1. Introduction
Ion channels play crucial roles in tumor pathophysiology, contributing to the regulation of membrane potential, cell cycle progression, cellular osmolarity, motility, invasion, migration, and proliferation [
1,
2]. Among these, potassium (K⁺) channels—encoded by approximately 77 genes—represent the most diverse and abundant group in excitable cells. These channels are essential for maintaining ionic homeostasis, selectively permitting potassium ion flux across membranes in response to various environmental stimuli, while restricting the movement of other ions.
Based on their activation mechanisms, ion channels are generally classified into voltage-gated, ligand-gated, and mechanically gated types. The term “ion channel” often refers specifically to voltage-gated channels, whose activity is predominantly regulated by membrane potential. These channels are named according to the ions that most readily permeate them.
Recently, cancer has increasingly been considered a channelopathy, and there is growing interest in targeting ion channel modulation as a novel therapeutic approach, particularly in breast cancer [
1].
Potassium channel proteins are involved in a wide range of physiological and pathological processes [
2]. Notably, dysregulation and abnormal expression of potassium channels have been observed in several types of cancer, including breast [
3], colorectal [
4], prostate [
5], lung [
6], liver [
7], and glioma [
8]. Several subtypes, such as KCNN4 [
9], KCNA1 [
10], Kv11.1 [
11], KCNK9 [
11,
12], KCNE1 [
13], and GIRK1 [
14], have been implicated in the malignant transformation and progression of breast cancer. Additionally, KCNK6 has been reported to be overexpressed in both breast cancer [
15] and thyroid carcinoma [
16].
Hou et al. demonstrated that KCNK6 expression is elevated in breast cancer cells, leading to weakened cell adhesion [
17]. Pharmacological inhibition of this channel enhanced cellular adhesion and biophysical function, suggesting that upregulation of KCNK6 may facilitate cancer cell proliferation, migration, and invasion. Similarly, Sun et al. reported that KCNK1 is overexpressed in breast cancer and associated with poor prognosis [
18]. Silencing of KCNK1 reduced cell proliferation and migration while enhancing sensitivity to paclitaxel chemotherapy.
In MCF-7 breast cancer cells, the “membrane potential” model proposed by Wonderlin et al. suggests that both proliferation and cell cycle progression are tightly linked to K⁺ channel activity [
19]. Inhibition of these channels causes membrane depolarization, which in turn suppresses cell proliferation. Electrophysiological studies have revealed that at least six distinct K⁺ currents—differing in their dependence on voltage, intracellular Ca²⁺, and ATP—are present in MCF-7 cells [
20,
21,
22,
23].
However, the precise mechanisms by which non-selective K⁺ channel blockers such as 4-aminopyridine (4-AP) induce cell death in breast cancer cells remain unclear. 4-Aminopyridine (4-AP) blocks potassium channels, leading to cell depolarization and calcium influx. Due to this property, it enhances neurotransmitter release by increasing Ca²⁺ influx in the presynaptic region and accelerates axonal conduction, making it useful in the treatment of central and peripheral nervous system disorders such as Multiple Sclerosis (MS), spinal cord injury (SCI), botulism, and Lambert-Eaton syndrome [
24].
Given 4-AP’s action as a potassium channel blocker, the altered expression of potassium channels in cancer cells, and the effects of channel blockade on cell proliferation [
13,
25,
26], studies have shown that 4-AP inhibits cancer cell proliferation and induces apoptosis [
27].
In this study, we hypothesized that blocking K⁺ channels with 4-AP alters membrane potential and calcium signaling, leading to regulated cell death. To investigate this, we employed pharmacological inhibitors—including cycloheximide (CHX), Z-VAD-FMK, and 2-APB—to dissect the involvement of apoptotic and calcium-dependent cell death pathways. The effects of 4-AP on cell viability, membrane potential, and intracellular calcium concentration were systematically evaluated.
2. Results
2.1. Determination of IC50 Values and the Investigation of Cell Death Mechanisms Induced by 4-AP in L929 and MCF-7 Cell Lines
The IC50 values of 4-aminopyridine (4-AP) for L929 (healthy fibroblast) and MCF-7 (breast cancer) cell lines were determined using the trypan blue exclusion method with a hemocytometer. A concentration range of 1–5 mM was used to determine the IC50 values. The IC50 value of 4-AP was found to be 4 mM for MCF-7 cells and 5 mM for L929 cells. The higher IC50 observed in L929 cells indicates lower sensitivity to 4-AP compared to MCF-7 cells. L929 cells, which do not exhibit overexpression of voltage-gated potassium channels, were selected as the healthy control line. The considerable difference in IC50 values between the two cell lines suggests that 4-AP exhibits selective cytotoxicity, potentially related to differential ion channel expression.
In subsequent experiments, the effects of 4-AP on cell death mechanisms were investigated using cycloheximide (CHX), z-VAD-FMK, and 2-APB based on concentrations previously reported in the literature.
