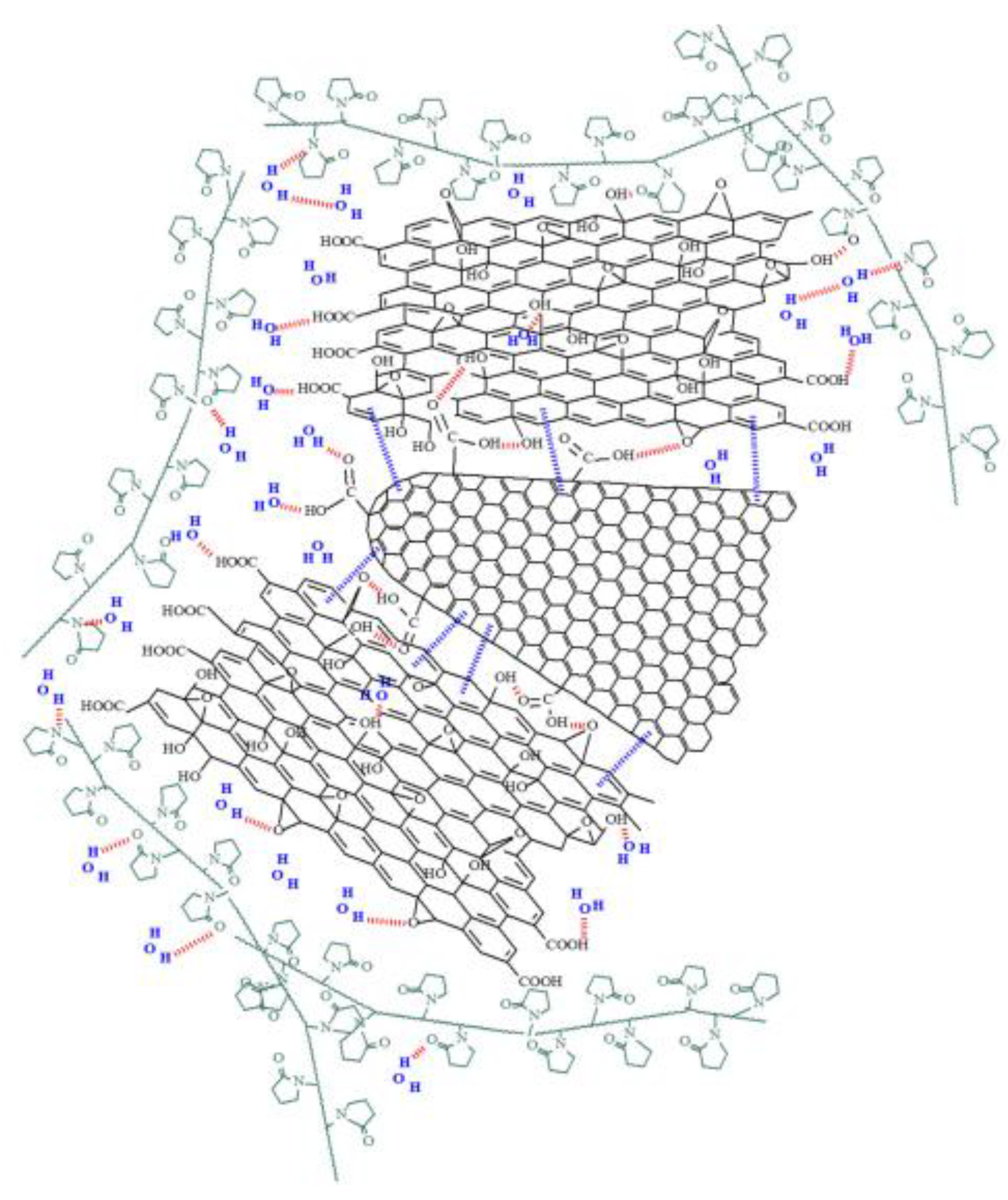
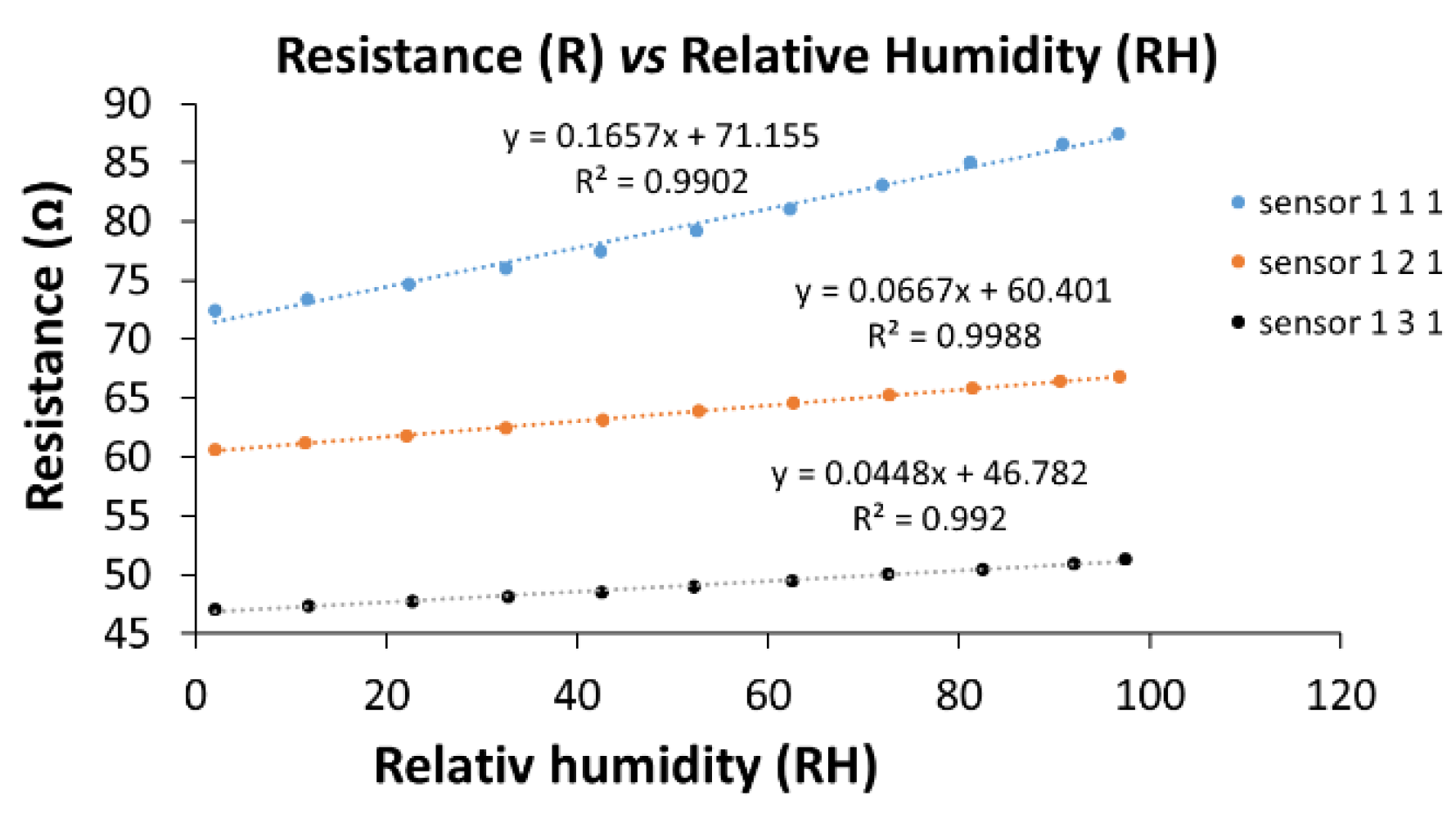
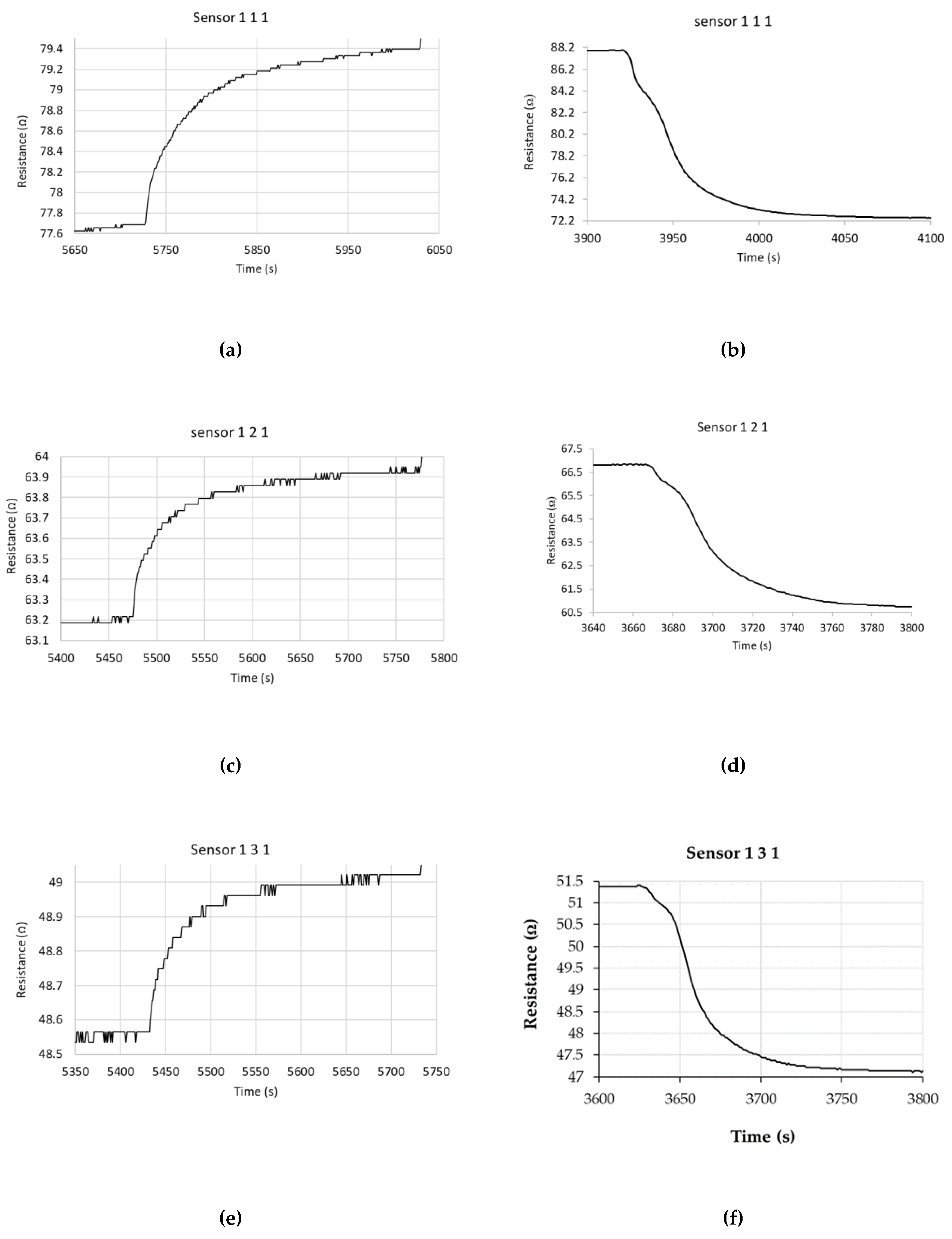
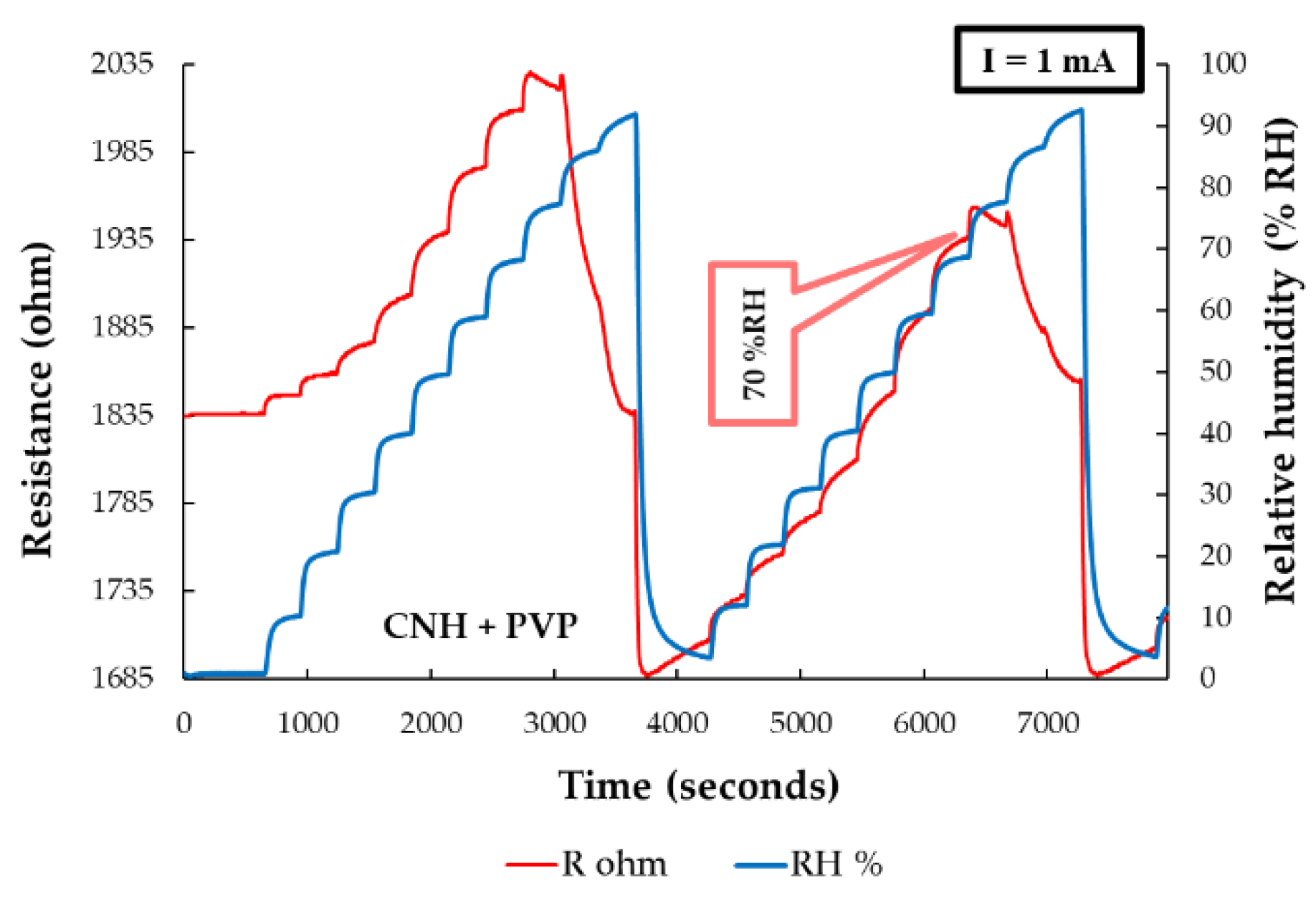
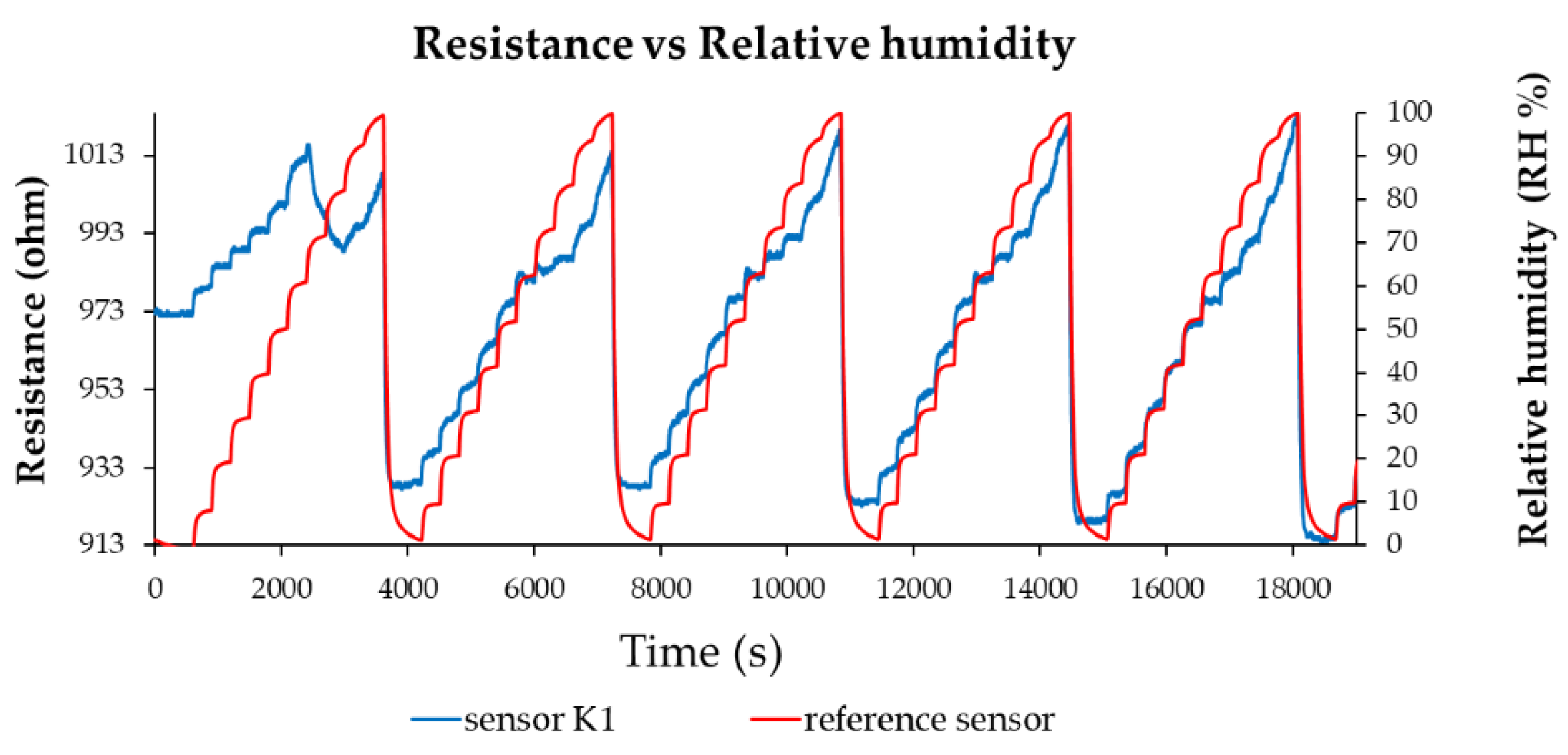
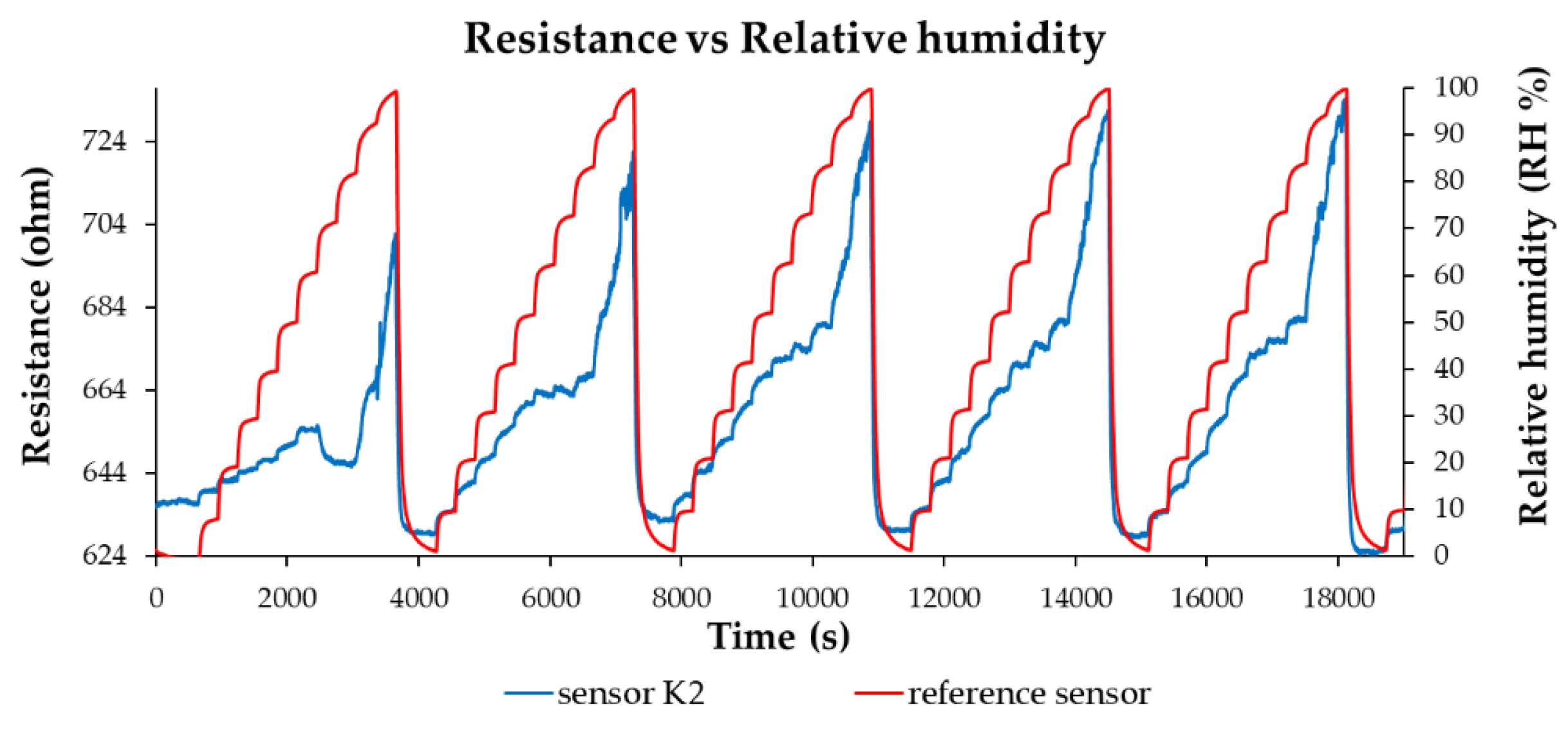
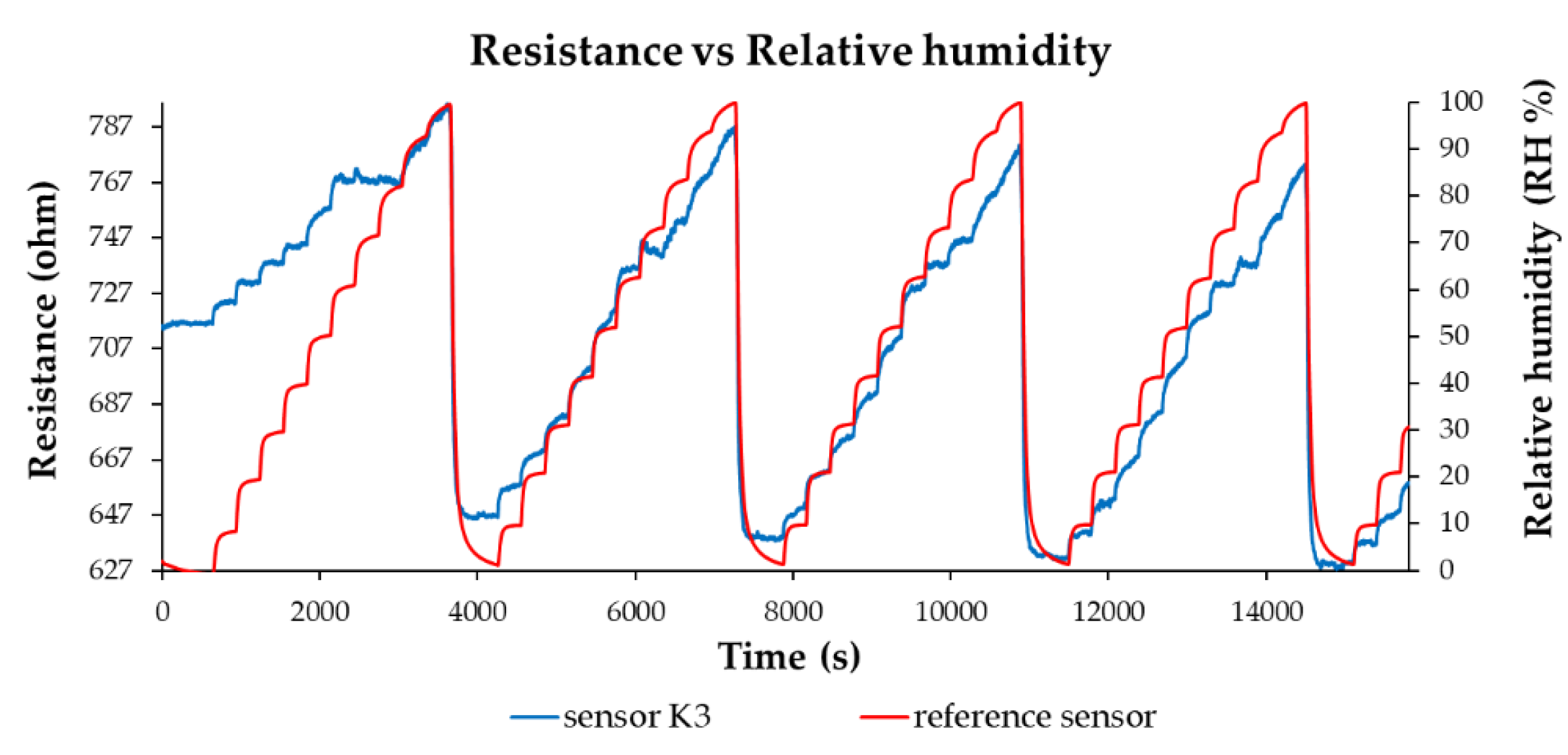
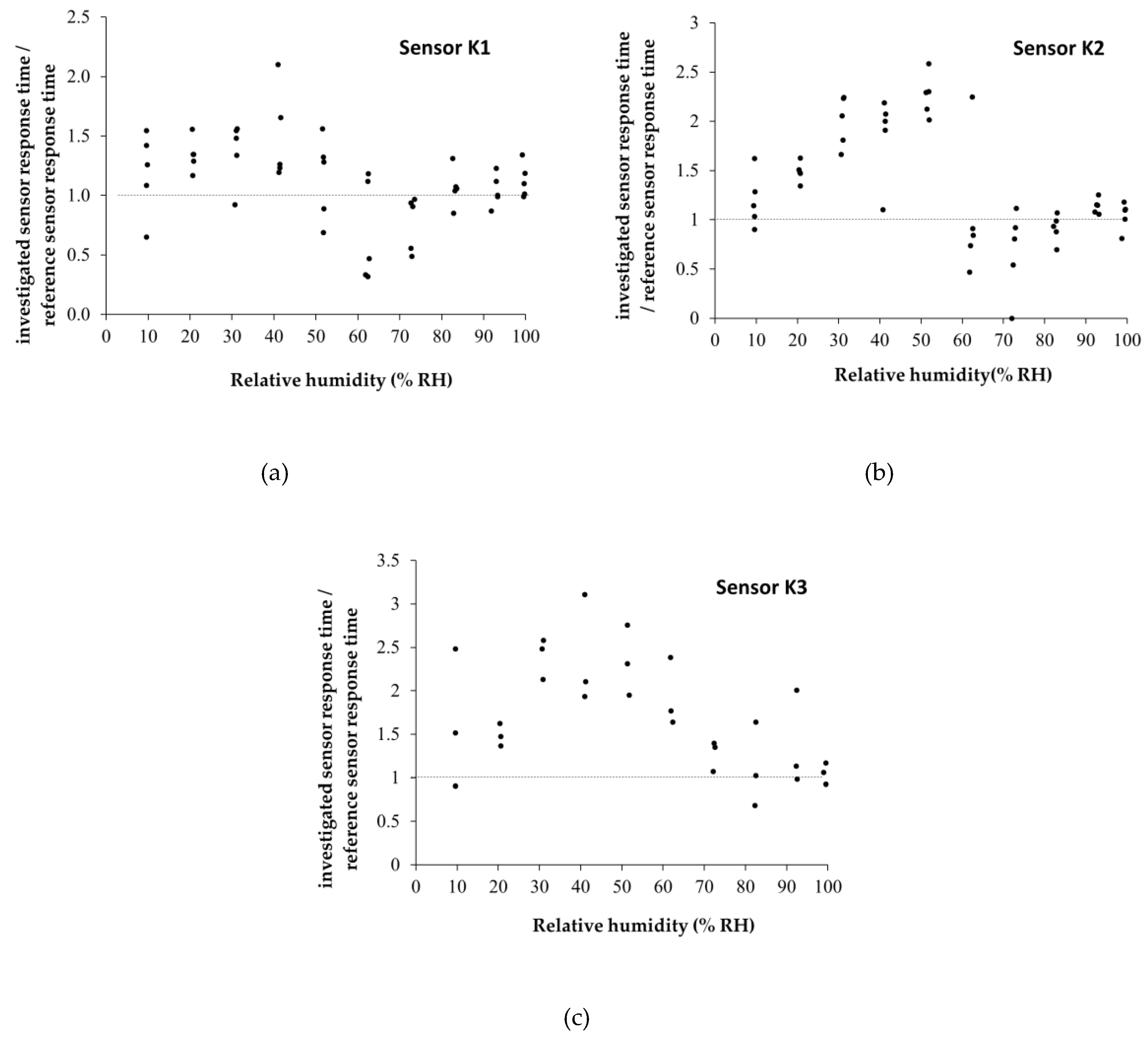
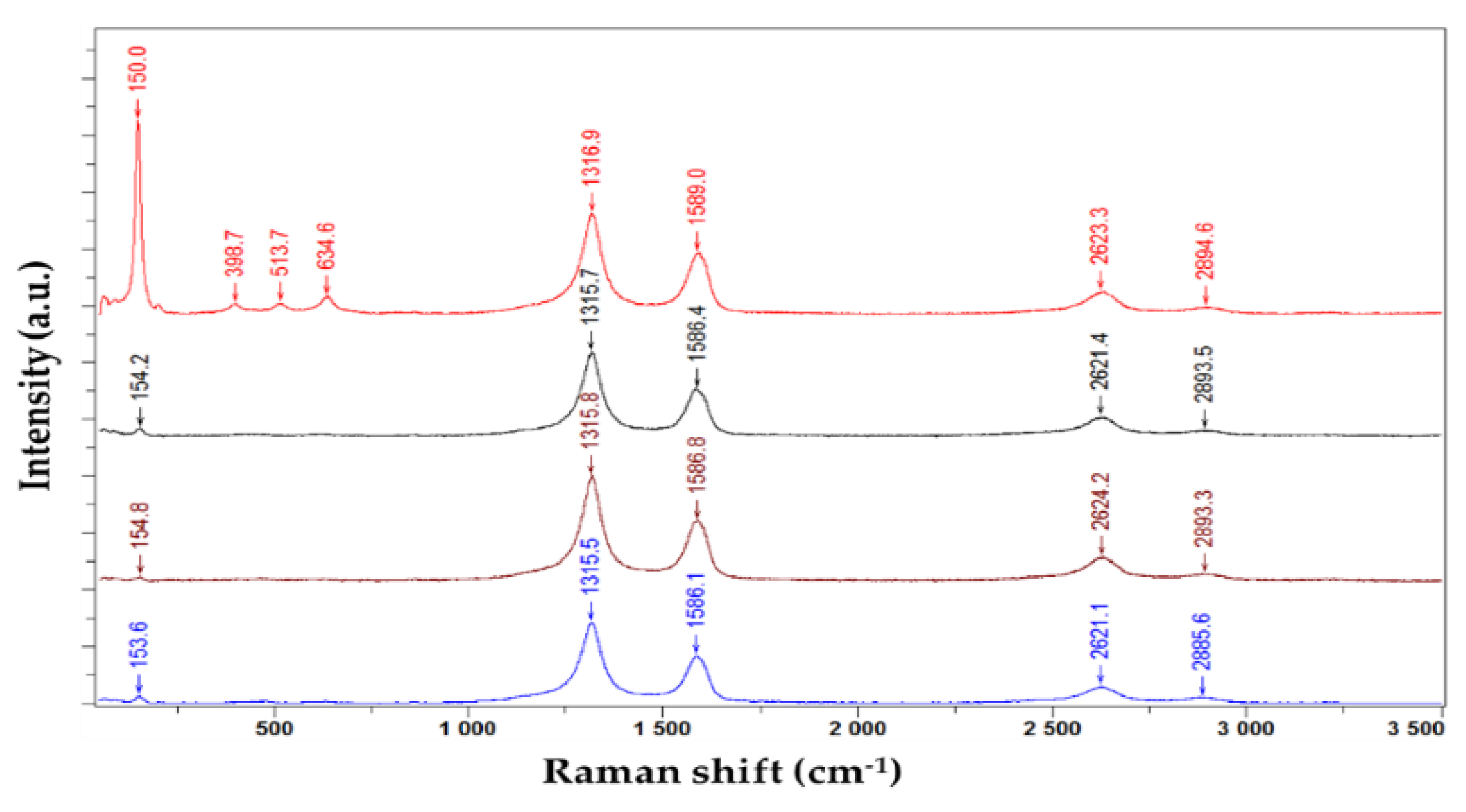
