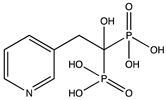
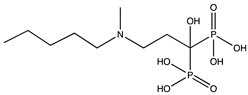
1. Introduction
Lipophilicity is a fundamental physicochemical property that contributes to the absorption, distribution, metabolism, excretion, and toxicity of the pharmaceuticals. This affects the solubility and permeability of the pharmaceuticals and determines their potency and selectivity. It is therefore essential to monitor and consider the lipophilic properties of pharmaceuticals during their development in order to improve quality and enhance the likelihood of therapeutic success [
1].
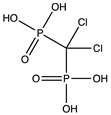
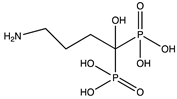
In this study, we focused on a class of pharmaceuticals derived from bisphosphonates. Bisphosphonates are commonly used oral medications for osteoporosis treatment [
2]. The pharmacological function of these active compounds is determined by their P–C–P backbone, where two phosphate groups are covalently linked to a carbon atom. The poor lipophilicity of bisphosphonates limits their absorption from the gastrointestinal tract. Furthermore, bisphosphonates exhibit a short plasma half-life and are rapidly eliminated in urine [
3]. The incorporation of hydrophobic side chains into the bisphosphonate structure allows to overcome these limitations, offering potential benefits in various therapeutic areas, including cancer and parasitic infections. Thus, a method that can distinguish lipophilicity of bisphosphonates would be a valuable tool for early-stage physicochemical screening in pharmaceutical research.
The n-octanol : water partition coefficient K
o : w (P) is a measure of lipophilicity in the absence of relevant speciation and refers to the neutral form of a substance. K
o : w is defined as the ratio of concentrations in an organic phase, typically n-octanol, and an aqueous phase [
4].
Polyaniline (PANI) is a conductive polymer whose structure and properties are highly sensitive to synthesis conditions [
5]. In particular, the choice of doping acid and the presence of salts during chemical oxidative polymerization can significantly influence the morphology of the resulting PANI and the types or levels of dopant ions incorporated. Previous studies have shown that different protonic acids yield PANI with distinct microstructures – for example, PANI doped with small inorganic acids like HCl tends to form aggregated granular or globular structures, whereas larger organic sulfonate dopants can produce more fibrous or networked morphologies [
5]. The acidity of the reaction medium also plays a crucial role: higher acid concentrations generally increase the extent of protonation and can lead to well-defined globular PANI particles, while milder conditions or the absence of added acid may result in mixed morphologies [
6] (e.g. fibers, plates) or smaller aggregates [
7]. In addition to acids, inert salts in the polymerization mixture (providing different anions and ionic strengths) have been reported to modulate PANI nanostructure formation by affecting nucleation and growth – for instance, adding NaCl or other salts can alter fiber diameters or aggregation tendencies of PANI [
8].
In membrane-based PANI composites, such as PANI-coated PVC membranes, these effects are compounded by the interaction of PANI growth with the substrate surface. Understanding how polymerization conditions like salt type (chloride vs sulfate) and the use of an acid dopant influence the surface morphology and elemental composition of PANI-based membranes is important for tailoring their properties (e.g. conductivity, homogeneity, and functional group content) for applications.
The successful use of the potentiometric sensors, either individually or as part of a sensor array, particularly in real sample analysis, has motivated a number of research laboratories to pursue their development [
9,
10,
11,
12]. We have recently shown that polyaniline (PANI) as an outer layer on a plasticized PVC membrane surface promotes the transport of highly hydrated sulfates from aqueous medium [
13,
14]. This phenomenon resulted from stronger PANI hydration in the presence of highly hydrated anions, which facilitated their transport through the inter-particle space of the PANI layer [
15].
In the present work, we propose the use of a novel sensor array based on ion-selective electrodes (ISEs) to evaluate the lipophilicity of bisphosphonate pharmaceuticals. The sensor array consisted of ISEs based on a poly(vinyl chloride) membranes coated with polyaniline layers, each exhibiting different selectivity toward analyte lipophilicity. We present a comparative analysis of a series of PANI-coated PVC plasticized with 2-nitrophenyl octhyl ether (NPOE) membranes, prepared either with or without the ion-exchanger tridodecylmethylammonium chloride (TDDMACl), under varying polymerization conditions. The influence of salt type (NaCl or Na₂SO₄) and acid usage (polymerization with or without added HCl) on the surface morphology (analyzed by scanning electron microscopy, SEM) and elemental composition (from energy-dispersive X-ray spectroscopy, EDS) of the PANI membranes was examined, including a comparison of polymer layers formed from aniline base and aniline hydrochloride in the presence of NaCl. By comparing these samples and integrating reference data from the literature, we aim to clarify how the presence of different anions and the acidity of the polymerization medium affect dopant incorporation (Cl, S, etc.), PANI’s distribution on the membrane, and the resulting polymer microstructure. Key findings are compared with prior reports on PANI doping to provide an understanding and to highlight novel observations. The signals recorded from the sensor array were processed using Principal Component Analysis (PCA).
2. Materials and Methods
2.1. Chemicals
The ISMs were prepared using PVC, NPOE, TDDMACl and tetrahydrofuran (THF) (Fluka, Selectophore, Switzerland). The deposition of polyaniline layer onto surface of PVC-ISMs was carried out from mixtures prepared from aniline hydrochloride (Fluka, Switzerland), ammonium peroxodisulfate (Lachema, Czech Republic) in the medium of hydrochloric acid mixed with either sodium chloride or sodium sulfate (Lachema, Czech Republic). Standard solutions of bisphosphonates were prepared from sodium salts of trihydrate alendronate (TGI, Japan), ibandronate (TGI, Japan), risedronate (Zentiva, Czech republic), clodronate (Merk, USA) (
Table 1).
As commercial bisphosphonates were purchased Ibandronát Mylan, Risendros, Fosavance, Bonefos.
Table 2 presents the composition of the commercial bisphosphonate-based pharmaceuticals.
2.2. Instrumentation
The wettability of the surface of the PANI-modified ISMs was measured using a Biolin Scientific Attension Theta Flex instrument (Biolin Scientific, UK) and the results were processed using the OneAttension software. Potentiometric measurements were performed using a pH meter (Labio, pHI 04). The electromotive force (EMN) was measured for experimental ISE set against a reference chloride-silver electrode (Ag / AgCl, 3 mol L−1 KCl).
