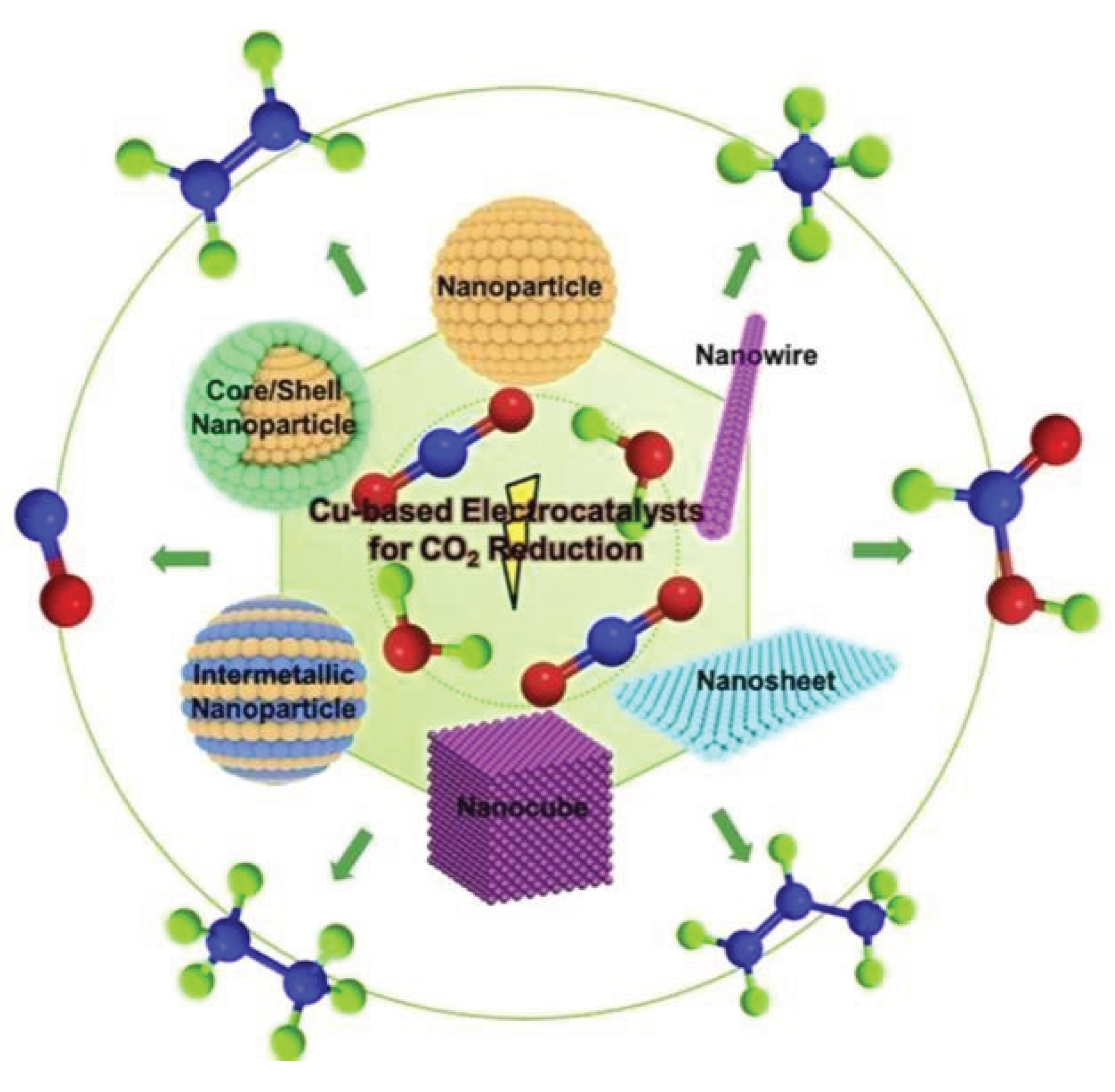
1. Introduction
Climate change and its associated impacts have led to profound environmental and economic consequences on a global scale. Among all greenhouse gases, carbon dioxide (CO
2) is the most significant contributor, playing a central role in ecosystem disruption and global warming. The increasing urgency of the climate crisis has driven extensive research into strategies for mitigating greenhouse gas emissions, with a particular focus on CO
2 due to its high atmospheric concentration and long-term persistence [
1,
2]. Effective management of CO
2 emissions involves not only reducing its release at the source but also capturing and converting it into valuable products such as fuels, chemicals, and construction materials. Conventional catalysts used for CO
2 reduction often rely on precious metals like gold and silver, which, despite their high catalytic efficiency, are expensive and scarce, limiting their practicality for large-scale applications [
3].
This review focuses on the development and application of earth-abundant metal catalysts as a sustainable and cost-effective alternative for CO
2 reduction. Metals such as copper, iron, nickel, zinc, manganese, and cobalt are readily available, economically viable, and exhibit a lower environmental impact, making them suitable candidates for scalable catalytic processes [
4,
5,
6,
7,
8,
9,
10,
11]. These metals possess unique electronic structures and catalytic properties that enable the efficient electrochemical or photochemical conversion of CO
2 into useful products, including hydrocarbons, alcohols, and organic acids [
6,
8,
10]. Recent advancements in materials science, particularly in alloy design, Nano structuring, and surface modification, have significantly enhanced the catalytic performance of these earth-abundant metals. Such innovations have improved their selectivity, activity, and stability, bringing them closer to industrial applicability [
12].
However, challenges remain. These include catalyst deactivation, limited selectivity toward specific products, and the complexity of post-reaction product separation and purification. Overcoming these limitations is essential for realizing the full potential of earth-abundant metals in CO
2 conversion technologies. Therefore, this chapter provides a comprehensive overview of the role of earth-abundant metals in the electrochemical reduction of CO
2, reviewing recent advances, mechanistic insights, and current research trends [
13].
By emphasizing the promise of these materials, this work contributes to the broader goal of advancing sustainable energy technologies. Ultimately, the development of efficient, scalable CO2 reduction methods based on earth-abundant metals can support global efforts to lower atmospheric CO2 concentrations and mitigate the adverse impacts of climate change.
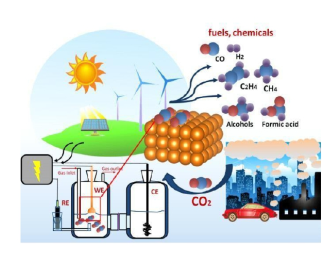
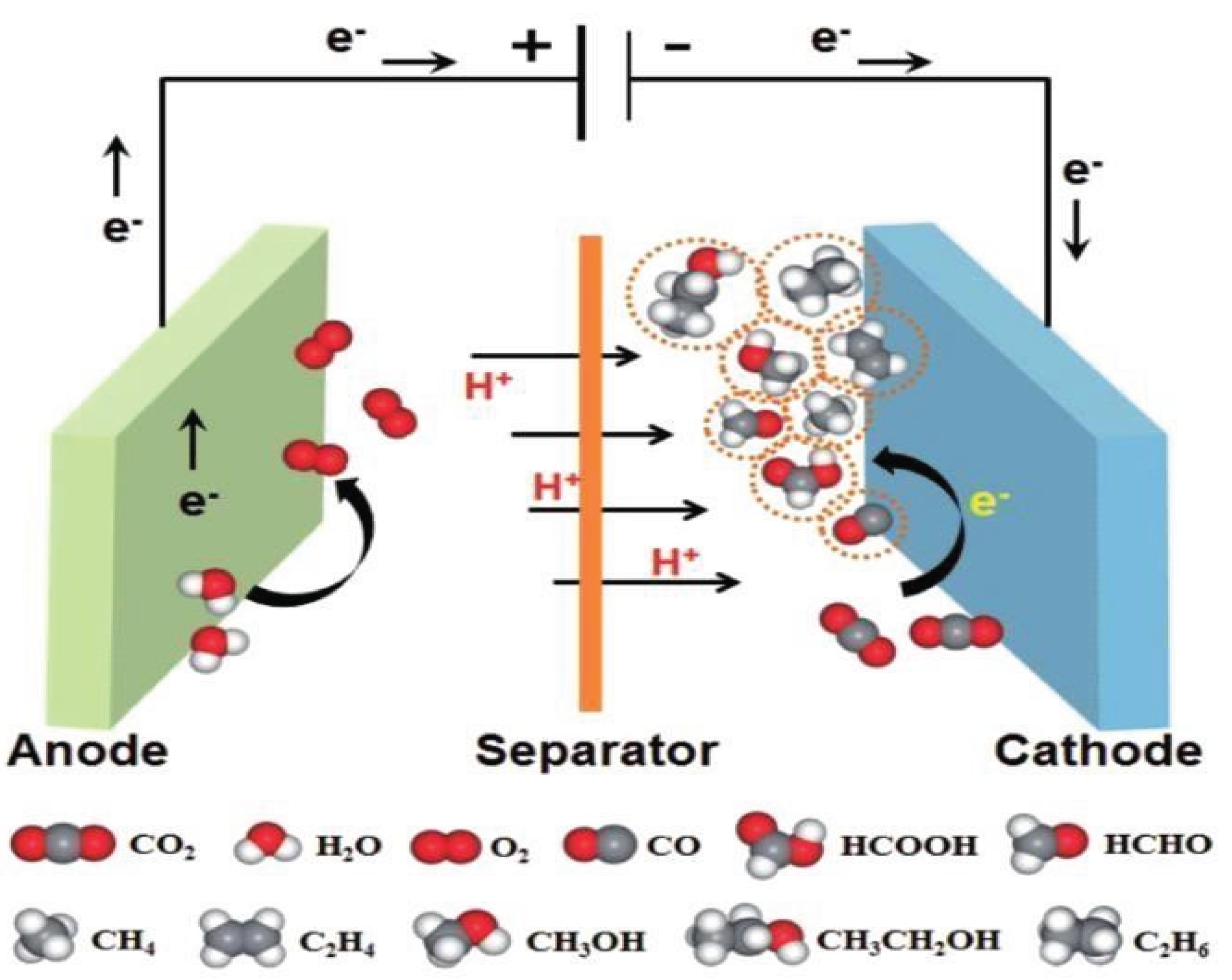
Figure 1.
Electrochemical reduction of CO
2 (Reproduced with permission from European Chemical Societies) [
14].
Figure 1.
Electrochemical reduction of CO
2 (Reproduced with permission from European Chemical Societies) [
14].
2. CO2 Reduction Technologies
As global awareness of climate change intensifies, the development of effective strategies to mitigate carbon dioxide (CO2) emissions has become a critical priority. CO2 is a major greenhouse gas primarily emitted through human activities such as the combustion of fossil fuels, industrial processes, and land-use changes. Its accumulation in the atmosphere contributes significantly to global warming, leading to widespread environmental, economic, and social consequences.
Given the dramatic rise in atmospheric CO
2 levels, there is an urgent need not only to reduce emissions at the source but also to capture and utilize existing CO
2. Currently, three major strategies are being explored to address this challenge (
Figure 2) [
15]:
CO2 Capture and Storage (CCS)
CO2 Capture and Utilization (CCU)
CO2 Conversion into Value-Added Products
This section explores various innovative technologies for CO2 conversion and reduction, including thermochemical, electrochemical, photoelectrochemical, photocatalytic, biological, and direct air capture approaches.
2.1. Mechanism of CO2 Reduction
The mechanism of carbon dioxide (CO2) reduction involves a series of chemical reactions step by step that convert CO2into useful by products, such as hydrocarbons, alcohols, or other chemicals. The specific mechanism can vary depending on the method of reduction (e.g., thermo chemical, electrochemical, Photochemical and photocatalytic) and the catalysts used. Here we discuss the general mechanisms which involved in CO2 reduction.
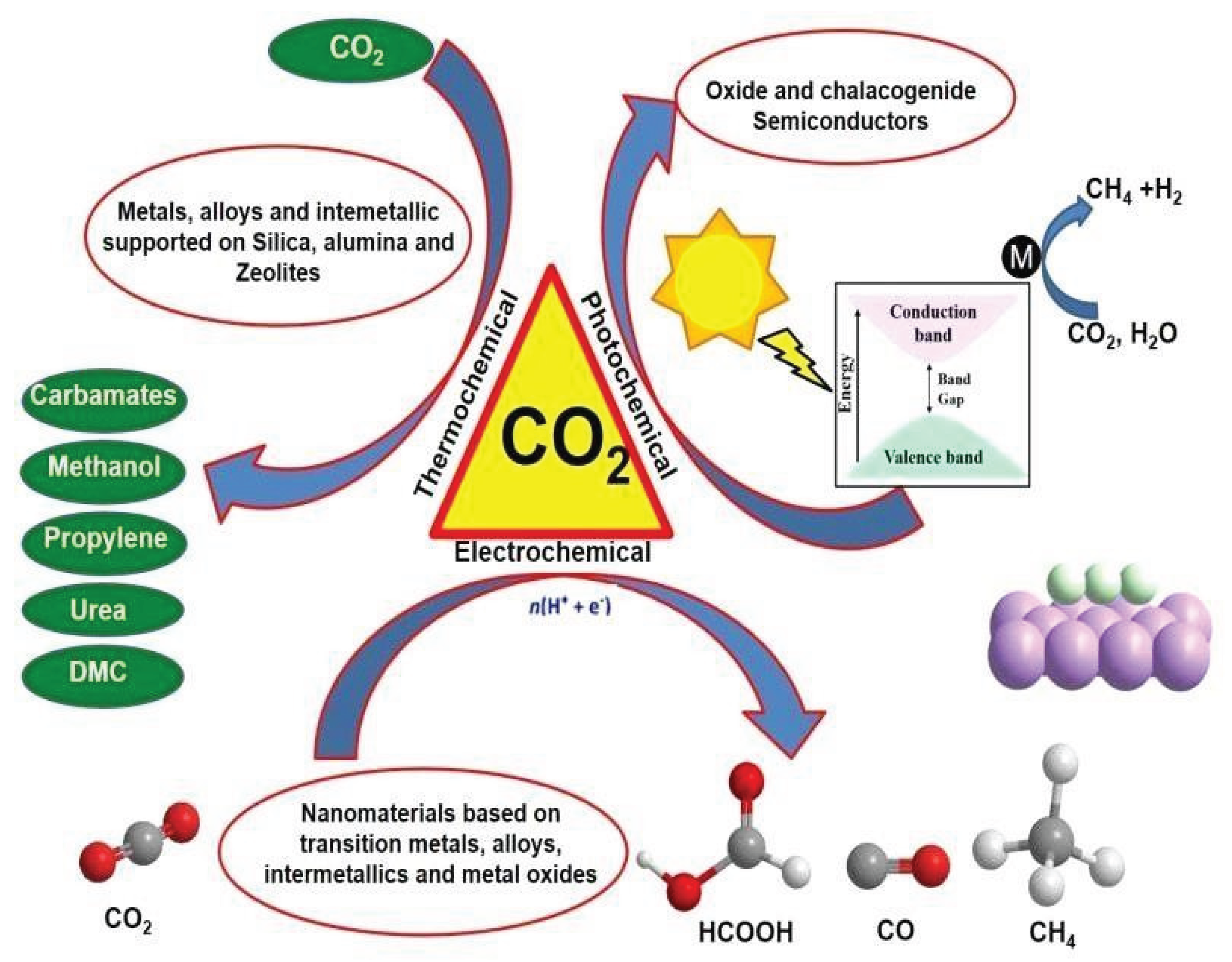
Figure 3.
Methods of CO2 reduction (Reproduced with permission from Jawaharlal Nehru Centre for Advanced Scientific Research).
Figure 3.
Methods of CO2 reduction (Reproduced with permission from Jawaharlal Nehru Centre for Advanced Scientific Research).
2.1.1. Thermochemical Processes
High-Temperature Reduction: CO
2 is reduced at high temperatures using energy sources such as solar or nuclear power. thermochemical CO
2 conversion uses catalysts and a combination of heat and pressure to convert CO
2 into valuable products such as fuels, chemicals, or other materials, aiding in carbon capture and utilization efforts shows in (
Figure 4). A direct approach to generate syngas from solar thermal energy involves the thermolysis of water and CO
2 molecules in one step.