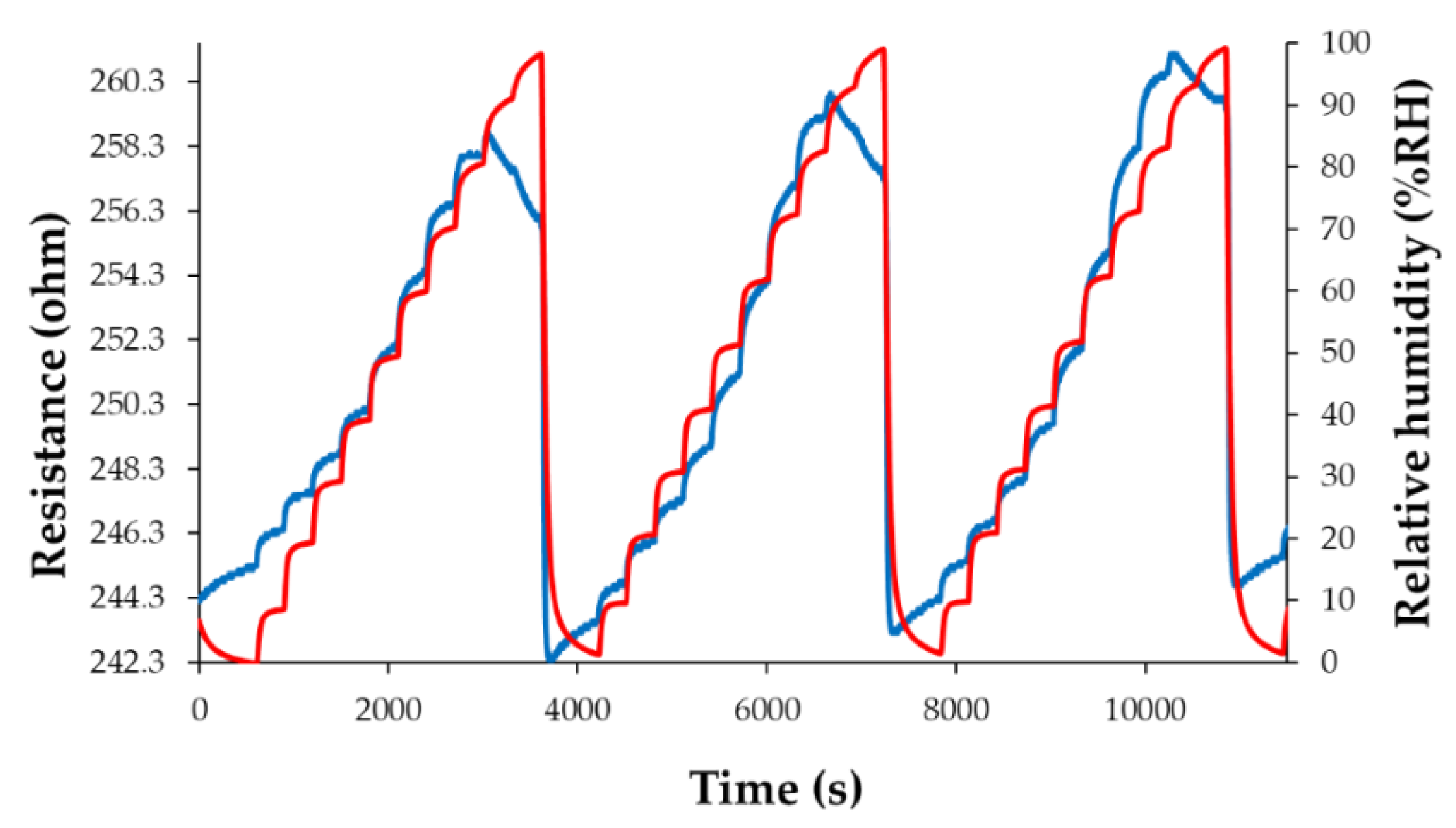
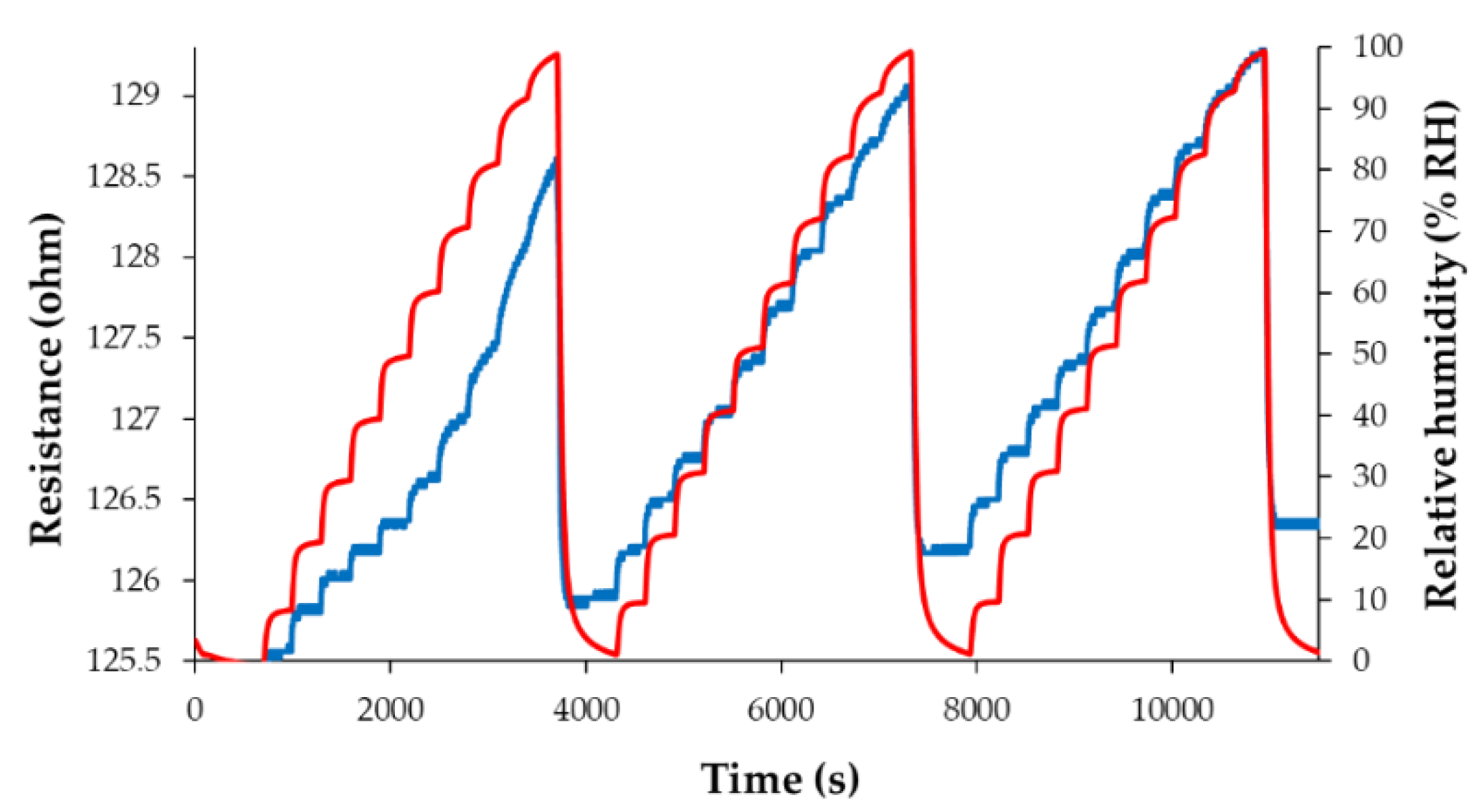
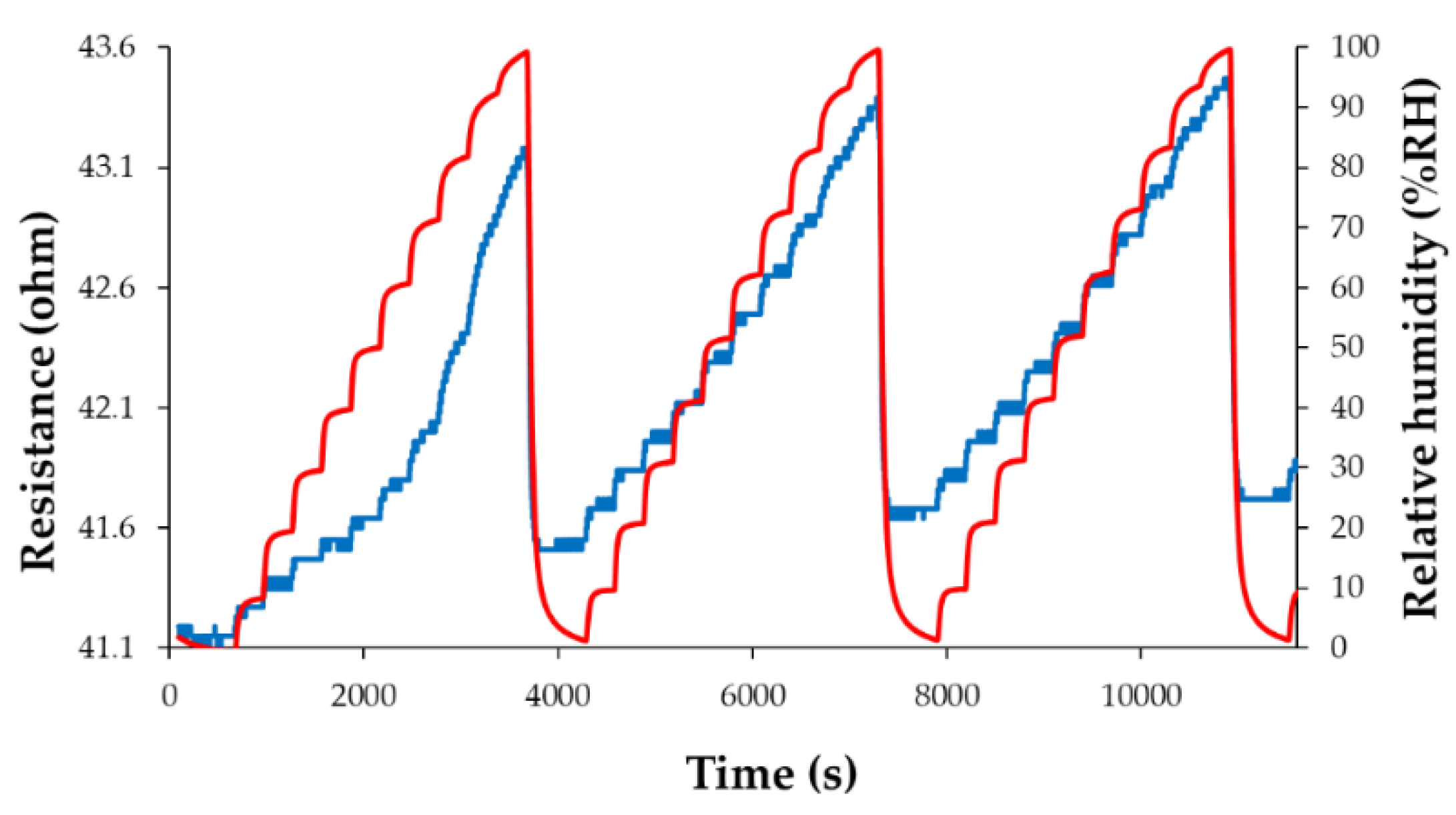
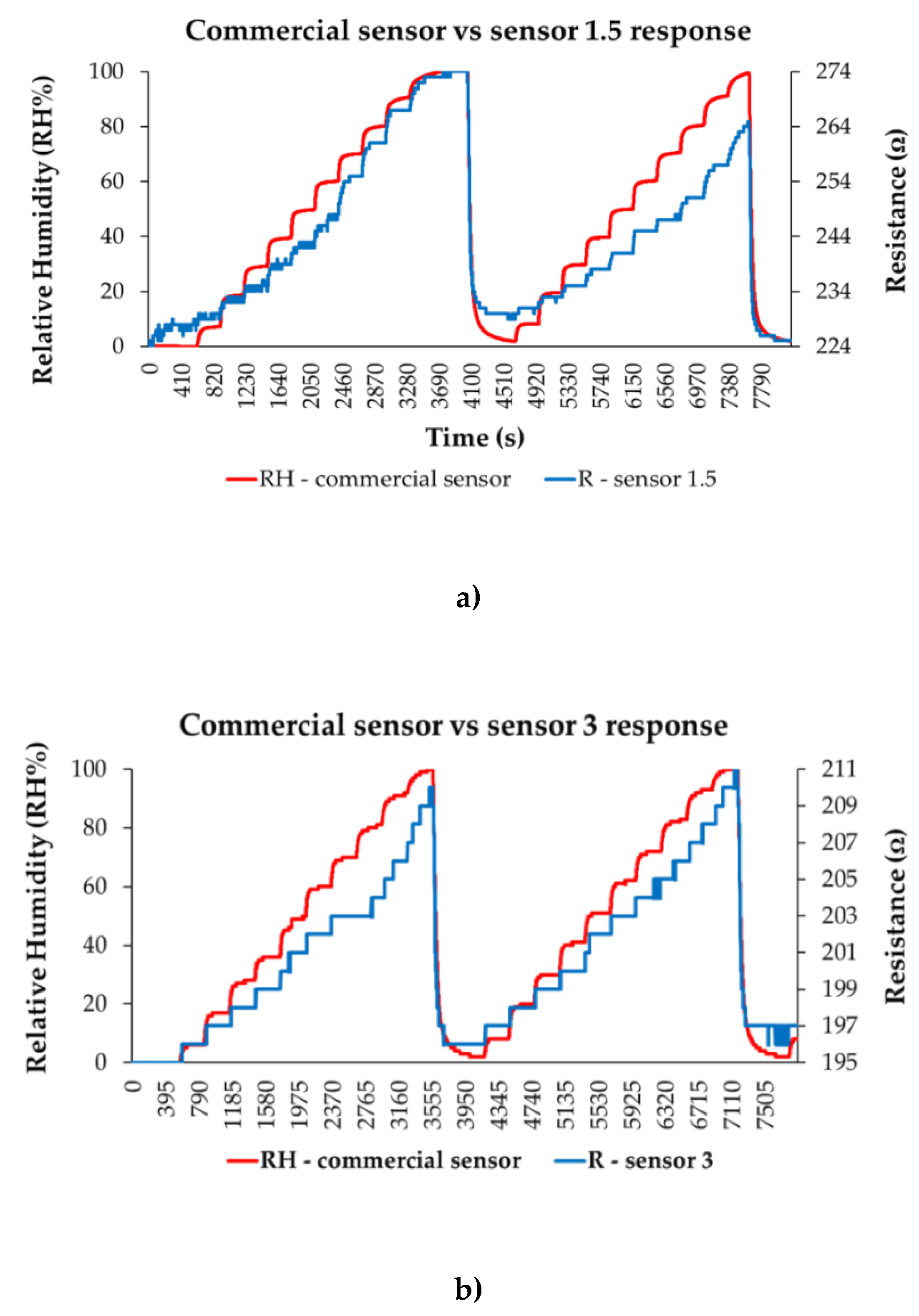
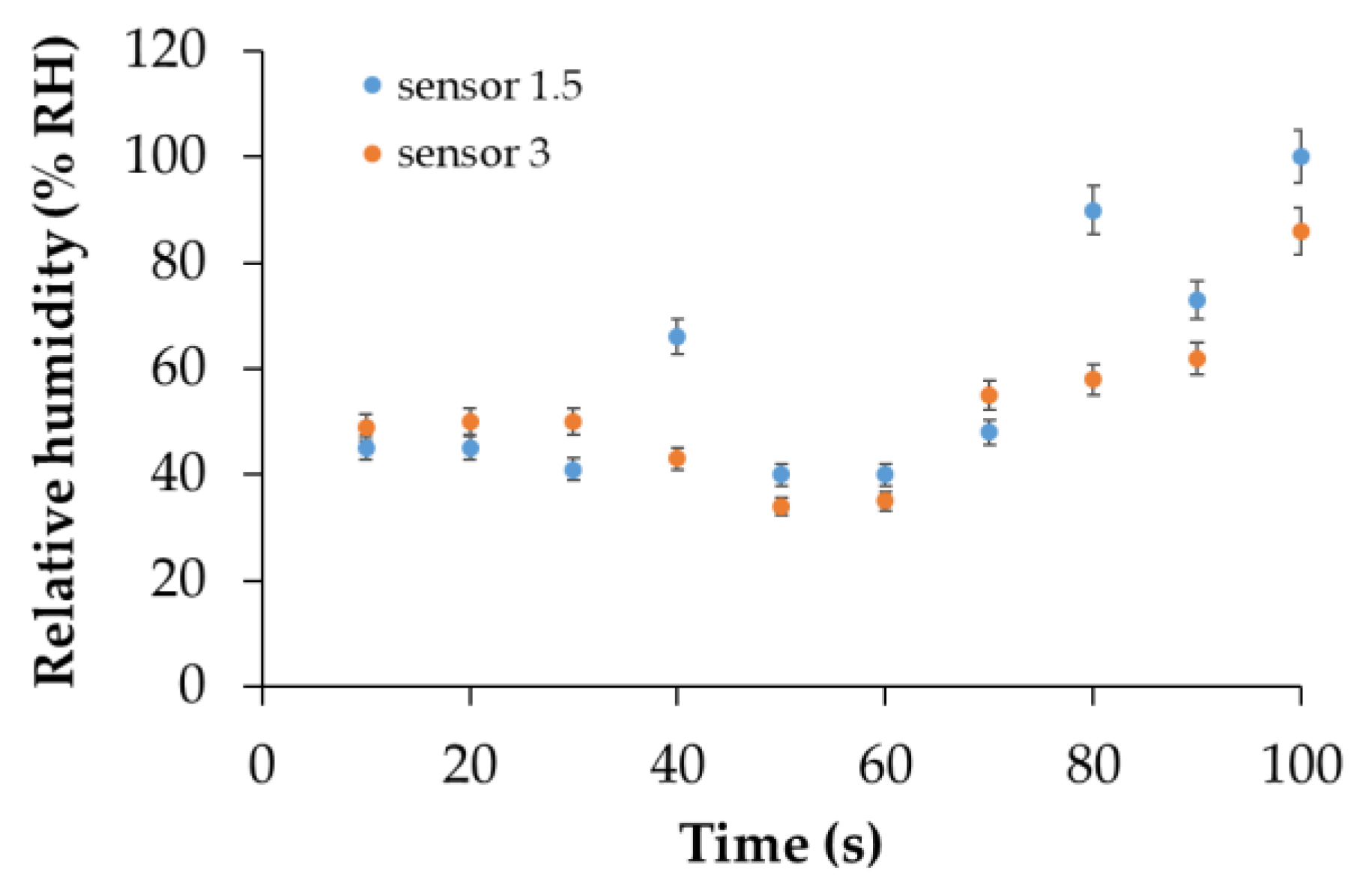
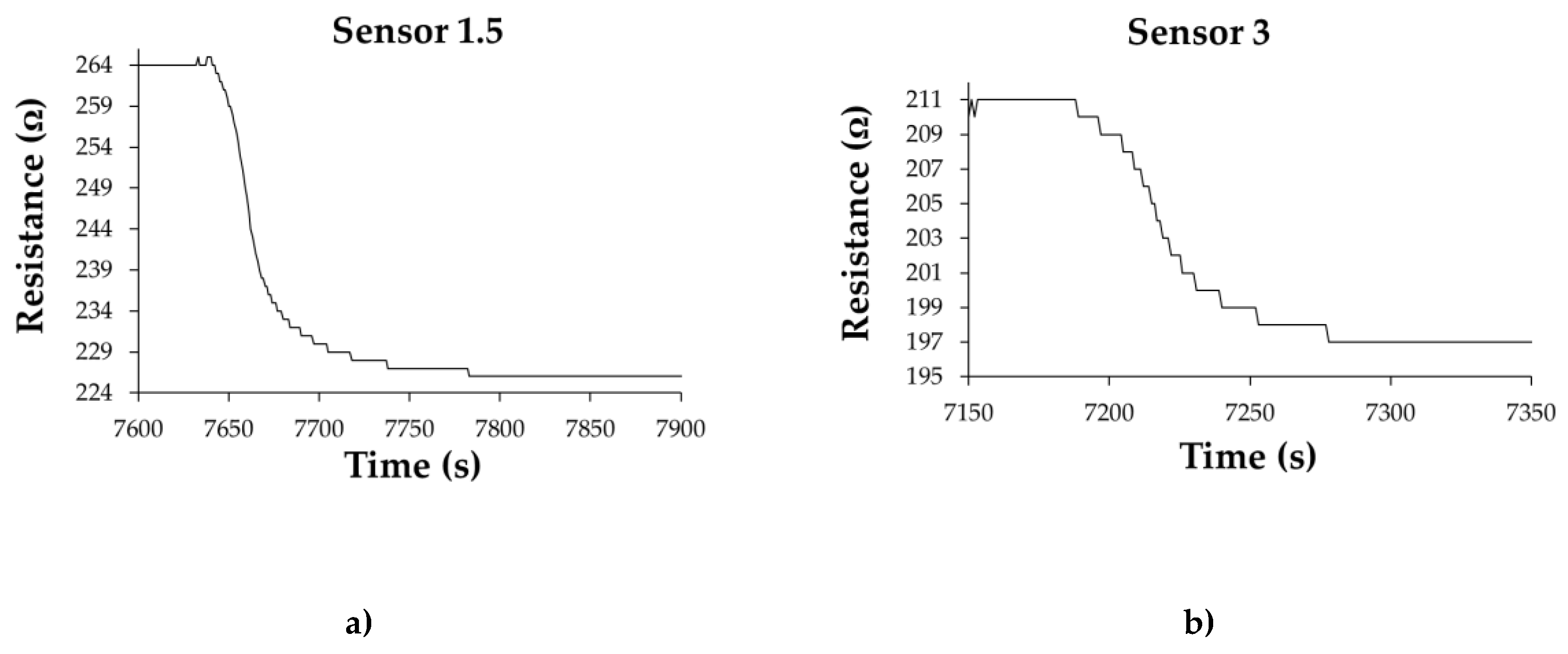
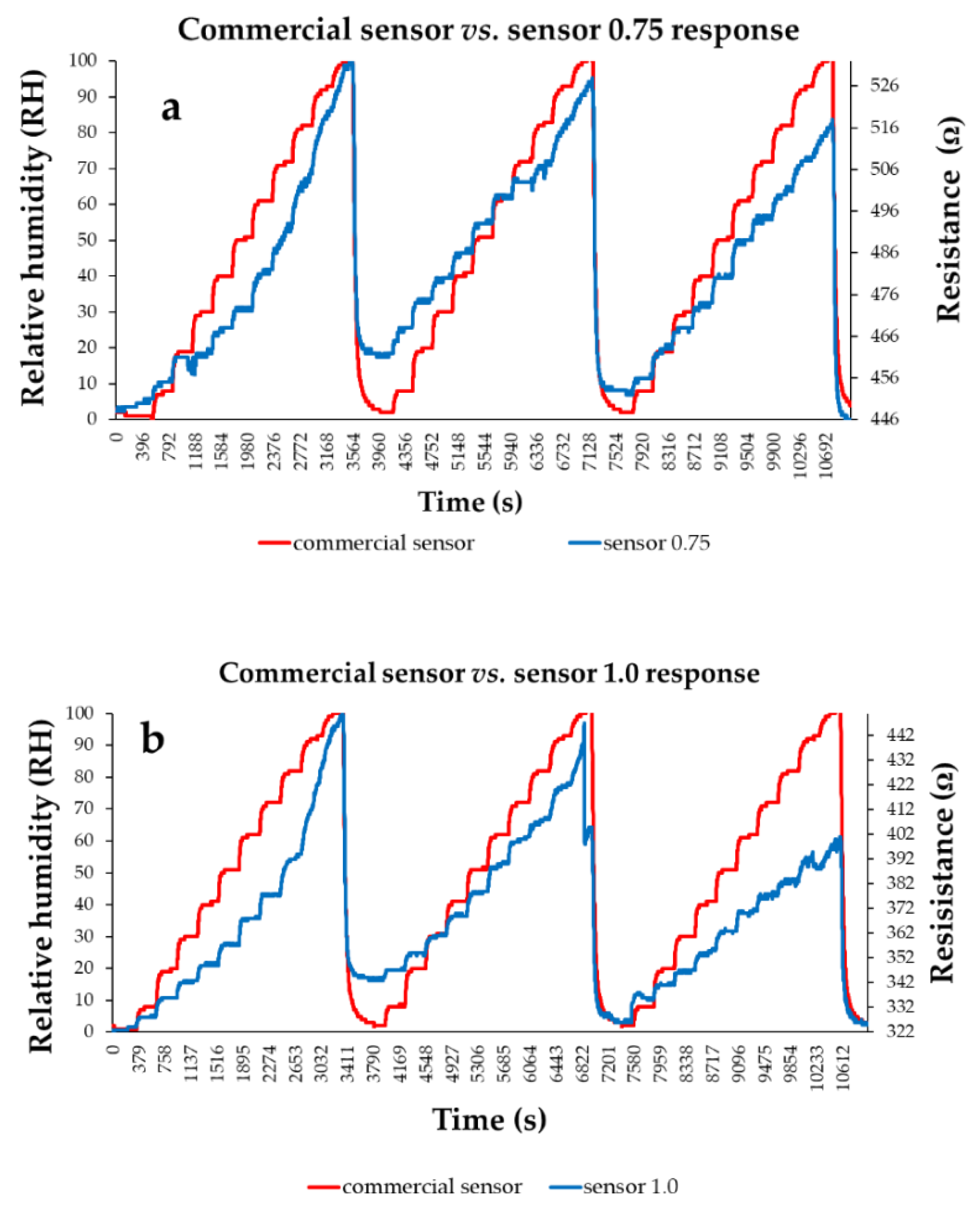
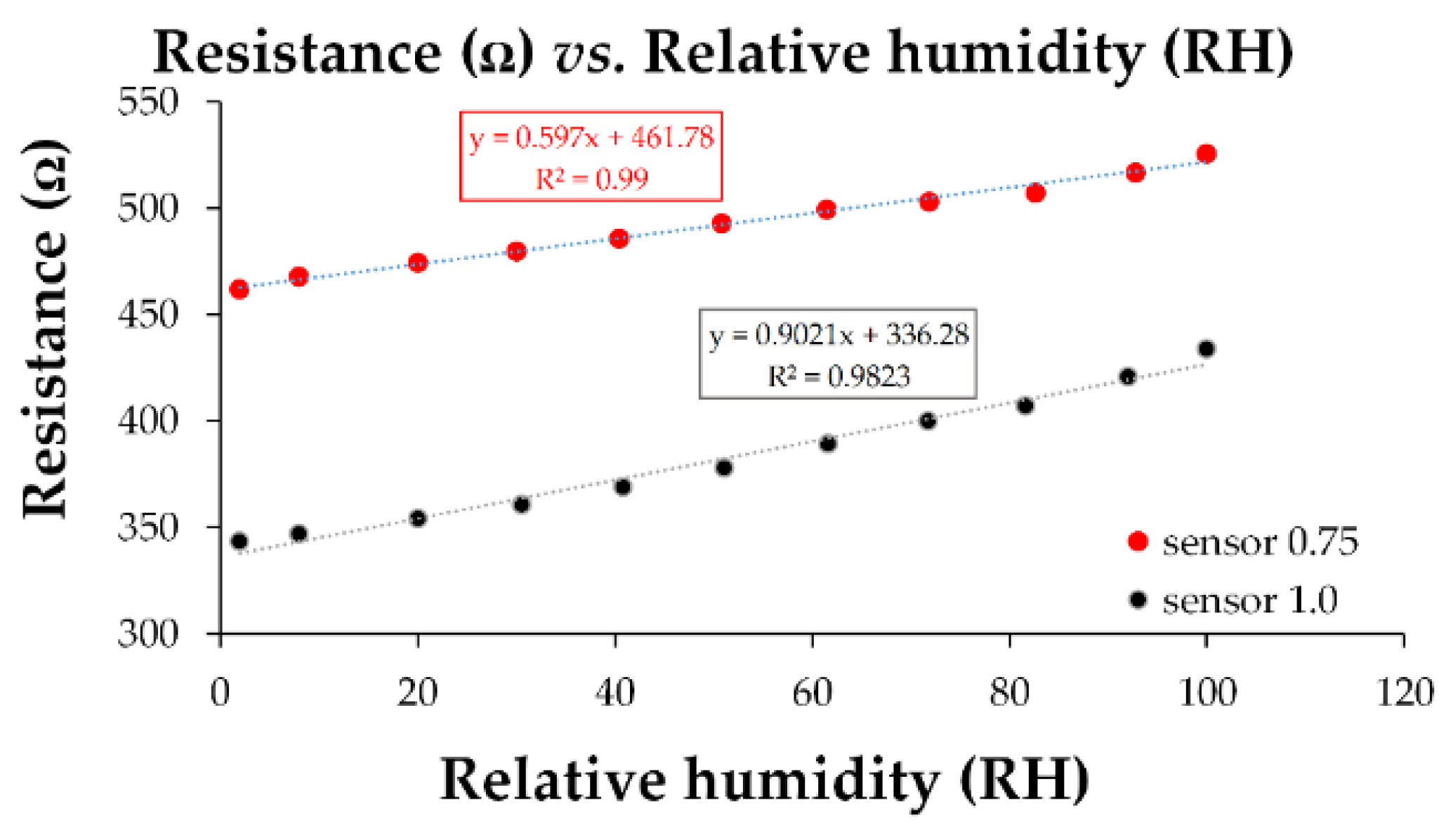