The surface morphology of the PANI-modified PVC membranes was analyzed using a MIRA 3 field emission scanning electron microscope (FE-SEM; TESCAN, Brno, Czech Republic) equipped with a secondary electron detector (Everhart–Thornley type). SEM images were captured at various magnifications (up to 100,000×) with an accelerating voltage of 5 kV, allowing qualitative assessment of surface roughness, particulate formations, and coating uniformity. Elemental composition and distribution were assessed using an energy-dispersive X-ray spectrometer Quantax 200 with an XFlash 6 detector (Bruker Nano GmbH, Berlin, Germany). EDS measurements were performed on the membrane surface, with an estimated interaction depth of approximately 400 nm and lateral interaction radius of about 200 nm. Elemental mapping confirmed the spatial distribution of dopant elements (e.g., Cl, Na, S), while semi-quantitative analysis (% by weight of C, N, O, Na, S, and Cl) was used to compare polymerization conditions.
2.3. Preparation and Modification of Ion-Selective Membrane Surface
Two compositions of ISMs were used in the present study, namely membranes without (membrane 1) and with an anion-exchanger (membrane 2). The content of anion-exchanger (TDDMACl) was 1 wt %, the ratio NPOE:PVC was 1:2 (wt %) for 0.1 g ISMs. The deposition of the PANI-polymeric layer was carried out using the chemical oxidation of aniline hydrochloride (ANI) with 0.08 mol L
-1 (NH
4)
2S
2O
8 in 1.5 mol L
-1 HCl at 0°C in the presence of PVC membrane with or without added inorganic salts (
Table 3).
After deposition of the PANI-layer the ISMs were removed from polymerization mixture, rinsed with 1.5 mol L-1 HCl aqueous solution, treated ultrasonically for 10 min in 1.5 mol L-1 HCl aqueous solution.
2.4. Potentiometric Measurements and Data Processing
Potentiometric response of the experimental ISEs was measured with standard and commercial samples of bisphosphonates. The concentrations of the standard solutions corresponded to those of the respective bisphosphonates in commercial pharmaceutical formulations: c(A) = 5.9 mmol L–1, c(I) = 2.9 mmol L–1, c (R) = 1.9 mmol L–1, c (C) = 5.5 mmol L–1. The electrodes were regenerated consequently 15 min in 10 mmol L–1 NaCl and then in distilled water. Data processing has been performed using principal component analysis (PCA).
3. Results and Discussion
3.1. Lipophilicity of Ion-Selective Membranes and Bisphosphonates
Contact angle measurements provide information on surface wettability, which reflects the lipophilicity of the surface. The main idea was to affect the surface lipophilicity of the PVC-membranes using the various polymerization mixture deposing the PANI layer and varying the membrane composition. Based on the measurement of contact angles (
Table 4), it is evident that the wettability of the surface is influenced by both the membrane formulation and the conditions of PANI polymerization. These findings clearly demonstrate that the lipophilicity of membrane surface was affected by the combined effects of membrane composition and the polymerizable mixture.
The addition of Na2SO4 to the polymerization mixture led to decreased contact angle values (membranes 1 C and 2C). Minor deviations observed between contact angles for ISMs based on TDDMACl and modified under similar conditions indicate the reproducibility of the PANI-layer coating.
