Submitted:
13 December 2024
Posted:
13 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
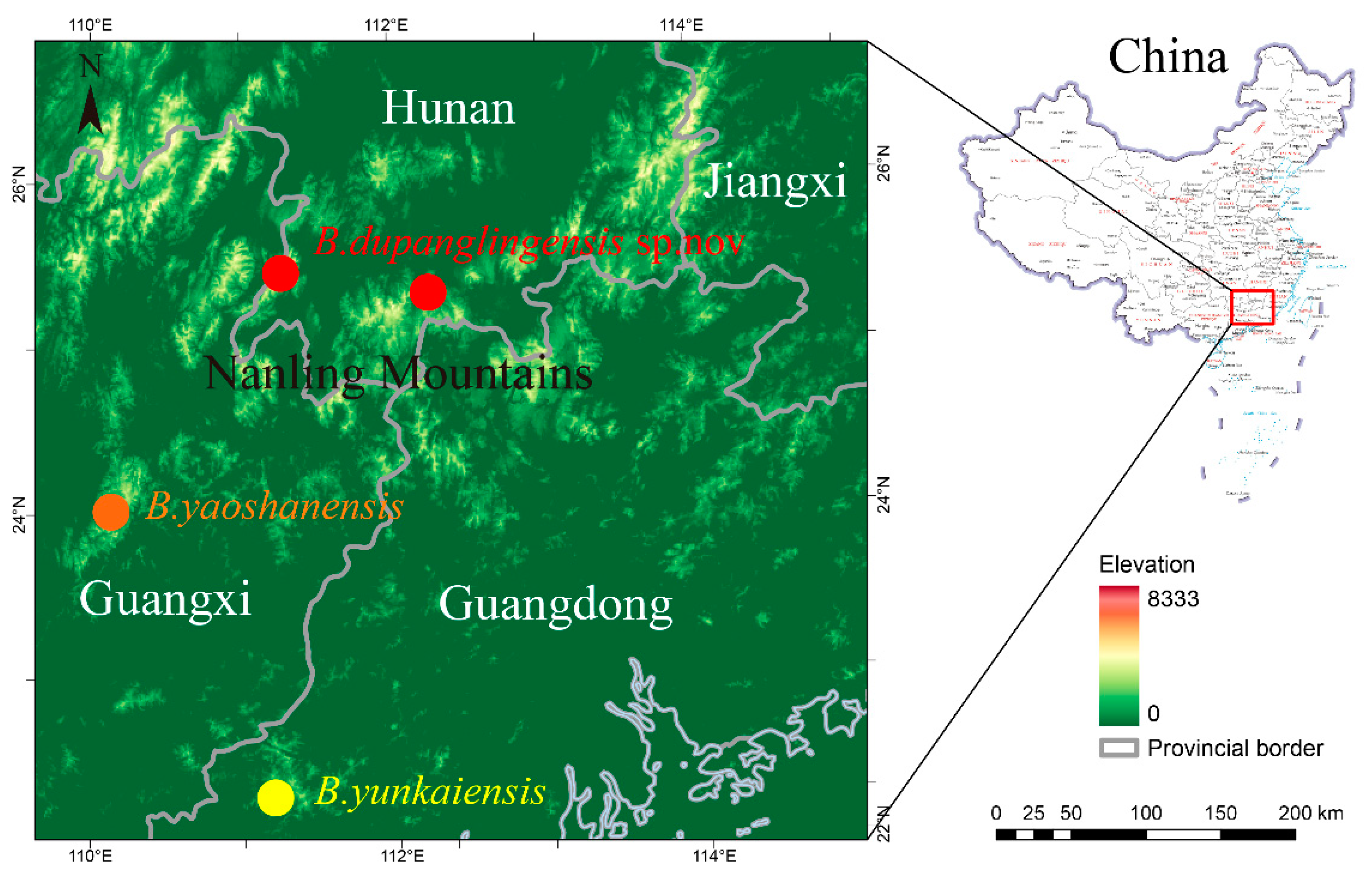
2.1. Sampling
2.2. Molecular and Phylogenetic Analyses
2.3. Morphological Analysis
3. Results
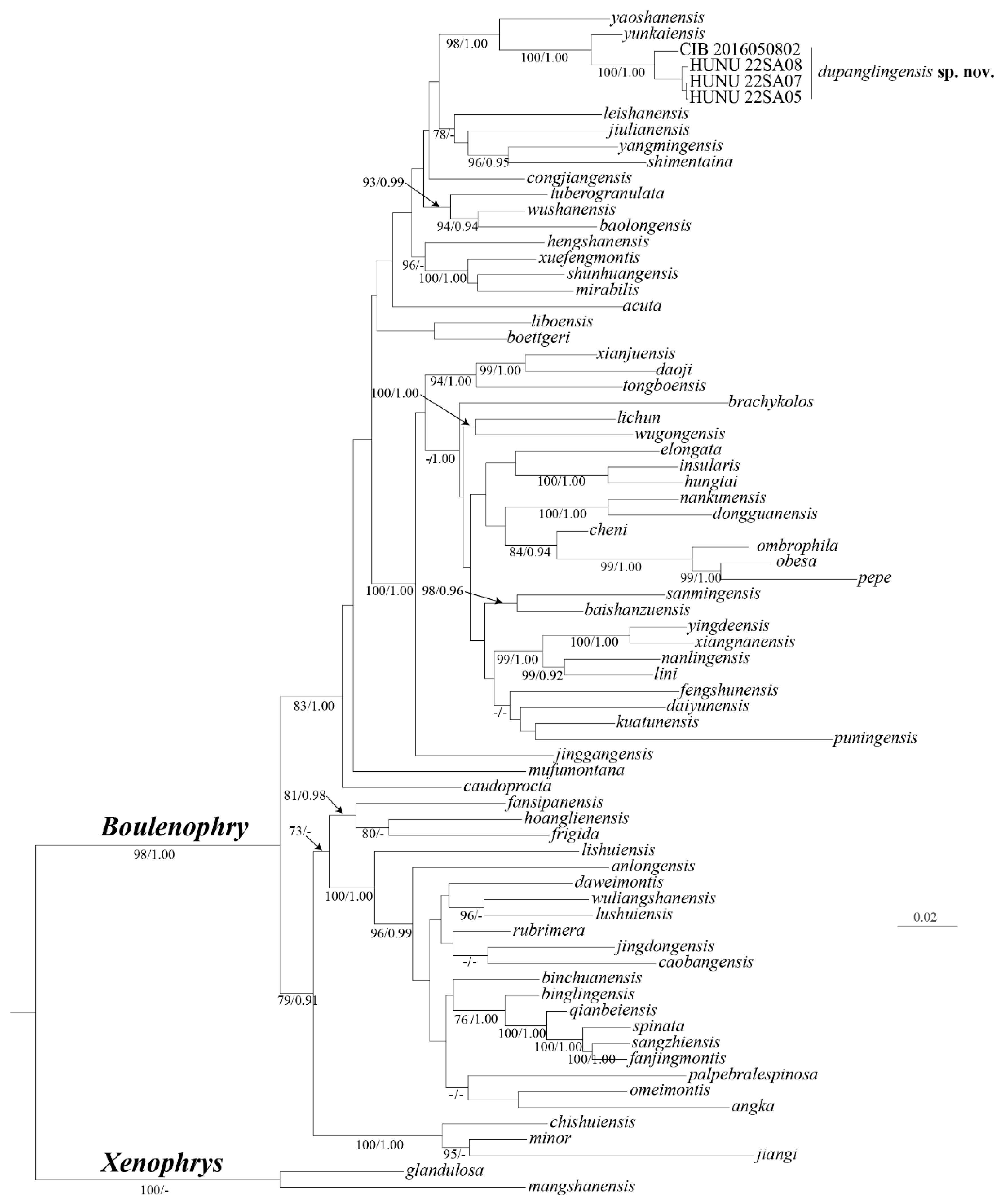
3.1. Molecular Phylogenetic Analyses
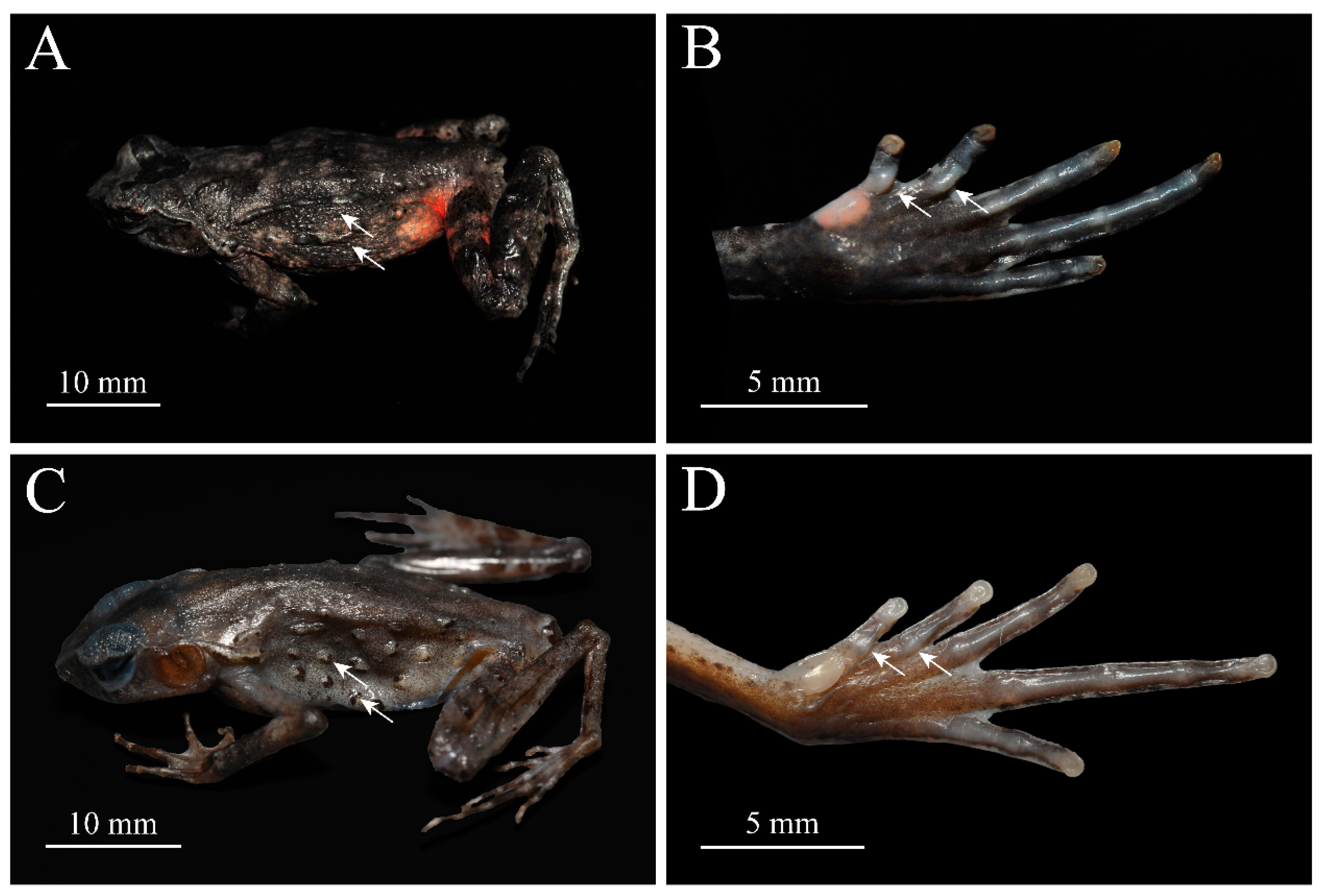
