1. Introduction
The Dendrocoelidae Hallez, 1892 is an important family of triclad freshwater worms belonging to suborder Continenticola [
1]. The main characteristic of this family is the alternative disposition of the longitudinal and circular muscular layers in the pharynx [
1,
2]. The family Dendrocoelidae consists of 22 genera with a large distribution, out of which only
Dendrocoelum and
Polycladodes are present in the Romanian fauna [
1,
3]. The knowledge on European Dendrocoelidae began with the description of
Dendrocoelum lacteum by Müller in 1774. A survey of the available literature [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11] brought the current count, up to 100 Palearctic species. The species once described, they were included into a system of supraspecific taxa, either at the rank of genus or subgenus:
Dendrocoelum,
Dendrocoelides,
Eudendrocoelum,
Neodendrocoelum,
Bolbodendrocoelum,
Paradendrocoelum,
Apodendrocoelum,
Palaeodendrocoelum,
Polycladodes (see paragraph 3). The most recent literature recognizes only the genera
Dendrocoelum and
Polycladodes [
1,
11],
Dendrocoelum s.l. being the reunion of the remaining taxa. In this paper, I use the term Dendrocoelidae for the only two genera present in the Romanian fauna,
Dendrocoelum and
Polycladodes.
The Dendrocoelidae of Romania were studied by several scientists, especially from a faunal and systematic point of view. The faunal approach was served by de Beauchamp, del Papa, Codreanu, Codreanu and Balcesco, Stocchino and coauthors [
3,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21] who identified and described new species. The systematics of the whole Palearctic Dendrocoelidae have been a matter of prolonged debate, with the revisions of many zoologists, for instance, Komárek, Stanković and Komárek, Kenk, de Beauchamp, Reisinger and Gourbault [
5]. Despite all the morphologically based revisions, the phylogenetic systematic remains unresolved, but it is expected to be continuously reshaped by modern tools as already prospected elsewhere [
31]. Significant research and research programmes using modern tools (barcoding, multilocus phylogenetic, genomic analyses, etc.) as well combined molecular and morphological analyses, explored and unravelled many conundrums of the Tricladida taxonomic biodiversity, evolution and evolutionary dynamics, speciation patterns, systematic, phylogeny at lower and higher level [
11,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37].
The aim of this paper is a general view on the Romanian Dendrocoelidae as part of the European/Palearctic group, with emphasis on the historical contribution of Radu Codreanu and Doina Balcesco. Their effort to learn about this group of worms is part of the historical effort to learn about the entire European/Palearctic group. Therefore, even though only the genera Dendrocoelum and Polycladodes are now recognized, the analysis and discussions on former Dendrocoelum s.l. divisions are considered by author, mandatory for this paper.
2. Methodology
The review of the literature was done using various databases: Romanian libraries, MNHN library (Muséum National d Histoire Naturelle Paris), Google and Google Scholar, Researchgate, BHL (Biodiversity Heritage Library), EurekaMag, NHBS, etc. Key words related to the topic of the article were used: Dendrocoelidae, Dendrocoelum species, systematic, planarian molecular phylogeny, etc. The Refference final list of all accessed articles was the starting point for new search, by article and journal title. Some articles were kindly provided to me by group specialists and colleagues.
3. Species Inventory
The species inventory—
Table 1—follows, in part, the system of Sluys et al. for the genera
Dendrocoelum and
Polycladodes, and Gourbault for the
Dendrocoelum s.l. subgenera [
1,
2]
The deposition of the specimen-types of the species authored by Codreanu and Balcesco is not recorded in any publication, suggesting loss or private property, thus, no material for further comparative studies. The search for the histological slides could require significant effort. This should be the subject of a separate study that may reveal either the type-specimens or the need of a recollection strategy and protocols for neotype designation.
4. The Classical Phylogenetic System, the Morphological Types and Taxa
With the increase in the number of species, the systematics of Dendrocoelidae has become complicated and underwent several changes, according to the point of view of different authors. Historically, species have been grouped into several morphological types defined by sets of morphological characters, the morphological types corresponding to so many supraspecific taxa, at the genus or subgenus rank. The taxa morphologically established were:
Dendrocoelum (s.l. and s.str.),
Polycladodes,
Dendrocoelides,
Eudendrocoelum,
Neodendrocoelum,
Paradendrocoelum,
Bolbodendrocoelum,
Apodendrocoelum and
Palaeodendrocoelum. The separation between them can be better discussed and understood from a historical perspective, as presented by Gourbault [