2.1.2. Electrochemical Reduction
Excessive emission of CO
2 resulting from over consumption of fossil fuels causes the green- house effect. The reduction of CO
2 not only addresses the issue of elevated CO
2 levels in the atmosphere but also enables the simultaneous production of valuable carbon-based chemicals and fuels. Electrochemical reduction of CO
2 is a encouraging technology for converting carbon dioxide into value-added chemicals and fuels using electricity. In this process electrochemical cells are employed to convert CO
2 into carbon monoxide (CO), methane (CH
4), formic acid (HCOOH), methanol (CH
3OH), ethylene(C
2H
4), ethanol (C
2H
5OH),
etc: and Catalysts also play a critical role in electrochemical reduction, enhancing reaction rates and selectivity towards desired products. Till date different types of metal based electrocatalysts such as Au, Zn, Ag, Cu, Mn, Sn, and Co have been investigated regarding electrochemical CO
2 reduction. As illustrated in
Figure 5, electrochemical CO
2 reduction systems consist of an anode, a cathode, an aqueous electrolyte solution saturated with CO
2, and a membrane, where the oxygen evolution reaction occurs at the anode and CO
2 reduction takes place at the cathode
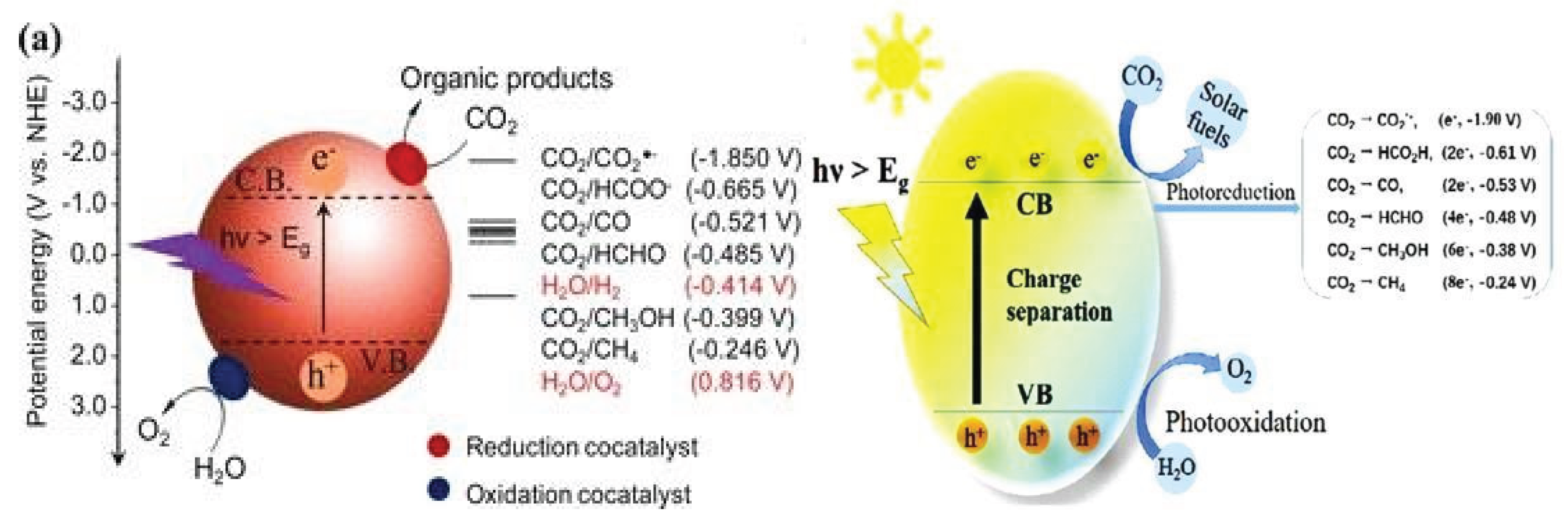
2.1.3. Photocatalytic Reduction
Photocatalytic reduction of CO2 is a promising technology that uses light energy, typically from the sun, to convert carbon dioxide into useful chemicals or fuels. Uses light energy (solar) to drive the reduction of CO2. Photocatalysts, such as semiconductors, metals, metal oxides or nanomaterials, are employed to facilitate the conversion of CO2 into useful chemicals under light irradiation.
Mechanism of photocatalytic reduction of CO
2 on a metal/semiconductor as photocatalyst involves several key steps, primarily generation electron-hole pair’s takes place due to expose of light on photocatalyst showed in (
Figure 6). Here’s a breakdown of the process:
Light adsorption (hv>Eg) by the photocatalysts: In this step photocatalysts adsorb photon light from sun light, promoting the generated electrons from valance band to conduction band and leaving behind holes in valance band so this step creation electron- hole pairs.
Charge separation: this step prevents the recombination of generated electron- hole pairs, through catalyst modification such as Surface modifications or the introduction of co-catalysts can enhance this separation.
Surface Reactions (Redox reaction): In this step reduction and oxidation both processes take place.
Reduction of CO
2: The conduction band electrons are utilized to reduce CO
2 molecules adsorbed on the catalyst surface, reduction pathway depends on various factors such as the catalyst used, reaction conditions, and the presence of other reactants (like water).
Oxidation Reactions: Holes in the valence band can oxidize water (H
2O) to produce protons (H
+) and oxygen (O
2), providing the necessary protons for the reduction of CO
2.
After the reduction, the products (like methanol or methane) desorb from the catalyst surface, allowing for new reactants (CO2 and H2O) to adsorb and continue the cycle.
2.2. Role of Earth-Abundant Metals in CO2 Reduction
Earth-abundant metals have emerged as vital components in the advancement of efficient and sustainable carbon dioxide (CO
2) reduction technologies. Metals such as iron (Fe), copper (Cu), nickel (Ni), zinc (Zn), and cobalt (Co) offer key advantages including wide natural abundance, low cost, and reduced environmental impact compared to traditionally employed precious metals. Owing to their diverse electronic structures and versatile catalytic properties, these metals are well-suited for a range of CO
2 conversion pathways, including electrochemical, thermochemical, and photocatalytic processes. In electrochemical CO
2 reduction, copper is particularly notable for its unique ability to catalyze the formation of hydrocarbons and oxygenates, positioning it as a central material for renewable fuel production. Nickel and cobalt have demonstrated considerable activity in the thermochemical hydrogenation of CO
2, enabling the conversion of CO
2 into valuable fuels and chemicals under elevated temperatures and pressures. In photocatalytic and photoelectrochemical systems, earth-abundant metals function effectively as co-catalysts, single-atom catalysts (SACs), or charge transfer mediators, thereby enhancing light harvesting, charge separation, and overall reaction efficiency. For example, cobalt-based frameworks such as DQTP COF-Co have exhibited high CO production rates in photocatalytic CO
2 reduction, illustrating their promise for solar-to-chemical energy conversion [
25,
26].
Beyond CO2 reduction, these metals also play crucial roles in broader energy-related catalytic processes, including the hydrogen evolution reaction (HER), oxygen reduction reaction (ORR), and water oxidation reaction (OER). Their incorporation into SACs, bimetallic alloys (e.g., Cu–Ni), and composite materials can harness synergistic interactions to enhance catalytic activity, selectivity, and long-term stability. Alloying strategies can effectively tune the electronic structure of active sites, while composite architectures facilitate improved charge transport and structural robustness under reaction conditions. Furthermore, the integration of these metals with conductive supports such as carbon-based materials or metal oxides enables the formation of well-dispersed and mechanically stable catalyst systems, improving reactant accessibility and electron mobility.
Recent research efforts have increasingly emphasized advanced catalyst design strategies, including nanostructuring, surface modification, and molecular-level tuning, to optimize the physicochemical characteristics of earth-abundant metal catalysts. These approaches aim to improve reaction kinetics, enhance product selectivity, and reduce catalyst deactivation. Coupling earth-abundant metals with visible-light photosensitizers or molecular catalysts represents a promising avenue for the development of next-generation CO2 reduction systems capable of operating under ambient conditions. Continued exploration of the unique catalytic properties of earth-abundant metals is paving the way for more efficient, selective, and scalable CO2 conversion technologies that align with global sustainability and circular economy objectives.
3. Earth – Abundant Metals as Catalysts
Recent studies have identified several earth-abundant metals including iron (Fe), copper (Cu), nickel (Ni), molybdenum (Mo), zinc (Zn), cobalt (Co), and manganese (Mn) as promising candidates for CO2 reduction catalysis. These metals have been incorporated into diverse catalyst architectures, such as single-atom catalysts (SACs), alloy and composite systems, and supported metal catalysts, each offering distinct advantages in terms of catalytic activity, product selectivity, and operational stability.
3.1. Molecular and Single Atom-Based Catalysts and Their Roles in CO2 Reduction
Earth-abundant metal-based molecular catalysts play a crucial role in the electrochemical reduction of carbon dioxide (CO
2) to valuable products, such as carbon monoxide (CO), methane (CH
4), and various hydrocarbons. These catalysts are gaining attention due to their potential for advancements in green chemistry and renewable energy technologies. These catalysts, composed of readily available elements such as iron, nickel, copper, and cobalt, not only present a cost-effective alternative to precious metal catalysts but also exhibit remarkable versatility in promoting the transformation of CO
2 into useful chemicals and fuels [
26].
Among various approaches for CO
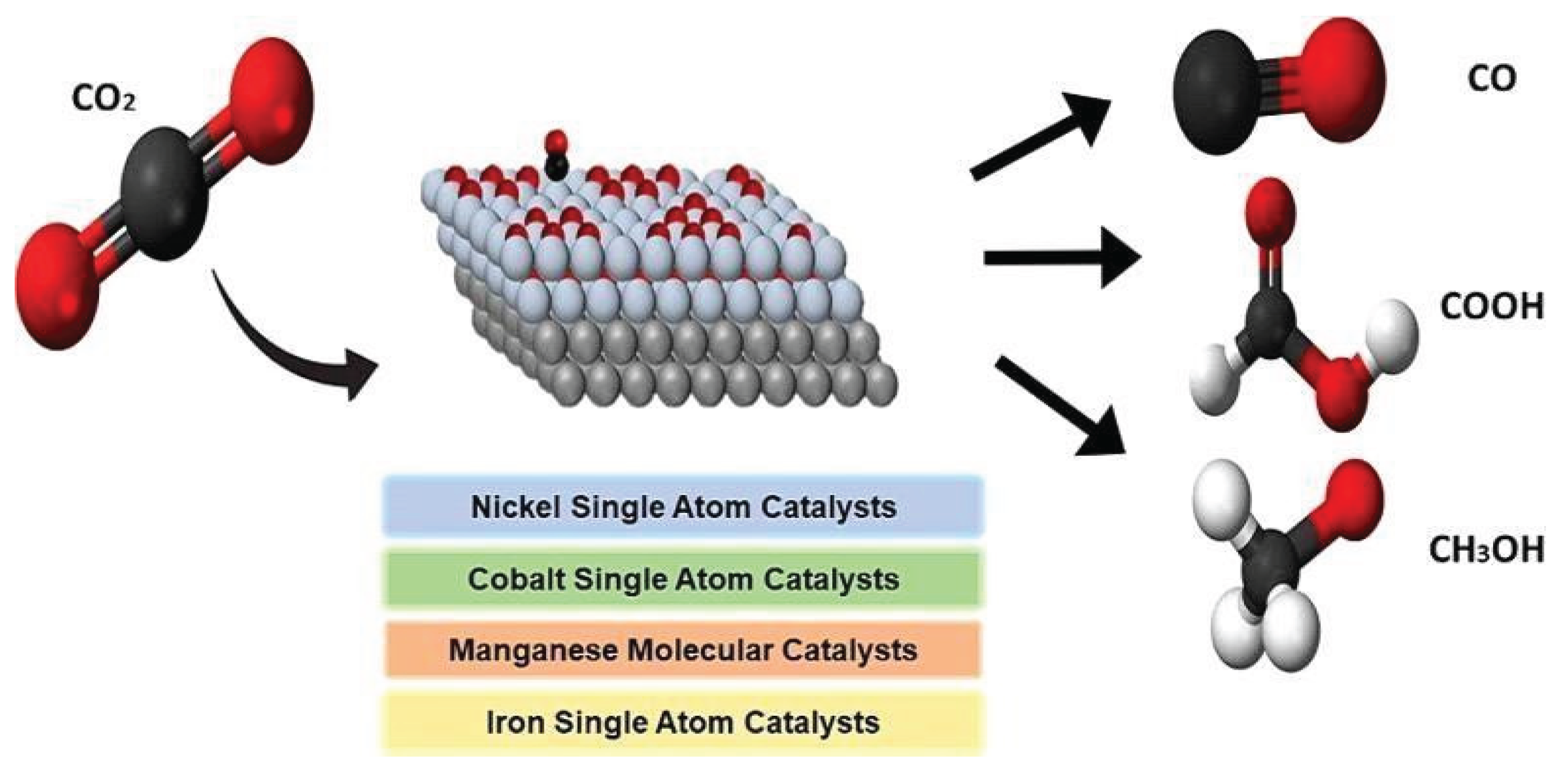
2, reduction Single-metal catalysts (SACs) play a promising role in the reduction of carbon dioxide (CO
2) due to their unique properties and high selectivity and stability. In single atom catalysts (SACs), metal atoms are dispersed on supports where they are coordinated with non-metals, demonstrating remarkable performance for CO
2 electroreduction due to their strong interactions with the support, optimal metal utilization, and exceptional catalytic activity. When using earth-abundant metals, as single atom- metal catalysts offer low-cost, sustainable and high-performance metals for fuel and chemical production. Due to the majority of metal sites being accessible at the catalyst’s surface and their distinct electronic structure, SACs exhibit high intrinsic catalytic activity [
26,
27]. In the photo and electrochemical CO
2 reduction, SACs exhibit encouraging performance in terms of catalytic activity, product selectivity, and stability. The most common metals used in SACs include Pt, Au, Pd, Rh and First-row transition metals Ni,Co, Fe, Cu, Zn, Bi, Mn and Sn (
Figure 7) [
28].
In this section of chapter discuss the use of Fe, Cu, Ni, Mn, Co, Mo and other metals as molecular and single atom catalysts (SACs) for CO2 electrochemical and photocatalytic reduction.
3.1.1. Iron Based Catalysts (Fe-SACs) for CO2 Reduction
Iron is the most earth- abundant transition metal has been extensively studied as a potential catalyst for various and diverse chemical reactions. Molecular iron-based complexes are among the most efficient catalysts for CO
2 reduction. Zhang et al. [
29] introduced a water-soluble iron–porphyrin complex (
Figure 8) as an electrocatalyst for the reduction of CO
2 to CO, achieving 90% CO production under an applied potential of −0.97 V vs. NHE. Solubility of Fe-porphyrin catalyst is attributed due to the four positively charged N,N,N,-trimetthyl-4-ammoniumphenyl substituents in porphyrin ring which showed excellent catalytic activity and selectivity toward electrocatalytic reduction of CO
2 to CO in aqueous solution. Busch et al. [
30] studies investigated the electrochemical reduction of CO
2 over the Fe-porphyrin catalyst system using DFT. They find the H
2 evolution reaction is suppressed by the high activation barrier over Fe-porphyrin. Fe(I)-porphyrin support the reduction of HCO
3– and H
2CO
3 reduction. Direct CO
2 reduction is hindered by a high activation barrier on Fe-porphyrin. Jung and Saito et al. [
31] report the synthesis of Fe- Complex with Bipyridyl which is supported by two phosphine molecules. Synthesized F- complex ligand catalysed photocatalytic reduction of CO
2 to CO and HCOOH combined with Ir(ppy)
3 as a photosensitizer. The bulky phosphine moieties stabilized the catalyst and enhanced its durability for up to 72 hours.
Among other metals Iron-based single atom metal catalysts (Fe-SACs) have emerged as promising materials for the electrochemical reduction of CO
2 due to their high catalytic activity, stability, high mechanical strength, strong conductivity and cost-effectiveness (
Figure 9)[
32]. Iron-based catalysts have also been investigated for their application in the Fischer-–Tropsch processes, which converts syngas into hydrocarbons and oxygenated hydrocarbons.