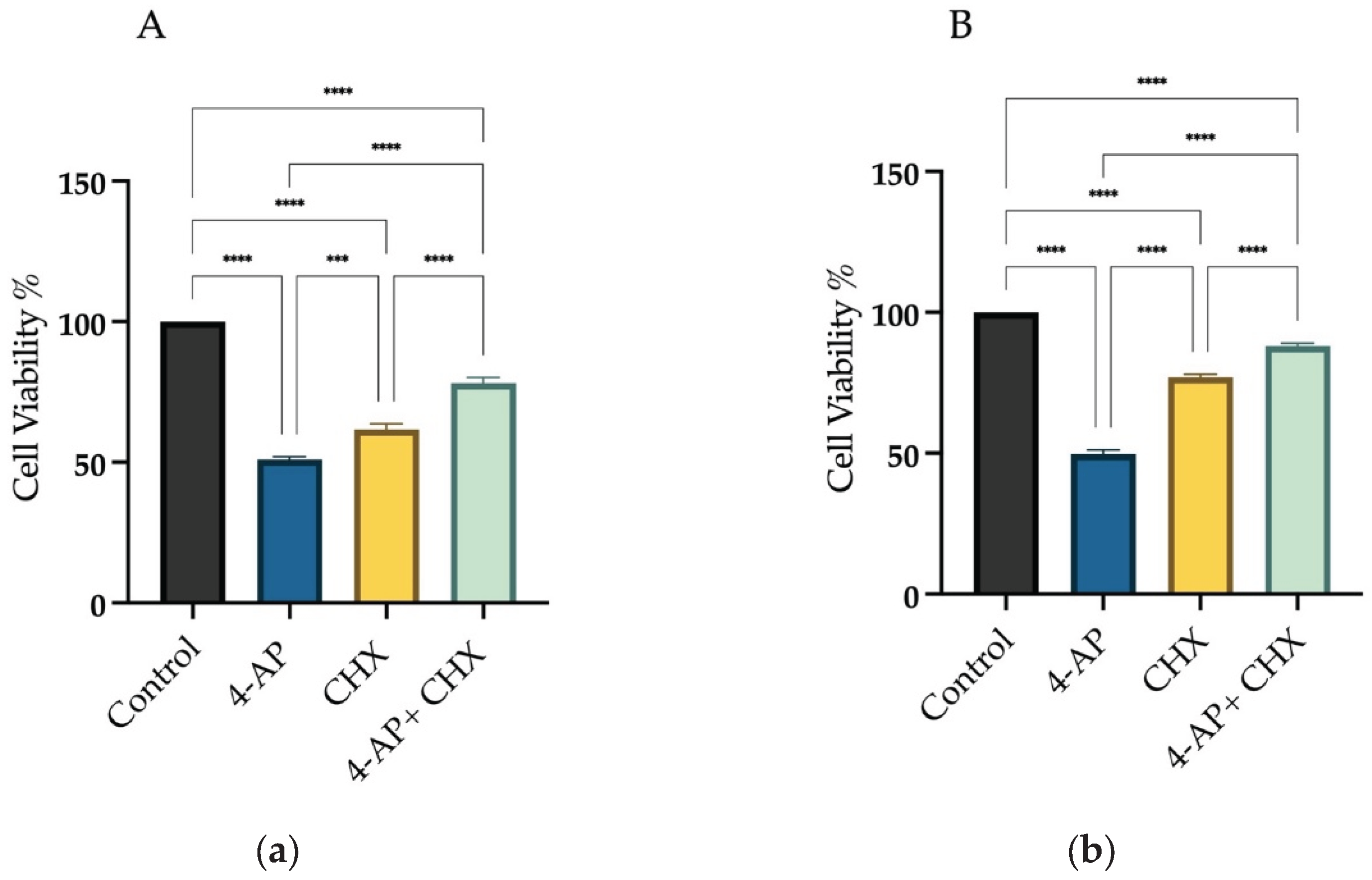
For CHX experiments, a 20 µM concentration was chosen. At this dose, cell viability was 61.6% ± 2.4 for L929 and 77% ± 1.5 for MCF-7. Cells were pretreated with CHX for 1 hour, and then treated with 4-AP for 24 hours. After the combined treatment, cell viability increased to 78% ± 2.2 in L929 and 88% ± 1.2 in MCF-7 cells (
Figure 1A,B). If 4-AP-induced cytotoxicity were solely due to paraptosis, these viability levels would be expected to be even higher, suggesting the involvement of other cell death mechanisms.
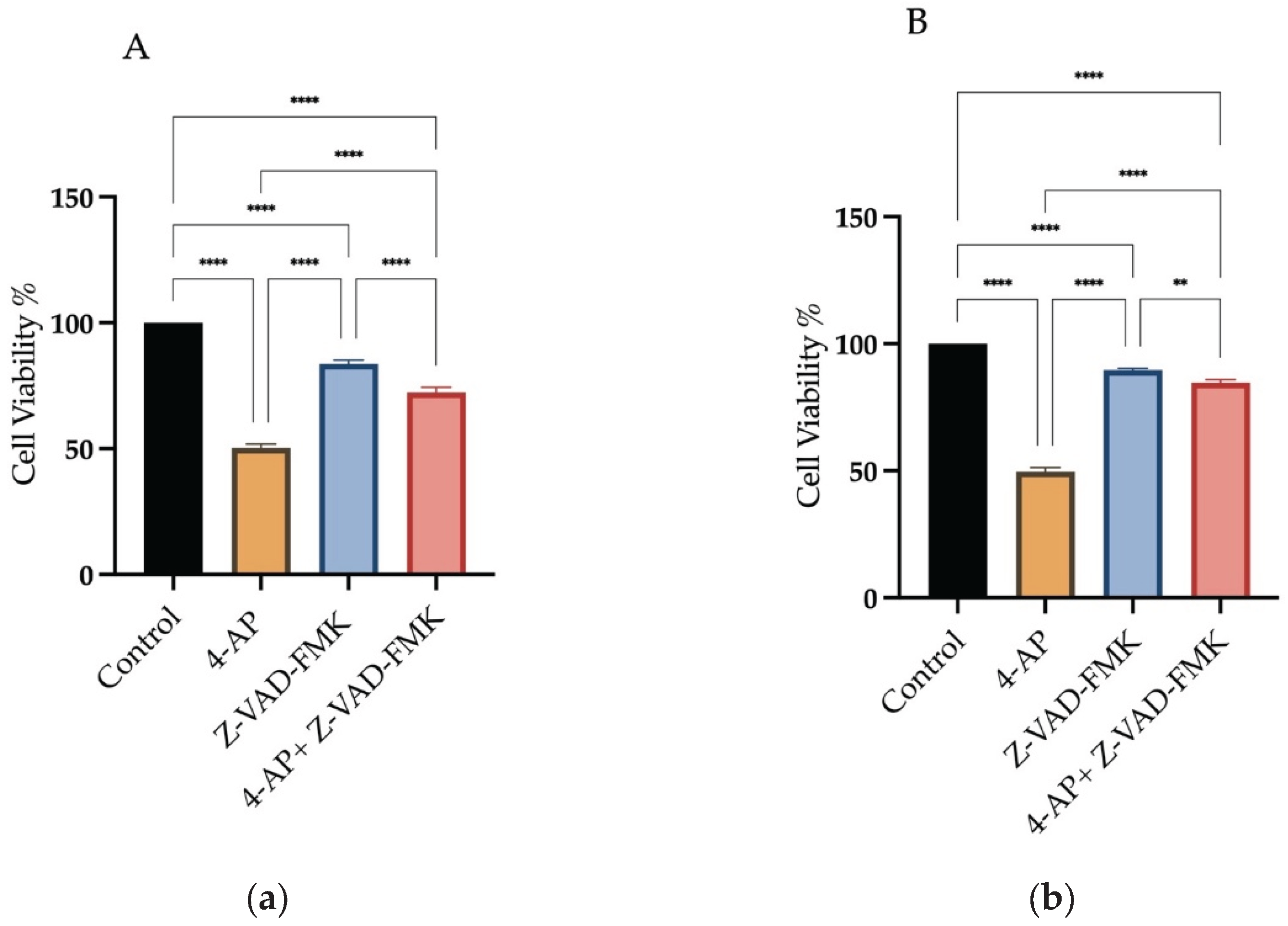
To explore the involvement of apoptosis, Z-VAD-FMK, a pan-caspase inhibitor, was used at a concentration of 50 µM. Treatment with Z-VAD-FMK alone resulted in cell viabilities of 83.6% ±1.9 for L929 and 91% ±0.9 for MCF-7. Following 1-hour pretreatment with Z-VAD-FMK, cells were incubated with 4-AP for 24 hours. Under these conditions, cell viability decreased to 72.3% ±2.4 in L929 and 85% ±1.2 in MCF-7 (
Figure 2A,B). These findings indicate that other cell death pathways may also be involved.