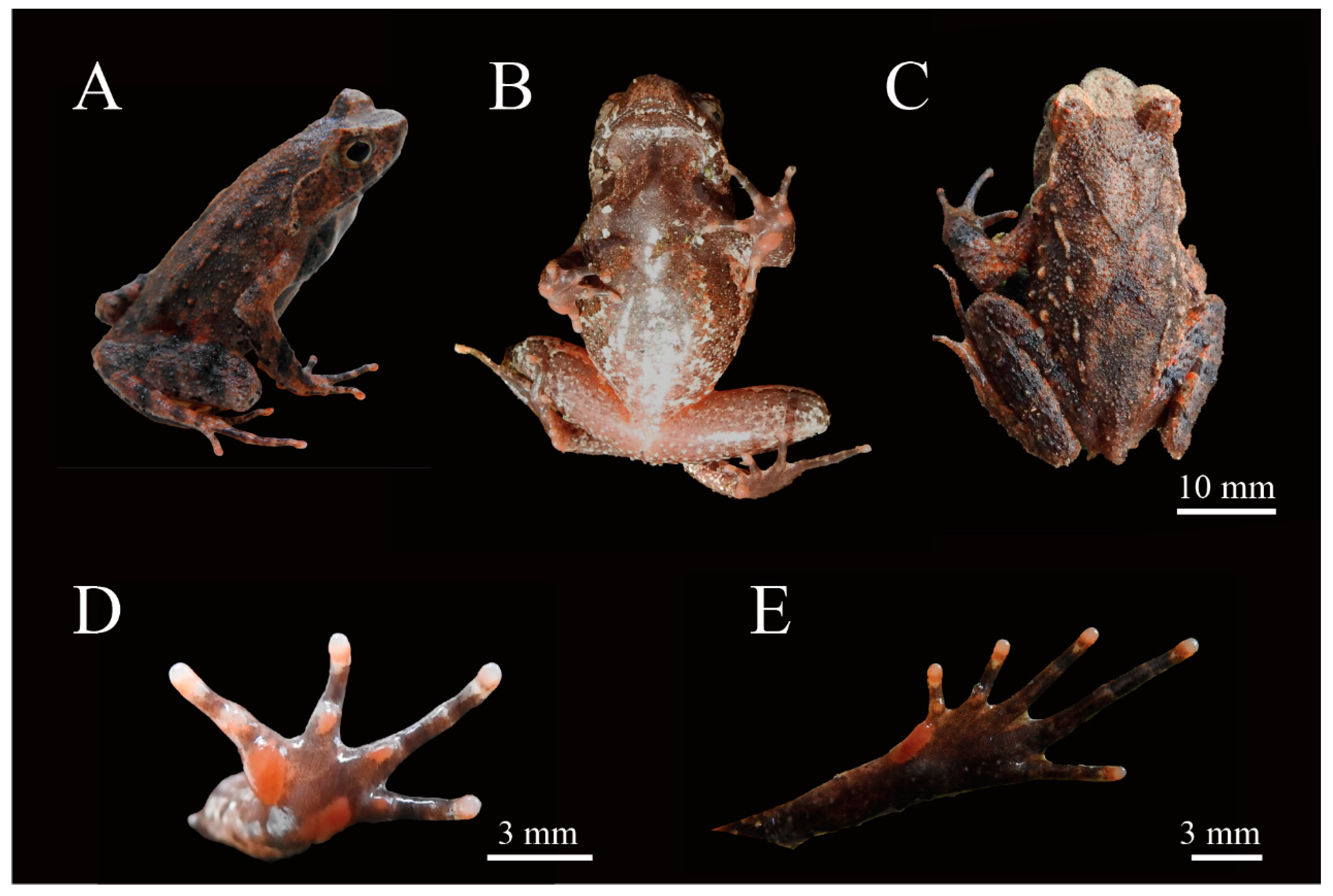
3.2. Morphological Comparisons
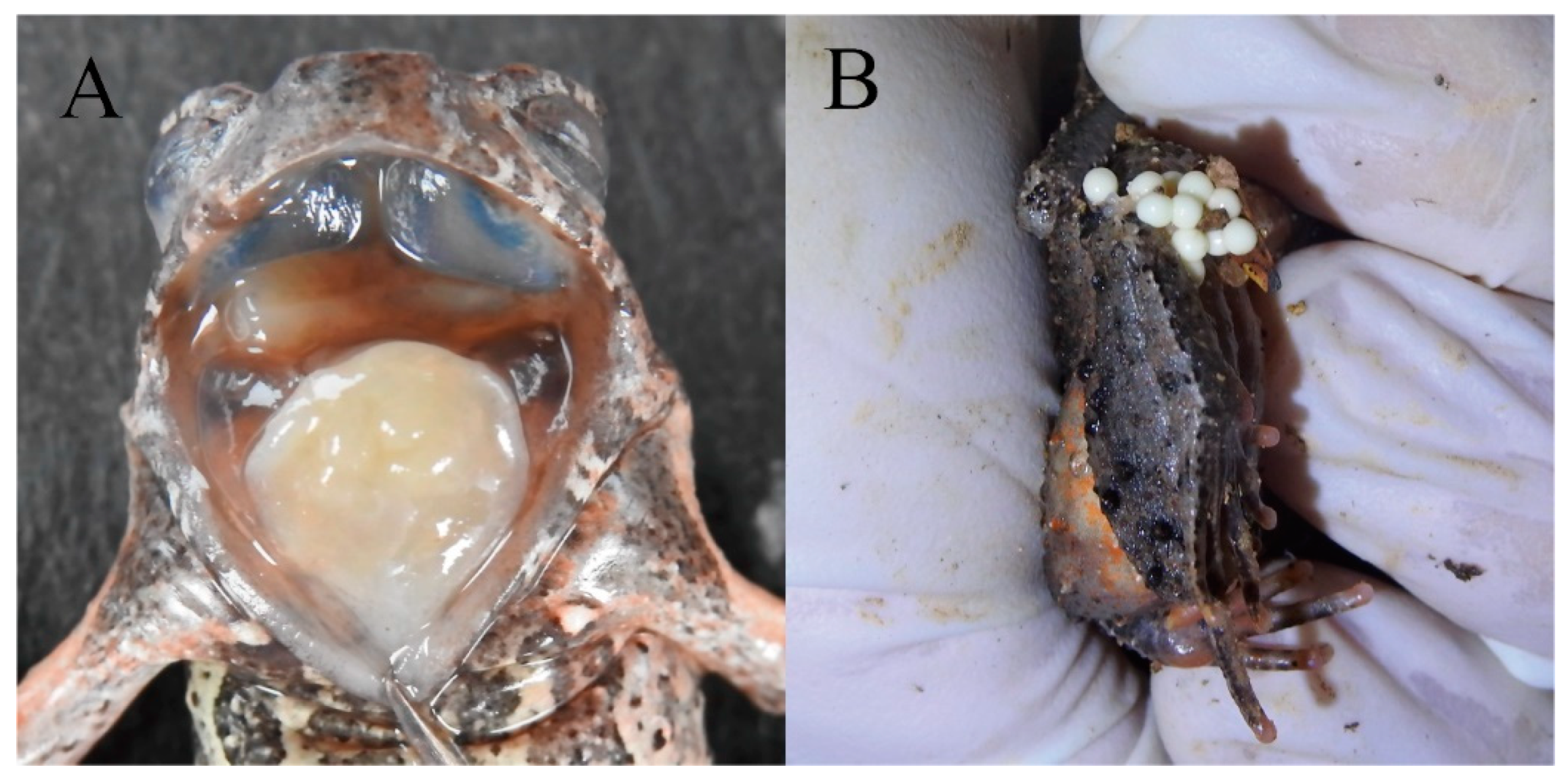
3.3. Taxonomic Account
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical statement
Acknowledgments
References
- AmphibiaWeb: information on amphibian biology and conservation, University of California, Berkeley, California. Available online: http://amphibiaweb.org/ (accessed on 18 Nov 2024).
- Bonaparte, C.L. Conspectus Systematum Herpetologiae et Amphibiologiae; Brill EJ, Leiden, 1850.
- Frost, D.R.; Amphibian Species of the World: an Online Reference. Version 6.2 (Date of access). Available online: doi.org/10.5531/db.vz.0001 (accessed on 18 Nov 2024).