Recent research still has focused on the developing of iron-based catalysts. Zhang et al. [
32] report the synthesis of series Fe-based catalysts which was obtained by fabrication through the hydrogen reduction on MgFeAl-layered double hydroxide nanosheets at temperature 300-700
oC. Synthesized catalysts showed photothermal reduction of CO
2 to C
2+ hydrocarbons under UV-vis light irradiations. Xu et al. [
33] prepared the nitrogen-doped graphene (Fe/NG) single-atom catalyst which offer efficient electrocatalytic reduction of CO
2 to CO. Fe/NG has a low reduction over potential with high Faradic efficiency up to 80%. Geng and Zeng et al. [
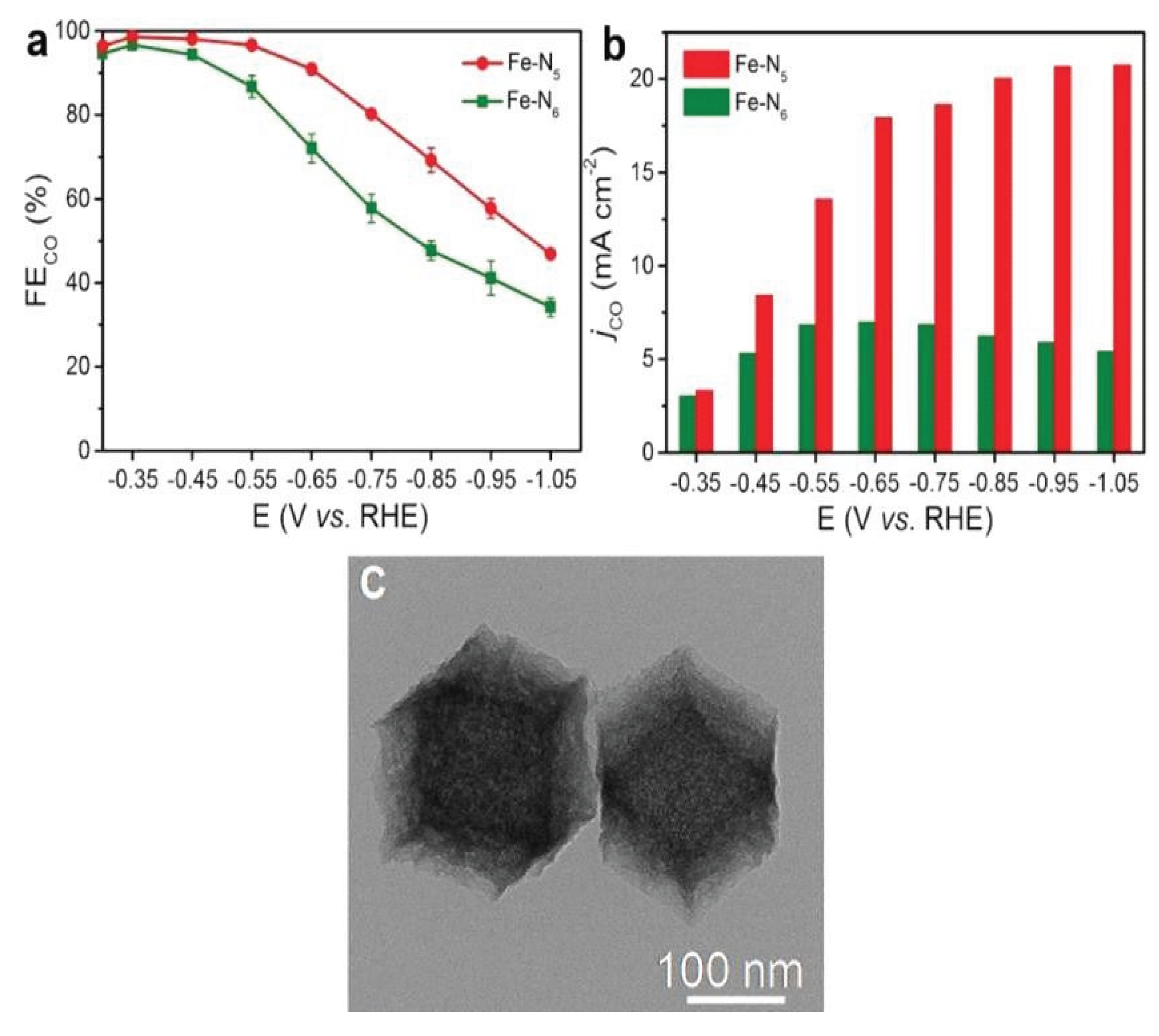
34] prepared highly active Fe single-atom catalysts (Fe–N
5/Fe–N
6) by tuning the coordination number of Fe with N towards CO
2 electroreduction. The faradaic efficiency for CO with Fe–N5 surpassed 90% within the range of −0.35 to −0.65 V versus the reversible hydrogen electrode (vs. RHE) during CO
2 electroreduction (
Figure 10). Cheng et al. [
35] report the electrocatalytic reduction of CO
2 using novel electrocatalysts Fe-N/P-C, synthesized catalysts Fe–N/P–C showed outstanding performance in the electrochemical reduction of CO2 to CO, with a high Faradaic efficiency of 98% and a high mass-normalized turnover frequency of 508.8 h–1 at a low overpotential of 0.34 V.
Fang et al. [
36] reported Fe–N–C electrocatalysts towards electrocatalytic reduction of CO
2 to CO with Faradaic efficiency of about 98% at −0.68 vs.
RHE. Zhang et al. [
37] research study focused on the electrocatalytic reduction of CO
2 using Fe-Based Single Atom Catalyst. In this catalyst, single Fe atom is coordinated with one S and three N atom, denoted as Fe−S
1N synthesized catalyst exhibited an outstanding performance for converting CO
2 to CO with Faradaic efficiency around 99.02% and exhibited a high intrinsic activity TOF of 7804.34 per h. Fe−S
1N
3 catalyst showed remarkable stability. Lyu et al. [
38] prepared single atom Fe-N-C based electrocatalyst via novel one-step calcination method. Synthesized showed excellent efficiency toward reduction of CO
2 to CO and H
2, with a total Faradaic efficiency of 100% and Tafel slope of 68 mv dec
-1. Fe-N-C material had promising catalytic activity and good stability. Ren et al. [
39] designed and synthesized a series of Fe- based single atom catalyst coordinated with B named as FeB
xC
y, results reveal a great potential of coordination tuning for reduction CO
2, and provide a new theoretical perspective for rational design of high activity, selective CO
2 reduction catalysts.
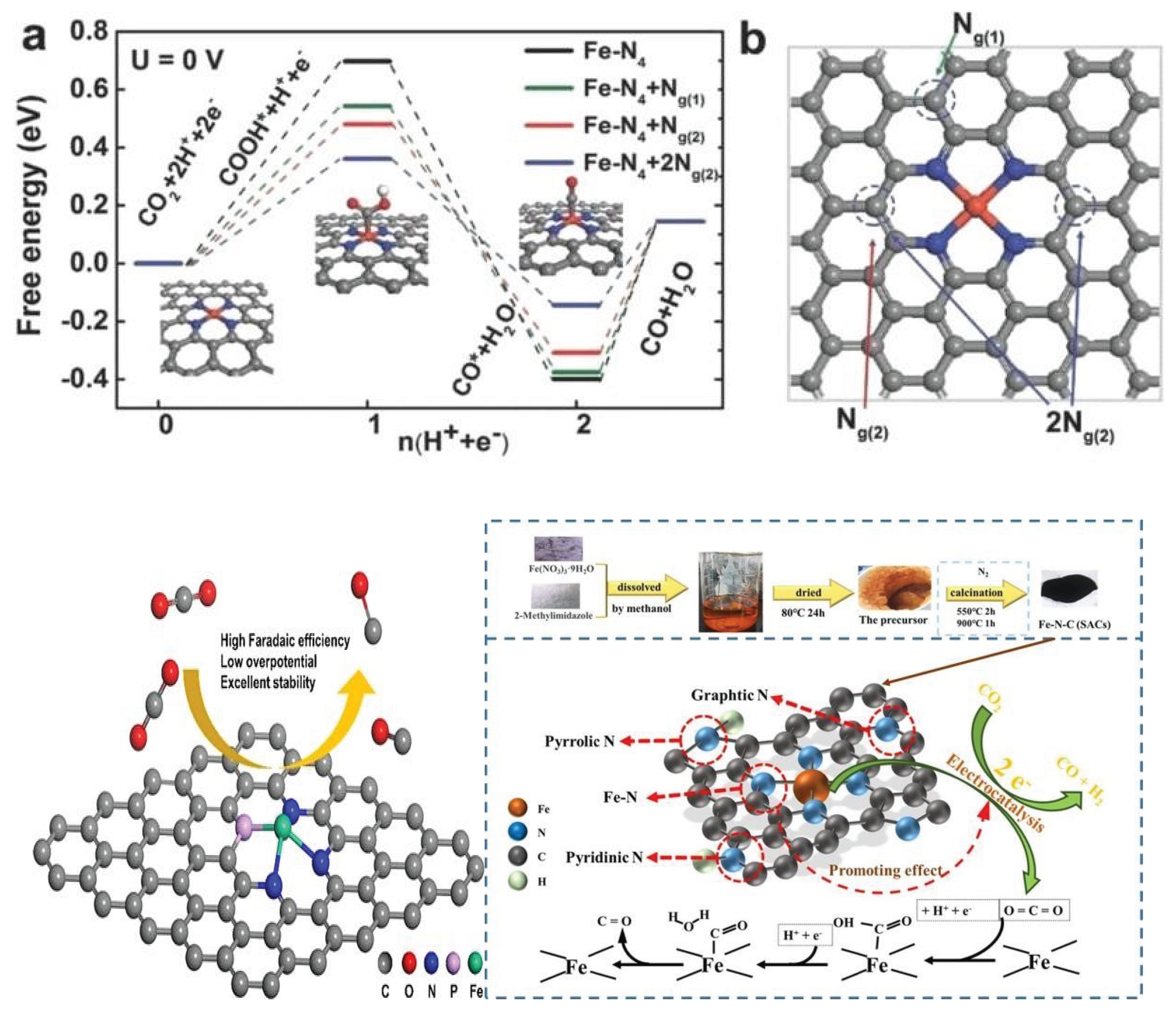
Figure 11.
Fe–N
4 as effective active sites for the CO
2RR to CO. (
a) Free energy vs. reaction path graph for the CO
2RR to CO on different Fe–N
4 centers for the Fe/NG catalyst. (
b) Top view-scheme of the Fe/NG catalyst highlighting the Fe–N
4 center (Fe atom in red, N atoms in blue) and the potential nitrogen-substitute atoms (c) (Reproduced with permission from Elsevier) [
36,
37,
38,
39].
Figure 11.
Fe–N
4 as effective active sites for the CO
2RR to CO. (
a) Free energy vs. reaction path graph for the CO
2RR to CO on different Fe–N
4 centers for the Fe/NG catalyst. (
b) Top view-scheme of the Fe/NG catalyst highlighting the Fe–N
4 center (Fe atom in red, N atoms in blue) and the potential nitrogen-substitute atoms (c) (Reproduced with permission from Elsevier) [
36,
37,
38,
39].
Table 1.
Iron based catalyst for CO2 reduction.
Table 1.
Iron based catalyst for CO2 reduction.
| Sr. No |
Name of Metal Catalyst |
Type of catalysts |
Conversion Form |
Conditions |
Faradaic efficiency |
References |
| 1. |
Iron–porphyrin complex |
Electrocatalyst |
CO2 to CO |
−0.97 V |
90% |
[30] |
| 2. |
Fe- Complex with Bipyridyl |
Electrocatalytic reduction |
CO2 to CO and HCOOH |
-0.91 V |
86% |
[31] |
| 3. |
Fe-SACs |
Electrocatalyst |
CO2 to CO |
-0.89 V |
92% |
[32] |
| 4. |
Fe/NG |
Electrocatalytic reduction |
CO2 to CO |
-0.57V |
80% |
[36] |
| 5. |
Fe–N6 |
Electroreduction |
CO2 to CO |
−0.35 to −0.65 V |
>90% |
[33] |
| 6. |
Fe–N/P–C |
Electrochemical reduction |
CO2 to CO |
0.34 V |
98% |
[35] |
| 7. |
Fe–N–C |
Electrocatalytic reduction |
CO2 to CO |
−0.68 |
98% |
[38] |
| 8. |
FeBxCy |
Electrocatalytic reduction |
CO2 to CO |
-0.45 V |
99.02% |
[39] |
3.1.2. Cupper (Cu) Based Catalysts for CO2 Reduction
Copper-based catalysts have emerged as promising candidates for the electrochemical and photochemical reduction of CO2 due to their unique electronic properties and ability to produce fuels and valuable chemicals.
Figure 12.
Different nanostructure form of Cu-Based electrocatalyst for CO2 reduction.
Figure 12.
Different nanostructure form of Cu-Based electrocatalyst for CO2 reduction.
Copper is an effective catalyst for catalytic reduction of CO
2 to produce high- molecular weight products such as alcohols and hydrocarbon. From the previous reported research work found that Cu as single atoms catalysts can selectively only for the CO formation in CO
2 reduction. Deng et al. [
40] report the Cu based single atoms catalyst named Cu-N-C with Cu–N3 coordination showed good electroreduction of CO
2 to CO with high faradaic efficiency of 98% at −0.67 V vs.
RHE as well as superior stability over 20h. The same catalyst achieved Faradaic Efficiency of 99.9% for CO at −0.67 V vs.
RHE when tested in an electrolyte cell flow, due to the three unoccupied N sites were spontaneously saturated by protons during the CO
2 reduction. With the help of DFT study find the reason for improved performance of the catalyst in the electrolyte cell with configuration. For instance, Xu et al. [
41] report the synthesis of Cu–N
4– NG through two step pyrolysis method, in this synthesis process single Cu atom lodged into 2D N-doped graphene. synthesized Cu–N
4–NG catalysts showed excellent selectivity toward electrocatalytic reduction of CO
2 in CO with Faradaic efficiency 80.6% for CO at −1.0 V vs.
RHE as compare to bulk Cu catalyst which showed low selectivity towards CO, while Cu–N
4– NG facilitate the higher CO
2 activation due to the presence of Cu–N
4 sites in catalyst, whereas the graphene substrate provides protons through the water dissociation, which involved in the CO
2 reduction process.
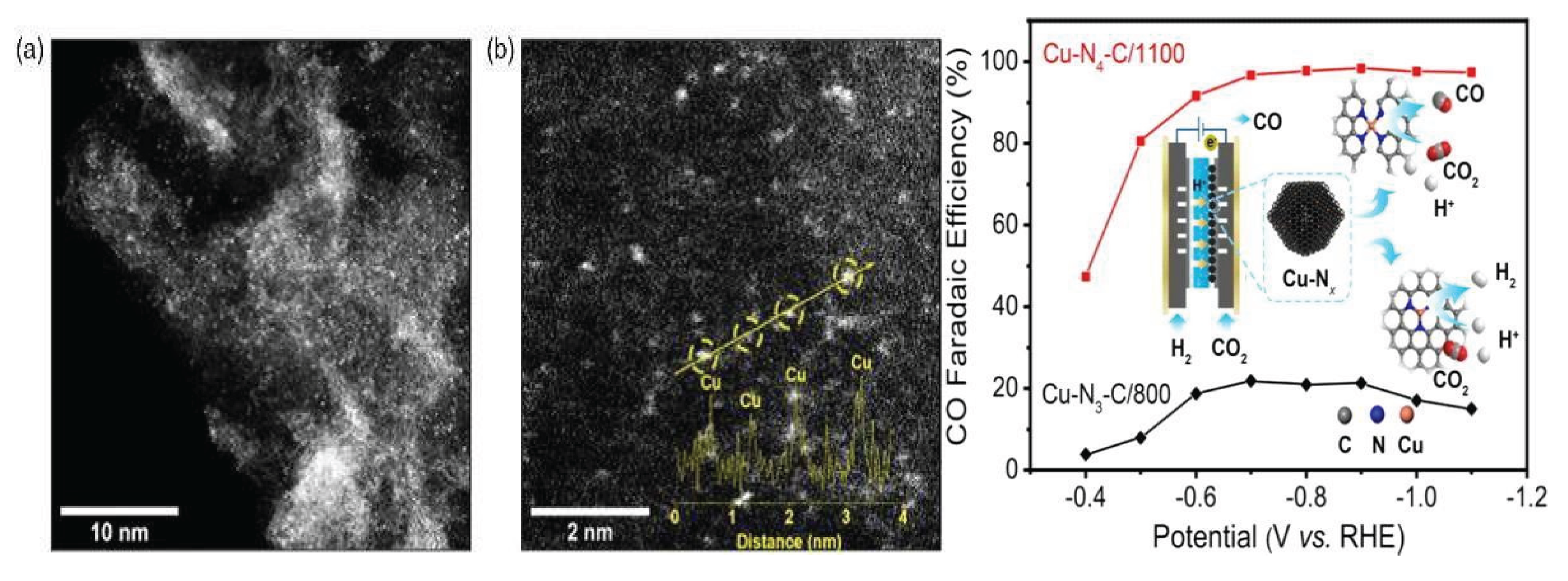
Cheng et al. [
42] demonstrated the effectiveness of the Cu–N
4 site for catalysing the CO
2 reduction reaction to CO, as it ensures optimal binding energy for the COOH and CO intermediates, thereby enhancing CO production. Notably, the authors successfully synthesized Cu single atoms dispersed on an N–C support using a MOF-assisted method (referred to as Cu-N
4-C/1100). The resulting catalyst achieved a maximum CO Faradaic efficiency of 98% at –0.9 V vs. RHE, maintaining high stability for at least 40 hours during the test. Chen et al. [
43] developed Cu single atoms on a nitrogenated carbon-based catalyst known as Cu–N–C. The Cu–N
3 sites in this catalyst were shown to significantly enhance CO* desorption. In a gas-tight H-type cell, Cu–N–C achieved a high CO Faradaic efficiency of 98% at −0.67 V vs. RHE, with impressive durability (Faradaic efficiency remaining above 90% over 20 hours of testing). The same catalyst was also evaluated in an electrolyte flow cell configuration, demonstrating an even higher CO Faradaic efficiency of 99.9% at −0.67 V vs. RHE, attributed to the improved rate of CO
2 diffusion. DFT calculations explained the enhanced performance of the catalyst: the Cu–N
3 site was positioned on an extended carbon plane with six nitrogen vacancies that stabilize the active site, while three unoccupied N sites were naturally saturated by protons during the CO
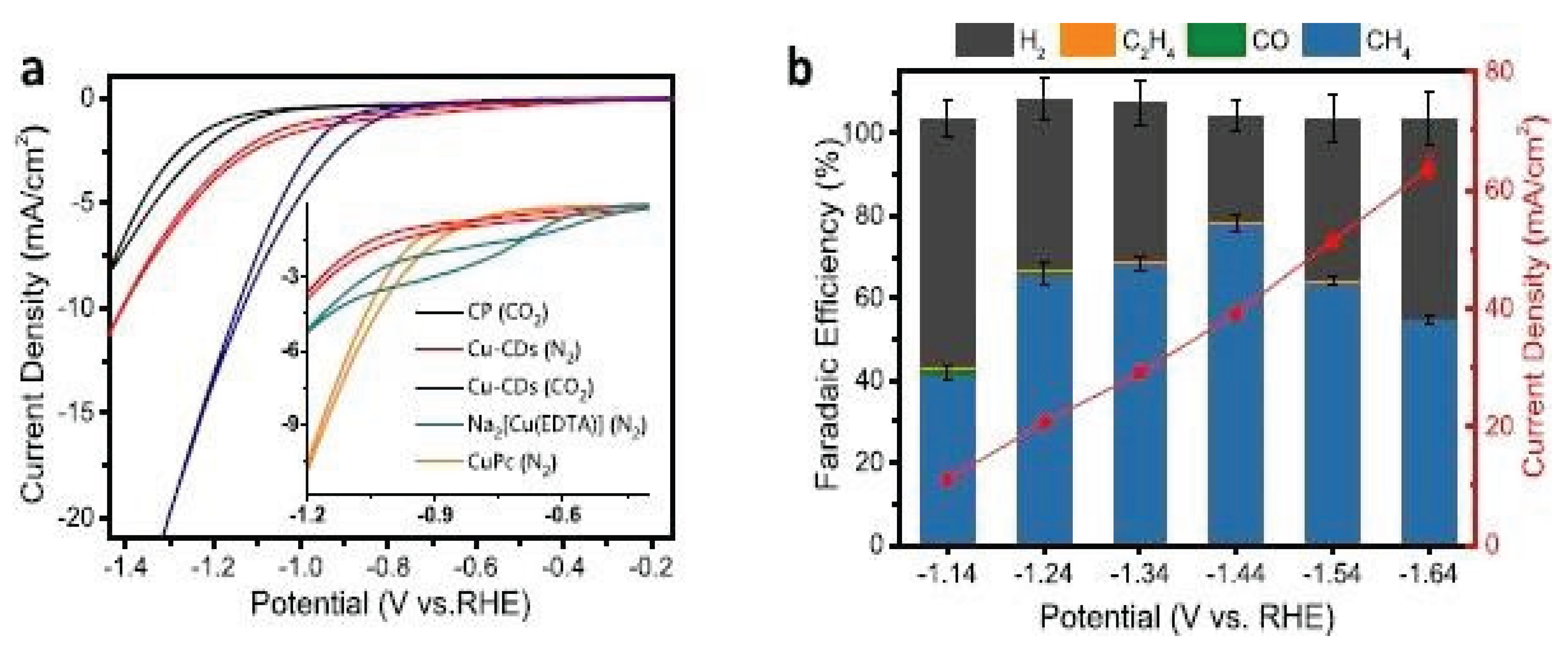
2 reduction process. Zhu et al. [
44] reported the development of single-atom Cu-embedded carbon dots (Cu-CDs) coordinated with two nitrogen and two oxygen atoms, marking the first introduction of N and O ligands. (
Figure 13).