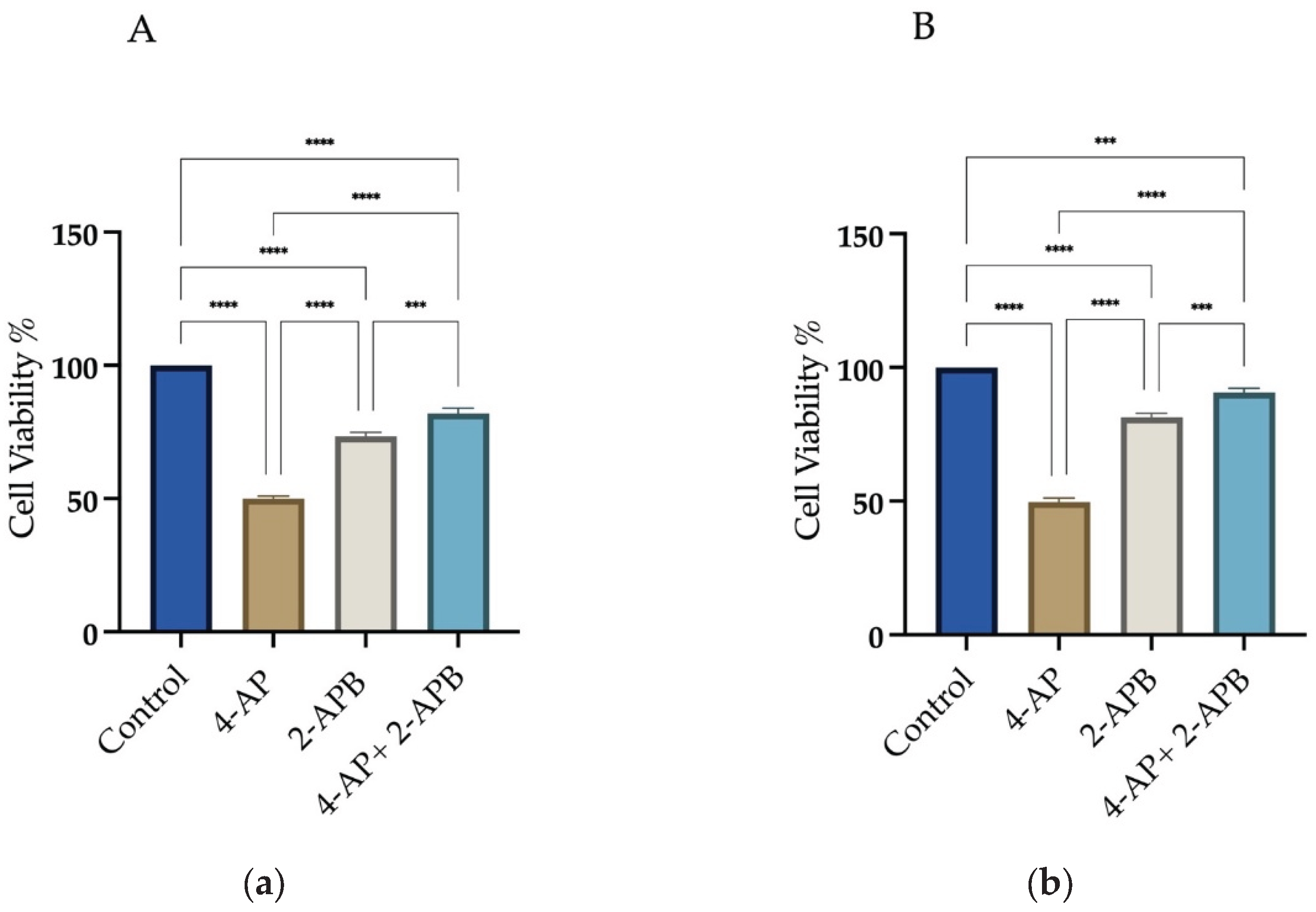
To assess the role of calcium signaling in 4-AP-induced cell death, 2-APB, an IP3 receptor inhibitor, was employed at a concentration of 10 µM. Treatment with 2-APB alone resulted in cell viabilities of 73.3% ±1.9 for L929 and 73% ±2.1 for MCF-7. Following 1-hour pretreatment with 2-APB and subsequent incubation with 4-AP for 24 hours, cell viability increased to 82% ±2.5 for L929 and 92% ±2.0 for MCF-7 (
Figure 3A,B). These findings suggest that IP3-mediated calcium signaling plays a significant role in 4-AP-induced cytotoxicity. The high cell viability observed after combined 2-APB and 4-AP treatment implies that inhibition of calcium signaling may prevent both apoptosis and paraptosis.