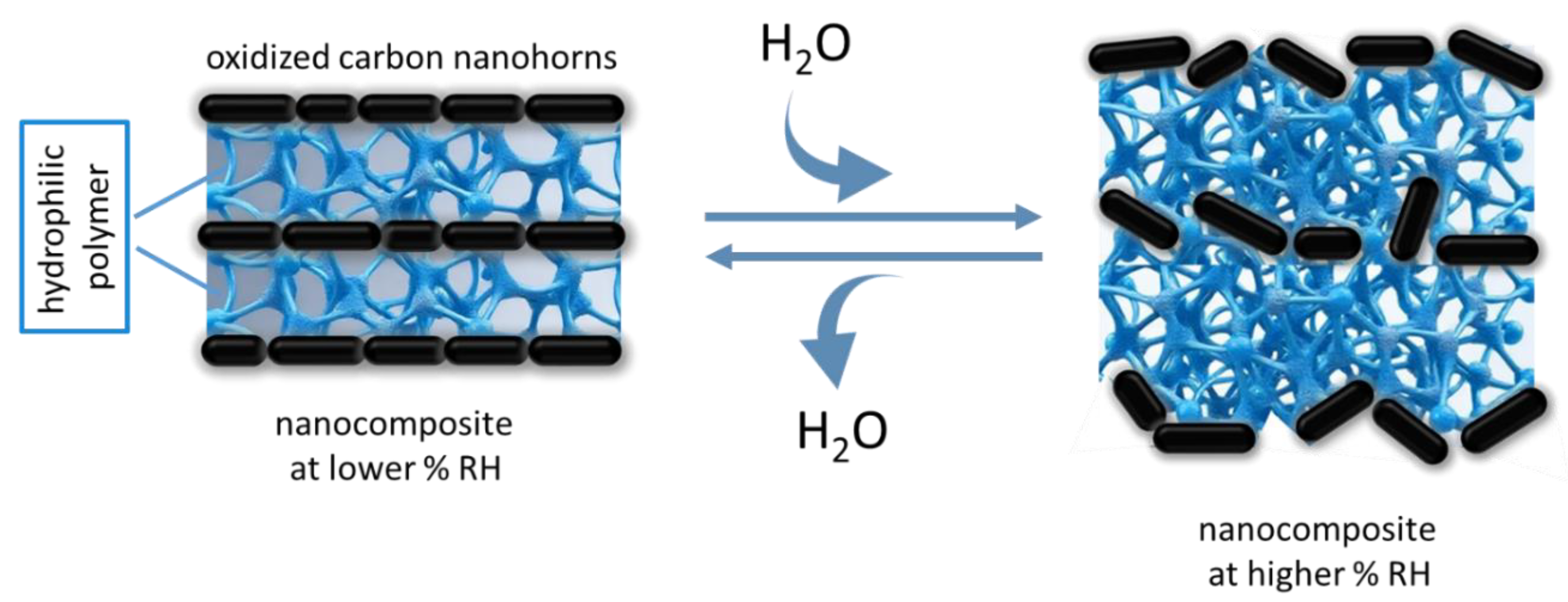
| Type of nanocarbonic material | Substrate | Measured RH range (%) | Reference |
|---|---|---|---|
| Graphene | Si/SiO2 | 1-96 | [26] |
| Graphene / ZnO | Glass | 15- 86 | [27] |
| Graphene / Poly(3,4-ethylenedioxythiophene)- polystyrene sulfonate | Si/SiO2, Kapton, PET, Paper | 30- 95 | [28] |
| Graphene oxide (GO) | Si/SiO2 | 40-88 | [29] |