As a result, the catalyst demonstrated exceptional selectivity for the electrochemical reduction of CO
2 to CH
4 across a wide potential range from −1.14 to −1.64 V vs. RHE (reversible hydrogen electrode), with over 99% of the CO
2 reduction products being CH
4. Additionally, Cu-CDs exhibited a high CH
4 Faradaic efficiency of 78% and a turnover frequency of 2370 h
−1 at −1.44 and −1.64 V, respectively. Density functional theory (DFT) calculations revealed that the separation of the CH
4 limiting potential from other products allows for the exclusive production of CH
4. Luo et al. [
45] prepared catalyst Cu
3N-derived Cu nanowires were shown to be an effective electrocatalyst for the electrochemical reduction of CO
2 to C
2 products. The synthesized catalyst displays outstanding activity and selectivity for producing C
2 products, achieving a maximum Faradaic efficiency of 86% at −1.0 V, and maintaining long-term stability over 28 hours of electrolysis. Cheng et al. [
46] synthesized Cu- N
x-C catalysts (x no of coordinated N atoms) via a Facile one step thermal pyrolysis method at different temperature range, author successfully synthesized Cu-N
4-C/1100 and Cu-N
3-C/800 through dispersion of single metal atom Cu on the N-C support. Prepared catalyst Cu-N
4- C/1100 showed excellent catalytic activity and selectivity for CO
2 reduction to CO as compare to Cu-N
3-C/800 catalyst, noticed 90% faradaic efficiency from −0.6 to −1.1 V
vs. RHE. At −0.9 V
vs. RHE achieved 98% faradaic efficiency. Cu-N
4-C/1100 exhibited superior stability up to 40 h during reduction process [
46].
Figure 14.
(a, b) showed the Sem micrograph of CuN
2O
2 catalyst and (c) showed the faradaic efficiency of Cu-N
3-C/800 and Cu-N
3-C/1100 catalysts. [
46,
47].
Figure 14.
(a, b) showed the Sem micrograph of CuN
2O
2 catalyst and (c) showed the faradaic efficiency of Cu-N
3-C/800 and Cu-N
3-C/1100 catalysts. [
46,
47].
Zhu et al. [
47] proposed synthesis of single atom Cu- embedded carbon dots and coordinated with two N and two O atoms, catalyst named CuN
2O
2, it was first time introducing O atom with Cu-N. The catalyst exhibited exceptional catalytic activity and selectivity for the electrochemical reduction CO
2 to CH
4 with 99% Faradaic efficiency over a wide potential range −1.14 to −1.64 V vs. RHE. While Faradaic efficiency of CH
4 achieved only 78% at −1.44 to −1.64 V when using Cu-CD as catalyst for CO
2 reduction. Synthesized CuN
2O
2 found a promising candidate towards electrochemical CO
2 reduction due to maximized atomic utilization. Liu et al. [
48] prepared electrocatalyst Cu
3N-derived Cu nanowires revealed high efficiency towards electrochemical reduction of CO
2 to C
2 products. The prepared catalyst showed maximum faradaic efficiency 86% of C
2 products at −1.0 V. The catalyst CuN
2O
2 demonstrates outstanding activity and selectivity for producing C
2 products, maintaining long-term stability over 28 hours.
Table 2.
Copper (Cu) based catalysts for CO2 reduction.
Table 2.
Copper (Cu) based catalysts for CO2 reduction.
| S.R. No. |
Name of Metal Catalyst |
Type of catalysts |
Conversion Form |
Conditions |
Faradaic efficiency |
References |
| 1. |
Cu-N-C |
electroreduction |
CO2 to CO |
−0.67 V |
98% |
[40] |
| 2. |
Cu–N4– NG |
electrocatalytic reduction |
CO2 in CO |
−1.0 V |
80.6% |
[41] |
| 3. |
Cu–N4 |
electrocatalytic reduction |
CO2 to CO |
–0.9 V |
98% |
[42] |
| 4. |
Cu-CDs |
electrochemical reduction |
CO2 to CH4 |
−1.64 V |
99% |
[44] |
| 5. |
Cu3N-derived Cu nanowires |
electrocatalyst |
CO2 to C2 |
−1.0 V |
86% |
[45] |
| 6. |
Cu- Nx-C catalysts |
electrocatalyst |
CO2 reduction to CO |
−1.1 V |
90% |
[46] |
| 7. |
CuN2O2
|
electrochemical reduction |
CO2 to C2 |
−1.0 V |
86% |
[48] |
3.1.3. Nickel Based Single Atom Catalysts (Ni-SACs) for CO2 Reduction
The electrochemical reduction of CO
2 to valuable chemicals is a critical area of research for addressing climate change. Nickel-based catalysts have gained significant attention due to their unique electronic properties, high activity, and selectivity for various reduction products, including hydrocarbons and alcohols. This literature review summarizes the recent advancements in the synthesis, and performance of Ni- based catalysts in CO
2 reduction. Wang et al. [
49] reports the synthesis of Ni single atom catalyst (Ni-SACs) on the support of carbon black particles, The Ni- single atomic sites exhibit an extraordinary performance for reduction of CO
2 to CO, with nearly 99% Faradaic efficiency for CO at 0.681 V in KHCO
3 aqueous solution. Xu and Mu et al. [
50] report Ni based molecular catalyst which showed outstanding performance under the photocatalytic reduction of CO
2 to CO under visible light irradiations. The author synthesized three Ni-based molecular catalysts known as Ni(II)-bipyridine complexes: Ni-1 ([NiCl
2(4,4′-dichloro-2,2′-bipyridine)2]), Ni-2 ([NiCl
2(4,4′-dibromo-2,2′- bipyridine)2]), and Ni-3 ([NiCl
2(4,4′-diphenyl-2,2′-bipyridine)2]). Among these Ni complexes, Ni-1 exhibited superior catalytic performance compared to traditional homogeneous Ni-bipyridine catalysts.
Hu et al. [
51] report a novel Ni based single atom catalyst via incorporation Ni single atom into carbon paperwork as a self-standing electrocatalyst for CO
2 reduction (
Figure 16). From the characterization results and DFT calculation confirm that the catalyst consists of one Ni atom which was coordinated with three N, and one S atoms on the surface of commercially available carbon paper. Catalyst showed excellent efficiency towards electroreduction of CO
2 with optimal selectivity (91%) at 0.6 V and activity (3.4mA cm
−2) for CO, also showed good stability up to 14h. Li et al. [
52] developed a Ni-N-C based sustainable catalyst of efficient electrochemical reduction of CO
2 to CO. For the synthesis of catalyst author using cheap cornstarch and doping with Nikel (Ni) with without need of acid was post-treatment. Synthesized cornstarch-based Ni- Catalyst exhibited high faradaic efficiency (FE) of 92% for CO production at –0.8 V vs RHE current density of CO 11.6 mA/cm
2. Author also discusses the electrochemical reduction of CO
2 to CO using Ni catalyst which was prepared by normal conventional method show low reduction efficiency with a lower FE (CO) at 81%. as compare to sustainable Ni-based catalyst. Wang et al. [
53] report the synthesis Ni-based single atom catalyst on the surface of commercially available carbon black particles through simple and scalable method (
Figure 15). Ni- SACs Catalyst showed promising efficiency towards reduction CO
2 to CO via traditional H-cell with faradic efficiency 99% at –0.681V RHE in aqueous solution of 0.5 M KHCO
3. (
Figure 15) Importantly, current densities exceeding 100 mA cm
−2 with nearly 100% CO production were achieved on an anion MEA, which is approximately 10 times higher than the current densities observed in an H-cell. An ultra-high CO/H
2 ratio of 114 was attained, defined as “relative selectivity” when CO selectivity approaches 100% and H
2 remains below 1% as measured by gas chromatography (GC), while sustaining a significant current of 74 mA cm
−2. Furthermore, after 20 hours of continuous operation at an average current density of approximately 85 mA cm
−2, the CO formation Faradaic efficiency remained around 100%, with H
2 levels still below 1%.
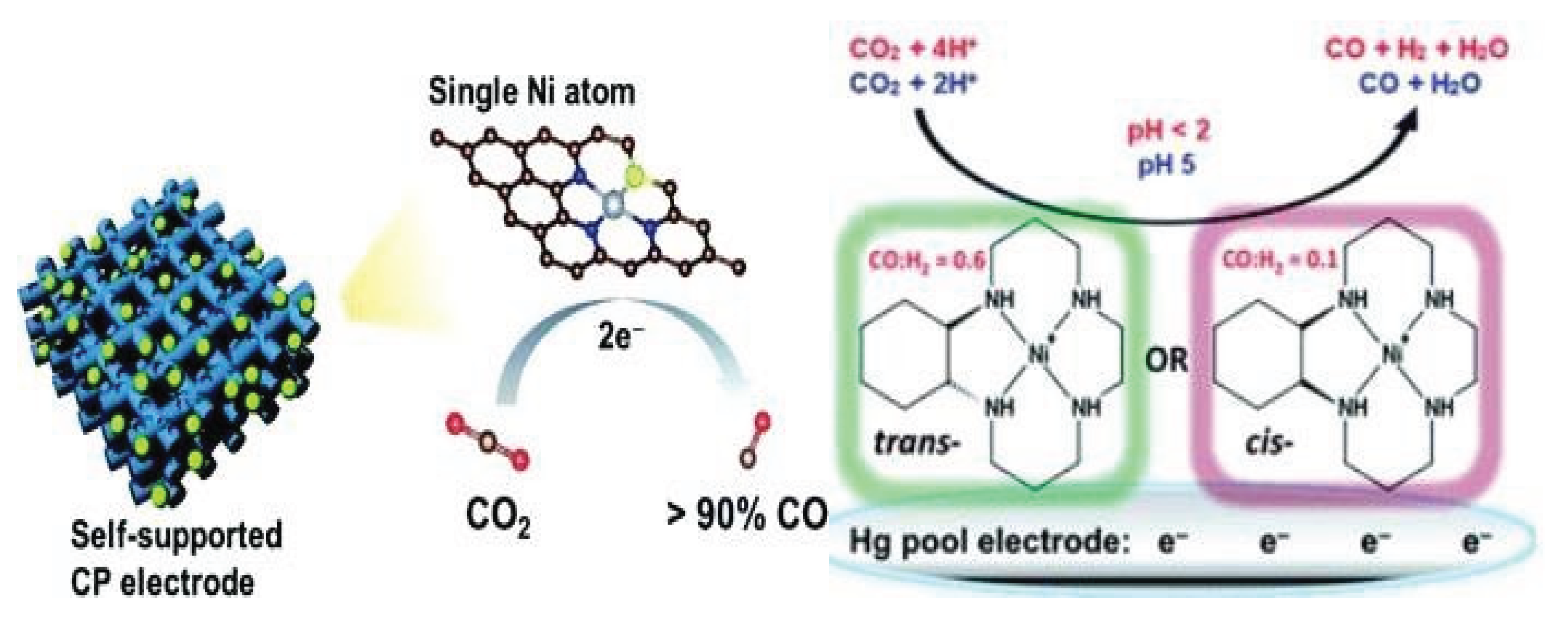
Fujita et al. [
54] prepared a series of Ni based molecular catalyst using support of macrocycle (Cyclam = 1,4,8,11- tetra azacyclotetradecane). Synthesized [Ni(cyclam)]
2+ material has been used as electrocatalyst for the electrocatalytic reduction of CO
2 to CO in aqueous solution at working electrode of mercury (
Figure 16).
Figure 16.
(a) Incorporation Ni single atom into carbon paperwork as a self-standing electrocatalyst for CO
2 reduction, [
51] (a) (b) [Ni(cyclam)]
2+ material used as electrocatalyst for the electrocatalytic reduction of CO
2 to CO in aqueous solution at working electrode of mercury. [
54].
Figure 16.
(a) Incorporation Ni single atom into carbon paperwork as a self-standing electrocatalyst for CO
2 reduction, [
51] (a) (b) [Ni(cyclam)]
2+ material used as electrocatalyst for the electrocatalytic reduction of CO
2 to CO in aqueous solution at working electrode of mercury. [
54].
Su et al. [
55], developed Ni-CTF catalyst modified with Covalent triazine frameworks (CTF). Ni-CFT showed much higher efficiency towards the electrocatalytic reduction of CO
2 as compared to Ni-porphyrin (using TPP: tetraphenyporphyrin) due to the low coordination number support in Ni-CFT. Ni-CFT exhibited high faradaic efficiency 90% of the CO during reduction of CO
2 at over potential −0.8 V
versus RHE. Another study of the same author reports the electrochemical reduction of CO
2 to CO using Ni-SACs named Ni-N-Gr catalysts. In this Ni atom dispersed on the N-doped graphene under rapid heat treatment at 900 °C, 1 min, Ni- SACs turned highly selective towards reduction of CO
2, with a CO FE of over 90% at −0.70 V vs.
RHE.
Yang et al. [
56] synthesized atomically dispersed Ni single atom of the surface of graphene via doping and without doping of S atom via pyrolysis method and designed catalyst as A–Ni–NG and A–Ni–NSG respectively. The prepared catalyst exhibits high reduction efficiency with CO FE of 97% at around −0.5 V and shoed good stability during test up to 100 hr with only 2% loss. Jiang et al. [
57] reports the synthesis of Ni single atoms coordinated in a graphene shell work as a active center for efficient artificial photosynthesis. Synthesized catalyst favors the CO
2 reduction to CO with high selectivity more than 90%. Author also reports the synthesis of Ni single atom catalyst via the doping of Ni atom in graphene nanosheet. Prepared electrocatalyst revealed high electrochemical reduction of CO
2 to CO with high FE 93.2 % at over potential at–0.82 V under significant current density ∼60 mA/mg. Yuan et al. [
58] proposed Semi- sacrificial template synthesis of Single atom Ni coordinated with N doped hollow carbon nanosphere as support. Prepared catalyst named as SA–Ni/N–CS showed efficient and stable electrocatalytic reduction of CO
2 (Figure 58) The SA–Ni/N–CS catalyst showed excellent faradaic efficiency 95.1% of CO at −0.8 V vs.