According to the calculated distribution coefficients (log D), the lipophilicity of bisphosphonates followed in order: Clodronate (-4.69) < Alendronate (-4.52) < Risedronate (-3.95) ≤ Ibandronate (-1.49) (
Table 1). A higher the logD value corresponds a more lipophilic substance. Among the bisphosphonates, ibandronate is the most lipophilic. The lipophilicity of clodronate and risedronate is comparable.
3.2. SEM and EDS Analysis
The surface morphology and elemental composition of ISMs coated with PANI were investigated using SEM and EDS. These analyses revealed significant differences in the structure and uniformity of the PANI layers, which were strongly influenced by the composition of the underlying membrane, the form of the aniline monomer, and the type of salt present during polymerization. Unmodified blank (PVC:NPOE) membranes exhibited a relatively smooth and featureless surface at the microscale, whereas PANI-coated membranes showed enhanced surface roughness and morphological diversity depending on the polymerization conditions.
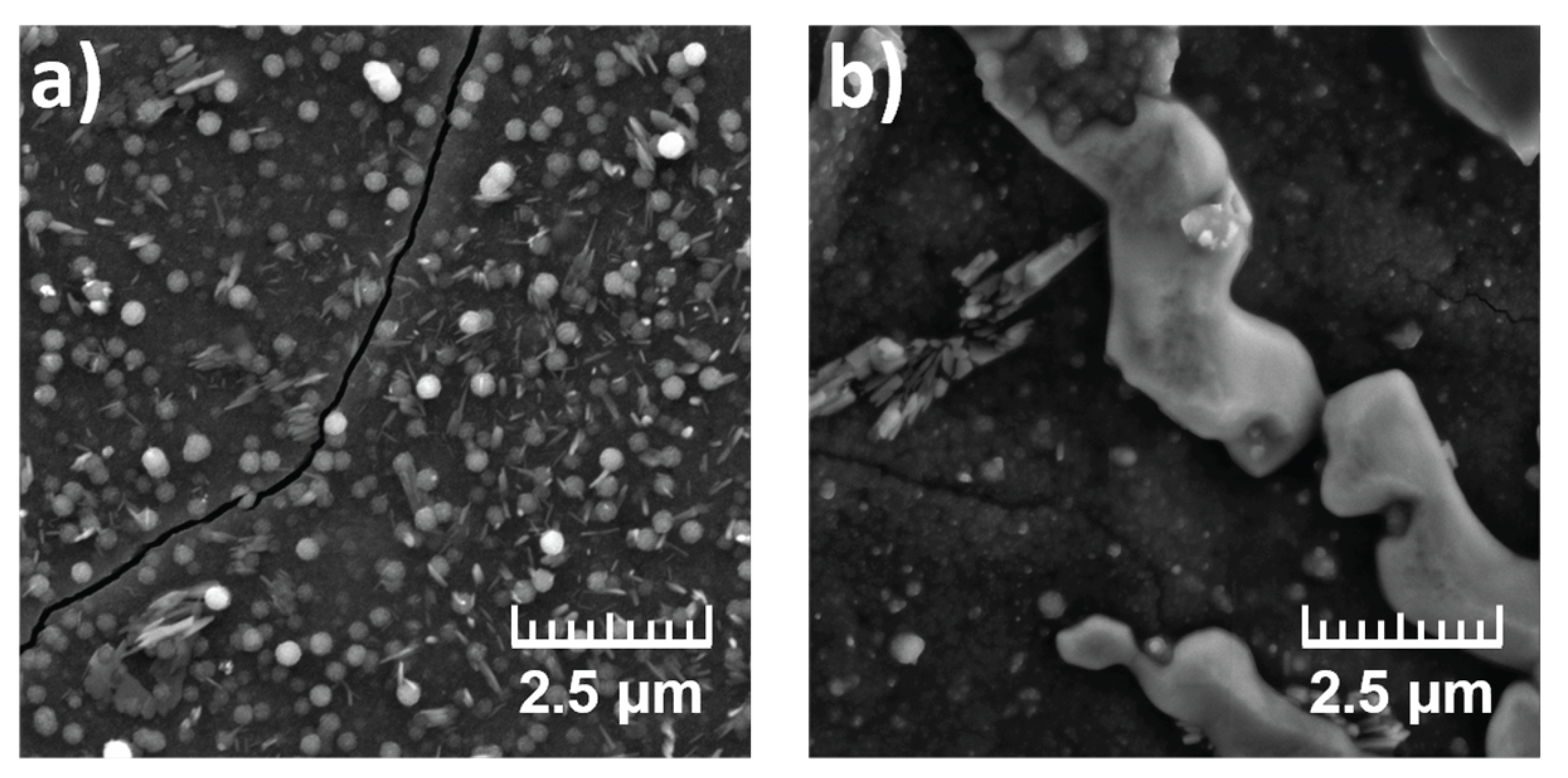
The influence of TDDMACl on PANI morphology was investigated using samples 1A and 2A. As illustrated in
Figure 1, comparison of samples 1A (PVC:NPOE without TDDMACl) and 2A (PVC:NPOE with TDDMACl) highlights the pronounced effect of membrane composition on PANI morphology. Sample 1A exhibited an irregular spherical or globular PANI formations, while sample 2A displayed a more compact layer, indicating improved layer formation. The presence of the cationic additive TDDMACl in 2A sample likely facilitated PANI nucleation and more uniform distribution of the polymer. EDS spectra (
Table 5) supported these observations, showing elevated nitrogen and chlorine signals in 2A, confirming successful PANI deposition and effective Cl doping. In addition, trace signals of sulfur in 1A sample may indicate the presence of residual oxidant (NH
4)
2S
2O
8 that did not fully react and may remain embedded within the polymer layer.
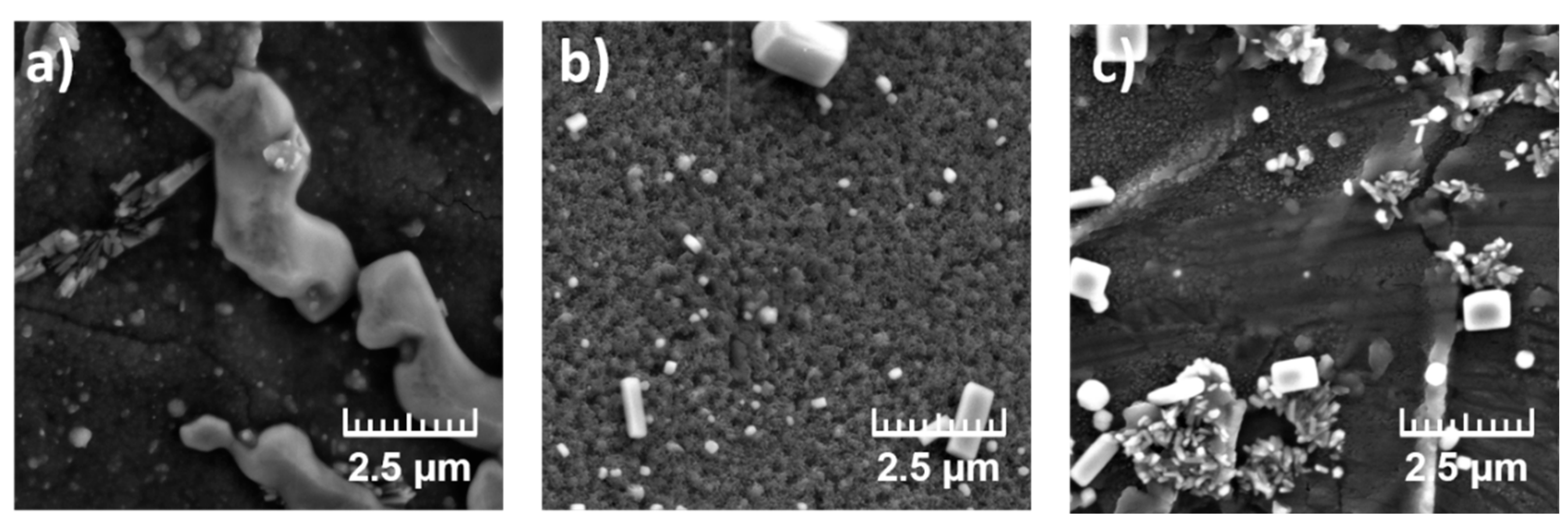
The influence of salt type on PANI morphology was evaluated using membranes 2A, 2B, and 2C, which shared the same PVC:NPOE:TDDMACl composition and were coated with PANI with differing salt additions in polymerization mixture (
Table 3). As shown in
Figure 2, sample 2A (no added salt) exhibited only partial PANI surface coverage, with small, spherical aggregates sparsely distributed across the membrane. In contrast, sample 2B, PANI deposited in the presence of NaCl, revealed a denser, more homogeneous granular morphology, with numerous packed clusters of PANI nodules and increased surface roughness. This suggests that the addition of NaCl, promoting chloride doping, enhances polymer nucleation and particle aggregation. EDS analysis confirmed elevated Cl content in sample 2B, indicating effective incorporation of the dopant and a thicker PANI layer. Sample 2C, PANI deposited in the presence of Na₂SO₄, displayed a distinctly different morphology characterized by a fine, more uniform layer with dispersed microstructures and crystalline domains. Compared to the chloride-doped coatings, the sulfate-doped PANI exhibited reduced clustering and a more evenly distributed morphology, likely due to altered nucleation kinetics in the presence of sulfate ions. These results underscore the critical role of salt and dopant anion type in shaping the final architecture of the PANI layer.