2].
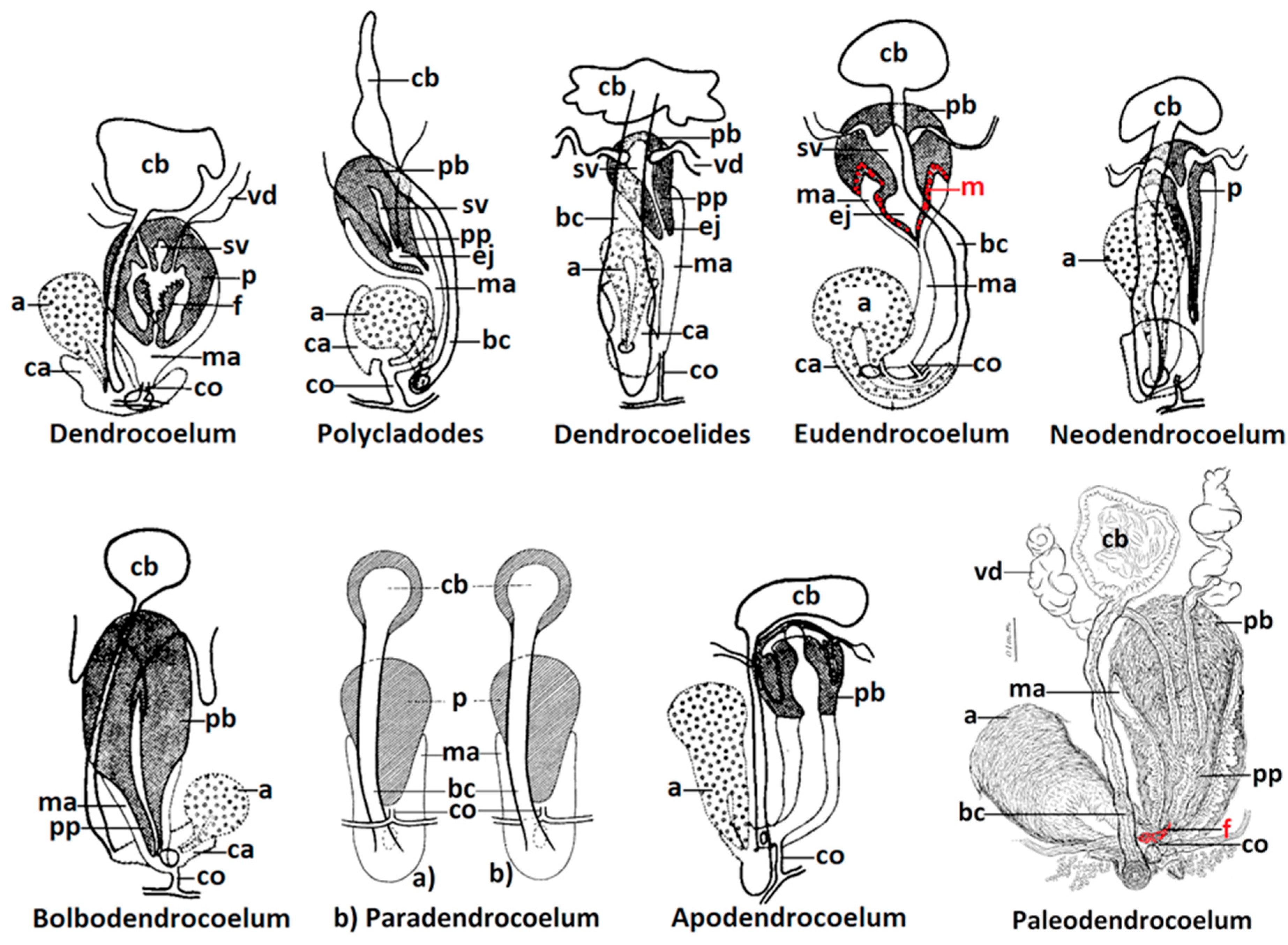
Dendrocoelum
This was created at the genus rank by Oersted, 1844, for the species
lacteum which is the type species. The systematics of Gourbault recognises the genus
Dendrocoelum (
Dendrocoelum s.l.) separated into eight subgenera including the subgenus
Dendrocoelum (
Dendrocoelum s. str.) with two species: the occulated
D. lacteum and the anophthalmic
D. infernale. [
2]. The distinctive character of the subgenus
Dendrocoelum (
Dendrocoelum s. str.) is the presence of a true flagellum which is defined as a long structure with longitudinal muscles covered by a vacuolated epithelium, derived from and attached to the inner epithelium of the penial papilla; it can be invaginated (inverted) or everted out of the seminal vesicle [
5,
9]. Other characters include: a globular penis with a large seminal vesicle, a short male atrium, the communication between the male atrium and the common atrium through an orifice and the opening of the common oviduct into the male atrium (
Figure 1)
Polycladodes
Polycladodes was created by Steinmann in 1910, at the genus level for the pluri-occulated species
Polycladodes alba (type species). The distinctive character of
Polycladodes is a supplementary longitudinal muscular layer into the external area of the pharynx, a character identifying the current genus rank [
1]. Other morphological characters extracted from Gourbault for
Polycladodes are the lack of a flagellum, a long and tubular male atrium, the communication between the male atrium and the common atrium through a short duct with no sphincter, the opening of the common oviduct into a short area at the meeting place of the male atrium and the common atrium [
2], (
Figure 1). Gourbault included four species in
Polycladodes: the pluri-occulated
P. album, with 15-30 eyes and the anophthalmic
P. caecum,
P. voinovi and
P. affine [
2].
Dendrocoelides
This taxon was created by de Beauchamp in 1919, at the genus level, for the species
Dendrocoelides regnardi. The distinctive character is the lack of flagellum
, opposed to that in
Dendrocoelum s.str. (
Figure 1).
Gourbault considered
Dendrocoelides at the subgenus rank and included in it 28 species out of which only two are occulated—
Dl. lescherae in France and
Dl. vaillanti in Algeria. Four species of this group (
Dl. lescherae, Dl. mrazeki panonicum, Dl. stenophalus and
Dl. racovitzai) possess invaginable penis papilla, that is a flagelliform papilla, thus, showing affinity with
Dendrocoelum [
2].
Eudendrocoelum
Eudendrocoelum was created by Komarek in 1926, at the genus level, to include three species
lacteum, infernale and
subterraneum. The distinctive character is the presence of a supplementary muscular circular layer starting at the base of the penis bulb, which thins along the wall of the penis papilla (
Figure 1). In 1932, de Beauchamp separated the species
lacteum and
infernale into the genus
Dendrocoelum. The shift of these two species from one taxon to another shows a clear intersection of
Eudendrocoelum with
Dendrocoelum. Gourbault, with respect to the flagellum, included in
Eudendrocoelum a mixture of 10 species: (i) flagellum absent in some species, showing affinity with
Dendrocoelides and (ii) a flagelliform structure, invaginated or not, present in some species:
E. sollaudi, remyi, gineti and
tubuliferum, showing affinity with
Dendrocoelum. Most species are unpigmented and anophthalmic. Only one species,
D. (E.) parvioculatum, has both eyes and a demi-invaginated penis papilla [
2].
Neodendrocoelum
This taxon was created by Komárek in 1926. The diagnosis given by Gourbault (1972) for
Neodendrocoelum at the subgenus rank includes the great development of penis and adenodactyl (
Figure 1), the histology of the oviducts and the glands of the genital pore. Gourbault included in
Neodendrocoelum 13 occulated species, most of them pigmented with a species-specific dorsal pattern, distributed mainly in former Yugoslavia, in Lake Ohrid and tributaries [
2]. Absent in Romania.
Bolbodendrocoelum
This taxon was created by de Beauchamp in 1932, at the subgenus level for the anophthalmic species
agile [
2]. Monospecific. The distinctive character is the overdeveloped penis bulb (
Figure 1). Absent in Romania.
Paradendrocoelum
This subgenus was created by Kenk in 1930 for the species
cavaticum, based on the position of the oviducts between the male atrium and the bursal canal (
Figure 1). Six hypogeic, anophthalmic species—
cavaticum,
spelaeum,
infernale,
tubuliferum,
hankói and
carpathicum were assigned by Kenk to
Paradendrocoelum [
38], one Romanian species—
P. alexandrinae was also assigned by Codreanu & Balcesco to
Paradendrocoelum [
21].
Apodendrocoelum
Apodendrocoelum was created by de Beauchamp in 1932, at the subgenus rank. The distinctive character is the greatly reduced penis papilla (
Figure 1).
Apodendrocoelum contains four anophthalmic species:
A. brachyphallus,
A. lipophallus in Romania,
A. puteale, and one species with insufficient diagnosis, in Germany [
2].