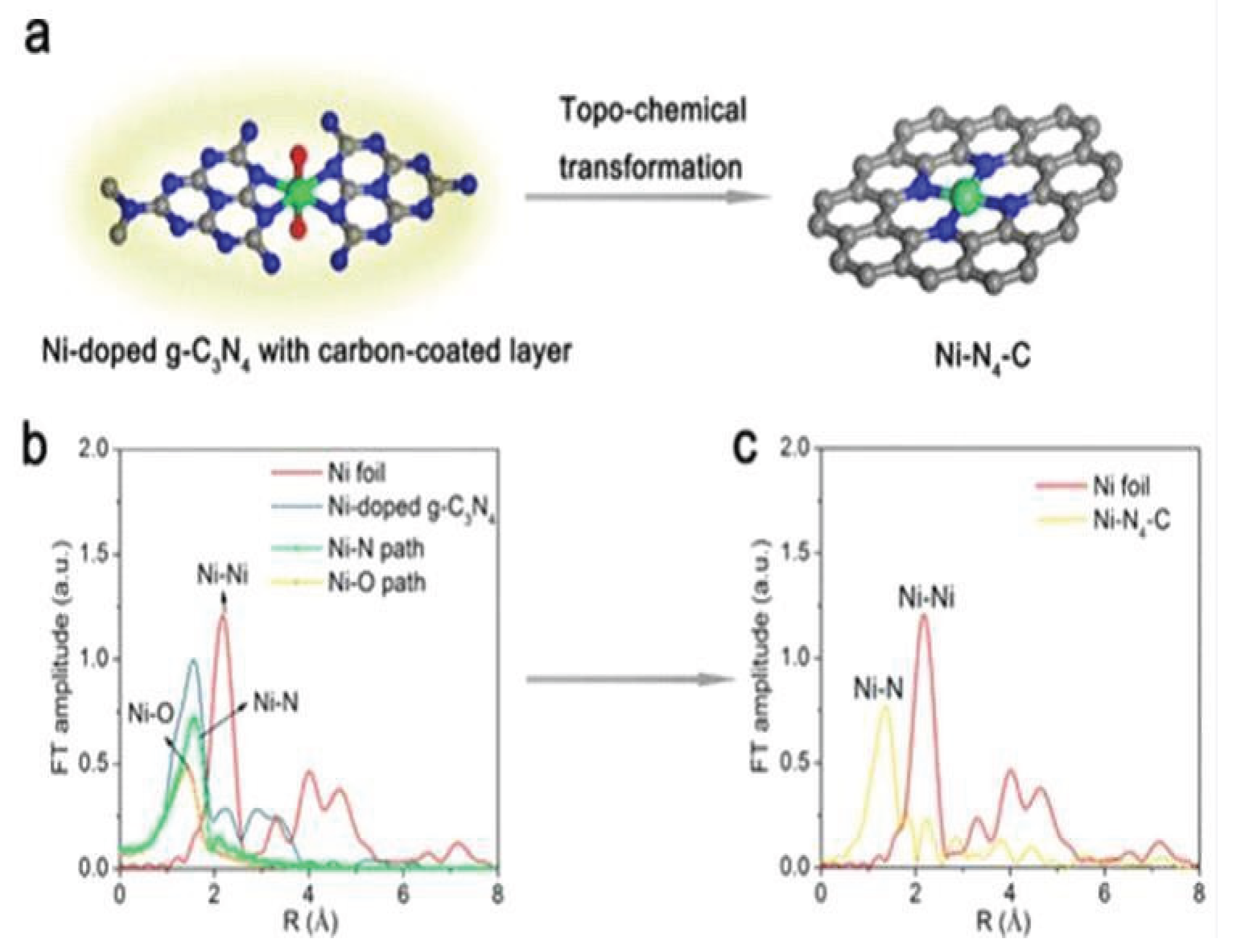
RHE with outstanding stability for 24 h with loss of any current. Li et al. [
59] developed a catalyst featuring exclusive Ni–N
4–C active moieties via a top-chemical transformation method (
Figure 17). The Ni–N
4–C catalyst achieved a maximum CO Faradaic efficiency of 99% at −0.81 V vs. RHE, with a total current density of 28.6 mA/cm
2, also adopted MOFs which assist the synthesis of Single Ni atom catalyst with exclusive Ni–N4–C active moieties via ion exchange between the Zn rod and Ni rod. Synthesized single Ni atom catalyst Ni–N
4–C exhibited a maximum faradaic efficiency 99% of CO at –0.81V vs. RHE and current density of 28.6 mA/cm
2.
Figure 17.
(a) Synthesis of Ni–N
4–C active moieties via a top-chemical transformation method [
59], and catalyst c Ni–N
4–C exhibited a maximum faradaic efficiency 99% of CO at –0.81V.
Figure 17.
(a) Synthesis of Ni–N
4–C active moieties via a top-chemical transformation method [
59], and catalyst c Ni–N
4–C exhibited a maximum faradaic efficiency 99% of CO at –0.81V.
Figure 18.
(a) N doped hollow carbon nanosphere as electrocatalyst for CO
2 reduction to CO [
58]. Prepared catalyst named as SA–Ni/N–CS N- doped carbon nanotube (Ni-NCTs) for electroreduction of CO
2 [
60].
Figure 18.
(a) N doped hollow carbon nanosphere as electrocatalyst for CO
2 reduction to CO [
58]. Prepared catalyst named as SA–Ni/N–CS N- doped carbon nanotube (Ni-NCTs) for electroreduction of CO
2 [
60].
Hou et al. [
60] successfully synthesized Ni based single atom catalyst via automatically dispersion of Ni atom on the surface of N- doped carbon nanotube (Ni-NCTs) for electroreduction of CO
2. Ni/NCTs catalyst showed nearly100% selectivity of CO at potential –0.6V RHE, with current density 34.3 mA/cm
2. Zhu et al. [
61] study introduced Ni-based catalyst for production of synthesis gas through electrocatalytic reduction of CO
2 In this Ni –based catalyst, Ni Single atom and Ni nanoparticles attached on support of N-doped carbon nanorods (named as Ni-CNRs and NiNPs-CNRs respectively) and both synthesized catalysts display excellent selectivity towards CO
2 reduction to CO and HER respectively.
Table 3.
Nickel based single atom catalysts (Ni-SACs) for CO2 reduction.
Table 3.
Nickel based single atom catalysts (Ni-SACs) for CO2 reduction.
| Sr. No |
Name of Metal Catalyst |
Type of catalysts |
Conversion Form |
Conditions |
Faradaic efficiency |
References |
| 1. |
Ni-SACs |
electrochemical reduction |
CO2 to CO |
0.681 V |
99% |
[49] |
| 2. |
Ni-N-C |
electrochemical reduction |
CO2 to CO. |
0.6 V |
98% |
[52] |
| 3. |
Ni- SACs |
electrochemical reduction |
CO2 to CO |
–0.681V |
99% |
[53] |
| 4. |
[Ni(cyclam)]2+ |
electrocatalyst |
CO2 to CO |
0.6 V |
99% |
[54] |
| 5. |
Ni-CTF |
electrocatalytic reduction |
CO2 to CO |
−0.8 V |
90% |
[55] |
| 6. |
SA–Ni/N–CS |
electrocatalytic reduction |
CO2 to CO |
−0.8 V |
95.1% |
[58] |
| 7. |
The Ni–N4–C |
electrocatalytic reduction |
CO2 to CO |
−0.81 V |
99% |
[59] |
3.1.4. Cobalt Based Single Atom Catalysts (Co-SACs) for CO2 Reduction
Cobalt-based catalysts are gaining recognition for their potential in CO
2 reduction, which is crucial for addressing climate change and producing renewable fuels. Recently, cobalt-based molecular catalysts have been investigated for there their application in the electrochemical and photochemical reduction of protons to hydrogen [
62,
63]. These catalysts have shown promising activity and stability under a wide range of various conditions; making them attractive, they are attractive candidates for the development of developing sustainable hydrogen production technologies. Cobalt-based catalysts have also been explored for their potential use in the electrocatalytic reduction of carbon dioxide to folate, a valuable chemical Products [
64].
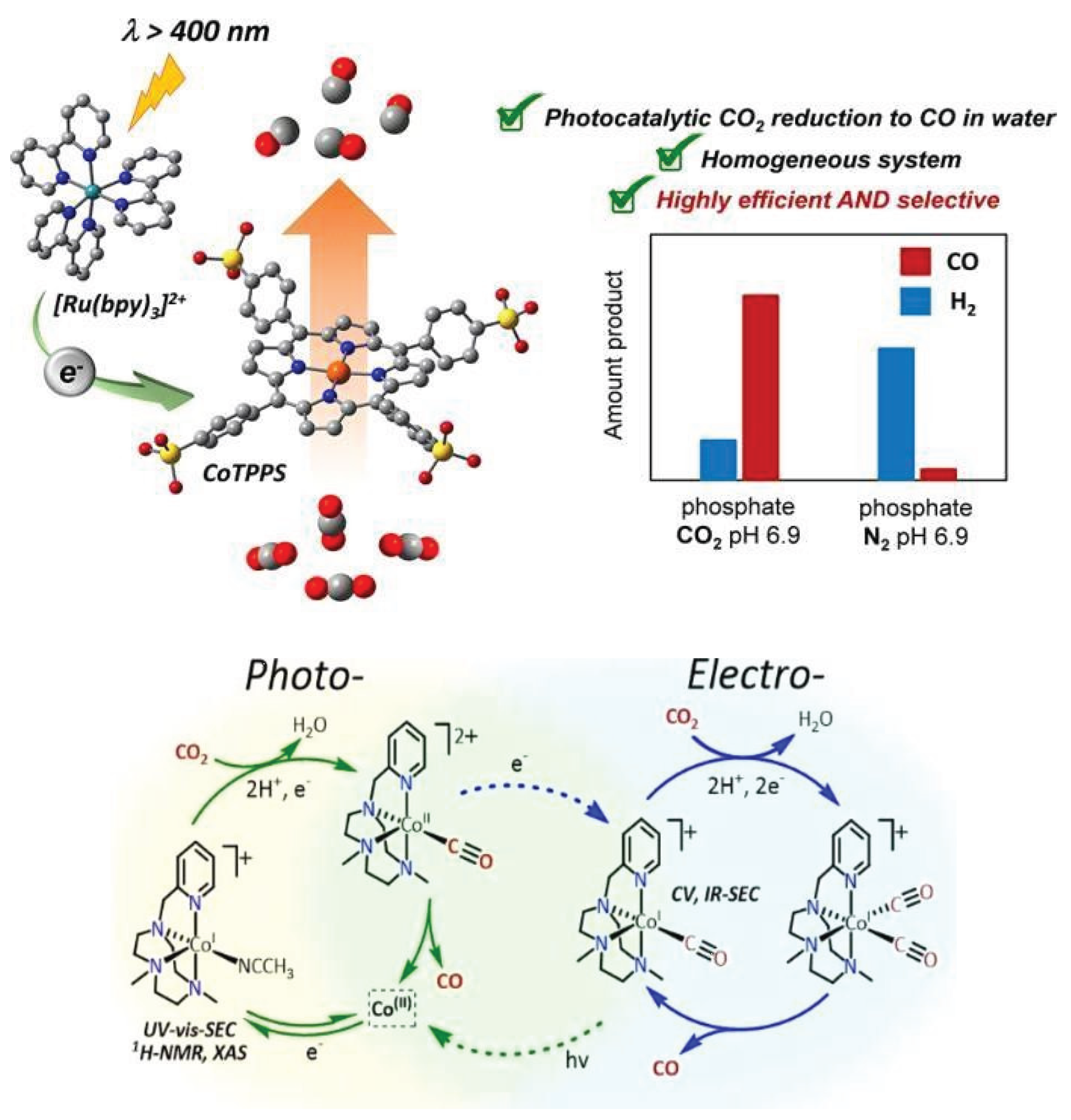
Sakai et al. [
65] reports the highly efficient cobalt porphyrin based molecular catalyst for photocatalytic reduction of CO
2. Synthesized cobalt porphyrin molecular catalyst named as CoTPPS ([{
meso-tetra(4-sulfonatophenyl) porphyrin to}cobalt (III)], work as catalyst in photocatalytic reduction of CO
2 to CO with 90% selectivity Ru(bpy)
3]
2+ as a photosensitizer using under visible light irradiation in aqueous solution. Robert et al. [
66] reports the CO
2 electrochemical catalytic reduction with a highly active cobalt phthalocyanine and CO production occurs with excellent selectivity (ca.95%), and good stability with a maximum partial current density of 165 mA cm
−2 (at −0.92 V vs. RHE), The CO
2RR was investigated by using a single-atom cobalt catalyst (Co-Typ-C) by Hou and coworkers [
67] via the pyrolysis of Co-terpyridine (TPY) organo-metallic complex. The Co-TPy catalyst noticed high Faradaic efficiency > 95% of CO with potential over range from −0.7– 1.0 V (vs RHE). Catalyst exhibited low FE CO with single Co catalyst without Typ ligand. Fernández et al. [
68] explained both electro- and photocatalytic CO
2 reduction electro- and photocatalytic CO
2 reduction using cobalt aminopyridine molecular catalyst. Recently Co coordinated with nitrogen doped carbon-based material received promising catalytic activity towards the CO
2RR to CO. Wang et al. [
69] synthesized a series of Co based catalyst via atomically dispersion Co on N doped carbon sheet and explore their performance towards electrocatalytic reduction of CO
2. From the reduction performance author noticed that the Co coordinated with two N atoms achieved superior catalytic activity and high selectivity with 94% FE of CO at an over potential –0.5 V.
Figure 19.
(a) molecular structure of cobalt porphyrin molecular catalyst named as Co-TPPS ([{
meso-tetra(4-sulfonatophenyl)porphyrin to}cobalt (III)], [
65] and (b) Electro- and photocatalytic CO
2.
Figure 19.
(a) molecular structure of cobalt porphyrin molecular catalyst named as Co-TPPS ([{
meso-tetra(4-sulfonatophenyl)porphyrin to}cobalt (III)], [
65] and (b) Electro- and photocatalytic CO
2.
Pan et al. [
70] synthesized Co single atoms anchored on polymer-derived hollow N-doped porous carbon spheres featuring Co–N
5 active canters (referred to as Co–N
5/HNPCSs), which demonstrated a CO Faradaic efficiency of 99.2% at −0.73 V vs. RHE and 99.4% at −0.79 V vs. RHE. Amal et al. [
71] report the synthesis of Co-single-atom-decorated, N-doped graphitic carbon shells encapsulating a Co NPs core (Co@CoNC-900) demonstrated the ability to maintain a stable H
2/CO ratio ranging from 0.25 to 1 within the −0.3 V to −0.8 V vs. RHE potential range. Song et al. [
72] proposed Electrochemical production of syngas using catalyst Co–C
2N
2 moieties, while other nitrogen functionalities like graphitic and pyridinic N, can promote hydrogen evolution reaction (HER). He et al. [
73] investigated the catalytic activity of different metal (e.g., Ag, Co, Pt, and Pd) based Single atom catalyst for electroreduction of CO
2 in to value added product. For the preparation of this catalyst single atom dispersed on the defective graphene support for electrochemical CO
2 conversion in to CO, HCHO, COOH, C1, CH
4 and CH
3OH using the selected five transition metals (i.e., Ag, Co, Cu, Pt, and Pd).
Figure 20.
(a) Synthesis scheme of polymer-derived hollow N-doped porous carbon spheres featuring Co–N
5 active canters (referred to as Co–N
5/HNPCSs) [
70], and (b) Faradaic efficiency of Co@CoNC-900. [
71].
Figure 20.
(a) Synthesis scheme of polymer-derived hollow N-doped porous carbon spheres featuring Co–N
5 active canters (referred to as Co–N
5/HNPCSs) [
70], and (b) Faradaic efficiency of Co@CoNC-900. [
71].
Table 4.
Cobalt based catalysts for CO2 reduction.
Table 4.