- Chen, J.M. , Zhou, W.W., Poyarkov, N. J., Stuart, B.L., Brown, R.M., Lathrop, A., Wang, Y.Y., Yuan, Z.Y., Jiang, K.; H.M., C., H.M., Suwannapoom, C.,; Nguyen, S.N., Duong, T.V., Papenfuss, T.J., Murphy, R.W., Zhang, Y.P., Che, J. A novel multilocus phylogenetic estimation reveals unrecognized diversity in Asian horned toads, genus Megophrys sensu lato (Anura: Megophryidae). Molecular Phylogenetics and Evolution 2017, 106, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y. , Chen, G.L., Zhu, T.Q., Zeng, Z.C., Lyu, Z.T, Wang, J., Messenger, K., Greenberg, A. J., Guo, Z., Yang, Z.H., Shi, S.H., Wang, Y.Y. Prevalence of cryptic species in morphologically uniform taxa - Fast speciation and evolutionary radiation in Asian frogs. Molecular Phylogenetics and Evolution 2018, 127, 723–731. [Google Scholar] [CrossRef]
- Mahony, S. , Foley, N.M. Biju, S. D. Teeling, E. C. Evolutionary History of the Asian Horned Frogs (Megophryinae): Integrative Approaches to Timetree Dating in the Absence of a Fossil Record. Molecular Biology and Evolution 2017, 34, 744–771. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A. , Ohler, A., Pyron, R.A. New concepts and methods for phylogenetic taxonomy and nomenclature in zoology, exemplified by a new ranked cladonomy of recent amphibians (Lissamphibia). Megataxa 2021, 5, 1–738. [Google Scholar] [CrossRef]
- Frost, D.R. , Grant, T., Faivovich, J., Bain, R. H., Haas, A., Haddad, C. F.B., De Sá, R. O., Channing, A., Wilkinson, M., Donnellan, S. C.,; Raxworthy, C.J., Campbell, J. A., Blotto, B. L., Moler, P., Drewes, R. C., Nussbaum, R. A., Lynch, J. D., Green, D. M., Wheeler, W. C. The amphibian tree of life. Bulletin of the American Museum of Natural History 2006, 8–370. [Google Scholar] [CrossRef]
- Li, S.Z. , Lu, N. N., Liu, J., Wang, B. Description of a new Megophrys Kuhl & Van Hasselt, 1822 (Anura, Megophryidae) from Guizhou Province, China. Zookeys 2020, 986, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.T. , Qi, S., Wang, J. Zhang, S. Y., Zhao, J., Zeng, Z. C., Wan, H., Yang, J. H., Mo, Y. M., Wang, Y. Y. Generic classification of Asian horned toads (Anura: Megophryidae: Megophryinae) and monograph of Chinese species. Zool Research 2023, 44, 380–450. [Google Scholar] [CrossRef] [PubMed]
- Beddard, F.E. Contributions to the knowledge of the anatomy of the batrachian family Pelobatid. Proceedings of the Zoological Society of London 1907, 871–911. [Google Scholar] [CrossRef]
- Boulenger, G.A. Descriptions of three new batrachians from Tonkin. Annals and Magazine of Natural History, Series 7 1903, 12, 186–188. [Google Scholar] [CrossRef]
- Fei, L. , Ye, C. Y. Amphibians of China; Chengdu Institute of Biology, Chinese Academy of Sciences. Science Press: Beijing, China, 2016; Volume 1. [Google Scholar]
- Günther, A.C.L.G. The reptiles of British India; Ray Society: London, 1864; pp. 414–415. [Google Scholar]
- Kuhl, H. , Van Hasselt, J. C. Uittreksels uit breieven van de Heeren Kuhl en van Hasselt, aan de Heeren C. J. Temminck, Th. van Swinderen en W. de Haan. Algemeene Konst-en Letter-Bode 1822, 7, 99–104. [Google Scholar]
- Tian, W.S. , Hu, Q. X. Taxonomic study on genus Megophrys, with descriptions of two new genera. Acta Herpetologica Sinica 1983, 2, 41–48. [Google Scholar]
- Li, Y.L. , Jin, M. J., Zhao, J., Liu, Z. Y., Wang, Y. Y., Pang, H. Description of two new species of the genus Megophrys (Amphibia: Anura: Megophryidae) from Heishiding Nature Reserve, Fengkai, Guangdong, China, based on molecular and morphological data. Zootaxa 2014, 3795, 449–471. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H. , Suwannapoom, C., Poyarkov, N. A., Jr., Pawangkhanant, P., Xu, K., Jin, J. Q., Murphy, R. W., Che, J. A new species of the genus Xenophrys Anura Megophryidae from northern Thailand. Zool Res 2019, 40, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. , Zhang, D.D., Lyu, Z.T., Wang, J., Li, Y. L., Liu, Z. Y., Chen, H.H., Rao, D.Q., Jin, Z.F., Zhang, C.Y., Wang, Y.Y. Review of the genus Brachytarsophrys (Anura: Megophryidae), with revalidation of Brachytarsophrys platyparietus and description of a new species from China. Zoological Research 2020, 41, 105–122. [Google Scholar] [CrossRef]
- Ye, C.Y. , Fei, L., Xie, F. A new species of Megophryidae–Megophyrys baolongensis from China (Amphibia, Anura). Acta Herpetologica Sinica 2007, 11, 38–41. [Google Scholar]
- Nguyen, T.Q. , Pham, C. T., Nguyen, T. T., Luong, A. M., Ziegler, T. A new species of Megophrys (Amphibia: Anura: Megophryidae) from Vietnam. Zootaxa 2020, 4722, 401–422. [Google Scholar] [CrossRef]
- Shen, Y.H. , Yang, D. D., Mo, X. Y., Li, H. H., Chen, D. The Fauna of Hunan: Amphibia; Hunan Science and Technology Press: Changsha, 2014. [Google Scholar]
- Luo, T. , Wang, Y., Wang, S., Lu, X., Wang, W., Deng, H., Zhou, J. A species of the genus Panophrys (Anura, Megophryidae) from southeastern Guizhou Province, China. Zookeys 2021, 1047, 27–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y. , Zhao, J., Yang, J.H., Zhou, Z., Chen, G.L., Liu, Y. Morphology, molecular genetics, and bioacoustics support two new sympatric Xenophrys toads (Amphibia: Anura: Megophryidae) in southeast China. PLoS One 2014, 9, e93075. [Google Scholar] [CrossRef]
- Xu, N. , Li, S. Z., Liu, J., Wei, G., Wang, B. A new species of the horned toad Megophrys Kuhl & Van Hasselt, 1822 (Anura, Megophryidae) from southwest China. Zookeys 2020, 943, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.T. , Zeng, Z. C., Wang, J., Liu, Z. Y., Huang, Y. Q., Li, W. Z., Wang, Y. Y. Four new species of Panophrys (Anura, Megophryidae) from eastern China, with discussion on the recognition of Panophrys as a distinct genus. Zootaxa 2021, 4927, 009–040. [Google Scholar] [CrossRef]
- Wang, J. , Lyu, Z. T., Liu, Z. Y., Liao, C. K., Zeng, Z. C., Zhao, J., Li, Y. L., Wang, Y. Y. Description of six new species of the subgenus Panophrys within the genus Megophrys (Anura, Megophryidae) from southeastern China based on molecular and morphological data. Zookeys, 2019; 851, 113–164. [Google Scholar] [CrossRef]
- Zeng, Z.C. , Wang, J., Chen, H.H., Xiao, W.W., Zhan, B.B., Li, Y.H., Lin, S.S. A New Species of the Genus <italic>Boulenophrys</italic> (Anura, Megophryidae) from Eastern Guangdong, China. Asian Herpetological Research 2024, 15, 12–21. [Google Scholar] [CrossRef]
- Zhang, L. , Liang, L.,Ran, H., Shen, Z.X. Megophrys binlingensis fanjingmontis: A New Subspecies of Megophryidae from Guizhou,China. Chinese Journal of Zoology 2012, 47, 135–138. [Google Scholar] [CrossRef]
- Tapley, B. , Cutajar, T., Nguyen, L.T., Nguyen, C.T., Harding, L., Portway, C., Van Luong, H. & Rowley, J.J. A new locality and elevation extension for Megophrys rubrimera (Tapley et al., 2017) in Bat Xat Nature Reserve, Lao Cai Province, northern Vietnam. Herpetology Notes 2018, 11, 865–868. [Google Scholar]
- Wang, J. , Zeng, Z. C., Lyu, Z. T., Qi, S., Liu, Z. Y., Chen, H. H., Lu, Y. H.,; Xiao, H.W., Lin, C. R., Chen, K., Wang, Y. Y. Description of three new Boulenophrys species from eastern Guangdong, China, emphasizing the urgency of ecological conservation in this region (Anura, Megophryidae). Zootaxa 2022, 5099, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Tapley, B. , Cutajar, T., Nguyen, L. T., Portway, C.,Mahony, S., Nguyen, C.T.,Harding, L.,Luong, H.V., Rowley, J. L. A new potentially Endangered species of Megophrys (Amphibia: Megophryidae) from Mount Ky Quan San, north-west Vietnam. Journal of Natural History 2020, 54, 2543–2575. [Google Scholar] [CrossRef]
- Qian, T.Y. , Hu, K., Mo, X.Y., Gao, Z.W., Zhang, N., Yang, D.D. A new species of Boulenophrys from central Hunan Province, China (Anura: Megophryidae). Vertebrate Zoology 2023, 73, 915–930. [Google Scholar] [CrossRef]
- Tapley, B. , Cutajar, T., Mahony, S., Nguyen, C.T., Dau, V.Q., Luong, A.M., Le, D.T., Nguyen, T.T., Nguyen, T.Q., Portway, C., Luong, H. V., Rowley, J. L. Two new and potentially highly threatened Megophrys Horned frogs (Amphibia: Megophryidae) from Indochina's highest mountains. Zootaxa 2018, 4508, 301–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Z.Y.; Lyu, Z.T.; Zeng, Z.C.; Wang, Y.Y. A new species of the genus Xenophrys (Amphibia: Anura: Megophryidae) from an offshore island in Guangdong Province, southeastern China. Zootaxa 2017, 4324, 541–556. [Google Scholar] [CrossRef]
- Liu, J. , Li, S. Z., Wei, G., Xu, N., Cheng, Y. L., Wang, B., Wu, J. A New Species of the Asian Toad Genus Megophrys sensu lato (Anura: Megophryidae) from Guizhou Province, China. Asian Herpetological Research 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Wang, Y.Y., Zhang, T. D., Zhao, J., Sung, Y. H., Yang, J. H., Pang, H., ; Zhang, Z. Description of a new species of the genus Xenophrys Gunther, 1864 (Amphibia: Anura: Megophryidae) from Mount Jinggang, China, based on molecular and morphological data. Zootaxa, 2012; 53–67.