2.2. Assessment of Intracellular Calcium Changes Following Drug Treatments
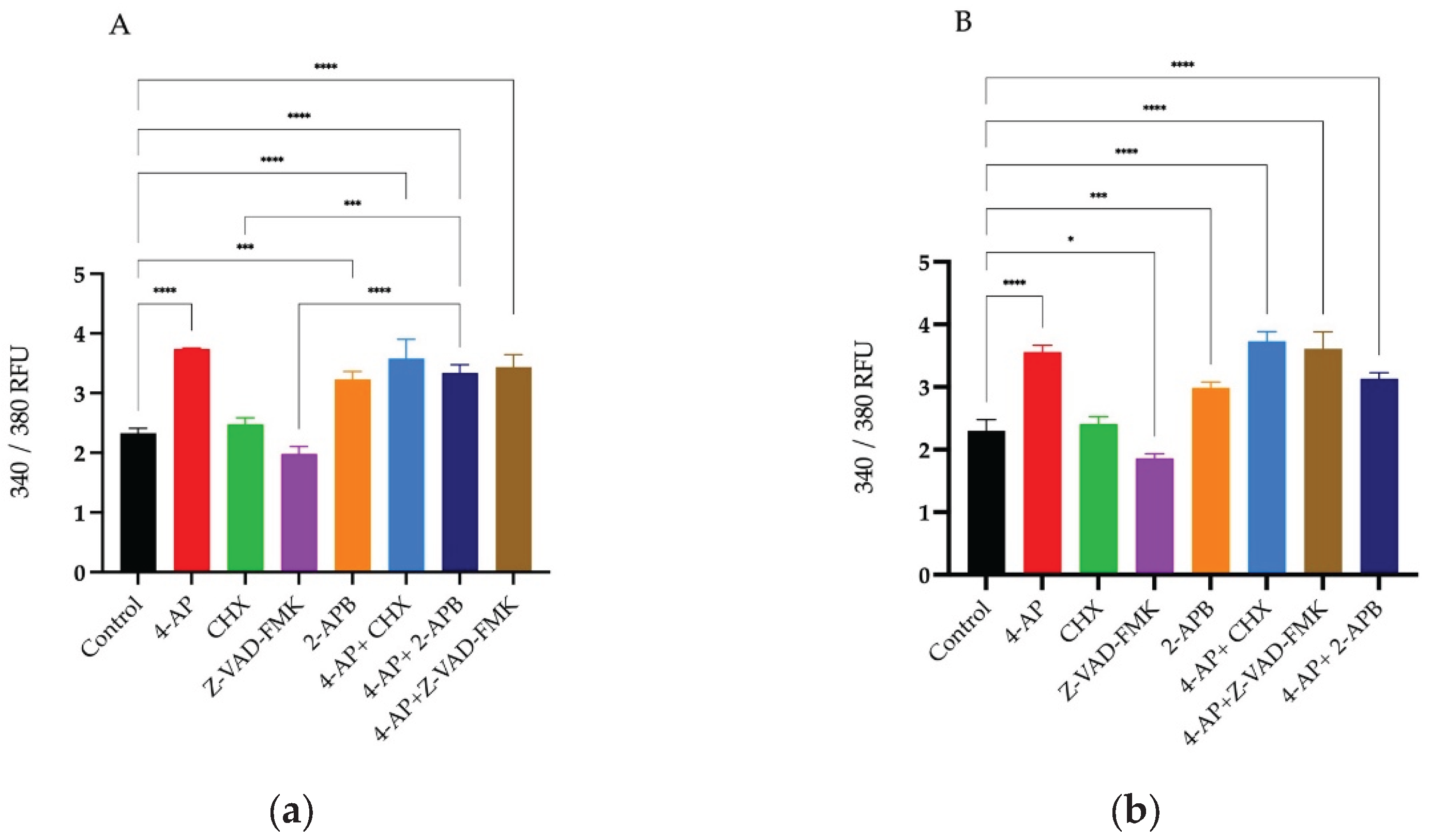
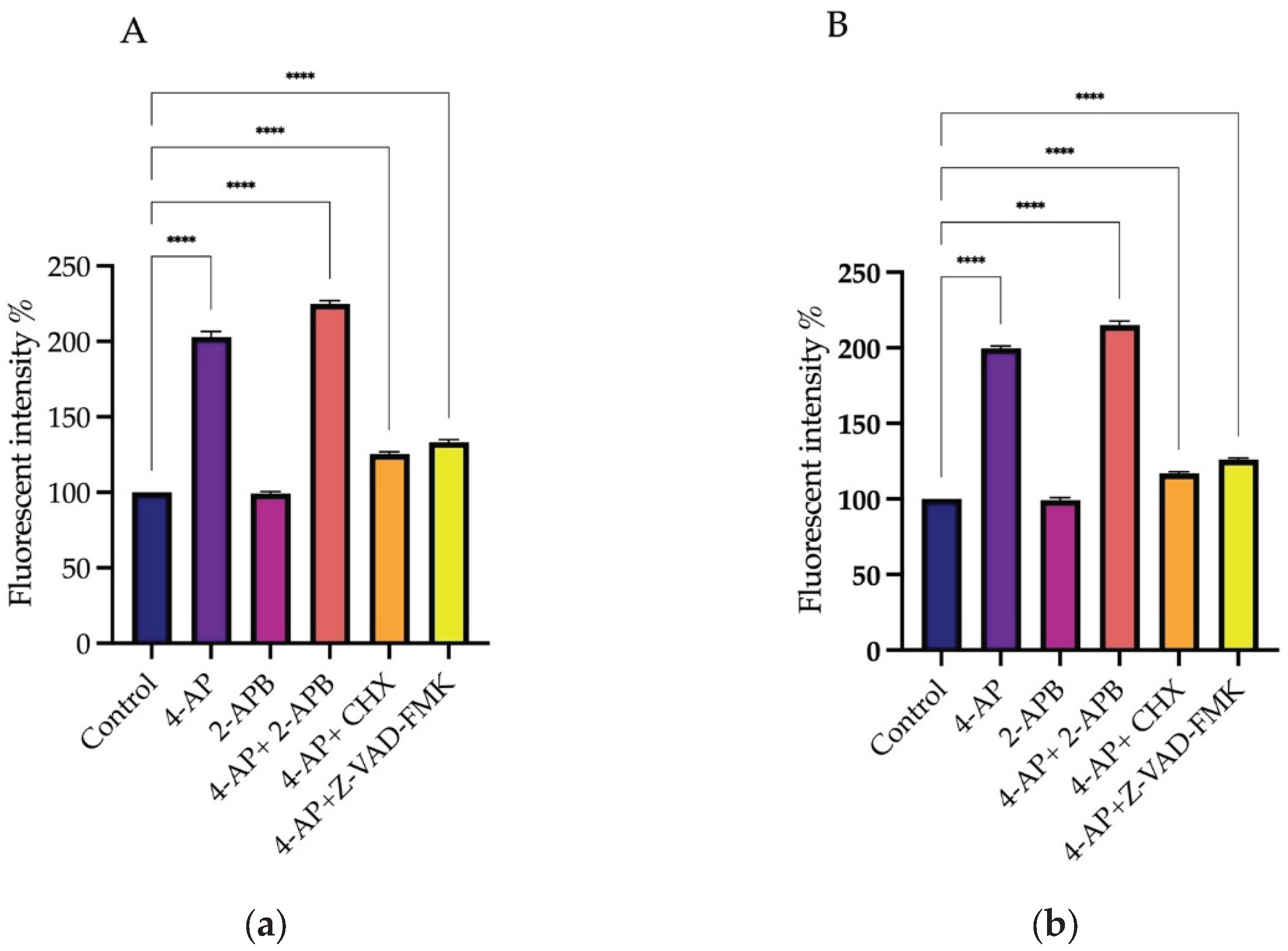
After drug treatments, changes in intracellular calcium levels were measured using the Screen Quest Fura-2 AM no-wash calcium assay kit. Fura-2 crosses the cell membrane and enters the cytoplasm. When Fura-2 binds to free calcium ions, its fluorescence emission wavelength shifts towards the blue region. When agonists or other signals stimulate the cell, receptor activation triggers the release of calcium, leading to an increase in the fluorescent intensity of Fura-2. For analysis of intracellular Ca²⁺ changes, Fura-2 measurements were taken immediately after drug addition for 20 hours. The results at 20th hour are presented as a histogram (
Figure 4A,B).