Palaeodendrocoelum
This genus was created by Codreanu in 1949-1950, based on external morphology (pigmentation, eyes, adhesive organ) combined with genital characters. The diagnosis given by Codreanu [
17] included: small size 9 mm x 1 mm, specific pigmentation, numerous eyes [
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31], with a particular disposition, differentiated and infra-nucleated adhesive organ, a non-invaginated flagellum, male and common atrium completely separated and the position of the oviducts between the male atrium and the bursal canal showing affinity with
Paradendrocoelum (
Figure 1).
A group of interest is represented by the species of Lake Ohrid. Kenk presented a group of 17 occulated
Dendrocoelum species, authored by Müller, Stanković, Stanković & Komárek, and Kenk [
5]. Of these species, only two bear a true flagellum (
D. lacteum, D. cruciferum); most of the others have various types of penial papilla—inversible, invertible, pseudoflagellum, etc. [
5]. Kenk showed the penis papilla variability which he attributed to physiological state, fixatives or even intrapopulational variability. Some of these species have a circular muscular layer at the base of the penis bulb (the wall of the penis papilla) [
5], showing affinity with
Eudendrocoelum. Nearly 10 of these 17 species were considered as belonging to
Neodendrocoelum by Gourbault, including
D. cruciferum, a species with a true flagellum [
2].
5. The Morphological Characters of Dendrocoelidae and Their Phylogenetic Value
The characters used in the old and more recent literature (for species diagnosis and for the system) concern both external and internal morphology.
External morphology considers body pigmentation, eyes and adhesive organs. Internal morphology concerns a multitude of characters, some of them already presented: pharynx musculature; position of the oviducts; the dorsal/ventral position of the testes; the communication of the vas deferens with the penis, the adenodactyl; the characteristics of the copulatory apparatus—location, the penis bulb (arrangement of the musculature, degree of development), the shape and size of the seminal vesicle, the type of flagellum when present; the degree and way of separation of the male and common atria; the bursal canal; the size ratio penis: adenodactyl; the development and disposition of the eosinophilic glands, etc.
The phylogenetic value of the morphological characters is visible especially when they are correlated with other aspects, for instance, the paleogeographic conditions and the geological ‘‘moment’’ of appearance, that is their oldness. For the systematic phylogeneticists, this is a complex work, requiring the establishment of the synapomorphies and autapomorphies [
39,
40].
The taxonomic value of some characters:
1) The position of the oviducts between the male atrium and the bursal canal was used to establish the taxon Paradendrocoelum.
The taxonomic value of this character is seen differently by different zoologists:
A) as invalid generic character.
Gourbault considered that the genus
Paradendrocoelum cannot be preserved because Codreanu showed evidence of the variability of this character “dans une même espèce” [
2], but no information about the species, journal and year of publication was given.
De Beauchamp considered the character as a secondary one, later in Dendrocoelidae evolution; thus, only a specific character and not a generic one [
17].
B) as a valid generic character.
For two reasons, Codreanu considered this character to be valuable:
a) this character is present in a group of hypogeic European species including
cavaticum sollaudi, hankoi, spelaeum and one unnamed sp. (affinities between species), in opposition to the group
sphaerophalus, carpathicum, tubuliferum and
infernale, species considered of evident different origins [
17]
b) as this character is present in all the Holarctic genera
Dendrocoelopsis,
Amyadenium,
Miodendrocoelum etc., it has the meaning of a primitive (primary) character, first in Dendrocoelidae evolution [
17].
2) The taxonomic value of the eyes and the biogeographical context (
Figure 2 Map)
Hypotheses and arguments:
A) Kenk and de Beauchamp considered that the eyes have no generic value; they disappear in the hypogeic species which are closely related. The lack of the eyes is seen as a regressive adaptative convergence in relation to subterranean life [
17].
Examples of related occulated and anophthalmic species belonging to the same genus are given to support this theory: the pluri-occulated
Polycladodes alba and the anophthalmic
Polycladodes affine [
17] and
Polycladodes voinovi; the occulated
Dendrocoelum lacteum and the anophthalmic
Dendrocoelum infernale [
17]; the pluri-occulated
Palaeodendrocoelum romanodanubialis and the anophthalmic
Palaeodendrocoelum getticum.
B) A second group of theories gives generic value to eyes. Species of Tertiary origin, relics in the actual fauna are occulated; the anophthalmic
Dendrocoelides are the result of the Quaternary glaciation. These theories are supported by Beclemișev, Stanković and Codreanu [
17].
a) The severe Glaciation in N and Central Europe was the driving force for speciation. Most epigeic Dendrocoelidae disappeared. Some species underwent a subterranean migration followed by adaptative loss of eyes. It is the case of the Romanian
Dendrocoelides anophthalmic species. After migration into the subterranean and adaptative eye loss, a process of diversification followed at the level of the copulatory apparatus (evolutive divergences), giving numerous anophthalmic endemic species, distinct at the level of genitalia [
18].
Figure 2.
The geographical distribution of the actual Dendrocoelidae and the biogeographical (paleo-geographical) context supporting the hypothesis of Dendrocoelides evolution and diversification, determined by the Quaternary Glaciation.
Figure 2.
The geographical distribution of the actual Dendrocoelidae and the biogeographical (paleo-geographical) context supporting the hypothesis of Dendrocoelides evolution and diversification, determined by the Quaternary Glaciation.
b) Attenuated Glaciation in S Europe determined the survival of some species (Tertiary species) in lakes, springs and rivers, as tertiary relics.
Palaeodendrocoelum romanodanubiale is a relic.
Palaeodendrocoelum has the genus rank [
17].
The age/oldness of the morphological characters in geological time is essential in tracing phylogenies. Regarding the eyes and copulatory apparatus, one question arises: which is the primary character (the primitive character), the eyes or the copulatory apparatus? This question generated two hypotheses:
Eyes first?
Codreanu considered the eyes as the primitive character [
17], while the lack of eyes means the loss of this character. In this situation, the genus/subgenus
Dendrocoelum s.str. should be considered the starting point in evolution. Such an evolutionary pattern involves the loss of the penial flagellum (
Dendrocoelum s.str. has a more complex copulatory apparatus, by the presence of the flagellum).
Copulatory apparatus first?
Gourbault considered only the penis important to establish the systematic position [
2]. The subgenus
Dendrocoelides (most species anophthalmic), having the simplest penis, represents the starting point in evolution, while the subgenus
Dendrocoelum s.str. has the highest evolutionary position due to the penial flagellum which is considered an evolutionary acquisition.
6. Discussion
The review of the literature reveals the gaps in knowledge.