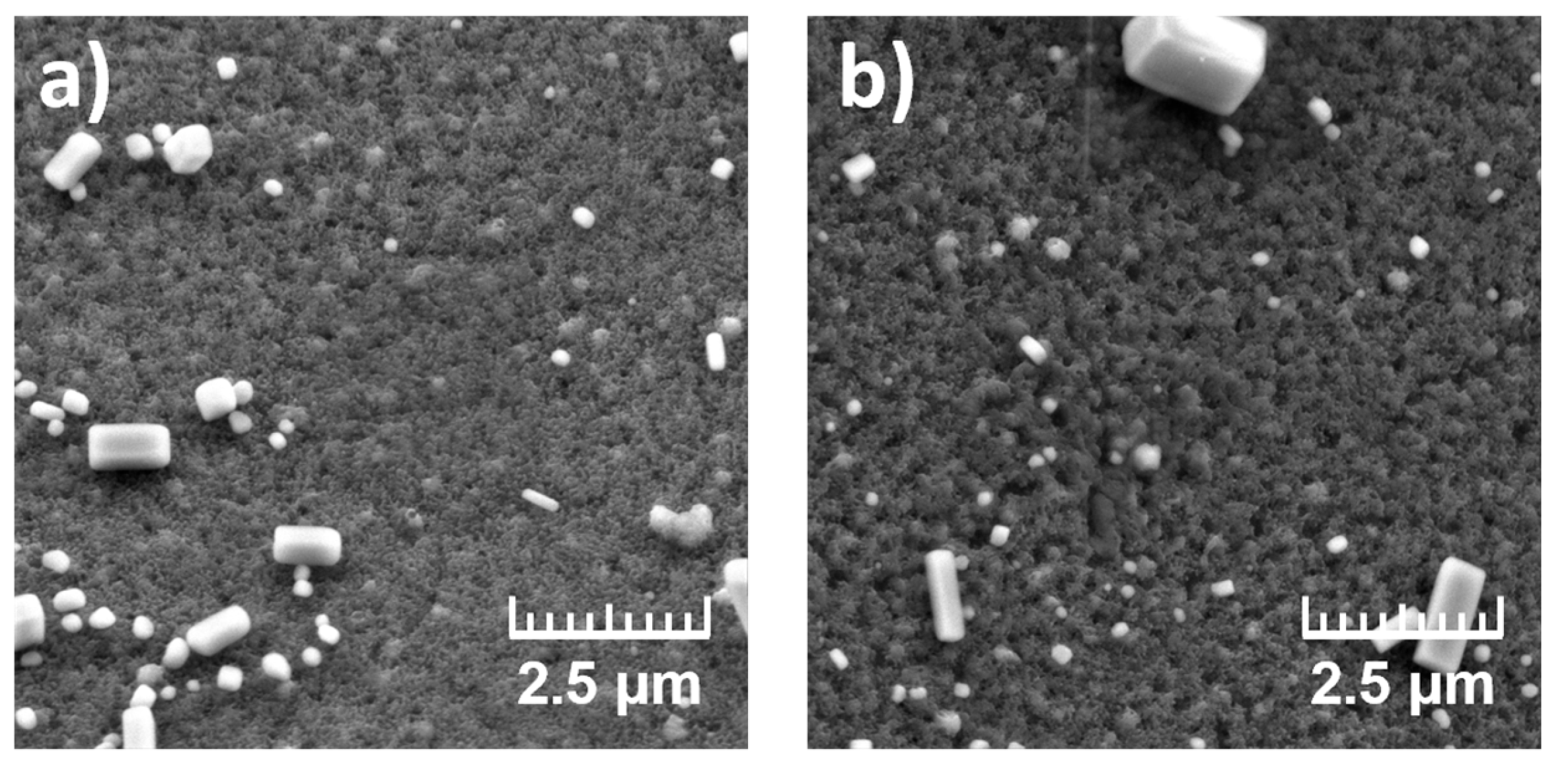
To evaluate the effect of monomer form on PANI morphology, samples 2B (aniline hydrochloride) and 2B* (aniline base with adding equimolar amount HCl), both polymerized in the presence of NaCl, were compared (
Figure 3). Both the PANI-coated surfaces exhibited polymer layers with high surface roughness and comparable structures. Only subtle morphological differences were observed, suggesting that the initial monomer form had limited influence on the overall surface appearance. However, EDS analysis revealed lower chlorine and nitrogen signals in 2B*, which may reflect a reduced degree of Cl doping or PANI incorporation compared to 2B.
Overall, the morphology of PANI coatings was strongly influenced by both the membrane composition and polymerization conditions. The incorporation of TDDMACl into the membrane matrix promoted more uniform PANI coverage, while the addition of NaCl in the polymerization mixture enhanced surface roughness due to improved nucleation and dopant incorporation. Substitution of NaCl with Na2SO4 yielded smoother coatings with finer features, suggesting altered nucleation behavior under sulfate doping. Although the monomer form (aniline base vs. aniline hydrochloride) had only a subtle impact on surface morphology, EDS confirmed reduced nitrogen and chlorine content when aniline base was used, implying lower PANI incorporation or doping efficiency. Together, these results highlight how small variations in membrane formulation and polymerization chemistry can be used to tailor the microstructure and surface properties of PANI-based membranes.
Table 5 summarizes the normalized weight percentage of key elements (C, N, O, Na, S, Cl) detected by EDS on the surface of the reference membrane and the PANI-coated samples prepared under different conditions. The reference blank (PVC:NPOE) membrane is composed primarily of carbon, oxygen and nitrogen and shows only minor traces of other elements. In contrast, all PANI-coated samples exhibit significant dopant-related elements (Cl, and in some cases Na and S), confirming the presence of the PANI polymer and the incorporation of the doping anions from the reaction medium.