The percentages of changes in intracellular Ca²⁺ concentration and cell viabilities after treatments are presented in the table relative to the control group (
Table 1). Briefly, 4-AP and its combinations caused an increase in intracellular Ca²⁺ concentration. CHX induced a slight increase, whereas Z-VAD-FMK led to a decrease in intracellular Ca²⁺ levels. Although 2-APB is an IP₃ receptor blocker, its treatment resulted in an unexpected increase in intracellular Ca²⁺ concentration. Both 2-APB alone and in combination with 4-AP increased intracellular Ca²⁺ levels. The combination effect of 4-AP and 2-APB can be explained by the blockade of voltage-gated potassium channels (VGKC) by 4-AP, which causes membrane depolarization. This depolarization likely opens voltage-gated calcium channels (VGCC), leading to an increase in intracellular Ca²⁺ concentration.
2.3. Membrane Potential Measurements: Effect of Drug Treatments on Membrane Polarization
Changes in membrane potential induced by drug treatments were measured using the DiBAC4(3) fluorescent dye. DiBAC4(3) is a hydrophobic dye that easily passes through the cell membrane, enabling detection of membrane potential alterations. In the absence of stimulation, the negative charge on the cytoplasmic side of the membrane prevents the ionized dye from entering the cell. Upon membrane depolarization triggered by stimulation, the dye binds to intracellular proteins and lipids, leading to an increase in fluorescence intensity. Conversely, during hyperpolarization, the fluorescence intensity of the dye decreases.
To determine changes in membrane potential following the inhibition of 4-AP-induced cell death, drugs and their combinations with 4-AP were administered, and DiBAC4(3) fluorescence measurements were performed using a microplate reader 15 minutes after treatments for 24 hours.
Measurements were taken in a time-dependent manner. In the L929 cell line, 4-AP treatment induced membrane depolarization. Since 4-AP blocks voltage-gated potassium channels (VGKCs), this blockade results in membrane depolarization. The combination of 4-AP and 2-APB also led to depolarization (
Figure 5A). The depolarization caused by 4-AP likely activated voltage-gated calcium channels (VGCCs), enhancing calcium influx and resulting in a greater degree of depolarization than 4-AP treatment alone. Combinations of 4-AP with CHX or Z-VAD-FMK also induced membrane depolarization; however, the depolarization levels were lower compared to 4-AP alone or the 4-AP + 2-APB combination. This difference may be attributed to intracellular accumulation of potassium ions without additional VGCC activation. Treatment with 2-APB alone did not induce membrane depolarization.
In the MCF-7 cell line, a similar pattern was observed. 4-AP treatment alone induced membrane depolarization, and its combination with 2-APB enhanced this effect. However, 4-AP combined with CHX or Z-VAD-FMK caused only a slight depolarization of the membrane (
Figure 5B).
3. Discussion
In this study, we demonstrated that 4-aminopyridine (4-AP), a known potassium channel blocker used in neurological disorders, induces cell death in MCF-7 breast cancer cells through both apoptotic and paraptotic pathways. Despite the development of chemotherapeutic agents, resistance to apoptosis remains a major limitation in effective cancer treatment [
28]. Therefore, triggering alternative cell death mechanisms, such as paraptosis, has become increasingly relevant in overcoming therapeutic resistance.
Our results showed that treatment with 4-AP caused vacuolization and cell swelling in MCF-7 cells, indicative of paraptotic or paraptosis-like cell death. These findings are in line with previous studies reporting that paraptosis is characterized by cytoplasmic vacuole formation, endoplasmic reticulum (ER) and mitochondrial swelling, and a dependence on protein synthesis [
29,
30]. The protective effect of the protein synthesis inhibitor cycloheximide (CHX) and therefore paraptotic cell death [
31], which increased MCF-7 cell survival from 53% to 88%, supports the involvement of a paraptotic mechanism.
Furthermore, we assessed whether apoptotic pathways were simultaneously involved in the 4-AP-induced cell death process. The application of the pan-caspase inhibitor z-vad-fmk significantly increased survival rates in both MCF-7 and L929 cells, suggesting that apoptosis also contributes to the observed cytotoxicity. These findings align with other studies reporting simultaneous activation of multiple cell death pathways in cancer cells exposed to various compounds, including celastrol, iturin A, and honokiol [
32,
33,
34].
Interestingly, although caspase-9 activity is reported to be required for paraptosis [
35], Sperandio et al. demonstrated that paraptosis is not inhibited by z-vad-fmk, indicating mechanistic complexity [
31]. Our findings that z-vad-fmk increases survival suggest partial overlap or co-activation of apoptotic and paraptotic pathways. To delineate the sequence and dominance of these pathways, future experiments using selective caspase-9 inhibitors and paraptosis-specific blockers such as AIP1/Alix are needed.
Another key observation in our study was the intracellular calcium (Ca²⁺) influx following 4-AP treatment. Using Fura-2-based analyses, we confirmed that 4-AP induced significant increases in intracellular Ca²⁺ levels. Pre-incubation with the IP3 receptor antagonist 2-APB improved survival rates (92% in MCF-7 cells), indicating that ER-derived Ca²⁺ release contributes to 4-AP-induced cell death. These findings are consistent with the notion that disruption of Ca²⁺ homeostasis is central to both apoptosis and paraptosis [
36].
However, we also observed that 2-APB alone induced Ca²⁺ elevation greater than 4-AP. This may be explained by its known activation of Orai3 channels in estrogen receptor-positive breast cancer cells, as previously reported [
37,
38]. Therefore, the context-dependent action of 2-APB and its concentration-specific effects on calcium channels must be carefully considered in interpreting these data [
39].