The Dendrocoelidae fauna of Romania consists of 21 species. Of these, one species has a wide European distribution—
Polycladodes album, the rest being endemic. More than half of the species (thirteen species) were authored by Codreanu and Codreanu & Balcesco [
15,
16,
17,
18,
19,
20,
21], species for which the deposition of the specimen-types is unknown. The diagnosis of some species is incomplete, with no figurative reconstruction of the copulatory apparatus, including
Dendrocoelum (Polycladodes) affine,
Dendrocoelum (Paradendrocoelum) alexandrinae and
Dendrocoelum (Palaeodendrocoelum)
getticum [
21]. These deficiencies impair the knowledge on the whole group.
Some of the morphological types presented are very distinct and unmistakable, making possible a clear diagnosis of the taxa—Bolbodendrocoelum and Apodendrocoelum. For the rest of the taxa (regardless of the genus or subgenus rank), a clear diagnosis is not possible, as one character of a set of characters is shared by more morphological types/taxa. Many supraspecific taxa include a mixture of species with a mixture of morphological characters which make them very difficult to order. This could be a sound reason to abandon these taxonomic divisions, at least temporarily. Some species may not be good species, and some others may not belong to the genus they were originally attributed. The taxa Paradendrocoelum and Palaeodendrocoelum seem to be the most problematic.
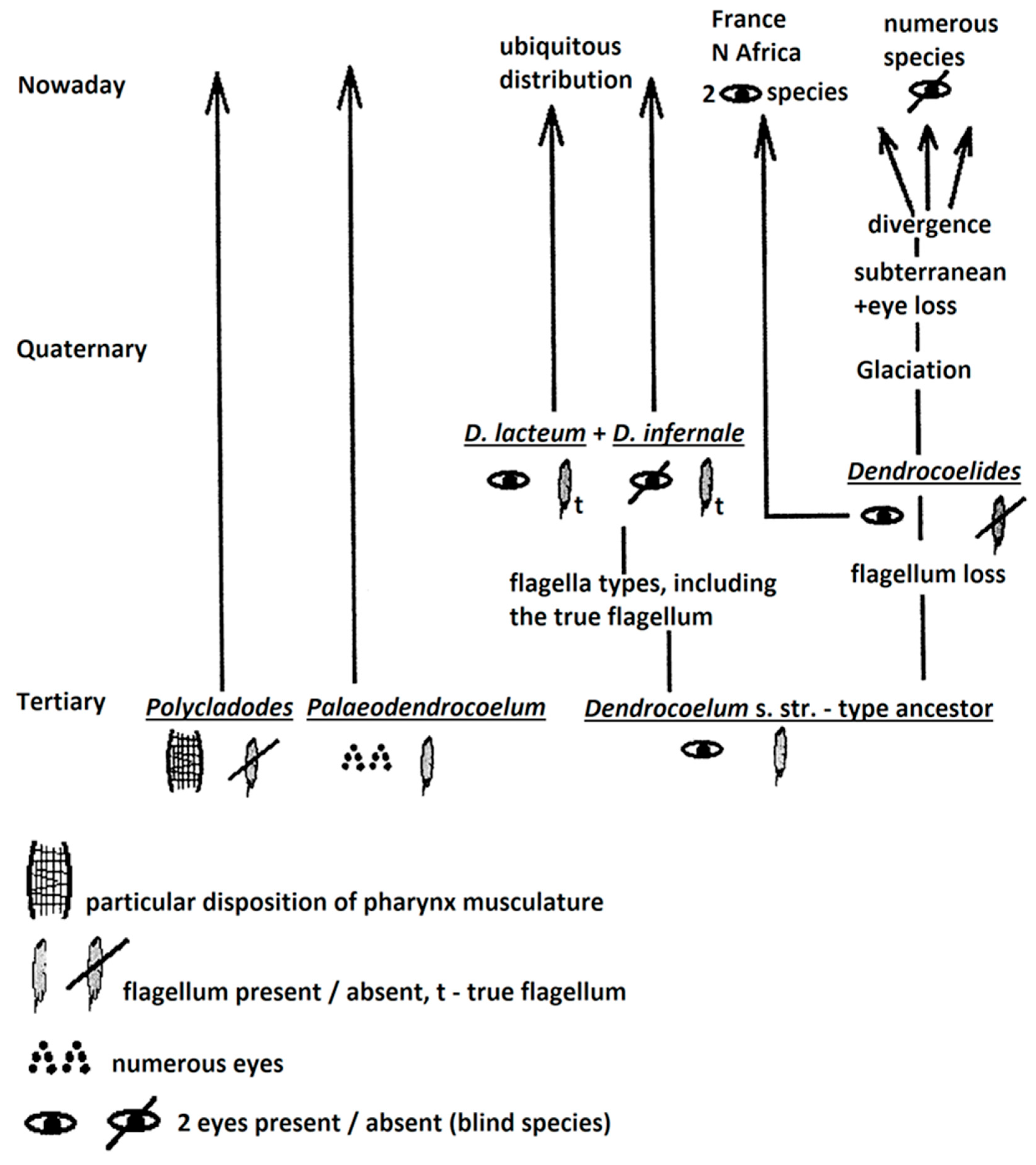
Using the eyes and the penial flagellum, and based on the hypotheses brought by all authors cited in this paper, I suggest two main evolutionary patterns:
1) one evolutionary pattern (
Figure 3) may consider three groups (taxa) of ancient origin:
Polycladodes and
Paleodendrocoelum (of Tertiary origin) and a
Dendrocoelum-type ancestor (of un-known geological origin), bi-occulated and with penial flagellum of un-known type. The evolution of the latter
Dendrocoelum-type ancestor may have followed two main directions:
1) a) flagellum preservation and its evolution in the various flagellar types found in the current fauna
1) b) the loss of the flagellum, character typical for Dendrocoelides. This type of ancestor (of pre-quaternary age) underwent Glaciation (see pages 8-9 of this paper, point 2) B)). Attenuated glaciation in some areas (S Europe, N Africa) determined the survival of two occulated species in France and N Africa; France and N Africa represent a margin of an ancient geographical range. Other Dendrocoelides species disappeared, others migrated into the subterranean where lost the eyes and suffered diversification, giving numerous endemic species distinct at the level of genitalia.
Figure 3.
Evolutionary pattern with a Dendrocoelum-type ancestor.
Figure 3.
Evolutionary pattern with a Dendrocoelum-type ancestor.