- Li, S.Z.; Xu, N.; Liu, J.; Jiang, J.P.; Wei, G.; Wang, B. A New Species of the Asian Toad Genus Megophrys sensu lato (Amphibia: Anura: Megophryidae) from Guizhou Province, China. Asian Herpetological Research 2018, 9, 224–239. [Google Scholar] [CrossRef]
- Lin, S.S. , Chen, H. H., Li, Y. H., Peng, Z. N., Zeng, Z. C., Wang, J. A new Boulenophrys species (Anura, Megophryidae) from the coastal hills of eastern Fujian Province, China. Zookeys 2024, 1216, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.C. , Li, D.H., Zhu, W.B., Wang, J., Jiang, J.P., Ye, C.Y., Fei, L., Wang, B. Description of a new toad of Megophrys Kuhl & Van Hasselt, 1822 (Amphibia: Anura: Megophryidae) from western Yunnan Province, China. Zootaxa 2021, 4942, 351–381. [Google Scholar] [CrossRef]
- Zhang, Y.N. , Li, G., Xiao, N., Li, J.Q., Pan, T., Wang, H., Zhang, B.W., Zhou, J. A New Species of the Genus Xenophrys (Amphibia: Anura: Megophryidae) from Libo County, Guizhou, China. Asian Herpetological Research 2017, 8, 75. [Google Scholar] [CrossRef]
- Wang, L. , Deng, X.J., Liu, Y., Wu, Q.Q., Liu, Z. A new species of the genus Megophrys (Amphibia: Anura: Megophryidae) from Hunan, China. Zootaxa 2019, 4695, 301–330. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.T. , Li, Y.Q., Zeng, Z.C., Zhao, J., Liu, Z.Y., Guo, G.X., Wang, Y.Y. Four new species of Asian horned toads (Anura, Megophryidae, Megophrys) from southern China. ZooKeys 2020, 942, 105–140. [Google Scholar] [CrossRef] [PubMed]
- Messenger, K.R. , Dahn, H.A., Liang, Y., Xie, P., Wang, Y., Lu, C. A new species of the genus Megophrys Gunther, 1864 (Amphibia: Anura: Megophryidae) from Mount Wuyi, China. Zootaxa 2019, 4554, 561–583. [Google Scholar] [CrossRef]
- Wang, J. , Lin, S.S., Gan, J.S., Chen, H.H., Yu, L.M., Pan, Z., Xiao, J.J., Zeng, Z.C. A new species of the genus Boulenophrys from South China (Anura, Megophryidae). Zootaxa 2024, 5514, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Su, H.J. , Shi, S.C., Wu, Y.Q., Li, G.R., Yao, X.G., Wang, B., Li, S.Z. Description of a new horned toad of Megophrys Kuhl & Van Hasselt, 1822 (Anura, Megophryidae) from southwest China. Zookeys, 2020; 131–159. [Google Scholar] [CrossRef]
- Tapley, B. , Cutajar, T., Mahony, S., Nguyen, C. T., Dau, V. Q., Nguyen, T. T., Luong, H. V., Rowley, J. J. L. The Vietnamese population of Megophrys kuatunensis (Amphibia: Megophryidae) represents a new species of Asian horned frog from Vietnam and southern China. Zootaxa 2017, 4344, 465–492. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.P. , Ye, C.Y., Fei, L. A New Horn Toad Megophrys sangzhiensis from Hunan, China (Amphibia,Anura). Zoological Research 2008, 29, 219–222. [Google Scholar] [CrossRef]
- Tian, Y.Z. , Gu, X. M., Sun, A. Q. A new species of Xenophrys in China (Amphibia: Pelobatidae). Acta Zootaxonomica Sinica 2000, 25, 462–466. [Google Scholar]
- Mo, X.Y. , Shen, Y.H., Li, H.H., Wu, X.S. A new species of Megophrys (Amphibia: Anura: Megophryidae) from the northwestern Hunan Province, China. Current Zoology 2010, 56, 432–436. [Google Scholar] [CrossRef]
- Fei, L. Atlas of Amphibians in China. Field Edition; Henan Science and Technology Press: Zhengzhou, p. 432.