Cobalt based catalysts for CO2 reduction.
| Sr.No. |
Name of Metal Catalyst |
Type of catalysts |
Conversion Form |
Conditions |
Faradaic efficiency |
References |
| 1. |
CoTPPS |
photocatalytic reduction |
CO2 to CO |
−0.92 V |
90% |
[65] |
| 2. |
CO2RR |
photocatalytic reduction |
CO2 to CO |
-0.94 V |
> 95% |
[68] |
| 3. |
Co–N5 |
photocatalytic reduction |
CO2 to CO |
−0.73 V |
99.2% |
[70] |
3.1.5. Zinc Based Single Atom Catalysts (Zn-SACs) for CO2 Reduction
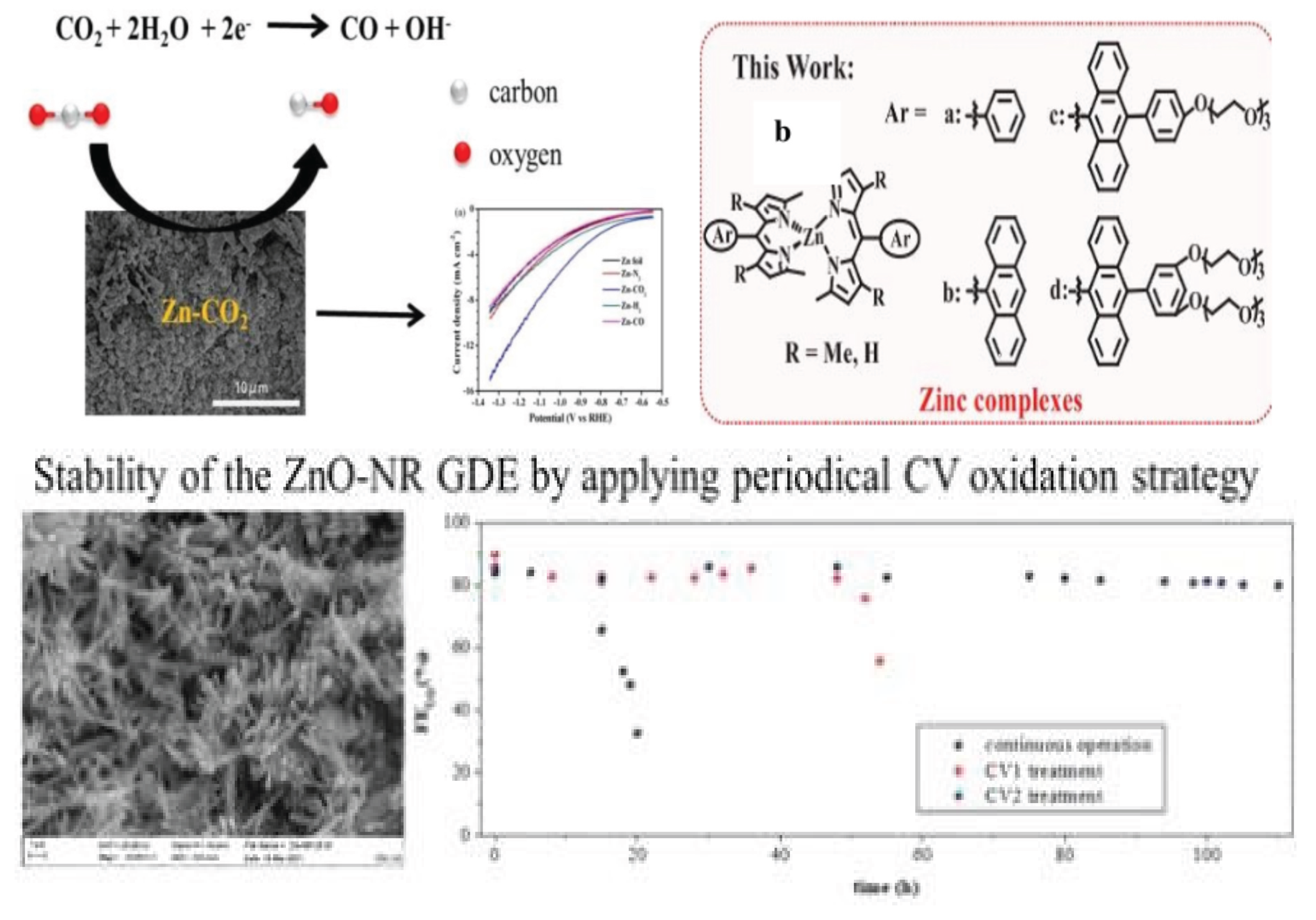
Zinc (Zn) is an earth-abundant metal that can be used as catalyst to reduce carbon dioxide (CO
2) to CO. Stamatelos et al. [
74] develop Zn-based catalysts for the reduction of CO
2 to CO with different structural form of Zn such as Zn-nanoparticles and ZnO nanorods. Results found that ZnO nanorods exhibited higher reduction of CO
2 as compared to Zn nanoparticles with faradaic efficiency 80% of CO in current density range of 50–160 mA cm
2 in both flow- cell and membrane electrode assembly (MEA) reactors. Yang et al. [
75] report highly efficient CO
2 electroreduction using Zn–N–C type single atom catalyst. In this synthesis Zn atom was dispersed on the surface of 4N coordinated carbon sheet (ZnN
x/C) and forming Zn–N
4 active sites for catalytic reduction. The prepared catalyst showed high catalytic performance for CO
2RR to CO, with CO FE of 95% at 0.43 V vs.
RHE, and exhibited good stability up to 75h without any current losses. Zhang et al. [
76] synthesized Zn–dipyrrin complexes (
Figure 18), used for the first time for CO
2 photo reduction and the influence of the SBCT efecton CO
2 reduction. Guo et al. [
77] reported the synthesis of a fibrous Zn catalyst (Zn-CO
2) that demonstrates high electrochemical activity and stability. The Zn-CO
2 catalyst achieves a Faradaic efficiency of 73.0% for CO at −1.2 V vs. RHE, (
Figure 18) with CO selectivity remaining nearly unchanged over 6 hours at that potential.
Figure 21.
(a) The Zn-CO
2 catalyst achieves a Faradaic efficiency [
80], (b) Structure of synthesized Zn–dipyrrin complexes [
76], and (c) Sem image of ZnO nanorods and showed higher reduction of CO
2 as compared to Zn nanoparticles with faradaic efficiency 80% [
74].
Figure 21.
(a) The Zn-CO
2 catalyst achieves a Faradaic efficiency [
80], (b) Structure of synthesized Zn–dipyrrin complexes [
76], and (c) Sem image of ZnO nanorods and showed higher reduction of CO
2 as compared to Zn nanoparticles with faradaic efficiency 80% [
74].
Table 5.
Zinc based catalysts for CO2 reduction.
Table 5.
Zinc based catalysts for CO2 reduction.
| Sr.No. |
Name of Metal Catalyst |
Type of catalysts |
Conversion Form |
Conditions |
Faradaic efficiency |
References |
| 1. |
Zn-nanoparticles |
electrocatalytic reduction |
CO2 to CO |
−0.77 V |
80% |
[77] |
| 2. |
Zn–dipyrrin complexes |
photo reduction |
CO2 to CO |
−1.2 V |
73.0% |
[79] |
3.1.6. Molybdenum Based Single Atom Catalysts (Mo-SACs) for CO2 Reduction
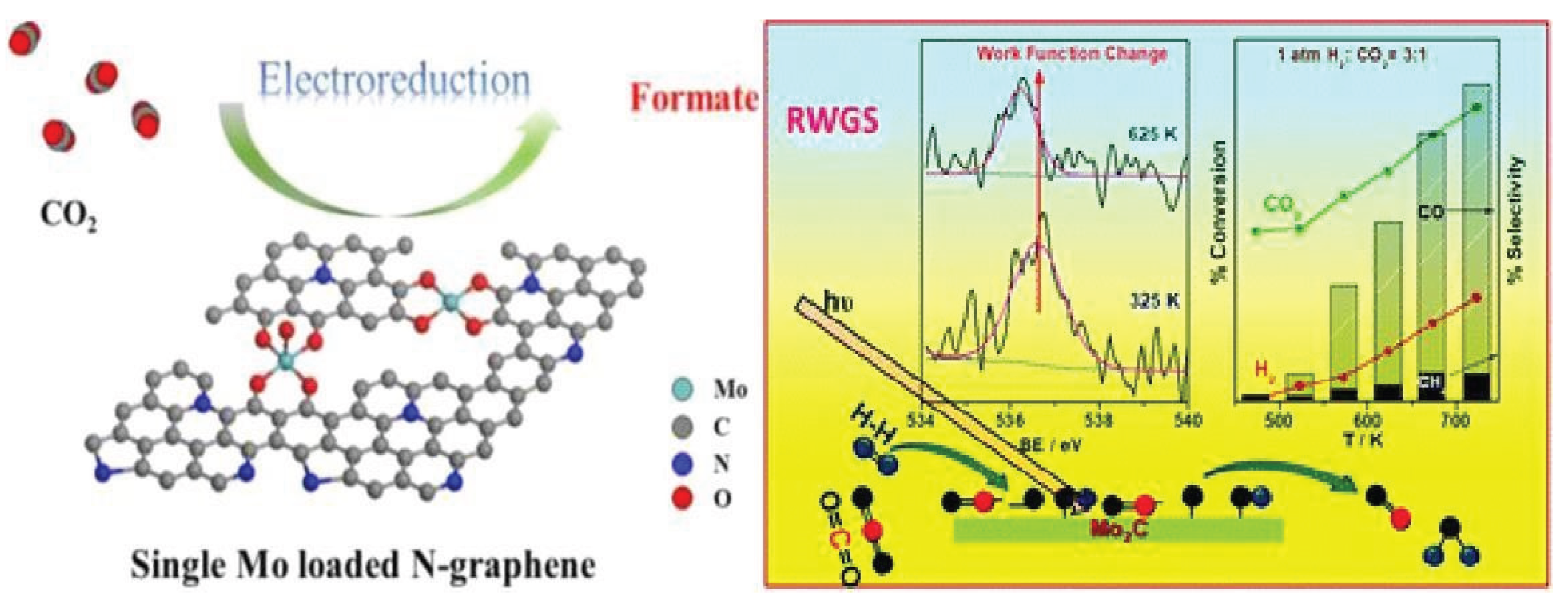
Huang et al. [
78] proposed Mo-NG electrocatalyst used for reduction of CO
2 into formate. In this electrocatalyst single Mo atom loaded on the N doped ultrathin graphene sheet (
Figure 22) Prepared electrocatalyst exhibited excellent electrocatalytic reduction of CO
2 into formate using 4% ionic liquid as an electrolyte. The faraday efficiency of CO was found 100% and formate yield of 747 mmol/(g
catal h). Author noticed that Mo-NG electrocatalyst showed higher reduction with ionic liquid than N doped graphene.
Khojinet al. [
79] reports the electrochemical reduction of CO
2 under to compartment using the M o S
2 catalyst within an ionic liquid with three electrode electrochemical cell. The MoS
2 catalyst exhibited a notably high current response of (65 mA cm
–2 at an over potential of 654 mV) along with strong selectivity for CO production with Faradaic efficiency of approximate 98%. Reddy et al. [
80] formulated low-cost Molybdenum carbide (Mo
2-C) catalyst for the reduction of CO
2 to CO (selectivity 68%). Zhuo et al. [
81] prepared metal carbides based 2D multilayered Mo2C catalyst for hydrogenation of CO
2 with high selectivity and stability. the synthesized two- dimensional (2D) multilayered 2D-Mo
2C material showed CO
2 hydrogenation the activity and product selectivity (CO, CH
4, C2–C5 alkanes, methanol, and dimethyl ether), (
Figure 23) highest selectivity towards CO with 94% faradaic efficiency at 430
oC and showed excellent stability up to 100 h without deactivation.
3.1.7. Other Metal-Based Catalysts for CO2 Reduction
There are several other metal-based catalysts which also shown promise effect for CO
2 reduction into value added products. Manganese was also the key element in the reduction of CO
2 Feng et al. [
82] Prepared single Mn based catalyst was embedded on N
4 coordinated graphitic carbon nitride (g- C
3N
4) on carbon nanotubes (named as Mn–C
3N
4/CNT). Synthesized Mn–C
3N
4/CNT electrocatalyst exhibited higher Faradaic efficiency 98% of CO in aqueous electrolyte with current density 14.0 mA/cm
2 at a low over potential of 0.44 V vs.
RHE, outperforming all the Mn–N
4-based SACs previously reported in the literature. Pan et al. [
83] synthesized Mn–N–C and Co–N–C catalysts by pyrolyzing a solid obtained from drying a water mixture of urea, citric acid, and a metal precursor. The Mn-based and Co-based single- atom catalysts demonstrated similar maximum CO Faradaic efficiencies (72% and 70%, respectively), although the overpotential required for the Mn–N–C to achieve maximum CO selectivity was 260 mV lower than that of the Co–N–C. Overall, both Mn and Co displayed lower catalytic performance for CO production compared to other single-atom catalysts.
Li et al. [
84] prepared a dual atom Ag
2–G electrocatalyst for electrochemical reduction of CO
2 to CO. Synthesized Ag
2–G catalyst showed superior properties toward reduction of CO
2 as compared to Ag-nanoparticles (Ag-NPs) and the single-atom Ag
1/graphene (Ag-G). The Ag
2–G active site consists of two adjacent Ag atoms; each is coordinated with three N atoms (AgN
3–AgN
3) further linked with the graphene matrix via Ag-C strong bond. Synthesized electrocatalyst Ag
2–G showed higher reduction of CO
2 to CO with FE CO up to 93.4% at over potential of −0.25 V vs.
RHE, with a current density of 11.87 mA/cm
2 at −0.7 V vs.
RHE, and catalyst exhibited good stability for 36 h of tests. He et al. [
85] prepared an N-doped carbon- supported Pd single-atom catalyst (referred to as Pd-NC) with a low Pd loading of 2.95 wt%. Through various characterization techniques and DFT calculations, the authors highlighted the unique coordination of Pd atoms with N atoms in the Pd–N
4 active centers, which aids in stabilizing and activating the adsorbed CO
2, thereby ensuring CO production at low overpotentials. Compared to commercial palladium on carbon (Pd/C), which shows high selectivity for the hydrogen evolution reaction (HER), the Pd-NC demonstrated moderate selectivity for the CO
2 reduction reaction (CO
2RR) to CO, achieving a CO Faradaic efficiency of 55% at −0.50 V vs. RHE. Podyacheva et al. [
86] reports the single Pd atom-based catalyst, in which Pd particles was dispersed on N-doped carbon-supported (named as Pd-NC) with a low Pd loading of 2.95 wt%. Through DFT calculation characterization results showed that the Pd atom was coordinated with four N atoms i.e., Pd–N
4 active centers, which helps stabilize and activate the adsorbed CO
2, hence guaranteeing CO generation at low overpotentials. From the reduction results found that the commercially available Pd/C exhibited higher selectivity towards CO
2RR to CO as compared to Pd-NC, While Pd-NC electrocatalyst showed a moderate selectivity for CO
2 reduction to CO with faradaic efficiency 55% at over potential –0.50 V vs
. RHE.
Table 6.
Other metal based catalysts for CO2 reduction.
Table 6.
Other metal based catalysts for CO2 reduction.
| Name of Metal Catalyst |
Type of catalysts |
Conversion Form |
Conditions |
Faradaic efficiency |
References |
| Mo-NG |
electrocatalyst |
CO2 into formate |
-0.96 |
100% |
[81] |
| Molybdenum carbide |
electrocatalyst |
CO2 to CO |
-0.92 |
98% |
[83] |
| Mn–C3N4/CNT |
electrocatalyst |
CO2 to CO |
0.44 V |
98% |
[85] |
| Ag2–G |
electrochemical reduction |
CO2 to CO |
−0.25 V |
93.4% |
. [87] |
3.2. Alloy and Dua Atom Catalysts: Towards Better Selectivity
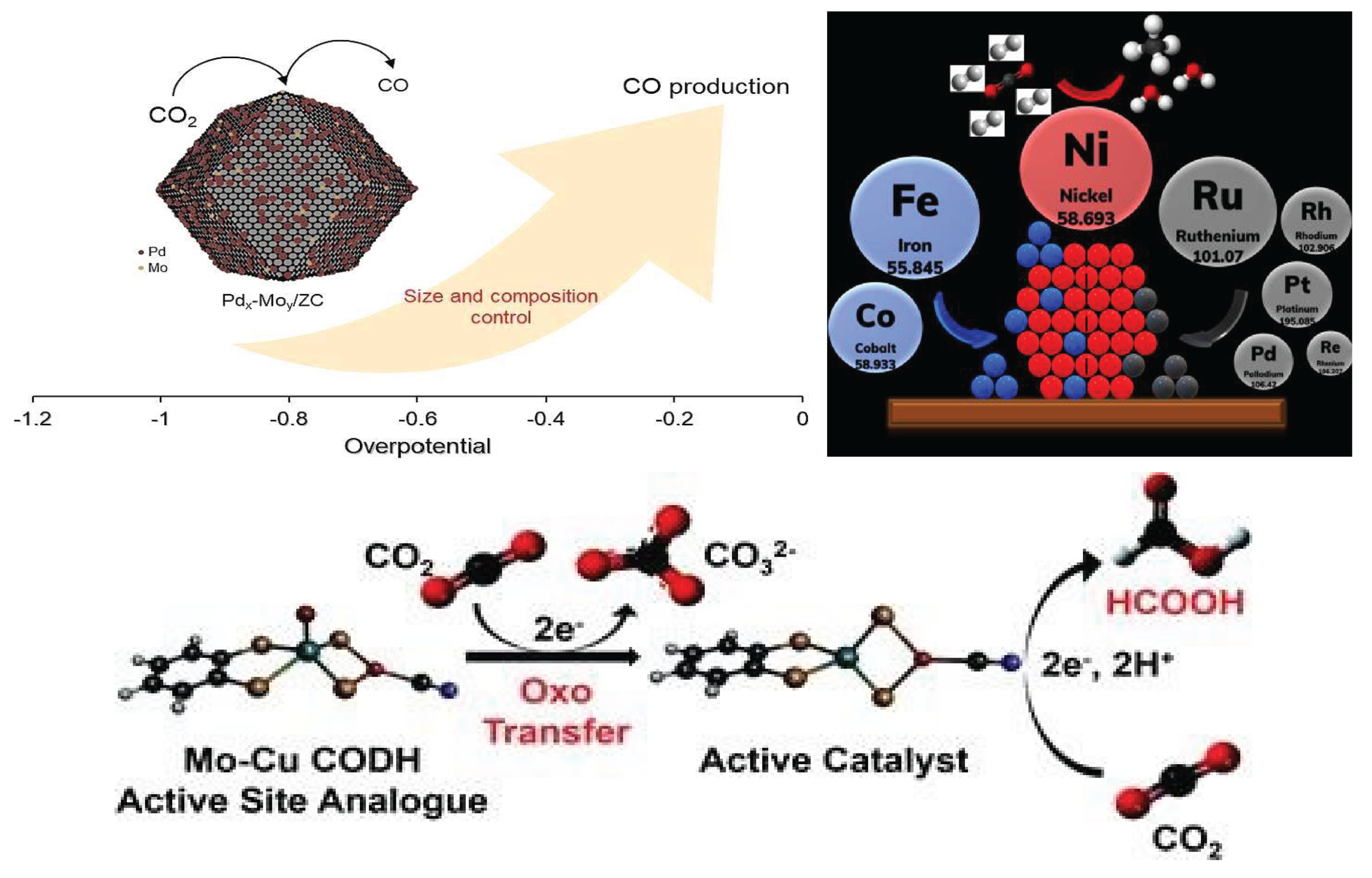
Lee et al. [
87] synthesized a Pd–Mo MMO catalyst by adjusting with its size and composition. prepared bimetallic Pd-Mo mixed metal oxides showed efficient properties towards the electroreduction of CO
2 to CO. From the result found that the CO
2 electrocatalytic performance increases as amount of Pd are increased in Pd–Mo MMO catalyst Pd–Mo MMO catalyst (
Figure 24). Mougel et al. [
88] investigated the electrocatalytic reduction of CO
2 to formate using a bimetallic complex of Mo-Cu, known as [(bdt)Mo
IVS
2Cu
ICN]
2− (where bdt = benzenedithiolate). In this complex, Mo and Cu atoms are linked by two sulfide ligands, mimicking the active site of the Mo–Cu carbon monoxide dehydrogenase (CODH
2) active site (
Figure 24). Infrared Spectro electrochemical (IR-SEC) studies combined with density functional theory (DFT) calculations revealed that the complex serves only as a pre-catalyst, with the active catalyst formed upon reduction in the presence of CO
2. The two-electron reduction of [(bdt)Mo
IVS
2Cu
ICN]
2− initiates the transfer of the oxo moiety to CO
2, resulting in the formation of CO and the complex [(bdt)Mo
IVS
2Cu
ICN]
2−, while an additional one-electron reduction is required to generate the active catalyst. Protonation of this catalyst produces a reactive MoVH hydride intermediate, which then reacts with CO
2 to yield format.