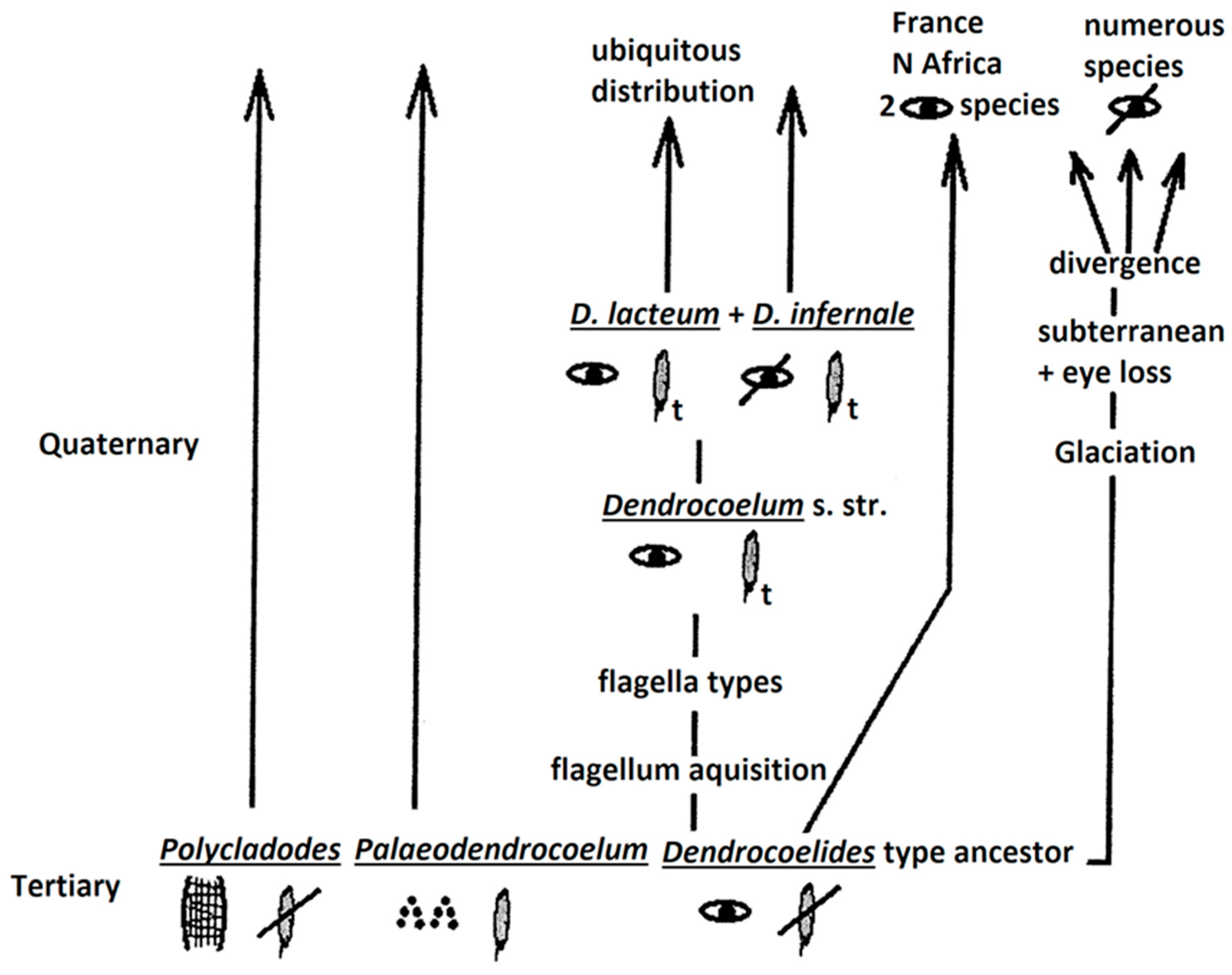
2) a second model (
Figure 4) may consider as well, three groups (taxa) of ancient origin, out of which
Polycladodes and
Paleodendrocoelum of Tertiary origin. The third group may be of the
Dendrocoelides-type, occulated and without penial flagellum, following two evolutionary paths:
2) a) flagellum acquisition, giving various types of flagella
2) b) evolution induced by the Glaciation, with the preservation of the two occulated species in the current fauna (in France and N Africa), also with the subterranean radiation.
Both patterns imply the followings: the lack of the eyes means their loss; a possible independent dual/multiple origin of the penial flagellum—in
Palaeodendrocoelum and the
Dendrocoelum-type via
Dendrocoelides ancestor (
Figure 4). Amongst invertebrates, molecular data have indicated independent evolution (dual origin) in even more unexpected cases, for example, the dual origin of striated musculature [
41].
Figure 4.
Evolutionary pattern with a Dendrocoelides-type ancestor.
Figure 4.
Evolutionary pattern with a Dendrocoelides-type ancestor.
Regarding the flagellar types, synthesised by Stocchino and coauthors [
9], some observations can be made: the histological structure of the penial flagellum is not known in all species; most species (thirteen) possess a pseudoflagellum, of these, ten species are occulated and found in Lake Ohrid. Two species out of four possessing a completely inverted penial papilla are occulated and are found in Lake Ohrid. The phylogenetic value of the penial papilla types (the flagellar types) should answer the following issues: the variability of the penis shape indicated by Kenk [
5]) (individual variability, variability in living and fixed worms); does the histological structure of the flagellum reflect a physiological state? (for instance, the vacuolated epithelium).
The natural history of this group is very difficult to be reconstructed on morphological grounds alone. The natural history may have had numerous morphological types lost during the geological times, not present in the actual fauna or not preserved in fossils. Additionally, it is completely unknown where and how many transitions from epigeic to hypogeic (and vice versa) occurred. The palaeogeographical evolution of European land, freshwater and brackish water could have taken place according to a model like the Glacial Sensitive Model (GSM) and ‘Sea-Level Sensitive’ dynamic model (SLS), models available for the marine island biogeography [
42], thus shaping the speciation process.
7. Conclusions
The natural history and phylogenetic systematic of Dendrocoelum s.l. should be re-investigated. The integrative approach paleo-bio-geography—morphology—biological features—genetics may lead to a better understanding of the group and may bring surprising outcomes.
8. Future Directions of Study
Specific future research needs and questions
The reinvestigation of the Romanian Dendrocoelidae should have different approaches and should aim to answer to the following questions:
1. the status of the describes species—some species may be synonymised; new species may be described.
2. the genus or subgenus level of Paradendrocoelum and Palaeodendrocoelum. In this regard, the species Paradendrocoelum alexandrinae and Palaeodendrocoelum getticum are of particular interest.
3. the anophthalmic species Palaeodendrocoelum getticum, Polycladodes voinovi and Polycladodes affine may be supportive for the validation of one of the hypotheses regarding the origin, the paleogeographic way of distribution (the migration route), also for clarifying the eye character status—homologous character (divergent evolution) or analogous character (convergent evolution)
4. the molecular support for timing of subterranean colonization, for eye/flagellum evolution hypotheses.