By elemental composition, carbon (C) is the most abundant element, followed by nitrogen (N), oxygen (O) and sulfur (S), consistent with the literature data concerning the composition of PANI coatings prepared through chemical polymerization [
16]. The carbon content decreased across PANI-coated membranes (53.6 –69.6 wt%) compared to the reference (84.9 wt%), likely due to accumulation of inorganic residues on the surface. The lowest carbon content was observed in sample 1A.
All PANI-coated samples exhibited elevated nitrogen content (~7.0–10.6 wt%) present in the aniline monomer units compared to the reference (~6.1 wt%), confirming successful PANI deposition. An unusually high oxygen content (14.8 wt%), along with elevated Na (5.8 wt%) and S (4.2 wt%) was found in sample 1A (no added salt), likely due to residual oxidant or sulfate salt retained on the layer. Other samples had moderate O levels (~3.0–7.7 wt%), likely from the underlying membrane or oxidant byproducts.
Chlorine levels were markedly higher in samples 1B (16.6 %) and 2B (23.7 %) both modified in the presence of NaCl, indicating substantial retention of chloride species, further enhanced by the the anion-exchanger TDDMACl. Samples deposited in the presence of Na2SO4 (1C: 0.4 wt% and 2C: 1.4 wt%) showed higher sulfur content compared to the uncoated samples (Sample 1: 0.1 wt% and Sample 2: 0.0 wt%). The better hydrated anions, such as sulfates, can provide more efficient hydration of the amine nitrogens of the PANI chains and, as consequence, can improve incorporation/doping through the PANI layer [
13,
17,
18]. In conclusion, EDS confirmed that both the choice of dopant and polymerization conditions significantly affect the elemental composition of the PANI layers.
3.3. Potentiometric Measurements
A priori, the discrimination of the tested pharmaceutical should be enabled by the polymeric layer deposited onto the plasticized PVC membrane. To achieve selective discrimination among pharmaceuticals, the PANI layer was deposited using different procedures, including the presence of different inorganic salts in the polymerization mixture.
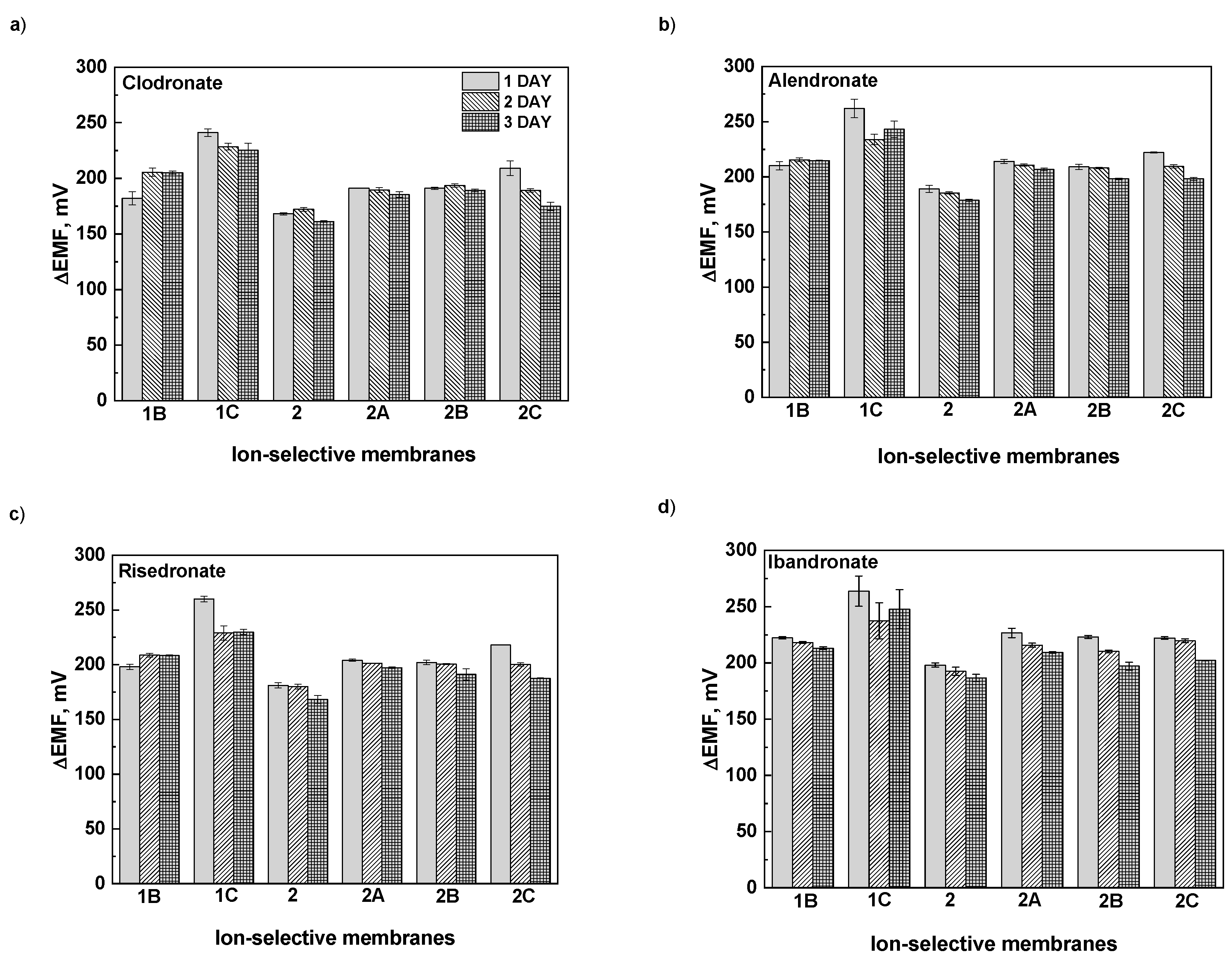
In the next step, the potentiometric responses of the prepared ISMs were measured and their signal stability overtime was evaluated (
Figure 4). For this experiment, ISMs with different surface properties and varying active components, namely 1B, 1C (blank, without ion-exchanger) and 2, 2A, 2B, 2C (with ion-exchanger) were selected. The concentration of bisphosphonate solutions corresponded to ones in commercials pharmaceuticals. Obviously, the contact of the ISMs in the sensor array with bisphosphonates cause an ion-exchange process onto phase boundary between the sample and PANI-modified surface. To ensure stable work of the sensor array, a regeneration procedure was implemented as follow. Between a series of repeated measurements, the ISMs were washed 1.0 mmol L
-1 NaCl solution. The concentration of solution for regeneration was selected in order to be high enough to allow such a recovery for a short time but not to be so high as to mask the response of the ions in the sample.