The observed ER and mitochondrial changes, combined with altered calcium signaling, support the idea that 4-AP simultaneously triggers both mitochondrial-mediated apoptosis and ER-related paraptosis. Nevertheless, temporal dynamics remain unclear. Confocal microscopy-based kinetic analyses of intracellular Ca²⁺ distribution, in combination with selective pathway inhibitors, will be crucial to clarify the sequence of events.
In conclusion, our findings suggest that 4-AP induces cancer cell death via dual activation of paraptotic and apoptotic pathways. This dual action may overcome apoptosis resistance in certain cancer types. Although 4-AP is unlikely to serve as a standalone anticancer agent, its ability to modulate ion channel activity and disrupt Ca²⁺ homeostasis makes it a promising candidate for combination therapy with conventional chemotherapeutics. As demonstrated by our previous findings that 4-AP in combination with paclitaxel increased cell death of both MCF-7 and MDA-MB-231 breast cancer cell lines [
40].
4. Materials and Methods
4.1. Cell Lines and the Growth Condition
The breast cancer cell line MCF-7 (ATCC HTB 22) and healthy mouse fibroblast cell line L929 (ATCC CCL-1) were used in this study. Cells were grown in DMEM medium containing L-glutamine (Capricorn Scientific GmbH, Germany) supplemented with 10% Fetal Bovine Serum (FBS; Capricorn Scientific GmbH, Germany), 1% penicillin/streptomycin (Capricorn Scientific GmbH, Germany) in a humidified atmosphere containing 5% CO2 at 37o C.
4.2. Cell Viability and Cytotoxicity of 4-AP, Z-VAD-FMK, CHX, 2-APB: Determination of IC50 Values
4-AP, Z-VAD-FMK, CHX, and 2-APB (Sigma Aldrich St. Louis MO, USA) stock solutions were prepared by dissolving in DMSO (Sigma Aldrich St. Louis MO, USA) and stored at –20o C. Growth medium was used for preparing the desired concentration from the stock solution. The final concentration of DMSO was 0.01% and the control groups were with and without DMSO. From cell survival point of view no difference was observed between the control without DMSO and the control with DMSO.
Determinations of viability and IC50 values were performed by the trypan blue exclusion method on hemocytometer. MCF-7 and L929 cells were seeded on 6 wells plates, cell numbers were 1.8x105 cells/well in 3 ml growth medium, and incubated overnight in a humidified atmosphere containing 5% CO2 at 37o C. After incubation, cells were treated with different concentrations of 4-AP, Z-VAD-FMK, CHX, and 2-APB and incubated for 24 h and then counted with a hemocytometer. Results were compared with control groups.
4.3. Determination of Intracellular Ca²⁺ Concentration
Activation of calcium channels or G protein-coupled receptors leads to changes in intracellular calcium concentrations, which can be homogeneously detected using the Fura-2 calcium indicator. Once inside the cell, esterases cleave the lipophilic and blocking groups of Fura-2, trapping the negatively charged fluorescent dye within the cell.
Changes in intracellular Ca²⁺ concentration were measured using the Screen Quest™ Fura-2 No-Wash Calcium Assay Kit (AAT Bioquest Inc., Pleasanton, CA, USA) with a microplate reader (Synergy H1, BioTek Instruments, VT, USA). Fura-2 AM was dissolved in DMSO and used according to the manufacturer’s protocol.
Cells were seeded in black 96-well plates at a density of 1 × 10⁴ cells per well in 100 µL of growth medium and incubated overnight. Intracellular calcium levels were detected by measuring fluorescence at dual excitation wavelengths of 340 nm and 380 nm, and an emission wavelength of 510 nm.
Fluorescence measurements were performed for 90 minutes immediately following drug addition to monitor dynamic calcium responses.
4.4. Measurement of Transmembrane Potential Using DiBAC₄(3)
The transmembrane potential was measured using the fluorescent dye Bis-(1,3-dibutyl barbituric acid) trimethine oxonol (DiBAC₄(3); AAT Bioquest Inc., Pleasanton, CA, USA) with a microplate reader (Synergy H1, BioTek Instruments, VT, USA). DiBAC₄(3) is a hydrophobic, slow-response dye that passively diffuses across the cell membrane. Upon incubation, the dye migrates from the extracellular aqueous medium into the lipid bilayer due to its hydrophobic nature. Unlike cationic carbocyanine dyes, DiBAC₄(3) is not absorbed by mitochondria and is primarily selective for changes in membrane potential.
The dye was prepared at a stock concentration of 20 mM in spectroscopic-grade DMSO. Cells were seeded in black 96-well plates at a density of 5 × 10⁴ cells per well in 100 µL of growth medium and incubated overnight. After incubation, the medium was removed and replaced with 100 µL of HBSS. Drug treatments were applied and cells were incubated for 2 hours. Following drug treatment, DiBAC₄(3) was added to each well to reach a final concentration of 2 µM in 100 µL HBSS, and the cells were incubated for 30 minutes at room temperature.
To remove excess dye, cells were washed with HBSS, and drug treatments were repeated. Fluorescence measurements were performed in HBSS medium with an excitation wavelength of 540 nm and an emission wavelength of 590 nm. Fluorescence intensity was recorded every 30 minutes over a 24-hour period to monitor dynamic changes in membrane potential.
4.5. Statistical Analyzes
Data in this study were obtained from at least three independent experiments. Results are presented as mean ± standard deviation (SD) in the figures. Statistical analyses were conducted using a two-tailed unpaired Student’s t-test for comparisons between two groups, and one-way analysis of variance (ANOVA) for comparisons among multiple groups, post-hoc analysis was performed using Tukey’s test. For all analyses, n ≥ 3. Differences were considered statistically significant at p < 0.05 and are indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Author Contributions
Conceptualization, E.M.C.A and P.I.; methodology, E.M.C.A.; software, E.M.C.A.; validation, E.M.C.A, P.I, and A.I.G.; formal analysis, E.M.C.A; investigation, E.M.C.A., P.I.; data curation, E.M.C.A.,P.I.; writing—original draft preparation, E.M.C.A.; writing—review and editing, E.M.C.A., A.I.G.; visualization, E.M.C.A.; supervision, A.I.G.; project administration, A.I.G.; funding acquisition, A.I.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by T.C. Marmara University Scientific Research Project Department, grant number SAG-C-YLP-130219-0050.