The Dendrocoelidae as freshwater flatworms in the era of genetics
The numerous modern genetic techniques, tools and protocols provide new and deeper insights into freshwater flatworms’ knowledge. This group of worms is characterised by the presence throughout their lives of neoblasts. Neoblasts are population of adult pluripotent stem cells involved in many processes—regeneration, turn-over of specialized cells, responses to external insults [
43]. The study of stem cells is focused on planarians (Dugesiidae) which became model system due to their high regenerative abilities. The literature in this field is impressive; numerous research articles and reviews bring into light a complex regulatory genetic network of a stem-cells system involved in developing, tissue and organ functioning, repairing various parts of a worm organism [
43,
44,
45,
46,
47,
48,
49].
Dendrocoelidae must have neoblasts and a stem-cell system (lost or degraded in evolution) but, so far, very much un-explored. Few papers treat regeneration in
Dendrocoelum lacteum alone [
50] or in other summative and comparative contexts [
49].
Two main topics seem to be important for
Dendrocoelides geological evolution—the neoblasts and the eye loss. Most
Dendrocoelides species of the actual fauna are endemic and cavernicolous. It is very well known and documented that the eye loss comes as an adaptation to dark environments in many invertebrate and vertebrate phyla, including planarians [
51,
52,
53]. Regarding the eyes, one question should be answered: how does a cavernicolous anophthalmic species will react when exposed to light for a longer period—will it develop eyes? What is the meaning of the acquisition or loss of eyes in this group of worms characterised by the presence of neoblasts?
In recent years, there has been increasing awareness of the role of the environment in producing phenotypes. An inherited genome can respond not only to mutations to produce new phenotypes but also to numerous environmental factors involving epigenetic control (for instance DNA methylation) [
54].
Other modern methodologies could bring new insights and outcomes in the study of this worms, like in other living beings, for various purposes and in different contexts—eDNA [
55], genome skimming [
56,
57,
58], microCT imaging [
59,
60,
61].
Conflict of Interests
Author declares no conflict of interests.
Acknowledgments
The author thanks Grigore Antipa Museum Bucharest for the opportunity to present the poster at Zoologycon 2024.
References
- Sluys, R.; Kawakatsu, M.; Riutort, M.; Baguña, J. A new higher classification of planarian flatworms (Platyhelminthes, Tricladida). Journal of Natural History 2009, 43, 1763–1777. [Google Scholar] [CrossRef]
- Gourbault, N. Recherches sur les Triclades Paludicoles hypogés. Mémoires du Muséum National d’Histoire Naturelle Paris, 1972; Série A, Tome LXXIII, 249 pp. + 3 planches.
- Stocchino, G.A.; Sluys, R.; Kawakatsu, M.; Sarbu, S.M.; Manconi, R. A new species of freshwater worm (Platyhelminthes, Tricladida, Dendrocoelidae) inhabiting a chemoautotrophic groundwater ecosystem in Romania. European Journal of Taxonomy 2017, 342, 1–21. [Google Scholar] [CrossRef]
- Del Papa, R. Dendrocoelum (Dendrocoelides bennazzii n. sp. From the cave of Stiffe (Abruzzo). Bolletino di Zoologia 1973, 40, 253–259. [Google Scholar] [CrossRef]
- Kenk, R. The planarians (Turbellaria: Tricladida paludicola) of Lake Ohrid in Macedonia. Smithsonian Contribution to Zoology 1978, 280, 1–56. [Google Scholar] [CrossRef]
- Villa-Farré, M.; Sluys, R.; Almagro, Í.; Handberg-Thorsager, M.; Romero, R. Freshwater planarians (Platyhelminthes, Tricladida) from the Iberian Peninsula and Greece: diversity and notes on ecology. Zootaxa 2011, 2779, 1–138. [Google Scholar] [CrossRef]
- Harrath, A.H.; Sluys, R.; Ghlala, A.; Alwasel, S. The first subterranean freshwater planarians from North Africa, with an analysis of adenodactyl structure in the genus Dendrocoelum (Platyhelminthes, Tricladida, Dendrocoelidae). Journal of Cave and Karst Studies 2012, 74, 48–7. [Google Scholar] [CrossRef]
- Sluys, R. A new, sibling species of cave flatworm from Switzerland (Platyhelminthes, Tricladida, Dendrocoelidae). Revue Suisse de Zoologie 2012, 119, 181–188. [Google Scholar] [CrossRef]
- Stocchino, G.A.; Sluys, R.; Marcia, P.; Manconi, R. Subterranean aquatic planarians of Sardinia, with a discussion on the penial flagellum and the bursal canal sphincter in the genus Dendrocoelum (Platyhelminthes, Tricladida, Dendrocoelidae). Journal of Cave and Karst Studies 2013, 75, 93–112. [Google Scholar] [CrossRef]
- Stocchino, Sluys R., Montanari A., Manconi R., A new species of stygobiont freshwater planarian (Platyhelminthes, Tricladida) from a chemoautotrophic ecosystem: the Frasassi karst in Italy. Zootaxa 2017, 4323, 547–560. [CrossRef]
- Vila-Farré, M. , Brand, J.N.; Boothe, T.; Maren Brockmeyer, M.; Ficze-Schmidt, F.; Grohme, M.A.; Weill, U.; Kluiver, K.H.; Gasiorowski, L.; Kauf, L.; Kanana, Y.; Bilandžija, H.; Riutort, M.; Rink, J.C. An integrative taxonomic approach reveals unexplored diversity in Croatian planarians. 2025. [Google Scholar] [CrossRef]
- Beauchamp, P. de. Nouvelle diagnoses de Triclades obscuricoles, Les Dendrocoelides de Transylvanie. Bull. Soc. Zool. Fr. 1929, 54, 20–28. [Google Scholar]
- Beauchamp, P. de. Turbellariés, Hirudinées, Branchiobdellidés. 2e série. Biospeologica LVIII. Arch. Zool. Exp. Gén. 1932, 73, 113–117. [Google Scholar]
- Beauchamp, P. de. Turbellariés 3e série. Biospeologica LXIX. Arch. Zool. Exp. Gén 1949, 86, 50–65. [Google Scholar]
- Codreanu, R. Polycladodes voinovi, n. sp., nouveau Triclade obscuricole de Roumanie. C. R. Soc. Biol. 1929, 101, 963–966. [Google Scholar]
- Codreanu, R. Dendrocoelum (Dendrocoelides) clujanum n. sp. Nouveau Triclade souterrain de Transylvanie. Anal. Acad. Române, Memor. Sect. stiinț. 1943, 3, 1–24. [Google Scholar]
- Codreanu, R. O nouă tricladă epigee relictă din Defileul Dunării: Palaeodendrocoelum danubialis N. G., N. SP. Anal. Acad. R. P. R. Ser. Geologie, Geografie 1950, 3, 599–622. [Google Scholar]
- Codreanu, R. , Balcesco, D. Sur trois Dendrocoelides aveugle nouveaux des sources du Banat (Roumanie). Rev. Roum. Biol.-Zoologie 1967, 12, 287–294. [Google Scholar]
- Codreanu, R. , Balcesco, D. Sur les rapports entre les sous-genres Paradendrocoelum Kenk 1930 et Dendrocoelides De Beauchamp 1919 d’après les espèces obscuricoles du Banat et de L’Olténie. Rev. Roum. Biol.-Zoologie 1967, 12, 337–349. [Google Scholar]
- Codreanu, R. , Balcesco, D. Sur deux nouveaux Dendrocoeles hypogés de Roumanie et certains effets de néoténie. Arch. Roum. Path. Exp. Microbiol. 1967, 46, 843–852. [Google Scholar]
- Codreanu, R. , Balcesco, D. Répartition des Dendrocoelides anophtalmes dans les Carpates de Courbure et dans la Plaine Roumaine. Livre de Centenaire E. Racovitza, 1970. [Google Scholar]
- Kuznedelov, K.D.; Novikova, O.A.; Naumova, T.V. Molecular genetic typification of planaria genus Bdellocephala (Dendrocoelidae, Tricladida, Turbellaria) from Lake Baikal and estimation of its specific variability. 1999, Abstract on Researchgate https://www.researchgate.net/publication/12456102.