- Wang, B. , Wu, Y. Q., Peng, J. W., Shi, S. C., Lu, N. N., Wu, J. A new Megophrys Kuhl & Van Hasselt (Amphibia, Megophryidae) from southeastern China. Zookeys 2020, 904, 35–62. [Google Scholar] [CrossRef]
- Qi, S. , Lyu, Z. T., Wang, J. Mo, Y. M., Zeng, Z. C., Zeng, Y. J., Dai, K. Y., Li, Y. Q., Grismer, L. L., Wang, Y. Y. Three new species of the genus Boulenophrys (Anura, Megophryidae) from southern China. Zootaxa 2021, 5072, 401–438. [Google Scholar] [CrossRef]
- Thompson, J.D. , Gibson, T. J., Plewniak, F., Jeanmougin, F., Higgins, D. G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic acids research 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Tamura, K. , Stecher, G., Kumar, S. MEGA11 Molecular Evolutionary Genetics Analysis Version 11. Molecular Biology and Evolution 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Lanfear, R. , Calcott, B., Ho, S.Y. W., Guindon, S. PartitionFinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses. Molecular Biology and Evolution 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Ronquist, F. , Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Hohna, S., Larget, B., Liu, L., Suchard, M.A., Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Systematic Biology 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Guindon, S. , Dufayard, J.F., Lefort, V., Anisimova, M., Hordijk, W., Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Systematic Biology 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.M. , Heyer, W.R. Variation and distribution in the tree-frog genus Phyllomedusa. Beitra¨ge zur Neotropischen Fauna 1967, 5, 111–131. [Google Scholar] [CrossRef]
- Gao, Z.W. , Qian, T. Y., Jiang, J. P., Hou, D. J., Deng, X. J., Yang, D. D. Species diversity and distribution of amphibians and reptiles in Hunan Province, China. Biodiversity Science 2022, 30, 21290. [Google Scholar] [CrossRef]


| ID | Boulenophrys species | References |
|---|---|---|
| 1 | B. acuta (Wang, Li & Jin, 2014) | Li et al. 2014[17] |
| 2 | B. angka (Wu, Suwannapoom, Poyarkov, Pawangkhanant, Xu, Jin, Murphy & Che, 2019) | Wu et al. 2019[18] |
| 3 | B. anlongensis (Li, Lu, Liu & Wang, 2020) | Li et al. 2020a[19] |
| 4 | B. baishanzuensis (Wu, Li, Liu, Wang & Wu, 2020) | Wu et al. 2020[18] |
| 5 | B. baolongensis (Ye, Fei & Xie, 2007) | Ye et al. 2007[20] |
| 6 | B. binchuanensis (Ye & Fei, 1995) | Fei and Ye 2016[13] |
| 7 | B. binlingensis (Jiang, Fei & Ye, 2009) | Fei and Ye 2016[13] |
| 8 | B. boettgeri (Boulenger, 1899) | Fei and Ye 2016[13] |
| 9 | B. brachykolos (Inger & Romer, 1961) | Fei and Ye 2016[13] |
| 10 | B. caobangensis (Nguyen, Pham, Nguyen, Luong & Ziegler, 2020) | Nguyen et al. 2020[21] |
| 11 | B. caudoprocta (Shen, 1994) | Fei and Ye 2016[22] |
| 12 | B. congjiangensis (Luo, Wang, Wang, Lu, Wang, Deng & Zhou, 2021) | Luo et al. 2021[23] |
| 13 | B. cheni (Wang & Liu, 2014) | Wang et al. 2014[24] |
| 14 | B. chishuiensis (Xu, Li, Liu, Wei & Wang, 2020) | Xu et al. 2020[25] |
| 15 | B. daiyunensis (Lyu, Wang & Wang, 2021) | Lyu et al. 2021[26] |
| 16 | B. daoji (Lyu, Zeng, Wang & Wang, 2021) | Lyu et al. 2023[10] |
| 17 | B. daweimontis (Rao & Yang, 1997) | Fei & Ye 2016[13] |
| 18 | B. dongguanensis (Wang & Wang, 2019) | Wang et al. 2019a[27] |
| 19 | B. elongata (Zeng, Wang, Chen, Xiao, Zhan, Li & Lin 2024) | Zeng et al. 2024[28] |
| 20 | B. fanjingmontis (Zhang, Liang, Ran & Shen 2012) | Zhang et al.2012[29] |
| 21 | B. fansipanensis (Tapley, Cutajar, Mahony, Nguyen, Dau, Luong, Le, Nguyen, Nguyen, Portway, Luong & Rowley, 2018) | Tapley et al. 2018[30] |
| 22 | B. fengshunensis (Wang, Zeng, Lyu, & Wang, 2022) | Wang et al. 2022[31] |
| 23 | B. frigida (Tapley, Cutajar, Nguyen, Portway, Mahony, Nguyen, Harding, Luong & Rowley, 2021) | Tapley et al. 2020[32] |
| 24 | B. hengshanensis (Qian, Hu, Mo, Gao, Zhang, & Yang, 2023) | Qian et al. 2023[33] |
| 25 | B. hoanglienensis (Tapley, Cutajar, Mahony, Nguyen, Dau, Luong, Le, Nguyen, Nguyen, Portway, Luong & Rowley, 2018) | Tapley et al. 2018[34] |
| 26 | B. hungtai (Wang, Zeng, Lyu, Xiao, & Wang, 2022) | Wang et al. 2022[31] |
| 27 | B. insularis (Wang, Liu, Lyu, Zeng & Wang, 2017) | Wang et al. 2017a[35] |
| 28 | B. jiangi (Liu, Li, Wei, Xu, Cheng, Wang & Wu, 2020) | Liu et al. 2020[36] |
| 29 | B. jingdongensis (Fei & Ye, 1983) | Fei & Ye 2016[13] |
| 30 | B. jinggangensis (Wang, 2012) | Wang et al. 2012[37] |
| 31 | B. jiulianensis (Wang, Zeng, Lyu & Wang, 2019) | Lyu et al. 2023[10] |
| 32 | B. kuatunensis (Pope, 1929) | Fei & Ye 2016; [13] |
| 33 | B. leishanensis (Li, Xu, Liu, Jiang, Wei & Wang, 2018) | Li et al. 2018[38] |
| 34 | B.lichun (Lin, Chen, Li, Peng, Zeng &Wang, 2024) | Lin et al. 2024[39] |