Wang et al. [
89] report the synthesis of bimetallic Zn-Cu electrocatalyst for electroreduction of CO
2. In this study catalyst Zn-Cu was prepared by facile galvanic-exchange synthesis procedure, and showed high selectivity (95%) for reduction CO
2 to CO, which is superior than that of pure Cu or Zn. Guola et al. [
90] reports the bimetallic Ni-Based catalyst with Fe, Co, Cu, Ru, Rh, Pt, Pd, Re for the CO
2 reduction into methane. Symes et al. [
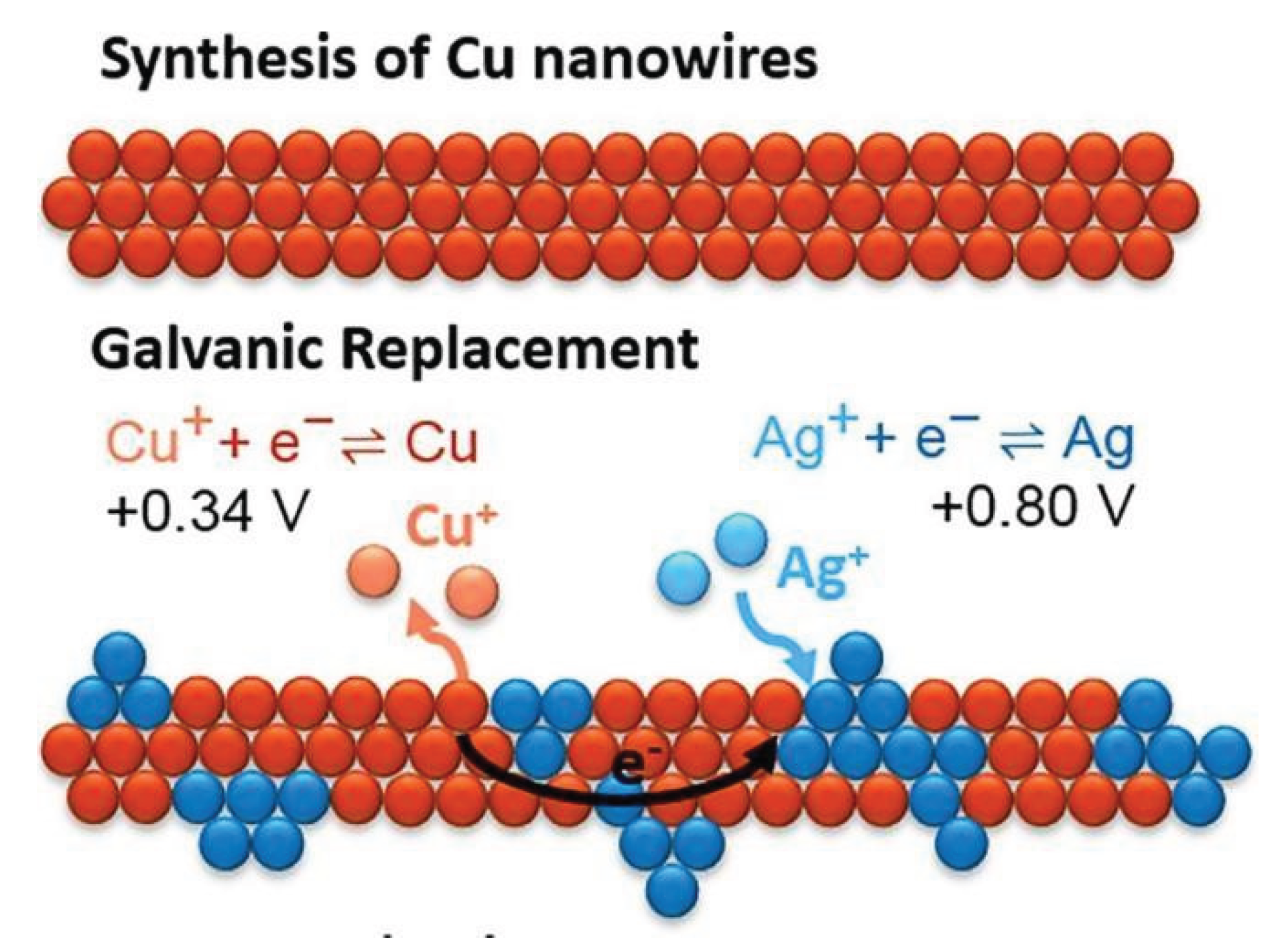
91] explained the electroreduction of CO
2 using Cu bimetallic catalyst. Author found that among the other metals Cu bimetallic based materials are most promising materials for CO
2 electro-reduction. Choi et al. Prepared bimetallic Cu-Ag nanowire by galvanic replacement (
Figure 25). Synthesized Cu-Ag catalyst showed excellent performance toward the production of CO by electrochemical reduction of CO
2.
4. Challenges and Future Directions
This chapter is focused on optimizing the performance of earth-abundant metal-based catalysts such as single atom-based catalyst, molecular catalyst and bimetallic catalyst through different techniques like nano-structuring and surface modification, which can enhance their catalytic activity and stability toward the catalytic reduction of CO2 into value added products. Now current research will be focused to exploring the unique properties of earth-abundant metals may lead to the discovery of new pathways for CO2 reduction that are more efficient or selective than existing methods. Research will be focused on designing more effective and stable catalyst from earth-abundant metals, also discuss the difficulties in scaling laboratory to industrial-scale processes. Analyze the environmental impact and economic viability of using earth-abundant metals compared to other options.
Future Directions
Further research is needed to improve the efficiency, stability and application of earth-abundant metal catalysts. Also focus on the development of more efficient synthesis methods, the exploration of novel alloy systems, and focus on the possible future advancements in catalyst design, process optimization, and scaling up. Highlight any new technologies or approaches that are emerging in reduction of CO2 into energy and fuels for creating a sustainable energy future.
Conclusions
In this chapter, we have explored the promising potential of earth-abundant metals as catalysts for the photochemical and electrochemical reduction of CO2. Through a comprehensive review of recent advancements, we highlighted various earth-abundant metal-based catalysts, including iron, copper, nickel, cobalt, zinc, molybdenum etc., which have demonstrated significant efficacy in converting CO2 into valuable chemical products.
The advantages of using these metals lie not only in their availability and cost-effectiveness but also in their ability to achieve high Faradic efficiencies and selectivity for specific products. We examined various strategies for enhancing the catalytic performance of these metals, such as optimizing their electronic structures through alloying, doping, and the use of nanostructure supports. Overall, the research on earth-abundant metals for CO2 reduction holds significant promise for the development of sustainable and economically viable technologies aimed at mitigating climate change and facilitating the transition to a carbon-neutral economy. Continued innovation in this area could pave the way for more effective catalysts that contribute to global efforts in reducing greenhouse gas emissions.
Author Contributions
Jannatun Zia, writing original draft, conceptualization, review, and methodology, while M.S.S.R. Tejaswini was responsible for writing comparative work, reviewing, visualization, and editing the article and Ufana Riaz review and prepared final draft.
Acknowledgment
Jannatun Zia acknowledges the Department of Chemistry, Siddhartha Academy of Higher Education, Deemed University, Vijayawada, A.P., India, for providing a suitable research laboratory to carry out the review work. M.S.S.R. Tejaswini acknowledges the Department of Chemistry, School of Applied sciences & Humanities, Vignan’s Foundation for Science, Technology &Research, Guntur, Andhra Pradesh India, for research collaboration.
Conflicts of Interest
The authors declare no competing interest.
References
- Nunes, L.J.R. The Rising Threat of Atmospheric CO2: A Review on the Causes, Impacts, and Mitigation Strategies. Environments 2023, 10, 66. [Google Scholar] [CrossRef]
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O, Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Singh, S. Recent Advances in Earth-Abundant Metal Catalysts for CO2 Reduction, Chem. Sus. Chem. 2020, 13, 14–33. [Google Scholar]
- Ludwig, J.R.; Schindler, C.S. Catalyst: Sustainable Catalysis. Chem 2017, 2, 313–316. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Y.; Hong, J. Electrochemical Reduction of Carbon Dioxide: Advances and Challenges. Chem. Rev. 2020, 119, 7439–7482. [Google Scholar]
- Gao, D.; Wang, C. Copper-Based Catalysts for CO2 Electroreduction: A Review. J. CO2 Util. 2021, 49, 101580. [Google Scholar]
- Tao, L.; Zhang, S. Earth-Abundant Metal Catalysts for CO2 Reduction: Design and Mechanism. Adv. Mater. 2020, 32, 2002946. [Google Scholar]
- Wang, H.; Chen, Y. The Role of Iron and Nickel in Electrochemical CO2 Reduction, Nat. Rev. Chem. 2022, 6, 244–261. [Google Scholar]
- Kwon, Y.; Lee, J. Towards Sustainable CO2 Reduction: Innovations in Earth-Abundant Metal Catalysts. ACS Sustain Chem. & Eng. 2023, 11, 1072–1085. [Google Scholar]
- Zhang, H.; Huang, X. Review of Electrocatalytic CO2 Reduction with Copper-Based Catalysts. FrChem. 2022, 6, 185. [Google Scholar]
- Schwabe, T.; Kauffmann, K. Sustainable Pathways for CO2 Utilization: Catalytic Approaches Using Earth-Abundant Metals, Chem. Eur. J. 2022, 28, 202200045. [Google Scholar]
- Liu, X.; Zhu, Y. Mechanistic Insights into CO2 Electroreduction on Earth-Abundant Metal Catalyst, J. Am. Chem. Soc. 2021, 143, 9657–9670. [Google Scholar]
- Raghu, K.; Narayanan, R. Metallic Catalysts for CO2 Reduction: A Review of Earth-Abundant Metals. Catal. 2020, 10, 758. [Google Scholar]
- Rotundo, L.; Gobetto, R.; Nervi, C. Electrochemical CO2 reduction with earth-abundant metal catalysts,Curr. Opin. Green. Sustain. Chem. 2021, 31, 100509. [Google Scholar]
- Bui, M. Carbon capture technologies for the power sector. Nat. Energy. 2018, 3, 420–431. [Google Scholar]
- Zhou, P. Direct air capture: A technology review, Renew. Sustain. Energy Rev. 2021, 135, 110220. [Google Scholar]
- Global Status of CCS Institute, Retrieved from Global CCS Institute website 2020.
- Gielen, D. The role of carbon capture and storage in the transition to a low-carbon energy system. Energy Econ. 2020, 87, 104559. [Google Scholar]
- Barton, J.R.; Ault, G.W. The role of energy storage in the future of the energy system, Renew. Sustain. Energy Rev. 2019, 113, 109270. [Google Scholar]
- Davidson, C.I. Sustainable bioenergy and carbon capture and storage. Nat. Clim. Change. 2018, 8, 963–971. [Google Scholar]
- Schembecker, G. Carbon Capture and Utilization: An Overview. J. CO2 Util. 2020, 41, 101203. [Google Scholar]
- Call, F.; Roeb, M.; Schmücker, M.; Bru, H. Thermogravimetric Analysis of Zirconia-Doped Ceria for Thermochemical Production of Solar Fuel. American J. Analyt. Chem. 2013, 4, 37–45. [Google Scholar] [CrossRef]
- Chen, Q.; Tsiakaras, P.; Shen, P. Electrochemical Reduction of Carbon Dioxide: Recent Advances on Au-Based Nano catalysts. Catal. 2022, 12, 1348. [Google Scholar] [CrossRef]
- Lingampalli, S.R.; Ayyub, M.M.; Rao, C.N. Recent Progress in the Photocatalytic Reduction of Carbon Dioxide. ACS Omega. 2017, 6, 1348. [Google Scholar] [CrossRef] [PubMed]
- Rotundo, L.; Gobetto, R.; Nervi, C. Electrochemical CO2 reduction with earth-abundant metal catalysts, Curr. Opin. Green Sustain. 2021, 31, 100509. [Google Scholar] [CrossRef]
- Fan, M.; Luo, Z.M.; Wang, J.W.; Aramburu-Trošelj, B.M.; Ouyang, G. Earth-abundant-metal complexes as photosensitizers in molecular systems for light-driven CO2 reduction, Coord. Chem. Rev. 2024, 500, 215529. [Google Scholar]
- Palade, E.A.; Gobetto, R.; Nervi, C. Molecular and single-atom catalysts based on earth-abundant transition metals for the electroreduction of CO2 to C1. Inorganica Chimica Acta 2024, 566, 122029. [Google Scholar] [CrossRef]
- Scarpa, D.; Sarno, M. Single-Atom Catalysts for the Electro-Reduction of CO2 to Syngas with a Tunable CO/H2 Ratio: A Review. Catal. 2022, 12, 275. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Chen, J.Y.; Siegbahn, P.E.M.; Liao, R.Z. Harnessing noninnocent porphyrin ligand to circumvent Fe-hydride formation in the selective Fe-catalyzed CO2 reduction in aqueous solution. ACS Catal. 2020, 10, 1–22. [Google Scholar] [CrossRef]
- Khakpour, R.; Laasonen, K.; Busch, M. Selectivity of CO2, carbonic acid and bicarbonate electroreduction over iron-porphyrin catalyst: a DFT study, Electrochim. Acta. 2023, 442, 141784. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Kamada, K.; Sekizawa, K.; Sato, S.; Morikawa, T.; Jung, J. Photocatalytic CO2 reduction using an iron by two phosphines for improving catalyst durability. Organometallics 2022, 41, 1865–1871. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Hua, Z.; Zhang, Z. Iron-based single-atom electrocatalysts: synthetic strategies and applications. RSC Adv. 2021, 11, 3079–3095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, S.; Wu, J.; Liu, M.; Yazdi, S.; Ren, M.; Sha, J.; Zhong, J.; Nie, K.; Jalilov, A.S.; Li, Z.; Li, H.; Yakobson, B.I.; Wu, Q.; Ringe, E.; Xu, H.; Ajayan, P.M.; Tour, J.M. Electrochemical CO2 Reduction with Atomic Iron-Dispersed on Nitrogen-Doped Graphene, Adv. Energy Mater. 2018, 8, 1703487. [Google Scholar] [CrossRef]
- Chen, H.; Guo, X.; Kong, X.; Xing, Y.; Liu, Y.; Yu, B.; Li, Q.X.; Geng, Z.; Si, R.; Zeng, J. Tuning the coordination number of Fe single atoms for the efficient reduction of CO2. Green Chem. 2020, 22, 7529. [Google Scholar] [CrossRef]
- Chen, S.; Liu, T.; Olanrele, S.O.; Lian, Z.; Si, C.; Chen, Z.; Li, B. Boosting electrocatalytic activity for CO2 reduction on nitrogen-doped carbon catalysts by co-doping with phosphorus. J. Energy Chem. 2021, 54, 143–150. [Google Scholar] [CrossRef]
- Wu, S.; Lv, X.; Ping, D.; Zhang, G.; Wang, S.; Wang, H.; Yang, X.; Guo, D.; Fang, S. Highly exposed atomic Fe–N active sites within carbon nanorods towards electrocatalytic reduction of CO2 to CO. Electrochim. Acta. 2020, 340, 135930. [Google Scholar] [CrossRef]
- Jin, Z.; Jiao, D.; Dong, Y.; Liu, L.; Fan, J.; Gong, M.; Ma, X.; Wang, Y.; Zhang, W.; Zhang, L.; Yu, Z.G.; Voiry, D.; Zheng, W.; Cui, X. Boosting Electrocatalytic Carbon Dioxide Reduction via Self-Relaxation of Asymmetric Coordination in Fe-Based Single Atom Catalyst. Angew. Chem. 2023, 136, 1–14. [Google Scholar]
- Lyu, H.; Ma, C.; Zhao, J.; Shen, B.; Tang, J. A novel one-step calcination tailored single-atom iron and nitrogen co-doped carbon material catalyst for the selective reduction of CO2 to CO. Sep. Purifi. Technol. 2022, 303, 122221. [Google Scholar] [CrossRef]
- Ren, M.; Guo, X.; Huang, S. Coordination-tuned Fe single-atom catalyst for efficient CO2 electroreduction: The power of B atom, Chem. Eng. J. 2022, 433, 134270. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Bu, Z.; Yang, F.; Luo, J.; An, Q.; Zeng, Z.; Wang, J.; Deng, S. Boosting CO2-to-CO conversion on a robust single-atom copper decorated carbon catalyst by enhancing intermediate binding strength. J. Mater. Chem. A 2021, 9, 1705–1712. [Google Scholar] [CrossRef]
- Xu, C.; Zhi, X.; Vasileff, A.; Wang, D.; Jin, B.; Zheng, Y.; Qiao, S.Z. Highly Selective Two-Electron Electrocatalytic CO2 Reduction on Single-Atom Cu Catalysts. Small Struct. 2020, 2, 2000058. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, X.; Li, X.; Nie, X.; Fan, S.; Feng, M.; Fan, Z.; Tan, M.; Che, Y.; He, G. Construction of atomically dispersed Cu-N4 sites via engineered coordination environment for high-efficient CO2 electroreduction. Chem. Eng. J. 2021, 407, 126842. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Bu, Z.; Yang, F.; Luo, J.; An, Q.; Zeng, Z.; Wang, J.; Deng, S. Boosting CO2-to-CO Conversion on a Robust Single-Atom Copper Decorated Carbon Catalyst by Enhancing Intermediate Binding Strength. J. Mater. Chem. A 2021, 9, 1705–1712. [Google Scholar] [CrossRef]