| Reduced graphene oxide / Poly (diallyldimethylammonium chloride) (PDAC) | Glass | 20- 70 | [30] |
| Multi-walled carbon nanotubes (MWCNTs) | Polyimide | 10- 90 | [31] |
| MWCNTs / Polyvinylpyrrolidone (PVP) | Quartz | 11- 94 | [32] |
| Pristine carbon nano-onions (CNOs) / PVP at 1/1 w/w ratio | Polyimide | 0-100 | [33] |
| Pristine CNOs / Polyvinyl Alcohol (PVA) at a 1/1 and 2/1 w/w ratios | Si/SiO2 | 5-95 | [34] |
| MWCNTs / Polyimide | Si3N4 | 10-95 | [35] |
| Hydrogenated amorphous carbon | Synthetic resin FR2 | 10-100 | [36] |
| Carbon-black / PVA | glass | 10,9 -73,7 | [37] |
| Shellac-derived carbon (SDC) | Si/SiO2 | 10-90 | [38] |
| Carbon nano coils (CNCs) | Liquid crystal polymer (LCP) | 4-95 | [39] |
| Porous carbon nanofiber | Cellulose | 13-97,3 | [40] |
| Carbon nanosheets and nanohoneycombs | Si (100) | 11-95 | [41] |
| Carbon quantum dots | Glass sheet | 7-95 | [42] |
| Pyrolyzed bamboo | α - alumina | 0-96 | [43] |
| Biochar | α-alumina | 5-100 | [44] |
| Graphene quantum dots | Si/SiO2 | 15-80 | [45] |
| Multi-walled carbon nanotubes (SWCNT) / Pt / P2O5 | Ceramic | 1-90 | [46] |
| SWCNTs / PVA filaments | Textile cloth | 60-100 | [47] |
| Chitosan / ZnO / SWCNTs | Polyimide |
11-97 |
[48] |
| Carbon nanodots | Polytetrafluoroethylene (PTFE) | 11-94 | [49] |
| Sensing layer composition (w/w) | Substrate | Sensitivity (ΔR/ΔRH) | Reference |
|---|---|---|---|
| CNHox | Si/SiO2 | 0.013-0.021 | [79] |
| CNHox/PVP 2/1 | Si/SiO2 | 0.017-0.025 | [83] |
| CNHox/PVP 1/1 | Si/SiO2 | 0.020-0.058 | [83] |
| GO/CNHox/PVP 1/3/1 | Si/SiO2 | 0.043-0.051 | [84,85] |
| GO/CNHox/PVP 1/2/1 | Si/SiO2 | 0.063-0.070 | [84,85] |
| GO/CNHox/PVP 1/1/1 | Si/SiO2 | 0.150-0.200 | [84,85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