| 35 | B. lushuiensis (Shi, Li, Zhu, Jiang, Jiang & Wang, 2021) | Shi et al. 2021[40] |
| 36 | B. liboensis (Zhang, Li, Xiao, Li, Pan, Wang, Zhang & Zhou, 2017) | Zhang et al. 2017[41] |
| 37 | B. lini (Wang & Yang, 2014) | Wang et al. 2014[24] |
| 38 | B. lishuiensis (Wang, Liu & Jiang, 2017) | Wang et al. 2017b[42] |
| 39 | B. minor (Stejneger, 1926) | Fei & Ye 2016[13] |
| 40 | B. mirabilis (Lyu, Wang & Zhao, 2020) | Lyu et al. 2020[43] |
| 41 | B. mufumontana (Wang, Lyu & Wang, 2019) | Wang et al. 2019a[27] |
| 42 | B. nankunensis (Wang, Zeng & Wang, 2019) | Wang et al. 2019a[27] |
| 43 | B. nanlingensis (Lyu, Wang, Liu & Wang, 2019) | Wang et al. 2019a[27] |
| 44 | B. obesa (Wang, Li & Zhao, 2014) | Li et al. 2014[17] |
| 45 | B. ombrophila (Messenger & Dahn, 2019) | Messenger et al. 2019[44] |
| 46 | B. omeimontis (Liu, 1950) | Fei & Ye 2016[13] |
| 47 | B. palpebralespinosa (Bourret, 1937) | Fei & Ye 2016[13] |
| 48 | B. pepe (Wang & Zeng, 2024) | Wang et al. 2024[45] |
| 49 | B. puningensis(Wang, Zeng, Lyu, Xiao, & Wang, 2022) | Wang et al. 2022[31] |
| 50 | B. qianbeinsis (Su, Shi, Wu, Li, Yao, Wang & Li, 2020) | Su et al. 2020[46] |
| 51 | B. rubrimera (Tapley, Cutajar, Mahony, Chung, Dau, Nguyen, Luong & Rowley, 2017) | Tapley et al. 2017[47] |
| 52 | B. sangzhiensis (Jiang, Ye & Fei, 2008) | Jiang et al. 2008[48] |
| 53 | B. sanmingensis (Lyu & Wang, 2021) | Lyu et al. 2023[26] |
| 54 | B. shimentaina (Lyu, Liu & Wang, 2020) | Lyu et al. 2023[10] |
| 55 | B. shuichengensis (Tian and Sun, 1995) | Tian et al. 2000[49] |
| 56 | B. shunhuangensis (Wang, Deng, Liu, Wu & Liu, 2019) | Wang et al. 2019b[42] |
| 57 | B. spinata (Liu & Hu, 1973) | Fei & Ye 2016[13] |
| 58 | B. tongboensis (Wang & Lyu, 2021) | Lyu et al. 2023[10] |
| 59 | B. tuberogranulatus (Shen, Mo & Li, 2010) | Mo et al. 2010[50] |
| 60 | B. wugongensis (Wang, Lyu & Wang, 2019) | Wang et al. 2019a[27] |
| 61 | B. wuliangshanensis (Ye & Fei, 1995) | Fei and Ye 2020[51] |
| 62 | B. wushanensis (Ye & Fei, 1995) | Fei and Ye 2020[51] |
| 63 | B. xiangnanensis (Lyu, Zeng & Wang, 2020) | Lyu et al. 2023[10] |
| 64 | B. xianjuensis (Wang, Wu, Peng, Shi, Lu & Wu, 2020) | Wang et al. 2020[52] |
| 65 | B. xuefengmontis (Lyu & Wang, 2023) | Lyu et al. 2023[10] |
| 66 | B. yangmingensis (Lyu, Zeng & Wang, 2020) | Lyu et al. 2023[10] |
| 67 | B. yaoshanensis (Qi, Mo, Lyu, Wang & Wang, 2021) | Qi et al. 2021[53] |
| 68 | B. yingdeensis (Qi, Lyu, Wang & Wang, 2021) | Qi et al. 2021[53] |
| 69 | B. yunkaiensis (Qi, Wang, Lyu & Wang, 2021) | Qi et al. 2021[53] |
| Species | SVL in males (in mm) | SVL in females (in mm) | horn-like tubercle at upper eyelid: slightly large (2), small (1) |
Vomerine teeth: present (1), or absent (0) |
Tongue: notched (1), or not notched (0) |
Heels overlapping (2) just meeting (1), or not meeting (0) |
lateral fringes on toes: wide (2), narrow (1), or absent (0) | Webs on toes: more than one-fourth (2), rudimentary (1), or lacking (0) | |
| 1 | B. dupanglingensissp. nov. | 37.8-40.2 | 41.8-45.9 | 1 | 0 | 0 | 2 | 0 | 1 |
| 2 | B. acuta | 27.1–33.0 | 28.1–33.6 | 2 | 0 | 0 | 0 | 1 | 1 |
| 3 | B. angka | 31.2–32.1 | 37.5–39.2 | 1 | 0 | 0 | 1 or 2 | 0 | 1 |
| 4 | B. anlongensis | 40.0–45.5 | 48.9–51.2 | 1 | 0 | 0 | 2 | 1 | 1 |
| 5 | B. baishanzuensis | 28.4–32.4 | / | 1 | 0 | 0 | 2 | 1 | 0 |
| 6 | B. baolongensis | 42.0–45.0 | / | 1 | 0 | 1 | 1 | 0 | 0 |
| 7 | B. binchuanensis | 32.0–36.0 | 40.2–42.5 | 1 | 0 | 0 or 1 | 1 | 2 | 1 |
| 8 | B. binlingensis | 45.1–51.0 | / | 1 | 0 | 1 | 2 | / | 1 |
| 9 | B. boettgeri | 34.5–37.8 | 39.7–46.8 | 1 | 0 | 1 | 1 | 2 | 1 |
| 10 | B. brachykolos | 33.7–39.3 | 33.9–45.9 | 1 | 0 | 0 | 0 | 0 | 1 |
| 11 | B. caobangensis | 34.9–38.9 | / | 1 | 0 | 0 | / | 0 | 1 |
| 12 | B. caudoprocta | 81.3 | / | 2 | 1 | 0 | 1 | / | 1 |
| 13 | B. congjiangensis | 28.6–33.4 | 38.4–40.2 | 1 | 0 | 0 | 2 | 1 | 1 |
| 14 | B. cheni | 26.2–29.5 | 31.8–34.1 | 1 | 0 | 1 | 2 | 2 | 1 |
| 15 | B. chishuiensis | 43.4–44.1 | 44.8–49.8 | 1 | 0 | 0 | 2 | 0 | 1 |
| 16 | B. daiyunensis | 27.6–28.7 | 33.7–35.6 | 1 | 1 | 0 | 1 or 2 | 1 | 1 |
| 17 | B. daoji | 32.6–33.6 | 37.5–41.4 | 1 | 0 | 0 | 0 | 1 | 1 |
| 18 | B. daweimontis | 34.0–37.0 | 40.0–46.0 | 1 | 1 | / | / | 0 | 0 |
| 19 | B. dongguanensis | 30.2–39.3 | / | 1 | 1 | 0 | 0 | 0 | 1 |
| 20 | B. elongata | 28.2–28.5 | 35.1–37.6 | 1 | 1 | 0 | 1 | 1 | 0 |
| 21 | B. fanjingmontis | 58.2–63.6 | 62.8–72.2 | 1 | 1 | 1 | 1 | 2 | 1 |
| 22 | B. fansipanensis | 30.9–44.3 | 41.7–42.5 | 1 | 1 | 0 or 1 | / | 0 | 0 |
| 23 | B. fengshunensis | 34.3–39.4 | 42.5–44.9 | 1 | 1 | 0 | 0 | 0 | 1 |
| 24 | B. frigida | 30.3–31.8 | / | 1 | 1 | 0 or 1 | / | 0 | 0 |
| 25 | B.hengshanensis | 35.7–41.2 | 37.5–50.2 | 1 | 0 | 0 | 0 | 0 | 0 |