- Cai, Y.; Fu, J.; Zhou, Y.; Chang, Y.C.; Min, Q.; Zhu, J.J.; Lin, Y.; Zhu, W. Insights on forming N, O-coordinated Cu single-atom catalysts for electrochemical reduction CO2 to methane. Nat. Commun. 2021, 12, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Shen, S.; Peng, X.; Bao, H.; Liu, X.; Luo, J. Selective Electroreduction of CO2 to C2 Products over Cu3N-Derived Cu Nanowires. Chem. Electro. Chem. 2019, 6, 2393–2397. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, X.; Li, X.; Nie, X.; Fan, S.; Feng, M.; Fan, Z.; Tan, M.; Che, Y.; He, G.G. Construction of atomically dispersed Cu-N4 sites via engineered coordination environment for high-efficient CO2 electroreduction. Chem. Eng. J. 2021, 407, 126842. [Google Scholar] [CrossRef]
- Fu, Y.C.J.; Zhou, Y.; Chang, Y.C.; Min, Q.; Zhu, J.; Lin, Y.; Zhu, W. Insights on forming N,O-coordinated Cu single-atom catalysts for electrochemical reduction CO2 to methane. Nat. Commun. 2021, 12, 586. [Google Scholar]
- Mi, Y.; Shen, S.; Peng, X.; Bao, H.; Liu, X. Selective Electroreduction of CO2 to C2 Products over Cu3N-Derived Cu Nanowires, Chem. Electro. Chem. 2019, 6, 2393–2397. [Google Scholar]
- Zheng, H.; Jiang, K.; Ta, N.; Hu, Y.; Zeng, J.; Liu, J.; Wang, H. Large-Scale and Highly Selective CO2 Electrocatalytic Reduction on Nickel Single-Atom Catalyst Tingting. Joule 2019, 3, 1–14. [Google Scholar] [CrossRef]
- Li, N.X.; Chen, Y.M.; Xu, Q.; Mu, W.H. Photocatalytic reduction of CO2 to CO using nickel(II)-bipyridine complexes with different substituent groups as catalysts. J. CO2 Util. 2023, 68, 102385. [Google Scholar] [CrossRef]
- Li, S.; Lu, X.; Frank, S.; Lock, N.; Roldan, A.; Hu, X.M.; Daasbjerg, K. Incorporation of nickel single atoms into carbon paper as self-standing electrocatalyst for CO2 reduction. J. Mater. Chem. A 2021, 9, 1583–1592. [Google Scholar] [CrossRef]
- Pellessier, J.; Gang, Y.; Li, Y. A Sustainable Synthesis of Nickel-Nitrogen-Carbon Catalysts for Efficient Electrochemical CO2 Reduction to CO. ES Mater. Manuf. 2021, 13, 66–75. [Google Scholar] [CrossRef]
- Zheng, T.; Jiang, K.; Ta, N.; Hu, Y.; Zeng, J.; Liu, J.; Wang, H. Large-Scale and Highly Selective CO2 Electrocatalytic Reduction on Nickel Single-Atom Catalyst. Joule. 2019, 3, 265–278. [Google Scholar] [CrossRef]
- Schneider, J.; Jia, H.; Kobiro, K.; Cabelli, D.E.; Muckermana, J.T.; Fujita, E. Nickel(ii) macrocycles: highly efficient electrocatalysts for the selective reduction of CO2 to CO. Energy Environ. Sci. 2012, 5, 9502–9510. [Google Scholar] [CrossRef]
- Su, P.; Iwase, K.; Harada, T.; Kamiya, K.; Nakanishi, S. Covalent triazine framework modified with coordinatively-unsaturated Co or Ni atoms for CO2 electrochemical reduction. Chem. Sci. 2018, 9, 3941–3947. [Google Scholar] [CrossRef]
- Yang, H.B.; Hung, S.F.; Liu, S.; Yuan, K.D.; Miao, S.; Zhang, L.P.; Huang, X.; Wang, H.Y.; Cai, W.Z.; Chen, R. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat. Energy. 2018, 3, 140–147. [Google Scholar] [CrossRef]
- Jiang, K.; Siahrostami, S.; Akey, A.J.; Li, Y. Transition-Metal Single Atoms in a Graphene Shell as Active Centers for Highly Efficient Artificial Photosynthesis. Chem. 2019, 3, 950–965. [Google Scholar] [CrossRef]
- Yuan, C.Z.; Zhan, L.Y.; Liu, S.; Chen, F.; Lin, H.; Wu, X.L.; Chen, J. Semi-sacrificial template synthesis of single-atom Ni sites supported on hollow carbon nanospheres for efficient and stable electrochemical CO2 reduction. Inorg. Chem. Front. 2020, 7, 1719–1725. [Google Scholar] [CrossRef]
- Li, X.; Bi, W.; Chen, M.; Sun, Y.; Ju, H.; Yan, W.; Zhu, J.; Wu, X.; Chu, W.; Wu, C. Exclusive Ni−N4 Sites Realize Near-Unity CO Selectivity for Electrochemical CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 14889–14892. [Google Scholar] [CrossRef]
- Hou, Y.; Liang, Y.L.; Shi, P.C.; Huang, Y.B.; Cao, R. Atomically dispersed Ni species on N-doped carbon nanotubes for electroreduction of CO2 with nearly 100% CO selectivity. Appl. Catal. B Environ. 2020, 271, 11892. [Google Scholar] [CrossRef]
- Zhu, W.; Fu, J.; Liu, J.; Chen, Y.; Li, X.; Huang, K.; Cai, Y.; He, Y.; Zhou, Y.; Su, D. Tuning single atom-nanoparticle ratios of Ni-based catalysts for synthesis gas production from CO2. Appl. Catal. B Environ. 2020, 264, 118502. [Google Scholar] [CrossRef]
- Suraj Gupta, Rohan Fernandes, Rupali Patel, Matjaž Spreitzer, Nainesh Patel, A review of cobalt-based catalysts for sustainable energy and environmental applications. Appl. Catal. 2023, 661, 119254. [CrossRef]
- Usman, M.; Humayun, M.; Garba, M.D.; Ullah, L.; Zeb, Z.; Helal, A.; Suliman, M.H.; Alfaifi, B.Y.; Iqbal, N.; Abdinejad, M.; Tahir, A.A.; Ullah, H. Electrochemical Reduction of CO2: A Review of Cobalt Based Catalysts for Carbon Dioxide Conversion to Fuels. Nanomater. 2021, 11, 2029. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Cometto, C.; Ishitani, O.; Robert, M. Electrons, Photons, Protons and Earth-Abundant Metal Complexes for Molecular Catalysis of CO2 Reduction. ACS Catal. 2017, 7, 70–88. [Google Scholar] [CrossRef]
- Call, A.; Cibian, M.; Takashi, K.Y.; Yamauchi, N.K.; Sakai, K. Highly Efficient and Selective Photocatalytic CO2 Reduction to CO in Water by a Cobalt Porphyrin Molecular Catalyst. ACS Catal. 2019, 9, 4867–4874. [Google Scholar] [CrossRef]
- Wang, M.; Torbensen, K.; Salvatore, D.; Ren, S.; Joulié, D.; Dumoulin, F.; Mendoza, D.; Lassalle-Kaiser, B.; Işci, U.; Berlinguette, C.P.; Robert, M. CO2 electrochemical catalytic reduction with a highly active cobalt phthalocyanine. Nat. Commun. 2019, 10, 3602. [Google Scholar] [CrossRef]
- Hou, P.; Song, W.; Wang, X.; Hu, Z.; Kang, P. Well-Defined Single-Atom Cobalt Catalyst for Electrocatalytic Flue Gas CO2 Reduction. Small. 2020, 16, 2001896. [Google Scholar] [CrossRef]
- Fernández, S.; Franco, F.; Casadevall, C.; Martin-Diaconescu, V.; Luis, J.M.; Lloret-Fillol, J. A Unified Electro- and Photocatalytic CO2 to CO Reduction Mechanism with Aminopyridine Cobalt Complexes. J. Am. Chem. Soc. 2020, 142, 120–133. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W.; Zhang, D.; Du, Y.; Qiao, S.; Wu, J.; Yin, Z. Surface strategies for catalytic CO2 reduction: from two-dimensional materials to nanoclusters to single atoms. Chem. Soc. Rev. 2019, 48, 5310–5349. [Google Scholar] [CrossRef]
- Pan, Y.; Lin, R.; Chen, Y.; Liu, S.; Zhu, W.; Cao, X.; Chen, W.; Wu, K.; Cheong, W.C.; Wang, Y. Design of Single-Atom Co–N5 Catalytic Site: A Robust Electrocatalyst for CO2 Reduction with Nearly 100% CO Selectivity and Remarkable Stability. J. Am. Chem. Soc. 2018, 140, 4218–4221. [Google Scholar] [CrossRef]
- Amal, A.; Wang, L.; Chen, W.; Zhang, D.; Du, Y.; Qiao, S.; Wu, J.; Yin, Z. Surface strategies for catalytic CO2 reduction: from two-dimensional materials to nanoclusters to single atoms. Chem. Soc. Rev. 2019, 48, 5310–5349. [Google Scholar]
- Song, X.; Zhang, H.; Yang, Y.; Zhang, B.; Zuo, M.; Cao, X.; Sun, J.; Lin, C.; Li, X.; Jiang, Z. Bifunctional Nitrogen and Cobalt Codoped Hollow Carbon for Electrochemical Syngas Production. Adv. Sci. 2018, 5, 1800177. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Jagvaral, Y. Electrochemical reduction of CO2 on graphene supported transition metals—Towards single atom catalysts. Phys. Chem. Chem. Phys. 2017, 19, 11436–11446. [Google Scholar] [CrossRef] [PubMed]
- Stamatelos, I.; Dinh, C.T.; Lehnert, W.; Shviro, M. Zn-Based Catalysts for Selective and Stable Electrochemical CO2 Reduction at High Current Densities. ACS Appl. Energy Mater. 2022, 6, 1–22. [Google Scholar] [CrossRef]
- Yang, F.; Song, P.; Liu, X.Z.; Mei, B.B.; Xing, W.; Jiang, Z.; Gu, L.; Xu, W.L. Highly efficient CO2 electroreduction on ZnN4-based single-atom catalyst. Angew. Chem. Int. Ed. 2018, 57, 12303–12307. [Google Scholar] [CrossRef]
- Stamatelos, I.; Scheepers, F.; Pasel, J.; Dinh, C.T.; Stolten, D. Ternary Zn-Ce-Ag catalysts for selective and stable electrochemical CO2 reduction at large-scale. Appl. Catal. B: Environ. Energy. 2024, 353, 124062. [Google Scholar] [CrossRef]
- Yang, F.; Song, P.; Liu, X.; Mei, B.; Xing, W.; Jiang, Z.; Gu, L.; Xu, W. Highly Efficient CO2 Electroreduction on ZnN4 -based Single-Atom Catalyst, Angew. Chem. Int. Ed. Eng. 2018, 57, 12303–12307. [Google Scholar] [CrossRef]
- Guo, M.; Li, X.; Huang, Y.; Li, L.; Li, J.; Lu, Y.; Zhang, L. CO2-Induced Fibrous Zn Catalyst Promotes Electrochemical Reduction of CO2 to CO. Catalysts 2021, 11, 477. [Google Scholar] [CrossRef]
- Guo, M.; Li, X.; Li, X.; Huang, Y.; Li, J.; Lu, Y.; Xu, Y.; Zhang, L. CO2-Induced Fibrous Zn Catalyst Promotes Electrochemical Reduction of CO2 to CO. Catalysts 2021, 11, 477. [Google Scholar] [CrossRef]
- Huang, P.; Cheng, M.; Zhang, H.; Zuo, M.; Xiao, C.; Xie, Y. Single Mo atom realized enhanced CO2 electro-reduction into formate on N-doped graphene. Nano Energy 2019, 61, 428–434. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Z.; Kountoupi, E.; Tsoukalou, A.; Abdala, P.M.; Florian, P. Alexey Fedorov & Christoph R. Müller, Two-dimensional molybdenum carbide 2D-Mo2C as a superior catalyst for CO2 hydrogenation. Nat. Commun. 2021, 12, 5510. [Google Scholar]
- Reddy, K.P.; Dama, S.; Mhamane, N.B.; Ghosalya, M.K.; Raja, T.; Satyanarayan, C.V.; Gopinath, C.S. Molybdenum carbide catalyst for the reduction of CO2 to CO: surface science aspects by NAPPES and catalysis studies. Dalton Trans. 2019, 48, 12199–12209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, Z.; Kountoupi, E.; Tsoukalou, A.; Abdala, P.M.; Florian, P.; Fedorov, A.; Müller, C.R. Two-dimensional molybdenum carbide 2D-Mo2C as a superior catalyst for CO2 hydrogenation. Nat. Commun. 2021, 12, 5510. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Gao, H.; Zheng, L.; Chen, Z.; Zeng, S.; Jiang, C.; Dong, H.; Liu, L.; Zhang, S.; Zhang, X. A Mn-N3 single-atom catalyst embedded in graphitic carbon nitride for efficient CO2 electroreduction. Nature Commun. 2020, 11, 4341. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Deng, W.; Justiniano, C.; Li, Y. Identification of champion transition metals centers in metal and nitrogen-codoped carbon catalysts for CO2 reduction. Appl. Catal. B Environ. 2018, 226, 463–472. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Cao, R.; Pan, Z.; He, H.; Zhou, K. Dual-atom Ag2/graphene catalyst for efficient electroreduction of CO2 to CO. Appl. Catal. B Environ. 2020, 268, 118747. [Google Scholar] [CrossRef]
- He, Q.; Lee, J.H.; Liu, D.; Liu, Y.; Lin, Z.; Xie, Z.; Hwang, S.; Kattel, S.; Song, L.; Chen, J.G. Accelerating CO2 Electroreduction to CO Over Pd Single-Atom Catalyst. Adv. Funct. Mater. 2020, 30, 2000407. [Google Scholar] [CrossRef]
- Podyacheva, O.Y.; Bulushev, D.A.; Suboch, A.N.; Svintsitskiy, D.A.; Lisitsyn, A.S.; Modin, E. Highly Stable Single-Atom Catalyst with Ionic Pd Active Sites Supported on N-Doped Carbon Nanotubes for Formic Acid Decomposition. Chem. sustain. Energy Environ. 2018, 11, 3724–3727. [Google Scholar] [CrossRef]
- Wijaya, D.T.; Lee, C.W. Pd–Mo bimetallic catalysts forelectrochemical reduction of carbon dioxideto carbon monoxide. J. Electroanalyt. Chem. 2024, 953, 118007. [Google Scholar] [CrossRef]
- Ahmed, M.; Tanya, T.; Subal, K.D.; Marc, F.; Mougel, V. A bioinspired molybdenum–copper molecular catalyst for CO2 electroreduction. Chem. Sci. 2020, 11, 5503. [Google Scholar]
- Wang, L.; Peng, H.; Lamaison, S.; Abild-Pederser, F.; Jaramillo, T.F.; Hahn, C. Bimetallic effects on Zn-Cu electrocatalysts enhance activity and selectivity for the conversion of CO2 to CO. Chem. Ctalyt. 2021, 1, 663–680. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. Bimetallic Ni-Based Catalysts for CO2 Methanation: A Review. Nanomaterials 2021, 11, 28. [Google Scholar] [CrossRef]
- Hannah, L.A.; Symes, M.D. Recent progress in CO2 reduction using bimetallic electrodes containing copper. Electrochem. Commun. 2022, 135, 107212. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).