It was found that the stability of the potentiometric signal was affected with both the composition of ISM/PANI-layer and the kind of measured bisphosphonate. The lowest values of RSD measured for 3 days were attained for ISM based on anion-exchanger: Clodronate (0.4 – 1.1 %), Alendronate (0,3 – 1,3%), Risedronate (0.1 – 1.0%) and Ibandronate (0.2 – 1.5%).
For ISMs based on anion-exchanger, the PANI layer deposited in absence (membrane 2A) and the presence of NaCl (membrane 2B) stabilized the potentiometric signal to bisphosphonates having log D < -1.49 (namely, Clodronate, Alendronate, Risedronate). It should be noted that the stabilized effect on the potentiometric response was observed for blank ISMs coated with PANI-layer in the presence of NaCl (membrane 1B), despite the absence of anion-exchanger. The PANI layer formed under these conditions (1B) is likely relatively thick, potentially enhancing ion-exchange capacity. The observed improvement is possibly explained by combination of number of factors, in particularly, the properties of the deposited PANI-layer (ion-exchange properties, quality and composition) and tested bisphosphonate (structure, lipophilicity).
Recently, it has been noted that the anion transport through the PANI on its hydration which is influenced by the structure of the anions present in the surrounding aqueous medium. The better hydrated anions possessing stronger water-structure-perturbing capacity can provide more efficient hydration of the amine nitrogen’s of the PANI chains and, as consequence, can improve the water permeability through the PANI layer [
13,
17,
18]. Comparing of the potentiometric signals of blank ISMs coated with the PANI layer prepared in the presence of Na2SO4 (membrane 1C) showed the effect of lipophilicity of bisphosphonate. The more reproducible signal was obtained for more hydrophilic bisphosphonate (Clodronate, log D = –4.69) that is able to hydrate the PANI chains.
3.4. Chemometric Processing
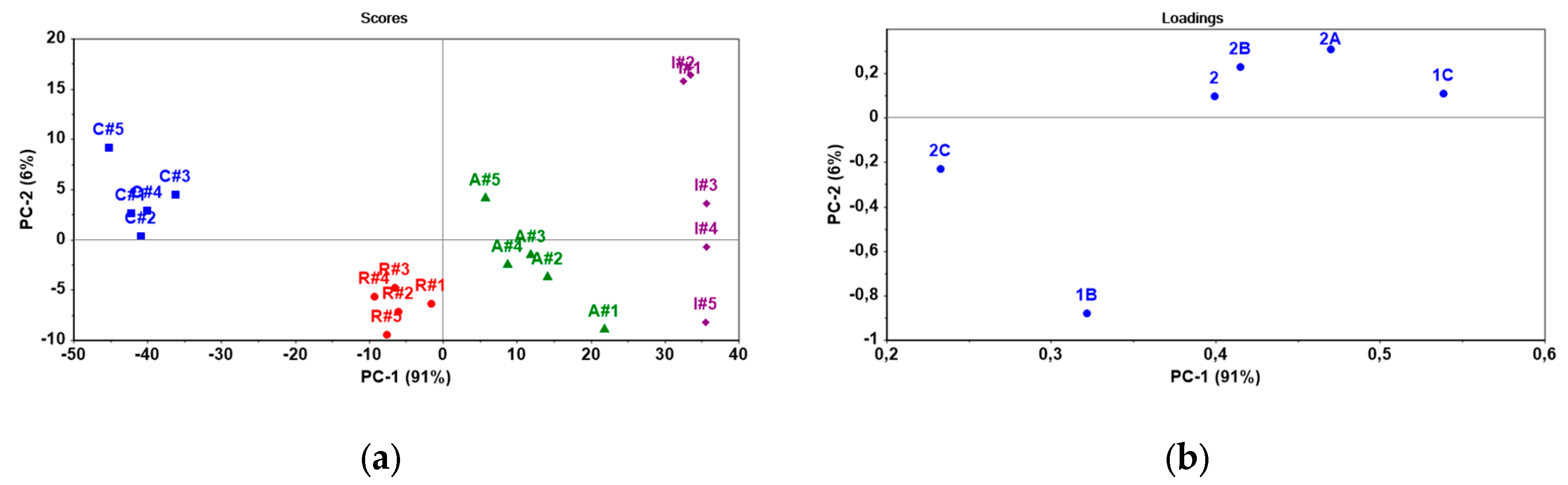
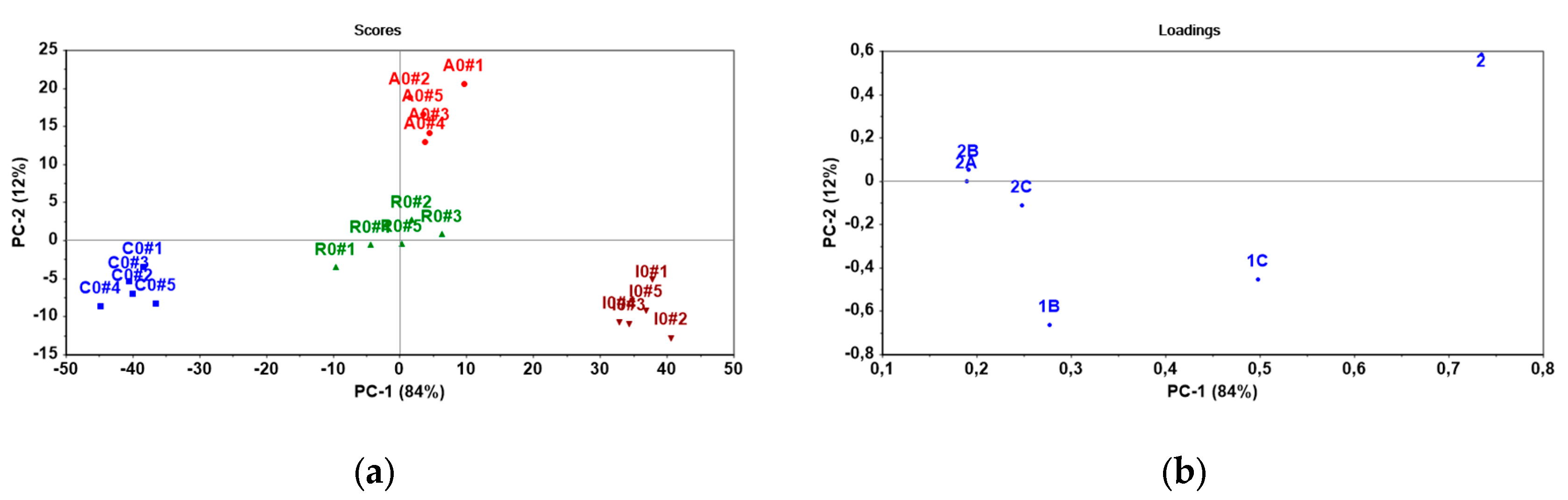
The sensor array consisted of 6 electrodes based on ISMs modified with PANI layers: 1B, 1C, 2, 2A, 2B and 2C. It was applied to discriminate between bisphosphonates differing in lipophilicity (
Table 1). It was tested the pure bisphosphonates as standards and commercial formulations (
Table 2). The potentiometric responses of the sensor array were processed by means of Principal Component Analysis (PCA), a multivariate technique commonly used for dimensionality reduction and pattern recognition in sensor data analysis [
19]. Here, the first two principal components (PC-1 and PC-2) were retained for interpretation as they capture the largest portion of variance in the data (
Figure 5 and
Figure 6).