| 26 | B. hoanglienensis | 37.4–47.6 | 59.6 | 1 | 1 | 1 | / | 0 | 1 or 0 |
| 27 | B. hungtai. | 25.8–33.3 | / | 1 | 0 | 0 | 0 | 0 | 0 |
| 28 | B. insularis | 36.8–41.2 | 47.1 | 1 | 1 | 1 | 0 | 0 | 1 |
| 29 | B. jiangi | 34.4–39.2 | 39.5–40.4 | 1 | 0 | 0 | 2 | 0 | 1 |
| 30 | B. jingdongensis | 53.0–56.5 | 63.5 | 1 | 1 | 1 | 2 | 2 | 2 |
| 31 | B. jinggangensis | 35.1–36.7 | 38.4–41.6 | 2 | 1 | 0 | 2 | 1 | 1 |
| 32 | B. jiulianensis | 30.4–33.9 | 34.1–37.5 | 1 | 1 | 1 | 2 | 0 | 1 |
| 33 | B. kuatunensis | 26.2–31.4 | 26.6–37.3 | 1 | 0 | 1 | 0 or 2 | 0 or 1 | 0 |
| 34 | B. leishanensis | 30.4–38.7 | 42.3 | 1 | 0 | 0 | 2 | 0 | 1 |
| 35 | B. lichun | 33.5–37.0 | 47.1 | 1 | 1 | 0 | 0 | 0 | 0 |
| 36 | B. liboensis | 60.5–67.7 | 60.8–70.6 | 2 | 1 | 1 | 2 | 2 | 1 |
| 37 | B. lini | 34.1–39.7 | 37.0–39.9 | 1 | 0 | 0 | 2 | 2 | 1 |
| 38 | B. lishuiensis | 30.7–34.7 | 36.9–40.4 | 1 | 0 | 0 | / | 0 | 0 |
| 39 | B. lushuiensis | 31.0–34.8 | / | 1 | 0 | 1 | / | 1 | 1 |
| 40 | B. minor | 34.5–41.2 | / | 1 | 0 | 1 | 1 or 2 | 0 | 1 |
| 41 | B. mirabilis | 55.8–61.4 | 68.5–74.8 | 2 | 0 | 0 | 2 | 1 | 1 |
| 42 | B. mufumontana | 30.1–30.8 | 36.3 | 1 | 0 | 0 | 2 | 1 | 1 |
| 43 | B. nankunensis | 29.9–34.9 | 39.4–41.9 | 1 | 1 | 0 | 0 | 0 | 1 |
| 44 | B. nanlingensis | 30.5–37.3 | / | 1 | 1 | 1 | 2 | 1 | 1 |
| 45 | B. obesa | 35.6 | 37.5–41.2 | 1 | 0 | 0 | 0 | 0 | 1 |
| 46 | B. ombrophila | 27.4–34.5 | 32.8–35.0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 47 | B. omeimontis | 56.0–59.5 | 68.0–72.5 | 1 | 1 | 1 | 2 | 1 | 1 |
| 48 | B. palpebralespinosa | 36.2–38.0 | / | 2 | 1 | 0 | 2 | 2 | 2 |
| 49 | B. pepe | 35.3–36.4 | / | 1 | 0 | 1 | 0 | 0 | 0 |
| 50 | B. puningensis | 31.7–34.6 | 37.8–38.3 | 1 | 1 | 0 | 0 | 0 | 1 |
| 51 | B. qianbeinsis | 49.3–58.2 | / | 1 | 1 | 1 | 2 | 2 | 2 |
| 52 | B. rubrimera | 26.6–30.8 | / | 1 | 1 | 0 or 1 | / | 1 | 0 |
| 53 | B. sangzhiensis | 54.7 | / | 1 | 1 | 1 | 2 | 1 | 1 |
| 54 | B. sanmingensis | 27.0–29.5 | 29.5 | 1 | 0 | 1 | 2 | 2 | 1 |
| 55 | B. shimentaina | 28.0–30.6 | / | 1 | 1 | 0 | 2 | 1 | 1 |
| 56 | B. shuichengensis | 102.0–118.3 | 99.8–115.6 | 2 | 0 | 1 | / | 2 | 2 |
| 57 | B. shunhuangensis | 30.3–33.7 | 37.6 | 1 | 0 | 0 | 2 | 0 | 1 |
| 58 | B. spinata | 47.2–54.4 | 54.0–55.0 | 1 | 0 | 1 | 2 | 2 | 2 |
| 59 | B. tongboensis | 26.5–31.5 | / | 1 | 1 | 1 | 2 | 0 | 0 |
| 60 | B. tuberogranulatus | 33.2–39.0 | 50.5 | 1 | 0 | 0 | / | 0 | 1 |
| 61 | B. wugongensis | 31.0–34.1 | 38.5–42.8 | 1 | 0 | 0 | 0 | 0 | 1 |
| 62 | B. wuliangshanensis | 27.3–31.6 | 41.3 | 1 | 0 | 0 or 1 | 2 | 0 | 0 |
| 63 | B. wushanensis | 30.4–35.5 | 38.4 | 1 | 0 | 0 | 1 | 0(in female), 2(in male) |
1 |
| 64 | B. xiangnanensis | 38.6–42.0 | 44.4 | 1 | 0 | 0 | 1 | 2 | 1 |
| 65 | B. xianjuensis | 31.0–36.3 | 41.6 | 1 | 0 | 0 | 2 | 1 | 1 |
| 66 | B. xuefengmontis | 37.0–38.3 | 45.3–48.9 | 1 | 0 | 0 | 1 or 2 | 0 | 0 |
| 67 | B. yaoshanensi | 32.5–42.6 | 46.6–47.4 | 1 | 0 | 0 | 1 or 2 | 0 | 1 |
| 68 | B. yangmingensis | 33.2–37.1 | 45.2 | 1 | 0 | 0 | 2 | 1 | 1 |
| 69 | B. yingdeensis | 33.2–35.3 | 36.3–45.8 | 1 | 1 | 0 | 1 or 2 | 0 | 1 |
| 70 | B. yunkaiensis | 35.3–40.0 | 45.3–46.1 | 1 | 0 | 0 | 1 or 2 | 0 | 1 |
| HUNU 22SA01* | HUNU 22SA02 | HUNU 22SA03 | HUNU 22SA04 | HUNU 22SA05 | HUNU 22SA06 | CIB2016050802 | HUNU 22SA07 | HUNU 22SA08 | HUNU 22SA09 | |
| Sex | Male | Male | Male | Male | Male | Male | Male | Female | Female | Female |
| SVL | 39.6 | 38.1 | 37.8 | 40.2 | 38.8 | 38.5 | 37.6 | 43.0 | 45.9 | 41.8 |
| HDL | 13.3 | 12.8 | 13.2 | 12.1 | 12.1 | 12.1 | 11.3 | 13.3 | 13.1 | 13.2 |
| HDW | 13.7 | 13.2 | 13.4 | 12.7 | 13.4 | 13.2 | 12.6 | 14.2 | 14.9 | 14.0 |
| SNT | 3.3 | 3.3 | 3.2 | 3.4 | 3.2 | 3.1 | 4.4 | 3.2 | 3.4 | 3.1 |
| IND | 4.4 | 4.1 | 3.7 | 4.2 | 4.2 | 3.9 | 4.2 | 4.4 | 4.7 | 4.4 |
| IOD | 3.1 | 3.3 | 3.3 | 3.7 | 3.2 | 3.3 | 3.8 | 3.6 | 3.8 | 3.7 |
| ED | 6.3 | 5.6 | 5.2 | 6.0 | 5.6 | 5.8 | 4.2 | 5.5 | 6.0 | 5.3 |
| TD | 3.3 | 3.2 | 2.9 | 2.9 | 3.1 | 3.1 | 2.3 | 2.6 | 3.1 | 3.0 |
| TED | 1.8 | 1.8 | 1.8 | 1.8 | 1.4 | 1.6 | 2.4 | 2.0 | 2.5 | 2.1 |
| HND | 9.7 | 9.2 | 9.7 | 9.5 | 9.2 | 9.2 | 8.4 | 10.3 | 11.8 | 9.8 |
| RAD | 8.0 | 7.4 | 7.8 | 7.7 | 7.6 | 7.2 | 8.9 | 8.0 | 8.3 | 7.1 |
| TIB | 15.1 | 14.8 | 14.9 | 15.5 | 14.8 | 14.4 | 14.6 | 15.7 | 18.6 | 15.8 |
| FTL | 23.8 | 23.1 | 23.2 | 23.6 | 23.2 | 22.8 | / | 25.4 | 27.3 | 26.7 |
| TD/ED | 0.52 | 0.57 | 0.48 | 0.48 | 0.55 | 0.53 | 0.54 | 0.53 | 0.47 | 0.57 |
| RAD/SVL | 0.20 | 0.19 | 0.21 | 0.19 | 0.20 | 0.19 | 0.24 | 0.19 | 0.18 | 0.17 |
| TIB/SVL | 0.38 | 0.39 | 0.39 | 0.39 | 0.38 | 0.37 | 0.39 | 0.37 | 0.41 | 0.38 |
| FTL/SVL | 0.60 | 0.61 | 0.61 | 0.59 | 0.60 | 0.59 | / | 0.59 | 0.59 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