- Kuznedelov, K.D.; Ishida, S.; Nishitani, S.-I. Genetic divergence of Japanese Turbellarians, studied by comparisons of partial 18S rRNA gene sequences I. On representatives on Dendrocoelidae (Platyhelminthes: Tricladida: Paludicola). Zoological Science 2000, 17, 491–496. [Google Scholar]
- Riutort, M.; Álvarez-Presas, M.; Lázaro, E.; Solà, E.; Paps, J. Evolutionary history of the Tricladida and the Platyhelminthes: an up-to-date phylogenetic and systematic account. Int. J. Dev. Biol. [CrossRef]
- Carranza, S.; Littlewood, D.T.J.; Clough, K.A. , Ruiz-Trillo, I.; Baguña, J.; Riutort, M. A robust molecular phylogeny of the Tricladida (Platyhelminthes: Seriata) with a discussion on morphological synapomorphies. Proc. R. Soc. Lond. B. 1998, 265, 631–640. [Google Scholar] [CrossRef]
- Baguñà, J.; Carranza, S.; Paps, J.; Ruiz-Trillo, I.; Riutort, M. Molecular taxonomy and phylogeny of the Tricladida. Chapter 6: 49—56 In: Press SASVC, editor. Interrelationships of the Platyhelminthes. London 2000.
- Álvarez-Presas, M.; Baguñà, J.; Riutort, M. Molecular phylogeny of land and freshwater planarians (Tricladida, Platyhelminthes): from freshwater to land and back. Mol Phylogenet Evol. 2008, 47, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, E.M.; Sluys, R.; Pala, M.; Stocchino, G.A.; Baguña, J.; Riutort, M. Molecular barcoding and phylogeography of sexual and asexual freshwater planarians of the genus Dugesia in the Western Mediterranean (Platyhelminthes, Tricladida, Dugesiidae). Mol. Phyl. Evol. 2009, 52, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Solà, E.; Sluys, R.; Gritzalis, K.; Riutort, M. Fluvial basin history in the northeastern Mediterranean region underlies dispersal and speciation patterns in the genus Dugesia (Platyhelminthes, Tricladida, Dugesiidae). Mol. Phyl. Evol. 2013, 66, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Presas, M.; Riutort, M. Planarian (Platyhelminthes, Tricladida) Diversity and Molecular Markers: A New View of an Old Group. Diversity 2014, 6, 323–338. [Google Scholar] [CrossRef]
- Sluys, R.; Riutort, M. Planarian diversity and phylogeny in Jochen C. Rink (ed.), Planarian Regeneration: Methods and Protocols, Methods in Molecular Biology 2018, 1774. [CrossRef]
- Inoue, K.; Pohl, A.L.; Sei, M.; Lang, B.K.; Berg, D.J. Use of species delimitation approaches to assess biodiversity in freshwater planaria (Platyhelminthes, Tricladida) from desert springs. Aquatic Conserv: Mar Freshw Ecosyst, 2: 30. [CrossRef]
- Leria, L.; Vila-Farré, M.; Álvarez-Presas, M.; Sánchez-Gracia, A.; Rozas, J.; Sluys, R.; Riutort, M. Cryptic species delineation in freshwater planarians of the genus Dugesia (Platyhelminthes, Tricladida): extreme intraindividual genetic diversity, morphological stasis, and karyological variability. Mol. Phyl. Evol. 2020. [CrossRef]
- Leria, L; Riutort, M.; Romero, R.; Ferrer, X.; Vila-Farré, M. Microplate tectonics and environmental factors as distribution drivers in Western Mediterranean freshwater planarians. Journal of Biogeography, 1124. [CrossRef]
- Benítez-Álvarez, L.; Leria, L.; Sluys, R.; Leal-Zanchet, A.M.; Riutort, M. Niche modelling and molecular phylogenetics unravel the colonisation biology of three species of the freshwater planarian genus Girardia (Platyhelminthes, Tricladida). Hydrobiologia 2023, 850, 3125–3142. [Google Scholar] [CrossRef]
- Dols-Serrate, D.; Stocchino, G.A.; Nuin-Villabona, P.; Sluys, R.; Riutort, M. New insights into the evolution and biogeography of freshwater planarians on islands in the Tyrrhenian Sea, Western Mediterranean Basin, with the integrative description of a new endemic species from Corsica (Platyhelminthes: Tricladida: Dugesia). Zoological Journal of the Linnean Society. [CrossRef]
- Sluys, R. The evolutionary terrestrialization of planarian flatworms (Platyhelminthes, Tricladida, Geoplanidae): a review and research programme. Zoosyst. Evol. 2019, 95(2), 543–556. [Google Scholar] [CrossRef]
- Kenk, R. Beiträge zum System der Probursalier (Tricladida paludicola). Zool. Anz. 1930, 89, 145–162. [Google Scholar]
- Ruppert, E.E.; Fox, R.S.; Barnes, R.D. Introduction to Invertebrates. In Invertebrate Zoology, a functional evolutionary approach, 7th ed.; Thomson Brooks/Cole, 2004; pp. 1–10.