All bisphosphonate standards formed distinct clusters, indicating differences in lipophilicity between individual compounds. The PC-1 and PC-2 describe 91 % and 6 % of variability, respectively (
Figure 5). The variability PC-1 that is responsible for the separation, is predominantly influenced by the response of ISMs 1C (θ = 53.0°), 2 (θ = 45.3°), 2A (θ = 44.4°) and 2B (θ = 41.9°). In the case of the PC-2, an important contribution is observed from ISMs 1B (θ = 85.1°). The potentiometric signal of a membrane should be regulated by the ion-exchange reaction occurring on the phase boundary membrane/solution. It is possible to propose that the observed separation results from combination of both the surface properties of ISM and the amount of ion-exchange sites in the PANI layer. The ion-exchange sites can provide TDDMACl as active component in membrane matrix and chloride as dopant of the PANI layer. Actually, a higher content of exchangable chloride was determined in the PANI layer of ISMs 1C (Cl: 14.6 wt%), 2A (Cl: 17.1 wt%) and 2B (Cl: 23.7 wt%) that influenced PC-1. In the case of 1A ISM, the PANI layer provided the high content of chloride (16.6 wt%), but its insufficient hydrophilicity (θ = 85.1°) may explain its limited contribution to the discrimination of bisphosphonates.
Further, the experimental sensor array was used to differentiate the commercial bisphosphonate-based pharmaceuticals (
Table 2 and
Figure 6). According to PC-1, pharmaceuticals presenting less and more lipophilic bisphosphonates, namely clodronate (log D = –4.69) and ibandronate (log D=–1.49) differ significantly, which is in agreement with the signal patterns obtained for their standards (
Figure 5a and
Figure 6a). As can be seen from
Figure 6a, risedronate (log D = –3.95) is presented middle position between less lipophilic clodronate (log D = –4.69) and more lipophilic ibandronate (log D=–1.49). From the point of view PC-1 (84% of variability), a considerable effect is observed from ISMs 2 (θ = 45.3°) and 1C (θ = 53.0°), while a weaker effect is from other ISMs: 2A (θ = 44.4°), 2B (θ = 41.9°) and 2C (θ = 29.0°). It is important to notice that potentiometric sensors respond towards ionized substances and thus the chemical composition of the employed ingredients and matrix will have immediate impact on the sensor responses and pattern recognition. The commercial pharmaceuticals include both lipophilic and hydrophilic ionic additives such as magnesium stearate and croscarmellose sodium that may influence the contributions of ISMs in discrimination of bisphosphonates. We can assume that selected combination of ISMs is suitable to distinguish lipophilicity of bisphosphonates in real samples as well.
4. Conclusions
This study presents a systematic investigation of how membrane composition, dopant type, and monomer form influence the surface morphology and elemental composition of PANI coatings on PVC:NPOE-based ISMs. Through combined SEM and EDS analysis, we demonstrated that both the presence of the cationic additive TDDMACl and the specific deposition conditions, including the type of salt, play a critical role in determining the structure and chemical makeup of the resulting PANI layers.
Three principal trends in surface morphology were observed. First, the inclusion of TDDMACl anion exchanger in the membrane modified PANI layer, producing more uniform coating compared to membranes without TDDMACl, which showed globular aggregates. Second, the type of salt used during deposition had a clear impact: NaCl promoted the formation of thick layers with high surface roughness and elevated chlorine content, while Na2SO4 led to finer, more homogeneous layers with detectable sulfur incorporation. Third, deposition with aniline base as opposed to fully protonated aniline hydrochloride resulted in comparable overall roughness but subtle morphological differences.
In conclusion, the interplay between membrane formulation (PVC:NPOE vs. PVC:NPOE:TDDMACl), dopant type (Cl- vs. SO42-), and monomer form (aniline or aniline hydrochloride) allows for controlled tuning of PANI layer morphology and composition. These structural differences are expected to influence functional performance. To demonstrate the analytical utility of these modifications, a potentiometric sensor array comprising PANI-modified ISMs was applied for classification of bisphosphonates differing in lipophilicity. Principal Component Analysis of the sensor responses revealed that electrodes with distinct membrane morphologies and compositions contributed differentially to the discrimination of both standard and commercial bisphosphonates. In particular, PC1 correlated with bisphosphonate lipophilicity, showing clear separation of analytes, and supporting the potential of membrane engineering for enhanced chemical sensing.
Future work could build on these findings by examining how these morphological and compositional differences translate to performance metrics (e.g. ion transport, sensor response) and by exploring other dopants or polymerization methods to further tailor PANI-based membranes.
Author Contributions
Conceptualization, T.V.S.; methodology, T.V.S.; investigation, A.S.C. and J.O.; resources, T.V.S and M.V.; data curation, T.V.S., A.S.C. and J.O.; writing—original draft preparation, T.V.S. and J.O.; writing—review and editing, T.V.S. and J.O.; visualization, T.V.S. and J.O.; supervision, T.V.S.; project administration, M.V.; funding acquisition, T.V.S. and M.V.