Institutional Review Board Statement
Ethical approval was not required for this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 4-AP |
4-Aminopyridine |
| 2-APB |
2-Aminoethoxydiphenyl borate |
| CHX |
Cycloheximide |
| DiBAC4(3) |
Bis-(1,3-dibutylbarbituric acid) trimethine oxonol |
| ER |
Endoplasmic reticulum |
| VGKC |
Voltage gated potassium channel |
| VGCC |
Voltage gated Calcium channel |
| IP3 |
Inositol 1,4,5-trisphosphate |
| MS |
Multiple Sclerosis |
| SCI |
spinal cord injury |
| DMEM |
Dulbecco’s Modified Eagle’s Medium |
| FBS |
Fetal bovine serum |
| DMSO |
Dimethyl sulfoxide |
| Orai3 |
Orai Calcium Release-Activated Calcium Modulator 3 |
References
- Pardo, L.A.; Stühmer, W. The Roles of K(+) Channels in Cancer. Nat Rev Cancer 2014, 14, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Fabian, A.; Hanley, P.J.; Stock, C. Role of Ion Channels and Transporters in Cell Migration. Physiol Rev 2012, 92, 1865–1913. [Google Scholar] [CrossRef]
- Ko, J.-H.; Ko, E.A.; Gu, W.; Lim, I.; Bang, H.; Zhou, T. Expression Profiling of Ion Channel Genes Predicts Clinical Outcome in Breast Cancer. Mol Cancer 2013, 12, 106. [Google Scholar] [CrossRef]
- Ishaque, N.; Abba, M.L.; Hauser, C.; Patil, N.; Paramasivam, N.; Huebschmann, D.; Leupold, J.H.; Balasubramanian, G.P.; Kleinheinz, K.; Toprak, U.H.; et al. Whole Genome Sequencing Puts Forward Hypotheses on Metastasis Evolution and Therapy in Colorectal Cancer. Nat Commun 2018, 9, 4782. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.M.; Krishan, A.; Chakarova, C.F.; Moya, L.; Chambers, S.K.; Hollands, M.; Illingworth, J.C.; Williams, S.M.G.; McCabe, H.E.; Shah, A.Z.; et al. MSR1 Repeats Modulate Gene Expression and Affect Risk of Breast and Prostate Cancer. Ann Oncol 2018, 29, 1292–1303. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Lin, B.; Chai, X.; Li, R.; Liao, Y.; Deng, X.; Liu, Q.; Yang, W.; Cai, Y.; et al. Phospholipid Phosphatase 4 Promotes Proliferation and Tumorigenesis, and Activates Ca2+-Permeable Cationic Channel in Lung Carcinoma Cells. Mol Cancer 2017, 16, 147. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zou, L.; Ma, K.; Yu, J.; Wu, H.; Wei, M.; Xiao, Q. Cell-Specific Mechanisms of TMEM16A Ca2+-Activated Chloride Channel in Cancer. Mol Cancer 2017, 16, 152. [Google Scholar] [CrossRef]
- Huang, X.; He, Y.; Dubuc, A.M.; Hashizume, R.; Zhang, W.; Reimand, J.; Yang, H.; Wang, T.A.; Stehbens, S.J.; Younger, S.; et al. EAG2 Potassium Channel with Evolutionarily Conserved Function as a Brain Tumor Target. Nat Neurosci 2015, 18, 1236–1246. [Google Scholar] [CrossRef]
- Steudel, F.A.; Mohr, C.J.; Stegen, B.; Nguyen, H.Y.; Barnert, A.; Steinle, M.; Beer-Hammer, S.; Koch, P.; Lo, W.; Schroth, W.; et al. SK4 Channels Modulate Ca2+ Signalling and Cell Cycle Progression in Murine Breast Cancer. Mol Oncol 2017, 11, 1172–1188. [Google Scholar] [CrossRef]
- Lallet-Daher, H.; Wiel, C.; Gitenay, D.; Navaratnam, N.; Augert, A.; Le Calvé, B.; Verbeke, S.; Carling, D.; Aubert, S.; Vindrieux, D.; et al. Potassium Channel KCNA1 Modulates Oncogene-Induced Senescence and Transformation. Cancer Res 2013, 73, 5253–5265. [Google Scholar] [CrossRef]
- Breuer, E.-K.; Fukushiro-Lopes, D.; Dalheim, A.; Burnette, M.; Zartman, J.; Kaja, S.; Wells, C.; Campo, L.; Curtis, K.J.; Romero-Moreno, R.; et al. Potassium Channel Activity Controls Breast Cancer Metastasis by Affecting β-Catenin Signaling. Cell Death Dis 2019, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Luo, L.; Lal, B.; Ma, X.; Chen, L.; Hann, C.L.; Fulton, A.M.; Leahy, D.J.; Laterra, J.; Li, M. A Monoclonal Antibody against KCNK9 K+ Channel Extracellular Domain Inhibits Tumour Growth and Metastasis. Nature Communications 2016, 7, 10339. [Google Scholar] [CrossRef] [PubMed]
- Becchetti, A. Ion Channels and Transporters in Cancer. 1. Ion Channels and Cell Proliferation in Cancer. American Journal of Physiology-Cell Physiology 2011, 301, C255–C265. [Google Scholar] [CrossRef]
- Stringer, B.K.; Cooper, A.G.; Shepard, S.B. Overexpression of the G-Protein Inwardly Rectifying Potassium Channel 1 (GIRK1) in Primary Breast Carcinomas Correlates with Axillary Lymph Node Metastasis. Cancer Res 2001, 61, 582–588. [Google Scholar]
- Williams, S.; Bateman, A.; O’Kelly, I. Altered Expression of Two-Pore Domain Potassium (K2P) Channels in Cancer. PLoS One 2013, 8, e74589. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, J.-F.; Wang, D.-M.; Zhang, J.; Zhang, W.-J.; Xue, G. The Correlation and Role Analysis of KCNK2/4/5/15 in Human Papillary Thyroid Carcinoma Microenvironment. J Cancer 2020, 11, 5162–5176. [Google Scholar] [CrossRef]
- Hou, X.; Tang, L.; Li, X.; Xiong, F.; Mo, Y.; Jiang, X.; Deng, X.; Peng, M.; Wu, P.; Zhao, M.; et al. Potassium Channel Protein KCNK6 Promotes Breast Cancer Cell Proliferation, Invasion, and Migration. Front Cell Dev Biol 2021, 9, 616784. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y.; Lan, H.; Jiang, T.; Wan, X.; Cheng, Y. Identification of KCNK1 as a Potential Prognostic Biomarker and Therapeutic Target of Breast Cancer. Pathology - Research and Practice 2023, 241, 154286. [Google Scholar] [CrossRef]
- Changes in Membrane Potential during the Progression of MCF-7 Human Mammary Tumor Cells through the Cell Cycle - PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/7559799/ (accessed on 2 July 2025).