- Sluys, R.; Kawakatsu, M. Towards a phylogenetic classification of dendrocoelid freshwater planarians (Platyhelminthes): a morphological and eclectic approach. Journal of Zoological Systematics and Evolutionary Research 2006, 44(4), 274–284. [Google Scholar] [CrossRef]
- Steinmetz, P.R.H.; Kraus, J.E.M.; Larroux, C.; Hammel, U.J.; Amon-Hassenzahl, A.; Houliston, E.; Wörheide, G.; Nickel, M.; Degnan, B.M.; Technau, U. Independent evolution of striated muscles in cnidarians and bilaterians. Nature 2012, 487, 231–234. [Google Scholar] [CrossRef]
- Ávila, S.P.; Melo, C.; Berning, B.; Sá, N.; Quartau, R.; Rijsdijk, K.F.; Ramalho, R.S.; Cordeiro, R.; De Sá, N.C.; Pimentel, A.; Baptista, L.; Medeiros, A.; Gil, A.; Johnson, M.E. Towards a ‘Sea-Level Sensitive’ dynamic model: impact of island ontogeny and glacio-eustasy on global patterns of marine island biogeography. Biological Reviews 2019, 94, 1116–1142. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.D.; Cebrià, F. Decoding stem cells: an overview on planarian stem cell heterogeneity and lineage progression. MDPI Biomolecules 2021, 11, 1532. [Google Scholar] [CrossRef] [PubMed]
- Lapan, S.W.; Reddien, P.W. Transcriptome Analysis of the Planarian Eye Identifies ovo as a Specific Regulator of Eye Regeneration. Cell Reports 2012, 2, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Deochand, M.E.; Birkholz, T.R.; Beane, S.W. (2016) Temporal regulation of planarian eye regeneration. Regeneration 2016. [CrossRef]
- Ivankovic, M.; Haneckova, R.; Thommen, A.; Grohme, M.A.; Vila-Farré, M.; Werner, S.; Rink, C.J. Model systems for regeneration: planarians. Development 2019, 146. [Google Scholar] [CrossRef]
- Ge, X.-Y.; Han, X.; Zhao, Y.-L.; Cui, G.-S.; Yang, Y.-G. An insight into planarian regeneration. Cell Proliferation 2022. [CrossRef]
- Wang, Q.; Liu, Y.; Jin, B.; Dong, Z.; Chen, G.; Liu, D. Djmek is involved in planarian regeneration by regulation of cell proliferation and apoptosis. Biochemical and Biophysical Research Communications 2020, 532, 355–361. [Google Scholar] [CrossRef]
- Vila-Farré, M.; Rozanski, A.; Ivanković, M.; Cleland, J.; Brand, J.N.; Thalen, F.; Grohme, M.A.; Kannen, S.; Grosbusch, A.L.; Vu, T.-K.H.; Prieto, C.E.; Carbayo, F.; Egger, B.; Bleidorn, C.; Rasko, J.E.J.; Jochen, C.R. Evolutionary dynamics of whole-body regeneration across planarian flatworms. Nature ecology & Evolution. [CrossRef]
- Liu, S.Y.; Selck, C.; Friedrich, B.; Lutz, R.; Vila-Farré, M.; Dahl, M.A.; Brandl, H.; Lakshmanaperumal, N.; Henry, I.; Rink, C.J. Reactivating head regrowth in a regeneration-deficient planarian species. Nature 2013, 500, 81–85. [Google Scholar] [CrossRef]
- Porter, M.L.; Sumner-Rooney, L. Evolution in the Dark: Unifying our Understanding of Eye Loss. Integrative and Comparative Biology 2018, 58, 367–371. [Google Scholar] [CrossRef]
- Sumner-Rooney, L.S. The Kingdom of the Blind: Disentangling Fundamental Drivers in the Evolution of Eye Loss. Integrative and Comparative Biology. [CrossRef]
- Saad, L.O.; Cooke, T.F.; Atabay, K.D.; Reddien, P.W.; Brown, F.D. (2024) Reduced adult stem cell fate specification led to eye reduction in cave planarians. Nature Communications 2024. [CrossRef]
- Gilbert, S.F.; Barresi, M.J. Development and the Environment. In Developmental Biology, 11th ed.; Sinauer New York Oxford University Press, 2018; pp. 763–784.
- Mauro, M.; Longo, F.; Valvo, L.V.; Vizzini, A.; Di Grigoli, A.; Radovic, S.; Arizza, V. , Vecchioni, L.; La Paglia, L.; Queiroz, V.; Ponte, M.; Gargano, C.; Ciaccio, P.S.F.; Vicari, D.; Vazzana, M. The Use of Environmental DNA as Preliminary Description of Invertebrate Diversity in Three Sicilian Lakes. Animals. [CrossRef]
- Dodsworth, S. Genome skimming for next-generation biodiversity analysis. RPLSC-1317 2015. [CrossRef]
- Ji, Y.; Yang, J.; Landis, J.B.; Wang, S.; Jin, L.; Xie, P. ; Haiyang Liu7, Jun-Bo Yang, J.-B.; Yi, T.-S. Genome Skimming Contributes to Clarifying Species Limits in Paris Section Axiparis (Melanthiaceae). Front. Plant Sci. 8320. [Google Scholar] [CrossRef]
- Taite, M.; Fernández-Álvarez, F.Á.; Braid, H.E.; Bush, S.L.; Bolstad, K.; J. Drewery, J.; Mills, S.; J.M. Strugnell, J.M.; Vecchione, M.; Villanueva, R.; J.R. Voight, J.R.; Allcock, A.L. Genome skimming elucidates the evolutionary history of Octopoda. L. Genome skimming elucidates the evolutionary history of Octopoda. Mol. Phyl. Evol. 2023, 182. [Google Scholar] [CrossRef]
- Lin, Y.-S.; 1, Chu, C. -C.; 1, Lin, J.-J.; 2, Chang, C.-C.; 1, Wang, C.-C.; Wang, C.-Y.;Tsui, P.-H. Optical coherence tomography: A new strategy to image planarian regeneration. Scientific Reports 2014, 4, 6316. [Google Scholar] [CrossRef] [PubMed]
- Carbayo, F.; Lenihan, J.W. Micro-computed tomography scan and virtual histological slide data for the land planarian Obama otavioi (Platyhelminthes). GigaScience. [CrossRef]
- Silva, M.S.; Carbayo, F. X-ray microcomputed tomography applied to the taxonomic study of rare material: redescriptions of seven of Schirch’s Brazilian species of land planarians (Geoplanidae, Platyhelminthes). ZooKeys 2020, 910, 1–42. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).