Funding
This work was supported by Institutional Resources (Department of Analytical Chemistry, UCT Prague, CZ; Grant Number: 402850061) and by OP JAC financed by ESIF and the MEYS (Project No. SENDISO - CZ.02.01.01/00/22_008/0004596)
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Dr. M. Člupek for discussion PCA analysis results. This work was supported by Institutional Resources (Department of Analytical Chemistry, UCT Prague, CZ; Grant Number: 402850061) and by OP JAC financed by ESIF and the MEYS (Project No. SENDISO - CZ.02.01.01/00/22_008/0004596).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PVC |
poly(vinyl chloride) |
| ISMs |
ion-selective membranes |
| PANI |
polyaniline |
| PCA |
principal component analysis |
| NPOE |
2-nitrophenyl octhyl ether |
| TDDMACl |
tridodecylmethylammonium chloride |
| SEM |
scanning electron microscopy |
| EDS |
energy-dispersive X-ray spectroscopy |
References
- Arnott, J.A.; Planey, S.L. The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discov. 2012, 7(10), 863-875. [CrossRef]
- Watts, N.B.; Diab, D.L. Long-term use of bisphosphonates in osteoporosis. J. Clin. Endocrinol. Metab. 2010, 95 (4), 1555-1565. [CrossRef]
- Kimmel, D.B. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J. Dent. Res. 2007, 86(11), 1022-1033. [CrossRef]
- Quancard, J.; Bach, A.; Borsari, C.; Craft, R.; Gnamm, C.; Guéret, S.M.; Hartung, I.V.; Koolman, H.F.; Laufer, S.; Lepri, S.; Messinger, J.; Ritter, K.; Sbardella, G.; Lopez, A.U.; Willwacher, M.K.; Cox, B.; Young, R.J. The European Federation for Medicinal Chemistry and Chemical Biology (EFMC) Best Practice Initiative: Hit to Lead. ChemMedChem 2025, 20(8), e202400931. [CrossRef]
- Gospodinova, N.P.; Ivanov, D.A.; Anokhin, D.V.; Mihai, I.; Vidal, L.; Brun, S.; Romanova, J.; Tadjer, A. Unprecedented route to ordered polyaniline: direct synthesis of highly crystalline fibrillar films with strong π-π stacking alignment. Macromol. Rapid Commun. 2009, 30, 29-33. [CrossRef]
- Golba, S.; Popczyk, M.; Miga, S.; Jurek-Suliga, J.; Zubko, M.; Kubisztal, J.; Balin, K. Impact of acidity profile on nascent polyaniline in the modified rapid mixing process—material electrical conductivity and morphological study. Materials 2020, 13, 5108. [CrossRef]
- Ahmat, D.O.; Jawad, Z.A.; Khosravi, V.; Yeap, S.P. Acidity’s impact on yield, morphological structure, and surface functionalities of polyaniline synthesised via oxidative polymerisation. J. Phys. Sci. 2023, 34(3), 67-79. [CrossRef]
- Wang, R.; Jing, Y. The effect of inorganic salt on the morphology and nucleation of polyaniline nanofibers synthesized via self-assembly. Des Monomers Polym. 2023, 26(1), 45-53. PMID: 36684708; PMCID: PMC9858426. [CrossRef]
- Gupta, V.K.; Arunima, N.; Singhal, B.; Agarwal S. Recent advances on potentiometric membrane sensors for pharmaceutical analysis. Comb. Chem. High Throughput Screen. 2011, 14(4), 284-302. PMID: 21375501. [CrossRef]
- Isildak, Ö.; Özbek, O. Application of potentiometric sensors in real samples. Crit. Rev. Anal. Chem. 2020, 51(3), 218-231. [CrossRef]
- Özbek, O.; Berkel, C.; Isildak, Ö. Applications of potentiometric sensors for the determination of drug molecules in biological samples. Crit. Rev. Anal. Chem.2022, 52(4), 768-779. Epub 2020 Sep 29. PMID: 32991203. [CrossRef]
- Ciosek-Skibińska, P.; Cal, K.; Zakowiecki, D.; Lenik, J. Potentiometric electronic tongue for the evaluation of multiple-unit pellet sprinkle formulations of rosuvastatin calcium. Materials (Basel). 2024, 17(20), 5016. PMID: 39459720; PMCID: PMC11509238. [CrossRef]
- Shishkanova, T.V.; Sykora, D.; Vinsova, H.; Kral, V.; Mihai, I., Gospodinova, N.P. A novel way to improve sulfate recognition. Electroanalysis 2009, 21 (17-18), 2010-2013. [CrossRef]
- Shishkanova, T.V.; Řezanková, K.; Řezanka P. Influence of surface properties on the deposition of a polyaniline film and detection of tumor markers. Chem. Pap. 2017, 71 (2), 489-494. [CrossRef]
- Gospodinova, N.P.; Ivanov, D.A.; Anokhin, D.V.; Mihai, I.; Vidal, L.; Brun, S.; Romanova, J.; Tadjer, A. Unprecedented route to ordered polyaniline: direct synthesis of highly crystalline fibrillar films with strong π-π stacking alignment. Macromol. Rapid Commun. 2009, 30, 29-33. [CrossRef]
- Anand, P.B.; Hasna, K.; Anilkumar, K.M.; Jayalekshmi, S. On the structural and optical properties of gold–polyaniline nanocomposite synthesized via a novel route. Polym Int 2012, 61 (12), 1733-1738. [CrossRef]
- Shishkanova, T. V.; Sapurina, I.; Stejskal, J.; Král, V.; Volf, R. Ion-selective electrodes: polyaniline modification and anion recognition. Anal. Chim. Acta 2005, 553(1-2), 160-168. [CrossRef]
- Shishkanova, T.V.; Rezacova, A.; Kral, V.; Gospodinova, N.P. Influence of polyaniline on the potentiometric determination of risedronate with ion-selective membranes. Anal. Methods 2010, 2 (10), 1614-1617. [CrossRef]
- Ciosek, P.; Wróblewski, W. Sensor arrays for liquid sensing - electronic tongue systems. Analyst 2007, 132, 963–978. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).