- Wegman, E.A.; Young, J.A.; Cook, D.I. A 23-pS Ca2+-Activated K+ Channel in MCF-7 Human Breast Carcinoma Cells: An Apparent Correlation of Channel Incidence with the Rate of Cell Proliferation. Pflügers Arch 1991, 417, 562–570. [Google Scholar] [CrossRef]
- Klimatcheva, E.; Wonderlin, W.F. An ATP-Sensitive K(+) Current That Regulates Progression through Early G1 Phase of the Cell Cycle in MCF-7 Human Breast Cancer Cells. J Membr Biol 1999, 171, 35–46. [Google Scholar] [CrossRef]
- Ouadid-Ahidouch, H.; Ahidouch, A. K+ Channel Expression in Human Breast Cancer Cells: Involvement in Cell Cycle Regulation and Carcinogenesis. J Membr Biol 2008, 221, 1–6. [Google Scholar] [CrossRef]
- Ouadid-Ahidouch, H.; Roudbaraki, M.; Delcourt, P.; Ahidouch, A.; Joury, N.; Prevarskaya, N. Functional and Molecular Identification of Intermediate-Conductance Ca2+-Activated K+ Channels in Breast Cancer Cells: Association with Cell Cycle Progression. American Journal of Physiology-Cell Physiology 2004, 287, C125–C134. [Google Scholar] [CrossRef] [PubMed]
- van der Bruggen, M.A.; Huisman, H.B.; Beckerman, H.; Bertelsmann, F.W.; Polman, C.H.; Lankhorst, G.J. Randomized Trial of 4-Aminopyridine in Patients with Chronic Incomplete Spinal Cord Injury. J Neurol 2001, 248, 665–671. [Google Scholar] [CrossRef]
- Lastraioli, E. Potassium Channels in Breast Cancer.
- Villalonga, N.; Ferreres, J.C.; Argilés, J.M.; Condom, E.; Felipe, A. Potassium Channels Are a New Target Field in Anticancer Drug Design. Recent Pat Anticancer Drug Discov 2007, 2, 212–223. [Google Scholar] [CrossRef]
- Kim, J.A.; Kang, Y.S.; Jung, M.W.; Kang, G.H.; Lee, S.H.; Lee, Y.S. Ca2+ Influx Mediates Apoptosis Induced by 4-Aminopyridine, a K+ Channel Blocker, in HepG2 Human Hepatoblastoma Cells. Pharmacology 2000, 60, 74–81. [Google Scholar] [CrossRef]
- Intrinsic Resistance to Chemotherapy in Breast Cancer - PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21118040/ (accessed on 3 July 2025).
- Wang, W.; Fang, H.; Groom, L.; Cheng, A.; Zhang, W.; Liu, J.; Wang, X.; Li, K.; Han, P.; Zheng, M.; et al. Superoxide Flashes in Single Mitochondria. Cell 2008, 134, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Di Domizio, A.; Limonta, P. The Emerging Role of Paraptosis in Tumor Cell Biology: Perspectives for Cancer Prevention and Therapy with Natural Compounds. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 2020, 1873, 188338. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, S.; Poksay, K.; de Belle, I.; Lafuente, M.J.; Liu, B.; Nasir, J.; Bredesen, D.E. Paraptosis: Mediation by MAP Kinases and Inhibition by AIP-1/Alix. Cell Death Differ 2004, 11, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.E.; Arbiser, J.L. Honokiol, a Multifunctional Antiangiogenic and Antitumor Agent. Antioxid Redox Signal 2009, 11, 1139–1148. [Google Scholar] [CrossRef]
- Wang, C.; Dai, S.; Zhao, X.; Zhang, Y.; Gong, L.; Fu, K.; Ma, C.; Peng, C.; Li, Y. Celastrol as an Emerging Anticancer Agent: Current Status, Challenges and Therapeutic Strategies. Biomedicine & Pharmacotherapy 2023, 163, 114882. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, X.; Lei, S.; Shao, D.; Jiang, C.; Shi, J.; Zhang, Y.; Liu, L.; Lei, S.; Sun, H.; et al. Iturin A-like Lipopeptides from Bacillus Subtilis Trigger Apoptosis, Paraptosis, and Autophagy in Caco-2 Cells. Journal of Cellular Physiology 2019, 234, 6414–6427. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, X.; Zhang, N.; Wang, L.; Hao, D.; Jiang, X.; He, G. Small-Molecule Compounds Target Paraptosis to Improve Cancer Therapy. Biomedicine & Pharmacotherapy 2019, 118, 109203. [Google Scholar] [CrossRef]
- Zhivotovsky, B.; Orrenius, S. Calcium and Cell Death Mechanisms: A Perspective from the Cell Death Community. Cell Calcium 2011, 50, 211–221. [Google Scholar] [CrossRef]
- Motiani, R.K.; Abdullaev, I.F.; Trebak, M. A Novel Native Store-Operated Calcium Channel Encoded by Orai3: Selective Requirement of Orai3 versus Orai1 in Estrogen Receptor-Positive versus Estrogen Receptor-Negative Breast Cancer Cells. J Biol Chem 2010, 285, 19173–19183. [Google Scholar] [CrossRef] [PubMed]
- DeHaven, W.I.; Smyth, J.T.; Boyles, R.R.; Bird, G.S.; Putney, J.W. Complex Actions of 2-Aminoethyldiphenyl Borate on Store-Operated Calcium Entry. J Biol Chem 2008, 283, 19265–19273. [Google Scholar] [CrossRef]
- Bootman, M.D.; Collins, T.J.; Mackenzie, L.; Roderick, H.L.; Berridge, M.J.; Peppiatt, C.M. 2-Aminoethoxydiphenyl Borate (2-APB) Is a Reliable Blocker of Store-Operated Ca2+ Entry but an Inconsistent Inhibitor of InsP3-Induced Ca2+ Release. FASEB J 2002, 16, 1145–1150. [Google Scholar] [CrossRef]
- Cüce-Aydoğmuş, E.M.; İnhan-Garip, G.A. Investigation of the Effects of Blocking Potassium Channels With 4-Aminopyridine on Paclitaxel Activity in Breast Cancer Cell Lines. Cancer Reports 2024, 7, e70072. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).